Abstract
Peer-reviewed probabilistic methods already predict the probability of an allergic reaction resulting from an accidental exposure to food allergens, however, the methods calculate it in different ways. The available methods utilize the same three major input parameters in the risk model: the risk is estimated from the amount of food consumed, the concentration of allergen in the contaminated product and the distribution of thresholds among allergic persons. However, consensus is lacking about the optimal method to estimate the risk of allergic reaction and the associated uncertainty. This study aims to compare estimation of the risk of allergic reaction and associated uncertainty using different methods and suggest improvements. Four cases were developed based on the previous publications and the risk estimations were compared. The risk estimation was found to agree within 0.5% with the different simulation cases. Finally, an uncertainty analysis method is also presented in order to evaluate the uncertainty propagation from the input parameters to the risk.
Subject terms: Risk factors, Public health
Introduction
Food allergy has been a growing public health concern over the last decade with an estimated prevalence of around 3–5% in adults and up to 8% in children1. People with food allergies need to avoid consuming food products containing the offending allergen2 to prevent allergic reactions. To support this, ingredient labelling should provide essential information to allergic individuals on which allergens are present in the food product as an ingredient. However, unexpected allergic reactions can still occur due to the unintended presence of allergen in food products, as a result of e.g. manufacturing processes. In order to alert allergic consumers and avoid potentially dangerous allergic reactions, food manufacturers use precautionary allergen labelling (PAL) when cross contamination is suspected or facilities are shared3.
The increasing use of PAL and a lack of understanding of its application has led people with food allergies to disregard the statements4, putting themselves at risk of a reaction. Good practice in the effective application of PAL by manufacturers dictates that it must rest on a risk assessment focussing on the health risk to the consumer, preferably quantitative5. An international workshop under the auspices of the Europrevall project agreed that probabilistic modelling was the most promising approach for assessing the risk from allergens at the population level6. Thus, the health consequences of unintended presence of allergens in food products i.e the probability of provoking a reaction in the susceptible population can be expressed quantitatively and this information can be used to evaluate possible risk mitigation measures, for example PAL or different forms of expressing it.
Several research groups have developed probabilistic risk assessment approaches for food allergens7–9. The approaches described have the same three input model variables: the consumption distribution (i.e. how much of the suspected contaminated product is consumed), the concentration distribution (i.e. how much allergen is in the contaminated product) and the threshold distribution (i.e. the range of minimum doses of allergen eliciting reactions in the relevant food-allergic population). One of the modelling methods is based on second order Monte Carlo simulations9 and the others are based on the combination of Bayesian inferences and second order Monte-Carlo simulations7,8. Based on these previous approaches, four different cases were developed to estimate the risk of an unexpected allergic reaction. This paper aims to compare the risk estimates under these four different cases and to understand the mechanism of uncertainty propagation from the input parameters to the risk when using different simulations methods. Specifically, a case was built based on log normal distributions for all the input variables. For this case, it is also possible to calculate mathematically the uncertainty around the risk estimate and to compare with results from simulations.
Materials and Methods
All four cases presented in the method have the same input distribution, namely the consumption distribution (X), the concentration distribution (Y) and the threshold distribution (Z). The probability of allergic reaction is then calculated from the three distributions (p(Z < XY)) at the population level and in a different way for each case. The differences between the four cases are summarized in Table 1.
Table 1.
Summary of the different case presented to assess the uncertainty propagation from the inputs to the risk of allergic reaction.
| Input | Distribution | Case A | Case B | Case C | Case D | |
|---|---|---|---|---|---|---|
| Frequentist – No uncertainty | Triple Log-Normal – Simulated | Triple Log-Normal – Calculated | Frequentist derived from case D | Bayesian | ||
|
Consumption (X) |
Parameter | No distribution |
|
|
Bootstrap | |
| Input |
Log-Normal
|
Log-Normal
|
Calculated |
Log-Normal
|
Empirical | |
|
Contamination (Y) |
Parameter | No distribution |
|
With vague prior parameters α = β = 10−3 |
||
| Input |
Log-Normal
|
Log-Normal
|
Calculated |
Exponential Y ~ Exp (λY) |
Exponential Y ~ Exp (λY) |
|
|
Threshold (Z) |
Parameter | No distribution |
|
|
Bayesian inferences with vague priors: aZ ~ Gamma (10−3, 10−3) bZ ~ Gamma (10−3, 10−3) |
|
| Input |
Log-Normal
|
Log-Normal
|
Calculated |
Weibull Z ~ Weibull (aZ, bZ) |
Weibull Z ~ Weibull (aZ, bZ) |
|
Mathematical formulation of the existing approaches
Mathematical formulation of the model developed by Spanjersberg et al.9
The probabilistic risk assessment method described in Spanjersberg et al.9 aims to take into consideration the variability and the uncertainty from input variables and can express the risk for the allergic user population (i.e. all individuals in the risk assessment simulation are allergic to the allergen of interest and consume the contaminated product). The allergic-user population risk can be expressed with a mathematical formulation and the three input distributions are stated in the article as:
where i(=1, …, n) is the iteration and k(=1, …, K) the replication.
The simulations are then repeated for n iterations and K replications from which the uncertainty and variability associated with the risk estimate are calculated. For each (i, k), Xik, Yik and Zik are simulated from the distributions, if XikYik > Zik, then Uik = 1, otherwise Uik = 0. Thus, the risk is calculated for each run k:
And the standard deviation is calculated for the K risks:
Originally the uncertainty and variability associated with the risk estimate were taken into account by adding uncertainty on the scale parameters (σ) of the Log-Normal consumption and concentration distributions and by adding variability among persons for the threshold distribution. However, the parameters do not vary from replication to replication (k). Increasing the number of simulations, i.e. increase in the number of iterations and/or replications, will lead to a more accurate estimate of the risk. However, the standard deviation calculated by this method expresses both the uncertainty of the numerical procedure and the uncertainty introduced by the input variables. Furthermore, the model was improved to take into account the uncertainty associated with the input parameters as described in Remington et al.8,10 but this is not further discussed in the current study.
Mathematical formulation of the model developed by Rimbaud et al.7
The probabilistic risk assessment described in Rimbaud et al.7 uses a combination of second order Monte-Carlo simulations and Bayesian inferences to estimate the risk of allergic reaction. The consumption (X), the contamination (Y) and the threshold (Z) distributions can also be used to express mathematically the risk in the allergic user population. In Bayesian analysis, the parameters characterizing the input distributions are themselves represented by distributions. Thus, the actual input distributions and the distribution of their prior parameters are defined below:
The distributions of the posterior parameters are evaluated either by direct calculation when prior distributions are conjugate or using Markov chain Monte-Carlo simulations. This results in parameter distributions from which K set of parameters are sampled in order to integrate the uncertainty in the risk estimation and n iterations are made for each set of parameters. The posterior parameter distributions are obtained using specific datasets that will be presented in the results section. Then, the risk is estimated at the individual level from the threshold dose distribution:
The risk is defined as the probability that the allergic consumer will react to the amount of allergen ingested. Thus, for each set of parameters, distributions of risks are obtained from the n individual results to describe the uncertainty and variability in risks. Case D is inspired by the Rimbaud et al.7 approach.
Methods comparison
The two approaches developed by Spanjersberg et al.9 and Rimbaud et al.7 have the same input variables namely consumption, concentration and threshold distribution. But, as described in sections 2.1.1 and 2.1.2, the two models use the input distribution in different ways to estimate the risk of allergic reaction. After mathematical review, it was highlighted that the method in Spanjersberg et al.9 is not able to separate the simulation uncertainty and the uncertainty integrated by the input parameters. However, the method described in Rimbaud et al.7 makes explicit the uncertainty propagation from the input variables to the risk calculation. More detailed investigation on uncertainty propagation with these two approaches are explained in section 2.4.
Besides the uncertainty around the risk estimates, the two methods also differ from the way the threshold curve is used. In Spanjersberg et al.9, for each individual a threshold is simulated from the density function and then directly compared to the amount of allergen ingested. The risk is calculated by counting the number of reactions among the simulated consumers. In contrast, in Rimbaud et al.7, the dose response curve (cumulative density function) is used to predict the chance of an allergic reaction for each consumer considering their exposure. The distribution of resulting risk estimates is used to estimate the overall risk of allergic reaction and its uncertainty. When the same threshold data are used, the difference in use of this distribution should not impact the risk estimation by the two methods and this is illustrated succinctly in Appendix 1 by estimating the risk using both the density and the cumulative density functions. Finally, this point will not be further addressed as it is not the main focus of the paper.
Mathematical formulation of the alternative frequentist approach and description of the cases under study
From a frequentist perspective, the risk is a deterministic function of the parameters from the three distributions, exemplified later in the article by the triple-log-normal case. The risk estimated from the three data sources is hence the same function with parameters estimated by sampling from each data source (the parameter estimates are then used in the risk function). Thus, the sample uncertainty of this risk estimate can be seen as a straightforward error propagation challenge for the risk function. Namely, the uncertainty which propagates from each sampling parameter estimate is evaluated through the non-linear risk function to provide an uncertainty evaluation of the risk estimate. In this paper, simulations were used to assess the uncertainty propagation with normal approximation for mean estimates and chi-square distributions for variance parameter estimates11.
Mathematical formulation of the alternative frequentist approach
Inspired from Spanjersberg et al.9, Monte-Carlo simulations that include parameter sampling can be used. The parameter distributions are defined from well-known distributions. The parameters of the consumption, concentration and threshold distribution are estimated from the data. It is assumed that their parameters are defined with the observations from a random sample of size l following the normal distribution N(μ, σ2):
The sample mean of the distributions parameter is sampled from a normal distribution with mean μl and standard deviation (uncertainty on the mean)11. So, the mean is sampled from: .
When a lognormal distribution is used, the sample variance S2 can be directly simulated from its specific distribution: for a sample of size l and with σ2 variance is follows a chi-square distribution: 11. So, the variance is sampled from: and is used as the scale parameter of the lognormal distribution.
In some cases, a model is fitted to the input data in order to estimate the distribution of the parameters and the associated uncertainty. The parameters are then sampled from normal distribution with the mean of the estimated parameter and standard deviation of the parameters estimated with the regression. So, the parameter a is sampled from: .
K different sets of parameters (number of replications) are sampled from these distributions as defined in Table 1 to assess the uncertainty around the risk.
Case A (Spansbjerg) and Case B (calculated and stimulated): Triple log-normal case
In case A (Spansbjerg), the risk calculation is based on from the probabilistic risk assessment described in Spanjersberg et al.9. Three log-normal distributions are used for the three input parameters (consumption – X, contamination – Y, threshold – Z) and no uncertainty around the three inputs was integrated in the risk calculation.
In Case B (triple log-normal), three log-normal distributions are again used to fit the three input parameters. Each lognormal distribution is described with its location μ and scale σ parameter. Additionally, the uncertainty is integrated by sampling the distribution parameters from normal and chi-squared distributions as defined in 2.2.1. The risk of allergic reaction can then be expressed mathematically and is then a function of 6 parameters:
In fact, in this case it becomes:
where φ is the probability density function of the standard normal distribution.
This risk expression will also be used to evaluate the uncertainty propagation both mathematically and with simulations. Thus, the comparison done in the triple log normal (Case B) will allow us to investigate the additivity of the different sources of uncertainty.
Case C: Frequentist derived from Rimbaud et al.7
Distributions were selected in order to compare the risk estimation and the uncertainty propagation estimated in Case C (frequentist inferences and simulations) with Case D (Rimbaud: simulations and Bayesian inferences). Thus, the actual input distributions and the distribution of their parameters for Case C are defined below:
K set of parameters are then sampled from the distribution of each parameters in order to integrate the uncertainty in the risk estimation and n iterations are made for each set of parameters.
Case D: Bayesian based on Rimbaud et al.7
Case D is designed in the same way as Rimbaud et al.7 and was previously described in 2.1.2.
Comparison of different cases to estimate the risk of an unexpected allergic reaction
General risk calculation
The four cases are formulated to estimate the risk of an unexpected allergic reaction. The cases are formulated in the same way, so the similarities and differences between the various ways of simulating from the distribution can be explicitly clarified and the consequences for the risk assessment can be formally assessed. Especially, the differences in the way the uncertainty propagates with all the simulation methods will be assessed. The consumption X follows a distribution noted FX, the concentration Y follows a distribution called FY and the threshold Z follows a distribution called FZ. The allergy outcome U is defined as follow:
U follows a Bernoulli distribution with probability pu, where pu = P(Z < XY). Formally it is assumed that the three random variables X, Y and Z are independent. The probability pu is mathematically a function of the three distributions: pu = f(Fx, Fy, Fz). The general principle of the risk modelling is presented in Fig. 1.
Figure 1.
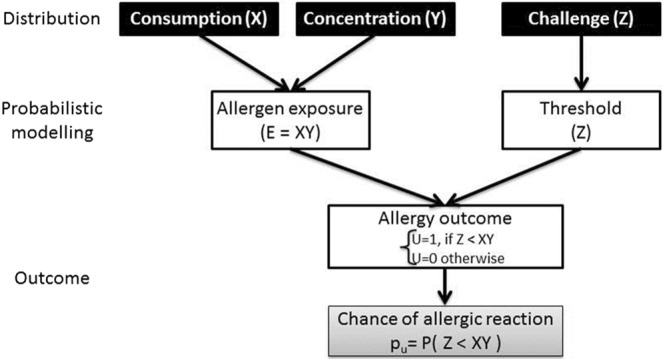
Risk estimation – general principle.
If these three distributions are given by parameters, then this is a function of the following:
Sampling distributions for the different cases
For each case, the distribution chosen for each input and the distribution of their parameters are presented in Table 1. The same simulation framework as described in 2.3.1 is used so the risk estimation can be directly compared.
Uncertainty propagation
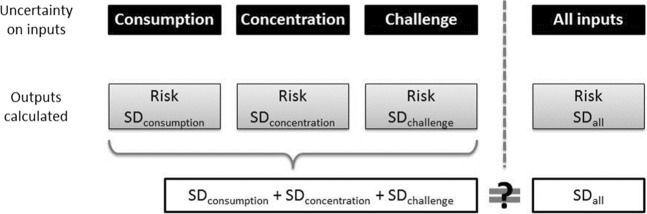
In order to assess how the uncertainty propagates from the consumption, contamination and threshold distributions separately to the risk, an adapted version of the uncertainty analysis described previously12 was used. This method is compliant with EFSA recommendations to evaluate sources of uncertainty individually13.
General principle – mathematical formulation
Propagation of the sampling uncertainty of all the distributions through the non-linear computation was investigated. This can actually be investigated for each distribution separately or for all of them jointly. Thus, it makes it possible to identify which input variable adds the most uncertainty to the risk. Formally, it amounts to considering the as a random variable as a function of the data:
where is a random sampling statistic which investigated the uncertainty induced by consumption data sampling, and the other two are held at the observed estimated parameters. Or, to investigate the uncertainty induced by the concentration data sampling:
Or, to investigate the uncertainty induced by the threshold data sampling:
Or all of them, which is how the risk of allergic reaction is usually calculated in probabilisitic modelling. The uncertainty induced by the three distributions is investigated:
Mathematical formulation of uncertainty propagation for Case B (Triple-log-normal)
The uncertainty around the risk can indeed be calculated with the multivariate propagation of error formula as the risk is estimated with three independent distributions14. The uncertainties should approximately add up on the variance scale in the triple log-normal model:
The derivative calculation is detailed in Appendix 2, and the variance of the risk was found to be:
where ni is the number of data points for each input data, and
and
Thus, the uncertainty around the risk can be calculated mathematically in the triple log-normal case, for each source of uncertainty separately and the three combined. So, it can be actually checked that the uncertainty from the three sources sum correctly.
Sampling scheme: uncertainty comparison of the input variables
Second order Monte Carlo simulations are used. The K different sets of parameters (replications) are sampled for the three distributions with parameters calculated from the study case distributions. n simulations are performed with the K different set of parameters. In order to assess the magnitude of the uncertainty from the three distributions individually, the uncertainty around the distribution of the input parameters is integrated for one input distribution at a time while the parameters from the other inputs are fixed. Then, the risk is calculated given the uncertainty from one input distribution at a time. As the inputs are assumed to be independent, the sum of the three uncertainties is compared to the uncertainty around the risk calculated with the uncertainty associated with the three input distributions at the same time. Thus, the sum of uncertainties can be compared to the uncertainty when the uncertainties around all input parameters distributions are integrated at the same time. The parameters sampling scheme is shown on Fig. 2.
Figure 2.
Uncertainty on the parameters sampling scheme (SD = standard deviation).
Simulations and software
Once the sampling distribution for each parameter had been selected, the risk was calculated for each set of parameters. The number of replications (K) to evaluate the uncertainty around the risk was set to 1 000 and the number of iterations (n) per replication was set to 10 000. The simulations were performed with R software version 3.2.215. The threshold distribution was fit to a survival model using the survival package (version 2.38.3) for the frequentist method and the JAGS software version 4.0.016 for the Bayesian method.
Data description
Concentration of unintended allergen distribution (Y)
In order to be able to compare the cases (A through D) for the risk modelling, peanut concentration data in cereal and nutrition bars were collected from peer-reviewed literature8. In total, the 24 data points collected were used as an input to the risk assessment.
Consumption (X)
The combined consumption of cereal bars in Netherlands, France and Denmark was used as an input distribution to the risk assessment17,18. As allergic reactions result from acute consumption on a single eating occasion, a conservative approach, i.e. the eating occasion with maximum consumption, was used.
Thresholds (Z)
A selection of the data described previously19 was used to describe the response to double blind placebo controlled food challenge (DBPCFC) with peanut protein. Thus, 158 NOAEL (No Observable Adverse Effect Level) and LOAEL (Lowest Observable Adverse Effect Level) values collected in publications20–31 were fitted to survival regression with Weibull or log-normal link function.
Results
Input distributions
Concentration of unintended allergen distribution (Y)
The mean and the standard deviation of the contamination distribution were 77 and 161 ppm respectively. Figure 3 shows the distribution of peanut concentration in cereal bars with the histogram and the log-normal distribution with mean and variance calculated from the concentration data.
Figure 3.
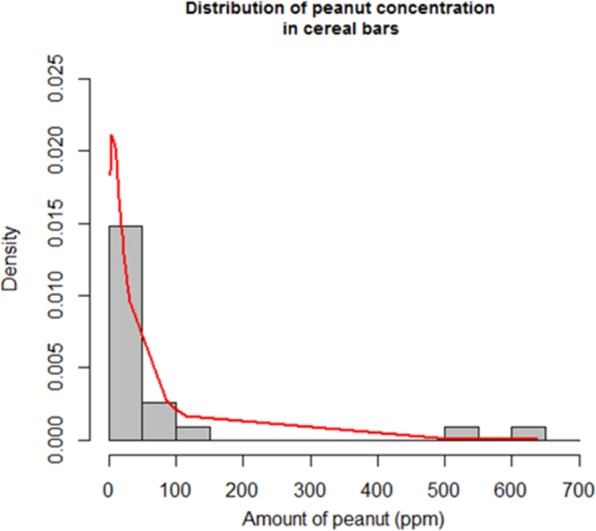
Distribution of peanut concentration in cereals bars (histogram and fitted log-normal distribution).
Table 2 shows no difference in the μY and σY parameters for the log normal distribution for cases A (Spansbjerg) and B (triple log-normal). μY is around 3.5 in both cases and σY around 1.3. Table 2 shows that the distribution of the λY parameter of the exponential distribution is similar when Monte Carlo simulations and Bayesian inferences are performed; the average λY is 0.013 in cases C and D.
Table 2.
Concentration parameters distribution for the cases A, B, C and D.
| Distribution | Parameter | Mean | SD | P2.5% | Median | P97.5% |
|---|---|---|---|---|---|---|
| Log normal | μY – case A | 3.504 | Not calculated | Not calculated | Not calculated | Not calculated |
| Log normal | σY – case A | 1.296 | Not calculated | Not calculated | Not calculated | Not calculated |
| Log normal | μY – case B | 3.496 | 0.273 | 2.942 | 3.492 | 4.042 |
| Log normal | σY – case B | 1.283 | 0.205 | 0.904 | 1.275 | 1.705 |
| Exponential | λY – case C | 0.013 | 0.001 | 0.010 | 0.013 | 0.016 |
| Exponential | λY – case D | 0.013 | 0.003 | 0.008 | 0.013 | 0.019 |
Consumption distribution (X)
With the 350 participants in the three combined Food Consumption surveys consuming cereal bars, the mean and the standard deviation of cereal bar consumption were 32 g and 28 g respectively. Furthermore, 95% of the consumption lies between 1.9 g and 291.0 g. Figure 4 shows the distribution of cereal bar consumption with the histogram and the log-normal distribution with mean and variance calculated from the consumption data.
Figure 4.
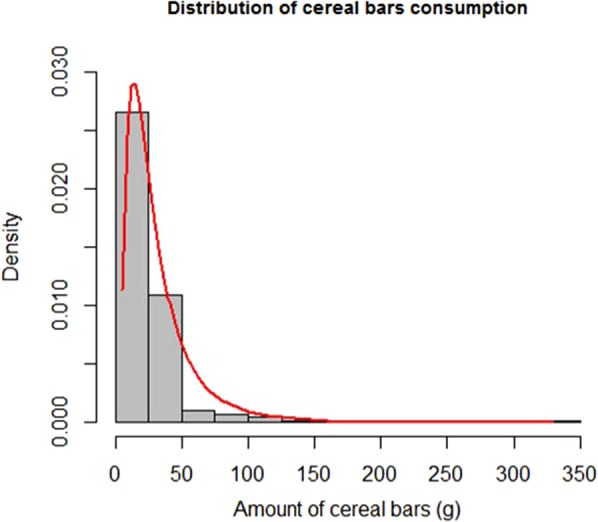
Distribution of cereal bars consumption (histogram and fitted log-normal distribution).
Summary statistics of the parameters of the log-normal distribution (μX and σX) are presented in Table 3. In cases A (Spansbjerg), B (triple log-normal) and C μx is around −3.7 and σX is around 0.7. In case D (Rimbaud), the empirical distribution is sampled, so no distribution parameters are calculated.
Table 3.
Consumption parameters distribution for the cases A, B and C.
| Distribution | Parameter | Mean | SD | P2.5% | Median | P97.5% |
|---|---|---|---|---|---|---|
| Log normal | μx – case A | −3.718 | Not calculated | Not calculated | Not calculated | Not calculated |
| Log normal | σX – case A | 0.745 | Not calculated | Not calculated | Not calculated | Not calculated |
| Log normal | μX – case B & C | −3.719 | 0.039 | −3.796 | −3.719 | −3.642 |
| Log normal | σX – case B & C | 0.747 | 0.028 | 0.691 | 0.746 | 0.801 |
Threshold distribution (Z)
The summary statistics of the parameters of the Log-Normal (cases A (Spansbjerg) and B(triple log-normal)) and Weibull (cases C and D (Rimbaud)) distribution parameters fitted with a survival model are presented in Table 4.
Table 4.
Threshold parameters distribution for the cases A, B, C and D.
| Distribution | Parameter | Mean | SD | P2.5% | Median | P97.5% |
|---|---|---|---|---|---|---|
| Log normal | μY – case A | 4.092 | Not calculated | Not calculated | Not calculated | Not calculated |
| Log normal | σY – case A | 2.982 | Not calculated | Not calculated | Not calculated | Not calculated |
| Log normal | μZ – case B | 4.088 | 0.238 | 3.612 | 4.088 | 4.55 |
| Log normal | σZ – case B | 2.987 | 0.171 | 2.668 | 2.984 | 3.324 |
| Weibull | aZ – case C | 0.382 | 0.027 | 0.331 | 0.381 | 0.438 |
| Weibull | bZ – case C | 229.621 | 55.099 | 142.773 | 223.298 | 351.067 |
| Weibull | aZ – case D | 0.379 | 0.027 | 0.328 | 0.379 | 0.433 |
| Weibull | bZ – case D | 225.075 | 51.906 | 139.852 | 219.042 | 341.439 |
In cases A (Spansbjerg) and B(triple log-normal), the estimated parameters for the log-normal distribution are similar with the average estimated coefficient for μZ is 4.09 and σZ is 2.98 in both cases. The distribution of the parameters aZ and bZ is similar between cases C and D (Rimbaud). The mean of distribution of aZ is 0.38 in cases C and D (Rimbaud) while the mean of distribution of bZ is 229.6 in case C and 225.1 in case D (Rimbaud).
Comparison of the calculated and simulated risk of reaction to peanut in cereal bars per eating occasion
In the peanut-allergic population consuming cereal bars, the probability of allergic reaction per eating occasion after consuming a contaminated cereal bar ranges from 9.79% to 14.69% in mean, depending on the way the risk of allergic reaction is estimated. When three log-normal distributions are used (cases A (Spansbjerg) and B (triple log-normal)), the average risk of allergic reaction is similar: around 9.8–9.9%. In case B (triple log-normal), the underlying distribution of the risk can be evaluated either with mathematical calculation or with simulations. In both estimations, the risks distributions are very similar as it can be noticed in Table 5. The average probability of allergic reaction in case B (triple log-normal) was actually calculated as 9.90% (CI 95: 6.07–14.74%) and simulated as 9.90% (CI 95: 5.99–14.71%), which, considering the simulation error, is identical. In cases C and D (Rimbaud), distributions other than log-normal were used to estimate the risk of allergic reaction and it can be seen that the average risk of allergic reaction is different (higher) than in cases A and B. However, the difference in risk estimates between cases C and D (Rimbaud) and cases A (Spansbjerg) and B (triple log-normal) was expected due to the due to the characteristics of the distributions used for the risk assessments. More importantly, the risk estimates in cases C and D (Rimbaud) are similar to each other when using the same distributions for the input parameters but different simulation methods (i.e. second order Monte Carlo simulations combined with frequentist inference in case C and with Bayesian inference in case D (Rimbaud)). The average risk estimate in both cases is around 14%: in case C, the risk is on average 13.76% (CI 95: 9.94–17.97%) while in case D (Rimbaud), the risk is on average 14.69% (CI 95: 10.48–19.41%). Different sampling methods (log-normal vs bootstrap) can explain the small difference in risk.
Table 5.
Risk distribution estimation for the 4 cases.
| Case | Mean | SD | 2.5% | Median | 97.5% |
|---|---|---|---|---|---|
| Case A | 9.79% | Not calculated | Not calculated | Not calculated | Not calculated |
| Case B – calculated | 9.90% | 2.20% | 6.07% | 9.75% | 14.74% |
| Case B – simulated | 9.90% | 2.23% | 5.99% | 9.74% | 14.71% |
| Case C – simulated | 13.76% | 2.09% | 9.94% | 13.65% | 17.97% |
| Case D – simulated | 14.69% | 2.31% | 10.48% | 14.66% | 19.41% |
Uncertainty propagation
Table 6 presents the uncertainty for cases B, C and D based on the input parameters when they are added individually or evaluated globally. In case B (triple log-normal), the individual uncertainties around the input parameters add up to similar values whether evaluated by calculation or simulation. Moreover, both the calculated global uncertainty estimates are similar to the uncertainty estimated by summing the individual uncertainties whether evaluated by calculation or simulation. Thus, it can be seen that the assumption that the three input distributions contribute independently to the uncertainty around the risk is correct. Furthermore, the rank order of the individual uncertainty inputs contributing to the uncertainty is of the same order in all cases for this example: the threshold distribution adds the most uncertainty to the risk, followed by the contamination distribution and then the consumption distribution. This observation is true whether log-normal (A, B) or Weibull (C, D) are used to fit the threshold data. The Weibull distribution contributes more uncertainty to the risk estimate than the log-normal distribution.
Table 6.
Risk variance (in 10−02%) calculated by summing the uncertainties around individual input parameters compared to global uncertainty across all parameters.
| Uncertainty on parameters | Case B | Case C | Case D | |
|---|---|---|---|---|
| Calculation | Simulation | |||
| Consumption (X) | 0.03 | 0.15 | 0.17 | 0.11 |
| Contamination (Y) | 2.23 | 2.29 | 0.35 | 1.14 |
| Threshold (Z) | 2.85 | 2.95 | 4.04 | 4.07 |
| Sum of uncertainty from individual parameters | 5.11 | 5.39 | 4.57 | 5.32 |
| All parameters | 5.28 | 5.37 | 4.36 | 5.37 |
In cases C and D, the uncertainty from the consumption distribution is similar when using a log-normal distribution or when bootstrapping in the empirical distribution. The uncertainty coming from the contamination distribution is different when using different sampling distributions for the parameter of the exponential distribution. Finally, the uncertainty from the threshold distribution is similar when using frequentist or Bayesian inference. The uncertainties are also additive in cases C and D. Furthermore, the rank order of the uncertainty contribution to the risk by each input is the same whether using a frequentist or a Bayesian approach.
Additional examples of cases B and C, using a large number of priority allergens and data from Taylor et al.19 can be found in Appendix 3. The data in Appendix 3 illustrate that the rank order of the contribution to overall uncertainty can vary depending on the allergen and total amount of data available on each of the inputs. As one would expect, there is less uncertainty from the threshold distribution when more data are available (full peanut/milk datasets) and there is more uncertainty from the threshold distribution in others with less data available (shrimp/sesame). Additionally, differences in the log-normal and Weibull threshold distribution estimates lead to more uncertainty being introduced from the use of the Weibull distribution for the threshold estimation (Appendix 3).
Conclusion/Discussion
In this paper, four different cases were compared to estimate the risk of allergic reaction for a single eating occasion following consumption of cereal bars containing unintended peanut and to understand the mechanism of uncertainty propagation from the input parameters (amount of product consumed, concentration of peanut in product, peanut thresholds) to the risk. Bayesian and frequentist cases used to estimate parameter distributions lead to similar estimates of risk and its associated uncertainty. Using a simplified case based on three log-normal distributions, simulations and analytical calculations were compared in order to demonstrate the additive nature of all sources of uncertainty. While the Bayesian and frequentist cases produced similar results, the better familiarity of food scientists with frequentist models model suggests preferential use of the frequentist simulation technique. Moreover, the food scientists might not be familiar to the prior distribution concept which might lead to a misuse of a Bayesian implementation if the food scientists are not trained.
In the triple-log-normal case, the risk can be easily expressed mathematically as a normal distribution with the three input distribution parameters. While the triple-log-normal was used as an example, in real-life it is recommended to test several distributions (log normal, log logistic, Weibull, exponential, gamma, normal, etc) and to select the ones best fitting the data. This applies to all input distributions (threshold, consumption, contamination, etc). The distributions were selected for illustrative purposes and the comparisons could have been done with other distribution models. Furthermore, analytical calculation cannot be done in all situations as the mathematical expression of the risk gets more complex by using other distributions or when more variables are included, for example the proportion of products that are contaminated or the prevalence of allergy. Thus, when the risk expression becomes more complex, the risk can be estimated with simulations.
Choices as to the number of iterations and replications were made for the risk assessment framework of this study (10 000 iterations and 1 000 replications). However, further possibilities were explored and risks were estimated for different number of iterations (from 1 000 to 1 000 000) and replications (25, 100, 1 000 and 10 000). The variances of the risk estimates were compared for the different situations, the computation error was assessed and the computation time was also calculated. Thus, based on this investigation, the number of iterations (10 000) and replications (1 000) were found to be the best compromise between the computation time and accuracy (data not shown).
Population-based consumption data and individual threshold data are collected in separate and unrelated studies. Thus, the data collection scheme does not allow the acquisition of the matching threshold of reaction to each individual consuming cereal bars. However, it could be conceived that that the consumption and threshold of reaction are dependent of the individual. The recent MIRABEL study32 measured threshold of reaction during an oral food challenge for allergic individuals, as well as their corresponding frequency of consumption and the amount of certain food products to determine if doses encountered in the community are near or above the estimated threshold doses.. Additional data of this nature are needed for further analyses to investigate the possible connection between consumption and threshold at an individual level and, if necessary, implement the results in future risk assessments.
Additionally, the amount of food allergen known to cause a reaction in an individual can change over time and can be affected by different intrinsic or extrinsic factors33. Further calculation was integrated in the method developed in Rimbaud et al.7 (comparable to case D) which allows the investigation of the heterogeneity of the risk of allergic reaction due to a changing threshold at individual level and could similarly be integrated into frequentist methods (cases B or C).
Furthermore, the current study focuses on simulations and sampling distributions suitable for allergen risk assessment, rather than suggesting the best distribution to be used for the input variables. As a future investigation, using one simulation method which takes into account the uncertainty around the’ parameters defining the distributions, the different distribution choices for each input (threshold, consumption, contamination, etc) could be compared to recommend the distribution that gives the estimation of the risk with the lowest uncertainty.
Sensitivity analysis had already been carried out with the model developed in Spanjersberg et al.9,34. With sensitivity analysis, the input variables that have the most influence on the model output (i.e. the risk of allergic reaction) were identified12. The estimation of the risk of allergic reaction increased when the threshold distribution was shifted to simulate a more potent allergen, while increasing the amount consumed did not yield the same increase in the number of predicted reactions. A sensitivity analysis concerning the concentration of allergen in the food consumed was not conducted in the current study. It is expected that the influence of the input variables on the risk will be the same for the different cases presented in this paper. Further, a sensitivity analysis could be performed with the different cases presented in this paper in order to compare how the risk is impacted by a shift of input distributions.
In conclusion we have compared the four different cases that could be used in food allergen probabilistic risk assessment and found that although different mathematical formulations have been used, the overall results obtained are very similar. In addition, we have demonstrated that all methods are able to propagate uncertainty. The sources of uncertainty can be compared for the different allergens and thus, additional data can be identified which will most efficiently contribute to a reduction of uncertainty on the risk estimation.
Supplementary information
Acknowledgements
This work was part of the iFAAM project (Integrated Approaches to Food Allergen and Allergy Risk Management, Grant Agreement No. 322147), the national food consumption data for France and Denmark were kindly provided by the work package partners: ANSES and DTU. The national food consumption data of the Netherlands was kindly provided by the National Institute for Public Health and the Environment in the Netherlands. The challenge data for peanut and soy was kindly provided by TNO and FARRP (University of Nebraska – Food Allergy Research and Resource Program). This paper was kindly reviewed and edited by René Crevel.
Author contributions
S.B., C.M., A.K., A.C., B.R., J.B. and P.B. conceived and designed the experiments; S.B. developed the methods and analyzed the data with input from C.M., A.K., A.C., B.R., J.B. and P.B.; C.M., A.K., A.C., B.R., J.B. and P.B. contributed materials and analysis tools; S.B. wrote the paper with input from C.M., A.K., A.C., B.R., J.B. and P.B.
Data availability
The data that support the findings of this study are available from the iFAAM project but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the iFAAM project.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-54844-1.
References
- 1.Gendel SM. Comparison of international food allergen labeling regulations. Regul. Toxicol. Pharmacol. 2012;63:279–285. doi: 10.1016/j.yrtph.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Fernández-Rivas, M. & Asero, R. Risk Management for Food Allergy. Risk Management for Food Allergy, 10.1016/B978-0-12-381988-8.00002-6 (Elsevier, 2014).
- 3.DunnGalvin A, et al. Precautionary allergen labelling: Perspectives from key stakeholder groups. Allergy Eur. J. Allergy Clin. Immunol. 2015;70:1039–1051. doi: 10.1111/all.12614. [DOI] [PubMed] [Google Scholar]
- 4.Barnett J, et al. Using ‘may contain’ labelling to inform food choice: a qualitative study of nut allergic consumers. BMC Public Health. 2011;11:734. doi: 10.1186/1471-2458-11-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.La Vieille, S. et al. Food allergies and advisory labelling (2008).
- 6.Madsen CB, et al. Approaches to risk assessment in food allergy: Report from a workshop “developing a framework for assessing the risk from allergenic foods”. Food Chem. Toxicol. 2009;47:480–489. doi: 10.1016/j.fct.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 7.Rimbaud L, Heraud F, La Vieille S, Leblanc JC, Crepet A. Quantitative risk assessment relating to adventitious presence of allergens in food: A probabilistic model applied to peanut in chocolate. Risk Anal. 2010;30:7–19. doi: 10.1111/j.1539-6924.2009.01322.x. [DOI] [PubMed] [Google Scholar]
- 8.Remington BC, Baumert JL, Marx DB, Taylor SL. Quantitative risk assessment of foods containing peanut advisory labeling. Food Chem. Toxicol. 2013;62:179–187. doi: 10.1016/j.fct.2013.08.030. [DOI] [PubMed] [Google Scholar]
- 9.Spanjersberg MQI, Kruizinga AG, Rennen MAJ, Houben GF. Risk assessment and food allergy: the probabilistic model applied to allergens. Food Chem. Toxicol. 2007;45:49–54. doi: 10.1016/j.fct.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 10.Remington BC, Taylor SL, Marx DB, Petersen BJ, Baumert JL. Soy in wheat - Contamination levels and food allergy risk assessment. Food Chem. Toxicol. 2013;62:485–491. doi: 10.1016/j.fct.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, R. Miller & Freund’s probability and statistics for engineers. (Prentice Hall, 2011).
- 12.Makowski D. Uncertainty and sensitivity analysis in quantitative pest risk assessments; practical rules for risk assessors. NeoBiota. 2013;18:157–171. doi: 10.3897/neobiota.18.3993. [DOI] [Google Scholar]
- 13.EFSA Scientific Committee. Guidance on Uncertainty in EFSA Scientific Assessment. The EFSA Journal, 10.2903/j.efsa.20YY.NNNN.
- 14.Navidi, W. Statistics for engineers and scientists. (McGraw-Hill, 2006).
- 15.R Core Team. R: A Language and Environment for Statistical Computing (2015).
- 16.Plummer, M. JAGS: A program for analysis of Bayesian graphical models using Gibbs sampling. In Proceedings of the 3rd International Workshop on Distributed Statistical Computing (2003).
- 17.Birot, S. et al. A procedure for grouping food consumption data for use in food allergen risk assessment. J. Food Compos. Anal., 10.1016/j.jfca.2017.01.008 (2017).
- 18.Birot S, et al. Food groups for allergen risk assessment: Combining food consumption data from different countries in Europe. Food Chem. Toxicol. 2018;118:371–381. doi: 10.1016/j.fct.2018.05.042. [DOI] [PubMed] [Google Scholar]
- 19.Taylor SL, et al. Establishment of Reference Doses for residues of allergenic foods: Report of the VITAL Expert Panel. Food Chem. Toxicol. 2014;63:9–17. doi: 10.1016/j.fct.2013.10.032. [DOI] [PubMed] [Google Scholar]
- 20.Anagnostou, K. et al. British Society for Allergy and Clinical Immunology Annual Conference 2009 Abstracts. In British Society for Allergy and Clinical Immunology Annual Conference 2009 Abstracts vol. 39, 1937–1958 (Blackwell Publishing Ltd, 2009).
- 21.Atkins FM, Steinberg SS, Metcalfe DD. Evaluation of immediate adverse reactions to foods in adult patients: II. A detailed analysis of reaction patterns during oral food challenge. J. Allergy Clin. Immunol. 1985;75:356–363. doi: 10.1016/0091-6749(85)90072-7. [DOI] [PubMed] [Google Scholar]
- 22.Blumchen K, et al. Oral peanut immunotherapy in children with peanut anaphylaxis. J. Allergy Clin. Immunol. 2010;126:83–91.e1. doi: 10.1016/j.jaci.2010.04.030. [DOI] [PubMed] [Google Scholar]
- 23.Clark AT, Ewan PW. Good prognosis, clinical features, and circumstances of peanut and tree nut reactions in children treated by a specialist allergy center. J. Allergy Clin. Immunol. 2008;122:286–289. doi: 10.1016/j.jaci.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Hourihane JO, et al. An evaluation of the sensitivity of subjects with peanut allergy to very low doses of peanut protein: A randomized, double-blind, placebo-controlled food challenge study. J. Allergy Clin. Immunol. 1997;100:596–600. doi: 10.1016/S0091-6749(97)70161-1. [DOI] [PubMed] [Google Scholar]
- 25.Leung DYM, et al. Effect of Anti-IgE Therapy in Patients with Peanut Allergy. N. Engl. J. Med. 2003;348:986–993. doi: 10.1056/NEJMoa022613. [DOI] [PubMed] [Google Scholar]
- 26.Lewis SA, Grimshaw KEC, Warner JO, Hourihane JO. The promiscuity of immunoglobulin E binding to peanut allergens, as determined by Western blotting, correlates with the severity of clinical symptoms. Clin. Exp. Allergy. 2005;35:767–773. doi: 10.1111/j.1365-2222.2005.02252.x. [DOI] [PubMed] [Google Scholar]
- 27.Nelson H, Lahr J, Rule R, Bock A, Leung D. Treatment of anaphylactic sensitivity to peanuts by immunotherapy with injections of aqueous peanut extract1. J. Allergy Clin. Immunol. 1997;99:744–751. doi: 10.1016/S0091-6749(97)80006-1. [DOI] [PubMed] [Google Scholar]
- 28.Nicolaou N, et al. Allergy or tolerance in children sensitized to peanut: Prevalence and differentiation using component-resolved diagnostics. J. Allergy Clin. Immunol. 2010;125:191–197.e13. doi: 10.1016/j.jaci.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Oppenheimer JJ, Nelson HS, Bock SA, Christensen F, Leung DYM. Treatment of peanut allergy with rush immunotherapy. J. Allergy Clin. Immunol. 1992;90:256–262. doi: 10.1016/0091-6749(92)90080-L. [DOI] [PubMed] [Google Scholar]
- 30.Patriarca G, et al. Oral Rush Desensitization in Peanut Allergy: A Case Report. Dig. Dis. Sci. 2006;51:471–473. doi: 10.1007/s10620-006-3157-4. [DOI] [PubMed] [Google Scholar]
- 31.Wainstein BK, Studdert J, Ziegler M, Ziegler JB. Prediction of anaphylaxis during peanut food challenge: usefulness of the peanut skin prick test (SPT) and specific IgE level. Pediatr. Allergy Immunol. 2010;21:603–611. doi: 10.1111/j.1399-3038.2010.01063.x. [DOI] [PubMed] [Google Scholar]
- 32.Crépet A, et al. Mirabel: An integrated project for risk and cost/benefit analysis of peanut allergy. Regul. Toxicol. Pharmacol. 2015;71:178–183. doi: 10.1016/j.yrtph.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 33.Turner PJ, et al. Can we identify patients at risk of life-threatening allergic reactions to food? Allergy Eur. J. Allergy Clin. Immunol. 2016;71:1241–1255. doi: 10.1111/all.12924. [DOI] [PubMed] [Google Scholar]
- 34.Kruizinga AG, et al. Probabilistic risk assessment model for allergens in food: sensitivity analysis of the minimum eliciting dose and food consumption. Food Chem. Toxicol. 2008;46:1437–1443. doi: 10.1016/j.fct.2007.09.109. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the iFAAM project but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of the iFAAM project.