Abstract
Background
Hypoxaemia is a common complication of pneumonia and a major risk factor for death, but less is known about hypoxaemia in other common conditions. We evaluated the epidemiology of hypoxaemia and oxygen use in hospitalised neonates and children in Nigeria.
Methods
We conducted a prospective cohort study among neonates and children (<15 years of age) admitted to 12 secondary-level hospitals in southwest Nigeria (November 2015–November 2017) using data extracted from clinical records (documented during routine care). We report summary statistics on hypoxaemia prevalence, oxygen use, and clinical predictors of hypoxaemia. We used generalised linear mixed-models to calculate relative odds of death (hypoxaemia vs not).
Findings
Participating hospitals admitted 23,926 neonates and children during the study period. Pooled hypoxaemia prevalence was 22.2% (95%CI 21.2–23.2) for neonates and 10.2% (9.7–10.8) for children. Hypoxaemia was common among children with acute lower respiratory infection (28.0%), asthma (20.4%), meningitis/encephalitis (17.4%), malnutrition (16.3%), acute febrile encephalopathy (15.4%), sepsis (8.7%) and malaria (8.5%), and neonates with neonatal encephalopathy (33.4%), prematurity (26.6%), and sepsis (21.0%). Hypoxaemia increased the adjusted odds of death 6-fold in neonates and 7-fold in children. Clinical signs predicted hypoxaemia poorly, and their predictive ability varied across ages and conditions. Hypoxaemic children received oxygen for a median of 2–3 days, consuming ∼3500 L of oxygen per admission.
Interpretation
Hypoxaemia is common in respiratory and non-respiratory acute childhood illness and increases the risk of death substantially. Given the limitations of clinical signs, pulse oximetry is an essential tool for detecting hypoxaemia, and should be part of the routine assessment of all hospitalised neonates and children.
Keywords: Hypoxaemia, Child, Neonate, Africa, Nigeria, Pulse oximetry, Oxygen
Research in context
Evidence before this study
Hypoxaemia is a well-recognised complication of pneumonia, increasing the risk of death four-fold and difficult to detect using clinical signs alone. Previous systematic reviews have collated data on the epidemiology of hypoxaemia in children with pneumonia, but fewer studies have addressed hypoxaemia in non-pneumonia cohorts or provided comparative data across age or diagnostic categories. With growing policy attention towards the scale up of pulse oximetry and oxygen therapy in low and middle-income countries (LMICs), policy-makers and program managers need data to inform decision-making. Current modelling estimates have addressed hypoxaemia, pulse oximetry and oxygen therapy primarily through the lens of child pneumonia. However, the burden of hypoxaemia, and the potential for reducing mortality through improved pulse oximetry and oxygen access, is much greater when non-pneumonia conditions are considered.
Added value of this study
This multi-centre cohort study was nested in the Nigerian Oxygen Implementation project, a large stepped wedge field trial evaluating pulse oximetry and improved oxygen systems. Our cohort included over 23,000 hospitalised children from birth to adolescence with various conditions, enabling us to describe the epidemiology of hypoxaemia in children and neonates admitted to secondary-level hospitals in low-altitude African towns. We found high hypoxaemia prevalence in pneumonia and other common childhood conditions, and particularly high prevalence in neonates. We found that hypoxaemia increased the risk of death across all age groups and all diagnostic categories, with higher risk in non-respiratory conditions. We found that clinical signs recorded during routine care were particularly poor at predicting hypoxaemia in children and neonates with non-respiratory conditions, having much lower sensitivity for hypoxaemia in non-respiratory conditions than respiratory conditions. The predictive value of the WHO combination of signs for hypoxaemia was reasonably good for child pneumonia, but was much poorer for non-respiratory conditions (particularly for children over the age of 1 year). We found that most hypoxaemic children required oxygen therapy for 2–3 days and calculated the mean annual oxygen requirements for small- and medium-sized hospitals that admit children.
Implications of all the available evidence
Hypoxaemia is common in respiratory and non-respiratory acute childhood illness and increases the risk of death substantially. Given the limitations of clinical signs recorded during routine care, pulse oximetry is an essential tool for detecting hypoxaemia, and should be part of the routine assessment of all hospitalised neonates and children.
1. Introduction
Hypoxaemia refers to low blood oxygen levels, typically defined as haemoglobin oxygen saturations less than 90% as measured by pulse oximetry (peripheral oxygen saturation, SpO2) or blood gas analysis (arterial oxygen saturation, SaO2) [1]. In childhood pneumonia, hypoxaemia is a common complication and an important marker of severity. Approximately 13% of children hospitalised with pneumonia in low- or middle-income countries are hypoxaemic [2] and hypoxaemia increases their risk of death approximately four-fold [3].
Multifaceted interventions to improve the detection of hypoxaemia and use of oxygen therapy can reduce inpatient child pneumonia mortality by approximately 35% [4], [5], [6]. Modelling estimates suggest that better use of pulse oximetry and oxygen therapy in the 12 highest mortality countries could prevent up to 148,000 child pneumonia deaths annually [7].
While hypoxaemia is less well studied in non-pneumonia cohorts, the available data suggests that hypoxaemia may be common in many non-respiratory conditions [2,[8], [9], [10], [11], [12], [13], [14], [15], [16], [17]], and that addressing oxygen access issues for these populations could deliver substantial mortality reductions [5,6].
Nigeria is a highly-populated, lower middle-income country in sub-Saharan Africa that contributes disproportionately to global child and neonatal mortality (2017: under-five mortality 100, neonatal mortality 33, per 1000 live births) [18]. The biggest childhood killers are pneumonia (18%), malaria (14%), prematurity (12%), perinatal complications (11%), diarrhoeal diseases (10%), and neonatal sepsis (5%) [19]. Recent global estimates show that Nigeria contributed one-third of U/5 malaria deaths and one-sixth of U/5 pneumonia deaths globally [19].
This multi-centre study aimed to describe the prevalence of hypoxaemia in children and neonates admitted to secondary-level hospitals in Nigeria with pneumonia, malaria, and other common childhood and neonatal conditions, using data recorded during routine care. In addition, we sought to determine: (i) the degree to which hypoxaemia predicts mortality; (ii) the duration of oxygen therapy required for hypoxaemic conditions; and (iii) which clinical signs best predict hypoxaemia - for children of different ages and with different conditions. This will inform estimates of the benefit of improved pulse oximetry and oxygen practices for children and neonates in resource-limited settings and assist with oxygen-related program planning.
2. Methods
2.1. Design
This prospective cohort study was nested within a stepped-wedge trial to improve oxygen use in 12 secondary-level hospitals in southwest Nigeria [20]. The first stage of this trial involved a needs-assessment [21] and retrospective clinical review. The second stage involved the introduction of pulse oximetry to participating hospitals, commencing in October/November 2015 [22]. The third stage involved the stepped introduction of comprehensive oxygen systems, with hospitals randomised to receive the intervention between March 2016 and March 2017. This paper reports prospective data from November 2015 to October 2017, using data extracted from clinical records.
2.2. Participants
We conducted this study in 12 small to medium-sized hospitals in southwest Nigeria, selected to be representative of secondary healthcare facilities that admit children [20]. This included a mix of government (n = 7) and mission (n = 5) hospitals of varying capacity (Table 1, more detail in Appendix 1). All hospitals were low-altitude (elevation 50–500 m above sea level).
Table 1.
Baseline characteristics, admissions, and pulse oximetry adoption patterns of 12 secondary-level hospitals in southwest Nigeria. Additional detail in Web Appendix 1.
| H1 | H2 | H3 | H4 | H5 | H6 | H7 | H8 | H9 | H10 | H11 | H12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hospital characteristics | ||||||||||||
| Hospital Type | Mission | Mission | State | State | State | Mission | State | State | State | Mission | Mission | State |
| Paediatric Beds child + neonatal |
70 (40+30) |
32 (20+12) |
25 (21+4) |
36 (16+20) |
60 (44+16) |
20 (15+5) |
48 (20+28) |
46 (22+24) |
13 (9 + 4) |
63 (38+25) |
14 (12+2) |
36 (26+10) |
| Access to Paediatrician Doctors in hospital Nurses in child/newborn wards |
Yesii 4 18 |
Noi 4 7 |
Yes 2 16 |
Yes 11 33 |
Yes 17 62 |
Yes 5 9 |
Yes 16 26 |
No 12 31 |
Noi 7 11 |
Yesii 6 18 |
Yesii 6 4 |
Noi 7 26 |
| Oxygen cylinders Oxygen concentrators |
Yesiv Yesv |
Yesiv No |
Yesiv Yesv |
Yes Yesv |
Yes No |
Yesiv Yes |
Yesiii Yesv |
Yes No |
Yesiv No |
Yes Yesv |
Yesiv Yesv |
Yesiv No |
| Pulse oximeters | 0 | 0 | 0 | 0 | 3v | 1 | 0 | 0 | 0 | 0 | 1v | 0 |
| Participants (total) | 4133 | 674 | 1655 | 2943 | 2176 | 635 | 4313 | 1790 | 495 | 3327 | 869 | 916 |
| Neonates <28 days | 2228 | 100 | 112 | 1322 | 406 | 102 | 1480 | 455 | 9 | 1170 | 89 | 0 |
| Infants 28 days to 1 year | 519 | 176 | 327 | 449 | 600 | 97 | 1085 | 388 | 117 | 543 | 131 | 187 |
| Children 1–4 years | 921 | 259 | 775 | 785 | 790 | 288 | 1669 | 582 | 235 | 990 | 312 | 455 |
| Children 5–14 years | 449 | 138 | 436 | 378 | 342 | 143 | 76 | 347 | 133 | 624 | 333 | 272 |
| Sex:% female | 44.8 | 44.7 | 46.9 | 42.2 | 42.0 | 41.6 | 44.6 | 43.8 | 45.3 | 43.7 | 42.9 | 40.6 |
| Median age, months (IQR) | 0 (0–20) | 15 (5–48) | 24 (11–60) | 5 (0–24) | 14 (2–39) | 24 (9–55) | 8 (0–18) | 13 (1–44) | 25 (12–60) | 11 (0–36) | 36 (11–90) | 24 (12–60) |
| Median LOS, days (IQR) Neonates <28 days Children |
4 (3–7) 5 (3–9) 3 (2–5) |
3 (2–5) 4 (2–5) 3 (2–4) |
3 (2–4) 3 (2–7) 3 (2–5) |
3 (2–6) 4 (2–6) 3 (2–5) |
8 (7–10) 11(10–13) 7 (7–9) |
3 (2–4) 4 (2–6) 3 (2–4) |
3 (2–5) 4 (2–6) 3 (2–4) |
7 (4–9) 10 (4–11) 6 (4–7) |
2 (1–2) 3 (3–4) 2 (1–2) |
4 (3–5) 5 (3–7) 4 (3–5) |
2 (1–3) 3 (2–4) 2 (1–3) |
3 (2–4) - 3 (2–4) |
| Pulse oximetry documented, n (%) | 3586 (88.4) | 559 (82.9) | 1415 (86.8) | 2365 (80.4) | 1911 (87.8) | 610 (96.1) | 2406 (57.2) | 1292 (74.4) | 364 (74.6) | 2965 (90.2) | 819 (94.3) | 585 (64.7) |
| Hypoxaemia prevalence Neonates <28 days Children |
16.9 20.2 12.5 |
16.6 44.3 12.1 |
12.9 20.6 12.3 |
16.2 25.9 8.9 |
11.3 17.5 9.8 |
11.0 32.7 6.7 |
20.1 30.6 13.7 |
13.2 18.6 11.1 |
4.4 33.3 4.2 |
11.3 16.8 8.0 |
7.5 7.9 7.4 |
9.6 - 9.6 |
| Deaths Case fatality rate (%) Neonates <28 days Children |
411 10.0 14.1 5.1 |
59 8.8 23.0 6.3 |
134 8.1 14.3 7.7 |
160 5.5 7.1 4.2 |
79 3.6 5.4 3.2 |
43 6.8 21.6 4.0 |
207 4.8 9.4 2.5 |
124 7.0 11.9 5.3 |
18 3.6 11.1 3.5 |
141 4.2 4.9 3.9 |
28 3.2 1.1 3.5 |
26 2.8 - 2.8 |
Neonate ≤28 days, Child 29 days-15 years. IQR = inter-quartile range, 25th/75th centiles. LOS = length of stay. (i) Family Medicine Consultant; (ii) part-time; (iii) piped system connected to large oxygen cylinder; (iv) not available in paediatric areas; (v) present but not fit for use.
We included all children aged under 15 years (including neonates) who were admitted to participating hospitals during the study period (November 2015 to October 2017, inclusive). This included neonates born in the facility as well as neonates (and children) referred in from elsewhere, without prejudice to gestational age, birthweight or diagnosis. Table 1 shows the participating hospital demographics.
2.3. Materials and procedures
We introduced pulse oximetry into routine clinical care for paediatrics (including neonates) using adapted WHO oxygen guidelines [1,23] (Appendix 2). The guidelines required all children (aged under 15 years) to have pulse oximetry performed on admission, and at least twice per day while receiving oxygen. Individual hospitals determined the specific pulse oximetry procedures (e.g. timing, location, personnel) based on local workflow and practices. Typically, patients presented to the children's outpatient or emergency area and a nurse would perform pulse oximetry alongside other vital signs prior to admission. In most hospitals, the admitting doctor would also perform pulse oximetry when completing the formal admission procedures.
We conducted hospital-based pulse oximetry training for nurses and doctors working in paediatric areas in October/November 2015 and supplied handheld Lifebox™ pulse oximeters (Acare Technology, Taiwan). Lifebox oximeters were developed for low-resource settings by the World Health Organization (WHO) and the World Federation of Societies for Anaesthesiologists, with rated accuracy of +/−2% [24,25]. We instructed healthcare workers to conduct pulse oximetry using an appropriately-sized probe applied to a well-perfused finger or toe, and to wait until there was a strong and steady waveform before recording the SpO2 reading. We provided supportive supervision through onsite project nurses in each hospital and regular visits from the project manager and/or nurse supervisor [20,22]. We introduced a comprehensive improved oxygen system to all hospitals at stepped intervals (3 hospitals every 4 months from March 2016 to March 2017), including oxygen concentrators (with reliable power), individually titratable flowmeter assemblies, appropriately-sized nasal prongs, and guidelines for using, cleaning and maintaining equipment (details in protocol [20]).
We collected data from the clinical records, including case notes and nursing observation charts. We did not provide additional training or standardization of healthcare worker diagnostic, treatment or documentation practices. Trained research nurses extracted data from case notes using a standardised data collection form. This included SpO2 on admission, oxygen use, as well as demographic data, diagnoses, symptoms and signs on admission, and clinical care practices.
2.4. Data analysis
Trained data entry clerks double-entered data from data collection forms using EpiData 3.1 [26], following standard data management procedures. We performed data cleaning and analysis using Stata 15.1 [27]. We defined hypoxaemia as SpO2 < 90%, irrespective of age or condition. We calculated hypoxaemia prevalence based on the SpO2 recorded on admission, in order to consistently represent the condition of the patient and to enable temporal correlation with the recorded admission signs. We calculated proportions and 95% confidence intervals for hypoxaemia prevalence and case fatality rates using complete case analysis approach (i.e. dropping missing data from analysis) [28]. We conducted sensitivity testing to evaluate the effect of missing data on the primary hypoxaemia prevalence outcome, by comparing prevalence estimates from periods with high missing data (earlier time periods) to periods with low missing data (later time periods) [28].
We calculated relative odds of death and 95% confidence intervals for patients with and without hypoxaemia, using generalised linear mixed-model (GLMM) analysis to adjust for clustering (at hospital level), time (in 4-month periods), age, sex, comorbid diagnoses, and the presence of the improved oxygen system [29,30]. We calculated median and inter-quartile ranges for oxygen use (duration, starting flow rate) and pre-treatment SpO2, restricting analysis to the post-intervention period to reflect clinical practice in the presence of adequate oxygen access. We calculated means for oxygen use (duration, starting flow rate) to enable the calculation of total oxygen demand. We calculated total volume of oxygen consumed by multiplying the mean duration of therapy by the starting flow rate (field observation showed that the flow rate was rarely altered after starting oxygen).
We calculated the sensitivity, specificity, positive and negative likelihood ratios, and area under the receiver operator characteristic (ROC) curve (AUC) for various clinical signs in predicting hypoxaemia (both individually and in combination) using the diagt program for Stata [31]. We provide tabular comparison of the predictive accuracy of various combinations of signs against the WHO combination: respiratory rate (RR) ≥70 breaths per minute (bpm), severe chest wall indrawing, grunting, inability to feed due to respiratory distress [23].
We report this data for various age groups (neonates <28 days, infants 28–364 days, young children 1–4 years, older children 5–14 years) and diagnoses. We report for the most common presenting clinical syndromes and diagnoses: acute lower respiratory infection (which includes pneumonia and bronchiolitis), acute febrile encephalopathy (which includes cerebral malaria, viral and bacterial encephalopathies), malaria, sepsis, acute watery diarrhoea, small/premature neonate (including premature and low birth weight), and neonatal jaundice. We also report additional conditions we considered of particular interest (e.g. HIV, severe acute malnutrition, asthma). We determined diagnostic case status for most conditions based on recorded admission symptoms, signs, and laboratory testing using standard case definitions (Table 2). If a sign was not recorded, we assumed that it was not present. We used the recorded admission diagnosis for diagnoses with more complex case definitions that required data not readily available from case notes (e.g. sepsis).
Table 2.
Diagnostic case definitions.
| Diagnosis | Diagnostic Definition and notes |
|---|---|
| ALRI, acute lower respiratory infection | Case definition (WHO) [23]: Cough or difficult breathing and any of: fast breathing, lower chest wall in-drawing. |
| Severe ALRI | Case definition (WHO) [23]: ALRI plus any of: central cyanosis, SpO2<90%, severe respiratory distress (e.g. grunting, very severe chest wall in-drawing), general danger sign (inability to breastfeed/drink, lethargy or unconscious, convulsions). |
| AFE, acute febrile encephalopathy | Case definition [65]: Fever and any of: seizures, altered conscious state. This clinical syndrome includes viral and bacterial encephalopathies, cerebral malaria, and non-infective encephalopathies. |
| Diarrhoea | Case definition (WHO) [23]: >3 loose stools per day, not >14 days duration. |
| Malaria | Case definition (WHO) [23]: Fever (or history of fever) and positive malaria test (rapid test or microscopy). |
| Sepsis | Clinical diagnosis [23]: Based on assessment of fever (or history of fever) and a sign of severe illness or haemodynamic instability (e.g. inability to breastfeed/drink, lethargy or unconscious, convulsions, shock) without other cause. |
| Seizures | Case definition [23]: Seizures (or history of seizures). |
| Meningitis / Encephalitis | Clinical diagnosis [23]: Based on assessment of stiff neck, pain on neck flexion, bulging fontanelle, photophobia, altered conscious state. |
| Haemoglobinopathy (typically sickle cell disease) | Clinical diagnosis [23]: Based on history of haemoglobinopathy (e.g. SS disease) and admission signs of crisis (e.g. limb pain, stroke, chest pain). |
| SAM, severe acute malnutrition. | Case definition [23]: Weight-for-height less than −3 z scores of median (WHO growth standards), MUAC<115 cm, or nutritionally-associated oedema (kwashiorkor). These parameters were not routinely assessed for all children, so likely underestimates the prevalence of SAM. |
| URTI (upper respiratory tract infection) | Clinical diagnosis: Based on assessment of upper airway obstruction/discharge, without signs of lung involvement. |
| Asthma | Clinical diagnosis [23]: Based on assessment of fast or difficult breathing, and wheezing, with bronchodilator response. |
| Trauma, Burns, Poisoning | Clinical diagnoses: Based on assessment of history (trauma, burns, poisoning). |
| Typhoid fever | Clinical diagnosis [23]: Based on assessment of fever (or history of fever) and gastrointestinal signs (e.g. constipation, vomiting, pain, hepatosplenomegaly), headache, cough, or rash, without other cause. |
| HIV (human immunodeficiency virus) | Clinical diagnosis [23]: Based on known history of HIV infection, positive HIV test result, or presentation with signs of HIV-related illness (e.g. pneumocystis jirovecii, oesophageal candidiasis) or possible HIV-related illness (e.g. oral thrush, recurrent infections). |
| Small | Case definition (WHO) [23]: <2500 g birth weight. Low birth weight (LBW) 1500–2499 g; Very Low Birth Weight (VLBW) 1000–1499 g; Extremely Low Birth Weight (ELBW) <1000 g. |
| Preterm | Case definition (WHO) [23]: <37 weeks gestational age. Gestational age typically estimated from last menstrual period and/or ultrasound scan, but was unknown for the majority of participants. |
| Neonatal sepsis (suspected) | Clinical diagnosis: Based on assessment of temperature (high or low), altered conscious state, other signs of possible infection (e.g. umbilical discharge), major risk factors for sepsis (e.g. maternal chorioamnionitis). |
| NE (Neonatal encephalopathy) | Clinical diagnosis [66]: Based on assessment of persisting altered conscious state and seizures or other neurobehavioural symptoms (e.g. abnormal posturing, hypotonia, weak or absent suck or reflexes). |
| Jaundice | Clinical diagnosis: Based on clinical assessment (e.g. skin colour, feeding) and laboratory confirmation with serum bilirubin. |
Wherever possible we determined case status using recorded admission symptoms, signs, and laboratory testing (“case definition”). For diagnoses for which we were unable to determine cases from the documented clinical data we relied on the recorded admission diagnosis (“clinical diagnosis”).
We present overall hypoxaemia prevalence findings in a bubble plot, illustrating prevalence (y-axis) and numbers affected (bubble size), across age groups (x-axis) and diagnoses (labelled bubbles). We report hypoxaemia prevalence and case fatality rates based on ‘any diagnosis’ (i.e. irrespective of whether it was identified as a primary or additional diagnosis, or whether it met criteria for multiple case definitions), with adjustment for comorbidity where relevant. For comparative purposes, we report additional analysis for ‘primary diagnosis’ and particular combinations of case definitions (e.g. ALRI with or without malaria) in Appendix 3. For reporting purposes we classified hospitals as small (<500 child admissions annually) and medium (500–2500 child admissions annually).
2.5. Role of the funding source
RI represented the funding agency and participated in site selection and methods meetings which informed the study design. The corresponding author, AAB, AIA, OBO, AGF, and TD had full access to all the data in the study. All authors had final responsibility for the decision to submit for publication.
3. Results
Participating hospitals admitted 16,453 children (aged 28 days or more) and 7473 neonates over the 24 month study period (November 2015 – October 2017). 23,846 (99.7%) had a documented clinical outcome and 18,877 (78.9%) had a documented SpO2 on admission.
3.1. Hypoxaemia prevalence
Hypoxaemia prevalence was 22.2% (95%CI 21.2–23.2) for neonates and 10.2% (9.7–10.8) for children aged ≥28 days (Table 3, Fig. 1). Hypoxaemia prevalence decreased with increasing age. Hypoxaemia prevalence varied between hospitals (median 12.1%, range 4.4–20.1%). Medium-sized hospitals typically admitted a higher proportion of hypoxaemic patients than small hospitals (15% vs 10%) and this was primarily accounted for by a higher proportion of neonates. Sensitivity testing showed that these prevalence estimate results remained robust to the effects of missing data.
Table 3.
Hypoxaemia on admission and corresponding case fatality rates and relative odds of death among children (aged <15 years) admitted to 12 secondary-level hospitals in southwest Nigeria over a 2 year period (Nov 2015–Oct 2017 inclusive).
| Cohort / Condition | No. (%) | No. hypoxaemic (%) | Hypoxaemia prevalence (95% CI) | Case fatality rate (95% CI) | Relative odds of death, hypoxaemic vs non-hypoxaemic, OR (95% CI) | |
|---|---|---|---|---|---|---|
| Crude | Adjusted [1] | |||||
| Total | 23,926 (100) | 2667 (100) | 14.1% (13.6–14.6) | 6.0% (5.7–6.3) | 8.4 (7.4–9.6) | 7.9 (7.0–9.0) |
| Neonates <28 days | 7473 (31.2) | 1363 (51.1) | 22.2% (21.2–23.2) | 9.9% (9.3–10.6) | 6.2 (5.2–7.3) | 5.6 (4.5–6.8) |
| Infants 28 days to 1 year | 4619 (19.3) | 543 (20.4) | 15.6% (14.4–16.9) | 5.6% (5.0–6.3) | 8.2 (6.1–11.1) | 7.7 (5.4–11.1) |
| Children 1–4 years | 8060 (33.7) | 537 (20.1) | 8.8% (8.1–9.5) | 3.8% (3.4–4.3) | 8.9 (6.7–11.9) | 7.5 (5.5–10.2) |
| Children 5–14 years | 3671 (15.3) | 220 (8.2) | 7.2% (6.3–8.2) | 3.4% (2.8–4.0) | 9.5 (6.0–14.8) | 7.6 (4.7–12.4) |
| Female | 10,468 (43.7) | 1147 (43.0) | 13.9% (13.2–14.7) | 5.9% (5.5–6.4) | 9.1 (7.5–11.0) | 8.7 (7.2–10.5) |
| Male | 13,436 (56.1) | 1519 (57.0) | 14.3% (13.6–15.0) | 6.0 (5.6–6.4) | 7.9 (6.7–9.4) | 7.3 (6.2–8.7) |
| Government (n = 7) | 14,292 (59.7) | 1507 (56.5) | 14.6% (13.9–15.3) | 5.3% (4.9–5.6) | 8.1 (6.8–9.7) | 8.0 (6.7–9.6) |
| Mission (n = 5) | 9646 (40.3) | 1160 (43.5) | 13.6% (12.9–14.3) | 7.1% (6.6–7.6) | 9.0 (7.5–10.7) | 8.2 (6.5–10.5) |
| Small [2] (n = 5) | 3590 (15.0) | 293 (11.0) | 10.0% (8.9–11.1) | 4.9% (4.2–5.6) | 12.4 (8.5–18.0) | 11.4 (7.8–16.5) |
| Medium [2] (n = 7) | 20,348 (85.0) | 2374 (89.0) | 14.9% (14.3–15.5) | 6.2% (5.9–6.5) | 7.9 (6.9–9.1) | 12.2 (7.9–18.8) |
| Child diagnoses[3] | ||||||
| Total children | 16,453 (100) | 1304 (100) | 10.2% (9.7–10.8) | 4.2% (3.9–4.5) | 9.2 (7.7–11.0) | 7.3 (6.0–9.0) |
| Malaria | 6166 (37.5) | 428 (32.8) | 8.5% (7.7–9.3) | 2.9% (2.5–3.3) | 9.4 (6.6–13.3) | 6.6 (4.6–9.5) |
| AFE | 3526 (21.4) | 412 (31.6) | 15.4% (14.1–16.9) | 8.6% (7.7–9.6) | 7.2 (5.4–9.5) | 6.5 (4.8–8.9) |
| ALRI | 2073 (12.6) | 486 (37.3) | 28.0% (25.9–30.2) | 7.2% (6.1–8.4) | 6.0 (4.0–8.9) | 7.1 (4.6–10.9) |
| Diarrhoea | 2007 (12.2) | 86 (6.6) | 6.1% (4.9–7.5) | 3.8% (3.0–4.7) | 19.3 (10.5–35.0) | 16.2 (7.8–33.6) |
| Sepsis | 5147 (31.6) | 356 (27.3) | 8.7% (7.8–9.6) | 4.7% (4.1–5.3) | 8.6 (6.2–11.8) | 5.9 (4.1–8.3) |
| Seizures | 2081 (12.6) | 238 (18.3) | 14.3% (12.7–16.1) | 7.7% (6.6–8.9) | 6.8 (4.6–10.0) | 6.3 (4.2–9.3) |
| Meningitis/Encephalitis | 521 (3.2) | 75 (5.8) | 17.4% (13.9–21.3) | 14.1% (11.2–17.4) | 4.4 (2.3–8.2) | 4.5 (2.3–8.6) |
| Haemoglobinopathy | 739 (4.5) | 56 (4.3) | 9.1% (6.9–11.6) | 3.4% (2.2–5.0) | 4.8 (1.6–13.1) | 2.9 (0.9–9.2) |
| Malnutrition (SAM) | 324 (2.0) | 45 (3.5) | 18.0% (13.4–23.3) | 16.4% (12.5–20.9) | 6.5 (3.0–14.2) | 14.6 (4.6–46.6) |
| URTI | 680 (4.2) | 37 (2.8) | 6.8% (4.9–9.3) | 1.0% (0–2.1) | 9.8 (0.8–87.7) | – |
| Asthma | 109 (0.7) | 20 (1.5) | 20.4% (12.9–29.7) | 0.9% (0.0–5.0) | – | – |
| Trauma, Burns, Poisoning | 351 (2.2) | 20 (1.5) | 7.1% (4.4–10.8) | 2.0% (0.8–4.1) | 4.5 (0.1–58.5) | – |
| Typhoid | 371 (2.3) | 13 (1.0) | 4.1% (2.2–6.8) | 3.5% (1.9–5.9) | 16.6 (3.0–76.0) | 21.6 (1.9–241.3) |
| HIV | 21 (0.1) | 2 (0.2) | 10.5% (1.3–33.2) | 19.0% (5.4–41.9) | – | – |
| Neonatal diagnoses[4] | ||||||
| Total neonates | 7473 (100) | 1363 (100) | 22.2% (21.2–23.2) | 9.9% (9.3–10.6) | 6.2 (5.2–7.3) | 5.6 (4.5–6.8) |
| Small / Preterm | 1770 (23.7) | 387 (28.4) | 25.8% (23.6–28.1) | 17.5% (15.7–19.3) | 4.3 (3.2–5.7) | 4.6 (3.3–6.3) |
| Small (<2500 g) | 1399 (18.7) | 311 (22.8) | 26.4% (23.9–29.0) | 17.6% (15.7–19.7) | 4.9 (3.5–6.8) | 5.5 (3.8–7.8) |
| - LBW | 1105 (14.8) | 228 (16.7) | 24.3% (21.5–27.1) | 10.8% (9.0–12.7) | 7.1 (4.5–11.2) | 7.7 (4.8–12.6) |
| - VLBW | 231 (3.1) | 58 (4.3) | 30.7% (24.2–37.8) | 32.0% (26.1–38.5) | 2.7 (1.4–5.5) | 3.1 (1.4–6.9) |
| - ELBW | 63 (0.8) | 25 (1.8) | 52.1% (37.2–66.7) | 85.7% (74.6–93.3) | 0.7 (0.1–6.8) | – |
| Preterm (<37 wks) | 1326 (17.7) | 299 (21.9) | 26.6% (24.1–29.3) | 19.6% (17.5–21.9) | 3.5 (2.5–4.7) | 3.6 (2.5–5.1) |
| Neonatal encephalopathy | 2850 (40.4) | 821 (60.2) | 33.4% (31.6–35.3) | 13.2% (12.0–14.5) | 4.1 (3.2–5.3) | 4.2 (3.2–5.6) |
| Neonatal sepsis | 3884 (54.9) | 671 (49.2) | 21.0% (19.6–22.4) | 10.0% (9.0–10.9) | 5.9 (4.6–7.6) | 5.6 (4.2–7.4) |
| Jaundice | 1692 (24.06) | 115 (8.4) | 8.8% (7.3–10.5) | 4.4% (3.5–5.5) | 10.1 (5.8–17.6) | 8.0 (4.0–16.2) |
(1) Adjusted for clustering, time, age, sex, comorbidity, and presence of improved oxygen system using generalised linear mixed model (GLMM); (2) Hospital size: Small - <500 annual paediatric (U/15) admissions; Medium – 500–2500 annual paediatric (U/15) admissions; (3) Malaria, AFE, ALRI, Diarrhoeal, SAM diagnoses based on case definition, other diagnoses based on recorded admission diagnosis; (4) Small and preterm diagnoses based on recorded birthweight and gestation, other diagnoses based on recorded admission diagnosis. AFE = acute febrile encephalopathy (fever on history or examination PLUS altered conscious state or seizures); ALRI = acute lower respiratory infection (cough or difficult breathing PLUS fast breathing for age or lower chest wall in-drawing); Diarrhoea case definition = >3 loose stools per day, not for >14 days duration; ELBW = extremely low birth weight (<1000 g); Hypoxaemia = SpO2 < 90%; LBW = low birth weight (1500–2499 g); Small / Preterm = <2500 g birthweight or <37 weeks gestational age; SAM = severe acute malnutrition (ght-for-height less than −3 z scores of median (WHO growth standards), MUAC<115 mm, or nutritionally-associated oedema (kwashiakor)); URTI = upper respiratory tract infection; VLBW = very low birth weight (1000–1499 g).
Fig. 1.
Bubble plot showing hypoxaemia prevalence on admission (y-axis), of children (<15 years of age) admitted to 12 hospitals in southwest Nigeria (November 2015 to October 2017, inclusive): by condition (labelled bubbles) and age group (x-axis). Size (area) of bubbles represent the number of affected participants.
AFE = acute febrile encephalopathy; ALRI = acute lower respiratory infection; AS = asthma; D = diarrhoea; HG = haemoglobinopathy; HIV = HIV/AIDS; JN = neonatal jaundice; M = malaria; ME = meningitis/encephalitis; NE = neonatal encephalopathy; NSS = neonatal sepsis; PT/S = preterm/small; SAM = severe acute malnutrition; SS = sepsis; SZ = seizures; TY = typhoid; URTI = upper respiratory tract infection. Blue = respiratory condition; Red = other infectious condition; Yellow = non-infectious condition. Malaria, AFE, ALRI, Diarrhoeal, SAM diagnoses based on case definition, small and preterm diagnoses based on recorded birthweight and gestation, other diagnoses based on recorded admission diagnosis.
Fig. 1 is a bubble plot showing the prevalence of hypoxaemia in various conditions across age groups. Among children, hypoxaemia was most prevalent in those with acute lower respiratory tract infection (ALRI), affecting 1 in 3 children (36.1%) who met WHO criteria for severe disease based on clinical signs, and 1 in 6 (16.6%) children who had no other signs of severe disease. Hypoxaemia was highly prevalent in children with asthma (20.4%), meningitis/encephalitis (17.4%), malnutrition (16.3%), and acute febrile encephalopathy (AFE) (15.4%).
Hypoxaemia affected 1 in 7 (15.3%) children with complicated malaria and 1 in 20 (4.6%) children with uncomplicated malaria (Table 3, Fig. 1). In absolute numbers there were similar numbers of hypoxaemic children with diagnoses of ALRI (n = 486), confirmed malaria (n = 428), and acute febrile encephalopathy (n = 412). Together these three presentations accounted for almost three-quarters of hypoxaemia cases (948/1304, 72.7%).
Among neonates, hypoxaemia was highly prevalent in all three of the major causes of mortality – prematurity (26.6%), sepsis (21.0%), and neonatal encephalopathy (33.4%) (Table 3, Fig. 1). These presentations accounted for almost all of the neonatal hypoxaemia cases (1218/1363, 89.4%).
Table 3 presents hypoxaemia prevalence data for the most common diagnostic categories. We found minor differences between hypoxaemia prevalence when analysed by “any diagnosis” compared to “primary diagnosis” (see Appendix 3).
3.2. Hypoxaemia as a risk factor for mortality
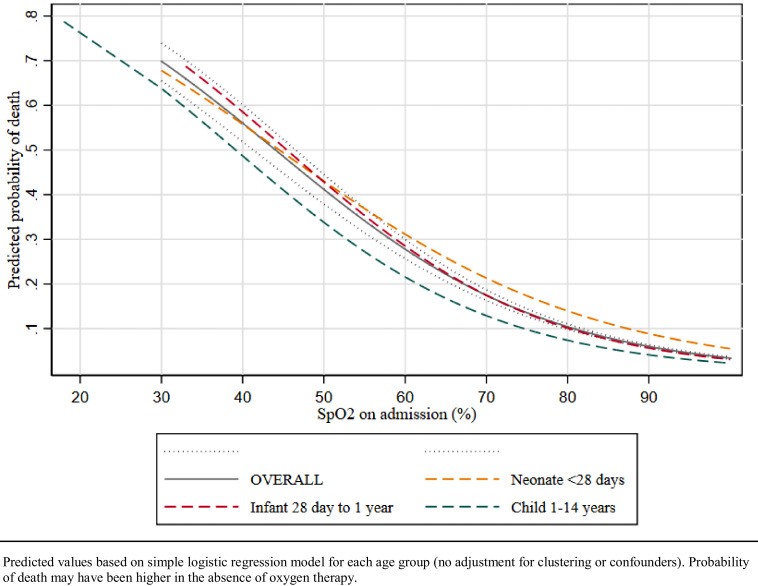
Table 3 presents data on the relative odds of death comparing hypoxaemic to non-hypoxaemic cohorts for the most common diagnoses. Hypoxaemia was a major predictor for mortality across all age groups and all diagnostic categories. The relative odds of death were higher in older children and those with conditions where hypoxaemia was less common (e.g. malaria versus ALRI). The risk of death was higher with lower admission SpO2 (Fig. 2).
Fig. 2.
Predicted probability of death according to oxygen saturation, among 23,938 neonates and children in 12 secondary-level hospitals in southwest Nigeria.
Predicted values based on simple logistic regression model for each age group (no adjustment for clustering or confounders). Probability of death may have been higher in the absence of oxygen therapy.
When adjusted for clustering, age, sex, comorbid diagnoses, and the presence of the improved oxygen system, neonates who were hypoxaemic on admission had 6-fold higher odds of death than those without hypoxaemia (aOR 5.6, 4.5–6.8), and hypoxaemic children had 7-fold higher odds of death than those without hypoxaemia (aOR 7.3, 6.0–9.0) (Table 3).
3.3. Oxygen therapy for hypoxaemia
Table 4 presents oxygen use data for various age and diagnostic categories. Children and neonates with hypoxaemia received oxygen for a median duration of 1.5 days (IQR 0.5–3.5) with little variation across age groups. Children with ALRI tended to require longer time on oxygen (median 2.5, IQR 1.5–3.5), as did preterm neonates (median 2.5, IQR 1.5–4.5). Among children and neonates with hypoxaemia, the median recorded SpO2 before starting oxygen therapy was 81% (IQR 67–88), with little variation across age groups or presenting conditions. Clinicians appeared to follow the guidelines regarding oxygen flow-rate, with neonates tending to be started on 0.5–1.0 litre per minute (LPM), younger children on 1.0–1.5 LPM and older children on 1.0–2.0 LPM. Based on these data, neonates with hypoxaemia can be expected to receive ∼3500 L of oxygen during admission, and children ∼5000 L. These rates are slightly higher for children with ALRI (∼5990 L), AFE (∼6480 L) and preterm neonates (∼4608 L).
Table 4.
Oxygen duration, starting flow rate, and pre-treatment oxygen saturation (SpO2) among hypoxaemic children and neonates admitted to participating hospitals.
| Oxygen duration (days) | Starting flow rate (LPM) | Pre-treatment oxygen saturation (%) | Post-treatment oxygen saturation (%) | Mean volume of oxygen required (L) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | IQR | Mean | Median | IQR | Mean | Median | IQR | Mean | Median | IQR | ||
| Overall | 2.8 | 1.5 | 0.5–3.5 | 1.1 | 1.0 | 0.5–1.0 | 74.6 | 81 | 67–88 | 94.6 | 96 | 94–98 | 4435 |
| Age group | |||||||||||||
| Neonates <28 days | 2.9 | 1.5 | 0.5–3.5 | 0.9 | 1.0 | 0.5–1.0 | 74.3 | 81 | 66–88 | 94.5 | 96 | 94–97 | 3758 |
| Infants 28 days to 1 year | 3.1 | 2.5 | 1.5–3.5 | 1.2 | 1.0 | 1.0–1.5 | 74.4 | 80 | 66.5–88 | 93.6 | 96 | 94–98 | 5356 |
| Children 1–4 years | 2.2 | 1.5 | 0.5–2.5 | 1.3 | 1.0 | 1.0–2.0 | 75.2 | 82 | 69–88 | 94.9 | 97 | 96–98 | 4118 |
| Children 5–14 years | 2.4 | 1.5 | 0.5–2.5 | 1.5 | 1.5 | 1.0–2.0 | 76.1 | 81.5 | 70.5–87 | 96.7 | 98 | 96–99 | 5184 |
| Condition | |||||||||||||
| ALRI | 3.2 | 2.5 | 1.5–3.5 | 1.3 | 1.0 | 1.0–2.0 | 74.8 | 81 | 68–88 | 94.3 | 96 | 94–98 | 5990 |
| AFE | 3.0 | 1.5 | 0.5–2.5 | 1.5 | 1.5 | 1.0–2.0 | 74.2 | 80.0 | 65–88 | 94.1 | 98 | 96–98 | 6480 |
| Malaria | 2.4 | 1.5 | 1.0–2.5 | 1.4 | 1.0 | 1.0–2.0 | 76.7 | 82 | 71–88 | 95.1 | 97 | 96–98 | 4838 |
| Diarrhoeal disease | 2.5 | 1.5 | 0.5–3.5 | 1.3 | 1.0 | 1.0–2.0 | 72.5 | 80 | 60–85 | 86.3 | 97 | 94–98 | 4680 |
| Sepsis | 2.6 | 1.5 | 0.5–3.5 | 1.2 | 1.0 | 1.0–2.0 | 75.8 | 82 | 68–88 | 93.9 | 97 | 95–98 | 4320 |
| Preterm | 4.0 | 2.5 | 1.5–4.5 | 0.8 | 0.5 | 0.5–1.0 | 75.6 | 82 | 68–89 | 95.4 | 96 | 94–97 | 4608 |
| Neonatal sepsis | 3.1 | 1.5 | 1.5–3.5 | 1.0 | 1.0 | 0.5–1.0 | 75.7 | 82.5 | 68–88 | 93.8 | 96 | 94–98 | 4464 |
| Neonatal encephalopathy | 2.7 | 1.5 | 1.5–3.5 | 0.9 | 1.0 | 0.5–1.0 | 73.8 | 80 | 66–88 | 94.3 | 96 | 94–97 | 3499 |
Analysis restricted to enhanced oxygen systems period to ensure limited supply did not prevent access. We report medians and interquartile ranges as the data is not normally distributed and report means for calculation total oxygen use (and easy comparison with previous studies). AFE = acute febrile encephalopathy; ALRI = acute lower respiratory infection; IQR = inter-quartile range, 25th75th centiles; LPM = litres per minute. Neonate = <28 days Mean volume of oxygen required assuming the starting flow rate is maintained throughout admission (i.e. mean oxygen duration x starting flow rate x 60 × 24).
3.4. Clinical signs to predict hypoxaemia
Table 5 shows the predictive value of individual clinical signs for hypoxaemia, recorded during routine care, in sick neonates, infants, younger and older children. In neonates, the most useful sign was tachypnoea with both RR ≥50 and ≥60 bpm cut-offs resulting in sensitivity and specificity between 46% and 69%. Severe respiratory distress and inability to feed had high specificity (93%|84%) but low sensitivity (34%|31%), while the highly specific signs of central cyanosis, decreased conscious state and hypotonia all had very low sensitivity. In infants, severe respiratory distress and tachypnoea (RR ≥50 bpm) both had moderately high sensitivity (40%|61%) and specificity (90%|74%). Tachypnoea (RR ≥40 bpm) remained useful in children aged 1–15 years while respiratory distress was specific but not sensitive (specificity 93–97%, sensitivity 18–27%) in older age groups.
Table 5.
Hypoxaemia prevalence, sensitivity, specificity, positive likelihood ratio (LR+) and negative likelihood ratio (LR-) for presenting clinical signs among children in 12 secondary-level hospitals in southwest Nigeria (Nov 2015 to Oct 2017 inclusive).
| Clinical sign [1] | Neonate <28 days | Infant 28 days-1 year | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | AUC | Sensitivity | Specificity | LR+ | LR- | N (%) | AUC | Sensitivity | Specificity | LR+ | LR- | |
| RR ≥70bpm | 915 | 0.561 | 25% | 88% | 1.98 | 1.75 | 301 | 0.602 | 26% | 94% | 4.57 | 0.784 |
| 15.1% | 22–27 | 87–89 | 8.9% | 22–30 | 93–95 | |||||||
| RR ≥60bpm | 2059 | 0.577 | 46% | 69% | 1.5 | 0.779 | 700 | 0.659 | 47% | 84% | 3.02 | 0.624 |
| 33.9% | 43–49 | 68–71 | 20.6% | 43–52 | 83–86 | |||||||
| RR ≥50bpm | 3279 | 0.557 | 63% | 49% | 1.22 | 0.764 | 1079 | 0.672 | 61% | 74% | 2.3 | 0.533 |
| 54.0% | 60–66 | 47–50 | 31.7% | 56–65 | 72–75 | |||||||
| RR ≥40bpm | 5180 | 0.504 | 86% | 15% | 1.01 | 0.945 | 2038 | 0.614 | 79% | 44% | 1.4 | 0.476 |
| 85.4% | 84–88 | 14–16 | 59.9% | 76–83 | 42–45 | |||||||
| Heart rate >160bpm | 781 | 0.563 | 23% | 90% | 2.25 | 0.861 | 741 | 0.579 | 35% | 81% | 1.83 | 0.805 |
| 12.9% | 20–25 | 89–91 | 21.8% | 31–39 | 79–82 | |||||||
| Severe respiratory distress | 510 | 0.636 | 34% | 93% | 5.03 | 0.709 | 510 | 0.651 | 40% | 90% | 4.03 | 0.665 |
| 8.4% | 31–37 | 93–94 | 15.0% | 36–44 | 89–91 | |||||||
| Central cyanosis | 168 | 0.533 | 8% | 99% | 6.15 | 0.933 | 24 | 0.507 | 2% | 100% | 3.86 | 0.986 |
| 2.8% | 6 to 9 | 98–99 | 0.7% | 1 to 3 | 99–100 | |||||||
| Unable to feed | 1202 | 0.573 | 31% | 84% | 1.89 | 0.826 | 663 | 0.508 | 20% | 81% | 1.09 | 0.98 |
| 19.8% | 28–33 | 83–85 | 19.5% | 17–24 | 80–83 | |||||||
| Seizures | 375 | 0.520 | 9% | 95% | 1.77 | 0.957 | 249 | 0.521 | 11% | 94 | 1.64 | 0.955 |
| 6.2% | 8 to 11 | 94–95 | 7.3% | 8 to14 | 93–94 | |||||||
| Decreased consciousness | 257 | 0.541 | 11% | 98% | 4.47 | 0.916 | 408 | 0.547 | 20% | 90% | 1.92 | 0.895 |
| 4.2% | 9 to 12 | 97–98 | 12.0% | 16–23 | 89–91 | |||||||
| Hypotonia | 214 | 0.524 | 7% | 98% | 2.96 | 0.951 | na | na | na | na | na | na |
| 3.5% | 6 to 9 | 97–98 | ||||||||||
| Shock | na | na | na | na | na | na | 12 | 0.506 | 1% | 100% | 7.63 | 0.989 |
| 0.4% | 1 to 3 | 100–100 | ||||||||||
| Severe dehydration | na | na | na | na | na | na | 194 | 0.513 | 8% | 95% | 1.51 | 0.972 |
| 5.7% | 6 to 10 | 94–96 | ||||||||||
| Clinical sign [1] | Young Child 1–4 years | Older Child 5–14 years | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (%) | AUC | Sensitivity | Specificity | LR+ | LR- | N (%) | AUC | Sensitivity | Specificity | LR+ | LR- | |
| RR ≥70bpm | 140 | 0.533 | 8% | 98% | 4.79 | 0.933 | 20 | 0.516 | 4% | 100% | 8.67 | 0.967 |
| 2.3% | 6 to 11 | 98–99 | 0.7% | 2 to 7 | 99–100 | |||||||
| RR ≥60bpm | 472 | 0.589 | 24% | 94% | 3.85 | 0.809 | 72 | 0.545 | 11% | 98% | 6.1 | 0.909 |
| 7.8% | 21–28 | 93–94 | 2.4% | 7 to 16 | 98–99 | |||||||
| RR ≥50bpm | 913 | 0.642 | 41% | 87% | 3.24 | 0.675 | 161 | 0.579 | 20% | 96% | 4.74 | 0.835 |
| 15.2% | 37–45 | 86–88 | 5.3% | 15–26 | 95–97 | |||||||
| RR ≥40bpm | 2360 | 0.657 | 68% | 64% | 1.86 | 0.505 | 566 | 0.634 | 44% | 83% | 2.59 | 0.677 |
| 39.2% | 64–72 | 62–65 | 18.8% | 37–51 | 82–85 | |||||||
| Heart rate >160bpm | 740 | 0.578 | 27% | 89% | 2.45 | 0.824 | 62 | 0.531 | 8% | 98% | 4.89 | 0.937 |
| 12.3% | 23–30 | 88–90 | 2.1% | 5 to 12 | 98–99 | |||||||
| Severe respiratory distress | 511 | 0.602 | 27% | 93% | 4.12 | 0.781 | 124 | 0.576 | 18% | 97% | 6.14 | 0.843 |
| 8.5% | 23–31 | 93–94 | 4.1% | 13–24 | 96–98 | |||||||
| Central cyanosis | 32 | 0.507 | 2% | 100% | 4.73 | 0.985 | 21 | 0.518 | 4% | 100% | 9.66 | 0.963 |
| 0.5% | 1 to 3 | 99–100 | 0.7% | 2 to 8 | 99–100 | |||||||
| Unable to feed | 1355 | 0.476 | 18% | 77% | 0.784 | 1.06 | 589 | 0.482 | 16% | 81% | 0.814 | 1.05 |
| 22.5% | 15–21 | 76–79 | 19.6% | Nov-21 | 79–82 | |||||||
| Seizures | 1056 | 0.545 | 26% | 84 | 1.55 | 0.892 | 347 | 0.544 | 20% | 89% | 1.82 | 0.901 |
| 17.5% | 22–29 | 83–85 | 11.5% | 15–25 | 88–90 | |||||||
| Decreased consciousness | 1073 | 0.595 | 35% | 84% | 2.2 | 0.775 | 467 | 0.577 | 30% | 86% | 2.08 | 0.821 |
| 17.8% | 31–39 | 83–85 | 15.5% | 24–36 | 85–87 | |||||||
| Hypotonia | na | na | na | na | na | na | na | na | na | na | na | na |
| Shock | 20 | 0.503 | 1% | 100% | 3.47 | 0.993 | 5 | 0.509 | 2% | 100% | 51.5 | 0.982 |
| 0.3% | 0–2 | 100–100 | 0.2% | 0–5 | 100–100 | |||||||
| Severe dehydration | 193 | 0.504 | 4% | 97 | 1.27 | 0.991 | 48 | 0.516 | 5% | 99% | 3.39 | 0.968 |
| 3.2% | 2 to 6 | 96–97 | 1.6% | 2 to 8 | 98–99 | |||||||
1 – assumes that if a sign is not documented, it was not present. AUC = the area under the ROC curve; LR+ positive likelihood ratio (LR+ = sensitivity/(1-specificity)); LR- negative likelihood ratio (LR- = specificity/(1-sensitivity)). A positive LR >10 and a negative LR <0.1 are considered to exert highly significant changes in probability, such as to alter clinical management. na = not applicable. Decreased consciousness = confusion, unconscious, difficult to wake. Severe respiratory distress = any of: severe chest wall in-drawing, grunting, gasping, tracheal tug, head nodding. Signs of shock = cold hands, capillary refill >3 s, fast and weak pulse, low or unmeasurable blood pressure. Signs of severe dehydration = sunken eyes, decreased skin turgor.
Table 6 shows the predictive value of various combinations of clinical signs. The WHO model (consisting of any of the following: RR ≥70 bpm, central cyanosis, severe chest wall in-drawing, grunting, or inability to feed) would have identified the majority of neonates and children with hypoxaemia (sensitivity 57%) with reasonable specificity (72%). The predictive value could be increased by using age-specific cut-offs for tachypnoea (neonate ≥70 bpm; infant ≥60 bpm; young child ≥50 bpm; older child ≥40 bpm) (Model 2 – sensitivity 65%, specificity 67%). Further modification using conscious state (Model 3) and tachycardia (Heart rate ≥160 beats per min, Model 4) provided marginal improvements in sensitivity at the cost of specificity.
Table 6.
Hypoxaemia prevalence, sensitivity, specificity, positive likelihood ratio (LR+) and negative likelihood ratio (LR-) for combinations of clinical signs among children in 12 secondary-level hospitals in southwest Nigeria (Mar 2016-Mar 2017).
| Model | Population | N (%) | AUC | Sensitivity | Specificity | LR+ | LR– |
|---|---|---|---|---|---|---|---|
| Model 1 (WHO) | Overall | 6017 (32.0) | 0.646 | 57% | 72% | 2.05 | 0.596 |
| - Neonate | 2292 (37.3) | 0.675 | 65% | 71% | 2.2 | 0.503 | |
| - Infant | 1220 (35.1) | 0.659 | 62% | 70% | 2.05 | 0.546 | |
| - Young child | 1785 (29.2) | 0.574 | 43% | 72% | 1.53 | 0.795 | |
| - Older child | 701 (22.9) | 0.553 | 33% | 78% | 1.48 | 0.865 | |
| - ALRI | 1011 (58.2) | 0.610 | 74% | 48% | 1.42 | 0.541 | |
| - AFE | 912 (34.2) | 0.579 | 48% | 68% | 1.5 | 0.768 | |
| - Malaria | 1502 (29.8) | 0.581 | 45% | 72% | 1.6 | 0.774 | |
| - Preterm | 644 (42.9) | 0.632 | 63% | 64% | 1.73 | 0.587 | |
| - Neonatal encephalopathy | 1281 (40.1) | 0.672 | 67% | 67% | 2.05 | 0.488 | |
| - Neonatal sepsis | 1190 (48.5) | 0.615 | 64% | 59% | 1.56 | 0.613 | |
| Model 2 | Overall | 7132 (37.9) | 0.656 | 65% | 67% | 1.93 | 0.532 |
| - Neonate | 2292 (37.3) | 0.675 | 65% | 71% | 2.2 | 0.503 | |
| - Infant | 1466 (42.2) | 0.677 | 72% | 63% | 1.96 | 0.442 | |
| - Young child | 2265 (37.0) | 0.628 | 60% | 65% | 1.74 | 0.608 | |
| - Older child | 1090 (35.7) | 0.614 | 57% | 66% | 1.67 | 0.655 | |
| - ALRI | 1388 (80.0) | 0.581 | 92% | 25% | 1.21 | 0.343 | |
| - AFE | 1191 (44.6) | 0.612 | 64% | 59% | 1.55 | 1.42 | |
| - Malaria | 1964 (39.0) | 0.626 | 62% | 63% | 1.69 | 0.6 | |
| - Preterm | 644 (42.9) | 0.632 | 63% | 64% | 1.73 | 0.587 | |
| - Neonatal encephalopathy | 1281 (40.1) | 0.672 | 67% | 67% | 2.05 | 0.488 | |
| - Neonatal sepsis | 1190 (48.5) | 0.615 | 64% | 59% | 1.56 | 0.613 | |
| Model 3 | Overall | 8162 (43.4) | 0.655 | 70% | 61% | 1.8 | 0.494 |
| - Neonate | 2326 (37.9) | 0.491 | 66% | 70% | 2.19 | 0.491 | |
| - Infant | 1650 (47.5) | 0.675 | 77% | 58% | 1.83 | 0.397 | |
| - Young child | 2824 (46.1) | 0.653 | 74% | 57% | 1.71 | 0.458 | |
| - Older child | 1341 (43.9) | 0.628 | 68% | 58% | 1.61 | 0.557 | |
| - ALRI | 1423 (82.0) | 0.575 | 93% | 22% | 1.19 | 1.15 | |
| - AFE | 2027 (75.9) | 0.574 | 88% | 26% | 1.2 | 0.442 | |
| - Malaria | 2408 (47.8) | 0.637 | 73% | 55% | 1.6 | 0.497 | |
| - Preterm | 652 (43.5) | 0.635 | 64% | 64% | 1.74 | 0.574 | |
| - Neonatal encephalopathy | 1295 (40.5) | 0.676 | 68% | 67% | 2.06 | 0.475 | |
| - Neonatal sepsis | 1205 (49.1) | 0.616 | 65% | 59% | 1.56 | 0.604 | |
| Model 4 | Overall | 9573 (50.9) | 0.653 | 77% | 54% | 1.66 | 0.429 |
| - Neonate | 2948 (48.0) | 0.669 | 74% | 60% | 1.84 | 0.431 | |
| - Infant | 2035 (58.5) | 0.657 | 85% | 46% | 1.59 | 0.322 | |
| - Young child | 3189 (52.1) | 0.646 | 79% | 51% | 1.59 | 0.421 | |
| - Older child | 1370 (44.8) | 0.633 | 70% | 57% | 1.62 | 1.47 | |
| - ALRI | 1501 (86.5) | 0.560 | 95% | 17% | 1.14 | 0.293 | |
| - AFE | 2128 (79.7) | 0.568 | 91% | 22% | 1.18 | 0.39 | |
| - Malaria | 2756 (54.7) | 0.637 | 80% | 48% | 1.52 | 0.422 | |
| - Preterm | 816 (54.4) | 0.635 | 74% | 53% | 1.57 | 0.487 | |
| - Neonatal encephalopathy | 1646 (51.5) | 0.651 | 75% | 55% | 1.67 | 0.448 | |
| - Neonatal sepsis | 1406 (57.2) | 0.620 | 73% | 51% | 1.49 | 0.528 |
Model 1 (WHO) = Respiratory rate ≥70 breaths per minute (bpm), Central cyanosis, Severe respiratory distress (any of: severe chest wall in-drawing, grunting, gasping, tracheal tug, head nodding), Inability to feed.
Model 2 = Model 1 with age-specific tachypnoea (Neonate ≥70 bpm; Infant ≥60 bpm; Young child ≥50 bpm; Older child ≥40 bpm).
Model 3 = Model 2 plus Decreased conscious state (unconscious/difficult to rouse/confused).
Model 4 = Model 3 plus Heart rate ≥160 beats per minute.
ALRI = acute lower respiratory infection; AFE = acute febrile encephalopathy; AUC = the area under the ROC curve; LR+ positive likelihood ratio (LR+ = sensitivity/(1-specificity)); LR- negative likelihood ratio (LR- = specificity/(1-sensitivity)). A positive LR >10 and a negative LR <0.1 are considered to exert highly significant changes in probability, such as to alter clinical management.
4. Discussion
Our study reports on hypoxaemia and oxygen use in hospitalised children, across multiple sites, multiple clinical conditions, and from birth to mid-adolescence, using data recorded during routine care. Our data fill important evidence gaps, showing that hypoxaemia is highly prevalent in hospitalised children aged 0–15 years with both respiratory and non-respiratory conditions, and is a major independent risk factor for death. Our data on hypoxaemia prevalence and oxygen use is important for national and global efforts to scale up pulse oximetry and oxygen therapy, providing essential data to inform quantification estimates on oxygen requirements and potential clinical impact.
4.1. Hypoxaemia prevalence
4.1.1. Hypoxaemia in pneumonia
Our hypoxaemia prevalence estimates for children admitted with ALRI were higher than reported by Subhi et al. in a 2009 systematic review [2] (28% in our study, versus median of 13% in the systematic review), especially when compared with the 8 included studies from Africa (all ≤10%). Our study only included low-altitude secondary-level hospitals, whereas most of the studies in the 2009 review reporting higher hypoxaemia prevalence were from tertiary-level facilities and/or higher elevations. Our findings are consistent with more recent studies from large African hospitals (ALRI hypoxaemia prevalence 28–43%) [32], [33], [34], including tertiary hospitals in southwest Nigeria (42–49%) [9,35]. We are aware of similar prevalence rates (51%) in a recent survey of 30 hospitals of various sizes in northern Nigeria (personal correspondence, Chizoba Fashanu/CHAI Nigeria, 25 August 2018).
Our findings and these recent studies suggest that hypoxaemia prevalence in children with ALRI may be higher than previously recognised in Africa. This may mean that modelling efforts have underestimated the extent of hypoxaemia and the potential impact of scaling up pulse oximetry and oxygen therapy [7].
4.1.2. Hypoxaemia in other conditions
Our data show higher hypoxaemia prevalence in other conditions than most studies in the 2009 hypoxaemia review and more recent studies (malaria 8.5% versus 2.9–17.1%, meningitis 17.1 vs 2.7–14.6, malnutrition 16.3 vs 1.8–8.3, seizures 14.3 vs 11.8, diarrhoea 6.1 vs 0–4.7) [2,[8], [9], [10], [11], [12], [13], [14], [15]], but similar results to a major tertiary hospital in the same region of Nigeria [8,9]. Our data show hypoxaemia prevalence among neonates is high (22%), with similar overall rates to other studies from Africa (17–41%) [2,9,10,14,15] and particularly high prevalence among preterm neonates (27%) and those with neonatal encephalopathy (33%).
Our study reported hypoxaemia prevalence at the point of admission, classified according to clinical signs and diagnoses (also recorded on admission). It is possible that respiratory illnesses were under-recognised. For example, pneumonia is challenging to diagnose clinically, particularly in settings with high HIV, malnutrition, and malaria prevalence (conditions which may share or hide the non-specific signs of pneumonia) – even with expert clinical assessment and chest radiography [36], [37], [38]. Diagnostic overlap and uncertainty is even more pronounced in the most severely unwell children, many of whom will have multiple pathological processes occurring simultaneously and clinical signs that evolve over time.
Given that we included comorbid diagnoses, the prevalence of hypoxaemia in those with non-respiratory illnesses may have been driven by comorbid respiratory illness (e.g. diarrhoea and pneumonia). Interestingly, our results remained robust when analysed by primary diagnosis (i.e. excluding secondary diagnoses), suggesting that hypoxaemia was real in many children and neonates presenting with a non-respiratory primary illness. This is understandable, as various pathologic processes can lead to hypoxaemia, including:
-
•
hypoventilation (e.g. seizures, acute encephalopathy),
-
•
systemic inflammation causing water retention, capillary leak and lung oedema (e.g. malaria, sepsis, systemic viral infections)
-
•
secretions leading to airway obstruction (e.g. seizures)
-
•
respiratory muscle weakness leading to atelectasis (e.g. malnutrition)
-
•
pulmonary hypertension causing shunting (e.g. neonatal illness)
Irrespective of the underlying cause of hypoxaemia, or whether respiratory illness were under-recognised, high overall prevalence of hypoxaemia strongly supports the routine use of pulse oximetry for all acutely unwell children and neonates on admission to hospital.
4.2. Hypoxaemia as a risk factor for death
Recent meta-analyses have reported that hypoxaemia (SpO2 < 90%) increased the odds of death five-fold (OR 5.47, 95% CI 3.93–7.63) in children with pneumonia [3] and three-fold in critically ill children in general (OR 3.1; 1.79–5.48) [39] (compared to those without hypoxaemia). Our study found similarly increased odds of death for hypoxaemia in children with ALRI (OR 6.0; 95% CI 4.0–8.9) as well as increased odds of death in other respiratory and non-respiratory conditions, across all age groups. This increased odds of death remained substantial when adjusted for age, co-morbidity and clustering – suggesting that hypoxaemia is a late-stage complication of many conditions that do not primarily involve the lungs.
4.3. Signs of hypoxaemia
Previous studies have explored the utility of various clinical signs to identify hypoxaemia in children with pneumonia [16,32,33,[40], [41], [42], [43], [44], [45], [46], [47], [48], [49]], but few have addressed this question in non-pneumonia cohorts or compared different diagnoses [9,10]. We found no individual or combination of clinical signs that reliably predict hypoxaemia and note significant difference in predictive ability of particular signs between respiratory and non-respiratory conditions (i.e. respiratory signs are more prevalent and sensitive for hypoxaemia in respiratory conditions, and less prevalent but more specific for hypoxaemia in non-respiratory conditions).
We found that the WHO combination of signs for hypoxaemia predicted hypoxaemia in neonates and infants moderately well. However, the WHO combination will miss most cases of hypoxaemia in children aged over 1 year and can be improved by using age-specific tachypnoea cut-offs. This modified WHO combination has moderately high sensitivity and specificity overall (65% sensitivity, 67% specificity) and very high sensitivity for hypoxaemia in ALRI (sensitivity 92%, specificity 25%). However, while it may be appropriate to use such combinations of clinical signs to guide oxygen therapy in hospitals where pulse oximetry is not available, pulse oximetry remains a far superior diagnostic tool.
4.4. Practical implications
Pulse oximetry and oxygen therapy are essential medical practices that are poorly available to hospitalised children globally [5,[50], [51], [52], [53], [54]]. International collaborations recently formed to address oxygen access in key high burden countries (e.g. United for Oxygen Alliance, Every Breath Counts coalition). Ethiopia and Nigeria have led the way in launching national strategies for the scale up of pulse oximetry and oxygen therapy. However, lack of data on which to base oxygen quantification estimates have hampered program planning and advocacy, and individual hospitals have struggled to know how they should respond.
At the hospital management level, our data show that hypoxaemia is common in many conditions – including all the biggest causes of child mortality in Nigeria. Hypoxaemic children typically require 3500–5000 L of oxygen over 2–3 days. Small hospitals (<500 child admissions annually) may admit up to 50 hypoxaemic children per year, requiring oxygen for 2–3 days, and consuming approximately 200,000 L of oxygen annually. Medium-sized hospitals (500–1500 child admissions annually) may admit 100–200 hypoxaemic children/neonates per year using up to 1 million litres of oxygen, and larger hospitals will likely admit new hypoxaemic children/neonates every day and use over 1 million litres of oxygen annually.
At a policy and planning level, our data support calls for the routine use of pulse oximetry in all neonates and children admitted to hospital, irrespective of diagnosis [22,55].We know healthcare workers (including low-level community-based healthcare workers) are able to use pulse oximetry effectively [6,22,52,[56], [57], [58]]. However, introducing pulse oximetry into routine care is challenging and requires a multi-faceted approach (e.g. clinical guidelines, equipment, education, record keeping, finances, and policies) [22]. Making pulse oximetry a “vital sign” (along with heart rate, respiratory rate and temperature) may facilitate faster adoption, but does require more oximeters and more measurement time [22]. Encouragingly, pulse oximeters are becoming more affordable (e.g. the Lifebox Foundation [25]) and, while time is an initial barrier to pulse oximetry uptake, nurses embrace it as a tool that enhances efficiency [22].
For researchers, future studies and modelling efforts regarding the utility of pulse oximetry and oxygen should consider the potential impact for neonates and children with non-pneumonia conditions.
4.5. Limitations
We report data from a large multi-centre study involving secondary level hospitals at low-altitude locations in southwest Nigeria. These data can inform oxygen estimates and program planning, but they will not negate the need for locally-acquired operational data (and we did not investigate other important indications for oxygen in anaesthetic, obstetric or adult patient care).
We relied on pulse oximetry measurements obtained during routine care by hospital healthcare workers using low-cost handheld pulse oximeters. Findings from our supervision visits and a targeted audit of nurses’ pulse oximetry practices showed high adherence to recommended procedures, low measurement failure rates, and consistency in the number and duration of attempts across different hospitals [22]. Lifebox oximeters do not have motion-resistance or low-perfusion technology, but have been validated for use in children and neonates and displayed equivalent accuracy to leading commercial brands in the US [24,59].
We used a single definition of hypoxaemia (SpO2 < 90%) irrespective of participant age or condition in keeping with WHO recommendations [23]. We recognise that particular clinical situations may warrant more liberal or conservative application of oxygen therapy due to the relative risk of tissue hypoxia (e.g. brain injury, severe anaemia) or the underlying cause and duration of hypoxaemia (e.g. congenital heart disease). We recognise that normal saturations can be lower for neonates immediately after birth and can vary by probe location (pre-ductal versus post-ductal). However, studies in similar settings have reported that more than 95% of SpO2 readings are ≥90% even in the first hours of life (irrespective of probe location) [60].
We extracted data from routine clinical care documents using an approach similar to others conducting implementation research through clinical research networks [61]. Our use of dedicated research nurses who extracted data immediately after discharge minimised the amount of missing data, and our audit of documentation practices prior to starting the study reassured us that documentation practices overall were excellent [21]. Nonetheless, some clinical signs may not have been identified and/or documented correctly, resulting in under-identification of some signs (and possibly over-identification of others). To address anticipated missing data on gestational age (prematurity was only recorded in 32% of neonates) we reported the composite “small/preterm” category (birth weight was recorded in 91%) [62,63].
We recognise the limits of our diagnostic classifications, and the particular challenges in pneumonia [64]. We aimed to increase reliability of diagnosis by (i) using standardised case definitions based on objectively recorded signs, symptoms, and investigations from the time of admission (rather than clinician diagnosis alone) and (ii) considering multiple diagnoses and adjusting for them in analysis (rather than selecting an arbitrary ‘primary’ diagnosis). For ALRI, we used the widely accepted WHO definition that is regarded as simple, sensitive, and generalizable with the main limitation being inclusion of a heterogenous group of aetiologies (viral, bacterial and other) [64]. Importantly, our prevalence estimates reflect hypoxaemia prevalence in real-life clinical contexts and are reported according to diagnoses and syndromes that frontline healthcare workers can identify. These estimates may be different in a more controlled trial setting. If our results are influenced by under-recognition of respiratory compromise this would further support the importance of using pulse oximetry as an objective tool to identify hypoxaemia in the high-risk population of hospitalised children.
Mortality is influenced by contextual factors related to case-mix and severity of illness (e.g. care-seeking, admission criteria) and quality of care (e.g. time, staffing, adherence to guidelines, equipment). We accounted for some of these variables through adjustment and stratified reporting, but will explore these issues more fully in a separate paper focussing on clinical outcomes.
We believe that our findings are representative of hypoxaemia as it is encountered among children and neonates admitted to small and medium-sized hospitals in Nigeria. While the precise estimates will vary in other regions, we believe our findings will have wide relevance to healthcare workers and managers in other LMICs, particularly in Africa.
5. Conclusions
Hypoxaemia is common in respiratory and non-respiratory acute childhood illness and increases the risk of death substantially. Given the limitations of clinical assessment, pulse oximetry is an essential tool for detecting hypoxaemia, and should be part of the routine assessment of all hospitalised neonates and children. Efforts to scale up pulse oximetry and oxygen therapy should consider the needs of, and potential impact on, neonates and children with non-pneumonia conditions.
Funding
Bill and Melinda Gates Foundation.
Ethics
This study obtained ethics approval from the University of Melbourne (1543797.1) and University of Ibadan/University College Hospital Ethics Committee, Ibadan, Nigeria (UI/EC/16/0413).
CRediT authorship contribution statement
Hamish Graham: Conceptualization, Data curation, Formal analysis, Writing - original draft. Ayobami A. Bakare: Data curation, Writing - review & editing. Adejumoke I. Ayede: Conceptualization, Data curation. Oladapo B. Oyewole: Data curation, Writing - review & editing. Amy Gray: Writing - review & editing. David Peel: Writing - review & editing. Barbara McPake: Writing - review & editing. Eleanor Neal: Writing - review & editing. Shamim A. Qazi: Writing - review & editing. Rasa Izadnegahdar: . Trevor Duke: Conceptualization, Writing - review & editing. Adegoke G. Falade: Conceptualization, Data curation, Writing - review & editing.
Declaration of Competing Interest
HG, AAB, AIA, OBO, DP, EN, TD, and AGF received payment for services on this project from the funder (Bill and Melinda Gates Foundation) and RI is an employee of the funder. HG reports consultancy grants from WHO for unrelated work during the conduct of the study. All other authors declare no competing interests.
Acknowledgements
We thank the participating hospitals and their clinical, technical and managerial staff: Adeoyo Maternity Hospital (Ibadan, Oyo state); Baptist Medical Centre (Saki, Oyo state); Mother and Child Hospital (Akure, Ondo state); Oluyoro Catholic Hospital (Ibadan, Oyo state); Oni Memorial Children's Hospital (Ibadan, Oyo state); Our Lady of Fatima Catholic Hospital (Osogbo, Osun state); Sacred Heart Hospital (Abeokuta, Ogun state); Seventh Day Adventist Hospital (Ife, Osun state); State Hospital Ijaeye (Abeokuta, Ogun state); State Hospital Oyo (Oyo, Oyo state); State Hospital Saki (Saki, Oyo state); State Specialist Hospital (Akure, Ondo state).
We thank all members of the Nigerian Oxygen Implementation team; representatives from the Federal Ministry of Health; representatives from the state Ministry of Health and Hospital Management Boards in Oyo, Ondo, Ogun, and Osun states; support staff at the Centre for International Child Health in Melbourne Australia.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2019.10.009.
Contributor Information
Hamish Graham, Email: Hamish.graham@rch.org.au.
Adejumoke I. Ayede, Email: idayede@yahoo.co.uk.
Amy Gray, Email: Amy.Gray@rch.org.au.
David Peel, Email: ashdownconsult@btconnect.com.
Barbara McPake, Email: barbara.mcpake@unimelb.edu.au.
Eleanor Neal, Email: Eleanor.Neal@mcri.edu.au.
Rasa Izadnegahdar, Email: Rasa.Izadnegahdar@gatesfoundation.org.
Trevor Duke, Email: Trevor.Duke@rch.org.au.
Appendix. Supplementary materials
Appendix 2 \elsamp #x2013; Oximetry Guidelines
Appendix 3 \elsamp #x2013; Supplemental results table: Hypoxaemia on admission and corresponding case fatality rates and relative odds of death among children (aged \elsamp #x003C;15 years) admitted to 12 secondary-level hospitals in southwest Nigeria over a 2 year period (Nov 2015 \elsamp #x2013; Oct 2017 inclusive) showing results by primary admission diagnosis and selected diagnostic combinations.
References
- 1.WHO . World Health Organization; Geneva: 2016. Oxygen therapy for children. [Google Scholar]
- 2.Subhi R., Adamson M., Campbell H., Weber M., Smith K., Duke T. The prevalence of hypoxaemia among ill children in developing countries: a systematic review. Lancet Infect Dis. 2009;9:219–227. doi: 10.1016/S1473-3099(09)70071-4. [DOI] [PubMed] [Google Scholar]
- 3.Lazzerini M., Sonego M., Pellegrin M.C. Hypoxaemia as a mortality risk factor in acute lower respiratory infections in children in low and middle-income countries: systematic review and meta-analysis. PLoS One. 2015;10(9) doi: 10.1371/journal.pone.0136166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duke T., Wandi F., Jonathan M. Improved oxygen systems for childhood pneumonia: a multihospital effectiveness study in Papua New Guinea. Lancet. 2008;372(9646):1328–1333. doi: 10.1016/S0140-6736(08)61164-2. [DOI] [PubMed] [Google Scholar]
- 5.Graham H., Tosif S., Gray A. Providing oxygen to children in hospitals: a realist review. Bull World Health Organ. 2017;95(4):288–302. doi: 10.2471/BLT.16.186676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray A.Z., Morpeth M., Duke T. Improved oxygen systems in district hospitals in Lao PDR: a prospective field trial of the impact on outcomes for childhood pneumonia and equipment sustainability. BMJ Open. 2017;1 doi: 10.1136/bmjpo-2017-000083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Floyd J., Wu L., Hay Burgess D., Izadnegahdar R., Mukanga D., Ghani A.C. Evaluating the impact of pulse oximetry on childhood pneumonia mortality in resource-poor settings. Nature. 2015;528(7580):S53–SS9. doi: 10.1038/nature16043. [DOI] [PubMed] [Google Scholar]
- 8.Adebola O., Babatunde O., Bose O. Hypoxemia predicts death from severe falciparum malaria among children under 5 years of age in Nigeria: the need for pulse oximetry in case management. Afr Health Sci. 2014;14(2):397–407. doi: 10.4314/ahs.v14i2.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Orimadegun A.E., Ogunbosi B.O., Carson S.S. Prevalence and predictors of hypoxaemia in respiratory and non-respiratory primary diagnoses among emergently ill children at a tertiary hospital in south western Nigeria. Trans R Soc Trop Med Hyg. 2013;107(11):699–705. doi: 10.1093/trstmh/trt082. [DOI] [PubMed] [Google Scholar]
- 10.Morgan M.C., Maina B., Waiyego M. Pulse oximetry values of neonates admitted for care and receiving routine oxygen therapy at a resource-limited hospital in Kenya. J Paediatr Child Health. 2018;54(3):260–266. doi: 10.1111/jpc.13742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wandi F., Peel D., Duke T. Hypoxaemia among children in rural hospitals in Papua New Guinea: epidemiology and resource availability – a study to support a national oxygen programme. Ann Trop Paediatr. 2006;26(4):277–284. doi: 10.1179/146532806X152791. [DOI] [PubMed] [Google Scholar]
- 12.Junge S., Palmer A., Greenwood B.M., Kim Mulholland E., Weber M.W. The spectrum of hypoxaemia in children admitted to hospital in the Gambia, West Africa. Trop Med Int Health. 2006;11:367–372. doi: 10.1111/j.1365-3156.2006.01570.x. [DOI] [PubMed] [Google Scholar]
- 13.Maitland K., Levin M., English M., Mithwani S., Peshu N., Marsh K., Newton C.R. Severe P. falciparum malaria in Kenyan children: evidence for hypovolaemia. QJM. 2003;96(6):427–434. doi: 10.1093/qjmed/hcg077. [DOI] [PubMed] [Google Scholar]
- 14.English M., Ngama M., Musumba C. Causes and outcome of young infant admissions to a Kenyan district hospital. Arch Dis Child. 2003;88:438–443. doi: 10.1136/adc.88.5.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber M.W., Carlin J.B., Gatchalian S., Lehmann D., Muhe L., Mulholland E.K. Predictors of neonatal sepsis in developing countries. Pediatr Infect Dis J. 2003;22:711–717. doi: 10.1097/01.inf.0000078163.80807.88. [DOI] [PubMed] [Google Scholar]
- 16.Duke T., Blaschke A.J., Sialis S., Bonkowsky J.L. Hypoxaemia in acute respiratory and non-respiratory illnesses in neonates and children in a developing country. Arch Dis Child. 2002;86:108–112. doi: 10.1136/adc.86.2.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chisti M.J., Duke T., Robertson C.F. Clinical predictors and outcome of hypoxaemia among under-five diarrhoeal children with or without pneumonia in an urban hospital, Dhaka, Bangladesh. Trop Med Int Health. 2012;17(1):106–111. doi: 10.1111/j.1365-3156.2011.02890.x. [DOI] [PubMed] [Google Scholar]
- 18.UN IGCME. United Nations Children's Fund (UNICEF); New York, US: 2018. Levels and trends in child mortality: report 2018. [Google Scholar]
- 19.WHO and Maternal and Child Epidemiology Estimation Group (MCEE). UNICEF data: monitoring the situation of children and women: cause of death. 2015. http://data.unicef.org(accessed 4 April 2017).
- 20.Graham H.R., Ayede A.I., Bakare A.A. Improving oxygen therapy for children and neonates in secondary hospitals in Nigeria: study protocol for a stepped-wedge cluster randomised trial. Trials. 2017;18(1):502. doi: 10.1186/s13063-017-2241-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Graham H., Ayede A.I., Bakare A. Oxygen for children and newborns in non-tertiary hospitals in South-West Nigeria: a needs-assessment. Afr J Med Med Sci. 2016;(45) [PubMed] [Google Scholar]
- 22.Graham H.R., Bakare A.A., Gray A. Adoption of paediatric and neonatal pulse oximetry by 12 hospitals in Nigeria: a mixed-methods realist evaluation. BMJ glob Health. 2018;3 doi: 10.1136/bmjgh-2018-000812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO . 2nd ed. World Health Organization; Geneva: 2013. Pocket book of hospital care for children: guidelines for the management of common childhood illnesses. [PubMed] [Google Scholar]
- 24.Dubowitz G., Breyer K., Lipnick M. Accuracy of the lifebox pulse oximeter during hypoxia in healthy volunteers. Anaesthesia. 2013;68:1220–1223. doi: 10.1111/anae.12382. [DOI] [PubMed] [Google Scholar]
- 25.Enright A., Merry A., Walker I., Wilson I. Lifebox: a global patient safety initiative. A&A Case Rep. 2016;6(12):366–369. doi: 10.1213/XAA.0000000000000335. [DOI] [PubMed] [Google Scholar]
- 26.Christiansen T., Lauritsen J. EpiData Association; Odense, Denmark: 2010. Comprehensive data management and basic statistical analysis system. Odense, Denmark: EpiData Association. [Google Scholar]
- 27.StataCorp . StataCorp LP; College Station, Texas: 2017. Stata statistical software: release 15. [Google Scholar]
- 28.Thabane L., Mbuagbaw L., Zhang S. A tutorial on sensitivity analyses in clinical trials: the what, why, when and how. BMC Med Res Methodol. 2013;13:92. doi: 10.1186/1471-2288-13-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hemming K., Haines T.P., Chilton P.J., Girling A.J., Lilford R.J. The stepped wedge cluster randomised trial: rationale, design, analysis, and reporting. BMJ Case Rep. 2015;350 doi: 10.1136/bmj.h391. h391-h. [DOI] [PubMed] [Google Scholar]
- 30.Davey C., Hargreaves J., Thompson J.A. Analysis and reporting of stepped wedge randomised controlled trials: synthesis and critical appraisal of published studies, 2010 to 2014. Trials. 2015;16:358. doi: 10.1186/s13063-015-0838-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seed P., Tobias A. DIAGT: Stata module to report summary statistics for diagnostic tests compared to true disease status. Stata Technical Bulletin. 2001;59:sbe36.1. [Google Scholar]
- 32.Bassat Q., Lanaspa M., Machevo S. Hypoxaemia in Mozambican children <5 years of age admitted to hospital with clinical severe pneumonia: clinical features and performance of predictor models. Trop Med Int Health. 2016;21(9):1147–1156. doi: 10.1111/tmi.12738. [DOI] [PubMed] [Google Scholar]
- 33.Wandeler G., Pauchard J.Y., Zangger E., Diawara H., Gehri M. Which clinical signs predict hypoxaemia in young Senegalese children with acute lower respiratory tract disease? Paediatr Int Child Health. 2015;35(1):65–68. doi: 10.1179/2046905514Y.0000000153. [DOI] [PubMed] [Google Scholar]
- 34.Salah E.T., Algasim S.H., Mhamoud A.S., NEOSA Husian. Prevalence of hypoxemia in under-five children with pneumonia in an emergency pediatrics hospital in Sudan. Indian J Crit Care Med. 2015;19:203. doi: 10.4103/0972-5229.154549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdulkadir M.B., Ibraheem R.M., Gobir A.A., Johnson W.B. Hypoxaemia as a measure of disease severity in young hospitalised Nigerian children with pneumonia: a cross-sectional study. SAJCH S Afr J Child Health. 2015;9(2):53–56. [Google Scholar]
- 36.Izadnegahdar R., Cohen A.L., Klugman K.P., Qazi S.A. Childhood pneumonia in developing countries. Lancet Respir Med. 2013;1(7):574–584. doi: 10.1016/S2213-2600(13)70075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Graham S. Challenges to improving case management of childhood pneumonia at health facilities in resource-limited settings. Bull World Health Organ. 2008;86(5):349–355. doi: 10.2471/BLT.07.048512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.English M., Punt J., Mwangi I., McHugh K., Marsh K. Clinical overlap between malaria and severe pneumonia in African children in hospital. Trans R Soc Trop Med Hyg. 1996;90:658–662. doi: 10.1016/s0035-9203(96)90423-x. [DOI] [PubMed] [Google Scholar]
- 39.Raman S., Prince N.J., Hoskote A., Ray S., Peters M.J. Admission PaO2 and mortality in critically ill children: a cohort study and systematic review. Pediatr Crit Care Med. 2016;17(10):e444–ee50. doi: 10.1097/PCC.0000000000000905. [DOI] [PubMed] [Google Scholar]
- 40.Ayieko P., English M. In children aged 2–59 months with pneumonia, which clinical signs best predict hypoxaemia. J Trop Pediatr. 2006;52:307–310. doi: 10.1093/tropej/fml036. [DOI] [PubMed] [Google Scholar]
- 41.Benet T., Picot V.S., Awasthi S. Severity of pneumonia in under 5-year-old children from developing countries: a multicenter, prospective, observational study. Am J Trop Med Hyg. 2017;97(1):68–76. doi: 10.4269/ajtmh.16-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duke T., Mgone J., Frank D. Hypoxaemia in children with severe pneumonia in Papua New Guinea. Int J Tuberc Lung Dis. 2001;5:511–519. [PubMed] [Google Scholar]
- 43.Margolis P.A., Ferkol T.W., Marsocci S. Accuracy of the clinical examination in detecting hypoxemia in infants with respiratory illness. J Pediatr. 1994;124(4):552–560. doi: 10.1016/s0022-3476(05)83133-6. [DOI] [PubMed] [Google Scholar]
- 44.Mwaniki M.K., Nokes D.J., Ignas J. Emergency triage assessment for hypoxaemia in neonates and young children in a Kenyan hospital: an observational study. Bull World Health Organ. 2009;87:263–270. doi: 10.2471/BLT.07.049148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Onyango F.E., Steinhoff M.C., Wafula E.M., Wariua S., Musia J., Kitonyi J. Hypoxaemia in young Kenyan children with acute lower respiratory infection. Br Med J. 1993;306:612–615. doi: 10.1136/bmj.306.6878.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smyth A., Hart H.C.C. Clinical predictors of hypoxaemia in children with pneumonia. Ann Trop Paediatr. 1998;18:31. doi: 10.1080/02724936.1998.11747923. [DOI] [PubMed] [Google Scholar]
- 47.Usen S., Webert M. Clinical signs of hypoxaemia in children with acute lower respiratory infection: indicators of oxygen therapy. Int J Tuberc Lung Dis. 2001;5(6):505–510. [PubMed] [Google Scholar]
- 48.Weber M.W., Usen S., Palmer A., Jaffar S., Mulholland E.K. Predictors of hypoxaemia in hospital admissions with acute lower respiratory tract infection in a developing country. Arch Dis Child. 1997;76:310–314. doi: 10.1136/adc.76.4.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang L., Mendoza-Sassi R., Santos J.C.H., Lau J. Accuracy of symptoms and signs in predicting hypoxaemia among young children with acute respiratory infection: a meta-analysis. Int J Tuberc Lung Dis. 2011;15(3):317–325. [PubMed] [Google Scholar]
- 50.Duke T., Graham S.M., Cherian M.N. Oxygen is an essential medicine: a call for international action. Int J Tuberc Lung Dis. 2010;14:1362–1368. [PMC free article] [PubMed] [Google Scholar]
- 51.Duke T., Peel D., Graham S., Howie S., Enarson P.M., Jacobson R. Oxygen concentrators: a practical guide for clinicians and technicians in developing countries. Ann Trop Paediatr. 2010;30:87–101. doi: 10.1179/146532810X12637745452356. [DOI] [PubMed] [Google Scholar]
- 52.Duke T., Subhi R., Peel D., Frey B. Pulse oximetry: technology to reduce child mortality in developing countries. Ann Trop Paediatr. 2009;29:165–175. doi: 10.1179/027249309X12467994190011. [DOI] [PubMed] [Google Scholar]
- 53.Ginsburg A.S., Van cleve W.C., Thompson M.I.W., English M. Oxygen and pulse oximetry in childhood pneumonia: a survey of healthcare providers in resource-limited settings. J Trop Pediatr. 2012;58:389–393. doi: 10.1093/tropej/fmr103. [DOI] [PubMed] [Google Scholar]
- 54.Ginsburg A.S., Izadnegahdar R., Klugman K.P. World Pneumonia Day 2016: pulse oximetry and oxygen. Lancet Glob Health. 2016:e893–e894. doi: 10.1016/S2214-109X(16)30296-0. [DOI] [PubMed] [Google Scholar]
- 55.McCollum E.D., Bjornstad E., Preidis G.A., Hosseinipour M.C., Lufesi N. Multicenter study of hypoxemia prevalence and quality of oxygen treatment for hospitalized Malawian children. Trans R Soc Trop Med Hyg. 2013;107(5):285–292. doi: 10.1093/trstmh/trt017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.King C., Boyd N., Walker I. Opportunities and barriers in paediatric pulse oximetry for pneumonia in low-resource clinical settings: a qualitative evaluation from Malawi and Bangladesh. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-019177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matai S., Peel D., Wandi F., Jonathan M., Subhi R., Duke T. Implementing an oxygen programme in hospitals in Papua New Guinea. Ann Trop Paediatr. 2008;28:71–78. doi: 10.1179/146532808X270716. [DOI] [PubMed] [Google Scholar]
- 58.McCollum E.D., King C., Deula R. Pulse oximetry implementation with rural frontline and community health workers during three years of child pneumonia care in two central Malawi districts: a prospective observational study. Bull World Health Organ. 2016;94(12):893–902. doi: 10.2471/BLT.16.173401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.King C., Mvalo T., Sessions K. Performance of a novel re-usable paediatric pulse oximeter probe. Pediatr Pulmonol. 2019;54:1052–1059. doi: 10.1002/ppul.24295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morgan M.C., Maina B., Waiyego M. Oxygen saturation ranges for healthy newborns within 24 h at 1800m. Arch Dis Child Fetal Neonatal Ed. 2017;102(3):266–268. doi: 10.1136/archdischild-2016-311813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.English M., Ayieko P., Nyamai R., Were F., Githanga D., Irimu G. What do we think we are doing? How might a clinical information network be promoting implementation of recommended paediatric care practices in Kenyan hospitals? Health Res Policy Syst. 2017;15(1):4. doi: 10.1186/s12961-017-0172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Villar J., Giuliani F., Fenton T.R., Ohuma E.O., Ismail L.C., Kennedy S.H. INTERGROWTH-21st very preterm size at birth reference charts. Lancet. 2016;387(10021):844–845. doi: 10.1016/S0140-6736(16)00384-6. [DOI] [PubMed] [Google Scholar]
- 63.Villar J., Ismail L.C., Victora C.G. International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857–868. doi: 10.1016/S0140-6736(14)60932-6. [DOI] [PubMed] [Google Scholar]
- 64.Mackenzie G. The definition and classification of pneumonia. Pneumonia. 2016;8:14. doi: 10.1186/s41479-016-0012-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Modi A., Atam V., Jain N., Gutch M., Verma R. The etiological diagnosis and outcome in patients of acute febrile encephalopathy: a prospective observational study at tertiary care center. Neurol India. 2012;60(2):168–173. doi: 10.4103/0028-3886.96394. [DOI] [PubMed] [Google Scholar]
- 66.Lee A.C., Kozuki N., Blencowe H. Intrapartum-related neonatal encephalopathy incidence and impairment at regional and global levels for 2010 with trends from 1990. Pediatr Res. 2013;74(Suppl 1):S50–S72. doi: 10.1038/pr.2013.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 2 \elsamp #x2013; Oximetry Guidelines
Appendix 3 \elsamp #x2013; Supplemental results table: Hypoxaemia on admission and corresponding case fatality rates and relative odds of death among children (aged \elsamp #x003C;15 years) admitted to 12 secondary-level hospitals in southwest Nigeria over a 2 year period (Nov 2015 \elsamp #x2013; Oct 2017 inclusive) showing results by primary admission diagnosis and selected diagnostic combinations.