Abstract
Statement of the Problem:
Sufficient bond strength of composite restoration leads to its durability and survival; therefore, preparation of dentin surface for higher bond strength is essential.
Purpose:
Our aim is to assess the deproteinizing effect of 3% bromelain enzyme and compare it to 4% titanium tetrafluoride (TiF4) and 5% sodium hypochlorite (NaOCl) regarding the shear bond strength (SBS) of composite resin to dentin.
Materials and Method:
In this experimental study, 40 intact extracted human maxillary premolars were selected, and the occlusal surfaces of the teeth were sectioned at a depth of 2 mm from dentinoenamel junction. The teeth were divided into 4 groups (n=10). In Group 1, the teeth were etched with 37% phosphoric acid gel. In Group 2, the teeth were etched and deproteinized with 5% NaOCl. In Group 3, the teeth were etched and deproteinized with 4% TiF4. In Group 4, the teeth were etched and deproteinized with 3% bromelain enzyme. In each specimen, composites with 3 mm diameter and 2 mm height were prepared and cured. The test specimens were then stored in distilled water at room temperature for 7 days before conducting the SBS test (MPa). By universal testing machine at a crosshead speed of 1 mm/min, the results were analyzed using one-way ANOVA and Tukey test.
Results:
One-way ANOVA test demonstrated that pretreatment of dentin with a bromelain enzyme, TiF4 solution, or NaOCl was not statistically different regarding SBS to dentin (p= 0.790).
Conclusion:
3% bromelain enzyme can be as effective as TiF4 and NaOCl and phosphoric acid 37% in terms of the SBS of composite resin to dentin.
Keywords: Sodium Hypochlorite , Shear Bond Strength , Bromelain , ToF4 , Composite
Introduction
Hybridization is a process in which a hybrid layer is formed on dentin surface through bonding phenomenon[1]. Hybrid layer is formed through demineralization of dentin as well as infiltration of resin monomers into collagen fibrils, and emerged after dentin demineralization[2]. To enable effective hybrid layer formation, smear layer should be removed prior to dentin surface bonding[3]. Different materials have been introduced for dentin pretreatment before adhesive application[4].
Irrigating solutions used during the bonding procedure may lead to alteration in the chemical and physical properties of dentin[5]. These materials react with organic tissue leading to change of the chemical structure and mechanical properties of dentin[6]. Sodium hypochlorite (NaOCl) has been used on dentin as a non-specific deproteinizing agent[7]. Given that NaOCl is cytotoxic, scientists seek for a new and ideal substance to deproteinize dentin[8-9]. It has been reported that application of 5% NaOCl causes a significant decrease in the shear bond strength (SBS) of nano-filled composite resins, and NaOCl has negative effects on bond strength[4]. Another study found that, there was not any statistically significant difference between control group and NaOCl-treated group[8].
Fluoridated materials can enhance dentin remineralization and decrease secondary caries formation[10]. Fluoride mainly affects longevity of restorations by inducing degradation of unprotected collagen. Titanium tetrafluoride (TiF4), a fluoridated product, has been suggested as an effective factor against dental caries formation[11-13]. It has been indicated that a tenacious titanium-rich coating is readily formed after application of TiF4 solution to enamel and cementum[14]. However, the use of TiF4 solution as a smear layer-modifying agent is a recent development, and further research is needed to approve its feasibility[15]. Different studies using TiF4 as pretreatment have reported different results [4,7,16]. It has been concluded that application of TiF4 on dentin does not significantly affect the micro-tensile and SBS of the composite resin[10,13].
Deproteinizing enzymes such as collagenase and bromelain enzyme are newer techniques to remove collagen network[17]. Bromelain is a proteolytic enzyme commercially obtained from the fruit or stem of pineapple. The function of proteases is to catalyze the hydrolysis of proteins to amino acids[18]. Bromelain is commonly used in medical supplements. Bromelain can act similarly to non-steroidal anti-inflammatory drugs and has been demonstrated to have fewer side effects. In Europe, it is orally or topically used for post-surgery wounds, trauma, and deep burns[19]. One study revealed that removal of unsupported collagen fiber with bromelain enzyme, after acid etching, improves bond strength[8].
There is insufficient information about the effect of 4% TiF4 and 3% bromelain enzyme[20] on the SBS of composite resins, and there was no comparison between these agents and 5% NaOCl. Therefore, we conducted this study to compare the SBS of restorative composites to dentin deproteinized by 5% NaOCl, 4% TiF4, and 3% bromelain enzyme.
Materials and Method
Sample
In this experimental study, forty intact extracted human maxillary premolars were selected and used with the approval of the Ethics Committee of Shiraz University of Medical Sciences, Shiraz, Iran (IR.SUMS. REC. 1395.S1191). They were completely sound premolars without any caries, cracks, and fractures. The teeth were hand-scaled and stored in 0.5 % thymol solution (Merck-Germany). Middle dentin was exposed by sectioning the crowns at a depth of 2 mm from dentinoenamel junction (DEJ) parallel to the occlusal surface, under water coolant, using a diamond disk (SP, Brazil)[21].
The roots of the sectioned teeth were embedded in acrylic resins of 1.5 cm diameter and 2.5 cm height, 1.0 mm below the cementoenamel junction. Then, the occlusal surfaces of the teeth were polished with a 600-grit silicon carbide paper (Starcke, Germany) under constant water spray to homogenize the dentin surface[21-22]. The teeth were randomly assigned to four groups of ten teeth (n=10) based on the dentin deproteinizing method:
For group 1, samples were etched for 15 seconds with 37% phosphoric acid gel (condac37; FGM, Brazil), rinsed for 15 seconds with water, and gently air dried so that a surface with a brilliant appearance to achieve wet-bonding remained on the dentin. Two consecutive coats of Adper single bond (3M, ESPE, St.Paul, USA) was gently applied on the dentin surface by a disposable micro-brush. Subsequently, it was gently dried for 2-5 seconds and light-cured for 10 seconds (according to the manufacturer’s instructions). Next, the nanofilled composite resin (Z350; 3M, ESPE, USA) was embedded in a Teflon mold of 3 mm diameter and 2 mm height over the central part of the prepared dentin. Later, we put a transparent matrix band on the occlusal surface of the composite and finger pressed it. Finally, additional composite was removed by the explorer.
For group 2, the procedure was similar to group 1 except that in this group, after acid application, 5% NaOCl (Merck, Germany) was actively applied by syringe for 2 minutes on the prepared surface. Next, the NaOCl-treated surface was thoroughly rinsed for 60 seconds. The rest of the procedure continued in the same way as group1.
For group 3, the procedure was similar to group 1; however, after acid application, 4% TiF4 (wt/v; PH: 1.2) (sigma Aldrich-USA) achieved by dissolving TiF4 in deionized distilled water by chemist, was actively applied by a disposable micro-brush for 1 minute. We then thoroughly rinsed the TiF4 treated surface for 30 seconds.
Finally, for group 4, the procedure was similar to group 1; however, after acid application, 3% bromelain enzyme (Salamat Parmoon Amin manufacturer, Iran) achieved by dissolving bromelain powder in deionized distilled water by chemist, was applied by a disposable micro-brush for 1 minute. Then, we thoroughly rinsed the bromelain treated surface for 30 seconds. The rest of the procedure continued in the same way as group 1.
In each specimen, the prepared composites were cured for 40 seconds (according to the manufacturer’s recommendation) by a halogen light polymerizing unit (Monitex Blue Luxcer M855) with the light intensity of 600 mW/cm2 as the device tip was perpendicular to composite surface.
The specimens were then stored in distilled water at room temperature for 7 days before being subjected to the SBS test (MPa). The shear bond test was performed at a cross-head speed of 1 mm/min and calculated in MPa, using universal testing machine (Zwick-Roell; Z020, Germany) (Figure 1). The results were analyzed using one-way ANOVA and Tukey test.
Figure1.
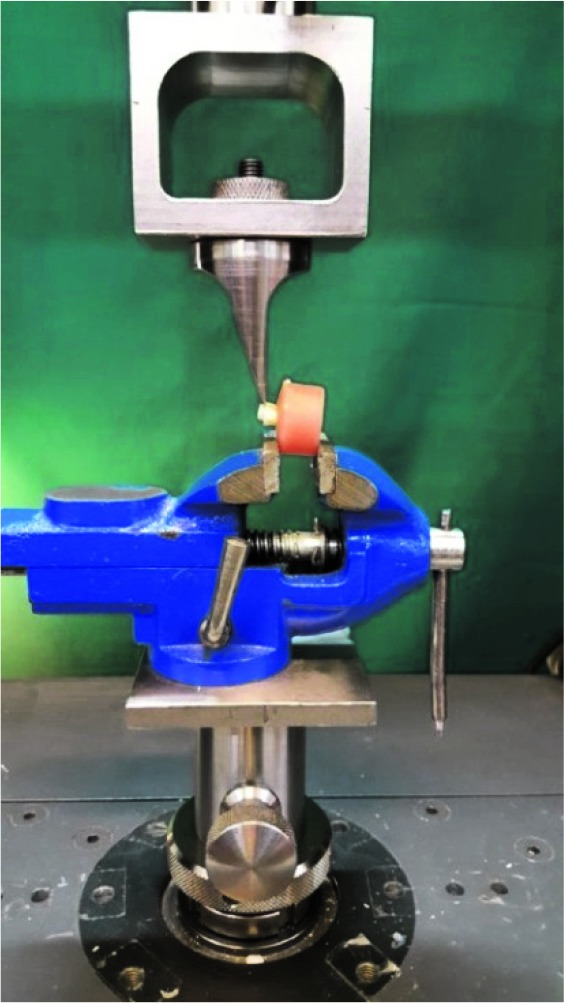
A sample in universal testing machine
Statistical analysis
Statistical analyses were conducted using the SPSS software (Version 19, Chicago, IL, USA). Values are presented as mean ± SD, and group differences were calculated by the parametric one-way ANOVA test (p< 0.05). The p value was 0.790 in our result, and there were no statistically significant differences between our groups; therefore, based on the statistical rules, there was no need for Tukey HSD test for the two-by-two comparisons of the groups.
Results
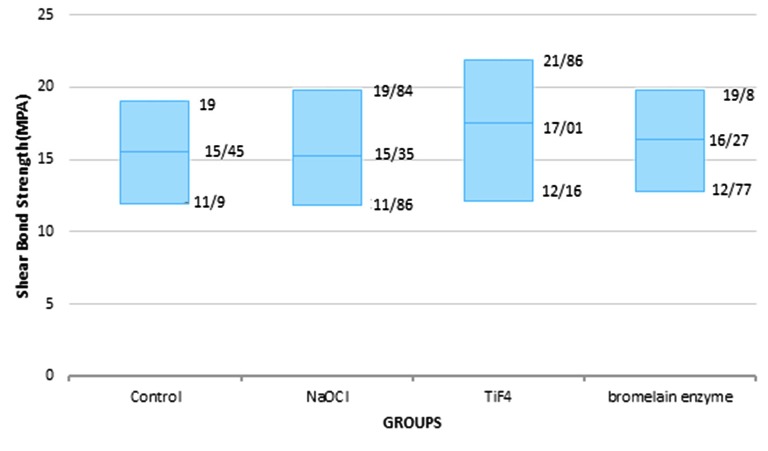
Table 1 demonstrates the mean and standard deviation as well as p-value of the SBS of composites to dentin, and Figure 2 shows mean±SD. One-way ANOVA test showed no statistically significant differences between th e groups (p= 0.790±SD). Therefore, bromelain, despite its low concentration, is as effective as the other three groups and the SBS was statistically similar in all the groups.
Table 1.
Mean and SD of SBS (MPa) of all four tested groups and p-value between groups (n=10)
| Groups | Mean±SD | p Value |
|---|---|---|
| 37% H3PO4 | 15.45±3.55 | 0.790 |
| 37% H3PO4+ NaOCl | 15.35±4.49 | |
| 37% H3PO4+TiF4 | 17.01±4.85 | |
| 37% H3PO4+Bromelain Enzyme | 16.27±3.53 |
Figure2.
Maximum and minimum of SBS of all groups
Discussion
In our study, NaOCl was used prior to bonding procedures. Previous studies indicated that after using it in the treatment of dentin, an increase in wettability was expected due to the hydrophilic surface produced by collagen removal[23-24].
It has been indicated that dehydration of collagen fibrils exposed by acid etching is associated with decrease in dentin matrix. These results are owing to incomplete infiltration of resin monomers into dentin. In other words, reduction occurs in dentin bond strength[25] Thus to preserve the collagen fibrils expanded prior to adhesive application, it is necessary to maintain the dentin moist[26].
Sharafeddin et al.[4] reported that using 5% NaOCl caused a significant decrease in the SBS of dentin to both nano-filled composite resin and silorane-based composite resin. Previous studies demonstrated that the use of 5% NaOCl significantly decreased the SBS of single bond and silorane adhesive to dentin[4,27-28]. In our study, the application of NaOCl was prior to etching. Moreover, in the previous study, the adhesive system for silorane-based composite resin was self-etch, but we used the total etches adhesive system. Decrease of bond strength using NaOCl in the previous study can be explained by production of oxygen after NaOCl decomposition into NaCl and O2[29-30]. The released oxygen in this chemical reaction prevents the polymerization of adhesive agents. These reactive residual free radicals in the NaOCl-treated dentin compete with the propagation of the vinyl-free radicals generated during light activation of the adhesive system, causing minimum infiltration of resin monomers and incomplete hybrid layer formation[4]. There is another possible explanation of lower bond strength, which is based on the deep or surface dentin treatment. Uceda-Gomez et al.[7] reported that the lower bond strength values were obtained in deep dentin rather than surface dentin. They attributed this phenomenon to smaller amount of intertubular dentin in deep dentin. In our study, deep occlusal substrate had much less intertubular dentin to penetration by resin tag; therefore, there is not a firm bonding to the tubule wall in this location. Hence, bonding efficacy was not seen in deep dentin.
The present study indicated that pretreatment with NaOCl resulted in the statistically similar SBS of the composites compared to the control group. Our result was in agreement with one study by Chauhan et al.[8]. They reported that the teeth, etched with 37% phosphoric acid for 15 seconds, rinsed with water, blot dried, and deproteinized with 5% NaOCl did not have statistically significant differences in SBS compared to the similar group without 5% NaOCl treatment.
One study conducted by Cecchin et al.[31] indicated that the use of 1% NaOCl alone, compared to dentin, led to higher tensile bond strength of the adhesive system. NaOCl (1%) was applied to dentin every 5 minutes for 1 hour. However, the solution concentration and the application time were different from those of our study, which may have caused different results.
It seems that NaOCl may affect bond strength differently, based on the chemical structure of the adhesive system, NaOCl concentration, and the type of the initiator in the adhesive system used.
Application of TiF4, as a dentin-pretreatment, was previously proposed in some studies[16,30]. In the study conducted by Bridi et al.[13], dentin was treated with a 2.5% TiF4 solution for 1 minute. They evaluated the micro-tensile bond strength of one- or two-step self-etching adhesive systems, and results demonstrated that pretreatment of dentin with a TiF4 solution did not affect the bond strength of either of the adhesive systems.
Application of TiF4 in the present study resulted in similar SBS to the control group. Our findings were in line with the study conducted by Devabhaktuni and Manjunath[10]. They applied TiF4 to dentin before and after phosphoric acid conditioning, and application of TiF4 had no influence on bond strength to dentin.
In addition, in the study conducted by Sharafeddin et al.[4], the authors concluded that the effect of 4% TiF4 on the nano-filled composite’s SBS to dentin was not statistically significant compared to the control group.
It is well known that low pH (about 1.2) of TiF4 is suitable to attach titanium to oxygen atom of the phosphate group[5]. This process causes formation of a titanium dioxide glaze-like layer on the surface to produce a globular titanium-rich surface layer[29,32]. This layer protects dentin demineralization from highly acidic pH (approximately 1.2) of the TiF4 solution. TiF4 was actively applied with a brush for 60 seconds[15]. One of the erosions inhibiting characteristics of TiF4 depends on its method of application on dentin. It seems that application with a micro-brush leads to surface wear rather than allowing the formation of the glaze-like surface layer[25]. This is the reason why 4% TiF4 pretreatment had no effect on SBS.
The application of 3% bromelain enzyme resulted in similar SBS to the control group. This result differs from the result of the study by Chauhan et al.[8]. They used the same procedure to determine effectiveness of bromelain enzyme pretreatment composite resin on SBS; however, there were some differences in type of incubation and bromelain enzyme concentration. Firstly, they used the teeth occlusal surfaces. Secondly, they did not mention the amount of enzyme concentration. In our study, however, we sectioned the teeth occlusal surfaces at a depth of 2 mm from DEJ. Moreover, we used 3% bromelain enzyme by a disposable micro-brush.
In another study, Dayem and Tameesh[17] assessed the deproteinizing effect of bromelain enzyme. Application of bromelain enzyme led to collagen network removal and significantly decreased the global leakage scores of the adhesive system. This is due to the ability of bromelain enzyme to efficiently remove the collagen network from acid etched dentin. This, subsequently, will lead to an increase of diffusion potential of the monomer to the intact dentin minimizing nano-leakage. The difference of their results from those of our study is due to some differences in methodology. Firstly, their method of evaluation was scanning electron microscopy. Secondly, dentin cavity preparation was on buccal and lingual surfaces. Moreover, they stored the teeth in 50% ethanol at 8ºc for 1 month.
There were only few studies assessing the effect of bromelain enzyme on the bond strength of composites to dentin since bromelain is a new material in restorative dentistry and is under investigation. Although application of these three solutions (NaOCl, TiF4 and bromelain enzyme) did not result in destruction of the etched dentin surface, it did not have any effect on the increase of SBS. It seems that preparation steps, type of adhesive systems, deproteinization with TiF4, and bromelain enzyme affect the SBS of composite resins. Further studies should be conducted to evaluate the effect of different concentrations of bromelain enzyme and TiF4 on deep and surface dentin. Moreover, Future studies should be considered with no acid etching.
Conclusion
Considering the limitations of this in vitro study, it can be concluded that, the pretreatment of premolar dentin with NaOCl, TiF4, and bromelain enzyme leads to equal SBS of composite resins. In other words, bromelain enzyme, a safe solution without any side effects, can successfully remove collagen network as effective as TiF4 or NaOCl, after acid etching.
Acknowledgement
The authors thank the vice-chancellor of Shiraz University of Medical Sciences, for supporting the research. The authors would like to thank the Biomaterial Research Center and Ms. Bagheri for testing specimens and Ms. Pakdel for preparing testing solutions. Additionally, the authors thank Dr. Mehrdad Vosough from the Dental Research Development Center, for the statistical analysis.
Conflict of Interest:The authors declare that they have no conflict of interest.
References
- 1.Nakabayashi N, Nakamura M, Yasuda N. Hybrid layer as a dentin-bonding mechanism. J Esthet Dent. 1991; 3: 133–138. doi: 10.1111/j.1708-8240.1991.tb00985.x. [DOI] [PubMed] [Google Scholar]
- 2.Van Meerbeek B, De Munck J, Yoshida Y, Inoue S, Vargas M, Vijay P, et al. Buonocore memorial lecture. Adhesion to enamel and dentin: current status and futurechallenges. Oper Dent . 2003; 28: 215–235. [PubMed] [Google Scholar]
- 3.Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J, Van Landuyt KL. State of the art of self-etch adhesives. Dent Mater. 2011; 27: 17–28. doi: 10.1016/j.dental.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 4.Sharafeddin F, Koohpeima F, Razazan N. The Effect of Titanium Tetrafluoride and Sodium Hypochlorite on the Shear Bond Strength of Methacrylate and Silorane Based Composite Resins: an In-Vitro Study. J Dent (Shiraz) 2017; 18: 82–87. [PMC free article] [PubMed] [Google Scholar]
- 5.Koohpeima F, Sharafeddin F, Jowkar Z, Ahmadzadeh S, Mokhtari MJ, Azarian B. Role of TiF4 in Microleakage of Silorane and Methacrylate-based Composite Resins in Class V Cavities. J Contemp Dent Pract. 2016; 17: 240–247. doi: 10.5005/jp-journals-10024-1834. [DOI] [PubMed] [Google Scholar]
- 6.Perdigão J, Lopes M, Geraldeli S, Lopes GC, García-Godoy F. Effect of a sodium hypochlorite gel on dentin bonding. Dent Mater. 2000; 16: 311–323. doi: 10.1016/s0109-5641(00)00021-x. [DOI] [PubMed] [Google Scholar]
- 7.Uceda-Gómez N, Reis A, Carrilho MR, Loguercio AD, Rodriguez Filho LE. Effect of sodium hypochlorite on the bond strength of an adhesive system to superficial and deep dentin. J Appl Oral Sci. 2003; 11: 223–228. doi: 10.1590/s1678-77572003000300012. [DOI] [PubMed] [Google Scholar]
- 8.Chauhan K, Basavanna RS, Shivanna V. Effect of bromelain enzyme for dentin deproteinization on bond strength of adhesive system. J Conserv Dent. 2015; 18: 360–363. doi: 10.4103/0972-0707.164029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salomão PMA, Oliveira FA, Rodrigues PD, Al-Ahj LP, Gasque KCDS, Jeggle P, et al. The cytotoxic effect of TiF4 and NaF on fibroblasts is influenced by the experimental model, fluoride concentration and exposure time. PLoS One. 2017; 12: e0179471. doi: 10.1371/journal.pone.0179471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devabhaktuni S, Manjunath M. Effect of 4% titanium tetrafluoride application on shear bond strength of composite resin: An in vitro study. J Conserv Dent. 2011; 14: 43–45. doi: 10.4103/0972-0707.80741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breschi L, Martin P, Mazzoni A, Nato F, Carrilho M, Tjäderhane L, et al. Use of a specific MMP-inhibitor (galardin) for preservation of hybrid layer. Dent Mater. 2010; 26: 571–578. doi: 10.1016/j.dental.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castellan CS, Bedran-Russo AK, Karol S, Pereira PN. Long-term stability of dentin matrix following treatment with various natural collagen cross-linkers. J Mech Behav Biomed Mater. 2011; 4: 1343–1350. doi: 10.1016/j.jmbbm.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bridi EC, Amaral FL, França FM, Turssi CP, Basting RT. Influence of dentin pretreatment with titanium tetrafluoride and self-etching adhesive systems on microtensile bond strength. Am J Dent. 2013; 26: 121–126. [PubMed] [Google Scholar]
- 14.Büyükyilmaz T, Ogaard B, Rølla G. The resistance of titanium tetrafluoride-treated human enamel to strong hydrochloric acid. Eur J Oral Sci. 1997; 105(5 Pt 2): 473–277. doi: 10.1111/j.1600-0722.1997.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 15.Magalhães AC, Kato MT, Rios D, Wiegand A, Attin T, Buzalaf MA. The effect of an experimental 4% Tif4 varnish compared to NaF varnishes and 4% TiF4 solution on dental erosion in vitro. Caries Res. 2008; 42: 269–274. doi: 10.1159/000135672. [DOI] [PubMed] [Google Scholar]
- 16.Hove LH, Holme B, Young A, Tveit AB. The protective effect of TiF4, SnF2 and NaF against erosion-like lesions in situ. Caries Res. 2008; 42: 68–72. doi: 10.1159/000112816. [DOI] [PubMed] [Google Scholar]
- 17.Dayem RN, Tameesh MA. A new concept in hybridization: Bromelain enzyme for deproteinizing dentin before application of adhesive system. Contemp Clin Dent. 2013; 4: 421–426. doi: 10.4103/0976-237X.123015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavan R, Jain S, Shraddha Kumar A. Properties and therapeutic application of bromelain: a review. Biotechnol Res Int. 2012; 2012: 976203. doi: 10.1155/2012/976203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muhammad ZA, Ahmad T. Therapeutic uses of pineapple-extracted bromelain in surgical care - A review. J Pak Med Assoc. 2017; 67: 121–125. [PubMed] [Google Scholar]
- 20.Pithon MM, Campos MS, Coqueiro Rda S. Effect of bromelain and papain gel on enamel deproteinisation before orthodontic bracketbonding. Aust Orthod J. 2016; 32: 23–30. [PubMed] [Google Scholar]
- 21.Cho SY, Kang HY, Kim KA, Yu MK, Lee KW. Effect of adhesive hydrophobicity on microtensile bond strength of low-shrinkage silorane resin to dentin. J Korean Acad Conserv Dent. 2011; 36:280–289. [Google Scholar]
- 22.Mathai V, Angelo MC, Jayakumar K. Effect of sodium hypochlorite on shear bond strength using three different adhesive systems: an in-vitro study. Int J Bioassays. 2013; 2: 637–640. [Google Scholar]
- 23.Slutzky-Goldberg I, Maree M, Liberman R, Heling I. Effect of sodium hypochlorite on dentin microhardness. J Endod. 2004; 30: 880–882. doi: 10.1097/01.don.0000128748.05148.1e. [DOI] [PubMed] [Google Scholar]
- 24.Sim TP, Knowles JC, Ng YL, Shelton J, Gulabivala K. Effect of sodium hypochlorite on mechanical properties of dentine and tooth surface strain. Int Endod J. 2001; 34: 120–132. doi: 10.1046/j.1365-2591.2001.00357.x. [DOI] [PubMed] [Google Scholar]
- 25.Wiegand A, Magalhães AC, Sener B, Waldheim E, Attin T. TiF(4) and NaF at pH 1.2 but not at pH 3.5 are able to reduce dentin erosion. Arch Oral Biol. 2009; 54: 790–795. doi: 10.1016/j.archoralbio.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Comar LP, Wiegand A, Moron BM, Rios D, Buzalaf MA, Buchalla W, et al. In situ effect of sodium fluoride or titanium tetrafluoride varnish and solution on cariou-sdemineralization of enamel. Eur J Oral Sci. 2012; 120: 342–348. doi: 10.1111/j.1600-0722.2012.00968.x. [DOI] [PubMed] [Google Scholar]
- 27.Manimaran VS, Srinivasulu S, Rajesh Ebenezar A, Mahalaxmi S, Srinivasan N. Application of a proanthocyanidin agent to improve the bond strength of root dentin treated with sodium hypochlorite. J Conserv Dent. 2011; 14: 306–308. doi: 10.4103/0972-0707.85822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chaharom MEE, Kahnamoii MA, Kimyai S, Moghaddam MH. Effect of sodium hypochlorite on the shear bond strength of fifth- and seventh-generation adhesives to coronal dentin. African Journal of Biotechnology. 2011; 10:342–348. [Google Scholar]
- 29.Sen BH, Büyükyilmaz T. The effect of 4% titanium tetrafluoride solution on root canal walls--a preliminary investigation. J Endod. 1998; 24: 239–243. doi: 10.1016/S0099-2399(98)80104-0. [DOI] [PubMed] [Google Scholar]
- 30.Schlueter N, Ganss C, Mueller U, Klimek J. Effect of titanium tetrafluoride and sodium fluoride on erosionprogression in enamel and dentine in vitro. Caries Res. 2007; 41: 141–145. doi: 10.1159/000098048. [DOI] [PubMed] [Google Scholar]
- 31.Cecchin D, Farina AP, Galafassi D, Barbizam JV, Corona SA, Carlini-Júnior B. Influence of sodium hypochlorite and edta on the microtensile bond strength of a self-etching adhesive system. J Appl Oral Sci. 2010; 18: 385–389. doi: 10.1590/S1678-77572010000400011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mundorff SA, Little MF, Bibby BG. Enamel dissolution: II. Action of Titanium tetrafluoride. J Dent Res. 1972; 51: 1567–1571. doi: 10.1177/00220345720510061001. [DOI] [PubMed] [Google Scholar]