Abstract
The use of lithium (Li) has dramatically increased during the last two decades due to the proliferation of mobile electronic devices and the diversification of electric-powered vehicles. Lithium is also prescribed as a medication against bipolar disorder. While Li can exert a toxic effect on living organisms, few studies have investigated the impact of anthropogenic inputs on Li levels in the environment. Here we report Li concentrations and Li isotope compositions of river, waste and tap water, and industrial products from the metropolitan city of Seoul. Results show that the large increase in population density in Seoul is accompanied by a large enrichment in aqueous Li. Lithium isotopes evidence a major release from Li-rich materials. Water treatment protocols are also shown to be inefficient for Li. Our study therefore highlights the need for a global Li survey and adequate solutions for minimizing their impact on ecosystems and city dwellers.
Subject terms: Geochemistry, Geochemistry
Lithium use in electronics has increased dramatically, but the environmental impacts are poorly understood. Here the authors show lithium in river and tap water in South Korea is coincident with population density, and that waste water treatment is ineffective at scrubbing this potential toxin.
Introduction
During the last two decades, industrial demands for lithium (Li) resulted in a dramatic increase in Li production and, in 2017, the world production of Li from minerals and brine was 43,000 t1. As the secondary Li-ion battery (LIB) is a major and growing industrial channel for the element, approximately 660 million cylindrical Li-ion cells were produced in 2012, of which Korea shared 21% of total LIB manufacturing capacity2. Lithium is also incorporated into alloys and is widely used as a therapeutic drug for treating bipolar disorder since its discovery in 19703. Although future demands will continue to grow and Li recycling may become an integral part of Li business4, there are still few disposal process guidelines for waste LIB. Furthermore, there is a gap in our knowledge concerning the impact of these materials on Li levels in the environment as well as in municipal waters. The biological effect of high Li levels on the diet of several organisms and human beings has however been already reported in several publications5–10. For aquatic organisms, most of published studies have shown that elevated aqueous Li levels induce toxic effects11,12. Concerning humans, a growing number of studies have reported an inverse relationship between Li concentrations in drinking water and suicide mortality indices (in the USA, Japan and Lithuania), consistent with its biological role in brain cells13–15. In contrast, elevated Li concentrations in drinking water may be deleterious and disturb Ca homeostasis during pregnancy16. Interestingly, Li isotopes (the ratio of 7Li/6Li) have been used by Earth scientists and geochemists since, when measured in rivers and soils, they provide key information on soil sustainability and weathering rate on continents, and therefore on the carbon cycle. They are considered as a key isotope proxy of unraveling why and how global climate could be regulated over geological timescale17–22. Thus, for all these reasons, it becomes increasingly important and urgent to quantify the amount of environmental Li that comes from anthropogenic activities. However, determining the conditions under which Li concentration or Li isotope signature can be impacted by anthropogenic activities remains a challenge.
Here, to test the effects of anthropogenic activities, we sampled and analyzed different types of water from the Han River (HR) basin. This river is the largest river system in South Korea, in terms of discharge and drainage area, and drains the Seoul Special Metropolitan City (Seoul), the capital and largest metropolis of South Korea. The population of the HR basin is estimated to be 12 million (Supplementary Table 1), of which more than 82.7% live in Seoul. Thus, this basin offers a unique opportunity to compare the upstream-inhabited part (although characterized by several dams) with the area of Seoul, located downstream of the Paldang Dam, and which is strongly impacted by urban and industrial activities. The downstream section of the Han River is also the main source of tap water for Seoul citizens. Our study provides the first Li isotope data of industrial products, allowing us to explain the significant Li-enrichment measured in the wastewaters, as well as the high Li contents in the Han River and tap water collected in the highly populated agglomeration of Seoul.
Results and Discussion
Water lithium and its isotopes upstream of the city of Seoul
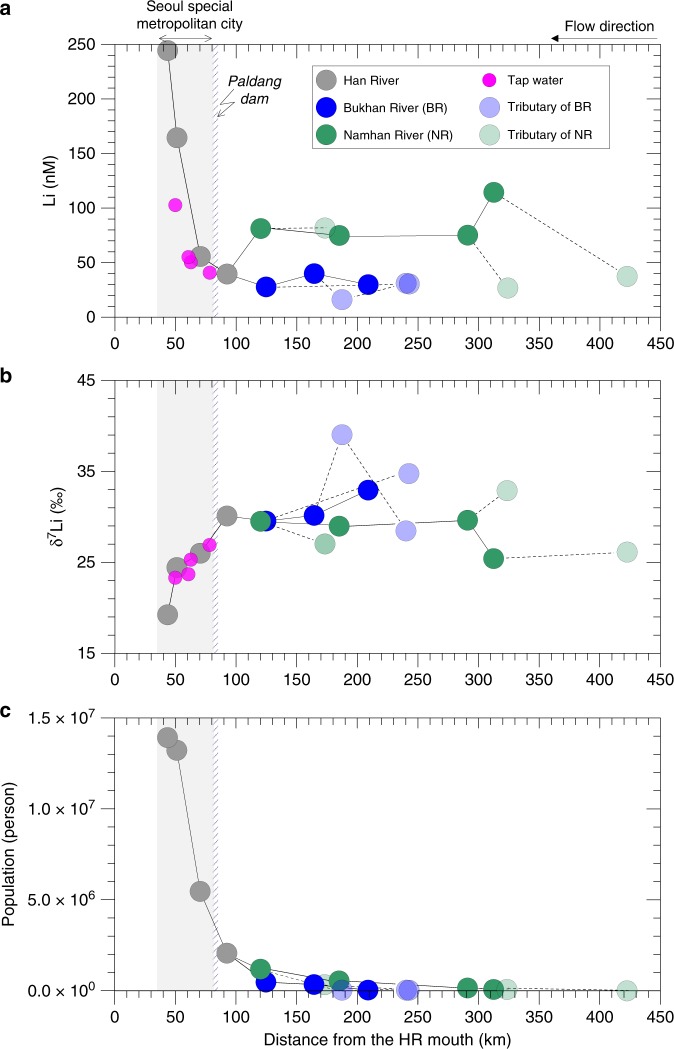
Compared to other rivers worldwide, the upper HR (HR1 in Fig. 1) and its two major tributaries (the Bukhan River, BR; and the Namhan River, NR) carry small amounts of dissolved Li ranging from 15.9 nM to 114 nM (see Methods section; Supplementary Table 2). This amount of Li is 2 to 16 times lower relative to the estimated global flow-weighted average (265 nM)17. Dissolved Li concentrations in each tributary are slightly variable over ~300 km down to the limit of Seoul city (Fig. 2). Lithium concentrations in the NR are systematically higher than those measured in the BR (Fig. 1), perhaps due to the occurrence of Li-rich shales. The lithium isotope compositions of these tributaries are significantly enriched in the heavy isotope (7Li), with δ7Li values all greater than 25‰. This finding is consistent with known mechanisms that fractionate Li isotopes during silicate weathering, such as 6Li-rich clay formation in soils19–23. The lithium isotope composition of both the BR and NR remains remarkably constant over ~340 km. This finding confirms the negligible impact of lithology on riverine δ7Li values, as generally found in rivers of mixed lithology basins, since the major source of riverine lithium remains weathering, and leaching of silicate rocks and minerals24,25. Although the topography and runoff are slightly different in both watersheds (Fig. 1), these differences do not result in significant differences in Li isotope compositions, suggesting that, on average, the leaching and neoformation rate in soils are roughly equivalent over the whole watershed26. After the confluence of both tributaries, Li concentrations and Li isotope compositions of the HR upstream and downstream of the Paldang Dam show the negligible impact of this dam on Li, through its water regulation system.
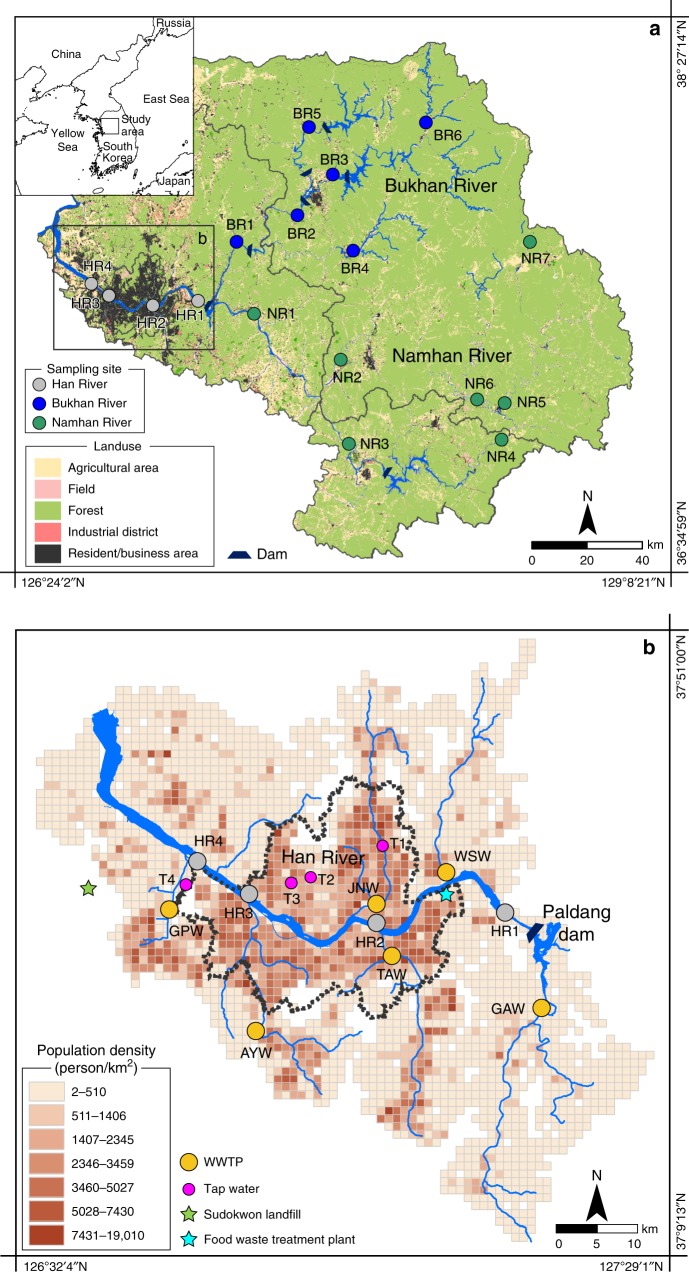
Fig. 1.
Map of the study area. Study area showing land use a, and population density and sampling sites b. Gyeongan (GAW), Wangsuk (WSW), Tan (TAW), Jungnang (JNW), Anyang (AYW), and Gulpo (GPW) display the location and name of wastewater treatment plants at which the wastewater was collected. Note that the HR4 site is located at ~30 km distance from the coastline.
Fig. 2.
Relationship between dissolved lithium and population. Spatial variation in Li concentration a and Li isotope composition b measured in river water and tap water as a function of the distance from the Han River mouth. Variation of population living in the HR basin c as a function of the distance from the Han River mouth. Half-transparent circles represent the tributary of each river.
Evidence for strong anthropogenic Li input downstream
In contrast to the upper watersheds, where all water Li levels are low (50.4 ± 29.2 nM, 1σ, n = 14), and the δ7Li values are high and constant (31.4 ± 3.9‰, 1σ, n = 14), the downstream part of the HR basin displays a strong and progressive evolution for both parameters (Fig. 2; Supplementary Table 2). When the HR crosses Seoul from East to West, Li concentrations abruptly increase by a factor of 6, while δ7Li values decrease significantly from 30.1‰ to 19.2‰. Both the changes in Li concentration and in δ7Li covary with large increase in the population density, which passes from 5 million people at the HR2 site (just after the Paldang Dam) to more than 14 million people at the HR4 site (Supplementary Table 1). This relationship suggests that anthropogenic activities related to increasing urban activities are responsible for the changes displayed by the HR.
The influents correspond to wastewaters coming from households, hospitals and industries within the city, and ultimately arriving at the wastewater treatment plants (WWTP). The effluents correspond to waters treated with various methods to minimize their impact on the environment, and drained back to the river (Supplementary Fig. 1). The Han River also represents the major reservoir of drinking (tap) waters, which are used by consumer households after rigorous purification processes (Supplementary Fig. 2). Thus, any component enriched in wastewaters can affect both the Han River and tap waters. Interestingly, there is no significant difference between influent and effluent wastewaters for both Li concentrations and Li isotope compositions (Fig. 3). This finding demonstrates the negligible effect of the various water treatment protocols used in these plants on the Li level and its isotope composition in waters27. At present, the classic treatment systems are not adapted to Li pollution since there is no significant removal of this element during water treatment.
Fig. 3.
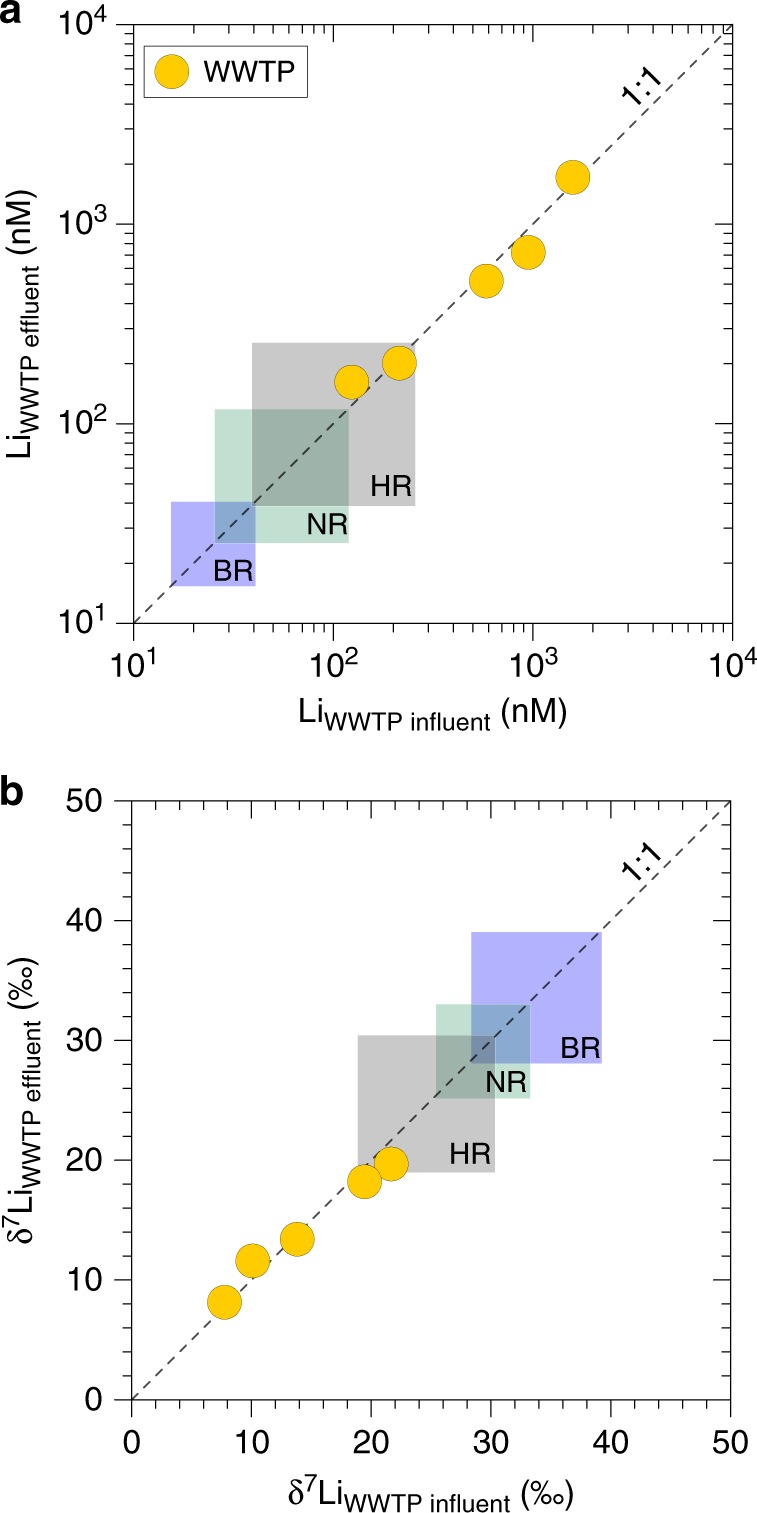
Lithium relationships in wastewaters. Li concentrations a and Li isotope compositions b of wastewater flowing in (influent) and flowing out (effluent) of wastewater treatment plants (WWTP, yellow circles). For comparison, the range of values obtained for the Han River and tributaries are also given (in squares).
If the first striking result is that all effluent wastewaters (leaving the treatment plants) are strongly enriched in Li (up to >1 mM), the second is that their δ7Li values are low (14.5 ± 4.3‰, 1σ, n = 6) and may therefore explain the decrease of the δ7Li value displayed by the HR in Seoul (Supplementary Table 3). As shown in Fig. 4, the relationship between Li concentration and Li isotopes can be explained by the release of isotopically light Li from WWTP. This appears consistent since the only landfill of the area (the Sudokwon landfill, Fig. 1b) is located at 35 km West of Seoul and its drainage waters cannot contribute significantly to our samples. Lithium concentrations of both the downstream HR waters and the effluent wastewaters correlate positively with the population density, especially when it approaches 100 person km−2 (Supplementary Fig. 3), supporting the link between population and volume of treated wastewater per unit population. We observe that this influence is more visible when the population density exceeds a certain threshold, typically higher than 100 person km−2, and that there is also an influence of the effluent water discharge rate from each WWTP on the HR Li level (see Supplementary Fig. 4).
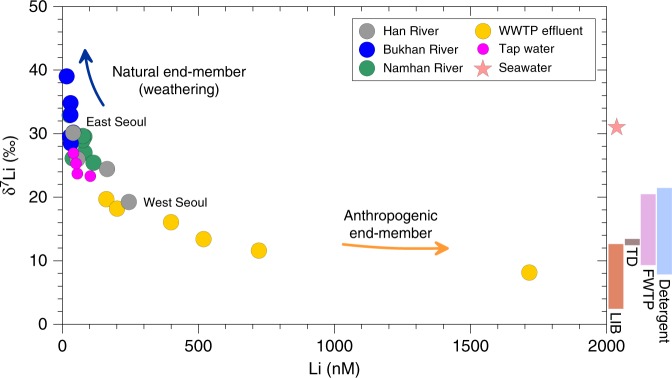
Fig. 4.
Li concentrations versus Li isotope compositions. Wastewater (yellow) plots towards a Li-rich anthropogenic end-member, while the Han River tributaries (BR in blue and NR in green), sampled upstream of the basin, plot towards a natural end-member, consistent with fractionating mechanisms during water-rock interactions (soil/rock weathering). The Han River crossing Seoul from East to West (in grey) evolves progressively towards the anthropogenic end-member represented by wastewaters and by the various Li-rich materials. LIB, TD and FWTP represent secondary Li-ion battery (LIB), therapeutic drug (TD) and food waste treatment plant (FWTP), respectively. Tap waters (in pink) follow the same trend and are consistent with the HR water from which they are sourced. Seawater data were taken from refs. 39,40.
We analyzed several tap water samples collected in Seoul Special Metropolitan City (Supplementary Table 4; Fig. 1). As shown in Fig. 2, Li concentrations and δ7Li values of tap water follow the same evolution from East to West, and are consistent with the values measured in the HR sampled in the same area. This finding strongly suggests that tap water is influenced by the same anthropogenic sources as the river, and confirms that the purification and wastewater treatment processes neither significantly lower Li level nor bias Li isotope composition. Altogether Li concentration and Li isotope composition show a binary mixing between a natural end-member (characterized by tributaries – BR and NR − draining rocks and soils upstream) and an anthropogenic end-member, consistent with isotopically light and strongly enriched wastewater (Fig. 4).
Although the total population of mobile phone subscribers in South Korea was >43 million in 2015 (i.e., 84% of total population in South Korea)28, only 1% of mobile phones were either exported or treated, due to no extended producer responsibility (EPR) regulations for LIB in Korea29–31. Therefore, it is likely that the high Li levels measured in waters would come from the release from LIB waste, along with other anthropogenic inputs such as therapeutic drug (Li carbonate), detergent and compost. In order to investigate further this possibility, we collected and analyzed several anthropogenic materials (Supplementary Table 5; Supplementary Figs. 3 and 4). As expected, the most enriched materials are the therapeutic drug, which contains about 10 wt% Li, and the LIB, which contain between 4.1 and 7.6 wt% Li. Both display systematically low δ7Li values, ranging from 2.4‰ to 13.3‰, consistent with the low δ7Li values displayed by the wastewaters. The other analyzed materials contain much less Li (0.53–2.92 µg g−1 d.w. for the detergents and <0.39 µg g−1 d.w. for the food wastewater and compost) and display, on average, slightly heavier δ7Li values (15.6‰). Thus, as shown in Fig. 4, this first isotopic investigation of Li-rich materials allows us to explain both the significant Li-enrichment of wastewaters and their low δ7Li values. Since treated and untreated waters are similarly enriched in Li, and explain the decrease of river δ7Li in Seoul when the population density is high (Figs. 2c and 4), Li isotopes confirm a major impact of the use of anthropogenic products on Li levels in river crossing the city and in municipal waters. Overall, our study shows that the large Li inputs observed in the Han River come from LIB, therapeutic drug, and food waste, all likely proportional to the population, combined with the inefficiency of wastewater treatment for Li-removal.
Compared to other trace metals32,33, such as Zn, Cu, Ni or Hg, for which contaminated zones are clearly identified and monitored, and whose impacts on aquatic organisms and plants have been carefully investigated for many years in ecotoxicology, there is little information on environmental Li and its toxic effect. By illustrating anthropogenic Li inputs in Seoul waters, our study highlights the need to estimate the environmental and health impact of Li-rich materials, particularly in highly populated areas. Understanding the biological and metabolic effects of high Li levels on aquatic ecosystems also remains to be investigated to fill the gap compared to other contaminants. Finally, this study highlights that in urban areas, Li isotopes are more sensitive to anthropogenic inputs rather than local weathering inputs and therefore should be used with caution as a weathering proxy.
Methods
Samples collection and field measurements
We collected 27 samples in July 2015 from 22 sites along a 422 km downstream transect between the uppermost reaches and the estuary of the Han River (Fig. 1). Upstream, the HR includes two major tributaries (i.e., the Bukhan River, BR; and the Namhan River, NR), which join at the Paldang Dam to form the main channel of the HR that crosses the capital city downstream. Due to the large population in Seoul, there are six major WWTPs that flow out to the HR. The wastewater going in (influent) and going out (effluent) of these plants was collected to estimate the impact of water treatment protocols on dissolved Li. Finally, we compared our results to several tap water samples from various locations in Seoul (Fig. 1) and to several anthropogenic sources. Sample locations were documented with a Garmin GPSMAP 60CSx handheld GPS meter. Temperature (±0.1 °C) and pH (±0.002) were measured in situ using an Orion 5-STAR portable meter equipped with an Orion 3-in-1 pH/ATC pH electrode. The electrode was calibrated twice per day using pH = 4.01, 7.00, and 10.01 buffers. Samples for dissolved cations, trace elements, and Li isotope measurements were passed through 0.2 μm filters, collected in I-CHEM LDPE bottles, and acidified to pH = 2 using concentrated, ultrapure HNO3. Samples for dissolved anions and total alkalinity (AT) were passed through 0.2 μm filters and collected in I-CHEM LDPE bottles.
Chemical analysis
Cation and trace element concentrations were measured using a Perkin Elmer Optima 8300 ICP-AES and a Thermo Elemental iCAPTM Q ICP-MS at the Korea Basic Science Institute (KBSI). Analyses of NRCC SLRS-4 and CRM TMDW-A were within ± 5% of certified values. Anion concentrations were measured using a Dionex ICS-1100 ion chromatograph equipped with a DionexTM IonPacTM AS14 anion-exchange column. The total carbonate alkalinity in μeq/L (AT = HCO3 + 2CO3) was measured using a Mettler Toledo T50A titrator with 0.01 M HCl acidimetric titration to an endpoint of pH = 4.5. The percent charge balance error (CBE), as one measure of the data quality, is given by the equation [CBE (%) = (TZ+ − TZ−)/(TZ+ + TZ−) × 100], where TZ+ = 2Ca2+ + 2Mg2+ + K+ + Na+, TZ− = Cl− + 2SO42− + NO3− + AT, and is on average better than ± 2% (Supplementary Table 2).
Lithium isotope analysis
Samples containing ~100 ng Li were dried in Teflon vessels, and the residues were treated with concentrated HNO3, dried, and re-dissolved in a 1:4 (v/v) mixture of 6 M HNO3 and 100% methanol. Lithium was separated from matrix elements using an AG 50 W−X8 resin (200–400 mesh)26. Then, the sample was dried and re-dissolved in 5% HNO3 (~40 ppb Li). Lithium isotope ratios were measured using a Neptune MC-ICP-MS upgraded with a large dry interface pump at the KBSI and the Korea Institute of Ocean Science & Technology (KIOST). Samples were introduced using a quartz dual cyclonic spray chamber and analyzed with a blank-standard-blank-sample-blank-standard-blank bracketing method. Sample intensities were matched to within 10% of the intensity of the standard. The sensitivity was ~90 V ppm−1 on mass 7 at a typical uptake rate of 100 μL min−1. Prior to isotopic analysis, each sample was checked for the yield and the concentration of matrix elements. The yields were approximately 100%, and the matrix concentration did not exceed 1.5% of the Li concentrations. The lithium isotopic composition is reported in delta notation relative to NIST RM 8545, where δ7Li = [(7Li/6Li)sample/(7Li/6Li)NIST RM 8545 – 1] × 1000. The accuracy and reproducibility of the whole method was validated using the USGS rock reference materials (BCR-2, BHVO-2, and BIR-1) and seawater standard (IAPSO). BCR-2 yielded +3.6 ± 1.7‰ (2σ, n = 14), BHVO-2 yielded +4.5 ± 0.0‰ (2σ, n = 2), BIR-1 yielded +4.1‰ (n = 1), and IAPSO yielded +31.2 ± 1.5‰ (2σ, n = 15), which were all in good agreement with reported values21,34–38.
Supplementary information
Acknowledgements
We thank Y.K. and K.R. for providing cathode materials for LIB and MC-ICP-MS at the KIOST. This project was supported by the National Research Council of Science & Technology (NST) grant by the Korea government (MSIP) (No. CAP-17-05-KIGAM) and also benefited from discussions initiated in the context of the ANR ISO2MET Grant (ANR-18-CES34-0002) started in January 2019.
Author contributions
J.-S.R. and N.V. designed the study, and led the writing of the manuscript. H.-B.C., W.-J.S. and J.-S.R. conducted the fieldwork and chemical analyses. All authors contributed equally to the data interpretation.
Data availability
All data generated or analyzed during this study are included with this published article in its Supplementary Information.
Competing interests
The authors declare no competing interests.
Footnotes
Peer reivew information Nature Communications thanks Jeroen Sonke, Paul Tomascak, and other, anonymous, reviewers for their contributions to the peer review of this work. Peer review reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-019-13376-y.
References
- 1.Ober, J. A. Mineral commodity summaries 2018, U.S. Geological Survey (2018).
- 2.A study on the prospect of lithium demand and supply, Ministry of Trade, Industry and Energy (2016).
- 3.Draaisma D. Lithium: the gripping history of a psychiatric success story. Nature. 2019;572:584–585. doi: 10.1038/d41586-019-02480-0. [DOI] [Google Scholar]
- 4.Mohr SH, Mudd GM, Giurco D. Lithium resources and production: critical assessment and global projections. Minerals. 2012;2:65–84. doi: 10.3390/min2010065. [DOI] [Google Scholar]
- 5.Freese D, Niehoff B, Søreide JE, Sartoris FJ. Seasonal patterns in extracellular ion concentrations and pH of the Arctic copepod Calanus glacialis. Limnol. Oceanogr. 2015;60:2121–2129. doi: 10.1002/lno.10158. [DOI] [Google Scholar]
- 6.Dwyer FJ, Burch SA, Ingersoll CG, Hunn JB. Toxicity of trace element and salinity mixtures to striped bass (Morone saxatilis) and Daphnia magna. Environ. Toxicol. Chem. 1992;11:513–520. doi: 10.1002/etc.5620110409. [DOI] [Google Scholar]
- 7.Roux M, Dosseto A. From direct to indirect lithium targets: a comprehensive review of omics data. Metallomics. 2017;9:1326–1351. doi: 10.1039/C7MT00203C. [DOI] [PubMed] [Google Scholar]
- 8.Nagato EG, et al. 1H NMR-based metabolomics investigation of Daphnia magna responses to sub-lethal exposure to arsenic, copper and lithium. Chemosphere. 2013;93:331–337. doi: 10.1016/j.chemosphere.2013.04.085. [DOI] [PubMed] [Google Scholar]
- 9.Tkatcheva V, et al. Lithium an emerging contaminant: Bioavailability, effects on protein expression, and homeostasis disruption in short-term exposure of rainbow trout. Aquat. Toxicol. 2015;161:85–93. doi: 10.1016/j.aquatox.2015.01.030. [DOI] [PubMed] [Google Scholar]
- 10.Aral H, Vecchio-Sadus A. Toxicity of lithium to humans and the environment—a literature review. Ecotoxicol. Environ. Saf. 2008;70:349–356. doi: 10.1016/j.ecoenv.2008.02.026. [DOI] [PubMed] [Google Scholar]
- 11.Kszos LA, Stewart AJ. Review of lithium in the aquatic environment: Distribution in the United States, toxicity and case example of groundwater contamination. Ecotoxicology. 2003;12:439–447. doi: 10.1023/A:1026112507664. [DOI] [PubMed] [Google Scholar]
- 12.Kszos LA, Beauchamp JJ, Stewart AJ. Toxicity of lithium to three freshwater organisms and the antagoistic effect of sodium. Ecotoxicology. 2003;12:427–437. doi: 10.1023/A:1026160323594. [DOI] [PubMed] [Google Scholar]
- 13.Blüml V, et al. Lithium in the public water supply and suicide mortality in Texas. J. Psychiatr. Res. 2013;47:407–411. doi: 10.1016/j.jpsychires.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Ohgami H, Terao T, Shiotsuki I, Ishii N, Iwata N. Lithium levels in drinking water and risk of suicide. Br. J. Psychiatry. 2009;194:464–465. doi: 10.1192/bjp.bp.108.055798. [DOI] [PubMed] [Google Scholar]
- 15.Liaugaudaite V, Mickuviene N, Raskauskiene N, Naginiene R, Sher L. Lithium levels in the public drinking water supply and risk of suicide: a pilot study. J. Trace Elem. Med. Biol. 2017;43:197–201. doi: 10.1016/j.jtemb.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 16.Harari F, Åkesson A, Casimiro E, Lu Y, Vahter M. Exposure to lithium through drinking water and calcium homeostasis during pregnancy: a longitudinal study. Environ. Res. 2016;147:1–7. doi: 10.1016/j.envres.2016.01.031. [DOI] [PubMed] [Google Scholar]
- 17.Huh Y, Chan LH, Zhang L, Edmond JM. Lithium and its isotopes in major world rivers: implications for weathering and the oceanic budget. Geochim. Cosmochim. Acta. 1998;62:2039–2051. doi: 10.1016/S0016-7037(98)00126-4. [DOI] [Google Scholar]
- 18.Misra S, Froelich PN. Lithium isotope history of Cenozoic seawater: changes in silicate weathering and reverse weathering. Science. 2012;335:818–823. doi: 10.1126/science.1214697. [DOI] [PubMed] [Google Scholar]
- 19.Penniston-Dorland S, Liu X-M, Rudnick RL. Lithium Isotope Geochemistry. Rev. Mineral. Geochem. 2017;82:165–217. doi: 10.2138/rmg.2017.82.6. [DOI] [Google Scholar]
- 20.Tomascak, P.B., Magna, T. & Dohmen, R. Advances in Lithium Isotope Geochemistry (Springer: Berlin, Heidelberg 2016).
- 21.Ryu J-S, Vigier N, Lee S-W, Lee K-S, Chadwick OA. Variation of lithium isotope geochemistry during basalt weathering and secondary mineral transformations in Hawaii. Geochim. Cosmochim. Acta. 2014;145:103–115. doi: 10.1016/j.gca.2014.08.030. [DOI] [Google Scholar]
- 22.Caves Rugenstein JK, Ibarra DE, von Blanckenburg F. Neogene cooling driven by land surface reactivity rather than increased weathering fluxes. Nature. 2019;571:99–102. doi: 10.1038/s41586-019-1332-y. [DOI] [PubMed] [Google Scholar]
- 23.Burton KW, Vigier N. Handbook of Environmental Isotope Geochemistry. Berlin, Heidelberg: Springer; 2012. Lithium isotopes as tracers in marine and terrestrial environments; pp. 41–59. [Google Scholar]
- 24.Kısakürek B, Widdowson M, James RH. Behaviour of Li isotopes during continental weathering: the Bidar laterite profile, India. Chem. Geol. 2004;212:27–44. doi: 10.1016/j.chemgeo.2004.08.027. [DOI] [Google Scholar]
- 25.Millot R, Vigier N, Gaillardet J. Behaviour of lithium and its isotopes during weathering in the Mackenzie Basin, Canada. Geochim. Cosmochim. Acta. 2010;74:3897–3912. doi: 10.1016/j.gca.2010.04.025. [DOI] [Google Scholar]
- 26.Bastian L, Revel M, Bayon G, Dufour A, Vigier N. Abrupt response of chemical weathering to Late Quaternary hydroclimate changes in northeast Africa. Sci. Rep. 2017;7:44231. doi: 10.1038/srep44231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia, Z., Li, S. & Wang, L. Assessment of soil heavy metals for eco-environment and human health in a rapidly urbanization area of the upper Yangtze Basin. Sci. Rep. 8, 10.1038/s41598-018-21569-6 (2018). [DOI] [PMC free article] [PubMed]
- 28.The Korea Ministry of Science and ICT; http://www.itstat.go.kr.
- 29.Korea Battery Recycling Association; http://www.kbra.net/epr/epr1.htm.
- 30.Song HT. Current status of the recycling of waste mobile phones and urgent problem. e-Recycling. 2004;8:7–9. [Google Scholar]
- 31.Lee, J.-c. Private communication with SK networks; http://www.sknetworks.co.kr (2006).
- 32.Ozaki, H. et al. Immutable heavy metal pollution before and after change in industrial waste treatment procedure. Sci. Rep. 9, 10.1038/s41598-019-40634-2 (2019). [DOI] [PMC free article] [PubMed]
- 33.Obrist D, et al. Tundra uptake of atmospheric elemental mercury drives Arctic mercury pollution. Nature. 2017;547:201–204. doi: 10.1038/nature22997. [DOI] [PubMed] [Google Scholar]
- 34.Choi MS, et al. Precise determination of the lithium isotope ratio in geological samples using MC-ICP-MS with cool plasma. J. Anal. Spectrom. 2013;28:505–509. doi: 10.1039/c2ja30293d. [DOI] [Google Scholar]
- 35.Elliott T, Thomas A, Jeffcoate A, Niu Y. Lithium isotope evidence for subduction-enriched mantle in the source of mid-ocean-ridge basalts. Nature. 2006;443:565–568. doi: 10.1038/nature05144. [DOI] [PubMed] [Google Scholar]
- 36.Huang KE, et al. Low-memory, small sample size, accurate and high-precision determinations of lithium isotopic ratios in natural materials by MC-ICP-MS. J. Anal. Spectrom. 2010;25:1019–1024. doi: 10.1039/b926327f. [DOI] [Google Scholar]
- 37.Liu X-M, Rudnick RL, McDonough WF, Cummings ML. Influence of chemical weathering on the composition of the continental crust: Insights from Li and Nd isotopes in bauxite profiles developed on Columbia River Basalts. Geochim. Cosmochim. Acta. 2013;115:73–91. doi: 10.1016/j.gca.2013.03.043. [DOI] [Google Scholar]
- 38.Ludwing T, et al. A secondary ion mass spectrometry (SIMS) re-evaluation of B and Li isotopic compositions of Cu-bearing elbaite from three global localities. Mineral. Mag. 2011;75:2485–2494. doi: 10.1180/minmag.2011.075.4.2485. [DOI] [Google Scholar]
- 39.Millot R, Guerrot C, Vigier N. Accurate and high-precision measurement of lithium isotopes in two reference materials by MC-ICP-MS. Geostand. Geoanal. Res. 2004;28:153–159. doi: 10.1111/j.1751-908X.2004.tb01052.x. [DOI] [Google Scholar]
- 40.Morozov NP. Geochemistry of rare alkaline elements in the oceans and seas. Oceanology. 1968;8:169–178. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included with this published article in its Supplementary Information.