Keywords: cannabis, dopamine, glutamate, nucleus accumbens, opioids, substance use disorders
Abstract
Drug consumption is driven by a drug’s pharmacological effects, which are experienced as rewarding, and is influenced by genetic, developmental, and psychosocial factors that mediate drug accessibility, norms, and social support systems or lack thereof. The reinforcing effects of drugs mostly depend on dopamine signaling in the nucleus accumbens, and chronic drug exposure triggers glutamatergic-mediated neuroadaptations in dopamine striato-thalamo-cortical (predominantly in prefrontal cortical regions including orbitofrontal cortex and anterior cingulate cortex) and limbic pathways (amygdala and hippocampus) that, in vulnerable individuals, can result in addiction. In parallel, changes in the extended amygdala result in negative emotional states that perpetuate drug taking as an attempt to temporarily alleviate them. Counterintuitively, in the addicted person, the actual drug consumption is associated with an attenuated dopamine increase in brain reward regions, which might contribute to drug-taking behavior to compensate for the difference between the magnitude of the expected reward triggered by the conditioning to drug cues and the actual experience of it. Combined, these effects result in an enhanced motivation to “seek the drug” (energized by dopamine increases triggered by drug cues) and an impaired prefrontal top-down self-regulation that favors compulsive drug-taking against the backdrop of negative emotionality and an enhanced interoceptive awareness of “drug hunger.” Treatment interventions intended to reverse these neuroadaptations show promise as therapeutic approaches for addiction.
Neuroscience research has revealed that addiction is a chronic, relapsing disease of the brain triggered by repeated exposure to drugs in those who are vulnerable because of genetics and developmental or adverse social exposures. As a result, the reward circuit’s capacity to respond to reward and motivate actions that are not drug related is decreased, the sensitivity of the emotional circuits to stress is enhanced, and the capacity to self-regulate is impaired. The result is compulsive drug seeking and drug taking despite severe harms and an inability to control the strong urges to consume the drug, even when there is a strong desire to quit. The changes in the brain responsible for these maladaptive behaviors can persist for months or even years after drug discontinuation but are amenable to treatment. Treatment should be aimed at improving self-regulation; helping to control craving and the emergence of distressing emotions, including depression and anxiety; and improving the sensitivity to alternative reinforcers. Addiction is a chronic disease, so its treatment should follow a sustained model of intervention, the intensity of which should be adjusted to the stage of the disease. Treatment should also be personalized and calibrated to the severity of the addiction, the presence of comorbidities, and the individual’s support systems. Crucially, addiction can be prevented, and both universal as well as tailored strategies can significantly reduce substance use disorder in the individual and in a population.
I. INTRODUCTION
There is an inherent need in all sentient beings to seek out positive and avoid negative stimuli, a universal formula that has evolved to maximize adaptive fitness and the chances of survival. The extent to which strategies for attaining or avoiding such stimuli are successful depends on complex interactions between an organism and its environment that are orchestrated by the nervous system. Neurobiology employs processes refined during evolution, such as homeostasis, sensory perception, associative and nonassociative learning, emotions, and decision-making, to shape an organism’s response to environmental stimuli and to maximize its ability to harness their predictable features and to adapt to unpredictable ones. Although types of stimuli vary from one species to another, there are striking similarities among different species, in their responses to positive (e.g., food and sex) and negative (e.g., pain and environmental threats) stimuli. This common representation, which reflects the critical role of such stimuli in boosting the odds of survival, is often reflected at the neurobiological level, whereby different species tap into similar brain structural, neurochemical, and functional strategies to tackle similar problems (77, 284).
Ingenuity has enabled humans to extract and refine highly reinforcing stimuli against which naturally occurring reinforcers cannot easily compete. The most notable example is our ability to purify and deliver drugs (e.g., high alcohol content beverages, cigarettes, syringes for drug injections, and more recently vaping devices) along with advances in chemistry that ushered new psychoactive compounds of unprecedented potency (e.g., synthetic opioids, cannabinoids, and stimulants). Access to these highly reinforcing drugs, when combined with promotive environments (e.g., the ubiquity of legal and illegal drugs, chronic stress, peer pressure) and individual vulnerabilities (e.g., preexisting mental illness, chronic pain, genetic predisposition, gender, young age), influence drug experimentation as well as the risk and prevalence of substance use disorders (SUD). The latest example of the potential consequences of such drug-promotive environments is the rising tide of opioid fatalities, initially fueled by misuse of prescription opioid analgesics, then by heroin, and now exacerbated by the misuse of very potent synthetic opioids such as fentanyl. The current opioid epidemic [estimated to have led to over 71,000 opioid overdose fatalities in 2017 (57) and with no signs of abating in 2018 (2)], combined with the high background mortality rate from alcohol (~88,000 annual deaths) (56, 310) and tobacco (>480,000 annual deaths) (58) use, highlights the devastating impact of drugs and addiction in our society.
The application of neuroscientific technologies in humans and laboratory animals has led to remarkable advances in our understanding of the neurobiological underpinnings of drug reinforcement and addiction. As a result, addiction, which has been viewed historically as a “moral deficiency,” is being increasingly regarded as a chronic relapsing disorder characterized by an urge to consume drugs and by the progressive loss of control over, and escalation in, drug intake despite repeated (unsuccessful) attempts to resist doing it (334). It is also recognized that addiction emerges in the context of complex biopsychosocial interactions between the pharmacological effects of a drug, individual vulnerabilities (e.g., genetics/epigenetics, developmental stage, existing pathology), inadequate social connectivity, and other sociocultural factors (e.g., normative behaviors regarding drug use, affordability and availability of drugs, legal status). Research on the mechanisms underlying the modulatory influence of adverse social environments, childhood experiences, and genetic variability is fundamental for helping us understand why not everyone who is exposed regularly to a drug becomes addicted (54, 231), and why some addicted individuals can recover while others do not (47, 210, 287).
II. DRUG REWARD
Dopamine (DA) lies at the center of drug reward (85, 182). Every drug with addiction potential increases DA, either through direct or indirect effects on DA neurons in the ventral tegmental area (VTA) with the consequent release of DA in the nucleus accumbens (NAc) (357) (FIGURE 1). Drugs of abuse increase DA through their initial action on different molecular targets and, depending on their pharmacological effects (TABLE 1), also engage additional neurotransmitters. Some of these, like the endogenous opioids (BOX 1) or the endogenous cannabinoids (BOX 2) (FIGURE 2), also contribute to the reinforcing effects of drugs through modulation of hedonic responses or inhibition of negative affective states (232). The significance of non-dopaminergic influences on reward processing has not been as extensively investigated as DA’s but should not be underestimated. In fact, dopamine-deficient (DD) mice showed conditioned place preference for cocaine [also shown for morphine in naive rats (157)], which appeared to be mediated by serotonin through a mechanism that involves DA neurons, presumably through their release of glutamate or neuropeptides like cholecystokinin and neurotensin (156). Also, studies in genetically engineered mice have shown that the mu opioid receptor (MOR) is not only the main target for heroin and other opioid drugs, but is also essential for the rewarding properties of nonopioid drugs, like alcohol, cocaine, and nicotine (62, 153).
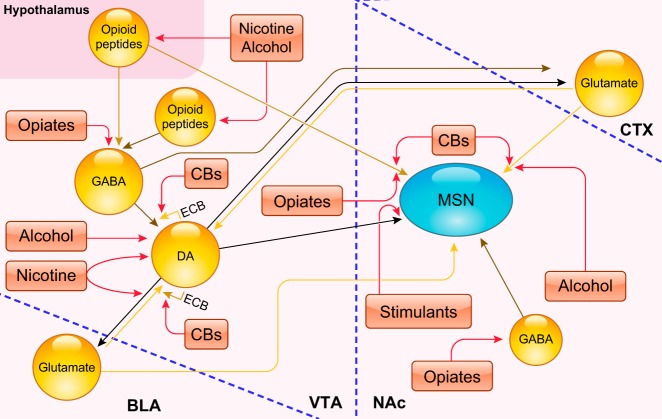
FIGURE 1.
Schematic representation of key target sites for various drugs of abuse across the reward circuitry. Ventral tegmental area (VTA) dopamine (DA)ergic neurons project to forebrain targets such as the basolateral amygdala (BLA), medial prefrontal area of the cortex (CTX) or mPFC, and nucleus accumbens (NAc). These neurons receive excitatory synaptic inputs from the mPFC (but also from lateral hypothalamus and pedunculopontine tegmental nucleus/dorsolateral tegmental nucleus; not shown). GABAergic neurons in the VTA target neighboring DAergic neurons as well as projecting to the mPFC and NAc (other inhibitory inputs to these DAergic neurons are likely to arise from extended amygdala output structures; not shown). GABAergic medium spiny neurons (MSNs) in the NAc, which project to either the globus pallidus externus/ventral pallidum (VP) predominantly via D2R-expressing but also D1R-expressing neurons or to the VTA/SN via D1R-expressing neurons, receive dopaminergic input from the VTA. They also receive excitatory inputs from the mPFC and the basolateral amygdala (BLA) (but also from the hippocampus and thalamus). The activity of MSNs is modulated by both cholinergic and fast-spiking GABAergic interneurons (not shown) (312). Drugs of abuse, despite diverse initial actions, produce some common effects on the VTA and NAc. Stimulants directly increase dopaminergic transmission in the NAc. Opiates increase DA indirectly by inhibiting GABAergic interneurons in the VTA, disinhibiting them and by stimulating mu opioid receptors (MOR) on NAc neurons (244). Nicotine stimulates DA neuron firing by its effects on ionotropic (nicotinic) acetylcholine receptors (314). Alcohol, among other effects, increases the firing of VTA DA neurons projecting to NAc via their disinhibition through the inhibition of GABA neurons (238). Cannabinoids (CBs) disrupt the normal endocannabinoid (ECB) signaling–from DAergic neurons on nearby glutamatergic (via retrograde suppression of excitation) and GABAergic (via retrograde suppression of inhibition) terminals–that is responsible for fine-tuning the activity of mesolimbic dopamine projections (31). [Modified from Nestler (244), with permission from Springer Nature.]
Table 1.
Pharmacological targets of main classes of drugs of abuse
| Drug Class | NT Mediators | Mechanism |
|---|---|---|
| Opioids | MOR → GABA↓ → DA↑ | Opioids, like morphine, heroin, or fentanyl, are agonists at MOR (214). Opioid stimulation of MOR in the VTA increases striatal DA release. |
| Alcohol | EtOH → MOR↑, NMDA↓, DA↑, GABA↑, ECS↑ | Unlike most addictive drugs that target specific receptors and transporters, EtOH affects a wide range of targets and indirectly increases DA in NAc (354). |
| Nicotine | nAChRs → DA↑ | Nicotine’s interaction with specific nAChRs (i.e., α4β2) leads to NAc DA release directly by increasing neuronal activity in VTA DA neurons (13, 24) or indirectly by activating modulatory (i.e., GABA or Glu) neurons in VTA (76, 114). |
| Stimulants | DAT/VMAT2 → DA↑ | Amphetamines block DAT and the VMAT2 (11, 96, 111), which increase synaptic levels of extracellular DA by DAT reversal and depletion of vesicular DA stores, which promotes DA release. Cocaine and methylphenidate block DAT inhibiting DA reuptake, thus increasing DA in NAc (171). |
| Cannabis | THC → Glu/GABA → DA↑↓ | THC activation of CB1 receptors regulates the presynaptic release of both GABA and glutamate, influencing the activity states of the mesolimbic DA system (92, 299) (see FIGURE 1) |
| Classic hallucinogens | 5-HT2ARs > DA↑; 5-HT2CRs > DA↓ | Indolamines (e.g., psilocybin, LSD, Mescaline) that display high-affinity agonist activity at serotonin 5-HT2 G protein-coupled receptor subtypes (5-HT2A, 5-HT2B, and 5-HT2C) (51). These drugs do not trigger compulsive drug taking and are therefore not considered addictive. Instead, these drugs are predominantly used to alter mental state. |
| Inhalants | Multiple agents and targets, including volatile substances like toluene, which modulates NMDA↓, 5-HT3↑, Gly↑, GABAA↑, nACh↓, and DA↑ (39, 119, 249) | Abused inhalants (other than nitrites) have a wide range of effects on neurotransmitter release and receptors, with a few similar actions as those of benzodiazepines, alcohol, and barbiturates (15) and have been shown to enhance striatal DA release and have direct reinforcing effects (166). |
| Benzodiazepines and barbiturates | GABA↑ > DA↑ | Benzodiazepines and barbiturates enhance GABA by increasing the frequency or the duration of the chloride ion channel opening at the GABAA receptor, respectively. Both drugs can increase the firing rate of DA neurons in VTA through disinhibition (86, 318). |
MOR, mu opioid receptors; VTA, ventral tegmental area; DA, dopamine; NMDA, N-methyl-d-aspartate; ECS, endogenous cannabinoid system; NAc, nucleus accumbens; nAChRs, nicotinic acetylcholine receptors; DAT, dopamine transporter; VMAT2, vesicular monoamine transporter 2; THC, tetrahydrocannabinol.
Box 1. The endogenous opioid system.
The endogenous opioid system modulates the mesolimbic DA system (107, 328) and is implicated in assigning hedonic values to rewards and in integrating reward‐related information to guide decision‐making and execution of goal‐directed behaviors (193). It consists of endogenous opioid peptides and their cognate receptors, namely, β-endorphins, enkephalins, and dynorphins, which signal preferentially through mu (MOR), delta (DOR), and kappa (KOR) opioid receptors, respectively. MOR are responsible for the rewarding effects of opioids and for analgesia, DOR are implicated in analgesia and anxiolysis, while KOR are implicated in the dysphorigenic responses associated with addiction (194) and in stress-induced relapse (136). MOR in the VTA and NAc, as well as in the basolateral amygdala, are implicated in opioids’ rewarding effects (FIGURES 1 and 2). The opioid system also modulates mood, with stimulation of MOR and KOR having predominantly antidepressant and dysphorigenic effects, respectively (250).
Box 2. The endogenous cannabinoid system.
The endogenous cannabinoid system (ECS) modulates other neurotransmitter systems including GABA, glutamate, and DA in key areas along the mesolimbic circuitry (209, 348). The ECS consists of endogenous cannabinoids [anandamide (AEA) and 2-arachidonoylglycerol (2-AG)] and their cognate receptors (CB1R and CB2R) (337). Recent studies corroborate the functional importance of the ECS in modulating reward circuitry (252). For example, activation of CB1R in cortical glutamatergic afferents inhibited DA release in the NAc and blunted reward-driven behaviors (225). In the VTA, 2-AG, and to a lesser extent AEA, released from DA neurons, retrogradely activate CB1R at VTA GABA inputs from GABAergic interneurons (FIGURE 2), or from pallidum or rostromedial tegmental nuclei terminals (252, 271). 2-AG also activates CB1Rs at VTA glutamate inputs arising from cortex (252). Cannabinoids also act in the NAc where medium spiny neurons (MSNs) are modulated by CB1R expressing GABAergic interneurons, and by CB1R expressing glutamate terminals originating from amygdala, hippocampus, and prefrontal cortex (3, 79, 152).
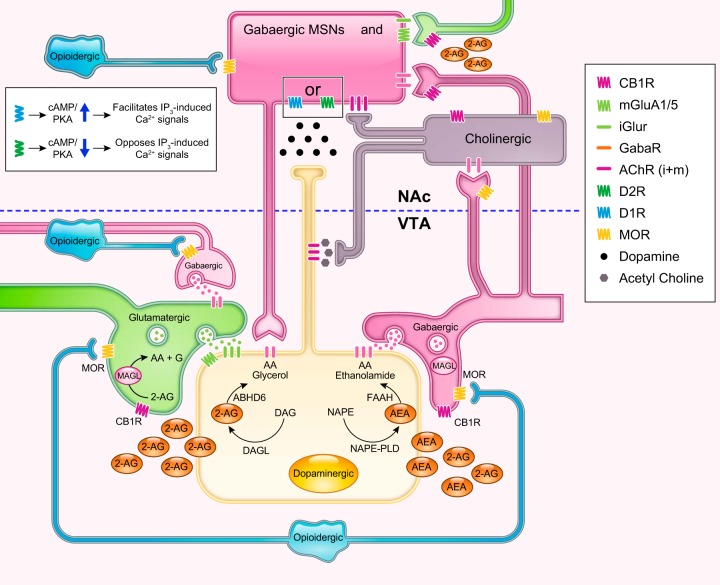
FIGURE 2.
Schematic simplified cartoon showing some of the indirect modulatory effects of midbrain (ventral tegmental area, VTA) opioid and endocannabinoid signals on dopaminergic transmission in nucleus accumbens (NAc). Reward-related stimuli conveyed through glutamatergic afferents (green) promote burst firing of dopamine (DA) neurons (yellow) mainly driven by ionotropic glutamate receptor (iGluR) binding activation at the dopaminergic cell. The level of activation is normally kept in check by GABAergic counterbalancing inputs (pink), but also by direct inhibitory GABAergic input inhibiting presynaptic glutamate release (66). Endogenous [released from opioidergic neurons (light blue), mostly projecting from the hypothalamus] or exogenous (natural or synthetic opioid like molecules) opioids activate endogenous mu opioid receptors (MOR) on GABAergic interneurons. The MOR is coupled to inhibitory G proteins, whose activation (by an endogenous peptide like endorphin or exogenous agonists like morphine and fentanyl) leads to a dissociation between the Gα and Gβγ subunits and the activation of intracellular effector pathways. One such pathway leads to the inhibition of GABA release as a result of increased conduction of potassium ions, which hyperpolarizes the cell making it less responsive to depolarizing inputs and inhibiting calcium influx (123). In addition, activation of MOR increases mitogen-activated protein kinase (MAPK) signaling while their phosphorylation activates the arrestin pathway (5), which has the ability to desensitize, activate, and control the trafficking of G protein-coupled receptors (GPCR) (140). A drop in GABAergic tone causes a net disinhibition of the neighboring dopaminergic neuron and the release of excess dopamine (black dots) onto direct and indirect medium spiny neurons [pink medium spiny neuron (MSN)], which reinforces the euphorigenic effects of opioids. Ionotropic GluR-mediated activation of the DA neuron leads to Ca2+ influx (via voltage-gated calcium channels), which is either facilitated or hampered in D1R vs D2R expressing MSN populations, respectively (317) (inset), leading to their differential roles in plasticity. At the same time, the Ca2+ influx, combined with activation of mGluA1/5, triggers the “on demand” production of 2-arachidonoylglycerol (2-AG) from diacylglycerol (DAG) [or anandamide (AEA) from N-acyl-phosphatidylethanolamines (NAPE)]. Retrograde 2-AG transmission through CB1 receptor binding on monoacylglycerol lipase (MAGL) containing afferent (GABA and Glu) neurons has the net effect of disinhibiting dopamine neurons and facilitating phasic DA release (63). This is because cannabinoids (e.g., tetrahydrocannabinol, 2-AG) operate as full agonists at GABA terminals [that display a high CB1R to vesicles ratio (188)] but as partial agonists at Glu terminals [where the CB1R-to-vesicles ratio is much lower (295, 296)]. As shown, AEA is assumed to be retrograde in spite of data showing that FAAH is predominately postsynaptic while NAPE PLD is presynaptic. The true nature of AEA neurotransmission remains unclear partly because there are other pathways for AEA synthesis. In the NAc, GABAergic projections, sent by the VTA, also synapse onto cholinergic interneurons (dark gray), thus inhibiting their excitatory input onto DA terminals. Activation of either CB1 or MOR on these GABA neurons can stimulate DA terminals (independently of VTA DA neuron activation) by disinhibiting ACh release while activation of these receptors, which are also expressed on ACh interneurons, could in theory have the opposite effect on DA levels in the accumbens (351). GABAergic and glutamatergic terminals in the NAc also have the capacity to modulate accumbal DA activity onto MSNs directly. Since these neurons also express MOR [but some also CB1R (226, 361)], their activation on GABA inputs could enhance DA release, while their inhibitory effects on glutamatergic inputs could reduce accumbal release of DA.
In turn, repeated dopaminergic stimulation from drug use induces neuroadaptations in multiple neurotransmitter systems, including the glutamatergic system, which enhances neuronal excitability and modulates neuroplasticity (286); the GABAergic system, which inhibits action potential transmission (168); and the opioid, endocannabinoid (232, 337, 351), cholinergic (78, 204), serotonin (36, 215), and noradrenergic (109) systems, which modulate affective, hedonic, and aversive circuits in the brain.
Midbrain DA neurons and their projections into the NAc and the dorsal striatum and their GABAergic outputs are implicated in motivating and sustaining reinforced behaviors (including towards food and drugs) but also in avoiding aversive stimuli or states (262). DA neurons in the VTA project to the NAc, which is a central hub of the reward circuit and a major driver of goal-directed actions that are sensitive to the current salience (estimated value) of an associated goal (281). Meanwhile, DA neurons in the substantia nigra (SN) project to the dorsal striatum and translate recurrent reward signals into habitual actions that become increasingly insensitive to actual or updated goal values and are selected instead based on prior experience with the reinforcement associated with that action. The repeated reward-associated behavior, over time, can eventually result in the emergence of habits (103), as the dorsal striatum gradually takes over from the ventral striatum. Additionally, following repeated drug exposures, habits might also result from a reduction in inputs from prefrontal cortex (PFC) into striatum that disrupt the control over action selection (269). However, the notion that “habit” formation is necessary for the establishment of addiction was recently questioned by a study that showed that rodents who had to solve an original problem before gaining access to cocaine did not transfer behavioral control from ventral to dorsal striatum even though they expressed addiction-like behaviors (300). The results from this study are consistent with observations that addicted individuals can display very creative solutions to procure the drug, while falling into ritualistic behaviors once they are consuming them. This indicates that behaviors in addiction are likely a complex combination of adaptive (mostly to procure the drug) and automatic stimulus responding.
VTA DA neurons also project to amygdala and hippocampus, which mediate emotional and memory associations, and to PFC regions, which mediate salience attribution and self-regulation, all of which participate in the reinforcing and conditioning that follow chronic drug consumption. DA neurons in VTA and SN are influenced by projections from multiple brain areas that control their tonic and phasic firing (112). Recent evidence points to significant diversity within the population of VTA DA neurons with respect to their afferent and efferent connectivity (235), their co-release of GABA or glutamate (or both), and the presynaptic receptors expressed in their terminals, which differentially modulate DA release in the presence of other neurotransmitters like GABA or acetylcholine (228). Diversity is also apparent in the cytoarchitectonic, neurochemical, and electrophysiological features of VTA DA neurons as well as in their sensitivity to rewarding versus aversive stimuli (159). Generally, tonic firing of DA neurons (1–8 Hz) sets the background dopaminergic tone, which is sufficient to stimulate the high-affinity DA D2 receptors (D2R), whereas phasic firing (<500 ms; >15 Hz) encodes responses to salient stimuli (rewarding, unexpected, novel, aversive) and results in higher DA levels (120) that are able to stimulate the low-affinity DA D1 receptors (D1R). Thus a drug like cocaine that blocks DA transport back into the terminal, promoting its accumulation in the extracellular space, while also increasing the frequency of DA release events in the NAc (9), triggers high DA levels that can activate both D1R and D2R.
The traditional model of the direct and indirect pathways in the dorsal striatum, with their opposing effects on facilitating or inhibiting movement, respectively, has been applied to reward processing by the NAc. Based on this model, NAc D1R-expressing medium spiny neurons (D1R-MSNs) in the direct pathway (midbrain projecting) are proposed to underlie reward and goal directed behaviors, whereas D2R-expressing MSNs (D2R-MSNs) in the indirect (ventral pallidum projecting) are proposed to be associated with avoidance behavior (155). However, recent studies question such a clear segregation of function and anatomic projections for both the dorsal and ventral striatum (187). For example, studies have shown that in the dorsal striatum both D1R-MSNs (direct) and D2R-MSNs (indirect) are activated when initiating an action (75). Moreover, in the NAc, these pathways are less segregated than in the dorsal striatum and D1R-MSNs project directly both to the midbrain and to the ventral pallidum, which is modulated both by D1R-MSNs and D2R-MSNs (277), and the D2R-MSNs that project to ventral pallidum directly disinhibit the thalamus (186). In the NAc, studies in rodents reported the existence of a subpopulation of neurons that coexpress D1R and D2R (145) and form a D1R-D2R complex that when activated inhibits basal natural and cocaine reward (146).
In the VTA, spontaneous firing of DA neurons sets tonic DA levels, which stimulate mainly D2R (also D3R, which have high affinity for DA) in NAc, upon which phasic DA firing can be superimposed resulting in higher DA levels that additionally stimulate D1R (262). Although an unexpected reward triggers phasic DA firing, its repeated presentation transforms it into an expected reward and causes the phasic firing of the DA neuron to occur upon exposure to the predictive cue (making it conditioned). In contrast, there is a pause in DA neuron firing when an expected reward does not materialize (making it discordant). In this way, when an outcome differs from what is expected, DA signals a “reward prediction error” regardless of its positive or negative valence, that recent studies suggest may reflect not just its scalar reward value but additional dimensions of the expected outcome, such as its characteristics or presentation sequence (189). Drug cues trigger phasic DA firing, which in the NAc binds to both D1R-expressing MSNs, where DA is stimulatory (increases cAMP and intracellular Ca signaling), and D2R-expressing MSNs where DA is inhibitory (decreases cAMP and intracellular Ca signaling) (213, 245, 297, 298), sparking the motivation to initiate reward-directed behaviors (346). Although it was believed that aversive stimuli or their cues, by reducing tonic activity of DA neurons and DA release in NAc, lowered D2R-inhibition of indirect pathway MSNs, leading to avoidance behavior, this, as discussed above, is now being questioned. Indeed, some DA neurons are activated, not inhibited by aversive stimuli (352), but further research is needed to characterize their projections into NAc and other brain regions (186). Additionally, both tonic and phasic firing stimulate the high-affinity D3R, which are highly expressed in NAc, where they colocalize with D1R potentiating their signaling (108) and possibly modulating drug reward and conditioning (117). The NAc also expresses D5R, which colocalize with D1R in MSNs (239), are also expressed in interneurons, and appear to play distinct roles in neuroplasticity relative to D1R (59). The D4R is also expressed in the NAc, and genetic studies have implicated its encoding gene (DRD4) in addiction vulnerability (255), whereas preclinical studies have shown that it modulates the pharmacological effects of drugs. However, it is clear that the functional differences between DA receptors, their colocalization, and interactions in NAc (including that between D3R and D5R, which has been minimally investigated) require further investigation.
Phasic DA firing and stimulation of D1R are needed for drug reward and for eliciting conditioned associations. On the other hand, DA stimulation of D2R signaling is associated with motivational drive (306) but, depending on the circumstances, can interfere with the reinforcing effects of drugs such as with exposure to multiple alternative reinforcers. Notably, positive reinforcement and maximal reward occur when both D1R and D2R are simultaneously stimulated in the NAc, but additional studies are needed to disentangle how each receptor subtype contributes to the overall effect (311).
III. DOPAMINE AND NEUROPLASTICITY
Drugs, via excessive and repeated dopaminergic stimulation, induce persistent neuroplastic adaptations in midbrain DA neurons and in their projections into NAc and also into dorsal striatum that are believed to underlie conditioning along with the enhanced incentive saliency to drug cues and behavioral inflexibility (128, 262, 293). When conditioning is established, DA neurons fire when exposed to the drug-predictive cues that precede the drug’s arrival, in effect predicting an imminent reward. Conditioning can be instantiated for many types of cues, including places and people associated with the drug experience, or mental states that predominated at the time when the drug was being consumed (depressed, bored, excited, stressed, etc.), all of which can subsequently awaken, by themselves, the motivation to seek the drug (289, 343). These neuroadaptations prominently involve glutamatergic inputs onto DA neurons in VTA and into MSNs in NAc setting up the stage for the respective behavioral changes in reward responsivity and habituation that characterize addiction, including a persistent risk of relapse that makes treatment so challenging (176, 290, 359). Some of the key drug-induced adaptations are similar to synaptic changes associated with learning including changes in dendritic morphology, ionotropic glutamate receptors (predominantly AMPA and NMDA receptors) that result in long-term potentiation (LTP) and long-term depression (LTD) (138, 176). Synaptic strength is modulated presynaptically through the regulation of glutamate release and postsynaptically by the insertion or removal of transmembrane glutamate ionotropic receptors (NMDA and AMPA) and by changes in their subunit composition, which modifies their efficacy. Glutamate release in NAc is decreased by activation of metabotropic glutamate receptors mGluA2/3 (362), adenosine A1 receptors (37), D2R (154), or cannabinoid CB1R (272, 316). Postsynaptically, trafficking of AMPA and NMDA receptors is regulated by D1R activation, which promote AMPA surface expression. The insertion of high calcium-permeable AMPA receptors that lack the GluA2 subunit is necessary for the expression of incubation of cocaine craving (106). Increases in NMDA receptors containing the GluN2B subunit have also been associated with neuroplasticity after chronic cocaine or heroin (160, 294, 349). Postsynaptic mGluR1 produce an LTD that reverses cocaine-induced increases of high calcium-permeable AMPA receptors in VTA (220) and in NAc where it reduced cue-induced cocaine craving (211).
Morphological changes occur in parallel to the strengthening of excitatory synapses, which is associated with larger synapses, whereas weakening results in smaller synapses and reduced dendritic spine density. In addition, recent studies in transgenic mice indicate that chronic administration of cocaine is also associated with specific structural plasticity in dopaminergic boutons in the NAc shell (91) (FIGURE 3).
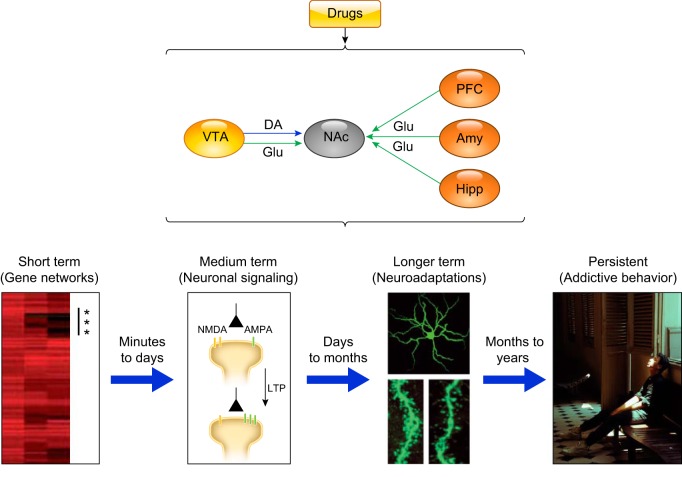
FIGURE 3.
Leading hypothesis of how a temporally coordinated cascade of drug-induced changes in synaptic activity hijack multipurpose learning processes to engender maladaptive and persistent addictive behaviors. VTA, ventral tegmental area; DA, dopamine; PFC, prefrontal cortex; Amy, amygdala; Hipp, hippocampus. Bottom figures depict (from left to right) a heat map of genes upregulated (*) in the nucleus accumbens (NAc) 1 h after acute cocaine administration to naive animals (275); examples of such transient transcription and epigenetic modulatory events include regulatory and signaling genes like fosB, ΔFosB, NFκB, CdK5, and MEF2. [From Robison and Nestler (275), with permission from Springer Nature.] Drug-induced changes in neuronal activity (e.g., changes in NMDA/AMPA receptor balance) lead to synaptic plasticity (e.g., LTP) in the reward circuitry (169). [From Jones and Bonci (169), with permission from Elsevier.] Morphological and functional changes, like increased dendritic spine density, which, if sustained, can lead to cytoskeletal and circuit remodeling, a phenomenon that correlates with synaptic strength and the strength of drug-associated memories in vivo, which, over a period of months and years, contributes to the orchestration and cementing of stereotypical addictive behaviors. [From Nestler. Dialogues Clin Neurosci 15: 431–443, 2013.]
However, our understanding of drug-induced neuroplasticity is evolving. For example, with the use of a modified reinstatement protocol, it was shown that cocaine-induced neuroplastic changes in dendritic spine morphology and AMPA/NMDA ratios were temporarily reversed by cocaine use (308). Also, while most glutamatergic synapses contain both AMPA and NMDA receptors, a small number of so-called silent (also referred to as “AMPA silent” or immature) synapses express NMDA receptors exclusively (203), and have been associated with chronic stress (315) and with addictive and neurodegenerative disorders (144). For example, silent synapses are produced in response to cocaine (in NAc) (89) and alcohol (in dentate gyrus) (23). However, these synapses do not contribute significantly to stimulus (i.e., drug) evoked excitatory postsynaptic currents. Thus it has been hypothesized that, by recapitulating some key aspects of the immature yet more “teachable” developing brain (89), silent synapses might provide a “metaplasticity” signal and prime the circuitry for any needed subsequent plastic changes (e.g., LTP or LTD) (217).
Furthermore, the identification of Maged1, implicated in the modulation of dendritic spine density and learning in the hippocampus (363), as a gene whose expression in PFC is essential for both extracellular DA release in NAc and behavioral sensitization to cocaine (82), illustrates another molecular process involved both in learning and in drug-induced neuroplasticity in PFC and NAc. Similarly, synapses from hippocampal glutamatergic terminals into the NAc were shown to also undergo LTP, which was necessary for the formation of reward-related contextual memories, although these neuroplastic changes did not appear to be DA-dependent (196).
There is also increasing evidence that in addition to Hebbian neuroplasticity, some forms of drug-induced neuroplasticity in NAc are homeostatic and interact with Hebbian neuroplastic changes (90, 161, 172, 290). For example, increases in synaptic strength following repeated cocaine exposure trigger homeostatic changes in membrane excitability in MSNs in the NAc, which appear to contribute to incubation of cocaine craving (349).
IV. NEUROCIRCUITRY OF ADDICTION
The percentage of laboratory animals that show addictive-like behaviors, or of people that become addicted to a drug after repeated exposure, varies as a function of the drug, being higher for drugs like heroin or methamphetamine and lower for drugs like alcohol or cannabis. For example, only between 15 and 20% of rats chronically exposed to cocaine will continue to compulsively prefer cocaine over other rewarding options (52), whereas the percentage of heroin-preferring rats can go as high as 50% under similar experimental conditions (198). These percentages, however, do vary across different rat strains, highlighting the role of genetics in modulating drugs’ effects. Epidemiological data are generally consistent with this picture. According to the best available estimates, the odds, in lifetime drug users, of ever becoming addicted to alcohol, cannabis, cocaine, or opioids (heroin) are ~1.5, 9, 17, and 23%, respectively (7). As of now, it is not clear what determines the transition from drug experimentation to addiction, which emerges when individuals lose their ability to overcome the strong urge to take the drug despite a conscious awareness of not wanting to do so and the recognition of their potentially catastrophic consequences. However, we do know this transition is associated with measurable disruptions in several brain circuits including those involved with conditioning, reward sensitivity, incentive motivation, self-monitoring/regulation, mood, and interoception. In this review, we use the term addiction in correspondence with the dimensional definition of moderate to severe SUD as per the Diagnostic and Statistical Manual of Mental Disorders (DSM-5) (TABLE 2).
Table 2.
Diagnostic criteria for substance use disorder based on DSM-5
| DSM 5 SUD Criteria |
|---|
| 1. Hazardous use |
| 2. Social/interpersonal problem related to use |
| 3. Neglected major roles because of use |
| 4. Withdrawal |
| 5. Tolerance |
| 6. Used larger amounts/longer |
| 7. Repeated attempts to quit/control use |
| 8. Much time spent using |
| 9. Physical/psychological problems related to use |
| 10. Activities given up in order to use |
| 11. Craving |
A mild substance use disorder (SUD) is diagnosed with 2–3 criteria, moderate with 4–5, and severe 6–7 criteria (147). DSM-5, Diagnostic and Statistical Manual of Mental Disorders.
A. Conditioning
A key process that initiates the transition into addiction and helps perpetuate it is the consolidation of conditioning to the drug. As addiction develops there is an expansion in the number of stimuli that become experientially linked (conditioned) to the drug, and thus a greater likelihood of being exposed to a drug-predictive cue. Any encounter with these cues can trigger bursts of DA in the NAc (259) and lead to further consolidation in dorsal striatum; this directs the attention to the drug-predictive cue and engenders the motivation to procure the drug. As a result, the motivational drive toward the drug now occurs before the drug is consumed and is triggered by the exposure to the drug-predictive cue. Upon drug consumption, the continued DA stimulation from the drug’s pharmacological effects promotes continued ingestion while further strengthening conditioned learning, thus perpetuating the cycle of relapse and drug-taking.
One of the changes believed to contribute to enhanced reactivity to drug-predictive cues in addiction is the disruption of the balance between D1R and D2R signaling in the ventral striatum. Overall, rodent studies provide support to the notion that strengthening of D1R-MSNs in NAc enhances cocaine reward, whereas strengthening of D2R-MSNs suppresses it (49, 208, 323). Similarly, a recent study reported that cue-induced reinstatement was intensified by either activating D1R-MSNs or reducing the activity of D2R-MSNs (151). Combined, the studies suggest that the motivation to take the drug in addiction is energized by drug-predictive cues and by drug-induced transient DA-induced stimulation of D1R (activating D1R-MSNs) concomitant to a weakening of signaling from D2R that is insufficient to counterbalance D1R-MSNs, thus facilitating compulsive intake. Using optical imaging in transgenic mice, we showed that in the dorsal striatum of naive mice, acute cocaine led to fast [Ca2+] increase in D1R-MSNs and to progressive [Ca2+] decreases in D2R-MSNs, consistent with DA stimulating D1R-MSNs and inhibiting D2R-MSNs (213). In contrast, in mice chronically exposed to cocaine, the [Ca2+] responses to acute cocaine were blunted but to a significantly greater extent in D2R-MSNs than in D1R-MSNs, unbalancing the relative signaling towards a predominance of D1R-MSNs over D2R-MSNs (251). However, studies are needed to assess if similar changes occur in NAc and their association with compulsive drug taking, particularly since there is evidence that in the NAc there is coexpression of D1R and D2R and of projections of both D1R-MSNs and D2R-MSNs into ventral pallidum.
Unbalancing D1R over D2R signaling with repeated drug exposure would favor cue-induced phasic DA firing and D1R signaling (driving drug-seeking) while undermining tonic DA firing and D2R signaling (which opposes prepotent responses). Specifically, upregulation of the low-affinity D1R would favor signaling from phasic DA responses, while decreasing the sensitivity to tonic DA responses due to a downregulation of the high-affinity D2R. In contrast, an upregulation of D2R would enhance the sensitivity to tonic DA release while attenuating phasic DA release via D2R autoreceptor inhibition and might explain why D2R upregulation inhibits compulsive cocaine taking (323). Unbalancing D1R over D2R would enhance the reinforcing values of drugs and drug-predictive cues while undermining the capacity for behavioral control, facilitating impulsive and compulsive drug consumption (213). In clinical studies, the enhanced sensitivity to conditioned drug-predictive cues has been associated with addiction severity (343) and with worse clinical outcomes (68, 185). Thus a better understanding of the relationship between D1R and D2R dynamics in NAc and dorsal striatum has significant translational implications for addiction treatment. For example, a rodent study that showed that optogenetic activation of glutamatergic inputs onto accumbal D2R-MSNs reduced cocaine self-administration suggests that strengthening signaling through D2R-MSNs could be beneficial for the treatment of addiction (34).
B. Reward and Motivation
In parallel to the enhanced sensitivity to the expectation of the drug’s rewarding effects (due to conditioning), there is a reduced sensitivity of the DA reward circuit to the actual consumption of the reward, which has been observed in drug addicted individuals and interestingly also among some obese individuals who display some phenotypic traits consistent with “food addiction” (326). This reduced sensitivity in drug-addicted individuals extends to non-drug rewards with the concomitant decrease in their motivational value, which contributes to the lack of interest in non-drug-associated activities characteristic of addiction. Brain imaging studies of drug-addicted individuals have helped characterize these adaptations by revealing decreased D2R expression and DA release in the striatum (both dorsal and ventral regions) (339; though see negative studies in Refs. 95, 175). Very few studies have evaluated D1R in addiction or in animal models of addiction, and the results are inconsistent. For example, chronic cocaine in non-human primates has been reported to decrease D1R in a specific subregion of the ventral striatum that encompasses the NAc (234), although they appear to recover after 90 days of abstinence (25), whereas neither electrophysiological studies in cocaine-exposed rats (227) nor brain imaging of cocaine users have found changes in D1R (224), even though the levels were predictive of cocaine intake in cocaine users (224). On the other hand, postmortem studies in methamphetamine users reported significant increases in D1R in NAc (360), whereas brain imaging studies showed no differences in D1R availability (247). These inconsistencies likely reflect in part the paucity of data, differences between methodologies (in vitro vs. in vivo measures), and differences in species and animal models used. Moreover, DA is a neuromodulator, so its effects are state-dependent, which may account in part for why DA stimulation can result in opposite effects depending on the context in which it occurs (291).
Imaging studies have also revealed decreased activation of brain reward regions to receipt of non-drug rewards, such as food, sexual stimuli, or money, in individuals addicted to drugs compared with controls (6, 33, 53, 99, 253). Such a reduced sensitivity to non-drug rewards is likely to impair an addicted individual’s capacity to be incentivized by naturally pleasurable activities and stimuli. Intriguingly, there is also a reduced reactivity of striatal and prefrontal regions to negative reinforcers, which is associated with worse outcomes (30). Decreased sensitivity to negative reinforcers could impair the capacity of the addicted person to feel deterred by negative outcomes (e.g., incarceration, loss of child custody).
C. Self-Regulation
The development of the powerful cue-conditioned cravings outlined above becomes even more deleterious when combined with growing deficits in the brain’s ability to inhibit maladaptive behaviors and prepotent responses. This is because deficits in self-control can contribute greatly to an individual’s inability to avoid risky or self-destructive behaviors, resist temptation (such as taking drugs), or delay gratifications (such as future payoff of engaging in a long-term recovery program), and thus increasing his/her vulnerability to addiction (335).
There is both preclinical and clinical evidence consistent with the notion that the weakening of self-control mechanisms correlates with impaired performance in PFC circuits secondary to drug-induced adaptations in striatal networks or sometimes by direct harm to PFC (320, 356). Brain imaging studies in humans have shown that, in the striatum, D2Rs are positively associated with baseline metabolic activity (marker of brain function) in frontal cortical regions and inversely associated with the sensitivity to the rewarding effects of psychostimulant drugs (102, 341, 342), whereas preclinical studies in rodents have shown that D2R upregulation interferes with drug consumption (323, 324). Clinical imaging studies also show that the reduced striatal D2R seen in human addiction is associated with decreased baseline metabolic activity in prefrontal regions including the orbitofrontal cortex (OFC), anterior cingulate cortex (ACC), and dorsolateral prefrontal cortex (DLPFC) (reviewed in Ref. 345; see also Refs. 333, 336, 340, 344). Since OFC, ACC, and DLPFC are implicated in salience attribution, inhibitory control/emotion regulation, and decision-making, respectively, it is reasonable to hypothesize that faulty modulation of these regions by striatal D2R is likely to underlie the enhanced motivational value of drugs, the significant loss of control over drug intake among addicted individuals (335), and the compulsive and impulsive drug intake seen in addiction (129). Moreover, subjects at high risk for alcoholism (positive family history) but who did not suffer from alcoholism showed upregulation of striatal D2R that was associated with normal baseline activity of the OFC, ACC, and DLPFC. This finding stands in sharp contrast to the hypoactivity seen in these same frontal regions of individuals affected by alcoholism and other addictions, prompting the hypothesis that striatal D2R upregulation could have protected unaffected individuals against alcoholism by regulating circuits involved in self-regulation (340). Indeed, prospective imaging studies of brain development are increasingly revealing that abnormalities in PFC constitute a vulnerability risk for SUD (see sect. V) (170).
Animal studies also corroborate the presence of neuroadaptations in mesocortical DA synapses in the PFC as well as in corticofugal glutamate synapses in the NAc in rodents withdrawn from chronic cocaine exposure (173). The former appears to involve a partial decoupling between Giα and D2R (40), and may contribute to an exaggerated reactivity towards drugs and drug-predictive cues and to a blunted response towards natural rewards. The latter relies on cellular adaptations leading to reduced levels of extracellular glutamate in NAc (12) that might also contribute to compulsive drug seeking.
The stimulation of D2R, through tonic DA firing (implicated in motivation), without concomitant phasic firing (implicated in associative learning) (142), can oppose drug consumption through its modulation of PFC regions involved with self-regulation (149). It is recognized that dopaminergic signaling via D2R in the PFC modulates its function, including inhibitory control and cognitive flexibility, where D2R signaling appears to be dependent not only on Gi but also Gs (enhancing the excitability of cortical pyramidal neurons) (274). Indeed, optogenetic stimulation of the prelimbic cortex in cocaine-exposed rats prevented compulsive cocaine seeking, whereas its inhibition enhanced it (64). Similarly, induction of tonic activity in VTA DA neurons, which project to infralimbic and prelimbic PFC, reduced ethanol self-administration (17). Moreover, the function of the PFC in addicted individuals has been shown to predict clinical outcomes, a disrupted connectivity between PFC and striatal regions being a consistent finding among individuals addicted to various drug classes (326). The importance of PFC regulation of striatal activity in addiction phenotype in rodents was recently shown in an optogenetic study that showed that increased connectivity between the OFC [presumably infralimbic PFC (192)] and the dorsal striatum predicted compulsive self-stimulation of VTA DA neurons despite receiving noxious electric shocks (254). In this study, optogenetic inhibition of the OFC projecting terminals into the dorsal striatum inhibited compulsive self-administration. The distinct projections from the various PFC regions to the dorsal and ventral striatum are likely to account for why, in contrast to these findings, the optogenetic stimulation of the prelimbic PFC decreased compulsive cocaine consumption, whereas its inhibition increased it (64). As such, the PFC has been a target of transcranial magnetic stimulation (TMS) (BOX 3) and transcranial direct electrical stimulation (tDCS) (BOX 4) interventions for the treatment of SUD, most of which have targeted the DLPFC. The PFC is also the target for behavioral interventions aimed at strengthening executive function and decreasing the incentive salience of drugs and drug cues, in part via exposure to alternative reinforcers as a means to facilitate and support recovery.
Box 3. TMS as a potential addiction treatment.
TMS is a noninvasive technique with the potential to reduce the long-term neurophysiological (and behavioral) changes induced by chronic drug use. Although it is premature to judge its effectiveness, initial results are encouraging. For example, in one pilot study (open label), high-frequency (excitatory) TMS delivered to the left DLPFC of patients with cocaine use disorder led to significant reductions in cocaine use and craving (322). Other preliminary results also support the idea that TMS could help patients control their cravings (263, 267) and cocaine consumption (35). The few studies exploring the use of TMS for the treatment of methamphetamine addiction have yielded promising but somewhat less consistent results (201, 206, 313). In addition, a recent smoking cessation trial using TMS targeting the DLPFC and insula, bilaterally, resulted in significantly reduced cigarette consumption and nicotine dependence scores that acted synergistically with concomitant cue exposure therapy (88). Clearly, more research and larger clinical studies will be needed to identify the source of some conflicting results (98), optimize TMS parameters for different indications, and ascertain the full therapeutic potential of TMS in addiction. However, the clear antidepressive properties of high-frequency TMS to the left DLPFC (45, 260), and the promise of low-frequency TMS (to OFC or supplementary motor area) for treating obsessive compulsive disorders (22), highlight its therapeutic potential for addiction, which shares key nosological features with these conditions. For these reasons, TMS has also emerged as a promising technique to treat patients with co-morbid SUD and other mental illnesses (73, 325, 331).
Box 4. tDCS as a potential addiction treatment.
tDCS is an alternative noninvasive brain modulation technique with therapeutic potential whereby some (hard to assess) portion of the current penetrates through the scalp affecting cortical excitability. Six of seven tDCS studies found significant reductions in alcohol-related craving or consumption after the treatment, whereas five of eight studies found significant reductions in nicotine cravings and/or consumption (reviewed in Ref. 70). Only two proof of principle studies investigated PFC targeting tDCS for reducing cocaine (18, 80) and while results were positive, the sample sizes were too small (11 and 17 subjects, respectively) and replication is needed. Reductions in craving were also reported in a study of 20 heroin-addicted individuals treated with tDCS targeting the fronto-temporal-parietal area (350). Similarly, bilateral tDCS targeting the DLPFC of methamphetamine users significantly decreased craving while modulating the functional connectivity of brain networks (DMN, executive control, and salience) (292).
Neuroimaging studies suggest the therapeutic effects of tDCS (as well as of TMS) could be mediated through its ability to modulate DA (69) in some of the brain areas where DA dysregulation could lead to impaired executive function and reward (113). However, much more work is required for understanding the mechanisms of action of transcranial stimulation techniques and their potential for treating addiction, including optimization of therapeutic protocols (stimulation frequency, dosing, location) and the possibility of personalized interventions based on the specific brain circuitry dysfunction of the addicted individual.
D. Negative Mood and Stress Reactivity
An important component of the addicted state is the behavioral shift that is typically observed from seeking the reward for its positive reinforcing value towards seeking it to avoid negative reinforcement (338). This state, which has been described as the “dark side” of addiction, is most evident during acute drug withdrawal and is associated with a high risk of relapse as a means to temporarily escape the experience of intense distress and negative emotionality (184). This distress is associated with reduced DA signaling in response to rewards (anhedonia) but also with an enhanced sensitivity of the brain’s stress system, including the extended amygdala, habenula, and hypothalamus (183, 273). Negative emotional states have been characterized in humans during acute and protracted abstinence from all major drugs of abuse (8), a phenomenon consistently replicated in animal studies (reviewed in Ref. 183) that contributes to the relapsing nature of addiction and likely also to its high comorbidity with depression, anxiety, and suicidality (1, 276).
Similar to typical stressors, like childhood neglect (122), acute exposure to drugs activates the hypothalamus-pituitary-adrenal (HPA) axis via the corticotropin releasing factor (CRF) (reviewed in Ref. 273), which stimulates production of ACTH in the anterior pituitary and, secondarily, cortisol by the adrenal cortex. In turn, HPA axis activation influences brain circuits involved in drug reward and the acquisition of drug-seeking behavior. Stress induces reinstatement of drug-seeking behavior in animal models of drug consumption, exemplifying the link between reward and stress systems (221). Molecules implicated in the regulation of stress-induced reinstatement include CRF, norepinephrine, DA, glutamate, dynorphin, hypocretin, neuropeptide Y, and others (221). These messengers act at various sites that include the bed nucleus of the stria terminalis (BNST), central amygdala, VTA, NAc, habenula (38, 266), dorsal raphe, locus coeruleus, and several PFC regions (221). CRF activation can translate an aversive event (e.g., social defeat, foot-shock) into robust DA increases in NAc, which though seemingly paradoxical, reflect the fact that CRF acts on a specific subset of DA VTA neurons (158) that are tuned to aversive rather than rewarding stimuli (159).
E. Interoceptive Awareness
The transition from flexible, goal-directed to reflexive, compulsive behaviors is also influenced by interoceptive and exteroceptive inputs. The insula, particularly its most anterior region, plays a major role in interoception through its involvement in sensing and integrating information about the internal physiological state (in the context of ongoing activity) and conveying it to the ACC, ventral striatum, and ventral medial PFC to initiate adaptive responses (256). The bidirectional communication between the insula and these limbic regions suggests a role in the integration of autonomic and visceral information (including information conveyed from the vagal nerve to the nucleus tractus solitarius) with emotive and motivational information that enables the conscious awareness of internal urges.
The relevance of the insula to addiction first emerged with a seminal study that showed that smokers with insular lesions (due to stroke) were able to quit smoking with remarkable ease, without cravings or relapse (243). Since then, multiple imaging studies have shown differential activation of the anterior insula during craving for nicotine (236), cocaine (278), and alcohol (285) and of the middle insula with cocaine and cigarette craving (20, 216). As such, insular reactivity has been proposed to offer a potential biomarker for relapse risk (164) and a target for TMS and tDCS as addiction treatments (94, 218) (BOXES 3 and 4).
The enhanced engagement of interoceptive processes in addiction also recruits the default mode network (DMN), which is also modulated by DA (242, 327). The DMN is involved in self-awareness and mind wandering, and its enhanced activation in the addictive state might redirect exaggerated attention towards the internal state of craving or discomfort. Not unexpectedly, imaging studies have revealed that addiction is associated with impairment within regions that are part of the DMN as well as between the DMN and other functional brain networks (364). This includes studies showing disrupted activity or connectivity of the ACC (part of the anterior DMN) and insula (202, 243). Additionally, neuroimaging studies have also revealed alterations in the precuneus (increased activation to drug-predictive cues and connectivity), a key region within the posterior DMN involved with the internal awareness of the perception of environmental stimuli (exteroception) and for self-monitoring in addicted individuals (84).
V. VULNERABILITY FACTORS
Repeated exposure to a drug of abuse is a prerequisite for the development of drug addiction, but its overt clinical manifestation depends heavily on interacting biological, environmental, and psychosocial factors.
A. Genetics and Epigenetics
Genetic variation plays a significant role in establishing interindividual differences in addiction risk. Studies focused on variability among identical and nonidentical siblings have produced a rough estimate of ~50% for the contribution of genetic differences to overall addiction risk. Genetic studies have reported an overlap in genetic variants that influence risk towards different classes of drugs (332), and the largest study to date on 1.2 million individuals that assessed common genes in alcohol and nicotine use has identified genes involved with dopaminergic and glutamatergic neurotransmission, genes involved with transcription and translation, and with brain development (205). They have also revealed that an important genetic contributor to SUDs appears to operate through a general purpose underlying mechanism (i.e., a shared predisposition) that influences a vulnerability for disorders characterized by pathological tendencies to violate social norms or to engage in oppositional behaviors (clustered as disorders with externalizing tendencies) (178), providing a link to the heterogeneous construct referred to as impulsivity (87). However, common genetic vulnerability has also been reported for SUD and internalizing disorders (282), providing a link for the frequent comorbidity between SUD and anxiety and depression (207, 268). In addition to these common genetic factors, studies have also identified genetic variants that are mostly specific for a given drug. Most notable are the genetic variants that encode for the alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) enzymes that lead to impaired metabolism of alcohol and that provide protection against alcoholism (74).
Multiple genome-wide association studies (GWAS) have identified genetic variants associated with specific drug addictions (28, 143, 279) (see Supplemental Table 1; https://github.com/rubenbaler/PRV-00014-2018R1/blob/master/ST.1.docx). However, like other complex biobehavioral disorders, addiction is a polygenic disease likely hinging on multiple genes and genetic networks (205, 229). Addiction-associated gene variants can impact the risk of abuse and addiction via direct or indirect influences on neurotransmitter systems, drug metabolic pathways, neural circuitry, cellular physiology, brain development, and personalities and traits (e.g., novelty seeking, impulsivity) that influence the behavioral responses to environmental stimuli. Although genetic research of addiction has not yet translated into novel therapeutics for SUD (105, 199, 261), there has been steady progress towards the identification of genetic biomarkers with translational potential for nicotine addiction (21, 29) and opioid use disorders (OUD) (32). Because genetic background can dramatically alter the phenotypic expressivity of different sets of genes, studies are needed to tease out modifier loci in diverse populations and the epigenetic modifications regulating them (104, 261, 264). Moreover, genetic findings offer clues as to which molecular networks might underlie the associations with addiction, thus expanding the array of potential targets for therapeutics development. This, coupled with systems biology analyses that examine Gene × Gene, Gene × Environment, and Gene × Environment × Development interactions are promising future strategies for therapeutics development and for identification of biomarkers.
Genetic studies have helped advance our understanding of the neurobiological processes involved in addiction. For example, the finding that a polymorphism in the α5 nicotinic receptor is associated with an increased risk of nicotine addiction triggered attention to the role of the habenula, which displays a substantial concentration of α5 nicotinic receptors (197) and also of MOR (118). This has revealed that the habenula is not only implicated in nicotine addiction but that it also participates in the negative affective states associated with the chronic use of various drugs of abuse, including alcohol (174) and opioids (222).
Our expanded understanding of how epigenetic modifications regulate the enhancement or silencing of gene expression has also led to studies that are beginning to characterize the effects of drugs on epigenetic marks in various brain regions as well as their involvement in the addiction process (261, 347). Arguably, much of the information comes from studies focused on the effects of repeated cocaine exposure on DNA methylation and posttranslational modifications of histone proteins that regulate the accessibility of DNA to the transcription machinery via a dynamic process of chromatin remodeling. For example, systemic administration of sodium butyrate, a general histone deacetylase (HDAC) inhibitor, facilitates extinction of a previously established cocaine-conditioned place preference in a mouse model of addiction (219). This result is consistent with previous studies showing that some of the behavioral effects of chronic cocaine are associated with the recruitment of HDACs to reduce histone acetylation and gene activity (270). Some of the drug effects in the modulation of gene expression are rather generalized, while others are gene and/or paradigm specific. For example, in the striatum, hyperacetylation of H4 at specific chromatin locations along the cFos gene promoter (with its concurrent transcriptional activation) occurs after acute but not chronic cocaine administration. In contrast, hyperacetylation of H3 at the Bdnf and Cdk5 promoters (with their concurrent transcriptional activation) is seen after chronic but not acute cocaine. Interestingly, the levels of H4 and H3 acetylation were found to increase in the NAc shell (not the core) after chronic but not acute cocaine self-administration (190). Epigenetic changes appear to contribute to different components of the addiction trajectory that might serve to uncover new candidates for medications development. Any targeted manipulation of epigenetic marks for therapeutic purposes, however, is presently a distant and challenging prospect.
B. Development
The transition from initial drug exposure to repeated use and subsequently to addiction depends, to a considerable extent, on age and developmental stage. While drug exposure alters brain function, the outcomes of that interaction change as a function of ongoing developmental and aging processes (126, 150). Certain ages and developmental periods (e.g., fetal, childhood, and adolescence) are characterized by broader or more rapid changes than others and, accordingly, exposure to drugs or adverse environmental stimuli during such critical time windows can have dire consequences for normal brain development and addiction vulnerability. A critical factor in the susceptibility of adolescents to risky behaviors, including drug-taking, pertains to the fact that PFC circuitry, which is necessary for self-regulation, is not fully developed until early adulthood (127). The neurobiological underpinnings of this critical transition are not fully understood, but an outline of significant and malleable events during brain development is beginning to emerge.
For example, the protracted period encompassing childhood and adolescence is characterized by increases in white matter volume and organization (195), while cortical gray matter shows a bimodal curve, increasing in volume until the onset of adolescence and then starting to decrease again (127). Brain maturation is also associated with an increased ability to synchronize neural oscillations in several frequency bands (329), and the trajectories of these processes are predictive of brain performance and cognitive abilities (83, 179, 329). Drugs of abuse can perturb these processes, an effect that has been most widely investigated in association with alcohol (139, 307) and cannabis (19, 100, 302) use. Delays in maturation of PFC networks due to drug exposures, genetics, or social deprivation appear to increase risky behaviors in adolescents (including drug-taking). Indeed, brain imaging studies in adolescents have begun to associate abnormalities in PFC function and structure with a higher risk for SUD, consistent with the role of PFC in self-regulation and its disruption as a factor contributing to vulnerability for drug use (170, 216).
C. Social Environment
Epidemiological studies have consistently recognized that environments with a high level of social stressors and poor social support (16, 48, 50, 330) along with easy accessibility to drugs (115, 124, 125) and lack of alternative reinforcers (180) lead to an elevated risk for drug experimentation and addiction. Neuroscientific studies have started to unveil how adverse social environments and lack of opportunities affect the human brain (46) and why early developmental stages may be the most sensitive to such detrimental influences. It is now recognized that brain development is influenced not just by genetic factors but also by environmental exposures (81, 181, 303). Adverse social environments during early childhood have been consistently associated with delayed maturation of prefrontal-limbic connectivity (133). For example, children with a history of early adversity display atypical coupling between amygdala and medial PFC. This abnormal connectivity pattern is partly driven by the actions of the stress hormone cortisol (121) and likely contributes to increased impulsivity (101) and SUD risk (233). The type of persistent social stress that can be triggered by having a subordinate rank among non-human primates (237) or by having poor social support systems in humans (355) has been associated with reduced striatal D2R expression and linked to higher risk for impulsivity and drug use (223, 237, 355). And, as already mentioned, easy access to drugs is an essential contributor to early drug experimentation and repeated consumption (115, 124, 125). Results of both animal (55, 288) and human (60, 309) studies provide compelling evidence that when exposure to drugs occurs during childhood or adolescence it can interfere with developmental brain trajectories, thus exacerbating adverse outcomes (72).
To better ascertain the effects of early drug exposure and the social environment upon developmental brain trajectories, National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism, together with other institutes at the National Institutes of Health, recently launched the Adolescent Brain Cognitive Development (ABCD) study, which will follow over 10,000 youth in the United States as they transition from childhood to adolescence to adulthood, while monitoring their physical and mental health, neurocognition, social environment, substance use, genetic and other biomarkers, and their structural and functional brain development (167).
VI. CLINICAL IMPLICATIONS
A. Prevention
Evidence suggests that prevention of SUDs must include universal components (TABLE 3), including the enhancement of protective factors (e.g., parental support, education) and reverse or reduce risk factors (e.g., deviant behavior, drug abusing peers, social neglect) (10, 148) and should address all forms of drug misuse, including underage use of legal (e.g., tobacco or alcohol), prescription (e.g., stimulant medications), and illicit (230) drugs. Such programs can be implemented in the family, school, and/or community environments.
Table 3.
Prevention matrix
| Risk Factors | Domain | Protective Factors | Reference Nos. |
|---|---|---|---|
| Early aggressive behavior, dysregulated stress response, positive attitudes toward substance use, genetic risk, psychiatric disorder | Individual | Social competence, self-regulatory skills, school readiness, supportive parenting | 41, 61, 135, 137, 233, 257, 258, 301 |
| Harsh or absent parenting | Family | Positive parenting | 42, 248 |
| Antisocial influence | Peer | Positive peer networks | 305 |
| Poor classroom management, poor control of drug availability | School | Positive school environment, teacher behavior management competence | 20, 110, 177, 191 |
| Laws, policies, and perceived norms for substance use | Community | Community-level strategies to prevent substance use | 44, 141 |
In addition, tailored prevention interventions should address the specific circumstances of those at higher risk, including people suffering from other mental illnesses or specific disadvantaged conditions. The strengthening of self-control is one example of a promising tailored individual-based intervention. When assessed early in life, poor self-control is a personal characteristic predictive of higher vulnerability for SUD as well as worse physical health, lower economic wealth, and greater criminal involvement (233). Importantly, various training approaches have been identified that can help enhance self-control and other dimensions of executive function when implemented among school-aged children; their usefulness when applied to adults, however, is unclear (116). Effective approaches include enhanced social-emotional and language-literacy programs (283), music education (165), and specific sports programs designed to build individual competences and promote enjoyment (162). A recent summary report paints a promising picture vis á vis the benefits of such interventions for improving self-control circuitry and function as a universal prevention strategy (241). Another promising prevention intervention is the use of mindfulness training to improve self-control, emotional regulation, and stress reactivity, which could also be harnessed for therapeutic purposes (see Ref. 321 and below). An exciting development in prevention research is the recent report that positive parenting (i.e., enhanced supportive parenting style) can overcome the negative effects of childhood poverty on brain development (43). This intervention, a randomized trial of the “Strong African American Families” program among parents and their 11-yr-old children blunted the reductions in specific brain volumes (e.g., left dentate gyrus and CA3 hippocampal subfields and left amygdala) previously associated with childhood poverty (212). This result suggests that enhanced early caregiving (like supportive parenting) could help buffer some of the detrimental effects of adverse social environments (43).
Several studies have also shown that it is possible to target abnormal stress reactivity in children impacted by early adversity and mitigate its impact. For example, studies of children at developmental risk (e.g., in foster care, maltreated, or who suffered parental depression or death) consistently show that psychosocial interventions (e.g., enriched environment, caregiver/parental training) can lower cortisol levels towards the range seen in a low-risk comparison group (304). Similarly, compared with institutional care as usual, high-quality foster care had a positive impact on the integrity of several white matter tracts (27), including those in the external capsule and corpus callosum, whose abnormalities had been associated with neglect-associated emergence of internalizing symptoms in middle childhood or early adolescence (26).
B. Treatment
Medications approved for the treatment of SUD are limited to opioid, nicotine, and alcohol use disorders (TABLE 4). There are several promising candidates for the treatment of stimulant and OUD that are undergoing clinical trials (see Supplementary TABLE 2; https://github.com/rubenbaler/PRV-00014-2018R1/blob/master/ST.2.docx), although for the most part research is still at the preclinical stages. On the other hand, the characterization of the various neuronal circuits disrupted in addiction identifies them as suitable targets for personalized interventions. For example, strengthening of self-control fronto-cortical circuitry might help prevent relapse (130, 320). The amygdala/hippocampus (mediating emotions, mood, and stress reactivity) and the insula (responsible for integrating and translating interoceptive signals) are also relevant as targets for addiction treatment (246, 339).
Table 4.
Current FDA-approved pharmacological treatments for SUD
| SUD | Current Status | FDA-Approved Medication | Promising Targets | Reference Nos. |
|---|---|---|---|---|
| Opioids (OUD) | Mu opioid receptor (MOR) drugs with different properties have been approved by the FDA and are recommended as first-line interventions, supported by high-quality evidence. Despite their effectiveness, relapse rates are very high (50% within 6 mo). | Maintenance medications: buprenorphine (partial agonist), methadone (full agonist), naltrexone (antagonist). Reverse opioid overdoses: naloxone. Control opioid withdrawal: lofexidine. | 93, 134, 353 | |
| α-Adrenergic agonists for opioid withdrawal. | ||||
| Nicotine | There are FDA-approved pharmacotherapies for smoking cessation. Despite their effectiveness, relapse rates are very high (75% within 1 yr). | Nicotine replacement therapies: patches, oral and nasal sprays, inhalers, and lozenges. Bupropion, varenicline. | Nortriptyline, clonidine | 131, 265 |
| Alcohol (AUD) | There are three FDA-approved medications for the treatment of AUD, displaying different mechanisms of action. | Disulfiram is an ADH inhibitor that blocks the breakdown of alcohol leading to a buildup of acetaldehyde that results in unpleasant side effects. Naltrexone is a MOR and KOR antagonist that reduces the pleasure a person with AUD can experience while drinking. Acamprosate is an NMDA receptor function booster that appears to reduce or stop severe alcohol withdrawal symptoms during detoxification. | Anticonvulsants: gabapentin, topiramate, and pregabalin. Antipsychotics: quetiapine and aripiprazole. Antidepressants: duloxetine and venlafaxine. Others: baclofen, ondansetron, and nalmefene. | 4 |
| Stimulants | No FDA-approved medications | NMDAR antagonist ketamine, A2AR antagonists, 5-HT2CR agonist lorcaserin, N-acetylcysteine, D3R antagonists, cannabidiol | 14, 71, 163 | |
| Cannabis (CUD) | No FDA-approved medications | CB1R agonists, gabapentin, N-acetylcysteine, FAAH inhibitors | 132, 280 |
Current United States Food and Drug Administration (FDA)-approved pharmacological treatments for substance use disorder (SUD) and other promising medications chosen based on reproducible preclinical findings that interfered with drug consumption, prevented relapse, or inhibited craving and/or on pilot clinical studies showing positive results in interfering with subjective drug reward, drug consumption, reducing craving, or withdrawal.
These could be translated into the next generation of non-medication-based interventions (i.e., targeted behavioral training, noninvasive modulation) designed to increase the effectiveness of control networks as a way to treat addiction, even among those without intention to quit (320). By the same token, TMS or tDCS could prove helpful in reducing craving by modulating insular activity (BOXES 3 and 4). Finally, there is experimental evidence suggesting that mindfulness-based techniques may positively impact cognitive processes (319) and mitigate addictive behaviors (97, 200, 321, 358). Indeed, preliminary imaging data showing that mindfulness activated the amygdala, striatum, ACC, PFC, and insula, which are regions that modulate emotion, self-regulation, and interoception, highlight its potential promise in addiction treatment (319).
VII. CONCLUSIONS
Significant advances in neuroscience have given us an understanding of the effects of drugs in the brain that result in addiction, which have led to the recognition that addiction is a chronic brain disorder that should be treated as done for any other medical condition. Similarly, our increased understanding of the neurobiological processes that are associated with vulnerability for drug experimentation and SUD, including the effects of exposure to adverse environmental conditions, is helping redefine our thinking around tailored prevention interventions for at-risk individuals. However, much work is still needed to capture the complexity of the effects of drugs and other rewards in our brains, to understand how they interact, and how they ultimately motivate behavior.
GRANTS
This work was supported by the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism.
DISCLOSURES
M. Michaelides is a cofounder and owns stock in Metis Laboratories, Inc. No conflicts of interest, financial or otherwise, are declared by the other authors.
ACKNOWLEDGMENTS
Address for reprint requests and other correspondence: N. D. Volkow, NSC Bldg., Rm. 5274, MS 9581, 6001 Executive Blvd., Rockville, MD 20852 (e-mail: nvolkow@nida.nih.gov).
REFERENCES
- 1.Abel KF, Skjærvø I, Ravndal E, Clausen T, Bramness JG. Perceived Self-Control is Related to Mental Distress in Patients Entering Substance Use Disorder Treatment. Subst Use Misuse 53: 1454–1462, 2018. doi: 10.1080/10826084.2017.1413114. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad FB, Escobedo LA, Rossen LM, Spencer MR, Warner M, Provisional Drug Overdose Death Counts PS. NVSS Vital Statistics Rapid Release, edited by National Center for Health Statistics Washington, DC: CDC/NCHS, 2019. [Google Scholar]
- 3.Ahmad T, Sun N, Lyons D, Laviolette SR. Bi-directional cannabinoid signalling in the basolateral amygdala controls rewarding and aversive emotional processing via functional regulation of the nucleus accumbens. Addict Biol 22: 1218–1231, 2017. doi: 10.1111/adb.12406. [DOI] [PubMed] [Google Scholar]
- 4.Akbar M, Egli M, Cho YE, Song BJ, Noronha A. Medications for alcohol use disorders: an overview. Pharmacol Ther 185: 64–85, 2018. doi: 10.1016/j.pharmthera.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Hasani R, Bruchas MR. Molecular mechanisms of opioid receptor-dependent signaling and behavior. Anesthesiology 115: 1363–1381, 2011. doi: 10.1097/ALN.0b013e318238bba6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alonso-Alonso M, Woods SC, Pelchat M, Grigson PS, Stice E, Farooqi S, Khoo CS, Mattes RD, Beauchamp GK. Food reward system: current perspectives and future research needs. Nutr Rev 73: 296–307, 2015. doi: 10.1093/nutrit/nuv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: basic findings from the National Comorbidity Survey. Drug Alcohol Depend 2: 244–268, 1994. doi: 10.1037/1064-1297.2.3.244. [DOI] [Google Scholar]
- 8.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (5th ed.) Washington, DC: American Psychiatric Press, 1994. [Google Scholar]
- 9.Aragona BJ, Cleaveland NA, Stuber GD, Day JJ, Carelli RM, Wightman RM. Preferential enhancement of dopamine transmission within the nucleus accumbens shell by cocaine is attributable to a direct increase in phasic dopamine release events. J Neurosci 28: 8821–8831, 2008. doi: 10.1523/JNEUROSCI.2225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arthur MW, Hawkins JD, Brown EC, Briney JS, Oesterle S, Abbott RD; Social Development Research Group . Implementation of the Communities That Care Prevention System by Coalitions in the Community Youth Development Study. J Community Psychol 38: 245–258, 2010. doi: 10.1002/jcop.20362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Avelar AJ, Juliano SA, Garris PA. Amphetamine augments vesicular dopamine release in the dorsal and ventral striatum through different mechanisms. J Neurochem 125: 373–385, 2013. doi: 10.1111/jnc.12197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci 6: 743–749, 2003. doi: 10.1038/nn1069. [DOI] [PubMed] [Google Scholar]
- 13.Balfour DJ. The neuronal pathways mediating the behavioral and addictive properties of nicotine. Handb Exp Pharmacol 192: 209–233, 2009. doi: 10.1007/978-3-540-69248-5_8. [DOI] [PubMed] [Google Scholar]
- 14.Ballesteros-Yáñez I, Castillo CA, Merighi S, Gessi S. The Role of Adenosine Receptors in Psychostimulant Addiction. Front Pharmacol 8: 985, 2018. doi: 10.3389/fphar.2017.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balster RL. Neural basis of inhalant abuse. Drug Alcohol Depend 51: 207–214, 1998. doi: 10.1016/S0376-8716(98)00078-7. [DOI] [PubMed] [Google Scholar]
- 16.Barton AW, Brody GH, Zapolski TCB, Goings TC, Kogan SM, Windle M, Yu T. Trajectory classes of cannabis use and heavy drinking among rural African American adolescents: multi-level predictors of class membership. Addiction 113: 1439–1449, 2018. doi: 10.1111/add.14200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bass CE, Grinevich VP, Gioia D, Day-Brown JD, Bonin KD, Stuber GD, Weiner JL, Budygin EA. Optogenetic stimulation of VTA dopamine neurons reveals that tonic but not phasic patterns of dopamine transmission reduce ethanol self-administration. Front Behav Neurosci 7: 173, 2013. doi: 10.3389/fnbeh.2013.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Batista EK, Klauss J, Fregni F, Nitsche MA, Nakamura-Palacios EM. A Randomized Placebo-Controlled Trial of Targeted Prefrontal Cortex Modulation with Bilateral tDCS in Patients with Crack-Cocaine Dependence. Int J Neuropsychopharmacol 18: pyv066, 2015. doi: 10.1093/ijnp/pyv066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker MP, Collins PF, Lim KO, Muetzel RL, Luciana M. Longitudinal changes in white matter microstructure after heavy cannabis use. Dev Cogn Neurosci 16: 23–35, 2015. doi: 10.1016/j.dcn.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beets MW, Flay BR, Vuchinich S, Snyder FJ, Acock A, Li KK, Burns K, Washburn IJ, Durlak J. Use of a social and character development program to prevent substance use, violent behaviors, and sexual activity among elementary-school students in Hawaii. Am J Public Health 99: 1438–1445, 2009. doi: 10.2105/AJPH.2008.142919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belsky DW, Moffitt TE, Baker TB, Biddle AK, Evans JP, Harrington H, Houts R, Meier M, Sugden K, Williams B, Poulton R, Caspi A. Polygenic risk and the developmental progression to heavy, persistent smoking and nicotine dependence: evidence from a 4-decade longitudinal study. JAMA Psychiatry 70: 534–542, 2013. doi: 10.1001/jamapsychiatry.2013.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berlim MT, Neufeld NH, Van den Eynde F. Repetitive transcranial magnetic stimulation (rTMS) for obsessive-compulsive disorder (OCD): an exploratory meta-analysis of randomized and sham-controlled trials. J Psychiatr Res 47: 999–1006, 2013. doi: 10.1016/j.jpsychires.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 23.Beroun A, Nalberczak-Skóra M, Harda Z, Piechota M, Ziółkowska M, Cały A, Pagano R, Radwanska K. Generation of silent synapses in dentate gyrus correlates with development of alcohol addiction. Neuropsychopharmacology 43: 1989–1999, 2018. doi: 10.1038/s41386-018-0119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertrand D, Terry AV Jr. The wonderland of neuronal nicotinic acetylcholine receptors. Biochem Pharmacol 151: 214–225, 2018. doi: 10.1016/j.bcp.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 25.Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Abstinence from chronic cocaine self-administration alters striatal dopamine systems in rhesus monkeys. Neuropsychopharmacology 34: 1162–1171, 2009. doi: 10.1038/npp.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bick J, Fox N, Zeanah C, Nelson CA. Early deprivation, atypical brain development, and internalizing symptoms in late childhood. Neuroscience 342: 140–153, 2017. doi: 10.1016/j.neuroscience.2015.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bick J, Zhu T, Stamoulis C, Fox NA, Zeanah C, Nelson CA. Effect of early institutionalization and foster care on long-term white matter development: a randomized clinical trial. JAMA Pediatr 169: 211–219, 2015. doi: 10.1001/jamapediatrics.2014.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bierut LJ, Madden PA, Breslau N, Johnson EO, Hatsukami D, Pomerleau OF, Swan GE, Rutter J, Bertelsen S, Fox L, Fugman D, Goate AM, Hinrichs AL, Konvicka K, Martin NG, Montgomery GW, Saccone NL, Saccone SF, Wang JC, Chase GA, Rice JP, Ballinger DG. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum Mol Genet 16: 24–35, 2007. doi: 10.1093/hmg/ddl441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bierut LJ, Tyndale RF. Preparing the Way: Exploiting Genomic Medicine to Stop Smoking. Trends Mol Med 24: 187–196, 2018. doi: 10.1016/j.molmed.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blair MA, Stewart JL, May AC, Reske M, Tapert SF, Paulus MP. Blunted Frontostriatal Blood Oxygen Level-Dependent Signals Predict Stimulant and Marijuana Use. Biol Psychiatry Cogn Neurosci Neuroimaging 3: 947–958, 2018. doi: 10.1016/j.bpsc.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bloomfield MA, Ashok AH, Volkow ND, Howes OD. The effects of Δ9-tetrahydrocannabinol on the dopamine system. Nature 539: 369–377, 2016. doi: 10.1038/nature20153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blum K, Chen ALC, Thanos PK, Febo M, Demetrovics Z, Dushaj K, Kovoor A, Baron D, Smith DE, Roy AK III, Fried L, Chen TJH, Chapman E Sr, Modestino EJ, Steinberg B, Badgaiyan RD. Genetic addiction risk score (GARS)™, a predictor of vulnerability to opioid dependence. Front Biosci (Elite Ed) 10: 175–196, 2018. doi: 10.2741/e816. [DOI] [PubMed] [Google Scholar]
- 33.Blum K, Gardner E, Oscar-Berman M, Gold M. “Liking” and “wanting” linked to Reward Deficiency Syndrome (RDS): hypothesizing differential responsivity in brain reward circuitry. Curr Pharm Des 18: 113–118, 2012. doi: 10.2174/138161212798919110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bock R, Shin JH, Kaplan AR, Dobi A, Markey E, Kramer PF, Gremel CM, Christensen CH, Adrover MF, Alvarez VA. Strengthening the accumbal indirect pathway promotes resilience to compulsive cocaine use. Nat Neurosci 16: 632–638, 2013. doi: 10.1038/nn.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolloni C, Panella R, Pedetti M, Frascella AG, Gambelunghe C, Piccoli T, Maniaci G, Brancato A, Cannizzaro C, Diana M. Bilateral Transcranial Magnetic Stimulation of the Prefrontal Cortex Reduces Cocaine Intake: A Pilot Study. Front Psychiatry 7: 133, 2016. doi: 10.3389/fpsyt.2016.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Borroto-Escuela DO, Carlsson J, Ambrogini P, Narváez M, Wydra K, Tarakanov AO, Li X, Millón C, Ferraro L, Cuppini R, Tanganelli S, Liu F, Filip M, Diaz-Cabiale Z, Fuxe K. Understanding the Role of GPCR Heteroreceptor Complexes in Modulating the Brain Networks in Health and Disease. Front Cell Neurosci 11: 37, 2017. doi: 10.3389/fncel.2017.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Borycz J, Pereira MF, Melani A, Rodrigues RJ, Köfalvi A, Panlilio L, Pedata F, Goldberg SR, Cunha RA, Ferré S. Differential glutamate-dependent and glutamate-independent adenosine A1 receptor-mediated modulation of dopamine release in different striatal compartments. J Neurochem 101: 355–363, 2007. doi: 10.1111/j.1471-4159.2006.04386.x. [DOI] [PubMed] [Google Scholar]
- 38.Boulos LJ, Darcq E, Kieffer BL. Translating the Habenula-From Rodents to Humans. Biol Psychiatry 81: 296–305, 2017. doi: 10.1016/j.biopsych.2016.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bowen SE, Batis JC, Paez-Martinez N, Cruz SL. The last decade of solvent research in animal models of abuse: mechanistic and behavioral studies. Neurotoxicol Teratol 28: 636–647, 2006. doi: 10.1016/j.ntt.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Bowers MS, McFarland K, Lake RW, Peterson YK, Lapish CC, Gregory ML, Lanier SM, Kalivas PW. Activator of G protein signaling 3: a gatekeeper of cocaine sensitization and drug seeking. Neuron 42: 269–281, 2004. doi: 10.1016/S0896-6273(04)00159-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brennan LM, Shaw DS, Dishion TJ, Wilson MN. The Predictive Utility of Early Childhood Disruptive Behaviors for School-Age Social Functioning. J Abnorm Child Psychol 43: 1187–1199, 2015. doi: 10.1007/s10802-014-9967-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brody GH, Chen YF, Beach SR, Philibert RA, Kogan SM. Participation in a family-centered prevention program decreases genetic risk for adolescents’ risky behaviors. Pediatrics 124: 911–917, 2009. doi: 10.1542/peds.2008-3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brody GH, Gray JC, Yu T, Barton AW, Beach SR, Galván A, MacKillop J, Windle M, Chen E, Miller GE, Sweet LH. Protective Prevention Effects on the Association of Poverty With Brain Development. JAMA Pediatr 171: 46–52, 2017. doi: 10.1001/jamapediatrics.2016.2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown EC, Hawkins JD, Rhew IC, Shapiro VB, Abbott RD, Oesterle S, Arthur MW, Briney JS, Catalano RF. Prevention system mediation of communities that care effects on youth outcomes. Prev Sci 15: 623–632, 2014. doi: 10.1007/s11121-013-0413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brunelin J, Poulet E, Boeuve C, Zeroug-vial H, d’Amato T, Saoud M. [Efficacy of repetitive transcranial magnetic stimulation (rTMS) in major depression: a review]. Encephale 33: 126–134, 2007. doi: 10.1016/S0013-7006(07)91542-0. [DOI] [PubMed] [Google Scholar]
- 46.Bucci M, Marques SS, Oh D, Harris NB. Toxic Stress in Children and Adolescents. Adv Pediatr 63: 403–428, 2016. doi: 10.1016/j.yapd.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Calabria B, Degenhardt L, Briegleb C, Vos T, Hall W, Lynskey M, Callaghan B, Rana U, McLaren J. Systematic review of prospective studies investigating “remission” from amphetamine, cannabis, cocaine or opioid dependence. Addict Behav 35: 741–749, 2010. doi: 10.1016/j.addbeh.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 48.Calcaterra SL, Beaty B, Mueller SR, Min SJ, Binswanger IA. The association between social stressors and drug use/hazardous drinking among former prison inmates. J Subst Abuse Treat 47: 41–49, 2014. doi: 10.1016/j.jsat.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Calipari ES, Bagot RC, Purushothaman I, Davidson TJ, Yorgason JT, Peña CJ, Walker DM, Pirpinias ST, Guise KG, Ramakrishnan C, Deisseroth K, Nestler EJ. In vivo imaging identifies temporal signature of D1 and D2 medium spiny neurons in cocaine reward. Proc Natl Acad Sci USA 113: 2726–2731, 2016. doi: 10.1073/pnas.1521238113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campbell-Sills L, Ursano RJ, Kessler RC, Sun X, Heeringa SG, Nock MK, Sampson NA, Jain S, Stein MB. Prospective risk factors for post-deployment heavy drinking and alcohol or substance use disorder among US Army soldiers. Psychol Med 48: 1624–1633, 2018. doi: 10.1017/S0033291717003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Canal CE, Murnane KS. The serotonin 5-HT2C receptor and the non-addictive nature of classic hallucinogens. J Psychopharmacol 31: 127–143, 2017. doi: 10.1177/0269881116677104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cantin L, Lenoir M, Augier E, Vanhille N, Dubreucq S, Serre F, Vouillac C, Ahmed SH. Cocaine is low on the value ladder of rats: possible evidence for resilience to addiction. PLoS One 5: e11592, 2010. doi: 10.1371/journal.pone.0011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carelli RM, West EA. When a good taste turns bad: neural mechanisms underlying the emergence of negative affect and associated natural reward devaluation by cocaine. Neuropharmacology 76, Pt B: 360–369, 2014. doi: 10.1016/j.neuropharm.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carim-Todd L, Mitchell SH, Oken BS. Impulsivity and Stress Response in Nondependent Smokers (Tobacco Chippers) in Comparison to Heavy Smokers and Nonsmokers. Nicotine Tob Res 18: 547–556, 2016. doi: 10.1093/ntr/ntv210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Carvalho AF, Reyes BA, Ramalhosa F, Sousa N, Van Bockstaele EJ. Repeated administration of a synthetic cannabinoid receptor agonist differentially affects cortical and accumbal neuronal morphology in adolescent and adult rats. Brain Struct Funct 221: 407–419, 2016. doi: 10.1007/s00429-014-0914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Centers for Disease Control and Prevention Alcohol-Related Disease Impact (ARDI). https://www.cdc.gov/alcohol/fact-sheets/alcohol-use.htm.
- 57.Centers for Disease Control and Prevention Provisional Drug Overdose Death Counts Atlanta, GA 30329–4027 USA. https://www.cdc.gov/drugoverdose/data/statedeaths.html.
- 58.Centers for Disease Control and Prevention Smoking & Tobacco Use: Tobacco-Related Mortality. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/tobacco_related_mortality/index.htm.
- 59.Centonze D, Grande C, Saulle E, Martin AB, Gubellini P, Pavón N, Pisani A, Bernardi G, Moratalla R, Calabresi P. Distinct roles of D1 and D5 dopamine receptors in motor activity and striatal synaptic plasticity. J Neurosci 23: 8506–8512, 2003. doi: 10.1523/JNEUROSCI.23-24-08506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chadwick B, Miller ML, Hurd YL. Cannabis Use During Adolescent Development: Susceptibility to Psychiatric Illness. Front Psychiatry 4: 129, 2013. doi: 10.3389/fpsyt.2013.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang H, Shaw DS, Dishion TJ, Gardner F, Wilson MN. Direct and indirect effects of the family check-up on self-regulation from toddlerhood to early school-age. J Abnorm Child Psychol 42: 1117–1128, 2014. doi: 10.1007/s10802-014-9859-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Charbogne P, Kieffer BL, Befort K. 15 years of genetic approaches in vivo for addiction research: ppioid receptor and peptide gene knockout in mouse models of drug abuse. Neuropharmacology 76, Pt B: 204–217, 2014. doi: 10.1016/j.neuropharm.2013.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci 27: 791–795, 2007. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, Bonci A. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature 496: 359–362, 2013. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- 65.Chen J, Papies EK, Barsalou LW. A core eating network and its modulations underlie diverse eating phenomena. Brain Cogn 110: 20–42, 2016. doi: 10.1016/j.bandc.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 66.Chen M, Zhao Y, Yang H, Luan W, Song J, Cui D, Dong Y, Lai B, Ma L, Zheng P. Morphine disinhibits glutamatergic input to VTA dopamine neurons and promotes dopamine neuron excitation. eLife 4: e09275, 2015. doi: 10.7554/eLife.09275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chhatbar PY, Kautz SA, Takacs I, Rowland NC, Revuelta GJ, George MS, Bikson M, Feng W. Evidence of transcranial direct current stimulation-generated electric fields at subthalamic level in human brain in vivo. Brain Stimul 11: 727–733, 2018. doi: 10.1016/j.brs.2018.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Childress AR, McLellan AT, Ehrman R, O’Brien CP. Classically conditioned responses in opioid and cocaine dependence: a role in relapse? NIDA Res Monogr 84: 25–43, 1988. [PubMed] [Google Scholar]
- 69.Cho SS, Strafella AP. rTMS of the left dorsolateral prefrontal cortex modulates dopamine release in the ipsilateral anterior cingulate cortex and orbitofrontal cortex. PLoS One 4: e6725, 2009. doi: 10.1371/journal.pone.0006725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Coles AS, Kozak K, George TP. A review of brain stimulation methods to treat substance use disorders. Am J Addict 27: 71–91, 2018. doi: 10.1111/ajad.12674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Collins GT, Gerak LR, France CP. The behavioral pharmacology and therapeutic potential of lorcaserin for substance use disorders. Neuropharmacology 142: 63–71, 2018. doi: 10.1016/j.neuropharm.2017.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Conrod PJ, Nikolaou K. Annual Research Review: On the developmental neuropsychology of substance use disorders. J Child Psychol Psychiatry 57: 371–394, 2016. doi: 10.1111/jcpp.12516. [DOI] [PubMed] [Google Scholar]
- 73.Conway KP, Green VR, Kasza KA, Silveira ML, Borek N, Kimmel HL, Sargent JD, Stanton C, Lambert E, Hilmi N, Reissig CJ, Jackson KJ, Tanski SE, Maklan D, Hyland AJ, Compton WM. Co-occurrence of tobacco product use, substance use, and mental health problems among adults: Findings from Wave 1 (2013-2014) of the Population Assessment of Tobacco and Health (PATH) Study. Drug Alcohol Depend 177: 104–111, 2017. doi: 10.1016/j.drugalcdep.2017.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Crabb DW, Matsumoto M, Chang D, You M. Overview of the role of alcohol dehydrogenase and aldehyde dehydrogenase and their variants in the genesis of alcohol-related pathology. Proc Nutr Soc 63: 49–63, 2004. doi: 10.1079/PNS2003327. [DOI] [PubMed] [Google Scholar]
- 75.Cui G, Jun SB, Jin X, Pham MD, Vogel SS, Lovinger DM, Costa RM. Concurrent activation of striatal direct and indirect pathways during action initiation. Nature 494: 238–242, 2013. doi: 10.1038/nature11846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.D’Souza MS, Markou A. Neuronal mechanisms underlying development of nicotine dependence: implications for novel smoking-cessation treatments. Addict Sci Clin Pract 6: 4–16, 2011. [PMC free article] [PubMed] [Google Scholar]
- 77.Daniel R, Pollmann S. A universal role of the ventral striatum in reward-based learning: evidence from human studies. Neurobiol Learn Mem 114: 90–100, 2014. doi: 10.1016/j.nlm.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dautan D, Souza AS, Huerta-Ocampo I, Valencia M, Assous M, Witten IB, Deisseroth K, Tepper JM, Bolam JP, Gerdjikov TV, Mena-Segovia J. Segregated cholinergic transmission modulates dopamine neurons integrated in distinct functional circuits. Nat Neurosci 19: 1025–1033, 2016. doi: 10.1038/nn.4335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davis MI, Crittenden JR, Feng AY, Kupferschmidt DA, Naydenov A, Stella N, Graybiel AM, Lovinger DM. The cannabinoid-1 receptor is abundantly expressed in striatal striosomes and striosome-dendron bouquets of the substantia nigra. PLoS One 13: e0191436, 2018. doi: 10.1371/journal.pone.0191436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Almeida Ramos R, Taiar I, Trevizol AP, Shiozawa P, Cordeiro Q. Effect of a Ten-Day Prefrontal Transcranial Direct Current Stimulation Protocol for Crack Craving: A Proof-of-Concept Trial. J ECT 32: e8–e9, 2016. doi: 10.1097/YCT.0000000000000302. [DOI] [PubMed] [Google Scholar]
- 81.De Araújo Costa Folha OA, Bahia CP, de Aguiar GPS, Herculano AM, Coelho NLG, de Sousa MBC, Shiramizu VKM, de Menezes Galvão AC, de Carvalho WA, Pereira A. Effect of chronic stress during adolescence in prefrontal cortex structure and function. Behav Brain Res 326: 44–51, 2017. doi: 10.1016/j.bbr.2017.02.033. [DOI] [PubMed] [Google Scholar]
- 82.De Backer JF, Monlezun S, Detraux B, Gazan A, Vanopdenbosch L, Cheron J, Cannazza G, Valverde S, Cantacorps L, Nassar M, Venance L, Valverde O, Faure P, Zoli M, De Backer O, Gall D, Schiffmann SN, de Kerchove d’Exaerde A. Deletion of Maged1 in mice abolishes locomotor and reinforcing effects of cocaine. EMBO Rep 19: e45089, 2018. doi: 10.15252/embr.201745089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Deoni SC, O’Muircheartaigh J, Elison JT, Walker L, Doernberg E, Waskiewicz N, Dirks H, Piryatinsky I, Dean DC III, Jumbe NL. White matter maturation profiles through early childhood predict general cognitive ability. Brain Struct Funct 221: 1189–1203, 2016. doi: 10.1007/s00429-014-0947-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.DeWitt SJ, Ketcherside A, McQueeny TM, Dunlop JP, Filbey FM. The hyper-sentient addict: an exteroception model of addiction. Am J Drug Alcohol Abuse 41: 374–381, 2015. doi: 10.3109/00952990.2015.1049701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85: 5274–5278, 1988. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Di Chiara G, Imperato A. Preferential stimulation of dopamine release in the nucleus accumbens by opiates, alcohol, and barbiturates: studies with transcerebral dialysis in freely moving rats. Ann N Y Acad Sci 473, 1 Neurochemical: 367–381, 1986. doi: 10.1111/j.1749-6632.1986.tb23629.x. [DOI] [PubMed] [Google Scholar]
- 87.Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O’Malley SS, Sher K. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict Biol 15: 217–226, 2010. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dinur-Klein L, Dannon P, Hadar A, Rosenberg O, Roth Y, Kotler M, Zangen A. Smoking cessation induced by deep repetitive transcranial magnetic stimulation of the prefrontal and insular cortices: a prospective, randomized controlled trial. Biol Psychiatry 76: 742–749, 2014. doi: 10.1016/j.biopsych.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 89.Dong Y, Nestler EJ. The neural rejuvenation hypothesis of cocaine addiction. Trends Pharmacol Sci 35: 374–383, 2014. doi: 10.1016/j.tips.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dong Y, Taylor JR, Wolf ME, Shaham Y. Circuit and Synaptic Plasticity Mechanisms of Drug Relapse. J Neurosci 37: 10867–10876, 2017. doi: 10.1523/JNEUROSCI.1821-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dos Santos M, Cahill EN, Bo GD, Vanhoutte P, Caboche J, Giros B, Heck N. Cocaine increases dopaminergic connectivity in the nucleus accumbens. Brain Struct Funct 223: 913–923, 2018. doi: 10.1007/s00429-017-1532-x. [DOI] [PubMed] [Google Scholar]
- 92.Draycott B, Loureiro M, Ahmad T, Tan H, Zunder J, Laviolette SR. Cannabinoid transmission in the prefrontal cortex bi-phasically controls emotional memory formation via functional interactions with the ventral tegmental area. J Neurosci 34: 13096–13109, 2014. doi: 10.1523/JNEUROSCI.1297-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dunlap B, Cifu AS. Clinical Management of Opioid Use Disorder. JAMA 316: 338–339, 2016. doi: 10.1001/jama.2016.9795. [DOI] [PubMed] [Google Scholar]
- 94.Dunlop K, Hanlon CA, Downar J. Noninvasive brain stimulation treatments for addiction and major depression. Ann N Y Acad Sci 1394: 31–54, 2017. doi: 10.1111/nyas.12985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dunn JP, Kessler RM, Feurer ID, Volkow ND, Patterson BW, Ansari MS, Li R, Marks-Shulman P, Abumrad NN. Relationship of dopamine type 2 receptor binding potential with fasting neuroendocrine hormones and insulin sensitivity in human obesity. Diabetes Care 35: 1105–1111, 2012. doi: 10.2337/dc11-2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Easton N, Steward C, Marshall F, Fone K, Marsden C. Effects of amphetamine isomers, methylphenidate and atomoxetine on synaptosomal and synaptic vesicle accumulation and release of dopamine and noradrenaline in vitro in the rat brain. Neuropharmacology 52: 405–414, 2007. doi: 10.1016/j.neuropharm.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 97.Enkema MC, Bowen S. Mindfulness practice moderates the relationship between craving and substance use in a clinical sample. Drug Alcohol Depend 179: 1–7, 2017. doi: 10.1016/j.drugalcdep.2017.05.036. [DOI] [PubMed] [Google Scholar]
- 98.Enokibara M, Trevizol A, Shiozawa P, Cordeiro Q. Establishing an effective TMS protocol for craving in substance addiction: is it possible? Am J Addict 25: 28–30, 2016. doi: 10.1111/ajad.12309. [DOI] [PubMed] [Google Scholar]
- 99.Enzi B, Lissek S, Edel MA, Tegenthoff M, Nicolas V, Scherbaum N, Juckel G, Roser P. Alterations of monetary reward and punishment processing in chronic cannabis users: an FMRI study. PLoS One 10: e0119150, 2015. doi: 10.1371/journal.pone.0119150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Epstein KA, Kumra S. Altered cortical maturation in adolescent cannabis users with and without schizophrenia. Schizophr Res 162: 143–152, 2015. doi: 10.1016/j.schres.2014.11.029. [DOI] [PubMed] [Google Scholar]
- 101.Ersche KD, Jones PS, Williams GB, Turton AJ, Robbins TW, Bullmore ET. Abnormal brain structure implicated in stimulant drug addiction. Science 335: 601–604, 2012. doi: 10.1126/science.1214463. [DOI] [PubMed] [Google Scholar]
- 102.Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci 363: 3125–3135, 2008. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Everitt BJ, Robbins TW. Drug Addiction: Updating Actions to Habits to Compulsions Ten Years On. Annu Rev Psychol 67: 23–50, 2016. doi: 10.1146/annurev-psych-122414-033457. [DOI] [PubMed] [Google Scholar]
- 104.Farris SP, Harris RA, Ponomarev I. Epigenetic modulation of brain gene networks for cocaine and alcohol abuse. Front Neurosci 9: 176, 2015. doi: 10.3389/fnins.2015.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ferguson LB, Harris RA, Mayfield RD. From gene networks to drugs: systems pharmacology approaches for AUD. Psychopharmacology (Berl) 235: 1635–1662, 2018. doi: 10.1007/s00213-018-4855-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galiñanes GL, Heng LJ, Tseng KY, Wolf ME. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca2+-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology 61: 1141–1151, 2011. doi: 10.1016/j.neuropharm.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fields HL, Margolis EB. Understanding opioid reward. Trends Neurosci 38: 217–225, 2015. doi: 10.1016/j.tins.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fiorentini C, Busi C, Spano P, Missale C. Dimerization of dopamine D1 and D3 receptors in the regulation of striatal function. Curr Opin Pharmacol 10: 87–92, 2010. doi: 10.1016/j.coph.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 109.Fitzgerald PJ. Elevated Norepinephrine May be a Unifying Etiological Factor in the Abuse of a Broad Range of Substances: Alcohol, Nicotine, Marijuana, Heroin, Cocaine, and Caffeine. Subst Abuse 7: 171–183, 2013. doi: 10.4137/SART.S13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Flay BR, Allred CG, Ordway N. Effects of the Positive Action program on achievement and discipline: two matched-control comparisons. Prev Sci 2: 71–89, 2001. doi: 10.1023/A:1011591613728. [DOI] [PubMed] [Google Scholar]
- 111.Fleckenstein AE, Volz TJ, Riddle EL, Gibb JW, Hanson GR. New insights into the mechanism of action of amphetamines. Annu Rev Pharmacol Toxicol 47: 681–698, 2007. doi: 10.1146/annurev.pharmtox.47.120505.105140. [DOI] [PubMed] [Google Scholar]
- 112.Floresco SB, West AR, Ash B, Moore H, Grace AA. Afferent modulation of dopamine neuron firing differentially regulates tonic and phasic dopamine transmission. Nat Neurosci 6: 968–973, 2003. doi: 10.1038/nn1103. [DOI] [PubMed] [Google Scholar]
- 113.Fonteneau C, Redoute J, Haesebaert F, Le Bars D, Costes N, Suaud-Chagny MF, Brunelin J. Frontal Transcranial Direct Current Stimulation Induces Dopamine Release in the Ventral Striatum in Human. Cereb Cortex 28: 2636–2646, 2018. doi: 10.1093/cercor/bhy093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fowler CD, Arends MA, Kenny PJ. Subtypes of nicotinic acetylcholine receptors in nicotine reward, dependence, and withdrawal: evidence from genetically modified mice. Behav Pharmacol 19: 461–484, 2008. doi: 10.1097/FBP.0b013e32830c360e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Freisthler B, Gruenewald PJ, Johnson FW, Treno AJ, Lascala EA. An exploratory study examining the spatial dynamics of illicit drug availability and rates of drug use. J Drug Educ 35: 15–27, 2005. doi: 10.2190/25QY-PBC3-B1EB-JB5Y. [DOI] [PubMed] [Google Scholar]
- 116.Friese M, Frankenbach J, Job V, Loschelder DD. Does Self-Control Training Improve Self-Control? A Meta-Analysis. Perspect Psychol Sci 12: 1077–1099, 2017. doi: 10.1177/1745691617697076. [DOI] [PubMed] [Google Scholar]
- 117.Galaj E, Harding W, Ranaldi R. Dopamine D1 and D3 receptor interactions in cocaine reward and seeking in rats. Psychopharmacology (Berl) 233: 3881–3890, 2016. doi: 10.1007/s00213-016-4420-9. [DOI] [PubMed] [Google Scholar]
- 118.Gardon O, Faget L, Chu Sin Chung P, Matifas A, Massotte D, Kieffer BL. Expression of mu opioid receptor in dorsal diencephalic conduction system: new insights for the medial habenula. Neuroscience 277: 595–609, 2014. doi: 10.1016/j.neuroscience.2014.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Garland EL, Howard MO. Volatile substance misuse: clinical considerations, neuropsychopharmacology and potential role of pharmacotherapy in management. CNS Drugs 26: 927–935, 2012. doi: 10.1007/s40263-012-0001-6. [DOI] [PubMed] [Google Scholar]
- 120.Garris PA, Ciolkowski EL, Pastore P, Wightman RM. Efflux of dopamine from the synaptic cleft in the nucleus accumbens of the rat brain. J Neurosci 14: 6084–6093, 1994. doi: 10.1523/JNEUROSCI.14-10-06084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, Hare TA, Bookheimer SY, Tottenham N. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci USA 110: 15638–15643, 2013. doi: 10.1073/pnas.1307893110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gerra G, Leonardi C, Cortese E, Zaimovic A, Dell’Agnello G, Manfredini M, Somaini L, Petracca F, Caretti V, Baroni C, Donnini C. Adrenocorticotropic hormone and cortisol plasma levels directly correlate with childhood neglect and depression measures in addicted patients. Addict Biol 13: 95–104, 2008. doi: 10.1111/j.1369-1600.2007.00086.x. [DOI] [PubMed] [Google Scholar]
- 123.Ghelardini C, Di Cesare Mannelli L, Bianchi E. The pharmacological basis of opioids. Clin Cases Miner Bone Metab 12: 219–221, 2015. doi: 10.11138/ccmbm/2015.12.3.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gillespie NA, Kendler KS, Prescott CA, Aggen SH, Gardner CO Jr, Jacobson K, Neale MC. Longitudinal modeling of genetic and environmental influences on self-reported availability of psychoactive substances: alcohol, cigarettes, marijuana, cocaine and stimulants. Psychol Med 37: 947–959, 2007. doi: 10.1017/S0033291707009920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gillespie NA, Neale MC, Kendler KS. Pathways to cannabis abuse: a multi-stage model from cannabis availability, cannabis initiation and progression to abuse. Addiction 104: 430–438, 2009. doi: 10.1111/j.1360-0443.2008.02456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Glisky EL. Changes in Cognitive Function in Human Aging. In: Brain Aging: Models, Methods, and Mechanisms, edited by Riddle DR. Boca Raton, FL: CRC, 2007. [PubMed] [Google Scholar]
- 127.Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF III, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA 101: 8174–8179, 2004. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Golden SA, Russo SJ. Mechanisms of psychostimulant-induced structural plasticity. Cold Spring Harb Perspect Med 2: a011957, 2012. doi: 10.1101/cshperspect.a011957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry 159: 1642–1652, 2002. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Goldstein RZ, Volkow ND. Oral methylphenidate normalizes cingulate activity and decreases impulsivity in cocaine addiction during an emotionally salient cognitive task. Neuropsychopharmacology 36: 366–367, 2011. doi: 10.1038/npp.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Gómez-Coronado N, Walker AJ, Berk M, Dodd S. Current and Emerging Pharmacotherapies for Cessation of Tobacco Smoking. Pharmacotherapy 38: 235–258, 2018. doi: 10.1002/phar.2073. [DOI] [PubMed] [Google Scholar]
- 132.Gorelick DA. Pharmacological Treatment of Cannabis-Related Disorders: A Narrative Review. Curr Pharm Des 22: 6409–6419, 2016. doi: 10.2174/1381612822666160822150822. [DOI] [PubMed] [Google Scholar]
- 133.Govindan RM, Behen ME, Helder E, Makki MI, Chugani HT. Altered water diffusivity in cortical association tracts in children with early deprivation identified with Tract-Based Spatial Statistics (TBSS). Cereb Cortex 20: 561–569, 2010. doi: 10.1093/cercor/bhp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Gowing L, Farrell M, Ali R, White JM. Alpha2-adrenergic agonists for the management of opioid withdrawal. Cochrane Database Syst Rev 5: CD002024, 2016. [DOI] [PubMed] [Google Scholar]
- 135.Graham AM, Pears KC, Kim HK, Bruce J, Fisher PA. Effects of a school readiness intervention on hypothalamus-pituitary-adrenal axis functioning and school adjustment for children in foster care. Dev Psychopathol 30: 651–664, 2018. doi: 10.1017/S0954579417001171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Graziane NM, Polter AM, Briand LA, Pierce RC, Kauer JA. Kappa opioid receptors regulate stress-induced cocaine seeking and synaptic plasticity. Neuron 77: 942–954, 2013. doi: 10.1016/j.neuron.2012.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gross HE, Shaw DS, Moilanen KL, Dishion TJ, Wilson MN. Reciprocal models of child behavior and depressive symptoms in mothers and fathers in a sample of children at risk for early conduct problems. J Fam Psychol 22: 742–751, 2008. doi: 10.1037/a0013514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Grueter BA, Rothwell PE, Malenka RC. Integrating synaptic plasticity and striatal circuit function in addiction. Curr Opin Neurobiol 22: 545–551, 2012. doi: 10.1016/j.conb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Guerri C, Pascual M. Mechanisms involved in the neurotoxic, cognitive, and neurobehavioral effects of alcohol consumption during adolescence. Alcohol 44: 15–26, 2010. doi: 10.1016/j.alcohol.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 140.Gurevich VV, Gurevich EV. GPCR Signaling Regulation: The Role of GRKs and Arrestins. Front Pharmacol 10: 125, 2019. doi: 10.3389/fphar.2019.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Guttmannova K, Skinner ML, Oesterle S, White HR, Catalano RF, Hawkins JD. The Interplay Between Marijuana-Specific Risk Factors and Marijuana Use Over the Course of Adolescence. Prev Sci 20: 235–245, 2019. doi: 10.1007/s11121-018-0882-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Hamid AA, Pettibone JR, Mabrouk OS, Hetrick VL, Schmidt R, Vander Weele CM, Kennedy RT, Aragona BJ, Berke JD. Mesolimbic dopamine signals the value of work. Nat Neurosci 19: 117–126, 2016. doi: 10.1038/nn.4173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hancock DB, Markunas CA, Bierut LJ, Johnson EO. Human Genetics of Addiction: New Insights and Future Directions. Curr Psychiatry Rep 20: 8, 2018. doi: 10.1007/s11920-018-0873-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hanse E, Seth H, Riebe I. AMPA-silent synapses in brain development and pathology. Nat Rev Neurosci 14: 839–850, 2013. doi: 10.1038/nrn3642. [DOI] [PubMed] [Google Scholar]
- 145.Hasbi A, O’Dowd BF, George SR. Heteromerization of dopamine D2 receptors with dopamine D1 or D5 receptors generates intracellular calcium signaling by different mechanisms. Curr Opin Pharmacol 10: 93–99, 2010. doi: 10.1016/j.coph.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hasbi A, Perreault ML, Shen MYF, Fan T, Nguyen T, Alijaniaram M, Banasikowski TJ, Grace AA, O’Dowd BF, Fletcher PJ, George SR. Activation of Dopamine D1-D2 Receptor Complex Attenuates Cocaine Reward and Reinstatement of Cocaine-Seeking through Inhibition of DARPP-32, ERK, and ΔFosB. Front Pharmacol 8: 924, 2018. doi: 10.3389/fphar.2017.00924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, Compton WM, Crowley T, Ling W, Petry NM, Schuckit M, Grant BF. DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry 170: 834–851, 2013. doi: 10.1176/appi.ajp.2013.12060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hawkins JD, Catalano RF, Arthur MW. Promoting science-based prevention in communities. Addict Behav 27: 951–976, 2002. doi: 10.1016/S0306-4603(02)00298-8. [DOI] [PubMed] [Google Scholar]
- 149.Heatherton TF, Wagner DD. Cognitive neuroscience of self-regulation failure. Trends Cogn Sci 15: 132–139, 2011. doi: 10.1016/j.tics.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hedman AM, van Haren NE, Schnack HG, Kahn RS, Hulshoff Pol HE. Human brain changes across the life span: a review of 56 longitudinal magnetic resonance imaging studies. Hum Brain Mapp 33: 1987–2002, 2012. doi: 10.1002/hbm.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Heinsbroek JA, Neuhofer DN, Griffin WC III, Siegel GS, Bobadilla AC, Kupchik YM, Kalivas PW. Loss of Plasticity in the D2-Accumbens Pallidal Pathway Promotes Cocaine Seeking. J Neurosci 37: 757–767, 2017. doi: 10.1523/JNEUROSCI.2659-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Hernandez G, Cheer JF. Effect of CB1 receptor blockade on food-reinforced responding and associated nucleus accumbens neuronal activity in rats. J Neurosci 32: 11467–11477, 2012. doi: 10.1523/JNEUROSCI.1833-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Herz A. Endogenous opioid systems and alcohol addiction. Psychopharmacology (Berl) 129: 99–111, 1997. doi: 10.1007/s002130050169. [DOI] [PubMed] [Google Scholar]
- 154.Higley MJ, Sabatini BL. Competitive regulation of synaptic Ca2+ influx by D2 dopamine and A2A adenosine receptors. Nat Neurosci 13: 958–966, 2010. doi: 10.1038/nn.2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hikida T, Kimura K, Wada N, Funabiki K, Nakanishi S. Distinct roles of synaptic transmission in direct and indirect striatal pathways to reward and aversive behavior. Neuron 66: 896–907, 2010. doi: 10.1016/j.neuron.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 156.Hnasko TS, Sotak BN, Palmiter RD. Cocaine-conditioned place preference by dopamine-deficient mice is mediated by serotonin. J Neurosci 27: 12484–12488, 2007. doi: 10.1523/JNEUROSCI.3133-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hnasko TS, Sotak BN, Palmiter RD. Morphine reward in dopamine-deficient mice. Nature 438: 854–857, 2005. doi: 10.1038/nature04172. [DOI] [PubMed] [Google Scholar]
- 158.Holly EN, DeBold JF, Miczek KA. Increased mesocorticolimbic dopamine during acute and repeated social defeat stress: modulation by corticotropin releasing factor receptors in the ventral tegmental area. Psychopharmacology (Berl) 232: 4469–4479, 2015. doi: 10.1007/s00213-015-4082-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Holly EN, Miczek KA. Ventral tegmental area dopamine revisited: effects of acute and repeated stress. Psychopharmacology (Berl) 233: 163–186, 2016. doi: 10.1007/s00213-015-4151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Huang YH, Lin Y, Mu P, Lee BR, Brown TE, Wayman G, Marie H, Liu W, Yan Z, Sorg BA, Schlüter OM, Zukin RS, Dong Y. In vivo cocaine experience generates silent synapses. Neuron 63: 40–47, 2009. doi: 10.1016/j.neuron.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Huang YH, Schlüter OM, Dong Y. Cocaine-induced homeostatic regulation and dysregulation of nucleus accumbens neurons. Behav Brain Res 216: 9–18, 2011. doi: 10.1016/j.bbr.2010.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Ishihara T, Mizuno M. Effects of tennis play on executive function in 6–11-year-old children: a 12-month longitudinal study. [Corrigendum in Eur J Sport Sci 18: 1045–1046, 2018.] Eur J Sport Sci 18: 741–752, 2018. doi: 10.1080/17461391.2018.1444792. [DOI] [PubMed] [Google Scholar]
- 163.Ivan Ezquerra-Romano I, Lawn W, Krupitsky E, Morgan CJA. Ketamine for the treatment of addiction: evidence and potential mechanisms. Neuropharmacology 142: 72–82, 2018. doi: 10.1016/j.neuropharm.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 164.Janes AC, Pizzagalli DA, Richardt S, Frederick BB, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biol Psychiatry 67: 722–729, 2010. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Jaschke AC, Honing H, Scherder EJA. Longitudinal Analysis of Music Education on Executive Functions in Primary School Children. Front Neurosci 12: 103, 2018. doi: 10.3389/fnins.2018.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jeon SY, Kim YJ, Kim YH, Shin J, Yun J, Han K, Park HK, Kim HS, Cha HJ. Abuse potential and dopaminergic effect of alkyl nitrites. Neurosci Lett 629: 68–72, 2016. doi: 10.1016/j.neulet.2016.06.057. [DOI] [PubMed] [Google Scholar]
- 167.Jernigan TL, Brown SA; ABCD Consortium Coordinators . Introduction. Dev Cogn Neurosci 32: 1–3, 2018. doi: 10.1016/j.dcn.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Jewett BE, Sharma S. Physiology, GABA. Treasure Island, FL: StatPearls, 2018. [Google Scholar]
- 168a.Johnston LD, Miech RA, O’Malley PM,Bachman JG, Schulenberg JE, Patrick ME. Monitoring the Future. National Survey Results on Drug Use: 1975–2017. Overview: Key Findings on Adolescent Drug Use. Ann Arbor, MI: Univ. of Michigan Institue for Social Research, 2018. [Google Scholar]
- 169.Jones S, Bonci A. Synaptic plasticity and drug addiction. Curr Opin Pharmacol 5: 20–25, 2005. doi: 10.1016/j.coph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 170.Jones SA, Morales AM, Lavine JB, Nagel BJ. Convergent neurobiological predictors of emergent psychopathology during adolescence. Birth Defects Res 109: 1613–1622, 2017. doi: 10.1002/bdr2.1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Kahlig KM, Galli A. Regulation of dopamine transporter function and plasma membrane expression by dopamine, amphetamine, and cocaine. Eur J Pharmacol 479: 153–158, 2003. doi: 10.1016/j.ejphar.2003.08.065. [DOI] [PubMed] [Google Scholar]
- 172.Kalivas PW. How do we determine which drug-induced neuroplastic changes are important? Nat Neurosci 8: 1440–1441, 2005. doi: 10.1038/nn1105-1440. [DOI] [PubMed] [Google Scholar]
- 173.Kalivas PW, Volkow N, Seamans J. Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron 45: 647–650, 2005. doi: 10.1016/j.neuron.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 174.Kang S, Li J, Zuo W, Fu R, Gregor D, Krnjevic K, Bekker A, Ye JH. Ethanol Withdrawal Drives Anxiety-Related Behaviors by Reducing M-type Potassium Channel Activity in the Lateral Habenula. Neuropsychopharmacology 42: 1813–1824, 2017. doi: 10.1038/npp.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Karlsson HK, Tuominen L, Tuulari JJ, Hirvonen J, Parkkola R, Helin S, Salminen P, Nuutila P, Nummenmaa L. Obesity is associated with decreased μ-opioid but unaltered dopamine D2 receptor availability in the brain. J Neurosci 35: 3959–3965, 2015. doi: 10.1523/JNEUROSCI.4744-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci 8: 844–858, 2007. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- 177.Kellam SG, Brown CH, Poduska JM, Ialongo NS, Wang W, Toyinbo P, Petras H, Ford C, Windham A, Wilcox HC. Effects of a universal classroom behavior management program in first and second grades on young adult behavioral, psychiatric, and social outcomes. Drug Alcohol Depend 95, Suppl 1: S5–S28, 2008. doi: 10.1016/j.drugalcdep.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kendler KS, Jacobson KC, Prescott CA, Neale MC. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulants, and opiates in male twins. Am J Psychiatry 160: 687–695, 2003. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- 179.Kharitonova M, Martin RE, Gabrieli JD, Sheridan MA. Cortical gray-matter thinning is associated with age-related improvements on executive function tasks. Dev Cogn Neurosci 6: 61–71, 2013. doi: 10.1016/j.dcn.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Khoddam R, Cho J, Jackson NJ, Leventhal AM. Diminished alternative reinforcement as a mechanism linking conduct problems and substance use in adolescence: a longitudinal examination. Addiction 113: 1139–1148, 2018. doi: 10.1111/add.14160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kolb B, Mychasiuk R, Muhammad A, Li Y, Frost DO, Gibb R. Experience and the developing prefrontal cortex. Proc Natl Acad Sci USA 109, Suppl 2: 17186–17193, 2012. doi: 10.1073/pnas.1121251109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Koob GF, Bloom FE. Cellular and molecular mechanisms of drug dependence. Science 242: 715–723, 1988. doi: 10.1126/science.2903550. [DOI] [PubMed] [Google Scholar]
- 183.Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW Jr, George O. Addiction as a stress surfeit disorder. Neuropharmacology 76, Pt B: 370–382, 2014. doi: 10.1016/j.neuropharm.2013.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Koob GF, Le Moal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci 8: 1442–1444, 2005. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- 185.Koob GF, Volkow ND. Neurocircuitry of addiction. [Erratum in Neuropsychopharmacology 35: 1051, 2010.] Neuropsychopharmacology 35: 217–238, 2010. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kupchik YM, Brown RM, Heinsbroek JA, Lobo MK, Schwartz DJ, Kalivas PW. Coding the direct/indirect pathways by D1 and D2 receptors is not valid for accumbens projections. Nat Neurosci 18: 1230–1232, 2015. doi: 10.1038/nn.4068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Kupchik YM, Kalivas PW. The Direct and Indirect Pathways of the Nucleus Accumbens Are Not What You Think. Neuropsychopharmacology 42: 369–370, 2017. doi: 10.1038/npp.2016.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Laaris N, Good CH, Lupica CR. Delta9-tetrahydrocannabinol is a full agonist at CB1 receptors on GABA neuron axon terminals in the hippocampus. Neuropharmacology 59: 121–127, 2010. doi: 10.1016/j.neuropharm.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Langdon AJ, Sharpe MJ, Schoenbaum G, Niv Y. Model-based predictions for dopamine. Curr Opin Neurobiol 49: 1–7, 2018. doi: 10.1016/j.conb.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.LaPlant Q, Nestler EJ. CRACKing the histone code: cocaine’s effects on chromatin structure and function. Horm Behav 59: 321–330, 2011. doi: 10.1016/j.yhbeh.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Lau C, Wong M, Dudovitz R. School Disciplinary Style and Adolescent Health. J Adolesc Health 62: 136–142, 2018. doi: 10.1016/j.jadohealth.2017.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Laubach M, Amarante LM, Swanson K, White SR. What, If Anything, Is Rodent Prefrontal Cortex? eNeuro 5: ENEURO.0315-18.2018, 2018. doi: 10.1523/ENEURO.0315-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Laurent V, Morse AK, Balleine BW. The role of opioid processes in reward and decision-making. Br J Pharmacol 172: 449–459, 2015. doi: 10.1111/bph.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Le Merrer J, Becker JA, Befort K, Kieffer BL. Reward processing by the opioid system in the brain. Physiol Rev 89: 1379–1412, 2009. doi: 10.1152/physrev.00005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Lebel C, Deoni S. The development of brain white matter microstructure. Neuroimage 182: 207–218, 2018. doi: 10.1016/j.neuroimage.2017.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.LeGates TA, Kvarta MD, Tooley JR, Francis TC, Lobo MK, Creed MC, Thompson SM. Reward behaviour is regulated by the strength of hippocampus-nucleus accumbens synapses. Nature 564: 258–262, 2018. doi: 10.1038/s41586-018-0740-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, Chen L, Chen L, Chen TM, Chin MC, Chong J, Crook BE, Czaplinska A, Dang CN, Datta S, Dee NR, Desaki AL, Desta T, Diep E, Dolbeare TA, Donelan MJ, Dong HW, Dougherty JG, Duncan BJ, Ebbert AJ, Eichele G, Estin LK, Faber C, Facer BA, Fields R, Fischer SR, Fliss TP, Frensley C, Gates SN, Glattfelder KJ, Halverson KR, Hart MR, Hohmann JG, Howell MP, Jeung DP, Johnson RA, Karr PT, Kawal R, Kidney JM, Knapik RH, Kuan CL, Lake JH, Laramee AR, Larsen KD, Lau C, Lemon TA, Liang AJ, Liu Y, Luong LT, Michaels J, Morgan JJ, Morgan RJ, Mortrud MT, Mosqueda NF, Ng LL, Ng R, Orta GJ, Overly CC, Pak TH, Parry SE, Pathak SD, Pearson OC, Puchalski RB, Riley ZL, Rockett HR, Rowland SA, Royall JJ, Ruiz MJ, Sarno NR, Schaffnit K, Shapovalova NV, Sivisay T, Slaughterbeck CR, Smith SC, Smith KA, Smith BI, Sodt AJ, Stewart NN, Stumpf KR, Sunkin SM, Sutram M, Tam A, Teemer CD, Thaller C, Thompson CL, Varnam LR, Visel A, Whitlock RM, Wohnoutka PE, Wolkey CK, Wong VY, Wood M, Yaylaoglu MB, Young RC, Youngstrom BL, Yuan XF, Zhang B, Zwingman TA, Jones AR. Genome-wide atlas of gene expression in the adult mouse brain. Nature 445: 168–176, 2007. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- 198.Lenoir M, Cantin L, Vanhille N, Serre F, Ahmed SH. Extended heroin access increases heroin choices over a potent nondrug alternative. Neuropsychopharmacology 38: 1209–1220, 2013. doi: 10.1038/npp.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Li MD, Wang J, Niu T, Ma JZ, Seneviratne C, Ait-Daoud N, Saadvandi J, Morris R, Weiss D, Campbell J, Haning W, Mawhinney DJ, Weis D, McCann M, Stock C, Kahn R, Iturriaga E, Yu E, Elkashef A, Johnson BA. Transcriptome profiling and pathway analysis of genes expressed differentially in participants with or without a positive response to topiramate treatment for methamphetamine addiction. BMC Med Genomics 7: 65, 2014. doi: 10.1186/s12920-014-0065-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Li W, Garland EL, Howard MO. Therapeutic mechanisms of Mindfulness-Oriented Recovery Enhancement for internet gaming disorder: reducing craving and addictive behavior by targeting cognitive processes. J Addict Dis 37: 5–13, 2018. doi: 10.1080/10550887.2018.1442617. [DOI] [PubMed] [Google Scholar]
- 201.Li X, Du L, Sahlem GL, Badran BW, Henderson S, George MS. Repetitive transcranial magnetic stimulation (rTMS) of the dorsolateral prefrontal cortex reduces resting-state insula activity and modulates functional connectivity of the orbitofrontal cortex in cigarette smokers. Drug Alcohol Depend 174: 98–105, 2017. doi: 10.1016/j.drugalcdep.2017.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Liang X, He Y, Salmeron BJ, Gu H, Stein EA, Yang Y. Interactions between the salience and default-mode networks are disrupted in cocaine addiction. J Neurosci 35: 8081–8090, 2015. doi: 10.1523/JNEUROSCI.3188-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature 375: 400–404, 1995. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 204.Lim SA, Kang UJ, McGehee DS. Striatal cholinergic interneuron regulation and circuit effects. Front Synaptic Neurosci 6: 22, 2014. doi: 10.3389/fnsyn.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Liu M, Jiang Y, Wedow R, Li Y, Brazel DM, Chen F, Datta G, Davila-Velderrain J, McGuire D, Tian C, Zhan X, Choquet H, Docherty AR, Faul JD, Foerster JR, Fritsche LG, Gabrielsen ME, Gordon SD, Haessler J, Hottenga JJ, Huang H, Jang SK, Jansen PR, Ling Y, Mägi R, Matoba N, McMahon G, Mulas A, Orrù V, Palviainen T, Pandit A, Reginsson GW, Skogholt AH, Smith JA, Taylor AE, Turman C, Willemsen G, Young H, Young KA, Zajac GJM, Zhao W, Zhou W, Bjornsdottir G, Boardman JD, Boehnke M, Boomsma DI, Chen C, Cucca F, Davies GE, Eaton CB, Ehringer MA, Esko T, Fiorillo E, Gillespie NA, Gudbjartsson DF, Haller T, Harris KM, Heath AC, Hewitt JK, Hickie IB, Hokanson JE, Hopfer CJ, Hunter DJ, Iacono WG, Johnson EO, Kamatani Y, Kardia SLR, Keller MC, Kellis M, Kooperberg C, Kraft P, Krauter KS, Laakso M, Lind PA, Loukola A, Lutz SM, Madden PAF, Martin NG, McGue M, McQueen MB, Medland SE, Metspalu A, Mohlke KL, Nielsen JB, Okada Y, Peters U, Polderman TJC, Posthuma D, Reiner AP, Rice JP, Rimm E, Rose RJ, Runarsdottir V, Stallings MC, Stančáková A, Stefansson H, Thai KK, Tindle HA, Tyrfingsson T, Wall TL, Weir DR, Weisner C, Whitfield JB, Winsvold BS, Yin J, Zuccolo L, Bierut LJ, Hveem K, Lee JJ, Munafò MR, Saccone NL, Willer CJ, Cornelis MC, David SP, Hinds DA, Jorgenson E, Kaprio J, Stitzel JA, Stefansson K, Thorgeirsson TE, Abecasis G, Liu DJ, Vrieze S; 23andMe Research Team; HUNT All-In Psychiatry . Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat Genet 51: 237–244, 2019. doi: 10.1038/s41588-018-0307-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Liu Q, Shen Y, Cao X, Li Y, Chen Y, Yang W, Yuan TF. Either at left or right, both high and low frequency rTMS of dorsolateral prefrontal cortex decreases cue induced craving for methamphetamine. Am J Addict 26: 776–779, 2017. doi: 10.1111/ajad.12638. [DOI] [PubMed] [Google Scholar]
- 207.Lo MT, Hinds DA, Tung JY, Franz C, Fan CC, Wang Y, Smeland OB, Schork A, Holland D, Kauppi K, Sanyal N, Escott-Price V, Smith DJ, O’Donovan M, Stefansson H, Bjornsdottir G, Thorgeirsson TE, Stefansson K, McEvoy LK, Dale AM, Andreassen OA, Chen CH. Genome-wide analyses for personality traits identify six genomic loci and show correlations with psychiatric disorders. Nat Genet 49: 152–156, 2017. doi: 10.1038/ng.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lobo MK, Covington HE III, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science 330: 385–390, 2010. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.López-Moreno JA, González-Cuevas G, Moreno G, Navarro M. The pharmacology of the endocannabinoid system: functional and structural interactions with other neurotransmitter systems and their repercussions in behavioral addiction. Addict Biol 13: 160–187, 2008. doi: 10.1111/j.1369-1600.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- 210.Lopez-Quintero C, Hasin DS, de Los Cobos JP, Pines A, Wang S, Grant BF, Blanco C. Probability and predictors of remission from life-time nicotine, alcohol, cannabis or cocaine dependence: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Addiction 106: 657–669, 2011. doi: 10.1111/j.1360-0443.2010.03194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Loweth JA, Scheyer AF, Milovanovic M, LaCrosse AL, Flores-Barrera E, Werner CT, Li X, Ford KA, Le T, Olive MF, Szumlinski KK, Tseng KY, Wolf ME. Synaptic depression via mGluR1 positive allosteric modulation suppresses cue-induced cocaine craving. Nat Neurosci 17: 73–80, 2014. doi: 10.1038/nn.3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, Nishino T, Barch D. The effects of poverty on childhood brain development: the mediating effect of caregiving and stressful life events. JAMA Pediatr 167: 1135–1142, 2013. doi: 10.1001/jamapediatrics.2013.3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Luo Z, Volkow ND, Heintz N, Pan Y, Du C. Acute cocaine induces fast activation of D1 receptor and progressive deactivation of D2 receptor striatal neurons: in vivo optical microprobe [Ca2+]i imaging. J Neurosci 31: 13180–13190, 2011. doi: 10.1523/JNEUROSCI.2369-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Macey TA, Lowe JD, Chavkin C. Mu opioid receptor activation of ERK1/2 is GRK3 and arrestin dependent in striatal neurons. J Biol Chem 281: 34515–34524, 2006. doi: 10.1074/jbc.M604278200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Macoveanu J. Serotonergic modulation of reward and punishment: evidence from pharmacological fMRI studies. Brain Res 1556: 19–27, 2014. doi: 10.1016/j.brainres.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 216.Mahmood OM, Goldenberg D, Thayer R, Migliorini R, Simmons AN, Tapert SF. Adolescents’ fMRI activation to a response inhibition task predicts future substance use. Addict Behav 38: 1435–1441, 2013. doi: 10.1016/j.addbeh.2012.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron 44: 5–21, 2004. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 218.Malik S, Jacobs M, Cho SS, Boileau I, Blumberger D, Heilig M, Wilson A, Daskalakis ZJ, Strafella AP, Zangen A, Le Foll B. Deep TMS of the insula using the H-coil modulates dopamine release: a crossover [11C]PHNO-PET pilot trial in healthy humans. Brain Imaging Behav 12: 1306–1317, 2018. doi: 10.1007/s11682-017-9800-1. [DOI] [PubMed] [Google Scholar]
- 219.Malvaez M, Sanchis-Segura C, Vo D, Lattal KM, Wood MA. Modulation of chromatin modification facilitates extinction of cocaine-induced conditioned place preference. Biol Psychiatry 67: 36–43, 2010. doi: 10.1016/j.biopsych.2009.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Lüscher C. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci 12: 1036–1041, 2009. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- 221.Mantsch JR, Baker DA, Funk D, Lê AD, Shaham Y. Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology 41: 335–356, 2016. doi: 10.1038/npp.2015.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Margolis EB, Fields HL. Mu Opioid Receptor Actions in the Lateral Habenula. PLoS One 11: e0159097, 2016. doi: 10.1371/journal.pone.0159097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Martinez D, Orlowska D, Narendran R, Slifstein M, Liu F, Kumar D, Broft A, Van Heertum R, Kleber HD. Dopamine type 2/3 receptor availability in the striatum and social status in human volunteers. Biol Psychiatry 67: 275–278, 2010. doi: 10.1016/j.biopsych.2009.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Martinez D, Slifstein M, Narendran R, Foltin RW, Broft A, Hwang DR, Perez A, Abi-Dargham A, Fischman MW, Kleber HD, Laruelle M. Dopamine D1 receptors in cocaine dependence measured with PET and the choice to self-administer cocaine. Neuropsychopharmacology 34: 1774–1782, 2009. doi: 10.1038/npp.2008.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Mateo Y, Johnson KA, Covey DP, Atwood BK, Wang HL, Zhang S, Gildish I, Cachope R, Bellocchio L, Guzmán M, Morales M, Cheer JF, Lovinger DM. Endocannabinoid Actions on Cortical Terminals Orchestrate Local Modulation of Dopamine Release in the Nucleus Accumbens. Neuron 96: 1112–1126.e5, 2017. doi: 10.1016/j.neuron.2017.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Mátyás F, Watanabe M, Mackie K, Katona I, Freund TF. Molecular architecture of the cannabinoid signaling system in the core of the nucleus accumbens. Ideggyogy Sz 60: 187–191, 2007. [PubMed] [Google Scholar]
- 227.Mayfield RD, Larson G, Zahniser NR. Cocaine-induced behavioral sensitization and D1 dopamine receptor function in rat nucleus accumbens and striatum. Brain Res 573: 331–335, 1992. doi: 10.1016/0006-8993(92)90783-6. [DOI] [PubMed] [Google Scholar]
- 228.Melchior JR, Ferris MJ, Stuber GD, Riddle DR, Jones SR. Optogenetic versus electrical stimulation of dopamine terminals in the nucleus accumbens reveals local modulation of presynaptic release. J Neurochem 134: 833–844, 2015. doi: 10.1111/jnc.13177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Merikangas KR, Avenevoli S. Implications of genetic epidemiology for the prevention of substance use disorders. Addict Behav 25: 807–820, 2000. doi: 10.1016/S0306-4603(00)00129-5. [DOI] [PubMed] [Google Scholar]
- 231.Miller PM, Hersen M, Eisler RM, Hilsman G. Effects of social stress on operant drinking of alcoholics and social drinkers. Behav Res Ther 12: 67–72, 1974. doi: 10.1016/0005-7967(74)90094-1. [DOI] [PubMed] [Google Scholar]
- 232.Mitchell MR, Berridge KC, Mahler SV. Endocannabinoid-Enhanced “Liking” in Nucleus Accumbens Shell Hedonic Hotspot Requires Endogenous Opioid Signals. Cannabis Cannabinoid Res 3: 166–170, 2018. doi: 10.1089/can.2018.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Moffitt TE, Arseneault L, Belsky D, Dickson N, Hancox RJ, Harrington H, Houts R, Poulton R, Roberts BW, Ross S, Sears MR, Thomson WM, Caspi A. A gradient of childhood self-control predicts health, wealth, and public safety. Proc Natl Acad Sci USA 108: 2693–2698, 2011. doi: 10.1073/pnas.1010076108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Moore RJ, Vinsant SL, Nader MA, Porrino LJ, Friedman DP. Effect of cocaine self-administration on striatal dopamine D1 receptors in rhesus monkeys. Synapse 28: 1–9, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 235.Morales M, Margolis EB. Ventral tegmental area: cellular heterogeneity, connectivity and behaviour. Nat Rev Neurosci 18: 73–85, 2017. doi: 10.1038/nrn.2016.165. [DOI] [PubMed] [Google Scholar]
- 236.Moran-Santa Maria MM, Hartwell KJ, Hanlon CA, Canterberry M, Lematty T, Owens M, Brady KT, George MS. Right anterior insula connectivity is important for cue-induced craving in nicotine-dependent smokers. Addict Biol 20: 407–414, 2015. doi: 10.1111/adb.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci 5: 169–174, 2002. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- 238.Morikawa H, Morrisett RA. Ethanol action on dopaminergic neurons in the ventral tegmental area: interaction with intrinsic ion channels and neurotransmitter inputs. Int Rev Neurobiol 91: 235–288, 2010. doi: 10.1016/S0074-7742(10)91008-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Muly EC, Maddox M, Khan ZU. Distribution of D1 and D5 dopamine receptors in the primate nucleus accumbens. Neuroscience 169: 1557–1566, 2010. doi: 10.1016/j.neuroscience.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 240.Muñoz-Cuevas FJ, Athilingam J, Piscopo D, Wilbrecht L. Cocaine-induced structural plasticity in frontal cortex correlates with conditioned place preference. Nat Neurosci 16: 1367–1369, 2013. doi: 10.1038/nn.3498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Murray DW, Rosanbalm K, Christopoulos C. Self-Regulation and Toxic Stress Report 3: A Comprehensive Review of Self Regulation Interventions from Birth Through Young Adulthood, edited by Office of Planning RaE Washington, DC: Administration for Children and Families, U.S. Department of Health and Human Services, 2016. [Google Scholar]
- 242.Nagano-Saito A, Liu J, Doyon J, Dagher A. Dopamine modulates default mode network deactivation in elderly individuals during the Tower of London task. Neurosci Lett 458: 1–5, 2009. doi: 10.1016/j.neulet.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 243.Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci 32: 56–67, 2009. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Nestler EJ. Is there a common molecular pathway for addiction? Nat Neurosci 8: 1445–1449, 2005. doi: 10.1038/nn1578. [DOI] [PubMed] [Google Scholar]
- 245.Neve KA, Seamans JK, Trantham-Davidson H. Dopamine receptor signaling. J Recept Signal Transduct Res 24: 165–205, 2004. doi: 10.1081/RRS-200029981. [DOI] [PubMed] [Google Scholar]
- 246.Noël X, Brevers D, Bechara A. A triadic neurocognitive approach to addiction for clinical interventions. Front Psychiatry 4: 179, 2013. doi: 10.3389/fpsyt.2013.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Okita K, Morales AM, Dean AC, Johnson MC, Lu V, Farahi J, Mandelkern MA, London ED. Striatal dopamine D1-type receptor availability: no difference from control but association with cortical thickness in methamphetamine users. Mol Psychiatry 23: 1320–1327, 2018. doi: 10.1038/mp.2017.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Osgood DW, Feinberg ME, Gest SD, Moody J, Ragan DT, Spoth R, Greenberg M, Redmond C. Effects of PROSPER on the influence potential of prosocial versus antisocial youth in adolescent friendship networks. J Adolesc Health 53: 174–179, 2013. doi: 10.1016/j.jadohealth.2013.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Páez-Martínez N, Ambrosio E, García-Lecumberri C, Rocha L, Montoya GL, Cruz SL. Toluene and TCE decrease binding to mu-opioid receptors, but not to benzodiazepine and NMDA receptors in mouse brain. Ann N Y Acad Sci 1139: 390–401, 2008. doi: 10.1196/annals.1432.031. [DOI] [PubMed] [Google Scholar]
- 250.Pan ZZ. mu-Opposing actions of the kappa-opioid receptor. Trends Pharmacol Sci 19: 94–98, 1998. doi: 10.1016/S0165-6147(98)01169-9. [DOI] [PubMed] [Google Scholar]
- 251.Park K, Volkow ND, Pan Y, Du C. Chronic cocaine dampens dopamine signaling during cocaine intoxication and unbalances D1 over D2 receptor signaling. J Neurosci 33: 15827–15836, 2013. doi: 10.1523/JNEUROSCI.1935-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Parsons LH, Hurd YL. Endocannabinoid signalling in reward and addiction. Nat Rev Neurosci 16: 579–594, 2015. doi: 10.1038/nrn4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Parvaz MA, Maloney T, Moeller SJ, Woicik PA, Alia-Klein N, Telang F, Wang GJ, Squires NK, Volkow ND, Goldstein RZ. Sensitivity to monetary reward is most severely compromised in recently abstaining cocaine addicted individuals: a cross-sectional ERP study. Psychiatry Res 203: 75–82, 2012. doi: 10.1016/j.pscychresns.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Pascoli V, Hiver A, Van Zessen R, Loureiro M, Achargui R, Harada M, Flakowski J, Lüscher C. Stochastic synaptic plasticity underlying compulsion in a model of addiction. Nature 564: 366–371, 2018. doi: 10.1038/s41586-018-0789-4. [DOI] [PubMed] [Google Scholar]
- 255.Patriquin MA, Bauer IE, Soares JC, Graham DP, Nielsen DA. Addiction pharmacogenetics: a systematic review of the genetic variation of the dopaminergic system. Psychiatr Genet 25: 181–193, 2015. doi: 10.1097/YPG.0000000000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Paulus MP, Tapert SF, Schulteis G. The role of interoception and alliesthesia in addiction. Pharmacol Biochem Behav 94: 1–7, 2009. doi: 10.1016/j.pbb.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Pears KC, Kim HK, Fisher PA. Decreasing Risk Factors for Later Alcohol Use and Antisocial Behaviors in Children in Foster Care by Increasing Early Promotive Factors. Child Youth Serv Rev 65: 156–165, 2016. doi: 10.1016/j.childyouth.2016.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Pears KC, Kim HK, Fisher PA, Yoerger K. Increasing pre-kindergarten early literacy skills in children with developmental disabilities and delays. J Sch Psychol 57: 15–27, 2016. doi: 10.1016/j.jsp.2016.05.004. [DOI] [PubMed] [Google Scholar]
- 259.Peciña S, Berridge KC. Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue-triggered ‘wanting’ for reward: entire core and medial shell mapped as substrates for PIT enhancement. Eur J Neurosci 37: 1529–1540, 2013. doi: 10.1111/ejn.12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Perera T, George MS, Grammer G, Janicak PG, Pascual-Leone A, Wirecki TS. The Clinical TMS Society Consensus Review and Treatment Recommendations for TMS Therapy for Major Depressive Disorder. Brain Stimul 9: 336–346, 2016. doi: 10.1016/j.brs.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Pierce RC, Fant B, Swinford-Jackson SE, Heller EA, Berrettini WH, Wimmer ME. Environmental, genetic and epigenetic contributions to cocaine addiction. Neuropsychopharmacology 43: 1471–1480, 2018. doi: 10.1038/s41386-018-0008-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Pignatelli M, Bonci A. Role of Dopamine Neurons in Reward and Aversion: A Synaptic Plasticity Perspective. Neuron 86: 1145–1157, 2015. doi: 10.1016/j.neuron.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 263.Politi E, Fauci E, Santoro A, Smeraldi E. Daily sessions of transcranial magnetic stimulation to the left prefrontal cortex gradually reduce cocaine craving. Am J Addict 17: 345–346, 2008. doi: 10.1080/10550490802139283. [DOI] [PubMed] [Google Scholar]
- 264.Ponomarev I, Wang S, Zhang L, Harris RA, Mayfield RD. Gene coexpression networks in human brain identify epigenetic modifications in alcohol dependence. J Neurosci 32: 1884–1897, 2012. doi: 10.1523/JNEUROSCI.3136-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Prochaska JJ, Benowitz NL. The Past, Present, and Future of Nicotine Addiction Therapy. Annu Rev Med 67: 467–486, 2016. doi: 10.1146/annurev-med-111314-033712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Proulx CD, Hikosaka O, Malinow R. Reward processing by the lateral habenula in normal and depressive behaviors. Nat Neurosci 17: 1146–1152, 2014. doi: 10.1038/nn.3779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Rapinesi C, Del Casale A, Di Pietro S, Ferri VR, Piacentino D, Sani G, Raccah RN, Zangen A, Ferracuti S, Vento AE, Angeletti G, Brugnoli R, Kotzalidis GD, Girardi P. Add-on high frequency deep transcranial magnetic stimulation (dTMS) to bilateral prefrontal cortex reduces cocaine craving in patients with cocaine use disorder. Neurosci Lett 629: 43–47, 2016. doi: 10.1016/j.neulet.2016.06.049. [DOI] [PubMed] [Google Scholar]
- 268.Regier DA, Rae DS, Narrow WE, Kaelber CT, Schatzberg AF. Prevalence of anxiety disorders and their comorbidity with mood and addictive disorders. Br J Psychiatry Suppl 173, S34: 24–28, 1998. doi: 10.1192/S0007125000293483. [DOI] [PubMed] [Google Scholar]
- 269.Renteria R, Baltz ET, Gremel CM. Chronic alcohol exposure disrupts top-down control over basal ganglia action selection to produce habits. Nat Commun 9: 211, 2018. doi: 10.1038/s41467-017-02615-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Renthal W, Nestler EJ. Epigenetic mechanisms in drug addiction. Trends Mol Med 14: 341–350, 2008. doi: 10.1016/j.molmed.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Riegel AC, Lupica CR. Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci 24: 11070–11078, 2004. doi: 10.1523/JNEUROSCI.3695-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci USA 99: 8384–8388, 2002. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Roberto M, Spierling SR, Kirson D, Zorrilla EP. Corticotropin-Releasing Factor (CRF) and Addictive Behaviors. Int Rev Neurobiol 136: 5–51, 2017. doi: 10.1016/bs.irn.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Robinson SE, Sohal VS. Dopamine D2 Receptors Modulate Pyramidal Neurons in Mouse Medial Prefrontal Cortex through a Stimulatory G-Protein Pathway. J Neurosci 37: 10063–10073, 2017. doi: 10.1523/JNEUROSCI.1893-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Robison AJ, Nestler EJ. Transcriptional and epigenetic mechanisms of addiction. Nat Rev Neurosci 12: 623–637, 2011. doi: 10.1038/nrn3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Roncero C, de Miguel A, Fumero A, Abad AC, Martín R, Bethencourt JM, Grau-López L, Rodríguez-Cintas L, Daigre C. Anxiety and Depression in Drug-Dependent Patients with Cluster C Personality Disorders. Front Psychiatry 9: 19, 2018. doi: 10.3389/fpsyt.2018.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Root DH, Melendez RI, Zaborszky L, Napier TC. The ventral pallidum: subregion-specific functional anatomy and roles in motivated behaviors. Prog Neurobiol 130: 29–70, 2015. doi: 10.1016/j.pneurobio.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Rotge JY, Cocker PJ, Daniel ML, Belin-Rauscent A, Everitt BJ, Belin D. Bidirectional regulation over the development and expression of loss of control over cocaine intake by the anterior insula. Psychopharmacology (Berl) 234: 1623–1631, 2017. doi: 10.1007/s00213-017-4593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Rutter JL. Symbiotic relationship of pharmacogenetics and drugs of abuse. AAPS J 8: E174–E184, 2006. doi: 10.1208/aapsj080121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Sabioni P, Le Foll B. Psychosocial and pharmacological interventions for the treatment of cannabis use disorder. F1000 Res 7: 173, 2018. doi: 10.12688/f1000research.11191.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron 76: 470–485, 2012. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Saraceno L, Munafó M, Heron J, Craddock N, van den Bree MB. Genetic and non-genetic influences on the development of co-occurring alcohol problem use and internalizing symptomatology in adolescence: a review. Addiction 104: 1100–1121, 2009. doi: 10.1111/j.1360-0443.2009.02571.x. [DOI] [PubMed] [Google Scholar]
- 283.Sasser TR, Bierman KL, Heinrichs B, Nix RL. Preschool Intervention Can Promote Sustained Growth in the Executive-Function Skills of Children Exhibiting Early Deficits. Psychol Sci 28: 1719–1730, 2017. doi: 10.1177/0956797617711640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Scaplen KM, Kaun KR. Reward from bugs to bipeds: a comparative approach to understanding how reward circuits function. J Neurogenet 30: 133–148, 2016. doi: 10.1080/01677063.2016.1180385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol 18: 121–133, 2013. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Scheefhals N, MacGillavry HD. Functional organization of postsynaptic glutamate receptors. Mol Cell Neurosci 91: 82–94, 2018. doi: 10.1016/j.mcn.2018.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Scherbaum N, Specka M. Factors influencing the course of opiate addiction. Int J Methods Psychiatr Res 17, Suppl 1: S39–S44, 2008. doi: 10.1002/mpr.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Schindler AG, Tsutsui KT, Clark JJ. Chronic alcohol intake during adolescence, but not adulthood, promotes persistent deficits in risk-based decision making. Alcohol Clin Exp Res 38: 1622–1629, 2014. doi: 10.1111/acer.12404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol 80: 1–27, 1998. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 290.Scofield MD, Heinsbroek JA, Gipson CD, Kupchik YM, Spencer S, Smith AC, Roberts-Wolfe D, Kalivas PW. The Nucleus Accumbens: Mechanisms of Addiction across Drug Classes Reflect the Importance of Glutamate Homeostasis. Pharmacol Rev 68: 816–871, 2016. doi: 10.1124/pr.116.012484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Seamans JK, Yang CR. The principal features and mechanisms of dopamine modulation in the prefrontal cortex. Prog Neurobiol 74: 1–58, 2004. doi: 10.1016/j.pneurobio.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 292.Shahbabaie A, Ebrahimpoor M, Hariri A, Nitsche MA, Hatami J, Fatemizadeh E, Oghabian MA, Ekhtiari H. Transcranial DC stimulation modifies functional connectivity of large-scale brain networks in abstinent methamphetamine users. Brain Behav 8: e00922, 2018. doi: 10.1002/brb3.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Shan Q, Ge M, Christie MJ, Balleine BW. The acquisition of goal-directed actions generates opposing plasticity in direct and indirect pathways in dorsomedial striatum. J Neurosci 34: 9196–9201, 2014. doi: 10.1523/JNEUROSCI.0313-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Shen H, Moussawi K, Zhou W, Toda S, Kalivas PW. Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. Proc Natl Acad Sci USA 108: 19407–19412, 2011. doi: 10.1073/pnas.1112052108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Shen M, Piser TM, Seybold VS, Thayer SA. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. J Neurosci 16: 4322–4334, 1996. doi: 10.1523/JNEUROSCI.16-14-04322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Shen M, Thayer SA. Delta9-tetrahydrocannabinol acts as a partial agonist to modulate glutamatergic synaptic transmission between rat hippocampal neurons in culture. Mol Pharmacol 55: 8–13, 1999. doi: 10.1124/mol.55.1.8. [DOI] [PubMed] [Google Scholar]
- 297.Shen W, Flajolet M, Greengard P, Surmeier DJ. Dichotomous dopaminergic control of striatal synaptic plasticity. Science 321: 848–851, 2008. doi: 10.1126/science.1160575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Sibley DR, Monsma FJ Jr, Shen Y. Molecular neurobiology of dopaminergic receptors. Int Rev Neurobiol 35: 391–415, 1993. doi: 10.1016/S0074-7742(08)60573-5. [DOI] [PubMed] [Google Scholar]
- 299.Silveira MM, Arnold JC, Laviolette SR, Hillard CJ, Celorrio M, Aymerich MS, Adams WK. Seeing through the smoke: human and animal studies of cannabis use and endocannabinoid signalling in corticolimbic networks. Neurosci Biobehav Rev 76, Pt B: 380–395, 2017. doi: 10.1016/j.neubiorev.2016.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Singer BF, Fadanelli M, Kawa AB, Robinson TE. Are Cocaine-Seeking “Habits” Necessary for the Development of Addiction-Like Behavior in Rats? J Neurosci 38: 60–73, 2018. doi: 10.1523/JNEUROSCI.2458-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Sitnick SL, Shaw DS, Gill A, Dishion T, Winter C, Waller R, Gardner F, Wilson M. Parenting and the Family Check-Up: Changes in Observed Parent-Child Interaction Following Early Childhood Intervention. J Clin Child Adolesc Psychol 44: 970–984, 2015. doi: 10.1080/15374416.2014.940623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Skosnik PD, Cortes-Briones JA, Hajós M. It’s All in the Rhythm: The Role of Cannabinoids in Neural Oscillations and Psychosis. Biol Psychiatry 79: 568–577, 2016. doi: 10.1016/j.biopsych.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 303.Slaker M, Barnes J, Sorg BA, Grimm JW. Impact of Environmental Enrichment on Perineuronal Nets in the Prefrontal Cortex following Early and Late Abstinence from Sucrose Self-Administration in Rats. PLoS One 11: e0168256, 2016. doi: 10.1371/journal.pone.0168256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Slopen N, McLaughlin KA, Shonkoff JP. Interventions to improve cortisol regulation in children: a systematic review. Pediatrics 133: 312–326, 2014. doi: 10.1542/peds.2013-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Snyder F, Flay B, Vuchinich S, Acock A, Washburn I, Beets M, Li KK. Impact of a social-emotional and character development program on school-level indicators of academic achievement, absenteeism, and disciplinary outcomes: a matched-pair, cluster randomized, controlled trial. J Res Educ Eff 3: 26–55, 2010. doi: 10.1080/19345740903353436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Soares-Cunha C, Coimbra B, David-Pereira A, Borges S, Pinto L, Costa P, Sousa N, Rodrigues AJ. Activation of D2 dopamine receptor-expressing neurons in the nucleus accumbens increases motivation. Nat Commun 7: 11829, 2016. doi: 10.1038/ncomms11829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Spear LP. Effects of adolescent alcohol consumption on the brain and behaviour. [Correction in Nat Rev Neurosci 19: 439, 2018.] Nat Rev Neurosci 19: 197–214, 2018. doi: 10.1038/nrn.2018.10. [DOI] [PubMed] [Google Scholar]
- 308.Spencer S, Garcia-Keller C, Roberts-Wolfe D, Heinsbroek JA, Mulvaney M, Sorrell A, Kalivas PW. Cocaine Use Reverses Striatal Plasticity Produced During Cocaine Seeking. Biol Psychiatry 81: 616–624, 2017. doi: 10.1016/j.biopsych.2016.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, Pfefferbaum A. Brain development in heavy-drinking adolescents. Am J Psychiatry 172: 531–542, 2015. doi: 10.1176/appi.ajp.2015.14101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Stahre M, Roeber J, Kanny D, Brewer RD, Zhang X. Contribution of excessive alcohol consumption to deaths and years of potential life lost in the United States. Prev Chronic Dis 11: 130293, 2014. doi: 10.5888/pcd11.130293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Steinberg EE, Boivin JR, Saunders BT, Witten IB, Deisseroth K, Janak PH. Positive reinforcement mediated by midbrain dopamine neurons requires D1 and D2 receptor activation in the nucleus accumbens. PLoS One 9: e94771, 2014. doi: 10.1371/journal.pone.0094771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Stuber GD, Britt JP, Bonci A. Optogenetic modulation of neural circuits that underlie reward seeking. Biol Psychiatry 71: 1061–1067, 2012. doi: 10.1016/j.biopsych.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Su H, Zhong N, Gan H, Wang J, Han H, Chen T, Li X, Ruan X, Zhu Y, Jiang H, Zhao M. High frequency repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex for methamphetamine use disorders: a randomised clinical trial. Drug Alcohol Depend 175: 84–91, 2017. doi: 10.1016/j.drugalcdep.2017.01.037. [DOI] [PubMed] [Google Scholar]
- 314.Subramaniyan M, Dani JA. Dopaminergic and cholinergic learning mechanisms in nicotine addiction. Ann N Y Acad Sci 1349: 46–63, 2015. doi: 10.1111/nyas.12871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Suvrathan A, Bennur S, Ghosh S, Tomar A, Anilkumar S, Chattarji S. Stress enhances fear by forming new synapses with greater capacity for long-term potentiation in the amygdala. Philos Trans R Soc Lond B Biol Sci 369: 20130151, 2014. doi: 10.1098/rstb.2013.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Swanson CJ, Baker DA, Carson D, Worley PF, Kalivas PW. Repeated cocaine administration attenuates group I metabotropic glutamate receptor-mediated glutamate release and behavioral activation: a potential role for Homer. J Neurosci 21: 9043–9052, 2001. doi: 10.1523/JNEUROSCI.21-22-09043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Swapna I, Bondy B, Morikawa H. Differential Dopamine Regulation of Ca(2+) Signaling and Its Timing Dependence in the Nucleus Accumbens. Cell Rep 15: 563–573, 2016. doi: 10.1016/j.celrep.2016.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Tan KR, Brown M, Labouèbe G, Yvon C, Creton C, Fritschy JM, Rudolph U, Lüscher C. Neural bases for addictive properties of benzodiazepines. Nature 463: 769–774, 2010. doi: 10.1038/nature08758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Tang YY, Hölzel BK, Posner MI. The neuroscience of mindfulness meditation. [Erratum in Nat Rev Neurosci 16: 312, 2015.] Nat Rev Neurosci 16: 213–225, 2015. doi: 10.1038/nrn3916. [DOI] [PubMed] [Google Scholar]
- 320.Tang YY, Posner MI, Rothbart MK, Volkow ND. Circuitry of self-control and its role in reducing addiction. Trends Cogn Sci 19: 439–444, 2015. doi: 10.1016/j.tics.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 321.Tang YY, Tang R, Posner MI. Mindfulness meditation improves emotion regulation and reduces drug abuse. Drug Alcohol Depend 163, Suppl 1: S13–S18, 2016. doi: 10.1016/j.drugalcdep.2015.11.041. [DOI] [PubMed] [Google Scholar]
- 322.Terraneo A, Leggio L, Saladini M, Ermani M, Bonci A, Gallimberti L. Transcranial magnetic stimulation of dorsolateral prefrontal cortex reduces cocaine use: a pilot study. Eur Neuropsychopharmacol 26: 37–44, 2016. doi: 10.1016/j.euroneuro.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Thanos PK, Michaelides M, Umegaki H, Volkow ND. D2R DNA transfer into the nucleus accumbens attenuates cocaine self-administration in rats. Synapse 62: 481–486, 2008. doi: 10.1002/syn.20523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Thanos PK, Volkow ND, Freimuth P, Umegaki H, Ikari H, Roth G, Ingram DK, Hitzemann R. Overexpression of dopamine D2 receptors reduces alcohol self-administration. J Neurochem 78: 1094–1103, 2001. doi: 10.1046/j.1471-4159.2001.00492.x. [DOI] [PubMed] [Google Scholar]
- 325.Tirado Muñoz J, Farré A, Mestre-Pintó J, Szerman N, Torrens M. Dual diagnosis in depression: treatment recommendations. Adicciones 30: 66–76, 2018. doi: 10.20882/adicciones.868. [DOI] [PubMed] [Google Scholar]
- 326.Tomasi D, Volkow ND. Striatocortical pathway dysfunction in addiction and obesity: differences and similarities. Crit Rev Biochem Mol Biol 48: 1–19, 2013. doi: 10.3109/10409238.2012.735642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Tomasi D, Volkow ND, Wang R, Telang F, Wang GJ, Chang L, Ernst T, Fowler JS. Dopamine transporters in striatum correlate with deactivation in the default mode network during visuospatial attention. PLoS One 4: e6102, 2009. doi: 10.1371/journal.pone.0006102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Trigo JM, Martin-García E, Berrendero F, Robledo P, Maldonado R. The endogenous opioid system: a common substrate in drug addiction. Drug Alcohol Depend 108: 183–194, 2010. doi: 10.1016/j.drugalcdep.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 329.Uhlhaas PJ, Roux F, Singer W, Haenschel C, Sireteanu R, Rodriguez E. The development of neural synchrony reflects late maturation and restructuring of functional networks in humans. Proc Natl Acad Sci USA 106: 9866–9871, 2009. doi: 10.1073/pnas.0900390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Valentine SE, Shipherd JC. A systematic review of social stress and mental health among transgender and gender non-conforming people in the United States. Clin Psychol Rev 66: 24–38, 2018. doi: 10.1016/j.cpr.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Van Emmerik-van Oortmerssen K, van de Glind G, Koeter MW, Allsop S, Auriacombe M, Barta C, Bu ET, Burren Y, Carpentier PJ, Carruthers S, Casas M, Demetrovics Z, Dom G, Faraone SV, Fatseas M, Franck J, Johnson B, Kapitány-Fövény M, Kaye S, Konstenius M, Levin FR, Moggi F, Møller M, Ramos-Quiroga JA, Schillinger A, Skutle A, Verspreet S, van den Brink W, Schoevers RA; IASP Research Group . Psychiatric comorbidity in treatment-seeking substance use disorder patients with and without attention deficit hyperactivity disorder: results of the IASP study. Addiction 109: 262–272, 2014. doi: 10.1111/add.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Vink JM. Genetics of Addiction: Future Focus on Gene × Environment Interaction? J Stud Alcohol Drugs 77: 684–687, 2016. doi: 10.15288/jsad.2016.77.684. [DOI] [PubMed] [Google Scholar]
- 333.Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry 158: 2015–2021, 2001. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- 334.Volkow ND, Collins FS. The Role of Science in Addressing the Opioid Crisis. N Engl J Med 377: 391–394, 2017. doi: 10.1056/NEJMsr1706626. [DOI] [PubMed] [Google Scholar]
- 335.Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. Cereb Cortex 10: 318–325, 2000. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- 336.Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse 14: 169–177, 1993. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- 337.Volkow ND, Hampson AJ, Baler RD. Don’t Worry, Be Happy: Endocannabinoids and Cannabis at the Intersection of Stress and Reward. Annu Rev Pharmacol Toxicol 57: 285–308, 2017. doi: 10.1146/annurev-pharmtox-010716-104615. [DOI] [PubMed] [Google Scholar]
- 338.Volkow ND, Koob GF, McLellan AT. Neurobiologic Advances from the Brain Disease Model of Addiction. N Engl J Med 374: 363–371, 2016. doi: 10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Volkow ND, Morales M. The Brain on Drugs: From Reward to Addiction. Cell 162: 712–725, 2015. doi: 10.1016/j.cell.2015.07.046. [DOI] [PubMed] [Google Scholar]
- 340.Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, Wong C, Ma Y, Logan J, Goldstein R, Alexoff D, Thanos PK. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry 63: 999–1008, 2006. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- 341.Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding YS, Pappas N. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry 156: 1440–1443, 1999. [DOI] [PubMed] [Google Scholar]
- 342.Volkow ND, Wang GJ, Fowler JS, Thanos PP, Logan J, Gatley SJ, Gifford A, Ding YS, Wong C, Pappas N. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study [Correction in Synapse 47: 295, 2003.]. Synapse 46: 79–82, 2002. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- 343.Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J Neurosci 26: 6583–6588, 2006. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, Alexoff D, Ding YS, Wong C, Ma Y, Pradhan K. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage 42: 1537–1543, 2008. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Volkow ND, Wang GJ, Tomasi D, Baler RD. Unbalanced neuronal circuits in addiction. Curr Opin Neurobiol 23: 639–648, 2013. doi: 10.1016/j.conb.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Volkow ND, Wise RA, Baler R. The dopamine motive system: implications for drug and food addiction. Nat Rev Neurosci 18: 741–752, 2017. doi: 10.1038/nrn.2017.130. [DOI] [PubMed] [Google Scholar]
- 347.Walker DM, Nestler EJ. Neuroepigenetics and addiction. Handb Clin Neurol 148: 747–765, 2018. doi: 10.1016/B978-0-444-64076-5.00048-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Wang H, Lupica CR. Release of endogenous cannabinoids from ventral tegmental area dopamine neurons and the modulation of synaptic processes. Prog Neuropsychopharmacol Biol Psychiatry 52: 24–27, 2014. doi: 10.1016/j.pnpbp.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Wang J, Ishikawa M, Yang Y, Otaka M, Kim JY, Gardner GR, Stefanik MT, Milovanovic M, Huang YH, Hell JW, Wolf ME, Schlüter OM, Dong Y. Cascades of Homeostatic Dysregulation Promote Incubation of Cocaine Craving. J Neurosci 38: 4316–4328, 2018. doi: 10.1523/JNEUROSCI.3291-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Wang Y, Shen Y, Cao X, Shan C, Pan J, He H, Ma Y, Yuan TF. Transcranial direct current stimulation of the frontal-parietal-temporal area attenuates cue-induced craving for heroin. J Psychiatr Res 79: 1–3, 2016. doi: 10.1016/j.jpsychires.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 351.Wenzel JM, Cheer JF. Endocannabinoid Regulation of Reward and Reinforcement through Interaction with Dopamine and Endogenous Opioid Signaling. Neuropsychopharmacology 43: 103–115, 2018. doi: 10.1038/npp.2017.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Wenzel JM, Rauscher NA, Cheer JF, Oleson EB. A role for phasic dopamine release within the nucleus accumbens in encoding aversion: a review of the neurochemical literature. ACS Chem Neurosci 6: 16–26, 2015. doi: 10.1021/cn500255p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Wheeler E, Jones TS, Gilbert MK, Davidson PJ; Centers for Disease Control and Prevention (CDC) . Opioid Overdose Prevention Programs Providing Naloxone to Laypersons - United States, 2014. MMWR Morb Mortal Wkly Rep 64: 631–635, 2015. [PMC free article] [PubMed] [Google Scholar]
- 354.Wiers CE, Cabrera E, Skarda E, Volkow ND, Wang GJ. PET imaging for addiction medicine: from neural mechanisms to clinical considerations. Prog Brain Res 224: 175–201, 2016. doi: 10.1016/bs.pbr.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 355.Wiers CE, Shokri-Kojori E, Cabrera E, Cunningham S, Wong C, Tomasi D, Wang GJ, Volkow ND. Socioeconomic status is associated with striatal dopamine D2/D3 receptors in healthy volunteers but not in cocaine abusers. Neurosci Lett 617: 27–31, 2016. doi: 10.1016/j.neulet.2016.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res 34: 1306–1318, 2010. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res 14: 169–183, 2008. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Witkiewitz K, Bowen S, Harrop EN, Douglas H, Enkema M, Sedgwick C. Mindfulness-based treatment to prevent addictive behavior relapse: theoretical models and hypothesized mechanisms of change. Subst Use Misuse 49: 513–524, 2014. doi: 10.3109/10826084.2014.891845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Wolf ME. Synaptic mechanisms underlying persistent cocaine craving. Nat Rev Neurosci 17: 351–365, 2016. doi: 10.1038/nrn.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Worsley JN, Moszczynska A, Falardeau P, Kalasinsky KS, Schmunk G, Guttman M, Furukawa Y, Ang L, Adams V, Reiber G, Anthony RA, Wickham D, Kish SJ. Dopamine D1 receptor protein is elevated in nucleus accumbens of human, chronic methamphetamine users. Mol Psychiatry 5: 664–672, 2000. doi: 10.1038/sj.mp.4000760. [DOI] [PubMed] [Google Scholar]
- 361.Wright WJ, Schlüter OM, Dong Y. A Feedforward Inhibitory Circuit Mediated by CB1-Expressing Fast-Spiking Interneurons in the Nucleus Accumbens. Neuropsychopharmacology 42: 1146–1156, 2017. doi: 10.1038/npp.2016.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Xi ZX, Baker DA, Shen H, Carson DS, Kalivas PW. Group II metabotropic glutamate receptors modulate extracellular glutamate in the nucleus accumbens. J Pharmacol Exp Ther 300: 162–171, 2002. doi: 10.1124/jpet.300.1.162. [DOI] [PubMed] [Google Scholar]
- 363.Yang J, Lai B, Xu A, Liu Y, Li X, Zhao Y, Li W, Ji M, Hu G, Gao X, Gao J. Maged1 co-interacting with CREB through a hexapeptide repeat domain regulates learning and memory in mice. Mol Neurobiol 51: 8–18, 2015. doi: 10.1007/s12035-014-8677-x. [DOI] [PubMed] [Google Scholar]
- 364.Zhang R, Volkow ND. Brain default-mode network dysfunction in addiction. Neuroimage 200: 313–331, 2019. [DOI] [PubMed] [Google Scholar]