Abstract
Background
There is recent interest in treating locally advanced rectal cancer (LARC) patients with total neoadjuvant therapy (TNT). However, whether TNT is associated with improved overall survival (OS) remains unknown. This study compares outcomes following TNT and following neoadjuvant chemoradiation therapy (nCRT) in patients with LARC, clinically defined cT3/4 or node positive disease, using the National Cancer Database.
Methods
LARC patients diagnosed between 2004–2015 were included. TNT was defined as multi-agent chemotherapy given at least 2 months before RT followed by pre-operative chemoradiation therapy and definitive surgery without adjuvant chemotherapy. nCRT was defined as pre-operative RT and chemotherapy started within 2 weeks from each other followed by definitive surgery with or without adjuvant chemotherapy. Kaplan-Meier curve with logrank test and multivariable Cox proportional hazards regression modelling were used to analyse the primary endpoint of overall survival (OS). Multivariable logistic regression modelling was used for secondary outcomes to determine if TNT is associated with pathological complete response (pCR), defined as ypT0N0, and negative circumferential resection margin (CRM).
Findings
Data from 372 TNT patients and 707 nCRT patients were analysed after a 2:1 propensity matching with replacement. Kaplan-Meier curve showed that OS with TNT was comparable to that with nCRT (p = 0•16). The 5-year OS rates for TNT and nCRT were 73•6% vs. 78•5% (p = 0•20). Multivariable Cox proportional hazards regression modelling confirmed no difference in OS between TNT and nCRT (HR = 1•21, p = 0•25). With TNT, 16•9% patients achieved pCR, whereas 13•1% patients achieved pCR with nCRT (p = 0•12). TNT was not found to be significantly associated with pCR (OR = 1•36, p = 0•13) or negative CRM (OR = 1•77, p = 0•19) in multivariable logistic regression modelling.
Interpretation
With results from current clinical trials pending, our data suggested that TNT and nCRT resulted in similar survival, while TNT led to higher pCR and CRM negative rate, albeit not statistically significant.
Keywords: Total neoadjuvant therapy, neoadjuvant chemoradiation, locally advanced rectal cancer, National Cancer Database
Research in context
Evidence before this study
There has been a rising interest in treating locally advanced rectal cancer (LARC) with total neoadjuvant therapy (TNT) where the adjuvant chemotherapy is delivered preoperatively. However, whether patients’ outcomes such as overall survival are improved when using TNT compared to standard neoadjuvant chemoradiation therapy followed by surgery with or without adjuvant chemotherapy (nCRT) is not currently known. Most studies are retrospective in nature from single institutions and describe the use of TNT in a highly selected patient population. Furthermore, there is no published randomised evidence to guide the use of TNT for LARC. Therefore, evidence we considered prior to our analysis were extrapolated from articles queried from Pubmed. Our search criteria included all Pubmed listed articles until February of 2019, which reported on the use of TNT for LARC. Search terms utilised include “rectal cancer” AND “total neoadjuvant therapy” or “neoadjuvant chemotherapy” and a filter limiting species to “humans”. The current evidence suggests that TNT may result in improved pathological complete response (pCR) with inconclusive data on overall survival benefit.
Added value of this study
In this large study, we demonstrate that was no difference in overall survival between patients with LARC receiving TNT and those receiving nCRT. Furthermore, we utilised multivariate and propensity-based matching to account for potential biases associated with retrospective data. By utilizing a national wide cancer database, our data best represents the LARC population. Of further significance, we were able to demonstrate that while TNT resulted in slightly higher pCR or negative circumferential resection margin (CRM) rates, this was not statistically significant.
Implications of all the available evidence
While the use on TNT in the management of LARC is currently on the rise, there was no clear improvement in OS or pCR rates when using TNT in this and other studies. As TNT can result in overtreatment for some patients with LARC, our data suggests awaiting definitive randomised trial results showing a clear benefit for TNT before its routine use in all patients with LARC.
1. Introduction
It is estimated that 44,180 new cases of rectal cancer will be diagnosed in the United States in 2019 [1]. One standard of care for locally advanced rectal cancer involves chemoradiation therapy (CRT) delivered in the neoadjuvant setting with preoperative 5-fluorouracil- or capecitabine-based chemotherapy combined with standard fractionated radiotherapy (RT), total mesorectal excision, and adjuvant chemotherapy, henceforth referred to as nCRT [2,3]. However, a significant proportion of patients treated with curative neoadjuvant CRT and surgery do not received their planned adjuvant chemotherapy, with less than 50% of eligible patients receiving the full course adjuvant chemotherapy, due to patient refusal or post-operative complications [2,[4], [5], [6], [7], [8]]. As a result, there has been an interest in the possibility of delivering chemotherapy before the initiation of neoadjuvant CRT, referred to as total neoadjuvant therapy (TNT). Two on-going clinical trials are exploring the potential benefits of this regimen. In the PROSPECT trial, patients in the study arm receive six cycles of FOLFOX chemotherapy and are then re-staged to determine if they still need pre-operative CRT based on their response to the FOLFOX [9]. This trial aims to prevent the use of radiation therapy in a subset of patients [9,10]. In the NRG- GI002 phase II TNT trial, patients receive eight cycles of mFOLFOX6 followed by RT in combination with either capecitabine or capecitabine + veliparib [10]. Other potential benefits of TNT in addition to improved compliance include early eradication of micrometastasis, higher rates of clinical complete response and improving rates of negative circumferential resection margin (CRM). While these trials will provide invaluable information regarding TNT regimen, they may not answer the question “is TNT better than standard treatment”. We therefore conducted a large retrospective analysis using the National Cancer Database (NCDB) to determine adoption of TNT and compare its outcomes to nCRT in patients with locally advanced rectal adenocarcinoma, clinically defined as cT3/4 or node positive disease.
2. Materials andmethods
2.1. Patient population
A total of 264,257 patients with rectal cancer diagnosed between 2004 and 2015 were identified using the NCDB. No patient identifiers or otherwise identifiable health information is provided through the NCBD. We limited our analysis to patients with invasive adenocarcinoma who had locally advanced disease, which was defined as clinical stage M0 and T3 or T4 or any T stage with nodal involvement. We further excluded patients if they were <18 years old, did not receive chemotherapy or RT before surgery, did not receive surgery within a year of RT, diagnosis was not confirmed histologically, did not receive treatments at the reporting facility, or received only ablative surgical procedures or local excision. To minimise immortality bias, patients were excluded if last contacts were made within 8 weeks of definitive surgery.
Given the limited data available in the NCDB (no information on specific chemotherapy agents or number of cycles e.g.), patients were considered to have received TNT if they received RT and multi-agent chemotherapy before definitive surgery, RT was started at least two months after the induction of chemotherapy, and no chemotherapy was given after definitive surgery, based on comparable studies in the past [11,12]. Patients were considered to have received nCRT if they received RT and chemotherapy before definitive surgery, and RT and chemotherapy were started less than 2 weeks apart. Patients not meeting either of these two definitions were not included in the analysis.
2.2. Propensity score matching
We utilised propensity score matching to minimise effects of selection bias between treatment types by balancing characteristics of patients receiving TNT and nCRT. Variables included in propensity matching were age, gender, year of diagnosis, race and ethnicity, Charlson-Deyo Comorbidity Score, clinical T and N stage, tumour grade, facility type, urban status, insurance status, and income. These variables chosen to generate the propensity scores were determined through Cox proportional hazards modelling and multivariable logistic regression as important confounders due to their association with either OS or pCR. Matching was performed with the nearest neighbour approach using STATA v. 14•2 (StataCorp, College Station, TX), with a calliper of 0•001.
Given the different numbers of patients receiving each type of treatment, two-to-one matching with replacement was performed as this provides improved statistical power compared to a one-to-one match. A ten-to-one matching was included as a sensitivity analysis to test if added statistical power through including more patients receiving nCRT in the analysis would change the results. Two other one-way sensitivity analyses were performed to evaluate the impact on the results from varying definitions of the nCRT comparison group. In the first one, the definition of nCRT was narrowed to only include patients who received adjuvant chemotherapy, whereas in the other sensitivity analysis the nCRT cohort was limited to only include those who received multi-agent chemotherapy in either the neoadjuvant or the adjuvant setting.
2.3. Statistical analysis
Multivariable Cox proportional hazards regression and Kaplan-Meier curves with both logrank and Wilcoxon–Breslow tests were used to evaluate any difference in OS between TNT and nCRT. Multivariable logistic regression was used to determine the association between treatment type and pCR rates and between treatment type and negative CRM rates. Cox proportional hazards regression was performed to determine the association between pCR and OS in each cohort. The proportional hazards assumption was tested and verified in the post-propensity matching cohort based on Schoenfeld residuals. T-tests and Chi-square tests were used to evaluate the distribution of patient characteristics and potential confounders between the TNT and nCRT treatment types, before and after propensity matching. OS rates at different time points were compared between the two treatment groups using Z-tests comparing two proportions.
3. Results
A total of 42,118 patients were identified to have locally advanced adenocarcinoma of the rectum diagnosed between 2004 and 2015 and met our inclusion criteria (Fig. 1). Among those patients, 421 received treatment that met our criteria for TNT, whereas treatment for 38,584 patients met the criteria for nCRT (Fig. 1). The characteristics of patients receiving the two treatment types were summarised in Table 1. The mean age of patients receiving TNT was 54•4 y, whereas that of patients receiving nCRT was 59•9 y (p < 0•001). TNT was more frequently used after 2010 with 78•8% patients receiving TNT diagnosed after 2010 while this proportion was 51•3% in those receiving nCRT (p < 0•001). Patients receiving TNT had better Charlson-Deyo co-morbidity score (≥2 1•4% vs. 3•7%, p < 0•01), but a more advanced T stage (T4 16•4% vs. 6•3%, p < 0•001) and N stage (N+ 69•9% vs. 44•9%, p < 0•001). A significantly higher proportion of patients receiving TNT were treated at an academic facility compared to those receiving nCRT (71•0% vs. 34•8%, p < 0•001).
Fig. 1.
CONSORT diagram illustrating the patient selection for this study. TNT was defined as (1) RT started at least 60 days after chemotherapy (2) Multi-agent chemotherapy (3) No chemotherapy after surgery. nCRT was defined as (1) Not receiving TNT (2) RT started within 14 days of chemotherapy induction.
Table 1.
Patient characteristics in the unmatched cohort.
| Baseline characteristics | All patients | TNT | nCRT | p-value |
|---|---|---|---|---|
| (n = 39,005) | (n = 421) | (n = 38,584) | ||
| Age (y), mean ± SD | 59.9 ± 12.1 | 54.4 ± 11.5 | 59.9 ± 12.1 | <0.001 |
| Sex, n (%) | ||||
| Male | 24,266 (62.2) | 262 (62.2) | 24,004 (62.2) | 0.99 |
| Female | 14,739 (37.8) | 159 (37.8) | 14,580 (37.8) | |
| Year of diagnosis, n (%) | ||||
| 2004–2005 | 5,446 (14.0) | 35 (8.3) | 5,411 (14.0) | <0.001 |
| 2006–2007 | 6,586 (16.9) | 30 (7.1) | 6,556 (17.0) | |
| 2008–2009 | 6,832 (17.5) | 24 (5.7) | 6,808 (17.6) | |
| 2010–2011 | 7,186 (18.4) | 72 (17.1) | 7,114 (18.4) | |
| 2012–2013 | 8,344 (21.4) | 152 (36.1) | 8,192 (21.2) | |
| 2014–2015 | 4,611 (11.8) | 108 (25.6) | 4,503 (11.7) | |
| Race, n (%) | ||||
| non-Hispanic White | 32,279 (82.8) | 329 (78.2) | 31,950 (82.8) | 0.009 |
| non-Hispanic Black | 2,930 (7.5) | 29 (6.9) | 2,901 (7.5) | |
| Hispanic | 1,958 (5.0) | 34 (8.1) | 1,924 (5.0) | |
| Asian | 1,128 (2.9) | 17 (4.0) | 1,111 (2.9) | |
| Other/Unknown | 710 (1.8) | 12 (2.8) | 698 (1.8) | |
| Charlson-Deyo co-morbidity score, n (%) | ||||
| 0 | 31,206 (80.0) | 359 (85.3) | 30,847 (80.0) | 0.008 |
| 1 | 6,366 (16.3) | 56 (13.3) | 6,310 (16.4) | |
| ≥2 | 1,433 (3.7) | 6 (1.4) | 1,427 (3.7) | |
| T stage, n (%) | ||||
| ≤T2 | 1,974 (5.1) | 16 (3.8) | 1,958 (5.1) | <0.001 |
| T3 | 29,190 (74.9) | 302 (71.7) | 28,888 (74.9) | |
| T4 | 2,502 (6.4) | 69 (16.4) | 2,433 (6.3) | |
| Tx | 5,323 (13.6) | 34 (8.1) | 5,289 (13.7) | |
| N stage, n (%) | ||||
| N0 | 15,505 (39.8) | 90 (21.4) | 15,415 (40.0) | <0.001 |
| N1 | 15,179 (38.9) | 226 (53.7) | 14,953 (38.8) | |
| N2 | 2,437 (6.2) | 68 (16.2) | 2,369 (6.1) | |
| Nx | 5,878 (15.1) | 37 (8.8) | 5,841 (15.1) | |
| Grade, n (%) | ||||
| Well/moderately differentiated | 29,106 (74.6) | 324 (77.0) | 28,782 (74.6) | 0.48 |
| Poorly differentiated/anaplastic | 4,245 (10.9) | 44 (10.5) | 4,201 (10.9) | |
| Unknown | 5,654 (14.5) | 53 (12.6) | 5,601 (14.5) | |
| Total radiation dose (Gy), median (25th-75th percentile) | 50.40 (50.40-50.40) |
50.40 (50.00-50.40) |
50.40 (50.40-50.40) |
<0.001 |
| Type of facility, n (%) | ||||
| Non-academic | 24,147 (64.9) | 110 (29.0) | 24,037 (65.2) | <0.001 |
| Academic | 13,080 (35.1) | 269 (71.0) | 12,811 (34.8) | |
| Urban status, n (%) | ||||
| Metropolitan area | 30,159 (77.3) | 347 (82.4) | 29,812 (77.3) | <0.001 |
| Metropolitan-adjacent area | 6,790 (17.4) | 24 (5.7) | 6,766 (17.5) | |
| Rural area | 992 (2.5) | 4 (1.0) | 988 (2.6) | |
| Unknown | 1,064 (2.7) | 46 (10.9) | 1,018 (2.6) | |
| Median income quartiles, n (%) | ||||
| <$38,000 | 6,681 (17.3) | 45 (10.8) | 6,636 (17.4) | <0.001 |
| $38,000-$47,999 | 9,680 (25.0) | 74 (17.7) | 9,606 (25.1) | |
| $48,000-$62,999 | 10,626 (27.5) | 96 (23.0) | 10,530 (27.5) | |
| ≥$63,000 | 11,689 (30.2) | 203 (48.6) | 11,486 (30.0) | |
| Medical insurance type | ||||
| Not insured | 1,776 (4.6) | 14 (3.3) | 1,762 (4.6) | <0.001 |
| Private insurance/managed care | 20,204 (51.8) | 292 (69.4) | 19,912 (51.6) | |
| Medicaid | 2,469 (6.3) | 36 (8.6) | 2,433 (6.3) | |
| Medicare | 13, 434 (34.4) | 67 (15.9) | 13,367 (34.6) | |
| Other government | 563 (1.4) | 8 (1.9) | 555 (1.4) | |
| Insurance status unknown | 559 (1.4) | 4 (1.0) | 555 (1.4) | |
| Circumferential resection margin, n (%) | ||||
| Positive | 1,027 (6.1) | 10 (4.7) | 1,017 (6.1) | 0.39 |
| Negative | 15,827 (93.9) | 203 (95.3) | 15,624 (93.9) |
Bold font indicates statistically significant difference with p < 0.05.
In the multivariable logistic regression model assessing which factors were associated with patient assignment of each treatment type, diagnosis after 2012, clinical stage >2, treatment at academic facilities, and higher median income quartiles were associated with higher likelihood of receiving TNT (p < 0•001 for all). Age, gender, Charlson-Deyo co-morbidity score, and tumour grade were not associated with the likelihood of receiving TNT (Supplementary Table 1).
Univariable Cox proportional hazards regression showed that female gender, diagnosis after 2010, treatment at an academic facility, and higher median income quartile were each associated with better survival outcome, whereas age greater than 65 years, the presence of comorbid conditions, high tumour grade, and non-metropolitan residency correlated with worse survival outcome (all p < 0•001, Supplementary Table 2).
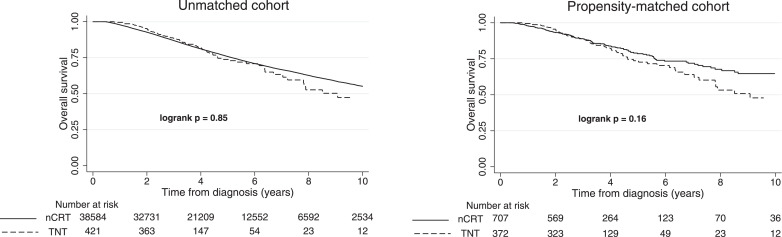
There was no difference in OS between TNT and nCRT in the unmatched cohort (logrank p = 0•85; Wilcoxon-Breslow p = 0•59, Fig. 2), with 10,415 deaths (27%) among patients receiving nCRT and 84 deaths (20%) among those receiving TNT. The 3-year, 4-year, and 5-year OS rates in each arm were 88•4% vs. 86•9%, 81•7% vs. 81•1%, and 73•8% vs. 75•8% (reported as TNT vs. nCRT, all p > 0•36), respectively, with a median follow-up time of 30•9 months for patients receiving TNT and 48•2 months for those receiving nCRT. After propensity matching, 707 nCRT patients and 372 TNT patients were identified as the matched cohort, balanced in the distribution of demographics and disease characteristics (Table 2). In the matched cohort, TNT was not associated with improved OS (logrank p = 0•16; Wilcoxon-Breslow p = 0•80, Fig. 2), with 122 deaths among patients receiving nCRT (17%) and 75 deaths among those receiving TNT (20%). The 3-year, 4-year, and 5-year OS rates for patients receiving TNT and nCRT were 88•5% vs. 88•9%, 82•0% vs. 83•9%, and 73•6% vs. 78•5%, respectively (all p > 0•20). The median follow-up time was 31•0 months for patients receiving TNT and 33•7 months for those receiving nCRT. Median time from diagnosis to surgery was 8•4 months (IQR: 7•2–9•2 months) for TNT compared to 4•4 months (IQR: 3•9–5•2 months) for nCRT. The median elapsed time between the initiation of chemotherapy to the start of RT was 17 weeks in patients receiving TNT.
Fig. 2.
The overall survival of patients receiving TNT and nCRT for the unmatched and matched cohorts. Number of patients at risk zero, two, four, six, eight, and ten years from diagnosis are displayed at the bottom. No statistically significant difference was found in either cohort (logrank p = 0•85; Wilcoxon-Breslow p = 0•59 for the unmatched cohort, and logrank p = 0•16; Wilcoxon-Breslow p = 0•80 for the matched cohort).
Table 2.
Patient characteristics in the propensity matched cohort.
| Patient characteristics | All patients | TNT | nCRT | p-value |
|---|---|---|---|---|
| (n = 1,079) | (n = 372) | (n = 707) | ||
| Age (y), mean ± SD | 57.0 ± 9.8 | 56.8 ± 9.6 | 57.1 ± 10.0 | 0.61 |
| Sex, n (%) | ||||
| Male | 668 (61.9) | 231 (62.1) | 437 (61.8) | 0.93 |
| Female | 411 (38.1) | 141 (37.9) | 270 (38.2) | |
| Year of diagnosis, n (%) | ||||
| 2004–2005 | 85 (7.9) | 30 (8.1) | 55 (7.8) | 0.88 |
| 2006–2007 | 91 (8.4) | 28 (7.5) | 63 (8.9) | |
| 2008–2009 | 71 (6.6) | 21 (5.6) | 50 (7.1) | |
| 2010–2011 | 165 (15.3) | 60 (16.1) | 105 (14.8) | |
| 2012–2013 | 397 (36.8) | 137 (36.8) | 260 (36.8) | |
| 2014–2015 | 270(25.0) | 96 (25.8) | 174 (24.6) | |
| Race, n (%) | ||||
| non-Hispanic White | 850 (78.8) | 294 (79.0) | 556 (78.6) | 0.67 |
| non-Hispanic Black | 66 (6.1) | 27 (7.3) | 39 (5.5) | |
| Hispanic | 89 (8.2) | 28 (7.5) | 61 (8.6) | |
| Asian | 52 (4.8) | 15 (4.0) | 37 (5.2) | |
| Other/Unknown | 22 (2.0) | 8 (2.2) | 14 (2.0) | |
| Charlson-Deyo co-morbidity score, n (%) | ||||
| 0 | 908 (84.2) | 314 (84.4) | 594 (84.0) | 0.98 |
| 1 | 154 (14.3) | 52 (14.0) | 102 (14.4) | |
| ≥2 | 17 (1.6) | 6 (1.6) | 11 (1.6) | |
| T stage, n (%) | ||||
| ≤T2 | 38 (3.5) | 15 (4.0) | 23 (3.2) | 0.88 |
| T3 | 771 (71.5) | 265 (71.2) | 506 (71.6) | |
| T4 | 193 (17.9) | 64 (17.2) | 129 (18.2) | |
| Tx | 77 (7.1) | 28 (7.5) | 49 (6.9) | |
| N stage, n (%) | ||||
| N0 | 282 (26.1) | 88 (23.7) | 194 (27.4) | 0.57 |
| N1 | 543 (50.3) | 196 (52.7) | 347 (49.1) | |
| N2 | 160 (14.8) | 56 (15.0) | 104 (14.7) | |
| Nx | 94 (8.7) | 32 (8.6) | 62 (8.8) | |
| Grade, n (%) | ||||
| Well/moderately differentiated | 844 (78.2) | 284 (76.3) | 560 (79.2) | 0.56 |
| Poorly differentiated/anaplastic | 104 (9.6) | 39 (10.5) | 65 (9.2) | |
| Unknown | 131 (12.1) | 49 (13.2) | 82 (11.6) | |
| Total radiation dose (Gy), median (25th-75th percentile) | 50.40 (50.14-50.40) |
50.40 (50.00-50.40) |
50.40 (50.40-50.40) |
<0.001 |
| Type of facility, n (%) | ||||
| Non-academic | 331 (30.7) | 108 (29.0) | 223 (31.5) | 0.40 |
| Academic | 748 (69.3) | 264 (71.0) | 484 (68.5) | |
| Urban status, n (%) | ||||
| Metropolitan area | 900 (83.4) | 313 (84.1) | 587 (83.0) | 0.81 |
| Metropolitan-adjacent area | 64 (5.9) | 23 (6.2) | 41 (5.8) | |
| Rural area | 10 (0.9) | 4 (1.1) | 6 (0.8) | |
| Unknown | 105 (9.7) | 32 (8.6) | 73 (10.3) | |
| Median income quartiles, n (%) | ||||
| <$38,000 | 125 (11.6) | 41 (11.0) | 84 (11.9) | 0.82 |
| $38,000-$47,999 | 205 (19.0) | 66 (17.7) | 139 (19.7) | |
| $48,000-$62,999 | 255 (23.6) | 91 (24.5) | 164 (23.2) | |
| ≥$63,000 | 494 (45.8) | 174 (46.8) | 320 (45.3) | |
| Medical insurance type | ||||
| Not insured | 39 (3.6) | 12 (3.2) | 27 (3.8) | 0.81 |
| Private insurance/managed care | 722 (66.9) | 254 (68.3) | 468 (66.2) | |
| Medicaid | 97 (9.0) | 32 (8.6) | 65 (9.2) | |
| Medicare | 193 (17.9) | 64 (17.2) | 129 (18.2) | |
| Other government | 23 (2.1) | 7 (1.9) | 16 (2.3) | |
| Insurance status unknown | 5 (0.5) | 3 (0.8) | 2 (0.3) | |
| Circumferential resection margin, n (%) | ||||
| Positive | 40 (6.3) | 9 (4.7) | 31 (6.9) | 0.30 |
| Negative | 598 (93.7) | 95.3 (95.3) | 417 (93.1) |
Limited information regarding toxicity is available through the NCDB. In the propensity-matched cohort (n = 372 for TNT and n = 707 for nCRT), the 90-day mortality rate after surgery was 0.54% for TNT and 0.57% for nCRT (p = 0.95).
On multivariable analysis, there was no statistically significant difference in OS between TNT and nCRT (HR = 1•21, p = 0•25; Table 3). In this multivariable Cox proportional hazards regression model, higher median income quartile was associated with better OS (p = 0•002), whereas age ≥65 years was associated with worse OS (p = 0•001). The associations between survival and gender, Charlson-Deyo co-morbidity score, clinical stage, tumour grade, and type of facility were not significant (Table 3).
Table 3.
Multivariable Cox proportional hazards regression for overall survival in the matched cohort.
| All patients | ||
|---|---|---|
| (n = 1,079) | ||
| Variable | Hazard ratio | p-value |
| (95% CIs) | ||
| Treatment group (TNT vs. nCRT) | 1.21 (0.87, 1.69) | 0.25 |
| Age (≥ 65 yr vs. < 65 yr) | 1.86 (1.30, 2.65) | 0.001 |
| Sex (female vs. male) | 0.85 (0.60, 1.19) | 0.34 |
| Charlson-Deyo co-morbidity score | ||
| 0 | 1.0 (ref) | 0.30 |
| 1 | 1.37 (0.91, 2.08) | |
| ≥2 | 1.29 (0.46, 3.59) | |
| Clinical stage (> 2 vs. ≤ 2) | 1.39 (0.96, 1.99) | 0.077 |
| Grade | ||
| Well/moderately differentiated | 1.0 (ref) | 0.099 |
| Poorly differentiated/anaplastic | 1.52 (0.95, 2.43) | |
| Unknown | 0.76 (0.44, 1.30) | |
| Type of facility (academic vs. non-academic) | 0.96 (0.68, 1.35) | 0.80 |
| Median income quartiles | ||
| <$38,000 | 1.0 (ref) | 0.002 |
| $38,000-$47,999 | 0.90 (0.55, 1.47) | |
| $48,000-$62,999 | 0.48 (0.28, 0.82) | |
| ≥$63,000 | 0.51 (0.32, 0.82) | |
Bold font indicates statistically significant difference with p < 0.05.
Among patients receiving TNT, 16•9% achieved pCR compared to 13•1% among patients receiving nCRT (p = 0•12). Multivariable logistic regression did not find that TNT was significantly associated with pCR (OR = 1•36, p = 0•13). The negative CRM rate was 95•3% among patients receiving TNT, compared to 93•1% among patients receiving nCRT (p = 0•30). In multivariable logistic regression, TNT was found to be associated with higher rate of negative CRM (OR = 1•77), but this difference was not significant statistically (p = 0•19). In both cohorts, having pCR at time of surgery was associated with better OS (HR = 0•11, p = 0•002 in the nCRT cohort and HR=0•10, p = 0•025 in the TNT cohort).
In the sensitivity analysis with a ten-to-one propensity matching, 372 patients receiving TNT were matched with 2946 patients receiving nCRT. Similar to the two-to-one match, a multivariable analysis showed no statistically significant difference in OS between patients receiving TNT and nCRT (HR = 1•16, p = 0•28; Supplementary Table 3).
In a second sensitivity analysis limiting the nCRT cohort to patients who received both neoadjuvant CRT and adjuvant chemotherapy, 330 patients receiving TNT were matched with 564 patients receiving nCRT. On multivariable analysis, there was no statistically significant difference in OS between patients receiving TNT and nCRT (HR = 0•86, p = 0•39; Supplementary Table 4).
In a third sensitivity analysis with the nCRT cohort consisting of patients receiving multi-agent chemotherapy in both the neoadjuvant and adjuvant setting, 323 patients receiving TNT were matched with 537 patients receiving nCRT. On multivariable analysis, there was no statistically significant difference in OS between patients receiving TNT and nCRT (HR = 1•36, p = 0•12; Supplementary Table 5).
4. Discussion
In this large retrospective cohort study using the NCDB, we found no significant difference in OS between patients with locally advanced rectal adenocarcinoma who were treated with TNT and those who were treated with nCRT. This result persisted after patients were propensity matched for characteristics that could confound the association with OS and to account for treatment selection bias. We also found a slightly higher pCR and negative CRM rate among patients receiving TNT, but these differences were not statistically significant and did not translate into better OS.
These results are consistent with those reported in the Spanish GCR-3 phase II randomised trial [13] and another phase II randomised trial reported by Maréchal et al. [14], where no difference was found in pCR and OS. Authors of GCR-3 pointed out that the study might be underpowered to detect any significant difference in OS. Although this may be true, our study did not find a statistically significant difference despite including a larger number of patients. This trial protocol mandated adjuvant chemotherapy in the nCRT treatment, which when tested in sensitivity analysis did not alter the results in our study. Of note, the study by Maréchal et al was closed prematurely due to perceived futility and the group receiving TNT experienced higher Grade 3/4 toxicity compared to the nCRT group [14]. The pCR rates in our study (TNT 16•9% and nCRT 13•1%) are also comparable to those reported in the GCR-3 study (TNT 14•3% and nCRT 13•5%) as well as the retrospective study by Cercek et al. (TNT 18% and nCRT 17%) [11,13]. The European EXPERT-C trial also showed a similar pCR rate (15% in the study arm with 4 cycles of capecitabine/oxaliplatin as induction chemotherapy) [15].
Given the lack of clear improvement in pCR rates, CRM rates and OS for the use of TNT in this and other studies, the results from the on-going PROSPECT and NRG-GI002 clinical trials will be crucial in helping refine the TNT regimen to maximise the benefits of preoperative therapy. While current national guidelines such as NCCN allow TNT for any T3-4 or node positive rectal cancer patients, it is possible that some of the lower risk patients maybe overtreated using TNT, especially the stage II rectal cancer patients who may not benefit as much from the addition of chemotherapy.
TNT can be defined in a few different ways depending on when chemotherapy is given in relation to chemoradiation, but prior to surgery. Due to the limited information available in the NCDB regarding chemotherapy agents and timing between chemotherapy and radiation, our definition follows that of TNT with induction chemotherapy followed by chemoradiation before surgery. A phase II trial from the German Rectal Cancer Study Group comparing two different TNT sequences demonstrated a higher pCR rate after TNT with consolidation chemotherapy compared to TNT with induction chemotherapy, although this could have been impacted by the prolonged interval between completion of chemoradiation and surgery [16]. Similar results were also found in another non-randomised trial where there were higher pCR rates with increasing number of cycles of chemotherapy, following chemoradiation [17].
Limitations of our study include its retrospective nature and lack of detailed information regarding specific chemotherapy agents used and the number of cycles administered. Another limitation is the potential selection bias associated with treatment assignment. We accounted for this source of bias by propensity matching, balancing potential confounders between the two treatment groups. Year of diagnosis, clinical stage, facility type, urban status, income and insurance type were found to be associated with receiving TNT and none of those characteristics are different between the two treatment groups after propensity matching. Nevertheless, there are some factors that were not captured in the NCDB, such as tumour location in rectum, depth of extramural tumour involvement and vascular invasion, treating centre expertise, and thus cannot be fully accounted for by propensity matching. TNT was more commonly performed at academic centres (71% vs. 35%) in typically healthier patients (Charlson co-morbidity score of zero in 85% vs. 80% of patients). These factors would tend to favour TNT over nCRT, but despite this we still do not find a significant difference in survival between the two treatment groups.
In conclusion, our study found no survival benefit or significant improvement in pCR rates associated with TNT treatment compared to nCRT.
Ethical approval
This study is a retrospective analysis of the National Cancer Database and is thus exempt from ethics approval.
Funding
None.
CRediT authorship contribution statement
Shaoyu Zhu: Conceptualization, Data curation, Writing - original draft, Writing - review & editing. N. Patrik Brodin: Conceptualization, Data curation, Writing - original draft, Writing - review & editing. Keara English: Conceptualization, Data curation, Writing - original draft, Writing - review & editing. Nitin Ohri: Conceptualization, Data curation, Supervision, Writing - review & editing. Jennifer W. Chuy: Conceptualization, Data curation, Supervision, Writing - review & editing. Lakshmi N. Rajdev: Conceptualization, Data curation, Supervision, Writing - review & editing. Rahul Narang: Conceptualization, Data curation, Supervision, Writing - review & editing. Shalom Kalnicki: Conceptualization, Data curation, Supervision, Writing - review & editing. Chandan Guha: Conceptualization, Data curation, Supervision, Writing - review & editing. Madhur K. Garg: Conceptualization, Data curation, Supervision, Writing - review & editing. Rafi Kabarriti: Conceptualization, Data curation, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
NO receives research support from Merck and receive consulting fees from Merck and AstraZeneca unrelated to this manuscript.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2019.09.009.
Appendix. Supplementary materials
References
- 1.Key statistics for colorectal cancer American cancer society2019 [cited 2019 2/13]. Available from:https://www.cancer.org/cancer/colon-rectal-cancer/about/key-statistics.html.
- 2.Rana N, Chakravarthy AB, Kachnic LA. Neoadjuvant treatment for locally advanced rectal cancer: new concepts in clinical trial design. Curr Treat Opt Oncol. 2017;18(2):13. doi: 10.1007/s11864-017-0454-4. [DOI] [PubMed] [Google Scholar]
- 3.Hong TS, Ryan DP. Total neoadjuvant therapy for locally advanced rectal cancer-the new standard of care? JAMA Oncol. 2018;4(6) doi: 10.1001/jamaoncol.2018.0070. [DOI] [PubMed] [Google Scholar]
- 4.Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15(2):184–190. doi: 10.1016/S1470-2045(13)70599-0. [DOI] [PubMed] [Google Scholar]
- 5.Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 6.Rodel C, Liersch T, Becker H, Fietkau R, Hohenberger W, Hothorn T. Preoperative chemoradiotherapy and postoperative chemotherapy with fluorouracil and oxaliplatin versus fluorouracil alone in locally advanced rectal cancer: initial results of the German CAO/ARO/AIO-04 randomised phase 3 trial. Lancet Oncol. 2012;13(7):679–687. doi: 10.1016/S1470-2045(12)70187-0. [DOI] [PubMed] [Google Scholar]
- 7.Khrizman P, Niland JC, ter Veer A, Milne D, Bullard Dunn K, Carson WE., 3rd Postoperative adjuvant chemotherapy use in patients with stage II/III rectal cancer treated with neoadjuvant therapy: a national comprehensive cancer network analysis. J Clin Oncol. 2013;31(1):30–38. doi: 10.1200/JCO.2011.40.3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayden DM, Pinzon MC, Francescatti AB, Edquist SC, Malczewski MR, Jolley JM. Hospital readmission for fluid and electrolyte abnormalities following ileostomy construction: preventable or unpredictable? J Gastrointestinal Surg. 2013;17(2):298–303. doi: 10.1007/s11605-012-2073-5. [DOI] [PubMed] [Google Scholar]
- 9.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 10.Shin SW. The current practice of transarterial chemoembolization for the treatment of hepatocellular carcinoma. Korean J Radiol. 2009;10(5):425–434. doi: 10.3348/kjr.2009.10.5.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cercek A, Roxburgh CSD, Strombom P, Smith JJ, Temple LKF, Nash GM. Adoption of total neoadjuvant therapy for locally advanced rectal cancer. JAMA Oncol. 2018;4(6) doi: 10.1001/jamaoncol.2018.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ludmir EB, Palta M, Willett CG, Czito BG. Total neoadjuvant therapy for rectal cancer: an emerging option. Cancer. 2017;123(9):1497–1506. doi: 10.1002/cncr.30600. [DOI] [PubMed] [Google Scholar]
- 13.Fernandez-Martos C, Garcia-Albeniz X, Pericay C, Maurel J, Aparicio J, Montagut C. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trialdagger. Ann Oncol. 2015;26(8):1722–1728. doi: 10.1093/annonc/mdv223. [DOI] [PubMed] [Google Scholar]
- 14.Marechal R, Vos B, Polus M, Delaunoit T, Peeters M, Demetter P. Short course chemotherapy followed by concomitant chemoradiotherapy and surgery in locally advanced rectal cancer: a randomized multicentric phase II study. Ann Oncol. 2012;23(6):1525–1530. doi: 10.1093/annonc/mdr473. [DOI] [PubMed] [Google Scholar]
- 15.Dewdney A, Cunningham D, Tabernero J, Capdevila J, Glimelius B, Cervantes A. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C) J Clin Oncol. 2012;30(14):1620–1627. doi: 10.1200/JCO.2011.39.6036. [DOI] [PubMed] [Google Scholar]
- 16.Fokas E, Allgauer M, Polat B, Klautke G, Grabenbauer GG, Fietkau R. Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ARO/AIO-12. J Clin Oncol. 2019 doi: 10.1200/JCO.19.00308. JCO1900308. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Aguilar J, Chow OS, Smith DD, Marcet JE, Cataldo PA, Varma MG. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol. 2015;16(8):957–966. doi: 10.1016/S1470-2045(15)00004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.