Abstract
Social interactions require decoding of subtle rapidly changing emotional cues in others to facilitate socially appropriate behaviour. It is possible that impairments in the ability to detect and decode these signals may increase the risk for aggression. Therefore, we examined violent offenders with schizophrenia spectrum disorders (SSD) and compared these with healthy controls on a computerized paradigm of briefly presented double masked faces exhibiting 7 basic emotions. Our hypotheses were that impaired semantic understanding of emotion words and low cognitive ability would yield lowest emotion recognition. SSD exhibited lower accuracy of emotion perception than controls (46.1% compared with 64.5%, p = 0.026), even when considering the unbiased hit rate (22.4% compared with 43%, Z = 2.62, p < 0.01). Raw data showed uncommon but significant misclassifications of fear as sad, disgust as sad, sad as happy and angry as surprise. Once guessing and presentation frequencies were considered, only overall accuracy differed between SSD and healthy controls. There were significant correlations between cognitive ability, antipsychotic dose, speed and emotion accuracy in the SSD group. In conclusion, that there were no specific emotion biases in the SSD group compared to healthy controls, but particular individuals may have greater impairments in facial emotion perception, being influenced by intellectual ability, psychomotor speed and medication dosages, rather than specifically emotion word understanding. This implies that both state and trait factors influence emotion perception in the aggressive SSD group and may reveal one source of potential misunderstanding of social situations which may lead to boundary violations and aggression.
Keywords: Emotion processing, Schizophrenia, Psychosis, Facial affect recognition, Aggression
1. Introduction
The ability to perceive and interpret other's emotions is a critical part of living a full and inclusive community life. Being impaired in this ability has been said to account for some of the psychosocial handicap associated with schizophrenia (Fett et al., 2011; Irani et al., 2012). Impairments in facial emotion recognition is present both in ultra-high-risk individuals and first episode schizophrenia individuals (Barbato et al., 2015; Lee et al., 2015) as well as in first-degree relatives of these (Allott et al., 2015), making it, along with other measures of neurocognition, an endophenotype candidate of schizophrenia (Seidman et al., 2015).
Most recent studies examining facial emotion recognition in schizophrenia have used paradigms where i) the face is presented in a static form until the respondent makes a forced choice of which emotion is portrayed, for example (Ruocco et al., 2014), or ii) as a sequence of pictures exhibiting ever more apparent emotions where the person has to judge the point at which the face expresses a particular emotion, for example (Huang et al., 2011). Both of these paradigms use longer duration stimuli and do not tap into the fleeting emotion portrayals that often occur in real life. Understanding and perceiving these subtle shifts act as “stop” or “go” signals in two-way communications, sometimes more subconsciously than consciously. Impairment in the ability to detect these subtle changes may lead to interpersonal boundary violations which, we hypothesize, may lead in some individuals to other transgressions in interpersonal situations. One of the models of socialized behaviour, the violence inhibition mechanism, posits that sad facial affects (distress cues) function as a human submission response which act as a stop signal for aggressive behaviour (Blair, 2001). If sad or fearful facially expressed emotions are not correctly identified, then this would be a possible mechanism for interpersonal aggression.
The network of areas in the brain processing emotions and facial affect are different to the neurocognitive areas and it has thus been assumed that general intellectual capacity will not determine emotion recognition capacity. Yet many studies in schizophrenia have found associations between intellectual ability and measures of social cognition (Sergi et al., 2007, Andric et al., 2016;). Recent emotion recognition studies on individuals with schizophrenia have reported a mean IQ of 90–105 but have not reported specifically on relationships between intellectual function and emotion perception at lower cognitive abilities (Huang et al., 2011; Comparelli et al., 2013; Ruocco et al., 2014). Studies on intellectual disability, whilst fewer, suggest increasing impairment with lower IQ's (Scotland et al., 2015).
Recent studies have identified a relationship between various symptoms of schizophrenia and alexithymia (the inability to identify the meaning and experience of emotion words) (Todarello et al., 2005; Fogley et al., 2014; Ospina et al., 2019) as well as with impaired facial emotion recognition (Tang et al., 2016). Yet, to the best of our knowledge studies examining facial emotion recognition in schizophrenia have rarely assessed the semantic understanding of emotion words prior to facial affect testing (Carra et al., 2017). In that study, subjects who did not know the meaning of the words from the test were excluded from participation. This may tease apart emotion recognition difficulties from poor understanding of the emotion word label, but there is a clear need to know how frequently impaired semantic understanding interferes with emotion labelling, and if this is indeed a source of impairments in perceiving-interpreting emotional interpersonal interactions.
Given the above issues, we wished to use a more ecologically valid model of dynamic emotion portrayals to examine possible relationships between cognitive, empathic measures and emotion recognition in individuals with schizophrenia spectrum disorders (SSD) who have had a history of aggressive behaviour. By using computer-presented brief facial affects based on the traditional Ekman faces (Ekman and Friesen, 1971) with double masking (before and after the target emotional face) we decided to test affect recognition in SSD persons that have all been aggressive and compare these to healthy controls. We decided to include patients who exhibit lower intellectual abilities in order to better reflect the population of patients who exhibit aggression directed towards others. Our hypotheses were that impaired semantic understanding and low cognitive ability would impair facial affect recognition.
2. Methods
2.1. Participants
Individuals with SSD, here defined as schizophrenia, psychotic bipolar disorder, schizoaffective disorder or autism spectrum disorders with psychotic episodes, were recruited as part of the Stockholm Forensic Care Project, established to investigate possible links between known epidemiological risk factors for aggression in those with SSD, and cognition, social cognition, and biological measures. This sub-study is a cross-sectional cohort study of patients hospitalized under compulsory forensic psychiatric care legislation. In order to be representative of the population of persons who commit aggressive acts, participants may have a history of substance abuse (in remission for at least 3 months before testing), comorbid ADHD, personality disorder and/or mild intellectual disability. The range of offences include threatening behaviour, assault, grievous bodily harm and manslaughter/murder. Other offences may include property theft, robbery, arson or deliberate fire-setting, but in these cases prior interpersonal violence has always been observed which warrants their inclusion in the research project. Participants are aged between 20 and 61 years. Individuals with neurological disorders, brain damage prior to diagnosis with psychosis, untreated endocrine disorders, moderate intellectual disability or acute psychosis were excluded from participation. Persons are in a period of mental state stability when consenting to the study as well as while doing tests. No treatment is altered prior to testing. Healthy controls were recruited for a validation study of the briefly presented double masked stimuli of emotion recognition paradigm by the Stockholm University and Södertörn University and were used in this study as the comparison group.
2.2. Measures
Using case record review supplemented by semi-structured interviews, symptoms of psychiatric disorders were rated by experienced psychiatrists according to psychotic and affective sections of WHO's Schedule for Clinical Assessment in Neuropsychiatry 2.1 (World Health Organisation, 1999) and diagnoses made according to DSM-5 (American Psychiatric Association, 2013). Type and extent of prior substance use was rated according to case record and interview. Current medication was noted, and doses of antipsychotic medication converted according to Andreasen's model to haloperidol equivalents per day (Andreasen et al., 2010). The seriousness of aggressive acts were analysed according to Cornell's rating guide (Cornell, 1996) based on i) crime report from police for crimes the person has been sentenced for, ii) reports in the forensic psychiatric care assessment about previous crimes the person has been sentenced for, iii) case records of observable aggressive incidents in hospital services prior to forensic psychiatric care, iv) self-reports. Threats were coded as corresponding to 1, assaults as 2 and 3, assaults with weapons (which all yielded serious or severe injury) as 4 and 5, manslaughter and murder as 6. None presented in the 7 category of the effect of aggression. The number of verified, observed aggressive incidents targeting people were counted from the above sources.
Actual psychotic symptoms were rated by an experienced psychiatrist according to the Scale for the assessment of positive symptoms (SAPS) (Andreasen, 1984), and the Scale for the assessment of negative symptoms (SANS) (Andreasen, 1983).
2.2.1. Investigations
Individuals with SSD were asked to identify the correct synonym for 14 emotion words. There was a choice of 3 words for each emotion for example for anger – pride, rage, despair; for fear – dread, anger and pleasure. Ninety forward facing natural coloured face photographs of a total of 16 individuals were shown against a neutral background. There were equal numbers of young to middle aged males and females, all Caucasians taken from the Radboud Faces database (Langner et al., 2010). The 90 faces began by exhibiting a neutral expression and then switched for 200 milliseconds into one of 7 emotions: fear, anger, happy, sad, surprise, disgust or contempt. The face returned to neutral and after total presentation time 4.2 s the screen changed into exhibiting the 7 words from which the participant had to choose the correct response by using the mouse to click on the word that most closely matched what they perceived. All participants were instructed to choose the first response that came to them and not to try to reason it through.
A psychologist administered Information and Matrix reasoning subtests of Wechsler intelligence test (WAIS-IV) to the SSD individuals blind to the results of the affect recognition test. Age-normed scaled scores were calculated. A computerized finger tapping test (Inquisit 5 lab, preprogramed finger tapping freeware, Millisecond program) was performed as a measure of psychomotor speed. This measured the number of taps on the space bar in a 10 s period with a number of trials separated by rest periods yielding an average score for right and left index fingers.
Because comparison subjects were recruited for a validation study of the affect recognition task prior to being included as subject in the current study, they had not performed WAIS tests, answered the synonym test or performed the finger tapping speed test.
Ethics: All procedures are in accordance with Vetenskapsrådets ethical guidelines and the Helsinki declaration. Approval 2014/827–31/4 and 2017/ 219–32 for the probands. For the healthy controls research protocol and procedures, including ethical considerations, were reviewed and approved by the Psychology Department at the Stockholm University.
2.3. Statistical analysis
Demographic variables and self-rating scale scores are shown as mean and SD, or median and range, or total number of individuals and percentages. t-tests and chi-squared were used to compare groups when appropriate. Data for the briefly doubled masked face are presented both as unprocessed hit rate and difference between proportions ascertained. Additional data was statistically analysed using the unbiased hit rate of Wagner, 1993 (Wagner, 1993), a method which takes simultaneous account of both stimulus and judgement performance by combining these two conditional probabilities into an estimate of the joint probability that a stimulus is i) correctly identified (given that it is presented) and ii) that a response is correctly used (given that it is used). The unbiased hit rate according to the formula
was calculated in SPSS (version 24). Hu is based on the frequency data from the emotion confusion matrix. Each Hu value is calculated by squaring the frequency value and then dividing it with the product of its row sum and column sum (Wagner, 1993). Given that we could not compare healthy controls and our study population on other variables, we performed within group correlations, either Spearman rank correlations or parametric correlations were used to ascertain relationships within the aggressive subgroup. A general linear model was used to ascertain the best model to establish which factors most influenced overall accuracy in the SSD group. All statistics, other than the Hu corrections, were performed in Statistica 13.2 (Tibco™).
3. Results
3.1. Demographics
Results are shown in Table 1. As can be seen the patient sample is relatively poorly educated with almost 50% not having completed post primary school training. WAIS-IV scaled subtest scores indicated an IQ equivalent to 75–90, if one assumes all the subtests were even. Of the SSD group 14 (23.7%) had information subtest score < 7. Eighty percent had a diagnosis of schizophrenia or schizoaffective disorder and most are treated with depot antipsychotics of either typical or atypical form. Treatment with clozapine solely or adjunctively is less common than within the Forensic psychiatric service, reflecting the non-participation of the most unwell and resistive group of patients in the research project (personal communication). The healthy controls were, however, drawn from a higher socioeconomic group, were better educated and younger than the patient group so matching instead had to be by gender.
Table 1.
Demographic data.
| Aggressive SSD n = 59 (range) |
Healthy controls n = 88 (range) |
|
|---|---|---|
| Sex – males/females | 47/12 | 62/26 |
| Age (years) | 37.4 ± 11.0 (20–61) | 25.4 (18–45)⁎ |
| Education completed | ||
| - primary school | 28 (47.4) | 1 (1.1)⁎⁎ |
| - secondary school | 24 (40.7) | 26 (29.6) |
| - vocational or university training | 7 (11.9) | 61 (69.3)⁎⁎ |
| Attended Swedish school <age 16 | 46 (77) | |
| Information (WAIS-IV) | 9.0 ± 3.6 (3–15) | – |
| Matrix Reasoning (WAIS-IV) | 7.5 ± 3.5 (2–16) | – |
| History of Substance Abuse – none | 15 (25.4) | – |
| - cannabis | 8 (13.6) | |
| - opiates | 2 (3.4) | |
| - stimulants | 0 | |
| - alcohol | 8 (13.6) | |
| - polysubstance abuse | 26 (44.0) | |
| Diagnosis | – | |
| - Schizophrenia/schizoaffective disorder | 47b (79.7) | |
| - Bipolar disorder | 4 (6.8) | |
| - Delusional disorder and other psychotic disorders (not drug induced) | 8 (13.5) | |
| Antipsychotics | – | |
| - typical | 28 (47.4) | |
| - atypical | 17 (28.8) | |
| - clozapine and depot injection | 6 (10.2) | |
| - combinations of typical and atypical | 8 (13.6) | |
| Antidepressants | 11 (18.6) | – |
| Mood stabilizers - lithium | 2 (3.4) | |
| - others | 8 (13.6) | |
| Duration of Illness (years) | 10.6 ± 8.1 (0.5–31) | – |
| Haloperidol equivalent dose (mg/day)a | 12 (0.5–70) | – |
| SANS | 24.5 ± 13.3 (4–60) | – |
| SAPSa | 3 (0–53) | – |
| Type of aggressive acts - threats | 9 (15.2) | – |
| - assaults | 28 (47.4) | |
| - assault with weapons | 19 (32.3) | |
| - manslaughter/murder | 3 (5.1) | |
| Number of assaults/threats | ||
| 1–2 | 13 (22.0) | – |
| 3–5 | 17 (28.8) | |
| 6–9 | 12 (20.4) | |
| >10 | 17 (28.8) |
Median, SD.
7 had comorbid autism spectrum disorder
t-test p < 0.0001.
χ2p > 0.0001.
3.2. SSD individuals and healthy controls briefly presented double masked emotion stimuli
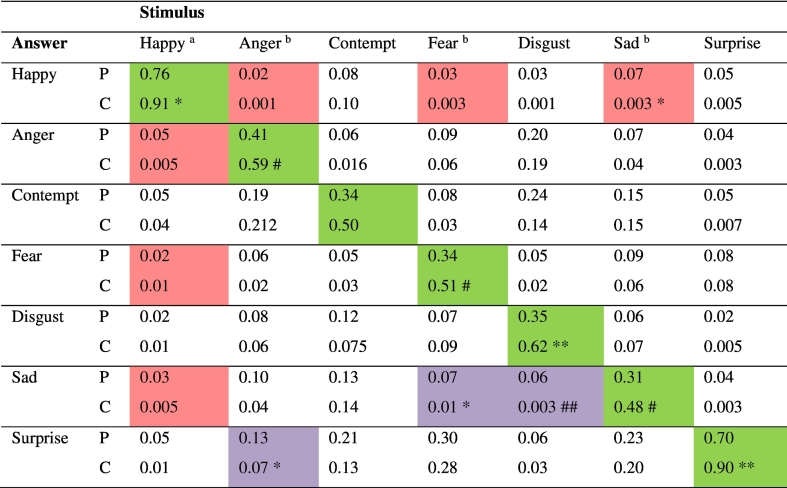
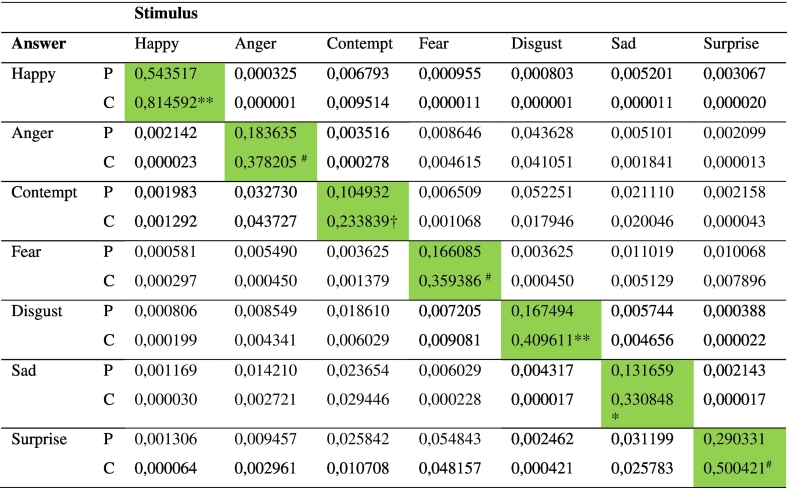
Significantly, probands were much worse in perceiving the correct emotion than healthy controls (Table 2). Only “happy” and “surprise” were correctly interpreted by more than half the SSD sample. Raw misidentification patterns of emotions are shown in the misclassification matrix (Table 3). The most significant but still uncommon misclassifications in the SSD group compared with controls were fear misclassified as sad, disgust as sad, sad as happy and angry as surprise. Given that happiness was the only positive valence emotion there were significant and unsurprising misclassifications to negative valence emotions in this forced test, yet it is more surprising that negative emotions were perceived as happy in 19% of responses compared with 8% in the control group (Z = 1.98, p = 0.048). However, correcting for the biased hit rate, all differences disappeared other than for overall accuracy (Table 4).
Table 2.
Total accuracy of emotion recognition, empathy scores, synonym understanding and motor speed.
| Probands % and range or score and SD (range) | Healthy controls | |
|---|---|---|
| Total accuracy | 46.1 (5.6–80) | 64.5 (31,4–88) |
| - Surprise | 70.9 (0−100) | 90.1 (50–100) |
| - Sad | 31.5 (0–84.6) | 47.6 (10−100) |
| - Disgust | 34.6 (0–92.3) | 61.8 (10–100) |
| - Fear | 34.1 (0–76.9) | 51.4 (10–90) |
| - Contempt | 35.0 (0–100) | 50.5 (10–90) |
| - Angry | 41.5 (0–92.3) | 58.6 (40–100) |
| - Happy | 77.8 (0–100) | 91.5 (50–100) |
| Synonyms of feeling wordsa | 13.5 (0–14) | – |
| Finger tapping speed dominant handb | 60.2 ± 12.7 (20.6–86.2) | – |
| Finger tapping speed nondominant | 52.5 ± 11.4 (23.6–79.8) | – |
Median.
per 10 s block.
Table 3.
Misclassification matrix of emotion responses to stimulus pictures- raw data.
P=Probands = 59. total of 767 presentations per emotion except happy = 708 presentations. C=Controls = 88, total of 880 presentations per emotion. Proportion with answer of total presentations. 2 tailed Z-test for difference between proportions probands compared with controls **p < 0.002, *p < 0.02, ## p < 0.04. # p < 0.05 a happy rated as sad, fearful or angry z = 2.13. p = 0.032. b angry, fear or sad rated as happy z = 2.32, p = 0.023.
Table 4.
Hu corrected responses to stimuli.
P = probands, C = healthy controls. Z test for difference in proportions ** p < 0.002, * p < 0.01, # p < 0.02, † p < 0.05.
3.3. Correlations within the aggressive schizophrenia spectrum disorder group
Not having had a Swedish education before the age of 16 was coupled with lower overall accuracy, lower fear and surprise accuracy (t-values between 2.07 and 2.20, p-values between 0.03 and 0.04).
The most significant associations with overall raw data emotion perception accuracy were with WAIS information (r = 0.47, p < 0.001) and WAIS matrix reasoning (r = 0.46, p < 0.001). Finger tapping speed in the dominant hand correlated with accuracy (r = 0.30, p = 0.03) showing association between psychomotor speed and the ability to perceive accurately stimuli of 200 ms duration. There were significant negative correlations between antipsychotic dosages and accuracy (Spearman rank coefficient − 0.51, p < 0.001) and positive symptoms and accuracy (Spearman rank coefficient − 0.35, p < 0.008). Spearman correlation coefficient between antipsychotic dose and current positive symptoms was 0.34, p < 0.008. The number of correct synonyms picked for emotion words correlated significantly with accuracy score (Spearman rank 0.40, p < 0.002). The number of correctly named synonyms also correlated strongly with WAIS information score (Spearman rank 0.49 p < 0.001) and matrix reasoning (Spearman rank 0.58 p < 0.001).
On the synonym test, fear was most commonly labelled anger (13 of 59 probands) and anger was labelled despair (10 of 59). We did not include fear as a synonym for anger in the questionnaire. In analysing if this resulted in impairments in interpreting the emotion in the tests, misunderstanding the word anger as fear resulted in significantly lower accuracy in labelling angry faces as anger (28% cf. 48%, t-value 2.48, p = 0.016) and a trend to lower accuracy for fear faces. In those labelling the word anger as despair, a trend was seen to lower accuracy of anger yet no effect on perceiving sadness. In other words, the impairments in linguistic understanding is partially a separate construct from that of identifying the emotion.
Given that there were a number of correlations between cognitive measures, speed and antipsychotic dosages a general linear model of best fit was performed, and results shown in Table 5. It can be seen that antipsychotic dose and information subscale continued to exert independent effects on overall emotion perception accuracy and that there was a trend to matrix reasoning to also affect the accuracy. In this model psychomotor speed lost its separate effect on accuracy. The semantic difficulties in understanding emotion words was related to intellectual resources and when added to the model reduced the overall adjusted R2.
Table 5.
Best explanatory general linear model for accuracy of briefly presented emotion stimuli in schizophrenia spectrum disorder group.
| F | Univariate p-value |
Adj R2 | Whole model P-value |
|
|---|---|---|---|---|
| Daily antipsychotic dose | 6.11 | 0.017 | 0.36 | <0.001 |
| WAIS matrix reasoning | 3.81 | 0.056 | ||
| WAIS information | 4.60 | 0.036 | ||
| Finger tapping speed | 1.64 | 0.206 |
4. Discussion
Using briefly presented double masked facial emotion portrayals which more closely mimics everyday subtle interpersonal cues we were able to see that most individuals with schizophrenia spectrum disorders who have been aggressive have marked difficulties in perceiving the correct emotion compared with heathy individuals. We found, in line with our hypothesis, very strong correlations of emotion perception with intellectual function. We found impairments were more significant in those with higher antipsychotic dosages. The profile of misinterpretations yielded certain statistically significant but uncommon misclassifications: fear was more commonly misclassified as sad, disgust as sad, sad as happy and angry as surprise. When examining the unbiased hit rate, there were no preferences for specific misclassifications in the SSD group. To some extent the findings relate to semantic misunderstanding of words as well as motor slowness in the SSD subjects.
The impairments shown by the patient group compared with controls is consistent with other recent studies in the area of facial affect recognition in schizophrenia (Premkumar et al., 2008; Comparelli et al., 2013; Ruocco et al., 2014; Maat et al., 2015; Song et al., 2015; Romero-Ferreiro et al., 2016;) where most studies have reported a 5–20% drop in accuracy compared with controls on longer duration emotion stimuli. In our study, the difference in accuracy ranged from 14% (happy) to 27% (disgust), with an average of 18%. It is noteworthy that in our sample even the healthy controls struggled to get high precision for emotions other than happy and surprise, so no ceiling effects were noted.
The strong association between emotion perception accuracy and intellectual function confirms findings in other studies in schizophrenia, for example (Sergi et al., 2007; Premkumar et al., 2008). It is likely in our study that the number of persons with premorbid extrapolated IQ <70, partially drove this result. This distinguishes our study from others where intellectual disability has been excluded when studying social cognition in schizophrenia. Yet it is this group of double and even triple diagnosis (the third being substance abuse) that present with increased likelihood of aggression in clinical and societal situations. The results confirm that intellectual reserves are needed in order to detect and interpret subtle emotional cues and that mental health professionals need to be aware of the inability to detect these cues in the day to day dealings with patients. Whilst emotion perception has been viewed as a separate construct, studies have suggested that underlying visual processing impairments (Belge et al., 2017) may underlie emotion perception impairments, which may partially explain why other cognitive measures are co-correlated and may also explain the lack of specific emotion misclassifications.
Psychomotor speed was associated with the ability to perceive briefly presented emotion stimuli but lost its significance when intellectual ability was factored in. In our study both measures of intelligence – information subscale of WAIS which has been shown to be relatively preserved in schizophrenia and the more illness sensitive matrix reasoning subscale (Fuentes-Dura et al., 2019) were associated with the ability to correctly perceive emotion. Contrary to the study by Sergi et al. (2007) but in line with the study by Buck et al. (2016), we found associations between emotion perception and positive but not negative symptoms. Negative symptoms of schizophrenia have previously been hypothesized to correlate with emotion recognition given that social withdrawal is an aspect of the construct. The Sergi et al. (2007) found strongest association between neurocognition and social cognition measures, intermediate correlations between neurocognition and negative symptoms but still significant associations between negative symptoms and social cognition. The reason we have not found this may be that all of our SSD subjects are in long stay inpatient units and score relatively high on negative symptoms, which may not be fully reflective of actual symptoms but a response to a low stimulus environment. To ascertain if this is the case, we would need to re-score the patients once in an outpatient environment.
In our study, antipsychotic dosages were significantly associated with impairments in facial emotion perception. In a cross-sectional study it is of course impossible to ascertain if this is a causal relationship or a reflection of the severity of illness. It is significant in our sample, that despite higher doses of antipsychotics, there remained positive symptoms even during a period of stability. Undoubtedly, the medication dosages also help explain the association with psychomotor speed we observed. In the review conducted by Hempel et al. (2010) it was difficult to determine what effect, if any, antipsychotics had on emotion perception, partly due to the different paradigms the studies used. Some of the studies used acutely psychotic patients and found no adverse effect of medication once treated, while other studies used short washout periods before switches of medication. Given the evidence that emotion perception is an endophenotype marker and stable over the course of the illness (Comparelli et al., 2013) it appears be affected by state factors such as psychotic symptoms (Maat et al., 2015).
Studies using Ekman faces examining misattributions in schizophrenia between anger and fear (Premkumar et al., 2008), between these and disgust (Barkl et al., 2014) or between fear and sadness (Cohen et al., 2009) have found significant misattribution errors between these. In this study, which included more emotions and corrected for unbiased hit rate we found misattributions occurring between all emotions, including across valency. This lends support to the theory that the deficits are either related to basic visual processing (Belge et al., 2017) or to a more general alexithymic deficit that impairs general emotion processing not just in understanding of emotion words.
A significant limitation of the study is that the community controls had not performed the same WAIS tests and ratings that the study subjects had which meant that the 2 groups could not be compared regarding the associations we found in the aggressive SSD group. Recruitment of a specific control group is underway and will be used to validate the current study and extend the work to the healthy community population. Furthermore, there was a significant difference in education between the groups and, likely intellectual function, such that the difference in emotion recognition may solely be related to this. Other limitations of our methodology include the sole use of young and middle-aged Caucasian persons in the briefly presented double-masked emotion presentation paradigm which may affect how non-Caucasians arriving in Sweden in adolescence and later interpret the emotion portrayals, possibly giving artificially low results. Additionally, whilst the faces begin and end as neutral it is possible that this condition may not have been perceived as neutral, thereby colouring the scoring in individuals with schizophrenia, in line with other studies (Ruocco et al., 2014; Silver et al., 2009). In fact, some but not all studies suggest that neutral faces activate the amygdala in individuals with schizophrenia (Filkowski and Haas, 2017), a finding which has been thought to underlie the threat-bias exhibited in some individuals with schizophrenia (Huang et al., 2011).
A strength of our study is the inclusion of patients who have prior substance abuse, now in remission in a protected environment as well as those with intellectual challenges creating ecologically valid and generalisable findings to the group of persons who commit aggressive acts, mostly during psychotic episodes. Yet the limitation is that we are unable to state which of the factors underlie the findings of impaired emotion perception other than by statistical associations. Another strength is that interviewers and raters of symptoms were blind to the emotion perception test results. Additionally, variation in diagnosis or symptom ratings were minimised by having only 2 raters of aggression and 2 raters of symptom and diagnoses, with scoring done by consensus. Whether the results relate specifically to schizophrenia spectrum diagnoses or to aggression is yet to be determined with the addition of a non-aggressive SSD group.
5. Conclusion
In this study we have demonstrated substantial impairments in emotion recognition on a paradigm of briefly presented double masked facial pictures in individuals with schizophrenia spectrum disorders compared to healthy controls. Importantly, there were misclassifications between all emotions studied, not confined to anger, fear and sadness. General intellectual abilities, speed and antipsychotic dosages contributed to greater frequency of misclassifications in the SSD group highlighting the interrelationships between fast mentation and recognizing fleeting emotions in others faces, a prerequisite for nuanced social communications in being able to judge subtle stop signals in interpersonal situations.
Contributors
Authors L Högman, H Fischer, M Kristiansson and A Johansson were instrumental in designing the study. Anette Johansson wrote the grant applications to enable the study to be performed. Anette Johansson trained test‑leaders to administer DMASC-MC. A Johansson and Charlotte Holst undertook diagnostic and symptom ratings. A Johansson and L Högman undertook the statistical analysis. Anette Johansson wrote the first draft of the manuscript. All authors reviewed and accepted the final manuscript.
Funding
The study has been supported by grants from the Swedish Society of Medicine (SLS-589661), Fredrik and Ingrid Thuring's fund (2015-00127, 2016-00264) as well as Stockholm County Council (ALF project 20160031 and 20180112). None of the sources has had a say in content or in the writing of the results.
Declaration of competing interest
None of the authors have conflict of interests.
Acknowledgements
We kindly thank the psychologists working at the North Section of Forensic Care Stockholm as well as Dr. Charlotte Holst and Malin Källman, study coordinator, for providing some of the data used in this article.
Contributor Information
Lennart Högman, Email: lhn@psychology.su.se.
Marianne Kristiansson, Email: Marianne.kristiansson@ki.se.
Håkan Fischer, Email: hakan.fischer@psychology.su.se.
Anette GM Johansson, Email: Anette.Johansson.2@ki.se.
References
- Allott K.A., Rice S., Bartholomeusz C.F., Klier C., Schlogelhofer M., Schafer M.R., Amminger G.P. Emotion recognition in unaffected first-degree relatives of individuals with first-episode schizophrenia. Schizophr. Res. 2015;161(2–3):322–328. doi: 10.1016/j.schres.2014.12.010. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . American Psychiatric Association; Washington DC: 2013. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. [Google Scholar]
- Andreasen N.C. 1983. The Scale for the Assessment of Negative Symptoms (SANS) University of Iowa, Iowa City. [Google Scholar]
- Andreasen N.C. University of Iowa; Iowa City: 1984. The Scale for the Assessment of Positive Symptoms (SAPS) [Google Scholar]
- Andreasen N.C., Pressler M., Nopoulos P., Miller D., Ho B.C. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol. Psychiatry. 2010;67(3):255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andric S., Maric N.P., Mihaljevic M., Mirjanic T., van Os J. Familial covariation of facial emotion recognition and IQ in schizophrenia. Psychiatry Res. 2016;246:52–57. doi: 10.1016/j.psychres.2016.09.022. [DOI] [PubMed] [Google Scholar]
- Barbato M., Liu L., Cadenhead K.S., Cannon T.D., Cornblatt B.A., McGlashan T.H., Perkins D.O., Seidman L.J., Tsuang M.T., Walker E.F., Woods S.W., Bearden C.E., Mathalon D.H., Heinssen R., Addington J. Theory of mind, emotion recognition and social perception in individuals at clinical high risk for psychosis: findings from the NAPLS-2 cohort. Schizophr. Res. Cogn. 2015;2(3):133–139. doi: 10.1016/j.scog.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkl S.J., Lah S., Starling J., Hainsworth C., Harris A.W., Williams L.M. Facial emotion identification in early-onset psychosis. Schizophr. Res. 2014;160(1–3):150–156. doi: 10.1016/j.schres.2014.10.035. [DOI] [PubMed] [Google Scholar]
- Belge J.B., Maurage P., Mangelinckx C., Leleux D., Delatte B., Constant E. Facial decoding in schizophrenia is underpinned by basic visual processing impairments. Psychiatry Res. 2017;255:167–172. doi: 10.1016/j.psychres.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Blair R.J. Neurocognitive models of aggression, the antisocial personality disorders, and psychopathy. J. Neurol. Neurosurg. Psychiatry. 2001;71(6):727–731. doi: 10.1136/jnnp.71.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck B.E., Healey K.M., Gagen E.C., Roberts D.L., Penn D.L. Social cognition in schizophrenia: factor structure, clinical and functional correlates. J. Ment. Health. 2016;25(4):330–337. doi: 10.3109/09638237.2015.1124397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carra G., Nicolini G., Lax A., Bartoli F., Castellano F., Chiorazzi A., Gamba G., Bava M., Crocamo C., Papagno C. Facial emotion recognition in schizophrenia: An exploratory study on the role of comorbid alcohol and substance use disorders and COMT Val158Met. Hum. Psychopharmacol. 2017;32(6) doi: 10.1002/hup.2630. [DOI] [PubMed] [Google Scholar]
- Cohen A.S., Nienow T.M., Dinzeo T.J., Docherty N.M. Attribution biases in schizophrenia: relationship to clinical and functional impairments. Psychopathology. 2009;42(1):40–46. doi: 10.1159/000173702. [DOI] [PubMed] [Google Scholar]
- Comparelli A., Corigliano V., De Carolis A., Mancinelli I., Trovini G., Ottavi G., Dehning J., Tatarelli R., Brugnoli R., Girardi P. Emotion recognition impairment is present early and is stable throughout the course of schizophrenia. Schizophr. Res. 2013;143(1):65–69. doi: 10.1016/j.schres.2012.11.005. [DOI] [PubMed] [Google Scholar]
- Cornell D.G. Curry School of Education, University of Virginia; 1996. Coding Guide for Violent Incidents: Instrumental Versus Hostile/Reactive Aggression. [Google Scholar]
- Ekman P., Friesen W.V. Constants across cultures in the face and emotion. J. Pers. Soc. Psychol. 1971;17(2):124–129. doi: 10.1037/h0030377. [DOI] [PubMed] [Google Scholar]
- Fett A.K., Viechtbauer W., Dominguez M.D., Penn D.L., van Os J., Krabbendam L. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci. Biobehav. Rev. 2011;35(3):573–588. doi: 10.1016/j.neubiorev.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Filkowski M.M., Haas B.W. Rethinking the use of neutral faces as a baseline in fMRI neuroimaging studies of Axis-I psychiatric disorders. J. Neuroimaging. 2017;27(3):281–291. doi: 10.1111/jon.12403. [DOI] [PubMed] [Google Scholar]
- Fogley R., Warman D., Lysaker P.H. Alexithymia in schizophrenia: associations with neurocognition and emotional distress. Psychiatry Res. 2014;218(1–2):1–6. doi: 10.1016/j.psychres.2014.04.020. [DOI] [PubMed] [Google Scholar]
- Fuentes-Dura I., Ruiz J.C., Dasi C., Navarro M., Blasco P., Tomas P. WAIS-IV performance in patients with schizophrenia. J. Nerv. Ment. Dis. 2019;207(6):467–473. doi: 10.1097/NMD.0000000000000997. [DOI] [PubMed] [Google Scholar]
- Hempel R.J., Dekker J.A., van Beveren N.J., Tulen J.H., Hengeveld M.W. The effect of antipsychotic medication on facial affect recognition in schizophrenia: a review. Psychiatry Res. 2010;178(1):1–9. doi: 10.1016/j.psychres.2008.07.025. [DOI] [PubMed] [Google Scholar]
- Huang J., Chan R.C., Gollan J.K., Liu W., Ma Z., Li Z., Gong Q.Y. Perceptual bias of patients with schizophrenia in morphed facial expression. Psychiatry Res. 2011;185(1–2):60–65. doi: 10.1016/j.psychres.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irani F., Seligman S., Kamath V., Kohler C., Gur R.C. A meta-analysis of emotion perception and functional outcomes in schizophrenia. Schizophr. Res. 2012;137(1–3):203–211. doi: 10.1016/j.schres.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner O., Dotsch R., Bijlstra G., Wigboldus D.H.J., Hawk S.T., van Knippenberg A. Presentation and validation of the Radboud Faces Database. Cognit. Emot. 2010;24(8):1377–1388. [Google Scholar]
- Lee S.Y., Bang M., Kim K.R., Lee M.K., Park J.Y., Song Y.Y., Kang J.I., Lee E., An S.K. Impaired facial emotion recognition in individuals at ultra-high risk for psychosis and with first-episode schizophrenia, and their associations with neurocognitive deficits and self-reported schizotypy. Schizophr. Res. 2015;165(1):60–65. doi: 10.1016/j.schres.2015.03.026. [DOI] [PubMed] [Google Scholar]
- Maat A., van Montfort S.J., de Nijs J., Derks E.M., Kahn R.S., Linszen D.H., van Os J., Wiersma D., Bruggeman R., Cahn W., de Haan L., Krabbendam L., Myin-Germeys I., Investigators G. Emotion processing in schizophrenia is state and trait dependent. Schizophr. Res. 2015;161(2–3):392–398. doi: 10.1016/j.schres.2014.11.027. [DOI] [PubMed] [Google Scholar]
- Ospina L.H., Shanahan M., Perez-Rodriguez M.M., Chan C.C., Clari R., Burdick K.E. Alexithymia predicts poorer social and everyday functioning in schizophrenia and bipolar disorder. Psychiatry Res. 2019;273:218–226. doi: 10.1016/j.psychres.2019.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar P., Cooke M.A., Fannon D., Peters E., Michel T.M., Aasen I., Murray R.M., Kuipers E., Kumari V. Misattribution bias of threat-related facial expressions is related to a longer duration of illness and poor executive function in schizophrenia and schizoaffective disorder. Eur. Psychiatry. 2008;23(1):14–19. doi: 10.1016/j.eurpsy.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Ferreiro M.V., Aguado L., Rodriguez-Torresano J., Palomo T., Rodriguez-Jimenez R., Pedreira-Massa J.L. Facial affect recognition in early and late-stage schizophrenia patients. Schizophr. Res. 2016;172(1–3):177–183. doi: 10.1016/j.schres.2016.02.010. [DOI] [PubMed] [Google Scholar]
- Ruocco A.C., Reilly J.L., Rubin L.H., Daros A.R., Gershon E.S., Tamminga C.A., Pearlson G.D., Hill S.K., Keshavan M.S., Gur R.C., Sweeney J.A. Emotion recognition deficits in schizophrenia-spectrum disorders and psychotic bipolar disorder: findings from the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Schizophr. Res. 2014;158(1–3):105–112. doi: 10.1016/j.schres.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotland J.L., Cossar J., McKenzie K. The ability of adults with an intellectual disability to recognise facial expressions of emotion in comparison with typically developing individuals: a systematic review. Res. Dev. Disabil. 2015;41-42:22–39. doi: 10.1016/j.ridd.2015.05.007. [DOI] [PubMed] [Google Scholar]
- Seidman L.J., Hellemann G., Nuechterlein K.H., Greenwood T.A., Braff D.L., Cadenhead K.S., Calkins M.E., Freedman R., Gur R.E., Gur R.C., Lazzeroni L.C., Light G.A., Olincy A., Radant A.D., Siever L.J., Silverman J.M., Sprock J., Stone W.S., Sugar C., Swerdlow N.R., Tsuang D.W., Tsuang M.T., Turetsky B.I., Green M.F. Factor structure and heritability of endophenotypes in schizophrenia: findings from the Consortium on the Genetics of Schizophrenia (COGS-1) Schizophr. Res. 2015;163(1–3):73–79. doi: 10.1016/j.schres.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergi M.J., Rassovsky Y., Widmark C., Reist C., Erhart S., Braff D.L., Marder S.R., Green M.F. Social cognition in schizophrenia: relationships with neurocognition and negative symptoms. Schizophr. Res. 2007;90(1–3):316–324. doi: 10.1016/j.schres.2006.09.028. [DOI] [PubMed] [Google Scholar]
- Silver H., Bilker W., Goodman C. Impaired recognition of happy, sad and neutral expressions in schizophrenia is emotion, but not valence, specific and context dependent. Psychiatry Res. 2009;169(2):101–106. doi: 10.1016/j.psychres.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Song Y., Xiang Y.T., Huang Y., Wang X., Wang X., Zhang F., Kwan J.S., Chan O.C., Wang Z., Ungvari G.S., Correll C.U., Zaroff C. Impairments in negative facial emotion recognition in Chinese schizophrenia patients detected with a newly designed task. J. Nerv. Ment. Dis. 2015;203(9):718–724. doi: 10.1097/NMD.0000000000000358. [DOI] [PubMed] [Google Scholar]
- Tang X.W., Yu M., Duan W.W., Zhang X.R., Sha W.W., Wang X., Zhang X.B. Facial emotion recognition and alexithymia in Chinese male patients with deficit schizophrenia. Psychiatry Res. 2016;246:353–359. doi: 10.1016/j.psychres.2016.09.055. [DOI] [PubMed] [Google Scholar]
- Todarello O., Porcelli P., Grilletti F., Bellomo A. Is alexithymia related to negative symptoms of schizophrenia? A preliminary longitudinal study. Psychopathology. 2005;38(6):310–314. doi: 10.1159/000088919. [DOI] [PubMed] [Google Scholar]
- Wagner H.R. On measuring performance in category judgment studies of nonverbal behavior. J. Nonverbal Behav. 1993;17(1):3–28. [Google Scholar]
- World Health Organisation . WHO – Assessment, Classification and Epidemiology; Geneva: 1999. Schedules for Clinical Assessment in Neuropsychiatry (SCAN 2.1) [Google Scholar]