Abstract
Background
Crowdsourcing may be an effective strategy to develop test promotion materials. We conducted an online randomized controlled trial (RCT) to evaluate a crowdsourced intervention to promote hepatitis B virus (HBV) and hepatitis C virus (HCV) testing among men who have sex with men (MSM) in China.
Methods
MSM never previously tested for hepatitis were recruited through social media. Eligible men were randomized to receive an online crowdsourced intervention or no testing promotion materials. Outcomes including self-reported and confirmed HBV and HCV test uptake were assessed after four weeks. Odds ratios (OR) with 95% confidence intervals (95% CI) of men achieving primary and secondary outcomes between the intervention and control arms were calculated.
Findings
556 eligible men were enrolled. Overall, 17•4% (97/556) of men self-reported HBV and HCV testing and 7•9% (44/556) confirmed HBV and HCV test uptake. The intervention was seen by 72•1% and 29•0% of men in the intervention and control arms, respectively. In intention-to-treat analysis, confirmed HBV and HCV test uptake was similar between the two arms, both when using a missing=failure approach (OR 0•98, 95% CI 0•53–1•82) or multiple imputation (OR 1•46, 95% CI 0•72–2•95).
Interpretation
This RCT extends the literature by developing and evaluating an intervention to spur hepatitis testing in a middle-income country with a high burden of hepatitis. Overall test uptake among MSM in China was similar to previous interventions promoting hepatitis testing in high-income countries. We found frequent intervention sharing, complicating interpretation of the results, and the role of crowdsourcing to promote hepatitis testing remains unclear.
Research in context.
Evidence before this study
Crowdsourcing may be an effective strategy to develop test promotion materials. Public challenge contests have been used to generate messages to promote HIV testing, and previous randomized controlled trials (RCTs) have found that these messages effectively increase HIV test uptake among men who have sex with men (MSM).
Added value of this study
Few studies have evaluated interventions to increase hepatitis testing in low- and middle-income countries (LMICs), and none have targeted MSM or used a crowdsourcing approach. This RCT addresses an important gap by developing and evaluating a crowdsourced intervention to spur hepatitis testing among MSM in a LMIC with a high burden of hepatitis.
Implications of all the available evidence
With nearly 20% of all enrolled men reporting first-time hepatitis test uptake, results from our RCT suggest online strategies that utilize social media and mobile applications may promote hepatitis testing among MSM in LMICs. However, high levels of intervention sharing complicate the interpretation of our results. Further research is needed to optimize crowdsourcing as a community-based testing promotion intervention. Future online evaluations of educational interventions should be designed to better capture and account for intervention sharing.
CRediT authorship contribution statement
Thomas Fitzpatrick: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Weiming Tang: Conceptualization, Investigation, Project administration, Data curation, Formal analysis. Katie Mollan: Methodology, Data curation, Formal analysis. Xin Pan: Investigation, Project administration. Po-Lin Chan: Conceptualization. Kali Zhou: Methodology. Yu Cheng: Conceptualization, Methodology, Supervision. Linghua Li: Investigation, Project administration, Supervision. William CW Wong: Conceptualization. Joseph D. Tucker: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing.
Alt-text: Unlabelled box
1. Introduction
Viral hepatitis is a leading cause of death worldwide with 1•45 million deaths annually, comparable to HIV and tuberculosis [1]. An estimated 257 million and 71 million people are chronically infected with hepatitis B virus (HBV) and hepatitis C virus (HCV), respectively, with most of those infected living in low- and middle-income countries (LMICs) [2]. Over 80% of all people living with chronic viral hepatitis are unaware they are infected and thus unable to benefit from highly effective oral antiviral therapies [2]. Expanding hepatitis testing, particularly to at-risk populations, is essential to achieve World Health Organization (WHO) goals of hepatitis elimination by 2030. The WHO recommends all persons at increased risk of infection receive HBV surface antigen and HCV antibody testing [3]. This recommendation includes men who have sex with men (MSM), a group with higher prevalence of HBV and HCV compared to the general population in both high-income countries and LMICs [4], [5], [6], [7], [8].
The burden of viral hepatitis in China is particularly high. One-third of all people living with HBV and nearly 10% of all people living with HCV are in China [9]. Similar to many LMICs, China has low rates of HBV and HCV testing, including among MSM. A recent nationwide survey found that only 41% of MSM had been tested for HCV, and 38% of MSM without HBV vaccination had been tested for HBV [10]. Interventions to increase HBV and HCV testing among at-risk groups, including MSM in China, are urgently needed to diagnose infected individuals and link them to treatment.
Crowdsourcing may be an effective strategy to develop hepatitis test promotion materials [11]. Public challenge contests solicit slogans, images, or strategies from the public (including, but not limited to, at-risk groups) to address a particular problem [12]. By actively engaging affected communities, crowdsourcing contests may generate more culturally appropriate and locally relevant materials than traditional social health marketing approaches [13]. Public challenge contests have been used to generate test promotion messaging in both high-income countries and LMICs, including messaging tailored to mainland China [14]. Test promotion materials developed through crowdsourcing contests in China have been shown to effectively promote HIV testing among Chinese MSM [15,16], and this strategy could also be used to generate hepatitis testing promotion materials.
In 2017, 13 public health organizations across China launched a contest to solicit images and videos to spur HBV and HCV testing [17]. We conducted a randomized controlled trial (RCT) to evaluate the impact of a crowdsourced intervention developed through the contest on first-time HBV and HCV test uptake among MSM in China.
2. Methods
A detailed description of trial methods and study design has been published [17]. This RCT was registered at ClinicalTrial.gov (NCT03482388).
2.1. Trial design
A recruitment announcement was promoted through social media accounts operated by a large gay dating application (Blued) and several community-based organizations (CBOs) that serve MSM in China (Danlan Gongyi, SESH, Qingtong, Jinan Caihong, Yantai Caihong, Jining Caihong). The recruitment announcement linked men to an online survey that collected information on sociodemographic and other baseline characteristics. After completing the baseline survey, men were asked to add the study's official WeChat profile. WeChat is social media application used to share images and videos, send private messages, and conduct electronic money transfers. With over 900 million daily active users, WeChat is the most widely-used mobile phone application in China [18]. Men were considered enrolled once their WeChat profile had been added and linked to a baseline survey using a unique mobile phone number and WeChat account.
Once enrolled, men were sent a WeChat message informing them that HBV surface antigen (HBsAg) and HCV antibody (anti-HCV IgG) testing costs would be reimbursed if they submitted a photo of an HBV and HCV test report to researchers through WeChat within four weeks. Participants were then randomly assigned to receive either a crowdsourced intervention to promote HBV and HCV testing or to receive no testing promotion material. After three weeks, an online follow-up survey was sent to all men through WeChat to assess primary and secondary outcomes. Men who self-reported not receiving HBV and/or HCV testing were asked to select reasons for not testing from a preformed list. Men were given one week to complete the follow-up survey.
2.2. Participants
Participants had to report being born biologically male, age 16 years or older, residence in mainland China, and having had previous anal sex with another man to be eligible for inclusion. Exclusion criteria included self-reported previous HBV vaccination, HBV testing, or HCV testing. All men agreed to an online informed consent before entering the baseline survey.
2.3. Intervention
A crowdsourced intervention was delivered to men in the intervention arm through WeChat. The intervention involved two components: (1) a multimedia component delivered two images and two videos promoting HBV and HCV testing; (2) a participatory component invited men to compose and submit suggestions for how to better tailor the two images and two videos to MSM in China. There were no restrictions on participants forwarding the images or videos to others.
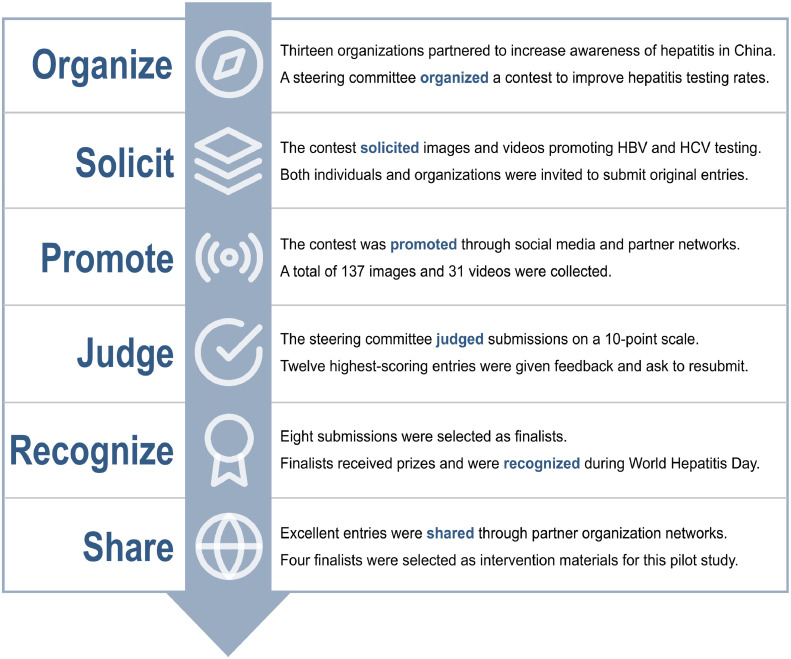
The images and videos used in the multimedia component were developed using a crowdsourcing approach. Thirteen organizations, including universities, government departments, and CBOs, launched a public challenge contest to spur HBV and HCV testing in China in 2017. An open call for submissions was distributed through social media and partner organization networks. Individuals and organizations were invited to submit original images or one-minute videos promoting HBV and HCV testing. A total of 168 submissions were collected between February and May 2017. Hepatitis experts and community members scored each entry for capacity to promote hepatitis testing, creativity, and potential to be shared widely on social media. The 12 highest scoring entries were selected as semifinalists and provided with feedback for improvement. After resubmission, eight finalists were awarded with official commendations and prizes. To select the finalists most appropriate for MSM in China, 60 MSM were recruited through local CBO social media accounts and asked to score each finalist image or video. The two highest scoring images and videos were selected as intervention materials. The crowdsourcing approach used to develop the intervention images and videos is illustrated in Fig. 1.
Fig. 1.
Development of images and videos to promote HBV and HCV testing through a public challenge contest. Contest implementation is presented in six stages: (1) organizing a steering committee, (2) soliciting entries, (3) promoting the contest, (4) judging entries, (5) recognizing excellent entries, and (6) sharing entries. The six stages of contest development are adapted from Wisdom of the Crowds: Methods for Organizing Crowdsourcing Challenge Contests for Health.
One image or video was sent to men in the intervention arm through WeChat every other day after enrollment. Each image or video had an associated invitation to submit suggestions on how each image or video could be improved to more effectively spur HBV and HCV testing among MSM in China. Suggestions were assessed for eligibility by two individuals and then evaluated by a four-member judging panel. Suggestions needed to be at least 50 characters and provide an actionable recommendation to be eligible for scoring. The judging panel included one physician, one gay man, one person living with chronic viral hepatitis, and one social media expert. Individuals who submitted the eight highest scoring suggestions were awarded prizes.
2.4. Outcomes
The primary outcome was first-time HBsAg and anti-HCV IgG test uptake confirmed through a submitted photo of test results at four weeks post-enrollment. The gender and age reported on the baseline survey and submitted photo of test results had to match for men to achieve the primary outcome. Confirmed HBsAg and anti-HCV IgG test uptake as independent component endpoints were secondary outcomes. Additional secondary outcomes included self-reported HBsAg test uptake, anti-HCV IgG test uptake, HIV test uptake, chlamydia test uptake, gonorrhea test uptake, syphilis test uptake, and HBV vaccination uptake. All self-reported outcomes were assessed at four weeks post-enrollment through a follow-up survey. Definitions of primary and secondary outcomes and methods of outcome assessment are detailed in Supplementary Table 1.
2.5. Sample size
In a pilot study conducted in November 2017, 8•8% (3/34) of men in the intervention arm received confirmed HBV and HCV testing while 0•0% (0/31) of men in the control arm received confirmed HBV and HCV testing [17]. A total sample of 674 men would be needed to achieve 80% power for an exact test if testing rates in the intervention and control arms were 8•5% and 3•0%, respectively. The final sample size was rounded to 700 to increase power.
2.6. Randomization
SAS software University Edition (Cary, North Carolina, USA) was used to generate a random allocation sequence that assigned participants to the intervention or control arms in a 1:1 ratio using permuted blocks. The allocation sequence was applied to participants in the order in which they were enrolled. The study's primary investigator (TF) generated the random allocation sequence that assigned participants to the intervention or control arms, and a study research assistant (PX) enrolled participants. Because WeChat was used to distribute intervention materials, investigators were aware of randomization assignment. Participants could not be blinded to randomization assignment because they were aware of whether they received the intervention materials.
2.7. Statistical methods
Descriptive statistics were used to summarize baseline characteristics and outcomes of men in the intervention and control arms. Logistic regression was performed to estimate odds ratios (OR) with 95% confidence intervals (95% CI) for the proportion of men achieving primary and secondary outcomes between the intervention and control arms (intention-to-treat analysis). Missing outcome data were first accounted for using a missing=failure approach in which all participants lost to follow-up were included and assumed to have not achieved the primary and secondary outcomes. Multiple imputation was also then performed using the fully conditional specification method in SAS software University Edition. Twenty imputed datasets were generated using a logit model that included baseline variables (age, current residence, household registration, education level, occupation, monthly income), intervention assignment, and outcome variables.
An as-exposed analysis was also performed in which participants were assigned to fully exposed, partially exposed, and not exposed groups based on whether they reported seeing all four intervention materials, one to three intervention materials, or no intervention materials during the four-week study period, respectively. Men lost to follow-up were assumed to have not been exposed to intervention materials and were assigned to the not exposed group. Adjusted ORs with 95% CIs of the proportion of men achieving primary and secondary outcomes were calculated comparing the fully exposed and not exposed groups as well as partially exposed and not exposed groups. Potential baseline confounders pre-specified prior to analyses (age, current residence, household registration, level of education, occupation, and income) were adjusted for in multivariable logistic regression. Again, both a missing=failure approach and multiple imputation were used to account for outcomes among men lost to follow-up.
2.8. Role of the funding source
Funders played no role in study design or in the collection, analysis, or interpretation of data.
3. Results
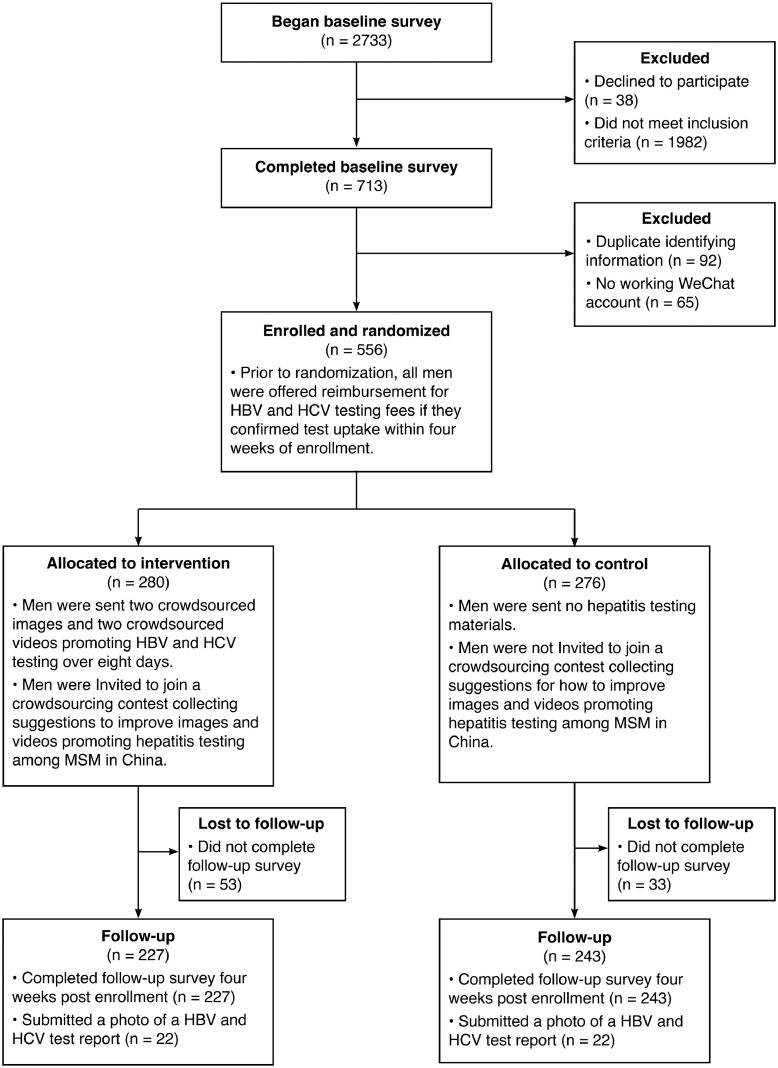
Participants were recruited over seven days from 09 May 2018 to 15 May 2018. The baseline survey was begun 2733 times, with 713 completed surveys meeting eligibility criteria. After excluding 157 surveys that could not be linked to a working WeChat account or reported duplicate identifying information, 556 eligible men were enrolled. Based on the random allocation sequence, 280 men were assigned to the intervention arm and 276 men were assigned to the control arm.
After four weeks, 470 men (84•5%) completed the follow-up survey. In the intervention and control arms 53 (18•9%) and 33 (12•0%) men were lost to follow-up, respectively. Besides randomization allocation, men with follow-up and lost to follow-up also differed significantly in terms of mean age (25•3 and 27•0 years old, respectively), proportion who were students (34•5% and 20•9%, respectively), and proportion who self-identified as male (92•6% and 84•9%, respectively). A total of 172 eligible suggestions were entered into the intervention's participatory component, with 36•1% (101/280) and 0•0% (2/276) of men in the intervention and control arms submitting at least one suggestion, respectively. Study recruitment, enrollment, and follow-up are outlined in Fig. 2.
Fig. 2.
Flow diagram of enrollment, randomization, and follow-up for a nationwide online randomized controlled trial to evaluate the impact of a crowdsourced intervention on hepatitis test uptake among men who have sex with men in China, 2018.
Mean age of enrolled participants was 25•6 years. The majority of men lived in an urban area (83•1%) and self-identified as gay (79•1%). At least one survey response was collected from each of mainland China's 31 provinces and administrative regions. Guangdong and Shandong Provinces were most represented, with 18•5% and 12•6% of men living in each province, respectively. Most men reported using a condom during their last anal sex encounter (70•7%) and had received HIV testing (68•9%). Men living with HIV accounted for 7•6% of all enrolled participants (Table 1).
Table 1.
Baseline sociodemographic characteristics, risk factors for hepatitis infection, and STI testing behaviors among 556 men enrolled in a nationwide online randomized controlled trial to evaluate the impact of a crowdsourced intervention on hepatitis test uptake among men who have sex with men in China, 2018.
| Total (n = 556) | Intervention (n = 280) | Control (n = 276) | ||||
| No. / Mean | % / SD | No. / Mean | % / SD | No. / Mean | % / SD | |
| Sociodemographic characteristics | ||||||
| Age | ||||||
| Years | 25•6 | 7•0 | 25•2 | 7•0 | 25•9 | 7•0 |
| Province | ||||||
| Guangdong | 103 | 18•5% | 51 | 18•2% | 52 | 18•8% |
| Shandong | 70 | 12•6% | 32 | 11•4% | 38 | 13•8% |
| Liaoning | 46 | 8•3% | 24 | 8•6% | 22 | 8•0% |
| Beijing | 41 | 7•4% | 19 | 6•8% | 22 | 8•0% |
| Other | 296 | 53•2% | 154 | 55•0% | 142 | 51•4% |
| Current Residence | ||||||
| Urban | 462 | 83•1% | 232 | 82•9% | 230 | 83•3% |
| Rural | 94 | 16•9% | 48 | 17•1% | 46 | 16•7% |
| Household Registration | ||||||
| Urban | 281 | 50•5% | 137 | 48•9% | 144 | 52•2% |
| Rural | 275 | 49•5% | 143 | 51•1% | 132 | 47•8% |
| Education Level | ||||||
| High school or below | 128 | 23•0% | 71 | 25•4% | 57 | 20•7% |
| Technical school | 181 | 32•6% | 91 | 32•5% | 90 | 32•6% |
| College | 219 | 39•4% | 107 | 38•2% | 112 | 40•6% |
| Advanced degree | 28 | 5•0% | 11 | 3•9% | 17 | 6•2% |
| Occupation | ||||||
| Student | 180 | 32•4% | 89 | 31•8% | 91 | 33•0% |
| Office worker / white collar | 123 | 22•1% | 56 | 20•0% | 67 | 24•3% |
| Service / retail | 106 | 19•1% | 56 | 20•0% | 50 | 18•1% |
| Other | 147 | 26•4% | 79 | 28•2% | 68 | 24•6% |
| Monthly Income (USD) | ||||||
| <220 | 149 | 26•8% | 70 | 25•0% | 79 | 28•6% |
| 220–439 | 131 | 23•6% | 64 | 22•9% | 67 | 24•3% |
| 440–732 | 168 | 30•2% | 89 | 31•8% | 79 | 28•6% |
| 733–1171 | 78 | 14•0% | 44 | 15•7% | 34 | 12•3% |
| >1172 | 30 | 5•4% | 13 | 4•6% | 17 | 6•2% |
| Marital status | ||||||
| Unmarried | 485 | 87•2% | 243 | 86•8% | 242 | 87•7% |
| Engaged / married | 52 | 9•4% | 27 | 9•6% | 25 | 9•1% |
| Separated / divorced | 19 | 3•4% | 10 | 3•6% | 9 | 3•3% |
| Sexual Orientation | ||||||
| Gay | 440 | 79•1% | 216 | 77•1% | 224 | 81•2% |
| Bisexual | 98 | 17•6% | 55 | 19•6% | 43 | 15•6% |
| Heterosexual | 4 | 0•7% | 2 | 0•7% | 2 | 0•7% |
| Unsure / other | 14 | 2•5% | 7 | 2•5% | 7 | 2•5% |
| Risk factors for hepatitis infection | ||||||
| HIV status | ||||||
| Positive | 42 | 7•6% | 23 | 8•2% | 19 | 6•9% |
| Negative or unknown | 514 | 92•4% | 257 | 91•8% | 257 | 93•1% |
| Number male partners | ||||||
| Past 12 months | 4•1 | 14•9 | 4•0 | 9•3 | 4•2 | 19•0 |
| Anal sex position | ||||||
| Insertive | 202 | 36•3% | 101 | 36•1% | 101 | 36•6% |
| Versatile | 106 | 19•1% | 51 | 18•2% | 55 | 19•9% |
| Receptive | 248 | 44•6% | 128 | 45•7% | 120 | 43•5% |
| Condom use during last anal sex | ||||||
| Yes | 393 | 70•7% | 198 | 70•7% | 195 | 70•7% |
| No | 163 | 29•3% | 82 | 29•3% | 81 | 29•3% |
| Previous injection drug use | ||||||
| Yes | 15 | 2•7% | 11 | 3•9% | 4 | 1•4% |
| No | 541 | 97•3% | 269 | 96•1% | 272 | 98•6% |
| Heard of HBV previously | ||||||
| Yes | 480 | 86•3% | 236 | 84•3% | 244 | 88•4% |
| No | 76 | 13•7% | 44 | 15•7% | 32 | 11•6% |
| Heard of HCV previously | ||||||
| Yes | 351 | 63•1% | 179 | 63•9% | 172 | 62•3% |
| No | 205 | 36•9% | 101 | 36•1% | 104 | 37•7% |
| Previous STI testing | ||||||
| Previous HIV testing | ||||||
| Yes | 383 | 68•9% | 184 | 65•7% | 199 | 72•1% |
| No | 173 | 31•1% | 96 | 34•3% | 77 | 27•9% |
| Previous syphilis testing | ||||||
| Yes | 205 | 36•9% | 104 | 37•1% | 101 | 36•6% |
| No | 351 | 63•1% | 176 | 62•9% | 175 | 63•4% |
| Previous chlamydia testing | ||||||
| Yes | 47 | 8•5% | 24 | 8•6% | 23 | 8•3% |
| No | 509 | 91•5% | 256 | 91•4% | 253 | 91•7% |
| Previous gonorrhea testing | ||||||
| Yes | 76 | 13•7% | 36 | 12•9% | 40 | 14•5% |
| No | 480 | 86•3% | 244 | 87•1% | 236 | 85•5% |
3.1. Intervention exposure and sharing
Among the 280 men assigned to the intervention arm, 72•1% (202/280) saw at least one intervention image or video and 35•7% (100/280) saw all four intervention materials during the study period. 52•9% (148/280) of men in the intervention arm shared intervention materials with others. In the control arm, 29•0% (80/276) and 6•9% (19/276) of men saw at least one intervention image or video and all four intervention materials, respectively, and 22•1% (61/276) shared intervention materials with others. Intervention exposure and sharing behaviors are summarized in Supplementary Table 2.
3.2. Self-reported and confirmed test uptake
Overall, 17•4% (97/556) of men self-reported receiving first-time HBV and HCV testing. Among the 346 HBV non-testers, the most common reasons for not testing were not having enough time to test (56•1%, 194/346), not feeling at risk of HBV infection (46•8%, 162/346), and not knowing where to get tested (27•8%, 96/346). Reasons for not receiving HCV testing were similar. Among the 367 HCV non-testers, 53•1% (195/367) reported not having enough time to test, 43•1% (158/367) did not feel at risk of HCV infection, and 27•8% (102/367) did not know where to receive testing.
Among enrolled men, 7•9% (44/556) confirmed first-time HBV and HCV test uptake. In total, 53 and 57 photos of HBV and HCV test reports were submitted during the study period, respectively. 83•6% (92/110) of submitted test reports were accepted as confirmation of test uptake, with 18 photos deemed ineligible because age or sex on the test report did not correspond with age or sex reported on the baseline survey. All 44 men with confirmed HBV and HCV test uptake also self-reported HBV and HCV test uptake on the follow-up survey.
3.3. Intention-to-treat analysis
Among men randomly assigned to the intervention and control arms, 7•9% (22/280) and 8•0% (22/276) confirmed HBV and HCV test uptake, respectively. The estimated odds of confirmed HBV and HCV test uptake were similar between the intervention and control arms using a missing=failure approach (OR 0•98, 95% CI 0•53–1•82) and were further from the null with multiple imputation (OR 1•46, 95% CI 0•72–2•95). In the intervention and control arms 16•1% (45/280) and 18•8% (52/276) of men self-reported HBV and HCV test uptake, respectively. In intention to treat analysis, the intervention did not have improved secondary outcomes compared to control, including self-reported HBV and HCV test uptake (Table 2).
Table 2.
Intention to treat analysis of primary and secondary outcomes for a nationwide online randomized controlled trial to evaluate the impact of a crowdsourced intervention on hepatitis test uptake among men who have sex with men in China, 2018.
| Total | Intervention | Control | ||||||||
| (n = 556) | (n = 280) | (n = 276) | ||||||||
| No. | % | No. | % | No. | % | OR1 | 95% CI | OR2 | 95% CI | |
| Primary Outcome | ||||||||||
| Confirmed HBV and HCV test uptake | 44 | 7•9% | 22 | 7•9% | 22 | 8•0% | 0•98 | 0•53–1•82 | 1•46 | 0•72–2•95 |
| Secondary Outcomes | ||||||||||
| Self-reported HBV and HCV test uptake | 97 | 17•4% | 45 | 16•1% | 52 | 18•8% | 0•82 | 0•53–1•28 | 0•94 | 0•57–1•55 |
| Confirmed HBV test uptake | 48 | 8•6% | 23 | 8•2% | 25 | 9•1% | 0•90 | 0•50–1•62 | 1•29 | 0•66–2•51 |
| Confirmed HCV test uptake | 44 | 7•9% | 22 | 7•9% | 22 | 8•0% | 0•98 | 0•53–1•82 | 1•46 | 0•72–2•95 |
| Self-reported HBV test uptake | 124 | 22•3% | 59 | 21•1% | 65 | 23•6% | 0•87 | 0•58–1•29 | 0•94 | 0•60–1•49 |
| Self-reported HCV test uptake | 103 | 18•5% | 48 | 17•1% | 55 | 19•9% | 0•83 | 0•54–1•28 | 0•96 | 0•59–1•58 |
| HBV vaccination uptake | 39 | 7•0% | 18 | 6•4% | 21 | 7•6% | 0•83 | 0•43–1•60 | 0•94 | 0•43–2•06 |
| HIV test uptake | 217 | 39•0% | 114 | 40•7% | 103 | 37•3% | 1•15 | 0•82–1•62 | 1•41 | 0•95–2•08 |
| Chlamydia test uptake | 27 | 4•9% | 14 | 5•0% | 13 | 4•7% | 1•06 | 0•49–2•31 | 1•36 | 0•62–2•96 |
| Gonorrhea test uptake | 35 | 6•3% | 17 | 6•1% | 18 | 6•5% | 0•93 | 0•47–1•84 | 1•06 | 0•51–2•20 |
| Syphilis test uptake | 116 | 20•9% | 62 | 22•1% | 54 | 19•6% | 1•17 | 0•78–1•76 | 1•44 | 0•90–2•30 |
| Visit with a physician after hepatitis test | 53 | 9•5% | 27 | 9•6% | 26 | 9•4% | 1•03 | 0•58–1•81 | 1•07 | 0•55–2•05 |
1 Odds ratios with 95% confidence intervals for intention to treat analysis using a missing=failure approach to account for men lost to follow-up.
2 Odds ratios with 95% confidence intervals for intention to treat analysis using multiple imputation to account for men lost to follow-up.
3.4. As-exposed analysis
21•4% (119/556), 29•3% (163/556), and 49•3% (274/556) of men saw all four intervention materials, one to three intervention materials, and no intervention materials in the four weeks after enrollment, respectively. As-exposed analysis found no meaningful difference in confirmed HBV and HCV test uptake comparing men who were fully exposed and partially exposed to men who were not exposed. Self-reported HBV and HCV test uptake was higher among fully exposed and partially exposed men compared to non-exposed men when using a missing=failure approach. However, these associations were not statistically significant when employing multiple imputation. Notably, of the six baseline variables that were pre-specified as potential confounders and adjusted for in multivariable logistic regression, four were not significantly associated with the primary outcome (age, current residence, occupation, and income), and two were significantly associated with the primary outcome (household registration, level of education). Results of as-exposed analyses are summarized in Table 3.
Table 3.
As-exposed analysis of primary and secondary outcomes for a nationwide online randomized controlled trial to evaluate the impact of a crowdsourced intervention on hepatitis test uptake among men who have sex with men in China, 2018.
| Not exposed | Partially exposed | Fully exposed | ||||||||
| (n = 274) | (n = 163) | (n = 119) | ||||||||
| No. | % | No. | % | No. | % | aOR1 | 95% CI | aOR2 | 95% CI | |
| Primary Outcome3 | ||||||||||
| Confirmed HBV and HCV test uptake | 22 | 8•0% | 9 | 5•5% | 13 | 10•9% | ||||
| Partially exposed | 0•70 | 0•30–1•61 | 0•45 | 0•19–1•07 | ||||||
| Fully exposed | 1•43 | 0•67–3•06 | 0•90 | 0•40–1•99 | ||||||
| Secondary Outcomes3 | ||||||||||
| Self-reported HBV and HCV test uptake | 30 | 10•9% | 34 | 20•9% | 33 | 27•7% | ||||
| Partially exposed | 2•36 | 1•34–4•14 | 1•27 | 0•72–2•25 | ||||||
| Fully exposed | 3•14 | 1•76–5•59 | 1•66 | 0•92–2•99 | ||||||
| Confirmed HBV test uptake | 25 | 9•1% | 9 | 5•5% | 14 | 11•8% | ||||
| Partially exposed | 0•59 | 0•26–1•33 | 0•39 | 0•17–0•90 | ||||||
| Fully exposed | 1•30 | 0•63–2•70 | 0•83 | 0•38–1•80 | ||||||
| Confirmed HCV test uptake | 22 | 8•0% | 9 | 5•5% | 13 | 10•9% | ||||
| Partially exposed | 0•70 | 0•30–1•61 | 0•45 | 0•19–1•07 | ||||||
| Fully exposed | 1•43 | 0•67–3•06 | 0•90 | 0•40–1•99 | ||||||
| Self-reported HBV test uptake | 40 | 14•6% | 44 | 27•0% | 40 | 33•6% | ||||
| Partially exposed | 2•21 | 1•33–3•67 | 1•24 | 0•74–2•07 | ||||||
| Fully exposed | 2•75 | 1•62–4•66 | 1•51 | 0•87–2•60 | ||||||
| Self-reported HCV test uptake | 32 | 11•7% | 37 | 22•7% | 34 | 28•6% | ||||
| Partially exposed | 2•48 | 1•43–4•30 | 1•34 | 0•76–2•37 | ||||||
| Fully exposed | 3•06 | 1•73–5•40 | 1•61 | 0•89–2•90 | ||||||
| HBV vaccination uptake | 5 | 1•8% | 16 | 9•8% | 18 | 15•1% | ||||
| Partially exposed | 8•00 | 2•59–24•75 | 1•87 | 0•64–5•46 | ||||||
| Fully exposed | 10•12 | 3•35–30•60 | 2•47 | 0•86–7•13 | ||||||
| HIV test uptake | 71 | 25•9% | 87 | 53•4% | 59 | 49•6% | ||||
| Partially exposed | 3•34 | 2•19–5•12 | 1•72 | 1•10–2•69 | ||||||
| Fully exposed | 2•72 | 1•71–4•32 | 1•40 | 0•85–2•29 | ||||||
| Chlamydia test uptake | 6 | 2•2% | 7 | 4•3% | 14 | 11•8% | ||||
| Partially exposed | 3•04 | 0•88–10•51 | 0•70 | 0•15–3•27 | ||||||
| Fully exposed | 7•53 | 2•46–23•04 | 2•03 | 0•61–6•77 | ||||||
| Gonorrhea test uptake | 5 | 1•8% | 15 | 9•2% | 15 | 12•6% | ||||
| Partially exposed | 7•01 | 2•28–21•59 | 2•13 | 0•72–6•28 | ||||||
| Fully exposed | 8•58 | 2•82–26•13 | 2•68 | 0•98–7•31 | ||||||
| Syphilis test uptake | 30 | 10•9% | 46 | 28•2% | 40 | 33•6% | ||||
| Partially exposed | 3•57 | 2•09–6•08 | 1•85 | 1•10–3•11 | ||||||
| Fully exposed | 4•04 | 2•31–7•06 | 2•07 | 1•19–3•59 | ||||||
| Visit with a physician after hepatitis test | 7 | 2•6% | 22 | 13•5% | 24 | 20•2% | ||||
| Partially exposed | 7•77 | 3•01–20•09 | 2•49 | 1•07–5•82 | ||||||
| Fully exposed | 10•18 | 4•01–25•83 | 3•25 | 1•43–7•42 | ||||||
1 Adjusted odds ratios with 95% confidence intervals for as-exposed analysis using a missing=failure approach to account for loss to follow-up. Multivariable logistic regression adjusts for age, current residence, household registration, level of education, occupation, and income.
2 Adjusted odds ratios with 95% confidence intervals for as-exposed analysis using multiple imputation to account for loss to follow-up. Multivariable logistic regression adjusts for age, current residence, household registration, level of education, occupation, and income.
3 Reference group is men not exposed to intervention materials during the four-week study period.
4. Discussion
In this nationwide online RCT the overall rate of self-reported hepatitis test uptake among MSM in China was similar to rates in previous interventions promoting hepatitis testing among at-risk populations in high-income countries. Nearly half of men who self-reported HBV and HCV test uptake also confirmed testing by submitting a photo of their test report through a mobile phone application. Men assigned to the intervention frequently shared crowdsourced test promotion materials with others, complicating interpretation of the results. This study extends the existing literature by applying crowdsourcing to the development of hepatitis test promotion materials and ascertaining hepatitis test uptake using a novel confirmatory method. In addition, nearly all previous trials to increase hepatitis testing have been conducted in high-income countries [19]. This RCT addresses an important gap by developing and evaluating an intervention to spur hepatitis testing in a LMIC with a high burden of hepatitis.
Among all 556 enrolled men, 22•3% (95% CI 18•9% to 26•0%) reported first-time HBV testing. This proportion of HBV test uptake is similar to, or higher than, that reported in other evaluations of community-based strategies to promote hepatitis testing. A recent meta-analysis pooled results from six RCTs investigating lay health worker educational interventions to encourage HBV testing and found an overall testing rate of 12•6%, with 19•0% and 6•5% of participants in the intervention and control arms reporting first-time HBV test uptake, respectively [19]. Notably, all six RCTs assessed HBV test uptake six months after enrollment, a substantially longer follow-up period than the four weeks allowed in our trial. 18•5% of all enrolled men in this RCT self-reported first-time HCV testing. Few community-based interventions to increase HCV testing have been evaluated, and none have reported first-time HCV test uptake as an outcome [19,20]. Non-controlled studies, however, have found comparable rates of HCV testing when using online platforms to promote testing among high-risk populations in high-income countries [21].
In this nationwide online trial 46% of men who self-reported first-time HBV and HCV testing confirmed test uptake by submitting an electronic photo of their test reports. Most community-based interventions to spur hepatitis testing have relied on self-reported outcomes [19]. However, the validity of self-reported hepatitis testing is not well established. Social desirability bias may motivate non-testers to inaccurately report testing behaviors, and the complexities of HBV and HCV testing items may leave study participants uncertain as to whether they have been appropriately screened for infection. Three previous RCTs investigating community-based educational interventions to promote HBV screening verified test uptake by first obtaining consent for medical records release and then contacting providers for testing records [22], [23], [24]. While rates of confirmed HBV test uptake were similar, with 46%–31% of self-reported testing being confirmed, these RCTs were limited in geographic scope, only enrolling participants in a single municipality [22], [23], [24]. In contrast, HBV and HCV test uptake was confirmed for men living in 16 of China's 31 provinces and administrative regions in this nationwide RCT. As increasing emphasis is placed on the importance of developing and evaluating regional and national strategies to increase hepatitis testing [3,25], utilizing mobile applications to confirm test uptake across large geographic regions may represent an important strategy to be employed in future implementation research.
There are several potential reasons why we found no significant difference in HBV and HCV test uptake between the intervention and control arms in this RCT. First, the crowdsourced intervention materials may not have effectively encouraged hepatitis testing. However, this explanation alone does not account for why nearly 20% of all participants, who had never received hepatitis testing previously, reported receiving first-time HBV and HCV testing within four weeks of enrollment. Alternatively, rates of intervention sharing were high, with more than half of men in the intervention arm having shared, and nearly 30% of men in the control arm having seen, the crowdsourced materials during the four-week study period. Sharing of educational interventions has reduced effect sizes and biased results toward the null in previous community-based trials [26,27]. While we attempted to adjust for sharing by performing an as-exposed analysis, reassigning men based on self-reported exposure to intervention materials does not account for the impact of other social influence processes that may have resulted from the intervention, including behavioral modeling and peer-to-peer persuasion [27]. Cluster randomization or more comprehensive measures of sharing behaviors may better adjust for sharing in future research of health promotion interventions, particularly those utilizing online social networks where messaging can be readily and widely disseminated.
Follow-up time allotted for testing may also have impacted outcome measures. More than half of non-testers reported they did not have enough time to receive hepatitis testing during the four-week study period. Test uptake was not assessed until three months post-enrollment in a recent cluster stepped-wedge RCT that found a crowdsourced intervention increased HIV testing among MSM in China [16]. Evaluations that have shown community-based educational interventions to successfully promote HBV and HCV testing among at-risk populations have assessed test uptake after six months or longer [24,[28], [29], [30], [31]]. The four-week follow-up period in this RCT may therefore have been too short to detect the effect of the intervention. Additionally, cost of testing has been identified as an important barrier to both HBV and HCV testing in LMICs [25,32], and economic incentives have been shown to increase test uptake for HIV and other sexually transmitted infections in several contexts [33]. Offering testing reimbursement to all enrolled men may have promoted testing across both arms of this trial, thus masking the effect of the crowdsourced intervention. Finally, slightly less than half of non-testers in both the intervention and control arms did not seek testing because they did not feel at risk of hepatitis infection. This suggests the intervention materials, which were designed for the general Chinese population, may not have been sufficiently tailored to the unique concerns of MSM to effectively spur test uptake.
While strengths of this RCT include an innovative community-based intervention, novel method of outcome ascertainment, and large geographic scope, several limitations deserve mention. More than 10% of completed baseline surveys were excluded due to duplicate identifying information (e.g., mobile number, WeChat account, IP address). As a result, our trial was smaller than anticipated, enrolling 556 rather than 700 eligible MSM, thus reducing power to detect a difference in outcomes between the intervention and control arms. To protect participant confidentiality, we did not require men to provide their name nor any official form of identification prior to enrollment. It is possible some men were enrolled more than once. Additionally, concealment or fabrication of demographic or health information to meet inclusion and exclusion criteria for study enrollment is an increasingly recognized challenge in clinical trials, particularly among heavily researched populations [34]. Online clinical trials, where researchers do not interact with potential enrollees in person, may be especially impacted by these concerns, and additional screening strategies may be required to exclude professional subjects who conceal or fabricate information [35].
The WHO has set the goal of eliminating hepatitis B and C by 2030, and scaling up testing among at-risk populations is a key strategy needed to achieve this ambitious target [2]. Although community testing programs have been identified as an important component of efforts to expand access to testing [25], few previous studies have examined interventions to increase hepatitis testing outside of clinics and healthcare facilities. This RCT addresses an important gap in the literature by investigating a community-based intervention to increase hepatitis testing in a LMIC with a high burden of hepatitis. With nearly 20% of all enrolled men reporting first-time HBV and HCV test uptake, results from our trial suggest online strategies that utilize social media and mobile applications can promote hepatitis testing among MSM in LMICs. However, high levels of intervention sharing complicate the interpretation of our results. Further research is needed to evaluate and optimize crowdsourcing as a community-based testing promotion intervention, and future online trials of educational interventions should be designed to better capture and account for intervention sharing.
Role of funding source
This work is supported in part by the Doris Duke Charitable Foundation through a grant supporting the Doris Duke International Clinical Research Fellows Program at University of North Carolina at Chapel Hill. TF is a Doris Duke International Research Fellow. This work is also supported by the National Institutes of Health (National Institute of Allergy and Infectious Diseases 1R01AI114310-01), UNC CFAR (P30 AI50410), and SESH Global (www.seshglobal.org). This work also receives administrative assistance from the Guangzhou Eighth People's Hospital, UNC Chapel Hill, and UNC – Project China. The listed funders played no role in the development or implementation of this study. TF had full access to all data in the study and had final responsibility for the decision to submit for publication.
Ethics committee approval
The study protocol was approved by IRB bodies at Sun Yat-sen University and University of North Carolina at Chapel Hill prior to launching (IRB Number: 18-0251). All participants completed and agreed to an online consent form prior to survey initiation.
Declaration of Competing Interest
JDT and WT are unpaid advisors at SESH Global LLC. The other authors declare that they have no competing interests.
Acknowledgments
Acknowledgments
Authors would like to acknowledge the following persons for their contributions to this research project and manuscript: Tianqi Zhang, Angel Xiaohan Ji, Yang Zhao, Jason Ong, Cai Weiping, and Wei Shufang.
Data sharing statement
Individual participant data that underlie the results reported in this article, after de-identification, will be available and shared. Study protocol, statistical analysis plan, and informed consent forms will also be available on request. Data will be available beginning nine months and ending 36 months following article publication. Researchers who provide a methodologically sound proposal can request study data and relevant documentation. Proposals should be directed to tsfitz@uw.edu to gain access, and data requestors will need to sign a data access agreement.
Footnotes
Funding: Doris Duke Charitable Foundation, National Institutes of Health
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2019.10.007.
Appendix. Supplementary materials
References
- 1.Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. Lancet. 2016;388(10049):1081–1088. doi: 10.1016/S0140-6736(16)30579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization; Geneva: 2017. Global hepatitis report 2017. [Google Scholar]
- 3.World Health Organization; Geneva: 2017. WHO guidelines on hepatitis B and C testing. [Google Scholar]
- 4.Chow EP, Tucker JD, Wong FY, Nehl EJ, Wang Y, Zhuang X. Disparities and risks of sexually transmissible infections among men who have sex with men in China: a meta-analysis and data synthesis. PloS one. 2014;9(2):e89959. doi: 10.1371/journal.pone.0089959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Houdt R, Bruisten SM, Speksnijder AG, Prins M. Unexpectedly high proportion of drug users and men having sex with men who develop chronic hepatitis B infection. J Hepatol. 2012;57(3):529–533. doi: 10.1016/j.jhep.2012.04.030. [DOI] [PubMed] [Google Scholar]
- 6.Alonso M, Gutzman A, Mazin R, Pinzon CE, Reveiz L, Ghidinelli M. Hepatitis C in key populations in Latin America and the Caribbean: systematic review and meta-analysis. Int J Public Health. 2015;60(7):789–798. doi: 10.1007/s00038-015-0708-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nadol P, O'Connor S, Duong H, Mixson-Hayden T, Tram TH, Xia GL. High hepatitis C virus (HCV) prevalence among men who have sex with men (MSM) in Vietnam and associated risk factors: 2010 Vietnam Integrated Behavioural and Biologic Cross-Sectional Survey. Sex Transm Infect. 2016 doi: 10.1136/sextrans-2015-052518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pitasi MA, Bingham TA, Sey EK, Smith AJ, Teshale EH. Hepatitis B virus (HBV) infection, immunity and susceptibility among men who have sex with men (MSM), Los Angeles County, USA. AIDS Behav. 2014;18(Suppl 3):248–255. doi: 10.1007/s10461-013-0670-2. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization: Regional Office for the Western Pacific; Manila: 2014. WHO technical consultation on a comprehensive National Hepatitis Programme in China with a focus on viral hepatitis B and C treatment. [Google Scholar]
- 10.Fitzpatrick T, Pan SW, Tang W, Guo W, Tucker JD. HBV and HCV test uptake and correlates among men who have sex with men in China: a nationwide cross-sectional online survey. Sex Transm Infect. 2018 doi: 10.1136/sextrans-2018-053549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization; Geneva: 2018. Crowdsourcing in health and health research: a practical guide. [Google Scholar]
- 12.Tucker JD, Fenton KA. Innovation challenge contests to enhance HIV responses. Lancet HIV. 2018;5(3):e113–e1e5. doi: 10.1016/S2352-3018(18)30027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang Y, Kim JA, Liu F, Tso LS, Tang W, Wei C. Creative contributory contests to spur innovation in sexual health: 2 cases and a Guide for Implementation. Sex Transm Dis. 2015;42(11):625–628. doi: 10.1097/OLQ.0000000000000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan SW, Stein G, Bayus B, Tang W, Mathews A, Wang C. Systematic review of innovation design contests for health: spurring innovation and mass engagement. BMJ Innovations. 2017 doi: 10.1136/bmjinnov-2017-000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang W, Han L, Best J, Zhang Y, Mollan K, Kim J. Crowdsourcing HIV test promotion videos: a noninferiority randomized controlled trial in China. Clin Infect Dis. 2016;62(11):1436–1442. doi: 10.1093/cid/ciw171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang W, Wei C, Cao B, Wu D, Li KT, Lu H. Crowdsourcing to expand HIV testing among men who have sex with men in China: A closed cohort stepped wedge cluster randomized controlled trial. PLoS Med. 2018;15(8) doi: 10.1371/journal.pmed.1002645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzpatrick T, Zhou K, Cheng Y, Chan PL, Cui F, Tang W. A crowdsourced intervention to promote hepatitis B and C testing among men who have sex with men in China: study protocol for a nationwide online randomized controlled trial. BMC Infect Dis. 2018;18(1):489. doi: 10.1186/s12879-018-3403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.2017 WeChat Data Report: WeChat lifestyle update for tencent global partners conference: Tencent Holdings Limited; 2017[Available from: http://blog.wechat.com/2017/11/09/the-2017-wechat-data-report/].
- 19.Zhou K, Fitzpatrick T, Walsh N, Kim JY, Chou R, Lackey M. Interventions to optimise the care continuum for chronic viral hepatitis: a systematic review and meta-analyses. Lancet Infect Dis. 2016;16(12):1409–1422. doi: 10.1016/S1473-3099(16)30208-0. [DOI] [PubMed] [Google Scholar]
- 20.Bajis S, Dore GJ, Hajarizadeh B, Cunningham EB, Maher L, Grebely J. Interventions to enhance testing, linkage to care and treatment uptake for hepatitis C virus infection among people who inject drugs: a systematic review. Int J Drug Policy. 2017;47:34–46. doi: 10.1016/j.drugpo.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Zuure FR, Davidovich U, Coutinho RA, Kok G, Hoebe CJ, van den Hoek A. Using mass media and the Internet as tools to diagnose hepatitis C infections in the general population. Am J Prev Med. 2011;40(3):345–352. doi: 10.1016/j.amepre.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Taylor VM, Hislop TG, Tu SP, Teh C, Acorda E, Yip MP. Evaluation of a hepatitis B lay health worker intervention for Chinese Americans and Canadians. J Community Health. 2009;34(3):165–172. doi: 10.1007/s10900-008-9138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor VM, Gregory Hislop T, Bajdik C, Teh C, Lam W, Acorda E. Hepatitis B ESL education for Asian immigrants. J Community Health. 2011;36(1):35–41. doi: 10.1007/s10900-010-9279-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor VM, Bastani R, Burke N, Talbot J, Sos C, Liu Q. Evaluation of a hepatitis B lay health worker intervention for Cambodian Americans. J Community Health. 2013;38(3):546–553. doi: 10.1007/s10900-012-9649-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Easterbrook P, Johnson C, Figueroa C, Baggaley R. HIV and hepatitis testing: global progress, challenges, and future directions. AIDS Rev. 2016;18(1):3–14. [PubMed] [Google Scholar]
- 26.Simmons N, Donnell D, Ou SS, Celentano DD, Aramrattana A, Davis-Vogel A. Assessment of contamination and misclassification biases in a randomized controlled trial of a social network peer education intervention to reduce HIV risk behaviors among drug users and risk partners in Philadelphia, PA and Chiang Mai, Thailand. AIDS Behav. 2015;19(10):1818–1827. doi: 10.1007/s10461-015-1073-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Latkin CA, Knowlton AR. Social network assessments and interventions for health behavior change: a critical review. Behav Med. 2015;41(3):90–97. doi: 10.1080/08964289.2015.1034645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bastani R, Glenn BA, Maxwell AE, Jo AM, Herrmann AK, Crespi CM. Cluster-randomized trial to increase hepatitis B testing among Koreans in Los Angeles. Cancer Epidemiol Biomarkers Prev. 2015;24(9):1341–1349. doi: 10.1158/1055-9965.EPI-14-1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen MS, Fang DM, Stewart SL, Ly MY, Lee S, Dang JH. Increasing hepatitis B screening for hmong adults: results from a randomized controlled community-based study. Cancer Epidemiol Biomarkers Prev. 2013;22(5):782–791. doi: 10.1158/1055-9965.EPI-12-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Juon HS, Lee S, Strong C, Rimal R, Kirk GD, Bowie J. Effect of a liver cancer education program on hepatitis B screening among Asian Americans in the Baltimore-Washington metropolitan area, 2009-2010. Prev Chronic Dis. 2014;11 doi: 10.5888/pcd11.130258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roux P, Rojas Castro D, Ndiaye K, Debrus M, Protopopescu C, Le Gall JM. Increased uptake of HCV testing through a community-based educational intervention in difficult-to-reach people who inject drugs: results from the ANRS-AERLI study. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0157062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ishizaki A, Bouscaillou J, Luhmann N, Liu S, Chua R, Walsh N. Survey of programmatic experiences and challenges in delivery of hepatitis B and C testing in low- and middle-income countries. BMC Infect Dis. 2017;17(Suppl 1):696. doi: 10.1186/s12879-017-2767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee R, Cui RR, Muessig KE, Thirumurthy H, Tucker JD. Incentivizing HIV/STI testing: a systematic review of the literature. AIDS Behav. 2014;18(5):905–912. doi: 10.1007/s10461-013-0588-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devine EG, Waters ME, Putnam M, Surprise C, O'Malley K, Richambault C. Concealment and fabrication by experienced research subjects. Clin Trials. 2013;10(6):935–948. doi: 10.1177/1740774513492917. [DOI] [PubMed] [Google Scholar]
- 35.Devine EG, Peebles KR, Martini V. Strategies to exclude subjects who conceal and fabricate information when enrolling in clinical trials. Contemp Clin Trials Commun. 2017;5:67–71. doi: 10.1016/j.conctc.2016.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.