Abstract
Serodiagnosis of Leishmania infantum infection in dogs relies on the detection of antibodies against leishmanial crude extracts or parasitic defined antigens. The expansion of canine leishmaniasis from geographical areas of Brazil in which the infection is endemic to regions in which the disease is emerging is occurring. This fact makes necessary the analysis of the serodiagnostic capabilities of different leishmanial preparations in distinct geographical locations. In this article sera from dogs infected with Leishmania and showing the clinical form of the disease, were collected in three distinct Brazilian States and were tested against soluble leishmanial antigens or seven parasite individual antigens produced as recombinant proteins. We show that the recognition of soluble leishmanial antigens by sera from these animals was influenced by the geographical location of the infected dogs. Efficacy of the diagnosis based on this crude parasite preparation was higher in newly endemic regions when compared with areas of high disease endemicity. We also show that the use of three of the recombinant proteins, namely parasite surface kinetoplastid membrane protein of 11 kDa (KMP-11), and two members of the P protein family (P2a and P0), can improve the degree of sensitivity without adversely affecting the specificity of the diagnostic assays for canine leishmaniasis, independently of the geographical area of residence. In addition, sera from dogs clinically healthy but infected were also assayed with some of the antigen preparations. We demonstrate that the use of these proteins can help to the serodiagnosis of Leishmania infected animals with subclinical infections. Finally, we propose a diagnostic protocol using a combination of KMP-11, P2a y P0, together with total leishmanial extracts.
Abbreviations: BB, blocking buffer; CanL, Canine visceral leishmaniasis; EDCB, ELISA denaturant coating buffer; ELISA, enzyme-linked immunosorbent assay; HSP, Heat shock protein; KMP-11, Kinetoplastid-membrane protein of 11 kDa; LR, Likelihood ratio; MS, Mato Grosso do Sul State (Brazil); PBS, phosphate saline buffer; PI, Piaui State (Brazil); ROC, Receiver Operating Characteristic; RR, Relative reactivity; RT, Room temperature; SC, Santa Catarina State (Brazil); SLA, Soluble leishmanial antigen; VL, Visceral leishmaniosis; WB, Washing buffer
Keywords: Leishmania, Canine leishmaniasis, Serodiagnosis, Antibodies, Recombinant proteins
Graphical abstract
Highlights
-
•
Serodiagnosis of canine leishmaniasis based on SLA is compromised in regions of high endemicity.
-
•
The use of individual antigens improves serodiagnosis of canine leishmaniasis.
-
•
Sera from clinically ill and subclinically infected dogs possess specific antibodies for KMP-11, LiP2a or LiP0 proteins.
-
•
The best criterion of positivity is to have reactivity for at least one of the next antigens: SLA, KMP-11, LiP2a, LiP0.
1. Introduction
Canine leishmaniasis (CanL) is a potentially fatal zoonotic disease caused by infection with Leishmania infantum (syn. L. chagasi (Mauricio et al., 2000)). Infected dogs can develop different forms of the disease ranging from clinically healthy animals (subclinical infection) to animals showing the clinical form of the disease (clinically ill). Depending on the number and severity of the disease manifestations and the pathological abnormalities there are different stages of the clinical disease, ranging from mild to very severe CanL (Solano-Gallego et al., 2009; Solano-Gallego et al., 2011). Infected animals typically develop a specific humoral response against crude preparation of parasite proteins (SLA; soluble leishmanial antigens). The titer of anti-Leishmania antibodies are usually higher in canine patients showing the most severe forms of the disease (Maia and Campino, 2008; Noli and Saridomichelakis, 2014; Solano-Gallego et al., 2017). Subclinically infected dogs include animals at the initial stage of the disease that will evolve towards the clinical form, showing a concomitant increase in the magnitude of the humoral response against parasite antigens (Nieto et al., 1999; Leandro et al., 2001; Fernandez-Cotrina et al., 2013). In addition, dogs that are subclinically infected will remain healthy for many years, showing a limited humoral response against parasite antigens after mounting an effective cell-mediated immunity that prevent parasite proliferation, (Baneth et al., 2008; Noli and Saridomichelakis, 2014; Abbehusen et al., 2017; Hosein et al., 2017).
The correct diagnosis of CanL continues to be an unresolved question, since there is not a current gold standard method to detect the 100% of Leishmania infected individuals. Some approaches are based on detection of the parasite in biological samples by cytological assays (cell or tissue staining, immunochemistry or parasite culture) and molecular techniques for detection of the parasite DNA (Solano-Gallego et al., 2017). The presence of circulating anti-Leishmania specific antibodies in the blood of infected dogs has allowed the development of serologic assays for diagnosis of CanL. They include, among others, direct agglutination test, indirect fluorescent antibody test or enzyme-linked immunosorbent assays (ELISA). ELISA is an immunological test that uses simple methodologies and can therefore be used in the field diagnosis of the disease. In addition, it can be also employed for characterizing the diagnostic capacities of different antigens to further develop qualitative immune-chromatographic rapid tests that do not require laboratory equipment for their use (Travi et al., 2018). Different antigenic sources are employed in these diagnostic methods, including SLA, as well as different parasite antigenic fractions, individual recombinant proteins or small peptides containing defined antigenic determinants (Travi et al., 2001; Coelho et al., 2009; Solano-Gallego et al., 2009; Ker et al., 2013; Rodriguez-Cortes et al., 2013; Solano-Gallego et al., 2017). Some of these antigens are parasite-specific proteins like the kinetoplastid-membrane protein of 11 kDa (KMP-11) (Berberich et al., 1997), or members of intracellular protein families such as histones (Soto et al., 1999), heat shock proteins (HSP) (Angel et al., 1996; Quijada et al., 1996a; Oliveira et al., 2011), ribosome related factors including the acidic ribosomal protein family (Soto et al., 2009) or the recombinant K39 protein that contains an extensive repetitive domain located in the C-terminal region of the leishmanial kinesin protein (Scalone et al., 2002). Although these antigens are proteins commonly conserved in different organisms, in leishmaniasis patients the immune response is elicited specifically towards the parasite proteins. This is due to the localization of the main epitopes in protein regions that contain specific amino acids for the parasite (Requena et al., 2000).
Human visceral leishmaniases (VL) and CanL are endemic in European and African countries of the Mediterranean basin, Middle-East, Asia and in Latin America (Dantas-Torres et al., 2012; Pigott et al., 2014) being domestic dogs the main reservoir for the infection to human (Pennisi, 2015). During the last years several reports indicate that there has been an expansion of human VL and CanL from its historical endemic regions to traditionally non-endemic areas such North Europe and North America countries (Dujardin et al., 2008; Petersen, 2009; Ready, 2010¸ Mattin et al., 2014). Evolution of human VL cases in Brazil perfectly reflects the disease expansion from regions with high number of cases (Northern States) to recently colonized areas (Southern States) (Harhay et al., 2011; Reis et al., 2017). In this work we have first evaluated the diagnostic properties of the SLA and different Leishmania antigenic proteins using sera collections of clinically ill dogs obtained in three geographical distant regions of Brazil with differences in the prevalence of CanL. Secondly, we have determined the diagnostic properties of some of the protein preparations for the detection of subclinically infected animals. On the basis of our results we propose the combination of three recombinant proteins besides SLA for serodiagnosis of CanL.
2. Materials and methods
2.1. Parasites and dogs
Leishmania infantum (MHOM/BR/2000/MER-STRAIN2) promastigotes were cultured in Schneider's medium supplemented with 10% heat-inactivated fetal bovine serum, 2 mM l-glutamine, 200 U/ml penicillin, 100 μg/ml streptomycin and 50 μg/ml gentamicin, at pH 7.4.
Serum samples were collected in the city of Teresina, state of Piaui (PI) in the Northeast region; in the cities of Camapuã and Campo Grande, state of Mato Grosso do Sul (MS) in the Central-West region; and in the city of Florianopolis, state of Santa Catarina (SC) in the South region of Brazil (Supplementary Fig. 1). For the first objective, sera from clinically ill CanL (PI, n = 46; MS, n = 57; SC, n = 52) dogs were collected at the three State locations. For the second objective, sera from Leishmania subclinically infected animals were collected in PI (n = 46) and MS (n = 35) and analyzed as a single group. For animal classification, the clinical profile of the animals included in the study was evaluated taking into account different clinical sign as previously reported (Silva et al., 2017). Absence of the clinical signs was scored as 0. According to the severity of the presented clinical signal, 1 point was assigned to the milder signal and 2 points for the most severe clinical signs. Subclinically infected animals were classified as having a total score of up to 3 points, being the group of clinically ill dogs those showing a clinical score higher than 3 points. For all the animals, the presence of amastigote forms was confirmed by direct observation after Giemsa staining of lymph nodes or bone marrow aspirates. Sera from healthy animals (clinical score = 0 and Leishmania negative) were collected in PI (n = 45), MS (n = 39) and SC (n = 82). Finally, sera from Leishmania negative animals affected by canine monocytic ehrlichiosis were collected in PI (n = 46) and MS (n = 30). Ehrlichia canis infection was monitored with the ALERE ERLIQUIOSE Ac TEST KIT (Bionote Inc., Gyeonggi-do, Korea) following manufacturer instructions.
This project was approved by the Animal Experimentation Ethics Committee of the Federal University of Piauí under protocol number 092/15, as well as the consent of the owners of the dogs to carry out the samples for analysis.
2.2. Antigen preparation
Freezed-thaw SLA was prepared from stationary phase promastigotes of L. infantum as previously described (Souza et al., 2013). Recombinant proteins were expressed in bacteria (Escherichia coli) transformed with pQE plasmids (Qiagen, Hilden, Germany) recombinant for the next L. infantum coding regions: KMP-11 (Fuertes et al., 2001); H2A (Iborra et al., 2004); HSP83 (Angel et al., 1996); HSP70 (Souza et al., 2013); LiP2a and LiP2b (Iborra et al., 2007); LiP0 (Iborra et al., 2003). Gene expression and protein purification of the different his-tagged recombinant proteins were performed by affinity chromatography using Ni-NTA resin (Qiagen), under denaturant conditions as described (Garde et al., 2018). Proteins were stored at −20 °C in ELISA denaturant coating buffer (EDCB: 3 M urea, 0.5 M NaCl, 5 mM imidazol, 1 mM 2-mercaptoethanol in 20 mM Tris HCl pH 8).
2.3. Qualitative ELISA
Microtiter immunoassay plates MaxiSorp™ (Nunc, Roskilde, Denmark) were coated with L. chagasi SLA (0.2 μg per well; 100 μl total volume) or each one of the recombinant proteins (0.1 μg per well) in EDCB buffer for 12 h at 4 °C. After coating, four washes were performed in 200 μl of washing buffer (WB: phosphate saline buffer [PBS] + 0.5% Tween 20). Free binding well sites were blocked with WB supplemented with 5% (w/v) non-fat milk (blocking buffer: BB) for 1 h at room temperature (RT). After, plates were incubated with 100 μl of canine sera (1:400 dilution in BB) for 2 h at RT. Then, wells were washed with WB as indicated above and incubated with 100 μl of secondary antibody (anti-dog IgG antibody (Sigma, St. Louis, USA) horseradish peroxidase conjugated; 1:6000 dilution in BB) for 1 h at RT. After 4 washes, reaction was developed with 100 μl of H2O2-ortophenylenediamine solution (Sigma) for 20 min in the dark, and stopped by addition of 50 μl of H2SO4 2 N. Absorbance values were determined at 490 nm in an ELISA microplate reader.
In all the plates the same negative control (canine sera from a healthy dog living in a non-endemic region for Leishmania) was always included to calculate the relative reactivity (RR) of each sample. RR was defined as the ratio between the absorbance of a given sample and the negative control sample taken from a selected healthy dog. As another technical control all plates also included a positive sera obtained from a CanL dog selected because of its reactivity (O.D.450 > 0.5) to all antigens assayed.
2.4. Statistical analysis
The statistical analysis was made using the GraphPad Prism software. The ELISAs cut-off values were calculated by comparison of the RR from the CanL sera (clinically ill or subclinically infected) and healthy (or E. canis infected dogs when indicated) using the Receiver Operating Characteristic (ROC) analysis. This test also allowed the determination of sensitivity, specificity and the Likelihood ratio (LR) defined as the ratio of expected ELISA result in dogs with CanL to the dogs without the disease (Simundic, 2009). D'Agostino and Pearson test was employed to analyze the Gaussian distribution of the samples. The Mann-Whitney non-parametric test was employed to assess the existence of significant differences between two groups. P-values lower than 0.05, 0.01 or 0.001 were represented as *, ** or ***, respectively. The Kruskal-Wallis non-parametric test was employed to analyze more than two groups. P-values lower than 0.01 or 0.001 were represented as ++ or +++.
3. Results
3.1. Anti-leishmanial humoral response in clinically ill dogs
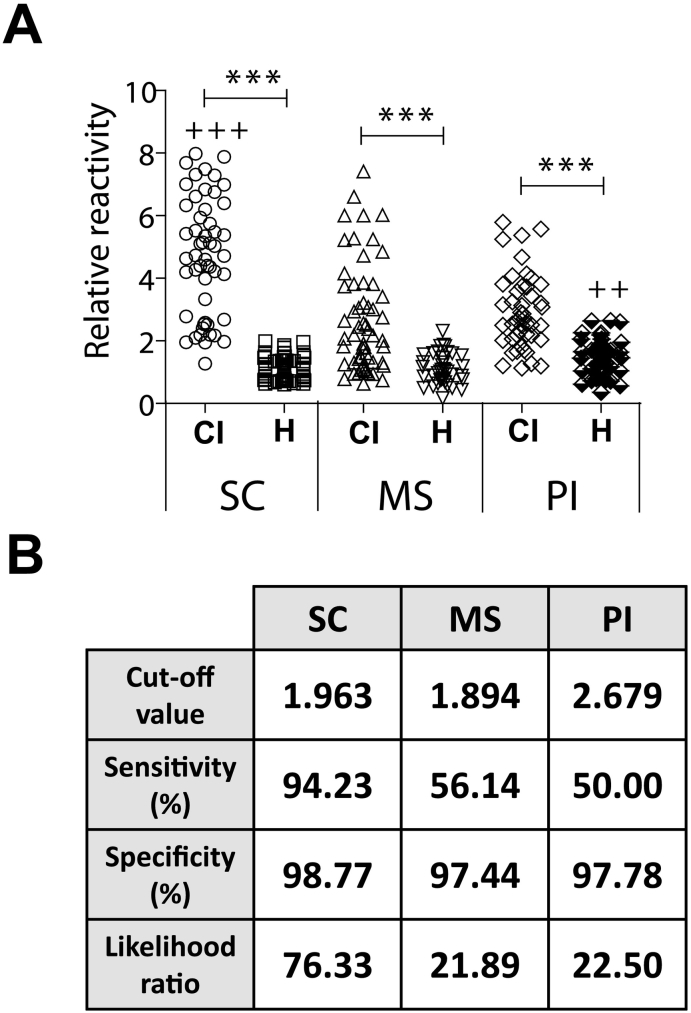
Our first objective was to analyze the reactivity against SLA of sera collections taken in three different States of Brazil: SC, MS, PI. The RR against SLA of the sera from clinically ill CanL animals, independently of the sampling place, was significantly higher than the RR calculated using sera from healthy animals residing in the same equivalent geographical regions (Fig. 1A). Interestingly, the RR found for SC sera was significantly higher than that found for MS and PI. On the contrary, the RR value for PI healthy samples was significantly higher than the equivalent sera from the other regions. These differences are reflected in the sensitivity value of the SLA-based assay, being the highest value for the SC samples and the lowest for the PI sera (Fig. 1B). Thus, diagnostic accuracy for SLA determined by the LR was very low, especially in MS and PI (Fig. 1B).
Fig. 1.
Sera from clinically ill CanL patients collected in different geographical locations show differences in the recognition of Leishmania soluble leishmanial antigens (SLA). Sera from CanL clinically ill dogs (CI) or healthy dogs (H) collected in Santa Catarina (SC), Mato Grosso do Sul (MS) or Piaui (PI) were assayed by ELISA against soluble leishmanial antigen (SLA). In the graph it is shown the relative reactivity (RR) defined as the absorbance of a given sera divided by the absorbance of a control healthy sera included in all plates. The symbol *** indicates significant differences (P < 0.001) between clinically ill CanL and healthy sera from the same location (Mann Whitney test). The symbol +++ indicates a significant increase (P < 0.001) between the clinically ill CanL from SC with regard the equivalent sera from the other two locations and the symbol ++ indicates a significant increase in the RR values of healthy sera taken in PI with regard the equivalent sera from SC and MS (Kruskal-Wallis test) (A). Table showing the diagnostic parameters calculated by a ROC analysis (B).
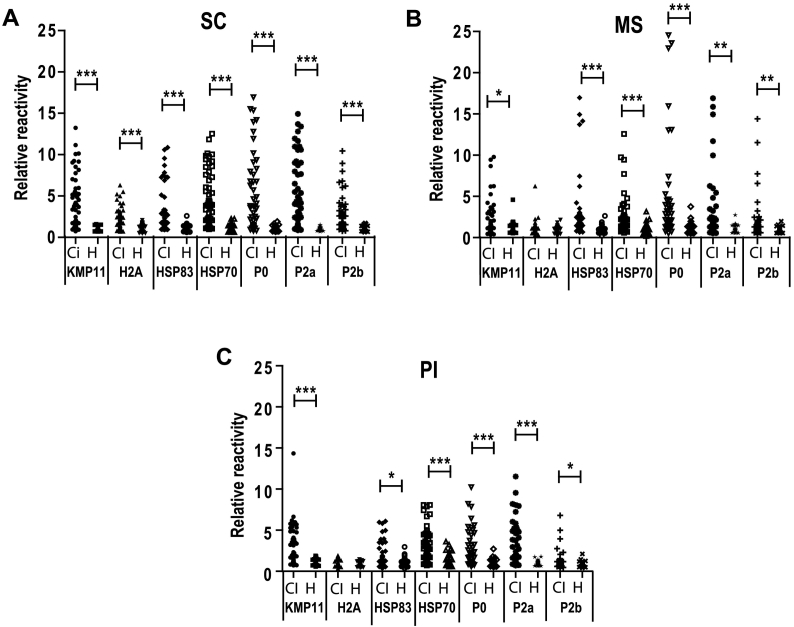
We next use seven individual antigenic proteins of Leishmania produced as heterologous recombinant proteins in bacteria, to perform ELISAs using the same sera collections (sera from clinically ill CanL animals and healthy dogs, respectively). All the proteins were recognized as antigenic when analyzed with SC sera. The RR values for the sera of clinically ill dogs were significantly higher than the RR of the healthy sera (Fig. 2A). A similar behavior was found for the sera from MS (Fig. 2B) or PI (Fig. 2C) except for the histone H2A, which was not recognized. The highest percentages of sensitivity for each one of the antigens were observed for sera taken in SC, resulting also in the highest value of the LR when compared to data from MS and PI sera data (Table 1). None of the assays performed with the recombinant proteins and sera from SC of MS (Table 1) reach the LR found for the SLA (Fig. 1B). This situation changes when data of the sera collected in PI were analyzed. For this sera collection, the KMP-11 surface protein and the LiP0 ribosomal protein showed slightly higher sensitivity percentages (60% and 54.35%, respectively) or LR values (27 and 24.46, respectively) (Table 1) than the SLA (50% of sensitivity and 22.5 of LR, respectively) (Fig. 1B). We conclude that the use of SLA-based methods can compromise the results of the diagnosis and that the individual antigens diagnostic performances can be of interest to improve these obtained with SLA.
Fig. 2.
Reactivity of sera from clinically ill CanL patients against single Leishmania recombinant antigenic proteins. Different Leishmania antigens purified as recombinant proteins after the expression of their coding regions in E. coli were employed for coating ELISA plates: Kinetoplastid membrane protein of 11 kDa (KMP11), histone H2A, the heat shock proteins of 70 kDa (HSP70) or 83 kDa (HSP83) and the acidic ribosomal proteins P2a, P2b and P0. Sera from CanL clinically ill dogs (CI) or healthy dogs (H) collected in Santa Catarina (SC) (A), Mato Grosso do Sul (MS) (B) or Piaui (PI) (C) were assayed by ELISA. The scatter plots show the relative reactivity of the sera represented individually. Symbols * (P < 0.05), ** (P < 0.05) and *** (P < 0.001) indicate significant differences between clinically infected CanL and healthy sera from the same geographical location (Mann Whitney test).
Table 1.
Detailed information about diagnostic properties of the Leishmania antigens obtained from the ROC curve analysis.
| Antigen | Sera origin | Cut-off | Sensitivity (%) | Specificity (%) | Likelihood ratio |
|---|---|---|---|---|---|
| KMP-11 | SC | 1.56 | 78.85 | 98.78 | 64.65 |
| MS | 1.97 | 39.13 | 97.22 | 14.09 | |
| PI | 1.81 | 60.00 | 97.78 | 27.00 | |
| H2A | SC | 1.94 | 57.69 | 98.78 | 50.46 |
| MS | 1.83 | 7.02 | 97.44 | 2.74 | |
| PI | 1.38 | 19.57 | 97.83 | 9.00 | |
| HSP83 | SC | 1.69 | 59.62 | 98.78 | 48.88 |
| MS | 1.94 | 45.61 | 97.44 | 17.79 | |
| PI | 2.21 | 28.26 | 97.73 | 12.4345 | |
| HSP70 | SC | 1.94 | 75.00 | 97.56 | 30.75 |
| MS | 2.24 | 33.33 | 97.94 | 13.00 | |
| PI | 3.31 | 47.83 | 97.78 | 21.52 | |
| P0 | SC | 1.78 | 61.54 | 98.78 | 50.46 |
| MS | 2.42 | 40.35 | 97.44 | 15.74 | |
| PI | 1.81 | 54.35 | 97.78 | 24.46 | |
| P2a | SC | 1.43 | 69.23 | 98.78 | 58.35 |
| MS | 1.69 | 38.60 | 97.44 | 15.05 | |
| PI | 1.48 | 60.87 | 95.35 | 13.09 | |
| P2b | SC | 1.58 | 59.62 | 98.78 | 48.88 |
| MS | 1.62 | 35.42 | 96.67 | 10.63 | |
| PI | 1.49 | 15.22 | 97.83 | 7.00 |
3.2. Reactivity of subclinically infected CanL sera against the antigenic preparations
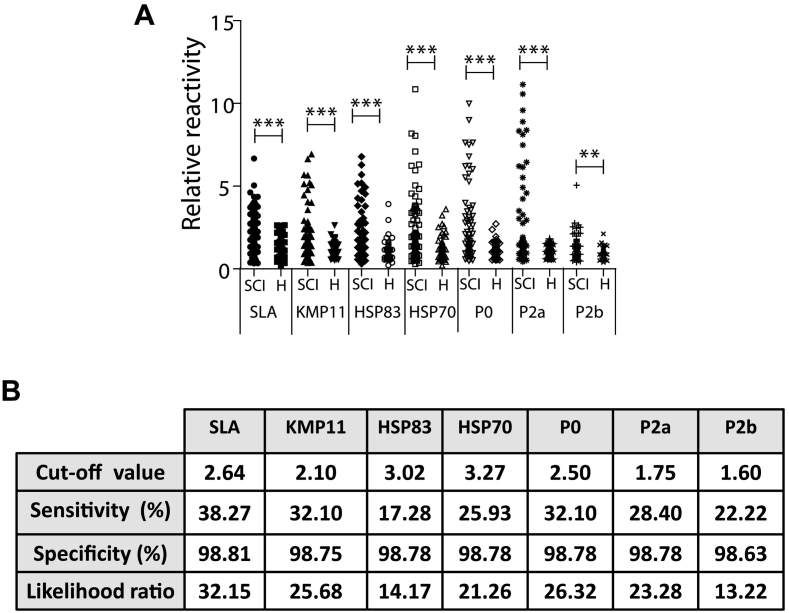
The second objective of this work was to assay the reactivity of the SLA or the recombinant proteins except H2A (due to the lack of antigenicity when sera from MS and PI were assayed) with the sera obtained from clinically healthy animals infected with Leishmania. With this purpose, sera were collected from subclinically infected animals in MS and PI and analyzed as a single group. The median of the RR values of the subclinically infected sera were significantly higher than the median value of the RR of the sera collected from healthy animals living in both locations for all antigenic samples tested, i.e. SLA and recombinant proteins (Fig. 3A). Sensitivity percentages range from the 38.27% for the SLA to the 17.28% for the HSP83 recombinant protein. The LR for the SLA was higher than those found for the individual antigens, being the HSP83 and the LiP2b the protein preparations that obtained the lowest LR values (Fig. 3B).
Fig. 3.
Reactivity of sera from CanL subclinically infected animals against SLA and the antigenic recombinant proteins. Sera from CanL dogs that are subclinically infected (SCI) or healthy dogs (H) were assayed by ELISA against SLA or against the indicated Leishmania antigens. A scatter plot indicating the relative reactivity values is shown. Symbols ** (P < 0.05) and *** (P < 0.001) indicate significant differences between subclinically infected CanL and healthy sera (Mann Whitney test) (A). Table showing the diagnostic parameters calculated by a ROC analysis (B).
3.3. Differential diagnosis of CanL and ehrlichiosis
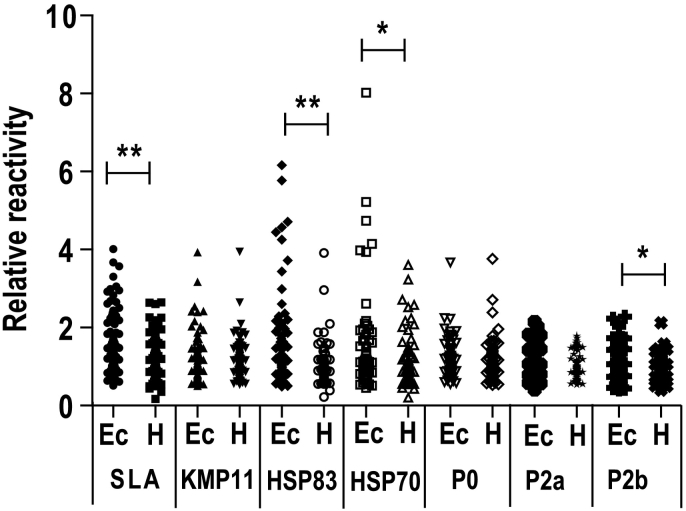
Next, we made a comparative analysis between sera from dogs infected with E. canis and the sera of healthy animals to test the specificity of the recognition of the parasite antigenic preparations (SLA and recombinant proteins, except H2A). Both sera groups were collected in PI and MS and grouped independently of the locations they were obtained. Four antigenic preparations including SLA, the HSP70 and HSP83, as well as the LiP2b protein presented higher RR values for sera of animals affected by ehrlichiosis than sera for co-residing healthy animals (Fig. 4). For SLA the RR values were homogeneously distributed along the ordinate axis, showing a RR median value significantly increased with regard the median of the RR of the healthy data. On the other hand, for the heat shock proteins, besides the incremented RR value for most of the sera population, the results showed high RR values for some individual sera (Fig. 4), suggesting the presence of highly cross-reactive antigenic determinants in these proteins, despite the evolutionary distance between Leishmania and Ehrlichia genus. On the other hand, no cross-reactivity was found for the surface located KMP-11 antigen or the acidic ribosomal proteins P0 and P2a. For these three proteins similar RR values were found in the sera from E. canis infected animals and the healthy ones (Fig. 4).
Fig. 4.
Serologic cross-reactivity of Ehrlichia canis infected dogs' sera and Leishmania antigens. Sera from E. canis infected dogs (Ec) and healthy animals (H) were assayed by ELISA against SLA or against the indicated Leishmania antigens. A scatter plot indicating the relative reactivity values is shown. Symbols * (P < 0.001) or ** (P < 0.05) indicate significant differences between samples from dogs suffering ehrlichiosis and healthy animals (Mann Whitney test).
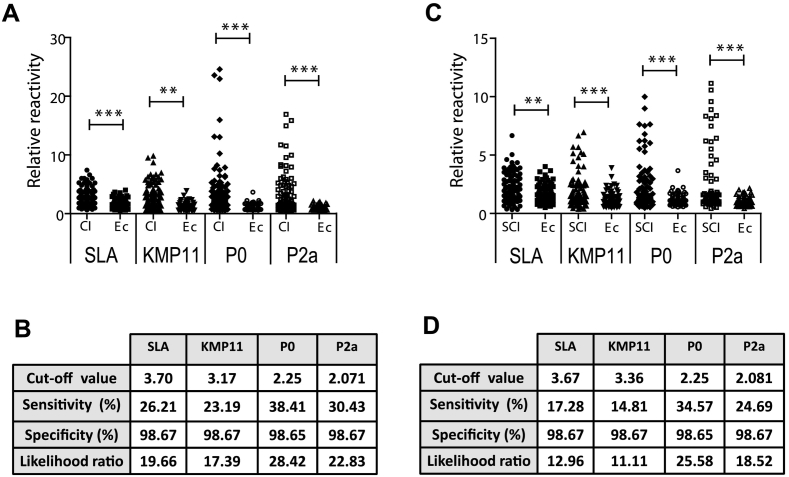
Thus, we decided to analyze the diagnostic value of these three individual proteins in comparison to SLA, using clinically ill (Fig. 5A and B) and subclinically infected (Fig. 5C and D) sera from PI and MS endemic areas. Leishmania-negative data were obtained from the sera of animals suffering mononuclear ehrlichiosis and collected in the same locations, simulating the worst conditions to perform a specific diagnosis of CanL. The median values of the RR were significantly higher in clinically ill (Fig. 5A) and subclinically infected (Fig. 5C) CanL serum groups when compared with sera from E. canis infected animals. The highest LR for diagnosis of clinically ill (Fig. 5B) or subclinically infected (Fig. 5D) CanL was found for the LiP2a and LiP0 acidic ribosomal proteins, being the lowest the LR of the KMP-11.
Fig. 5.
Comparative diagnostic performance of SLA and the selected leishmanial antigenic preparations. Sera from clinically ill (CI) or subclinically infected (SCI) dogs affected by CanL and dogs infected by E. canis (Ec) were assayed by ELISA against SLA or against Leishmania KMP-11, P0 and P2a antigens. A scatter plot showing the relative reactivity values (A) or a table showing the diagnostic parameters (B) from CanL clinically ill animals are shown. In (C) and (D) equivalent information for the CanL subclinically infected collection is included. Symbols ** (P < 0.05) and *** (P < 0.001) indicate significant differences between the sera from CanL animals and sera from E. canis infected dogs (Mann Whitney test).
In spite of these statistical significant differences, the main problem was the low sensitivity. Thus, protein with the highest LR, LiP0, was only able to diagnose up to 38% of the CanL animals showing clinical signs and up to 34.57% of the subclinically infected animals. In order to solve the low sensitivity value of the diagnostic based on single proteins we propose to establish the next criterion of positivity: a CanL patient will be one who gives a positive response to at least one of the three candidates. This condition was based on the high degree of heterogeneity found in the recognition of the KMP-11, the LiP0 and the LiP2a antigens by each one of the individual sera from the highly endemic areas (Supplementary Fig. 2A for clinically ill CanL sera and Supplementary Fig. 2B for subclinically infected CanL sera). By the use of the cut-off values shown in Fig. 5 to classify each serum as positive or negative, a percentage of 56.28% (59/103) of the clinically ill and 39.5% (32/81) of the subclinically infected CanL sera were diagnosed as positive. Including the SLA beside the recombinant proteins we were able to rescue an additional sera in the clinically ill group 58.25% (60/103) (Supplementary Fig. 2A) and two sera for subclinically infected group CanL 41.97% (34/81) (Supplementary Fig. 2B). Finally we determined the number of sera diagnosed as positive from non-endemic area (SC) using the same criterion and the cut-off value obtained using the E. canis infected dogs simulating the worst diagnostic conditions. As it is shown in the Supplementary Fig. 2C, recombinant proteins were able to diagnose the 75% (39/52) of the positive sera and the SLA alone rescued 76.92% (40/52). The best results were obtained when the diagnostic criterion is based on the reactivity towards any of the four antigen preparations: 82.69% (43/52). Thus, even in non-endemic regions the inclusion of the recombinant proteins improves the diagnostic sensitivity of the SLA. We propose to use the three selected antigens in combination with SLA for obtaining the best results for a high sensitive serodiagnosis.
4. Discussion
In this work we have made a comparative analysis of two different antigenic sources, SLA (a soluble leishmanial total extract preparation) and a series of different individual parasite antigens, obtained by a biotechnological approach as heterologous recombinant protein produced in bacteria. Regarding the SLA preparation and in coincidence with other authors assaying sera collected in the New or the Old World, our results shown that most of the CanL clinically ill animals possess antibodies against total parasite antigen preparations (Boarino et al., 2005; Porrozzi et al., 2007; Coelho et al., 2009; Fraga et al., 2014; Laurenti et al., 2014). We found a 94% of assay sensitivity for SC sera collection when the results of the CanL clinically ill sera results are compared to these obtained for healthy dogs from the same geographical area (Fig. 1). Interestingly, the sensitivity values of our ELISA test based on in-house SLA preparation were significantly affected by the geographic location of canine patients. In this sense, the percentages of positive animals residing in MS or PI diminished to percentages of 56% or 50%, respectively. This decrease was related to the lower reactivity value of the sera from these CanL clinically ill animals (both in MS and PI) and to an increase in the non-specific signal of the sera of healthy animals living in the most Northern State (PI). The existence of differences in the diagnostic capacities of a given test in different geographical areas also occurred when a SLA-based commercial kit was assayed for sera samples from different Brazilian region, with sensitivity values ranging from 70% to 91% (Lira et al., 2006; Laurenti et al., 2014; Fraga et al., 2016; de Carvalho et al., 2018). This sensitivity differences may limit the usefulness of the SLA-based methods.
Another drawback for the use of SLA for the CanL serodiagnosis is the existence of cross reactions between the total proteins of Leishmania and the serum of animals harboring other pathogens, including protozoan parasites (dogs infected with Babesia, or Trypanosoma) or bacteria like E. canis (Lira et al., 2006; da Silva et al., 2013; Fraga et al., 2014; Laurenti et al., 2014). One of the limitations of our work is the lack of cross-reactivity data between the SLA and the sera of animals infected with the most common pathogens in South America (recently reviewed in (Maggi and Kramer, 2019)) except Ehrichia. Employing the sera from dogs affected by monocytic ehrlichiosis, a highly endemic disease in several regions of Brazil (Maia and Campino, 2008), we detected a positive cross-reactivity, being the median value of the reactivity against SLA of these sera significantly higher than the reactivity detected for the sera of healthy animal (Fig. 4). This finding results in an increase of the cut-off value for positive/negative discrimination, negatively affecting diagnostic tests sensitivity (Porrozzi et al., 2007; Alves et al., 2012). Our data demonstrate that the maintenance of a high value of specificity (98.7%) provoked a concomitant decrease in the sensitivity of the SLA-based assay to diagnose CanL clinically ill animals. Regarding serodiagnosis of CanL in dogs that are subclinically infected, and in accordance with our results, the diagnostic properties of SLA-based methods give raise to lower sensitivity values than these observed for clinically ill dogs, which decrease more markedly when cross-reactivity parameters are also taken into consideration (Ferreira Ede et al., 2007; Laurenti et al., 2014; Zhao et al., 2016; de Carvalho et al., 2018). In our case, the sensitivity value fell from 38.27% (Fig. 3) to 17.28% (Fig. 5D) when considering the cross-reactivity parameter to calculate the cut-off.
Molecular methods can be employed for detecting clinically ill and subclinically infected animals because of their high sensitivity (Albuquerque et al., 2017; Mendonca et al., 2017; de Carvalho et al., 2018; Riboldi et al., 2018). However, development of serological improved systems would allow an easier monitoring of large number of samples in endemic areas. In this sense it is likely that the characterization of individual antigens can help to improve the serodiagnosis of CanL cases, because of the improvement of the sensitivity values without increasing the specificity (Carvalho et al., 2017; Magalhaes et al., 2017; Dias et al., 2018). In this regard we found that all the recombinant proteins included in this work, except histone H2A, were recognized by the canid sera from the three different Brazilian States. The lack of sera recognition for H2A in MS or PI should be taken as an example regarding the variability in the quality of the humoral response of leishmaniasis patients in different parts of the world. These differences in antigen recognition may be influenced by differences in animal nutrition (Calder et al., 2006) or changes in the immune system capacities due to previous contact or co-infection with other pathogens (Lescano et al., 2012; Murphy et al., 2013), that could be more habitual in the most endemic regions for Leishmania (PI and MS). We have found the H2A histone is recognized by approximately the 58% of the Leishmania-infected dogs in SC (Table 1), in accordance with the positive reactivity found in the 78% of the CanL sera collected in Spain (Soto et al., 1995). Also, this protein is recognized by the sera from human patients suffering VL in Tunisia (Maalej et al., 2003) or tegumentary leishmaniasis in the New World (Souza et al., 2013). However, the data obtained in this work discourages its use in a serological diagnostic test. Interestingly, our results demonstrate that such differences in sensitivity of the clinically ill sera due to geographical reasons were not observed for certain individual antigens, especially KMP-11 and the acidic ribosomal proteins P0 and P2a (Table 1). In addition to this property, no cross-reactivity was found against these proteins when assayed with the sera form E. canis infected dogs. Our data also dampen the use of the HSP70 and HSP83 heat shock proteins for serodiagnosis. In spite of being recognized by infected animals in the three locations studied in this work, as well as by a high number of Leishmania-infected human or canine patients in different parts of the world (Celeste et al., 2004; Angel et al., 1996; Quijada et al., 1996a; Rafati et al., 2007; Abanades et al., 2012; Souza et al., 2013), a positive reactivity with sera from dogs affected with ehrlichiosis was found. An interesting alternative to not discard these proteins, would be to explore the location of their cross-reactive antigenic determinants to produce non cross-reactive recombinant proteins (or synthetic peptides) containing the specific epitopes (Quijada et al., 1996b). Finally, the use of the P2b for diagnosis was discarded because of its cross-reactivity with the sera from dogs affected by ehrlichiosis and the low percentages of sera recognizing this antigen especially in the most endemic areas (Fig. 4 and Table 1, respectively).
One of the main findings of our work is the characterization of the diagnostic properties of the recombinant version of the KMP-11, the P2a and the P0 proteins. In this sense, as it is deduced from data shown in Fig. 5, P2a and P0 performance for the diagnosis of CanL for both, clinically ill or subclinically infected animals was higher than this detected for the SLA, when cut-off is calculated using as control group the sera from dogs affected by mononuclear ehrlichiosis. Diagnostic performance of the KMP-11 offered similar values than the SLA. However, the main drawback observed for the use of the three selected individual antigens for diagnosis (either in the clinically ill or in the subclinically infected forms) is related to the low value of sensitivity observed when the specificity of the assay is maintained up to 98%. Coinciding with other authors, it looks like the use of single recombinant antigen is insufficient to constructs serodiagnostic tests able to detect high percentages of the leishmaniasis patients (Maalej et al., 2003; Porrozzi et al., 2007; Oliveira et al., 2011). The high degree of variability in the humoral response of patients and infected dogs against individual parasite proteins (Porrozzi et al., 2007; Goto et al., 2009) offers the possibility of improving the sensitivity degree by using combination of recombinant proteins or chimeric recombinant proteins in which the antigenic determinants of different parasite proteins are fused (Soto et al., 1998; Faria et al., 2015). This is the case of a rapid test (DPP) based on recombinant fusion products containing the antigenic regions of different parasite antigen that have shown high sensibility for diagnosing clinically ill CanL cases (Grimaldi Jr et al., 2012; Morales-Yuste et al., 2012; da Silva et al., 2013; Rodriguez-Cortes et al., 2013; Marcondes et al., 2013; Laurenti et al., 2014; Fraga et al., 2016; Riboldi et al., 2018), although its sensitivity decreases in canine patients showing low clinical symptoms or in subclinically infected cases (Grimaldi Jr et al., 2012; Lopes et al., 2017). As it is deduced from data shown in the Supplementary Fig. 2, a high degree of heterogeneity was found for the recognition of the individual antigen by each of the sera assayed. Thus, complementarity found in the immunoreactivity of the sera against the three recombinant antigens and SLA allowed us the increase of the number of sera diagnosed as positive when the criterion of positivity is to have a positive value (over the most restricted cut-off) for any of the selected individual antigens. If SLA is also included (with cut-off values maintaining a high specificity degree to discriminate animals affected by ehrlichiosis), we were able to diagnose up to a 58.25% of the clinically ill dogs (approx. 42% of the subclinically infected canine samples) living in the most endemic regions maintaining a high value of sensitivity for clinically ill dogs living in the new colonized regions (82.69% for SC, using restrictive cut-off values). It has to be highlighted that the seropositivity values obtained here are lower than those reported for other antigenic preparations (Laurenti et al., 2014; Faria et al., 2015; Fraga et al., 2016; de Carvalho et al., 2018). This is due to our proposal of maintaining a high value of specificity for not to diagnose false positive animals, a necessary property for a serodiagnostic test. Before moving to the field, the specificity in the recognition of recombinant proteins should be analyzed using sera from animals infected by other pathogens that coexist with Leishmania (Maggi and Kramer, 2019) including the study of cross-reactivity with dogs infected with T. cruzi (Roque et al., 2013; Freitas et al., 2018).
5. Conclusion
From the results obtained in this work it can be concluded that the combined use of different antigenic preparations improves the sensitivity and specificity of CanL diagnosis. This conclusion is valid to confirm the disease in animals that present clinical signs compatible with leishmaniasis. The proposed strategy has also been shown to be able to increase the number of subclinically infected animals diagnosed because of the presence of anti-Leishmania antibodies. Undoubtedly, the percentages of positivity must be improved before moving to the field. We plan to study new recombinant proteins that can act cooperatively to these studied in this work in order to achieve better diagnostic performances for confirming the disease in clinically infected animals, as well as identifying dogs subclinically infected.
The following are the supplementary data related to this article.
Geographical location of the Brazilian States included in the study. The cities where the samples were taken are highlighted.
Individualized analysis of serum reactivity. Schematic representation of the positivity against the indicated antigens from the next sera: CanL clinically ill sera (MS + PI) (A), CanL subclinically infected sera (MS+PI) (B) and CanL clinically ill sera from SC (C). The shaded squares indicated these sera over the value of cut-off showed in Fig. 5B for clinically ill and Fig. 5D for subclinically infected. The cut-off value for panel (C) was taken from Fig. 5A for the SLA and the recombinant proteins. At the left, grey arrows indicate sera positive only for the SLA preparation (A–C). In (C), +++ indicate sera negative for SLA and positive for at least one of the recombinant proteins.
Acknowledgements
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (Brazil) within the call “CNPq/MS/SCTIE/DECIT N° 32/2014 - Pesquisas sobre Leishmanioses” grant number reference 467389/2014-4. Institutional grants from the Fundación Ramón Areces and Banco de Santander to the CBMSO are also acknowledged. TC received scholarship from Fundação de Amparo a Pesquisa do Estado de Santa Catarina – FAPESC.
References
- Abanades D.R., Arruda L.V., Arruda E.S., Pinto J.R., Palma M.S., Aquino D., Caldas A.J., Soto M., Barral A., Barral-Netto M. Immunodominant antigens of Leishmania chagasi associated with protection against human visceral leishmaniasis. PLoS Negl.Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbehusen M.M.C., Almeida V.D.A., Solca M.D.S., Pereira L.D.S., Costa D.J., Gil-Santana L., Bozza P.T., Fraga D.B.M., Veras P.S.T., Dos-Santos W.L.C., Andrade B.B., Brodskyn C.I. Clinical and immunopathological findings during long term follow-up in Leishmania infantum experimentally infected dogs. Sci. Rep. 2017;7:15914. doi: 10.1038/s41598-017-15651-8. https://www.nature.com/articles/s41598-017-15651-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albuquerque A., Campino L., Cardoso L., Cortes S. Evaluation of four molecular methods to detect Leishmania infection in dogs. Parasit. Vectors. 2017;10:57. doi: 10.1186/s13071-017-2002-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves A.S., Mouta-Confort E., Figueiredo F.B., Oliveira R.V., Schubach A.O., Madeira M.F. Evaluation of serological cross-reactivity between canine visceral leishmaniasis and natural infection by Trypanosoma caninum. Res. Vet. Sci. 2012;93:1329–1333. doi: 10.1016/j.rvsc.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Angel S.O., Requena J.M., Soto M., Criado D., Alonso C. During canine leishmaniasis a protein belonging to the 83-kDa heat-shock protein family elicits a strong humoral response. Acta Trop. 1996;62:45–56. doi: 10.1016/s0001-706x(96)00020-4. [DOI] [PubMed] [Google Scholar]
- Baneth G., Koutinas A.F., Solano-Gallego L., Bourdeau P., Ferrer L. Canine leishmaniosis - new concepts and insights on an expanding zoonosis: part one. Trends Parasitol. 2008;24:324–330. doi: 10.1016/j.pt.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Berberich C., Requena J.M., Alonso C. Cloning of genes and expression and antigenicity analysis of the Leishmania infantum KMP-11 protein. Exp. Parasitol. 1997;85:105–108. doi: 10.1006/expr.1996.4120. [DOI] [PubMed] [Google Scholar]
- Boarino A., Scalone A., Gradoni L., Ferroglio E., Vitale F., Zanatta R., Giuffrida M.G., Rosati S. Development of recombinant chimeric antigen expressing immunodominant B epitopes of Leishmania infantum for serodiagnosis of visceral leishmaniasis. Clin. Diagn. Lab. Immunol. 2005;12:647–653. doi: 10.1128/CDLI.12.5.647-653.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calder P.C., Krauss-Etschmann S., de Jong E.C., Dupont C., Frick J.S., Frokiaer H., Heinrich J., Garn H., Koletzko S., Lack G., Mattelio G., Renz H., Sangild P.T., Schrezenmeir J., Stulnig T.M., Thymann T., Wold A.E., Koletzko B. Early nutrition and immunity - progress and perspectives. Br. J. Nutr. 2006;96:774–790. https://www.cambridge.org/core/journals/british-journal-of-nutrition/article/early-nutrition-and-immunity-progress-and-perspectives/C952EBB0A43BD282C723C1E7A7FD94E5 [PubMed] [Google Scholar]
- Carvalho A., Costa L.E., Salles B.C.S., Santos T.T.O., Ramos F.F., Lima M.P., Chavez-Fumagalli M.A., Silvestre B.T., Portela A.S.B., Roatt B.M., Silveira J.A.G., Goncalves D.U., Magalhaes-Soares D.F., Duarte M.C., Menezes-Souza D., Coelho E.A.F. An ELISA immunoassay employing a conserved Leishmania hypothetical protein for the serodiagnosis of visceral and tegumentary leishmaniasis in dogs and humans. Cell. Immunol. 2017;318:42–48. doi: 10.1016/j.cellimm.2017.06.001. [DOI] [PubMed] [Google Scholar]
- Celeste B.J., Angel S.O., Castro L.G., Gidlund M., Goto H. Leishmania infantum heat shock protein 83 for the serodiagnosis of tegumentary leishmaniasis. Br. J. Med. Biol. Res. 2004;37:1591–1593. doi: 10.1590/s0100-879x2004001100001. https://www.ncbi.nlm.nih.gov/pubmed/15517072?dopt=Citation [DOI] [PubMed] [Google Scholar]
- Coelho E.A., Ramirez L., Costa M.A., Coelho V.T., Martins V.T., Chavez-Fumagalli M.A., Oliveira D.M., Tavares C.A., Bonay P., Nieto C.G., Abanades D.R., Alonso C., Soto M. Specific serodiagnosis of canine visceral leishmaniasis using Leishmania species ribosomal protein extracts. Clin. Vaccine Immunol. 2009;16:1774–1780. doi: 10.1128/CVI.00295-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva D.A., Madeira Mde F., Abrantes T.R., Filho C.J., Figueiredo F.B. Assessment of serological tests for the diagnosis of canine visceral leishmaniasis. Vet. J. 2013;195:252–253. doi: 10.1016/j.tvjl.2012.06.010. [DOI] [PubMed] [Google Scholar]
- Dantas-Torres F., Solano-Gallego L., Baneth G., Ribeiro V.M., de Paiva-Cavalcanti M., Otranto D. Canine leishmaniosis in the old and new worlds: unveiled similarities and differences. Trends Parasitol. 2012;28:531–538. doi: 10.1016/j.pt.2012.08.007. [DOI] [PubMed] [Google Scholar]
- de Carvalho F.L.N., Riboldi E.O., Bello G.L., Ramos R.R., Barcellos R.B., Gehlen M., Halon M.L., Romao P.R.T., Dallegrave E., Rossetti M.L.R. Canine visceral leishmaniasis diagnosis: a comparative performance of serological and molecular tests in symptomatic and asymptomatic dogs. Epidemiol. Infect. 2018;146:571–576. doi: 10.1017/S0950268818000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias D.S., Martins V.T., Ribeiro P.A.F., Ramos F.F., Lage D.P., Tavares G.S.V., Mendonca D.V.C., Chavez-Fumagalli M.A., Oliveira J.S., Silva E.S., Gomes D.A., Rodrigues M.A., Duarte M.C., Galdino A.S., Menezes-Souza D., Coelho E.A.F. Antigenicity, immunogenicity and protective efficacy of a conserved Leishmania hypothetical protein against visceral leishmaniasis. Parasitol. 2018;145:740–751. doi: 10.1017/S0031182017001731. [DOI] [PubMed] [Google Scholar]
- Dujardin J.C., Campino L., Canavate C., Dedet J.P., Gradoni L., Soteriadou K., Mazeris A., Ozbel Y., Boelaert M. Spread of vector-borne diseases and neglect of leishmaniasis, Europe. Emerg. Infect. Dis. 2008;14:1013–1018. doi: 10.3201/eid1407.071589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faria A.R., de Castro Veloso L., Coura-Vital W., Reis A.B., Damasceno L.M., Gazzinelli R.T., Andrade H.M. Novel recombinant multiepitope proteins for the diagnosis of asymptomatic Leishmania infantum-infected dogs. PLoS Negl. Trop. Dis. 2015;9 doi: 10.1371/journal.pntd.0003429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Cotrina J., Iniesta V., Belinchon-Lorenzo S., Munoz-Madrid R., Serrano F., Parejo J.C., Gomez-Gordo L., Soto M., Alonso C., Gomez-Nieto L.C. Experimental model for reproduction of canine visceral leishmaniosis by Leishmania infantum. Vet. Parasitol. 2013;192:118–128. doi: 10.1016/j.vetpar.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Ferreira Ede C., de Lana M., Carneiro M., Reis A.B., Paes D.V., da Silva E.S., Schallig H., Gontijo C.M. Comparison of serological assays for the diagnosis of canine visceral leishmaniasis in animals presenting different clinical manifestations. Vet. Parasitol. 2007;146:235–241. doi: 10.1016/j.vetpar.2007.02.015. [DOI] [PubMed] [Google Scholar]
- Fraga D.B., da Silva E.D., Pacheco L.V., Borja L.S., de Oliveira I.Q., Coura-Vital W., Monteiro G.R., Oliveira G.G., Jeronimo S.M., Reis A.B., Veras P.S. A multicentric evaluation of the recombinant Leishmania infantum antigen-based immunochromatographic assay for the serodiagnosis of canine visceral leishmaniasis. Parasit. Vectors. 2014;7:136. doi: 10.1186/1756-3305-7-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraga D.B., Pacheco L.V., Borja L.S., Tuy P.G., Bastos L.A., Solca Mda S., Amorim L.D., Veras P.S. The rapid test based on leishmania infantum chimeric rK28 protein improves the diagnosis of canine visceral leishmaniasis by reducing the detection of false-positive dogs. PLoS Negl. Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas Y.B.N., Souza C., Magalhaes J.M.E., Sousa M.L.R., d'Escoffier L.N., Valle T.Z.D., Goncalves T.C.M., Gil-Santana H.R., Kazimoto T.A., Amora S.S.A. Natural infection by Trypanosoma cruzi in triatomines and seropositivity for Chagas disease of dogs in rural areas of Rio Grande do Norte, Brazil. Rev Soc Bras Med Trop. 2018;51:190–197. doi: 10.1590/0037-8682-0088-2017. [DOI] [PubMed] [Google Scholar]
- Fuertes M.A., Perez J.M., Soto M., Lopez M.C., Alonso C. Calcium-induced conformational changes in Leishmania infantum kinetoplastid membrane protein-11. J. Biol. Inorg. Chem. 2001;6:107–117. doi: 10.1007/s007750000175. [DOI] [PubMed] [Google Scholar]
- Garde E., Ramirez L., Corvo L., Solana J.C., Martin M.E., Gonzalez V.M., Gomez-Nieto C., Barral A., Barral-Netto M., Requena J.M., Iborra S., Soto M. Analysis of the antigenic and prophylactic properties of the Leishmania translation initiation factors eIF2 and eIF2B in natural and experimental leishmaniasis. Front. Cell. Infect. Microbiol. 2018;8:112. doi: 10.3389/fcimb.2018.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto Y., Howard R.F., Bhatia A., Trigo J., Nakatani M., Netto E.M., Reed S.G. Distinct antigen recognition pattern during zoonotic visceral leishmaniasis in humans and dogs. Vet. Parasitol. 2009;160:215–220. doi: 10.1016/j.vetpar.2008.10.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi G., Jr., Teva A., Ferreira A.L., dos Santos C.B., Pinto I., de-Azevedo C.T., Falqueto A. Evaluation of a novel chromatographic immunoassay based on dual-path platform technology (DPP(R) CVL rapid test) for the serodiagnosis of canine visceral leishmaniasis. Trans. R. Soc. Trop. Med. Hyg. 2012;106:54–59. doi: 10.1016/j.trstmh.2011.10.001. [DOI] [PubMed] [Google Scholar]
- Harhay M.O., Olliaro P.L., Costa D.L., Costa C.H. Urban parasitology: visceral leishmaniasis in Brazil. Trends Parasitol. 2011;27:403–409. doi: 10.1016/j.pt.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Hosein S., Blake D.P., Solano-Gallego L. Insights on adaptive and innate immunity in canine leishmaniosis. Parasitol. 2017;144:95–115. doi: 10.1017/S003118201600055X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iborra S., Soto M., Carrion J., Nieto A., Fernandez E., Alonso C., Requena J.M. The Leishmania infantum acidic ribosomal protein P0 administered as a DNA vaccine confers protective immunity to Leishmania major infection in BALB/c mice. Infect. Immun. 2003;71:6562–6572. doi: 10.1128/IAI.71.11.6562-6572.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iborra S., Soto M., Carrion J., Alonso C., Requena J.M. Vaccination with a plasmid DNA cocktail encoding the nucleosomal histones of Leishmania confers protection against murine cutaneous leishmaniosis. Vaccine. 2004;22:3865–3876. doi: 10.1016/j.vaccine.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Iborra S., Abanades D.R., Parody N., Carrion J., Risueno R.M., Pineda M.A., Bonay P., Alonso C., Soto M. The immunodominant T helper 2 (Th2) response elicited in BALB/c mice by the Leishmania LiP2a and LiP2b acidic ribosomal proteins cannot be reverted by strong Th1 inducers. Clin. Exp. Immunol. 2007;150:375–385. doi: 10.1111/j.1365-2249.2007.03501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ker H.G., Coura-Vital W., Aguiar-Soares R.D., Roatt B.M., das Dores Moreira N., Carneiro C.M., Machado E.M., Teixeira-Carvalho A., Martins-Filho O.A., Giunchetti R.C., Araujo M.S., Coelho E.A., da Silveira-Lemos D., Reis A.B. Evaluation of a prototype flow cytometry test for serodiagnosis of canine visceral leishmaniasis. Clin. Vaccine Immunol. 2013;20:1792–1798. doi: 10.1128/CVI.00575-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenti M.D., de Santana Leandro M.V., Jr., Tomokane T.Y., De Lucca H.R., Aschar M., Souza C.S., Silva R.M., Marcondes M., da Matta V.L. Comparative evaluation of the DPP((R)) CVL rapid test for canine serodiagnosis in area of visceral leishmaniasis. Vet. Parasitol. 2014;205:444–450. doi: 10.1016/j.vetpar.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Leandro C., Santos-Gomes G.M., Campino L., Romao P., Cortes S., Rolao N., Gomes-Pereira S., Rica Capela M.J., Abranches P. Cell mediated immunity and specific IgG1 and IgG2 antibody response in natural and experimental canine leishmaniosis. Vet. Immunol. Immunopathol. 2001;79:273–284. doi: 10.1016/s0165-2427(01)00270-7. [DOI] [PubMed] [Google Scholar]
- Lescano S.A., Nakhle M.C., Ribeiro M.C., Chieffi P.P. IgG antibody responses in mice coinfected with Toxocara canis and other helminths or protozoan parasites. Rev. Inst. Med. Trop. Sao Paulo. 2012;54:145–152. doi: 10.1590/s0036-46652012000300006. [DOI] [PubMed] [Google Scholar]
- Lira R.A., Cavalcanti M.P., Nakazawa M., Ferreira A.G., Silva E.D., Abath F.G., Alves L.C., Souza W.V., Gomes Y.M. Canine visceral leishmaniosis: a comparative analysis of the EIE-leishmaniose-visceral-canina-bio-manguinhos and the IFI-leishmaniose-visceral-canina-bio-manguinhos kits. Vet. Parasitol. 2006;137:11–16. doi: 10.1016/j.vetpar.2005.12.020. [DOI] [PubMed] [Google Scholar]
- Lopes E.G., Seva A.P., Ferreira F., Nunes C.M., Keid L.B., Hiramoto R.M., Ferreira H.L., Oliveira T., Bigotto M.F.D., Galvis-Ovallos F., Galati E.A.B., Soares R.M. Serological and molecular diagnostic tests for canine visceral leishmaniasis in Brazilian endemic area: one out of five seronegative dogs are infected. Epidemiol. Infect. 2017;145:2436–2444. doi: 10.1017/S0950268817001443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maalej I.A., Chenik M., Louzir H., Ben Salah A., Bahloul C., Amri F., Dellagi K. Comparative evaluation of ELISAs based on ten recombinant or purified Leishmania antigens for the serodiagnosis of Mediterranean visceral leishmaniasis. Am. J. Trop. Med. Hyg. 2003;68:312–320. https://www.ncbi.nlm.nih.gov/pubmed/12685637 [PubMed] [Google Scholar]
- Magalhaes F.B., Castro Neto A.L., Nascimento M.B., Santos W.J.T., Medeiros Z.M., Lima Neto A.S., Costa D.L., Costa C.H.N., Dos Santos W.L.C., Pontes de Carvalho L.C., Oliveira G.G.S., de Melo Neto O.P. Evaluation of a new set of recombinant antigens for the serological diagnosis of human and canine visceral leishmaniasis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0184867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi R.G., Kramer F. A review on the occurrence of companion vector-borne diseases in pet animals in Latin America. Parasit. Vectors. 2019;12:145. doi: 10.1186/s13071-019-3407-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maia C., Campino L. Methods for diagnosis of canine leishmaniasis and immune response to infection. Vet. Parasitol. 2008;158:274–287. doi: 10.1016/j.vetpar.2008.07.028. [DOI] [PubMed] [Google Scholar]
- Marcondes M., de Lima V.M., de Araujo Mde F., Hiramoto R.M., Tolezano J.E., Vieira R.F., Biondo A.W. Longitudinal analysis of serological tests officially adopted by the Brazilian Ministry of Health for the diagnosis of canine visceral leishmaniasis in dogs vaccinated with Leishmune®. Vet. Parasitol. 2013;197:649–652. doi: 10.1016/j.vetpar.2013.07.013. [DOI] [PubMed] [Google Scholar]
- Mattin M.J., Solano-Gallego L., Dhollander S., Afonso A., Brodbelt D.C. The frequency and distribution of canine leishmaniosis diagnosed by veterinary practitioners in Europe. Vet. J. 2014;200:410–419. doi: 10.1016/j.tvjl.2014.03.033. [DOI] [PubMed] [Google Scholar]
- Mauricio I.L., Stothard J.R., Miles M.A. The strange case of Leishmania chagasi. Parasitol. Today. 2000;16:188–189. doi: 10.1016/s0169-4758(00)01637-9. [DOI] [PubMed] [Google Scholar]
- Mendonca I.L., Batista J.F., Werneck G.L., Soares M.R.A., Costa D.L., Costa C.H.N. Serological tests fail to discriminate dogs with visceral leishmaniasis that transmit Leishmania infantum to the vector Lutzomyia longipalpis. Rev. Soc. Bras. Med. Trop. 2017;50:483–488. doi: 10.1590/0037-8682-0014-2017. [DOI] [PubMed] [Google Scholar]
- Morales-Yuste M., Morillas-Marquez F., Diaz-Saez V., Baron-Lopez S., Acedo-Sanchez C., Martin-Sanchez J. Epidemiological implications of the use of various methods for the diagnosis of canine leishmaniasis in dogs with different characteristics and in differing prevalence scenarios. Parasitol. Res. 2012;111:155–164. doi: 10.1007/s00436-011-2812-7. [DOI] [PubMed] [Google Scholar]
- Murphy L., Pathak A.K., Cattadori I.M. A co-infection with two gastrointestinal nematodes alters host immune responses and only partially parasite dynamics. Parasite Immunol. 2013;35:421–432. doi: 10.1111/pim.12045. [DOI] [PubMed] [Google Scholar]
- Nieto C.G., Garcia-Alonso M., Requena J.M., Miron C., Soto M., Alonso C., Navarrete I. Analysis of the humoral immune response against total and recombinant antigens of Leishmania infantum: correlation with disease progression in canine experimental leishmaniasis. Vet. Immunol. Immunopathol. 1999;67:117–130. doi: 10.1016/s0165-2427(98)00213-x. [DOI] [PubMed] [Google Scholar]
- Noli C., Saridomichelakis M.N. An update on the diagnosis and treatment of canine leishmaniosis caused by Leishmania infantum (syn. L. chagasi) Vet. J. 2014;202:425–435. doi: 10.1016/j.tvjl.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Oliveira G.G., Magalhaes F.B., Teixeira M.C., Pereira A.M., Pinheiro C.G., Santos L.R., Nascimento M.B., Bedor C.N., Albuquerque A.L., dos-Santos W.L., Gomes Y.M., Moreira E.D., Jr., Brito M.E., Pontes de Carvalho L.C., de Melo Neto O.P. Characterization of novel Leishmania infantum recombinant proteins encoded by genes from five families with distinct capacities for serodiagnosis of canine and human visceral leishmaniasis. Am. J. Trop. Med. Hyg. 2011;85:1025–1034. doi: 10.4269/ajtmh.2011.11-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennisi M.G. Leishmaniosis of companion animals in Europe: an update. Vet. Parasitol. 2015;208:35–47. doi: 10.1016/j.vetpar.2014.12.023. [DOI] [PubMed] [Google Scholar]
- Petersen C.A. Leishmaniasis, an emerging disease found in companion animals in the United States. Top Companion Anim. Med. 2009;24:182–188. doi: 10.1053/j.tcam.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigott D.M., Bhatt S., Golding N., Duda K.A., Battle K.E., Brady O.J., Messina J.P., Balard Y., Bastien P., Pratlong F., Brownstein J.S., Freifeld C.C., Mekaru S.R., Gething P.W., George D.B., Myers M.F., Reithinger R., Hay S.I. Global distribution maps of the leishmaniases. elife. 2014;3 doi: 10.7554/eLife.02851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrozzi R., Santos da Costa M.V., Teva A., Falqueto A., Ferreira A.L., dos Santos C.D., Fernandes A.P., Gazzinelli R.T., Campos-Neto A., Grimaldi G., Jr. Comparative evaluation of enzyme-linked immunosorbent assays based on crude and recombinant leishmanial antigens for serodiagnosis of symptomatic and asymptomatic Leishmania infantum visceral infections in dogs. Clin. Vaccine Immunol. 2007;14:544–548. doi: 10.1128/CVI.00420-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quijada L., Requena J.M., Soto M., Alonso C. During canine viscero-cutaneous leishmaniasis the anti-Hsp70 antibodies are specifically elicited by the parasite protein. Parasitol. 1996;112(3):277–284. doi: 10.1017/s0031182000065793. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8728991 [DOI] [PubMed] [Google Scholar]
- Quijada L., Requena J.M., Soto M., Gomez L.C., Guzman F., Patarroyo M.E., Alonso C. Mapping of the linear antigenic determinants of the Leishmania infantum hsp70 recognized by leishmaniasis sera. Immunol. Let.t. 1996;52:73–79. doi: 10.1016/0165-2478(96)02585-0. [DOI] [PubMed] [Google Scholar]
- Rafati S., Gholami E., Hassani N., Ghaemimanesh F., Taslimi Y., Taheri T., Soong L. Leishmania major heat shock protein 70 (HSP70) is not protective in murine models of cutaneous leishmaniasis and stimulates strong humoral responses in cutaneous and visceral leishmaniasis patients. Vaccine. 2007;25:4159–4169. doi: 10.1016/j.vaccine.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Ready P.D. Leishmaniasis emergence in Europe. Euro Surveill. 2010;15:19505. https://www.eurosurveillance.org/content/10.2807/ese.15.10.19505-en [PubMed] [Google Scholar]
- Reis L.L.D., Balieiro A., Fonseca F.R., Goncalves M.J.F. Changes in the epidemiology of visceral leishmaniasis in Brazil from 2001 to 2014. Rev. Soc. Bras. Med. Trop. 2017;50:638–645. doi: 10.1590/0037-8682-0243-2017. http://www.ncbi.nlm.nih.gov/pubmed/20403308 [DOI] [PubMed] [Google Scholar]
- Requena J.M., Alonso C., Soto M. Evolutionarily conserved proteins as prominent immunogens during Leishmania infections. Parasitol. Today. 2000;16:246–250. doi: 10.1016/s0169-4758(00)01651-3. [DOI] [PubMed] [Google Scholar]
- Riboldi E., Carvalho F., Romao P.R.T., Barcellos R.B., Bello G.L., Ramos R.R., de Oliveira R.T., Junior J.P.A., Rossetti M.L., Dallegrave E. Molecular method confirms canine Leishmania infection detected by serological methods in non-endemic area of Brazil. Korean J. Parasitol. 2018;56:11–19. doi: 10.3347/kjp.2018.56.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Cortes A., Ojeda A., Todoli F., Alberola J. Performance of commercially available serological diagnostic tests to detect Leishmania infantum infection on experimentally infected dogs. Vet. Parasitol. 2013;191:363–366. doi: 10.1016/j.vetpar.2012.09.009. [DOI] [PubMed] [Google Scholar]
- Roque A.L., Xavier S.C., Gerhardt M., Silva M.F., Lima V.S., D'Andrea P.S., Jansen A.M. Trypanosoma cruzi among wild and domestic mammals in different areas of the Abaetetuba municipality (Para State, Brazil), an endemic Chagas disease transmission area. Vet. Parasitol. 2013;193:71–77. doi: 10.1016/j.vetpar.2012.11.028. [DOI] [PubMed] [Google Scholar]
- Scalone A., De Luna R., Oliva G., Baldi L., Satta G., Vesco G., Mignone W., Turilli C., Mondesire R.R., Simpson D., Donoghue A.R., Frank G.R., Gradoni L. Evaluation of the Leishmania recombinant K39 antigen as a diagnostic marker for canine leishmaniasis and validation of a standardized enzyme-linked immunosorbent assay. Vet. Parasitol. 2002;104:275–285. doi: 10.1016/s0304-4017(01)00643-4. [DOI] [PubMed] [Google Scholar]
- Silva K.R., Mendonca V.R., Silva K.M., Nascimento L.F., Mendes-Sousa A.F., Pinho F.A., Barral-Netto M., Barral A.M., Cruz M.D. Scoring clinical signs can help diagnose canine visceral leishmaniasis in a highly endemic area in Brazil. Mem. Inst. Oswaldo Cruz. 2017;112:53–63. doi: 10.1590/0074-02760160305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simundic A.M. Measures of diagnostic accuracy: basic definitions. EJIFCC. 2009;19:203–211. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4975285/ [PMC free article] [PubMed] [Google Scholar]
- Solano-Gallego L., Koutinas A., Miro G., Cardoso L., Pennisi M.G., Ferrer L., Bourdeau P., Oliva G., Baneth G. Directions for the diagnosis, clinical staging, treatment and prevention of canine leishmaniosis. Vet. Parasitol. 2009;165:1–18. doi: 10.1016/j.vetpar.2009.05.022. [DOI] [PubMed] [Google Scholar]
- Solano-Gallego L., Miro G., Koutinas A., Cardoso L., Pennisi M.G., Ferrer L., Bourdeau P., Oliva G., Baneth G., The LeishVet, G LeishVet guidelines for the practical management of canine leishmaniosis. Parasit Vectors. 2011;4:86. doi: 10.1186/1756-3305-4-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solano-Gallego L., Cardoso L., Pennisi M.G., Petersen C., Bourdeau P., Oliva G., Miro G., Ferrer L., Baneth G. Diagnostic challenges in the era of canine Leishmania infantum vaccines. Trends Parasitol. 2017;33:706–717. doi: 10.1016/j.pt.2017.06.004. [DOI] [PubMed] [Google Scholar]
- Soto M., Requena J.M., Quijada L., Garcia M., Guzman F., Patarroyo M.E., Alonso C. Mapping of the linear antigenic determinants from the Leishmania infantum histone H2A recognized by sera from dogs with leishmaniasis. Immunol. Lett. 1995;48:209–214. doi: 10.1016/0165-2478(95)02473-5. [DOI] [PubMed] [Google Scholar]
- Soto M., Requena J.M., Quijada L., Alonso C. Multicomponent chimeric antigen for serodiagnosis of canine visceral leishmaniasis. J. Clin. Microbiol. 1998;36:58–63. doi: 10.1128/jcm.36.1.58-63.1998. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=9431920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto M., Requena J.M., Quijada L., Perez M.J., Nieto C.G., Guzman F., Patarroyo M.E., Alonso C. Antigenicity of the Leishmania infantum histones H2B and H4 during canine viscerocutaneous leishmaniasis. Clin. Exp. Immunol. 1999;115:342–349. doi: 10.1046/j.1365-2249.1999.00796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto M., Ramirez L., Pineda M.A., Gonzalez V.M., Entringer P.F., Indiani de Oliveira C., Nascimento I.P., Souza A.P., Corvo L., Alonso C.P.B., Brodskyn C., Barral A., Barral-Netto M., Iborra S. Searching genes encoding Leishmania antigens for diagnosis and protection. Scholarly Res. Exchange. 2009;2009 [Google Scholar]
- Souza A.P., Soto M., Costa J.M., Boaventura V.S., de Oliveira C.I., Cristal J.R., Barral-Netto M., Barral A. Towards a more precise serological diagnosis of human tegumentary leishmaniasis using Leishmania recombinant proteins. PLoS One. 2013;8 doi: 10.1371/journal.pone.0066110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travi B.L., Tabares C.J., Cadena H., Ferro C., Osorio Y. Canine visceral leishmaniasis in Colombia: relationship between clinical and parasitologic status and infectivity for sand flies. Am. J. Trop. Med. Hyg. 2001;64:119–124. doi: 10.4269/ajtmh.2001.64.119. https://www.ncbi.nlm.nih.gov/pubmed/11442205 [DOI] [PubMed] [Google Scholar]
- Travi B.L., Cordeiro-da-Silva A., Dantas-Torres F., Miro G. Canine visceral leishmaniasis: diagnosis and management of the reservoir living among us. PLoS Negl. Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao G.H., Yin K., Zhong W.X., Xiao T., Wei Q.K., Cui Y., Liu G.Z., Xu C., Wang H.F. Epidemiological investigation of asymptomatic dogs with Leishmania infection in southwestern China where visceral leishmaniasis is intractable. Korean J Parasitol. 2016;54:797–801. doi: 10.3347/kjp.2016.54.6.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Geographical location of the Brazilian States included in the study. The cities where the samples were taken are highlighted.
Individualized analysis of serum reactivity. Schematic representation of the positivity against the indicated antigens from the next sera: CanL clinically ill sera (MS + PI) (A), CanL subclinically infected sera (MS+PI) (B) and CanL clinically ill sera from SC (C). The shaded squares indicated these sera over the value of cut-off showed in Fig. 5B for clinically ill and Fig. 5D for subclinically infected. The cut-off value for panel (C) was taken from Fig. 5A for the SLA and the recombinant proteins. At the left, grey arrows indicate sera positive only for the SLA preparation (A–C). In (C), +++ indicate sera negative for SLA and positive for at least one of the recombinant proteins.