Abstract
We aimed to evaluate the pooled incidence of central vein sign on T2*-weighted images from patients with multiple sclerosis (MS), and to determine the diagnostic performance of this central vein sign for differentiating MS from other white matter lesions and provide an optimal cut-off value. A computerized systematic search of the literature in PUBMED and EMBASE was conducted up to December 14, 2018. Original articles investigating central vein sign on T2*-weighted images of patients with MS were selected. The pooled incidence was obtained using random-effects model. The pooled sensitivity and specificity were obtained using a bivariate random-effects model. An optimal cut-off value for the proportion of lesions with a central vein sign was calculated from those studies providing individual patient data. Twenty-one eligible articles covering 501 patients with MS were included. The pooled incidence of central vein sign at the level of individual lesion in patients with MS was 74% (95% CI, 65–82%). The pooled sensitivity and pooled specificity for the diagnostic performance of the central vein sign were 98% (95% CI, 92–100%) and 97% (95% CI, 91–99%), respectively. The area under the HSROC curve was 1.00 (95% CI, 0.99–1.00). The optimal cut-off value for the proportion of lesions with a central vein sign was found to be 45%. Although various T2*-weighted images have been used across studies, the current evidence supports the use of the central vein sign on T2*-weighted images to differentiate MS from other white matter lesions.
Subject terms: Diagnostic markers, Multiple sclerosis
Introduction
The accurate diagnosis of multiple sclerosis (MS) is clinically important to avoid inappropriate management or unnecessary invasive biopsy. MRI is the most commonly performed investigation able to support a clinical diagnosis of MS, and MRI might be useful for ruling out MS-mimicking pathologies1,2. However, even though the 2017 McDonald criteria have been published, there are still challenging cases and misdiagnoses, which are prevalent problems in MS3,4. Therefore, there is still a need for more accurate MRI criteria that can exclude other MS-mimicking white matter lesions.
The “central vein sign” which is considered to be a MRI-detectable central vein inside white matter lesion identified as a hypointensity relative to the surrounding lesion on T2*-weighted images, has been introduced as a biomarker of inflammatory demyelination1,5. This central vein sign has been investigated in various neurological conditions, including MS, cerebral small vessel disease, neuromyelitis optica spectrum disorder (NMOSD), inflammatory vasculopathies, and migraine, and evidence has accumulated that the central vein sign may allow the accurate differentiation of MS from other white matter lesions6–26.
The North American Imaging in Multiple Sclerosis Cooperative mentioned that, as the differential diagnosis of MS is broad, the pooling of data from multiple centers would be a realistic strategy for conducting a systematic and well-powered evaluation of the central vein sign on T2*-weighted images5. To our knowledge, the incidence of central vein sign on T2*-weighted images from patients with MS, and its diagnostic performance for differentiating MS from other white matter lesions, have not yet been systematically reviewed. Therefore, we performed a systematic review and meta-analysis to evaluate the pooled incidence of central vein sign on T2*-weighted imaging of patients with MS. In addition, we aimed to determine the diagnostic performance of the central vein sign for differentiating MS from other white matter lesions and provide an optimal cut-off value for this differentiation.
Materials and Methods
This systematic review and meta-analysis was conducted according to the Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies (PRISMA-DTA) statement27,28.
Literature Search
A computerized systematic search of the literature in PUBMED and EMBASE was conducted to find published original articles investigating the central vein sign on T2*-weighted imaging of patients with MS. The search term combined synonyms of “multiple sclerosis” and “central vein” as follows: ((“multiple sclerosis”)) AND ((“central vein”) OR (vein in lesion) OR (perivenular)). The databases were searched for articles published up to December 14, 2018. The search was restricted to English-language publications. A manual search was also performed to find additional relevant articles. EndNote X8 was used for literature handling.
Eligibility criteria
Studies were selected if all of the following inclusion criteria were met: (1) patients with MS; (2) patients underwent MRI including T2*-weighted images; and (3) provision of sufficient information for the incidence of central vein sign on T2*-weighted images or the reconstruction of 2 × 2 tables for determination of the diagnostic performance of central vein sign for diagnosis of MS.
Studies were excluded if any of the following exclusion criteria were satisfied: (1) conference abstracts; (2) review articles; (3) case reports or case series including fewer than five patients; (4) letters, editorials, and short surveys; (5) studies with a partially overlapping patient cohort, and (6) animal studies. For studies with a partially overlapping study population, the study including the largest number of patients was selected. Authors of potentially eligible articles that did not provide sufficient information were contacted for the provision of further data.
Data extraction and quality assessment
The incidence of central vein sign on T2*-weighted images from patients with MS and the diagnostic performance of the central vein sign for differentiating MS from other white matter lesions were extracted from the eligible articles. Central vein sign on T2*-weighted imaging was defined as follows: (1) the vein should appear as a thin line or dot; (2) when technically possible, the vein should be visualized in at least two perpendicular planes; and (3) the vein can run partially or entirely through the lesion, but must be located centrally, regardless of the lesion’s shape5. Two by two tables (true positive, false positive, false negative, true negative) for determination of the diagnostic performance of the central vein sign for differentiating MS from other white matter lesions such as small vessel disease, CNS inflammatory vasculopathies, or NMOSD were also constructed. If the diagnostic performances of multiple MRI sequences were separately evaluated, the results with the highest performance were selected. If a two by two table could not be acquired, the authors were contacted for provision of further data by E-mail.
The following information was extracted from the eligible studies: (1) the institution, the study period, study design (retrospective or prospective design), consecutive or non-consecutive patient enrollment, and the reference standard; (2) the number of MS patients, mean age, age range, and female to male ratio; (3) the magnetic field strength of the scanner, scanner manufacturer, scanner model, MRI sequence, and cut-off values for the proportion of lesions with central vein sign used to diagnose MS; and (4) the number of MRI readers, and blindness to the reference standard.
Quality assessment was performed using the Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2) criteria29. The literature search, study selection, data extraction, and quality assessment were performed by two reviewers (C.H.S., S.J.K.).
Statistical analyses
The pooled incidence of central vein sign on T2*-weighted images of MS was calculated with the inverse variance method for calculating weights and the DerSimonian-Liard random-effects model30–32. Heterogeneity was assessed by Higgins inconsistency index (I2) test, with values greater than 50% taken as indicating substantial heterogeneity33. Publication bias was assessed by a funnel plot, and the statistical significance was assessed by Egger’s test34. Meta-regression was conducted to explain the effects of study heterogeneity. The following covariates were considered: (1) study design (prospective study vs. other); (2) MRI sequence (studies including FLAIR* [combined FLAIR and T2*-weighted images]35 vs. other); (3) reader (radiologist vs. other); (4) reader blindness to the reference standard; and (5) patient age (age ≤41 [median value] vs. age >41). Subgroup analyses according to the strength of the MRI scanner (7, 3, and 1.5-Tesla) were also performed.
The pooled sensitivity and specificity and their 95% confidence intervals [CI] for the diagnostic performance of central vein sign on T2*-weighted images for differentiating MS from other white matter lesions were calculated using a bivariate random-effects model30–32. A coupled forest plot of sensitivity and specificity and a hierarchical summary receiver operating characteristic (HSROC) curve with 95% confidence and prediction regions were plotted. Heterogeneity was assessed by the following methods: (1) Cochran’s Q-test (p < 0.05 indicating the presence of heterogeneity); (2) Higgins I2 test (a value >50% indicating the presence of heterogeneity);33 (3) visual assessment of the coupled forest plot for the presence of a threshold effect, i.e., a positive correlation between sensitivity and false positive rate; and (4) the Spearman correlation coefficient between sensitivity and false positive rate (a value >0.6 indicating a threshold effect)36. Publication bias was assessed by Deeks’ funnel plot, with the statistical significance being assessed by Deeks’ asymmetry test37. A meta-regression was conducted to explain the effects of study heterogeneity, with the following covariates being utilized for the bivariate meta-regression model: (1) study design (prospective study vs. other); (2) MRI sequence (studies including FLAIR*35 vs. other); (3) reader (radiologist vs. other); (4) reader blindness to the reference standard; and (5) patient age (age ≤41 [median value] vs. age >41). Subgroup analysis was conducted on those studies using a proportion of lesions with central vein sign as a cut-off value.
An optimal cut-off value for the proportion of lesions with central vein sign was calculated from those studies providing individual patient data. The individual patient data were extracted from the articles, and when not reported, Plot Digitizer 2.6.8 (plotdigitizer.sourceforge.net) was used to estimate the data from plots indicating the proportion of lesions with central vein sign. The sensitivity and specificity of the central vein sign and the corresponding cut-off value for the proportion of lesions with a central vein sign were estimated using the Youden index. The Youden index is defined as sensitivity + specificity – 1, with it having a minimum value of −1 and a maximum value of + 1, with a value of + 1 indicating the optimal value for an algorithm38.
Statistical analyses were performed by one reviewer (C.H.S., with 6 years of experience in performing systematic reviews and meta-analysis) using the “metafor” and “mada” packages in R v.3.4.1 (R Foundation for Statistical Computing, Austria), and the “metandi” and “midas” modules in STATA 15.0 (StataCorp, College Station, USA).
Results
Literature search
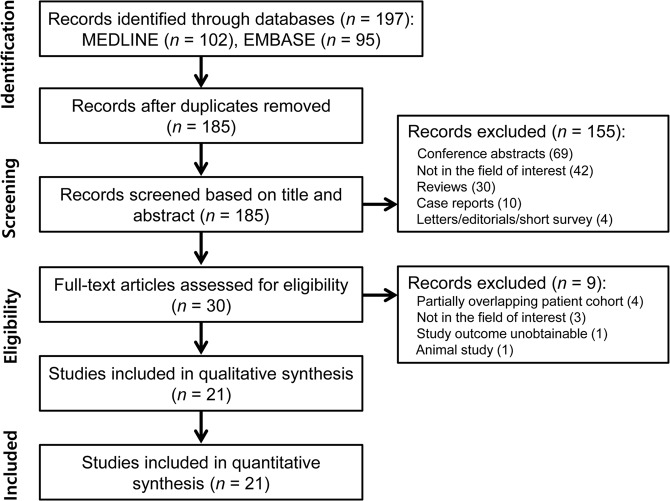
The details of the study selection process are illustrated in Fig. 1 and Supplementary materials. Finally, 21 eligible articles encompassing 501 patients with MS were included in the analyses6–26.
Figure 1.
Flow diagram for the literature selection process.
Characteristics of the included studies
The characteristics of the eligible studies are shown in Table 1. Six studies had a prospective design8,10,15,18,21,23 and six studies had a retrospective design7,14,16,17,22,25, with the other studies not reporting the design. Patient enrollment was conducted in a consecutive fashion in only three studies18,20,25, with this detail not being reported in the others. The eligible studies included numbers of MS patients ranging from 8 to 68. Fifteen studies6–10,12,14–17,20,21,23–25 used the revised 2010 McDonald criteria39, two studies13,26 used the revised 2005 McDonald criteria40, and the other four studies11,18,19,22 did not mention the criteria used to diagnose MS.
Table 1.
Characteristics of the eligible studies.
| Author (year of publication) | Duration of patient recruitment | Institution | Study design | MS patients (n) | Mean age (years) | Female:Male |
|---|---|---|---|---|---|---|
| Al-Zandi SH, et al.6 (2018) | 2016.4–2017.3 | Al-Imamain Al-Kadhymain Medical City, Iraq | NA | 30 | 40.8 (22–58) | 7:23 |
| Campion T, et al.7 (2017) | NA | The Royal London Hospital, UK | retrospective | 25 | 41 | 14:11 |
| Cortese R, et al.8 (2018) | NA | Walton Centre and the National Hospital for Neurology and Neurosurgery, UK | prospective | 18 | 41.8 | 4:14 |
| Darwish EAF, et al.9 (2018) | NA | Ain Shams University, Egypt | NA | 9 | 33 (26–45) | 2:7 |
| Gabr RE, et al.10 (2018) | NA | University of Texas Health Science Center at Houston, USA | prospective | 15 | 43 (26–62) | 6:9 |
| Gaitan MI, et al.11 (2013) | NA | National Institutes of Health, USA | NA | 8 | 37.1 | 2:6 |
| George IC, et al.12 (2016) | NA | National Institutes of Health, USA | NA | 68 | 46 | 36:32 |
| Grabner G, et al.13 (2011) | NA | Medical University of Vienna, Austria | NA | 8 | NA | NA |
| Hosseini Z, et al.14 (2018) | NA | University of Western Ontario, Canada | retrospective | 17 | 39.4 (26–46) | 6:11 |
| Kau T, et al.15 (2013) | NA | Klinikum Klagenfurt, Austria | prospective | 5 | 47 (20–57) | 1:4 |
| Lamot U, et al.16 (2017) | NA | University Medical Centre Ljubljana, Slovenia | retrospective | 34 | 39.6 (21–66) | 9:25 |
| Lane JI, et al.17 (2015) | 18 months | Mayo Clinic, USA | retrospective | 21 | 46 | 5:16 |
| Lummel N, et al.18 (2011) | NA | University of Munich, Germany | prospective | 15 | 48.4 | 3:12 |
| Luo J, et al.19 (2014) | NA | Washington University, USA | NA | 30 | 51.5 (27–70) | 14:16 |
| Maggi P, et al.20 (2018) | 2015.1–2017.6 | Multicenter (four academic research hospitals) | NA | 52 | 41 (20–65) | 18:34 |
| Mistry N, et al.21 (2016) | NA | Nottingham University Hospitals NHS Trust, UK | prospective | 23 | 45.5 (25–66) | 13:10 |
| Oztoprak B, et al.22 (2016) | 2013.9–2014.9 | Cumhuriyet University School of Medicine, Turkey | retrospective | 38 | 34.3 | 5:33 |
| Sinnecker T, et al.23 (2012) | NA | NeuroCure Clinical Research Center, Charité – Universitaetsmedizin Berlin, Germany | prospective | 18 | 41 (27–53) | 7:11 |
| Solomon AJ, et al.24 (2018) | NA | University of Vermont, USA | NA | 20 | 43.5 | 2:18 |
| Sparacia G, et al.25 (2018) | 2016.12–2017.4 | University of Palermo, Italy | retrospective | 19 | 36.9 (19–53) | 9:10 |
| Tallantyre EC, et al.26 (2011) | 2007.8–2009.8 | Nottingham University Hospitals NHS Trust, UK | NA | 28 | 46.5 | 16:12 |
MS = multiple sclerosis, NA = not available.
The MRI characteristics are shown in Table 2. Twelve studies used a 3-Tesla scanner6–10,12,15,16,18,19,21,24, four studies used a 7-Tesla scanner11,14,23,26, three studies used a 1.5-Tesla scanner17,22,25, one study used either 7- or 3-Tesla scanners13, and one study used either 3- or 1.5-Tesla scanners20. All studies used T2*-based MRI sequences; eight studies used FLAIR and susceptibility-weighted imaging (SWI)9,13–18,22, five studies used FLAIR*7,10,12,20,24, four studies used T2*-weighted images11,21,23,26, three studies used SWI6,19,25, and one study used proton density-weighted imaging, T2-weighted imaging, and SWI8.
Table 2.
MRI characteristics of the eligible studies.
| Magnetic field strength | Scanner model, manufacturer | MRI sequences | MRI readers | MRI reader blindness to the reference standard | |
|---|---|---|---|---|---|
| Al-Zandi SH, et al.6 (2018) | 3 T | Achieva, Philips | SWI | 2 radiologists | NA |
| Campion T, et al.7 (2017) | 3 T | Achieva TX, Philips | FLAIR* | 2 neuroradiologists, 1 radiology resident | yes |
| Cortese R, et al.8 (2018) | 3 T | Achieva, Philips | PD, T2WI, SWI | 2 neuroradiologists | yes |
| Darwish EAF, et al.9 (2018) | 3 T | Magnetom Skyra, Siemens | FLAIR, SWI | 2 neuroradiologists | yes |
| Gabr RE, et al.10 (2018) | 3 T | Ingenia, Philips | FLAIR* | 1 neuroradiologist | NA |
| Gaitan MI, et al.11 (2013) | 7 T | NA, Siemens | T2*-weighted images | NA | NA |
| George IC, et al.12 (2016) | 3 T | NA, Philips | T2-FLAIR + FLAIR* | 2 neurologists | yes |
| Grabner G, et al.13 (2011) | 3 T, 7 T | Tim Trio, 7 T system, Siemens | FLAIR (3 T), SWI (7 T) | 1 radiologist | NA |
| Hosseini Z, et al.14 (2018) | 7 T | NA, Agilent Technologies | FLAIR, SWI | 1 neuroradiologist, 1 radiology resident | NA |
| Kau T, et al.15 (2013) | 3 T | Achieva, Philips | FLAIR, SWI | 2 readers | yes |
| Lamot U, et al.16 (2017) | 3 T | Magnetom Trio, Siemens | FLAIR, T2WI, SWI | 2 neuroradiologists | yes |
| Lane JI, et al.17 (2015) | 1.5 T | Avanto or Espree, Siemens | FLAIR, SWI | 3 neuroradiologists | yes |
| Lummel N, et al.18 (2011) | 3 T | Signa HDxt, GE | FLAIR, SWAN | 2 neuroradiologists | yes |
| Luo J, et al.19 (2014) | 3 T | Trio, Siemens | SWI | NA | NA |
| Maggi P, et al.20 (2018) | 3 T or 1.5 T | Best or Achieva, Philips | FLAIR* | 2 neurologists | yes |
| Mistry N, et al.21 (2016) | 3 T | Achieva, Philips | T2*-weighted images | 1 neurologist, 1 neuroradiologist | yes |
| Oztoprak B, et al.22 (2016) | 1.5 T | Magnetom Aera, Siemens | FLAIR, SWI | 2 radiologists | NA |
| Sinnecker T, et al.23 (2012) | 7 T | Magnetom, Siemens | T2*-weighted images | 1 neuroradiologist, 1 trained observer | yes |
| Solomon AJ, et al.24 (2018) | 3 T | NA, Philips | FLAIR* | 3 neurologists | yes |
| Sparacia G, et al.25 (2018) | 1.5 T | Achieva, Philips | SWI | 2 neuroradiologists | yes |
| Tallantyre EC, et al.26 (2011) | 7 T | Achieva, Philips | T2*-weighted images | 1 primary observer | yes |
SWI = susceptibility-weighted imaging, NA = not available, SWAN = susceptibility-weighted angiography.
Quality assessment
The quality of the 21 eligible studies was considered as moderate, with more than four of the seven domains being satisfied (Supplementary Fig. 1). The details of the quality assessment are described in Supplementary materials.
Incidence of the central vein sign on T2*-weighted imaging of patients with MS
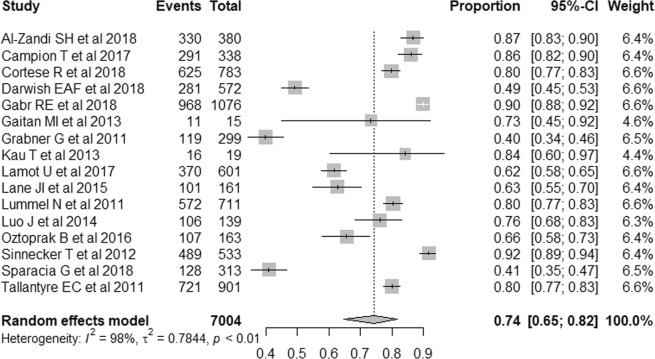
Sixteen original articles evaluated the incidence of central vein sign on T2*-weighted images of patients with MS6–11,13,15–19,22,23,25,26. The individual incidences of central vein sign on T2*-weighted images at the level of individual lesion varied from 40% to 92%, while the pooled incidence of central vein sign on T2*-weighted images was 74% (95% CI, 65–82%; Fig. 2). Heterogeneity was present among these values (I2 = 98%). A meta-regression was performed to explore the effects of heterogeneity, and among the various covariates analyzed, the study design showed statistical significance (p = 0.01). Prospective studies showed a significantly higher pooled incidence of central vein sign on T2*-weighted images (86%; 95% CI, 80–91%) than retrospective studies (67%; 95% CI, 56–77%). Other covariates including MRI sequence (p = 0.08), reader (p = 0.51), reader blindness to the reference standard (p = 0.93), and age (p = 0.31) did not show statistically significant differences. There was no publication bias (p = 0.63; Supplementary Fig. 2).
Figure 2.
Forest plots showing the pooled incidence of central vein sign on T2*-weighted images in patients with MS. Numbers are estimates with 95% confidence intervals [CI] in parentheses.
In the subgroup analyses, the pooled incidence of central vein sign on T2*-weighted images was 84% (95% CI, 70–93%) in studies using 7-Tesla, 79% (95% CI, 68–87%) in studies using 3-Tesla, and 56% (95% CI, 39–72%) in studies using 1.5-Tesla. Although there were no statistical differences between 7-Tesla and 3-Tesla (p = 0.53), and between 3-Tesla and 1.5-Tesla (p = 0.05), there was a statistical difference between 7-Tesla and 1.5-Tesla (p = 0.01).
Eleven original articles evaluated the incidence of central vein sign on T2*-weighted images of patients with non-MS6–9,15–18,23,25,26. The pooled incidence of central vein sign on T2*-weighted images was 33% (95% CI, 18–52%). Heterogeneity was present (I2 = 99%). In the subgroup analyses, the pooled incidences of central vein sign were 26% (95% CI, 13–44%) in studies using 7-Tesla and 38% (95% CI, 18–63%) in studies using 3-Tesla. There were no statistical differences between 7-Tesla and 3-Tesla (p = 0.59). The pooled incidences of central vein sign were 36% (95% CI, 16–63%) in studies included patients with small vessel disease and 33% (95% CI, 28–38%) in studies included patients with NMOSD. There were no statistical differences between small vessel disease and NMOSD (p = 0. 91).
Diagnostic performance of the central vein sign on T2*-weighted Images for diagnosis of MS
Twelve original articles evaluated the overall diagnostic performance of the central vein sign on T2*-weighted images for differentiating MS from other white matter lesions6–9,12,14,15,20,21,23,24,26. Four studies included patients with small vessel disease as a comparison group6,7,21,26, two studies included patients with NMOSD8,23, two studies included patients with CNS inflammatory vasculopathies9,20, two studies included healthy controls12,14, one study included non-MS white matter lesions15, and one study included patients with migraine who had been erroneously diagnosed with MS24.
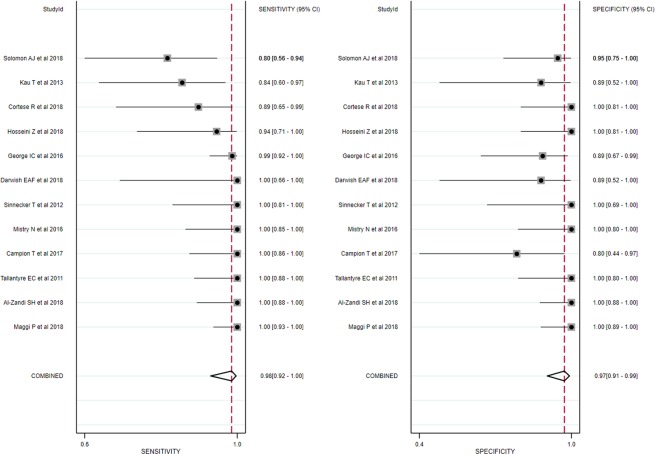
Ten of the twelve studies used a cut-off parameter based on the proportion of lesions with central vein sign on T2*-weighted images6–9,12,14,20,21,23,26, and the patients with MS showed significantly higher proportions of lesions with central vein sign than did the patients with other white matter lesions. One study used just the presence of a central vein sign15, and one study used a simplified three-lesion algorithm24. The individual sensitivities and specificities both varied from 80% to 100%. The pooled sensitivity was 98% (95% CI, 92–100%), and the pooled specificity was 97% (95% CI, 91–99%; Fig. 3). The area under the HSROC curve was 1.00 (95% CI, 0.99–1.00; Fig. 4).
Figure 3.
Coupled forest plots of the sensitivity and specificity of the central vein sign on T2*-weighted images for differentiating MS from other white matter lesions. Numbers are estimates with 95% confidence intervals [CI] in parentheses.
Figure 4.
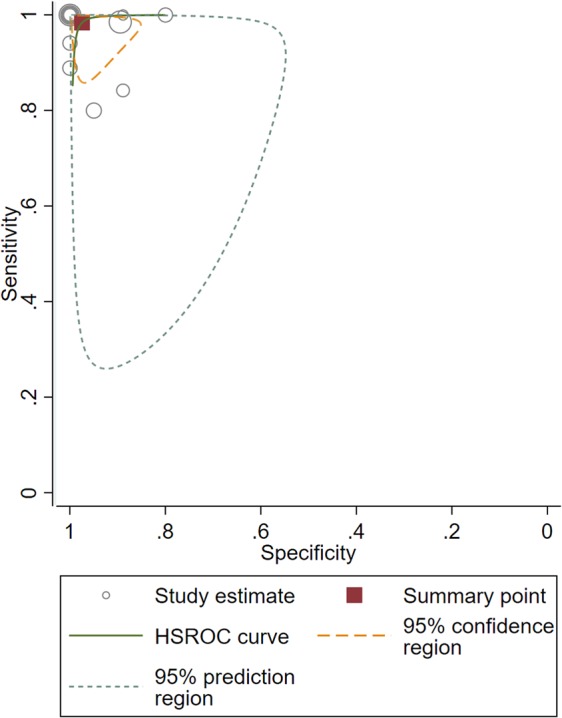
Hierarchical summary receiver operating characteristic (HSROC) curve of the diagnostic performance of the central vein sign on T2*-weighted images for differentiating MS from other white matter lesions.
Both the Q-test (Q = 2.636, p = 0.13) and the Higgins I2 statistic (I2 = 24%) demonstrated that the possibility of heterogeneity was low across the studies. The coupled forest plot revealed no evidence of a threshold effect (Fig. 3), and the Spearman correlation coefficient was −0.092 (95% CI, −0.632–0.509), also indicating no threshold effect. The Deeks’ funnel plot demonstrated that publication bias was present (p < 0.01; Supplementary Fig. 3).
In the meta-regression, none of the covariates, including study design (p = 0.28), MRI sequence (p = 0.88), reader (p = 0.22), reader blindness to the reference standard (p = 0.46), and age (p = 0.36) significantly affected the heterogeneity. In the subgroup analysis, studies using a proportion of lesions with a central vein sign as a cut-off value6–9,12,14,20,21,23,26 also showed high sensitivity (99% [95% CI, 95–100%]) and specificity (99% [95% CI, 89–100%]), with the area under the HSROC curve being 0.99 (95% CI, 0.99–1.00). The Q-test (Q = 2.215, p = 0.17) and the Higgins I2 statistic (I2 = 10%) demonstrated that the possibility of heterogeneity was low.
Diagnostic performance using individual patient data
Eight studies provided individual patient data, including the proportion of lesions with a central vein sign on T2*-weighted images used for differentiating MS from other white matter lesions7–9,14,20,21,23,26. These studies included a total of 245 patients, with 177 patients (72.2%) having MS. Three studies had a prospective design8,21,23 and five studies had a retrospective design7,9,14,20,26. Four studies used a 3-Tesla scanner7–9,21, three studies used a 7-Tesla scanner14,23,26, and one study used either 3- or 1.5-Tesla20. Three studies used T2*-weighted images21,23,26, two studies used FLAIR and SWI9,14, two studies used FLAIR*7,20, and one study used proton density-weighted imaging, T2-weighted imaging, and SWI8. Three studies included patients with small vessel disease as a comparison group7,21,26, two studies included patients with NMOSD8,23, two studies included patients with CNS inflammatory vasculopathies9,20, and two studies included healthy controls14.
The individual cut-off values ranged from 30% to 67%, with a median value of 45%. The area under the ROC curve of the proportion of lesions with central vein sign for the diagnosis of MS was 0.994 (95% CI, 0.975–1.000; Fig. 5). The optimal cut-off value was 45% using the Youden index, resulting in a sensitivity of 97% (95% CI, 94–99%) and specificity of 99% (95% CI, 92–100%).
Figure 5.
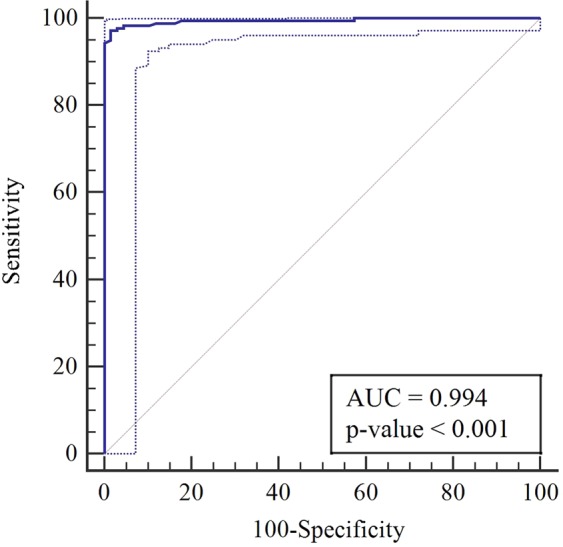
Receiver operating characteristic curve of the diagnostic performance of central vein sign on T2*-weighted images for differentiating MS from other white matter lesions according to individual patient data.
Discussion
The current study revealed a high incidence (74%) of central vein sign on T2*-weighted images of patients with MS, and also revealed that the central vein sign has excellent diagnostic performance for differentiating MS from other white matter lesions, with a pooled sensitivity of 98% and a pooled specificity of 97%. Using individual patient data, the optimal cut-off value for the proportion of lesions with central vein sign on T2*-weighted images was found to be 45%. Although various T2*-weighted images have been used across studies, the current evidence supports the use of the central vein sign on T2*-weighted images to differentiate MS from other white matter lesions.
The differentiation of MS from other white matter lesions can sometimes be challenging, both clinically and radiologically. The proportion of lesions exhibiting the central vein sign is thought to be useful for differentiating MS from some of its mimics1. Our results also showed excellent diagnostic performance for differentiating MS from other white matter lesions according to the proportion of lesions exhibiting the central vein sign. In terms of pathophysiology, the inflammatory demyelination in MS spreads in the parenchyma with perivenular extension41. However, cerebral small vessel disease is thought to contribute to the chronic ischemic damage presenting at the arteriole42, and inflammatory vasculopathies affect medium and small arteries, and are characterized by inflammatory infiltrates of the vessel wall, fibrinoid necrosis, and thrombosis with ischemic change43. As the central vein sign is based on a pathological background, the central vein sign may become a promising biomarker for differentiating MS from other white matter lesions.
The current study highlights the fact that the determination of an optimal cut-off value for the proportion of lesions with a central vein sign on T2*-weighted images is clinically and radiologically important if standardized T2*-weighted images are to be used in daily clinical practice. We found that individual cut-off values ranged from 30% to 67%, and that the optimal cut-off value using individual patient data was 45%, resulting in a sensitivity of 97% and specificity of 99%. Although our results were outstanding, the application of this optimal cut-off value requires time-consuming lesion counting and frequency estimation, which may be difficult to conduct in daily clinical practice. A recent study showed the possibility of a fully automated method for detecting the central vein sign, demonstrating a promising performance44. However, further studies are needed to validate fully automated methods for detecting the central vein sign.
The North American Imaging in Multiple Sclerosis Cooperative mentioned that imaging of veins in the brain can be performed using T2*-based MRI sequences at any magnetic field strength (1.5, 3, or 7-Tesla)5. In addition, high-resolution isotropic T2*-weighted 3D EPI is currently the most promising sequence, and FLAIR* has the potential to become a standard clinical protocol5. However, these sequences have not been widely used because of the difficulty in optimizing protocols and post-processing. Therefore, standardization of T2*-weighted imaging is crucial. We found that five studies using FLAIR* demonstrated excellent diagnostic performance for diagnosing MS7,10,12,20,24. To generate FLAIR* images, co-registration, interpolation, and multiplication processes are needed35. For widespread dissemination of FLAIR*, manufacturer-provided software for direct automated image post-processing on the scanner is necessary.
Although our study results showed the area under the HSROC curve of 0.99 for diagnosing MS using the central vein sign, there are several issues should be considered. Our study is vulnerable to inclusion bias because of the selection of controls. Various comparison groups such as small vessel disease, NMOSD, CNS inflammatory vasculopathies, healthy controls, and non-MS white matter lesions were included. In addition, a previous study showed that the specificity for diagnosing MS using brain MRI with American Academy of Neurology criteria was only 29%, which indicated an increased risk of false-positive diagnosis of MS45. Therefore, careful clinical application should be made using our results in daily clinical practice.
This study has several limitations. First, only 6 of 21 eligible studies were of a prospective design8,10,15,18,21,23 and 20 studies were single institution studies. In addition, patient number of the included studies were relatively small (median 20, range, 5–68). Second, 13 of 21 eligible studies were case-control designs, which are vulnerable to selection bias6–9,12,14–17,21,23,24,26. Third, publication bias was present across the included studies (p < 0.01). Therefore, there may be a possibility that the diagnostic performance of central vein sign on T2*-weighted images for diagnosing MS may be overestimated. Last but not least, although all studies used T2*-based MRI sequences, various T2*-weighted images including SWI or FLAIR*, or T2*-weighted images were used. However, all the eligible studies represented the full extent of the currently available evidence. To overcome these limitations, we conducted this systematic review and meta-analysis using recent robust methodology, including hierarchical logistic regression modeling30–32, and reported this study according to prestigious guidelines such as the Handbook for Diagnostic Test Accuracy Reviews published by the Cochrane Collaboration46, the Agency for Healthcare Research and Quality (AHRQ)47, and PRISMA48.
In conclusion, although various T2*-weighted images have been used across studies, the current evidence supports the use of the central vein sign on T2*-weighted images to differentiate MS from other white matter lesions.
Supplementary information
Acknowledgements
No funding was received for this study.
Author contributions
Chong Hyun Suh and Sang Joon Kim have made substantial contributions to the conception or design of the work, the acquisition, analysis, or interpretation of data, have drafted the work or substantively revised it. Seung Chai Jung, Choong Gon Choi, and Ho Sung Kim have made substantial contributions to have substantively revised it.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-019-54583-3.
References
- 1.Geraldes R, et al. The current role of MRI in differentiating multiple sclerosis from its imaging mimics. Nature reviews. Neurology. 2018;14:199–213. doi: 10.1038/nrneurol.2018.14. [DOI] [PubMed] [Google Scholar]
- 2.Filippi M, et al. MRI criteria for the diagnosis of multiple sclerosis: MAGNIMS consensus guidelines. The Lancet. Neurology. 2016;15:292–303. doi: 10.1016/S1474-4422(15)00393-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solomon AJ, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: A multicenter study. Neurology. 2016;87:1393–1399. doi: 10.1212/WNL.0000000000003152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solomon, A. J., Naismith, R. T. & Cross, A. H. Misdiagnosis of multiple sclerosis: Impact of the 2017 McDonald criteria on clinical practice. Neurology (2018). [DOI] [PMC free article] [PubMed]
- 5.Sati P, et al. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nature reviews. Neurology. 2016;12:714–722. doi: 10.1038/nrneurol.2016.166. [DOI] [PubMed] [Google Scholar]
- 6.Al-Zandi SH, Fayadh NAH, Al-Waely NKN. Central vein sign detected by SWI at 3T MRI as a discriminator between multiple sclerosis and leukoaraiosis. Egyptian Journal of Radiology and Nuclear Medicine. 2018;49:158–164. doi: 10.1016/j.ejrnm.2017.09.003. [DOI] [Google Scholar]
- 7.Campion T, et al. FLAIR* to visualize veins in white matter lesions: A new tool for the diagnosis of multiple sclerosis? European radiology. 2017;27:4257–4263. doi: 10.1007/s00330-017-4822-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cortese R, et al. Value of the central vein sign at 3T to differentiate MS from seropositive NMOSD. Neurology. 2018;90:e1183–e1190. doi: 10.1212/WNL.0000000000005256. [DOI] [PubMed] [Google Scholar]
- 9.Darwish EAF, Ibrahim YA, Zamzam DA. Value of central vein sign in discriminating multiple sclerosis plaques from other white matter lesions. Egyptian Journal of Radiology and Nuclear Medicine. 2018;49:165–171. doi: 10.1016/j.ejrnm.2017.12.008. [DOI] [Google Scholar]
- 10.Gabr RE, et al. Interleaved susceptibility-weighted and FLAIR MRI for imaging lesion-penetrating veins in multiple sclerosis. Magnetic resonance in medicine. 2018;80:1132–1137. doi: 10.1002/mrm.27091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gaitan MI, et al. Multiple sclerosis shrinks intralesional, and enlarges extralesional, brain parenchymal veins. Neurology. 2013;80:145–151. doi: 10.1212/WNL.0b013e31827b916f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.George IC, et al. Clinical 3-tesla FLAIR* MRI improves diagnostic accuracy in multiple sclerosis. Multiple sclerosis (Houndmills, Basingstoke, England) 2016;22:1578–1586. doi: 10.1177/1352458515624975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grabner G, et al. Analysis of multiple sclerosis lesions using a fusion of 3.0 T FLAIR and 7.0 T SWI phase: FLAIR SWI. Journal of magnetic resonance imaging: JMRI. 2011;33:543–549. doi: 10.1002/jmri.22452. [DOI] [PubMed] [Google Scholar]
- 14.Hosseini Z, et al. Morphology-Specific Discrimination between MS White Matter Lesions and Benign White Matter Hyperintensities Using Ultra-High-Field MRI. AJNR. American journal of neuroradiology. 2018;39:1473–1479. doi: 10.3174/ajnr.A5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kau T, et al. The “central vein sign”: is there a place for susceptibility weighted imaging in possible multiple sclerosis? European radiology. 2013;23:1956–1962. doi: 10.1007/s00330-013-2791-4. [DOI] [PubMed] [Google Scholar]
- 16.Lamot U, Avsenik J, Sega S, Surlan Popovic K. Presence of central veins and susceptibility weighted imaging for evaluating lesions in multiple sclerosis and leukoaraiosis. Multiple sclerosis and related disorders. 2017;13:67–72. doi: 10.1016/j.msard.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 17.Lane JI, et al. Characterization of multiple sclerosis plaques using susceptibility-weighted imaging at 1.5 T: can perivenular localization improve specificity of imaging criteria? Journal of computer assisted tomography. 2015;39:317–320. doi: 10.1097/RCT.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 18.Lummel N, et al. Presence of a central vein within white matter lesions on susceptibility weighted imaging: a specific finding for multiple sclerosis? Neuroradiology. 2011;53:311–317. doi: 10.1007/s00234-010-0736-z. [DOI] [PubMed] [Google Scholar]
- 19.Luo J, et al. Gradient echo magnetic resonance imaging correlates with clinical measures and allows visualization of veins within multiple sclerosis lesions. Multiple sclerosis (Houndmills, Basingstoke, England) 2014;20:349–355. doi: 10.1177/1352458513495935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maggi P, et al. Central vein sign differentiates Multiple Sclerosis from central nervous system inflammatory vasculopathies. Annals of neurology. 2018;83:283–294. doi: 10.1002/ana.25146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mistry N, et al. Imaging central veins in brain lesions with 3-T T2*-weighted magnetic resonance imaging differentiates multiple sclerosis from microangiopathic brain lesions. Multiple sclerosis (Houndmills, Basingstoke, England) 2016;22:1289–1296. doi: 10.1177/1352458515616700. [DOI] [PubMed] [Google Scholar]
- 22.Oztoprak B, Oztoprak I, Yildiz OK. The effect of venous anatomy on the morphology of multiple sclerosis lesions: a susceptibility-weighted imaging study. Clinical radiology. 2016;71:418–426. doi: 10.1016/j.crad.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Sinnecker T, et al. Distinct lesion morphology at 7-T MRI differentiates neuromyelitis optica from multiple sclerosis. Neurology. 2012;79:708–714. doi: 10.1212/WNL.0b013e3182648bc8. [DOI] [PubMed] [Google Scholar]
- 24.Solomon AJ, et al. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Multiple sclerosis (Houndmills, Basingstoke, England) 2018;24:750–757. doi: 10.1177/1352458517726383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sparacia G, et al. Multiple sclerosis: High prevalence of the ‘central vein’ sign in white matter lesions on susceptibility-weighted images. The neuroradiology journal. 2018;31:356–361. doi: 10.1177/1971400918763577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tallantyre EC, et al. A comparison of 3T and 7T in the detection of small parenchymal veins within MS lesions. Investigative radiology. 2009;44:491–494. doi: 10.1097/RLI.0b013e3181b4c144. [DOI] [PubMed] [Google Scholar]
- 27.Frank RA, Bossuyt PM, McInnes MDF. Systematic Reviews and Meta-Analyses of Diagnostic Test Accuracy: The PRISMA-DTA Statement. Radiology. 2018;289:313–314. doi: 10.1148/radiol.2018180850. [DOI] [PubMed] [Google Scholar]
- 28.McInnes MDF, et al. Preferred Reporting Items for a Systematic Review and Meta-analysis of Diagnostic Test Accuracy Studies: The PRISMA-DTA Statement. Jama. 2018;319:388–396. doi: 10.1001/jama.2017.19163. [DOI] [PubMed] [Google Scholar]
- 29.Whiting PF, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Annals of internal medicine. 2011;155:529–536. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 30.Suh CH, Park SH. Successful Publication of Systematic Review and Meta-Analysis of Studies Evaluating Diagnostic Test Accuracy. Korean journal of radiology. 2016;17:5–6. doi: 10.3348/kjr.2016.17.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim KW, et al. Systematic Review and Meta-Analysis of Studies Evaluating Diagnostic Test Accuracy: A Practical Review for Clinical Researchers-Part I. General Guidance and Tips. Korean journal of radiology. 2015;16:1175–1187. doi: 10.3348/kjr.2015.16.6.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J, et al. Systematic Review and Meta-Analysis of Studies Evaluating Diagnostic Test Accuracy: A Practical Review for Clinical Researchers-Part II. Statistical Methods of Meta-Analysis. Korean journal of radiology. 2015;16:1188–1196. doi: 10.3348/kjr.2015.16.6.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clinical research ed.) 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Egger M, Davey Smith G, Schneider M, Minder C. BMJ (Clinical research ed.) 1997. Bias in meta-analysis detected by a simple, graphical test; pp. 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sati P, et al. FLAIR*: a combined MR contrast technique for visualizing white matter lesions and parenchymal veins. Radiology. 2012;265:926–932. doi: 10.1148/radiol.12120208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deville WL, et al. Conducting systematic reviews of diagnostic studies: didactic guidelines. BMC Med Res Methodol. 2002;2:9. doi: 10.1186/1471-2288-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deeks JJ, Macaskill P, Irwig L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. Journal of clinical epidemiology. 2005;58:882–893. doi: 10.1016/j.jclinepi.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::AID-CNCR2820030106>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 39.Polman CH, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Annals of neurology. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Polman CH, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald Criteria”. Annals of neurology. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 41.Gaitan MI, et al. Evolution of the blood-brain barrier in newly forming multiple sclerosis lesions. Annals of neurology. 2011;70:22–29. doi: 10.1002/ana.22472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bot JC, et al. Differentiation of multiple sclerosis from other inflammatory disorders and cerebrovascular disease: value of spinal MR imaging. Radiology. 2002;223:46–56. doi: 10.1148/radiol.2231010707. [DOI] [PubMed] [Google Scholar]
- 43.Kuhlmann T, Lassmann H, Bruck W. Diagnosis of inflammatory demyelination in biopsy specimens: a practical approach. Acta neuropathologica. 2008;115:275–287. doi: 10.1007/s00401-007-0320-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dworkin JD, et al. Automated Integration of Multimodal MRI for the Probabilistic Detection of the Central Vein Sign in White Matter Lesions. AJNR. American journal of neuroradiology. 2018;39:1806–1813. doi: 10.3174/ajnr.A5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nielsen JM, et al. Overdiagnosis of multiple sclerosis and magnetic resonance imaging criteria. Annals of neurology. 2005;58:781–783. doi: 10.1002/ana.20632. [DOI] [PubMed] [Google Scholar]
- 46.Deeks, J. J., Bossuyt, P. M. & Gatsonis, C. (eds.) 2013 Cochrane handbook for systematic reviews of diagnostic test accuracy version 1.0.0. The Cochrane Collaboration. http://srdta.cochrane.org/handbook-dta-reviews. Accessed 9 Oct 2017.
- 47.Trikalinos TA, et al. Chapter 8: meta-analysis of test performance when there is a “gold standard”. Journal of general internal medicine. 2012;27(Suppl 1):S56–66. doi: 10.1007/s11606-012-2029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liberati A, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of internal medicine. 2009;151:W65–94. doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.