Abstract
Besides clinical characteristics, easy-accessible laboratory markers could be of value to refine risk stratification in peripheral artery disease. In the current study, we investigated whether α-hydroxybutyrate dehydrogenase (HBDH) is associated with atherothrombotic events in 83 stable patients undergoing infrainguinal angioplasty and stenting. The primary endpoint was defined as the composite of the first occurrence of nonfatal myocardial infarction, nonfatal stroke or transient ischemic attack and cardiovascular death within 2 years after angioplasty and stenting, and occurred in 6 patients (7.2%). HBDH levels at baseline were significantly higher in patients who subsequently developed the primary endpoint (126 U/L [116–137 U/L] vs. 105 U/L [95–120 U/L]; p = 0.04). ROC curve analysis revealed that HBDH could distinguish between patients without and with future atherothrombotic events. A HBDH concentration ≥ 115 U/L was identified as the best threshold to predict the composite endpoint, providing a sensitivity of 83.3% and a specificity of 71.4%, and was therefore defined as high HBDH. High HBDH was seen in 28 patients (33.7%). Ischemic events occurred significantly more often in patients with high HBDH than in patients with lower HBDH levels (5 vs. 1 patients, p = 0.007). In conclusion, HBDH is associated with the occurrence of atherothrombotic events after infrainguinal angioplasty with stent implantation. Future trials are warranted to study the predictive role of HBDH for ischemic outcomes and to investigate underlying mechanisms.
Subject terms: Peripheral vascular disease, Restenosis
Introduction
Patients with peripheral artery disease (PAD) are at an increased risk of atherothrombotic events like myocardial infarction and stroke1. Moreover, previous studies have shown that those undergoing angioplasty and stenting for PAD frequently suffer target vessel restenosis or reocclusion2–4. While the latter are predominantly a consequence of intimal hyperplasia5, atherothrombotic events are often initiated by plaque rupture with subsequent platelet and coagulation activation6, and may therefore be prevented by more intense antithrombotic therapy7,8. Indeed, a new antithrombotic regimen consisting of platelet inhibition with low-dose aspirin and inhibition of thrombin generation with a very low dose of the factor Xa antagonist rivaroxaban recently yielded promising results in patients with stable PAD9. In detail, rivaroxaban 2.5 mg twice daily in additon to 100 mg aspirin significantly reduced the occurrence of atherothrombotic events compared to 100 mg aspirin alone in 7470 PAD patients of the COMPASS (Cardiovascular Outcomes for People Using Anticoagulation Strategies) trial. However, the decrease in ischemic outcomes was achieved at the cost of an increased major bleeding risk9. Consequently, the new treatment regimen should only be prescribed in PAD patients at high risk of atherothrombotic events. In order to optimally select PAD patients in need of intensified antithrombotic therapy, it seems crucial to identify factors associated with adverse ischemic outcomes. Besides clinical characteristics, easy-accessible laboratory markers could be of value to refine risk stratification in PAD.
α-Hydroxybutyrate dehydrogenase (HBDH) is a marker of cell death particularly reflecting renal, red blood cell and myocardial damage10–14. Since chronic kidney disease, anemia and myocardial injury predispose patients to adverse events following percutaneous coronary intervention10,15–20, we hypothesized that HBDH may be associated with ischemic outcomes following infrainguinal angioplasty and stenting for stable PAD.
Methods
Patients
In this prospective cohort study, 83 patients undergoing successful infrainguinal angioplasty with endovascular stent implantation were enrolled consecutively at the Division of Vascular Medicine of the Medical University of Vienna21. All patients had intermittent claudication classified as Rutherford stages of PAD 2–3 due to sonographically confirmed infrainguinal artery stenosis and occlusion, respectively. All patients received long-term aspirin therapy (100 mg/day), and 75 mg of clopidogrel per day for three months following angioplasty and stenting. Clinical follow-up was assessed 1 and 2 years after the percutaneous intervention.
Exclusion criteria were a known aspirin or clopidogrel intolerance (allergic reactions, gastrointestinal bleeding), a therapy with vitamin K antagonists (warfarin, phenprocoumon, acenocoumarol) or direct oral anticoagulants (dabigatran, rivaroxaban, apixaban and edoxaban), a treatment with ticlopidine, dipyridamol or nonsteroidal antiinflammatory drugs, a family or personal history of bleeding disorders, malignant paraproteinemias, myeloproliferative disorders or heparin-induced thrombocytopenia, severe hepatic failure, known qualitative defects in thrombocyte function, a major surgical procedure within one week before enrollment, a platelet count <100.000 or >450.000/µl and a haematocrit <30%22.
The study protocol was approved by the Ethics Committee of the Medical University of Vienna in accordance with the Declaration of Helsinki and written informed consent was obtained from all study participants.
Blood sampling
As previously described22, blood was drawn by aseptic venipuncture from an antecubital vein using a 21-gauge butterfly needle (0.8 × 19 mm; Greiner Bio-One, Kremsmünster, Austria) one day after the percutaneous intervention. To avoid procedural deviations all blood samples were taken by the same physician applying a light tourniquet, which was immediately released and the samples were mixed adequately by gently inverting the tubes.
Measurement of HBDH, lactate dehydrogenase (LDH) and free haemoglobin
HBDH, LDH and free haemoglobin were measured in the central laboratory of the Medical University of Vienna according to standardized protocols.
Clinical endpoints
Clinical follow-up was assessed at regular visits of the study participants to the outpatient department of the Division of Vascular Medicine at the Medical University of Vienna and via telephone calls, respectively. The primary endpoint was defined as the composite of the first occurrence of nonfatal myocardial infarction, nonfatal stroke or transient ischemic attack, and cardiovascular death within 2 years after angioplasty and stenting21. Target vessel restenosis > 80% or reocclusion as assessed by duplex sonography was defined as secondary endpoint.
Sample size calculation and statistical analysis
A sample size calculation was based on the observed mean ± SD (108 ± 26 U/L) of HBDH in a former population of 30 stable PAD patients (15 male, 15 female; age 64 years (58–71 years)) 24 hours after angioplasty and stenting23–25. We calculated that we needed to include 80 patients to be able to detect a 30% relative difference of HBDH between patients without and with the primary endpoint with a power of 83% (using a two-sided alpha level of 0.05). To compensate for loss to follow-up we included 3 additional patients24.
Statistical analysis was performed using the Statistical Package for Social Sciences (IBM SPSS version 25, Armonk, New York, USA). Median and interquartile range of continuous variables are shown. Categorical variables are given as number (%). We performed Mann Whitney U tests to detect differences of continuous variables. The chi-square test and the Fisher´s exact test were used to detect differences in categorical variables, respectively24. Receiver operating characteristic (ROC) curve analysis was used to determine the ability of HBDH to distinguish between patients without and with the primary endpoint25. The p-value was determined using the DeLong test. The optimal cut-off value was calculated by determining the HBDH level that provided the greatest sum of sensitivity and specificity. A survival curve was generated using the Kaplan-Meier method, and the difference between the groups was assessed by the log-rank test. Two-sided p-values < 0.05 were considered statistically significant21,24,25.
Results
Characteristics of the overall study population and of patients without and with the primary endpoint are shown in Table 1. HBDH in the overall study population was 106 U/L (95–123 U/L). Of note, none of the included patients had suffered myocardial infarction within 6 months prior to infrainguinal angioplasty and stenting. Moreover, a history of myocardial infarction was present in 18.2% and 16.7% of patients without and with the primary endpoint, respectively (Table 1; p = 1). Twenty-eight (33.7%) and 23 (27.7%) patients of the study population had documented stable coronary artery disease (CAD) and cerebrovascular disease (CVD) at study inclusion, respectively. The presence of stable CAD and CVD at baseline did not differ significantly between patients without and with the primary endpoint (Table 1; both p ≥ 0.7). Polyvascular disease was documented in 41 patients (49.4%) at study inclusion (10 patients with PAD, CAD and CVD; 18 patients with PAD and CAD; 13 patients with PAD and CVD; Table 1). The presence of polyvascular disease at baseline did not differ significantly between patients without and with the primary endpoint (38 (49.4%) vs. 3 (50%) patients; p = 1).
Table 1.
Clinical, laboratory and procedural characteristics of the overall study population, and of patients without and with the primary endpoint.
| Characteristics | overall (n = 83) | no primary endpoint (n = 77) | primary endpoint (n = 6) | p |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 65 (58–74) | 64 (58–72) | 73 (66–81) | 0.2 |
| Male sex, n (%) | 51 (61.4) | 48 (62.3) | 3 (50) | 0.7 |
| Body mass index, kg/m2 | 26.8 (25.7–29) | 26.7 (24.5–28.7) | 27.1 (26.4–29) | 0.6 |
| Medical history | ||||
| Hypertension | 78 (94) | 72 (93.5) | 6 (100) | 1 |
| Hypercholesterolemia | 77 (92.8) | 71 (92.2) | 6 (100) | 1 |
| Diabetes mellitus | 29 (34.9) | 28 (36.4) | 1 (16.7) | 0.7 |
| Active smoking | 35 (42.2) | 34 (44.2) | 1 (16.7) | 0.4 |
| Previous myocardial infarction | 15 (18.1) | 14 (18.2) | 1 (16.7) | 1 |
| CAD | 28 (33.7) | 26 (33.8) | 2 (33.3) | 1 |
| CVD | 23 (27.7) | 21 (27.3) | 2 (33.3) | 0.7 |
| PAD, CAD + CVD | 10 (12) | 9 (11.7) | 1 (16.7) | 0.6 |
| PAD + CAD | 18 (21.7) | 17 (22.1) | 1 (16.7) | 1 |
| PAD + CVD | 13 (15.7) | 12 (15.6) | 1 (16.7) | 1 |
| Laboratory data | ||||
| Haemoglobin, g/dL | 13.7 (12.5–14.7) | 13.7 (12.6–14.7) | 12.3 (11–14.6) | 0.3 |
| Haematocrit, % | 40.2 (37–42.9) | 40.4 (37.2–43.2) | 35.9 (33.8–42.9) | 0.2 |
| White blood cell count, G/L | 8.7 (6.8–10.5) | 9 (6.8–10.5) | 7.5 (6.7–8.4) | 0.2 |
| Platelet count, G/L | 210 (181–237) | 210 (181–230) | 231 (207–249) | 0.5 |
| HBDH, U/L | 106 (95–123) | 105 (95–120) | 126 (116–137) | 0.04 |
| Lactate dehydrogenase, U/L | 167 (145–190) | 167 (145–187) | 179 (166–229) | 0.2 |
| Free haemoglobin, mg/dL | 2.1 (1.4–3.3) | 2.1 (1.4–3.4) | 1.7 (1.3–2) | 0.2 |
| Serum creatinine, mg/dL | 1 (0.9–1.2) | 1 (0.9–1.1) | 1.2 (1–1.4) | 0.2 |
| C-reactive protein, mg/dL | 1.1 (0.4–1.8) | 1.1 (0.4–1.8) | 1.3 (0.4–4.3) | 0.4 |
| Procedure | ||||
| Stent implantation | 83 (100) | 77 (100) | 6 (100) | 1 |
| Number of stents/patient | 2 (1–2) | 2 (1–2) | 2 (1–2) | 0.8 |
| Medication pre-intervention | ||||
| Clopidogrel | 83 (100) | 77 (100) | 6 (100) | 1 |
| Aspirin | 83 (100) | 77 (100) | 6 (100) | 1 |
| Statins | 74 (89.2) | 68 (88.3) | 6 (100) | 1 |
| ACE inhibitors/ARB | 72 (86.7) | 66 (85.7) | 6 (100) | 1 |
| Beta blockers | 51 (61.4) | 47 (61) | 4 (66.7) | 1 |
| Calcium channel blockers | 34 (41) | 31 (40.3) | 3 (50) | 0.7 |
| Proton pump inhibitors | 39 (47) | 37 (48.1) | 2 (33.3) | 0.7 |
Continuous data are shown as median (interquartile range). Dichotomous data are shown as n (%).
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blockers; CAD, coronary artery disease; CVD, cerebrovascular disease; HBDH, α-hydroxybutyrate dehydrogenase; PAD, peripheral artery disease.
The primary endpoint occurred in 6 patients (7.2% of the study population) within 2 years after peripheral angioplasty with stent implantation and comprised 2 nonfatal myocardial infarctions, 2 nonfatal strokes, 1 transient ischemic attack and 1 cardiovascular death. Target vessel restenosis or reocclusion occurred in 28 patients (33.7%) during follow-up.
HBDH levels at baseline were significantly higher in patients who subsequently developed the primary endpoint (Fig. 1; Table 1; 126 U/L [116–137 U/L] vs. 105 U/L [95–120 U/L]; p = 0.04). In contrast, LDH and free haemoglobin levels did not differ significantly between patients without and with atherothrombotic events during follow-up (Table 1; LDH: 167 U/L [145–187 U/L] vs. 179 U/L [166–229 U/L]; free haemoglobin: 2.1 mg/dL [1.4–3.4 mg/dL] vs. 1.7 mg/dL [1.3–2 mg/dL]; both p ≥ 0.2). HBDH, LDH and free haemoglobin were similar in patients without and with target vessel restenosis or reocclusion within 2 years after angioplasty and stenting (all p > 0.6).
Figure 1.
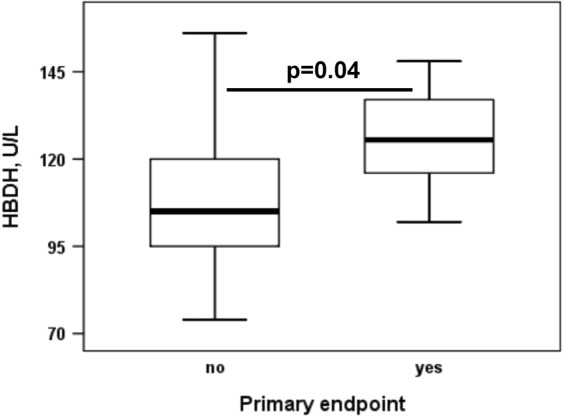
α-Hydroxybutyrate dehydrogenase (HBDH) in patients without and with the primary endpoint. The boundaries of the box show the lower and upper quartile of data, and the line inside the box represents the median. Whiskers are drawn from the edge of the box to the highest and lowest values that are outside the box but within 1.5 times the box length.
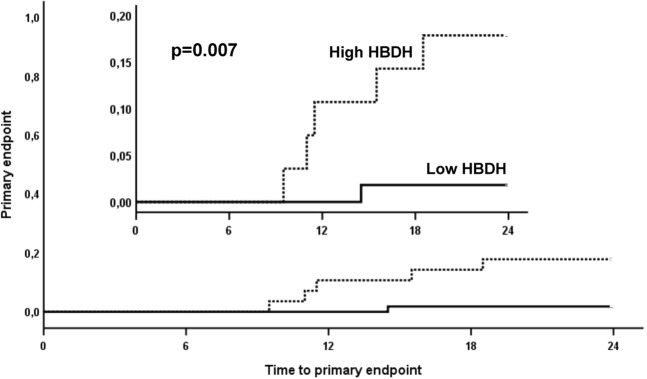
ROC curve analysis revealed that HBDH could distinguish between patients without and with future atherothrombotic events (Fig. 2; area under the curve = 0.753, 95% CI 0.61–0.9; p = 0.04). A HBDH concentration ≥ 115 U/L was identified as the best threshold to predict the composite endpoint, providing a sensitivity of 83.3% and a specificity of 71.4%, and was therefore defined as high HBDH. High HBDH was seen in 28 patients (33.7% of the study population). Ischemic events occurred significantly more often in patients with high HBDH than in patients with lower HBDH levels (Fig. 3; 5 vs. 1 patients, p = 0.007 with the log-rank test).
Figure 2.
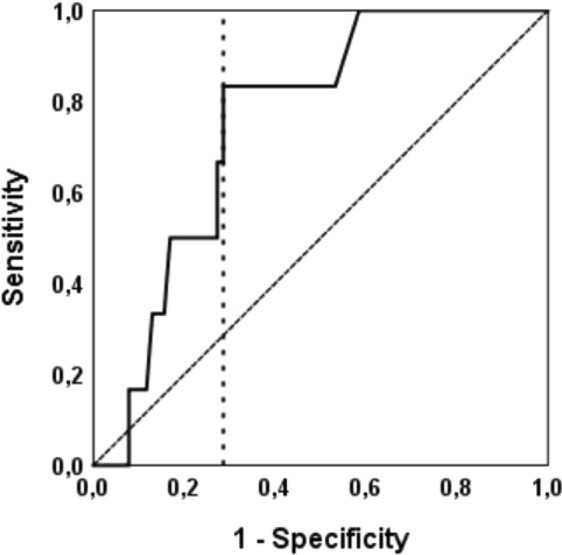
Receiver-operating characteristic curve for the analysis of the predictive value of α-hydroxybutyrate dehydrogenase (HBDH) for the primary endpoint. An area under the curve of 0.753 was observed (95% CI 0.61–0.9; p = 0.04). The cut-off value for high HBDH is shown as vertical dotted line originating from the x-axis.
Figure 3.
Kaplan-Meier analysis for the cumulative incidence of the primary endpoint in patients with high and low α-hydroxybutyrate dehydrogenase (HBDH). High HBDH is indicated by the dotted line, low HBDH is indicated by the solid line.
Discussion
Our study is the first to investigate the association of markers of cell death with clinical outcomes following infrainguinal angioplasty and stenting for PAD. High levels of HBDH were linked to an increased risk of atherothrombotic events over 2 years, whereas target lesion restenosis and reocclusion did not occur more frequently in patients with high HBDH. Plasma concentrations of LDH and free haemoglobin were not linked to atherothrombotic events and target lesion restenosis or reocclusion, respectively.
HBDH was investigated as a potential risk marker because it reflects renal, red blood cell and myocardial injury10–14. In order to study whether cell death in general or red cell damage alone are linked to ischemic outcomes, we decided to also measure LDH and free haemoglobin concentrations, respectively. Our results, however, point towards HBDH as most promising risk predictor of atherothrombotic events among these 3 markers of cell injury. Of note, serum creatinine, haemoglobin and haematocrit alone did not differ significantly between patients without and with the primary endpoint in our study (Table 1). However, serum creatinine was numerically higher and both haemoglobin and haematocrit were numerically lower in patients who subsequently developed the primary endpoint. It may therefore be speculated that by combining information on subclinical kidney, red blood cell and myocardial injury, HBDH may represent a more sensitive risk marker for atherothrombotic outcomes than the respective laboratory values alone.
Considering the inclusion of patients with manifest atherosclerotic cardiovascular disease, we observed a rather low rate of atherothrombotic events during follow-up. This may be due to 1. the inclusion of stable PAD patients undergoing elective infrainguinal angioplasty and stenting because of intermittent claudication and 2. optimal medical treatment and risk factor management following the percutaneous intervention. In detail, all patients received state-of-the-art antiplatelet, antihypertensive and lipid-lowering therapy and had regular check-ups at the outpatient department every 6 months2. As a next step, it would be interesting to study the predictive value of HBDH for atherothrombotic outcomes in high-risk patients. Among PAD patients, especially those suffering critical limb ischemia may benefit from adequate risk stratification in order to receive an individually tailored treatment approach and follow-up strategy. Finally, HBDH may also be linked to the prognosis of patients with chronic or acute ischemic heart disease and cerebrovascular disease, respectively.
The lack of a significant association between HBDH levels and target vessel restenosis or reocclusion may be explained by differences in the underlying pathomechanisms: Target vessel restenosis usually occurs due to intimal hyperplasia potentially resulting from chronic inflammation26. In contrast, myocardial infarction, stroke and cardiovascular death primarily arise from a prothrombotic environment6. Our finding of an association between HBDH and atherothrombotic events suggests that HBDH levels might mirror the latter. However, large clinical trials confirming HBDH as predictor of thrombotic outcomes are needed before HBDH can be considered for risk stratification in PAD.
Due to the small sample size, our study should be considered hypothesis-generating only. Moreover, we exclusively enrolled stable PAD patients who underwent elective infrainguinal angioplasty and stenting due to intermittent claudication. Therefore, our results cannot be extrapolated to patients with critical limb ischemia. Elevated HBDH levels may be attributable to individual reactions to the peripheral interventions. Alternatively, HBDH might have already been increased before angioplasty and stenting in some patients, reflecting increased cell turnover or damage. Unfortunately, all markers of cell death were only determined 24 hours after the intervention. Consequently, we cannot provide preprocedural HBDH values or data on the variability of HBDH levels over time. This time point was chosen because (1) 24 hours after the elective procedures, all patients were still at the inpatient ward, and (2) we sought to investigate whether or not a single postprocedural HBDH measurement may be used for risk stratification. Further, the AUC of 0.753 observed in the ROC curve cannot be considered a strong classifier. Finally, we do not provide mechanistic data supporting the above-discussed speculations.
In conclusion, HBDH is associated with the occurrence of atherothrombotic events after infrainguinal angioplasty with stent implantation. Future trials are warranted to study the predictive role of HBDH for adverse outcomes in patients with critical limb ischemia and other manifestations of cardiovascular disease, and to investigate underlying mechanisms.
Author contributions
Silvia Lee: statistical analysis, writing the manuscript. Renate Koppensteiner: critical revision and final approval. Christoph W. Kopp: critical revision and final approval. Thomas Gremmel: study design, patient enrollment, statistical analysis, writing the manuscript.
Data availability
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Welten GM, et al. Long-term prognosis of patients with peripheral arterial disease: a comparison in patients with coronary artery disease. J Am Coll Cardiol. 2008;51:1588–1596. doi: 10.1016/j.jacc.2007.11.077. [DOI] [PubMed] [Google Scholar]
- 2.Aboyans V, et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS) Eur Heart J. 2018;39:763–816. doi: 10.1093/eurheartj/ehx095. [DOI] [PubMed] [Google Scholar]
- 3.Norgren L, et al. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II) J Vasc Surg. 2007;45(Suppl S):S5–67. doi: 10.1016/j.jvs.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 4.Schillinger M, et al. Balloon angioplasty versus implantation of nitinol stents in the superficial femoral artery. N Engl J Med. 2006;354:1879–1888. doi: 10.1056/NEJMoa051303. [DOI] [PubMed] [Google Scholar]
- 5.Gur I, et al. Clinical outcomes and implications of failed infrainguinal endovascular stents. J Vasc Surg. 2011;53(discussion 667):658–666. doi: 10.1016/j.jvs.2010.09.069. [DOI] [PubMed] [Google Scholar]
- 6.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 7.Pultar Joseph, Wadowski Patricia P., Panzer Simon, Gremmel Thomas. Oral antiplatelet agents in cardiovascular disease. Vasa. 2019;48(4):291–302. doi: 10.1024/0301-1526/a000753. [DOI] [PubMed] [Google Scholar]
- 8.Gremmel T, Michelson AD, Frelinger AL, III, Bhatt DL. Novel aspects of antiplatelet therapy in cardiovascular disease. Res Pract Thromb Haemost. 2018;2:439–449. doi: 10.1002/rth2.12115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anand Sonia S, Bosch Jackie, Eikelboom John W, Connolly Stuart J, Diaz Rafael, Widimsky Peter, Aboyans Victor, Alings Marco, Kakkar Ajay K, Keltai Katalin, Maggioni Aldo P, Lewis Basil S, Störk Stefan, Zhu Jun, Lopez-Jaramillo Patricio, O'Donnell Martin, Commerford Patrick J, Vinereanu Dragos, Pogosova Nana, Ryden Lars, Fox Keith A A, Bhatt Deepak L, Misselwitz Frank, Varigos John D, Vanassche Thomas, Avezum Alvaro A, Chen Edmond, Branch Kelley, Leong Darryl P, Bangdiwala Shrikant I, Hart Robert G, Yusuf Salim, SALA JORGELINA, CARTASEGNA LUIS, VICO MARISA, HOMINAL MIGUEL ANGEL, HASBANI EDUARDO, CACCAVO ALBERTO, ZAIDMAN CESAR, VOGEL DANIEL, HRABAR ADRIAN, SCHYGIEL PABLO OMAR, CUNEO CARLOS, LUQUEZ HUGO, MACKINNON IGNACIO J., AHUAD GUERRERO RODOLFO ANDRES, COSTABEL JUAN PABLO, BARTOLACCI INES PALMIRA, MONTANA OSCAR, BARBIERI MARIA, GOMEZ VILAMAJO OSCAR, GARCIA DURAN RUBEN OMAR, SCHIAVI LILIA BEATRIZ, GARRIDO MARCELO, INGARAMO ADRIAN, BORDONAVA ANSELMO PAULINO, PELAGAGGE MARIA JOSE, NOVARETTO LEONARDO, ALBISU DI GENNERO JUAN PABLO, IBANEZ SAGGIA LUZ MARIA, ALVAREZ MOIRA, VITA NESTOR ALEJANDRO, MACIN STELLA MARIS, DRAN RICARDO DARIO, CARDONA MARCELO, GUZMAN LUIS, SARJANOVICH RODOLFO JUAN, CUADRADO JESUS, NANI SEBASTIAN, LITVAK BRUNO MARCOS RAUL, CHACON CAROLINA, MAFFEI LAURA ELENA, GRINFELD DIEGO, VENSENTINI NATALIA, MAJUL CLAUDIO RODOLFO, LUCIARDI HECTOR LUCAS, GONZALEZ COLASO PATRICIA DEL CARMEN, FERRE PACORA FREDY ANTONI, VAN DEN HEUVEL PAUL, VERHAMME PETER, ECTOR BAVO, DEBONNAIRE PHILIPPE, VAN DE BORNE PHILIPPE, LEROY JEAN, SCHROE HERMAN, VRANCKX PASCAL, ELEGEERT IVAN, HOFFER ETIENNE, DUJARDIN KARL, INDIO DO BRASIL CLARISSE, PRECOMA DALTON, ABRANTES JOSE ANTONIO, MANENTI EULER, REIS GILMAR, SARAIVA JOSE, MAIA LILIA, HERNANDES MAURO, ROSSI PAULO, ROSSI DOS SANTOS FABIO, ZIMMERMANN SERGIO LUIZ, RECH RAFAEL, ABIB JR EDUARDO, LEAES PAULO, BOTELHO ROBERTO, DUTRA OSCAR, SOUZA WEIMAR, BRAILE MARIA, IZUKAWA NILO, NICOLAU JOSE CARLOS, TANAJURA LUIZ FERNANDO, SERRANO JUNIOR CARLOS VICENTE, MINELLI CESAR, NASI LUIZ ANTONIO, OLIVEIRA LIVIA, DE CARVALHO CANTARELLI MARCELO JOSE, TYTUS RICHARD, PANDEY SHEKHAR, LONN EVA, CHA JAMES, VIZEL SAUL, BABAPULLE MOHAN, LAMY ANDRE, SAUNDERS KEVIN, BERLINGIERI JOSEPH, KIAII BOB, BHARGAVA RAKESH, MEHTA PRAVINSAGAR, HILL LAURIE, FELL DAVID, LAM ANDY, AL-QOOFI FAISAL, BROWN CRAIG, PETRELLA ROBERT, RICCI JOSEPH A, GLANZ ANTHONY, NOISEUX NICOLAS, BAINEY KEVIN, MERALI FATIMA, HEFFERNAN MICHAEL, DELLA SIEGA ANTHONY, DAGENAIS GILLES R, DAGENAIS FRANCOIS, BRULOTTE STEEVE, NGUYEN MICHEL, HARTLEIB MICHAEL, GUZMAN RANDOLPH, BOURGEOIS RONALD, RUPKA DENNIS, KHAYKIN YAARIV, GOSSELIN GILBERT, HUYNH THAO, PILON CLAUDE, CAMPEAU JEAN, PICHETTE FRANCIS, DIAZ ARIEL, JOHNSTON JAMES, SHUKLE PRAVIN, HIRSCH GREGORY, RHEAULT PAUL, CZARNECKI WLODZIMIERZ, ROY ANNIE, NAWAZ SHAH, FREMES STEPHEN, SHUKLA DINKAR, JANO GABRIEL, COBOS JORGE LEONARDO, CORBALAN RAMON, MEDINA MARCELO, NAHUELPAN LEONARDO, RAFFO CARLOS, PEREZ LUIS, POTTHOFF SERGIO, STOCKINS BENJAMIN, SEPULVEDA PABLO, PINCETTI CHRISTIAN, VEJAR MARGARITA, TIAN HONGYAN, WU XUESI, KE YUANNAN, JIA KAIYING, YIN PENGFEI, WANG ZHAOHUI, YU LITIAN, WU SHULIN, WU ZONGQUI, LIU SHAO WEN, BAI XIAO JUAN, ZHENG YANG, YANG PING, YANG YUN MEI, ZHANG JIWEI, GE JUNBO, CHEN XIAO PING, LI JUNXIA, HU TAO HONG, ZHANG RUIYAN, ZHENG ZHE, CHEN XIN, TAO LIANG, LI JIANPING, HUANG WEIJIAN, FU GUOSHENG, LI CHUNJIAN, DONG YUGANG, WANG CHUNSHENG, ZHOU XINMIN, KONG YE, SOTOMAYOR ARISTIDES, ACCINI MENDOZA JOSE LUIS, CASTILLO HENRY, URINA MIGUEL, AROCA GUSTAVO, PEREZ MARITZA, MOLINA DE SALAZAR DORA INES, SANCHEZ VALLEJO GREGORIO, FERNANDO MANZUR J, GARCIA HENRY, GARCIA LUIS HERNANDO, ARCOS EDGAR, GOMEZ JUAN, CUERVO MILLAN FRANCISCO, TRUJILLO DADA FREDY ALBERTO, VESGA BORIS, MORENO SILGADO GUSTAVO ADOLFO, ZIDKOVA EVA, LUBANDA JEAN-CLAUDE, KALETOVA MARKETA, KRYZA RADIM, MARCINEK GABRIEL, RICHTER MAREK, SPINAR JINDRICH, MATUSKA JIRI, TESAK MARTIN, MOTOVSKA ZUZANA, BRANNY MARIAN, MALY JIRI, MALY MARTIN, WIENDL MARTIN, FOLTYNOVA CAISOVA LENKA, SLABY JOSEF, VOJTISEK PETR, PIRK JAN, SPINAROVA LENKA, BENESOVA MIROSLAVA, CANADYOVA JULIA, HOMZA MIROSLAV, FLORIAN JINDRICH, POLASEK ROSTISLAV, COUFAL ZDENEK, SKALNIKOVA VLADIMIRA, BRAT RADIM, BRTKO MIROSLAV, JANSKY PETR, LINDNER JAROSLAV, MARCIAN PAVEL, STRAKA ZBYNEK, TRETINA MARTIN, DUARTE YAN CARLOS, POW CHON LONG FREDDY, SANCHEZ MAYRA, LOPEZ JOSE, PERUGACHI CARMITA, MARMOL RICARDO, TRUJILLO FREDDY, TERAN PABLO, TUOMILEHTO JAAKKO, TUOMILEHTO HENRI, TUOMINEN MARJA-LEENA, TUOMILEHTO HENRI, KANTOLA ILKKA, STEG GABRIEL, ABOYANS VICTOR, LECLERCQ FLORENCE, FERRARI EMILE, BOCCARA FRANCK, MESSAS EMMANUEL, MISMETTI PATRICK, SEVESTRE MARIE ANTOINETTE, CAYLA GUILLAUME, MOTREFF PASCAL, STOERK STEFAN, DUENGEN HANS-DIRK, STELLBRINK CHRISTOPH, GUEROCAK OSMAN, KADEL CHRISTOPH, BRAUN-DULLAEUS RUEDIGER, JESERICH MICHAEL, OPITZ CHRISTIAN, VOEHRINGER HANS-FRIEDRICH, APPEL KARL-FRIEDRICH, WINKELMANN BERNHARD, DORSEL THOMAS, NIKOL SIGRID, DARIUS HARALD, RANFT JURGEN, SCHELLONG SEBASTIAN, JUNGMAIR WOLFGANG, DAVIERWALA PIROZE, VORPAHL MARC, BAJNOK LASZLO, LASZLO ZOLTAN, NOORI EBRAHIM, VERESS GABOR, VERTES ANDRAS, ZSARY ANDRAS, KIS ERNO, KORANYI LASZLO, BAKAI JUDIT, BODA ZOLTAN, POOR FERENC, JARAI ZOLTAN, KEMENY VENDEL, BARTON JOHN, MCADAM BRENDAN, MURPHY ANDREW, CREAN PETER, MAHON NIALL, CURTIN RONAN, MACNEILL BRIAIN, DINNEEN SEAN, HALABI MAJDI, ZIMLICHMAN REUVEN, ZELTSER DAVID, TURGEMAN YOAV, KLAINMAN ELIEZER, LEWIS BASIL, KATZ AMOS, ATAR SHAUL, ZIMLICHMAN REUVEN, NIKOLSKY EUGENIA, BOSI STEFANO, NALDI MONICA, FAGGIANO POMPILIO, ROBBA DEBORA, MOS LUCIO, SINAGRA GIANFRANCO, COSMI FRANCO, OLTRONA VISCONTI LUIGI, CARMINE DE MATTEIS, DI PASQUALE GIUSEPPE, DI BIASE MATTEO, MANDORLA SARA, BERNARDINANGELI MARINO, PICCINNI GIOVANNI CARLO, GULIZIA MICHELE MASSIMO, GALVANI MARCELLO, VENTURI FLAVIO, MOROCUTTI GIORGIO, BALDIN MARIA GRAZIA, OLIVIERI CARLO, PERNA GIAN PIERO, CIRRINCIONE VINCENZO, KANNO TAKAYASU, DAIDA HIROYUKI, OZAKI YUKIO, MIYAMOTO NAOMASA, HIGASHIUE SHINICHI, DOMAE HIROSHI, HOSOKAWA SHINOBU, KOBAYASHI HIROO, KURAMOCHI TAKEHIKO, FUJII KENSHI, MIZUTOMI KAZUAKI, SAKU KEIJIRO, KIMURA KAZUO, HIGUCHI YOSHIHARU, ABE MITSUNORI, OKUDA HARUHITO, NODA TOSHIYUKI, MITA TERUAKI, HIRAYAMA ATSUSHI, ONAKA HARUHIKO, INOKO MORIAKI, HIROKAMI MITSUGU, OKUBO MUNENORI, AKATSUKA YUTAKA, IMAMAKI MIZUHO, KAMIYA HARUO, MANITA MAMORU, HIMI TOSHIHARU, UENO HIDEKI, HISAMATSU YUJI, AKO JUNYA, NISHINO YASUHIRO, KAWAKAMI HIDEO, YAMADA YUTAKA, KORETSUNE YUKIHIRO, YAMADA TAKAHISA, YOSHIDA TETSURO, SHIMOMURA HIDEKI, KINOSHITA NORIYUKI, TAKAHASHI AKIHIKO, YUSOFF KHALID, WAN AHMAD WAN AZMAN, ABU HASSAN MUHAMMAD RADZI, KASIM SAZZLI, ABDUL RAHIM AIZAI AZAN, MOHD ZAMRIN DIMON, MACHIDA MASAHARU, HIGASHINO YORIHIKO, UTSU NORIAKI, NAKANO AKIHIKO, NAKAMURA SHIGERU, HASHIMOTO TETSUO, ANDO KENJI, SAKAMOTO TOMOHIRO, PRINS F.J., LOK DIRK, MILHOUS JOHANNES GERT-JAN, VIERGEVER ERIC, WILLEMS FRANK, SWART HENK, ALINGS MARCO, BREEDVELD ROB, DE VRIES KEES-JAN, VAN DER BORGH ROGER, OEI FANNY, ZOET-NUGTEREN STIENEKE, KRAGTEN HANS, HERRMAN JEAN PAUL, VAN BERGEN PAUL, GOSSELINK MARCEL, HOEKSTRA EDUARD, ZEGERS ERWIN, RONNER EELKO, DEN HARTOG FRANK, BARTELS GERARD, NIEROP PETER, VAN DER ZWAAN COEN, VAN ECK JACOB, VAN GORSELEN EDWIN, GROENEMEIJER BJORN, HOOGSLAG PIETER, DE GROOT MARC ROBERT, LOYOLA ALDRIN, SULIT DENNIS JOSE, REY NANNETTE, ABOLA MARIA TERESA, MORALES DANTE, PALOMARES ELLEN, ABAT MARC EVANS, ROGELIO GREGORIO, CHUA PHILIP, DEL PILAR JOSE CARLO, ALCARAZ JOHN DENNIS, EBO GERALDINE, TIRADOR LOUIE, CRUZ JOSEFINA, ANONUEVO JOHN, PITARGUE ARTHUR, JANION MARIANNA, GUZIK TOMASZ, GAJOS GRZEGORZ, ZABOWKA MACIEJ, RYNKIEWICZ ANDRZEJ, BRONCEL MARLENA, SZUBA ANDRZEJ, CZARNECKA DANUTA, MAGA PAWEL, STRAZHESKO IRINA, VASYUK YURY, SIZOVA ZHANNA, POZDNYAKOV YURY, BARBARASH OLGA, VOEVODA MIKHAIL, POPONINA TATIANA, REPIN ALEXEY, OSIPOVA IRINA, EFREMUSHKINA ANNA, NOVIKOVA NINA, AVERKOV OLEG, ZATEYSHCHIKOV DMITRY, VERTKIN ARKADIY, AUSHEVA AZA, COMMERFORD PATRICK, SEEDAT SAADIYA, VAN ZYL LOUIS, ENGELBRECHT JAN, MAKOTOKO ELLEN MAKONLI, PRETORIUS CATHARINA ELIZABETH, MOHAMED ZAID, HORAK ADRIAN, MABIN THOMAS, KLUG ERIC, BAE JANG-HO, KIM CHEOLHO, KIM CHONG-JIN, KIM DONG-SOO, KIM YONG JIN, JOO SEUNGJAE, HA JONG-WON, PARK CHUL SOO, KIM JANG YOUNG, KIM YOUNG-KWON, JARNERT CHRISTINA, MOOE THOMAS, DELLBORG MIKAEL, TORSTENSSON INGEMAR, ALBERTSSON PER, JOHANSSON LARS, AL-KHALILI FARIS, ALMROTH HENRIK, ANDERSSON TOMMY, PANTEV EMIL, TENGMARK BENGT-OLOV, LIU BO, RASMANIS GUNDARS, WAHLGREN CARL-MAGNUS, MOCCETTI TIZIANO, PARKHOMENKO ALEXANDER, TSELUYKO VIRA, VOLKOV VOLODYMYR, KOVAL OLENA, KONONENKO LYUDMYLA, PROKHOROV OLEKSANDR, VDOVYCHENKO VALERIY, BAZYLEVYCH ANDRIY, RUDENKO LEONID, VIZIR VADYM, KARPENKO OLEKSANDR, MALYNOVSKY YAROSLAV, KOVAL VALENTYNA, STOROZHUK BORYS, COTTON JAMES, VENKATARAMAN ASOK, MORIARTY ANDREW, CONNOLLY DEREK, DAVEY PATRICK, SENIOR ROXY, BIRDI INDERPAUL, CALVERT JOHN, DONNELLY PATRICK, TREVELYAN JASPER, CARTER JUSTIN, PEACE AARON, AUSTIN DAVID, KUKREJA NEVILLE, HILTON THOMAS, SRIVASTAVA SUNNY, WALSH RONALD, FIELDS RONALD, HAKAS JOSEPH, PORTNAY EDWARD, GOGIA HARINDER, SALACATA ABRAHAM, HUNTER JOHN J., BACHARACH J MICHAEL, SHAMMAS NICOLAS, SURESH DAMODHAR, SCHNEIDER RICKY, GURBEL PAUL, BANERJEE SUBHASH, GRENA PAUL, BEDWELL NOEL, SLOAN STEPHEN, LUPOVITCH STEVEN, SONI ANAND, GIBSON KATHLEEN, SANGRIGOLI RENEE, MEHTA RAJENDRA, I-HSUAN TSAI PETER, GILLESPIE EVE, DEMPSEY STEPHEN, HAMROFF GLENN, BLACK ROBERT, LADER ELLIS, KOSTIS JOHN B., BITTNER VERA, MCGUINN WILLIAM, BRANCH KELLEY, MALHOTRA VINAY, MICHAELSON STEPHEN, VACANTE MICHAEL, MCCORMICK MATTHEW, ARIMIE RALUCA, CAMP ALAN, DAGHER GEORGE, KOSHY N. MATHEW, THEW STEPHEN, COSTELLO FREDERICK, HEIMAN MARK, CHILTON ROBERT, MORAN MICHAEL, ADLER FREDRIC, COMEROTA ANTHONY, SEIWERT ANDREW, FRENCH WILLIAM, SEROTA HARVEY, HARRISON ROBERT, BAKAEEN FAISAL, OMER SHUAB, CHANDRA LOKESH, WHELAN ALAN, BOYLE ANDREW, ROBERTS-THOMSON PHILIP, ROGERS JAMES, CARROLL PATRICK, COLQUHOUN DAVID, SHAW JAMES, BLOMBERY PETER, AMERENA JOHN, HII CHRIS, ROYSE ALISTAIR, SINGH BHUWAN, SELVANAYAGAM JOSEPH, JANSEN SHIRLEY, LO WINGCHI, HAMMETT CHRISTOPHER, POULTER ROHAN, NARASIMHAN SESHASAYEE, WIGGERS HENRIK, NIELSEN HENRIK, GISLASON GUNNAR, KOBER LARS, HOULIND KIM, BOENELYKKE SOERENSEN VIBEKE, DIXEN ULRIK, REFSGAARD JENS, ZEUTHEN ELISABETH, SOEGAARD PETER, HRANAI MARIAN, GASPAR LUDOVIT, PELLA DANIEL, HATALOVA KATARINA, DROZDAKOVA ERIKA, COMAN IOAN, DIMULESCU DOINA, VINEREANU DRAGOS, CINTEZA MIRCEA, SINESCU CRINA, ARSENESCU CATALINA, BENEDEK IMRE, BOBESCU ELENA, DOBREANU DAN, GAITA DAN, IANCU ADRIAN, ILIESIU ADRIANA, LIGHEZAN DANIEL, PETRESCU LUCIAN, PIRVU OCTAVIAN, TEODORESCU IULIA, TESLOIANU DAN, VINTILA MARIUS MARCIAN, CHIONCEL OVIDIU. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. The Lancet. 2018;391(10117):219–229. doi: 10.1016/S0140-6736(17)32409-1. [DOI] [PubMed] [Google Scholar]
- 10.Ohlmann P, et al. Prognostic value of C-reactive protein and cardiac troponin I in primary percutaneous interventions for ST-elevation myocardial infarction. Am Heart J. 2006;152:1161–1167. doi: 10.1016/j.ahj.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 11.Dissmann R, Linderer T, Schroder R. Estimation of enzymatic infarct size: direct comparison of the marker enzymes creatine kinase and alpha-hydroxybutyrate dehydrogenase. Am Heart J. 1998;135:1–9. doi: 10.1016/S0002-8703(98)70335-7. [DOI] [PubMed] [Google Scholar]
- 12.Kemp M, Donovan J, Higham H, Hooper J. Biochemical markers of myocardial injury. Br J Anaesth. 2004;93:63–73. doi: 10.1093/bja/aeh148. [DOI] [PubMed] [Google Scholar]
- 13.Karayalcin G, Lanzkowsky P, Kari AB. Serum alpha-hydroxybutyrate dehydrogenase levels in children with sickle cell disease. Am J Pediatr Hematol Oncol. 1981;3:169–171. doi: 10.1097/00043426-198100320-00010. [DOI] [PubMed] [Google Scholar]
- 14.Apostolov I, et al. Acute changes of serum markers for tissue damage after ESWL of kidney stones. Int Urol Nephrol. 1991;23:215–220. doi: 10.1007/BF02550414. [DOI] [PubMed] [Google Scholar]
- 15.Fox CS, et al. Use of evidence-based therapies in short-term outcomes of ST-segment elevation myocardial infarction and non-ST-segment elevation myocardial infarction in patients with chronic kidney disease: a report from the National Cardiovascular Data Acute Coronary Treatment and Intervention Outcomes Network registry. Circulation. 2010;121:357–365. doi: 10.1161/CIRCULATIONAHA.109.865352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Surana SP, Riella LV, Keithi-Reddy SR, Charytan DM, Singh AK. Acute coronary syndrome in ESRD patients. Kidney Int. 2009;75:558–562. doi: 10.1038/ki.2008.233. [DOI] [PubMed] [Google Scholar]
- 17.Stigant C, Izadnegahdar M, Levin A, Buller CE, Humphries KH. Outcomes after percutaneous coronary interventions in patients with CKD: improved outcome in the stenting era. Am J Kidney Dis. 2005;45:1002–1009. doi: 10.1053/j.ajkd.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Giustino G, et al. Impact of Anemia on Platelet Reactivity and Ischemic and Bleeding Risk: From the Assessment of Dual Antiplatelet Therapy With Drug-Eluting Stents Study. Am J Cardiol. 2016;117:1877–1883. doi: 10.1016/j.amjcard.2016.03.034. [DOI] [PubMed] [Google Scholar]
- 19.Pilgrim T, et al. Additive effect of anemia and renal impairment on long-term outcome after percutaneous coronary intervention. PLoS One. 2014;9:e114846. doi: 10.1371/journal.pone.0114846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang X, et al. Impact of anemia on long-term ischemic events and bleeding events in patients undergoing percutaneous coronary intervention: a system review and meta-analysis. J Thorac Dis. 2015;7:2041–2052. doi: 10.3978/j.issn.2072-1439.2015.11.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gremmel Thomas, Koppensteiner Renate, Ay Cihan, Panzer Simon. Residual thrombin generation potential is inversely linked to the occurrence of atherothrombotic events in patients with peripheral arterial disease. European Journal of Clinical Investigation. 2014;44(3):319–324. doi: 10.1111/eci.12236. [DOI] [PubMed] [Google Scholar]
- 22.Lee S, Ay C, Kopp CW, Panzer S, Gremmel T. Impaired glucose metabolism is associated with increased thrombin generation potential in patients undergoing angioplasty and stenting. Cardiovasc Diabetol. 2018;17:131. doi: 10.1186/s12933-018-0774-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gremmel T, et al. Response to antiplatelet therapy and platelet reactivity to thrombin receptor activating peptide-6 in cardiovascular interventions: Differences between peripheral and coronary angioplasty. Atherosclerosis. 2014;232:119–124. doi: 10.1016/j.atherosclerosis.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 24.Gremmel T, et al. In vivo and protease-activated receptor-1-mediated platelet activation but not response to antiplatelet therapy predict two-year outcomes after peripheral angioplasty with stent implantation. Thromb Haemost. 2014;111:474–482. doi: 10.1160/TH13-07-0558. [DOI] [PubMed] [Google Scholar]
- 25.Gremmel T, et al. Serum Cholinesterase Levels Are Associated With 2-Year Ischemic Outcomes After Angioplasty and Stenting for Peripheral Artery Disease. J Endovasc Ther. 2016;23:738–743. doi: 10.1177/1526602816655521. [DOI] [PubMed] [Google Scholar]
- 26.Ross R. Atherosclerosis–an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and analysed during the current study are available from the corresponding author on reasonable request.