Abstract
Background
Total knee arthroplasty (TKA) is one the most common elective procedures in the world. Post-operative infection is one of its most devastating complications, often necessitating multiple additional surgeries. We aimed to describe the relationship between surgical duration and risk of deep infection following primary elective TKA.
Methods
In this cohort study we analyses primary TKAs done between 2009 and 2016 in Ontario, Canada. We utilized restricted cubic splines to identify a threshold of surgical duration that was associated with an increased risk for infection requiring surgery. Patients with a ‘short’ duration of surgery were matched to those with a ‘long’ duration on patient age (±3 years), patient sex, severe obesity (BMI > 40), the primary surgeon, the hospital and the type of anesthetic.
Findings
In 92,343 primary TKAs, the median surgical duration was 106 min. We identified a cut-point of 100 min that was associated with an increased risk for infection. Subsequently, 17,815 TKA recipients with a ‘long’ procedure length were matched to those with a ‘short’ procedure length. ‘Long’ procedures had a higher rate of deep infection (1.1% versus 0.6%, p < 0.0001). This was equal to a relative risk of 1.81 (p < 0.0001).
Interpretation
In a cohort of TKA recipients, we found that procedure lengths longer than 100 min were associated with a significantly increased risk of deep infection requiring surgery. This time threshold serves a useful time-point to identify patients that require closer surveillance.
Keywords: Infection, Surgical duration, Arthroplasty
Research in context
Evidence before this study
Periprosthetic infection after knee replacement is a relatively rare but potentially devastating complication, often requiring multiple surgical interventions. Risk factors for infection can be broadly divided into patient and provider (surgeon and hospital) factors. A recent meta-analysis from 2016 comprising 66 studies and >500,000 patients (both hip and knee replacement) described several risk factors for infection, including patient age, male sex, and co-morbidities (e.g.: obesity, rheumatoid arthritis, diabetes mellitus). We searched MEDLINE, Embase and Web of Science from March 2016 to April 2019 for observational studies, randomized control trials, meta-analyses and systematic reviews reporting on duration of surgery and risk for infection after joint replacement. We restricted our search by language (English only). We identified multiple review papers that identified prolonged duration as a risk factor for infection. We also identified a recent observational study that specifically examined the role for surgical duration in the risk for infection, however it was limited by a heterogeneous definition for infection, an inability to validate a threshold for increased risk, nor could they quantify this risk. They did not look at knee replacements.
Added value of this study
Using a single observational cohort of 92,343 patients receiving their first total knee replacement, we evaluated the impact of surgical duration on the risk for deep infection requiring additional surgery. The median surgical duration was 106 min, and we identified a threshold for infection at 100 min. We matched ‘long’ (100+ minutes) and ‘short’ (<100 min) procedures by patient age (±3 years), patient sex, severe obesity (BMI > 40), the primary surgeon, the hospital and the type of anesthetic. After matching, ‘long’ procedures had an 80% increased risk for periprosthetic infection. This threshold serves two purposes: 1) as a potential modifiable factor to mitigate the risk for infection, and 2) to identify patients at increased risk for infection that require additional monitoring or potentially earlier intervention.
Implications of all the available evidence
Total knee replacements are one of the most common elective procedures worldwide. Although the rates of periprosthetic infection are relatively low, the burden of this complication will show a commensurate increase. Knowledge of factors that affect the risk for infection will aid physicians in mitigating this risk.
1. Introduction
Total knee arthroplasty (TKA) is being performed with ever increasing frequency in North America and across the world. Over 600,000 TKAs are performed annually in North America [1], and over 75,000 are performed annually in the United Kingdom [2]. Future projections suggest that the demand for primary TKA in North America and the United Kingdom over the next two decades will grow by 673% and 150%, respectively [2,3]. With these numbers in mind, limiting complications is crucial for patient outcomes and controlling healthcare costs.
Deep infection remains one of the most expensive [4,5] and challenging complications and accounts for 24% of all early revisions [6]. Several risk factors for deep infection have been identified include elevated body-mass index, diabetes mellitus, hypertension and rheumatoid arthritis [5]. However, these factors are largely non-modifiable, particularly if the patient's arthritis is severe enough that delaying surgery is inappropriate. One potentially modifiable factor is surgical duration – several studies have demonstrated that longer surgeries are associated with increased risk for infection [7], [8], [9]. However, these studies have generally assumed a linear relationship between duration of surgery and infection, or were unable to control for several important confounders (e.g.: surgeon volume, hospital volume). The Centers for Disease Control (CDC) chose a cut-off of 122 min (the 75th percentile) to identify knee replacements at increased risk for infection. However, it is not known whether this threshold accurately reflects the point at which the risk for infection increases, or if one exists.
The specific objectives of this study were: 1) to describe the relationship between surgical duration and the risk for deep infection requiring surgery and to identify a cut-point that predicts differential risk for this complication, if one exists; and 2) to quantify any increased risk of infection in cases with longer surgical durations, after controlling for relevant confounders [10].
2. Methods
2.1. Study sample
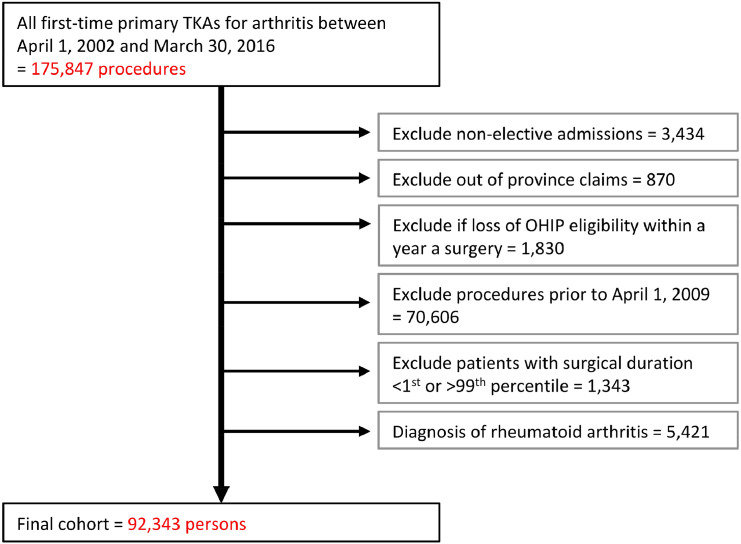
We defined a cohort of consecutive adults (>18 years of age) who received their first primary elective TKA for osteoarthritis between April 1, 2009 and March 31, 2016 (Fig. 1). Ontario has a solely single-payer healthcare system which covers medically necessary procedures, including total knee arthroplasty. There is not private coverage for this procedure, so we are able to identify all knee replacements (and subsequent complications, including revisions) that occurred in this province. We started enrolment in 2009 as surgical start and end times were measured from this point onwards. To maintain a relatively homogenous patient population, we excluded patients with rheumatoid arthritis, those with non-elective admissions, those that received bilateral TKA procedures, those who moved out of the province in the year following surgery, those with a length of stay greater than seven days, and patients with an atypical surgical duration (under 30 min or over 190 min, the 1st and 99th percentile, respectively).
Fig. 1.
Selection of patients for inclusion.
2.2. Data sources
Data utilized included hospital discharge abstracts from the Canadian Institute for Health Information Discharge Abstract Database (CIHI-DAD) and physician claims from the Ontario Health Insurance Plan (OHIP). We identified patients using specific procedure and diagnostic codes (CIHI: 1VG53LA, OHIP: R441) from the Canadian version of the 10th revision of the International Statistical Classification of Diseases and the Canadian Classification of Health Interventions (ICD-10-CA/CCI). Patient demographics, including age and sex were accessed via the OHIP Registered Persons Databases (RPDB) [11]. These definitions have been validated and are the same used by the Ministry of Health and Long-Term Care [12].
2.3. Main exposure: surgical duration
Surgical duration was determined by subtracting the start-time of each procedure from its end-time. During the study period, the start and end-times of every procedure were captured, reflecting the entry and exit times into the operating room [13].
2.4. Primary outcome: deep infection requiring surgery
The primary study outcome was the occurrence of deep infection requiring surgery (i.e. irrigation and debridement with liner exchange, single or two-stage revision) within one year. Deep infections were identified using a combination of ICD-10-CA diagnostic codes and physician billing codes. Specifically, infections were defined as the first occurrence of: an ICD-10-CA diagnostic code for intra-articular infection, with a confirmatory code for an irrigation and debridement; an OHIP fee code for a spacer insertion; and/or revision arthroplasty, identified using ICD-10-CA/CCI procedure codes accompanied by the supplementary status attribute “R” with an appropriate surgeon billing code (CCI codes: 1VG55LAP, 1VG53LAS, 1VG35LA-K8, 1VG52, 1VG87, 1VG57; OHIP fee codes: R412, R414K, R417, R496, R497, R442, R424, R248).
2.5. Covariates of interest
We measured and controlled for several factors that have previously been shown to affect the risk of occurrence of complications following joint replacement. These included patient age, sex, socioeconomic status and co-morbidities. Using validated algorithms, we identified patients with a history of pre-existing cardiovascular disease [14], congestive heart failure, diabetes [15], hypertension [16], and chronic obstructive pulmonary disease (COPD) [17]. Physician billing codes were used to identify patients that had been counseled on smoking cessation.
Additional co-morbidities listed on hospital discharge abstracts in the three years before the index TKA admission were categorized according to an adaptation of the Charlson Co-morbidity Index. Adjusted Clinical Groups (ACGs), based on diagnosis codes from hospitalizations and physician visits in the two years before the index TKA admission were used to classify patients as ‘frail’. Obesity was identified by the occurrence of a physician billing code for patients with a body-mass index over 40.
Neighbourhood income quintile was used as a surrogate measure for socioeconomic status and living conditions. Neighbourhood income quintiles categorize small geographic areas into five roughly equal population groups, with the lowest quintile referring to the least affluent neighbourhoods [18]. For each TKA, surgeon volume was defined as the number of knee arthroplasty procedures (both primary and revision) performed by the primary surgeon in the 365 days prior to the index procedure. Hospital volume was similarly defined. Use of a general anesthetic was identified from the DAD.
2.6. Describing the relationship between surgical duration and risk of deep infection
The logistic regression for the occurrence of a deep infection was created, incorporating several patient and provider factors. These included patient socio-demographics (age, sex, rural residence, income quintile), co-morbidities, and provider factors (surgeon volume, hospital volume, and use of a general anesthetic). Additionally, surgical duration was represented by a restricted cubic spline with four knots [19] to allow for a non-linear relationship between duration of surgery and the occurrence of deep infection. The resultant function of surgical duration was then examined to identify an inflection point, if any, to dichotomize surgical duration into clinically meaningful categories. If a range was observed within which inflection occurred, multivariable logistic regression was used to determine the area under the curve (AUC) for the models relating surgical duration cut-points to the risk of deep infection. The duration with the maximum AUC was selected as the cut-point.
2.7. Statistical analyses and matching
Patients in the cohort were then classified according to whether the duration of their surgery was ‘short’ or ‘long’ based on our findings of the spline analysis. Baseline cohort characteristics were calculated using proportions and medians as appropriate, and were compared between groups using the Wilcoxon rank sum tests for continuous variables and the chi-square test for categorical variables. A logistic regression predicting a ‘long’ duration of surgery was estimated with the following covariates: patient socio-demographics (age, sex, rural residence, income quintile) and patient health status (Charlson score, frailty, hypertension, COPD, CHF, diabetes, coronary artery disease, obesity, prior counseling on smoking cessation).
TKA recipients with ‘long’ surgical durations were matched to those with ‘short’ durations based on patient age (±3 years), patient sex, severe obesity (BMI > 40), the primary surgeon, the hospital and the type of anesthetic (general anesthesia versus spinal anesthesia). We estimated standardized differences for patient socio-demographics, co-morbidity, obesity and type of anesthesia after matching, with a standardized difference of 10% or more considered indicative of imbalance. The occurrence of a deep infection was compared between the two groups after matching, using methods appropriate for the analysis of matched data. The hazard ratio (HR) for occurrence of a deep infection or death within one year was determined using a hazards model that took pair-matching into account by using robust variance estimation.
All analyses were performed at ICES (www.ices.on.ca) using SAS version 9.3 for UNIX (SAS Institute, Cary NC). The type I error probability was set to 0.05 for all analyses. Use of the data in this study was authorized under section 45 of Ontario's Personal Health Information Protection Act, which does not require review by a Research Ethics Board.
2.8. Secondary analysis
We estimated the risk for deep infection associated with ‘long’ procedures after shortening and increasing the threshold to 90 and 110 min, respectively.
3. Results
3.1. Patient characteristics
Between April 1, 2009 and March 31, 2016, we identified 92,343 eligible TKA recipients (Fig. 1, Table 1). The median surgical duration was 106 min (IQR 91–124 min).
Table 1.
Characteristics of eligible TKA recipients.
| Number of patients | N = 92,343 |
|---|---|
| Demographics | |
| Age (y) [Median (IQR)] | 68 (61–75) |
| Female [N (%)] | 56,807 (61.5%) |
| Rural residence [N (%)] | 15,451 (16.7%) |
| Income quintile [N (%)] | |
| Lowest | 15,839 (17.2%) |
| 2 | 19,151 (20.7%) |
| 3 | 18,944 (20.5%) |
| 4 | 19,018 (20.6%) |
| Highest | 19,173 (20.8%) |
| Co-morbidities [N (%)] | |
| Coronary artery disease | 2959 (3.2%) |
| Congestive heart failure | 4781 (5.2%) |
| Chronic obstructive pulmonary disease | 17,280 (18.7%) |
| Counseled about smoking cessation | 3879 (4.2%) |
| Diabetes | 27,095 (29.3%) |
| Hypertension | 67,833 (73.5%) |
| Frail | 5483 (5.9%) |
| BMI > 40 | 1500 (1.6%) |
| Charlson score [N (%)] | |
| 0 | 65,628 (71.1%) |
| 1 | 16,901 (18.3%) |
| 2 | 6345 (6.9%) |
| 3 or more | 3469 (3.8%) |
| Admission characteristics | |
| Hospital volume [Median (IQR)] | 411 (301–589) |
| Surgeon volume [Median (IQR)] | 99 (69–135) |
| General anesthetic [N (%)] | 15,921 (17.2%) |
| Duration of surgery [Median (IQR)] | 106 (91–124) |
3.2. Cubic splines describing the relationship between surgical duration and risk of deep infection
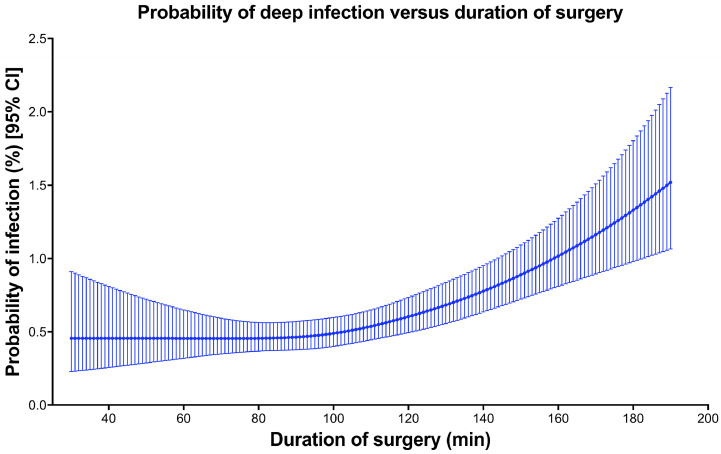
Increased age, male sex, low income quintile and high co-morbidity were all associated with an increased risk for infection (Table 2). The restricted cubic spline for the risk of a deep surgical site infection was flat until an inflection point at approximately 100 min of surgery, after which rates increased rapidly with increased surgical duration (Fig. 2). Receiver operating characteristic (ROC) curves were generated relating surgical duration (with cut-points of 80, 85, 90, 95, 100, 105, 110, 120 min) to the risk for deep infection requiring surgery within one year of surgery. The optimal cut-point was found to be 100 min, with an AUC of 0.605.
Table 2.
Logistic regression for the occurrence of a deep infection after surgery.
| OR (95% CI) | p-value | |
|---|---|---|
| Demographics | ||
| Age (y) | 0.98 (0.97–0.99) | 0.0002 |
| Female | 0.56 (0.47–0.66) | <0.0001 |
| Income quintile | ||
| Lowest | 1.35 (1.03–1.77) | 0.0321 |
| 2 | 1.21 (0.93–1.58) | 0.4058 |
| 3 | 1.14 (0.87–1.49) | 0.9157 |
| 4 | 0.99 (0.75–1.31) | 0.1368 |
| Highest | REF | |
| Co-morbidities | ||
| Congestive heart failure | 1.68 (1.26–2.25) | 0.0004 |
| Chronic obstructive pulmonary disease | 1.55 (1.29–1.87) | <0.0001 |
| Diabetes | 1.24 (1.01–1.53) | 0.0432 |
| Frail | 1.73 (1.31–2.29) | 0.0001 |
| Charlson score [N (%)] | ||
| 0 | REF | |
| 1 | 0.99 (0.77–1.26) | 0.0792 |
| 2 | 1.43 (1.06–1.92) | 0.0435 |
| 3 or more | 1.27 (0.87–1.85) | 0.487 |
| Admission characteristics | ||
| Hospital volume | 1 (1–1) | 0.5701 |
| Surgeon volume | 1.00 (0.99–1.00) | 0.3365 |
| General anesthetic | 1.22 (0.99–1.49) | 0.0544 |
| Duration of surgery | Fig. 2 | |
Fig. 2.
Probability of deep infection versus duration of surgery.
3.3. Factors associated with ‘long’ surgical durations
The majority of patients in the cohort had ‘long’ operations (N = 56,590, 61%) (Table 2). Factors that were positively associated with long procedures included male sex (adjusted OR 1.38, 95%CI 1.34–1.43, p < 0.0001), elevated BMI (adjusted OR 2.14, 95%CI 1.89–2.44, p < 0.0001), hypertension (adjusted OR 1.11, 95%CI 1.07–1.14, p < 0.0001), frailty (adjusted OR 1.12, 95%CI 1.05–1.19, p = 0.0007), use of a general anesthetic (adjusted OR 1.54, 95%CI 1.48–1.60, p < 0.0001), living in a rural area (adjusted OR 1.58, 95%CI 1.52–1.65, p < 0.0001), and having the surgery at a teaching hospital (adjusted OR 2.23, 95%CI 2.15–2.32, p < 0.0001). Factors that reduced the risk of long procedures included age (adjusted OR 0.86 for each additional ten years, 95% 0.84–0.87, p < 0.0001), surgeon volume (adjusted OR 0.87 for each additional ten cases/year, 95% 0.87–0.88, p < 0.0001), hospital volume (adjusted OR 0.84 for each additional hundred cases/year, 95% 0.84–0.85, p < 0.0001).
3.4. Matching
A total of 17,815 patients (50%) who received a TKA with a ‘short’ surgical duration (<100 min) were successfully matched to one with a ‘long’ surgical duration (Table 3). After matching, the absolute standardized differences were less than 10% for patient socio-demographics, co-morbidity, obesity and type of anesthesia, indicating an adequate match (Table 3).
Table 3.
Comparison of TKA recipients, before and after matching.
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| Short cases | Long cases | p-value | Short cases | Long cases | Standardized difference | |
| Number of patients | N = 35,753 | N = 56,590 | N = 17,815 | N = 17,815 | ||
| Demographics | ||||||
| Age (y) [Median (IQR)] | 68 (62–75) | 67 (61–74) | <0.001 | 68 (61–75) | 68 (61–74) | 0.01 |
| Female [N (%)] | 23,500 (65.7%) | 33,307 (58.9%) | <0.001 | 11,237 (63.1%) | 11,237 (63.1%) | 0 |
| Rural residence [N (%)] | 4725 (13.2%) | 10,726 (19.0%) | <0.001 | 2914 (16.4%) | 2924 (16.4%) | 0 |
| Income Quintile [N (%)] | <0.001 | |||||
| Lowest | 5796 (16.2%) | 10,043 (17.7%) | 3099 (17.4%) | 3149 (17.7%) | 0.01 | |
| 2 | 7453 (20.8%) | 11,698 (20.7%) | 3731 (20.9%) | 3715 (20.9%) | 0 | |
| 3 | 7495 (21.0%) | 11,449 (20.2%) | 3635 (20.4%) | 3665 (20.6%) | 0 | |
| 4 | 7361 (20.6%) | 11,657 (20.6%) | 3670 (20.6%) | 3617 (20.3%) | 0.01 | |
| Highest | 7563 (21.2%) | 11,610 (20.5%) | 3634 (20.4%) | 3626 (20.4%) | 0 | |
| Co-morbidities [N (%)] | ||||||
| Coronary artery disease | 1095 (3.1%) | 1864 (3.3%) | 0.052 | 552 (3.1%) | 566 (3.2%) | 0 |
| Congestive Heart Failure | 1746 (4.9%) | 3035 (5.4%) | 0.001 | 809 (4.5%) | 944 (5.3%) | 0.04 |
| Chronic Obstructive Pulmonary Disease | 6292 (17.6%) | 10,988 (19.4%) | <0.001 | 3195 (17.9%) | 3319 (18.6%) | 0.02 |
| Counseled about smoking cessation | 1478 (4.1%) | 2401 (4.2%) | 0.422 | 834 (4.7%) | 684 (3.8%) | 0.04 |
| Diabetes | 10,026 (28.0%) | 17,069 (30.2%) | <0.001 | 5060 (28.4%) | 5414 (30.4%) | 0.04 |
| Hypertension | 25,936 (72.5%) | 41,897 (74.0%) | <0.001 | 12,711 (71.3%) | 13,376 (75.1%) | 0.08 |
| Frail | 2013 (5.6%) | 3470 (6.1%) | 0.002 | 950 (5.3%) | 1092 (6.1%) | 0.03 |
| BMI >40 | 344 (1.0%) | 1156 (2.0%) | <0.001 | 127 (0.7%) | 127 (0.7%) | 0 |
| Charlson Score [N (%)] | <0.001 | |||||
| 0 | 26,076 (72.9%) | 39,552 (69.9%) | 12,969 (72.8%) | 12,463 (70.0%) | 0.06 | |
| 1 | 6241 (17.5%) | 10,660 (18.8%) | 3119 (17.5%) | 3380 (19.0%) | 0.04 | |
| 2 | 2279 (6.4%) | 4066 (7.2%) | 1162 (6.5%) | 1271 (7.1%) | 0.02 | |
| 3 or more | 1157 (3.2%) | 2312 (4.1%) | 565 (3.2%) | 701 (3.9%) | 0.04 | |
| Type of anesthesia | ||||||
| General Anesthesia [N (%)] | 4579 (12.8%) | 11,342 (20.0%) | <0.001 | 2426 (13.6%) | 2426 (13.6%) | 0 |
| Complication | Short cases | Long cases | p-value | Short cases | Long cases | p-value |
|---|---|---|---|---|---|---|
| Deep infection [N (%)] | 236 (0.7%) | 603 (1.1%) | <0.001 | 108 (0.6%) | 195 (1.1%) | <0.0001 |
3.5. Outcomes after matching
Patients with a ‘long’ surgical duration (≥100 min) had a higher rate of deep infection within a year of their surgery than patients with a ‘short’ duration (1.1% versus 0.6%, p < 0.0001; HR 1.81, 95%CI 1.43–2.29, p < 0.0001). The number needed to harm was 204 persons (95%CI 147–336).
3.6. Secondary analysis
After setting the threshold for a ‘long’ procedure to 90 min, we successfully matched 13,171 ‘short’ and ‘long’ procedures. After matching, ‘long’ procedures remained at increased risk for infection (HR 1.67, 95%CI 1.27–2.19, p = 0.0002).
After setting the threshold to 110 min, we successfully matched 18,223 ‘short’ and ‘long’ procedures. After matching, ‘long’ procedures remained at increased risk for infection (HR 1.72, 95%CI 1.31–2.25, p < 0.0001).
4. Discussion
This study suggests a threshold of surgical duration for the risk of a deep infection requiring additional surgery following total knee arthroplasty. As expected, increased surgical duration was associated increased risk of a subsequent deep infection. Through the use of restricted cubic splines, we found that the relationship between surgical duration and risk for deep infection was not linear. The risk of deep infection starts relatively low, but noticeably increased at approximately 100 min. This increased risk persisted after controlling for several patient and provider factors. Our surgical duration threshold of 100 min is considerably lower than the 120-minute threshold used by the CDC to identify patients at increased risk of infection [10].
Most past research suggests that increased duration is associated with an increased risk for infection. The most likely explanation is the increased likelihood of contamination of the surgical site, either directly or from secondarily contaminated instruments. Another possibility is that patients with increased co-morbidities (such as obesity) are more complex and require a longer surgical duration. As such, our finding may reflect the underlying condition of the patient. We mitigated this by choosing a relatively homogenous elective procedure (primary total knee arthroplasty for osteoarthritis), and balancing for known confounders (obesity, Charlson score, and various other co-morbidities).
Most recently, Surace et al. used cubic splines to examine the relationship between duration and risk for infection following total hip arthroplasty [9], and did not find as dramatic a change in slope as the splines generated for this study – this may reflect the different anatomy of the sites being studied. Hips have a thicker soft-tissue envelope that may mitigate the risk for infections relative to knees. Additionally, Surace et al. were unable to control for factors that affect operative times as well as the risk for post-operative infection, such as surgeon volume and hospital volume. As such, any association between operative time and infection risk may actually represent the effect of surgeon skill. In the current study we matched on both the primary surgeon and the hospital, increasing the likelihood that increased duration is truly associated with increased risk for infection. The current study also examined the effect on outcomes of decreasing operative time. This permits a quantification of the harm of increased operative time vs. shortened operative time.
Several patient and provider factors were additionally associated with increased surgical duration. As expected, men (who tend to be more muscular) and obese patients (who have a larger soft-tissue envelope) tended to have longer procedures. Rural patients also had longer procedure times, but this may reflect a greater likelihood of their procedures being done in lower-volume centers. Longer procedure duration was associated with lower surgeon and hospital volumes.
While surgical duration is technically modifiable, there are limitations to the degree to which our findings may be applied. Although we limited our cohort to first-time primary TKAs performed for osteoarthritis, this is still a heterogeneous cohort with variable surgical complexity. Despite appropriate pre-operative planning, surgeons may not be able to prevent a procedure from ‘running long.’ However, the identified threshold could serve as a time at which to give a second dose of antibiotics, as well as identify patients that require closer monitoring for infection.
Strengths of our study include the use of population-based administrative data to assemble a large sample of first-time total knee arthroplasty patients. Furthermore, we were able to consider several important patient, hospital, and surgeon predictors of post-surgical infection. Our use of restricted cubic splines allowed us to visualize the relation between surgical duration and subsequent deep infection requiring surgery, enabling selection of a cut point for surgical duration. By hard-matching on type of anesthetic, surgeon, and hospital we also were able to control for intrinsic and potentially unmeasurable provider factors between groups. After matching, our cohorts were balanced by several characteristics (such as age, sex, comorbidity, rurality, various indices of socioeconomic status).
However, our study has several limitations. Foremost, surgical duration was defined based on operating room entry and exit times. The data on surgery times has high face validity – basic tests of their quality included that 1) start times always occurred after the hospital admission time and that 2) “sensible” surgical durations are predicted by surgery start and end times [13]. However, the surgical duration we utilize in this study does not solely include the time from incision to closure, but also captures time required positioning the patient and inducing anesthesia. As there is likely to be variation according to type of anesthesia and presence or absence of separate ‘block rooms’ for induction of spinal or regional anesthesia, we hard-matched according to type of anesthetic and hospital. However, it is possible that by hard-matching on these factors, we have systematically eliminated procedures with surgical durations that were ‘modifiable’ (i.e. by having a more experienced surgeon in a better operating room), leaving procedures with durations that are less modifiable. However, we believe that even in situations in which surgical duration cannot be modified, the identification of a threshold will allow for improved surveillance. One additional consideration is that even if the surgical site is not open at the ‘start time’ we capture, the surgical trays containing the instruments which are used during the procedure are open and exposed by the time the patient is brought into the operating room. The ‘closing’ time that is captured is closer to the recommended definition of the end of the procedure used by the CDC [20].
We were also limited in our ability to measure and control for several known confounders, including implant type, use of bone cement, body-mass index and smoking status. We controlled for variations in surgical practice (e.g.: type of implants and use of cement) by matching by surgeon and hospital. We identified patients with a body-mass index over 40 using physician billing codes, but were unable to identify patients with a BMI over 30 or 35. Billing codes were also used to identify patients that were counseled for smoking cessation, but while this code may be specific for patients that have a smoking history, it is not sensitive. Nor does it tell us anything about the severity of a patient's smoking habit. However, both obesity and smoking are strongly associated with other factors that were measured and balanced between matched groups, including: diabetes [21], hypertension [22], congestive heart failure [23], chronic obstructive pulmonary disease [24], frailty [25], and various indices of socioeconomic status [26].
In summary, among first-time primary elective TKA recipients, a surgical duration greater than 100 min was associated with higher risk for a deep infection requiring surgery within one year. This risk persisted after matching and controlling for various relevant confounders, including patient and provider factors. Surgical duration is a potentially modifiable factor that can be utilized to lower the risk for infection, possibly by preferentially referring patients to high-volume centers. This time threshold requires further prospective study to clarify its validity, but at this point may serve to identify patients that require closer surveillance for infection after knee replacement.
Disclaimer
The opinions, results and conclusions reported in this paper are those of the authors and are independent from the data providers and funding sources. No endorsement by CIHI, ICES or the Ontario Ministry of Health and Long-Term Care is intended or should be inferred.
CRediT authorship contribution statement
Bheeshma Ravi: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing - original draft, Writing - review & editing. Richard Jenkinson: Conceptualization, Investigation, Methodology, Writing - original draft. Sven O'Heireamhoin: Investigation, Methodology, Writing - original draft. Peter C. Austin: Methodology, Validation, Writing - review & editing. Suriya Aktar: Methodology, Data curation. Timothy S. Leroux: Conceptualization, Writing - original draft. Michael Paterson: Methodology, Validation. Donald A. Redelmeier: Conceptualization, Supervision, Methodology, Writing - review & editing.
Declaration of Competing Interest
The authors do not have any conflicts of interest.
Acknowledgements
This study was supported by a grant from the Canadian Institutes of Health Research and by ICES (grant no. 201701CMA), a non-profit research institute funded by the Ontario Ministry of Health and Long-Term Care (grant no. 384308). Parts of this material are based on data and information compiled and provided by the Canadian Institute for Health Information (CIHI). Dr. Austin is supported in part by a Mid-Career Investigator Award from the Heart and Stroke Foundation. None of the funders had any role in study design, data collection, data analysis, interpretation, or writing of the report.
References
- 1.Agency for Healthcare Research and Quality. AHRQ study: joint replacement to become the most common elective surgical procedure in the next decades 2016 [Available from:https://www.ahrq.gov/news/newsletters/e-newsletter/503.html.
- 2.Culliford D., Maskell J., Judge A., Cooper C., Prieto-Alhambra D., Arden N.K. Future projections of total hip and knee arthroplasty in the UK: results from the UK clinical practice research datalink. Osteoarthr Cartil. 2015;23(4):594–600. doi: 10.1016/j.joca.2014.12.022. 1522-9653 (Electronic) [DOI] [PubMed] [Google Scholar]
- 3.Kurtz S., Ong K., Lau E., Mowat F., Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Jt Surg Am. 2007;89(4):780–785. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 4.Kapadia B.H., McElroy M.J., Issa K., Johnson A.J., Bozic K.J., Mont M.A. The economic impact of periprosthetic infections following total knee arthroplasty at a specialized tertiary-care center. J Arthroplast. 2014;29(5):929–932. doi: 10.1016/j.arth.2013.09.017. 1532-8406 (Electronic) [DOI] [PubMed] [Google Scholar]
- 5.Lenguerrand E., Whitehouse M.R., Beswick A.D., Kunutsor S.K., Foguet P., Porter M. Risk factors associated with revision for prosthetic joint infection following knee replacement: an observational cohort study from England and Wales. Lancet Infect Dis. 2019;19(6):589–600. doi: 10.1016/S1473-3099(18)30755-2. 1474-4457 (Electronic) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le D.H., Goodman S., Maloney W., Huddleston J.I. Current modes of failure in TKA: infection, instability, and stiffness predominate. Clin Orthop Relat Res. 2014;472(7):2197–2200. doi: 10.1007/s11999-014-3540-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng H., Chen B.P., Soleas I.M., Ferko N.C., Cameron C.G., Hinoul P. Prolonged operative duration increases risk of surgical site infections: a systematic review. Surg Infect. 2017;18(6):722–735. doi: 10.1089/sur.2017.089. 1557-8674 (Electronic) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anis H.K., Sodhi N., Klika A.K., Mont M.A., Barsoum W.K., Higuera C.A. Is operative time a predictor for post-operative infection in primary total knee arthroplasty? J Arthroplast. 2018 doi: 10.1016/j.arth.2018.11.022. pii: S0883-5403(18)31150-1Epub ahead of print](1532-8406 (Electronic) [DOI] [PubMed] [Google Scholar]
- 9.Surace P., Sultan A.A., George J., Samuel L.T., Khlopas A., Molloy R.M. The association between operative time and short-term complications in total hip arthroplasty: an analysis of 89,802 surgeries. J Arthroplast. 2019;34(3):426–432. doi: 10.1016/j.arth.2018.11.015. 1532-8406 (Electronic) [DOI] [PubMed] [Google Scholar]
- 10.Dudeck M.A., Horan T., Peterson K., Allen-Bridson K., Morrell G., Pollock D. National healthcare safety network (NHSN) report, data summary for 2009, device-associated module. Am J Infect Control. 2011;39(5):349–367. doi: 10.1016/j.ajic.2011.04.011. 1527-3296 (Electronic) [DOI] [PubMed] [Google Scholar]
- 11.Kralj B. Ontario Medical Association; Toronto: 2005. Measuring “Rurality” for purposes of health care planning: an empirical measure for Ontario. [Google Scholar]
- 12.Health Quality Ontario & Ministry of Health and Long-Term Care. Quality-based procedures clinical handbook for primary hip and knee replacement. Available from:http://www.hqontarioca/evidence/publications-and-ohtac-recommendations/clinical-handbooks2013.
- 13.Redelmeier D.A., Thiruchelvam D., Daneman N. Introducing a methodology for estimating duration of surgery in health services research. J Clin Epidemiol. 2008;61(9):882. doi: 10.1016/j.jclinepi.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 14.Ko D.T., Mamdani M., Alter D.A. Lipid-lowering therapy with statins in high-risk elderly patients: the treatment-risk paradox. JAMA. 2004;291(15):1864–1870. doi: 10.1001/jama.291.15.1864. [DOI] [PubMed] [Google Scholar]
- 15.Hux J.E., Ivis F., Flintoft V., Bica A. Diabetes in Ontario: determination of prevalence and incidence using a validated administrative data algorithm. Diabetes Care. 2002;25(3):512–516. doi: 10.2337/diacare.25.3.512. [DOI] [PubMed] [Google Scholar]
- 16.Tu K., Campbell N.R., Chen Z.L., Cauch-Dudek K.J., McAlister F.A. Accuracy of administrative databases in identifying patients with hypertension. Open Med. 2007;1(1):e18–e26. [PMC free article] [PubMed] [Google Scholar]
- 17.Gershon A., Wang C., Guan J., Vasilevska-Ristovska J., Cicutto L., To T. Identifying individuals with physcian diagnosed COPD in health administrative databases. COPD. 2009;6(5):388–394. doi: 10.1080/15412550903140865. [DOI] [PubMed] [Google Scholar]
- 18.Information CIfH . CIHI; Ottawa, Ont.: 2008. Reducing gaps in health: a focus on socio-economic status in urban canada. [Google Scholar]
- 19.Marrie R.A., Dawson N.V., Garland A. Quantile regression and restricted cubic splines are useful for exploring relationships between continuous variables. J Clin Epidemiol. 2009;62(5):511–517. doi: 10.1016/j.jclinepi.2008.05.015. e1. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention. Instructions for completion of denominator for procedure form (CDC 57.121)2019 [Available from:https://www.cdc.gov/nhsn/forms/instr/57_121.pdf.
- 21.Hu F.B. Sedentary lifestyle and risk of obesity and type 2 diabetes. Lipids. 2003;38(2):103–108. doi: 10.1007/s11745-003-1038-4. [DOI] [PubMed] [Google Scholar]
- 22.Colosia A.D.P.R., Khan S. Prevalence of hypertension and obesity in patients with type 2 diabetes mellitus in observational studies: a systematic literature review. Diabetes Metab Syndr Obes. 2013;6:327–338. doi: 10.2147/DMSO.S51325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kenchaiah S., Evans J.C., Levy D., Wilson P.W.F., Benjamin E.J., Larson M.G. Obesity and the risk of heart failure. N Engl J Med. 2002;347(5):305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
- 24.O'Donnell D.E.O.D.C., Webb K.A., Guenette J.A. Respiratory consequences of mild-to-moderate obesity: impact on exercise performance in health and in chronic obstructive pulmonary disease. Pulm Med. 2012;2012 doi: 10.1155/2012/818925. doi: 101155/2012/818925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hubbard R.E., Lang I.A., Llewellyn D.J., Rockwood K. Frailty, body mass index, and abdominal obesity in older people. J Gerontol Ser A-Biol Sci Med Sci. 2010;65(4):377–381. doi: 10.1093/gerona/glp186. [DOI] [PubMed] [Google Scholar]
- 26.White H., Matheson F., Moineddin R., Dunn J., Glazier R. Neighbourhood deprivation and regional inequalities in self-reported health among Canadians: are we equally at risk? Health Place. 2011;17(1):361–369. doi: 10.1016/j.healthplace.2010.11.016. Epub Dec 8. [DOI] [PubMed] [Google Scholar]