Abstract
Objective
This observational study was designed to understand the usage pattern of ticagrelor in real-life clinical practice among a large number of acute coronary syndrome (ACS) patients undergoing percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG), or medical management (MM). The study also recorded clinical events, i.e., bleeding, dyspnea, and cardiovascular (CV) events, reported by the investigator during the follow-up period.
Methods
The ACS patients aged ≥18 years hospitalized for ACS and were prescribed ticagrelor upon discharge or ≤1 month and patients who underwent PCI, CABG, or MM for ACS were enrolled. The subjects were followed up for a period of up to 12 months. The data were collected on a case report form.
Result
The study recruited 2997 subjects from 49 sites in India. Approximately half of the ACS subjects had ST segment elevation myocardial infarction (48.9%), and PCI was used as management in 92.4% subjects. The mean (±SD) duration of use of ticagrelor was 314 (±110.2) days over a period of 12 months. Of 136 subjects (4.5%) who experienced any clinical events, CV deaths were reported in 20 (0.7%), myocardial infraction in 19 (0.6) subjects and ischemic stroke in 23 (0.8%) subjects, and severe dyspnea was reported in 68 (2.2%) subjects. Out of 33 bleeding cases, 25 (0.8%) subjects had thrombolysis in myocardial infarction (TIMI) minimal, seven (0.2%) had TIMI minor, and one TIMI major. Platelet inhibition and patient outcomes (PLATO) major was reported in two subjects and CABG bleed in one subject. The incidence of PLATO defined major and minimal bleeding were lower in subjects undergoing fibrinolysis than overall population.
Conclusion
Ticagrelor has been used across ACS types and in different management strategies in real world settings in India. The incidence of clinical events was lower as compared with data in literature. ClinicalTrials.gov Identifier: NCT02408224
Keywords: ACS, CAD, STEMI, Ticagrelor, Acute coronary syndrome, PCI
1. Introduction
Acute coronary syndrome (ACS), a complication of coronary artery disease (CAD), is one of the leading causes of mortality across the globe. ACS is a group of diseases comprising ST segment elevation myocardial infarction (STEMI), non-ST segment elevation myocardial infraction (NSTEMI), and unstable angina (UA).1 Together, CAD and ACS account for around seven million deaths every year worldwide.2, 3 In India, cardiac diseases affect approximately 45 million patients, of whom more than 30 million have CAD.4 In India, cardiovascular (CV) risk factors associated with ACS are on upsurge making it a major public health problem.5
The most commonly prescribed medications in ACS patients include aspirin (162–325 mg), morphine, nitroglycerine, β-blockers, angiotensin-converting enzyme inhibitors, P2Y12 inhibitors, and statins. The most commonly used thienopyridine in ACS patients is clopidogrel.6 However, the drug causes irreversible platelets inhibition and also has limitation that it requires hepatic conversion to an active metabolite and thus has delayed action. The drug also has interpatient variation in conversion rate because of pharmacogenomics differences. These factors necessitate the need for a new antiplatelet agent which does not have these imitations.
Ticagrelor, a reversible and direct-acting oral antagonist of cyclopentyl triazolopyrimidines class provides faster P2Y12 inhibition than available alternatives. Ticagrelor binds reversibly and noncompetitively to the P2Y12 receptor. Ticagrelor 90 mg bid has demonstrated good efficacy and an acceptable tolerability profile among patients with ACS in randomized controlled trials.7, 8
Ticagrelor has been used in India since 20129{, #99}{cdsco.nic.in/writereaddata/New%20Drug%20Update%202012.docs, #98}. However, retrospective analysis of available data has not been possible because of poor infrastructure and lack of electronic case recording formats in Indian hospitals. As such, this study was designed to understand the usage pattern of ticagrelor in real life clinical practice in a sample of 3000 ACS patients undergoing percutaneous coronary intervention (PCI), coronary artery bypass graft (CABG), or medical management (MM) in India.
1.1. Aims and objectives
The primary objective of the study was to understand the usage pattern of ticagrelor in ACS patients undergoing PCI, CABG, or MM in a clinical practice setting in India.
Other objectives included observing the usage of ticagrelor in patients preloaded with clopidogrel, recording clinical events (bleeding, dyspnea, and CV events) reported by the investigator during the follow-up period and observing the usage of ticagrelor in patients who received fibrinolytic therapy for current ACS event to record the various risk factors and reasons of discontinuation of ticagrelor therapy.
1.2. Patients and methods
This noninterventional, prospective, observational, and multicenter study enrolled ACS patients of either gender aged ≥18 years who were hospitalized for ACS and had been prescribed ticagrelor upon discharge or ≤1 month and patient underwent PCI, CABG, or MM for ACS. The subjects were assessed for the proportion of enrolled patients with different types of ACS, namely STEMI, NSTEMI, and UA. The diagnosis of STEMI was based on the criteria of chest discomfort, with ST-segment elevation, presumed left bundle branch block (LBBB), and elevated creatine kinase-muscle/brain (CK-MB) and troponin; NSTEMI is chest discomfort, lack of persistent ST elevation, lack of presumed LBBB and elevated cardiac enzymes, and UA are symptoms of angina at rest or on minimal exercise and transient ST-T changes.
Patients with medical history of intracranial hemorrhage, the presence of serious co-morbidities, who were not fit to receive ticagrelor, and the pregnant women were excluded from the study. Patients were followed up for a period of up to 12 months (according to the label). Because this was an observational study, the therapy was not prescribed for the purpose of inclusion of the patients in this study. The patients were included in the study who were being prescribed ticagrelor according to the prescribing information as part of routine patient care. The study objectives and methodology had been published earlier.10
The study was planned to enroll a total of 3000 ACS patients. The duration of participation was 12 months with visits at 1, 3, 6, and 12 months; if subjects were not able to come to the site, the telephonic visit was conducted. Once the subjects met eligibility criteria and signed the informed consent, they were enrolled in the study. The data were collected on a case report form. Subject data about demographic characteristics, disease characteristics, medical history, vital signs, physical examination, laboratory investigations, angiography findings, and management of ACS were recorded at baseline. Data regarding clinical events (bleeding, dyspnea, and CV events), ticagrelor discontinuation, and ongoing current treatment were captured at all follow-up visits.
The study was performed in accordance with ethical principles that were consistent with the Declaration of Helsinki, International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use—Good Clinical Practice and the applicable legislation on noninterventional studies after obtaining written informed consent from the subjects.
1.2.1. Statistical analysis
Statistical analysis wherever specified was performed using SAS, version 9.4, (SAS Institute, Cary, NC). This article summarizes the findings of an observational study and presents primary and secondary endpoint data from the analysis. Categorical variables were summarized with the frequency and percentage of patients in each category. Continuous variables were summarized descriptively with the number of patients, mean, standard deviation, minimum, median, and maximum values.
2. Results
2.1. Patient demographics and baseline characteristics
A total of 3001 subjects were enrolled in the study. Four subjects in the study were not on ticagrelor and were excluded from the analysis population. All the analysis for the study were performed on 2997 subjects. The subject disposition of 2997 subjects who fulfilled the eligibility criteria is presented in Table 1. Majority of (77%) subjects completed the study. The mean (SD) age of study population was 57.8 (10.1) years with a mean (SD) weight of 69.6 (11.3) kg. A clear predominance of males (80.1%) was documented in the study population (Table 2).
Table 1.
Subject disposition.
| Parameters | Ticagrelor (N = 2997) n (%) |
|---|---|
| Subjects completed the study | 2326 (77.6) |
| Subjects discontinued the study | 671 (22.4) |
| Reason for discontinuation | |
| Death | 31 (1.0) |
| Subject withdrew consent | 123 (4.1) |
| Subject lost to follow-up | 71 (2.4) |
| Significant safety concern (Illness or Surgery) | 3 (0.1) |
| Adverse event or abnormality | 38 (1.3) |
| Protocol deviations/violations | 4 (0.1) |
| Other | 358 (11.9) |
| Missing | 43 (1.4) |
Percentages are based on the number of subjects enrolled.
N = Total number of subjects enrolled; n = number of subjects in the specific category.
Table 2.
Details of demographics, intervention used, risk factors, and their association with ACS.
| Parameters |
Patients (N = 2997) |
| Age in years Mean (SD) | 57.8 (10.9) |
| Gender | n (%) |
| Male | 2402 (80.1) |
| Female | 595 (19.9) |
| Weight in kg* | |
| Mean (SD) | 69.6 (11.3) |
| Type of ACS | n (%) |
| STEMI | 1466 (48.9) |
| NSTEMI | 689 (23.0) |
| UA | 842 (28.1) |
| Intervention used for patient | n (%) |
| PCI | 2769 (92.4%) |
| CABG | 102 (3.4%) |
| MM |
126 (4.2%) |
| Risk Factors and their association with ACS | |
| Age categories (18–60 years) | n (%) |
| STEMI | 946 (64.5) |
| NSTEMI | 396 (57.5) |
| UA | 450 (53.4) |
| Age categories (>60 years) | n (%) |
| STEMI | 520 (35.5) |
| NSTEMI | 293 (42.5) |
| UA | 392 (46.6) |
| History of diabetes | n (%) |
| STEMI | 641 (43.7) |
| NSTEMI | 325 (47.2) |
| UA | 388 (46.1) |
| History of no diabetes | n (%) |
| STEMI | 825 (56.3) |
| NSTEMI | 364 (52.8) |
| UA | 454 (53.9) |
| History of renal impairment | n (%) |
| STEMI | 20 (1.4) |
| NSTEMI | 11 (1.6) |
| UA | 5 (0.6) |
| History of no renal impairment | n (%) |
| STEMI | 1446 (98.6) |
| NSTEMI | 678 (98.4) |
| UA | 837 (99.4) |
| History of smoking/nicotine uptake | n (%) |
| STEMI | 209 (14.3) |
| NSTEMI | 98 (14.2) |
| UA | 63 (7.5) |
| History of no smoking/nicotine uptake | n (%) |
| STEMI | 1257 (85.7) |
| NSTEMI | 591 (85.8) |
| UA | 779 (92.5) |
Percentages are based on the total number of subjects enrolled.
N = Total number of subjects in the treatment group; n = number of subjects in the specific category; ACS = acute coronary syndrome; STEMI-ST = segment elevation myocardial infarction; NSTEMI = non-ST segment elevation myocardial infarction; UA = unstable angina; MM = medical management; PCI = percutaneous coronary intervention; CABG= coronary artery bypass graft.
The history of hypertension was reported in 54.6% of patients followed by diabetes mellitus (45.2%), dyslipidemia (9.2%), and renal impairment (1.2%). Few of the patients also had a history of transient ischemic attack (0.2%) and nonhemorrhagic stroke (0.7%).
2.2. Primary analysis
Out of 2997 subjects, 48.9% had STEMI while NSTEMI were reported in 23% and UA 28.1%. The type of ACS and summary of management of ACS is presented in Table 2, Table 3 respectively. Single stent was used in 57.1% of subjects, whereas two stents were used in 30.1% subjects. There were 85% of subjects in whom drug eluting stent was used. The subjects with one coronary stenosis or occlusion were more than one third (41.4%) followed by two vessels (34.0%) and three coronary stenosis or occlusion (21.9%). The subjects with history of coronary stenosis or occlusion had 90–100% culprit coronary stenosis or occlusion in 73.2% subjects, and 41.2% subjects had 70–89% nonculprit vessels with single blockade.
Table 3.
Summary of ACS management by type and usage pattern of ticagrelor in ACS patients.
| Parameters | STEMI (N = 1466) | NSTEMI (N = 689) | UA (N = 842) |
|---|---|---|---|
| ACS management, n (%) | |||
| Percutaneous coronary intervention | 1363 (93.0) | 622 (90.3) | 784 (93.1) |
| Coronary artery bypass graft | 36 (2.5) | 32 (4.6) | 34 (4.0) |
| Medical management | 67 (4.6) | 35 (5.1) | 24 (2.9) |
| Whether stent was used | n (%) | ||
| Yes | 2801 (93.5) | ||
| No | 91 (3.0) | ||
| NA | 105 (3.5) | ||
| Number of stents used | n (%) | ||
| 1 | 1711 (57.1) | ||
| 2 | 843 (28.1) | ||
| 3 | 191 (6.4) | ||
| 4 | 45 (1.5) | ||
| >4 | 11 (0.4) | ||
| Stent type | |||
| BMS | 169 (5.6) | ||
| DES | 2547 (85.0) | ||
| BVS | 115 (3.8) | ||
| Missing | 195 (6.5) | ||
| Duration of treatment with ticagrelor (in days) | |||
| Mean (SD) n = 2993 | 314.1 (110.2) | ||
| Median | 366.0 | ||
| (Min, Max) | (1.0, 414.0) | ||
| Ticagrelor interrupted/discontinued, n (%) | |||
| Ticagrelor not interrupted | 2437 (81.3) | ||
| Ticagrelor interrupted | 13 (0.4) | ||
| Ticagrelor discontinued | 547 (18.3) | ||
| Reason for interruption/discontinued, n (%) | |||
| Bleeding | 19 (0.6) | ||
| CV events | 17 (0.6) | ||
| Dyspnea | 27 (0.9) | ||
| Nonavailability | 7 (0.2) | ||
| Nonaffordability | 397 (13.2) | ||
| Subject unwillingness | 88 (2.9) | ||
STEMI = ST-segment elevation myocardial infarction; NSTEMI = non-ST segment elevation myocardial infarction; UA = unstable angina; CV= cardiovascular. N = Total number of subjects in the treatment group; n = number of subjects in the specific category; NA = not available: SD = standard deviation. BMS = bare metal stent; DES = drug eluting stent; BVS = bioresorbable vascular scaffold; ACS = acute coronary syndrome.
2.3. Secondary analysis
2.3.1. To record various risk factors and their association with ACS and usage of ticagrelor
The duration of treatment with ticagrelor (in days) was calculated for 2993 subjects. The mean (SD) duration was 314 (110.2) days over 12 months. The uninterrupted use of ticagrelor was documented in 2437 (81%) subjects participated in the study (Table 3).
The majority of diabetic subjects had STEMI (641 out of 1354) in the study followed by UA (388) and NSTEMI (325). The risk factor and their association with ACS is presented in Table 2.
2.3.2. Details of clinical events incidence rate: bleeding, dyspnea, and CV events
There were 136 (4.5%) subjects who experienced any clinical event. There were 20 (0.7%) subjects who had CV deaths, five reported myocardial infraction (MI), and five ischemic stroke (IS). Bleeding and dyspnea were reported in 32 and 71 subjects, respectively. Two subjects experienced an event of bleeding with PLATO-major severity grade (Table 4).
Table 4.
Details of clinical events incidence rate: bleeding, dyspnea, and CV events.
| Clinical events | Ticagrelor (N = 2997) |
|---|---|
| Patient experienced any clinical event, n (%) | |
| Yes | 136 (4.5) |
| No | 2861 (95.5) |
| Event typea, n (%) | |
| MI | 19 (0.6) |
| IS | 23 (0.8) |
| CV death | 20 (0.7) |
| Missing | 76 (2.5) |
| Incidence of stent thrombosis, n (%) | |
| No | 126 (4.2) |
| Possible | 4 (0.1) |
| Probable | 4 (0.1) |
| Definite | 4 (0.1) |
| Bleeding, n (%) | |
| No | 104 (3.5) |
| CABG bleeds | 1 (0.0) |
| Non-CABG: GI | 5 (0.2) |
| Non-CABG: Gum bleeds | 1 (0.0) |
| Non-CABG: Petechiae/ecchymosis | 10 (0.3) |
| Non-CABG: Epistaxis | 4 (0.1) |
| Non-CABG: Hematuria | 1 (0.0) |
| Non-CABG: Others | 10 (0.3) |
| TIMI bleeding severity scale, n (%) | |
| TIMI—Minimal | 25 (0.8) |
| TIMI—Minor | 7 (0.2) |
| TIMI—Major | 1 (0.0) |
| Plato Bleeding Severity Scale, n (%) | |
| PLATO-Minimal | 20 (0.7) |
| PLATO-Minor | 10 (0.3) |
| PLATO-Major | 2 (0.1) |
| Dyspnea, n (%) | |
| Mild | 34 (1.1) |
| Moderate | 25 (0.8) |
| Severe | 11 (0.4) |
| Severe requiring discontinuation | 1 (0.0) |
| Episodes of dyspnea | |
| N | 68 |
| Mean (SD) | 2.5 (1.4) |
| Median | 2.0 |
| (Min, Max) | (1.0, 6.0) |
Percentages are based on the total number of subjects enrolled.
CABG = coronary artery bypass graft; IS= ischemic stroke; MI = myocardial infraction; TIMI = thrombolysis in myocardial infarction; PLATO = Platelet Inhibition and Patient Outcomes; N = total number of subjects in the treatment group; n = number of subjects with nonmissing values in the specific category; ACS = acute coronary syndrome.
Two subjects had multiple events.
The analysis of events of bleeding in subjects taking concurrent medication compared with overall population was performed. There were 21 subjects who experienced the event of bleeding while on concurrent medications. However, when compared the incidence with overall population experiencing the same event, it was not statistically significant (p 0.109) (Table 7).
Table 7.
Incidence of bleeding events and concomitant medications.
| Category | Ticagrelor (N = 2997) | p valueb |
|---|---|---|
| Bleeding in overall population | 32 (1.1) | 0.1092 |
| Bleeding in patients with concurrent medications | 21 (0.7) | |
| Unfractionated heparin | 20 (95.2) | |
| Low molecular weight heparin | 8 (38.1) | |
| Fondaparinux | 0 (0.0) | |
| GP 2b/3a inhibitors—Tirofiban | 6 (28.6) | |
| GP 2b/3a inhibitors—Abxicimab | 3 (14.3) | |
| GP 2b/3a Inhibitors—Eptifibatide | 0 (0.0) | |
| Bleeding in patients without concurrent medicationsa | 11 (0.4) |
Percentages in the bleeding main categories are based on the “all enrolled” patients.
Percentages in the medication subcategories are based on the total number of patients in the “bleeding in patients with concurrent medications” row.
Concurrent medications include “unfractionated heparin”, “low molecular weight heparin”, “fondaparinux”, “GP 2b/3a inhibitors—tirofiban”, “GP 2b/3a Inhibitors—Abxicimab”, “GP 2b/3a Inhibitors—Eptifibatide”.
P value will be calculated using Chi-square/Fisher Exact test at 5% level of significance.
2.3.3. Details of thrombolytic given for current episode for ACS management and duration of ticagrelor load
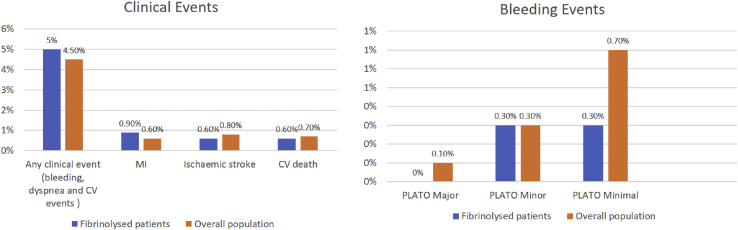
Overall, 338 (11.3%) subjects received thrombolytic treatment for their current episode, and over half of those patients (55.9%) received streptokinase as fibrinolytic therapy (Table 5). The time between fibrinolysis and ticagrelor loading in a large proportion of patients (69.5%) was ≥24 h, and nearly half of them (48.8%) were administered with ticagrelor in Cath lab after angiography. The subjects who were received clopidogrel before ticagrelor at the baseline were 35.6%. Streptokinase was the most common fibrinolytic used followed by tenecteplase. Incidence of any clinical event was numerically similar in fibrinolyzed subgroup as compared with overall population with numerical increase in the incidence of myocardial infarction (MI). Incidence of PLATO defined major and minimal bleeding were lower in patients undergoing fibrinolysis than overall population (Table 5 and Fig. 1).
Table 5.
Details of thrombolytic given for current episode for ACS management and clopidogrel received previously for ACS management.
| Details of thrombolytics | All enrolled (N = 2997) |
|---|---|
| Thrombolytic given for current episode, n (%) | |
| Yes | 338 (11.3) |
| No | 2659 (88.7) |
| Fibrinolytic used, n (%)a | |
| Streptokinase | 189 (55.9) |
| Urokinase | 3 (0.9) |
| Tenecteplase | 75 (22.2) |
| Alteplase | 3 (0.9) |
| Others | 68 (20.1) |
| Patient received Clopidogrel previously, n (%) | |
| Yes | 1067 (35.6%) |
| No | 1930 (64.4%) |
| Dose of Clopidogrel used, n (%)b | |
| 75 | 677 (63.4%) |
| 150 | 83 (7.8%) |
| 300 | 273 (25.6%) |
| 600 | 34 (3.2%) |
ACS = acute coronary syndrome.
Percentage was based on number of patients, who were given thrombolytic for current episode.
Percentage was based on number of patients who received clopidogrel.
Fig. 1.
Details of clinical events and bleeding events in fibrinolyzed patients vs. overall study population. MI = myocardial infraction; PLATO = Platelet Inhibition and Patient Outcomes; CV = cardiovascular.
2.3.4. Details of ticagrelor discontinuation and reason
Ticagrelor was interrupted in 13 (0.4%) subjects, whereas 81% of subjects continued it with no interruption. Nonaffordability of the drug was the reason for interruption and discontinuation in 397 (13.2%) subjects (Table 3).
2.3.5. Background/concomitant medication
Almost the entire study population (99.2%) received concomitant medication. The most prescribed medication included aspirin (99.2%) followed by unfractionated heparin (41.2%) and low molecular weight heparin (20.1%) (Table 6).
Table 6.
Summary of concomitant medication (all enrolled subjects).
| Concommitant medication | Ticagrelor (N = 2997) | |
|---|---|---|
| Medication Name | n | (%) |
| Any concomitant medication taken | 2974 | (99.2) |
| Aspirin | 2972 | (99.2) |
| Unfractionated heparin | 1236 | (41.2) |
| Low molecular weight heparin (LMWH) | 601 | (20.1) |
| Fondaparinux | 197 | (6.6) |
| GP 2b/3a inhibitors—Tirofiban | 171 | (5.7) |
| GP 2b/3a inhibitors—Abxicimab | 82 | (2.7) |
| GP 2b/3a inhibitors—Eptifibatide | 56 | (1.9) |
| Bivalirudin | 12 | (0.4) |
N = Total number of subjects in the treatment group.
n = number of subjects in the specific category.
3. Discussion
CV diseases are major causes of morbidity and mortality in the Indian subcontinent, causing more than 25% of deaths. It has been predicted that these diseases will increase rapidly in India, and this country will represent more than half the cases of heart disease in the world within the next 15 years.11 ACS remains a vital health issue worldwide. ACS needs close patient monitoring and attention as it imposes significant restrictions on the patient's quality of life. Its pathogenesis is attributable to many etiologies including physical inactivity, smoking, elevated low-density lipoprotein, hypertension, and genetic factors.12
The present study was a large observational study conducted in tertiary care hospitals across India with 2997 subjects. The results suggested that in >90% of patients with STEMI or NSTEMI or UA underwent PCI as an intervention in the management of ACS. PCI can restore the flow of blood into the myocardium in >90% of patients if performed by a skilled provider at a proficient PCI facility.13, 14 A tertiary care hospital in Delhi completed a registry study in STEMI patients from Feb 2013 to May 2015. Out of a total of 371 enrolled patients, 96.4% of patients underwent successful primary PCI.15 The majority of the patients in the TREASURE study population used a drug-eluting stent. A single stent was used in more than half study population. However, a maximum of ≥4 stents were used in around 02% of patients.
The evaluation of risk factors for ACS such as smoking, diabetes mellitus, and renal impairment performed in the study. The researchers found that smoking is a common risk factor in patients with <40 years. A retrospective analysis of PLATO study on Asian population demonstrated that patients with a history of smoking were between 38% and 41%.16 However, the overall patient population with a history of smoking was significantly lower in the present study. The enrolled patients who presented a history of diabetes mellitus constituted >40% population in all categories, i.e., STEMI, NSTEMI, and UA. However, the studies reported a lower percentage of the diabetic population with ACS.15, 16, 17
There was a low incidence of clinical events (4.5%) reported in the study which included 20 (0.7%) of CV deaths and 19 (0.6) of MI and 23 (0.8) of IS. SWEDEHEART registry conducted in Sweden with 45,000 subjects, published the composite of all-cause death, readmission with MI, or stroke with ticagrelor vs. clopidogrel as 11.7 vs. 22.3% [adjusted hazard ratio (HR) 0.85; 95% confidence interval: 0.78–0.93] at 24 months.18 In an another multicenter, double-blind, and randomized controlled trial of ticagrelor vs. clopidogrel for the prevention of CV events in 18,624 patients, the incidence of CV deaths with clopidogrel were reported to be 4.0%.7
A small fraction of the study population experienced clinical events of bleeding during the study duration as compared with results published in previous studies. The PLATO study demonstrated 11.6% of cases of major bleeding with ticagrelor, while in the present study, two subjects experienced PLATO major bleeding.7 The percentage of the population with TIMI-minimal bleeding was also less than one percent, and single patient reported TIMI-major event. The researchers demonstrated that fibrinolytic therapy contributed to TIMI events.19 The incidence of PLATO defined major and minimal bleeding were lower in patients undergoing fibrinolysis than overall population in TREASURE study. Streptokinase (6%) was the most commonly prescribed fibrinolytic among all. A multicenter, randomized, and double-blind trial conducted in 18,624 patients from October 2006 through July 2008. The interventions studied were of ticagrelor and clopidogrel. In our study, the bleeding events were significantly lesser than reported in the PLATO study.7 The event of dyspnea occurred in 68 (2%) patients with ticagrelor. The reason for discontinuation of ticagrelor among 27 (0.9%) patients was dyspnea. The event of dyspnea was reported significantly less in the present study as compared with the abovementioned study (13.8%).7 More than 60% of subjects with the reported event of bleeding, prescribed concomitant medication such as unfractioned heparin, GP 2b/3a inhibitors, and low molecular weight heparin. The concomitant medications could have an association with the risks of bleeding. Though the incidence of bleeding reported in the present study was lower than the literature.16
Antiplatelet therapy is of high importance in ACS patients. The majority of the population using ticagrelor continued with the drug in an uninterrupted fashion. The reason for discontinuation of ticagrelor in 13.2% of patients was reported to be nonaffordability of the drug. Whereas, only a small fraction of patients switched/discontinued the treatment because of CV events, dyspnea, or nonavailability. The proportion of patients restarting ticagrelor intake after discontinuation or switching was reported to be low. Almost the entire study population was found to be taking concomitant medications.
The limitations of the study were that majority of sites from where data has been collected are tertiary care hospitals, and management of the CV events with PCI, CABG, and MM may not represent the true incidence. Also, the study design wherein there were subjects who were followed up at 30 days may have contributed for underrepresentation of CV events. Also, another limitation of study was that evaluation of use of morphine and its effects on activity of ticagrelore was not performed.
4. Conclusion
Ticagrelor has been used across ACS types and in different management strategies in real world settings in India. Majority of the patients who had STEMI and underwent PCI were prescribed ticagrelor. Moreover, the most commonly used stent was drug eluting stent. The major reason for discontinuation of drug was nonaffordability of treatment.
Conflicts of interest
All authors have none to declare.
Disclosures
Ethics
All necessary ethical approvals were taken for conducting of this study.
Funding support
This study was funded by AstraZeneca Pharma India Ltd.
Author contribution
All authors have contributed in the concept, data analysis, execution, interpretation, statistics and manuscript preparation.
Acknowledgments
The authors would like to thank AstraZeneca Pharma India Ltd., Bangalore and Tech-Observer India Pvt. Ltd., New Delhi, the Contract Research Organization for supervising the study, providing administrative and medical writing support.
References
- 1.Misiriya K.J., Sudhayakumar N., Khadar S.A., George R., Jayaprakasht V.L., Pappachan J.M. The clinical spectrum of acute coronary syndromes: experience from a major center in Kerala. J Assoc Phys India. 2009;57:377–383. [PubMed] [Google Scholar]
- 2.Ohira T., Iso H. Cardiovascular disease epidemiology in Asia. Circ J. 2013;77(7):1646–1652. doi: 10.1253/circj.cj-13-0702. [DOI] [PubMed] [Google Scholar]
- 3.Chan M.Y., Du X., Eccleston D., Ma C. Acute coronary syndrome in the Asia-Pacific region. Int J Cardiol. 2016;202:861–869. doi: 10.1016/j.ijcard.2015.04.073. [DOI] [PubMed] [Google Scholar]
- 4.Kaul U., Bhatia V. Perspective on coronary interventions & cardiac surgeries in India. Indian J Med Res. 2010;132(5):543–548. [PMC free article] [PubMed] [Google Scholar]
- 5.WHO factsheet: cardiovascular diseases (CVDs) 2016. http://www.who.int/mediacentre/factsheets/fs317/en/ Available from: [Google Scholar]
- 6.Husted S V.G.J. Ticagrelor: the first reversibly binding oral P2Y12 receptor antagonist. Cardiovasc Ther. 2009;27(4):259–274. doi: 10.1111/j.1755-5922.2009.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallentin L B.R., Budaj A., Cannon C.P. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. NEJM. 2009;361(11):1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 8.Bonaca MP G.S., Bhatt D.L., Steg P.G. Prevention of stroke with ticagrelor in patients with prior myocardial infarction: insights from PEGASUS-TIMI 54 (prevention of cardiovascular events in patients with prior heart attack using ticagrelor compared to placebo on a background of aspirin-thrombolysis in myocardial infarction 54) Circ J. 2016;134(12):861–871. doi: 10.1161/CIRCULATIONAHA.116.024637. [DOI] [PubMed] [Google Scholar]
- 9.List of Approved Drugs from 01-01-2012 to till Date. 2012. http://cdsco.nic.in/writereaddata/LIST-OF-APPROVED-DRUG-2012(1).pdf Available from: [Google Scholar]
- 10.Gandhi A., Kumar A., Nageshwaran G., Kotak B., Raza A. A non-inTeRventional prospEctive observationAl Study to Understand the usage pattern of Ticagrelor in Indian patients with acute coronaRy syndromE (TREASURE) Indian Heart J. 2014;66(Supplement 2):S8. [Google Scholar]
- 11.Gupta R., Joshi P., Mohan V., Reddy K.S., Yusuf S. Epidemiology and causation of coronary heart disease and stroke in India. Heart. 2008;94(1):16–26. doi: 10.1136/hrt.2007.132951. [DOI] [PubMed] [Google Scholar]
- 12.Worthley S.G., Osende J., Helft G., Badimon J.J., Fuster V. Pathogenesis and acute coronary syndromes. Mt Sinai J Med. 2001;68(3):167. [PubMed] [Google Scholar]
- 13.Magid D.J., Calonge B., Rumsfeld J.S. Relation between hospital primary angioplasty volume and mortality for patients with acute MI treated with primary angioplasty vs thrombolytic therapy. J Am Med Assoc. 2000;284(24):3131–3138. doi: 10.1001/jama.284.24.3131. [DOI] [PubMed] [Google Scholar]
- 14.Canto J.G., Every N., Magid D.J. The volume of primary angioplasty procedures and survival after acute myocardial infarction. National Registry of Myocardial Infarction 2 Investigators. NEJM. 2000;342(21):1573–1580. doi: 10.1056/NEJM200005253422106. [DOI] [PubMed] [Google Scholar]
- 15.Dubey G., Verma S.K., Bahl V. Primary percutaneous coronary intervention for acute ST elevation myocardial infarction: outcomes and determinants of outcomes: a tertiary care center study from North India. Indian Heart J. 2017;69:294–298. doi: 10.1016/j.ihj.2016.11.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang H.J., Clare R., Gao R., Held C. PLATO investigators., ticagrelor versus clopidogrel in Asian patients with acute coronary syndrome: a retrospective analysis from the platelet inhibition and patient outcomes (PLATO) trial. Am Heart J. 2015;169(6):899–905. doi: 10.1016/j.ahj.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 17.Lu Y., Li Y., Yao R. Clinical effect of ticagrelor administered in acute coronary syndrome patients following percutaneous coronary intervention. Exp Ther Med. 2016;11(6):2177–2184. doi: 10.3892/etm.2016.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahlen A., Varenhorst C., Lagerqvist B., Renlund Henrik, Omerovic Elmir, Erlinge David, Wallentin Lars, James Stefan K., Tomas Jernberg. Outcomes in patients treated with ticagrelor or clopidogrel after acute myocardial infarction: experiences from SWEDEHEART registry. Eur Heart J. 2016;37(44):3335–3342. doi: 10.1093/eurheartj/ehw284. [DOI] [PubMed] [Google Scholar]
- 19.Mehran R., Rao S.V., Bhatt D.L. Standardized bleeding definitions for cardiovascular clinical trials. A consensus report from the bleeding academic Research consortium. Circ J. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]