Peterman and Prekeris review abscission and discuss the diverse roles for the postmitotic midbody in regulating polarity, tumorigenesis, and stemness.
Abstract
Abscission, the final stage of cell division, requires well-orchestrated changes in endocytic trafficking, microtubule severing, actin clearance, and the physical sealing of the daughter cell membranes. These processes are highly regulated, and any missteps in localized membrane and cytoskeleton dynamics often lead to a delay or a failure in cell division. The midbody, a microtubule-rich structure that forms during cytokinesis, is a key regulator of abscission and appears to function as a signaling platform coordinating cytoskeleton and endosomal dynamics during the terminal stages of cell division. It was long thought that immediately following abscission and the conclusion of cell division, the midbody is either released or rapidly degraded by one of the daughter cells. Recently, the midbody has gained prominence for exerting postmitotic functions. In this review, we detail the role of the midbody in orchestrating abscission, as well as discuss the relatively new field of postabscission midbody biology, particularly focusing on how it may act to regulate cell polarity and its potential to regulate cell tumorigenicity or stemness.
Introduction
Mitotic cell division is a very important event in the life of a cell. From DNA synthesis to nuclear envelope breakdown and separation of the chromosomes, the entire process of mitotic cell division is highly regulated. Accordingly, any defects in the mechanisms governing cell division lead to aberrant separation of genetic material as well as other cytosolic components. A series of checkpoints used by the cell ensures the proper replication of DNA and its subsequent separation into each daughter cell. Historically, these checkpoints include the DNA damage checkpoint and the mitotic spindle checkpoint. Since numerous excellent reviews have been written about regulation of mitotic spindle and cytokinetic furrow formation (Pollard, 2010, 2017; London and Biggins, 2014; Amon, 1999; D’Avino et al., 2005; Forth and Kapoor, 2017), in this review we focus on the machinery driving abscission, emerging new roles of the midbody as a key regulator of abscission, and postmitotic midbody roles in regulating cell differentiation and fate.
Midbody and cell division
Midbody formation
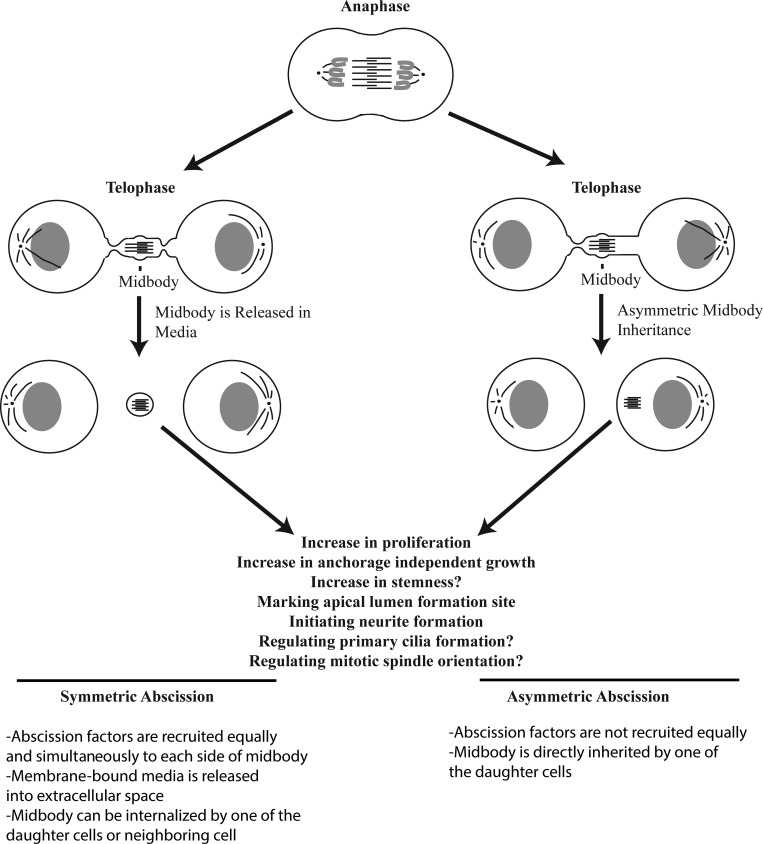
Upon formation and contraction of the actomyosin contractile ring, the antiparallel central spindle microtubules are compacted to a microtubule-dense structure that resides within the intercellular bridge, still connecting two daughter cells (Fig. 1). First visualized by Walther Flemming in the late 1800s, the midbody has since garnered attention for its role as a scaffold for several proteins necessary to facilitate abscission. The antiparallel arrangement of the microtubules (Schiel et al., 2011; Sherman et al., 2016; Mierzwa and Gerlich, 2014) as well as the presence of multiple microtubule cross-linkers such as PRC1, result in a very dense, microtubule-rich structure. Other midbody components, such as Citron kinase and the centralspindlin complex (composed of MKLP1 and CYK-4), also act as microtubule organizers and regulators of other cytokinetic players, including RhoA (D’Avino, 2017; White and Glotzer, 2012). Interestingly, abscission always occurs either on one side (asymmetric abscission) or both sides (symmetric abscission) of the midbody (Fig. 1). Furthermore, it is now well established that the midbody is not just a passive barrier for finishing cytokinesis, but also plays an active role in recruiting and activating various abscission-regulating proteins, as well as regulating abscission timing (abscission checkpoint) and determining the location of the abscission site.
Figure 1.
Symmetric versus asymmetric abscission leads to different fates of the midbody. In abscission (left), cells release the postmitotic midbody into extracellular space. It can then be engulfed by one of the daughter cells or a cell in the surrounding area, lending to a potential mechanism for lateral transfer of information by the postmitotic midbody. In symmetric abscission, the postmitotic midbody is membrane bound. In asymmetric abscission, the process occurs on only one side of the midbody, leading to inheritance of the postmitotic midbody. This midbody is not membrane bound. Symmetric versus asymmetric abscission may be a cell type–specific phenomenon, and more work should be performed to fully answer this question.
Midbody and ESCRT complex
While it has been originally described as a protein complex that mediates multivesicular body formation and lysosomal degradation, the endosomal sorting complex required for transport (ESCRT) complex has now been implicated in a variety of cellular functions. This includes abscission, particularly due to its positioning proximal to the midbody in the intracellular bridge connecting the daughter cells (Henne et al., 2011). The ESCRT complex is primarily composed of four complexes: ESCRT-0, -I, -II, and -III and the AAA-ATPase VPS4 (Fededa and Gerlich, 2012). A large body of work has described how the ESCRT complex is recruited and ultimately performs its membrane scission function during abscission. First, the ESCRT-I component TSG101 and/or ALIX interact directly with the midbody protein CEP55 (Christ et al., 2016; Yang et al., 2008; Elia et al., 2011). Following the recruitment of TSG101/ALIX proteins, the ESCRT-III complex is then targeted to the midbody and, eventually, the abscission site. Work detailing multivesicular body biogenesis and viral budding suggested that ESCRT-III is the principal ESCRT complex that achieves the actual membrane scission (Christ et al., 2017). Indeed, during abscission, ESCRT-III is the complex recruited last, and only after ESCRT-III recruitment does abscission occur (Elia et al., 2011). It is also worth noting that actin depolymerization and clearance from the intercellular bridge must occur before ESCRT-III can mediate final abscission (see Midbody and regulation of actin dynamics). Superresolution microscopy, electron microscopy, and several in vitro studies all suggest that ESCRT-III forms filamentous spirals that are capable of associating with membrane, and the formation of these spirals drives membrane scission (Mierzwa et al., 2017; Goliand et al., 2018; Guizetti et al., 2011; Cashikar et al., 2014).
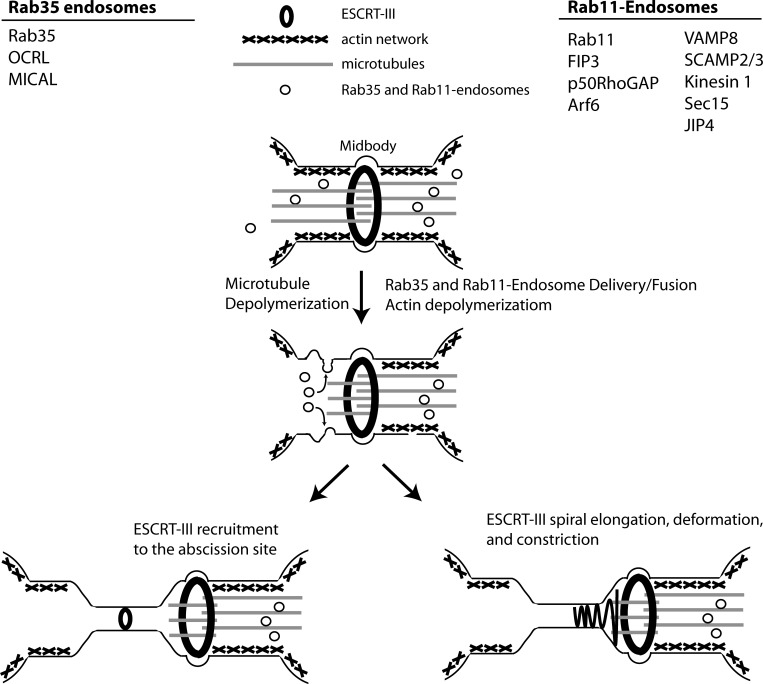
It is not disputed that the ESCRT complexes are important for abscission, since knockdown of ESCRT-III components in vertebrate cells inhibits the process (Lafaurie-Janvore et al., 2013; Christ et al., 2016). However, how the ESCRT-III complex drives scission during telophase remains to be fully understood. The intercellular bridge that forms after conclusion of actomyosin ring–mediated ingression is still 1–3 µm in diameter, which is far greater than membrane tubes that typically recruit ESCRT-III (20–100 nm; Bajorek et al., 2009; Lata et al., 2008). Consequently, it was suggested that endosomal trafficking is required to decrease this diameter (via a process known as secondary ingression) to thin the intercellular bridge to the diameter suitable for the ESCRT-III complex (Schiel et al., 2011, 2012). In the same work, it was demonstrated that CHMP4B, a member of the ESCRT-III complex, is recruited to the abscission site from the midbody only after completion of the secondary ingression (Fig. 2; Schiel et al., 2012). Alternatively, it was proposed that midbody-localized ESCRT-III forms a filamentous spiral of a gradually decreasing diameter that binds to the plasma membrane, creating membrane deformation and driving the secondary ingression and eventual abscission (Fig. 2; Elia et al., 2011; Guizetti et al., 2011).
Figure 2.
Midbody-mediated abscission concludes cell division. In late telophase, the midbody recruits and tethers Rab11 and Rab35 endosomes. Concurrent with this, microtubules are severed in a spastin-dependent manner, and actin is depolymerized via delivery of Rab11 and Rab35 cargo proteins. From here, two models exist. After secondary ingression, either ESCRT-III filaments can be recruited to the future abscission site (left) or ESCRT-III filaments can elongate and constrict from the midbody (right). Ultimately, ESCRT-III filaments facilitate membrane scission and the conclusion of cell division.
Interestingly, proteins other than ESCRTs can also function in the regulation of secondary ingression sites. A recent study suggested that nonmuscle myosin IIA also contributes to the formation of the secondary ingression site, after which ESCRT-III filaments are able to slide to the abscission site (Wang et al., 2019). Anillin, a major component of the cytokinetic ring, and septins, cytoskeletal filaments sensitive to membrane curvature, have also been implicated in the regulation of secondary ingression sites. These studies suggest that anillin is responsible for the recruitment of septins to the sites of abscission, where septins can be modified, form a ring, and regulate the recruitment of various ESCRT proteins (Ribet et al., 2017; Renshaw et al., 2014; Karasmanis et al., 2019). Lastly, work performed in Caenorhabditis elegans embryos suggested that the ESCRT-III complex is not always directly required for abscission but rather can play a role in the removal of excess membrane from intercellular bridge (König et al., 2017). Additionally, the ESCRT-III protein CHMP4C was found to be phosphorylated by Aurora B, and depletion of CHMP4C circumvented an abscission checkpoint (Capalbo et al., 2012). Collectively, multiple models have been proposed for the necessity of the ESCRT complex in abscission, and further studies will be needed to fully elucidate the mechanisms of ESCRT-mediated abscission.
Midbody and regulation of microtubule severing
Central spindle microtubules on either side of the midbody must be severed for cell division to be completed. Indeed, loss of the microtubule-severing enzyme spastin results in abscission defects (Vietri et al., 2015). One of the mechanisms for the recruitment of spastin to the abscission site are the posttranslational modifications of microtubule tails. It has been suggested that these marks, deposited by tubulin tyrosine ligase-like proteins, aid in the recruitment of spastin to microtubules (Roll-Mecak and McNally, 2010; Valenstein and Roll-Mecak, 2016; McNally and Roll-Mecak, 2018). Additional evidence suggests that ESCRT-III complex proteins themselves can aid in the recruitment of spastin. Indeed, the ESCRT-III protein CHMP1B was found to bind directly to spastin and target it to the midbody, and non-midbody roles of the ESCRT-III protein IST1 have been found to recruit spastin to recycling tubules and the mitotic spindle (Allison et al., 2013; Yang et al., 2008). Further work should be performed to determine which tubulin tyrosine ligase-like proteins are responsible for depositing these posttranslational modifications during telophase, as well as if any other ESCRT proteins or other midbody-localized proteins are capable of targeting spastin to the midbody.
Midbody and regulation of actin dynamics
In addition to microtubules, the midbody and intercellular bridge contain actin as remnants of the actomyosin contractile ring. Importantly, abscission can occur only if actin filaments are depolymerized and cleared, since the sub–plasma membrane actomyosin network blocks ESCRT assembly at the abscission site (Schiel et al., 2012; Dambournet et al., 2011). Actin depolymerization relies on the targeted delivery to the midbody of the specialized endosomes that deliver various actin regulators to the abscission site. Most notably, Rab11/FIP3- and Rab35-containing endosomes have all been observed to play major roles in abscission, predominantly by regulating actin depolymerization, allowing ESCRT-III recruitment and determining the formation of the future abscission site (Schiel et al., 2012; Dambournet et al., 2011; Kouranti et al., 2006; Fig. 2). In the case of the Rab11/FIP3 complex, the proteins p50RhoGAP and Scamp2/3 are delivered to sites of abscission to aid in actin clearance by inactivating RhoA (Schiel et al., 2012). Rab35 delivers the proteins MICAL-1 and OCRL to regulate actin oxidation and phosphatidylinositol 4,5-bisphosphate dephosphorylation, ultimately leading to increased actin depolymerization (Dambournet et al., 2011; Kouranti et al., 2006). While the importance of Rab35 and Rab11 endosomes in regulating abscission is becoming firmly established, many questions remain. Why do cells need multiple endosomes (both Rab35 and Rab11) to regulate actin depolymerization? What is the cross-talk between ESCRTs, septins, and endosomes? Do endosomes play other roles during cytokinesis in addition to delivering actin regulators?
Postmitotic roles of the midbody
While the importance of the midbody in mediating abscission is now well established, its role after completion of mitotic cell division remains to be fully defined. In some cases, cells can retain intracellular postmitotic midbodies (Crowell et al., 2014; Peterman et al., 2019). Furthermore, proteomic analyses of isolated midbodies have shown that, in addition to microtubules and abscission regulators (such as ESCRTs, Aurora B, Plk1, Rab11, and Rab35), midbodies contain numerous signaling proteins and transcription factors (Capalbo et al., 2019; Skop et al., 2004; Peterman et al., 2019). Finally, intracellular accumulation of postmitotic midbodies, also known as midbody remnants, were shown to correlate with increases in cell division rates and stemness (Kuo and Chen et al., 2011). It was proposed that postmitotic midbodies may in fact play a role in regulating cell proliferation, stem cell differentiation, and fate determination. Study of the functions of postmitotic midbodies is a very new and somewhat controversial field. Here, we summarize and discuss the latest findings for the involvement of postmitotic midbodies in controlling cell polarization, differentiation, and proliferation.
The midbody as a polarity cue during apical lumen formation
Epithelial tissues are polarized with an apical surface facing the lumen and basolateral surfaces connecting to adjoining cells and extracellular matrix. How these cells achieve this polarity during tissue morphogenesis has been a focus of research for quite some time. During de novo lumen formation, we now have a good understanding that the midbody can act as a symmetry-breaking structure and polarity cue. It has been shown that, in MDCK cell 3D tissue culture models, the midbody formed during first cell division marks a site of nascent apical lumen formation (Li et al., 2014). Consistent with this hypothesis, the apical membrane initiation site (AMIS) forms a ring-like structure around the midbody. The midbody-associated AMIS then acts as a targeting platform for the delivery of apical cargo via Rab11 and Rab35 endosomes (Mangan et al., 2016; Klinkert et al., 2016). The targeting of these endosomes is mediated, at least in part, by the Exocyst complex, which primarily functions to tether these endosomes near the AMIS during telophase. Interestingly, the Exocyst complex and Rab11 endosomes also play important roles in mediating the delivery of proteins necessary for abscission, suggesting that abscission and cell polarity determination are linked cellular events (Jones et al., 2014; Armenti et al., 2014). In particular, the delivery of polarity proteins such as gp135 and the Crumbs complex help to mediate the initial formation of the apical surface (Mangan et al., 2016; Klinkert et al., 2016). Depletion of the Rab11 effector protein FIP5 or Rab35 leads to many luminal defects, including the formation of multiple lumens as well as inverted polarity. Interestingly, recent work also demonstrated that protein phosphatase PRL-3 regulates apical lumen formation in MDCK cells, presumably by regulating midbody localization (Lujan et al., 2017). A function for midbodies as an apical lumen polarity cue is not limited to MDCK cells. In hepatocytes, the polarity proteins Par3 and Mdr (a marker for bile canaliculus in hepatocytes) and the tight junction protein ZO1 are all localized to the midbody just before abscission (Wang et al., 2014).
Since postmitotic midbody biology is still a relatively new field, there are a number of outstanding questions in relation to its function in apicobasal polarity regulation, including what other proteins are involved, the ultimate fate of the postmitotic midbodies, and how these midbodies might be signaling to cells. Since all aforementioned studies have been done using tissue culture cells, it also remains unclear whether midbodies also affect apicobasal polarity in vivo. A recent study has shown that during C. elegans development, midbodies translocate and align at the intestinal lumen formation site (Bai et al., 2018 Preprint). Similarly, the abscission site marks the apical lumen site during neural tube formation in zebrafish (Girdler et al., 2013; Distel et al., 2011). Work in Drosophila melanogaster suggests that the midbody is an important structure in maintaining apical polarity, further cementing the idea that the midbody acts as a platform for polarity proteins just before and during lumen formation (Daniel et al., 2018; Le Bras and Le Borgne, 2014). Finally, during division of frog epithelia, midbodies also migrate and associate with tight junctions and apical pole (Higashi et al., 2016). However, while these are all intriguing observations, the causal relationship between postmitotic midbodies and luminogenesis in vivo remains to be established and will require further studies.
The postmitotic midbody as a spatial landmark for other polarized structures
A function of postmitotic midbodies in regulating the positioning of polarized structures is not limited to epithelial cells. For example, a symmetry-breaking event in neurons is needed to define where cells form an axon. It was previously thought that centrosome positioning defines the axon formation site, but in flies, neurite sprout formation was seen to be independent of centrioles. Instead, the positioning of the postmitotic midbody (rich in the proteins RhoA and Aurora A) dictated the positioning of neurite sprout formation (Pollarolo et al., 2011). A similar function for postmitotic midbodies was observed in MDCK cells, where surface-bound postmitotic midbodies appear to regulate formation of the primary cilia. Removal of postabscission midbodies from the apical surfaces of these cells impaired ciliogenesis, suggesting that the midbody may contribute to licensing ciliogenesis by communicating with the centrosome/basal body (Bernabé-Rubio et al., 2016). A question left unanswered by both of these studies was if the postmitotic midbody is sufficient to induce neural sprout formation or ciliogenesis. Since the purification of postmitotic midbodies has been achieved and used to examine other cellular processes (Peterman and Prekeris, 2017; Lujan et al., 2017), a similar approach could be used to see if addition of midbodies could induce either ectopic/multiple neurite sprouts or ciliogenesis in nonciliated cells.
Mitotic spindle positioning often plays an important role in determining or maintaining cell polarity. It is known that, in early C. elegans embryos, morphological gradients coupled with cortical rotational flow can determine the anterior-posterior axis. It was also found that cortical rotational flow in the 2–4-cell embryo could position the postmitotic midbody (from first cell division) in such a way that it could regulate mitotic spindle orientation for the subsequent division. Laser ablation of these postmitotic midbodies at the 2–4-cell stage resulted in a loss of embryonic polarity, suggesting that the midbodies contribute to establishment of polarity in these early embryos (Singh and Pohl, 2014). However, it remains controversial whether midbodies truly affect mitotic spindle orientation, since work from a different laboratory failed to confirm these findings (Ou et al., 2014). Thus, additional work will be needed to fully characterize a potential role for midbodies in cell polarity and fate determination in early C. elegans embryos.
The postmitotic midbody in cancer and stem cells
A longstanding idea in the field of midbody biology is whether it plays any part in regulating stemness or tumorigenicity. Indeed, a small body of work has examined the postmitotic midbody in these processes, but the data have not always been consistent. One study suggested that postabscission midbodies accumulate within stem and cancer cells, and that retention of these midbodies through inhibition of autophagy could lead to increased tumorigenicity (Kuo et al., 2011). Similar to this work, we also identified a protein responsible for postabscission midbody degradation, named FYCO1, and found that loss of FYCO1 increased intracellular postmitotic midbody accumulation and increased the invasive capacity of squamous cell carcinoma (Dionne and Peterman et al., 2017). Accumulation of postmitotic midbodies was also shown to increase proliferation and anchorage-independent growth of HeLa and MDA-MB-231 cells (Kuo et al., 2011; Peterman et al., 2019). Finally, it was suggested that some stem cell populations release their midbodies more frequently, and that differentiation of stem cells resulted in an increase in postmitotic midbody release (Ettinger et al., 2011). Thus, it is almost certain that the function and fate of the postmitotic midbodies is a context-dependent phenomenon. Indeed, a study in D. melanogaster suggested that the gender of the fly will actually dictate whether germ stem cells accumulate or release postmitotic midbodies. It was shown that in male flies, germ stem cells retain the mother centrosome but release the postmitotic midbody, while female stem cells retain both the centrosome and the midbody (Salzmann et al., 2014).
The postmitotic midbody as an intracellular signaling organelle
Collectively, several recent studies have shed light on new functions for the postmitotic midbody in maintaining or regulating stemness and tumorigenicity. As discussed, the most notable of these studies describe how postmitotic midbodies can be seen accumulating in cancer or stem cells in a context-dependent fashion. Yet, if and how these postmitotic midbodies signal has remained largely a mystery. Multiple studies in the last decade proposed two different modes of midbody accumulation, namely midbody inheritance and midbody uptake (Crowell et al., 2014; Kuo et al., 2011). Midbody inheritance is thought to be caused by asymmetric abscission at only one side of the midbody (Fig. 1). This results in one of the daughter cells retracting the intracellular bridge and “inheriting” midbody remnants. In contrast, during symmetric abscission, the intercellular bridge is cut at both sides of the midbody, releasing the postmitotic midbody into extracellular space, upon which one of the daughter cells or nearby cell can engage the midbody and engulf it via a phagocytosis-like mechanism (midbody internalization; Fig. 1). This internalization results in a double membrane–bound organelle that can be retained within the cytosol from several hours to a couple of days (Peterman et al., 2019). Importantly, these membrane-bound midbody remnants are capable of stimulating cell proliferation and anchorage-independent growth (Peterman et al., 2019). Which mode of midbody accumulation is more common? Recent high-spatiotemporal-resolution analyses of abscission revealed that symmetric abscission appears to be the dominant mode of cell division, suggesting that postmitotic midbodies likely accumulate within cells predominantly via midbody internalization (Crowell et al., 2014). It is also worth noting, however, that most abscission studies have been performed using HeLa cell lines, and so one cannot discount the possibility that midbody inheritance may be a predominant midbody accumulation mechanism in other cell lines or can occur in some specialized context in vivo. Indeed, a recent report suggested that in MDCK cells, postmitotic midbodies sometimes remain connected to one of the daughter cells by a thin intercellular bridge (Bernabé-Rubio et al., 2016).
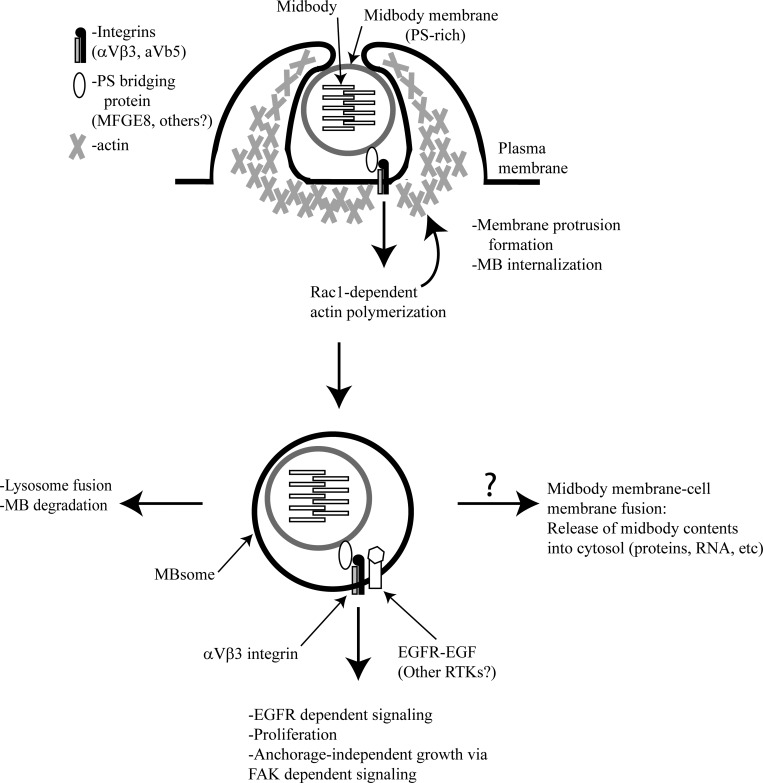
How do cells internalize postmitotic midbodies? This process so far has been most studied in C. elegans embryos. During C. elegans development, the Rac1 GTPase was shown to be necessary for midbody internalization, indicating an actin-dependent mechanism (Chai et al., 2012). Similar experiments from our laboratory and others have shown that Rac1 and actin-dependent polymerization orchestrates midbody internalization in vertebrate cells (Crowell et al., 2014; Peterman et al., 2019). Based on these studies, it has been proposed that postmitotic midbodies are recognized by a specialized “midbody receptor.” Importantly, midbody internalization in C. elegans appears to depend on apoptotic corpse receptors that bind to phosphatidylserine (PS), a lipid that is normally maintained on the inner leaflet of the membrane (Crowell et al., 2014). Since in apoptotic bodies PS is flipped to the outer leaflet and is recognized by phagocytes, PS is often referred to as an “eat-me” signal (Dupuy and Caron 2008; Flannagan et al., 2012). Recently, in mammalian cells, MFG-E8 was identified as an adaptor protein to bridge PS on the outer leaflet of postmitotic midbodies and αVβ3 integrins on the cell surface (Peterman et al., 2019). Consistent with this hypothesis, a lipidomic analysis of the midbodies has shown that their membranes are highly enriched in PS, and it also was shown that PS accumulates at the outer leaflet of postmitotic midbodies (Atilla-Gokcumen et al., 2014; Peterman et al., 2019). Collectively, several studies have demonstrated that Rac1 and an actin-dependent mechanism are responsible for internalization of postmitotic midbodies and that extracellular midbody recognition appears to be driven by a PS-dependent eat-me signal (Fig. 3).
Figure 3.
The postmitotic midbody regulates cell proliferation. The extracellular, membrane-bound postmitotic midbody is recognized by engulfing cells via a PS/MFGE8/integrin complex. Rac1-dependent actin polymerization is required for postmitotic midbody internalization (top). Once internalized, the postmitotic midbody now exists as a double-membrane structure termed the MBsome (bottom). It facilitates EGFR- and FAK-dependent signaling to modulate cell proliferation and anchorage-independent growth and is ultimately degraded. It is unknown if the contents of these internalized postmitotic midbodies ever reach the cytosol of the cell. Please note that this schematic representation of MBsomes was modified from the figure published in Peterman et al. (2019).
While the role of postmitotic midbodies as signaling organelles or polarity cues is becoming well established, much less is known about the molecular machinery that mediates midbody-dependent signaling. Since internalized postmitotic midbodies are encapsulated in a double membrane, one would expect that in these cases signaling is mediated via some sort of transmembrane receptor. Consistent with this hypothesis, it was recently shown that PS and MFG-E8 bind to αVβ3 integrins and activate them, and that these activated integrin heterodimers continue to signal from internalized midbodies via FAK-dependent pathways (Peterman et al., 2019). Importantly, cells containing internalized postmitotic midbodies appear to acquire anchorage independence, presumably due to continuous integrin-dependent inside-in signaling from the midbodies (Peterman et al., 2019). Similar types of inside-in signaling has been described in some cancer cells that acquire anchorage independence due to integrin signaling from signaling endosomes (Hamidi and Ivaska, 2018); thus, internalized postmitotic midbodies are also referred to as midbody-associated signaling endosomes or MBsomes (Peterman et al., 2019).
Cooperation between integrins and receptor tyrosine kinases (RTKs) is required to obtain a full complement of RTK signaling (Guo et al., 2006; Barrow-McGee et al., 2016). It has also been shown that inducing integrin aggregation leads to RTK-mediated phosphorylation, and FAK and integrins can cooperate to further promote RTK signaling (Miyamoto et al., 1996; Sieg et al., 2000). Moreover, epidermal growth factor receptor (EGFR) activity is maintained after endocytic internalization from a specialized subset of organelles, known as signaling endosomes (Sorkin and von Zastrow, 2009). We recently have shown that activated EGFR is present at the MBsome and is required for MBsome-dependent increase in proliferation (Fig. 3; Peterman et al., 2019). This raises an interesting possibility that EGFR and possibly other RTKs may contribute to MBsome signaling, although further studies will be needed to elucidate this. It also remains unclear how activated EGFR accumulates at the MBsome. One possibility is that activated EGFRs are internalized and clustered with αVβ3 integrins during internalization of postmitotic midbodies. However, it is also conceivable that EGFR is actively transported to the forming MBsome after midbody internalization.
Summary and future directions
The midbody is well established as a structure that, during mitosis, orchestrates mitotic cell division, including the recruitment of ESCRT proteins and endosomes and mediating the function of the abscission checkpoint. Furthermore, the postmitotic midbody has now been suggested to function as a signaling organelle (MBsome) that is capable of modulating cell polarity and proliferation. While it is now clear that midbodies regulate many cellular functions, many questions remain to be answered, starting with why cells want to accumulate postabscission midbodies, in vivo or in vitro? Work from our laboratory and others has shown that accumulation of postmitotic midbodies modulates stemness, tumorigenicity, and proliferation. It is possible that the accumulation and maintenance of postabscission midbodies would lead to a transient increase in proliferation. This, of course, would be important during various stages of development and organogenesis. For example, the transit-amplifying and Lgr5+ stem cells in the intestinal crypt must maintain the cells that rapidly turn over in the villus by continuously dividing (Barker et al., 2007; Haegebarth and Clevers, 2009), and so midbody accumulation in these cells might allow for a proliferative advantage. This also raises an intriguing possibility that professional phagocytes may regulate cell proliferation by internalizing postabscission midbodies so that other cells do not engulf them. Phagocytes would be in an excellent position to do so, as they readily express the machinery needed to recognize PS on the outer membrane leaflets of midbodies.
We have recently proposed that MBsomes propagate inside-in signaling (Peterman et al., 2019), and such a model does not require for any internal components of the midbody to enter the cytosol of the cell. However, it is possible that eventually the midbody membrane fuses with endocytic membrane, thereby allowing internal postmitotic midbody components to play an active role in regulating cell proliferation, differentiation, and polarity (Fig. 3). Multiple proteomics analyses of the midbody have been performed, and a variety of proteins capable of influencing these processes, including transcription factors, have been found in postmitotic midbodies (Skop et al., 2004; Peterman et al., 2019; Capalbo et al., 2019). Furthermore, it is possible that coding and noncoding RNAs exist in the midbody, and proteins necessary for messenger RNA processing and translation have been observed in midbody proteomics, providing a possible alternative route for midbody signaling (Zheng et al., 2010; Skop et al., 2004; Gnazzo et al., 2016; Peterman et al., 2019; Capalbo et al., 2019). As speculative as some of these may be, some of the most pivotal experiments that remain to be performed are in vivo studies that concretely show that the midbody is capable of acting as a signaling organelle. Some works have shown a role for the midbody in cell polarity, but none have shown a causative role for the midbody in regulating cell proliferation in vivo. While the notion of signaling from postmitotic midbodies has shown great promise in a number of different contexts, this is a new and emerging field that is still relatively unexplored.
Acknowledgments
We apologize to any of our peers whose work could not be cited because of space constraints.
This work was funded by National Institutes of Health grants DK064380 and GM122768 to R. Prekeris.
The authors declare no competing financial interests.
References
- Allison R., Lumb J.H., Fassier C., Connell J.W., Ten Martin D., Seaman M.N.J., Hazan J., and Reid E.. 2013. An ESCRT-spastin interaction promotes fission of recycling tubules from the endosome. J. Cell Biol. 202:527–543. 10.1083/jcb.201211045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amon A. 1999. The spindle checkpoint. Curr. Opin. Genet. Dev. 9:69–75. 10.1016/S0959-437X(99)80010-0 [DOI] [PubMed] [Google Scholar]
- Armenti S.T., Chan E., and Nance J.. 2014. Polarized exocyst-mediated vesicle fusion directs intracellular lumenogenesis within the C. elegans excretory cell. Dev. Biol. 394:110–121. 10.1016/j.ydbio.2014.07.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atilla-Gokcumen G.E., Muro E., Relat-Goberna J., Sasse S., Bedigian A., Coughlin M.L., Garcia-Manyes S., and Eggert U.S.. 2014. Dividing cells regulate their lipid composition and localization. Cell. 156:428–439. 10.1016/j.cell.2013.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X., Lee P.-Y., Chen C.-Y., Simmons J.R., Nebenfuehr B., Mitchell D., Klebanow L.R., Mattson N., Sorensen C.G. Turpin B.-C. Chen, et al. . 2018. Aurora B Is Required for Programmed Variations of Cytokinesis during Morphogenesis in the C. Elegans Embryo. bioRxiv. doi:10.1101/319657 (Preprint posted August 27, 2018). [Google Scholar]
- Bajorek M., Schubert H.L., McCullough J., Langelier C., Eckert D.M., Stubblefield W.M., Uter N.T., Myszka D.G., Hill C.P., and Sundquist W.I.. 2009. Structural basis for ESCRT-III protein autoinhibition. Nat. Struct. Mol. Biol. 16:754–762. 10.1038/nsmb.1621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J., and Clevers H.. 2007. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 449:1003–1007. 10.1038/nature06196 [DOI] [PubMed] [Google Scholar]
- Barrow-McGee R., Kishi N., Joffre C., Ménard L., Hervieu A., Bakhouche B.A., Noval A.J., Mai A., Guzmán C., Robbez-Masson L., et al. . 2016. Beta 1-integrin-c-Met cooperation reveals an inside-in survival signalling on autophagy-related endomembranes. Nat. Commun. 7:11942 10.1038/ncomms11942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernabé-Rubio M., Andrés G., Casares-Arias J., Fernández-Barrera J., Rangel L., Reglero-Real N., Gershlick D.C., Fernández J.J., Millán J., Correas I., et al. . 2016. Novel Role for the Midbody in Primary Ciliogenesis by Polarized Epithelial Cells. J. Cell Biol. 214:259–273. 10.1083/jcb.201601020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capalbo L., Bassi Z.I., Geymonat M., Todesca S., Copoiu L., Enright A.J., Callaini G., Riparbelli M.G., Yu L., Choudhary J.S., et al. . 2019. The midbody interactome reveals unexpected roles for PP1 phosphatases in cytokinesis. Nat. Commun. 10:4513 10.1038/s41467-019-12507-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capalbo L., Montembault E., Takeda T., Bassi Z.I., Glover D.M., and D’Avino P.P.. 2012. The chromosomal passenger complex controls the function of endosomal sorting complex required for transport-III Snf7 proteins during cytokinesis. Open Biol. 2:120070 10.1098/rsob.120070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashikar A.G., Shim S., Roth R., Maldazys M.R., Heuser J.E., and Hanson P.I.. 2014. Structure of Cellular ESCRT-III Spirals and Their Relationship to HIV Budding. eLife. 3:e02184 10.7554/eLife.02184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y., Tian D., Yang Y., Feng G., Cheng Z., Li W., and Ou G.. 2012. Apoptotic regulators promote cytokinetic midbody degradation in C. elegans. J. Cell Biol. 199:1047–1055. 10.1083/jcb.201209050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christ L., Raiborg C., Wenzel E.M., Campsteijn C., and Stenmark H.. 2017. Cellular Functions and Molecular Mechanisms of the ESCRT Membrane-Scission Machinery. Trends Biochem. Sci. 42:42–56. 10.1016/j.tibs.2016.08.016 [DOI] [PubMed] [Google Scholar]
- Christ L., Wenzel E.M., Liestøl K., Raiborg C., Campsteijn C., and Stenmark H.. 2016. ALIX and ESCRT-I/II function as parallel ESCRT-III recruiters in cytokinetic abscission. J. Cell Biol. 212:499–513. 10.1083/jcb.201507009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell E.F., Gaffuri A.L., Gayraud-Morel B., Tajbakhsh S., and Echard A.. 2014. Engulfment of the midbody remnant after cytokinesis in mammalian cells. J. Cell Sci. 127:3840–3851. 10.1242/jcs.154732 [DOI] [PubMed] [Google Scholar]
- D’Avino P.P. 2017. Citron kinase - renaissance of a neglected mitotic kinase. J. Cell Sci. 130:1701–1708. 10.1242/jcs.200253 [DOI] [PubMed] [Google Scholar]
- D’Avino P.P., Savoian M.S., and Glover D.M.. 2005. Cleavage furrow formation and ingression during animal cytokinesis: a microtubule legacy. J. Cell Sci. 118:1549–1558. 10.1242/jcs.02335 [DOI] [PubMed] [Google Scholar]
- Dambournet D., Machicoane M., Chesneau L., Sachse M., Rocancourt M., El Marjou A., Formstecher E., Salomon R., Goud B., and Echard A.. 2011. Rab35 GTPase and OCRL phosphatase remodel lipids and F-actin for successful cytokinesis. Nat. Cell Biol. 13:981–988. 10.1038/ncb2279 [DOI] [PubMed] [Google Scholar]
- Daniel E., Daud M., Kolotuev I., Charish K., Auld V., and Le Borgne R.. 2018. Coordination of Septate Junctions Assembly and Completion of Cytokinesis in Proliferative Epithelial Tissues. Curr. Biol. 28:1380–1391.e4. 10.1016/j.cub.2018.03.034 [DOI] [PubMed] [Google Scholar]
- Dionne L.K., Peterman E., Schiel J., Gibieža P., Skeberdis V.A., Jimeno A., Wang X.-J., and Prekeris R.. 2017. FYCO1 Regulates Accumulation of Post-Mitotic Midbodies by Mediating LC3-Dependent Midbody Degradation. J. Cell Sci. 130:4051–4062. 10.1242/jcs.208983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Distel M., Hocking J.C., and Köster R.W.. 2011. In vivo cell biology using Gal4-mediated multicolor subcellular labeling in zebrafish. Commun. Integr. Biol. 4:336–339. 10.4161/cib.4.3.15037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuy A.G., and Caron E.. 2008. Integrin-dependent phagocytosis: spreading from microadhesion to new concepts. J. Cell Sci. 121:1773–1783. 10.1242/jcs.018036 [DOI] [PubMed] [Google Scholar]
- Elia N., Sougrat R., Spurlin T.A., Hurley J.H., and Lippincott-Schwartz J.. 2011. Dynamics of Endosomal Sorting Complex Required for Transport (ESCRT) Machinery during Cytokinesis and Its Role in Abscission. Proc. Natl. Acad. Sci. USA. 108:4846–4851. 10.1073/pnas.1102714108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger A.W., Wilsch-Bräuninger M., Marzesco A.M., Bickle M., Lohmann A., Maliga Z., Karbanová J., Corbeil D., Hyman A.A., and Huttner W.B.. 2011. Proliferating versus differentiating stem and cancer cells exhibit distinct midbody-release behaviour. Nat. Commun. 2:503 10.1038/ncomms1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fededa J.P., and Gerlich D.W.. 2012. Molecular control of animal cell cytokinesis. Nat. Cell Biol. 14:440–447. 10.1038/ncb2482 [DOI] [PubMed] [Google Scholar]
- Flannagan R.S., Jaumouillé V., and Grinstein S.. 2012. The cell biology of phagocytosis. Annu. Rev. Pathol. 7:61–98. 10.1146/annurev-pathol-011811-132445 [DOI] [PubMed] [Google Scholar]
- Forth S., and Kapoor T.M.. 2017. The mechanics of microtubule networks in cell division. J. Cell Biol. 216:1525–1531. 10.1083/jcb.201612064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girdler G.C., Araya C., Ren X., and Clarke J.D.W.. 2013. Developmental Time Rather than Local Environment Regulates the Schedule of Epithelial Polarization in the Zebrafish Neural Rod. Neural Dev. 8:5 10.1186/1749-8104-8-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnazzo M.M., Uhlemann E.E., Villarreal A.R., Shirayama M., Dominguez E.G., and Skop A.R.. 2016. The RNA-binding protein ATX-2 regulates cytokinesis through PAR-5 and ZEN-4. Mol. Biol. Cell. 27:3052–3064. 10.1091/mbc.e16-04-0219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goliand I., Adar-Levor S., Segal I., Nachmias D., Dadosh T., Kozlov M.M., and Elia N.. 2018. Resolving ESCRT-III Spirals at the Intercellular Bridge of Dividing Cells Using 3D STORM. Cell Reports. 24:1756–1764. 10.1016/j.celrep.2018.07.051 [DOI] [PubMed] [Google Scholar]
- Guizetti J., Schermelleh L., Mäntler J., Maar S., Poser I., Leonhardt H., Müller-Reichert T., and Gerlich D.W.. 2011. Cortical constriction during abscission involves helices of escrt-iii–dependent filaments. Science. 331:1616–1620. 10.1126/science.1201847 [DOI] [PubMed] [Google Scholar]
- Guo W., Pylayeva Y., Pepe A., Yoshioka T., Muller W.J., Inghirami G., and Giancotti F.G.. 2006. Beta 4 integrin amplifies ErbB2 signaling to promote mammary tumorigenesis. Cell. 126:489–502. 10.1016/j.cell.2006.05.047 [DOI] [PubMed] [Google Scholar]
- Haegebarth A., and Clevers H.. 2009. Wnt signaling, lgr5, and stem cells in the intestine and skin. Am. J. Pathol. 174:715–721. 10.2353/ajpath.2009.080758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamidi H., and Ivaska J.. 2018. Every step of the way: integrins in cancer progression and metastasis. Nat. Rev. Cancer. 18:533–548. 10.1038/s41568-018-0038-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne W.M., Buchkovich N.J., and Emr S.D.. 2011. The ESCRT pathway. Dev. Cell. 21:77–91. 10.1016/j.devcel.2011.05.015 [DOI] [PubMed] [Google Scholar]
- Higashi T., Arnold T.R., Stephenson R.E., Dinshaw K.M., and Miller A.L.. 2016. Maintenance of the Epithelial Barrier and Remodeling of Cell-Cell Junctions during Cytokinesis. Curr. Biol. 26:1829–1842. 10.1016/j.cub.2016.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T.A., Nikolova L.S., Schjelderup A., and Metzstein M.M.. 2014. Exocyst-mediated membrane trafficking is required for branch outgrowth in Drosophila tracheal terminal cells. Dev. Biol. 390:41–50. 10.1016/j.ydbio.2014.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karasmanis E.P., Hwang D., Nakos K., Bowen J.R., Angelis D., and Spiliotis E.T.. 2019. A Septin Double Ring Controls the Spatiotemporal Organization of the ESCRT Machinery in Cytokinetic Abscission. Curr. Biol. 29:2174–2182.e7. 10.1016/j.cub.2019.05.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkert K., Rocancourt M., Houdusse A., and Echard A.. 2016. Rab35 GTPase couples cell division with initiation of epithelial apico-basal polarity and lumen opening. Nat. Commun. 7:11166 10.1038/ncomms11166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- König J., Frankel E.B., Audhya A., and Müller-Reichert T.. 2017. Membrane remodeling during embryonic abscission in Caenorhabditis elegans. J. Cell Biol. 216:1277–1286. 10.1083/jcb.201607030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouranti I., Sachse M., Arouche N., Goud B., and Echard A.. 2006. Rab35 regulates an endocytic recycling pathway essential for the terminal steps of cytokinesis. Curr. Biol. 16:1719–1725. 10.1016/j.cub.2006.07.020 [DOI] [PubMed] [Google Scholar]
- Kuo T.C., Chen C.T., Baron D., Onder T.T., Loewer S., Almeida S., Weismann C.M., Xu P., Houghton J.M., Gao F.B., et al. . 2011. Midbody accumulation through evasion of autophagy contributes to cellular reprogramming and tumorigenicity. Nat. Cell Biol. 13:1214–1223. 10.1038/ncb2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafaurie-Janvore J., Maiuri P., Wang I., Pinot M., Manneville J.B., Betz T., Balland M., and Piel M.. 2013. ESCRT-III assembly and cytokinetic abscission are induced by tension release in the intercellular bridge. Science. 339:1625–1629. 10.1126/science.1233866 [DOI] [PubMed] [Google Scholar]
- Lata S., Schoehn G., Jain A., Pires R., Piehler J., Gottlinger H.G., and Weissenhorn W.. 2008. Helical structures of ESCRT-III are disassembled by VPS4. Science. 321:1354–1357. 10.1126/science.1161070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bras S., and Le Borgne R.. 2014. Epithelial cell division - multiplying without losing touch. J. Cell Sci. 127:5127–5137. 10.1242/jcs.151472 [DOI] [PubMed] [Google Scholar]
- Li D., Mangan A., Cicchini L., Margolis B., and Prekeris R.. 2014. FIP5 phosphorylation during mitosis regulates apical trafficking and lumenogenesis. EMBO Rep. 15:428–437. 10.1002/embr.201338128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- London N., and Biggins S.. 2014. Signalling dynamics in the spindle checkpoint response. Nat. Rev. Mol. Cell Biol. 15:736–747. 10.1038/nrm3888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan P., Rubio T., Varsano G., and Köhn M.. 2017. Keep it on the edge: The postmitotic midbody as a polarity signal unit. Commun. Integr. Biol. 10:e1338990 10.1080/19420889.2017.1338990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan A.J., Sietsema D.V., Li D., Moore J.K., Citi S., and Prekeris R.. 2016. Cingulin and actin mediate midbody-dependent apical lumen formation during polarization of epithelial cells. Nat. Commun. 7:12426 10.1038/ncomms12426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally F.J., and Roll-Mecak A.. 2018. Microtubule-severing enzymes: From cellular functions to molecular mechanism. J. Cell Biol. 217:4057–4069. 10.1083/jcb.201612104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mierzwa B., and Gerlich D.W.. 2014. Cytokinetic abscission: molecular mechanisms and temporal control. Dev. Cell. 31:525–538. 10.1016/j.devcel.2014.11.006 [DOI] [PubMed] [Google Scholar]
- Mierzwa B.E., Chiaruttini N., Redondo-Morata L., von Filseck J.M., König J., Larios J., Poser I., Müller-Reichert T., Scheuring S., Roux A., and Gerlich D.W.. 2017. Dynamic subunit turnover in ESCRT-III assemblies is regulated by Vps4 to mediate membrane remodelling during cytokinesis. Nat. Cell Biol. 19:787–798. 10.1038/ncb3559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S., Teramoto H., Gutkind J.S., and Yamada K.M.. 1996. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J. Cell Biol. 135:1633–1642. 10.1083/jcb.135.6.1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou G., Gentili C., and Gönczy P.. 2014. Stereotyped Distribution of Midbody Remnants in Early C. Elegans Embryos Requires Cell Death Genes and Is Dispensable for Development. Cell Res. 24:251–253. 10.1038/cr.2013.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterman E., and Prekeris R.. 2017. Understanding Post-Mitotic Roles of the Midbody during Cell Differentiation and Polarization. Methods Cell Biol. 137:173–186. 10.1016/bs.mcb.2016.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterman E., Gibieža P., Schafer J., Skeberdis V.A., Kaupinis A., Valius M., Heiligenstein X., Hurbain I., Raposo G., and Prekeris R.. 2019. The post-abscission midbody is an intracellular signaling organelle that regulates cell proliferation. Nat. Commun. 10:3181 10.1038/s41467-019-10871-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T.D. 2010. Mechanics of cytokinesis in eukaryotes. Curr. Opin. Cell Biol. 22:50–56. 10.1016/j.ceb.2009.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T.D. 2017. Nine unanswered questions about cytokinesis. J. Cell Biol. 216:3007–3016. 10.1083/jcb.201612068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollarolo G., Schulz J.G., Munck S., and Dotti C.G.. 2011. Cytokinesis remnants define first neuronal asymmetry in vivo. Nat. Neurosci. 14:1525–1533. 10.1038/nn.2976 [DOI] [PubMed] [Google Scholar]
- Renshaw M.J., Liu J., Lavoie B.D., and Wilde A.. 2014. Anillin-dependent organization of septin filaments promotes intercellular bridge elongation and Chmp4B targeting to the abscission site. Open Biol. 4:130190 10.1098/rsob.130190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribet D., Boscaini S., Cauvin C., Siguier M., Mostowy S., Echard A., and Cossart P.. 2017. SUMOylation of human septins is critical for septin filament bundling and cytokinesis. J. Cell Biol. 216:4041–4052. 10.1083/jcb.201703096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll-Mecak A., and McNally F.J.. 2010. Microtubule-severing enzymes. Curr. Opin. Cell Biol. 22:96–103. 10.1016/j.ceb.2009.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzmann V., Chen C., Chiang C.Y., Tiyaboonchai A., Mayer M., and Yamashita Y.M.. 2014. Centrosome-dependent asymmetric inheritance of the midbody ring in Drosophila germline stem cell division. Mol. Biol. Cell. 25:267–275. 10.1091/mbc.e13-09-0541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiel J.A., Park K., Morphew M.K., Reid E., Hoenger A., and Prekeris R.. 2011. Endocytic membrane fusion and buckling-induced microtubule severing mediate cell abscission. J. Cell Sci. 124:1411–1424. 10.1242/jcs.081448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiel J.A., Simon G.C., Zaharris C., Weisz J., Castle D., Wu C.C., and Prekeris R.. 2012. FIP3-endosome-dependent formation of the secondary ingression mediates ESCRT-III recruitment during cytokinesis. Nat. Cell Biol. 14:1068–1078. 10.1038/ncb2577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman S., Kirchenbuechler D., Nachmias D., Tamir A., Werner S., Elbaum M., and Elia N.. 2016. Resolving new ultrastructural features of cytokinetic abscission with soft-X-ray cryo-tomography. Sci. Rep. 6:27629 10.1038/srep27629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieg D.J., Hauck C.R., Ilic D., Klingbeil C.K., Schaefer E., Damsky C.H., and Schlaepfer D.D.. 2000. FAK integrates growth-factor and integrin signals to promote cell migration. Nat. Cell Biol. 2:249–256. 10.1038/35010517 [DOI] [PubMed] [Google Scholar]
- Singh D., and Pohl C.. 2014. Coupling of rotational cortical flow, asymmetric midbody positioning, and spindle rotation mediates dorsoventral axis formation in C. elegans. Dev. Cell. 28:253–267. 10.1016/j.devcel.2014.01.002 [DOI] [PubMed] [Google Scholar]
- Skop A.R., Liu H., Yates J. III, Meyer B.J., and Heald R.. 2004. Dissection of the mammalian midbody proteome reveals conserved cytokinesis mechanisms. Science. 305:61–66. 10.1126/science.1097931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin A., and von Zastrow M.. 2009. Endocytosis and signalling: intertwining molecular networks. Nat. Rev. Mol. Cell Biol. 10:609–622. 10.1038/nrm2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenstein M.L., and Roll-Mecak A.. 2016. Graded Control of Microtubule Severing by Tubulin Glutamylation. Cell. 164:911–921. 10.1016/j.cell.2016.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vietri M., Schink K.O., Campsteijn C., Wegner C.S., Schultz S.W., Christ L., Thoresen S.B., Brech A., Raiborg C., and Stenmark H.. 2015. Spastin and ESCRT-III coordinate mitotic spindle disassembly and nuclear envelope sealing. Nature. 522:231–235. 10.1038/nature14408 [DOI] [PubMed] [Google Scholar]
- Wang K., Wloka C., and Bi E.. 2019. Non-muscle Myosin-II Is Required for the Generation of a Constriction Site for Subsequent Abscission. iScience. 13:69–81. 10.1016/j.isci.2019.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Yanger K., Stanger B.Z., Cassio D., and Bi E.. 2014. Cytokinesis defines a spatial landmark for hepatocyte polarization and apical lumen formation. J. Cell Sci. 127:2483–2492. 10.1242/jcs.139923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E.A., and Glotzer M.. 2012. Centralspindlin: at the heart of cytokinesis. Cytoskeleton (Hoboken). 69:882–892. 10.1002/cm.21065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D., Rismanchi N., Renvoisé B., Lippincott-Schwartz J., Blackstone C., and Hurley J.H.. 2008. Structural basis for midbody targeting of spastin by the ESCRT-III protein CHMP1B. Nat. Struct. Mol. Biol. 15:1278–1286. 10.1038/nsmb.1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R., Shen Z.. Tripathi V., Xuan Z., Freier S.M., Bennett C.F., Prasanth S.G., Prasanth K.V.. 2010. Polypurine-repeat-containing rnas: a novel class of long non-coding rna in mammalian cells. J. Cell Sci. 123:3734–3744. 10.1242/jcs.070466 [DOI] [PMC free article] [PubMed] [Google Scholar]