Abstract
Staphylococcus aureus is recognized as an important pathogen causing a wide spectrum of diseases. Here we examined the antimicrobial effects of the lectin isolated from leaves of Schinus terebinthifolia Raddi (SteLL) against S. aureus using in vitro assays and an infection model based on Galleria mellonella larvae. The actions of SteLL on mice macrophages and S. aureus-infected macrophages were also evaluated. SteLL at 16 µg/mL (8 × MIC) increased cell mass and DNA content of S. aureus in relation to untreated bacteria, suggesting that SteLL impairs cell division. Unlike ciprofloxacin, SteLL did not induce the expression of recA, crucial for DNA repair through SOS response. The antimicrobial action of SteLL was partially inhibited by 50 mM N-acetylglucosamine. SteLL reduced staphyloxathin production and increased ciprofloxacin activity towards S. aureus. This lectin also improved the survival of G. mellonella larvae infected with S. aureus. Furthermore, SteLL induced the release of cytokines (IL-6, IL-10, IL-17A, and TNF-α), nitric oxide and superoxide anion by macrophagens. The lectin improved the bactericidal action of macrophages towards S. aureus; while the expression of IL-17A and IFN-γ was downregulated in infected macrophages. These evidences suggest SteLL as important lead molecule in the development of anti-infective agents against S. aureus.
Subject terms: Lectins, Applied microbiology
Introduction
Staphylococcus aureus is recognized as an important pathogen causing a wide spectrum of diseases including cutaneous and blood stream infections1,2. This versatility is ensured by the high ability of this microorganism to acquire drug resistance and to produce virulence factors that are regulated by complex genetic networks2–5. The multiple virulence factors identified in S. aureus play different roles during the infection such as adhesion, host lesions and evasion of the immune system, even from professional phagocytes such as macrophages6–8.
It has been reported that the dissemination of S. aureus is associated to the capacity of this bacterium to survive and replicate inside the phagocytes (in both phagosome and/or cytoplasm), and modulate important cellular mechanisms such as autophagy, apoptosis and pyronecrosis9–11. S. aureus is also able to release several effector molecules to suppress or enhance cytokine production (including IL-1β, IL-17 and TNF) as well as to damage immune cells and host tissues6,12–14. Thus, compounds able to improve and/or regulate the cellular immune response have been pointed out as promising lead drugs for treatment of microbial infections and also to improve the general understanding related to host-pathogen interactions15–19.
Plants are important sources of molecules to be used for drug development due their high chemical variability and diverse action mechanisms20,21. Among plant derived compounds, lectins have displayed a wide range of biotechnological applications, including antimicrobial and immunomodulatory actions16,19,22–27. Previously, the isolation of SteLL, an N-acetylglucosamine-binding (NAG) lectin, from leaves of Schinus terebinthifolia Raddi (Anacardiaceae) was reported.
SteLL is a 14 kDa glycoprotein and the antimicrobial activity of this protein was reported towards both Gram-positive and Gram-negative bacteria and Candida albicans25. SteLL also affected the survival and nutrition of the beetle Sitophilus zeamais adults28. Recently, the antitumoral activity of SteLL was shown in sarcoma 180-bearing mice. The authors reported that the treatment with SteLL did not induce hematological changes nor genotoxic effects in mice, advocating for the safety of in vivo use of this lectin29.
This present work provides insights into the in vitro effects of SteLL on S. aureus and evaluates the phenotypic response induced by this lectin in macrophages uninfected and/or infected by S. aureus. In addition, the in vivo activity of this lectin against S. aureus is reported using Galleria mellonella larvae (Lepidoptera: Pyralidae) as infection model.
Results
SteLL induced changes in the cell size/DNA content ratio of S. aureus
As previously reported25, SteLL was able to inhibit the growth of all S. aureus strains tested (S. aureus 8325-4, S. aureus ATCC 6538, S. aureus ATCC 29312) with a MIC (minimum inhibitory concentration) of 2 µg/mL. A flow cytometry-based assay was performed to assess the effects of SteLL on cell size (seen by mean light scattering detected in LS1) and DNA content (seen by the fluorescence intensity in FL2 channel) of S. aureus. Ciprofloxacin (inhibitor of DNA replication) and chloramphenicol (inhibitor of protein synthesis) were used as controls in this assay (Table 1 and Fig. 1).
Table 1.
Effects of SteLL on cell size and DNA content of S. aureus 8325-4 during exponential growth.
| Untreated cells | CAM (1 × MIC) |
CIP (1 × MIC) |
SteLL (2 × MIC) |
SteLL (8 × MIC) |
|
|---|---|---|---|---|---|
| Cell mass | 426.1 ± 36.1a | 470.0 ± 26.6a | 638.0 ± 44.7b | 492.3 ± 62.2a | 560.6 ± 65.2b |
| DNA content | 303.8 ± 41.5a | 562.2 ± 31.0b | 264.4 ± 36.4a | 383.1 ± 73.1a | 422.5 ± 74.4b |
| DNA/mass ratio | 0.71 | 1.20 | 0.41 | 0.78 | 0.75 |
Cell mass and DNA content was measured by flow cytometry using forward scattering (at LS1 detector) and fluorescence intensity (at FL2 channel) and are expressed by arbitrary units (a.u.). Legend: CAM: Chloramphenicol; CIP: Ciprofloxacin; SteLL: Schinus terebinthifolia leaf lectin. In each row the values with significant differences (p < 0.05) are indicated by different letters.
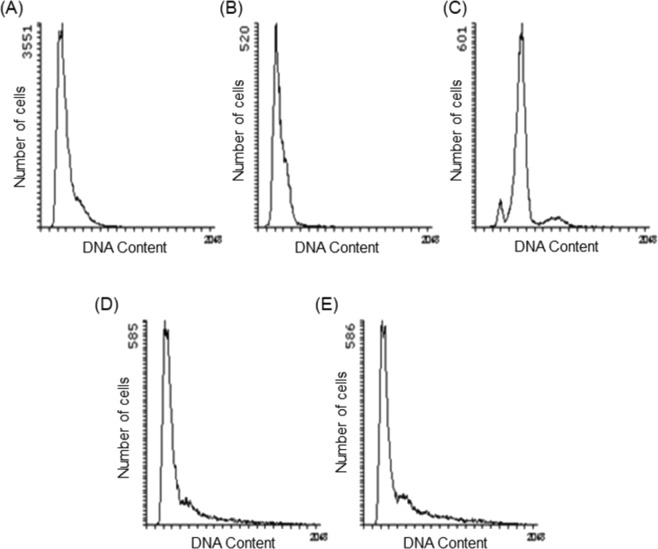
Figure 1.
Effects of SteLL and selected antimicrobials on DNA content of S. aureus 8325-4. (A) Exponentially growing cells; (B) Cells treated with 0.78 µg/mL ciprofloxacin for 3 h; (C) Cells treated with 12.5 µg/mL chloramphenicol for 3 h; (D) Cells treated with 2 × MIC SteLL (4 µg/mL) for 3 h; (E) Cells treated with 8 × MIC (16 µg/mL) SteLL for 3 h.
The exposure of S. aureus to ciprofloxacin at MIC resulted in significantly increase in cell mass, while the DNA content decreased when compared with untreated cells. This resulted in a decrease in the cellular DNA concentration (DNA/mass ratio = 0.41) compared to untreated bacteria (DNA/mass ratio = 0.71). Bacteria treated with chloramphenicol did not alter cell size as cell division requires protein synthesis and hence was blocked. In these bacteria, the cellular DNA content increased (DNA/mass ratio = 1.20), which is consistent with a DNA replication arrest specifically at the level of initiation. Consequently, runout chromosome synthesis was observed and the bacterial cells end up with integral numbers of fully replicated chromosomes (Fig. 1C).
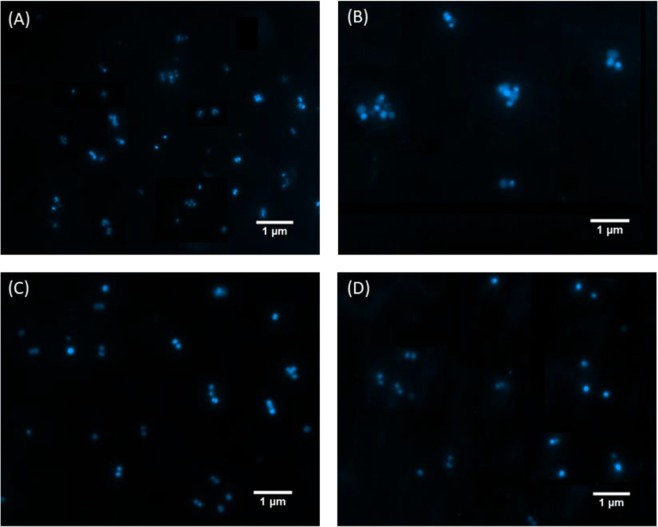
Cells treated with SteLL did not follow any of these profiles (Fig. 1D,E). A significant increase (31%) on cell size was observed in bacteria treated with SteLL at 8 × MIC (16 µg/mL), this was accompanied by a similar increase in DNA content (39%), and consequently the DNA concentration remained almost unchanged in these cells (DNA/mass ratio = 0.75) (Table 1). The effects of SteLL on S. aureus cell size were also confirmed by fluorescence microscopy (Fig. 2), where cells treated with the lectin (Fig. 2C,D) appeared with increased size when compared with control cells (Fig. 2A).
Figure 2.
Effects of SteLL on cell size of S. aureus 8325-4. (A) Exponentially growing cells; (B) Cells treated with 0.78 µg/mL ciprofloxacin for 3 h; (C) Cells treated with 2 × MIC SteLL (4 µg/mL) for 3 h; (D) Cells treated with 8 × MIC (16 µg/mL) SteLL for 3 h.
We also evaluated whether sub-inhibitory concentration of SteLL (0.5 × MIC) had any effect on the SOS response, a pathway associated with acquisition of drug resistance and virulence phenotypes. After 3 h of treatment, recA transcription determined from a recA-lacZ transcriptional fusion was not altered by SteLL (Fig. 3). On the other hand, ciprofloxacin (a DNA damaging agent) induced more than 5-fold increase in recA transcription. These results revealed that SteLL, unlike ciprofloxacin, did not affect DNA integrity and thus did not induce any SOS-related mutagenic pathways.
Figure 3.
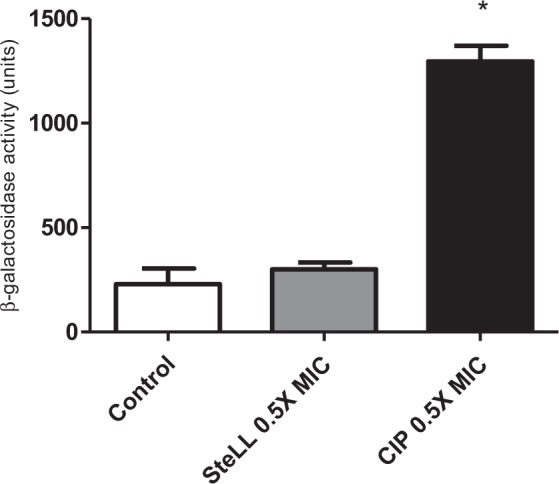
Effects of SteLL on recA expression of S. aureus. The expression of recA were performed using a derivative S. aureus 8325-4 strain carrying a recA::lacZ fusion. β-galactosidase activity was measured using ONPG. (*) Indicates significant differences in relation to control cells (p < 0.05).
SteLL increased ciprofloxacin activity against S. aureus
Checkerboard assays were performed to evaluate the interaction of SteLL with two selected antibiotics: ciprofloxacin and ampicillin. The MIC values for these two drugs towards S. aureus 8325-4 were 0.78 µg/mL and 25 µg/mL, respectively. A synergistic activity was observed when combining SteLL with ciprofloxacin (ΣFIC: 0.47); while an additive effect was found between SteLL and ampicillin (ΣFIC: 0.53).
We subsequently performed time-kill assays on S. aureus strain 8325-4 using SteLL (2 × MIC and 8 × MIC), ciprofloxacin (2 × MIC), and combinations of these two agents (2 × MIC SteLL + 2 × MIC ciprofloxacin and 8 × MIC SteLL + 2 × MIC ciprofloxacin). Both SteLL concentrations delayed the onset of growth. After 7.5 h, the reductions of bacterial counts by both SteLL concentrations were approximately 2 log CFU/mL (relative to untreated cells). In contrast, ciprofloxacin (2 × MIC) strongly reduced the bacterial growth in a time-dependent manner (Fig. 4A,B).
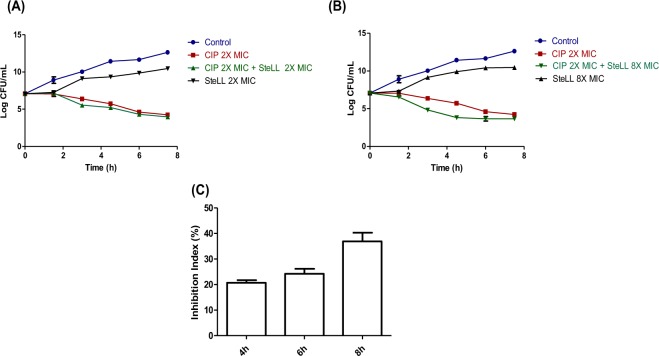
Figure 4.
Antimicrobial action of SteLL against S. aureus. (A) Time-kill curves for 2 × MIC SteLL (4 µg/mL) alone or in combination with 2 × MIC ciprofloxacin. (B) Time-kill curves for 8 × MIC SteLL (16 µg/mL) alone or in combination with 2 × MIC ciprofloxacin. (C) Effects of N-acetylglucosamine (NAG) on antimicrobial action of 8 × MIC SteLL (16 µg/mL).
When SteLL was combined at 2 × MIC, it did not induce any effect on ciprofloxacin (2 × MIC) bactericidal action (ΔLC were less than 1 log CFU/mL when compared with ciprofloxacin-treated cells). On the other hand, the combination of ciprofloxacin at 2 × MIC and SteLL at 8 × MIC was more effective than ciprofloxacin alone. For this combination, significant reductions were observed at all evaluated times, with the maximal effect observed after 4.5 h of incubation. At this time a ΔLC of 1.38 log CFU/mL relative to cells treated with ciprofloxacin alone. Thus, at this concentration SteLL exhibited an additive bactericidal effect resulting in a faster reduction in viable bacteria during the first hours of incubation (Fig. 4B).
In order to evaluate the involvement of carbohydrate-binding domain, we compared the effects of SteLL (8 × MIC) on S. aureus growth in the presence of 50 mM NAG. The antimicrobial action of SteLL was partially inhibited by NAG with inhibition index (IN%) of 20.67 ± 1.07, 24.19 ± 1.97 and 36.91 ± 3.35 after incubation of 4 h, 6 h and 8 h (Fig. 4C).
SteLL inhibits staphyloxanthin production
Next, we evaluated whether sub-inhibitory SteLL concentrations (0.065 × MIC, 0.125 × MIC, 0.25 × MIC and 0.5 × MIC) could inhibit the production of staphyloxanthin, the golden pigment of S. aureus ATCC 29312. SteLL induced a dose-dependent reduction in staphyloxanthin production relative to untreated bacteria (Fig. 5A). The reductions ranged from 48.78% to 82.88% (Fig. 5B). The treatment with the highest tested concentration of SteLL (0.5 × MIC) reduced the staphyloxanthin content to around 18% (in comparation to untreated bacteria), resulting in almost colorless S. aureus cells.
Figure 5.
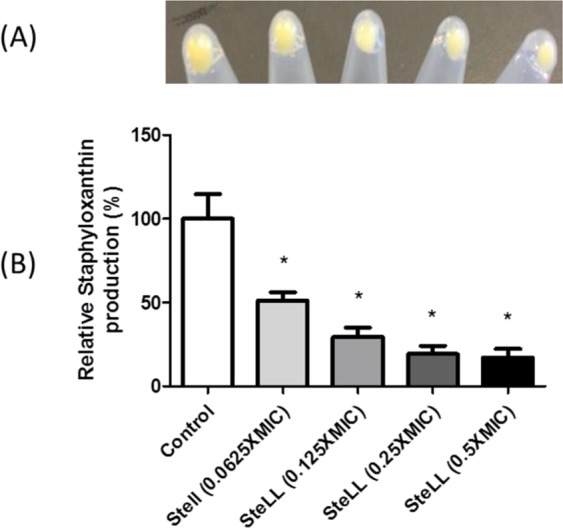
Effects of SteLL on staphyloxanthin production by S. aureus ATCC 29312. (A) Qualitative assay; (B) Quantitative assay. (*) Indicates significant differences in relation to control cells (p < 0.05).
SteLL increased the release of nitric oxide and superoxide by mice macrophages
The macrophage viability was not affected by any tested SteLL concentrations (2–16 µg/mL) (data not shown). On the other hand, the treatment of macrophages with different concentrations of this lectin resulted in a significant increase in NO production in relation to untreated cells (Fig. 6A). Maximum NO production was observed in the presence of SteLL at 8 µg/mL and 16 µg/mL. At these concentrations, the levels of NO were similar (p > 0.05) to those produced by M1 macrophages.
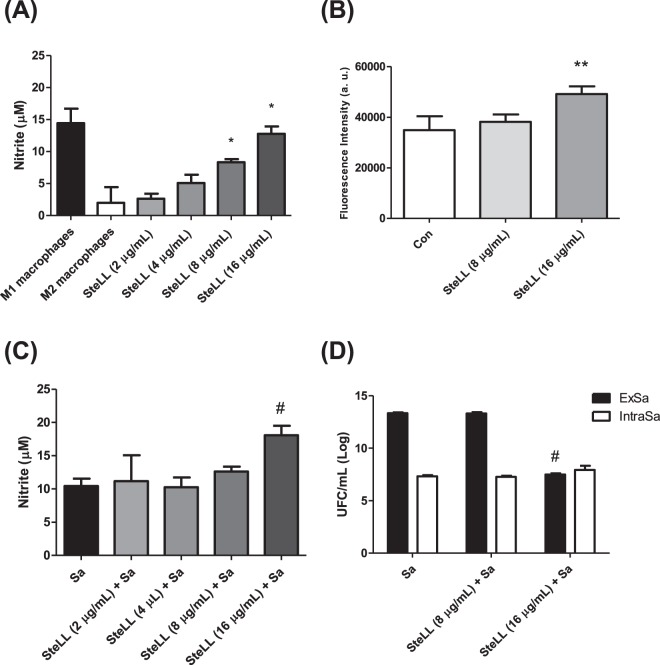
Figure 6.
Effects of SteLL on different responses of mice peritoneal macrophages. (A) Nitric oxide release by mice macrophages induced by SteLL. (B) Production of mitochondrial superoxide anion by mice peritoneal macrophages induced by SteLL. (C) Nitric oxide release by S. aureus-infected macrophages induced by SteLL. (D) Effects of SteLL on bactericidal activity of mice peritoneal macrophages towards extracellular and (ExSa) intracellular (IntraSa) S. aureus. M1 macrophages: macrophages treated with LPS + INF-γ. M2 macrophages: macrophages treated with IL-4 + IL-13. Con: untreated cells; Sa: S. aureus. (*) Indicates significant differences in relation to M2 macrophages (p < 0.05). (**) Indicates significant differences in relation to untreated macrophages (p < 0.05). (#) Indicates significant differences in relation to S. aureus-infected cells without SteLL treatment (p < 0.05).
The induction of mitochondrial superoxide by SteLL was evaluated using the MitoSOX fluorescent probe. The macrophages were incubated for 30 min with SteLL at 8 µg/mL and 16 µg/mL. As shown in Fig. 6B, only the treatment with SteLL at 16 µg/mL significantly enhanced superoxide production by the macrophages (increase of 40%).
SteLL increased the bactericide activity of mice macrophages
Next, we evaluated the effects of SteLL on S. aureus-infected macrophages. SteLL at 16 µg/mL significantly increased the NO levels released by S. aureus-infected macrophages (p > 0.05) (Fig. 6C). The highest tested concentration of SteLL (16 µg/mL) also induced a significant decrease in bacterial load in the supernatant (7.49 ± 0.33 log CFU/mL) in relation to untreated S. aureus-infected macrophages (13.35 ± 0.24 log CFU/mL) (Fig. 6D). However, the intracellular amount of S. aureus was not altered by SteLL treatment (Fig. 6D). The same response was observed in macrophages infected with S. aureus ATCC 6538 and treated with SteLL (Supplementary Fig. 1). These results indicated that the reduction of bacteria in supernatant of SteLL-treated macrophages is related with the increased release of reactive species.
SteLL modulated cytokine release by uninfected macrophages or Staphylococcus aureus-uninfected macrophages
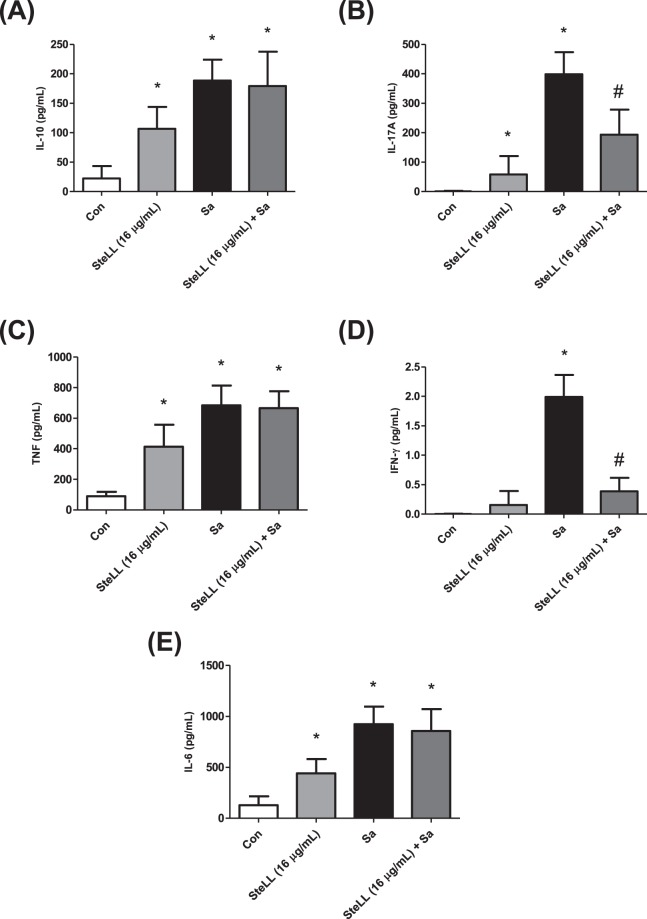
We selected the most active concentration of SteLL (16 µg/mL) to evaluate its influence on cytokine release pattern of macrophages uninfected and infected with S. aureus. For uninfected macrophages, the treatment with this dose resulted in a significant enhancement of IL-6, IL-10, IL-17A, and TNF-α compared to untreated cells (p > 0.05) (Fig. 7A,B,C,E). The levels of IFN-γ were also higher in the supernant of SteLL-treated cells, although no statitical differences were found when compared with the values obtained for control cells (Fig. 7D). The lectin did not influence the levels of IL-4 and IL-12 (data not shown).
Figure 7.
Effects of SteLL on cytokine release of mice peritoneal macrophages infected or not with S. aureus. (A) IL-10; (B) IL-17A; (C) TNF; (D) INF-γ; (E) IL-6. Con: untreated cells; Sa: S. aureus. (*) Indicates significant differences in relation to control cells (p < 0.05). (#) Indicates significant differences in relation to S. aureus infected cells (p < 0.05).
The macrophages infected with S. aureus expressed high levels of IL-6, IL-10, IL-17A, and TNF-α (Fig. 7). The roles of theses cytokines in pathogenesis of S. aureus have been described previously30–32. The level of IFN-γ was also increased by S. aureus infection (about 2-fold). SteLL was able to downregulate the expression of IL-17A (Fig. 7B) and IFN-γ (Fig. 7D) by infected macrophages, while the levels of the other tested cytokines were also reduced albeit not in a significant manner. It is important to highlight that SteLL did not totally inhibit the release of IL-17A and IFN-γ; in fact, their levels were reduced to approximately what was found in the supernatant of control cells.
SteLL protected Galleria mellonella larvae against Staphylococcus aureus infection
To finally show the treatment efficacy of SteLL, we employed an infection assay using G. mellonella larvae. This model has been widely used to study microbial pathogenesis33 and to assess the in vivo activity of antimicrobial agents34,35. Uninfected larvae inoculated with PBS or SteLL exhibited similar survival curves (p > 0.05). On the other hand, infection with S. aureus 8325-4 reduced the larval viability by 30%, 80% and 100%, on day 1, 2 and 3, respectively. The median survival of this group was 2 days.
The single-dose treatment using 0.2 mg/kg SteLL (corresponding to the administration of 10 µL of a SteLL solution at 2 × MIC) increased the survival of S. aureus-infected insects. For this group, the median survival was not possible to be defined, and only a 30% reduction in survival was recorded after 3 days (Fig. 8A). The survival curves of SteLL-treated larvae and S. aureus-infected group were significantly different (p < 0.05).
Figure 8.
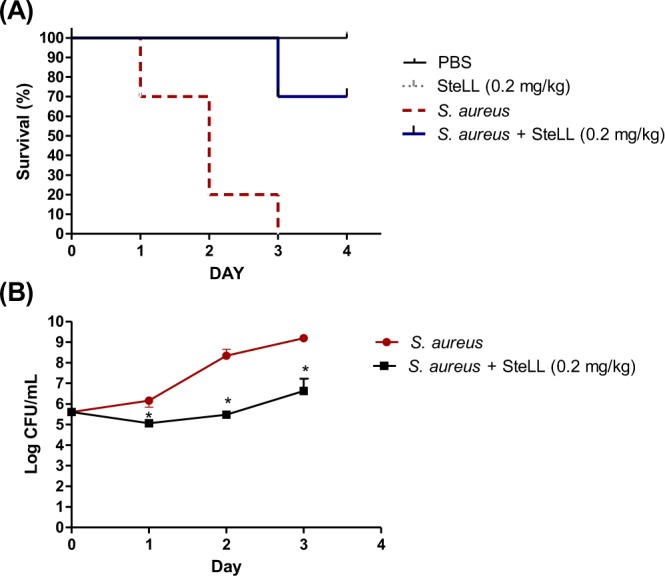
Effects of SteLL on survival (A) and bacteria load in hemolymph (B) of G. mellonella larvae infected with S. aureus. In all experiments the larvae were infected with a S. aureus 8325-4 suspension (10 μL of 1.0 × 105 CFU/mL) and treated with SteLL at 0.2 mg/kg. (*) Indicates significant differences in relation to control cells (p < 0.05).
Next, we evaluated the number of S. aureus colonies in the hemolymph of larvae. The untreated larvae infected with S. aureus exhibited increased levels of bacteria in hemolymph during the experiment (Fig. 8B). The treatment with SteLL was able to significantly inhibit the bacteria growth (p < 0.05). During the first two days the bacterial load in SteLL-treated animals remained the same as at time of inoculation and only a small increase was observed in the third day (about 1 log CFU/mL). This effect is in accordance to the effect of SteLL in the time kill assay (Fig. 3). In this sense, SteLL treatment reduced the S. aureus proliferation in larvae hemolymph resulting in increased animal survival.
Discussion
In this study, we evaluated the in vitro effects of SteLL on S. aureus and the responses induced by this protein in two models of infections (using macrophages and G. mellonella). SteLL is purified from leaves of S. terebinthifolia, a medicinal plant widely used in Northeastern Brazilian (where it is popularly known as “Aroeira da praia”) to treat skin wounds and inflammation36. Besides SteLL, other products derived from S. terebinthifolia have exhibited antimicrobial activity such as essential oils and some purified compounds37–39.
We employed a flow cytometry-based method to analyze whether SteLL could induce any effect on cell size and DNA content40. Proper cell cycle control is essential to ensure the generation of two identical daughter cells as result of cell division41–43. Perturbations in cell cycle regulation are therefore deleterious for bacterial proliferation; and thus the proteins involved in this pathway constitute potential targets for drug action44. We observed that SteLL induced significant increases in both the cell size and DNA content after 3 hours of incubation. These effects were confirmed by fluorescence microscopy and may indicate impairment of cell division (since both DNA and cell mass are increased), a kind of response that has been described for other antimicrobial agents such as targocil, a cell wall stressor45.
The ability to bind N-acetylglucosamine residues has been associated with the bacteriostatic properties of chitin-binding lectins (such as SteLL)25,26,46, since high amount of peptidoglycan in S. aureus cell wall provides multiples targets for interactions. We demonstrated that 50 mM NAG partially inhibited the antimicrobial action of SteLL. Thus, it is possible to hypothesize that SteLL binds N-acetylglucosamine residues present in cell wall and disturbs the process of cell division.
In addition, SteLL treatment did not induce the expression of recA which is instrumental in triggering the SOS response. RecA detects ssDNA generated by DNA degradation or inhibition of DNA replication, stimulates LexA autocleavage which in turn leads to derepression of a number of LexA regulated genes. These genes encode enzymes involved in DNA repair and mutagenesis (reviewed by Simmons et al.47). Taken together, these findings indicated that SteLL may inhibit bacterial growth by impairing division without affecting DNA structure.
Since SteLL inhibits the growth of S. aureus, we evaluated whether this lectin could affect the activity of antibiotics in clinical use. The combinatory effects of SteLL and the antibiotics ciprofloxacin or ampicillin were assessed using checkboard and time-kill experiments. Initially, we found that this lectin improved the action of ampicillin (β-lactam) and ciprofloxacin (quinolone) through additive and synergistic effects, respectively. SteLL at 16 µg/mL (8 × MIC) could also increase the bactericidal properties of ciprofloxacin, however, this action was only observed during the first hours. The synergistic interactions with antibiotics have been reported only for few plant lectins, including the lectins extracted from Alpinia purpurata (ApuL)24 and Vatairea macrocarpa (VML)48.
Another effect of SteLL on S. aureus physiology is the inhibition of staphyloxanthin production, a carotenoid pigment encoded by the crtOPQMN operon. Staphyloxanthin has been associated with the protection against oxidant attack promoted by immune cells49, which brought a new light in the use of this pigment as target for drug development34,50. Some plant derived compounds have inhibitory effects on staphyloxanthin34,51,52, however, this is the first report of a similar action for a plant lectin.
Given the well known ability of plant lectins to alter the phenotic responses of immune cells, we examined the effects of SteLL on macrophages and S. aureus-infected macrophages. The results showed that SteLL induced the the release of cytokines (IL-6, IL-10, IL-17A, and TNF-α) and reactive species (nitric oxide, superoxide anion) by uninfected macrophagens. The ability of plant lectins to alter the macrophage responses have been demonstrated by other authors18,19,53. The effects of SteLL on nitric oxide and superoxide anion production may be related to the improvement of macrophages bactericidal action, since these reactive species play essential roles in the defense against infectious diseases54,55.
Importantly, SteLL modulated the expression of two proinflammatory cytokines (IL-17A and IFN-γ) in infected macrophages. In the context of S. aureus infectious, IL-17A is essential for antimicrobial peptides production and bacterial clearance56, while IFN-γ increase the macrophages response against S. aureus57. Intriguingly, the overproduction of both cytokines can also exacerbate the severity of some infections14,58. For example, S. aureus phenol-soluble modulins that induce high levels of IL-17 leading to skin inflammatory response13.
The excess of IFN-γ is also related to the harmful inflammation state associated with the damage of essential organs (such as liver and kidney)59. Recent evidences suggested that IFN-γ favored the outgrowth of S. aureus58. Thus, inhibition of the expression of both IFN-γ and IL-17A has been reported as a beneficial effect in several models of S. aureus-induced infection59,60.
Although the molecular mechanism underlying the suppression of IL-17A and IFN-γ induced by lectin treatment in macrophages infected with S. aureus remains to be elucidated, it is possible that decreased levels of these cytokines help to attenuate the deleterious effects of persistent inflammatory responses at the site of infection. Furthermore, because the non-infected cells treated with SteLL presented different cytokine profiles than those found in the S. aureus-infected cells treated with SteLL, it is likely that bacterial components also contribute to modulation of the macrophage response.
These paradoxical effects on the production of inflammatory mediators when comparing uninfected and infected hosts have been observed for other plant lectins16,18,19. For instance, the lectin isolated from Cratylia mollis (Cramoll) has been described as pro-inflammatory agent in in vitro and in vivo models18,61–63; however the treatment with this protein was shown to reduce the release of cytokines (such as TNF-α, IL-6) in experimental models of infection induced by S. aureus (using peritoneal cells)18 and Cryptococcus gatti (using mice)64.
Similarly, the lectin from Canavalia brasiliensis (ConBr) induced different responses in Salmonella enteritidis-infected and uninfected macrophages. The exposition of uninfected macrophages to ConBr resulted in high levels of mRNA transcripts for IL-6 (in relation to untreated and uninfected macrophages). However, S. enteritidis-infected macrophages treated with this lectin exhibited lower levels of IL-6 gene transcription when compared to untreated S. enteritidis-infected cells. ConBr treatment also suppressed the transcription of IL-10 gene in macrophages infected with S. enteritidis19.
Material and Methods
S. aureus strains
S. aureus 8325-4, S. aureus ATCC 6538 and S. aureus ATCC 29312 were used for antimicrobial evaluation. The S. aureus 8325-4 derivative strain carrying a recA::lacZ transcriptional fusion in its chromosome65 was kindly shared by Prof. Dr. Hanne Ingmer. The S. aureus ATCC 29312 was used for staphyloxanthin quantification, since S. aureus 8325-4 is a weak producer due a natural deletion in rsbU66.
Lectin purification
SteLL was purified from dried leaves of S. terebinthifolia (collected in Recife, Brazil) using the methodology reported by Gomes et al.25. The leaves were obtained from specimens grown in the campus of the ‘Universidade Federal de Pernambuco’ at Recife, Brazil (8°02′55.9″S 34°56′48.4″W). The plant collection was authorized by ‘Instituto Chico Mendes de Conservação da Biodiversidade’ from Brazilian Ministry of Environment (license number: 36301).
For SteLL purification, the powder from dried leaves (20 g) was suspended in a saline solution (0.15 M NaCl) and submitted to agitation at 4 °C. After 16 h, the filtered extract was centrifuged (3000 × g for 15 min) and then submitted to chitin column (Sigma-Aldrich, MO, USA). The elution was performed using acetic acid (1 M) and SteLL was obtained after dialysis (10 kDa cut-off membrane; Sigma-Aldrich) against distilled water (4 h, 4 °C) and in sequence against 0.15 M NaCl (4 h, 4 °C). The protein concentration was determined according to Lowry et al. using a standard curve of bovine serum albumin (31.25‒500 μg/mL)67.
Antibacterial activity and combinatory effects with antibiotics
MIC determination
The antimicrobial activity of SteLL was confirmed by determination of the Minimum Inhibitory Concentration (MIC) against S. aureus strains (S. aureus 8325-4, S. aureus ATCC 6538 and S. aureus ATCC 25923) using broth microdilution assay25. Briefly, serial dilutions of SteLL were prepared in 96-wells plates containing Luria-Bertani (LB) broth to obtain concentrations ranging from 128 to 0.25 µg/mL. Following, each well received 10 μL of a microbial suspension (resulting in a bacterial load of approximately 1.0 × 107 CFU/mL for each well). Bacterial growth was detected measuring the optical density at 600 nm (OD600).
The antimicrobial action of SteLL was also evaluated in the presence of NAG, to evaluate the participation of carbohydrate-binding domain. For this, SteLL (16 µg/mL) was pre-incubated with 50 mM NAG. After 1 h, the bacteria were added as described for MIC determination. The bacterial growth was determined after 3 h, 6 h and 9 h of incubation. The inhibition index (IN%) was calculated using the following equation:
Where, BAC is the growth of untreated bacteria; BACSteLL is the growth of bacteria in the presence of SteLL; BACNAG is the growth of untreated bacteria in presence of NAG; BACSteLL+NAG BACSteLL is the growth of bacteria in the presence of SteLL and NAG.
Combinatory effects
The interaction between SteLL and drugs (ciprofloxacin or ampicillin) were evaluated using checkerboard assay. Fractional inhibitory concentration index (FICI) was assessed algebraically by the sum of the single FIC values for each sample present in the well:
where “L” is the concentration (µg/mL) of SteLL in a given well, and MICL represents the control MIC of SteLL alone. “D” is the concentration of the tested drug in a given well, and MICD represents the control MIC of the tested drug alone. FICI mean (ΣFIC) is derived by averaging the FICI values along the growth–no growth interface. Data interpretation: ΣFIC ≤ 0.5: synergism (syn); 0.5 < ΣFIC ≤ 1: addition (add); 1 < ΣFIC < 4: noninteraction (non); ΣFIC ≥ 4: antagonism (ant)68.
Time-kill studies
Overnight cultures of S. aureus 8325-4 were diluted 1:100 in LB broth and placed in a shaking water bath at 37 °C. When the cultures reached an OD600 of 0.1 they were distributed in fresh LB broth containing increasing concentrations of SteLL (2 × and 8 × MIC) or ciprofloxacin (2 × MIC) alone or combined. The cell growth was monitored by plating 4 µL of 10-fold-diluted suspensions from each tube in quadruplicate and following the OD600 at 0, 1.5 h, 3.0 h, 4.5 h, 6.0 h and 7.5 h. The plates were incubated at 37 °C for 24 h. After this period, the colonies were counted for the calculation of CFU/mL. The bactericidal combinatory effects was assessed by variation on Log CFU/mL (ΔLC) and the effects were recorded as synergistic if ΔLC ≥ 2 log CFU/mL; additive if ΔLC was between 1 and 2 log CFU/mL); indifference if ΔLC = ±1 log CFU/mL; or antagonism if ΔLC > −1 log CFU/mL69.
Effects of SteLL on cell size and DNA content
The possible effects of SteLL on cell size and DNA content of S. aureus were evaluated using flow cytometry and fluorescence microcopy. For flow cytometry, exponentially growing cells of S. aureus 8325-4 were treated with SteLL (at 4 µg/mL and 16 µg/mL, corresponding to 2 × MIC and 8 × MIC, respectively), ciprofloxacin (0.78 µg/mL; 1 × MIC), or chloramphenicol (12.5 µg/mL; 1 × MIC) for 3 h. Bacteria were centrifuged (9000 × g for 8 min at 4 °C) and fixed by resuspending in 100 µL Tris (10 mM; pH 7.5) and adding 1 mL of ethanol (77%). For staining, each sample (100 µL) was centrifuged as above and the cell pellet was then resuspended in 170 µL of a solution of ethidium bromide (20 µg/mL) and mithramycin (90 µg/mL). For each sample, a minimum of 15,000 cells were analyzed using an Apogee A10 instrument. Cell mass and DNA content were determined by the measurement of forward scattering (at LS1 detector) and fluorescence intensity (at FL2 channel), respectively40.
For fluorescence microscopy, S. aureus strain 8325-4 was grown exponentially at 37 °C in LB. SteLL (2 × MIC or 8 × MIC) or ciprofloxacin (1 × MIC) were added and samples were taken for DAPI (4′,6-diamidino-2-phenylindole) staining after 3 h. The images were recorded by fluorescence microscopic system (Axio Imager Z2, Carl Zeiss, Germany).
SOS response assay
The induction of SOS response was measured using a derivative S. aureus 8325-4 strain carrying a recA::lacZ fusion. Bacteria were grown exponentially in LB medium until an OD600 between 0.1 and 0.2. Cells were treated with SteLL or ciprofloxacin (both at 0.5 × MIC) for 3 h70. Cells were permeabilizated by toluene and β-galactosidase activity was measured using ONPG (ortho-Nitrophenyl-β-galactoside; Sigma-Aldrich).
Staphyloxanthin inhibition assay
Overnight cultures of S. aureus ATCC 29312 (a strong staphyloxanthin producer) were diluted (1:100) in LB medium and samples (1 mL) of this suspension were incubated with sub-inhibitory concentrations of SteLL (0.0625 × MIC, 0.125 × MIC, 0.25 × MIC, 0.5 × MIC). After overnight incubation at 37 °C, the tubes were centrifuged (9000 × g for 10 min), suspended with 1 mL of phosphate-buffered saline (PBS) and re-centrifuged. Bacteria cells were then photographed. Next, an assay to quantify carotenoid pigments (including staphyloxanthin) was performed. For this, each pellet was resuspended in methanol (0.2 mL) and incubated for 3 min at 55 °C. The methanol phase (supernatant) and cell debris were separated by centrifugation (9000 × g for 10 min) and the pellets were submitted to entire pigment extraction procedure three more times. Finally, the absorbance of methanol extract was determined at 465 nm34.
Assays with mice peritoneal macrophages
Isolation of mice peritoneal macrophages
Peritoneal macrophages were obtained from inbred strains of C57BL/6 mice of both sexes at 8–10 weeks of age. Exudate cells were harvested by peritoneal lavage using 10 mL of ice-cold sterile phosphate-buffered saline (PBS) (pH 7.2). After centrifugation at 120 × g for 5 min, the cell pellets were suspended in RPMI-1640 medium supplemented with bovine calf serum (10%; v/v), penicillin and streptomycin (100 U/mL) (all from Sigma-Aldrich). For all assays, macrophages (1 × 106 cells/mL) were cultured in 24- or 96-well plates, and non-adherent cells were removed. All animal experiments were performed according to the ethical standards of the CEUMA University and were approved by the ethics committee for animal experimentation of this institution (CEUA-CEUMA) (Protocol of Approval N° 107/14), which follows the principles of care with laboratory animals.
Determination of nitric oxide (NO) production and cell viability
For both assays, macrophages (1 × 106 cells/mL) were seeded in 96-well plates for 24 h at 37 °C and 5% CO2. The cells were then treated with SteLL (2, 4, 8 and 16 µg/mL) for another 24 h. Next, the supernatant was used for determination of NO production, and the adherent cells were assessed by the MTT assay (below). Untreated cells were used as negative control. LPS (Escherichia coli; 2000 ng/mL; Sigma-Aldrich) + INF-γ (100 ng/mL; BD Pharmingen) were used as inductors of macrophages activation (M1 macrophages), while IL-4 (400 ng/mL; BD Pharmingen) + IL-13 (400 ng/mL; BD Pharmingen) were used for induction of alternative activation (M2 macrophages). The assays were performed following the protocols described below in quadruplicate in two independent experiments. The results are expressed as the mean ± standard deviation (S.D.).
NO production: The measurement of NO production by peritoneal macrophages was determined using the Griess assay. Briefly, a 50 μL sample from the supernatant of each well was mixed with 50 μL of Griess reagent in a 96-wells plate. After incubation for 15 min at room temperature, the optical density was determined at 540 nm with a microplate reader (Benchmark Plus, Bio-Rad, CA, US). The nitrite concentration (μmol/106 cells) was quantified by extrapolation from a sodium nitrite standard curve for each experiment.
MTT assay: Cell viability was evaluated using the MTT assay, which measures the metabolic conversion of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) salt to colored formazan dye. At the end of the incubation period, the medium was removed, and fresh RPMI medium containing 5 mg/mL MTT solution was added, and the sample was incubated for 3 h. Subsequently, the medium was removed, and the intracellular formazan product was dissolved in DMSO. The optical density (OD) was measured at 595 nm. Cell viability was expressed as the % of viable cells compared to the control.
Mitochondrial superoxide production
Mitochondrial superoxide anion production by the macrophages was evaluated using a MitoSOX™ Red Mitochondrial Superoxide Indicator (Molecular Probes). Peritoneal macrophages were treated with SteLL (8 and 16 µg/mL) for 30 min. After trypsinization and washing with PBS, MitoSOX™ Reagent (1 mL, 5 μM) was added to the culture samples and incubated for 10 min at 37 °C, protected from light. The cells were washed three times with warm PBS and analyzed using a BD Accuri C6 Flow Cytometer (BD Biosciences, San Jose, CA, USA). The events (at least 10,000) were analyzed with FlowJo 7.6.1 software (TreeStar-Ashland, OR, USA).
Macrophage infection and treatment with SteLL
Overnight cultures of S. aureus 8325-4 were centrifuged at 10,000 × g for 10 min, washed twice with PBS, and resuspended in PBS. In parallel, peritoneal macrophages (1 × 106 cells/mL) were seeded in a 96-wells plate for 24 h and infected with S. aureus 8325-4 at a ratio of 10:1 (bacteria/cells) in the presence or absence of lectin (2, 4, 8 and 16 µg/mL). After 24 h, the supernatant was assayed for nitric oxide as described above.
Bacterial killing assay
The effects of SteLL on the bactericidal effect of peritoneal macrophages were evaluated towards intracellular and extracellular bacteria. To quantify extracellular bacteria, aliquots of 4 µL of 10-fold-diluted suspensions from cell supernatants were added to agar plates. For the measurement of intracellular bacteria, the supernatants were removed and each well was washed 5× with ice-cold PBS containing trypan blue in order to remove extracellular bacteria. Following, the cells lysed after washing in 0.1 mL sterile water71. The cell lysates were 10-fold-diluted and added in agar plates. All plates were incubated at 37 °C for 24 h. After this period, the colonies were counted for the calculation of CFU/mL.
Measurement of cytokine release by macrophages
The levels of cytokines (TNFα, IL-6, IL-17A, IFN-γ, IL-4, IL-12 and IL-10) in the supernatant of macrophages were determined by using Mouse cytometric bead array (CBA) cytokine kits (BD Biosciences, Brazil) according with the manufacturer’s instructions. Analysis was performed on a BD Accuri 6 flow cytometer. Results were calculated in CBA FCAP Array software (BD Biosciences, Brazil) and expressed as pg/mL.
In vivo infection model with Galleria mellonella
Survival assay
G. mellonella larvae (~200 mg) were randomly distributed in three experimental groups (n = 10). Two groups were infected by injection of 10 μL of a fresh S. aureus 8325-4 suspension (1 × 105 CFU/mL) into the last left proleg, followed by incubation at 37 °C. After 2 h, one group of animals received 10 μL of 4 μg/mL SteLL (2 × MIC) dissolved in PBS (resulting in a dose of 0.2 mg/kg). The second group of animals was treated with PBS. The larvae were incubated at 37 °C, and the larval viability was determined daily for 4 days.
Quantification of S. aureus in G. mellonella hemolymph
G. mellonella larvae were infected with S. aureus and treated as described above. Each day, a total of 5 larvae were cut in the cephalocaudal direction with a scalpel blade and squeezed to remove the hemolymph. Each sample was 10-fold-diluted in PBS and 4 µL was plated on LB agar. After 24 h-incubation at 37 °C, the colonies were enumerated, and the results were expressed as CFU/mL.
Statistics analysis
All experiments were performed in quadruplicates and in at least two independent assays. Plotting of data was performed using GraphPad Prism 5. Experiments with p < 0.05 were considered significant and are stated in the results section. The survival plots for in vivo infection were performed using Kaplan-Meier analysis on pooled data for repetitive experiments. Statistical analysis was carried out with log-rank (Mantel-Cox) test for comparison of survival curves. Experiments with p < 0.05 were considered significant and are stated in the results section.
Conclusion
Altogether, the findings of this study suggest that the SteLL impairs cell division of S. aureus without provoking DNA damage. The lectin also alters bacteria metabolism resulting in a reduced staphyloxanthin production. These effects may be responsible for the anti-infective activity of SteLL in G. mellonella. Furthermore, we demonstrate that SteLL increase the production of cytokines by uninfected macrophages and modulate the release of IL-17A and IFN-γ in S. aureus-infected macrophages. Taken together, these findings from both in vitro and in vivo studies suggest SteLL as a promising lead for the development of new anti-infective agents against S. aureus.
Supplementary information
Acknowledgements
We would like thank Dr. Claudener Souza Teixeira from Universidade Federal do Maranhão (Chapadinha, Brazil) for sharing N-acetylglucosamine for the inhibition assay. We are also grateful for Prof. Dr. Hanne Ingmer from University of Copenhagen for kindly sharing the report strain. This work was funded by Fundação de Amparo à Pesquisa e Desenvolvimento Científico do Maranhão (UNIVERSAL-00998/16, COOPI-02860/16, BEPP-02241/18, BM-02751/16, BIC-00682/19), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Science without borders program; Process: 12179-13-2) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (Process number: 426950/2018-6).
Author contributions
L.C.N.S., P.M.G.P., T.H.N., A.L.O. and K.A.K. conceived the study and performed the study design. T.H.N. and P.M.P.G. performed the purification of SteLL. I.M.S.F.L., C.M.B.F., H.S.M., D.M.S., B.S.C., and L.C.N.S. performed the microbiological assays. I.M.S.F.L., L.C.N.S., A.Z., D.M.S., L.S.S., B.S.C., S.L.V. and E.M.S. performed the assays with mouse macrophages. I.M.S.F.L., L.C.N.S., P.M.G.P., T.H.N., A.L.O. and K.A.K. wrote the paper. All author discussed the results and approved the final version of this manuscript.
Data availability
The data used to build all graphs to support the findings of this study are available from the corresponding author upon request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-54616-x.
References
- 1.Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG., Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee AS, et al. Methicillin-resistant Staphylococcus aureus. Nat Rev Dis Primers. 2018;4:18033. doi: 10.1038/nrdp.2018.33. [DOI] [PubMed] [Google Scholar]
- 3.Bonar EA, et al. Joint Genomic and Proteomic Analysis Identifies Meta-Trait Characteristics of Virulent and Non-virulent Staphylococcus aureus Strains. Front Cell Infect Microbiol. 2018;8:313. doi: 10.3389/fcimb.2018.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choudhary KS, et al. The Staphylococcus aureus Two-Component System AgrAC Displays Four Distinct Genomic Arrangements That Delineate Genomic Virulence Factor Signatures. Front Microbiol. 2018;9:1082. doi: 10.3389/fmicb.2018.01082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choe D, et al. Genome-scale analysis of Methicillin-resistant Staphylococcus aureus USA300 reveals a tradeoff between pathogenesis and drug resistance. Sci Rep. 2018;8:2215. doi: 10.1038/s41598-018-20661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Jong NWM, et al. Identification of a staphylococcal complement inhibitor with broad host specificity in equid Staphylococcus aureus strains. J Biol Chem. 2018;293:4468–4477. doi: 10.1074/jbc.RA117.000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerlach D, et al. Methicillin-resistant Staphylococcus aureus alters cell wall glycosylation to evade immunity. Nature. 2018;563:705–709. doi: 10.1038/s41586-018-0730-x. [DOI] [PubMed] [Google Scholar]
- 8.Niemann S, et al. Panton-Valentine Leukocidin associated with S. aureus osteomyelitis activates platelets via neutrophil secretion products. Sci Rep. 2018;8:2185. doi: 10.1038/s41598-018-20582-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fraunholz M, Sinha B. Intracellular Staphylococcus aureus: live-in and let die. Front Cell Infect Microbiol. 2012;2:43. doi: 10.3389/fcimb.2012.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grosz M, et al. Cytoplasmic replication of Staphylococcus aureus upon phagosomal escape triggered by phenol-soluble modulin alpha. Cell Microbiol. 2014;16:451–465. doi: 10.1111/cmi.12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horn J, Stelzner K, Rudel T, Fraunholz M. Inside job: Staphylococcus aureus host-pathogen interactions. Int J Med Microbiol. 2018;308:607–624. doi: 10.1016/j.ijmm.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 12.Berends, E. T. M. et al. Staphylococcus aureus Impairs the Function of and Kills Human Dendritic Cells via the LukAB Toxin. MBio10, 10.1128/mBio.01918-18 (2019). [DOI] [PMC free article] [PubMed]
- 13.Nakagawa S, et al. Staphylococcus aureus Virulent PSMalpha Peptides Induce Keratinocyte Alarmin Release to Orchestrate IL-17-Dependent Skin Inflammation. Cell Host Microbe. 2017;22:667–677 e665. doi: 10.1016/j.chom.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li J, Casanova JL, Puel A. Mucocutaneous IL-17 immunity in mice and humans: host defense vs. excessive inflammation. Mucosal Immunol. 2018;11:581–589. doi: 10.1038/mi.2017.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hancock RE, Nijnik A, Philpott DJ. Modulating immunity as a therapy for bacterial infections. Nat Rev Microbiol. 2012;10:243–254. doi: 10.1038/nrmicro2745. [DOI] [PubMed] [Google Scholar]
- 16.Jandu JJB, et al. Targeting the Immune System with Plant Lectins to Combat Microbial Infections. Front Pharmacol. 2017;8:671. doi: 10.3389/fphar.2017.00671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaufmann SHE, Dorhoi A, Hotchkiss RS, Bartenschlager R. Host-directed therapies for bacterial and viral infections. Nat Rev Drug Discov. 2018;17:35–56. doi: 10.1038/nrd.2017.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.da Silva LC, et al. Immunomodulatory effects of pCramoll and rCramoll on peritoneal exudate cells (PECs) infected and non-infected with Staphylococcus aureus. Int J Biol Macromol. 2015;72:848–854. doi: 10.1016/j.ijbiomac.2014.09.045. [DOI] [PubMed] [Google Scholar]
- 19.Batista J, et al. Plant lectins ConBr and CFL modulate expression toll-like receptors, pro-inflammatory cytokines and reduce the bacterial burden in macrophages infected with Salmonella enterica serovar Typhimurium. Phytomedicine. 2017;25:52–60. doi: 10.1016/j.phymed.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 20.Dos Santos BS, et al. Application of Omics Technologies for Evaluation of Antibacterial Mechanisms of Action of Plant-Derived Products. Front Microbiol. 2016;7:1466. doi: 10.3389/fmicb.2016.01466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Farha MA, Brown ED. Strategies for target identification of antimicrobial natural products. Nat Prod Rep. 2016;33:668–680. doi: 10.1039/c5np00127g. [DOI] [PubMed] [Google Scholar]
- 22.da Silva LC, Correia MT. Plant lectins and Toll-like receptors: implications for therapy of microbial infections. Front Microbiol. 2014;5:20. doi: 10.3389/fmicb.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ayaz Ahmed KB, Raman T, Veerappan A. Jacalin capped platinum nanoparticles confer persistent immunity against multiple Aeromonas infection in zebrafish. Sci Rep. 2018;8:2200. doi: 10.1038/s41598-018-20627-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferreira GRS, et al. Antimicrobial potential of Alpinia purpurata lectin (ApuL): Growth inhibitory action, synergistic effects in combination with antibiotics, and antibiofilm activity. Microb Pathog. 2018;124:152–162. doi: 10.1016/j.micpath.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 25.Gomes FS, Procopio TF, Napoleao TH, Coelho LC, Paiva PM. Antimicrobial lectin from Schinus terebinthifolius leaf. J Appl Microbiol. 2013;114:672–679. doi: 10.1111/jam.12086. [DOI] [PubMed] [Google Scholar]
- 26.da Silva PM, et al. Punica granatum sarcotesta lectin (PgTeL) impairs growth, structure, viability, aggregation, and biofilm formation ability of Staphylococcus aureus clinical isolates. International journal of biological macromolecules. 2019;123:600–608. doi: 10.1016/j.ijbiomac.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 27.Liedke SC, et al. Characterization of the antifungal functions of a WGA-Fc (IgG2a) fusion protein binding to cell wall chitin oligomers. Sci Rep. 2017;7:12187. doi: 10.1038/s41598-017-12540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camaroti JRSL, et al. Sitophilus zeamais adults have survival and nutrition affected by Schinus terebinthifolius leaf extract and its lectin (SteLL) Industrial crops and products. 2018;116:81–89. doi: 10.1016/j.indcrop.2018.02.065. [DOI] [Google Scholar]
- 29.Ramos DBM, et al. Evaluation of antitumor activity and toxicity of Schinus terebinthifolia leaf extract and lectin (SteLL) in sarcoma 180-bearing mice. J Ethnopharmacol. 2019;233:148–157. doi: 10.1016/j.jep.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 30.Cruciani M, et al. Staphylococcus aureus Esx Factors Control Human Dendritic Cell Functions Conditioning Th1/Th17 Response. Front Cell Infect Microbiol. 2017;7:330. doi: 10.3389/fcimb.2017.00330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krishna S, Miller LS. Innate and adaptive immune responses against Staphylococcus aureus skin infections. Semin Immunopathol. 2012;34:261–280. doi: 10.1007/s00281-011-0292-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krakauer, T. Staphylococcal Superantigens: Pyrogenic Toxins Induce Toxic Shock. Toxins (Basel)11, 10.3390/toxins11030178 (2019). [DOI] [PMC free article] [PubMed]
- 33.Jonsson R, Struve C, Jenssen H, Krogfelt KA. The wax moth Galleria mellonella as a novel model system to study Enteroaggregative Escherichia coli pathogenesis. Virulence. 2017;8:1894–1899. doi: 10.1080/21505594.2016.1256537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva LN, et al. Myricetin protects Galleria mellonella against Staphylococcus aureus infection and inhibits multiple virulence factors. Sci Rep. 2017;7:2823. doi: 10.1038/s41598-017-02712-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferro TA, et al. Cinnamaldehyde Inhibits Staphylococcus aureus Virulence Factors and Protects against Infection in a Galleria mellonella Model. Front Microbiol. 2016;7:2052. doi: 10.3389/fmicb.2016.02052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carvalho M, Melo A, Aragão C, Raffin F, Moura T. Schinus terebinthifolius Raddi: chemical composition, biological properties and toxicity. Revista Brasileira de Plantas Medicinais. 2013;15:158–169. doi: 10.1590/S1516-05722013000100022. [DOI] [Google Scholar]
- 37.Estevao LRM, et al. Schinus terebinthifolius Raddi (Aroeira) leaves oil attenuates inflammatory responses in cutaneous wound healing in mice 1. Acta Cir Bras. 2017;32:726–735. doi: 10.1590/s0102-865020170090000005. [DOI] [PubMed] [Google Scholar]
- 38.da Silva JHS, et al. Anti-Escherichia coli activity of extracts from Schinus terebinthifolius fruits and leaves. Nat Prod Res. 2018;32:1365–1368. doi: 10.1080/14786419.2017.1344657. [DOI] [PubMed] [Google Scholar]
- 39.Salem MZM, et al. Antibacterial activity of extracted bioactive molecules of Schinus terebinthifolius ripened fruits against some pathogenic bacteria. Microb Pathog. 2018;120:119–127. doi: 10.1016/j.micpath.2018.04.040. [DOI] [PubMed] [Google Scholar]
- 40.Lobner-Olesen A, Skarstad K, Hansen FG, von Meyenburg K, Boye E. The DnaA protein determines the initiation mass of Escherichia coli K-12. Cell. 1989;57:881–889. doi: 10.1016/0092-8674(89)90802-7. [DOI] [PubMed] [Google Scholar]
- 41.Westfall CS, Levin PA. Bacterial cell size: multifactorial and multifaceted. Annual review of microbiology. 2017;71:499–517. doi: 10.1146/annurev-micro-090816-093803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Corrigan RM, Abbott JC, Burhenne H, Kaever V, Gründling A. c-di-AMP is a new second messenger in Staphylococcus aureus with a role in controlling cell size and envelope stress. PLoS pathogens. 2011;7:e1002217. doi: 10.1371/journal.ppat.1002217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jorge AM, Hoiczyk E, Gomes JP, Pinho MG. EzrA contributes to the regulation of cell size in Staphylococcus aureus. PLoS One. 2011;6:e27542. doi: 10.1371/journal.pone.0027542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deforet M, van Ditmarsch D, Xavier JB. Cell-Size Homeostasis and the Incremental Rule in a Bacterial Pathogen. Biophys J. 2015;109:521–528. doi: 10.1016/j.bpj.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Campbell J, et al. An antibiotic that inhibits a late step in wall teichoic acid biosynthesis induces the cell wall stress stimulon in Staphylococcus aureus. Antimicrob Agents Chemother. 2012;56:1810–1820. doi: 10.1128/AAC.05938-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coelho L, et al. Lectins as antimicrobial agents. Journal of applied microbiology. 2018;125:1238–1252. doi: 10.1111/jam.14055. [DOI] [PubMed] [Google Scholar]
- 47.Simmons, L. A., Foti, J. J., Cohen, S. E. & Walker, G. C. The SOS Regulatory Network. EcoSal Plus2008, 10.1128/ecosalplus.5.4.3 (2008). [DOI] [PubMed]
- 48.Santos VF, et al. The Galactose-Binding Lectin Isolated from Vatairea macrocarpa Seeds Enhances the Effect of Antibiotics Against Staphylococcus aureus-Resistant Strain. Probiotics Antimicrob Proteins. 2019 doi: 10.1007/s12602-019-9526-z. [DOI] [PubMed] [Google Scholar]
- 49.Xiong, Y. Q., Yang, S. J., Tong, S. Y., Alvarez, D. N. & Mishra, N. N. The role of Staphylococcal carotenogenesis in resistance to host defense peptides and in vivo virulence in experimental endocarditis model. Pathog Dis73, 10.1093/femspd/ftv056 (2015). [DOI] [PMC free article] [PubMed]
- 50.Ni S, et al. Novel Staphyloxanthin Inhibitors with Improved Potency against Multidrug Resistant Staphylococcus aureus. ACS Med Chem Lett. 2018;9:233–237. doi: 10.1021/acsmedchemlett.7b00501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubini D, et al. Chitosan extracted from marine biowaste mitigates staphyloxanthin production and biofilms of Methicillin- resistant Staphylococcus aureus. Food Chem Toxicol. 2018;118:733–744. doi: 10.1016/j.fct.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 52.Colasso, A. H. M. et al. The latex of Euphorbia tirucalli inhibits staphyloxanthin production and protects Tenebrio molitor larvae against Staphylococcus aureus infection. Nat Prod Res, 1–4, 10.1080/14786419.2019.1582036 (2019). [DOI] [PubMed]
- 53.Duarte CEM, et al. A new TRAF-like protein from B. oleracea ssp. botrytis with lectin activity and its effect on macrophages. Int J Biol Macromol. 2017;94:508–514. doi: 10.1016/j.ijbiomac.2016.10.061. [DOI] [PubMed] [Google Scholar]
- 54.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 55.Nathan C, Cunningham-Bussel A. Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat Rev Immunol. 2013;13:349–361. doi: 10.1038/nri3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Archer NK, et al. Interleukin-17A (IL-17A) and IL-17F Are Critical for Antimicrobial Peptide Production and Clearance of Staphylococcus aureus Nasal Colonization. Infect Immun. 2016;84:3575–3583. doi: 10.1128/IAI.00596-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greenlee-Wacker MC, Nauseef WM. IFN-gamma targets macrophage-mediated immune responses toward Staphylococcus aureus. J Leukoc Biol. 2017;101:751–758. doi: 10.1189/jlb.4A1215-565RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Domenico EG, et al. Inflammatory cytokines and biofilm production sustain Staphylococcus aureus outgrowth and persistence: a pivotal interplay in the pathogenesis of Atopic Dermatitis. Sci Rep. 2018;8:9573. doi: 10.1038/s41598-018-27421-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang X, et al. Micheliolide provides protection of mice against Staphylococcus aureus and MRSA infection by down-regulating inflammatory response. Sci Rep. 2017;7:41964. doi: 10.1038/srep41964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dey S, Bishayi B. Riboflavin along with antibiotics balances reactive oxygen species and inflammatory cytokines and controls Staphylococcus aureus infection by boosting murine macrophage function and regulates inflammation. J Inflamm (Lond) 2016;13:36. doi: 10.1186/s12950-016-0145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.de Melo CM, et al. Immunomodulatory response of Cramoll 1,4 lectin on experimental lymphocytes. Phytother Res. 2010;24:1631–1636. doi: 10.1002/ptr.3156. [DOI] [PubMed] [Google Scholar]
- 62.de Melo CM, et al. Cramoll 1,4 lectin increases ROS production, calcium levels, and cytokine expression in treated spleen cells of rats. Mol Cell Biochem. 2010;342:163–169. doi: 10.1007/s11010-010-0480-z. [DOI] [PubMed] [Google Scholar]
- 63.de Oliveira PS, et al. Cratylia mollis 1, 4 lectin: a new biotechnological tool in IL-6, IL-17A, IL-22, and IL-23 induction and generation of immunological memory. Biomed Res Int. 2013;2013:263968. doi: 10.1155/2013/263968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jandu JJ, et al. Treatment with pCramoll Alone and in Combination with Fluconazole Provides Therapeutic Benefits in C. gattii Infected Mice. Front Cell Infect Microbiol. 2017;7:211. doi: 10.3389/fcimb.2017.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gottschalk S, et al. The antimicrobial lysine-peptoid hybrid LP5 inhibits DNA replication and induces the SOS response in Staphylococcus aureus. BMC Microbiol. 2013;13:192. doi: 10.1186/1471-2180-13-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Olivier AC, Lemaire S, Van Bambeke F, Tulkens PM, Oldfield E. Role of rsbU and staphyloxanthin in phagocytosis and intracellular growth of Staphylococcus aureus in human macrophages and endothelial cells. The Journal of infectious diseases. 2009;200:1367–1370. doi: 10.1086/606012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 68.da Silva AP, et al. Antimicrobial Activity and Phytochemical Analysis of Organic Extracts from Cleome spinosa Jaqc. Front Microbiol. 2016;7:963. doi: 10.3389/fmicb.2016.00963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zuo GY, et al. Antimicrobial activity and synergy of antibiotics with two biphenyl compounds, protosappanins A and B from Sappan Lignum against methicillin-resistant Staphylococcus aureus strains. J Pharm Pharmacol. 2015;67:1439–1447. doi: 10.1111/jphp.12433. [DOI] [PubMed] [Google Scholar]
- 70.Vestergaard M, Paulander W, Ingmer H. Activation of the SOS response increases the frequency of small colony variants. BMC Res Notes. 2015;8:749. doi: 10.1186/s13104-015-1735-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lin Y, et al. Microbicidal Phagocytosis of Nucleus Pulposus Cells Against Staphylococcus aureus via the TLR2/MAPKs Signaling Pathway. Front Immunol. 2019;10:1132. doi: 10.3389/fimmu.2019.01132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used to build all graphs to support the findings of this study are available from the corresponding author upon request.