Abstract
Aims/hypothesis
Exposure to environmental pollution has been consistently linked to diabetes incidence in humans, but the potential causative mechanisms remain unclear. Given the critical role of regulated insulin secretion in maintaining glucose homeostasis, environmental chemicals that reach the endocrine pancreas and cause beta cell injury are of particular concern. We propose that cytochrome P450 (CYP) enzymes, which are involved in metabolising xenobiotics, could serve as a useful biomarker for direct exposure of islets to pollutants. Moreover, functional CYP enzymes in islets could also impact beta cell physiology. The aim of this study was to determine whether CYP1A enzymes are activated in islets following direct or systemic exposure to environmental pollutants.
Methods
Immortalised liver (HepG2) and rodent pancreatic endocrine cell lines (MIN6, βTC-6, INS1, α-TC1, α-TC3), as well as human islets, were treated in vitro with known CYP1A inducers 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and 3-methylcholanthrene (3-MC). In addition, mice were injected with either a single high dose of TCDD or multiple low doses of TCDD in vivo, and islets were isolated 1, 7 or 14 days later.
Results
CYP1A enzymes were not activated in any of the immortalised beta or alpha cell lines tested. However, both 3-MC and TCDD potently induced CYP1A1 gene expression and modestly increased CYP1A1 enzyme activity in human islets after 48 h. The induction of CYP1A1 in human islets by TCDD was prevented by cotreatment with a cytokine mixture. After a systemic single high-dose TCDD injection, CYP1A1 enzyme activity was induced in mouse islets ~2-fold, ~40-fold and ~80-fold compared with controls after 1, 7 and 14 days, respectively, in vivo. Multiple low-dose TCDD exposure in vivo also caused significant upregulation of Cyp1a1 in mouse islets. Direct TCDD exposure to human and mouse islets in vitro resulted in suppressed glucose-induced insulin secretion. A single high-dose TCDD injection resulted in lower plasma insulin levels, as well as a pronounced increase in beta cell death.
Conclusions/interpretation
Transient exposure to TCDD results in long-term upregulation of CYP1A1 enzyme activity in islets. This provides evidence for direct exposure of islets to lipophilic pollutants in vivo and may have implications for islet physiology.
Electronic supplementary material
The online version of this article (10.1007/s00125-019-05035-0) contains peer-reviewed but unedited supplementary material, which is available to authorised users.
Keywords: Diabetes, Drug metabolism enzymes, Islets, Pollutants, Toxicology, Xenobiotics
Introduction
Persistent organic pollutants (POPs) are lipophilic and resistant to degradation, resulting in widespread environmental dispersion and bioaccumulation [1, 2]. Chronic human exposure to POPs is associated with adverse health outcomes [3–8], including increased diabetes risk [9–25] and clinical measures of poor insulin secretion [26–28]. However, the mechanisms underlying these associations remain poorly understood.
The pancreas is not traditionally considered a target organ for environmental chemicals, yet mounting evidence suggests that pollutants, particularly POPs, may impact beta cell function [26, 29–31]. For example, reduced insulin secretion was observed in isolated islets 24 h after in vivo exposure to dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin; TCDD) [30, 31]. Dioxin/dioxin-like compounds are a broad class of POPs that act as agonists for the aryl hydrocarbon receptor (AhR), which activates AhR target genes, including CYP1A1 and CYP1A2 [32, 33]. The primary role for cytochrome P450 (CYP) enzymes is xenobiotic metabolism and detoxification, but the reactive metabolites generated by CYP-mediated oxidation can be highly toxic. These metabolites are generally unstable and act locally to cause oxidative stress and DNA/protein damage [32–34]. Although CYP enzymes mainly act in the liver, non-hepatic CYP enzymes have also been described [35, 36]. Interestingly, non-hepatic tissues typically accumulate substantially lower concentrations of xenobiotics than liver, but can be disproportionately sensitive to their effects. For example, following TCDD administration in mice, TCDD concentrations were 100 times higher in liver than lung, but CYP1A1 activity was two times higher in lung than liver [37]. We hypothesised that CYP enzymes would be inducible in the endocrine pancreas and serve as a useful tool to elucidate whether environmental chemicals directly target islet cells in vivo.
Previous data supported our idea that CYP1A enzymes might be inducible in the endocrine pancreas. A modest increase in CYP1A1 protein was detected by western blot in immortalised MIN-6 beta cells following 24 h TCDD treatment [38]. ‘CYP1A-like’ proteins were reportedly induced in pancreas sections from rats following in vivo 3-methylcholanthrene (3-MC) exposure, but these data relied on a promiscuous polyclonal antibody [39]. Most notably, O-dealkylation of 7-ethoxyrosorufin (EROD), an established assay for CYP1A1 activity, was increased in pancreatic microsomes from 3-MC-injected rats compared with controls [40]. It remains unclear whether CYP1A1/1A2 are upregulated and/or functional in islets, particularly human tissues. Here, we investigated whether CYP1A genes are induced in mouse and/or human islets following direct in vitro exposure to xenobiotics, TCDD and 3-MC, or systemic exposure in vivo. We also used enzyme activity assays to determine whether islets harbour functional CYP1A enzymes capable of substrate metabolism.
Methods
Cell culture
HepG2 cells (kindly provided by T. Kieffer, University of British Columbia), an immortalised human liver cell line, were cultured in high-glucose (25 mmol/l) DMEM (DMEM-HG; #10-013-CV, Corning, Corning, NY, USA; or #D6429, Sigma-Aldrich, St Louis, MO, USA) with 10% (vol./vol.) heat-inactivated FBS (Sigma-Aldrich #F1051). INS-1 cells (kindly provided by C. Wollheim, University Medical Center, Geneva, Switzerland), an immortalised rat beta cell line, were cultured in RPMI 1640 (Corning #10-041-CV) with 10% (vol./vol.) FBS, 50 μmol/l 2-mercaptoethanol (Sigma-Aldrich), 10 mmol/l HEPES (#BP310, Thermo Fisher Scientific, Waltham, MA, USA) and 1 mmol/l sodium pyruvate (Sigma-Aldrich #S8636). MIN6 cells (kindly provided by J. Miyazaki, Osaka University Graduate School of Medicine, Osaka, Japan), an immortalised mouse beta cell line, were cultured in DMEM-HG with 10% (vol./vol.) FBS. βTC-6 cells (#CRL-11506, ATCC, Manassas, VA, USA), an immortalised mouse beta cell line, were cultured in DMEM-HG with 15% (vol./vol.) FBS. α-TC3 and α-TC1 clone nine cells (kindly provided by T. Kieffer), immortalised mouse alpha cell lines, were cultured in DMEM-HG (Sigma-Aldrich #D6429A) with 10% (vol./vol.) FBS. All cell lines were confirmed to be free of mycoplasma using the MycoAlert Mycoplasma Detection Kit (#CA11006554; Lonza, Basel, Switzerland).
Mouse islet isolation and culture
Islets were isolated from mice by pancreatic duct injection with collagenase (1000 U/ml; Sigma-Aldrich #C7657) dissolved in Hanks’ balanced salt solution (HBSS: 137 mmol/l NaCl, 5.4 mmol/l KCl, 4.2 mmol/l NaH2PO4, 4.1 mmol/l KH2PO4, 10 mmol/l HEPES, 1 mmol/l MgCl2, 5 mmol/l dextrose, pH 7.2). Pancreases were incubated at 37°C for 12 min, vigorously agitated and the collagenase reaction quenched by adding cold HBSS with 1 mmol/l CaCl2. The pancreas tissue was washed three times in HBSS+CaCl2 (centrifuging for 1 min at 1000 g in between washes) and resuspended in Ham’s F-10 (#SH30025.01, HyClone, GE Healthcare Bio-sciences, Pittsburgh, PA, USA; or Corning #10-070-CV) containing 0.5% (wt/vol.) BSA (Sigma-Aldrich #10775835001), 100 U/ml penicillin and 100 μg/ml streptomycin (Corning #30002CI). Pancreas tissue was filtered through a 70 μm cell strainer and islets were handpicked under a dissecting scope to >95% purity.
Human islet culture
Human islets for experiments in Figs. 3a, b, d, e (H216), 3c (H219), 3f–h (H210, H211, H220) and 4d (H215) were obtained from the Ike Barber Human Islet Transplant Laboratory (Vancouver, BC, Canada). Organ donor purity ranged from 60% to 90% islets. Human islets for experiments in Fig. 4a–c (R161) were obtained from the Alberta Diabetes Institute IsletCore (Edmonton, AB, Canada) and islet purity was 95%. All human islets were cultured in CMRL medium (Gibco #11530-037; Thermo Fisher Scientific) with 10% (vol/vol) FBS, 100 U/ml penicillin, 100 μg/ml streptomycin and 2 mmol/l l-glutamine (Sigma-Aldrich #59202C). Research with human islets was approved by the Research Ethics Boards at the University of British Columbia and Carleton University. Refer to the electronic supplementary material (ESM) for the Human Islets Checklist.
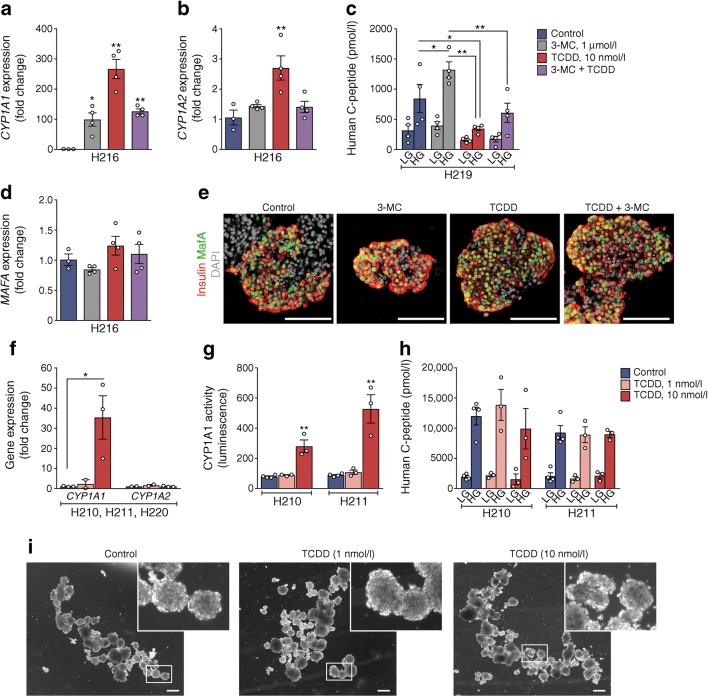
Fig. 3.
Functional CYP1A1 enzymes are induced in human islets following xenobiotic exposure in vitro. Human islets were treated with vehicle, or 3-MC (1 μmol/l) ± TCDD (1 or 10 nmol/l), for 48 h in vitro. (a, b, d) Gene expression of CYP1A1, CYP1A2 and MAFA, expressed as the fold change relative to control. *p < 0.05, **p < 0.01 vs control (one-way ANOVA with Dunnet’s post hoc test). (c) Human C-peptide secretion after 1 h in LG (2.9 mmol/l) and HG (16.7 mmol/l) buffer. *p < 0.05, **p < 0.01 (two-way repeated measures ANOVA with Tukey’s post hoc test). (e) Representative images of human islets with immunofluorescence staining for insulin (red), MafA (green) and DAPI (grey). Scale bars represent 100 μm. (f) Gene expression of CYP1A1 and CYP1A2, expressed as fold change relative to control. *p < 0.05 vs control (one-way ANOVA with Dunnet’s post hoc test). (g) CYP1A1 enzyme activity. **p < 0.01 vs control (one-way ANOVA with Dunnet’s post hoc test). (h) Human C-peptide secretion after 1 h in LG and HG buffer. (i) Representative brightfield images showing gross morphology of human islets after 48 h in vitro treatments. Scale bars represent 100 pixels. All data are presented as mean ± SEM. Individual data points represent technical replicates from a single organ donor (a, b, d: H216; c: H219; g, h: H210, H211), except for (f) where data points represent biological replicates from three organ donors (H210, H211, H220)
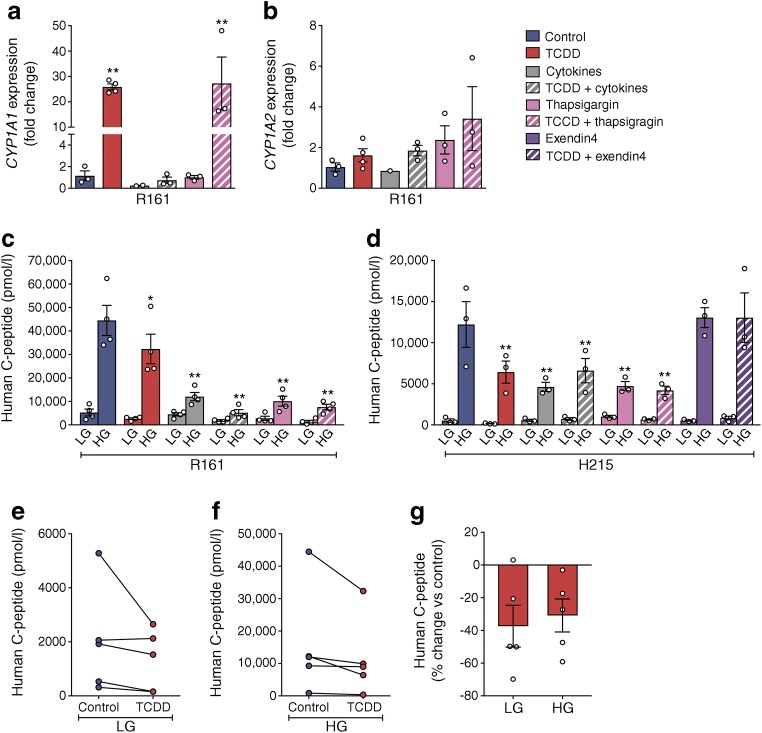
Fig. 4.
Cotreatment with cytokines, but not thapsigargin, interferes with CYP1A1 induction in human islets. Human islets were treated with media containing DMSO (control), 10 nmol/l TCDD alone, cytokines (1000 U/ml IFNγ, 50 ng/ml TNF-α, 10 ng/ml IL1β) ± 10 nmol/l TCDD, 1 μmol/l thapsigargin ± 10 nmol/l TCDD, or 10 nmol/l exendin-4 ± 10 nmol/l TCDD for 48 h in vitro. (a, b) Gene expression of CYP1A1 (a) and CYP1A2 (b), expressed as fold change relative to control. **p < 0.01 vs control (one-way ANOVA with Dunnet’s post hoc test). (c, d) Human C-peptide after a 1 h incubation in LG (2.9 mmol/l) and HG (16.7 mmol/l) buffer. *p < 0.05, **p < 0.01 vs control (two-way repeated measures ANOVA with Dunnet’s post hoc test). (e–g) Compiled human C-peptide secretion data from five different organ donors, expressed as the mean human C-peptide concentration following LG (e) and HG (f) conditions, as well as the percentage change for TCDD-exposed islets relative to their respective control islets (g). A Wilcoxon matched-pairs signed rank test was performed to compare human C-peptide secretion for each organ donor with and without TCDD exposure (LG: p = 0.125, HG: p = 0.063). All data are presented as mean ± SEM. Individual data points represent technical replicates from a single organ donor (a–c: R161; d: H215), except for (e–g) where data points represent biological replicates from five different organ donors (H210, H211, H215, H219 and R161)
In vitro cell treatments
To determine whether CYP1A1 could be induced in islets, we selected doses that are well established to maximally induce CYP1A1 in liver cells without causing toxicity. The upper doses reflect the half maximal inhibitory concentration (EC50) for each chemical. Since TCDD is 1000 times more potent than 3-MC [41–43], we used lower doses of TCDD (1–10 nmol/l) compared with 3-MC (100–1000 nmol/l). Adherent cell lines and isolated pancreatic islets (mouse and human) were treated for 48 h in vitro with 0.1 μmol/l or 1.0 μmol/l 3-MC (Sigma-Aldrich #46434-2ML-R, 100 ng/μl solution in acetonitrile), 1 nmol/l or 10 nmol/l TCDD (Sigma-Aldrich #48599, 10 μg/ml solution in toluene) or the appropriate vehicle control (acetonitrile, toluene or DMSO) in culture medium. Medium was refreshed after 24 h. Where indicated, human islets were also treated with 1 μmol/l thapsigargin (Sigma-Aldrich), a cytokine mixture (TNF-α, 50 ng/ml, #510-RT-010, R&D Systems, Minneapolis, MN, USA; IFNγ, 1000 U/ml, #213-10156-1AF, RayBiotech, Peachtree Corners, GA, USA; IL-1β, 10 ng/ml, #JM-4128-10, MBL International Corporation, Woburn, MA, USA) or 10 nmol/l exendin-4 (Sigma-Aldrich #E7144)
CYP enzyme activity assay
Enzyme activity was measured in various adherent cell lines (HepG2, MIN6, βTC-6, INS1, α-TC1, α-TC3) and isolated mouse and human islets using the P450-Glo CYP1A1 Assay (#V8752; Promega, Madison, WI, USA) and CYP1A2 Assay (#V8772, Promega). All assays were performed in 96-well white-walled plates with clear bottoms (#655098; Greiner Bio-One, Kremsmünster, Austria) using the lytic method, as described by the manufacturer. For adherent cell lines, the assay was performed on cells at 70–90% confluence. For islets, 50 mouse or human islets were handpicked into each well of the 96-well plate.
In vitro glucose-stimulated insulin secretion assays
To assess beta cell function, 25–50 mouse or human islets per condition were transferred to a pre-warmed (37°C) 24-well plate containing Krebs–Ringer bicarbonate buffer (KRBB) (115 mmol/l NaCl, 5 mmol/l KCl, 24 mmol/l NaHCO3, 2.5 mmol/l CaCl2, 1 mmol/l MgCl2, 10 mmol/l HEPES, 0.1% (wt/vol.) BSA, pH 7.4) with 2.9 mmol/l glucose (low glucose, LG) for a 40 min pre-incubation at 37°C. Islets were then transferred to 500 μl of LG KRBB for 1 h, followed by transfer to 500 μl of KRBB with 16.7 mmol/l glucose (high glucose, HG) for 1 h at 37°C. The LG KRBB and HG KRBB samples were centrifuged, and the supernatant stored at −30°C until use. To measure insulin content, islets were transferred to an acid-ethanol solution of 1.5% (vol./vol.) HCl in 70% (vol./vol.) ethanol at 4°C overnight and then neutralised with 1 mol/l Tris base (pH 7.5) before long-term storage at −30°C. Concentrations of human C-peptide (#10-1141-01; Mercodia, Uppsala, Sweden) and mouse insulin (#80-INSMS or 80-INSMSH; ALPCO, Salem, NH, USA) were measured by ELISA.
Animals
All mice received ad libitum access to a standard irradiated diet (Teklad Diet #2918; Harlan Laboratories, Madison, WI, USA) and were maintained on a 12 h light/dark cycle throughout the study. All experiments were approved by the University of British Columbia or Carleton University Animal Care Committees and carried out in accordance with the Canadian Council on Animal Care guidelines. Prior to beginning experimental protocols, animals were randomly assigned to treatment groups and matched for body weight and blood glucose levels (ensuring that these variables were not significantly different between groups).
Cohort 1
As outlined in Fig. 5a, 8-week-old male C57Bl/6 mice (Jackson Laboratory, Bar Harbour, ME, USA) received a single i.p. injection of corn oil (25 ml/kg, vehicle control; n = 4), 20 μg/kg TCDD (n = 4), 100 μg/kg TCDD (n = 4) or 200 μg/kg TCDD (n = 4). Liver was flash frozen in liquid nitrogen and islets were isolated from all mice (as described above) 24 h after injection.
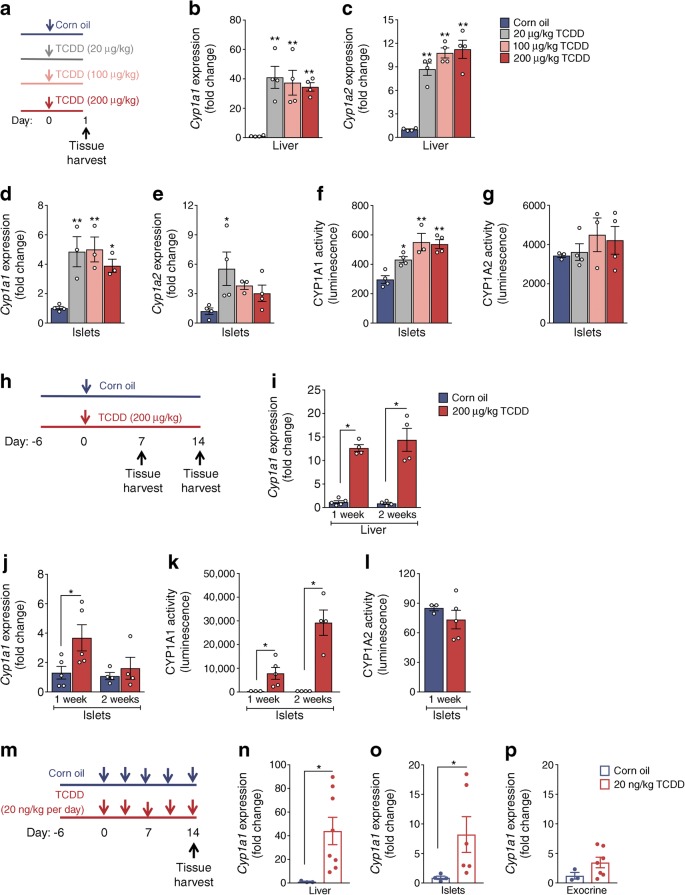
Fig. 5.
CYP1A1 gene expression and enzyme activity are induced in mouse islets in vivo. (a–g) Male C57Bl/6 mice were injected i.p. with either corn oil (vehicle) or TCDD (20 μg/kg, 100 μg/kg or 200 μg/kg) and euthanised 24 h later to harvest liver and isolate islets (schematic timeline shown in [a]). (b, d) Cyp1a1 and (c, e) Cyp1a2 gene expression expressed as fold change relative to controls in liver (b, c) and isolated islets (d, e). (f) CYP1A1 and (g) CYP1A2 enzyme activity was measured in islets at 24 h. (h–l) Male C57Bl/6 mice were injected i.p. with either corn oil or TCDD (200 μg/kg) and euthanised either 7 or 14 days later (schematic timeline shown in [h]). (i, j) Cyp1a1 gene expression was measured in liver (i) and isolated islets (j) at 1 and 2 weeks post injection (expressed as fold change relative to control). (k) CYP1A1 and (l) CYP1A2 enzyme activity in isolated islets at 1 and 2 weeks post injection. (m–p) Male C57Bl/6 mice were injected i.p. with either corn oil or TCDD (20 ng/kg per day) twice per week and euthanised 14 days later (schematic timeline shown in [m]). (n–p) Cyp1a1 gene expression was measured in liver (n), isolated islets (o) and pancreatic exocrine tissue (p) on day 14 (expressed as fold change relative to control). All data are presented as mean ± SEM. Individual data points represent biological replicates from different mice. (b–g) *p < 0.05, **p < 0.01 vs control, two-way repeated measures ANOVA with Dunnet’s post hoc test. (i–l, n–p) *p < 0.05, **p < 0.01 vs control, unpaired two-tailed t test
Cohort 2
As outlined in Figs. 5h, 6a, 8-week-old male C57Bl/6 mice (Jackson Laboratory) received a single i.p. injection of corn oil (25 ml/kg, vehicle control; n = 24) or 200 μg/kg TCDD (n = 22). On days 7 (n = 9–10 per group) and 14 (n = 9–11 per group) following injection, islets were isolated from a subset of mice for ex vivo glucose-stimulated insulin secretion assays (25 islets per mouse), RNA isolation (~150–200 islets per mouse) and CYP1A enzyme activity assays (50 islets per mouse per assay). Liver was flash frozen in liquid nitrogen on days 7 (n = 5 per group) and 14 (n = 9–11 per group). Whole pancreas and liver were harvested from a different subset of mice on day 7 (n = 3 per group) and stored in 4% (vol./vol.) paraformaldehyde (PFA) for 24 h, followed by long-term storage in 70% (vol./vol.) ethanol.
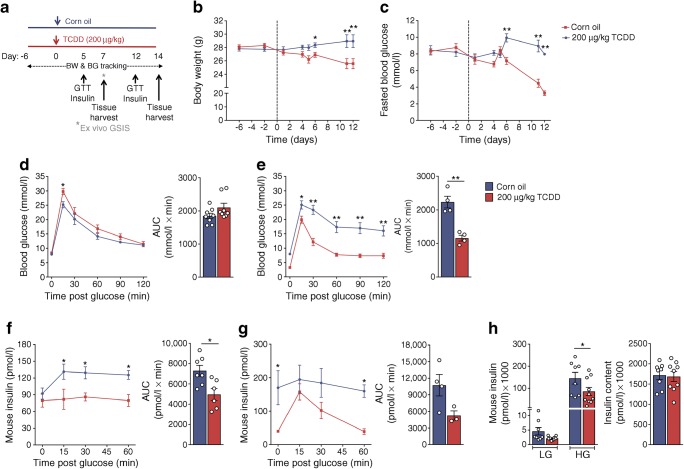
Fig. 6.
A single high-dose TCDD injection leads to suppressed plasma insulin levels in vivo. Male C57Bl/6 mice were injected i.p. with either corn oil (vehicle) or TCDD (200 μg/kg) and euthanised either 7 or 14 days later (schematic timeline shown in [a]). BG, blood glucose; BW, body weight. (b) Body weight and (c) blood glucose were measured after a 4 h morning fast throughout the study. (d, e) Blood glucose and (f, g) plasma insulin were measured during a GTT on days 5 (d, f) and 12 (e, g). (h) On day 7 islets were isolated and glucose-stimulated insulin secretion (GSIS) was measured ex vivo after 1 h in LG (2.9 mmol/l) and 1 h in HG (16.7 mmol/l), followed by total insulin content measurement. All data are presented as mean ± SEM. Individual data points represent biological replicates from different mice. *p < 0.05, **p < 0.01 vs control; (b, c) two-way ANOVA with Šídák multiple comparisons test, (d–h) two-way repeated measures ANOVA with Šídák multiple comparisons test, (d–g) unpaired two-tailed t test for AUC
Cohort 3
As outlined in Fig. 5m and ESM Fig. 2a, 8-week-old male C57Bl/6 mice received multiple i.p. injections of corn oil (25 ml/kg, vehicle control) or a low-dose of TCDD (20 ng/kg per day) twice per week. This dose was previously shown to induce CYP1A1 in liver and lung [44, 45]. Furthermore, chronic administration of 46 ng/kg TCDD per day resulted in circulating TCDD concentrations of ~8.8 pg/g in rats [44], which is within the range of background dioxin levels reported in the USA (≤10 pg/g) and corresponds to individuals in the upper quartile (≥5.2 pg/g) with increased diabetes prevalence [16]. In our study, the first group of mice (Fig. 5m–p; Jackson Laboratory) was treated at the University of Ottawa Roger Guindon Hall vivarium (n = 5 control, n = 8 TCDD); liver was flash frozen and islets were isolated after 2 weeks (i.e. five injections). A second group of mice (ESM Fig. 2) was generated by in-house breeding and then treated in the Modified Barrier Facility at the University of British Columbia (n = 4 per group) for metabolic assessment.
Cyp1a1/1a2 double knockout mice
Mice with a global deletion of both Cyp1a1 and Cyp1a2, originally generated and characterised by D. Nebert [46], were generously provided by F. Gonzalez (University of Cincinnati). Male mice were used for ex vivo experiments with isolated islets (Fig. 7) and as a negative control for CYP1A1 immunofluorescence staining of pancreas and liver tissues (ESM Fig. 1).
Fig. 7.
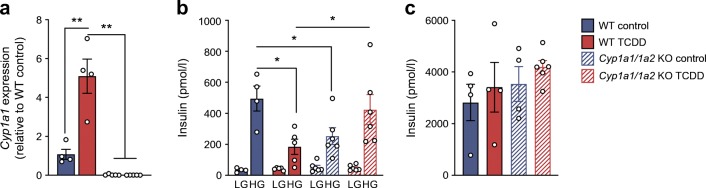
TCDD suppressed glucose-stimulated insulin secretion in male WT islets but not Cyp1a1/1a2 KO islets. Islets were isolated from male WT mice and mice with a global KO of Cyp1a1 and Cyp1a2 (Cyp1a1/1a2 KO) and treated ex vivo for 48 h with 10 nmol/l TCDD. (a) Cyp1a1 gene expression was expressed relative to WT control levels. (b) Insulin secretion was measured ex vivo after 1 h in LG (2.9 mmol/l) and 1 h in HG (16.7 mmol/l), followed by (c) total insulin content in islets. All data are presented as mean ± SEM. Individual data points represent biological replicates from different mice. *p < 0.05, **p < 0.01; (a) one-way ANOVA with Tukey’s multiple comparisons test, (b) two-way repeated measures ANOVA with Tukey’s multiple comparisons test
Metabolic assessments
All metabolic analyses were performed in conscious, restrained mice and blood samples were collected via saphenous vein using heparinised microhematocrit tubes at the indicated time points. Blood glucose levels were measured using a handheld glucometer (Lifescan, Burnaby, BC, Canada).
Body weight and blood glucose levels were assessed weekly or bi-weekly following a 4 h morning fast. For all other metabolic tests, time 0 indicates the blood sample collected after fasting and prior to administration of glucose or insulin. For GTTs, mice received an i.p. bolus of glucose (2 g/kg; Vetoquinol, Lavaltrie, QC, Canada) following a 6 h morning fast. During the GTT, blood was collected at the indicated time points for measuring plasma insulin levels by ELISA (ALPCO mouse ultrasensitive insulin ELISA, #80-INSMSU-E01). For ITTs, mice received an i.p. bolus of insulin (0.7 U/kg, Novolin ge Toronto #02024233; Novo Nordisk Canada, Mississauga, ON, Canada) after a 4 h morning fast. For all tests, mice from different treatment groups were randomly distributed throughout the experiment to ensure that timing of blood collection was not a factor in our analysis.
Quantitative real-time PCR
RNA was isolated from cultured cell lines using the RNeasy Mini Kit (#74104; Qiagen, Hilden, Germany), human or mouse islets using the RNeasy Micro Kit (#74004; Qiagen) and liver using TRIzol reagent (#15596018; Invitrogen, Carlsbad, CA, USA) or the Qiagen RNeasy Mini Kit, according to the manufacturer instructions. DNase treatment was performed prior to cDNA synthesis with the iScript gDNA Clear cDNA Synthesis Kit (#1725035; Bio-Rad, Mississauga, ON, Canada). Quantitative real-time PCR (qPCR) was performed using the SsoFast EvaGreen Supermix (#1725200; Bio-Rad) or SsoAdvanced Universal SYBR Green Supermix (#1725271, Bio-Rad) and run on a CFX96 or CFX384 (Bio-Rad). Hprt/HPRT or Ppia/PPIA were used as the reference genes. Data were analysed using the ΔΔCt method. Primer sequences are listed in ESM Table 1.
Immunofluorescent staining and image quantification
Human islets were washed with PBS, resuspended in 4% (wt/vol.) PFA and stored overnight at 4°C. The following day, islets were washed twice with PBS, resuspended in 200 μl pre-warmed 2% (wt/vol.) agarose and cooled briefly at −20°C. The agarose pellet was resuspended in 4% (wt/vol.) PFA for 1 h at room temperature and then transferred to 70% (vol/vol.) ethanol for long-term storage at 4°C until paraffin embedding. Whole pancreas tissue was fixed in 4% (vol./vol.) PFA for 24 h and stored in 70% (vol./vol.) ethanol prior to paraffin embedding.
Paraffin sections (5 μm thickness) were prepared by Wax-it Histology Services (Vancouver, BC, Canada) or the University of Ottawa Heart Institute Histology Core Facility (Ottawa, ON, Canada). Briefly, slides were deparaffinised with sequential 5 min incubations in xylene (×3), 100% (vol./vol.) ethanol (×2), 95% (vol./vol.) ethanol (×1) and 70% (vol./vol.) ethanol (×1), and then transferred to PBS for 10 min on a shaker. Heat-induced epitope retrieval (HIER) was performed in citrate buffer (10 mmol/l sodium citrate, 0.05% (vol./vol.) Tween 20, pH 6.0) for 10 min at 95°C using the EZ Retriever microwave system (#MW014-MO; BioGenex, Fremont, CA, USA), unless indicated otherwise below. Slides were transferred to deionised water for a 5 min rinse and then to PBS for 5 min. Tissue sections were circumscribed with an ImmEdge Pen (#H-4000; Vector Laboratories, Burlingame, CA, USA) and then incubated at room temperature for a minimum of 30 min with Dako serum-free protein block (#X090930-2; Agilent, Santa Clara, CA, USA). Next, primary antibodies were diluted as indicated below for each antibody and added to each section; slides were stored in a humid chamber overnight at 4°C. The following day, slides were washed 3×10 min in PBS on a shaker and incubated in secondary antibodies for 1 h in a humid chamber at room temperature, protected from light. Finally, slides were washed 3×10 min in PBS on a shaker, and then coverslips were mounted with VECTASHIELD HardSet Mounting Medium with DAPI (#H-1500, Vector Laboratories) for counterstaining.
The following primary antibodies were used in this study: rabbit anti-MAFA (MAF bZIP transcription factor A) (15 min HIER, 1:1000, shared by T. Kieffer), rabbit anti-somatostatin (1:500, Sigma-Aldrich #HPA019472), rabbit anti-insulin (C27C9, 1:200, Cell Signaling Technology, Danvers, MA, USA; #3014), mouse anti-insulin (L6B10, 1:250, Cell Signaling Technology #8138BF) and mouse anti-glucagon (1:1000, Sigma-Aldrich #G2654). Numerous primary antibodies for CYP1A1 were tested, and these are outlined in ESM Table 2. The following secondary antibodies were used: goat anti-rabbit IgG (H+L) secondary antibody, Alexa Fluor 594 (1:1000, Invitrogen #A11037); and goat anti-mouse IgG (H+L) secondary antibody, Alexa Fluor 488 (1:1000, Invitrogen #A11029). All antibodies were diluted using Dako Background Reducing Antibody Diluent solution (#S302283-2, Agilent).
To measure apoptosis, pancreas and liver sections were stained with the Molecular Probes Click-iT Plus TUNEL Assay using Alexa Fluor 488 dye (Invitrogen #C10617), according to the manufacturers instructions. Pancreas sections were counterstained with rabbit anti-insulin (C27C9, 1:200, Cell Signaling #3014) and goat anti-rabbit IgG (H+L) secondary antibody, Alexa Fluor 594 (1:1000, Invitrogen #A11037). No antigen retrieval was used for counterstaining.
For islet morphology quantification, a minimum of eight islets were imaged with an EVOS FL Cell Imaging System (Invitrogen); the islet area, insulin-immuonoreactive area and glucagon-immunoreactive area were measured for each islet using ImageJ software (https://imagej.nih.gov/ij/index.html). Islet cell apoptosis was quantified as the percentage of insulin+ and insulin− islet cells that colocalised with TUNEL+ nuclei. Liver apoptosis was quantified in at least five images per mouse and expressed as the percentage of TUNEL+ cells in the field of view. Images were captured and quantified by a researcher blinded to the treatment groups.
H&E staining of pancreas and liver tissues was performed using standard protocols by Wax-it Histology Services. Colour images were taken with an Axio Observer 7 microscope (ZEISS, Oberkochen, Germany).
Statistical analysis
All statistics were performed using GraphPad Prism software (GraphPad Software, La Jolla, CA, USA). Specific statistical tests are indicated in the figure legends. For all analyses, p < 0.05 was considered statistically significant. Data are presented as mean ± SEM, unless indicated otherwise.
Results
TCDD levels in pancreas decline slower than other tissues in vivo
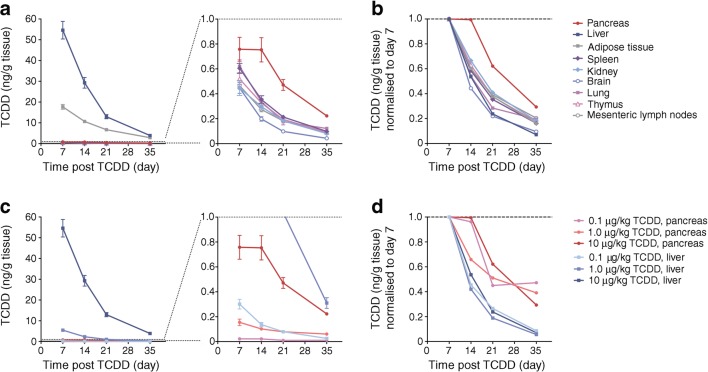
To determine how much TCDD reaches the pancreas, we used data from a biodistribution study that measured TCDD tissue concentrations after TCDD administration in mice [37]. After 7 days, the TCDD concentration in pancreas was 72 times lower than liver and 23 times lower than adipose, but higher than other non-classical target tissues (Fig. 1a). However, TCDD levels declined slower in the pancreas than all other tissues (Fig. 1b, d). In liver, TCDD levels were 72 times higher than pancreas 7 days after 10 μg/kg TCDD administration, but only 17 times higher after 35 days (Fig. 1c), and this gap diminished further at lower doses. On day 35, TCDD levels were only five times higher in liver than pancreas after a 1.0 μg/kg dose and 2.4 times higher after a 0.1 μg/kg dose (Fig. 1c). Between days 7 and 35 the concentration of TCDD declined by 91–94% in liver, compared with only 50–70% in pancreas (Fig. 1d). These data indicate that TCDD may be more stable in the pancreas than classical target tissues.
Fig. 1.
TCDD levels are more stable in the pancreas than in other tissues. Data from a published biodistribution study [37] were re-analysed to compare TCDD levels in the pancreas with other mouse tissues at 7, 14, 21 and 35 days following oral administration of TCDD (0.1, 1.0 or 10 μg/kg) to female B6C3F1 mice (n = 5 mice per group). (a, b) Concentration of TCDD (ng/g tissue) in various tissues after administration of 10 μg/kg TCDD. (c, d) Concentration of TCDD (ng/g tissue) in liver and pancreas after oral administration of 0.1, 1.0 or 10 μg/kg TCDD. Data are presented as mean ± SD (a, c) and mean TCDD concentration normalised to day 7 (b, d)
CYP1A enzymes are not induced in immortalised beta or alpha cell lines
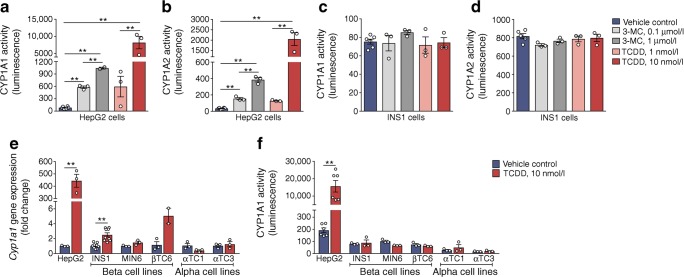
To test our hypothesis that CYP1A enzymes are inducible in pancreatic endocrine cells, we first treated INS1 cells and HepG2 liver cells with CYP1A inducers TCDD and 3-MC. Both chemicals profoundly increased CYP1A1 and CYP1A2 enzyme activity in HepG2 cells (Fig. 2a, b), but not in INS1 cells (Fig. 2c, d). A modest but statistically significant ~2.4-fold increase in Cyp1a1 gene expression was detected in TCDD-exposed INS1 cells, but this was unremarkable compared with the ~440-fold increase in HepG2 cells (Fig. 2e). Moreover, changes in gene expression did not result in measureable increases in enzyme activity in INS1 cells or any other pancreatic endocrine cell lines tested (Fig. 2f).
Fig. 2.
Xenobiotics do not induce CYP1A enzymes in immortalised pancreatic endocrine cell lines. Liver cells (HepG2), beta cell lines (INS1, MIN6, βTC6) and alpha cell lines (αTC1 and αTC3) were treated for 48 h with media containing vehicle (control), 3-MC (0.1 or 1.0 μmol/l) or TCDD (1.0 or 10 nmol/l). (a–d) Following treatment, CYP1A1 and CYP1A2 enzyme activity was measured in HepG2 (a, b) and INS1 cells (c, d). **p < 0.01, one-way ANOVA with Tukey’s post hoc test. (e) Cyp1a1 gene expression and (f) CYP1A1 enzyme activity were measured after exposure to vehicle (DMSO) or 10 nmol/l TCDD in all immortalised cell lines. **p < 0.01, unpaired two-tailed t test. All data are presented as mean ± SEM and individual data points represent technical replicates of cells treated independently in different wells
Functional CYP1A1 enzymes are strongly induced in human islets
Since immortalised cell lines are not always an appropriate model, we next tested our hypothesis in primary human islets. Remarkably, both 3-MC and TCDD potently induced CYP1A1 expression in human islets (~100-fold and ~270-fold, respectively; Fig. 3a), while CYP1A2 was only modestly induced ~2.7-fold by TCDD (Fig. 3b). Interestingly, glucose-induced C-peptide secretion was increased by 3-MC, but inhibited by TCDD (Fig. 3c). Neither gene nor protein expression of MafA, a critical beta cell transcription factor, was affected by chemical exposure (Fig. 3d, e).
In three additional organ donors, CYP1A1, but not CYP1A2, was significantly upregulated by 10 nmol/l TCDD (Fig. 3f). Importantly, this was associated with a significant increase in CYP1A1 enzyme activity (Fig. 3g), demonstrating that human islets harbour functional xenobiotic metabolism enzymes. In these donors, C-peptide secretion was not impacted by TCDD exposure (Fig. 3h). Islets appeared to be generally healthy, confirming that these doses of TCDD were not cytotoxic (Fig. 3i).
To determine whether other stressors induce CYP1A expression or interact with TCDD, human islets were cotreated with cytokines (to mimic inflammatory stress) or thapsigargin (to mimic endoplasmic reticulum stress). TCDD induced CYP1A1 ~26-fold with or without thapsigargin (Fig. 4a). However, cotreatment with cytokines completely prevented CYP1A1 induction by TCDD (Fig. 4a). CYP1A2 gene expression was unchanged by any combination of stressors (Fig. 4b). TCDD significantly suppressed glucose-induced C-peptide secretion in two organ donors (Fig. 4c, d), and this effect was prevented by cotreatment with exendin-4 (Fig. 4d). Exposure to cytokines or thapsigargin also suppressed C-peptide secretion, irrespective of cotreatment with TCDD (Fig. 4c, d).
Given the variable insulin secretory responses from different organ donors (Figs. 3c, h, 4c, d), we compiled these results in Fig. 4e–g. Despite the biological variability in C-peptide production among donors, a consistent pattern of decreased C-peptide secretion was observed for each organ donor following TCDD treatment, particularly under HG conditions (p = 0.063; Fig. 4f). The change in glucose-induced C-peptide secretion by TCDD-treated (vs control) human islets ranged from −3% to −60% among the five donors (Fig. 4g).
TCDD causes long-term CYP1A1 induction in mouse islets in vivo
Our next question was whether functional CYP1A enzymes could be induced in islets following systemic TCDD exposure in vivo rather than direct exposure ex vivo. Mice were injected with a single high dose of TCDD (20, 100 or 200 μg/kg) or corn oil, and tissues harvested 24 h later (Fig. 5a). As expected, Cyp1a1 and Cyp1a2 were induced ~40-fold and ~10-fold, respectively, in the liver following TCDD exposure (Fig. 5b, c). In islets, Cyp1a1 was induced ~5-fold by TCDD (all doses; Fig. 5d). Cyp1a2 was only induced by 20 μg/kg TCDD (Fig. 5e). Most importantly, a dose-dependent increase in CYP1A1 enzyme activity (but not CYP1A2) was detected in islets 24 h post TCDD (Fig. 5f, g).
To assess the longevity of CYP1A1 activation, we followed a second cohort of mice for 2 weeks after a high-dose TCDD injection (200 μg/kg; Fig. 5h). Cyp1a1 was induced ~12-fold in TCDD-exposed liver at 1 and 2 weeks post injection (Fig. 5i), compared with the ~35-fold increase after 24 h (Fig. 5b). In islets, Cyp1a1 was elevated ~4-fold at 1 week post TCDD, but unchanged at 2 weeks (Fig. 5j). However, while Cyp1a1 gene levels were declining in islets, CYP1A1 enzyme activity increased dramatically (Fig. 5k). Whereas only a 1.9-fold increase in CYP1A1 activity was detected in TCDD-exposed islets at 24 h (Fig. 5f), CYP1A1 activity increased ~40-fold and ~80-fold in islets at 1 and 2 weeks, respectively, after the single TCDD injection (Fig. 5k). Islet CYP1A2 activity was unchanged (Fig. 5l).
We performed immunofluorescence staining on pancreas sections from TCDD-injected mice to localise CYP1A1 expression within islets, but unfortunately the CYP1A1 antibodies we tested were unreliable (ESM Table 1). Immunofluorescence was either undetectable in TCDD-exposed liver and pancreas or the same pattern of immunoreactivity was observed in both wild-type (WT) and Cyp1a1/1a2 knockout (KO) mice, indicating non-specific immunoreactivity (representative examples shown in ESM Fig. 1). Therefore, we can conclude that CYP1A1 is upregulated in whole islets, but cannot comment on whether it is induced in beta cells specifically.
Finally, we investigated whether a more physiologically relevant TCDD dosing protocol would induce CYP1A1 enzymes in islets. Mice were injected two times per week with 20 ng/kg TCDD (Fig. 5m), which is 10,000 times lower than the single high-dose model (Fig. 5a–l). After 2 weeks Cyp1a1 levels were upregulated ~40-fold in liver (Fig. 5n) and ~8-fold in islets (Fig. 5o). This was comparable to the degree of Cyp1a1 induction 24 h after a single high-dose TCDD injection (Fig. 5b, d). Cyp1a1 levels did not change in TCDD-exposed pancreatic exocrine tissue (Fig. 5p).
TCDD exposure suppressed glucose-stimulated insulin secretion
Activation of CYP1A1 enzymes in islets strongly suggests that islets are directly exposed to TCDD in vivo. Therefore, we investigated the impact of TCDD on beta cell function and survival rate. We first characterised the mice exposed to a single high dose of TCDD (Fig. 6a) in which islet CYP1A1 enzyme activity was dramatically upregulated at 1 and 2 weeks (Fig. 5k). These mice can only be maintained for ~2 weeks, as their health begins to decline thereafter [47]. Beginning at 6 days post TCDD, we observed modest but significant body weight loss (~5%; Fig. 6b) and a drastic decline in fasting blood glucose levels (Fig. 6c). However, on day 5 TCDD-exposed mice had slightly higher blood glucose levels 15 min after a glucose challenge with no overall change in glucose tolerance (Fig. 6d). By day 12, TCDD-exposed mice were profoundly hypoglycaemic during the GTT (Fig. 6e). Plasma insulin levels were signficantly lower in TCDD-exposed mice during the GTT on both days (Fig. 6f, g).
In contrast to the single high-dose TCDD, glucose homeostasis was largely unaffected by multiple low-dose TCDD exposures for 25 days (ESM Fig. 2). Fasting glucose levels were modestly decreased in TCDD-exposed mice (ESM Fig. 2c), but there was no difference in glucose tolerance (ESM Fig. 2e), body weight (ESM Fig. 2b), insulin sensitivity (ESM Fig. 2d) or plasma insulin levels during the GTT (ESM Fig. 2f).
To better understand whether the low plasma insulin levels following high-dose TCDD (Fig. 6f, g) were caused by impaired beta cell function vs adaptation to other effects of TCDD (e.g. hepatic toxicity, increased insulin clearance), islets were isolated on day 7. Interestingly, glucose-stimulated insulin secretion was suppressed in islets from TCDD-injected mice compared with controls ex vivo (Fig. 6h). Next, islets were isolated from Cyp1a1/Cyp1a2 KO or WT mice and treated with TCDD ex vivo to determine whether induction of CYP1A1 in islets might be involved in suppressing insulin secretion. As expected, Cyp1a1 was induced by TCDD in WT islets and non-detectable in KO islets, irrespective of treatment (Fig. 7a). Consistent with our findings in vivo (Fig. 6) and in human islets (Figs. 3c, 4c–g), TCDD suppressed glucose-stimulated insulin secretion in WT islets (Fig. 7b). In contrast, TCDD did not suppress insulin secretion in Cyp1a1/1a2 KO islets, although control KO islets had unexpectedly lower insulin secretion than WT islets (Fig. 7b), suggesting a role for basal CYP1A1/CYP1A2 in regulating beta cell function. Total insulin content was not affected by genotype or TCDD (Fig. 7c).
TCDD causes beta cell death and suppression of anti-apoptotic genes in islets
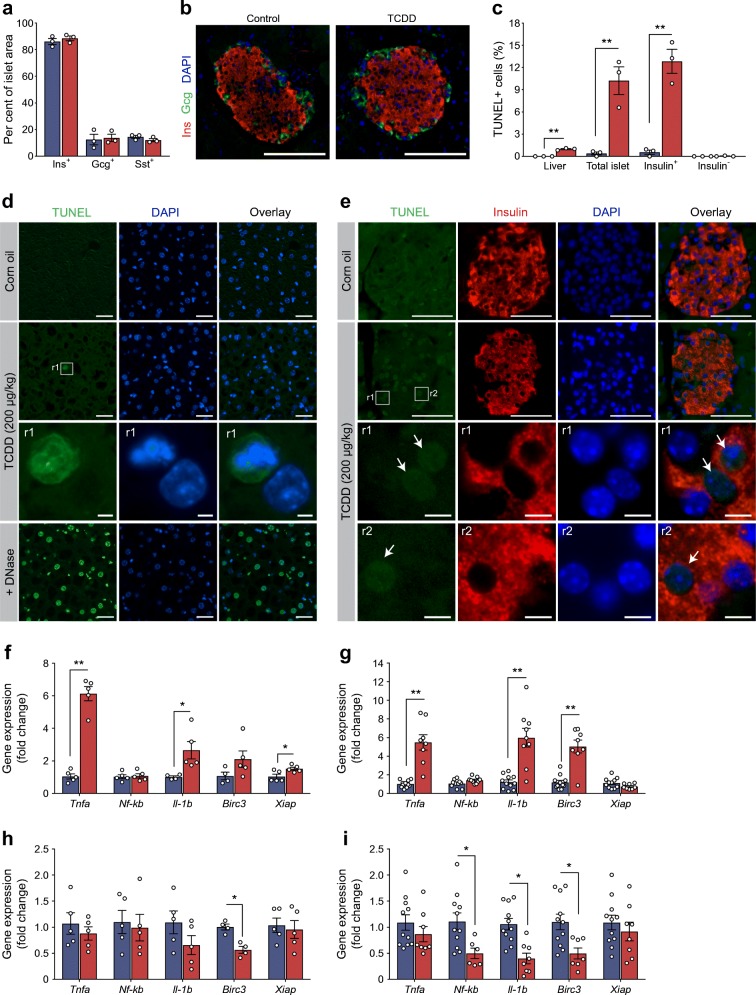
Reduced plasma insulin can also be caused by beta cell loss, so we next assessed pancreas histology. Surprisingly, pancreas morphology appeared relatively normal by H&E staining, unlike the liver, which showed obvious signs of inflammation and cell damage (ESM Fig. 3). Likewise, there was no change in endocrine cell composition within islets (percentage insulin+, glucagon+ and somatostatin+ area) at 7 days post TCDD (Fig. 8a, b). There was, however, a remarkably high percentage of TUNEL+ islet cells, nearly all of which were insulin+ rather than insulin− (Fig. 8c, e). On average, ~13% of insulin+ cells were TUNEL+ (Fig. 8c, e), compared with only ~1% TUNEL+ liver cells in the same mice (Fig. 8c, d). At both 7 and 14 days post TCDD, the liver had significant induction of proinflammatory cytokines (Tnfa, Il-1b), as well as inhibitors of apoptosis Birc3 and Xiap (Fig. 8f, g). In stark constrast, TCDD significantly suppressed Birc3 expression in islets on days 7 and 14 (Fig. 8h, i), as well as Il-1b and Nf-kb on day 14 (Fig. 8i).
Fig. 8.
TCDD causes beta cell death in vivo. Male C57Bl/6 mice were injected i.p. with either corn oil (vehicle) or TCDD (200 μg/kg) and euthanised either 7 or 14 days later. (a) The percentage of total islet area that was immunoreactive for insulin (Ins+), glucagon (Gcg+) and somatostatin (Sst+) was quantified in pancreas sections collected at 7 days post injection. (b) Representative images of islets with insulin (red) and glucagon (green) immunoreactivity are shown. Scale bars represent 100 μm. (c) The percentages of liver cells and islet cells that coexpressed TUNEL were quantified in tissue sections at 1 week post injection. Within islets, TUNEL+ cells were further delineated into insulin+ and insulin− cells. (d, e) Representative images of liver (d) and pancreas (e) showing TUNEL (green), insulin (red) and DAPI (blue) as individual channels and overlay images. Inset regions within the white boxes (labelled r1 and r2) are shown magnified below. Scale bars represent 50 μm, except for inset regions, which are 5 μm. Images from liver sections treated with DNase as a postive control are also provided (d). Arrows in (e) indicate TUNEL+ cells. (f–i) Expression of genes involved in inflammation and apoptosis was measured in liver (f, g) and isolated islets (h, i) on day 7 (f, h) and day 14 (g, i). Gene levels are expressed as fold change relative to control. All data are presented as mean ± SEM and individual data points represent biological replicates from different mice. *p < 0.05, **p < 0.01 vs control, unpaired two-tailed t test
Discussion
Our study demonstrates that xenobiotic metabolising CYP enzymes are activated within pancreatic islets following pollutant exposure. Specifically, CYP1A1 gene expression and enzyme activity were induced in human and mouse islets following direct TCDD exposure in vitro, and in mouse islets following systemic TCDD exposure in vivo. The enzyme activity assay measures CYP1A1-mediated metabolism of a proluciferin substrate into d-luciferin, indicating that the upregulated CYP1A1 gene is indeed translated into functional enzymes capable of producing metabolites. To our knowledge, this is the first evidence of functional CYP enzymes in the endocrine pancreas. Remarkably, the activity level of CYP1A1 enzymes in islets increased for at least 2 weeks after a single high-dose TCDD injection, even though Cyp1a1 gene expression in islets had returned to baseline. Moreover, this pathway was activated in islets by both a supraphysiological dose of TCDD in vivo and a biologically relevant, multiple low-dose exposure protocol.
These findings have several important implications. First and foremost, local activation of CYP1A1 in islets provides strong evidence that environmental pollutants reach pancreatic endocrine cells in vivo at sufficient levels to directly alter cell signalling. Given the highly specialised nature of endocrine cells, the signalling pathways activated by dioxin in islets will likely differ from classical target tissues. This was apparent in our analysis of inflammatory signalling pathways in liver vs islets from TCDD-exposed mice. In liver, TCDD profoundly upregulated proinflammatory cytokines, along with anti-apoptotic genes Birc3 and Xiap, likely as a protective response. However, TCDD significantly downregulated Birc3, an inhibitor of beta cell apoptosis [48], at 1 and 2 weeks in islets and did not induce cytokines. Birc3 activates Nf-kb [48], so the subsequent decrease in Nf-kb and ll-1b at 2 weeks is consistent with TCDD suppressing Birc3 in islets. Nf-κb is also reportedly anti-apoptotic in beta cells [49], whereas Il-1b can be either pro-apoptotic or protective depending on timing and concentration [50–52]. The absence of an inflammatory and anti-apoptotic response in islets was associated with a dramatic increase in beta cell apoptosis, compared with only a modest increase in liver cell death in TCDD-exposed mice. Although a detailed mechanism remains to be elucidated, our data show that while TCDD induces CYP1A1 in liver and islets, the consequences of chemical exposure are profoundly different at both molecular and cellular levels.
Our study also shows that a single transient chemical exposure results in long-term activation of CYP1A1 enzymes within islets. The relatively slow decline of TCDD levels in pancreas [37], combined with the long-term induction of CYP1A1 activity in islets, suggests that pancreatic cells could be a ‘sink’ for long-term storage of lipophilic chemicals. Furthermore, the induction of Cyp1a1 in islets, but not exocrine tissue, suggests that whole pancreas measurements of TCDD may underestimate islet exposure. We speculate that the dense intra-islet vascular network, designed to ensure rapid delivery of oxygen and nutrients to endocrine cells, also promotes delivery of xenobiotics to islets. Furthermore, the degree of CYP1A1 activation in islets was surprisingly high (~5–8-fold lower than liver) considering the relatively small proportion of TCDD that reaches the pancreas. This supports both our hypothesis that islets are exposed to disproportionately high levels of TCDD and conclusions by Diliberto and colleagues [37] that some tissues require lower levels of TCDD to ellicit a response. Whether islets are exposed to more TCDD than predicted by biodistribution studies, or are highly sensitive to TCDD, our data conclusively show that CYP1A1 enzymes are activated locally following environmental chemical exposure.
Neither thapsigargin nor cytokines activated AhR signalling in human islets, but TCDD was surprisingly unable to induce CYP1A1 in the presence of proinflammatory cytokines. This could have important implications for how individuals with islet inflammation, including those with type 2 diabetes [53–56], respond to environmental chemicals that activate AhR signalling. For instance, if activation of CYP1A1 in islets is an important defence pathway, then islets with increased inflammation may be more sensitive to injury caused by environmental chemicals. On the other hand, if upregulation of CYP1A1 in islets is detrimental (for example, due to the formation of reactive metabolites), then islets in a proinflammatory state might be somewhat protected from the toxic effects of TCDD or other AhR ligands. Indeed, our data suggest that CYP1A enzymes may play a non-conventional role in islets. Direct TCDD exposure suppressed glucose-stimulated insulin secretion in WT mouse islets, but not in Cyp1a1/1a2 KO islets, suggesting that CYP1A1 induction might inhibit insulin secretion. However, Cyp1a1/1a2 KO control islets also showed an unexpected decrease in insulin secretion compared with WT controls, pointing to a possible role for basal CYP1A1 in regulating beta cell function. More research is required to understand the role of CYP1A1 enzymes in islet physiology and possible interactions with proinflammatory cytokines.
The single high dose of TCDD used in these studies (200 μg/kg) was selected to study CYP1A1 activation in islets and was not ideal for assessing the impact of TCDD on beta cell function. After 1 week, TCDD-exposed mice displayed weight loss and hypoglycaemia, likely resulting from hepatotoxicity. This is consistent with previous reports [47, 57, 58] and confounds our interpretation of potential direct effects of TCDD on islets. However, on day 5, when blood glucose levels were still within a healthy range, TCDD-exposed mice displayed reduced plasma insulin levels during a GTT. Suppressed insulin secretion was also detected in isolated islets ex vivo on day 7 post TCDD, which is consistent with previous findings in isolated islets 24 h after a high dose of TCDD in vivo [30, 31]. Furthermore, when human islets were exposed directly to TCDD, three of five donors had significantly lower C-peptide secretion and, on average, all donor islets secreted less C-peptide if they had been exposed to TCDD vs DMSO. Mouse islets also showed a similar response to direct TCDD treatment ex vivo. Together, these data suggest that TCDD directly affects islet function, possibly due to direct impairment of insulin secretion by beta cells or via indirect paracrine mechanisms (i.e. secretion of counterregulatory hormones). The impact of TCDD on islet physiology should be further assessed in longer-term studies at more physiologically relevant concentrations.
Exposure to dioxin-like pollutants is consistently associated with increased type 2 diabetes incidence and beta cell dysfunction in humans [24, 25, 27, 28], yet the pancreas has been largely overlooked as a potential target tissue. We show that systemic TCDD administration upregulates CYP1A1 enzymes in islets, indicating that the endocrine pancreas is directly exposed to TCDD in vivo. We expect that other dioxin-like pollutants will activate the AhR pathway in islets in a similar manner, although presumably with less potency than TCDD. The role of CYP1A1 in islets generally and beta cells specificially remains to be determined, but, at minimum, this enzyme serves as a useful biomarker for direct exposure of the endocrine pancreas to POPs in vivo.
Electronic supplementary material
(PDF 21724 kb)
Acknowledgements
We are extremely grateful to T.J. Kieffer (UBC) for his mentorship and financial support of the experiments conducted at UBC. We also thank Z. Ao and G. Warnock from the Irving K. Barber Human Islet Isolation Laboratory (Vancouver, BC, Canada) and P. MacDonald from the University of Alberta IsletCore (Edmonton, AB, Canada) for providing human islets. Finally, we thank the following individuals from UBC for their technical assistance: R. Baker for help designing qPCR primers, A. Asadi for paraffin embedding human islets, S. Erener for guidance with islet stress experiments and T. Webber for cell culture support.
Abbreviations
- 3-MC
3-Methylcholanthrene
- AhR
Aryl hydrocarbon receptor
- CYP
Cytochrome P450
- CYP1A1
Cytochrome P450 1A1
- CYP1A2
Cytochrome P450 1A2
- DMEM-HG
High-glucose DMEM
- HBSS
Hanks’ balanced salt solution
- HG
High glucose
- HIER
Heat-induced epitope retrieval
- KO
Knockout
- KRBB
Krebs–Ringer bicarbonate buffer
- LG
Low glucose
- MafA
MAF bZIP transcription factor A
- PFA
Paraformaldehyde
- POPs
Persistent organic pollutants
- qPCR
Quantitative real-time PCR
- TCDD
2,3,7,8-Tetrachlorodibenzo-p-dioxin
- WT
Wild type
Contribution statement
JEB and MI conceived the experimental design. All authors were involved with acquistion, analysis and interpretation of data. JEB wrote the manuscript. All authors contributed to manuscript revisions and approved the final version of the article. JEB is the guarantor of this work.
Funding
This research was funded by a National Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant to JEB (#RGPIN-2017-06265) and the Canadian Foundation for Innovation John R. Evans Leaders Fund and Ontario Research Fund (#37231). This work was also supported by a CIHR Foundation Grant to T. J. Kieffer (#332793). MPH was supported by an NSERC CGS M and an Ontario Graduate Scholarship (OGS). SF and CC were funded by a Carleton University Dean’s Summer Research Internship and SF also received NSERC USRA funding.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.McGoldrick DJ, Murphy EW. Concentration and distribution of contaminants in lake trout and walleye from the Laurentian Great Lakes (2008-2012) Environ Pollut. 2016;217:85–96. doi: 10.1016/j.envpol.2015.12.019. [DOI] [PubMed] [Google Scholar]
- 2.Hung H, Katsoyiannis AA, Guardans R. Ten years of global monitoring under the Stockholm Convention on Persistent Organic Pollutants (POPs): trends, sources and transport modelling. Environ Pollut. 2016;217:1–3. doi: 10.1016/j.envpol.2016.05.035. [DOI] [PubMed] [Google Scholar]
- 3.Kumar J, Lind L, Salihovic S, van Bavel B, Ingelsson E, Lind PM. Persistent organic pollutants and liver dysfunction biomarkers in a population-based human sample of men and women. Environ Res. 2014;134:251–256. doi: 10.1016/j.envres.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 4.Perkins JT, Petriello MC, Newsome BJ, Hennig B. Polychlorinated biphenyls and links to cardiovascular disease. Environ Sci Pollut Res Int. 2016;23(3):2160–2172. doi: 10.1007/s11356-015-4479-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gascon M, Morales E, Sunyer J, Vrijheid M. Effects of persistent organic pollutants on the developing respiratory and immune systems: a systematic review. Environ Int. 2013;52:51–65. doi: 10.1016/j.envint.2012.11.005. [DOI] [PubMed] [Google Scholar]
- 6.Hertz-Picciotto I, Park HY, Dostal M, Kocan A, Trnovec T, Sram R. Prenatal exposures to persistent and non-persistent organic compounds and effects on immune system development. Basic Clin Pharmacol Toxicol. 2008;102(2):146–154. doi: 10.1111/j.1742-7843.2007.00190.x. [DOI] [PubMed] [Google Scholar]
- 7.Boas M, Feldt-Rasmussen U, Main KM. Thyroid effects of endocrine disrupting chemicals. Mol Cell Endocrinol. 2012;355(2):240–248. doi: 10.1016/j.mce.2011.09.005. [DOI] [PubMed] [Google Scholar]
- 8.Birnbaum LS, Tuomisto J. Non-carcinogenic effects of TCDD in animals. Food Addit Contam. 2000;17(4):275–288. doi: 10.1080/026520300283351. [DOI] [PubMed] [Google Scholar]
- 9.Bertazzi PA, Consonni D, Bachetti S, et al. Health effects of dioxin exposure: a 20-year mortality study. Am J Epidemiol. 2001;153(11):1031–1044. doi: 10.1093/aje/153.11.1031. [DOI] [PubMed] [Google Scholar]
- 10.Wang SL, Tsai PC, Yang CY, Guo YL. Increased risk of diabetes and polychlorinated biphenyls and dioxins: a 24-year follow-up study of the Yucheng cohort. Diabetes Care. 2008;31(8):1574–1579. doi: 10.2337/dc07-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vena J, Boffetta P, Becher H, et al. Exposure to dioxin and nonneoplastic mortality in the expanded IARC international cohort study of phenoxy herbicide and chlorophenol production workers and sprayers. Environ Health Perspect. 1998;106(Suppl 2):645–653. doi: 10.1289/ehp.98106645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calvert GM, Sweeney MH, Deddens J, Wall DK. Evaluation of diabetes mellitus, serum glucose, and thyroid function among United States workers exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Occup Environ Med. 1999;56(4):270–276. doi: 10.1136/oem.56.4.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yi SW, Hong JS, Ohrr H, Yi JJ. Agent Orange exposure and disease prevalence in Korean Vietnam veterans: the Korean veterans health study. Environ Res. 2014;133:56–65. doi: 10.1016/j.envres.2014.04.027. [DOI] [PubMed] [Google Scholar]
- 14.Kim JS, Lim HS, Cho SI, Cheong HK, Lim MK. Impact of Agent Orange exposure among Korean Vietnam veterans. Ind Health. 2003;41(3):149–157. doi: 10.2486/indhealth.41.149. [DOI] [PubMed] [Google Scholar]
- 15.Henriksen GL, Ketchum NS, Michalek JE, Swaby JA. Serum dioxin and diabetes mellitus in veterans of Operation Ranch Hand. Epidemiology. 1997;8(3):252–258. doi: 10.1097/00001648-199705000-00005. [DOI] [PubMed] [Google Scholar]
- 16.Longnecker MP, Michalek JE. Serum dioxin level in relation to diabetes mellitus among Air Force veterans with background levels of exposure. Epidemiology. 2000;11(1):44–48. doi: 10.1097/00001648-200001000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Kuo CC, Moon K, Thayer KA, Navas-Acien A. Environmental chemicals and type 2 diabetes: an updated systematic review of the epidemiologic evidence. Curr Diab Rep. 2013;13(6):831–849. doi: 10.1007/s11892-013-0432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porta M. Persistent organic pollutants and the burden of diabetes. Lancet. 2006;368(9535):558–559. doi: 10.1016/S0140-6736(06)69174-5. [DOI] [PubMed] [Google Scholar]
- 19.Thayer KA, Heindel JJ, Bucher JR, Gallo MA. Role of environmental chemicals in diabetes and obesity: a National Toxicology Program workshop review. Environ Health Perspect. 2012;120(6):779–789. doi: 10.1289/ehp.1104597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee DH, Lee IK, Song K, et al. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999–2002. Diabetes Care. 2006;29(7):1638–1644. doi: 10.2337/dc06-0543. [DOI] [PubMed] [Google Scholar]
- 21.Pal S, Blais JM, Robidoux MA, et al. The association of type 2 diabetes and insulin resistance/secretion with persistent organic pollutants in two First Nations communities in northern Ontario. Diabetes Metab. 2013;39(6):497–504. doi: 10.1016/j.diabet.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 22.Everett CJ, Frithsen IL, Diaz VA, Koopman RJ, Simpson WM, Jr, Mainous AG., 3rd Association of a polychlorinated dibenzo-p-dioxin, a polychlorinated biphenyl, and DDT with diabetes in the 1999–2002 National Health and Nutrition Examination Survey. Environ Res. 2007;103(3):413–418. doi: 10.1016/j.envres.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Lee DH, Porta M, Jacobs DR, Jr, Vandenberg LN. Chlorinated persistent organic pollutants, obesity, and type 2 diabetes. Endocr Rev. 2014;35(4):557–601. doi: 10.1210/er.9013-1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor KW, Novak RF, Anderson HA, et al. Evaluation of the association between persistent organic pollutants (POPs) and diabetes in epidemiological studies: a national toxicology program workshop review. Environ Health Perspect. 2013;121(7):774–783. doi: 10.1289/ehp.1205502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roh E, Kwak SH, Jung HS, et al. Serum aryl hydrocarbon receptor ligand activity is associated with insulin resistance and resulting type 2 diabetes. Acta Diabetol. 2015;52(3):489–495. doi: 10.1007/s00592-014-0674-z. [DOI] [PubMed] [Google Scholar]
- 26.Lee YM, Ha CM, Kim SA, et al. Low-dose persistent organic pollutants impair insulin secretory function of pancreatic β-cells: human and in vitro evidence. Diabetes. 2017;66(10):2669–2680. doi: 10.2337/db17-0188. [DOI] [PubMed] [Google Scholar]
- 27.Jorgensen ME, Borch-Johnsen K, Bjerregaard P. A cross-sectional study of the association between persistent organic pollutants and glucose intolerance among Greenland Inuit. Diabetologia. 2008;51(8):1416–1422. doi: 10.1007/s00125-008-1066-0. [DOI] [PubMed] [Google Scholar]
- 28.Dirinck EL, Dirtu AC, Govindan M, Covaci A, Van Gaal LF, Jorens PG. Exposure to persistent organic pollutants: relationship with abnormal glucose metabolism and visceral adiposity. Diabetes Care. 2014;37(7):1951–1958. doi: 10.2337/dc13-2329. [DOI] [PubMed] [Google Scholar]
- 29.Mailloux R, Fu A, Florian M, et al. A Northern contaminant mixture impairs pancreas function in obese and lean JCR rats and inhibits insulin secretion in MIN6 cells. Toxicology. 2015;334:81–93. doi: 10.1016/j.tox.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Novelli M, Piaggi S, De Tata V. 2,3,7,8-Tetrachlorodibenzo-p-dioxin-induced impairment of glucose-stimulated insulin secretion in isolated rat pancreatic islets. Toxicol Lett. 2005;156(2):307–314. doi: 10.1016/j.toxlet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 31.Kurita H, Yoshioka W, Nishimura N, Kubota N, Kadowaki T, Tohyama C. Aryl hydrocarbon receptor-mediated effects of TCDD on glucose-stimulated insulin secretion in mice. J Appl Toxicol. 2009;29:689–694. doi: 10.1002/jat.1459. [DOI] [PubMed] [Google Scholar]
- 32.Wells PG, Lee CJJ, McCallum GP, Perstin J, Harper PA. Receptor- and reactive intermediate-mediated mechanisms of teratogenesis. In: Uetrecht J, editor. Adverse drug reactions. Handbook of Experimental Pharmacology. Berlin, Heidelberg: Springer; 2010. [DOI] [PubMed] [Google Scholar]
- 33.Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem Pharmacol. 2000;59(1):65–85. doi: 10.1016/s0006-2952(99)00310-x. [DOI] [PubMed] [Google Scholar]
- 34.Ma Q, Lu AY. CYP1A induction and human risk assessment: an evolving tale of in vitro and in vivo studies. Drug Metab Dispos. 2007;35(7):1009–1016. doi: 10.1124/dmd.107.015826. [DOI] [PubMed] [Google Scholar]
- 35.Miksys S, Tyndale RF. Cytochrome P450-mediated drug metabolism in the brain. J Psychiatry Neurosci. 2013;38(3):152–163. doi: 10.1503/jpn.120133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gundert-Remy U, Bernauer U, Blomeke B, et al. Extrahepatic metabolism at the body’s internal-external interfaces. Drug Metab Rev. 2014;46(3):291–324. doi: 10.3109/03602532.2014.900565. [DOI] [PubMed] [Google Scholar]
- 37.Diliberto JJ, Akubue PI, Luebke RW, Birnbaum LS. Dose-response relationships of tissue distribution and induction of CYP1A1 and CYP1A2 enzymatic activities following acute exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in mice. Toxicol Appl Pharmacol. 1995;130(2):197–208. doi: 10.1006/taap.1995.1025. [DOI] [PubMed] [Google Scholar]
- 38.Lai KP, Wan HT, Ng AH, Li JW, Chan TF, Wong CK. Transcriptomic and functional analyses on the effects of dioxin on insulin secretion of pancreatic islets and β-cells. Environ Sci Technol. 2017;51(19):11390–11400. doi: 10.1021/acs.est.7b02830. [DOI] [PubMed] [Google Scholar]
- 39.Clarke J, Flatt PR, Barnett CR. Cytochrome P450 1A-like proteins expressed in the islets of Langerhans and altered pancreatic β-cell secretory responsiveness. Br J Pharmacol. 1997;121(3):389–394. doi: 10.1038/sj.bjp.0701139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kessova IG, DeCarli LM, Lieber CS. Inducibility of cytochromes P-4502E1 and P-4501A1 in the rat pancreas. Alcohol Clin Exp Res. 1998;22:501–504. doi: 10.1111/j.1530-0277.1998.tb03679.x. [DOI] [PubMed] [Google Scholar]
- 41.Chen YH, Tukey RH. Protein kinase C modulates regulation of the CYP1A1 gene by the aryl hydrocarbon receptor. J Biol Chem. 1996;271(42):26261–26266. doi: 10.1074/jbc.271.42.26261. [DOI] [PubMed] [Google Scholar]
- 42.Xu H, Li C, Li Y, et al. Generation of Tg(cyp1a:gfp) transgenic zebrafish for development of a convenient and sensitive in vivo assay for aryl hydrocarbon receptor activity. Mar Biotechnol (NY) 2015;17:831–840. doi: 10.1007/s10126-015-9669-1. [DOI] [PubMed] [Google Scholar]
- 43.Riddick DS, Huang Y, Harper PA, Okey AB. 2,3,7,8-Tetrachlorodibenzo-p-dioxin versus 3-methylcholanthrene: comparative studies of Ah receptor binding, transformation, and induction of CYP1A1. J Biol Chem. 1994;269(16):12118–12128. [PubMed] [Google Scholar]
- 44.National Institutes of Health (2006) NTP technical report on the toxicology and carcinogenesis studies of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in female Harlan Sprague-Dawley rats (gavage studies). NIH publication no. 06-4468. U.S. Department of Health and Human Services, Public Health Service, National Institutes of Health, Research Triangle Park. Available from https://ntp.niehs.nih.gov/ntp/htdocs/lt_rpts/tr521.pdf. Accessed October 2019
- 45.Bell DR, Clode S, Fan MQ, et al. Relationships between tissue levels of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), mRNAs, and toxicity in the developing male Wistar(Han) rat. Toxicol Sci. 2007;99(2):591–604. doi: 10.1093/toxsci/kfm179. [DOI] [PubMed] [Google Scholar]
- 46.Dragin N, Uno S, Wang B, Dalton TP, Nebert DW. Generation of ‘humanized’ hCYP1A1_1A2_Cyp1a1/1a2(-/-) mouse line. Biochem Biophys Res Commun. 2007;359(3):635–642. doi: 10.1016/j.bbrc.2007.05.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Uno S, Dalton TP, Sinclair PR, et al. Cyp1a1(-/-) male mice: protection against high-dose TCDD-induced lethality and wasting syndrome, and resistance to intrahepatocyte lipid accumulation and uroporphyria. Toxicol Appl Pharmacol. 2004;196(3):410–421. doi: 10.1016/j.taap.2004.01.014. [DOI] [PubMed] [Google Scholar]
- 48.Tan BM, Zammit NW, Yam AO, et al. Baculoviral inhibitors of apoptosis repeat containing (BIRC) proteins fine-tune TNF-induced nuclear factor kappaB and c-Jun N-terminal kinase signalling in mouse pancreatic beta cells. Diabetologia. 2013;56(3):520–532. doi: 10.1007/s00125-012-2784-x. [DOI] [PubMed] [Google Scholar]
- 49.Kim S, Millet I, Kim HS, et al. NF-κB prevents β cell death and autoimmune diabetes in NOD mice. Proc Natl Acad Sci U S A. 2007;104(6):1913–1918. doi: 10.1073/pnas.0610690104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Maedler K, Schumann DM, Sauter N, et al. Low concentration of interleukin-1β induces FLICE-inhibitory protein-mediated beta-cell proliferation in human pancreatic islets. Diabetes. 2006;55(10):2713–2722. doi: 10.2337/db05-1430. [DOI] [PubMed] [Google Scholar]
- 51.Arous C, Ferreira PG, Dermitzakis ET, Halban PA. Short term exposure of beta cells to low concentrations of interleukin-1beta improves insulin secretion through focal adhesion and actin remodeling and regulation of gene expression. J Biol Chem. 2015;290(10):6653–6669. doi: 10.1074/jbc.M114.611111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Papaccio G, Graziano A, D’Aquino R, Valiante S, Naro F. A biphasic role of nuclear transcription factor (NF)-κB in the islet β-cell apoptosis induced by interleukin (IL)-1β. J Cell Physiol. 2005;204(1):124–130. doi: 10.1002/jcp.20276. [DOI] [PubMed] [Google Scholar]
- 53.Ehses JA, Perren A, Eppler E, et al. Increased number of islet-associated macrophages in type 2 diabetes. Diabetes. 2007;56(9):2356–2370. doi: 10.2337/db06-1650. [DOI] [PubMed] [Google Scholar]
- 54.Richardson SJ, Willcox A, Bone AJ, Foulis AK, Morgan NG. Islet-associated macrophages in type 2 diabetes. Diabetologia. 2009;52(8):1686–1688. doi: 10.1007/s00125-009-1410-z. [DOI] [PubMed] [Google Scholar]
- 55.Butcher MJ, Hallinger D, Garcia E, et al. Association of proinflammatory cytokines and islet resident leucocytes with islet dysfunction in type 2 diabetes. Diabetologia. 2014;57(3):491–501. doi: 10.1007/s00125-013-3116-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Solimena M, Schulte AM, Marselli L, et al. Systems biology of the IMIDIA biobank from organ donors and pancreatectomised patients defines a novel transcriptomic signature of islets from individuals with type 2 diabetes. Diabetologia. 2018;61(3):641–657. doi: 10.1007/s00125-017-4500-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chapman DE, Schiller CM. Dose-related effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in C57BL/6J and DBA/2J mice. Toxicol Appl Pharmacol. 1985;78(1):147–157. doi: 10.1016/0041-008x(85)90314-x. [DOI] [PubMed] [Google Scholar]
- 58.Smith AG, Clothier B, Carthew P, et al. Protection of the Cyp1a2(-/-) null mouse against uroporphyria and hepatic injury following exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol Appl Pharmacol. 2001;173(2):89–98. doi: 10.1006/taap.2001.9167. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 21724 kb)
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.