Abstract
Background
Context-specific evidence of the spectrum of type 2 diabetes (T2D) burden is essential for setting priorities and designing interventions to reduce associated morbidity and mortality. However, there are currently limited data on the burden of T2D complications and comorbidity in sub-Saharan Africa (SSA).
Methods
T2D complications and comorbidities were assessed in 2,784 participants with diabetes enrolled from tertiary health centres and contextualised in 3,209 individuals without diabetes in Nigeria, Ghana and Kenya. T2D complications and comorbidities evaluated included cardiometabolic, ocular, neurological and renal characteristics.
Findings
The most common complications/comorbidities among the T2D participants were hypertension (71%; 95% CI 69–73), hyperlipidaemia (34%; 95% CI 32–36), and obesity (27%; 95% CI 25–29). Additionally, the prevalence of cataracts was 32% (95% CI 30–35), diabetic retinopathy 15% (95% CI 13–17), impaired renal function 13% (95% CI 12–15), and erectile dysfunction (in men) 35% (95% CI 32–38). T2D population-attributable fraction for these comorbidities ranged between 6 and 64%.
Interpretation
The burden of diabetes complications and comorbidity is substantial in SSA highlighting the urgent need for innovative public health strategies that prioritise promotion of healthy lifestyles for prevention and early detection of T2D. Also needed are strategies to strengthen health care system capacities to provide treatment and care for diabetes complications.
Keywords: Type 2 Diabetes, Sub-Sahara Africa, Complications, Co-morbidity, Epidemiology
1. Research in context
1.1. Evidence before this study
Type 2 diabetes (T2D) is the most common metabolic-endocrine disorder affecting adults. Its multisystemic nature implies that complications and comorbidities can affect many organ systems, especially in the absence of good glycaemic control. Currently, there is inadequate data on the burden and risk factors associated with T2D complications and comorbidities in sub-Saharan Africa (SSA). Except for the Diabcare Africa study, most of the published studies have small sample sizes, were conducted in a single hospital and/or did not evaluate associated risk factors. In addition, only a subset of participants included in these studies had data for some complications/comorbidities; for example, 45 percent of patients had fasting lipids in the largest study to date. The data from these studies indicate that hypertension is the most common T2D complication/comorbidity in SSA (up to 65% of patients), ocular complications (cataracts and retinopathy) were present in 14–18% and signs of neuropathy were found in up to 48% of patients.
1.2. Added value of this study
The findings of the present study provide a richer context for the assessment of burden and risk factors for T2D complications/comorbidities in SSA by using a large and well-characterised sample to estimate prevalence of a set of variables that were assessed by clinical examination, laboratory assays and medical history. The study identified risk factors for T2D complications/comorbidities and specifically investigated the effect of study site while adjusting for other covariates to identify heterogeneity between sites which may reflect differential access to care and differences in lifestyle/behavioural characteristics. The prevalence of the metabolic syndrome was particularly high as it was observed in three-quarters of T2D patients. Since many complications/comorbidities (e.g. cataracts) seen in T2D can be due to other disorders, the specific enrolment of controls in the present study facilitated the estimation of risk conferred by T2D on specific comorbidities as well as calculation of population attributable fraction (PAF) of the comorbidity due to T2D. The PAF provides an estimate of the proportion by which the burden of the comorbidity would be reduced in the population if T2D were absent or controlled.
1.3. Implication of all the available evidence
The findings of the present study combined with existing evidence indicate that the burden of T2D complications/comorbidity in SSA is high and specific strategies are needed to limit their deleterious impact on lifestyle, morbidity and mortality. Good glycaemic control and achieving patient weight loss will mitigate the two most critical risk factors for T2D complications/comorbidity. In this regard, increasing the capacity to monitor glycaemic control in SSA is crucial. Regular screening for hyperlipidaemia, renal function, ocular and foot complications in all T2D patients (rather than the current one-half to two-thirds of patients even in major specialist centres) is important and the adoption of a consensus screening checklist may facilitate this goal. Further research is needed to identify the factors responsible for the differences between sites reported by this and previous studies, as there may be the need for region-specific interventions. New research initiatives are needed to generate reliable data on other aspects of T2D complications such as the age of onset, rate of progression and impact on quality of life.
2. Background
The burden of type 2 diabetes (T2D) is substantial and growing across sub-Saharan Africa (SSA), with an estimated diabetes prevalence of 7.1% in 2014 representing a 129% increase since 1980 [1], [2]. Additionally, projections by the International Diabetes Federation (IDF) show that, whilst all regions of the world will experience increases in T2D prevalence, the greatest increase between now and 2045 will take place in SSA [3]. A key feature of T2D is the presence of complications and comorbidity which have implications for prognosis, overall disease burden and treatment options [4], [5], [6], [7], [8], [9]. Thus, T2D care ought to integrate the prevention and management of comorbidities such as hypertension and dyslipidaemia, as well as complications including stroke, ophthalmological and neurological complications, among others [2], [10], [11], [12]. This requires a strong evidence base of the key T2D complications and comorbidities in order to determine intervention priorities and assess the effectiveness of such interventions in specific populations. However, with the notable exception of the Diabcare Africa Study [13], most studies of T2D complications and comorbidities from Africa done so far only focus on one or a few specific complications and/or are in limited sample sizes. Indeed, the recent Lancet and Endocrinology Commission report on diabetes in SSA noted the absence of reliable data on the prevalence, age of onset, rate of progression and other aspects of T2D complications in SSA [14]. This study aimed to estimate the burden of T2D complications and comorbidities, identify their risk factors and estimate their population attributable fraction in sub-Saharan Africans enrolled in a large multi-country study.
3. Methods
3.1. Study participants and data collection
Participants included in the present investigation were drawn from a case-control study — the Africa America Diabetes Mellitus (AADM) Study. The AADM study, which enrolled participants between 2000 and 2016, was designed to assess the environmental and genetic determinants of T2Dwere [15], [16]. Individuals aged 18 years or older were enrolled from Nigeria (Enugu, Ibadan and Lagos), Ghana (Accra and Kumasi) and Kenya (Eldoret) using a standardized consent and data collection protocol. Individuals with T2D were enrolled from medical centres while individuals without T2D were enrolled from surrounding communities of the respective medical centres. Details of study procedures and enrolment have been described elsewhere [15], [16].
Briefly, potential participants were identified by research staff at outpatient clinics and community centres who explained the study objectives and procedures. Informed consent was obtained from willing individuals that met the inclusion/exclusion criteria followed by data collected on demographic, social, lifestyle factors and clinical data including medical history, anthropometry, blood pressure and cardiometabolic parameters as previously described [15], [16]. Data were collected by trained research assistants and physicians . Neurological examination including tests of motor reflexes and sensation were conducted by physicians. Eye examination that involved pupillary dilation, applanation tonometry and fundoscopy were conducted by specialist ophthalmologists. The study received ethical approval from the Institutional Review Board at each Africa study site, Howard University and the United States National Institutes of Health.
3.2. Laboratory assays
Fasting samples obtained after an overnight fast of at least 8 h were used to measure clinical biomarkers using an auto-analyser, COBAS Integra 400 plus (Roche Diagnostics, Indianapolis, IN). Fasting plasma glucose was measured using an enzymatic method with hexokinase. HDL-cholesterol, LDL-cholesterol and triglycerides (TG) were determined enzymatically with methods standardized to in-house and other appropriate reference methods (CDC reference methods for HDL-cholesterol and isotope dilution mass spectrometry (ID-MS) for TG from the manufacturer). Creatinine was measured using a modified Jaffé reaction. eGFR was estimated using the Modification of Diet in Renal Diseases (MDRD) formula.
3.3. Definitions
T2D was defined according to the American Diabetes Association criteria of fasting plasma glucose concentration (FPG) ≥ 7.0 mmol/L, or 2-h post load value in the oral glucose tolerance test ≥ 11.1 mmol/l on more than one occasion, or pharmacological treatment for T2D confirmed by a review of medical records. Glycaemic control by fasting glucose levels (controlled glucose) among individuals with T2D was defined as fasting plasma glucose <6.1 mmol/L, the American College of Endocrinologists fasting glucose threshold for satisfactory diabetic control [17]. The complications and comorbidity evaluated included: complications by medical history (diabetic coma, visual problems, non-healing ulcers, amputation, stocking/glove numbness, transient ischemic attack/stroke, erectile dysfunction—ED), ocular complications as evaluated by ophthalmologic examination (cataract, retinal detachment, maculopathy, glaucoma, diabetic retinopathy), neurological complication by clinical examination (abnormal ankle reflex, abnormal knee reflex, abnormal touch, abnormal pain, abnormal vibration sense) and cardiometabolic complications including elevated triglycerides (≥2.26 mmol/L), total cholesterol (TC ≥ 6.22 mmol/L) and low-density lipoprotein (LDL ≥ 4.14 mmol/L), and low high-density lipoprotein (HDL < 1.03/1.3 mmol/L, men/women); [18], [19] hypertension defined as systolic blood pressure (SBP) ≥ 140 mmHg or diastolic blood pressure (DBP) ≥ 90 mmHg, or treatment for hypertension; overweight defined as body mass index (BMI) 25.0–29.9 kg/m2, obese as BMI ≥30 kg/m2; and metabolic syndrome (MS) was defined according to the International Diabetes Federation harmonized criteria [20]. We used the guidelines of the National Kidney Foundation Kidney Disease Outcome Quality Initiative that defined moderate CKD as eGFR <60 ml/min/1.73 m2 to denote impaired renal function. We also defined high serum creatinine as serum creatinine >177 µmol/L. Diabetic retinopathy was diagnosed only if a participant had a minimum of one microaneurysm in any field, as well as exhibiting haemorrhages (dot, blot, or flame shaped), and maculopathy (with or without clinically significant oedema) [21].
3.4. Statistical analysis
Continuous variables were summarized using means and standard deviations (SD), or median and interquartile range (IQR) in case of deviation from normal distribution tested by the Shapiro-Wilk test. Categorical variables were summarized using proportions expressed as percentages. Student's t-test and Mann-Whitney U test were used to compare continuous variables between two groups; while categorical variables were compared using chi-squared tests. Adjusted prevalence was obtained as marginal predictions from logistic regression models with covariates set to their mean values. Logistic regression models were used to assess the association between each comorbidity/complication and potential correlates among individuals with T2D. We used the I2-statistic from the random effects meta-analysis model to assess heterogeneity in prevalence across study sites. We computed population-attributable fraction (PAF) due to T2D from multivariable logistic regression models adjusting for age and sex. All statistical tests were two sided and P < 0.05 considered significant. Analyses were performed in STATA 15 (StataCorp. 2017. Stata Statistical Software: Release 15. College Station, TX: StataCorp LLC).
4. Results
4.1. Characteristics of T2D cases and controls
We studied 5993 individuals (Table 1) including 2784 T2D cases (39% men) who had a mean age of 56 (SD 11) years and median known duration of T2D of 5 (IQR 2–10) years. Among cases, only 3% were current smokers and 15% were former smokers. Contrastingly, a larger proportion, 77%, were current consumers of alcohol and 20% were former alcohol consumers. Twenty-three percent of the cases had post-secondary education. The median fasting blood glucose level among cases was 9.8 (IQR 5.9–12.5) mmol/L. Glycaemic control as assessed by fasting glucose was low with only 27% of cases achieving controlled levels of blood glucose at enrolment despite nearly all cases being on diabetes treatment. Among the 2784 T2D cases, 112 were diagnosed during the study and were not previously on any T2D treatment. Among the 2672 previously diagnosed cases, 74% were on a combination of lifestyle intervention (diet and physical activity) and oral medication only, 18% used insulin in addition to lifestyle intervention and/or oral medication, 4% were on lifestyle intervention only, and 4% were not on any intervention. Metformin and sulfonylurea were the most common class of drugs, used by 83% of cases taking oral medication (42% metformin + sulfonylurea, 19% metformin only, and 22% sulfonylurea only) (Supplementary Table 1). Participants self-reported to be taking their medications at the time of the study.
Table 1.
Characteristics of participants in the study of T2D complications and comorbidity in sub-Saharan Africa.
| Characteristics | Number of participants (Controls/cases) | T2D Cases | Controls | aP value | |
|---|---|---|---|---|---|
| Sex | 5993 (3209/2784) | Men | 39 (38–41) | 40 (39–42) | 0.426 |
| Age | 5993 (3209/2784) | Mean (SD) | 56 (11) | 45 (16) | <0.001 |
| Education | 5971 (3200/2771) | Post-secondary | 23 (21–24) | 26 (25–28) | 0.001 |
| Smoking | 5945 (3181/2764) | Never smoked regularly | 82 (80–83) | 87 (86–88) | |
| Former regular smoker | 15 (14–17) | 9 (8–10) | |||
| Current regular smoker | 3 (2–4) | 4 (4–5) | <0.001 | ||
| Alcohol | 5509 (3092/2417) | Never consumed regularly | 3 (2–3) | 7 (6–7) | |
| Former regular consumer | 20 (19–22) | 28 (26–30) | |||
| Current regular consumer | 77 (75–79) | 65 (64–67) | <0.001 | ||
| Comorbidity | 5954 (3184/2770) | Overweight | 36 (35–38) | 28 (26–29) | <0.001 |
| 5955 (3184/2770) | Obesity | 27 (25–28) | 23 (22–25) | 0.005 | |
| 5904 (3164/2740) | Clinically defined hypertension | 70(68–71) | 40 (38–42) | <0.001 | |
| 5618 (2993/2625) | Raised TG | 12 (11–13) | 4 (4–5) | <0.001 | |
| 5618 (2993/2625) | Raised TC | 22 (21–24) | 15 (14–16) | <0.001 | |
| 5583 (2986/2597) | Raised LDL-cholesterol | 24 (23–26) | 18 (17–20) | <0.001 | |
| 5618(2993/2625) | Hyperlipidaemia (Raised TC, TG or LDL) | 28 (27–30) | 20 (19–22) | <0.001 | |
| 5617 (3209/2784) | Low HDL-cholesterol | 60 (58–62) | 52 (50–54) | <0.001 | |
| 5993(3209/2784) | Metabolic syndrome | 77 (75–78) | 25 (24–27) | <0.001 | |
| 4800 (2204/2596) | eGFR < 60 mL/min/1.73 m2 | 13 (12–15) | 5 (4–6) | <0.001 | |
| 4800 (2596/2204) | Creatinine > 177 µmol/L | 2.4 (1.8–3.1) | 0.4 (0.2–0.8) | <0.001 |
Data are percent (95% CI) except where otherwise stated; SD Standard deviation;.
P-values are from Chi-square test; T2D Type two diabetes; TG triglycerides (Raised TG, TG ≥ 2.26 mmol/L); TC total cholesterol (Raised TC, TC ≥ 6.22 mmol/L); LDL Low-density lipoprotein (Raised LDL, LDL ≥ 4.14 mmol/L); HDL High-density lipoprotein (Low HDL, HDL < 1.03 (men); 1.3 (women)); eGFR estimated glomerular filtration rate.
Study participants also included 3209 controls (40% men) with a mean age of 45 (SD 16) years. Controls were more likely to have never smoked, less likely to be current consumers of alcohol and had lower prevalence of obesity, hypertension, hyperlipidaemia and low eGFR when compared with cases. In other words, cases had a more adverse lifestyle, metabolic and kidney function risk profile compared with controls (Table 1).
4.2. Prevalence of T2D complications and comorbidities
The prevalence of the evaluated T2D complications and comorbidities is shown in Table 2. Prevalence of cardiometabolic parameters was 71% for hypertension, 27% for obesity, 22% for hypercholesterolemia and 11% for hypertriglyceridemia. Dyslipidaemia for any of the lipid sub-fractions was observed in 34% while three-quarters (78%) had the metabolic syndrome by IDF criteria. Ocular complications of cataracts, glaucoma and diabetic retinopathy were found in 32%, 4% and 15%, respectively. Neurological complications such as abnormal ankle reflex and abnormal vibration sensation were more common (33% and 12%, respectively) in contrast to abnormal pain and abnormal touch sensation (4% and 5%, respectively). On the other hand, stocking/glove numbness by medical history was far more frequent (46%). ED was reported by 35% of the men (Table 2).
Table 2.
Complications and comorbidities of T2D in sub-Saharan Africa by glycaemic control.
| Controlled | All T2D cases | No. of T2D cases not controlled/No. of T2D cases | T2D cases not controlled | T2D cases controlled | aP-value |
|---|---|---|---|---|---|
| Participant characteristics | |||||
| Age, Mean (SD) | 56 (11) | 1805/684 | 55 (11) | 58 (11) | <0.001 |
| Sex (Percent men) | 39 (37–41) | 1805/684 | 38 (36–40) | 43 (39–46) | 0.029 |
| Duration of T2D, Median (IQR) | 5(2–10) | 1805/684 | 5 (2–10) | 3 (1–8) | <0.001 |
| Glucose, Median (IQR) | 9.8 (5.9–12.5) | 1805/684 | 9.9 (7.4–14.0) | 4.7 (4.3–5.1) | <0.001 |
| Cardiometabolic and renal complications | |||||
| Obesity | 27 (25–29) | 1799/678 | 25 (23–27) | 24 (21–28) | 0.863 |
| Clinically defined hypertension | 71 (69–73) | 1802/684 | 72(69–74) | 76 (72–79) | 0.041 |
| Raised TG | 11 (10–13) | 1789/677 | 12 (10–13) | 10 (8–13) | 0.370 |
| Raised TC | 22 (20–24) | 1789/677 | 23 (21–25) | 19 (16–22) | 0.075 |
| Raised LDL-cholesterol | 24 (23–26) | 1772/669 | 25 (23–27) | 21 (18–25) | 0.110 |
| Low HDL-cholesterol | 60 (58–62) | 1789/677 | 61 (59–63) | 58 (54–61) | 0.147 |
| Hyperlipidaemia (Raised TC, TG or LDL) | 34 (32–36) | 1789/677 | 34 (32–36) | 32 (28–35) | 0.246 |
| Metabolic syndrome | 78 (77–80) | 1805/684 | 81 (79–83) | 80 (76–83) | 0.318 |
| eGFR < 60 mL/min/1.73 m2 | 13 (12–15) | 1522/536 | 11 (10–13) | 12 (10–16) | 0.515 |
| Creatinine > 177 µmol/L | 2.4 (1.8–3.1) | 1522/536 | 1.5 (1.0–2.2) | 2.6 (1.6–4.3) | 0.059 |
| Ocular complications | |||||
| Cataracts | 32 (30–35) | 1306/428 | 29 (27–32) | 30 (26–35) | 0.680 |
| Retinal detachment | 0.2 (0.0–0.6) | 1151/385 | 0.1 (0.0–0.6) | 0.1 (0.0–1.4) | 0.760 |
| Maculopathy | 14 (12–16) | 1152/378 | 13 (11–15) | 12 (9–16) | 0.538 |
| Glaucoma | 4 (4–5) | 1805/684 | 4 (3–5) | 4 (3–6) | 0.856 |
| Diabetic retinopathy | 15 (13–17) | 1157/389 | 14 (12–17) | 12 (9–15) | 0.165 |
| Neurological complications | |||||
| Abnormal ankle reflex | 33 (30–37) | 554/106 | 34 (30–38) | 29 (21–38) | 0.327 |
| Abnormal knee reflex | 76 (72–79) | 557/106 | 76 (73–80) | 77 (68–84) | 0.909 |
| Abnormal touch | 5 (4–7) | 558/108 | 4 (3–6) | 0 (0–4) | 0.004 |
| Abnormal pain | 4 (3–6) | 559/108 | 3 (2–5) | 2 (1–7) | 0.778 |
| Abnormal vibration sense | 12 (10–15) | 558/107 | 10 (8–13) | 5 (2–10) | 0.037 |
| Complications/comorbidity by medical history | |||||
| Diabetic coma | 8 (7–9) | 1758/663 | 7 (6–8) | 8 (6–11) | 0.256 |
| Visual problems | 47 (45–50) | 1724/649 | 48 (46–50) | 46 (42–50) | 0.440 |
| Non-healing ulcers | 5 (4–6) | 1732/655 | 5 (4–6) | 4 (3–6) | 0.427 |
| Amputation | 1 (1–2) | 1765/665 | 1 (1–1) | 1 (1–2) | 0.321 |
| Stocking/glove numbness | 46 (44–48) | 1770/668 | 46 (43–48) | 48 (44–51) | 0.434 |
| TIA/Stroke | 3 (2–3) | 1572/566 | 3 (2–3) | 3 (2–4) | 0.965 |
| bErectile dysfunction | 35 (32–38) | 662/279 | 38 (34–42) | 27 (22–33) | 0.002 |
No. Number; Cases not controlled refers to cases whose glucose in not controlled; Cases controlled refers to cases with controlled glucose; T2D type two diabetes; SD standard deviation; IQR inter quartile range; TG triglycerides (Raised TG, TG ≥ 2.26 mmol/L); TC total cholesterol (Raised TC, TC ≥ 6.22 mmol/L); LDL Low-density lipoprotein (Raised LDL, LDL ≥ 4.14 mmol/L); HDL High-density lipoprotein (Low HDL, HDL <1.03 (men); 1.3 (women)); MS metabolic syndrome.
Data are percentage (95% CI) except for age, duration of T2D and glucose.
Adjusted for age, sex and duration of T2D, except as indicated;.
Men only; eGFR estimated glomerular filtration rate; Proportion of individuals with uncontrolled glucose is 73% (71–74). No. Cases not controlled, and No. Cases controlled do not always add up to 2784 because of missing glucose data.
4.3. Prevalence of T2D complications and comorbidities by glycaemic control
The median blood glucose in controlled and uncontrolled cases (i.e. glycaemic control measured by fasting blood glucose) was 4.7 (IQR 4.3–5.1) mmol/L and 9.9 (IQR 7.4–14.0) mmol/L, respectively (Table 2). Cases with controlled glucose were older on average, had a shorter known duration of T2D, and comprised a higher proportion of men, when compared with cases with uncontrolled glucose. Therefore, in subsequent analyses we adjusted for age, sex and duration of T2D in comparisons between cases with controlled and uncontrolled blood glucose levels. The adjusted prevalence of many T2D complications and comorbidities did not differ by glycaemic control for most parameters with a few key exceptions as noted for hypertension (76% versus 72%, P = 0.041), ED in men (27% versus 38%, P = 0.002), abnormal touch (0%versus4%, p = 0.004) and abnormal vibration sense (5% versus 10%, P = 0.037) (Table 2).
4.4. Risk factors for T2D complications and comorbidity
Independent associations of individual comorbidity or complication with age, sex, BMI and known duration of T2D, adjusted for study site and glucose control are shown in Tables 3 and 4. The results indicate that hypertension was associated with older age, higher BMI and longer known duration of T2D; while obesity was associated with the female sex and shorter known duration of T2D. Additionally, raised TG was associated with higher BMI; whereas raised TC and raised LDL were associated with the female sex, higher BMI and longer known duration of T2D.
Table 3.
Odds ratio associated with risk factors for clinically-assessed T2D cardiometabolic comorbidity and complications.
| Complication/comorbidity | Risk factor | uORa | 95% CI | P-value | aORb | 95% CI | P-value | aORc | 95% CI | P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| Obesity | Age (Years) | 0.99 | (0.99–1.00) | 0.169 | 1.00 | (0.99–1.01) | 0.794 | 1.00 | (0.99–1.01) | 0.965 |
| Sex (Ref = males) | 3.78 | (3.08–4.64) | <0.001 | 3.86 | (3.11–4.80) | <0.001 | 3.84 | (3.08–4.80) | <0.001 | |
| Duration of T2D | 0.97 | (0.95–0.98) | <0.001 | 0.97 | (0.95–0.98) | <0.001 | 0.97 | (0.95–0.99) | <0.001 | |
| Accra, Ghana | 2.48 | (2.02–3.04) | <0.001 | 2.19 | (1.60–3.00) | <0.001 | 2.19 | (1.60–3.01) | <0.001 | |
| Kumasi, Ghana | 0.36 | (0.25–0.51) | <0.001 | 0.37 | (0.24–0.57) | <0.001 | 0.39 | (0.24–0.63) | <0.001 | |
| Enugu, Nigeria | 0.47 | (0.36–0.63) | <0.001 | 0.62 | (0.43–0.90) | 0.012 | 0.62 | (0.43–0.91) | 0.013 | |
| Ibadan, Nigeria | 1.33 | (1.12–1.59) | 0.001 | 1.42 | (1.06–1.89) | 0.018 | 1.46 | (1.09–1.96) | 0.011 | |
| Lagos, Nigeria | 0.70 | (0.51–0.96) | 0.027 | 1.00 | (0.66–1.52) | 0.988 | 0.98 | (0.64–1.49) | 0.912 | |
| Hypertension | Age (Years) | 1.07 | (1.06–1.07) | 0.000 | 1.06 | (1.05–1.07) | <0.001 | 1.06 | (1.05–1.07) | <0.001 |
| Sex (Ref = males) | 1.14 | (0.97–1.35) | 0.113 | 0.95 | (0.78–1.15) | 0.581 | 0.96 | (0.79–1.17) | 0.694 | |
| BMI (Kg/m2) | 1.08 | (1.07–1.10) | 0.000 | 1.10 | (1.08–1.12) | <0.001 | 1.10 | (1.08–1.12) | <0.001 | |
| Duration of T2D | 1.03 | (1.02–1.05) | 0.000 | 1.02 | (1.00–1.03) | 0.029 | 1.02 | (1.00–1.03) | 0.034 | |
| Accra, Ghana | 0.79 | (0.64–0.97) | 0.022 | 0.43 | (0.31–0.60) | <0.001 | 0.44 | (0.31–0.62) | <0.001 | |
| Kumasi, Ghana | 0.65 | (0.50–0.83) | 0.001 | 0.55 | (0.38–0.79) | 0.001 | 0.61 | (0.41–0.91) | 0.013 | |
| Enugu, Nigeria | 0.86 | (0.69–1.08) | 0.187 | 0.61 | (0.43–0.85) | 0.004 | 0.62 | (0.44–0.87) | 0.006 | |
| Ibadan, Nigeria | 1.45 | (1.22–1.74) | 0.000 | 0.61 | (0.45–0.83) | 0.002 | 0.63 | (0.46–0.87) | 0.004 | |
| Lagos, Nigeria | 0.72 | (0.55–0.95) | 0.019 | 0.50 | (0.34–0.74) | 0.001 | 0.52 | (0.35–0.76) | 0.001 | |
| Raised TG | Age (Years) | 1.00 | (1.00–1.01) | 0.405 | 1.00 | (0.99–1.01) | 0.496 | 0.99 | (0.98–1.01) | 0.230 |
| Sex (Ref = males) | 0.88 | (0.73–1.06) | 0.177 | 0.96 | (0.77–1.20) | 0.715 | 0.78 | (0.58–1.04) | 0.090 | |
| BMI (Kg/m2) | 1.02 | (1.01–1.04) | 0.008 | 1.03 | (1.02–1.05) | <0.001 | 1.05 | (1.02–1.07) | <0.001 | |
| Duration of T2D | 0.98 | (0.97–1.00) | 0.031 | 0.98 | (0.97–1.00) | 0.07 | 0.98 | (0.96–1.00) | 0.166 | |
| Accra, Ghana | 0.47 | (0.36–0.62) | 0.000 | 0.10 | (0.07–0.14) | <0.001 | 0.06 | (0.04–0.10) | <0.001 | |
| Kumasi, Ghana | 0.57 | (0.40–0.81) | 0.002 | 0.12 | (0.08–0.18) | <0.001 | 0.07 | (0.03–0.14) | <0.001 | |
| Enugu, Nigeria | 0.41 | (0.30–0.56) | 0.000 | 0.09 | (0.06–0.14) | <0.001 | 0.06 | (0.04–0.11) | <0.001 | |
| Ibadan, Nigeria | 0.86 | (0.70–1.05) | 0.135 | 0.18 | (0.14–0.24) | <0.001 | 0.13 | (0.09–0.11) | <0.001 | |
| Lagos, Nigeria | 0.57 | (0.39–0.82) | 0.002 | 0.11 | (0.07–0.17) | <0.001 | 0.09 | (0.05–0.17) | <0.001 | |
| Raised TC | Age (Years) | 1.01 | (1.00–1.02) | 0.013 | 1.01 | (1.00–1.02) | 0.267 | 1.01 | (1.00–1.02) | 0.272 |
| Sex (Ref = males) | 1.66 | (1.36–2.02) | 0.000 | 1.90 | (1.53–2.37) | <0.001 | 1.83 | (1.46–2.28) | <0.001 | |
| BMI (Kg/m2) | 1.03 | (1.01–1.04) | 0.002 | 1.02 | (1.00–1.04) | 0.024 | 1.02 | (1.00–1.04) | 0.019 | |
| Duration of T2D | 1.02 | (1.01–1.03) | 0.006 | 1.02 | (1.00–1.03) | 0.011 | 1.02 | (1.00–1.03) | 0.024 | |
| Accra, Ghana | 0.77 | (0.60–0.98) | 0.037 | 0.29 | (0.21–0.40) | <0.001 | 0.27 | (0.19–0.37) | <0.001 | |
| Kumasi, Ghana | 0.61 | (0.43–0.87) | 0.006 | 0.25 | (0.17–0.38) | <0.001 | 0.23 | (0.15–0.37) | <0.001 | |
| Enugu, Nigeria | 0.66 | (0.50–0.88) | 0.004 | 0.31 | (0.22–0.44) | <0.001 | 0.29 | (0.21–0.41) | <0.001 | |
| Ibadan, Nigeria | 0.92 | (0.76–1.13) | 0.437 | 0.37 | (0.28–0.48) | <0.001 | 0.37 | (0.28–0.49) | <0.001 | |
| Lagos, Nigeria | 1.01 | (0.74–1.39) | 0.946 | 0.41 | (0.28–0.61) | <0.001 | 0.39 | (0.27–0.58) | <0.001 | |
| Raised LDL-cholesterol | Age (Years) | 1.00 | (0.99–1.02) | 0.368 | 1.00 | (0.99–1.01) | 0.873 | 1.01 | (1.00–1.02) | 0.136 |
| Sex (Ref = males) | 1.47 | (1.13–1.90) | 0.003 | 1.60 | (1.21–2.11) | 0.001 | 1.79 | (1.45–2.22) | <0.001 | |
| BMI (Kg/m2) | 1.01 | (0.99–1.03) | 0.249 | 1.01 | (0.99–1.04) | 0.262 | 1.02 | (1.01–1.04) | 0.008 | |
| Duration of T2D | 1.01 | (0.99–1.03) | 0.304 | 1.01 | (0.99–1.03) | 0.329 | 1.02 | (1.00–1.03) | 0.017 | |
| Accra, Ghana | 0.64 | (0.45–0.90) | 0.011 | 0.30 | (0.19–0.47) | <0.001 | 0.57 | (0.41–0.79) | 0.001 | |
| Kumasi, Ghana | 0.74 | (0.47–1.15) | 0.182 | 0.39 | (0.24–0.66) | <0.001 | 0.62 | (0.42–0.94) | 0.022 | |
| Enugu, Nigeria | 1.00 | (0.71–1.39) | 0.977 | 0.57 | (0.38–0.85) | 0.006 | 0.61 | (0.44–0.85) | 0.004 | |
| Ibadan, Nigeria | 0.97 | (0.75–1.26) | 0.842 | 0.51 | (0.36–0.72) | <0.001 | 0.59 | (0.44–0.78) | <0.001 | |
| Lagos, Nigeria | 1.03 | (0.68–1.56) | 0.897 | 0.6 | (0.37–0.97) | 0.038 | 0.89 | (0.61–1.29) | 0.529 | |
| Low HDL-cholesterol | Age (Years) | 1.00 | 0.99–1.00 | 0.399 | 1.00 | 0.99–1.00 | 0.281 | 1.00 | 0.99–1.00 | 0.368 |
| Sex (Ref = males) | 1.91 | 1.62–2.24 | <0.001 | 1.75 | 1.47–2.10 | <0.001 | 1.73 | 1.44–2.07 | <0.001 | |
| BMI (Kg/m2) | 1.05 | 1.04–1.07 | <0.001 | 1.04 | 1.03–1.06 | <0.001 | 1.04 | 1.03–1.06 | <0.001 | |
| Duration of T2D | 0.97 | 0.96–0.99 | <0.001 | 0.98 | 0.97–0.99 | 0.002 | 0.98 | 0.96–0.99 | <0.001 | |
| Accra, Ghana | 0.51 | 0.42–0.62 | <0.001 | 0.77 | 0.58–1.03 | 0.078 | 0.76 | 0.57–1.01 | 0.62 | |
| Kumasi, Ghana | 1.85 | 1.38–2.47 | <0.001 | 2.87 | 2.01–4.10 | <0.001 | 2.50 | 1.73–3.60 | <0.001 | |
| Enugu, Nigeria | 1.00 | 0.80–1.24 | 0.97 | 1.68 | 1.26–2.25 | <0.001 | 1.67 | 1.25–2.23 | 0.001 | |
| Ibadan, Nigeria | 2.08 | 1.74–2.48 | <0.001 | 2.71 | 2.10–3.51 | <0.001 | 2.71 | 2.10–3.51 | <0.001 | |
| Lagos, Nigeria | 0.87 | 0.66–1.13 | 0.296 | 1.54 | 1.10–2.17 | 0.012 | 1.51 | 1.07–2.13 | 0.018 | |
| eGFR < 60 mL/min/1.73 m2 | Age (Years) | 1.05 | (1.04–1.07) | <0.001 | 1.05 | (1.03–1.06) | <0.001 | 1.05 | (1.03–1.06) | <0.001 |
| Sex (Ref = males) | 1.23 | (0.95–1.58) | 0.118 | 1.47 | (1.10–1.95) | 0.008 | 1.4 | (1.05–1.87) | 0.022 | |
| BMI (Kg/m2) | 1.00 | (0.97–1.02) | 0.801 | 1.01 | (0.98–1.04) | 0.407 | 1.01 | (0.98–1.04) | 0.412 | |
| Duration of T2D | 1.04 | (1.58–2.76) | <0.001 | 1.02 | (1.00–1.04) | 0.018 | 1.02 | (1.01–1.04) | 0.013 | |
| Accra, Ghana | 0.42 | (0.29–0.60) | <0.001 | 0.27 | (0.17–0.43) | <0.001 | 0.28 | (0.18–0.45) | <0.001 | |
| Kumasi, Ghana | 0.39 | (0.23–0.67) | 0.001 | 0.30 | (0.16–0.53) | <0.001 | 0.14 | (0.06–0.33) | <0.001 | |
| Enugu, Nigeria | 1.32 | (0.98–1.78) | 0.068 | 0.82 | (0.57–1.20) | 0.310 | 0.85 | (0.59–1.25) | 0.413 | |
| Ibadan, Nigeria | 2.09 | (1.58–2.76) | <0.001 | 0.91 | (0.64–1.30) | 0.610 | 0.94 | (0.66–1.35) | 0.746 | |
| Lagos, Nigeria | 0.21 | (0.10–0.42) | <0.001 | 0.14 | (0.07–0.30) | <0.001 | 0.15 | (0.07–0.31) | <0.001 | |
| Creatinine > 177 µmol/L | Age (Years) | 1.05 | (1.02–1.08) | <0.001 | 1.03 | (1.00–1.06) | 0.036 | 1.03 | (1.00–1.06) | 0.049 |
| Sex (Ref = males) | 0.36 | (0.20–0.63) | <0.001 | 0.41 | (0.22–0.77) | 0.005 | 0.41 | (0.22–0.78) | 0.007 | |
| BMI (Kg/m2) | 0.96 | (0.91–1.02) | 0.201 | 1 | (0.94–1.07) | 0.940 | 1 | (0.94–1.07) | 0.897 | |
| Duration of T2D | 1.07 | (1.04–1.10) | <0.001 | 1.05 | (1.02–1.08) | 0.004 | 1.06 | (1.02–1.09) | 0.001 | |
| Accra, Ghana | 0.61 | (0.29–1.31) | 0.205 | 0.78 | (0.27–2.25) | 0.645 | 0.84 | (0.29–2.45) | 0.746 | |
| Kumasi, Ghana | 0.3 | (0.07–1.26) | 0.1 | 0.56 | (0.12–2.67) | 0.466 | 0.31 | (0.04–2.49) | 0.269 | |
| Enugu, Nigeria | 1.46 | (0.78–2.76) | 0.24 | 1.58 | (0.65–3.83) | 0.316 | 1.68 | (0.68–4.10) | 0.259 | |
| Ibadan, Nigeria | 2.4 | (1.34–4.28) | 0.003 | 1.86 | (0.81–4.27) | 0.143 | 1.84 | (0.79–4.26) | 0.157 | |
| Lagos, Nigeria | 0.32 | (0.08–1.32) | 0.115 | 0.36 | (0.08–1.72) | 0.202 | 0.38 | (0.08–1.80) | 0.223 |
uOR, unadjusted odds ratio from logistic regression model.
aOR, adjusted odds ratio from logistic regression model including all variables listed.
aOR, adjusted odds ratio from logistic regression model including all variables listed, plus glucose control (yes/no). The reference site is Eldoret (Kenya). eGFR estimated glomerular filtration rate.
Table 4.
Odds ratio associated with risk factors for clinically-assessed ocular complications of T2D in sub-Saharan Africa.
| Complication/comorbidity | Risk factor | uORa | 95% CI | P-value | aORb | 95% CI | P-value | aORc | 95% CI | P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| Cataracts | Age (Years) | 1.09 | (1.07–1.10) | <0.001 | 1.09 | (1.08–1.10) | <0.001 | 1.09 | (1.08–1.10) | <0.001 |
| Sex (Ref = males) | 0.94 | (0.78–1.14) | 0.529 | 1.15 | (0.91–1.44) | 0.236 | 1.22 | (0.96–1.55) | 0.100 | |
| BMI (Kg/m2) | 1.06 | (1.05–1.08) | <0.001 | 0.97 | (0.95–0.99) | 0.005 | 0.97 | (0.95–0.99) | 0.014 | |
| Duration of T2D | 1.56 | (1.22–1.99) | <0.001 | 1.03 | (1.01–1.05) | <0.001 | 1.03 | (1.01–1.05) | 0.001 | |
| Accra, Ghana | 0.69 | (0.51–0.95) | 0.024 | 3.24 | (2.27–4.62) | <0.001 | 3.36 | (2.35–4.82) | <0.001 | |
| Kumasi, Ghana | 0.7 | (0.54–0.90) | 0.007 | 1.28 | (0.85–1.94) | 0.243 | 1.18 | (0.73–1.91) | 0.504 | |
| Enugu, Nigeria | 0.95 | (0.75–1.19) | 0.627 | 1.08 | (0.76–1.55) | 0.667 | 1.18 | (0.82–1.70) | 0.367 | |
| Ibadan, Nigeria | 2.43 | (1.79–3.31) | <0.001 | 1.09 | (0.79–1.51) | 0.603 | 0.94 | (0.66–1.32) | 0.715 | |
| Lagos, Nigeria | 0.72 | (0.56–0.92) | 0.008 | 4.52 | (2.98–6.85) | <0.001 | 4.81 | (3.13–7.38) | <0.001 | |
| Glaucoma | Age (Years) | 1.03 | (1.02–1.05) | <0.001 | 1.03 | (1.01–1.05) | 0.001 | 1.03 | (1.01–1.05) | 0.004 |
| Sex (Ref = males) | 0.56 | (0.39–0.80) | 0.002 | 0.66 | (0.44–0.97) | 0.036 | 0.55 | (0.36–0.83) | 0.004 | |
| BMI (Kg/m2) | 0.96 | (0.93–0.99) | 0.023 | 0.98 | (0.94–1.02) | 0.228 | 0.98 | (0.94–1.02) | 0.359 | |
| Duration of T2D | 1.03 | (1.01–1.06) | 0.005 | 1.02 | (0.99–1.05) | 0.134 | 1.02 | (0.99–1.05) | 0.170 | |
| Accra, Ghana | 0.91 | (0.56–1.48) | 0.697 | 0.32 | (0.18–0.55) | <0.001 | 0.31 | (0.18–0.54) | <0.001 | |
| Kumasi, Ghana | 0.67 | (0.34–1.34) | 0.259 | 0.2 | (0.09–0.44) | <0.001 | 0.18 | (0.07–0.45) | <0.001 | |
| Enugu, Nigeria | 0.45 | (0.22–0.88) | 0.021 | 0.14 | (0.07–0.29) | <0.001 | 0.12 | (0.06–0.26) | <0.001 | |
| Ibadan, Nigeria | 0.23 | (0.13–0.42) | <0.001 | 0.08 | (0.04–0.15) | <0.001 | 0.06 | (0.03–0.13) | <0.001 | |
| Lagos, Nigeria | 1.13 | (0.61–2.08) | 0.702 | 0.30 | (0.16–0.59) | <0.001 | 0.30 | (0.16–0.59) | <0.001 | |
| Diabetic retinopathy | Age (Years) | 1.02 | (1.01–1.04) | <0.001 | 1.01 | (1.00–1.03) | 0.095 | 1.02 | (1.00–1.03) | 0.031 |
| Sex (Ref = males) | 0.91 | (0.69–1.19) | 0.493 | 1.07 | (0.80–1.43) | 0.657 | 1.06 | (0.78–1.44) | 0.712 | |
| BMI (Kg/m2) | 1.07 | (1.06–1.09) | <0.001 | 0.97 | (0.94–1.00) | 0.063 | 0.97 | (0.94–1.00) | 0.058 | |
| Duration of T2D | 1.3 | (0.92–1.82) | 0.135 | 1.07 | (1.05–1.09) | <0.001 | 1.07 | (1.05–1.09) | <0.001 | |
| Accra, Ghana | 0.6 | (0.36–1.00) | 0.048 | 1.12 | (0.74–1.71) | 0.586 | 1.09 | (0.71–1.66) | 0.689 | |
| Kumasi, Ghana | 0.77 | (0.53–1.12) | 0.168 | 0.52 | (0.30–0.92) | 0.024 | 0.61 | (0.32–1.18) | 0.141 | |
| Enugu, Nigeria | 0.72 | (0.51–1.01) | 0.059 | 0.63 | (0.40–0.98) | 0.041 | 0.61 | (0.39–0.96) | 0.032 | |
| Ibadan, Nigeria | 1.41 | (0.90–2.19) | 0.13 | 0.5 | (0.33–0.76) | 0.001 | 0.45 | (0.28–0.71) | 0.001 | |
| Lagos, Nigeria | 1.42 | (1.05–1.93) | 0.022 | 1.08 | (0.65–1.80) | 0.764 | 1.11 | (0.66–1.86) | 0.706 |
uOR, unadjusted odds ratio from logistic regression model.
aOR, adjusted odds ratio from logistic regression model including all variables listed.
aOR, adjusted odds ratio from logistic regression model including all variables listed, plus glucose control (yes/no). The reference site is Eldoret (Kenya).
Cataracts was associated with older age, lower BMI and longer known duration of T2D; diabetic retinopathy, and ED were associated with older age and longer known duration of T2D; and glaucoma was associated with older age and male sex (Table 4).
Among complications assessed by medical history (Table 5), visual problems were associated with older age, lower BMI and longer known duration of T2D; diabetic coma with lower BMI and longer known duration of T2D; non-healing ulcers with female sex; amputation with known duration of T2D; stocking or glove numbness with older age, female sex and longer known duration of T2D; and stroke with older age.
Table 5.
Odds ratio associated with risk factors for medical history-ascertained T2D complications and comorbidity in sub-Saharan Africa.
| Complication/comorbidity | Risk factor | uORa | 95% CI | P-value | aORb | 95% CI | P-value | aORc | 95% CI | P-value |
|---|---|---|---|---|---|---|---|---|---|---|
| Diabetic coma | Age (Years) | 1.02 | (1.01–1.03) | 0.004 | 1.01 | (1.00–1.02) | 0.161 | 1.01 | (1.00–1.03) | 0.100 |
| Sex (Ref = males) | 0.84 | (0.63–1.12) | 0.246 | 0.95 | (0.70–1.30) | 0.756 | 1.00 | (0.72–1.38) | 0.983 | |
| BMI (Kg/m2) | 0.96 | (0.93–0.99) | 0.004 | 0.96 | (0.93–0.99) | 0.005 | 0.96 | (0.93–0.99) | 0.008 | |
| Duration of T2D | 1.05 | (1.03–1.07) | <0.001 | 1.04 | (1.02–1.06) | <0.001 | 1.04 | (1.02–1.07) | <0.001 | |
| Accra, Ghana | 1.25 | (0.88–1.78) | 0.218 | 2.05 | (1.17–3.59) | 0.012 | 2.10 | (1.19–3.68) | 0.010 | |
| Kumasi, Ghana | 0.69 | (0.41–1.16) | 0.160 | 1.13 | (0.57–2.22) | 0.731 | 1.13 | (0.54–2.37) | 0.748 | |
| Enugu, Nigeria | 1.19 | (0.81–1.76) | 0.375 | 1.66 | (0.93–2.96) | 0.086 | 1.67 | (0.93–3.00) | 0.084 | |
| Ibadan, Nigeria | 1.30 | (0.97–1.74) | 0.080 | 1.85 | (1.12–3.05) | 0.016 | 1.79 | (1.08–2.97) | 0.025 | |
| Lagos, Nigeria | 0.61 | (0.34–1.12) | 0.110 | 0.92 | (0.44–1.91) | 0.815 | 0.97 | (0.46–2.03) | 0.937 | |
| Visual problems | Age (Years) | 1.00 | (1.00–1.01) | 0.195 | 1.01 | (1.00–1.02) | 0.002 | 1.02 | (1.01–1.03) | <0.001 |
| Sex (Ref = males) | 0.95 | (0.81–1.11) | 0.537 | 0.98 | (0.82–1.17) | 0.82 | 0.99 | (0.82–1.19) | 0.889 | |
| BMI (Kg/m2) | 0.95 | (0.94–0.97) | <0.001 | 0.97 | (0.95–0.98) | <0.001 | 0.97 | (0.95–0.98) | <0.001 | |
| Duration of T2D | 1.03 | (1.02–1.04) | <0.001 | 1.03 | (1.02–1.04) | <0.001 | 1.03 | (1.01–1.04) | <0.001 | |
| Accra, Ghana | 1.34 | (1.10–1.65) | 0.004 | 1.63 | (1.23–2.17) | 0.001 | 1.65 | (1.24–2.21) | 0.001 | |
| Kumasi, Ghana | 3.02 | (2.30–3.98) | <0.001 | 3.19 | (2.27–4.47) | <0.001 | 3.64 | (2.49–5.31) | <0.001 | |
| Enugu, Nigeria | 2.79 | (2.20–3.53) | <0.001 | 2.58 | (1.91–3.49) | <0.001 | 2.62 | (1.93–3.56) | <0.001 | |
| Ibadan, Nigeria | 0.51 | (0.27–0.38) | <0.001 | 0.51 | (0.40–0.66) | <0.001 | 0.43 | (0.33–0.56) | <0.001 | |
| Lagos, Nigeria | 0.87 | (0.66–1.13) | 0.295 | 0.92 | (0.661.28) | 0.622 | 0.91 | (0.65–1.29) | 0.609 | |
| Non-healing ulcers | Age (Years) | 1.00 | (0.98–1.01) | 0.742 | 1.00 | (0.98–1.01) | 0.603 | 1.00 | (0.98–1.02) | 0.775 |
| Sex (Ref = males) | 0.71 | (0.50–1.00) | 0.052 | 0.64 | (0.43–0.93) | 0.020 | 0.57 | (0.38–0.87) | 0.009 | |
| BMI (Kg/m2) | 0.97 | (0.94–1.01) | 0.102 | 0.99 | (0.96–1.03) | 0.730 | 1.01 | (0.97–1.05) | 0.763 | |
| Duration of T2D | 1.02 | (1.00–1.05) | 0.044 | 1.03 | (1.00–1.06) | 0.027 | 1.02 | (1.00–1.05) | 0.098 | |
| Accra, Ghana | 1.17 | (0.75–1.81) | 0.494 | 2.10 | (1.00–4.37) | 0.049 | 2.05 | (0.98–4.29) | 0.058 | |
| Kumasi, Ghana | 2.52 | (1.63–3.88) | 0.000 | 4.17 | (2.01–8.63) | <0.001 | 4.70 | (2.20–10.06) | <0.001 | |
| Enugu, Nigeria | 1.11 | (0.68–1.80) | 0.688 | 1.90 | (0.90–4.04) | 0.100 | 1.84 | (0.86–3.94) | 0.117 | |
| Ibadan, Nigeria | 0.88 | (0.52–1.13) | 0.549 | 1.48 | (0.74–2.94) | 0.263 | 1.07 | (0.52–2.22) | 0.847 | |
| Lagos, Nigeria | 0.55 | (0.25–1.19) | 0.127 | 0.93 | (0.36–2.45) | 0.888 | 0.81 | (0.30–2.24) | 0.691 | |
| Amputation | Age (Years) | 1.03 | (0.99–1.06) | 0.118 | 1.02 | (0.98–1.06) | 0.368 | 1.02 | (0.98–1.06) | 0.282 |
| Sex (Ref = males) | 0.57 | (0.28–1.17) | 0.124 | 0.62 | (0.28–1.37) | 0.239 | 0.65 | (0.29–1.46) | 0.298 | |
| BMI (Kg/m2) | 0.96 | (0.89–1.03) | 0.230 | 0.97 | (0.89–1.05) | 0.391 | 0.98 | (0.90–1.06) | 0.548 | |
| Duration of T2D | 1.08 | (1.04–1.12) | <0.001 | 1.07 | (1.02–1.12) | 0.003 | 1.07 | (1.03–1.12) | 0.002 | |
| Accra, Ghana | 2.07 | (0.94–4.55) | 0.070 | 1.31 | (0.46–3.76) | 0.611 | 1.37 | (0.47–3.97) | 0.565 | |
| Kumasi, Ghana | 0.93 | (0.28–3.09) | 0.907 | 0.73 | (0.18–2.96) | 0.663 | 1.01 | (0.25–4.16) | 0.984 | |
| Enugu, Nigeria | 0.94 | (0.32–2.70) | 0.903 | 0.65 | (0.19–2.25) | 0.492 | 0.70 | (0.20–2.45) | 0.574 | |
| Ibadan, Nigeria | 0.85 | (0.25–1.37) | 0.729 | 0.37 | (0.12–1.12) | 0.077 | 0.34 | (0.11–1.10) | 0.072 | |
| Lagos, Nigeriad | ||||||||||
| Stocking/glove numbness | Age (Years) | 1.02 | (1.01–1.02) | <0.001 | 1.02 | (1.01–1.02) | <0.001 | 1.02 | (1.01–1.03) | <0.001 |
| Sex (Ref = males) | 1.17 | (1.00–1.37) | 0.047 | 1.21 | (1.02–1.44) | 0.03 | 1.26 | (1.05–1.50) | 0.012 | |
| BMI (Kg/m2) | 0.98 | (0.96–0.99) | 0.001 | 0.99 | (0.97–1.00) | 0.126 | 0.99 | (0.97–1.00) | 0.100 | |
| Duration of T2D | 1.03 | (1.02–1.04) | <0.001 | 1.03 | (1.02–1.04) | <0.001 | 1.02 | (1.01–1.04) | <0.001 | |
| Accra, Ghana | 0.76 | (0.62–0.93) | 0.008 | 0.89 | (0.67–1.17) | 0.402 | 0.90 | (0.67–1.19) | 0.446 | |
| Kumasi, Ghana | 2.75 | (2.11–3.59) | <0.001 | 2.80 | (2.02–3.89) | <0.001 | 2.91 | (2.03–4.19) | <0.001 | |
| Enugu, Nigeria | 1.91 | (1.52–2.39) | <0.001 | 1.91 | (1.43–2.56) | <0.001 | 1.88 | (1.40–2.53) | <0.001 | |
| Ibadan, Nigeria | 0.96 | (0.63–0.87) | 0.669 | 0.86 | (0.67–1.09) | 0.210 | 0.77 | (0.60–0.98) | 0.035 | |
| Lagos, Nigeria | 0.50 | (0.38–0.67) | <0.001 | 0.59 | (0.42–0.83) | 0.003 | 0.60 | (0.43–0.86) | 0.005 | |
| TIA/Stroke | Age (Years) | 1.03 | (1.01–1.06) | 0.003 | 1.04 | (1.02–1.07) | 0.001 | 1.04 | (1.01–1.07) | 0.003 |
| Sex (Ref = males) | 1.14 | (0.70–1.86) | 0.592 | 1.10 | (0.65–1.88) | 0.721 | 1.10 | (0.62–1.96) | 0.747 | |
| BMI (Kg/m2) | 0.99 | (0.95–1.04) | 0.711 | 0.99 | (0.94–1.04) | 0.742 | 0.99 | (0.93–1.04) | 0.627 | |
| Duration of T2D | 1.00 | (0.97–1.04) | 0.961 | 0.99 | (0.95–1.03) | 0.512 | 0.99 | (0.95–1.03) | 0.709 | |
| Accra, Ghana | 1.32 | (0.76–2.31) | 0.325 | 5.68 | (1.62–19.91) | 0.007 | 5.71 | (1.62–20.10) | 0.007 | |
| Kumasi, Ghana | 1.89 | (1.04–3.43) | 0.038 | 7.89 | (2.22–28.06) | 0.001 | 6.94 | (1.83–26.32) | 0.004 | |
| Enugu, Nigeria | 1.61 | (0.91–2.85) | 0.099 | 6.16 | (1.76–21.53) | 0.004 | 6.24 | (1.78–21.84) | 0.004 | |
| Ibadan, Nigeria | 0.79 | (0.44–1.41) | 0.425 | 3.23 | (0.93–11.26) | 0.066 | 2.37 | (0.65–8.72) | 0.193 | |
| Lagos, Nigeria | 0.67 | (0.27–1.69) | 0.400 | 3.15 | (0.74–13.34) | 0.12 | 2.53 | (0.56–11.48) | 0.229 | |
| Erectile dysfunctione | Age (Years) | 1.02 | (1.00–1.03) | 0.007 | 1.02 | (1.00–1.03) | 0.007 | 1.02 | (1.01–1.04) | 0.001 |
| BMI (Kg/m2) | 0.98 | (0.95–1.01) | 0.186 | 0.99 | (0.96–1.03) | 0.647 | 1.00 | (0.97–1.04) | 0.804 | |
| Duration of T2D | 1.05 | (1.03–1.07) | <0.001 | 1.05 | (1.03–1.07) | <0.001 | 1.04 | (1.02–1.07) | <0.001 | |
| Accra, Ghana | 0.98 | (0.67–1.44) | 0.906 | 1.10 | (0.68–1.78) | 0.709 | 1.03 | (0.63–1.68) | 0.904 | |
| Kumasi, Ghana | 3.38 | (2.08–5.49) | 0.000 | 3.62 | (2.02–6.48) | <0.001 | 3.52 | (1.88–6.57) | <0.001 | |
| Enugu, Nigeria | 1.33 | (0.94–1.88) | 0.106 | 1.30 | (0.83–2.04) | 0.243 | 1.27 | (0.81–1.99) | 0.306 | |
| Ibadan, Nigeria | 0.61 | (0.33–0.58) | 0.001 | 0.51 | (0.34–0.77) | 0.001 | 0.39 | (0.25–0.60) | <0.001 | |
| Lagos, Nigeria | 1.59 | (1.08–2.35) | 0.020 | 1.62 | (0.99–2.64) | 0.055 | 1.54 | (0.93–2.55) | 0.095 |
uOR, unadjusted odds ratio from logistic regression model.
aOR, adjusted odds ratio from logistic regression model including all variables listed.
aOR, adjusted odds ratio from logistic regression model including all variables listed, plus glucose control (yes/no).
No amputations reported.
Men only. The reference site is Eldoret (Kenya).
4.5. Prevalence of T2D complications and comorbidity by site
We assessed the variation in prevalence across study sites of the most common complications and comorbidities (Figs. 1 and 2). After adjusting for the effect of age, sex and known duration of T2D, there was significant variation in the prevalence of hypertension, obesity, raised TG, raised TC and raised LDL across sites as assessed by the I2 statistic (I2 ranging from 74% to 98%) – Fig. 1. A closer examination revealed that this difference was primarily driven by one site (Eldoret, Kenya) for raised TG and raised TC. Other features also showed substantial variation between sites, including the prevalence of visual problems, from 29% in Ibadan (Nigeria) to 74% in Kumasi (Ghana); stocking/glove numbness, from 33% in Lagos (Nigeria) to 70% in Kumasi (Ghana); ED, from 22% in Ibadan (Nigeria) to 67% in Kumasi (Ghana), and cataract from 21% in Eldoret (Kenya) to 55% in Lagos (Nigeria) – Fig. 2. The prevalence of diabetic retinopathy, abnormal knee jerk reflex and abnormal ankle jerk reflex varied less than the preceding complications and comorbidities.
Fig. 1.
Prevalence of selected comorbidities and complications of type 2 diabetes across sites in sub-Saharan Africa.
Fig. 2.
Prevalence of selected comorbidities and complications of type 2 diabetes across sites in sub-Saharan Africa.
4.6. Sensitivity analysis comparing prevalence of comorbidities between T2D cases and non-diabetes controls
The design of this study which enrolled both cases and controls enabled the conduct of sensitivity analysis comparing T2D cases with controls to evaluate the impact of non-diabetes risk factors on comorbid parameters. To aid the interpretation of comparisons, we assessed potential differences in the socio-demographic and behavioural characteristics of cases and controls; the distribution of sex, education, smoking and alcohol consumption was similar between the groups, whereas cases were older with mean age 56 (SD 11) years compared with 45 (SD 16) years among controls (Table 1). The age- and sex-adjusted prevalence of hypertension, overweight, raised TG and raised TC was higher in cases than controls. Similarly, comorbidities including visual problems, jaundice, stroke, cancer and kidney disease, though less common, were more prevalent in cases compared with controls. More crude- and age-sex adjusted results of the sensitivity analysis are shown in the Supplementary Table 2.
4.7. Population-attributable fraction of comorbidities
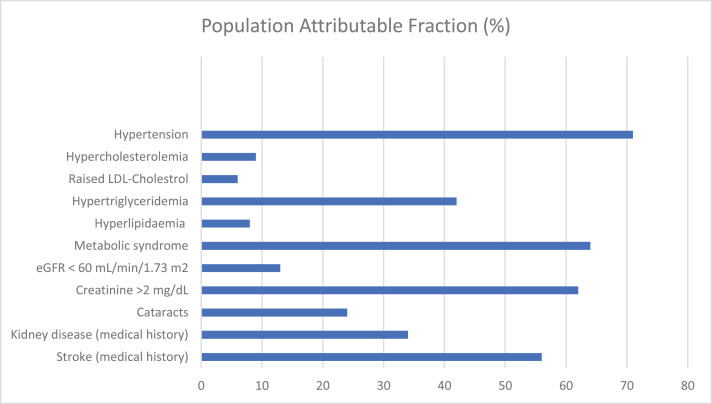
As previously noted, most T2D-associated complications and comorbidities can be due to other causes. To estimate the effect of T2D on the prevalence of the measures we studied, we used the population-attributable fraction (PAF). The population attributable fraction (PAF) is a measure that is widely used to assess the public health impact of risk factors or exposures in populations. Conceptually, it is defined as the fraction of all occurrence of a disease or other adverse condition in a population that is attributable to a specific exposure. Therefore, in the present study, PAF provides an estimate of the proportional reduction in the prevalence of the comorbidities that would occur if T2D were reduced to an alternative ideal exposure scenario (e.g. no T2D). Our findings show that the PAF due to T2D varies between 6% and 64% for most comorbidities studied, with it being higher for such conditions as hypertension, hypertriglyceridemia and the metabolic syndrome (Fig. 3).
Fig. 3.
Population-attributable fraction (PAF) of T2D cardiometabolic complications and comorbidities in sub-Saharan Africa: the AADM Study.
5. Discussion
T2D is currently one of the most important non-communicable disorders in Africa. While there are increasingly better estimates of T2D prevalence in SSA, reliable data on the burden of T2D complications and comorbidity still lags far behind [14], [22]. Estimating the burden and risk factors for T2D complications and comorbidity in SSA is critical because the limited available data shows that only half of people with T2D are diagnosed and only about one in nine patients receive the advice and medication needed to minimize complications [14]. Our findings indicate that the most common T2D comorbidities were hypertension (affecting about three in four persons), visual problems (affecting one in two persons), hyperlipidaemia (raised TC, LDL or TG) and obesity (each affecting about one in three T2D cases). A longer known duration of T2D, higher BMI and older age were important risk factors for most of the complications and comorbidities. These findings have implications for the objective prioritisation of complications for screening among T2D cases for efficient utilisation of scarce health resources in resource limited settings such as SSA.
The large sample size of the present study provided opportunity to present estimates of prevalence of T2D complications and comorbidity with greater precision. Having a control sample of non-T2D adults allowed for the computation of PAFs to facilitate the interpretation of the relative effect of T2D on the prevalence of the complications and comorbidities while the multi-site design enabled us to perform between-site comparisons.
Our findings show important similarities and differences with earlier reports of T2D comorbidity in SSA. Similar to our study, hypertension and raised TC were among the most common T2D comorbidities reported in the Diabcare Africa Study [13]. By contrast the Diabcare Africa study reported a much lower prevalence of raised TG than we report in the current study. Differences in sex distribution, glycaemia control and study design may partly explain the differences between the two studies. Sex differences in the relative risk of diabetes complications may be due to sex differences in health seeking behaviour as well as sexual dimorphism in body composition and fat distribution in addition to other cardiometabolic pathways [23]. Additionally, the extent of glycaemic control between the present study and the Diabcare Africa Study cannot be directly compared because of differences in assessment methods. Therefore, it is possible that glycaemic control differed between the studies and explains some of the observed differences in prevalence of complications. Further, information on comorbidities in Diabcare Africa Study was limited to individuals who had at least one specialist visit in the 12 months period preceding the study. Thus, the relevant sub sample from which frequency of comorbidities was estimated was substantially smaller than the overall study sample. In contrast, all the above comorbidities (apart from retinopathy by fundoscopy) were screened for in all participants in the current study.
The co-occurrence of T2D, hypertension, overweight and lipid dysregulation (the constellation of features labelled “the metabolic syndrome” [24]) is well established and is understood to be due to the overlap in the underlying aetiology and disease pathways reflecting shared environmental and genetic risk factors [25]. The conditions often interact and reinforce each other leading to a vicious cycle [26], [27]. In this regard, the observation that three-quarters of T2D patients in this study meet the IDF definition of the metabolic syndrome is particularly troubling. Therefore, T2D may exacerbate the effects of the above comorbidities and have important implications for cardiovascular disease risk. For example, the high co-occurrence of hypertension and hyperlipidaemia suggests an increased risk of coronary heart disease and stroke among individuals with T2D relative to the general population [28]. We note that, the observed prevalence of obesity and hyperlipidaemia in the current study, though still undesirable, was lower than has been reported in other populations and environmental contexts [29], [30], [31]. The reason for this is not clear but perhaps reflects population differences in diet, physical activity, or genetic differences leading to a different pathophysiological mechanism linking these comorbid conditions in this population. Future studies are needed to better define and explain these observations.
An example of a comorbidity that is due to several interacting risk factors is ED. Globally, ED is a well-known T2D complication [32] often with devastating effect on the lifestyle of men and is a cause of noncompliance for some ED-implicated antihypertensive medication including some centrally acting agents, beta-blockers and diuretics [33]. In the context of diabetes, ED could result from endothelial dysfunction and microangiopathy due to T2D, from hypertension (which is a frequent comorbidity in T2D) or from antihypertensive treatment [33]. In this regard, it is important to note that in the present study, 68% of T2D men also had hypertension, of whom 34% were on either alpha-methyldopa (a centrally-acting agent), a potassium-sparing diuretic, a thiazide diuretic and/or a beta-blocker. It is therefore not surprising that 35% of men with T2D in this study suffered erectile dysfunction. Risk factors for erectile dysfunction in this study include age, duration of T2D, glycaemic control and study site. Therefore, men with both T2D and hypertension need careful control of both conditions with attention to avoiding (if possible) medications that may increase the risk of this distressing comorbidity.
Ocular complications (including cataract, diabetic retinopathy and maculopathy), neurological complications (including numbness, abnormal knee jerk reflex and abnormal ankle jerk reflex), and erectile dysfunction were the most prevalent T2D complications, with substantial heterogeneity across the three countries, and were associated with longer known duration of T2D, older age and lower BMI. The association of T2D comorbid conditions and complications with older age and longer duration of disease observed in this study is consistent with accumulation of environmental risks and hyperglycaemic injury to the vascular system. Our results show similarities with those of the Diabcare Africa Study, the only major study to have considered T2D complications across more than one SSA country, as well as those of other single-centre studies that identified retinopathy, cataract, neuropathy and microalbuminuria as the most common complications of T2D, with variation in prevalence across countries [13], [34], [35], [36], [37], [38], [39], [40]. Our study adds to this evidence by providing more precise estimates of prevalence being, to our knowledge, the largest study of T2D complications and comorbidity in SSA to date. In addition, the identification of risk factors (including study site) help to depict a more complete picture of T2D complications and comorbidity on the continent.
The observed preponderance of microvascular over macrovascular complications despite a high prevalence of hypertension is consistent with observations in other African ancestry populations. In other studies, it is likely to be due to, at least in part, the short average known duration of T2D prior to diagnosis [41], [42]. In our study settings, T2D is likely to be diagnosed late and unlikely to be adequately controlled after diagnosis as shown by the low rates of glycaemic control observed in the current study [3]. Therefore, the observed high prevalence of microvascular complications may be due to late initiation of diabetes treatment (because of late diagnoses) and poor glycaemia control, which together mean that the chances of developing complications are much higher than indicated by the reported known duration of T2D alone.
In policy terms, there are three key implications of the present study. Firstly, the prevalence of T2D complications and comorbidities is high in SSA populations. Therefore, regular screening is essential to facilitate early detection and intervention. Regular screening for hyperlipidaemia, renal function, ocular and foot complications in all T2D patients (rather than the current one-half to two-thirds of patients even in major specialist centres) is important and the adoption of a consensus screening checklist may facilitate this goal. In the face of limited access to specialists, training of primary/community health workers on how to screen individuals at increased disease risk, and adapting existing health programmes for other diseases (such as HIV) which are well established in many SSA countries for diabetes management may also be useful [22], [43], [44]. Secondly, increasing the capacity to monitor glycaemic control in SSA is crucial. Use of HbA1c to monitor glycaemic control is still not routine in much of SSA, including the three countries in which our study was conducted. Thirdly, there is much heterogeneity between and within-countries in the frequency and risk factors for many T2D complications and comorbidities. This may indicate the need for local prioritisation of resources for screening and intervention.
Further research is needed to provide additional insight into the variation observed between the study sites in the three countries with respect to prevalence and risk factors of T2D complications and comorbidities. While a potential explanation may be differences in accuracy of medical examinations and data verification between sites, we sought to minimize these factors by careful standardization of data collection procedures. Other potential explanations include differences in access to clinical care, healthcare resources, diagnostic criteria, and definitions used in routine clinical practice between sites [45]. We did not measure these factors and could not verify their impact on heterogeneity in the present study, empirical data are needed to investigate these issues. Larger studies that includes more SSA geographical regions and adds more potential explanatory variables will be most useful in providing a detailed description of variation in T2D complications and comorbidity across Africa.
The present study has several important strengths, including a large sample size, standardized data collection procedures, inclusion of non-T2D controls and the use of multiple modalities to assess T2D complications and comorbidity. However, there are also a few limitations. T2D cases included in this study were enrolled from medical centres which may limit the generalizability of our findings to the general population, especially in a continent where it is estimated that half of all T2D cases are undiagnosed [14]. The cross-sectional design of the current study means that a temporal relationship between T2D and complications is not established. Finally, use of fasting glucose as an indicator of glycaemic control is sub-optimal compared to HbA1C. However, HbA1c assays are still not routinely available for most of SSA. Population based prospective studies are needed to overcome these limitations.
In summary, our findings point to a high prevalence of complications and comorbidity in patients with T2D in SSA despite reported treatment and a relatively short reported known duration of T2D diagnosis. Age, BMI, known duration of T2D diagnosis, glycaemic control and study site were key risk factors for the evaluated complications and comorbidities. Importantly, these findings suggest the need to strengthen interventions that promote early detection of T2D and ensure optimal glycaemia control to reduce T2D complications and comorbidity in SSA. Such interventions need to prioritize the maintenance of healthy blood pressure, weight and lipid levels, as well as strengthen health system capacities to detect and treat neurological and ophthalmological complications of T2D.
Authors’ contributions
Conceptualization and Funding acquisition: CR, AA, GD, and FC; Investigation: CR, AA, OF, GO, BE, KA, JA, WB, AA, JA, TJ, JO, and CA; Methodology, Data curation and Formal analysis: KE, AD, AB, GC, JZ, and DS; Writing - original draft: KE and AA; Writing - review and editing: CR, AA, and KE. All authors reviewed and approved the manuscript.
CRediT authorship contribution statement
Kenneth Ekoru: . Ayo Doumatey: . Amy R. Bentley: . Guanjie Chen: . Jie Zhou: . Daniel Shriner: . Olufemi Fasanmade: . Godfrey Okafor: . Benjamin Eghan: . Kofi Agyenim-Boateng: . Jokotade Adeleye: . Williams Balogun: . Albert Amoah: . Joseph Acheampong: . Thomas Johnson: . Johnnie Oli: . Clement Adebamowo: . Francis Collins: Conceptualization, Funding acquisition. Georgia Dunston: Conceptualization, Funding acquisition. Adebowale Adeyemo: Conceptualization, Funding acquisition, Investigation. Charles Rotimi: Conceptualization, Funding acquisition, Investigation.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgments
Acknowledgements
The authors acknowledge with thanks and sadness for the untimely passing of our friend and colleague, Prof Duncan Ngare, who was the lead investigator for the Kenya site. We are also thankful to the participants in the AADM project, their families and their physicians. The study was supported in part by the Intramural Research Program of the National Institutes of Health (NIH) in the Center for Research on Genomics and Global Health (CRGGH). The CRGGH is supported by the National Human Genome Research Institute (NHGRI), the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the Office of the Director at the NIH (1ZIAHG200362). Support for participant recruitment and initial genetic studies of the AADM study was provided by NIH grant No. 3T37TW00041-03S2 from the Office of Research on Minority Health.
Funding
The funders had no role in study design, data collection, data analysis, interpretation, or writing of the paper.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2019.09.001.
Contributor Information
Adebowale Adeyemo, Email: adeyemoa@mail.nih.gov.
Charles Rotimi, Email: rotimic@mail.nih.gov.
Appendix. Supplementary materials
References
- 1.NCD Risk Factor Collaboration (NCD-RisC) Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4.4 million participants. Lancet (London, England) 2016;387(10027):1513–1530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jaffar S., Gill G. The crisis of diabetes in sub-Saharan Africa. Lancet Diabetes Endocrinol. 2017;5(8):574–575. doi: 10.1016/S2213-8587(17)30219-X. [DOI] [PubMed] [Google Scholar]
- 3.International Diabetes Federation . 8th edn. International Diabetes Federation; Brussels: 2017. Diabetes Atlas.http://www.idf.org/diabetesatlas Available from: [Google Scholar]
- 4.Beckman J.A., Creager M.A., Libby P. Diabetes and atherosclerosis: epidemiology, pathophysiology, and management. JAMA. 2002;287(19):2570–2581. doi: 10.1001/jama.287.19.2570. [DOI] [PubMed] [Google Scholar]
- 5.Kerr E.A., Heisler M., Krein S.L. Beyond comorbidity counts: how do comorbidity type and severity influence diabetes patients' treatment priorities and self-management? J Gen Intern Med. 2007;22(12):1635–1640. doi: 10.1007/s11606-007-0313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caughey G.E., Roughead E.E., Vitry A.I., McDermott R.A., Shakib S., Gilbert A.L. Comorbidity in the elderly with diabetes: identification of areas of potential treatment conflicts. Diabetes Res Clin Pract. 2010;87(3):385–393. doi: 10.1016/j.diabres.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 7.Gijsen R., Hoeymans N., Schellevis F.G., Ruwaard D., Satariano W.A., van den Bos G.A. Causes and consequences of comorbidity: a review. J Clin Epidemiol. 2001;54(7):661–674. doi: 10.1016/s0895-4356(00)00363-2. [DOI] [PubMed] [Google Scholar]
- 8.Piette J.D., Kerr E.A. The impact of comorbid chronic conditions on diabetes care. Diabetes Care. 2006;29(3):725–731. doi: 10.2337/diacare.29.03.06.dc05-2078. [DOI] [PubMed] [Google Scholar]
- 9.Fortin M., Soubhi H., Hudon C., Bayliss E.A., van den Akker M. Multimorbidity's many challenges. BMJ. 2007;334(7602):1016–1017. doi: 10.1136/bmj.39201.463819.2C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rothe U., Muller G., Schwarz P.E. Evaluation of a diabetes management system based on practice guidelines, integrated care, and continuous quality management in a Federal State of Germany: a population-based approach to health care research. Diabetes Care. 2008;31(5):863–868. doi: 10.2337/dc07-0858. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization . WHO; Geneva: 2008. 2008-2013, Action plan for the global strategy to prevention and control of noncommunicable diseases. World Health Assembly Resolution 2008. [Google Scholar]
- 12.Laiteerapong N., Huang E.S., Chin M.H. Prioritization of care in adults with diabetes and comorbidity. Ann N Y Acad Sci. 2011;1243:69–87. doi: 10.1111/j.1749-6632.2011.06316.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sobngwi E., Ndour-Mbaye M., Boateng K.A. Type 2 diabetes control and complications in specialised diabetes care centres of six sub-Saharan African countries: the Diabcare Africa study. Diabetes Res Clin Pract. 2012;95(1):30–36. doi: 10.1016/j.diabres.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Atun R., Davies J.I., Gale E.A.M. Diabetes in sub-Saharan Africa: from clinical care to health policy. Lancet Diabetes Endocrinol. 2017;5(8):622–667. doi: 10.1016/S2213-8587(17)30181-X. [DOI] [PubMed] [Google Scholar]
- 15.Rotimi C.N., Dunston G.M., Berg K. In search of susceptibility genes for type 2 diabetes in West Africa: the design and results of the first phase of the AADM study. Ann Epidemiol. 2001;11(1):51–58. doi: 10.1016/s1047-2797(00)00180-0. [DOI] [PubMed] [Google Scholar]
- 16.Rotimi C.N., Chen G., Adeyemo A.A. A genome-wide search for type 2 diabetes susceptibility genes in West Africans: the Africa America Diabetes Mellitus (AADM) study. Diabetes. 2004;53(3):838–841. doi: 10.2337/diabetes.53.3.838. [DOI] [PubMed] [Google Scholar]
- 17.Lebovitz H.E., Austin M.M., Blonde L. ACE/AACE consensus conference on the implementation of outpatient management of diabetes mellitus: consensus conference recommendations. Endocrine Pract Official J Am College Endocrinol Am Assoc Clin Endocrinol. 2006;12(Suppl 1):6–12. doi: 10.4158/EP.12.S1.6. [DOI] [PubMed] [Google Scholar]
- 18.Third report of the national cholesterol education program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult treatment panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 19.Adebamowo S.N., Adeyemo A.A., Tekola-Ayele F. Impact of type 2 diabetes on impaired kidney function in sub-saharan african populations. Front Endocrinol. 2016;7:50. doi: 10.3389/fendo.2016.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alberti K.G., Eckel R.H., Grundy S.M. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American Heart Association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 21.Rotimi C.N., Chen G., Adeyemo A.A. Genomewide scan and fine mapping of quantitative trait loci for intraocular pressure on 5q and 14q in West Africans. Invest Ophthalmol Vis Sci. 2006;47(8):3262–3267. doi: 10.1167/iovs.05-1537. [DOI] [PubMed] [Google Scholar]
- 22.Glezeva N., Chisale M., McDonald K., Ledwidge M., Gallagher J., Watson C.J. Diabetes and complications of the heart in Sub-Saharan Africa: an urgent need for improved awareness, diagnostics and management. Diabetes Res Clin Pract. 2018;137:10–19. doi: 10.1016/j.diabres.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 23.Peters S.A.E., Woodward M. Sex differences in the burden and complications of diabetes. Curr Diab Rep. 2018;18(6):33. doi: 10.1007/s11892-018-1005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarwar N., Gao P., Seshasai S.R. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet (London, England) 2010;375(9733):2215–2222. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheung B.M.Y., Li C. Diabetes and hypertension: is there a common metabolic pathway? Curr Atheroscler Rep. 2012;14(2):160–166. doi: 10.1007/s11883-012-0227-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.National high blood pressure education program working group report on hypertension in diabetes. Hypertension (Dallas, Tex: 1979. 1994;23(2):145–158. discussion 59-60. [PubMed] [Google Scholar]
- 27.Sowers J.R., Epstein M. Diabetes mellitus and associated hypertension, vascular disease, and nephropathy. An update. Hypertension (Dallas, Tex: 1979) 1995;26(6 Pt 1):869–879. doi: 10.1161/01.hyp.26.6.869. [DOI] [PubMed] [Google Scholar]
- 28.James P.A., Oparil S., Carter B.L. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the eighth joint national committee (JNC 8) Jama. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- 29.Suh D.C., Choi I.S., Plauschinat C., Kwon J., Baron M. Impact of comorbid conditions and race/ethnicity on glycemic control among the us population with type 2 diabetes, 1988-1994 to 1999-2004. J Diabetes Complicat. 2010;24(6):382–391. doi: 10.1016/j.jdiacomp.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Alonso-Morán E., Orueta J.F., Esteban J.I.F. The prevalence of diabetes-related complications and multimorbidity in the population with type 2 diabetes mellitus in the basque country. BMC Public Health. 2014;14:1059. doi: 10.1186/1471-2458-14-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jelinek H.F., Osman W.M., Khandoker A.H. Clinical profiles, comorbidities and complications of type 2 diabetes mellitus in patients from United Arab Emirates. BMJ Open Diabetes Res Care. 2017;5(1) doi: 10.1136/bmjdrc-2017-000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maiorino M.I., Bellastella G., Esposito K. Diabetes and sexual dysfunction: current perspectives. Diabetes Metab Syndr Obes. 2014;7:95–105. doi: 10.2147/DMSO.S36455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manolis A., Doumas M. Antihypertensive treatment and sexual dysfunction. Curr Hypertens Rep. 2012;14(4):285–292. doi: 10.1007/s11906-012-0276-5. [DOI] [PubMed] [Google Scholar]
- 34.Mbanya J.C., Sobngwi E. Diabetes in Africa. Diabetes microvascular and macrovascular disease in Africa. J Cardiovasc Risk. 2003;10(2):97–102. doi: 10.1097/01.hjr.0000060842.48106.78. [DOI] [PubMed] [Google Scholar]
- 35.Hall V., Thomsen R.W., Henriksen O., Lohse N. Diabetes in Sub Saharan Africa 1999-2011: epidemiology and public health implications. A systematic review. BMC Public Health. 2011;11:564. doi: 10.1186/1471-2458-11-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen D.B., Allain T.J., Glover S. A survey of the management, control, and complications of diabetes mellitus in patients attending a diabetes clinic in Blantyre, Malawi, an area of high HIV prevalence. Am J Trop Med Hyg. 2010;83(3):575–581. doi: 10.4269/ajtmh.2010.10-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Glover S.J., Burgess P.I., Cohen D.B. Prevalence of diabetic retinopathy, cataract and visual impairment in patients with diabetes in sub-Saharan Africa. Br J Ophthalmol. 2012;96(2):156–161. doi: 10.1136/bjo.2010.196071. [DOI] [PubMed] [Google Scholar]
- 38.Unadike B.C., Eregie A., Ohwovoriole A.E. Prevalence of hypertension amongst persons with diabetes mellitus in Benin City, Nigeria. Niger J Clin Pract. 2011;14(3):300–302. doi: 10.4103/1119-3077.86772. [DOI] [PubMed] [Google Scholar]
- 39.Olamoyegun M., Ibraheem W., Iwuala S., Audu M., Kolawole B. Burden and pattern of micro vascular complications in type 2 diabetes in a tertiary health institution in Nigeria. Afr Health Sci. 2015;15(4):1136–1141. doi: 10.4314/ahs.v15i4.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jingi A.M., Noubiap J.J., Ellong A., Bigna J.J., Mvogo C.E. Epidemiology and treatment outcomes of diabetic retinopathy in a diabetic population from Cameroon. BMC Ophthalmol. 2014;14:19. doi: 10.1186/1471-2415-14-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grant R.W. Invited commentary: untangling the web of diabetes causality in African Americans. Am J Epidemiol. 2007;166(4):388–390. doi: 10.1093/aje/kwm187. discussion 91-2. [DOI] [PubMed] [Google Scholar]
- 42.Levitt N.S. Diabetes in africa: epidemiology, management and healthcare challenges. Heart (British Cardiac Society) 2008;94(11):1376–1382. doi: 10.1136/hrt.2008.147306. [DOI] [PubMed] [Google Scholar]
- 43.Katz I., Schneider H., Shezi Z. Managing type 2 diabetes in Soweto-The South African chronic disease outreach program experience. Prim Care Diabetes. 2009;3(3):157–164. doi: 10.1016/j.pcd.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 44.Allain T.J., van Oosterhout J.J., Douglas G.P. Applying lessons learnt from the 'DOTS' tuberculosis model to monitoring and evaluating persons with diabetes mellitus in Blantyre, Malawi. Trop Med Int Health. 2011;16(9):1077–1084. doi: 10.1111/j.1365-3156.2011.02808.x. [DOI] [PubMed] [Google Scholar]
- 45.Litwak L., Goh S.Y., Hussein Z., Malek R., Prusty V., Khamseh M.E. Prevalence of diabetes complications in people with type 2 diabetes mellitus and its association with baseline characteristics in the multinational A1chieve study. Diabetol Metab Syndr. 2013;5(1):57. doi: 10.1186/1758-5996-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.