Abstract
Background and Aim
Large bowel functional symptoms are common in patients with inflammatory bowel disease (IBD) who are in disease remission. The efficacy of pelvic floor muscle training for symptoms of evacuation difficulty or fecal incontinence is well established in patients without organic bowel disease but is unknown in these patients. This study aimed to systematically evaluate the published evidence in this group of patients.
Methods
A systematic review was conducted of articles evaluating pelvic floor muscle training, with or without biofeedback, to improve bowel function in patients with quiescent IBD, including those with an ileoanal pouch. The outcome of interest was improved bowel function measured by bowel diary, patient report, or validated questionnaire in randomized controlled studies, cohort studies, or case series.
Results
Two randomized controlled trials, four retrospective case series, and one prospective study met eligibility criteria. Pelvic floor muscle training for patients with quiescent IBD improved symptoms in 51 of 76 (68%) patients with evacuation difficulty and 20 of 25 (80%) patients with fecal incontinence. Pelvic floor muscle training for patients with an ileoanal pouch, prior to stoma closure, did not appear to reduce the risk or severity of fecal incontinence following stoma closure. Studies were limited by small numbers, study design, methodological quality, and lack of long‐term follow‐up.
Conclusion
Pelvic floor muscle training appears to be of therapeutic value in some patients with quiescent IBD and evacuation difficulty or fecal incontinence. The effectiveness of this approach warrants further investigation.
Keywords: pelvic floor, biofeedback, inflammatory bowel disease, ileoanal pouch, fecal incontinence, dyssynergic defecation, constipation
The efficacy of pelvic floor muscle training for symptoms of evacuation difficulty or fecal incontinence is well established in patients without organic bowel disease but not in patients with inflammatory bowel disease (IBD). This study aimed to systematically evaluate the published evidence in this group of patients. Pelvic floor muscle training appears to be of therapeutic value in some patients with quiescent IBD and evacuation difficulty or fecal incontinence.
Introduction
Inflammatory bowel diseases (IBD) are chronic relapsing and remitting inflammatory diseases of the gastrointestinal tract. Most patients achieve drug‐induced disease remission, but approximately 15% of those with ulcerative colitis (UC) require removal of the colon within 10 years of diagnosis.1 Proctocolectomy with ileoanal pouch formation is the most commonly applied surgical treatment, designed to avoid the negative physical and psychosocial effects of a permanent stoma.2
Many patients continue to experience troublesome bowel symptoms, including fecal urgency, increased bowel frequency, fecal incontinence, constipation (low bowel frequency or impaired rectal evacuation), abdominal pain, or bloating, despite apparent drug‐ or surgically induced disease remission.3, 4, 5 Fecal incontinence is a key concern for people with IBD.6, 7 The prevalence of fecal incontinence in patients with IBD ranges from 24 to 74% and occurs during active and quiescent disease phases.8, 9, 10, 11, 12 Incontinence rates in patients with a pouch vary from 4 to 55% overnight and 4 to 40% during the day.13, 14 Constipation occurs in 26% of those with UC and 6% in those with Crohn's disease during remission.3 Evacuation difficulty has been reported in 9–40% of patients with an ileoanal pouch,15, 16, 17 increasing with age.15 Despite the high prevalence, these symptoms are underreported by patients and underrecognized by clinicians.9, 18, 19
A complex interaction of physiological and psychological factors is most likely involved in the generation and perpetuation of functional bowel symptoms following disease remission.3, 20, 21, 22 Alterations in gut motility, rectal or pouch compliance (stiffness), sensitivity, and contractility occurring in response to the inflammatory process or pouch surgery are implicated in symptom generation.23, 24, 25, 26 Patients with fistulizing Crohn's disease or an ileoanal pouch may have poor anal sphincter function, further compromising bowel function.27, 28 Psychological stress affects gut motility, visceral sensation, and immune factors and can exacerbate or perpetuate symptoms.20, 29, 30 Persistent symptoms are associated with anxiety, depression, health‐care utilization, absenteeism, and impaired health‐related quality of life.3, 31, 32, 33, 34
Normal pelvic floor muscle function is integral to the maintenance of bowel control (continence) and evacuation of stool (defecation).35 Pelvic floor muscle dysfunction may be a learned “maladaptive” behavior in response to unpleasant stimuli such as abdominal or anorectal pain, loose stools, and fecal urgency, which are common in patients with IBD.36 Defecation is impaired when the pelvic floor and anal sphincter muscles contract or fail to relax adequately during evacuation. This is referred to as dyssynergia, paradoxical puborectalis contraction, or nonrelaxing pelvic floor muscle dysfunction.37, 38 Pelvic floor muscle dysfunction has been identified in over half the patients with an ileoanal pouch39, 40 and between 45 and 97% of patients with quiescent IBD and symptoms of evacuation difficulty.41, 42
Functional bowel symptoms are therefore a major problem for patients with IBD, but their management has received little attention. Typically, treatment is empirical and includes drug therapy, dietary modification, or psychological therapies.43 None of these therapies directly target the maladaptive toileting behavior or pelvic floor muscle dysfunction. Pelvic floor muscle training with biofeedback has been suggested as a treatment option for patients with IBD, but the efficacy of this approach is unclear.40, 41, 42, 44 Pelvic floor muscle training, with or without biofeedback, has been extensively investigated and used successfully to treat bowel dysfunction in patients without IBD.45, 46, 47 However, there are very limited data supporting efficacy in the setting of IBD. This review aimed to systematically evaluate the evidence for pelvic floor muscle training in the management of bowel symptoms suggestive of dysfunction in patients with IBD in disease remission.
Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta‐analyses guidelines.48
Literature search strategy
Six electronic databases (MEDLINE 1946–2018, EMBASE 1980–2018, CINAHL 1982–2018, PEDro 1999–2018, PsycINFO 1946–2018, and the Cochrane Library 2018) were searched systematically in March 2018. Conference abstracts from the following journals were also searched: Journal of Crohn's Colitis, Inflammatory Bowel Diseases, Diseases of the Colon and Rectum, Colorectal Disease, Gut; Journal of Gastroenterology and Hepatology; and the United European Gastroenterology Journal.
The search strategy used combinations of the following MeSH headings and keywords: inflammatory bowel disease, Crohn or Crohn's disease, ulcerative colitis, proctocolectomy restorative, colonic pouches, ileoanal reservoir, ileal pouch anal anastomosis, ileoanal pouch, J pouch, IPAA, biofeedback psychology, electromyography (EMG), physical therapy modalities, physiotherapy, physical therapy, behavior therapy, rehabilitation, pelvic floor muscle, levator ani, puborectalis, fecal incontinence, constipation, and defecation. Articles were limited to those published in full in English. Reference lists of selected articles and conference abstracts between 2013 and 2018 were also checked, and relevant abstracts were followed up to determine if the data had been published in a full paper.
Study selection criteria
Studies were included if they met the following eligibility criteria:
Study design
Randomized controlled trials (RCTs), cohort studies, or case series reports.
Participants
Adults ≥18 years of age with IBD in disease remission, defined clinically, endoscopically, or histologically, or with an ileoanal pouch but no pouch inflammation. Patients were included with symptoms of fecal urgency or incontinence and evacuation difficulty or constipation. Patients with an ileoanal pouch before stoma closure were also included to determine whether behavioral treatment prior to stoma closure would prevent or reduce bowel symptoms after stoma closure.
Intervention
Pelvic floor, Kegel, or anal sphincter muscle exercises with or without biofeedback. Training methods vary and can include exercises focused on strength training, sensory training, and/or coordination or simulated defecation training. “Biofeedback” is just one of the training tools used to provide information to the patient about muscle performance and changes made with the training program.
Outcomes
The primary outcome reported was bowel function using any of the following measures: bowel diary, patient rating of improvement, or a validated questionnaire.
One author (Angela J Khera) screened all titles and abstracts to identify potential studies for inclusion. Two reviewers (Angela J Khera and Janet W Chase) independently evaluated the abstracts and full texts of all retrieved papers to decide eligibility. A third reviewer (Michael A Kamm) resolved any disagreements.
Data extraction
Extracted data were recorded on a review‐specific form and included the first author's name, publication year, study design, number of participants, age, gender, IBD diagnosis, presenting symptoms, details of the intervention type, outcome measures, training frequency, duration of training program, dropouts, follow‐up period, and results.
Quality appraisal
Methodological quality was assessed independently by two reviewers (Janet W Chase and Angela J Khera) using the Methodological Index for Non‐Randomized Studies (MINORS) tool49 and the Cochrane Risk of Bias Tool50 for RCTs. MINORS is a validated tool assessing nonrandomized studies using eight criteria, each allocated a score of 0–2 per item (0 = not reported, 1 = reported but inadequate, 2 = reported and adequate). Items include study aim, inclusion criteria, nature of data collection, end‐points, blinding of assessment, follow‐up period, dropout reporting, and study size calculation. The Cochrane risk‐of‐bias tool for randomized trials assesses seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. “High,” “low,’ or “unclear” risk of bias was determined by set criteria within each domain.50 Papers were assigned an overall quality rating (poor, fair, good, excellent) based on the assessed criteria and reviewer consensus.
Results
Study selection
Following the electronic database search, a total of 4450 studies were identified, and a further 4 were found from hand searching. Titles and abstracts were screened after duplicates were removed, leaving nine studies to be assessed for eligibility. Seven studies meeting eligibility criteria were finally included for review (Fig. 1).
Figure 1.
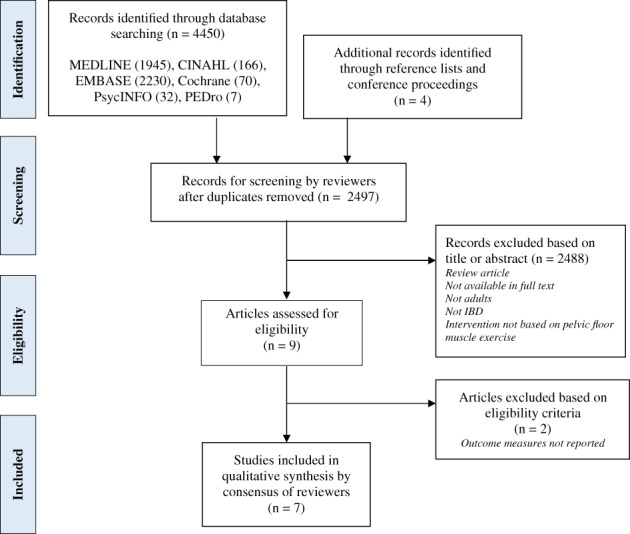
Diagram of study selection process. IBD, inflammatory bowel disease.
Study characteristics
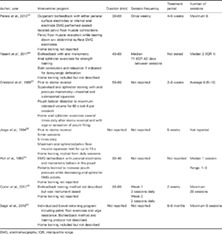
Study design
Two RCTs,51, 52 one prospective observational study,53 and four retrospective case series39, 42, 54, 55 were included.
Participants
A total of 227 participants (females 58%) were included in the studies, of whom 134 had an intervention including pelvic floor or anal sphincter muscle training. Thirty‐one participants who had training had IBD in remission,42, 54 and 103 had an ileoanal pouch.39, 51, 52, 53, 55 Thirty‐three participants were in control groups,51, 52 57 were not referred for therapy,39, 42 and 3 dropped out before treatment commenced.42, 51
Participants in the RCTs (n = 66) had ileoanal pouch surgery for UC and were asymptomatic as training occurred prior to stoma reversal.51, 52 Participants in the nonrandomized trials presented with symptoms including evacuation difficulty (n = 76), fecal incontinence (n = 25), abdominal pain (n = 1), and pruritus (n = 1).39, 42, 54, 55 Anal sphincter or pelvic floor muscle function was assessed prior to training with anal physiological testing including manometry, balloon expulsion or anal EMG.
Screening for IBD activity occurred in both IBD studies, and all patients were cleared of active left‐sided disease, with both endoscopy and histology, prior to pelvic floor muscle training.42, 54 The exclusion of pouchitis prior to training was not uniformly described. One study reported that physical examination was performed to exclude physical abnormalities but did not explicitly state that pouchitis was excluded.53 Quinn et al.39 performed endoscopy to assess pouch inflammation but did not state whether those treated with biofeedback had active pouchitis or not. Details of screening for pouchitis prior to biofeedback treatment were not reported in another study.55 Participant characteristics are listed in Table 1.
Table 1.
Participant characteristics
| Author, year | Participants, n | Diagnosis, n | Male: Female | Age, years | Symptoms and investigations |
|---|---|---|---|---|---|
| Perera et al., 201342 | Total 30 | CD 24 | 6: 24 | Mean (SD) 42.1 (12.75) | Evacuation difficulty Dyssynergic defecation demonstrated by anal manometry and balloon expulsion testing |
| Training 22/30 | |||||
| 23 referred for therapy; | UC 6 | ||||
| 22 attended | |||||
| Vasant et al., 201754 | Total 9 | CD 6 | 2: 7 | Median 53 (IQR 7) | Fecal incontinence Anal manometry findings: |
| 9/9 external anal sphincter weakness | |||||
| Training 9/9 | UC 3 | 6/9 internal sphincter weakness | |||
| 8/9 rectal hypersensitivity | |||||
| Oresland et al., 198851 | Total 40 | Pouch (UC 40) | 18: 20 | Training Mean 36(range,19–58) | Asymptomatic (prestoma reversal) Anal manometry performed pre‐ and postoperatively up to 12 months after ileostomy closure |
| Training 18/20 | |||||
| Two withdrawn with postoperative complications | Control Mean 38 (range,18–51) | ||||
| Control 20/20 | |||||
| Jorge et al., 199452 | Total 26 | Pouch (UC 26) | 16: 10 | Training Mean 33 (range, 17–56) | Asymptomatic (prestoma reversal) Anal manometry performed prior to the pouch procedure and again prior to ileostomy closure |
| Training 13/13 | Control Mean 38 (range, 24–69) | ||||
| Control 13/13 | |||||
| Hull et al., 199553 | Total 13 | Pouch (UC 4, CD 4, others 5) | 7: 6 | Not reported | Evacuation dysfunction Paradoxical puborectalis contraction demonstrated on EMG |
| Training 13/13 | |||||
| Quinn et al., 201739 | Total (with pelvic floor dysfunction) 83/111 | Pouch (UC 100, others 11) | 49: 62 | Median 44 (range, 15–75) | Evacuation difficulty Pelvic floor dyssynergia identified by one or more of the following: anal manometry, balloon expulsion testing, defecography, or anal EMG |
| Training 33/83 No details on other 50 | Diagnosis not reported separately for training group; CD excluded | Not reported separately for training group | Not reported separately for training group | ||
| Segal et al., 201855 | Total 26 | Pouch (UC 23, others 3) | 8: 18 | Median 49 (range, 36–74) | Fecal incontinence 26 Evacuation difficulty 8 (Other symptoms 2) |
| Training 26/26 | Assessment methods not reported |
CD, Crohn's disease; EMG, electromyography; IQR, interquartile range; UC, ulcerative colitis.
Intervention
The intervention varied in the type of training delivered, the duration, and the frequency and number of sessions (Table 2). The pelvic floor muscles, particularly puborectalis, and the anal sphincter muscles act as a functional unit and are considered together in this review. Four studies provided details of biofeedback‐assisted training using EMG, anal pressure, or balloon manometry, while two studies did not provide any details about the type of biofeedback used.39, 55 Training involved pelvic floor exercises alone in one study.52 Programs included strength training,51, 52, 54 simulated defecation training,42, 53, 54 repeated pouch balloon dilations,51 or urge resistance training.55
Table 2.
Intervention
| Author, year | Intervention program | Duration (min) | Session frequency | Treatment period | Number of sessions |
|---|---|---|---|---|---|
| Perera et al., 201342 | Outpatient biofeedback with either perianal surface electrodes or internal anal electrode EMG performed seated | 30–60 | Once weekly | 4–6 weeks | Maximum 6 |
| Isolated pelvic floor muscle contractions | |||||
| Pelvic floor muscle relaxation while bearing down +/− abdominal surface EMG electrodes | |||||
| Home training not reported | |||||
| Vasant et al., 201754 | Biofeedback with anal manometry | 45–60 |
Median 71 (IQR 42) days between sessions |
Not stated | Median 2 (IQR 1) |
| Anal sphincter exercises for strength training | |||||
| Both contraction and relaxation if indicated for dyssynergic defecation | |||||
| Home training included but not described | |||||
| Oresland et al., 198851 | Prior to stoma reversal | 50–60 | Not reported | 2–8 weeks | Average 8 (5–10) |
| Supervised anal sphincter training with anal pressure manometry—maximal and submaximal squeezes | |||||
| Pouch balloon dilatation to maximum tolerated volume for 60 s (×4–6 per session) | |||||
| Home anal sphincter exercises several times daily after stoma reversal and with urge or sensation of pouch filling | |||||
| Jorge et al., 199452 |
Prior to stoma reversal 5‐min sessions 5 times daily |
Not reported | Not reported | 5 weeks | Not reported |
| Maximum anal sphincter/pelvic floor muscle squeezes held for up to 10 s | |||||
| Home training implied from daily sessions | |||||
| Hull et al., 199553 | EMG biofeedback with perianal electrodes and manometry balloon in the pouch | 30–45 | Not reported | Not reported |
Median 1 session Range, 1–3 |
| Patients learned to increase pouch pressure while decreasing anal sphincter EMG activity | |||||
| Home training not reported | |||||
| Quinn et al., 201739 | Biofeedback training method not described but was instrument based | 30–60 |
Week 1 3 sessions daily |
2 weeks |
Maximum 25 sessions |
| Home training not reported |
Week 2 2 sessions daily |
||||
| Segal et al., 201855 | Individualized bowel retraining program including pelvic floor exercises and urge resistance. Biofeedback method and training protocol not described. | Not reported | Not reported | 6–8 months | Maximum 6 sessions |
| Home training included but not described |
EMG, electromyography; IQR, interquartile range.
The number of training sessions ranged from 1 to 25, delivered over periods that varied from 2 weeks to 8 months. Sessions typically lasted 30–60 min. Home training was not reported by two studies,39, 53 and little detail of the home training regime was provided by the remaining five studies. Segal et al.55 was the only study to describe any additional treatment strategies provided to participants as part of the training program. These were modifications to diet and fluid intake, toileting posture, and defecation technique, as well as pelvic floor myofascial release techniques. Only one study reported the professional discipline of the therapist delivering the intervention, that is, nurse, physiotherapist, or physician.54
Outcome measures
Outcome was assessed by patient report of improvement,39, 42, 53, 54, 55 a gastrointestinal‐specific questionnaire,42, 51, 52, 55 manometric measures of anorectal or anal‐pouch function,51, 52 EMG,51, 53 bowel diary,51, 54 or health‐care utilization.42 The questionnaires used included the short inflammatory bowel disease questionnaire (SIBDQ),42 the Oresland functional score,51 Cleveland fecal incontinence score,52 and the International Consultation on Incontinence—Bowel questionnaire (ICIQ‐B).55 Only the SIBDQ and ICIQ‐B included assessment of health‐related quality of life. Outcome assessment occurred at a wide range of intervals, from immediately following treatment to 15 months later.
Risk of bias and study quality
Percentage agreement and Cohen's kappa statistic56 were used to determine the interrater agreement of quality assessment using the MINORs (Table 3) and Cochrane Risk of Bias (Table 4) tools. The kappa coefficient was 0.76, indicating substantial agreement.57 Reviewers had complete agreement on 46 of 54 items (85.2%) and reached consensus on the remaining 8 items before deciding the final study quality rating (poor, fair, good, excellent).
Table 3.
Assessment of non‐randomized study quality (MINORS)
MINORS criteria†
|
| MINORS† | Quality assessment | |
|---|---|---|
| IBD | ||
| Perera et al.42 | 8 | Fair |
| Vasant et al.54 | 7 | Fair |
| Pouch | ||
| Hull et al.53 | 8 | Fair |
| Quinn et al.39 | 6 | Poor |
| Segal et al.55 | 6 | Poor |
Each item is scored 0 (not reported), 1 (reported but inadequate), or 2 (reported and adequate) with an ideal score of 16.
MINORS, methodological index for nonrandomized studies.
Table 4.
Assessment of randomized study quality (Cochrane risk of bias)
| Jorge et al.52 | Oresland et al.51 | Domain† |
|---|---|---|
| ? | ? | Random sequence generation |
| ? | ? | Allocation concealment |
| ? | ? | Blinding of participants and personnel |
| ? | ? | Blinding of outcome assessment |
| ? | + | Incomplete outcome data |
| + | + | Selective reporting |
| ? | ? | Other sources of bias |
| Poor | Fair | Quality Assessment |
Each domain is rated (?) = unclear risk of bias, (+) = low risk of bias, or (‐‐) = high risk of bias based on the specific criteria within each domain.
The nonrandomized studies (Table 3) were limited by small numbers of participants and lack of a nonexposed cohort, blinded assessment, intention‐to‐treat analysis, missing data, or long‐term follow‐up.39, 42, 53, 54, 55 The two randomized trials (Table 4) were also limited by small numbers and lack of detail about random allocation method, allocation concealment, blinding of personnel, and blinding of outcome assessment.51, 52 Follow‐up time was inadequate in one of the randomized trials, and dropout rate was not reported.52 Neither of these studies stated what exposure the control group had during the study period.
Due to these limitations, four studies were rated “fair”42, 51, 53, 54 and three “poor”39, 52, 55 for overall quality.
Outcomes
Evacuation difficulty—IBD
Perera et al.42 examined the outcome of biofeedback‐assisted pelvic floor training in patients with quiescent IBD and persistent evacuation problems (Table 5). Although 30 patients were identified, only 22 patients underwent biofeedback‐assisted training. Patients had a mean disease duration of 14.4 ± 12.5 years. Most patients were females with Crohn's disease (67%). Outcome was assessed in four ways at the completion of treatment: physical therapist report of correction of dyssynergic defecation pattern, patient‐reported improvement, the shortened form of the inflammatory bowel disease questionnaire (SIBDQ), and health‐care utilization (the number of IBD‐related medical visits in the 6 months before and after treatment). Two patients dropped out of the treatment for unstated reasons and were not included in the analysis. Of the 20 remaining patients, 16 (80%) reported symptomatic improvement. The overall change in SIBDQ score was not significant, although a small proportion (30%) of patients had a clinically significant (≥7‐point) score reduction. The bowel‐related health‐care visits were significantly reduced in the 6 months following treatment compared to the 6 months prior to treatment. Six patients also had fecal urgency and/or fecal incontinence, but their outcome is not reported separately.
Table 5.
Pelvic floor muscle training outcomes (IBD)
| Author, year, country and study type | Participants completed and dropouts | Measure | Preintervention Mean (SD) | Postintervention, Mean (SD) | P‐value | Follow‐up | Outcome |
|---|---|---|---|---|---|---|---|
|
Perera et al., 201342 USA Retrospective case review |
20/22 2 patients did not complete therapy – no details provided |
SIBDQ HCU • number of health‐care visits in the 6 months prior to and following training Self‐report by therapist • correction of dyssynergic pattern Patient report of improvement in symptoms |
SIBDQ Score 38.6 (14.1) HCU—Visits 4.7 (4.2) |
SIBDQ Score 40.3 (12.8) HCU – Visits 2.7 (1.6) |
0.85 0.003 * |
At completion of treatment and 6 months later |
Nil significant change in health‐ related quality of life Significant reduction in HCU following training Therapist report not stated Improved 16/20 (80%) |
|
Vasant et al., 201754 UK Retrospective case review |
8/9 1 dropout after 2 sessions (non‐responder) |
Bowel diary • episodes of fecal incontinence per week Patient report of full continence or improvement in symptoms |
Incontinence episodes per week, 11.5 |
Incontinence episodes per week, 0.0 | 0.003 * | At completion of treatment |
Improved 8/9 (89%) Fully continent 5/9 (56%) |
P < 0.05.
HCU, health‐care utilization; IBD, inflammatory bowel disease; SIBDQ, short inflammatory bowel disease questionnaire.
Evacuation difficulty—Ileoanal pouch
Three studies investigated the outcome of biofeedback training in patients with an ileoanal pouch and symptoms of evacuation difficulty (Table 6).39, 53, 55 Twelve patients with demonstrated paradoxical puborectalis contraction (dyssynergia) on EMG underwent biofeedback training using anal surface EMG and a pressure balloon in the pouch (Table 2).53 Eleven patients were followed up an average of 8 months after the completion of training. Of the 11 patients, 9 (82%) reported improvement, defined as a patient report of normal defecation and a normal EMG pattern (abolition of dyssynergia). All 11 patients had a normal defecation pattern on repeat EMG after treatment, although 2 did not report symptomatic benefit.
Table 6.
Pelvic floor muscle training outcomes (pouch)
| Author, year, country, and study type | Participants completed and dropouts | Measure | Preintervention Mean (SD) | Postintervention, Mean (SD) | P‐value | Follow‐up | Outcome |
|---|---|---|---|---|---|---|---|
|
Oresland et al., 198851 Sweden Randomized controlled trial |
Training 18/20 Control 20/20 2 in training group withdrawn due to postoperative complication |
Maximum pouch volume Maximum anal resting and squeeze pressures Bowel frequency per 24 h —bowel diary Oresland functional score |
4 weeks after pouch construction 75ml Maximum resting pressure 50 mmHg Maximum squeeze pressure 170 mmHg 1 week after stoma closure Training group 7.3 (2.5) Control group 7.5 (2.5) Actual scores not stated |
Prior to stoma reversal Training group 136 (34) ml Control group 108 (57) ml 12 months after stoma closure Maximum volume both groups 265 ml At 12 months Maximum resting pressure Training group 56 (17) mmHg Control group 50 (15) mmHg Maximum squeeze pressure both groups = 200 mmHg 6 months after stoma closure Training group 4.9 (1.6) Control group 5.4 (1.8) Actual scores not stated |
NS NS NS NS NS NS |
Before stoma closure 1, 3, 6, and 12 months poststoma reversal |
Training prior to stoma reversal did not have a significant effect on maximum pouch volume, maximum anal squeeze pressure, or maximum anal resting pressure Training prior to stoma reversal did not affect functional outcome |
|
Jorge et al., 199452 USA Randomized controlled trial |
Training 13/13 Control 13/13 Dropouts not reported |
Anal sphincter pressures Cleveland Fecal Incontinence score |
Anal resting pressure Control 65 (15) mmHg Training 75 (25) mmHg Anal Squeeze pressure Control 128 (52) mmHg Training 97 (48) mmHg Control 0.2 (0.1)Training 0.2 (1.2) |
Anal resting pressure Control 44 (14) mmHg Training 48 (18) mmHg Anal Squeeze pressure Control 110 (48) mmHg Training 86 (44) mmHg Control 2.8 (1.6) Training 2.0 (1.2) |
0.20 0.300.07 |
Within 1 month of stoma reversal | Training prior to stoma reversal did not affect anal pressures or functional outcome soon after stoma reversal |
|
Hull et al.,199553 USA Prospective case series |
12/13 1 patient was lost to follow‐up |
Patient report of symptom resolution and normal EMG | None reported | None reported | Not reported |
Average follow‐up 8 months Range, 1–15 |
Improved 9/12 (75%) No change 2/12 (17%) All 11 normalized EMG |
|
Quinn et al., 201739 USA Retrospective case series |
22/33 7 dropped out due to pain with treatment 3 with time constraints 1 due to lack of progress |
Patient rating 15‐point Likert scale −7 “a great deal worse” 0 “no change” + 7 “a very great deal better” Physician rating of improvement “significant improvement” “mild–moderate improvement” “no change” |
Not reported |
Change in patient rating scale +4.6 |
Not reported | At completion of training |
Significant improvement 5/22 (23%) Mild–moderate improvement 15/22 (68%) No change 2/22 (9%) |
|
Segal et al., 201855 UK Retrospective case series |
24/24 Objective data available for only 9/24 patients |
Subjective improvement rating by 2 independent reviewers from patient reports in the medical record ICIQ‐B questionnaire Bowel pattern Bowel control Nonscored Quality of life St Marks tool for ED |
Not relevant FI Group n = 5/16 Median (range)62 (49–62) 82 (33–102) 22 (17–35) 80 (62–98) ED Group n = 4/8 Incomplete emptying 4/4 (100%) Straining 4/4 (100%) Pain 4/4 (100%) Bloating 3/4 (75%) Laxatives 1/4 (25%) |
Not reported 46 (39–62) 53 (11–76) 29 (12–29) 41 (30–55) Incomplete emptying 4/4 (100%) Straining 2/4 (50%) Pain 1/4 (25%) Bloating 2/4 (50%) Laxatives 0/4 (0%) |
Not reported 0.12 0.21 0.35 0.01 * Not reported |
Median follow‐up 3 months from last biofeedback session Range, 1–6 months |
FI Much improved 4/16 25% Some improvement 8/16 50% No improvement 4/16 25% ED Much improved 4/8 50% Some improvement 2/8 25% No improvement 2/8 25% Combined outcome (FI and ED) Improved 75% |
P < 0.05.
ED, evacuation disorder; EMG, electromyography; FI, fecal incontinence; ICIQ, International Consultation on Incontinence questionnaire‐bowel; NS, not significant.
Eighty‐three patients with an ileoanal pouch and symptoms of evacuation difficulty were identified with nonrelaxing pelvic floor muscle dysfunction by Quinn et al.39 (Table 6). Of these patients, 33 had biofeedback training, with 22 (67%) patients completing the training program. Seven patients ceased treatment due to pain during therapy. The biofeedback method was not described but may have been invasive (electrodes or balloons inserted per anum), and training was intensive, occurring over a 2‐ week period. Three others withdrew due to time limitations and one due to lack of improvement. The outcome was recorded at the end of the 2‐week training period with no longer‐term follow‐up. Of 22 patients who completed therapy, 20 (91%) had symptomatic improvement as assessed by both the patient and physician.
Segal et al.55 used two independent reviewers to determine improvement from reports in the medical record for eight patients with an ileoanal pouch and problems with evacuation. The kappa coefficient for interrater reliability was high (0.94). Specific details of the biofeedback‐assisted pelvic floor training program were not reported, but six (75%) of eight patients were reported to have improved at a median of 3 months from their last training session. A tool for assessing evacuation disorders was also used by these researchers but was only completed by four of the eight patients at the completion of treatment. Symptoms of abdominal pain, bloating, and straining reduced, but incomplete emptying was unchanged (Table 6).
Fecal incontinence—IBD
The outcome of biofeedback‐assisted pelvic floor muscle training in a group of nine patients with quiescent IBD (Table 5) was measured by patient report of symptomatic improvement and fecal incontinence episodes per week using a bowel diary.54 Only patients who had completed biofeedback training were included in this study. The authors did not state whether there were other patients who had not completed training and had been excluded. Patients were divided into responders and nonresponders according to outcome. Responders were those achieving continence or reporting significant improvement. Eight responders (89%) achieved a significant reduction in the median number of fecal incontinence episodes Five patients (56%) achieved full continence. The single nonresponder dropped out of the treatment after two sessions. Two patients were found to have a dyssynergic defecation pattern on manometry testing, and both improved with treatment. Six patients had documented reports of performing home exercises as instructed. There was no long‐term follow‐up.
Segal et al.55 included 16 patients with an ileoanal pouch and fecal incontinence in their retrospective case review. The outcome of the biofeedback training program, at a median of 3 months following treatment completion, was assessed using two independent reviewers to determine improvement from reports in the medical record, patient report of improvement, or the ICIQ‐B questionnaire (Table 6). Symptom improvement was reported in 12 (75%) of 16 cases, but ICIQ‐B scores were only available for 5 (31%) of the 16 participants. The quality‐of‐life domain of the ICIQ‐B was the only domain in this questionnaire that changed significantly (P = 0.01).
Fecal incontinence—Ileoanal pouch
The RCTs recruited consecutive patients with an ileoanal pouch prior to stoma reversal and assessed the effect of different training protocols on anal sphincter muscle function and pouch function after reversal.51, 52 Jorge et al.52 randomized 26 patients, with 13 patients in each group, to the training or control group. Those in the training group were asked to perform anal sphincter (pelvic floor) exercises five times daily for up to 5 weeks prior to stoma reversal (Table 6). The authors did not state if patients were shown how to perform the exercises correctly, and biofeedback was not used. Patients were assessed at baseline and within a month of stoma reversal using anal manometry and the Cleveland fecal incontinence score. There were no significant differences between groups in anal resting pressure (P = 0.20) or anal squeeze pressure (P = 0.30). The training group had a lower mean fecal incontinence score (2.0) than the control group (2.8), but this did not reach significance (P = 0.07).
The second randomized trial used repeated progressive pouch dilatations with a balloon and biofeedback‐guided anal sphincter exercises for 2–8 weeks prior to stoma reversal.51 Forty patients were randomized, with 20 patients in the training group and 20 in the control group. Two patients were lost from the training group due to surgical complications. Outcomes were assessed at multiple time points for up to 12 months following stoma reversal. These included bowel frequency using a daily diary, a questionnaire (the Oresland score) devised to assess functional outcome (lower score equals better outcome), anal sphincter pressures, and maximum pouch volume (Table 6). Pouch volume, anal resting pressure, anal squeeze pressure, and bowel frequency did not differ significantly between groups at any time point. The training group had a lower Oresland score than the control group at 6 and 12 months following stoma reversal but, again, did not reach significance.
Summary
The total number of patients receiving anal sphincter or pelvic floor muscle training for evacuation problems was 76, with 61 (80%) of these 76 completing training and 51 (84%) of these 61 reported as improved. The improvement rate for the total cohort when including treatment dropouts was 67% (51 of 76), 65% (35 of 54) for those with an ileoanal pouch, and 73% (16 of 22) for those with quiescent IBD.
The total number of patients receiving anal sphincter or pelvic floor muscle training for fecal incontinence was 25, with 24 (96%) of these 25 completing training and 20 (83%) of 24 reporting as improved. The improvement rate for this cohort, including dropouts, was 20 (80%) of 25 patients.
Pelvic floor muscle training prior to stoma reversal in patients with an ileoanal pouch did not significantly reduce fecal incontinence or improve pouch function following stoma closure compared to the control groups.
Discussion
This review aimed to systematically evaluate the evidence for pelvic floor muscle training in the management of impaired evacuation or fecal incontinence in patients with quiescent IBD. Although pelvic floor muscle training is well validated in the non‐IBD setting, its application in the IBD population has been neglected. Only two RCTs and five nonrandomized studies were considered eligible after a comprehensive literature search.
The nonrandomized studies reported a decrease in bowel symptoms (fecal incontinence or defecation difficulty) after training in 65–80% of patients.39, 42, 53, 54, 55 While outcomes immediately following treatment were encouraging, there were significant limitations in some of the studies. In one study, less than half (33 of 83) of the patients identified with nonrelaxing pelvic floor muscle dysfunction had biofeedback training.39 It is unknown why 50 were excluded and whether those treated had pouchitis or not. One third did not complete treatment, seven due to pain. The type of intervention was not described, and treatment dropouts were not included in the final analysis.
Another study did not describe whether their screening process excluded pouchitis or other types of pouch dysfunction prior to treatment.55 Objective data were missing in the final analysis, with most data coming from patient reports found in the medical record.
Long‐term follow‐up (≥12 months) to determine whether treatment effect was sustained was reported in just one study.52 The manometric measures of anal sphincter function in those with fecal incontinence did not improve with training despite symptomatic improvement. This lack of correlation between symptomatic improvement and physiological measures following biofeedback training is consistent with previous studies in non‐IBD patients.58, 59
The RCTs51, 52 failed to show that pelvic floor or anal sphincter muscle training in patients with an ileoanal pouch, prior to stoma closure, reduces the risk, or severity, of fecal incontinence poststoma reversal. These studies may have been limited by small participant numbers as, in both studies, outcomes tended to favor the intervention group but did not reach significance. Oresland et al.51 used pouch balloon dilatation for pouch stretching, which may also have been a means of improving the awareness of pouch contents or improving pelvic floor muscle response to urge or sense of pouch fullness. In both controlled, randomized trials, it was unclear what exposure the control groups may have had during the study to personnel, medication, or self‐initiated pelvic floor exercises.
Limitations of the studies included in this systematic review are study design, small participant numbers, missing data, and lack of blinded assessment and long‐term follow‐up. It is possible that the effects observed were due to natural recovery or other factors such as patient education and support, medications, or interaction with a therapist. Patient adherence to the training protocols was not reported. There was wide variation in training protocols and follow‐up duration. There was insufficient evidence to determine whether pelvic floor muscle exercises alone are as effective as biofeedback‐assisted training or whether one training protocol is more effective than another.
A recent systematic review and meta‐analysis on the prevalence, diagnosis, and management of dyssynergic defecation in patients with IBD and symptoms of defecatory dysfunction concluded that symptoms of evacuation difficulty in patients with quiescent IBD do respond to biofeedback training.5 That systematic review included patients with an ileoanal pouch from a single center, possibly a single patient cohort, published in three separate abstracts,41, 60, 61 all of which were included in the meta‐analysis. The review did not include details about patient selection, treatment provided, outcome measures used, follow‐up periods, dropout rates, or the criteria used to assess study quality.
A second systematic review and meta‐analysis by the same research team on the prevalence, diagnosis, and management of fecal incontinence in patients with IBD did not report on pelvic floor muscle training and/or biofeedback.12
We have not conducted a meta‐analysis as there were insufficient studies to do so. The existing studies varied too much in their methodologies to be combined into one analysis. Studies should include full descriptions of the interventions delivered. This allows clinicians to implement the interventions more effectively and for researchers to replicate them in future studies, providing more meaningful outcome analyses.
There are good clinical reasons for offering pelvic floor muscle training, with or without biofeedback, to patients with mild or quiescent IBD and persistent bowel symptoms despite the lack of published evidence. Published guidelines for the management of fecal incontinence recognize that both IBD and bowel surgery increase the risk of developing fecal incontinence.62, 63 The sensorimotor function of the anorectum may be affected by the inflammatory process, with alterations in the sensory perception of rectal contents and the ability to contain or expel contents.23, 24 Surgical procedures or perianal fistulae may further compromise anal sphincter function.
Pelvic floor muscle training is not purely strength training. It incorporates exercises for improving the awareness of muscle contraction and relaxation, endurance, and coordination with abdominal and diaphragm muscles for the normal functions of continence and effective defecation. Nonrelaxing pelvic floor muscle dysfunction may develop in response to pain, urgency, or diarrhea as a protective mechanism.36 The muscles develop abnormal behavior through prolonged periods of holding on, which may eventually compromise their ability to contract and relax effectively. Muscle contraction strength in shortened, tight, or tense muscles is diminished.64 This can affect both continence and the ability to evacuate effectively. Symptoms do not correlate well with underlying pathophysiology, and there is no single standardized test for diagnosing pelvic floor muscle dysfunction. It is widely accepted that a combination of tests is required and includes skilled digital examination, anal manometry, balloon expulsion testing, EMG, defecography, or ultrasound.45, 65 Nonrelaxing pelvic floor muscle dysfunction has been demonstrated in patients with IBD,41, 42 and pelvic floor muscle training, often assisted by biofeedback, is the key therapy recommended.37, 38, 66 Noninvasive forms of biofeedback such as external EMG or real‐time ultrasound imaging may be preferable in this patient cohort to prevent patients withdrawing from therapy due to discomfort.60 It is a safe and effective treatment in the non‐IBD population with results maintained in the long term.45
Pelvic floor muscle training, with or without biofeedback, is often combined with other conservative interventions, including education, dietary and medication advice, toileting behavior modifications, urge resistance or deferral techniques, lifestyle changes, emotional support, and practical management strategies. Usually referred to as behavioral treatment, this package of care is tailored by the therapist to address individual patient symptoms. The education and psychological support provided by a therapist during training sessions as well as the skill and experience of the therapist may be key factors contributing to the efficacy of treatment.58, 67, 68, 69 There is only one published study investigating behavioral treatment in the management of bowel dysfunction in patients with quiescent IBD, a study in patients with an ileoanal pouch.55
In conclusion, this review suggests that symptomatic benefit can be achieved with pelvic floor muscle training in patients with quiescent IBD and bowel dysfunction, but the current evidence is limited. Despite the limitations of the current evidence, pelvic floor muscle training is a safe intervention that can be provided to patients with IBD or an ileoanal pouch without risk of serious adverse effects. Patients most likely to benefit have fecal incontinence or impaired evacuation and demonstrate pelvic floor or anal sphincter muscle dysfunction. Active inflammation and anal or anastomotic strictures should be excluded. Training programs that are individualized to target existing symptoms and muscle deficits and that adhere to exercise training principles are recommended. Given the prevalence and impact of functional bowel symptoms in patients with quiescent IBD and the potential benefit of gut‐directed behavioral treatment, including pelvic floor muscle training, prospective trials that may include standardized pelvic floor muscle assessment, health‐related quality of life measures, and long‐term follow‐up are urgently needed. This could help develop better‐targeted therapies for patients with IBD and persistent bowel symptoms despite drug‐ or surgically induced remission.
Acknowledgments
Angela J Khera was supported via an Australian Government Research Training Program Scholarship. Assistance with the literature search strategy was kindly provided by Anna Lovang (St Vincent's Hospital) and Lorena Romero (Alfred Health).
Declaration of conflict of interest: None.
Author contribution: Michael A. Kamm and Angela J Khera devised the concept. Angela J Khera conducted the literature search, screened and assessed all studies, performed data extraction and statistical analysis, and drafted the manuscript. Janet W Chase reviewed the data extracted and reviewed and assessed the eligible studies and data extracted. Michael A. Kamm, Janet W Chase, Michael Salzberg, and Alexander JV Thompson provided critical revision of the manuscript. All authors approved the final version of the manuscript.
References
- 1. Frolkis AD, Dykeman J, Negron ME et al Risk of surgery for inflammatory bowel diseases has decreased over time: a systematic review and meta‐analysis of population‐based studies. Gastroenterology. 2013; 145: 996–1006. [DOI] [PubMed] [Google Scholar]
- 2. Knowles SR, Tribbick D, Connell WR, Castle D, Salzberg M, Kamm MA. Exploration of health status, illness perceptions, coping strategies, and psychological morbidity in stoma patients. J. Wound Ostomy Continence Nurs. 2014; 41: 573–80. [DOI] [PubMed] [Google Scholar]
- 3. Farrokhyar F, Marshall JK, Easterbrook B, Irvine EJ. Functional gastrointestinal disorders and mood disorders in patients with inactive inflammatory bowel disease: prevalence and impact on health. Inflamm. Bowel Dis. 2006; 12: 38–46. [DOI] [PubMed] [Google Scholar]
- 4. Peyrin‐Biroulet L, Germain A, Patel AS, Lindsay JO. Systematic review: outcomes and post‐operative complications following colectomy for ulcerative colitis. Aliment. Pharmacol. Ther. 2016; 44: 807–16. [DOI] [PubMed] [Google Scholar]
- 5. Rezaie A, Gu P, Kaplan GG, Pimentel M, Al‐Darmaki AK. Dyssynergic defecation in inflammatory bowel disease: a systematic review and meta‐analysis. Inflamm. Bowel Dis. 2018; 24: 1065–73. [DOI] [PubMed] [Google Scholar]
- 6. Dibley L, Norton C. Experiences of fecal incontinence in people with inflammatory bowel disease: self‐reported experiences among a community sample. Inflamm. Bowel Dis. 2013; 19: 1450–62. [DOI] [PubMed] [Google Scholar]
- 7. Casati J, Toner B, De Rooy E, Drossman DA, Maunder R. Concerns of patients with inflammatory bowel disease a review of emerging themes. Dig. Dis. Sci. 2000; 45: 26–31. [DOI] [PubMed] [Google Scholar]
- 8. Flor L, Minguez M, Tosca J, Anton R, Bosca‐Watts MM, Mora F. Fecal incontinence (FI) in patients with inflammatory bowel disease (IBD). Probably as important as prevalent. sa1129. Gastroenterology. 2014; 146: s207–7. [Google Scholar]
- 9. Norton C, Dibley LB, Bassett P. Faecal incontinence in inflammatory bowel disease: associations and effect on quality of life. J. Crohns Colitis. 2013; 7: e302–11. [DOI] [PubMed] [Google Scholar]
- 10. Duncan J, Sebepos‐Rogers G, Poole‐Wilson O et al Pwe‐080 prevalence of faecal incontinence in adults with inflammatory bowel disease. Gut. 2013; 62: A162–3. [Google Scholar]
- 11. Enck P, Bielefeldt K, Rathmann W, Purrmann J, Tschöpe D, Erckenbrecht JF. Epidemiology of faecal incontinence in selected patient groups. Int. J. Colorectal Dis. 1991; 6: 143–6. [DOI] [PubMed] [Google Scholar]
- 12. Gu P, Kuenzig ME, Kaplan GG, Pimentel M, Rezaie A. Fecal incontinence in inflammatory bowel disease: a systematic review and meta‐analysis. Inflamm. Bowel Dis. 2018; 24: 1280–90. [DOI] [PubMed] [Google Scholar]
- 13. Lovegrove RE, Heriot AG, Constantinides V et al Meta‐analysis of short‐term and long‐term outcomes of J, W and S ileal reservoirs for restorative proctocolectomy. Colorectal Dis. 2007; 9: 310–20. [DOI] [PubMed] [Google Scholar]
- 14. Fazio VW, Kiran RP, Remzi FH et al Ileal pouch anal anastomosis: analysis of outcome and quality of life in 3707 patients. Ann. Surg. 2013; 257: 679–85. [DOI] [PubMed] [Google Scholar]
- 15. Bengtsson J, Borjesson L, Lundstam U, Oresland T. Long‐term function and manovolumetric characteristics after ileal pouch‐anal anastomosis for ulcerative colitis. Br. J. Surg. 2007; 94: 327–32. [DOI] [PubMed] [Google Scholar]
- 16. Wheeler JM, Banerjee A, Ahuja N, Jewell DP, Mortensen NJ. Long‐term function after restorative proctocolectomy. Dis. Colon Rectum. 2005; 48: 946–51. [DOI] [PubMed] [Google Scholar]
- 17. Sagar PM, Lewis W, Holdsworth PJ, Johnston D, Mitchell C, MacFie J. Quality of life after restorative proctocolectomy with a pelvic ileal reservoir compares favorably with that of patients with medically treated colitis. Dis. Colon Rectum. 1993; 36: 584–92. [DOI] [PubMed] [Google Scholar]
- 18. Bliss DZ, Norton C, Vodusek DB. Raising awareness about fecal incontinence. Neurourol. Urodyn. 2010; 29: 612–15. [DOI] [PubMed] [Google Scholar]
- 19. Brandsborg S, Chen TY, Nicholls RJ, Laurberg S. Difference between patients' and clinicians' perception of pouch dysfunction and its impact on quality of life following restorative proctocolectomy. Colorectal Dis. 2015; 17: O136–40. [DOI] [PubMed] [Google Scholar]
- 20. Van Oudenhove L, Levy RL, Crowell MD, Drossman DA et al Biopsychosocial aspects of functional gastrointestinal disorders. Gastroenterology. 2016; 150: 1355–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gracie DJ, Williams CJ, Sood R et al Poor correlation between clinical disease activity and mucosal inflammation, and the role of psychological comorbidity, in inflammatory bowel disease. Am. J. Gastroenterol. 2016; 111: 541–51. [DOI] [PubMed] [Google Scholar]
- 22. Jonefjall B, Ohman L, Simren M, Strid H. IBS‐like symptoms in patients with ulcerative colitis in deep remission are associated with increased levels of serum cytokines and poor psychological well‐being. Inflamm. Bowel Dis. 2016; 22: 2630–40. [DOI] [PubMed] [Google Scholar]
- 23. Bassotti G, Antonelli E, Villanacci V, Salemme M, Coppola M, Annese V. Gastrointestinal motility disorders in inflammatory bowel diseases. World J. Gastroenterol. 2014; 20: 37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rao SS, Read NW, Davison PA, Bannister JJ, Holdsworth CD. Anorectal sensitivity and responses to rectal distention in patients with ulcerative colitis. Gastroenterology. 1987; 93: 1270–5. [DOI] [PubMed] [Google Scholar]
- 25. Loening‐Baucke V, Metcalf AM, Shirazi S. Rectosigmoid motility in patients with quiescent and active ulcerative colitis. Am. J. Gastroenterol. 1989; 84: 34–9. [PubMed] [Google Scholar]
- 26. Sunde ML, Ricanek P, Oresland T, Jahnsen J, Naimy N, Færden AE. Determinants of optimal bowel function in ileal pouch‐anal anastomosis ‐ physiological differences contributing to pouch function. Scand. J. Gastroenterol. 2017; 53: 8–14. [DOI] [PubMed] [Google Scholar]
- 27. Vollebregt PF, Visscher AP, van Bodegraven AA, Felt‐Bersma RJF. Validation of risk factors for fecal incontinence in patients with Crohn's disease. Dis. Colon Rectum. 2017; 60: 845–51. [DOI] [PubMed] [Google Scholar]
- 28. Tomita R. Ano‐neorectal function using manometry on patients with soiling at 10 years or more after ileal J pouch‐anal anatomosis for ulcerative colitis. Hepatogastroenterology. 2009; 56: 1326–30. [PubMed] [Google Scholar]
- 29. Tribbick D, Salzberg M, Ftanou M et al Prevalence of mental health disorders in inflammatory bowel disease: an Australian outpatient cohort. Clin. Exp. Gastroenterol. 2015; 8: 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guthrie E, Jackson J, Shaffer J, Thompson D, Tomenson B, Creed F. Psychological disorder and severity of inflammatory bowel disease predict health‐related quality of life in ulcerative colitis and Crohn's disease. Am. J. Gastroenterol. 2002; 97: 1994–9. [DOI] [PubMed] [Google Scholar]
- 31. Simren M, Axelsson J, Gillberg R, Abrahamsson H, Svedlund J, Björnsson ES. Quality of life in inflammatory bowel disease in remission: the impact of IBS‐like symptoms and associated psychological factors. Am. J. Gastroenterol. 2002; 97: 389–96. [DOI] [PubMed] [Google Scholar]
- 32. Gracie DJ, Williams CJ, Sood R et al Negative effects on psychological health and quality of life of genuine irritable bowel syndrome‐type symptoms in patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2017; 15: 376–384 e5. [DOI] [PubMed] [Google Scholar]
- 33. Makkar R, Graff LA, Bharadwaj S, Lopez R, Shen B. Psychological factors in irritable pouch syndrome and other pouch disorders. Inflamm. Bowel Dis. 2015; 21: 2815–24. [DOI] [PubMed] [Google Scholar]
- 34. Barnes EL, Herfarth HH, Sandler RS et al Pouch‐related symptoms and quality of life in patients with ileal pouch‐anal anastomosis. Inflamm. Bowel Dis. 2017; 23: 1218–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bajwa A, Emmanuel A. The physiology of continence and evacuation. Best Pract. Res. Clin. Gastroenterol. 2009; 23: 477–85. [DOI] [PubMed] [Google Scholar]
- 36. Butrick CW. Pathophysiology of pelvic floor hypertonic disorders. Obstet. Gynecol. Clin. North Am. 2009; 36: 699–705. [DOI] [PubMed] [Google Scholar]
- 37. Faubion SS, Shuster LT, Bharucha AE. Recognition and management of nonrelaxing pelvic floor dysfunction. Mayo Clin. Proc. 2012; 87: 187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Whitehead WE, Bharucha AE. Diagnosis and treatment of pelvic floor disorders: what's new and what to do. Gastroenterology. 2010; 138: 1231–5 e1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Quinn KP, Tse CS, Lightner AL, Pendegraft RS, Enders FT, Raffals LE. Non‐relaxing pelvic floor dysfunction is an underestimated complication of ileal pouch‐anal anastomosis. Clin. Gastroenterol. Hepatol. 2017; 15: 1242–7. [DOI] [PubMed] [Google Scholar]
- 40. Khanna R, Li Y, Schroeder T et al Manometric evaluation of evacuatory difficulty (dyschezia) in ileal pouch patients. Inflamm. Bowel Dis. 2013; 19: 569–75. [DOI] [PubMed] [Google Scholar]
- 41. Tremaine WJ, Raffals LH, Bharucha AE, Timmons LJ, Pemberton JH, Camilleri M. 561 Inflammatory bowel disease and non‐relaxing pelvic floor dysfunction. Gastroenterology. 2013; 144: S‐104. [Google Scholar]
- 42. Perera LP, Ananthakrishnan AN, Guilday C et al Dyssynergic defecation: a treatable cause of persistent symptoms when inflammatory bowel disease is in remission. Dig. Dis. Sci. 2013; 58: 3600–5. [DOI] [PubMed] [Google Scholar]
- 43. Pezzone MA, Wald A. Functional bowel disorders in inflammatory bowel disease. Gastroenterol. Clin. North Am. 2002; 31: 347–57. [DOI] [PubMed] [Google Scholar]
- 44. Bondurri A, Maffioli A, Danelli P. Pelvic floor dysfunction in inflammatory bowel disease. Minerva Gastroenterol. Dietol. 2015; 61: 249–59. [PubMed] [Google Scholar]
- 45. Wald A, Bharucha AE, Cosman BC, Whitehead WE. ACG clinical guideline: management of benign anorectal disorders. Am. J. Gastroenterol. 2014; 109: 1141–57. [DOI] [PubMed] [Google Scholar]
- 46. Norton C, Thomas L, Hill J. Management of faecal incontinence in adults: summary of NICE guidance. BMJ. 2007; 334: 1370–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Norton C, Whitehead WE, Bliss DZ, Harari D, Lang J, Conservative Management of Fecal Incontinence in Adults Committee of the International Consultation on Incontinence . Management of fecal incontinence in adults. Neurourol.Urodyn. 2010; 29: 199–206. [DOI] [PubMed] [Google Scholar]
- 48. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Slim K, Nini E, Forestier DF, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non‐randomised studies (MINORS): development and validation of a new instrument. ANZ J. Surg. 2003; 73: 712–16. [DOI] [PubMed] [Google Scholar]
- 50. Higgins JP, Altman DG, Gotzsche PC et al The Cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Oresland T, Fasth S, Hulten L, Nordgren S, Swenson L, Åkervall S. Does balloon dilatation and anal sphincter training improve ileoanal‐pouch function? Int. J. Colorectal Dis. 1988; 3: 153–7. [DOI] [PubMed] [Google Scholar]
- 52. Jorge JMN, Wexner SD, Moragado PJ Jr, James K, Nogueras JJ, Jagelman DG. Optimization of sphincter function after the ileoanal reservoir procedure: a prospective, randomized trial. Dis. Colon Rectum. 1994; 37: 419–23. [DOI] [PubMed] [Google Scholar]
- 53. Hull TL, Fazio VW, Schroeder T. Paradoxical puborectalis contraction in patients after pelvic pouch construction. Dis. Colon Rectum. 1995; 38: 1144–6. [DOI] [PubMed] [Google Scholar]
- 54. Vasant DH, Limbdi JK, Solanki K, Radhakrishnan NV. Biofeedback therapy improves continence in quiescent inflammatory bowel disease patients with ano‐rectal dysfunction. J. Gastroenterol. Pancreatol. Liver Disord. 2016; 3: 1–4. [Google Scholar]
- 55. Segal JP, Chan H, Collins B, Faiz OD, Clark SK, Hart AL. Biofeedback in patients with ileoanal pouch dysfunction: a specialist centre experience. Scand. J. Gastroenterol. 2018; 53: 665–9. [DOI] [PubMed] [Google Scholar]
- 56. Cohen J. A coefficient of agreement for nominal scales. EPM. 1960; 20: 37–46. [Google Scholar]
- 57. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977; 33: 159–74. [PubMed] [Google Scholar]
- 58. Norton C, Chelvanayagam S, Wilson‐Barnett J, Redfern S, Kamm MA. Randomized controlled trial of biofeedback for fecal incontinence. Gastroenterology. 2003; 125: 1320–9. [DOI] [PubMed] [Google Scholar]
- 59. Jorge JM, Habr‐Gama A, Wexner SD. Biofeedback therapy in the colon and rectal practice. Appl. Psychophysiol. Biofeedback. 2003; 28: 47–61. [DOI] [PubMed] [Google Scholar]
- 60. Quinn KP, Lightner A, Tse CS, Enders F, Raffals L. Non‐relaxing pelvic floor dysfunction and pouchitis in patients with ileal‐pouch anal anastomosis. Gastroenterology. 2016; 150: S‐176. [Google Scholar]
- 61. Raffals LH, Bharucha AE, Timmons LJ, Pemberton JH, Camilleri M, Tremaine WJ. Mo1236 Ileal pouch anal anastomosis and non‐relaxing pelvic floor dysfunction. Gastroenterology. 2014; 146: S‐594. [Google Scholar]
- 62. National Collaborating Centre for Acute Care . Faecal Incontinence: The Management of Faecal Incontinence in Adults. London: National Collaborating Centre for Acute Care, 2007; 1–146. [PubMed] [Google Scholar]
- 63. Bliss DJ, Mimura T, Berghmans B et al Assessment and conservative management of faecal incontinence and quality of life in adults. In: Abrams P, Cardozo L, Khoury S, Wein A. (eds). Incontinence, 6th edn. Plymouth: Health Publications, 2017; 1993–2083. [Google Scholar]
- 64. Gordon AM, Huxley AF, Julian FJ. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J. Physiol. 1966; 184: 170–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Rao SSC, Patcharatrakul T. Diagnosis and treatment of dyssynergic defecation. J. Neurogastroenterol. Motil. 2016; 22: 423–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Butrick CW. Pelvic floor hypertonic disorders: identification and management. Obstet. Gynecol. Clin. North Am. 2009; 36: 707–22. [DOI] [PubMed] [Google Scholar]
- 67. Rao SS. Biofeedback therapy for constipation in adults. Best Pract. Res. Clin. Gastroenterol. 2011; 25: 159–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shim LS, Jones M, Prott GM, Morris LI, Kellow JE, Malcolm A. Predictors of outcome of anorectal biofeedback therapy in patients with constipation. Aliment. Pharmacol. Ther. 2011; 33: 1245–51. [DOI] [PubMed] [Google Scholar]
- 69. Ilnyckyj A, Fachnie E, Tougas G. A randomized‐controlled trial comparing an educational intervention alone vs education and biofeedback in the management of faecal incontinence in women. Neurogastroenterol. Motil. 2005; 17: 58–63. [DOI] [PubMed] [Google Scholar]


