Abstract
Background
Paclitaxel-coated balloons (DCB) are suitable to reduce the risk of restenosis after angioplasty of atherosclerotic femoropopliteal lesions. However, numerous types of DCBs are distinguished by drug density and coating. Conflicting evidence exists about the risk of mortality. This study sought to evaluate benefit and risk of DCB angioplasty compared to plain old balloon angioplasty (POBA).
Methods
Randomised trials published between January 1, 2005 and February 3, 2019 were identified by searching MEDLINE, CENTRAL, and Clinical.trials.gov. Studies on DCB versus POBA for the treatment of femoropopliteal artery disease were included, and those focused on in-stent restenosis or critical limb ischemia were excluded. Random-effects meta-analysis was conducted to assess the main outcomes of freedom from target lesion revascularisation (FfTLR) and all-cause mortality.
Findings
Of 552 identified records, 14 studies including 2504 patients were eligible. DCB significantly increased the risk of FfTLR with substantial heterogeneity (12-month: risk ratio [RR] 1·24 [95% CI 1·14–2·27], I2 = 66%; 24-month RR 1·39 [95% CI 1·39–1·52], I2 = 21%). The risk of 24-month all-cause mortality was increased after DCB (random-effects model: RR 1·53 [95% CI 0·94–2·50], p = 0·09; fixed-effect model: RR 1·74 [95% CI 1·08–2·81], p = 0·02).
Interpretation
Efficacy of DCB differs substantially across studies. Effect size depends on the type of DCB, treatment strategy, and lesion complexity. The risk of 2-year all-cause mortality at 2 years was increased, but without evidence of causation.
Keywords: Angioplasty, Intermittent claudication, Meta-analysis, Paclitaxel, Peripheral artery disease
1. Introduction
Growing prevalence of atherosclerotic peripheral artery disease (PAD) with more than 202 million patients worldwide is associated with increased disability and mortality over the last decades [1,2]. In patients with intermittent claudication, the femoropopliteal artery segment is most frequently involved [3]. Nowadays, endovascular strategies have been established as first-line therapy for revascularisation. In particular, paclitaxel-coated balloon (DCB) angioplasty yielded encouraging results as compared with plain old balloon angioplasty (POBA) in the treatment of femoropopliteal lesions. Paclitaxel, applied to the inner side of the artery wall during balloon inflation, is intended to prevent neointimal proliferation, the main cause of restenosis [4]. To date, there are around 15 different types of DCBs on the market that differ in paclitaxel density, excipient, and integrity of the coating, resulting in different drug tissue concentrations and maintenance [5].
Previous meta-analyses of randomised controlled trials (RCT) on DCB angioplasty versus POBA stated a significant reduction of the incidence of binary restenosis and target lesion revascularisation with DCB angioplasty without a difference in death or amputation up to five years. However, these meta-analyses included only a fraction of current RCTs [6], [7], [8] and included trials that focused on in-stent restenosis or infrapopliteal disease [9], [10], [11]. Moreover, due to a recent meta-analysis on 28 RCTs that reports on an increased risk of late all-cause mortality after treatment with paclitaxel-coated balloons and stents, conflicting evidence exists about safety [12].
This study was initiated to update findings from previous studies and to focus on de-novo femoropopliteal artery disease. The study aimed to evaluate the efficacy of DCB angioplasty with particular regard to heterogeneity across studies and its potential sources. The study also aimed to examine the effect of DCB angioplasty on all-cause mortality.
2. Methods
2.1. Search strategy and selection criteria
This systematic review and meta-analysis was conducted according to the Preferred Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [13]. Randomised controlled clinical trials reporting on efficacy and safety of DCB angioplasty compared POBA for the treatment of de-novo femoropopliteal artery disease were included. Only trials registered in a clinical trial registry and published in a peer-reviewed journal were eligible. Studies on aortoiliac or infrapopliteal disease, or those limited to critical limb ischemia or in-stent restenosis, or permitted the use of angioplasty devices other than paclitaxel-coated balloons, uncoated balloons, or bare-metal stents, as well as additional drugs not yet part of routine clinical practice were excluded.
We searched MEDLINE, the Cochrane controlled register of trials (CENTRAL), and web-based platforms of specialised journals. In a further step, we checked ClinicalTrials.gov and grey literature for study protocols and reports. Initial search period was between January 1, 2005 and August 28, 2018. No language restriction was imposed. The search was developed and conducted by CK and UT. Start time of the search was chosen because the first in vivo testing of DCB was published in 2004 [4]. We updated the search using the same criteria on February 3, 2019. Titles were screened by two, and abstracts by three reviewers who subsequently assessed full-text versions of selected articles for eligibility. Differences of opinion were resolved through discussion. Full MEDLINE search strategy can be found in the appendix (Table 1, p2).
2.2. Data analysis
Data were extracted by one reviewer (CK) and double-checked by a second reviewer (UT). At a later time, extracted data were independently checked and verified by the remaining three authors (TL, RA, NE) to assure accuracy. Duplicates of data were excluded by selecting the publication that provided the most data. Extracted data included: (1) study characteristics; (2) patient, lesion, and procedure characteristics; (3) outcomes of four categories. The first category included clinical efficacy outcomes of 12- and 24-month FfTLR, the incidence of improvement of at least one Rutherford category, the walking impairment questionnaire (WIQ) score, and the EuroQol 5 Dimensions (EQ-5D) score on quality of life at 12 months. The second category included the safety outcomes of 12- and 24-month all-cause mortality and major or minor amputations. The third category included the morphologic efficacy outcomes of six-month late lumen loss (LLL), and 12- and 24-month primary patency. LLL is defined as the change in minimum lumen diameter from the final angiogram to follow-up, and primary patency refers to the absence of recurrent target lesion stenosis > 50% by imaging that is obtained without the need for additional or secondary surgical or endovascular procedures. The fourth category included the hemodynamic efficacy outcome measure of the 12- and 24-month target limb ankle-brachial index (ABI). This meta-analysis and review primarily assessed the first two outcome categories of clinical efficacy and safety. Post-hoc subgroup analyses were conducted of the main efficacy outcome of 12-month FfTLR and the safety outcome of 24-month all-cause mortality. Detailed information on extracted data is reported in the appendix (Tables 2–4, pp 3–8).
To assess the validity of included studies, the risk of bias was assessed open-label by two reviewers (CK, UT) at study and outcome level according to the Cochrane Collaboration's risk of bias assessment tool [14]. The following modifications were applied: (1) unblinded follow-up for subjective outcome measures (e.g. FfTLR) without adjudication by a clinical-events committee was declared as unknown risk of detection bias; (2) the existence of significant differences in baseline patient, lesion, or procedure characteristics was declared as high risk of bias; (3) incomplete reporting of relevant data on loss to follow-up was declared as unclear risk of attrition bias. Reporting bias within studies was assessed by comparison of the reported outcomes with outcomes listed in study protocols or the methods sections of study reports. Finally, information on risk of bias did not cause exclusion of studies or any adjustment of data synthesis. To detect publication bias we visually evaluated the symmetry of a funnel plot regarding 12-month FfTLR and identified studies registered or presented at congresses but not published (appendix Figs. 1 and 2, pp 12–13).
Summary estimates were either risk ratios (RR) or numbers needed to treat (NNT) with their corresponding 95% confidence intervals (CI) for categorical outcome measures, or difference in means for continuous outcome measures. Differences between means of continuous variables were assessed with the t-test, and categorical variables were compared by using Fisher's exact test or Chi-squared test including Yate's correction. The random-effects Mantel-Haenszel meta-analysis method was applied to determine risk ratios of dichotomous outcomes, and the random-effects inverse variance method to determine mean differences of continuous outcomes. Log risk ratios were assessed by random-effects inverse variance meta-regression. Heterogeneity between studies was evaluated by using I2 statistic (0–40%: heterogeneity might not be important, 30–60%: may represent moderate, 50–90% substantial, 75–100% considerable heterogeneity; importance depending on magnitude, strength, and evidence of the treatment effect). Post-hoc sensitivity analysis was conducted to assess robustness of results from the random-effects compared to the fixed-effect analysis (appendix Tables 5–7, pp 9–11). A two-sided p value < 0·05 was considered statistically significant. We used SPSS Statistics (Version 25.0. IBM, Armonk, NY, USA), Review Manager (Version 5.3, The Nordic Cochrane Center, Copenhagen), and Comprehensive Meta-Analysis (Version 3, Biostat, NY, USA) for analysis. This study is registered with ClinicalTrials.gov, Identifier: NCT02927574.
2.3. Role of funding source
There was no funding source for this study. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
3. Results
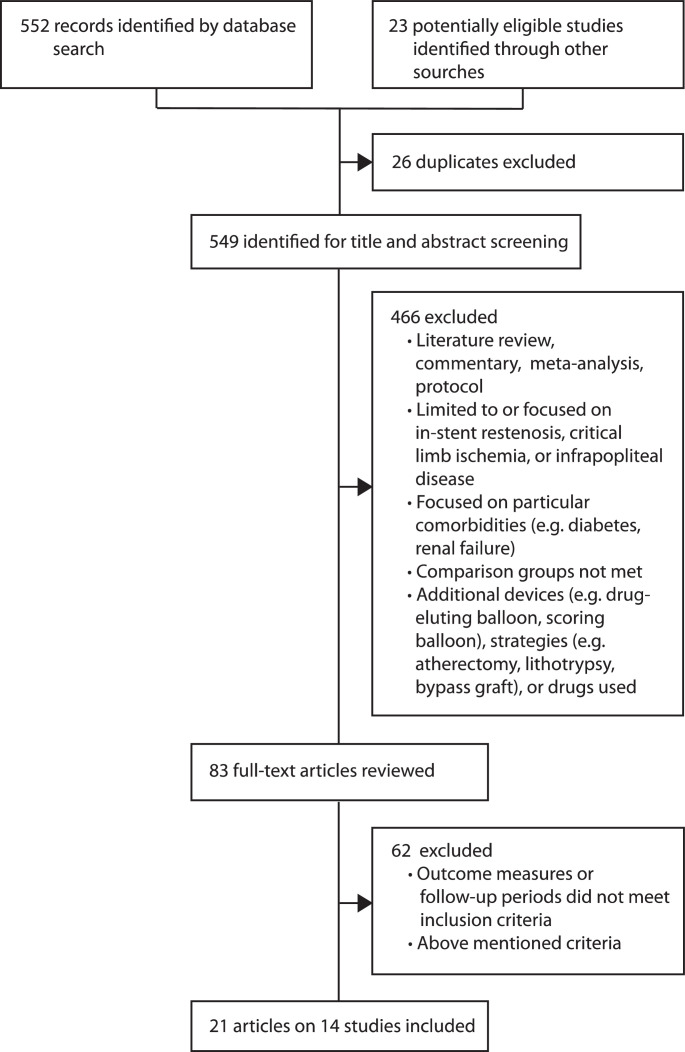
552 articles were identified by search of which 83 relevant publications were selected for full-text review. As a result, 21 publications based on 14 randomised controlled trials, done between 2009 and 2015 were included into this meta-analysis and review (Fig. 1) [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]. Eligible studies conducted in eight countries evaluated 2504 patients with 2571 femoropopliteal lesions treated with nine different DCB types. Patients were randomly assigned to either DCB angioplasty (1524 patients) or POBA (980 patients). Primary outcome of eligible studies was 6-month late lumen loss, 6-month diameter stenosis, 1-year primary patency, or 1-year binary restenosis. The ILLUMENATE EU study additionally defined the composite of procedure related death at 30 days and freedom from target limb major amputation and clinically driven TLR at 12 months as primary safety outcome [22]. In all included studies, DCB angioplasty turned out to be superior to POBA with respect to the primary endpoint (Table 1). Characteristics of the included studies are summarised in Table 1 and characteristics of patients, lesions, and procedures in Table 2 and the appendix Tables 2–4, pp 3–8.
Fig. 1.
Study selection process.
Table 1.
Characteristics of randomised controlled studies included in the meta-analysis.
| Countries (Number of centers) | Enrolment | Primary endpoint (Result) | Latest follow-up | Number of patients (DCB/POBA) | Type of DCB (Manufacturer) | Paclitaxel density (Excipient) | Freedom from 12-month TLR |
All-cause death at 12 months |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DCB | POBA | DCB | POBA | ||||||||
| AcoArt I Jia et al. [15] Xu et al. (2018) [16] |
China (10) | 2013–2014 | Late lumen loss at 6 months (DCB superior) | 2 years | 200 (100/100) |
Orchid® (Acotec Scientific) | 3·0 µg/mm2 (Magnesium stearate) | 93% (90/97) |
60% (58/96) |
2% (2/97) |
2% (2/96) |
|
p < 0·0001 |
p = 0·992 |
||||||||||
| BIOLUX P-1 Scheinert et al. [17] |
Austria Germany (5) |
2010–2011 | Late lumen loss at 6 months (DCB superior) | 1 year | 60 (30/30) |
Passeo-18 Lux® (Biotronik) |
3·0 µg/mm2 (Butyryl-tri-n-hexyl citrate) |
84% (21/25) |
58% (14/24) |
0% (0/25) |
8% (2/26) |
|
p = 0·047 |
p = 0·490 |
||||||||||
| CONSEQUENT Tepe et al. [18] Albrecht et al. [19] |
Germany (10) |
2013–2015 | Late lumen loss at 6 months (DCB superior | 2 years | 153 (78/75) |
Sequent Please OTW® (B.Braun Melsungen) | 3·0 µg/mm2 (Resveratrol) | 82% (60/73) |
62% (43/69) |
3% (2/73) |
1% (1/69) |
|
p = 0·014 |
p = 0·593 |
||||||||||
| DEBATE SFA Liistro et al. [20] |
Italy (1) |
2010–2011 | Binary Restenosis at 1 year (DCB superior) | 1 year | 104 (53/51) |
IN.PACT Admiral® (Medtronic) | 3·5 µg/mm2 (Urea) | 83% (44/53) |
65% (33/51) |
4% (2/53) |
2% (1/51) |
|
p = 0·057 |
p = 0·973 |
||||||||||
| FemPac Werk et al. [21] |
Germany (2) |
2010–2012 | Late lumen loss at 6 months (DCB superior) |
2 years | 87 (45/42) |
Paccocath® coating (Bavaria Medizin Technologie) |
3·0 µg/mm2 (Iopromide) |
NR | NR | NR | NR |
| ILLUMENATE EU Schroeder et al. [22] Brodmann et al. [23] |
Austria Germany (18) |
2012–2015 | Primary patency at 1 year (DCB superior) Composite safety outcome* at 30 days and 1 year (DCB superior) |
2 years | 294 (222/72) |
Stellarex® (Spectranetics) | 2·0 µg/mm2 (Polyetylene glycol) | 94% (193/205) |
83% (50/60) |
1% (2/198) |
2% (1/61) |
|
p = 0·016 |
p = 0·688 |
||||||||||
| ILLUMENATE Pivotal Krishnan et al. [24] |
USA (43) |
2013–2015 | Primary patency at 1 year (DCB superior) | 1 year | 300 (200/100) |
Stellarex® (Spectranetics) |
2·0 µg/mm2 (Polyetylene glycol) |
92% 83% (174/189) (79/95) |
3% (5/192) |
2% (2/96) |
|
|
p = 0·039 |
p = 0·787 |
||||||||||
| IN.PACT SFA Tepe et al. [25] Laird et al. [26] Schneider et al. [27] |
Austria Belgium Germany Italy Switzerland USA (57) |
2010–2013 | Primary patency at 12 months (DCB superior) | 3 years | 311 (220/111) |
IN.PACT Admiral® (Medtronic) | 3·5 µg/mm2 (Urea) | 98% 79% (202/207) (85/107) |
2% (4/207) |
0% (0/107) |
|
|
p < 0·0001 |
p = 0·148 |
||||||||||
| ISAR-STATH Ott et al. [28] |
Germany (2) |
2009–2013 | Diameter stenosis at 6 months (DCB superior) | 2 years | 100 (48/52) |
IN.PACT Admiral® (Medtronic) | 3·5 µg/mm2 (Urea) | 87% (34/39) |
72% (33/46) |
NR | NR |
|
p = 0·142 |
|||||||||||
| LEVANT I Scheinert et al. [29] |
Belgium Germany USA (9) |
2009 | Late lumen loss at 6 months (DCB superior) | 2 years | 101 (49/52) |
Lutonix® (Bard) |
2·0 µg/mm2 (Polysorbate and Sorbitol) | 71% (32/45) |
67% (28/42) |
4% (2/45) |
10% (4/42) |
|
p = 0·829 |
p = 0·609 |
||||||||||
| LEVANT 2 Rosenfield et al. [30] |
Austria, Belgium, Germany USA (54) |
2011–2012 | Primary patency at 1 year (DCB superior) | 1 year | 476 (316/160) |
Lutonix® (Bard) | 2·0 µg/mm2 (Polysorbate and Sorbitol) | 88% 85% (250/285) (121/143) |
2% (7/290) |
3% (4/144) |
|
|
p = 0·373 |
p = 0·820 |
||||||||||
| PACIFIER Werk et al. [31] |
Germany (3) |
2010–2011 | Late lumen loss at 6 months (DCB superior) | 2 years | 85 (44/47) |
IN.PACT Pacific® (Medtronic) | 3·0 µg/mm2 (Urea) | 93% (39/42) |
72% (31/43) |
0% (0/39) |
8% (3/40) |
|
p = 0·026 |
p = 0·248 |
||||||||||
| RANGER SFA Bausback et al. [32] Steiner et al. [33] |
Austria France Germany (10) |
2014–2015 | Late lumen loss at 6 months (DCB superior) | 1 year | 105 (71/34) |
Ranger® (Bosten Scientific, Hemoteq) | 2·0 µg/mm2 (Acety tri-n‑butyl citrat) | 88% (46/52) |
68% (19/28) |
3% (2/71) |
3% (1/34) |
|
p = 0·051 |
p = 0·555 |
||||||||||
| THUNDER Tepe at al [34] Tepe et al. [35] |
Germany (3) |
2004–2005 | Late lumen loss at 6 months (DCB superior) | 5 years | 102 (48/54) |
Paccocath® coating (Bavaria Medizin Technologie) | 3·0 µg/mm2 (Iopromide) | 90% (43/48) |
52% 28/54) |
NR | NR |
|
p < 0·0001 |
|||||||||||
Values are given as% (n/N).
Device or procedure related death at 30 days and freedom from target limb major amputation and clinically driven target lesion revascularisation at 12 months. DCB = drug coated balloon; NR = not reported; POBA = plain old balloon angioplasty; TLR = target lesion revascularisation.
Table 2.
Pooled patient, lesion, and procedure characteristics of included studies.
| Patients | DCB (n = 1524) | POBA (n = 980) | p value |
|---|---|---|---|
| Age (years) | 68·1 ± 9·4 (n = 1479) | 69.0 ± 9·3 (n = 938) | p = 0·021 |
| Male | 64·9% (989) | 66·7% (654) | p = 0·344 |
| Smokera | 56·4% (832/1476) | 58·4% (541/926) | p = 0·322 |
| Diabetes mellitus | 43·4% (662) | 45·3% (444) | p = 0·358 |
| Hypertension | 84·1% (1282) | 77·7% (761) | P < 0·0001 |
| Dyslipidemia | 72·7% (1108) | 67·8% (664) | p = 0·008 |
| Ankle-brachial indexb | 0·70 ± 0·25 (n = 1408) | 0·66 ± 0·28 (n = 904) | p = 0·0002 |
| Critical limb ischemiac | 10·5% (153/1453) | 13·5% (128/946) | p < 0·0001 |
| Lesions | DCB (n = 1568) | POBA (n = 1003) | pvalue |
|---|---|---|---|
| Total lesion length (mm) | 82·2 ± 64·3 (n = 1523) | 87·8 ± 68·0 (n = 961) | p = 0·041 |
| Reference vessel diameter (mm) | 4·8 ± 0·9 (n = 1523) | 4·7 ± 0·9 (n = 961) | p = 0·415 |
| Diameter stenosis (%) | 80·8 ± 15·8 (n = 1523) | 82·2 ± 17·4 (n = 961) | p = 0·045 |
| Calcification | 62·3% (390/626) | 59·7% (264/442) | p = 0·396 |
| Severe calcification | 19·0% (221/1162) | 16·6% (98/591) | p = 0·211 |
| Total occlusion | 23·8% (365/1534) | 32·4% (313/966) | P < 0·0001 |
| Procedures | DCB (n = 1568) | POBA (n = 1003) | pvalue |
|---|---|---|---|
| Predilation | 93·6% (1381/1474) | 86·4% (813/941) | P < 0·0001 |
| Postdilation | 32·1% (350/1090) | 28·3% (155/547) | p = 0·119 |
| Stent implantation | 16·2% (253/1561) | 25·0% (248/991) | P < 0·0001 |
| Residual DS < 30% | 96·1% (1309/1362) | 94·3% (746/791) | p = 0·054 |
| Final DS (%) | 23·8 ± 12·0 (n = 1068) | 23·7 ± 12·5 (n = 661) | p = 0·868 |
| Dissection | 35·1% (387/1104) | 41·2% (235/571) | p = 0·014 |
Values are given as mean ± SD or% (n).
Current or former smoker.
An ankle-brachial index of ≤ 0·9 is the threshold for the diagnosis of peripheral artery disease.
Ischemic rest pain and/or ulceration and/or gangrene. DCB = drug coated balloon; DS = diameter stenosis; POBA = plain old balloon angioplasty.
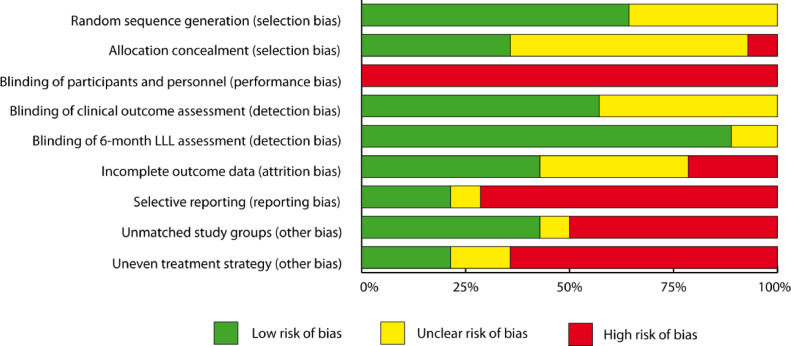
None of the studies blinded participants or personal, thus all studies contained a high risk of performance bias. A high risk of attrition bias due to incomplete outcome data was assigned to three studies [22,28,35] and more than half of all studies had evidence of high risk of reporting bias [15,17,18,20,22,24,25,28,30,33], bias from unmatched treatment groups [15,17,18,20,22,24,25,[29], [30], [31],34], or uneven treatment strategy [15,17,20,22,25,[29], [30], [31],34]. Detailed results on the risk of bias within studies are available on Fig. 2 and appendix Fig. 1, p 12. Visual inspection of the funnel plot showed minor evidence of publication bias with respect to the main efficacy outcome of freedom from 12-month TLR with four trials outside of the designated area of the 95% CI, and no evidence of publication bias concerning 12-month all-cause mortality (Egger test for asymmetry: p = 0.015 and p = 0.814, respectively), (appendix Fig. 2, p 13) [5,22,30,35]. One RCT was registered with ClinicalTrials.gov but not published (NCT02145065) and 24-month results from the LEVANT 2 trial have not been published to date.
Fig. 2.
Risk of bias.
Detection bias regarding the outcome measure of late lumen loss (LLL) was assessed from nine studies that provided results on LLL, all other risks of bias was assessed from all included studies.
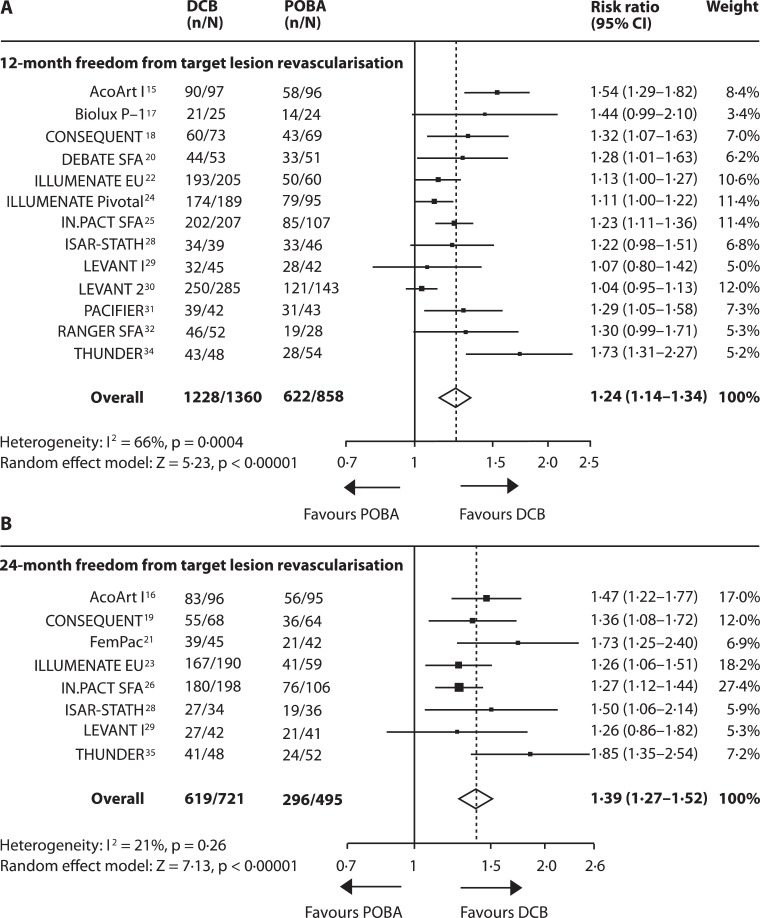
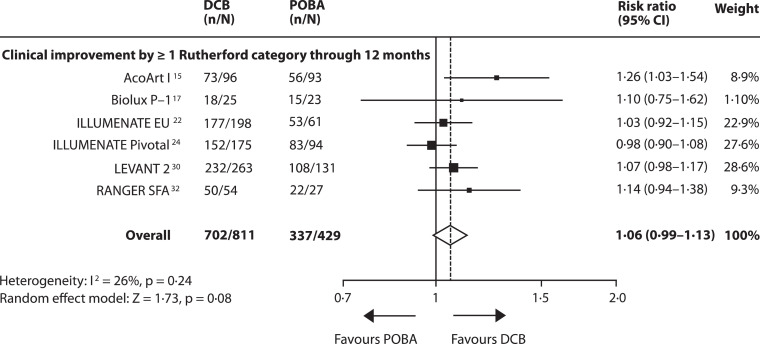
Data on 12-month FfTLR were available from 13 studies including 2218 patients [15], [16], [17], [18], [19], [20],[22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35]. Overall, DCB angioplasty significantly increased the risk of FfTLR by 24% (95% CI 14–34) compared to POBA with a number needed to treat (NNT) of 6 [95% CI 4–9]. However, heterogeneity across studies was substantial (I2 = 66%, p = 0·0004). Efficacy did not reach significance in three studies (ISAR-STATH, LEVANT 1 and LEVANT 2) [28], [29], [30]. The effect of DCB angioplasty on 24-month FfTLR was reported in 8 studies including 1216 patients [15,16,18,19,[21], [22], [23],25,28,29,34]. DCB angioplasty increased the risk of 24-month FfTLR by 39% (95% CI 27–52) and a NNT of 4 [95% CI 3–5], (Fig. 3A and B, appendix Figs. 3, p14). Risk estimates on FfTLR from fixed-effect analysis were similar to those from random-effects analysis (appendix Table 5, p 9). Long-term outcomes were recorded in the IN.PACT SFA study (FfTLR at 3 years: DCB 84·8%, POBA 68·%, p = 0·002) and the THUNDER study (FfTLR at 5 years: DCB group 79%, POBA group 44%, p = 0·0005). DCB angioplasty was associated with a non-significant 6·0% (95% CI −1 to 13) increase in risk of clinical improvement by at least one Rutherford category compared to POBA, (Fig. 4) [15,17,22,24,30,33]. Fixed-effect model showed a significantly increased clinical improvement with DCB (7·0% (95% CI 1–13), p = 0·01 (appendix Table 5, p 9). The WIQ score on walking impairment and the EQ-5D score on health related quality of life at 12 months were not significantly affected by the treatment strategy (WIQ: p = 0·80; EQ-5D: p = 0·11), appendix Fig. 4, p 15) [24,30,33].
Fig. 3.
Forest plots showing the effect of DCB angioplasty versus POBA on freedom from target lesion revascularisation.
Data are presented for the 12-month (A) and 24-month (B) follow-ups. DCB = drug coated balloon angioplasty; POBA = plain old balloon angioplasty.
Fig. 4.
Effect of DCB angioplasty on clinical improvement.
Forest plot illustrates 12-month incidence of clinical improvement by at least one Rutherford category after DCB angioplasty versus POBA. Rutherford classification: category 0 = asymptomatic, category 1 = mild, category 2 = moderate, category 3 = severe claudication, category 4 = ischemic rest pain, category 5 = ischemic ulceration, category 5 = ischemic gangrene, DCB = drug coated balloon angioplasty; POBA = plain old balloon angioplasty.
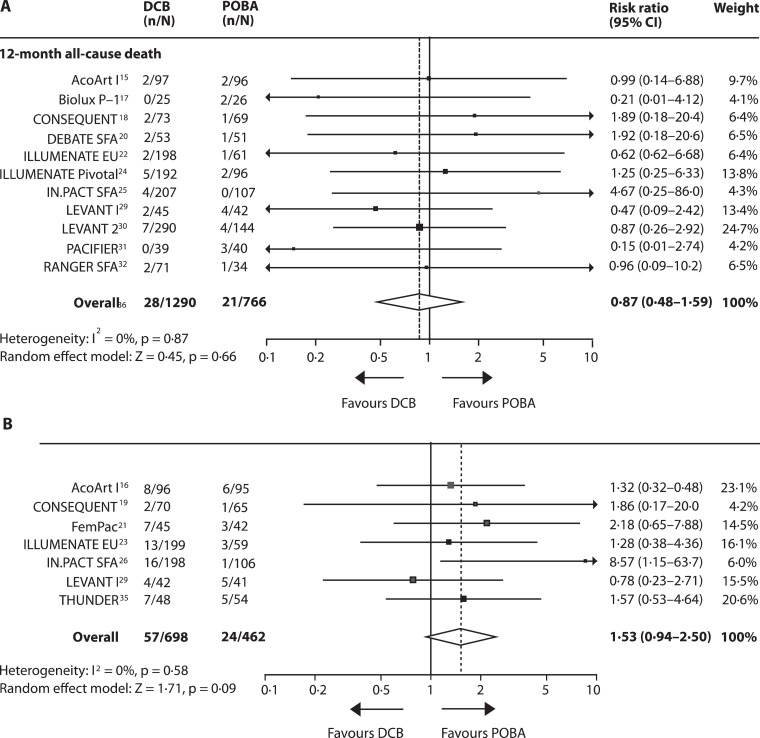
All-cause mortality at 12 months was reported in 11 studies [15,17,18,20,22,24,25,[29], [30], [31],33], including 2056 patients, and all-cause mortality at 24 months in 7 studies [16,19,21,23,27,29,35], including 1160 patients. At 12 months, no significant treatment effect on the risk of all-cause mortality was observed (risk reduction with DCB: 13% [95% CI −52 to 59, p = 0·66]; number needed to harm [NNH]: 285 [95% CI 62 NNH to ∞ to 108 NNT]. At 24 months, DCB angioplasty was associated with an increased risk of death by 53% (95% CI −6 to 150, p = 0·09) and a NNH of 26 (95% CI 16–79). No heterogeneity across studies was observed at 12 and 24 months (Fig. 5A and B, appendix Fig. 3, p14). Running the fixed-effect model for sensitivity analysis revealed a significant increase in risk of 24-month all-cause mortality with DCB (74% [95% CI 8–181], p = 0·02). Long-term data is available from the IN-PACT SFA study (3-year all-cause mortality: DCB 10·7%, POBA 1·9%, p = 0·006, none of the deaths were assigned device or procedure related) [27]. Risk of minor and major amputations was non-significantly increased in patients allocated to DCB angioplasty (12 months: RR 2.1 (95% CI 0·6–7·5), p = 0·26; 24 months: RR 2·3 (95% CI 0·7–7·9),p = 0·17; appendix Fig. 5, p 16).
Fig. 5.
Forest plots showing the effect of DCB angioplasty versus POBA on all-cause death.
Data are presented for the 12-month (A) and 24-month (B) follow-ups. DCB = drug coated balloon angioplasty; POBA = plain old balloon angioplasty.
DCB angioplasty reduced the weighted mean LLL significantly by 0·97 mm (95% CI −1·33 to −0·61), but with substantial heterogeneity across studies (I2 = 78%, p < 0·0001), (appendix Fig. 6, p 17) [15,17,18,21,28,29,32,34], and significantly increased the risk of primary patency by 45% (95% CI 27–66, p < 0·00,001) at 12 months and by 49% (95% CI 25–77, p < 0·00,001) at 24 months with moderate heterogeneity (appendix Fig. 7, p 18). The NNT was 3 (95% CI 2–3) and 4 (95% CI 3–7), respectively (appendix Fig. 3, p 14). Mean ABIs did not differ significantly between treatment strategies (12 months: p = 0·72; 24 months: p = 0·43), (appendix Fig. 8, p 19).
Post-hoc subgroup and meta-regression analyses concerning 12-month FfTLR identified ABI, lesion length, predilation strategy, and paclitaxel density as sources of heterogeneity. Poor hemodynamic condition and longer lesions were associated with a larger DCB efficacy (RR 1·5 versus 1·16; subgroup difference p = 0·002 and RR 1·44 versus 1·19; subgroup difference p = 0·002, respectively). Studies with a higher proportion of predilated lesions or a two-step predilation with DCB angioplasty achieved a larger DCB efficacy (RR 1·34 versus 1·21; subgroup difference p = 0·02). Finally, higher paclitaxel density went along with larger DCB efficacy (RR 1·09 [2 µg/mm2] versus 1·44 [3·0 µg/mm2] versus 1·23 [3·5 µg/mm2]; subgroup difference p < 0·00,001). Studies using DCBs with a higher paclitaxel density showed a tendency to a larger risk of 24-month mortality compared to POBA (RR 1·01 [2 µg/mm2] versus 1·61 [3·0 µg/mm2] versus 8·57 [3·5 µg/mm2]; subgroup difference p = 0·15).
Detailed results can be found in the appendix Figs. 9–23, pp 20–34. . In contrast to the random-effects model, the fixed-effect model revealed a trend for a higher efficacy of DCB in studies that provided balanced proportions of bailout stenting across groups compared to studies with higher stenting rates in POBA lesions (RR 1·21 versus 1·14; subgroup difference p = 0.09). The LEVANT 2 trial was weighted higher with the fixed-effect model (appendix Table 7, p 11).
4. Discussion
This study found a significant increase in the risk of FfTLR after DCB angioplasty compared to POBA up to two years, together with an increased trend to clinical improvement. However, risk of bias within studies and heterogeneity across studies was substantial, calling a class effect of DCB into question. In patients treated with DCB, the risk of all-cause mortality was similar to POBA at 12 months, and increased significantly at 24 months according to fixed-effect analysis but without evidence of causation.
Regarding FfTLR and clinical improvement this study confirmed the previously described favourable impact of DCB angioplasty in general [6], [7], [8], [9],11]. However, heterogeneity mainly due to a considerably stronger effect of DCB angioplasty compared to the overall cohort in two trials (ACOArt I, THUNDER) and a considerably lower effect in another two trials (ILLUMENATE Pivotal, LEVANT 2) was substantial. Stronger efficacy was associated with a lower mean ABI at baseline, longer lesions and a paclitaxel density of ≥ 3·0 µg/mm2 [15,24,30,35]. Heterogeneity was already mentioned by Giacoppo et al. [10]., who reported on a worse efficacy of DCB angioplasty in the LEVANT trials assuming a relation to the type of DCB. Moreover, two previously published meta-analyses found an association between efficacy and paclitaxel density of DCBs [12,36]. A dose-response relationship was also observed in this study, however, due to confounders, particularly lesion complexity and preparation, no causal link could be drawn. Heterogeneity may also be a result of different excipients, however, despite significant subgroup differences, there are too many excipients to conclude a causal link. Besides lesion and device related causes of heterogeneity, even the treatment strategy of predilation may have had potential impact on results. A relation between regular predilation and an increased DCB efficacy is supported by subgroup differences and is suggested by a lower DCB efficacy in two studies that stipulated randomisation only after predilation (LEVANT 2 and ILLUMENATE EU). No subgroup difference with respect to the stenting strategy or frequency was observed in this study. Finally, from current RCTs, effect size cannot necessarily be assigned to specific DCB types.
Effect size could also be influenced by bias within studies. In particular, none of the trials was blinded, with the consequence of a high risk of performance bias throughout all RCTs. Having regard to the device allocated treatment strategy might be in favour of DCB patients. This assumption is supported by the finding of a significantly higher incidence of predilation, and a higher, although not significant incidence of postdilation with DCB angioplasty, and, on the other hand, a greater frequency of dissections and bailout stenting procedures with POBA. Moreover, POBA patients were disadvantaged compared with DCB patients in terms of a lower ABI, a higher incidence of CLI, longer lesions, and more total occlusions. Therefore, in general, efficacy of DCB angioplasty might be overestimated.
None of the included RCTs raised concerns about safety. As with this study, no difference between DCB angioplasty and POBA in the risk of all-cause mortality occurred up to 12 months [6,7,10,11] and the same applies for the risk of amputations. However, severe adverse events and long-term follow-ups were rare, and only some of the RCTs convened a clinical events committee. A recent meta-analysis of trials, including a broad spectrum of PAD reported on a significantly increased risk of all-cause mortality as from 2 years up to 5 years after treatment with paclitaxel-coated balloons or paclitaxel-eluting stents. Moreover, authors addressed the possibility of a causal relationship between mortality and paclitaxel exposure. However, the meta-analysis included paclitaxel-eluting stents, which differ considerably from balloons regarding paclitaxel density and release kinetics [12]. In this study, RR of mortality and NNH at 24 months were similarly increased after DCB angioplasty compared to the meta-analysis mentioned above. The same applies for a trend to a paclitaxel dose-response relationship. A recently published executive summary of the U.S. Food and Drug Administration on this issue also reported on an increased long-term mortality with paclitaxel devices. However, no relationship to the paclitaxel dose could be detected and no underlying pathomechanism was identified [37]. Finally, so far, there is no sufficient evidence for a causal connection between death and paclitaxel coated balloon angioplasty.
The strength of this study resides in the fact that current RCTs and latest published follow-ups were included. Particular attention was drawn to risk of bias and sources of heterogeneity. Due to the present conflicting evidence on safety, this study additionally assessed risks of major safety outcomes. However, this study has some limitations. First, no patient level data were available. Thus, causes of death could not be specified. Second, safety events were rare and none of the included trials was powered to determine significance of differences. Third, potential small study effects limited the evidence base. Fourth, original studies do not provide mean changes and standard deviation of WIQ score, EQ-5D score, and ABI that would have allowed comparison of changes. Fifth, summary data are only provided up to two years. Unpublished long-term results were not considered. Sixth, the outcome of FfTLR is prone to a certain degree of subjectivity since the intervention is finally left to the discretion of investigator and patient. Sixth, calculation was based on proportions, not on Kaplan-Meier estimates. Thus, there was a risk of bias from loss off follow-up. Finally, limitations of original studies were transferred to this study. This study is not registered with PROSPERO.
Future research might benefit from blinded, randomised head to head comparisons between different DCB types under the same conditions to assess superiority of one over the other. One option to standardise treatment conditions would be to randomise patients only after predilation. Long-term data on large cohorts and regression analysis based on patient level data would be desirable. To evaluate, whether and in what manner paclitaxel might endanger health, mortality should be recorded meticulously and analysed on the basis of patient-level data. However, a possible causal relationship has to be ascertained by supplemental pharmacological studies. Results should have implications on treatment recommendation and reimbursement.
5. Research in context
5.1. Evidence before this study
Paclitaxel-coated balloon (DCB) angioplasty is suitable to effectively reduce the risk of binary restenosis and target lesion revascularisation of femoropopliteal lesions compared to standard balloon angioplasty up to 5 years of follow-up. However, clinical efficacy is not proven for all types of DCB. Moreover, there is conflicting evidence on safety of paclitaxel-coated devices due to recently published data on all-cause mortality from 2 years after intervention.
5.2. Added value of this study
This study suggests an overestimation of the effect size of DCB angioplasty due of a high risk of bias, mainly because no blinding and consequential differences in the treatment strategy. Moreover, substantial heterogeneity across studies contradicts the assumption of a DCB class effect. Mandatory predilation, lower ABI, longer lesions, and a higher paclitaxel density are associated with greater efficacy of DCB angioplasty. DCB angioplasty was related to an increased risk of all-cause mortality at 2 years, together with a trend to a paclitaxel dose-response relationship.
5.3. Implications of all the available evidence
Treatment recommendations and reimbursement should not apply generally but for every single DCB type according to its efficacy. Head to head comparisons of DCBs are desirable. Crucial safety events have to be recorded meticulously over the long term and should be analysed based on patient level data. Additional pharmacological studies on safety are needed.
Contributors
UT and CK proposed the hypothesis and idea for this systematic review and developed the analysis plan. CK did the literature search and CK and UT reviewed studies for inclusion. CK performed the data extraction and curation. CK, RA, NE, UT validated the data. TL and CK did the statistical analysis and CK wrote the first draft of the report with input from UT. CK, TL, RA, NE, UT reviewed and interpreted the results and edited the manuscript.
Funding
No funding.
Declaration of Competing Interest
All authors declare no competing interests.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.eclinm.2019.09.004.
Appendix. Supplementary materials
References
- 1.Fowkes F.G., Rudan D., Rudan I. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 2.Sampson U.K., Fowkes F.G., McDermott M.M. Global and regional burden of death and disability from peripheral artery disease: 21 world regions, 1990 to 2010. Glob Heart. 2014;9(1):145–158. doi: 10.1016/j.gheart.2013.12.008. e21. [DOI] [PubMed] [Google Scholar]
- 3.Diehm N., Shang A., Silvestro A. Association of cardiovascular risk factors with pattern of lower limb atherosclerosis in 2659 patients undergoing angioplasty. Eur J Vasc Endovasc Surg. 2006;31(1):59–63. doi: 10.1016/j.ejvs.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 4.Scheller B., Speck U., Abramjuk C., Bernhardt U., Bohm M., Nickenig G. Paclitaxel balloon coating, a novel method for prevention and therapy of restenosis. Circulation. 2004;110(7):810–814. doi: 10.1161/01.CIR.0000138929.71660.E0. [DOI] [PubMed] [Google Scholar]
- 5.Teichgraber U.K., Klumb C. Drug-coated balloon angioplasty in femoropopliteal arteries - is there a class effect? Zentralbl Chir. 2017;142(5):470–480. doi: 10.1055/s-0043-119895. [DOI] [PubMed] [Google Scholar]
- 6.Cassese S., Byrne R.A., Ott I. Paclitaxel-coated versus uncoated balloon angioplasty reduces target lesion revascularization in patients with femoropopliteal arterial disease: a meta-analysis of randomized trials. Circ Cardiovasc Interv. 2012;5(4):582–589. doi: 10.1161/CIRCINTERVENTIONS.112.969972. [DOI] [PubMed] [Google Scholar]
- 7.Fusaro M., Cassese S., Ndrepepa G. Paclitaxel-coated balloon or primary bare nitinol stent for revascularization of femoropopliteal artery: a meta-analysis of randomized trials versus uncoated balloon and an adjusted indirect comparison. Int J Cardiol. 2013;168(4):4002–4009. doi: 10.1016/j.ijcard.2013.06.081. [DOI] [PubMed] [Google Scholar]
- 8.Katsanos K., Kitrou P., Spiliopoulos S., Diamantopoulos A., Karnabatidis D. Comparative effectiveness of plain balloon angioplasty, bare metal stents, drug-coated balloons, and drug-eluting stents for the treatment of infrapopliteal artery disease: systematic review and bayesian network meta-analysis of randomized controlled trials. J Endovasc Ther. 2016;23(6):851–863. doi: 10.1177/1526602816671740. [DOI] [PubMed] [Google Scholar]
- 9.Kayssi A., Al-Atassi T., Oreopoulos G., Roche-Nagle G., Tan K.T., Rajan D.K. Drug-eluting balloon angioplasty versus uncoated balloon angioplasty for peripheral arterial disease of the lower limbs. Cochrane Database Syst Rev. 2016;(8) doi: 10.1002/14651858.CD011319.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giacoppo D., Cassese S., Harada Y. Drug-Coated balloon versus plain balloon angioplasty for the treatment of femoropopliteal artery disease: an updated systematic review and meta-analysis of randomized clinical trials. JACC Cardiovasc Interv. 2016;9(16):1731–1742. doi: 10.1016/j.jcin.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Jongsma H., Bekken J.A., de Vries J.P., Verhagen H.J., Fioole B. Drug-eluting balloon angioplasty versus uncoated balloon angioplasty in patients with femoropopliteal arterial occlusive disease. J Vasc Surg. 2016;64(5):1503–1514. doi: 10.1016/j.jvs.2016.05.084. [DOI] [PubMed] [Google Scholar]
- 12.Katsanos K., Spiliopoulos S., Kitrou P., Krokidis M., Karnabatidis D. Risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the leg: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2018;7(24) doi: 10.1161/JAHA.118.011245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins J.P., Altman D.G., Gotzsche P.C. The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jia X., Zhang J., Zhuang B. Acotec drug-coated balloon catheter: randomized, multicenter, controlled clinical study in femoropopliteal arteries: evidence from the acoart i trial. JACC Cardiovasc Interv. 2016;9(18):1941–1949. doi: 10.1016/j.jcin.2016.06.055. [DOI] [PubMed] [Google Scholar]
- 16.Xu Y., Jia X., Zhang J. Drug-Coated balloon angioplasty compared with uncoated balloons in the treatment of 200 Chinese patients with severe femoropopliteal lesions: 24-Month results of AcoArt I. JACC Cardiovasc Interv. 2018;11(23):2347–2353. doi: 10.1016/j.jcin.2018.07.041. [DOI] [PubMed] [Google Scholar]
- 17.Scheinert D., Schulte K.L., Zeller T., Lammer J., Tepe G. Paclitaxel-releasing balloon in femoropopliteal lesions using a BTHC excipient: twelve-month results from the BIOLUX P-I randomized trial. J Endovasc Ther. 2015;22(1):14–21. doi: 10.1177/1526602814564383. [DOI] [PubMed] [Google Scholar]
- 18.Tepe G., Gogebakan O., Redlich U. Angiographic and clinical outcomes after treatment of femoro-popliteal lesions with a novel paclitaxel-matrix-coated balloon catheter. Cardiovasc Intervent Radiol. 2017;40(10):1535–1544. doi: 10.1007/s00270-017-1713-2. [DOI] [PubMed] [Google Scholar]
- 19.Albrecht T., Waliszewski M., Roca C. Two-Year clinical outcomes of the consequent trial: can femoropopliteal lesions be treated with sustainable clinical results that are economically sound. Cardiovasc Intervent Radiol. 2018;41(7):1008–1014. doi: 10.1007/s00270-018-1940-1. [DOI] [PubMed] [Google Scholar]
- 20.Liistro F., Grotti S., Porto I. Drug-eluting balloon in peripheral intervention for the superficial femoral artery: the DEBATE-SFA randomized trial (drug eluting balloon in peripheral intervention for the superficial femoral artery) JACC Cardiovasc Interv. 2013;6(12):1295–1302. doi: 10.1016/j.jcin.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Werk M., Langner S., Reinkensmeier B. Inhibition of restenosis in femoropopliteal arteries: paclitaxel-coated versus uncoated balloon: femoral paclitaxel randomized pilot trial. Circulation. 2008;118(13):1358–1365. doi: 10.1161/CIRCULATIONAHA.107.735985. [DOI] [PubMed] [Google Scholar]
- 22.Schroeder H., Werner M., Meyer D.R. Low-Dose paclitaxel-coated versus uncoated percutaneous transluminal balloon angioplasty for femoropopliteal peripheral artery disease: one-Year results of the illumenate European randomized clinical trial (randomized trial of a novel paclitaxel-coated percutaneous angioplasty balloon) Circulation. 2017;135(23):2227–2236. doi: 10.1161/CIRCULATIONAHA.116.026493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brodmann M., Werner M., Meyer D.R. Sustainable antirestenosis effect with a low-dose drug-coated balloon: the illumenate European randomized clinical trial 2-Year results. JACC Cardiovasc Interv. 2018;11(23):2357–2364. doi: 10.1016/j.jcin.2018.08.034. [DOI] [PubMed] [Google Scholar]
- 24.Krishnan P., Faries P., Niazi K. Stellarex drug-coated balloon for treatment of femoropopliteal disease: twelve-month outcomes from the randomized illumenate pivotal and pharmacokinetic studies. Circulation. 2017;136(12):1102–1113. doi: 10.1161/CIRCULATIONAHA.117.028893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tepe G., Laird J., Schneider P. Drug-coated balloon versus standard percutaneous transluminal angioplasty for the treatment of superficial femoral and popliteal peripheral artery disease: 12-month results from the IN.PACT SFA randomized trial. Circulation. 2015;131(5):495–502. doi: 10.1161/CIRCULATIONAHA.114.011004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laird J.R., Schneider P.A., Tepe G. Durability of treatment effect using a drug-coated balloon for femoropopliteal lesions: 24-Month results of IN.PACT SFA. J Am Coll Cardiol. 2015;66(21):2329–2338. doi: 10.1016/j.jacc.2015.09.063. [DOI] [PubMed] [Google Scholar]
- 27.Schneider P.A., Laird J.R., Tepe G. Treatment effect of drug-coated balloons is durable to 3 years in the femoropopliteal arteries: long-Term results of the IN.PACT SFA randomized trial. Circ Cardiovasc Interv. 2018;11(1) doi: 10.1161/CIRCINTERVENTIONS.117.005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ott I., Cassese S., Groha P. Randomized comparison of paclitaxel-eluting balloon and stenting versus plain balloon plus stenting versus directional atherectomy for femoral artery disease (ISAR-STATH) Circulation. 2017;135(23):2218–2226. doi: 10.1161/CIRCULATIONAHA.116.025329. [DOI] [PubMed] [Google Scholar]
- 29.Scheinert D., Duda S., Zeller T. The LEVANT I (Lutonix paclitaxel-coated balloon for the prevention of femoropopliteal restenosis) trial for femoropopliteal revascularization: first-in-human randomized trial of low-dose drug-coated balloon versus uncoated balloon angioplasty. JACC Cardiovasc Interv. 2014;7(1):10–19. doi: 10.1016/j.jcin.2013.05.022. [DOI] [PubMed] [Google Scholar]
- 30.Rosenfield K., Jaff M.R., White C.J. Trial of a paclitaxel-coated balloon for femoropopliteal artery disease. N Engl J Med. 2015;373(2):145–153. doi: 10.1056/NEJMoa1406235. [DOI] [PubMed] [Google Scholar]
- 31.Werk M., Albrecht T., Meyer D.R. Paclitaxel-coated balloons reduce restenosis after femoro-popliteal angioplasty: evidence from the randomized Pacifier trial. Circ Cardiovasc Interv. 2012;5(6):831–840. doi: 10.1161/CIRCINTERVENTIONS.112.971630. [DOI] [PubMed] [Google Scholar]
- 32.Bausback Y., Willfort-Ehringer A., Sievert H. Six-month results from the initial randomized study of the ranger paclitaxel-coated balloon in the femoropopliteal segment. J Endovasc Ther. 2017;24(4):459–467. doi: 10.1177/1526602817710770. [DOI] [PubMed] [Google Scholar]
- 33.Steiner S., Willfort-Ehringer A., Sievert H. 12-Month Results from the first-in-human randomized study of the ranger paclitaxel-coated balloon for femoropopliteal treatment. JACC Cardiovasc Interv. 2018;11(10):934–941. doi: 10.1016/j.jcin.2018.01.276. [DOI] [PubMed] [Google Scholar]
- 34.Tepe G., Zeller T., Albrecht T. Local delivery of paclitaxel to inhibit restenosis during angioplasty of the leg. N Engl J Med. 2008;358(7):689–699. doi: 10.1056/NEJMoa0706356. [DOI] [PubMed] [Google Scholar]
- 35.Tepe G., Schnorr B., Albrecht T. Angioplasty of femoral-popliteal arteries with drug-coated balloons: 5-year follow-up of the thunder trial. JACC Cardiovasc Interv. 2015;8(1 Pt A):102–108. doi: 10.1016/j.jcin.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 36.Cassese S., Ndrepepa G., Fusaro M., Kufner S., Xhepa E., Fusaro M. Paclitaxel density and clinical efficacy of drug-coated balloon angioplasty for femoropopliteal artery disease. Meta-analysis and adjusted indirect comparison of 20 randomized trials. EuroIntervention. 2018 doi: 10.4244/EIJ-D-18-00550. [DOI] [PubMed] [Google Scholar]
- 37.FDA . Food and Drug Administration; Silver Spring: U.S.: 2019. Paclitaxel-coated drug-coated balloon and drug-eluting stent late mortality panel: executive summary.https://www.fda.gov/media/127698/download (accessed July 21, 2019) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.