Abstract
Vitamin D and its main active metabolite 1,25-dihydroxyvitamin D serve a crucial role in maintenance of a healthy calcium metabolism, yet have additional roles in immune and central nervous system cell homeostasis. Serum levels of 25-hydroxyvitamin D are a biomarker of future disease activity in patients with early relapsing–remitting multiple sclerosis (RRMS), and vitamin D supplementation in patients with low circulating 25-dihydroxyvitamin D levels has been anticipated as a potential efficacious treatment strategy. The results of the first large randomized clinical trials (RCTs), the SOLAR and CHOLINE studies, have now been published. The SOLAR study compared 14,000 IU of vitamin D3 (cholecalciferol) per day with placebo for 48 weeks in 232 randomized patients, whereas CHOLINE compared vitamin D3 100,000 IU every other week with placebo for 96 weeks in 129 randomized patients. All patients in both studies also used interferon-β-1a. None of the studies met their primary endpoints, which were no evidence of disease activity (NEDA-3) at 48 weeks in SOLAR and annualized relapse rate at 96 weeks in CHOLINE. Both studies did, however, suggest modest effects on secondary endpoints. Thus, vitamin D reduced the number of new or enlarging lesions and new T2 lesions in SOLAR, and the annualized relapse rate and number of new T1 lesions, volume of hypointense T1 lesions, and disability progression in the 90 patients who completed 96 weeks’ follow-up in CHOLINE. We conclude that none of the RCTs on vitamin supplementation in MS have met their primary clinical endpoint in the intention to treat cohorts. This contrasts the observation studies, where each 25 nmol/l increase in 25-hydroxyvitamin D levels were associated with 14–34% reduced relapse risk and 15–50% reduced risk of new lesions on magnetic resonnance imaging. This discrepancy may have several explanations, including confounding and reverse causality in the observational studies. The power calculations of the RCTs have been based on the observational studies, and the RCTs may have been underpowered to detect less prominent yet important effects of vitamin D supplementation. Although the effect of vitamin D supplementation is uncertain and less pronounced than suggested by observational studies, current evidence still support that people with MS should avoid vitamin D insufficiency, and preferentially aim for vitamin D levels around 100 nmol/L or somewhat higher.
Key Points
| A low vitamin D status predicts a higher risk of exacerbations and magnetic resonance imaging activity in people with early relapsing–remitting multiple sclerosis (RRMS). |
| Clinical trials on vitamin D supplementation in RRMS are negative on primary clinical endpoints. |
| The effect of vitamin D on multiple sclerosis activity is less pronounced than suggested by observational studies. |
| This discrepancy may reflect reverse causality or confounding in the observational studies, or differences in trial designs in terms of inclusion criteria, power for primary and secondary outcomes, disease-modifying therapy use, and duration and dose of vitamin D supplementation in the clinical trials. |
Introduction
Vitamin D is the precursor of a potent steroid hormone with multiple biological effects including immunomodulation and neuroprotection [1, 2]. The history of vitamin D in multiple sclerosis (MS) dates back to 1974, when Goldberg suggested that inadequate intake of vitamin D, calcium, and magnesium in genetically predisposed individuals leads to abnormal lipid composition and unstable myelin, predisposing to MS development later in adulthood [3]. Vitamin D receptors (VDRs) were discovered on human immune cells in 1983 [4], and immunomodulatory properties of vitamin D were reported in 1984 [5]. In 1986 the first clinical trial of vitamin D in MS reported that vitamin D supplementation reduced the relapse rate by 50% compared with pre-treatment levels [6].
As for other conditions, the role of vitamin D supplementation in MS can only be determined in randomized clinical trials (RCTs) [7]. A Cochrane report concluded that very low-quality evidence suggests no benefit of vitamin D for patient-important outcomes [8]. Previously published RCTs have been underpowered with heterogeneous patient selections and are thus largely inconclusive. Very recently, the results of two larger RCTs on vitamin D as add on to interferon (IFN)-β, SOLAR (Supplementation of VigantOL®oil versus placebo as Add-on in patients with relapsing–remitting multiple sclerosis receiving Rebif® treatment) and CHOLINE (Cholecalceferol in relapsing remitting MS: A randomized clinical trial), have been published [9, 10]. We review the role of vitamin D supplementation in MS in light of the results from RCTs, with particular focus on these recent advances.
Vitamin D Metabolism: Focus on Immune and Central Nervous System Cells
Vitamin D has a longstanding recognized vital role in maintenance of calcium homeostasis [11]. For its in vivo synthesis, vitamin D requires exposure of the skin to ultraviolet (UV) B light to contribute to a metabolism pathway mostly mediated by cytochrome P450 superfamily enzymes. Vitamin D comes in two isoforms: the plant-derived vitamin D2 (ergocalciferol) and animal-derived vitamin D3 (cholecalciferol). The most notable metabolites in this pathway are 25-hydroxyvitamin D [25(OH)D] and 1,25-dihydroxyvitamin D [1,25(OH)2D]. 25(OH)D is the most abundant vitamin D metabolite in the circulation. Although the kidney is the main source of circulating 1,25(OH)2D, hydroxylation into this active metabolite can also occur in a multitude of MS-relevant cell types inside and outside the central nervous system (CNS), including T cells, B cells, monocytes, macrophages, dendritic cells, microglia, astrocytes, and neurons [4, 12, 13] (reviewed by Smolders and colleagues [14, 15]). Since all these cells also express the VDR in resting and/or activated states, both auto- and paracrine effects of this activated vitamin D can be anticipated. In the context of MS, this can occur both in the lymph nodes and also in the CNS itself. 25(OH)D is encountered in the cerebrospinal fluid, as measured with mass spectrometry [16], and an upregulation of the enzymatic machinery to activate vitamin D and VDRs have been found in active MS lesions [17]. In vitro and in animal models of MS, 1,25(OH)2D is mostly attributed anti-inflammatory properties in activated lymphocytes, myeloid cells, and glia [14, 15]. In neurons, homeostatic functions have been observed. These observations make it tempting to interpret vitamin D as a promotor of a homeostatic state both in immune and CNS-resident cells.
Genetic and Epidemiological Evidence for Vitamin D as a Risk Factor for Multiple Sclerosis (MS) Development
In nested case–control studies, low circulating 25(OH)D levels when compared with controls have been found in military personnel prior to MS onset [18], in blood spots taken with the heel prick in newborns who develop MS in later life [19], and even in mothers during pregnancy with a child develops MS in later life [20]. The causal interpretation of this association is challenging. Several lines of evidence support an effect of vitamin D on the onset of first symptoms of MS. A low vitamin D intake was found in participants of the Nurses Health Initiatives studies who developed MS in later life [21]. Furthermore, several vitamin D-associated single nucleotide genetic polymorphisms (SNPs) have been associated with risk of MS in genome-wide association studies [22]. In Mendelian randomization studies a cumulative risk score of SNPs predicting low 25(OH)D levels was associated with MS, while an association of these SNPs by themselves with MS was lacking [23–25]. Although confounding from residual pleiotrophy, compensatory feedback interactions, or other unknown factors cannot be totally excluded, these studies strongly support a causal role for vitamin D on MS development.
Experimental Animal Models: Inflammation and Beyond
The in vitro effect of various dosages of 1,25(OH)2D on neural stem cell proliferation and their differentiation to oligodendrocytes has been studied [26]. Neural stem cells showed VDR expression, which was upregulated by 1,25(OH)2D, leading to enhanced proliferation of neural stem cells and enhanced differentiation into neurons and oligodendrocytes.
Effects in the Experimental Autoimmune Encephalomyelitis Model
The activated form of vitamin D3, 1,25(OH)2D3, is highly efficient in both disease prevention and treatment in experimental autoimmune encephalomyelitis (EAE) mice [27, 28]. Most research has focused on the effect of vitamin D on lymphocyte activation (reviewed by Hayes et al. [29]). A recent study in a myelin oligodendrocyte glycoprotein (MOG) EAE model has investigated the effect of 1,25(OH)2D on oligodendrocyte maturation and differentiation [30]. Intraperitoneal injection of 1,25(OH)2D was associated with elevated numbers of neural stem cells, oligodendrocyte precursor cells, as well as oligodendrocytes in disease lesions in the CNS, suggesting also a potential for remyelination and neural repair in this animal model.
Effects in Toxic Models on Demyelination
The first study on the effect of vitamin D on remyelination was performed in the ethidium bromide (EB) model for demyelination [31], where direct injection of EB leads to oligodendroglial damage and subsequent demyelination [32]. Rats were given 5 µg/kg of vitamin D3 for 2, 7, or 28 days following local EB injection. Vitamin D3 administration reduced the EB-induced damage and increased the endogenous remyelination. In 2011, Wergeland and colleagues [33] performed a study on vitamin D using the cuprizone model, where mice are fed with cuprizone, leading to oligodendrocyte death and a subsequent reversible demyelination [34]. The mice were given either a diet deficient of vitamin D3 (< 50 IU/kg) or were supplemented with low (500 IU/kg), high (6200 IU/kg), or very high (12,500 IU/kg) amounts of vitamin D3. High dosages of vitamin D3 significantly reduced the extent of white matter demyelination. In a follow-up study [35], mice receiving intraperitoneal injections of 1,25(OH)2D after induction of demyelination had significantly faster remyelination than mice receiving placebo. In another study of remyelination, mice received cuprizone for 5 weeks and were then either injected intraperitoneally with vitamin D diluted in olive oil or injected with olive oil only (SHAM) or were without any injection [36]. A significant increase in remyelination, as measured by MOG and CNPase (2′,3′-cyclic-nucleotide 3′-phosphodiesterase) expression, was seen in the vitamin D-injected group. Finally, high-dose vitamin D has been found to protect against axonal damage, as measured by loss of neurofilament-positive axons, in the cuprizone model if the vitamin D treatment started before induction of demyelination [37].
The mechanisms by which vitamin D can prevent demyelination and stimulate remyelination have been examined in some studies. Using proteomics, it was detected that 125 proteins were differentially regulated in brains from 1,25(OH)2D-treated mice during remyelination compared with placebo. The upregulated proteins were especially involved in calcium binding or in mitochondrial function [38]. Another study focused on the retinoid X receptor (RXR)-γ, which has been shown to be a positive regulator of oligodendrocyte progenitor cell differentiation [39]. The authors demonstrated that RXR–VDR signaling induced oligodendrocyte precursor cell differentiation that was enhanced by vitamin D.
Low Vitamin D Status Predicts a More Active Disease Course in Early MS
Several, but not all, cohort studies have found that increasing serum 25(OH)D levels are associated with low disease activity in clinically isolated syndrome (CIS) and relapsing–remitting MS (RRMS). The risk of subsequent relapses was 14–34% lower for each 25 nmol/L (10 ng/L) increase in 25(OH)D in subjects with CIS or RRMS (Table 1). This was particularly true for subjects with CIS [40, 41]. However, in other studies the effect was limited to subgroups with the youngest ages [42] or a specific genetic risk–allele constitution [43]. Notably, in the largest RRMS onset cohort studied, 25(OH)D levels did not predict subsequent long-term relapse rate [44]. The predictive value of 25(OH)D levels for risk of Expanded Disability Severity Scale (EDSS) progression is even less certain. This association was found in a cohort of CIS patients [40], but not convincingly in cohorts with (advanced) RRMS or progressive MS [42–45].
Table 1.
Association of 25-hydroxyvitamin D levels with relapse risk
| Study | Year | n | Follow-up (years; median) | Proportional decrease in IR/RR/HR for each 25 nmol/L increase in 25(OH)D (%)a | Specification |
|---|---|---|---|---|---|
| Simpson et al. [82] | 2010 | 145 | 0.5 | 23 | RRMS |
| Mowry et al. [86] | 2010 | 110 | 1.7 | 34 | Pediatric RRMS/CIS |
| Mowry et al. [43] | 2012 | 469 | 5 | 14 | Only HLA-DR15*01 single RRMS/CIS, not in whole cohort |
| Runia et al. [87] | 2012 | 73 | 1.7 | 27b | RRMS |
| Ascherio et al. [40] | 2014 | 465 | 5 | 26 | CIS |
| Fitzgerald et al. [44] | 2015 | 1482 | 2 | NR | RRMS, no effect ARR |
| Kuhle et al. [41] | 2015 | 1047 | 4.3 | 24c | CIS, not in multivariate |
| Muris et al. [42] | 2016 | 340 | 3 | 32 | Only RRMS < 37.5 years, not in whole cohort |
25(OH)D 25-hydroxyvitamin D, ARR annualized relapse rate, CIS clinical isolated syndrome, HLA human leukocyte antigen, HR hazard ratio, IR incidence risk, NR not reported, RR relative risk, RRMS relapsing–remitting multiple sclerosis
aIR, RR, and HR for a relapse (yes/no) values were linearly recalculated to correspond with a 25 nmol/L (10 ng/L) increase of 25(OH)D levels, except for Runia et al. [87] and Kuhle et al. [41]
bFor each doubling of 25(OH)D (non-linear relationship)
cOnly quartiles reported (median level 49.3 nmol/L)
Magnetic resonance imaging (MRI) is accepted as a more sensitive biomarker for inflammatory disease activity in MS than relapses. The risk of new/enlarging T2 and/or gadolinium-enhancing T1 lesions on MRI decreased 15–50% for each 25 nmol/L increase in 25(OH)D levels in patients with CIS or RRMS (Table 2). This association appears more robust and reproducible when compared with relapse risk. It has been observed in cohorts of patients treated with IFN-β [40, 44]; however, in another study with repeated measurements before and after initiation of IFN-β in the same patients, it was only observed prior to start, and not during the use of this drug [46]. Measures of total brain volume also showed a less marked reduction in CIS patients with higher 25(OH)D levels [40], but not in RRMS [44]. Radiological and neurophysiological measures of remyelination have not been associated with 25(OH)D levels yet, albeit that retinal nerve fiber layer thinning as assessed with optical coherence tomography after recovery of optic neuritis (from any cause) was more pronounced in subjects with the lowest 25(OH)D levels [47]. Altogether, these findings suggest that low 25(OH)D-levels early in disease characterize patients with a high risk of an active inflammatory disease course.
Table 2.
Randomized clinical trials with clinical outcomes
| Study, year | Patients | na | Months | Intervention | Primary outcomeb |
|---|---|---|---|---|---|
| Derakhshandi et al. [52], 2013 | Optic neuritis | 30/24 | 12 | D3 50,000 IU weekly/placebo | Conversion to RRMS negative |
| Salari et al. [53], 2015 | Optic neuritis | 74/52 | 5 | D3 50,000 IU weekly/placebo | Retinal nerve layer thickness negative |
| O’Connell et al. [54], 2017 | CIS | 32/29 | 5 | D3 5000 IU/10,000 IU/placebo | CD4 T cells negative (relapses/MRI negative) |
| Stein et al. [55], 2011 | RRMS | 23/20 | 6 | High dose/D2 1000 IU | GE/T2 lesions negative |
| Kampman et al. [56], 2012 | RRMS | 71/68 | 41 | D3 20,000 IU weekly/placebo | Relapses negative |
| Soilu-Hänninen et al. [62], 2012 | RRMS | 66/62 | 12 | D3 20,000 IU weekly/placebo | T2 lesion volume negative |
| Shaygannejad et al. [64], 2012 | RRMS | 50/50 | 12 | Calcitriol 0–5 µg/placebo | EDSS positive |
| Golan et al. [65], 2013 | RRMS | 45/30 | 12 | 4370 IUc/D3 800 IU | Flu symptoms negative (relapses/EDSS negative) |
| Achiron et al. [66], 2015 | RRMS | 158/143 | 6 | Alfacalcidiol 1 µg/placebo | Fatigue positive (relapses positive) |
| Sotirchos et al. [67], 2016 | RRMS | 40/35 | 6 | 10,400/D3 800 IU | CD4 T cells positive (relapses negative) |
| Hupperts et al. [9], 2019 | RRMS | 229/183 | 11 | D3 14,000 IU/placebo | NEDA-3 negative |
| Camu et al. [10], 2019 | RRMS | 129/90 | 21 | D3 100,000 IU twice monthly/placebo | Relapses negative |
CIS clinical isolated syndrome, D2 vitamin D2, D3 vitamin D3, EDSS Expanded Disability Severity Scale, GE gadolinium-enhancing T1 lesions, MRI magnetic resonance imaging, NEDA-3 no evidence on disease activity including relapses, EDSS, and MRI, RRMS relapsing–remitting multiple sclerosis
aRandomized/completed
bSecondary clinical outcomes in parentheses for studies with other primary outcomes
c75,000 IU every 3 weeks plus 800 IU daily
Several investigators explored correlations between 25(OH)D levels and a wide variety of general immunological outcomes in blood and cerebrospinal fluid of people with MS. The absence of a clear auto-antigen or single immunological biomarker that clearly defines the immune response in MS makes it difficult to fully appreciate the relevance of analytes tested. Additionally, small sample sizes give a high risk of bias, and the heterogeneity in assays used and subjects included makes reproducibility an issue. Two studies are of note to discuss separately. First, a large dataset of participants in the BENEFIT (Betaferon/Betaseron in Newly Emerging multiple sclerosis For Initial Treatment) study was analyzed for interaction between 25(OH)D status, MRI disease activity, and whole-blood mRNA expression profiles [48]. A network shift comprising IFN responses and lymph node-homing molecules was observed in people with MS with higher 25(OH)D levels. A limitation of this study is that small yet significant effects of 25(OH)D on relevant subfractions of cells may get lost in the whole-blood transcriptome. Second, an inverse correlation was observed between 25(OH)D levels and antibodies against the Epstein–Barr virus (EBV) nuclear antigen 1 (EBNA1) epitope in patients with MS [49]. A similar inverse correlation between 25(OH)D levels and EBV viral load has been reported [50]. The strong association between EBV seropositivity and MS onset is well-known, and anti-EBNA1 IgG antibodies have been identified as predictors of MS risk and disease activity in several but not all studies [51].
Randomized Controlled Trials with Clinical Results
Clinical Trials in Optic Neuritis and Clinically Isolated Syndrome (CIS)
Intervention studies with clinical endpoints in CIS and MS are shown in Table 2.
Derakhshandi et al. [52] compared 50,000 IU of vitamin D3 per week and placebo in patients with optic neuritis and serum vitamin D < 75 nmol/L. There were four dropouts in the placebo group and two in the vitamin D group, leaving only 11 and 13 patients, respectively, for the final analysis. End-of-study 25(OH)D levels were not reported. During the 12-month follow-up, five patients in the placebo group and none in the vitamin D group experienced a second demyelinating attack (p = 0.007). The authors also reported a significant effect on several MRI outcomes (cortical, juxtacortical, corpus callosal, new gadolinium-enhancing, and new T2 lesions, and black holes). Distribution of baseline MRI characteristics was not reported.
In another study from Iran, Salari et al. [53] compared 50,000 IU of vitamin D3 weekly with placebo in 74 patients with optic neuritis and baseline 25(OH)D < 60 nmol/L. A total of 52 patients completed the 24-week observation period. There was no effect of vitamin D treatment on retinal nerve fiber layer thickness, which was the primary endpoint. The effects on 25(OH)D levels and conversion to MS were not reported.
O’Connell et al. [54] compared 10,000 IU of vitamin D3, 5000 IU of vitamin D3, and placebo for 24 weeks in 29 patients with CIS and 37 healthy controls. For the CIS patients, mean vitamin D levels were around 50 nmol/L at baseline and increased by 18 nmol/L in the placebo group, 76 nmol/L in the vitamin D 5000 IU group, and 115 nmol/L in the vitamin D 10,000 IU group. There was no effect on the frequency of pro-inflammatory CD4 T cells, which was the primary endpoint. The only MS relapse during the study period occurred in the vitamin D 5000 IU arm. There was no effect on MRI outcomes (gadolinium-enhancing and new T2 lesions).
Clinical Trials in MS
In 2011, Stein and coworkers [55] performed a 6-month double-blind RCT of high-dose vitamin D2 6000 IU, versus 1000 IU of vitamin D2, with the aim of achieving serum 25(OH)D 130–175 nmol/L in 23 RRMS patients, of whom 19 were treated with IFN-β or glatiramer acetate [55]. Patients on immunomodulatory treatment had to have at least one relapse within the last 24 months. Brain MRI was performed at baseline and at months 4, 5, and 6, and primary endpoints were the cumulative number of new gadolinium-enhancing lesions and change in the volume of T2 lesions. Median 25(OH)D increased from 54 to 69 nmol/L (Δ 15 nmol/L) in the low-dose and from 59 to 120 nmol/L (Δ 61 nmol/L) in the high-dose vitamin D2 group. The primary outcome was negative, but after adjusting for entry EDSS, the exit EDSS was higher in the high-dose vitamin D group (p = 0.05), where four relapses were recorded versus two in the low-dose group (p = 0.04).
Kampman et al. [56] compared weekly supplementation with 20,000 IU of vitamin D3 and placebo for 96 weeks in 68 RRMS patients with EDSS ≤ 4.5 [56]. Approximately 50% of the patients received immunomodulatory drugs, mostly IFN-β. The trial was designed to study the effect on bone mass density, and no MRI outcomes were included. Neither bone mass density nor turnover were affected by vitamin D supplementation [57, 58]. The mean annualized relapse rate (ARR) at baseline was low (0.11 and 0.15 in the vitamin D and placebo groups, respectively). Mean 25(OH)D levels increased from 55.6 to 123.1 nmol/L (Δ 67.5 nmol/L) in the vitamin D group and from 57.3 to 61.8 nmol/L (Δ 4.5 nmol/L) in the placebo group. After 96 weeks, there was no significant difference in ARR (absolute difference 0.10, 95% confidence interval [CI] − 0.07 to 0.27), EDSS (absolute difference − 0.01, 95% CI − 0.35 to 0.35), or MS functional composite components, grip strength, or fatigue. Secondary studies revealed no effect of vitamin D on inflammation markers or neurofilament light chain in serum, except a non-significant reduction in neurofilament light chain levels among patients not using conventional disease-modifying treatment [59, 60]. Serum anti-EBNA1 antibody levels decreased significantly after 48 weeks’ supplementation, but not after 96 weeks [61].
Soilu-Hänninen et al. [62] compared weekly supplementation with 20,000 IU vitamin D3 with placebo for 1 year. All 66 patients had RRMS and had used IFN-β for at least 1 month prior to inclusion. As for the Kampman et al. [56] study, recent disease activity was not an inclusion criterion, but the ARR was higher (around 0.5 in both treatment arms). The primary endpoint was T2 burden. Mean serum 25(OH)D changed from 54 nmol/L at baseline to 110 nmol/L at 12 months in the vitamin D-treated group (Δ 56 nmol/L), and from 56 to 50 nmol/L in the placebo group. T2 lesion volume increased more in the placebo group (median change 287 mm3) than in the vitamin D group (median change 83 mm3). The vitamin D group also accumulated fewer new T2 lesions, but neither of these differences were statistically significant (p = 0.105 and 0.286, respectively). The ARR at study end was similar (0.26 and 0.28). The vitamin D group did, however, have significantly fewer T1 enhancing lesions (p = 0.004), as well as a tendency to reduced disability accumulation (p = 0.071) and improved timed tandem walk (p = 0.076). An array of soluble inflammatory mediators was analyzed, of which only the latency activated peptide of transforming growth factor (TGF)-β (47–55 pg/mL; p = 0.0249) was reported as a single regulated molecule within the vitamin D arm [63].
Shaygannejad et al. [64] compared calcitriol in escalating doses up to 0.5 µg/day and placebo in 50 RRMS patients. Recent disease activity was not an inclusion criterion. The patients had to be clinically stable during the last month before study entry, and to have 25(OH)D > 100 nmol/L at baseline. Further information about 25(OH)D levels throughout the study was not reported. ARR decreased significantly in both groups, and was 1.04 at baseline and 0.32 at study end in the vitamin D group and 1.04 and 0.40 in the placebo group. EDSS remained stable (1.6) in the vitamin D group and increased from 1.70 to 1.94 in the placebo group. When comparing between groups, only the difference in EDSS change was significant (p < 0.05).
In 2013, Golan et al. [65] performed a 1-year double-blind RCT of daily supplementation with 4370 versus 800 IU of vitamin D3 in 45 patients on IFN-β, with reduction in flu-like symptoms as the primary endpoint. Mean serum 25(OH)D increased from 48 to 68 nmol/L (Δ 20 nmol/L) and from 48.2 to 122.6 nmol/L (Δ 74.4 nmol/L) in the low- and high-dose groups, respectively. The primary endpoint was negative, and there was no difference in ARR at the end of the study (mean ± standard deviation 0.34 ± 0.27 vs. 0.51 ± 0.34 in the low- and high-dose groups, respectively). One patient in the high-dose group with ARR 0 at baseline had three relapses during the study. Circulating interleukin (IL)-17 increased in the placebo (p = 0.037) but not the vitamin D3 group.
Achiron et al. [66] compared the effect of 1 µg/day of the vitamin D3 metabolite alfacalcidol for 6 months in 158 MS patients with significant fatigue [66]. The majority of the patients had RRMS, with mean ± standard deviation disease duration 6.2 ± 5.5 years and EDSS score 2.9 ± 2.6. The effect on 25(OH)D levels was not reported. Alfacalcidol decreased the mean relative fatigue severity impact score, which was the primary endpoint, as compared with placebo (− 41.6% vs. − 27.4%; p = 0.007). There was also an effect on relapses, with eight relapses in the alfacalcidol-treated arm compared with 25 in the placebo arm (p = 0.006), but not on EDSS.
Sotirchos et al. [67] compared 10,400 and 800 IU of vitamin D3 daily for 6 months in 40 RRMS patients. Changes in the proportion of IFN-γ+ and IL-17+CD4+ T cells were the primary endpoints [67]. The majority of the patients were treated with either IFN-β, glatiramer acetate, natalizumab, or fingolimod. Mean 25(OH)D levels at baseline were 67.6 and 69.3 nmol/L in the low- and high-dose groups, and increased by 17.0 and 86.2 nmol/L, respectively. There was a reduction in the proportion of IL-17+CD4+ T cells, CD161+CD4+ T cells, and effector memory CD4+ T cells in the high-dose vitamin D group only. Relapses were the only clinical outcome reported, with one in each study arm.
SOLAR was a randomized, double-blind, multicenter study comparing vitamin D3 (6670 IU increasing to 14,000 IU per day after 4 weeks) with placebo for 48 weeks, including 232 RRMS patients aged 18–55 years from Denmark, Estonia, Finland, Germany, Italy, Latvia, Lithuania, The Netherlands, Norway, Portugal, and Switzerland [9]. Three patients did not receive study medication, leaving 229 patients for the intention-to-treat (ITT) analyses. Inclusion criteria were first clinical event occurring within 5 years, EDSS score ≤ 4.0, relapse or gadolinium-enhancing MRI lesion within the last 18 months, no or low vitamin D supplementation, and treatment with IFN-β-1a 44 µg three times weekly for 3–18 months. Median 25(OH)D levels at baseline and week 48 were 53 and 215 nmol/L (Δ 162 nmol/L) in the vitamin D group, and 54 and 49 nmol/L (Δ − 5 nmol/L) in the placebo group. Due to slow inclusion, the primary endpoint was changed during the study from new T2 lesions at week 48 and the proportion of relapse-free patients at week 96 [68] to no evidence of disease activity (NEDA-3), defined as no relapses, EDSS progression, or combined unique MRI activity (CUA; new gadolinium-enhancing or new/enlarging T2 lesions) at week 48. Overall, 36.3% of patients in the vitamin D group and 35.3% in the placebo group had NEDA-3 at 48 weeks (p = 0.80). For the secondary endpoints, vitamin D supplementation was associated with a 32% reduction in the number of CUA lesions compared with placebo (p = 0.0045) and change from baseline in total volume of T2 lesions at week 48 (3.57% vs. 6.07%; p = 0.035). The vitamin D group also tended to have a lower ARR (30%; p = 0.17) and risk of EDSS progression (odds ratio [OR] 0.77; p = 0.39). The proportion of relapse-free patients at week 48 was similar and there were no statistically significant differences between vitamin D3 and placebo for time to confirmed EDSS progression at week 48. No safety issues appeared.
A subgroup of 53 Dutch participants in SOLAR was analyzed in a substudy, SOLARIUM (SOLAR ImmUne Modulating effects). In the treatment arm of this subgroup, 25(OH)D levels increased from 60 to 231 nmol/L (Δ 171 nmol/L) and remained stable in the placebo group (54 and 60 nmol/L, respectively [Δ 6 nmol/L]). On the whole, there were no differences between treatment arms regarding proportions of regulatory T and B cell subsets or T cell cytokine profiles [69, 70]. A within-treatment arm 48-week reduction of IL-4-positive T cells (mean 3.7–2.9%; p = 0.04), a reduction of CD25 protein expression by regulatory T cells, and a reduction in soluble CD25 was observed in the placebo group but not in the vitamin D-supplemented group [69, 71]. Definitive interpretation of these data requires further study. In the vitamin D group, there was a reduction of anti-EBNA1 IgG levels (median 526–455; p < 0.001), which was not seen in the placebo-group [72].
CHOLINE was a multicenter, double-blind, placebo-controlled, parallel-group randomized controlled trial in RRMS patients across 27 centers in France comparing vitamin D3 (100,000 IU every other week) to placebo for 96 weeks [10]. A total of 181 patients were recruited, of whom 129 were randomly assigned to receive vitamin D3 (n = 63) or placebo (n = 66). Inclusion criteria were age 18–65 years, EDSS score from 0 to 5, at least one documented relapse during the previous 2 years, stable disease (no episode in the 30 days before screening), serum 25(OH)D concentration < 75 nmol/L, and treatment with subcutaneous IFN-β-1a 44 µg three times weekly for 4 ± 2 months. The primary endpoint was ARR at 96 weeks. Change in time of MRI parameters, EDSS, 3-level version of EQ-5D (EQ-5D-3L), Paced Auditory Serial Addition Task 3, second version (PASAT-3) scores, and safety were the secondary endpoints. Mean serum 25(OH)D levels in the vitamin D3 group raised from 49.19 nmol/L at baseline to 156.92 nmol/L at 2 years (Δ 107.73 nmol/L). ARR was reduced by the vitamin D3 treatment but the difference was statistically non-significant in the ITT sample (rate ratio [rR] = 0.799, 95% CI 0.481–1.32; p = 0.379), as well as on a per protocol basis (rR = 0.630, 95% CI 0.353–1.101; p = 0.111). Safety was good. A total of 39 patients could not reach the 2-year follow-up; thus, the completers’ population was composed of 90 subjects (45 vitamin D3 and 45 placebo). In those patients, treatment with vitamin D3 was associated with a lower ARR (rR = 0.403, 95% CI 0.208–0.814; p = 0.012), a reduction in the number of new T1-weighted lesions (rR = 0.494, 95% CI 0.267–0.913; p = 0.025), a lower volume of hypointense T1-weighted MRI lesions (− 312, 95% CI − 596 to − 29; p = 0.031), and a lower progression of EDSS (− 0.06 ± 0.78 vs. 0.32 ± 0.87, 95% CI − 0.614 to − 0.043; p = 0.026).
Safety
No RCTs revealed significant adverse events that can be attributed to even highly dosed vitamin D supplements in addition to other disease-modifying therapies (DMTs) [9, 10, 56, 62, 67]. Various notes of caution have been published regarding hypercalcemia as the main manifestation of vitamin D toxicity [11]. In one study, mice with EAE developed hypercalcemia after being supplemented with high doses of vitamin D [73]. Hypercalcemia was not seen in the human RCTs in MS, but has been described in an MS patient taking high doses of both vitamin D3 and calcium supplements [74]. An additional case report suggests relevant toxic effects of ultra-high doses of vitamin D exceeding 50,000 IU/day [75], but the added value of reaching these high 25(OH)D levels can be debated based on the spectrum of RCTs performed (see Sect. 6.2). There are no safety concerns for vitamin D3 supplements when targeting the (high) physiological range.
Interpretation of Randomized Controlled Trial Data
When assessing the studies performed thus far, a discrepancy between observational studies and trials becomes eminent. In the observation studies, each 25 nmol/L increase in 25(OH)D was associated with a 14–34% reduced relapse risk and 15–50% reduced risk of new MRI lesions (Tables 2 and 3). Average baseline 25(OH)D levels of 53 nmol/L were elevated with approximately 108 nmol/L in the supplementation studies (Fig. 1). If the reported associations in the observational studies could be directly extrapolated, this would imply a huge treatment effect of vitamin D, with an almost abrogation of disease activity. Clearly, this is not what has been observed. Although the MRI data from the new RCTs suggest that there is a beneficial effect of vitamin D [9, 10], no single study met its primary endpoint in the ITT cohorts. What happened?
Table 3.
Association of 25-hydroxyvitamin D levels with occurrence of magnetic resonance imaging lesions
| Study | Year | n | Follow-up (years) | Proportional decrease in OR/IR/RR/HR for each 25 nmol/L increase in 25(OH)D (%)a | Specification |
|---|---|---|---|---|---|
| Mowry et al. [43] | 2012 | 469 | 5 | 15 | T2, RRMS/CIS |
| 32 | GE, RRMS/CIS | ||||
| Løken-Amsrud et al. [46] | 2012 | 88 | 0.5 | 29 | T2, untreated RRMSb |
| 32 | GE, untreated RRMSb | ||||
| Ascherio et al. [40] | 2014 | 465 | 5 | 29–32 | T2/GE, CIS |
| Fitzgerald et al. [44] | 2015 | 1482 | 2 | 16 | T2/GE, RRMS |
| Cree et al. [45] | 2016 | 517 | 2 | 50 | GE, only in RRMS subgroup |
25(OH)D 25-hydroxyvitamin D, CIS clinically isolated syndrome, GE new gadolinium-enhancing T1 lesions, HR hazard ratio, IR incidence risk, MRI magnetic resonance imaging, OR odds ratio, RR relative risk, RRMS relapsing–remitting multiple sclerosis, T2 new T2 lesions
aOR, IR, RR, and HR for a new MRI lesion (yes/no) values were linearly recalculated to correspond with a 25 nmol/L (10 ng/L) increase in 25(OH)D levels
bNo effect of 25(OH) D during 1.5 subsequent years on interferon-β
Fig. 1.

Reported median/mean baseline 25-hydroxyvitamin D [25(OH)D] levels and the reported mean/median elevation of 25(OH)D levels in the supplementation arms of controlled vitamin D2/D3 supplementation studies. Studies are labeled with the first author names of the study reports [9, 10, 54–56, 62, 65, 67]. Dot size corresponds with sample size of the intention-to-treat cohorts (smallest n < 25, largest n > 200)
A first explanation is that associations between vitamin D status and MS outcomes are likely not entirely driven by a direct effect of vitamin D on the disease course of MS. There is a substantial body of evidence that 1,25(OH)2D is an anti-inflammatory molecule, and Mendelic randomization studies strongly support a qualitative effect of vitamin D on MS onset (see Sect. 3). However, these studies do not clarify the quantitative contribution of this effect to the associations observed. Reverse causality may operate at the same time (Fig. 2). Since inflammation lowers 25(OH)D levels [76], an active immune system more prone to accumulate in MS or subclinical disease activity may reduce 25(OH)D levels. Additionally, subclinical disease activity and disability may induce sun-avoiding behavior [77]. Furthermore, association studies can be confounded by other factors that elevate 25(OH)D and suppress MS activity, without the latter two being related. UVB exposure has direct immune-modulating effects, and low UVB/sun exposure has indeed been shown to be an independent risk factor for MS development and early progression [78]. UVB exposure is thus likely the main confounding factor for vitamin D in MS, and UVB phototherapy is currently also under investigation for disease-modifying properties in MS [79]. A higher body mass index (BMI) is associated with lower 25(OH)D levels but also with brain atrophy and a more detrimental disease course in MS [80, 81]. Furthermore, unknown factors in diet and general physical activity may affect desease course and also be associated with 25(OH)D levels.
Fig. 2.
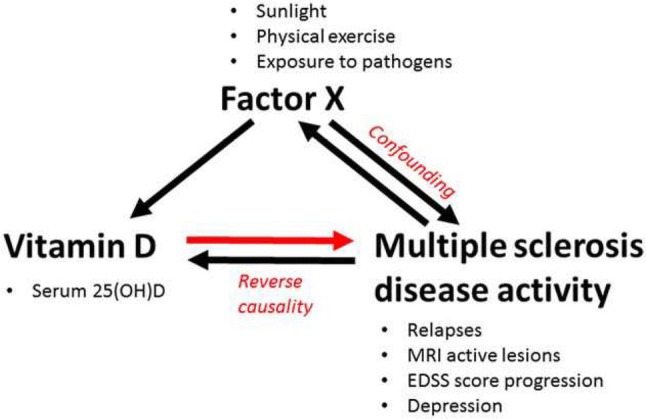
Interaction of vitamin D status with multiple sclerosis (MS) onset and MS disease activity. When an effect of vitamin D on the disease course of MS is assumed, the association may also be explained by an effect of these MS outcomes on vitamin D (reverse causality) or by an effect of another factor that suppresses MS and promotes vitamin D (confounding). Reprinted from Smolders et al. [97], with permission from Elsevier. 25(OH)D 25-hydroxyvitamin D, EDSS Expanded Disability Severity Scale, MRI magnetic resonance imaging
Second, since most RCTs on vitamin D have been powered based on results from observational studies, they are much smaller than studies on conventional immunomodulatory drugs and may lack the power to detect smaller effects of vitamin D supplements. Signals supporting this hypothesis come from secondary endpoints, where improved MRI endpoints are reported in vitamin D arms [9, 62], or in subgroups of protocol completers [10]. These small sample sizes do not allow post hoc analyses of subgroups which may have had the most benefit of supplements, as has been suggested in the observational studies (Tables 1 and 2). A meta-analysis with sensitivity analyses could suggest whether patients with the lowest 25(OH)D levels, the shortest disease duration (i.e., CIS vs. advanced RRMS), or the most active disease course of MS have more prominent treatment effects. The last subgroup may be suggested by the absence of significant effects of vitamin D supplements on dichotomized outcomes (i.e., relapse free and free from active lesions as included in NEDA-3) versus continuous outcomes (relapse rate and number of active lesions) in SOLAR [9]. The same could hypothetically account for participants with the highest anti-EBNA1 IgG levels, the only biomarker that is reduced after vitamin D3 supplements in independent RRMS cohorts [61, 72]. It should also be pointed out that the duration of the RCTs is 2 years or shorter, while observation studies have shown positive effects on disease course during a much longer follow-up [40]. This is, however, not likely to fully explain the discrepancies between the results from RCTs and observational studies, as several observational studies showed short-term results [43–45, 54]. Lastly, most large RCTs have so far either exclusively or predominantly included patients on IFN-β [9, 10, 56, 62, 65]. It is possible that the effect of vitamin D would be different in combination with another disease-modifying drug. However, it is not plausible that this will apply for drugs that reduce inflammatory disease activity more efficiently than IFN-β. Additionally, the largest observational studies showing associations between 25(OH)D levels and disease activity in MS also included predominantly IFN-β-treated participants [38, 42, 82, 83].
The studies performed thus far were helpful to downscale the enormous expectations of vitamin D supplements as a disease-modifying treatment in MS, and to provide at least some hopeful signals that a treatment effect may be present on selected outcomes in selected subgroups. However, no single RCT thus far was of sufficient size to pinpoint these subgroups. Currently, the most urgent need is a supplementation study that reaches its primary endpoint and confirms the main assumptions of its design. Until then, the relevance of vitamin D supplements for the management of active RRMS will remain a matter of debate.
In addition to the question of efficacy, there are other knowledge gaps to bridge. One main question is how much vitamin D should be supplemented, and which 25(OH)D levels should be targeted. Three observations are worthwhile to take into account when thinking about this issue. First, subjects with 25(OH)D levels exceeding 100 nmol/L did best on the outcomes investigated in the MS incidence studies, as was recently propagated by the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS) environmental risk factor workshop [84]. Second, an inverse association between relapse risk and 25(OH)D levels reached could only be observed for 25(OH)D levels up to 110 nmol/L in an uncontrolled study—above this, the association was lost [85]. Observational studies generally also did not include subjects with 25(OH)D levels exceeding 150 nmol/L [43, 46, 82, 86, 87]. Third, the largest vitamin D3 supplementation studies performed thus far did not show any convincing signals supporting a relevant benefit of targeting supraphysiological 25(OH)D levels [9] versus the (high) physiological range [62, 88]. Optimally, this observation should be subject to meta-analysis of RCT data. Based on these observations, targeting levels of 100 nmol/L would be a reasonable strategy, and no observations support higher levels [73].
Why and How to Pursue a Role of Vitamin D as a Disease-Modifying Therapy in MS?
A legitimate question that arises is whether it is worthwhile to pursue the research on potential benefits of vitamin D supplementation for people with MS. As elaborated in this review, treatment effects are likely small when compared to the expanding package of highly efficacious DMTs [74]. Efficacy can be weighed against various variables, including the efficacy of other DMTs. An alternative approach is to weigh efficacy against potential harmful effects of a treatment. Although not elaborated on, no RCTs revealed adverse events that can be attributed to vitamin D supplements. Efficacy can also be weighed against economic costs. Vitamin D3 supplements, even in high doses, are very inexpensive when compared to DMTs. Should the point estimate for ARR reduction as reported in the SOLAR trial hold true in future powered studies [9], the crude compound costs to achieve this reduction could be much lower than that of licensed first-line disease-modifying drugs, although these have larger effect sizes [75]. A favorable cost–benefit ratio regarding burden for patients in terms of adverse effects and for society in terms of financial costs could justify a less impressive efficacy. However, it should be underscored that the beneficial effect of vitamin D in MS remains unproven, and thus does not fulfill the criteria for reimbursement by public healthcare systems.
Luckily, several RCTs are still ongoing, and these will provide additional information on vitamin D3 supplementation in MS (summarized in Amato et al. [84]). However, it remains unlikely that these (small) studies will be able to completely bridge the aforementioned knowledge gaps regarding dosing, subgroups, 25(OH)D levels, and disease activity. These issues call for meta-analyses of original study data and, optimally, large-scale clinical trials of sufficient power to allow subgroup analyses. We propose that such a trial should include RRMS patients early in their disease, with clinical or MRI activity, and that 25(OH)D levels exceeding 100 nmol/L should be targeted in addition to conventional DMTs. Inclusion of patients with the lowest 25(OH)D levels will likely increase the odds of a trial achieving positive results on its primary endpoint. Although the evidence for low 25(OH)D levels as a biomarker for active MS is mounting, the current absence of significant effects on primary efficacy endpoints makes it ethically sound to compare vitamin D supplementation with placebo in such a trial [89].
In addition to established clinical and MRI endpoints, measuring biomarkers of end-organ damage in samples collected from the ongoing or already conducted studies would be of added value. Neurofilament light chain has been shown to reflect therapeutic effects in MS, and the serum concentration is tightly correlated with that in cerebrospinal fluid [81]. Vitamin D supplementation had no overall effect on neurofilament light chain in the only study published so far [60]. However, the baseline inflammatory activity in this study was low, and in spite of that there was a positive trend in the subgroup not treated with conventional disease-modifying drugs. Hopefully other studies can extend these data.
Advice for People with MS and Clinicians Based on Current Data
Low serum levels of 25(OH)D are frequently encountered in people with MS [83], and are useful for patients and clinicians to unveil. First, even at the start of their disease, people with MS report an increased tendency to fall and have more fractures than people without MS [90–92]. Low bone mineral density is frequent already at the time of diagnosis [93]. Although high-dose supplementation did not prevent bone loss in a cohort with relatively good vitamin D status [57, 58], correction of the lowest 25(OH)D levels may promote bone health and reduce fracture risk [11]. The optimal 25(OH)D level for bone health is not established, but it is generally accepted that vitamin D levels < 50 nmol/L are suboptimal [94]. When assessing vitamin D measurements in individual patients, seasonal variation should be taken into account. The serum level in Scandinavian MS patients is likely to be 50% lower in late winter/spring than in late summer [95], but this is probably different in other geographical areas where seasonal variations in UV exposure and sun habits are different. Second, since low 25(OH)D levels are a biomarker of higher subsequent disease activity (see Sect. 8), it could be used in prognostic considerations and treatment decisions in early MS. Third, although not conclusive, we presented reasonable data from observational studies and RCTs which suggest a benefit for patients who elevate their 25(OH)D to higher levels (see Sect. 8). This fits in the current concept of promoting general brain health [94], in which a healthy diet, active lifestyle, not being obese, and cessation of smoking and alcohol are promoted to make the CNS more resilient to the inflammatory attack of MS. Prevention of the lowest 25(OH)D ranges, associated with loss of bone mineral density and the most detrimental endpoints in MS, can be achieved with vitamin D3 1000–2000 IU/day. The earlier suggested optimal 25(OH)D levels exceeding 100 nmol/L [73, 84] were achieved in the context of a controlled study by supplementing people with RRMS with vitamin D3 4000 IU/day [96]. This would be reasonable advice for people with MS in northern parts of Europe and North America, whereas people in Australia and other more sunny areas may require less supplementation.
Compliance with Ethical Standards
Conflict of interest
Dr. Smolders received speaker and/or consultancy fees from Biogen, Merck, Novartis, and Sanofi-Genzyme. Dr Holmøy, Dr Torkildsen, and Dr Camu report no conflicts of interest that are directly relevant to this review.
Funding
No targeted funding was received for this review. The open access fee was paid by the University of Oslo.
References
- 1.Camu W, Tremblier B, Plassot C, Alphandery S, Salsac C, Pageot N, et al. Vitamin D confers protection to motoneurons and is a prognostic factor of amyotrophic lateral sclerosis. Neurobiol Aging. 2014;35:1198–1205. doi: 10.1016/j.neurobiolaging.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Peelen E, Knippenberg S, Muris A-H, Thewissen M, Smolders J, Tervaert JWC, et al. Effects of vitamin D on the peripheral adaptive immune system: a review. Autoimmun Rev. 2011;10:733–743. doi: 10.1016/j.autrev.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Goldberg P. Multiple Sclerosis: vitamin D and calcium as environmental determinants of prevalence. Part 1: sunlight, dietary factors and epidemiology. J Environ Stud. 1974;6:19–27. [Google Scholar]
- 4.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 5.Tsoukas CD, Provvedini DM, Manolagas SC. 1,25-dihydroxyvitamin D3: a novel immunoregulatory hormone. Science. 1984;224:1438–1440. doi: 10.1126/science.6427926. [DOI] [PubMed] [Google Scholar]
- 6.Goldberg P, Fleming MC, Picard EH. Multiple sclerosis: decreased relapse rate through dietary supplementation with calcium, magnesium and vitamin D. Med Hypotheses. 1986;21(2):193–200. doi: 10.1016/0306-9877(86)90010-1. [DOI] [PubMed] [Google Scholar]
- 7.Jorde R. RCTS are the only appropriate way to demonstrate the role of vitamin D in health. J Steroid Biochem Mol Biol. 2018;177:10–14. doi: 10.1016/j.jsbmb.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Jagannath VA, Filippini G, Di Pietrantonj C, Asokan GV, Robak EW, Whamond L, et al. Vitamin D for the management of multiple sclerosis. Cochrane Database Syst Rev. 2018;9:CD008422. doi: 10.1002/14651858.CD008422.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hupperts R, Smolders J, Di Vieth R, Holmøy T, Marhardt K, Schluep M, et al. Randomised trial of daily high-dose vitamin D3 in RRMS patients receiving sc interferon beta-1a. Neurology. 2018;93:1–11. doi: 10.1212/WNL.0000000000008445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camu W, Lehert P, Pierrot Deseilligny C, Hautecoeur P, Besserve A, Jean Deleglise A, et al. Cholecalciferol in relapsing-remitting MS. A randomized clinical trial (CHOLINE) Neurol Neuroimmunol Neuroinflamm. 2019;6(5):e597. doi: 10.1212/NXI.0000000000000597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 12.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1 alpha-hydroxylase in human brain. J Chem Neuroanat. 2005;29:21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 13.Prüfer K, Veenstra TD, Jirikowski GF, Kumar R. Distribution of 1,25-dihydroxyvitamin D3 receptor immunoreactivity in the rat brain and spinal cord. J Chem Neuroanat. 1999;16:135–145. doi: 10.1016/s0891-0618(99)00002-2. [DOI] [PubMed] [Google Scholar]
- 14.Smolders J, Moen SM, Damoiseaux J, Huitinga I, Holmøy T. Vitamin D in the healthy and inflamed central nervous system: access and function. J Neurol Sci. 2011;311:37–43. doi: 10.1016/j.jns.2011.07.033. [DOI] [PubMed] [Google Scholar]
- 15.Smolders J, Damoiseaux J. Vitamin D as a T-cell modulator in multiple sclerosis. Vitam Horm. 2011;86:401–428. doi: 10.1016/B978-0-12-386960-9.00018-6. [DOI] [PubMed] [Google Scholar]
- 16.Holmøy T, Moen SM, Gundersen TA, Holick MF, Fainardi E, Castellazzi M, et al. 25-Hydroxyvitamin D in cerebrospinal fluid during relapse and remission of multiple sclerosis. Mult Scler J. 2009;15:1280–1285. doi: 10.1177/1352458509107008. [DOI] [PubMed] [Google Scholar]
- 17.Smolders J, Schuurman KG, Van Strien ME, Melief J, Hendrickx D, Hol EM, et al. Expression of vitamin D receptor and metabolizing enzymes in multiple sclerosis-affected brain tissue. J Neuropathol Exp Neurol. 2013;72:91–105. doi: 10.1097/NEN.0b013e31827f4fcc. [DOI] [PubMed] [Google Scholar]
- 18.Munger KL, Levin LI, Hollis BW, Howard NS, Ascherio A. Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis. JAMA. 2006;296:2832. doi: 10.1001/jama.296.23.2832. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen NM, Munger KL, Koch-Henriksen N, Hougaard DM, Magyari M, Jørgensen KT, et al. Neonatal vitamin D status and risk of multiple sclerosis. Neurology. 2017;88:44–51. doi: 10.1212/WNL.0000000000003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munger KL, Åivo J, Hongell K, Soilu-Hänninen M, Surcel H-M, Ascherio A. Vitamin D status during pregnancy and risk of multiple sclerosis in offspring of women in the Finnish Maternity Cohort. JAMA Neurol. 2016;73:515. doi: 10.1001/jamaneurol.2015.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munger KL, Zhang SM, O’Reilly E, Hernán MA, Olek MJ, Willett WC, et al. Vitamin D intake and incidence of multiple sclerosis. Neurology. 2004;62:60–65. doi: 10.1212/01.wnl.0000101723.79681.38. [DOI] [PubMed] [Google Scholar]
- 22.Sawcer S, Hellenthal G, Pirinen M, Spencer CCA, Patsopoulos NA, Moutsianas L, et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature. 2011;476:214–219. doi: 10.1038/nature10251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gianfrancesco MA, Stridh P, Rhead B, Shao X, Xu E, Graves JS, et al. Evidence for a causal relationship between low vitamin D, high BMI, and pediatric-onset MS. Neurology. 2017;88:1623–1629. doi: 10.1212/WNL.0000000000003849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhead B, Bäärnhielm M, Gianfrancesco M, Mok A, Shao X, Quach H, et al. Mendelian randomization shows a causal effect of low vitamin D on multiple sclerosis risk. Neurol Genet. 2016;2:e97. doi: 10.1212/NXG.0000000000000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mokry LE, Ross S, Ahmad OS, Forgetta V, Smith GD, Leong A, et al. Vitamin D and risk of multiple sclerosis: a Mendelian randomization study. PLoS Med. 2015;12:e1001866. doi: 10.1371/journal.pmed.1001866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shirazi HA, Rasouli J, Ciric B, Rostami A, Zhang G-X. 1,25-Dihydroxyvitamin D3 enhances neural stem cell proliferation and oligodendrocyte differentiation. Exp Mol Pathol. 2015;98:240–245. doi: 10.1016/j.yexmp.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemire JM, Archer DC. 1,25-Dihydroxyvitamin D3 prevents the in vivo induction of murine experimental autoimmune encephalomyelitis. J Clin Investig. 1991;87:1103–1107. doi: 10.1172/JCI115072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantorna MT, Hayes CE, DeLuca HF. 1,25-Dihydroxyvitamin D3 reversibly blocks the progression of relapsing encephalomyelitis, a model of multiple sclerosis. Proc Natl Acad Sci USA. 1996;93:7861–7864. doi: 10.1073/pnas.93.15.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayes CE, Hubler SL, Moore JR, Barta LE, Praska CE, Nashold FE. Vitamin D actions on CD4+ T cells in autoimmune disease. Front Immunol. 2015;6:100. doi: 10.3389/fimmu.2015.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shirazi HA, Rasouli J, Ciric B, Wei D, Rostami A, Zhang G-X. 1,25-Dihydroxyvitamin D3 suppressed experimental autoimmune encephalomyelitis through both immunomodulation and oligodendrocyte maturation. Exp Mol Pathol. 2017;102:515–521. doi: 10.1016/j.yexmp.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goudarzvand M, Javan M, Mirnajafi-Zadeh J, Mozafari S, Tiraihi T. Vitamins E and D3 attenuate demyelination and potentiate remyelination processes of hippocampal formation of rats following local injection of ethidium bromide. Cell Mol Neurobiol. 2010;30:289–299. doi: 10.1007/s10571-009-9451-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blakemore WF. Ethidium bromide induced demyelination in the spinal cord of the cat. Neuropathol Appl Neurobiol. 1982;8:365–375. doi: 10.1111/j.1365-2990.1982.tb00305.x. [DOI] [PubMed] [Google Scholar]
- 33.Wergeland S, Torkildsen Ø, Myhr K-M, Aksnes L, Mørk SJ, Bø L. Dietary vitamin D3 supplements reduce demyelination in the cuprizone model. PLoS One. 2011;6:e26262. doi: 10.1371/journal.pone.0026262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torkildsen O, Brunborg LA, Myhr K-M, Bø L. The cuprizone model for demyelination. Acta Neurol Scand Suppl. 2008;188:72–76. doi: 10.1111/j.1600-0404.2008.01036.x. [DOI] [PubMed] [Google Scholar]
- 35.Nystad AE, Wergeland S, Aksnes L, Myhr K-M, Bø L, Torkildsen O. Effect of high-dose 1.25 dihydroxyvitamin D3 on remyelination in the cuprizone model. APMIS. 2014;122:1178–1186. doi: 10.1111/apm.12281. [DOI] [PubMed] [Google Scholar]
- 36.Mashayekhi F, Salehi Z. Administration of vitamin D3 induces CNPase and myelin oligodendrocyte glycoprotein expression in the cerebral cortex of the murine model of cuprizone-induced demyelination. Folia Neuropathol. 2016;54:259–264. doi: 10.5114/fn.2016.62535. [DOI] [PubMed] [Google Scholar]
- 37.Nystad AE, Torkildsen Ø, Wergeland S. Effects of vitamin D on axonal damage during de- and remyelination in the cuprizone model. J Neuroimmunol. 2018;321:61–65. doi: 10.1016/j.jneuroim.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 38.Oveland E, Nystad A, Berven F, Myhr K-M, Torkildsen Ø, Wergeland S. 1,25-Dihydroxyvitamin-D3 induces brain proteomic changes in cuprizone mice during remyelination involving calcium proteins. Neurochem Int. 2018;112:267–277. doi: 10.1016/j.neuint.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 39.de la Fuente AG, Errea O, van Wijngaarden P, Gonzalez GA, Kerninon C, Jarjour AA, et al. Vitamin D receptor–retinoid X receptor heterodimer signaling regulates oligodendrocyte progenitor cell differentiation. J Cell Biol. 2015;211:975–985. doi: 10.1083/jcb.201505119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ascherio A, Munger KL, White R, Köchert K, Simon KC, Polman CH, et al. Vitamin D as an early predictor of multiple sclerosis activity and progression. JAMA Neurol. 2014;71:306. doi: 10.1001/jamaneurol.2013.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuhle J, Disanto G, Dobson R, Adiutori R, Bianchi L, Topping J, et al. Conversion from clinically isolated syndrome to multiple sclerosis: a large multicentre study. Mult Scler J. 2015;21:1013–1024. doi: 10.1177/1352458514568827. [DOI] [PubMed] [Google Scholar]
- 42.Muris A-H, Smolders J, Rolf L, Klinkenberg LJ, van der Linden N, Meex S, et al. Vitamin D status does not affect disability progression of patients with multiple sclerosis over three year follow-up. PLoS One. 2016;11:e0156122. doi: 10.1371/journal.pone.0156122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mowry EM, Waubant E, McCulloch CE, Okuda DT, Evangelista AA, Lincoln RR, et al. Vitamin D status predicts new brain magnetic resonance imaging activity in multiple sclerosis. Ann Neurol. 2012;72:234–240. doi: 10.1002/ana.23591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fitzgerald KC, Munger KL, Köchert K, Arnason BGW, Comi G, Cook S, et al. Association of vitamin D levels with multiple sclerosis activity and progression in patients receiving interferon beta-1b. JAMA Neurol. 2015;72:1458. doi: 10.1001/jamaneurol.2015.2742. [DOI] [PubMed] [Google Scholar]
- 45.Cree BAC, Gourraud P-A, Oksenberg JR, Bevan C, Crabtree-Hartman E, Gelfand JM, University of California, San Francisco MS-EPIC Team et al. Long-term evolution of multiple sclerosis disability in the treatment era. Ann Neurol. 2016;80:499–510. doi: 10.1002/ana.24747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Løken-Amsrud KI, Holmøy T, Bakke SJ, Beiske AG, Bjerve KS, Bjørnarå BT, et al. Vitamin D and disease activity in multiple sclerosis before and during interferon-β treatment. Neurology. 2012;79:267–273. doi: 10.1212/WNL.0b013e31825fdf01. [DOI] [PubMed] [Google Scholar]
- 47.Burton JM, Eliasziw M, Trufyn J, Tung C, Carter G, Costello F. A prospective cohort study of vitamin D in optic neuritis recovery. Mult Scler. 2017;23:82–93. doi: 10.1177/1352458516642315. [DOI] [PubMed] [Google Scholar]
- 48.Munger KL, Köchert K, Simon KC, Kappos L, Polman CH, Freedman MS, et al. Molecular mechanism underlying the impact of vitamin D on disease activity of MS. Ann Clin Transl Neurol. 2014;1:605–617. doi: 10.1002/acn3.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wergeland S, Myhr K-M, Løken-Amsrud KI, Beiske AG, Bjerve KS, Hovdal H, et al. Vitamin D, HLA-DRB1 and Epstein-Barr virus antibody levels in a prospective cohort of multiple sclerosis patients. Eur J Neurol. 2016;23:1064–1070. doi: 10.1111/ene.12986. [DOI] [PubMed] [Google Scholar]
- 50.Pérez-Pérez S, Domínguez-Mozo MI, García-Martínez MÁ, Aladro Y, Martínez-Ginés M, García-Domínguez JM, et al. Study of the possible link of 25-hydroxyvitamin D with Epstein–Barr virus and human herpesvirus 6 in patients with multiple sclerosis. Eur J Neurol. 2018;25:1446–1453. doi: 10.1111/ene.13749. [DOI] [PubMed] [Google Scholar]
- 51.Lossius A, Johansen JN, Torkildsen Ø, Vartdal F, Holmøy T. Epstein-Barr virus in systemic lupus erythematosus, rheumatoid arthritis and multiple sclerosis—association and causation. Viruses. 2012;4:3701–3730. doi: 10.3390/v4123701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Derakhshandi H, Etemadifar M, Feizi A, Abtahi S-H, Minagar A, Abtahi M-A, et al. Preventive effect of vitamin D3 supplementation on conversion of optic neuritis to clinically definite multiple sclerosis: a double blind, randomized, placebo-controlled pilot clinical trial. Acta Neurol Belg. 2013;113:257–263. doi: 10.1007/s13760-012-0166-2. [DOI] [PubMed] [Google Scholar]
- 53.Salari M, Janghorbani M, Etemadifar M, Dehghani A, Razmjoo H, Naderian G. Effects of vitamin D on retinal nerve fiber layer in vitamin D deficient patients with optic neuritis: preliminary findings of a randomized, placebo-controlled trial. J Res Med Sci. 2015;20:372–378. [PMC free article] [PubMed] [Google Scholar]
- 54.O’Connell K, Sulaimani J, Basdeo SA, Kinsella K, Jordan S, Kenny O, et al. Effects of vitamin D3 in clinically isolated syndrome and healthy control participants: a double-blind randomised controlled trial. Mult Scler J Exp Transl Clin. 2017;3:2055217317727296. doi: 10.1177/2055217317727296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stein MS, Liu Y, Gray OM, Baker JE, Kolbe SC, Ditchfield MR, et al. A randomized trial of high-dose vitamin D2 in relapsing-remitting multiple sclerosis. Neurology. 2011;77:1611–1618. doi: 10.1212/WNL.0b013e3182343274. [DOI] [PubMed] [Google Scholar]
- 56.Kampman MT, Steffensen LH, Mellgren SI, Jørgensen L. Effect of vitamin D3 supplementation on relapses, disease progression, and measures of function in persons with multiple sclerosis: exploratory outcomes from a double-blind randomised controlled trial. Mult Scler J. 2012;18:1144–1151. doi: 10.1177/1352458511434607. [DOI] [PubMed] [Google Scholar]
- 57.Steffensen LH, Jørgensen L, Straume B, Mellgren SI, Kampman MT. Can vitamin D supplementation prevent bone loss in persons with MS? A placebo-controlled trial. J Neurol. 2011;258:1624–1631. doi: 10.1007/s00415-011-5980-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Holmøy T, Lindstrøm JC, Eriksen EF, Steffensen LH, Kampman MT. High dose vitamin D supplementation does not affect biochemical bone markers in multiple sclerosis—a randomized controlled trial. BMC Neurol. 2017;17:67. doi: 10.1186/s12883-017-0851-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Røsjø E, Steffensen LH, Jørgensen L, Lindstrøm JC, Šaltytė Benth J, Michelsen AE, et al. Vitamin D supplementation and systemic inflammation in relapsing-remitting multiple sclerosis. J Neurol. 2015;262:2713–2721. doi: 10.1007/s00415-015-7902-5. [DOI] [PubMed] [Google Scholar]
- 60.Holmøy T, Røsjø E, Zetterberg H, Blennow K, Lindstrøm JC, Steffensen LH, et al. Vitamin D supplementation and neurofilament light chain in multiple sclerosis. Acta Neurol Scand. 2019;139:172–176. doi: 10.1111/ane.13037. [DOI] [PubMed] [Google Scholar]
- 61.Røsjø E, Lossius A, Abdelmagid N, Lindstrøm JC, Kampman MT, Jørgensen L, et al. Effect of high-dose vitamin D3 supplementation on antibody responses against Epstein–Barr virus in relapsing-remitting multiple sclerosis. Mult Scler. 2017;23:395–402. doi: 10.1177/1352458516654310. [DOI] [PubMed] [Google Scholar]
- 62.Soilu-Hänninen M, Aivo J, Lindström B-M, Elovaara I, Sumelahti M-L, Färkkilä M, et al. A randomised, double blind, placebo controlled trial with vitamin D3 as an add on treatment to interferon β-1b in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2012;83:565–571. doi: 10.1136/jnnp-2011-301876. [DOI] [PubMed] [Google Scholar]
- 63.Åivo J, Hänninen A, Ilonen J, Soilu-Hänninen M. Vitamin D3 administration to MS patients leads to increased serum levels of latency activated peptide (LAP) of TGF-beta. J Neuroimmunol. 2015;280:12–15. doi: 10.1016/j.jneuroim.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 64.Shaygannejad V, Janghorbani M, Ashtari F, Dehghan H. Effects of adjunct low-dose vitamin D on relapsing-remitting multiple sclerosis progression: preliminary findings of a randomized placebo-controlled trial. Mult Scler Int. 2012;2012:1–7. doi: 10.1155/2012/452541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Golan D, Halhal B, Glass-Marmor L, Staun-Ram E, Rozenberg O, Lavi I, et al. Vitamin D supplementation for patients with multiple sclerosis treated with interferon-beta: a randomized controlled trial assessing the effect on flu-like symptoms and immunomodulatory properties. BMC Neurol. 2013;13:60. doi: 10.1186/1471-2377-13-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Achiron A, Givon U, Magalashvili D, Dolev M, Liraz Zaltzman S, Kalron A, et al. Effect of Alfacalcidol on multiple sclerosis-related fatigue: a randomized, double-blind placebo-controlled study. Mult Scler. 2015;21:767–775. doi: 10.1177/1352458514554053. [DOI] [PubMed] [Google Scholar]
- 67.Sotirchos ES, Bhargava P, Eckstein C, Van Haren K, Baynes M, Ntranos A, et al. Safety and immunologic effects of high- vs low-dose cholecalciferol in multiple sclerosis. Neurology. 2016;86:382–390. doi: 10.1212/WNL.0000000000002316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Smolders J, Hupperts R, Barkhof F, Grimaldi LM, Holmoy T, Killestein J, Rieckmann P, Schluep M, Vieth R. Hostalek U, Ghazi-Visser L, Beelke M; SOLAR study group.Efficacy of vitamin D3 as add-on therapy in patients with relapsing-remitting multiple sclerosis receiving subcutaneous interferon β- 1a: a Phase II, multicenter, double-blind, randomized, placebo-controlled trial. J Neurol Sci. 2011;311:44–49. doi: 10.1016/j.jns.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 69.Muris A-H, Smolders J, Rolf L, Thewissen M, Hupperts R, Damoiseaux J. Immune regulatory effects of high dose vitamin D3 supplementation in a randomized controlled trial in relapsing remitting multiple sclerosis patients receiving IFNβ; the SOLARIUM study. J Neuroimmunol. 2016;300:47–56. doi: 10.1016/j.jneuroim.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 70.Rolf L, Muris A-H, Bol Y, Damoiseaux J, Smolders J, Hupperts R. Vitamin D3 supplementation in multiple sclerosis: symptoms and biomarkers of depression. J Neurol Sci. 2017;378:30–35. doi: 10.1016/j.jns.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 71.Rolf L, Muris A-H, Theunissen R, Hupperts R, Damoiseaux J, Smolders J. Vitamin D supplementation and the IL-2/IL-2R pathway in multiple sclerosis: attenuation of progressive disturbances? J Neuroimmunol. 2017;314:50–57. doi: 10.1016/j.jneuroim.2017.11.007. [DOI] [PubMed] [Google Scholar]
- 72.Rolf L, Muris A-H, Mathias A, Du Pasquier R, Koneczny I, Disanto G, et al. Exploring the effect of vitamin D 3 supplementation on the anti-EBV antibody response in relapsing-remitting multiple sclerosis. Mult Scler J. 2017;10:1280–1287. doi: 10.1177/1352458517722646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Holmoy T, Kampman MT, Smolders J. Vitamin D in multiple sclerosis: implications for assessment and treatment. Expert Rev Neurother. 2012;12:1101–1112. doi: 10.1586/ern.12.99. [DOI] [PubMed] [Google Scholar]
- 74.Wingerchuk DM, Weinshenker BG. Disease modifying therapies for relapsing multiple sclerosis. BMJ. 2016;354:i3518. doi: 10.1136/bmj.i3518. [DOI] [PubMed] [Google Scholar]
- 75.Montalban X, Gold R, Thompson AJ, Otero-Romero S, Amato MP, Chandraratna D, et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult Scler J. 2018;24:96–120. doi: 10.1177/1352458517751049. [DOI] [PubMed] [Google Scholar]
- 76.Holowaychuk MK, Birkenheuer AJ, Li J, Marr H, Boll A, Nordone SK. Hypocalcemia and hypovitaminosis D in dogs with induced endotoxemia. J Vet Intern Med. 2012;26:244–251. doi: 10.1111/j.1939-1676.2012.00886.x. [DOI] [PubMed] [Google Scholar]
- 77.van der Mei IAF, Ponsonby A-L, Dwyer T, Blizzard L, Taylor BV, Kilpatrick T, et al. Vitamin D levels in people with multiple sclerosis and community controls in Tasmania, Australia. J Neurol. 2007;254:581–590. doi: 10.1007/s00415-006-0315-8. [DOI] [PubMed] [Google Scholar]
- 78.Simpson S, Jr, van der Mei I, Lucas RM, Ponsonby AL, Broadley S, Blizzard L, Ausimmune/AusLong Investigators Group. Taylor B. Sun exposure across the life course significantly modulates early multiple sclerosis clinical course. Front Neurol. 2018;9:16. doi: 10.3389/fneur.2018.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hart PH, Jones AP, Trend S, Cha L, Fabis-Pedrini MJ, Cooper MN, et al. A randomised, controlled clinical trial of narrowband UVB phototherapy for clinically isolated syndrome: the PhoCIS study. Mult Scler J Exp Transl Clin. 2018;4:205521731877311. doi: 10.1177/2055217318773112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mowry EM, Azevedo CJ, McCulloch CE, Okuda DT, Lincoln RR, Waubant E, et al. Body mass index, but not vitamin D status, is associated with brain volume change in MS. Neurology. 2018;91(24):e2256–e2264. doi: 10.1212/WNL.0000000000006644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khalil M, Teunissen CE, Otto M, Piehl F, Sormani MP, Gattringer T, et al. Neurofilaments as biomarkers in neurological disorders. Nat Rev Neurol. 2018;14:577–589. doi: 10.1038/s41582-018-0058-z. [DOI] [PubMed] [Google Scholar]
- 82.Simpson S, Taylor B, Blizzard L, Ponsonby A-L, Pittas F, Tremlett H, et al. Higher 25-hydroxyvitamin D is associated with lower relapse risk in MS. Ann Neurol. 2010;68:193–203. doi: 10.1002/ana.22043. [DOI] [PubMed] [Google Scholar]
- 83.Smolders J, Menheere P, Kessels A, Damoiseaux J, Hupperts R. Association of vitamin D metabolite levels with relapse rate and disability in multiple sclerosis. Mult Scler. 2008;14:1220–1224. doi: 10.1177/1352458508094399. [DOI] [PubMed] [Google Scholar]
- 84.Amato MP, Derfuss T, Hemmer B, Liblau R, Montalban X, Soelberg Sørensen P, et al. Environmental modifiable risk factors for multiple sclerosis: report from the 2016 ECTRIMS focused workshop. Mult Scler. 2017 doi: 10.1177/1352458516686847. [DOI] [PubMed] [Google Scholar]
- 85.Pierrot-Deseilligny C, Rivaud-Péchoux S, Clerson P, de Paz R, Souberbielle J-C. Relationship between 25-OH-D serum level and relapse rate in multiple sclerosis patients before and after vitamin D supplementation. Ther Adv Neurol Disord. 2012;5:187–198. doi: 10.1177/1756285612447090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mowry EM, Krupp LB, Milazzo M, Chabas D, Strober JB, Belman AL, et al. Vitamin D status is associated with relapse rate in pediatric-onset MS. Ann Neurol. 2010;67:618–624. doi: 10.1002/ana.21972. [DOI] [PubMed] [Google Scholar]
- 87.Runia TF, Hop WCJ, de Rijke YB, Buljevac D, Hintzen RQ. Lower serum vitamin D levels are associated with a higher relapse risk in multiple sclerosis. Neurology. 2012;79:261–266. doi: 10.1212/WNL.0b013e31825fdec7. [DOI] [PubMed] [Google Scholar]
- 88.Please provide complete reference.
- 89.Smolders J, Rolf L, Damoiseaux J, Hupperts R. On the ethics of not supplementing low 25-hydroxyvitamin D levels in a controlled study in relapsing remitting multiple sclerosis. J Neurol Sci. 2017;379:331. doi: 10.1016/j.jns.2017.06.009. [DOI] [PubMed] [Google Scholar]
- 90.Moen SM, Celius EG, Nordsletten L, Holmøy T. Fractures and falls in patients with newly diagnosed clinically isolated syndrome and multiple sclerosis. Acta Neurol Scand Suppl. 2011;124:79–82. doi: 10.1111/j.1600-0404.2011.01548.x. [DOI] [PubMed] [Google Scholar]
- 91.Bazelier MT, Bentzen J, Vestergaard P, Stenager E, Leufkens HG, van Staa T-P, et al. The risk of fracture in incident multiple sclerosis patients: the Danish National Health Registers. Mult Scler J. 2012;18:1609–1616. doi: 10.1177/1352458512442755. [DOI] [PubMed] [Google Scholar]
- 92.Bazelier MT, van Staa T-P, Uitdehaag BMJ, Cooper C, Leufkens HGM, Vestergaard P, et al. Risk of fractures in patients with multiple sclerosis: a population-based cohort study. Neurology. 2012;78:1967–1973. doi: 10.1212/WNL.0b013e318259e0ff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moen SM, Celius EG, Sandvik L, Nordsletten L, Eriksen EF, Holmoy T. Low bone mass in newly diagnosed multiple sclerosis and clinically isolated syndrome. Neurology. 2011;77:151–157. doi: 10.1212/WNL.0b013e3182242d34. [DOI] [PubMed] [Google Scholar]
- 94.Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium; Ross AC, Taylor CL, Yaktine AL, Del Valle HB, editors. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press (US); 2011. http://www.ncbi.nlm.nih.gov/pubmed/21796828. Accessed 6 Jun 2019. [PubMed]
- 95.Saltytė Benth J, Myhr K-M, Løken-Amsrud KI, Beiske AG, Bjerve KS, Hovdal H, et al. Modelling and prediction of 25-hydroxyvitamin D levels in Norwegian relapsing-remitting multiple sclerosis patients. Neuroepidemiology. 2012;39:84–93. doi: 10.1159/000339360. [DOI] [PubMed] [Google Scholar]
- 96.Rolf L, Damoiseaux J, Huitinga I, Kimenai D, van den Ouweland J, Hupperts R, et al. Stress-axis regulation by vitamin D3 in multiple sclerosis. Front Neurol. 2018;9:263. doi: 10.3389/fneur.2018.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smolders J, Hupperts R, Damoiseaux J. Chapter 8: the way forward for vitamin D in multiple sclerosis. In: Minagar A, editor. Neuroinflammation. 2. London: Elsevier; 2018. pp. 175–191. [Google Scholar]


