Abstract
Statement of the Problem:
Paxillin is a major cytoskeletal protein aberrantly deregulated in various human cancers and involved in tumor growth and invasion. However, the clinicopathological and prognostic significance of paxillin in salivary gland tumors (SGTs) is still unclear.
Purpose:
This study was conducted to evaluate the relationship between paxillin expression and clinicopathological features of patients with SGTs.
Materials and Method:
In this retrospective study, 50 paraffin-embedded tissue samples which were histologically confirmed as benign (pleomorphic adenoma, PA) or malignant (mucoepidermoid carcinoma (MEC), adenoid cystic carcinoma, ACC) SGTs, and 19 specimens from those with normal salivary gland (NSG) as a control group were assessed for paxillin expression using the immunohistochemistry. The paxillin expression in our samples was scored based on the extent and intensity of immunoreactivity and compared with histological type, clinical stage, and distant metastasis.
Results:
High paxillin expression was identified in 66% of SGTs whereas all patients with NSG showed low expression (p< 0.0001). Although the expression of paxillin in patients with benign and malignant tumors is similar, there is a significant difference between patients with PA, MEC, and ACC with that of the NSG (p< 0.0001). Paxillin expression was not correlated with clinicopathological features of patients.
Conclusion:
High expression of paxillin was observed in tumoral tissues compared with the controls that establish an important role of paxillin in SGTs but its prognostic role was unclear and need further evaluation.
Keywords: Mucoepidermoid Carcinoma , Paxillin , Pleomorphic Adenoma , Immunohistochemistry
Introduction
Salivary gland tumors (SGTs) are among the relatively rare group of neoplasms characterized by the wide range of biological behaviors and a variety of benign and malignant types. The annual incidence of SGTs is approximately 0.4 to 13.5 cases per 100,000 individuals, about 3-6% of all head and neck cancers[1-4]. SGTs are considered to have multifactorial etiology, and histopathologic characteristic complicates their diagnosis and management. The management requires distinctive surgical and adjuvant therapy, but the early detection plays an important role in successful therapy and reduces the severity of its impact on the patient's life[3-4]. Despite a low prevalence, a number of factors such as the complex histopathological diagnosis, grade of malignancies, varied clinical behaviors and risk of recurrence pose the major challenges for the pathologists and clinicians to achieve the effective treatment[3-5].
Paxillin is a 68-kDa focal adhesion-associated adaptor protein, which is originally identified as a tyrosine phosphorylated protein in chick embryo fibroblasts. Paxillin is localized at the intracellular surfaces where the cells adhere to the extracellular matrix. The function has not been well characterized, but it has been proposed that paxillin acts as an oncogene in many malignancies and involved in key signal transduction, cell motility, migration, proliferation, survival, angiogenesis, and apoptosis[6-10]. A number of studies have been evaluated the expression of paxillin and shows the deregulation and its role in the development of various human carcinomas such as the squamous cell carcinoma[11], esophageal squamous cell carcinoma[12], lung[13-14], breast[15], cervical[16], colorectal[9], and prostate cancers[17-18]. Furthermore, it was shown that paxillin level significantly associated with the progression and metastasis of malignancies. Patients with high paxillin expression had poorer prognosis and survival rate compared to those with low paxillin expression. However, the underlying mechanisms of paxillin overexpression remain unresolved. Therefore, paxillin might be a potential therapeutic target to suppress tumor progression[6,9,19-21]. To the best of our knowledge and among the different type of SGTs, only one study has evaluated the expression of paxillin in patients with salivary adenoid cystic carcinoma (ACC)[22]. However, the role and its expression in the other types of SGTs still need to be clearly elucidated. Therefore, the current study aimed to determine the expression levels of paxillin and its prognostic and clinicopathological significance in Iranian patients with benign and malignant SGTs.
Materials and Method
Study Population
A total of 50 paraffin-embedded SGTs tissues from patients who underwent surgery in the ENT department of Khalili Hospital and School of Dentistry both affiliated to Shiraz University of Medical Sciences, were enrolled in this retrospective study. All the patients had a histologically confirmed diagnosis of SGTs and received no treatment prior to surgery. The patient group consisted of 19 (38%) male and 31 (62%) females whose age ranged from 19 to 81 years (mean age of 47.6±17.3). Patients were divided into 2 groups of benign (n = 17) and malignant (n = 33). All of the benign tumors were pleomorphic adenoma (PA) while the malignant cases were as follows: 16 patients with mucoepidermoid carcinoma (MEC) and 17 patients with ACC.
On the other hand, 19 specimens including 8 male (42.1%) and 11 female (57.9%) with the mean age of 56.5±16.1 years (range 38-81 years) were also kept from those with normal salivary gland (NSG) adjacent to the tumoral tissues as a control group of this study. Relevant clinicopathological characteristics such as age, gender, tumor size, lymph node, and distant metastasis were obtained from the patients’ medical database. The common tumor-node-metastasis (TNM) staging system was used for classification of all histologic variants of SGTs based on the American Joint Committee on Cancer (AJCC). The research protocol was approved by the Ethics Committee of Shiraz University of Medical Sciences, and all participants gave written informed consent for using their samples for research purpose.
Immunohistochemistry
Immunohistochemical staining was used to detect paxillin expression with standard protocols. Briefly, formalin-fixed paraffin-embedded tissue blocks were cut serially into 4μm. Sections were deparaffinized with xylene, rehydrated through a graded alcohol series, and treated with 3% hydrogen peroxide in methanol to block endogenous peroxidase activity. Then, the sections were rinsed with phosphate-buffered saline and subjected to microwave antigen retrieval in 0.01 mol/L sodium citrate buffer (pH 6.0). An anti-paxillin antibody (Rabbit, Abcam Corporation, ab 32084, USA) was used as the primary antibody, followed by the streptavidin-horseradish peroxidase complex. Finally, binding was visualized with 3, 3 diaminobenzidine (DAB) chromogen and all the sections were counterstained with hematoxylin. HeLa cells (line from cervical cancer cells) were used as positive control and negative control was obtained by omission of the primary antibody.
To quantify the paxillin expression, both the percentage and intensity of immunoreactivity were assessed and scored. The percentage of staining was graded according to the percentage of cells that had positive immunoreactivity in every microscopic field: 0= less than 25%; 1=weak (25‑50%); 2= moderate (>50‑75%); and 3= strong (>75-100%). The staining intensity score ranging from 0 to 3 and graded as follows: 1= weak; 2= moderate; and 3= strong. By multiplying the scores for percentage and intensity, a total score was achieved (ranged from 0 to 9). According to the immunohistochemical results of paxillin staining, patients were divided into two groups of paxillin low-level expression and paxillin high-level expression. The score of 4 and more than 4 was rated as “high” paxillin expression whereas a score less than 4 was rated as “low” paxillin expression[8].
Statistical analysis
The statistical analysis was performed using the SPSS software package (version 21, SPSS Inc, Chicago, USA). The comparison of paxillin expression and association between clinicopathological characteristics and paxillin expression was analyzed by the chi-square and ANOVA test. A p value of less than 0.05 was considered statistically significant.
Results
Paxillin was mainly localized in the cytoplasm of tumor cells and all samples (100%) included in this study was expressed paxillin (Table 1).
Table 1.
Paxillin overexpression in benign and malignant salivary gland tumors in comparison with normal salivary gland tissues
| Types of lesion | Number of patients | High Paxillin expression, N(%) | Low Paxillin expression N(%) |
|---|---|---|---|
| ACC | 17 | 12(70.6) | 5(29.4) |
| MEC | 16 | 9(56.3) | 7(43.8) |
| PA | 17 | 12 (70.6) | 5(29.4) |
| Normal | 19 | 0 (0) | 19 (100) |
Paxillin expression was mainly seen in ductal cells of NSG. In PA and ACC both ductal and myoepithelial cells showed paxillin positivity (Figures 1 to 4).
Figure1.
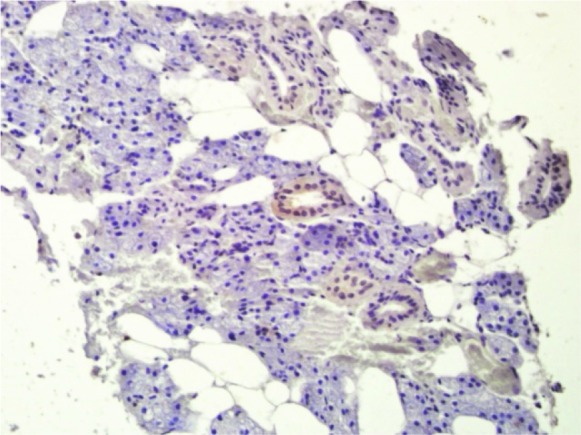
Cytoplasmic paxillin expression in normal salivary gland (200×)
Figure2.
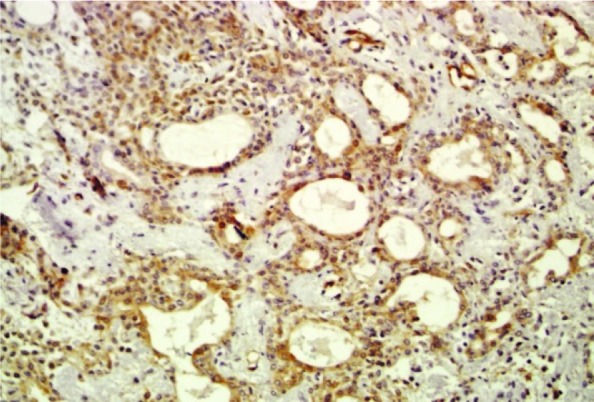
Cytoplasmic paxillin expression in pleomorphic adenoma (200×)
Figure3.
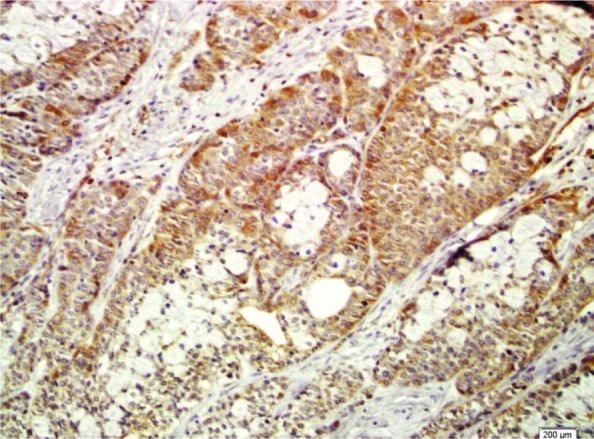
Cytoplasmic paxillin in mucoepidermoid carcinoma (200×)
Figure4.
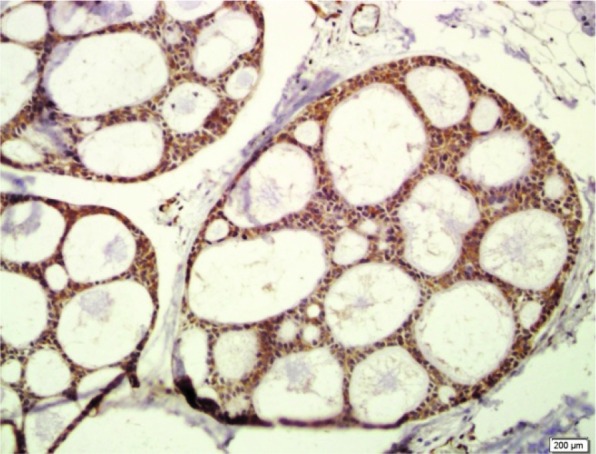
Cytoplasmic paxillin in adenoid cystic carcinoma (200×)
The expression of paxillin is higher in the SGTs than that in the NSG
Among the study groups, the percentage and intensity of paxillin expression were strong in 58% of SGTs cases (29 of 50). Most of the patients with NSG showed weak paxillin positivity (94.7%) and moderate paxillin intensity (68.4%) while the strong intensity diagnosed in only 2 (10.5%) cases. The paxillin total score was signi-ficantly higher in SGTs than NSG (Table 1) (p< 0.0001).
The relationship between paxillin expression and clinicopathological characteristics
Next, we analyzed the paxillin expression levels in patients with benign and malignant tumors and found no significant difference (p= 0.930). The expression levels of paxillin appeared to be similar among the 3 subgroups (PA, MEC and ACC) of SGTs (p> 0.05), but there was a significant difference between PA, MEC and ACC groups with that of the NSG (p< 0.0001) (Table 1). Our results indicated that 70.6%, 56.3% and 70.6% of patients with PA, MEC, and ACC rated as high paxillin expression, respectively. Immunohistochemistry analysis revealed that there were no differences between genders, age, tumor size, lymph node metastasis, and TNM stage regarding paxillin expression (Table 2) (p> 0.05).
Table 2.
Clinicopathologic characteristics of the 33 patients with malignant salivary gland tumors
| Variable | N (%) | Paxillin protein (High expression) N(%) | Paxillin protein (Low expression) N(%) | p value |
|---|---|---|---|---|
| T Status | ||||
| T1+T2 | 25(75.7) | 15(60) | 10(40) | 0.8 |
| T3+T4 | 8(24.3) | 6(75) | 2(25) | |
| N Status | ||||
| N0 | 24(72.7) | 16(66.6) | 8(33.4) | 0.2 |
| N1 | 9(27.3) | 5(55.5) | 4(44.5) | |
| M Status | ||||
| M0 | 30(90.9) | 18(60) | 12(40) | 0.4 |
| M1 | 3(9.1) | 3(100) | 0(0) | |
| Stage | ||||
| I+II | 22(66.6) | 15(68.1) | 7(31.9) | 0.2 |
| III+IV | 11(33.4) | 6(54.5) | 5(45.5) | |
Discussion
Paxillin serves as an important multifunctional protein with scaffolding role that provides the binding sites between the plasma membrane and the actin cytoskeleton. This platform is necessary for the integration and processing of adhesion and growth factor-related signals that are mainly involved in tumor migration, invasion, and metastasis[20,23-24]. Recent studies have reported that paxillin expression differs among various malignancies and promotes the tumor progression[22,25-26]. However, despite the extensive investigations of paxillin expression, little information is available in SGTs. The incidence of SGTs in Iranian population estimated to be 0.4- 4.9%[27-29]. Therefore, due to the prevalence and importance of SGTs, the current study was intended to evaluate the expression of paxillin in tissue samples of Iranian patients with benign and malignant SGTs.
Our study evaluated the expression of paxillin by the use of both the percentage and intensity of paxillin immunoreactivity. Paxillin was expressed in all of the tissue samples, but the SGTs showed the higher expression of paxillin in all three subgroups (PA, MEC, and ACC) compared with that of NSG, which suggested the crucial role of paxillin in tumorgenesis. We found high paxillin expression in patients with PA, MEC, and ACC (70.6%, 56.3% and 70.6%), respectively. To our knowledge, only one report has explored the expression of paxillin in patients with SGTs. Shi et al.[22] evaluated the 47 cases of salivary ACC and showed paxillin expression in 57.45% of patients which is lower than that of observed in our series. The difference between these two studies maybe related to different immunohistochemistry evaluation and sample size.
Paxillin was highly expressed in 66% of our patients, which is similar to the results of patients with hepatocellular carcinoma[26], but differs from urothelial bladder tumor[30]. On the other hand, all the patients with NSG rated as low paxillin expression. In accordance to our results, almost all previous studies such as those conducted on patients with laryngeal squamous cell carcinoma[20], esophageal squamous cell carcinoma[12], colorectal cancer[8], lung cancer[13], gastric cancer[6], and colorectal cancer[9] show the higher expression of paxillin in cancerous tissues compared with that of observed in their controls. Furthermore, paxillin could not differentiate between benign and malignant cases. Our results revealed that paxillin expression was not correlated with clinicopathological parameters of patients with SGTs. It is in contrast with the results of Shi et al.[22] in which paxillin expression was associated with clinical stage and distant metastasis, but not with histologic type. Similar results were obtained in patients with colorectal cancer[19,31], and hepatocellular carcinoma[26].
High expression of paxillin was significantly correlated with the advanced TNM stage in gastric cancer[6], prostate cancer[32], colorectal cancer[19], laryngeal squamous cell carcinoma[20], and salivary ACC[22]. However, the relationship between paxillin expression and advanced clinical stages was not reached in this study, because of small sample size of malignant tumors. So due to the relatively small number of samples, further investigation of its potential is highly warranted, especially when early detection of patients could improve the prognosis and survival rate.
In summary, the present study showed that paxillin expression of SGTs was significantly higher than NSG, which highlight the important role of paxillin expression in the development of tumors. However, it is not correlated with clinicopathologic factors of patients. Our study had some limitations. First, this is a retrospective study and not well controlled. In addition, this study concerned the small number of patients and relatively few clinical events, limiting our ability for precise conclusions. Further studies with larger sample size will be required to assess the potential roles of paxillin for the early detection of SGTs.
Conclusion
High expression of paxillin was observed in tumoral tissues compared with the controls that establish an important role of paxillin in SGTs but its prognostic role was unclear and need further evaluation.
Acknowledgement
The authors thank the Vice-Chancellery of Shiraz University of Medical Sciences for supporting this research (Grant #96-01-99-15769). This manuscript is based on the thesis of Aysuda Afshari for partial fulfillment of DDS degree. The authors thank Dr. M. Salehi from De-ntal Research Development Center, Shiraz Dental School for his statistical analysis.
Conflict of Interest:The authors declare no conflict of interests.
References
- 1.Melo GM, Cervantes O, Abrahao M, Covolan L, Ferreira ES, Baptista HA. A brief history of salivary gland surgery. Rev Col Bras Cir. 2017; 44: 403–412. doi: 10.1590/0100-69912017004004. [DOI] [PubMed] [Google Scholar]
- 2.Randy Todd D. The Molecular Biology of Benign and Malignant Salivary Gland Tumors. Salivary Gland Pathology: Diagnosis and Management. 2nd ed . USA: Wiely ; 2015. pp. 203–232. [Google Scholar]
- 3.Wang X, Luo Y, Li M, Yan H, Sun M, Fan T. Management of salivary gland carcinomas - a review. Oncotarget. 2017; 8: 3946–3956. doi: 10.18632/oncotarget.13952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peravali RK, Bhat HH, Upadya VH, Agarwal A, Naag S. Salivary gland tumors: a diagnostic dilemma! . J Maxillofac Oral Surg. 2015;( 14(Suppl 1)):438–442. doi: 10.1007/s12663-014-0665-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ettl T, Schwarz-Furlan S, Gosau M, Reichert TE. Salivary gland carcinomas. Oral Maxillofac Surg. 2012; 16: 267–283. doi: 10.1007/s10006-012-0350-9. [DOI] [PubMed] [Google Scholar]
- 6.Chen DL, Wang ZQ, Ren C, Zeng ZL, Wang DS, Luo HY, et al. Abnormal expression of paxillin correlates with tumor progression and poor survival in patients with gastric cancer. J Transl Med. 2013; 11: 277. doi: 10.1186/1479-5876-11-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao LJ, Zhao EH, Zhao S, Zheng X, Zheng HC, Takano Y, et al. Paxillin expression is closely linked to the pathogenesis, progression and prognosis of gastric carcinomas. Oncol Lett. 2014; 7: 189–194. doi: 10.3892/ol.2013.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du C, Wang X, Zhang J, Liu X, Zhu J, Liu Y. Paxillin is positively correlated with the clinicopathological factors of colorectal cancer, and knockdown of Paxillin improves sensitivity to cetuximab in colorectal cancer cells. Oncol Rep. 2016; 35: 409–417. doi: 10.3892/or.2015.4352. [DOI] [PubMed] [Google Scholar]
- 9.Chen DL, Wang DS, Wu WJ, Zeng ZL, Luo HY, Qiu MZ, et al. Overexpression of paxillin induced by miR-137 suppression promotes tumor progression and metastasis in colorectal cancer. Carcinogenesis. 2013; 34: 803–811. doi: 10.1093/carcin/bgs400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wozniak MA, Modzelewska K, Kwong L, Keely PJ. Focal adhesion regulation of cell behavior. Biochim Biophys Acta. 2004; 1692: 103–119. doi: 10.1016/j.bbamcr.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Wu DW, Chuang CY, Lin WL, Sung WW, Cheng YW, Lee H. Paxillin promotes tumor progression and predicts survival and relapse in oral cavity squamous cell carcinoma by microRNA-218 targeting. Carcinogenesis. 2014; 35: 1823–1829. doi: 10.1093/carcin/bgu102. [DOI] [PubMed] [Google Scholar]
- 12.Li BZ, Lei W, Zhang CY, Zhou F, Li N, Shi SS, et al. Increased expression of paxillin is found in human oesophageal squamous cell carcinoma: a tissue microarray study. J Int Med Res. 2008; 36: 273–278. doi: 10.1177/147323000803600209. [DOI] [PubMed] [Google Scholar]
- 13.Jagadeeswaran R, Surawska H, Krishnaswamy S, Janamanchi V, Mackinnon AC, Seiwert TY, et al. Paxillin is a target for somatic mutations in lung cancer: implications for cell growth and invasion. Cancer Res. 2008; 68: 132–142. doi: 10.1158/0008-5472.CAN-07-1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu DW, Cheng YW, Wang J, Chen CY, Lee H. Paxillin predicts survival and relapse in non-small cell lung cancer by microRNA-218 targeting. Cancer Res. 2010; 70: 10392–10401. doi: 10.1158/0008-5472.CAN-10-2341. [DOI] [PubMed] [Google Scholar]
- 15.Short SM, Yoder BJ, Tarr SM, Prescott NL, Laniauskas S, Coleman KA, et al. The expression of the cytoskeletal focal adhesion protein paxillin in breast cancercorrelates with HER2 overexpression and may help predict response to chemotherapy: a retrospective immunohistochemical study. Breast J. 2007; 13: 130–139. doi: 10.1111/j.1524-4741.2007.00389.x. [DOI] [PubMed] [Google Scholar]
- 16.McCormack SJ, Brazinski SE, Moore JL Jr, Werness BA, Goldstein DJ. Activation of the focal adhesion kinase signal transduction pathway in cervicalcarcinoma cell lines and human genital epithelial cells immortalized with humanpapillomavirus type 18. Oncogene. 1997; 15: 265–274. doi: 10.1038/sj.onc.1201186. [DOI] [PubMed] [Google Scholar]
- 17.Kasai M, Guerrero-Santoro J, Friedman R, Leman ES, Getzenberg RH, DeFranco DB. The Group 3 LIM domain protein paxillin potentiates androgen receptor transactivation in prostate cancer cell lines. Cancer Res. 2003; 63: 4927–4935. [PubMed] [Google Scholar]
- 18.Sen A, De Castro I, Defranco DB, Deng FM, Melamed J, Kapur P, et al. Paxillin mediates extranuclear and intranuclear signaling in prostate cancer proliferation. J Clin Invest. 2012; 122: 2469–2481. doi: 10.1172/JCI62044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yin H, Zhang Q, Wang X, Li T, Wan Y, Liu Y, et al. Role of paxillin in colorectal carcinoma and its relationship to clinicopathological features. Chin Med J (Engl) 2014; 127: 423–429. [PubMed] [Google Scholar]
- 20.Li L, Wang J, Gao L, Gong L. Expression of paxillin in laryngeal squamous cell carcinoma and its prognostic value. Int J Clin Exp Pathol. 2015; 8:9232–9239. [PMC free article] [PubMed] [Google Scholar]
- 21.Sun LH, Yang FQ, Zhang CB, Wu YP, Liang JS, Jin S, et al. Overexpression of Paxillin Correlates with Tumor Progression and Predicts PoorSurvival in Glioblastoma. CNS Neurosci Ther. 2017; 23: 69–75. doi: 10.1111/cns.12606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi J, Wang S, Zhao E, Shi L, Xu X, Fang M. Paxillin expression levels are correlated with clinical stage and metastasis in salivaryadenoid cystic carcinoma. J Oral Pathol Med. 2010; 39: 548–551. doi: 10.1111/j.1600-0714.2009.00859.x. [DOI] [PubMed] [Google Scholar]
- 23.Turner CE. Paxillin and focal adhesion signalling. Nat Cell Biol. 2000; 2: E231–E236. doi: 10.1038/35046659. [DOI] [PubMed] [Google Scholar]
- 24.Metalli D, Lovat F, Tripodi F, Genua M, Xu SQ, Spinelli M, et al. The insulin-like growth factor receptor I promotes motility and invasion of bladder cancer cells through Akt- and mitogen-activated protein kinase-dependent activation of paxillin. Am J Pathol. 2010; 176: 2997–3006. doi: 10.2353/ajpath.2010.090904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai HX, Yang LC, Song XH, Liu ZR, Chen YB, Dong GK. Expression of paxillin and FAK mRNA and the related clinical significance in esophagealcarcinoma. Mol Med Rep. 2012; 5: 469–472. doi: 10.3892/mmr.2011.664. [DOI] [PubMed] [Google Scholar]
- 26.Li HG, Xie DR, Shen XM, Li HH, Zeng H, Zeng YJ. Clinicopathological significance of expression of paxillin, syndecan-1 and EMMPRIN in hepatocellular carcinoma. World J Gastroenterol. 2005; 11: 1445–1451. doi: 10.3748/wjg.v11.i10.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taghavi N, Sargolzaei S, Mashhadiabbas F, Akbarzadeh A, Kardouni P. Salivary Gland Tumors: A 15- year Report from Iran. Turk Patoloji Derg. 2016; 32: 35–39. doi: 10.5146/tjpath.2015.01336. [DOI] [PubMed] [Google Scholar]
- 28.Jaafari-Ashkavandi Z, Ashraf MJ, Moshaverinia M. Salivary gland tumors: a clinicopathologic study of 366 cases in southern Iran. Asian Pac J Cancer Prev. 2013; 14: 27–30. doi: 10.7314/apjcp.2013.14.1.27. [DOI] [PubMed] [Google Scholar]
- 29.Shahsavari F, Khaniki M, Farbod M. Prevalence of Salivary Glands Lesions in Iran: A 10-Year Retrospective Study. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014; 119: e174. [Google Scholar]
- 30.Athanasopoulou A, Aroukatos P, Nakas D, Repanti M, Papadaki H, Bravou V. Decreased ezrin and paxillin expression in human urothelial bladder tumors correlate with tumor progression. Urol Oncol. 2013;31:836–42. doi: 10.1016/j.urolonc.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Zhao CJ, Du SK, Dang XB, Gong M. Expression of paxillin is correlated with clinical prognosis in colorectal cancer patients. Med Sci Monit. 2015; 21:1989–1995. doi: 10.12659/MSM.893832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng QS, Chen SH, Wu YP, Chen HJ, Chen H, Wei Y, et al. Increased Paxillin expression in prostate cancer is associated with advancedpathological features, lymph node metastases and biochemical recurrence. J Cancer. 2018; 9: 959–967. doi: 10.7150/jca.22787. [DOI] [PMC free article] [PubMed] [Google Scholar]


