Abstract
Background:
There are few treatment options for multiple sclerosis (MS) patients with advanced disability [expanded disability status scale (EDSS) ⩾ 6.0]. In 2010, we reported initial results of using intrathecal methotrexate (ITMTX) in patients with progressive MS. We now report on long-term use of ITMTX. We performed a retrospective chart analysis of patients who have had 18 or more treatments to establish the ongoing safety and tolerability of ITMTX. Thus, the objective of this study was to establish the safety and tolerability of long-term therapy with (ITMTX) in patients with treatment-resistant, progressive forms of MS.
Methods:
We studied 83 patients (67 secondary and 16 primary progressive) who received ITMTX 12.5 mg every 8–11 weeks for 3–10 years (range: 18–57 treatments). All patients were evaluated neurologically, and their EDSS was assessed at every treatment. In addition, all adverse events, frequency of infections, and any hospitalizations, were noted.
Results:
There were no deaths, hospitalizations, or other serious adverse effects related to ITMTX. Headaches occurred at least once in 12% of patients, and transient fatigue occurred in 53% of patients. As determined by EDSS, there was no significant change from baseline status to post-treatment scores in both primary progressive MS (PPMS) and secondary progressive (SPMS) patients.
Conclusions:
Pulsed ITMTX was well tolerated for up to 10 years in PPMS patients with no serious adverse effects. Although this was an open-label, retrospective analysis, and efficacy could not be studied, there was evidence of disease stabilization in many patients receiving ITMTX. It appears that long-term ITMTX is a safe therapeutic option in advanced progressive MS.
Keywords: intrathecal methotrexate, PPMS, progressive multiple sclerosis, SPMS
Introduction
Most patients with multiple sclerosis (MS) initially present with a relapsing-remitting course (RRMS), which, over time, may become secondary progressive (SPMS). Additionally, approximately 10–15% of patients have a progressive course from the onset of their disease, designated as primary progressive MS (PPMS).1 Multiple agents are currently Unites States Food and Drug Administration (FDA)-approved for the treatment of RRMS, and many more are in development.2 However, only one medication, mitoxantrone, is currently approved for SPMS, but significant safety concerns limit the widespread use of this drug.3–6 For PPMS, only Ocrelizumab is approved, and its efficacy may be most evident early in the inflammatory course of the disease.7–10 In clinical practice, a variety of MS medications are often prescribed for SPMS, with varying results. These treatments are more effective earlier in the disease course, likely due to their anti-inflammatory properties, but are increasingly ineffective for the later, presumably degenerative, phase of the disease.11–13
Methotrexate (MTX) is an antimetabolite that has been used since 1948 to treat cancer, predominantly leukemia and lymphoma, as well as a variety of autoimmune conditions, such as psoriasis and rheumatoid arthritis.14–16 The various adverse effects of MTX, when administered orally, are known to include ulcers of the gastrointestinal tract, bone marrow suppression, and infections.17 For the treatment of central nervous system cancers, intrathecal methotrexate (ITMTX) has been associated with leukoencephalopathy, especially when administered continuously through an Omaya reservoir. Use of ITMTX in this cancer population has been associated with infections, headaches following lumbar puncture (LP), and subdural hematoma formation.
MTX has been studied previously in patients with progressive MS. In 1995, Goodkin and colleagues published results of a randomized, double-blind, placebo-controlled trial of low-dose oral MTX (7.5 mg weekly) in 60 progressive MS patients over 2 years.18 They reported that the treatment was well tolerated, and some improvement was observed in upper extremity function. However, there was no positive change in overall expanded disability status scale (EDSS). A subgroup MRI analysis revealed significantly decreased T2 lesion volume in the MTX treated group.19 Olek and colleagues reported on using 20 mg weekly MTX administered subcutaneously in an open-label, prospective study.20 They also showed that MTX was safe and well tolerated, but did not report any efficacy. In 2001, Lugaresi and colleagues reported that, after a year of treatment with low-dose (7.5 mg) oral MTX, there appeared to be some disease stabilization, as only 2 out of 20 patients with progressive MS discontinued treatment because of continued worsening; however, an additional 2 patients had to stop treatment because of lymphadenopathy and elevated liver function tests, respectively.21 Finally, multiple studies have examined MTX treatment in combination with other available MS treatments.22,23 In these studies, weekly low-dose (7.5–20 mg) oral MTX was primarily added to interferon (IFN) treatment in RRMS patients, and showed radiographic and clinical benefit with low rates of adverse events.
The mechanism of action of MTX in MS is unknown. We examined the effect of methotrexate on the noninflammatory, cuprizone-induced model of demyelination.24 Cuprizone-treated mice were intraventricularly administered a low-dose of MTX, equivalent to the dose used in our clinical studies. MTX-treated mice exhibited reduced demyelination and decreased accumulation of GFAP+ reactive astrocytes in the corpus callosum. Furthermore, the low-dose of methotrexate administered in the mice did not inhibit CNS repair processes. This study suggests that the mechanism of action of ITMTX in progressive MS may be a result of inhibition of glial scar formation (sclerosis).
In 2010, our group published a retrospective, open-label chart review of 121 progressive MS patients treated with ITMTX every 8–11 weeks for up to 2 years (eight treatments).25 We reported stabilization or improved EDSS in 89% of SPMS and 82% of PPMS patients.26 We also did not observe any significant safety or tolerability issues.
Of the original cohort of 121 patients, 38 patients discontinued therapy as a result of various reasons unrelated to ITMTX tolerability. The current study is a retrospective chart analysis of the remaining 83 patients who have had 18 or more ITMTX treatments over 3–10 years, in order to establish the long-term safety and tolerability of this treatment in patients with advanced progressive MS.
Materials and methods
Patient selection
This study was approved by the institutional review board committee of St. Luke’s-Roosevelt Hospital, New York (NY, USA). Patient informed consent was obtained prior to each administration of MTX and prior to the review for this study.
The charts of patients treated with at least 18 doses of ITMTX between 2003 and 2014 were reviewed; 83 patients (67 SPMS and 16 PPMS) were included in the study. Patients were initially selected at the discretion of the treating physician for ITMTX therapy, if they fulfilled the following criteria: clinically definite, progressive MS (PPMS or SPMS), previously failed treatment with at least 3 FDA-approved medications for at least 1 year each, with continued deterioration as evidenced by worsening of EDSS by at least 0.5 points in patients with a baseline EDSS of 6 or greater; and by at least 1.0 point in patients with a baseline EDSS of 5.5 or less in the year preceding initiation of ITMTX. Exclusion criteria to ITMTX included pregnancy, active infection, and known allergy to MTX.
Treatment protocol
MTX at a dose of 12.5 mg was administered intrathecally via lumbar puncture procedure (56 patients) or via an access port of a Medtronic pump previously implanted for control of spasticity (27 patients). The dose of MTX was based on the dose used for patients with malignant disease; however, the frequency was less (three times a week in cancer patients compared with every 2 months for MS). Treatments were scheduled every 8–11 weeks (range of 18–57 treatments) to achieve compliance of patients needing spinal punctures every 8 weeks and preclinical studies that suggested dose effect that lasted for 6–8 weeks.24,25 Complete blood counts (CBC) and liver function tests (LFTs) were obtained intermittently throughout the treatment period. Patients had the option to discontinue treatment at any time. Patients did not receive folinic acid.
Patient assessment
Chart review included review of detailed neurological examinations performed every 6 months, documentation of EDSS, incidence of infection, as well as any other adverse events, including hospitalizations and post-LP headaches. CBCs, LFTs, and brain MRI scans were also performed as needed for routine neurological care, and reviewed. At the time of chart review, the Treatment Satisfaction Questionnaire for Medication (TSQM) was administered to each patient. The TSQM is a validated measure for patient medication satisfaction and includes 14 questions evaluating four domains: effectiveness, side effects, convenience, and global satisfaction.27,28 The domains are scored on a scale from 0 to 100, with 100 the highest score possible in that domain.
Results
Patient demographics
Of the 83 patients included in the study, two-thirds were female (Table 1). Age range at onset of MTX treatment was between 32 and 77 years, with an mean age of 55 years old and median age of 55.5. Disease duration at onset of MTX treatment ranged from 1 to 44 years, with a mean disease duration of 18.4 years and median of 16 years. Overall baseline EDSS ranged from 3.5 to 8.5, averaging 6.33. Baseline mean EDSS was 6.16 for PPMS patients and 6.40 for SPMS patients, respectively. Other treatments were occasionally used in combination with ITMTX for some or all of the study period. These include glatiramer acetate (seven patients), dimethyl fumarate (six patients), rituximab (six patients), IFN β-1a (four patients), intravenous immunoglobulin (three patients), IFN β-1b (one patient), and teriflunomide (one patient). Additionally, 11 of these patients had received at least one course of intravenous methylprednisolone, usually (10 of 11) within the first or second ITMTX administration over the study period. Fewer than five patients had previously received treatment with natalizumab. These patients had a 6-month washout period prior to receiving ITMTX. Incidentally, all of these patients were seronegative for JC virus antibody.
Table 1.
Patient demographics.
| Patient demographics | n |
|---|---|
| Total number of patients | 83 |
| Number of patients with over 6 years of treatment | 41 |
| Age range (at initiation of treatment) | 54.5 ± 10.0 (range 32–77) |
| Total males | 27 |
| Total females | 56 |
| SPMS | 67 |
| PPMS | 16 |
| Disease duration (years) | 18.39 ± 9.9 |
| Pretreatment EDSS | 6.33 ± 1.12 |
| Post-treatment EDSS | 6.70 ± 1.15 |
| Number of treatments | 2791 |
| Patient years | 497 |
Values reported as mean ± SD or n.
EDSS, Kurtze expanded disability status scale; PPMS, primary progressive multiple sclerosis; SPMS, secondary progressive multiple sclerosis.
Duration of treatment
Mean duration of ITMTX treatment at the time of review was 6 years (range 3–10.25). Mean number of treatments over that period was 34 (range 18–57). Total drug exposure was 497 patient-years. All patients received treatment with ITMTX for more than 2 years, and the longest duration of treatment was over 10 years. About 50% of patients (42 out of 83) received over 31 doses of ITMTX over a period of greater than 5 years.
Safety and tolerability
Transient fatigue was reported by 53% of patients at some point during the course of their treatment, dizziness was reported at least once in 41% of patients, blurred vision was reported at least once in 13% of patients, headaches occurred at least once in 12% of patients (possibly minimized by the use of a 25- or 27-gauge spinal needle), double vision was reported at least once in 7% of patients, and post-treatment nausea was reported at least once by 7% of patients (Table 2). No cases of MTX-associated leukoencephalopathy were observed, perhaps due to the less frequent administration than in MTX-treated oncology patients. There were no serious adverse events reported, and no hospitalizations related to this therapy. No central nervous system (CNS) or pump infections occurred and no deaths were reported. No patient discontinued treatment secondary to CBC or LFT abnormalities. No patient developed megaloblastic anemia. There were no neoplasms reported among the study subjects. Headaches did not occur in the 27 patients who received MTX via implanted access ports, but otherwise there were no discernible differences between patients receiving MTX via the direct spinal route or via the access port.
Table 2.
Adverse events.
| Side effect | n |
|---|---|
| Fatigue | 44 |
| Dizziness | 34 |
| Blurred vision | 11 |
| Headache | 10 |
| Nausea post-treatment | 6 |
| Double vision | 6 |
| Hair loss post-treatment | 2 |
| Back pain post-treatment | 1 |
| Low-grade fever post-treatment | 1 |
| Total | 115 |
There were numerous instances of urinary tract infections, some upper respiratory infections, and less than five patients reported having decubitus ulcers during the study period. However, these are considered relatively common events in advanced MS patients with significant disability, and they occur with comparable frequency to similarly disabled patients who are not on ITMTX. When there was evidence of an active infection, treatment with ITMTX was held until resolution of the infection. Treatment was not withheld due to infection for more than 7 days in any case.
EDSS assessment
The mean EDSS for all patients at initiation of therapy was 6.33 ± 1.12, and, after 18–57 treatments (3–10.25 years), the mean EDSS increased to 6.70 ± 1.15 (p = 0.033). Over the duration of the study, 50% of patients had improvement or stability in their EDSS scores.
When analyzed by MS subtype, there was no statistically significant difference between baseline and post-treatment EDSS in either the PPMS or SPMS group (Table 3). In SPMS patients, over an average of 5.96 years, the mean EDSS increase was 0.35 (p = 0.097). In PPMS patients, over an average of 6.67 years, the mean EDSS increase was 0.53 (p = 0.126). Furthermore, 36 out of 67 (54%) SPMS patients had no measurable clinical worsening, whereas only 6 out of 16 (38%) PPMS patients remained stable (Table 4). In the patients who progressed on treatment, the average worsening was 0.84 points in the SPMS group and 0.85 in the PPMS group.
Table 3.
Effect of ITMTX therapy on mean EDSS.
| MS type | Mean EDSS ± SD |
|
|---|---|---|
| EDSS prior to treatment | EDSS post treatment | |
| PPMS | 6.16 ± 0.86 | 6.69 ± 0.98* |
| SPMS | 6.36 ± 1.17 | 6.71 ± 1.19** |
EDSS, Kurtze expanded disability status scale; ITMTX, intrathecal methotrexate; PPMS, primary progressive multiple sclerosis; SPMS, secondary progressive multiple sclerosis.
p = 0.126 comparing baseline to post-treatment EDSS in PPMS.
p = 0.097 comparing baseline to post-treatment EDSS in SPMS.
Table 4.
EDSS trends subsequent To ITMTX therapy.
| MS type | Stable/improved EDSS | Declined EDSS |
|---|---|---|
| PPMS | 6 (37.5%) | 10 (62.5%) |
| SPMS | 36 (54%) | 31 (46%) |
| Total | 42 (50%) | 41 (50%) |
Values reported as n (%).
EDSS, Kurtze expanded disability status scale; ITMTX, intrathecal methotrexate; PPMS, primary progressive multiple sclerosis; SPMS secondary progressive multiple sclerosis.
Overall, no patient worsened by more than 2 EDSS points. Five patients had an EDSS increase of 1.5 and two patients had an EDSS increase of 2 over the entire study period.
Magnetic resonance imaging
All the patients had magnetic resonance imaging (MRI) of the brain performed during the study period, although, since this was a retrospective study, the studies were not performed at defined intervals or using the same protocol. None of these MRIs revealed Gadolinium enhancing lesions, and the few patients with multiple MRIs had no change in disease burden while on treatment. Furthermore, there was no radiologic evidence of chronic spinal fluid leakage or CNS infection.
Questionnaire
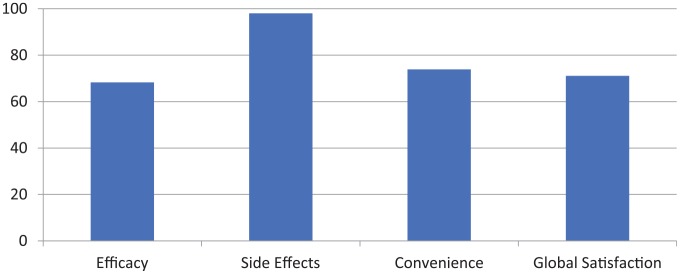
A total of 36 patients completed the TSQM. Mean scores for each domain are as follows, with 100 representing the highest score achievable: effectiveness 68.28, side effects/tolerability 98.05, convenience 73.87, and global satisfaction 71.09 (Figure 1).
Figure 1.
Treatment Satisfaction Questionnaire for Medication.
Discussion
This is a retrospective chart review on the use of administration of ITMTX in treatment-unresponsive, PPMS and SPMS patients at a single center. We report that, in our cohort of patients who have remained on this treatment for up to 10 years, ITMTX was well tolerated, with no major safety or tolerability issues. One of the noteworthy aspects of this study was the acceptance of patients to undergo repeated LP in order to receive ITMTX. Perhaps because of the dosing frequency relative to the much higher dosing frequency used in patients with CNS malignancies, significant adverse effects were not observed in our cohort.
MTX is associated with a number of side effects, usually related to the route of administration. Oral administration is known to cause a number of systemic side effects including gastrointestinal ulcers, megaloblastic anemia, pancytopenia, and a variety of infections. Intrathecal administration for oncologic conditions, which often entails higher doses administered in a continuous manner via Omaya reservoir, has been associated with leukoencephalopathy. In our experience, intermittent administration of 12.5 mg of MTX was not associated with any of these complications during the 2–10 year trial period.
This study is retrospective, open label, uncontrolled and not designed to study efficacy. However, the selection of severely disabled patients (mean pretreatment EDSS of 6.33) with long-standing MS (mean disease duration of 18.4 years) minimized the bias associated with remissions that occur early in the disease course. Additionally, the relatively long duration of the study of up to 10.25 years enabled us to observe any disease progression that may confound shorter duration studies. Our observations suggested that there may have been disease stabilization in a number of patients, especially those with SPMS. There was no worsening of EDSS in 54% of SPMS patients, and the mean EDSS increase in SPMS patients was 0.35 points over a mean of 5.96 years. While PPMS patients exhibited similar safety and tolerability results, only 6 out of 16 displayed stabilization of EDSS. The mean EDSS increase in PPMS patients was 0.53 points over a mean of 6.67 years. Although this study was not placebo controlled, ITMTX treated patients exhibited less disease progression than would be expected from the natural history of progressive MS. In 2000, Confavreux and colleagues reported that the median time of progression from an EDSS score of 6 to a score of 7 was 3.4 years, and that the time of progression was similar between PPMS and SPMS groups.29 In 2006, Confavreux again reported that the median time for progression from an EDSS score of 6–7 was 3.6 years (2.2–5.0) and 4.0 years (2.8–5.2) for SPMS and PPMS patients, respectively.30 Furthermore, Weinshenker and colleagues found that, in the first 5 years after diagnosis of progressive MS, the average rate of DSS change was 0.53 ± 0.02 points per year.31 However, these observations are based on different cohort of patients and are not necessarily comparable to our study subjects. Although the natural course of MS can vary, the degree of disease slowing in ITMTX treated patients over many years of observation suggests that the treatment may contribute to retarding disease progression in patients with progressive MS. Clearly to establish this, one would need to embark on a study similar to the Oratorio study with Ocrelizumab.10
Other factors could have affected our results, including the fact that some patients were concomitantly administered other treatments while transitioning to IT MTX, at the discretion of their treating neurologist. This is also a self-selecting sample of patients because only those patients who tolerated the treatment and perceived efficacy continued with repeated LP/side port access over the duration of the study. Although patients showed no radiographic progression during the study period, MRIs of patients with progressive forms of MS often do not change as frequently as patients’ MRIs early in the disease course. Finally, the TSQM scores validate our assertion of patient satisfaction, particularly with perceived side effects and tolerability.
Overall, in accordance with our previously published work, pulsed ITMTX therapy was well tolerated over a period of 2–10 years in progressive MS patients, with no serious adverse effects. Although this study was not controlled, there was a suggestion of disease stabilization, given the poor natural history of severe progressive MS. These findings support the use of ITMTX treatment as an extremely inexpensive and relatively safe treatment for progressive MS. Prospective, controlled studies are planned to evaluate the efficacy of this treatment.
Footnotes
Funding: The authors received no financial support for the research, authorship, and publication of this article.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethics statement: This study was approved by the institutional review board committee of St. Luke’s-Roosevelt Hospital, New York (approval number 11–110), and, therefore, was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Patient informed consent was obtained prior to each administration of MTX and prior to the review for this study.
ORCID iD: James W. Stark  https://orcid.org/0000-0002-2117-278X
https://orcid.org/0000-0002-2117-278X
Contributor Information
James W. Stark, From the International Multiple Sclerosis Management Practice, New York, NY, USA
Lena Josephs, Tisch Multiple Sclerosis Research Center of New York, New York, NY, USA.
Deirdre Dulak, Tisch Multiple Sclerosis Research Center of New York, New York, NY, USA.
Madison Clague, Tisch Multiple Sclerosis Research Center of New York, New York, NY, USA.
Saud A. Sadiq, International Multiple Sclerosis Management Practice and Tisch Multiple Sclerosis Research Center of New York, 521 West 57th St., 4th floor, New York, NY 10019, USA.
References
- 1. Sadiq SA. Multiple sclerosis. In: Rowland LP. (ed.) Merritt’s neurology. 11th ed. Philadelphia: Lippincott Williams and Wilkins, 2005, pp.941–963. [Google Scholar]
- 2. Ali R, Nicholas RS, Muraro PA. Drugs in development for relapsing multiple sclerosis. Drugs 2013; 73: 625–650. [DOI] [PubMed] [Google Scholar]
- 3. Hartung HP, Gonsette R, Koniq N, et al. Mitoxantrone in progressive multiple sclerosis: a placebo controlled, double-blind, randomized, multicentre trial. Lancet 2002; 360: 2018–2025. [DOI] [PubMed] [Google Scholar]
- 4. Sadiq SA, Rammal M, Sara G. Chronic myeloid leukemia associated with mitoxantrone treatment in a patient with MS. Mult Scler 2008; 14: 272–273. [DOI] [PubMed] [Google Scholar]
- 5. Rommer PS, Stüve O. Management of secondary progressive multiple sclerosis: prophylactic treatment—past, present, and future aspects. Curr Treat Options Neurol 2013; 15: 241–258. [DOI] [PubMed] [Google Scholar]
- 6. Marriott JJ, Miyasaki JM, Gronseth G, et al. Evidence report: the efficacy and safety of mitoxantrone (novantrone) in the treatment of multiple sclerosis: report of the therapeutics and technology assessment subcommittee of the American academy of neurology. Neurology 2010; 74: 1463–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wolinsky JS, Narayana PA, O’Connor PW, et al. Glatiramer acetate in primary progressive multiple sclerosis: results of a multinational, multicenter, double-blind, placebo-controlled trial. Ann Neurol 2007; 61: 14–24. [DOI] [PubMed] [Google Scholar]
- 8. Hawker K, O’Connor P, Freedman MS, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomised double-blind placebo-controlled multicenter trial. Ann Neurol 2009; 66: 460–471. [DOI] [PubMed] [Google Scholar]
- 9. Lublin F, Miller DH, Freedman MS, et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomized, double-blind, placebo-controlled trial. Lancet 2016; 387: 1075–1084. [DOI] [PubMed] [Google Scholar]
- 10. Montalban X, Hauser S, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med 2017; 376: 209–220. [DOI] [PubMed] [Google Scholar]
- 11. Bornstein MB, Miller A, Slagle S, et al. A placebo-controlled, double-blind, randomized, two-centered, pilot trial of Cop 1 in chronic progressive multiple sclerosis. Neurology 1991; 41: 533–539. [DOI] [PubMed] [Google Scholar]
- 12. Kappos L, Weinshenker B, Pozzilli C, et al. Interferon beta-1b in secondary progressive MS: a combined analysis of the two trials. Neurology 2004; 63: 1779–1787. [DOI] [PubMed] [Google Scholar]
- 13. Secondary progressive efficacy clinical trial of recombinant interferon-beta-1 in MS (SPECTRIMS) study group. Randomized controlled trial of interferon-beta-1a in secondary progressive MS: clinical results. Neurology 2001; 56: 1496–1504. [DOI] [PubMed] [Google Scholar]
- 14. Bleyer WA, Poplack DG. Prophylaxis and treatment of leukemia in the central nervous system and other sanctuaries. Semin Oncol 1985; 12: 131–148. [PubMed] [Google Scholar]
- 15. Cronstein BN. Low-dose methotrexate: a mainstay in the treatment of rheumatoid arthritis. Pharmacol Rev 2005; 57: 163–172. [DOI] [PubMed] [Google Scholar]
- 16. Ward JR. Historical perspective on the use of methotrexate for the treatment of rheumatoid arthritis. J Rheumatol 1985; 12(Suppl. 12): 3–6. [PubMed] [Google Scholar]
- 17. Cohen JA, Carter JL, Kinkel RP, et al. Therapy of relapsing multiple sclerosis. Treatment approaches for nonresponders. J Neuroimmunol 1999; 98: 29–36. [DOI] [PubMed] [Google Scholar]
- 18. Goodkin DE, Rudick RA, VanderBrug Medendorp S, et al. Low-dose (7.5 mg) oral methotrexate reduces the rate of progression in chronic progressive multiple sclerosis. Ann Neurol 1995; 37: 30–40. [DOI] [PubMed] [Google Scholar]
- 19. Goodkin DE, Rudick RA, VanderBrug Medendorp S, et al. Low-dose oral methotrexate in chronic progressive multiple sclerosis: analyses of serial MRIs. Neurology 1996; 47: 1153–1157. [DOI] [PubMed] [Google Scholar]
- 20. Olek MJ, Hohol MJ, Weiner HL. Methotrexate in the treatment of multiple sclerosis. Ann Neurol 1996; 39: 684. [DOI] [PubMed] [Google Scholar]
- 21. Lugaresi A, Caporale C, Farina D, et al. Low-dose oral methotrexate treatment in chronic progressive multiple sclerosis. Neurol Sci 2001; 22: 209–210. [DOI] [PubMed] [Google Scholar]
- 22. Calabresi PA, Wilterdink JL, Rogg JM, et al. An open-label trial of combination therapy with interferon β-1a and oral methotrexate in MS. Neurology 2002; 58: 314–317. [DOI] [PubMed] [Google Scholar]
- 23. Jeffery DR. Use of combination therapy with immunomodulators and immunosuppressants in treating multiple sclerosis. Neurology 2004; 28; 63(Suppl. 6): S41–S46. [DOI] [PubMed] [Google Scholar]
- 24. Mueller AM, Nassery A, Conlon H, et al. Effects of intraventricular methotrexate administration on Cuprizone-induced demyelination in mice. Front Mol Neurosci 2013; 6: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sadiq SA, Simon EV, Puccio LM. Intrathecal methotrexate in multiple sclerosis. J Neurol 2010; 257: 1806–1811. [DOI] [PubMed] [Google Scholar]
- 26. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology 1983; 33: 1444–1452. [DOI] [PubMed] [Google Scholar]
- 27. Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the treatment satisfaction questionnaire for medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes 2004; 2: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Atkinson MJ, Kumar R, Cappelleri JC, Hass SL. Hierarchical construct validity of the treatment satisfaction questionnaire for medication (TSQM version II) among outpatient pharmacy consumers. Value Health 2005; 8(Suppl. 1): S9–S24. [DOI] [PubMed] [Google Scholar]
- 29. Confavreux C, Vukusic S, Moreau T, et al. Relapses and progression of disability in multiple sclerosis. N Engl J Med 2000; 343: 1430–1438. [DOI] [PubMed] [Google Scholar]
- 30. Confavreux C, Vukusic S. Natural history of multiple sclerosis: a unifying concept. Brain 2006; 129: 606–616. [DOI] [PubMed] [Google Scholar]
- 31. Weinshenker BG, Bass B, Rice GP, et al. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain 1989; 112: 133–146. [DOI] [PubMed] [Google Scholar]