Abstract
Chronic non-communicable diseases share the pathomechanism of increased reactive oxygen species (ROS) production by nicotinamide adenine dinucleotide phosphate (NADPH) oxidases, known as Nox. The recent discovery that expression of Nox1, a Nox isoform that has been implicated in the pathogenesis of cardiovascular and kidney disease and cancer, is regulated by the expression and activity of G protein-coupled estrogen receptor (GPER) led to the identification of orally active small-molecule GPER blockers as selective Nox1 downregulators (NDRs). Preclinical studies using NDRs have demonstrated beneficial effects in vascular disease, hypertension, and glomerular renal injury. These findings suggest the therapeutic potential of NDRs, which reduce Nox1 protein levels, not only for cardiovascular disease conditions including arterial hypertension, pulmonary hypertension, heart failure with preserved ejection fraction (HFpEF), and chronic renal disease, but also for other non-communicable diseases, such as cerebrovascular disease and vascular dementia, Alzheimer’s disease, autoimmune diseases and cancer, in which elevated Nox1-derived ROS production plays a causal role.
Keywords: coronary artery disease; non-communicable diseases; chronic renal disease; heart failure; HFpEF, hypertension; NADPH Oxidase; oxidative stress; stroke; superoxide; vascular
1. Therapies for chronic non-communicable diseases: Unmet needs
Chronic, non-communicable diseases are responsible for more than half of global mortality [1, 2], with half of these deaths caused by only four diseases, predominantly affecting the arterial vasculature: arterial hypertension, diabetes, coronary artery disease, and stroke [3–6]. Despite continued efforts, the prevalence of these diseases continues to rise, largely because of the obesity pandemic and population aging [7], indicating unmet needs that require additional therapeutic options.
2. Reactive oxygen species in chronic diseases
Reactive oxygen species (ROS) are highly reactive, short-lived molecules that are formed under physiological conditions and act as second messengers, modulating rapid signaling and the activity of transcription factors [8]. ROS contribute to the regulation of vascular tone, cell proliferation and, if ROS bioactivity is abnormally increased under pathological conditions, to fibrosis, vasoconstriction, thrombosis, vascular stiffening and calcification. Part of this effect is brought about by decreasing nitric oxide (·NO) bioavailability through inactivation by ·O2− at a diffusion-limited rate [9], reducing or abrogating the vasoprotective properties of ·NO that include vasodilatation and inhibition of cell proliferation, as well as inhibition of inflammation and thrombosis [9]. Numerous pathologies, such as cerebral ischemia, arterial remodeling and vascular hypertrophy, ischemia-reperfusion injury in stroke and myocardial infarction, heart failure and proteinuric renal disease, have been linked to ROS-dependent mechanisms [10].
3. Dietary supplementation of “antioxidant vitamins” without effect
Over several decades, clinical studies have explored the theoretical benefits of non-specifically reducing ROS bioactivity through supplementation with antioxidant vitamins as “scavengers” of ROS. However, such antioxidants react with superoxide anion at a rate that is one billion times slower than its reaction with ·NO [11]. Not surprisingly therefore, multiple clinical trials using dietary supplementation of antioxidant vitamins, such as vitamin C or vitamin E, found little to no effect on cardiovascular morbidity or mortality [10–14]. The failure of these studies has been ascribed to the fact that many were observational cohort studies and not randomized trials, as well as to the type, dosage, treatment duration, and administration route of the antioxidant vitamins [12–14].
4. NADPH oxidases: key sources of reactive oxygen species
Non-communicable diseases are associated with the upregulation or activation of ROS-producing cellular enzymes [8], which include nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (Nox) [15], “uncoupled” endothelial ·NO synthase (generating ·O2− instead of ·NO) [9, 16, 17], xanthine oxidase, and mitochondrial respiratory enzymes [8]. Cellular ROS formation is endogenously controlled by anti-oxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase, as well as by antioxidant steroid hormones, such as estrogens (that also downregulate the Nox-associated protein Rac1) [18], calcitriol (“vitamin” D) [19], and antioxidant vitamins [10].
Under healthy conditions, Nox represent the main cellular source of ROS in the vasculature and are the only enzymes whose primary function is to produce ROS; Nox consist of a multienzyme complex (Figure 1) of which at least 7 mammalian isoforms have been identified, including five Nox proteins (Nox1-Nox5) and two dual oxidases, Duox1 and Duox2 [20]. All Nox isoforms catalyze the transfer of two electrons from NAPDH via their FAD domain and two iron-heme prosthetic groups to molecular oxygen [21]. Nox1, Nox2, Nox3, Nox 5, and the Duox oxidases are regulated via intracellular signal transduction, whereas Nox4 exhibits constitutive activity [11, 22–24]. Overall ROS production by Nox/Duox enzymes also depends on the expression levels of the enzymatic subunit in particular, as evidenced by decreased ROS production upon GPER inhibition, which decreases Nox1 protein levels [20, 25]. Autocrine regulation of Nox1-derived ROS is also essential for the proliferation of stem cells, indicating a novel “self-regulating” function of Nox1 [26, 27], a mechanism also implicated in the self-renewal of thyroid cancer cells [28]. Nox1 is induced by growth-stimulating and pro-inflammatory mediators such as angiotensin II and endothelin-1 [29], as well as by RNA-binding proteins, such as HuR (human antigen R) [30], which is tightly regulated by hydrogen sulfide (H2S) generated from cystathionine gamma lyase [31, 32].
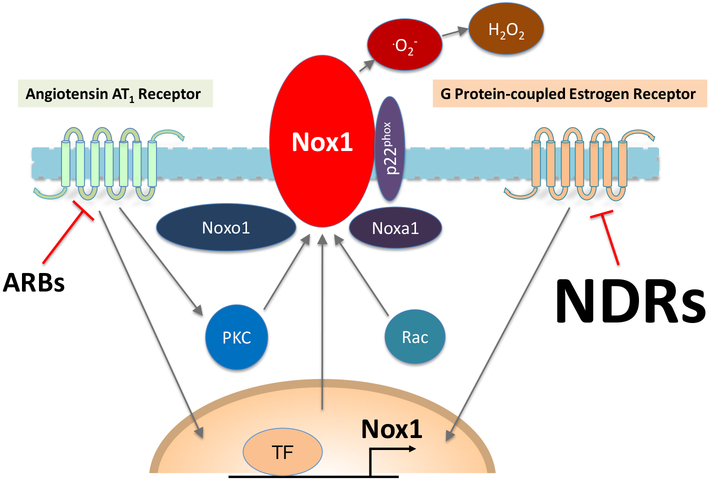
Figure 1. Schematic representation of the membrane-bound Nox1 enzyme complex and its regulation by angiotensin receptor blockers (ARBs) and Nox1 downregulators (NDRs).
ARBs inhibit angiotensin II-induced assembly and function of Nox1 and its specific activator (Noxo1, Rac1) and adaptor proteins (p22phox, Noxa1) through protein kinase C (PKC)-dependent and other rapid signaling pathways. NDRs reduce Nox1-dependent superoxide (O2−) production by downregulating Nox1 protein synthesis. H2O2, hydrogen peroxide; TF, transcription factor. Modified from reference [20] and reproduced with permission of Elsevier.
Nox1, Nox2, Nox3, and Nox5 (all considered homologues of Nox2/gp91(phox) [33, 34]), generate superoxide. Species- and tissue-specific expression and function of the different Nox isoforms have been identified, with Nox1 and Nox2 isoforms propagating disease. By contrast, Nox4 has protective effects in the cardiovascular system [24, 35–37] and mediates its effects via formation of H2O2, which acts as a vasodilator through endothelium-dependent hyperpolarization, thereby controlling blood pressure [38]. Moreover, Nox4 has a physiological role in maintaining myocardial function during exercise [39]. Nox5 is found only in the human but not in the rodent genome; it is regulated in a calcium-dependent fashion and highly expressed in the human kidney and vasculature [22, 34, 40]. Studies using overexpression of human Nox5 in rodents found remarkable effects on disease pathologies. Transgenic podocyte-specific overexpression of Nox5 in rodents with diabetic nephropathy resulted in podocyte injury, early-onset proteinuria and hypertension [41], suggesting an important role for human diseases as well. Similarly, in rodents, Nox5 overexpression that was limited to either endothelial or to vascular smooth muscle and mesangial cells was associated with glomerular injury, inflammation and fibrosis [42].
5. NADPH oxidases and drug discovery
Over the past decade, drug discovery programs have focused their attention on identifying small molecule compounds that directly interfere with ROS production at their enzymatic source instead of scavenging them [10, 11, 43]. We and others have recently reviewed the current status of the development of these new drugs, as well as their potential shortcomings. Recently, a combined Nox1/4 inhibitor (GKT-831, formerly GKT-137831) entered Phase I and Phase II clinical trials for diabetic nephropathy and primary biliary cholangitis [21, 22]; however, these trials failed to show any benefits. It is likely that the disappointing results were in part related to the fact that “protective” Nox4 was also blocked by the GKT-831 compound, in addition to the “pathogenic” Nox1 isoform. In fact, Guzik and associates recently concluded that there existed an unmet need for identifying new molecules that provide isoform-specific inhibition of Nox homologues and that efforts “must focus on generating small molecular weight inhibitors of NADPH oxidases, allowing the selective inhibition of dysfunctional NADPH oxidase homologs. This appears to be the most reasonable approach, potentially much more efficient than non-selective scavenging of all ROS by the administration of antioxidants.” [11]. So far, only few compounds have been identified that allow selective inhibition of one Nox isoform [20, 25]. Schramm et al. as well as Altenhöfer et al. have recently reviewed some of the Nox inhibitors that have been developed, including VAS2870, VAS3947, GK-136901, GKT-831 and S17834 [11, 21].
6. Dual-acting drugs
The majority of drugs that have resulted from discovery and screening efforts and later entered clinical application were initially designed to target solely one receptor or enzyme. However, several drugs inhibit more than one target and may even act as a combined agonist/antagonist. After the AT1 antagonist losartan (DuP 793) was licensed for clinical use, the active metabolite of losartan, EXP3174, was found to not only inhibit the AT1 receptor but also the thromboxane A2/prostaglandin H2 receptor [44, 45]. Similarly, the endothelin receptor antagonist darusentan was found to also reduce ACE activity in certain tissues under pathological conditions [46, 47]. In the field of steroid pharmacology, drugs such as selective estrogen receptor modulators (SERMs) or selective estrogen receptor downregulators (SERDs), which inhibit or reduce levels of nuclear estrogen receptors α and β [48], were subsequently found to also act as agonists of GPER [49]. Aside from these accidental discoveries, efforts in drug development have recently been directed toward the generation of dual-acting drugs. These include dual inhibitors of cyclooxygenase / thromboxane A2 receptor [50, 51], dual 5-HT6 receptor / D3 receptor antagonists [52], dual VEGF receptor / PDGF receptor antagonists [53], dual AT1 / ETA antagonists [54], dual AT1 / α1-receptor antagonists [55], dual muscarinic antagonists / β2 agonists [56, 57], and dual norepinephrine reuptake inhibitors / 5-HT receptor antagonists [58]. With regard to Nox, dual Nox1/Nox4 and pan-Nox inhibitors, which inhibit all Nox isoforms, have been synthesized. However, pharmacological inhibition of the “protective” Nox4 isoform is not desirable as it may abrogate its beneficial physiological and disease-protective effects [24, 35–37, 39]. Accordingly, Rajaram et al. have recently called for reinforcing safety in clinical drug development: “with the emergence of pharmacological NOX4 inhibitors in clinical trials, caution should be taken in identifying potential side effects in patients prone to acute kidney injury and cardiovascular disease” [37].
7. Nox1: An emerging therapeutic target
The human Nox1 gene is located on the X chromosome, and thus some of its effects, like other X-linked genes, may determine possible sex-dependent risks of disease [59]. Nox1 (Figure 1), highly expressed in organs rich in smooth muscle or smooth muscle-like cells, such as mesangial cells (initially designated «mitogenic oxidase» [60]), has recently emerged as a promising new therapeutic target [15, 20, 25, 61, 62]. Nox1 contributes to the pathogenesis and progression of chronic, non-communicable diseases, including vascular disease, heart failure, diabetes, proteinuric renal disease, cancer / tumor angiogenesis, and fibrosis [14, 15, 61]. We have recently reviewed drug discovery efforts to identify inhibitors that block one or more Nox isoforms [20]. These include non-selective small molecules as well as peptides such as Nox1ds, a synthetic, cell-permeable peptide containing an 11-amino acid sequence of the docking site of the Nox1 activator NoxA1. This peptide prevents assembly and subsequent activation of the Nox1 multienzyme complex, but has only been shown to have activity in vitro [20, 63].
8. Constitutive, ligand-independent activity of receptors
The concept of unliganded, constitutively active G protein-coupled receptors, originally introduced by Costa and Herz in 1989 [64], is now firmly rooted in receptor pharmacology and occurs through the spontaneous population of active receptor states [65, 66]. The paradigm that a steroid receptor strictly requires activation by its ligand ligand (e.g. phosphorylation by MAPK) has also been revised [67]. Another example are certain drugs, such as the AT1 receptor antagonist olmesartan, that mediate some of their effects via nuclear estrogen receptors even in the absence of their natural ligand estrogen [68, 69]. Ligand-independent activity of ERα has also been reported by Karas and associates, who showed that activation of unliganded ERα induces multiple genes associated with vascular injury, inhibiting endothelial cell proliferation and migration, and triggering VSMC proliferation, as well as inflammatory responses in both cell types, thereby counteracting estrogen-dependent effects mediated by ERα [70].
9. Constitutive ligand-independent regulation of Nox1 through GPER
GPER, previously known as G protein-coupled receptor 30 (GPR30) [71], was first cloned in 1991 as an orphan receptor [71] and subsequently shown to mediate rapid signaling in response to estrogen [72–75]. The creation of GPER-deficient mice and pharmacological antagonists [49, 76, 77] has facilitated the study of ligand-dependent and -independent (taken here as constitutive or basal activity in the absence of known or added specific agonists) functions of GPER. Over the last decade, we have observed that some of the effects of GPER do not require estrogens (at circulating levels seen in females) or pharmacological ligands, suggesting the possibility of “constitutive” effects mediated by this receptor [20].
The requirement of GPER expression and activity for the expression of Nox1 was a serendipitous finding, a discovery we have previously summarized in detail [20]. In brief, in view of the numerous studies using GRAs (G protein-coupled estrogen receptor agonists), we had hypothesized that with aging, ROS-related mechanisms would worsen functional responses in isolated arteries. However, contrary to our expectations, this was not the case: vascular function in aged animals lacking Gper was better than in aged wild-type animals and essentially resembled that in young animals [25, 78]. In line with these unexpected findings, we had observed that GPER deficiency reduces activation of the endothelin system in the myocardium, which normally increases with age partly due to ROS [79]. In a series of experiments, we subsequently established that expression of Nox1, whose activity depends on intracellular signaling events [8], is regulated by GPER as a hitherto unknown constitutive activator of Nox1 (but not of other Nox isoforms). Moreover, we established that GPER-dependent expression of Nox1 and the associated ROS production play an obligatory role in pathological processes associated with increased ROS activity [78, 79]. Such pathologies include cardiac fibrosis, diastolic heart failure (heart failure with preserved ejection fraction, HFpEF), arterial hypertension, and chronic renal disease, and likely other chronic non-communicable diseases [20].
In the process of this work, G36 [77] (Figure 2), originally developed as orally active small molecule GPER blocker from its precursor G15 [76], has emerged as the first ever Nox1 downregulator (NDR), capable of reducing Nox1 protein levels, thereby reducing the abundance and activity of this ROS-producing enzyme in the cardiovascular system and the kidney [20]. This mechanism of action of NDRs is different from that obtained with synthetic GPER agonists such as G-1 or its natural ligand 17β-estradiol, which increase the bioactivity of ·NO, thereby indirectly scavenging ROS (such as superoxide anion) [80], while also possibly exerting direct antioxidant effects [81]. By contrast, NDRs such as G36 lack direct antioxidant activity [25]. Thus, ligand-dependent activation of GPER by synthetic GPER agonists [82] or estrogen, as well as inhibition of constitutive/basal activity of GPER (through the reduction of Nox1 abundance) by NDRs [25] - albeit though different mechanisms and pathways - can both yield similar net beneficial effects on pathophysiology and end-organ injury [83]. Whether G36 acts as an inverse agonist to inhibit constitutive activity of GPER, or as a neutral antagonist to inhibit basal activity of GPER (due to low levels of endogenous agonist ligands, as in male mice in vivo or in estrogen-free medium in vitro) remains unclear. Nevertheless , G36 inhibits disease progression in estrogen-dependent pathologies such as endometrial cancer [84], by acting as a “classical” GPER antagonist, interfering with estrogen-stimulated endometrial epithelial cell proliferation [76, 77]. NDRs, as a new class of therapeutics, hold promise to be efficacious in a number of chronic non-communicable diseases, similar to what has been observed with downregulators of certain steroid receptors in hormone-dependent forms of cancer [48], where the abundance of the target protein is reduced by increasing its turnover [85]. The currently known pathological conditions that represent therapeutic targets for Nox1 downregulators are discussed in detail below. We will also briefly discuss other potential disease targets in which Nox1 has been identified to play a role.
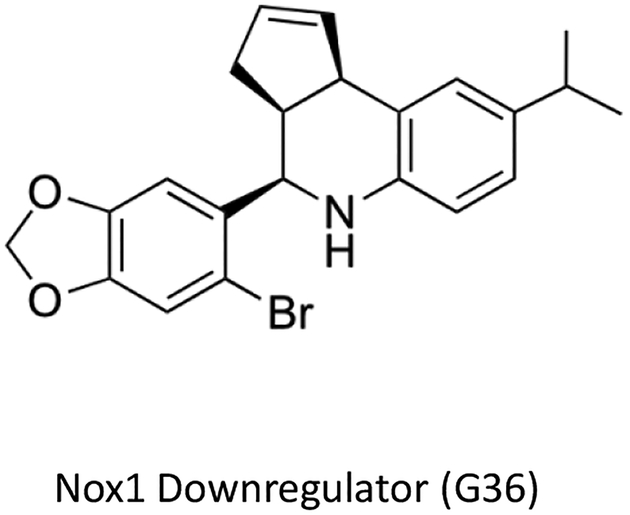
Figure 2. Chemical structure of the first selective Nox1 downregulator, G36.
The small molecule compound G36 is the first selective Nox1 downregulator to reduce expression and function of Nox1, which is under constitutive control of G protein-coupled estrogen receptor (GPER). G36 was originally identified as a GPER blocker and thus has dual functions, on the one hand inhibiting estrogen-dependent pathways involving GPER, while on the other inhibiting Nox1 expression and reducing Nox1 protein levels that are activated through constitutive GPER expression independently of estrogen.
10. Potential therapeutic applications of Nox1 downregulators
10.1. Arterial hypertension and vascular disease
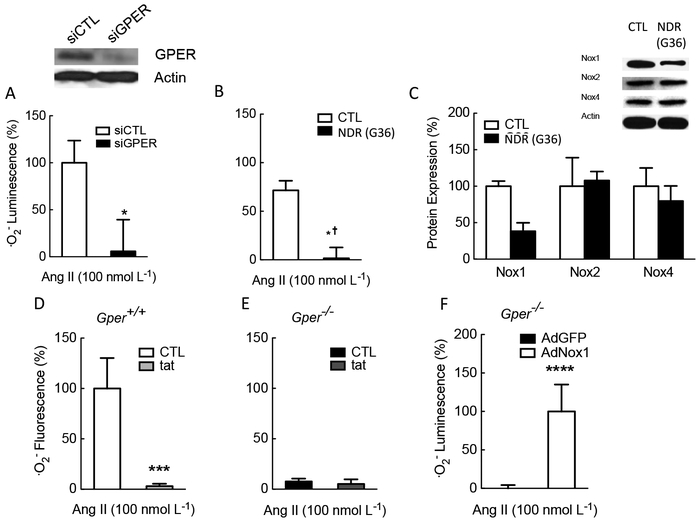
The first hint suggesting that GPER-dependent regulation of Nox1 could play a role in arterial hypertension came from experiments demonstrating that Gper deficiency largely abrogates ROS/superoxide production in response to angiotensin II, the prototypic inducer of Nox1 [33, 34, 60] and one of the main drivers of arterial hypertension and renal disease in humans (Figure 3). Angiotensin II directly enhances AT1-Nox1 binding to stimulate vascular smooth muscle cell growth [86]. Nox1-derived ROS are also one of the mechanisms underlying enhanced vasoconstriction in response to angiotensin II [87]. Consistent with the genetic ablation of GPER, its pharmacological inhibition with the Nox1 downregulator G36 was similarly effective [25] (Figure 3). As it was initially unclear whether this effect was specific to Nox1 or might also involve other isoforms of Nox, we performed experiments to determine the expression of different Nox proteins in vascular smooth muscle cells incubated with G36. As shown in Figure 3C, G36 reduced the abundance of only the Nox1 protein, but not that of Nox2 or Nox4, indicating its specificity and selectivity [25]. The effects of Nox inhibition on ·O2− production were confirmed by employing the peptide inhibitor of Nox1/Nox2, gp91ds-tat [88]. The results were similar to those obtained with either Gper-deficient mice or wild-type mice treated with G36, while angiotensin II had no effect at all in animals lacking the Gper gene (Figure 3). Finally, as a proof of principle to confirm that the reduced ROS production was only due to the lack of Nox1 expression, the human Nox1 gene was introduced into Gper-deficient cells using adenovirus-mediated gene transfer, a procedure that restored the cells’ ability to produce superoxide [Fig 3].
Figure 3. Regulation of Nox1-dependent superoxide production through constitutive expression/activation of GPER in murine and human vascular smooth muscle cells in vitro.
Treatment with siRNA targeting Gper abrogated Ang II-induced superoxide production (A). Comparable effects were observed using the Nox1 downregulator G36 (B). Selectivity of G36 for the Nox1 isoform was demonstrated by a reduction of Nox1 protein, but not Nox2 or Nox4 protein (C). In contrast to wild-type cells (D), in vascular smooth muscle cells derived from mice lacking Gper, Ang II was ineffective at stimulating superoxide production (E). Conversely, adenoviral introduction of human Nox1 into cells from animals genetically lacking murine Gper restored the cells’ ability to produce superoxide anion in response to Ang II (F). Ang II, angiotensin II; siCTL, scrambled siRNA, siGPER, siRNA against Gper; NDR, Nox1 downregulator. Gper+/+, wild-type; Gper−/−, gper-deficient; tat, Nox1/2 inhibitor gp91ds-tat; AdGFP, adenoviral vector carrying sequence for control protein GFP; AdNox1, adenoviral vector carrying Nox1 sequence. *p<0.05 vs. siCTL; *† and **p<0.05 vs CTL; ***p<0.05 vs solvent CTL; ****p<0.05 vs AdGFP control protein; Modified from reference [25] and reproduced with permission of the AAAS.
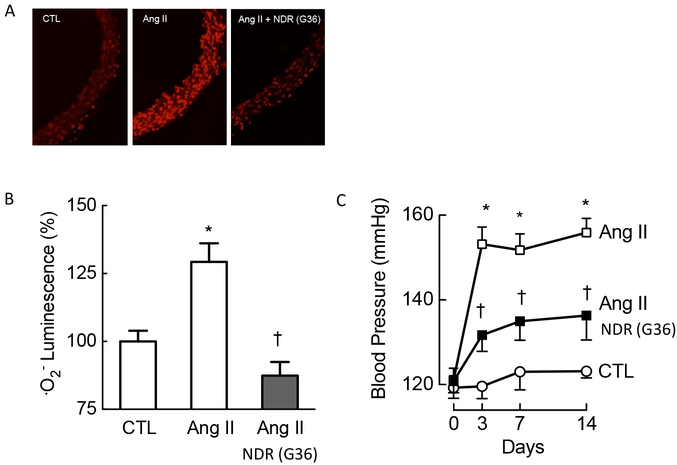
To this point, we had established that the activity of one of the principal drivers of Nox1, angiotensin II, depends on the basal activity of GPER, a concept that we deemed worthy of testing in vivo as well. In a well-established model of angiotensin II-induced arterial hypertension, we recapitulated the effects of angiotensin on arterial superoxide production in vivo. Interestingly, in animals lacking the Gper gene, angiotensin II failed to elicit any changes in blood pressure, reinforcing the obligatory role of this receptor in activating Nox1 and the associated superoxide production [20, 25]. However, in animals expressing the Gper gene, angiotensin II caused robust superoxide production and increased arterial blood pressure; the GPER antagonist G36 completely prevented this increase in ROS, and attenuated the arterial hypertension induced by angiotensin II [25] (Figure 4). Importantly, G36 treatment also prevented the impaired endothelium-dependent relaxation that develops after chronic angiotensin II infusion [25], a vascular dysfunction that is an independent prognostic marker of survival in patients with coronary artery disease [89].
Figure 4. Therapeutic effects of the Nox1 downregulator G36 in vivo in a model of chronic Nox1-dependent, AngII-induced hypertension.
Chronic treatment of wild-type mice with the NDR G36 completely prevented the Nox1-dependent increase of superoxide production (A, quantified in B), and had profound protective antihypertensive effects (C). Treatment was also associated with normalization of endothelium-dependent vascular function (not shown). CTL, control, Ang II, angiotensin II. *p<0.05 vs. CTL; †<0.05 vs. Ang II. Modified from reference [25] and reproduced with permission of the AAAS.
These findings indicated not only a role for GPER in the constitutive expression of Nox1, but also introduced a new therapeutic concept, namely downregulating the abundance of the Nox1 enzyme and concomitantly reducing the ROS it produces [20]. These findings were consistent with and corroborate previous observations by Gavazzi et al., who observed that animals lacking Nox1 had slightly lower blood pressures than wild-type control animals and that Nox1 knockout animals completely lack the increase in blood pressure in response to angiotensin II infusion [90], effects identical to those of Gper deletion in this model of hypertension [25]. It is likely that Nox1 downregulators would not only have potent antihypertensive effects in patients with arterial hypertension but that they would also reduce clinical sequelae of hypertension, such as atherosclerotic vascular disease, chronic renal disease, cerebrovascular disease, stroke and others.
Angiotensin II-dependent mechanisms, which include activation of Nox1 (Figure 1), play a disease-propagating role in atherosclerosis, coronary artery disease, and coronary plaque rupture, the main cause of myocardial infarction [91], whereas the Nox4 isoform has been demonstrated to protect from this disease, causing vasodilation, lowering blood pressure and protecting from atherosclerosis [24, 35]. In this context, it is worth noting that concomitant Nox4 inhibition appears to be undesirable from the therapeutic angle. Selective Nox1 downregulators provide a novel approach to selectively interfere with Nox1 without affecting other isoforms. Selective Nox1 downregulation could also have a role in the treatment of reperfusion injury (associated with substantial ROS production) following myocardial infarction or stroke [92].
10.2. Heart Failure
Heart failure is a clinical syndrome reflecting abnormalities in myocardial contraction or relaxation resulting in failure of the heart during systole (heart failure with reduced ejection fraction, HFrEF) or diastole (heart failure with preserved ejection fraction, HFpEF). Both, HFrEF [93] and HFpEF [25, 94], are associated with activation of ROS production; however, the individual molecular pathways involved have been identified only in part [15]. We have previously observed that activation of GPER conveys protective effects in the cardiovascular system, including inhibition of atherosclerosis in ovariectomized female mice [82], largely recapitulating the protective effects of endogenous estrogens. This is not surprising as GPER mediates part of its effects with estrogens as its ligands [49], and therefore the therapeutic effects of GPER agonists may be largely confined to females [82, 95]. In order to reduce ligand-dependent effects due to the cyclic release of circulating estrogens, we decided to use male animals (where plasma estrogen levels are only a fraction of that of cycling females) and estrogen-free in vitro conditions in order to study whether and how this receptor may exert “ligand-independent” effects in cardiovascular pathologies. Circulating serum levels of estrogen, as measured by high-sensitive gas chromatography-tandem mass spectrometry, are higher in female rodents than in males, and their regulation depend critically on the presence of ERα [96]. It is currently not known how intracrine extragonadal production of sex steroids, such as estrogens or testosterone (the substrate of estrogen-forming aromatase [97]), plays a role in physiology or disease; however, preclinical and clinical studies have found correlations between circulating estrogens levels and the absence or presence of diseases such as coronary atherosclerosis [98].
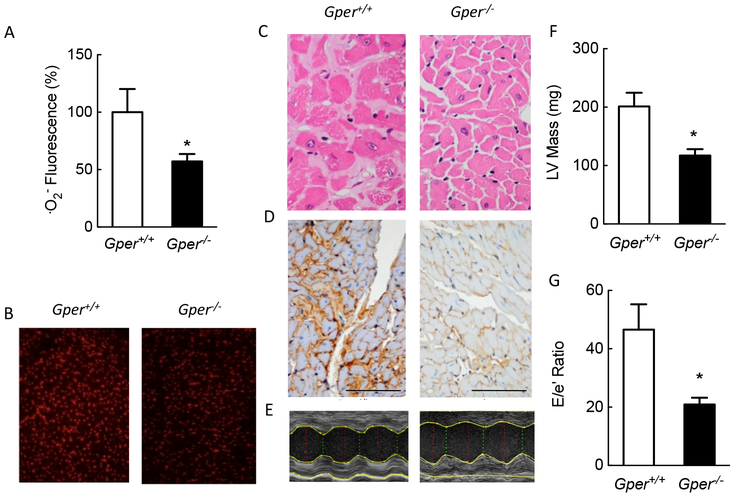
Aging in humans [99] and in rodents [25] is associated with myocardial fibrosis, inflammation and left ventricular hypertrophy, all contributing to diastolic dysfunction and diastolic heart failure. Given that in our previous studies we had only observed beneficial effects using GPER agonists (GRAs), we hypothesized that genetic deficiency of GPER would result in the aggravation of any pathologies that had already developed with aging at the functional, morphological, or molecular level. To exclude the effects of ovarian steroids as ligands, the hypothesis was tested in aged male animals expressing or lacking GPER. Much to our surprise, the results were contrary to what we had expected: aged animals deficient in GPER almost completely lacked the abnormalities that developed with age in wild-type mice, and in fact organs studied in the Gper−/− mice resembled those of 3-month old control mice used as controls, lacking left ventricular hypertrophy, myocardial fibrosis, and diastolic heart failure (Figure 5). Gper deficiency was associated with reductions in O2− production in the myocardium. Interestingly, similar to what we had observed in hypertensive animals infused with angiotensin II, abnormal endothelium-dependent vascular function that developed over two years in wild-type mice was not observed in Gper-deficient mice. Diastolic heart failure (HFpEF) is commonly found in elderly patients [99] and there is currently no drug treatment available to interfere with its progression. Interfering with the constitutive activator of Nox1, GPER, by using Nox1 downregulators, could prove to be beneficial for heart failure in patients. A direct role for Nox1 in the changes associated with aging has been corroborated by Meiljes et al., who recently reported that cellular aging in human and murine vascular cells is mediated by Nox1, and that genetic ablation of Nox1 protects against endothelial cell senescence through mechanisms involving thrombospondin-CD47 signaling [62]. More preclinical studies are required to test this concept experimentally, which is however complicated by the limited experimental models of HFpEF, particularly those including the aging heart in otherwise healthy rodents [25], and involving increased oxidative stress [94, 100, 101]. Nevertheless, the therapeutic principle offered by this new class of drugs holds promise for effective treatment of severe disease conditions, such as heart failure.
Figure 5. Constitutive GPER-dependent Nox1 expression is essential for myocardial fibrosis and heart failure with preserved ejection fraction (HFpEF).
Shown are data obtained from aged mice, resembling cardiac changes in humans. Aging was associated with increased myocardial superoxide production (A and B), cardiomyocyte hypertrophy (C) and fibrosis (C and D), left ventricular hypertrophy (E and F), and diastolic dysfunction consistent with heart failure with preserved ejection fraction (HFpEF) as determined by the E/é ratio using transthoracic echocardiography (G). Genetic ablation of Gper essentially prevented myocardial aging at the structural and functional level, completely abrogating ventricular hypertrophy, fibrosis, and HFpEF. Scale bar in panel D: 100 μm. *p<0.05 vs. Gper+/+ Modified from reference [25] and reproduced with permission of the AAAS.
10.3. Chronic renal disease
Chronic kidney disease is becoming one of the major factors contributing to morbidity and mortality due to the increasing prevalence of diabetes and hypertension, but also due to population aging. While certain drugs have been firmly established in renal medicine to slow the progression of proteinuria and chronic renal disease, they are predominantly inhibitors of the renin-angiotensin-system, its main effector angiotensin II being a key activator of Nox1 [102]. Angiotensin II leads to activation of proliferation-and inflammation-propagating pathways, such as the endothelin system, not only in the vasculature but also in the kidney [103]. Importantly, Nox1 has been recently shown to directly contribute to angiotensin II-induced ROS-mediated aldosterone synthesis in human and rat adrenal cells, an effect that can be abrogated by silencing the Nox1 gene [103]. There is still an unmet need for new therapies that could slow down or even reverse the process of glomerulosclerosis, as shown by pharmacological inhibition of the effects mediated by endothelin-1 [104, 105] or angiotensin II [106, 107]. Similar to the heart, aging in the kidney is associated with the development of pathologic anomalies, such as focal-segmental glomerulosclerosis (FSGS). FSGS is associated with upregulation of the cyclin-dependent kinase inhibitor p21waf1/cip1, which is regulated in an endothelin-dependent fashion [104]. This appears to be an important element in the disease process since endothelin-1, like angiotensin II, is one of the known activators of Nox1 [29]. Consistent with these previous observations, Zhu et al. have recently found that in experimental diabetic chronic kidney disease, Nox1 in the renal cortex promotes renal senescence through p21waf1/cip1[108]. Cha et al., using a “pan-Nox inhibitor” inhibiting all Nox isoforms (including Nox4), demonstrated a reduction in proteinuria, suggesting therapeutic potential of Nox inhibition in chronic renal disease [109]. We have begun to study the role of Nox1 and Nox1 downregulators in chronic renal disease. Similar to vascular smooth muscle cells, G36 reduces Nox1 abundance in cultured mesangial cells (manuscript in preparation) and protects cultured human podocytes, the gatekeepers of the glomerular filtration barrier [110], from injury in response to exogenous stimuli [111]. Moreover, similar to the heart, mice develop advanced focal segmental glomerulosclerosis with aging [111], while aged mice lacking the Gper gene are largely protected and their kidneys, for the most part, resemble those of young control animals [111]. Also, age-dependent functional abnormalities of the main renal artery are absent in Gper-deficient animals lacking the constitutive activation of Nox1 [112]. Further studies are underway to determine the mechanisms of how Nox1 downregulators can alleviate or even reverse acute or chronic renal injury in different forms of renal disease, including diabetic nephropathy, FSGS, and renovascular disease.
10.4. Nox1 as a therapeutic target in other chronic non-communicable diseases
Nox1 has been implicated in a number of non-communicable diseases that account for a considerable portion of morbidity and mortality in the general population. Possible clinical applications of Nox1 downregulators include pulmonary arterial hypertension or diseases associated with tissue fibrosis, including liver fibrosis [113] or radiation-induced pulmonary fibrosis, for which Choi et al. have recently shown protective effects of Nox1 inhibition [114]. Selective Nox1 downregulators may also have therapeutic efficacy in chronic inflammatory and autoimmune diseases such autoimmune (type 1) diabetes [115], autoimmune encephalomyelitis/multiple sclerosis [116], or forms of arthritis involving immune reactions [117], diseases all associated with an activation of Nox1. Important new areas of therapeutic application for Nox1 downregulators are neurodegenerative diseases that cause dementia, the most important ones being vascular dementia due to arterial hypertension or Alzheimer’s disease. A possible therapeutic role in the latter has been recently suggested by Nortley et al. who found that in experimental and clinical Alzheimer’s disease β-amyloid induces ROS in a Nox1/Nox2-dependent fashion [118, 119], resulting in pericyte vasoconstriction causing cerebral hypoperfusion, a pathomechanism that suggests therapeutic potential for NDRs [20, 21, 119]. Recent work by Fan et al. has implicated Nox-derived ROS in neuronal damage due to aging in mice, observations further corroborated in postmortem brain tissues of elderly adults [120]. These investigators observed that intrinsic production of angiotensin II within the brain increases with aging and that similar to deletion of the endogenous Nox1-activator GPER [25], deletion of Nox2 largely protected from aging-dependent pathologies [120]. Nox1 also contributes to islet cell dysfunction, and its inhibition reduces islet beta cell dysfunction following exposure to inflammatory cytokines in vitro, suggesting therapeutic potential for type 2 diabetes [121]. Other potential areas of clinical application of Nox1 downregulators include arterial thrombosis, thrombus formation and platelet aggregation, which is sensitive to Nox1 inhibition, possibly through direct interactions between Nox1 and the collagen receptor, GPVI [122], as well as different forms of cancer as either direct or adjuvant therapy [123–128].
11. Conclusion and perspectives
NDRs represent a new class of drugs that regulate the expression of a specific ROS-producing enzyme, thereby reducing its abundance and activity [20]. Nox1 is one of the key isoforms of NADPH oxidases involved in numerous non-communicable disease conditions. Its expression is controlled by the constitutive/basal expression and activity of GPER, a G protein-coupled receptor that previously had only been implicated in rapid and sustained effects mediated by its natural ligand estrogen [49]. NDRs provide several advantages over currently existing compounds targeting Nox pathways: inhibitors of Nox enzymes only interfere with their activity without affecting their expression, and therefore aspects such as dosage, pharmacokinetics and half-life of the drugs become important considerations, particularly with regard to clinical drug development. Moreover, several of the currently available Nox-targeting compounds that are or have been in development block more than one Nox isoform, particularly the “protective” isoform Nox4 [24, 35–37]. Aside from cardiovascular and renal disease conditions, for which preclinical studies suggest therapeutic efficacy of NDRs, future research should also be directed towards exploring the therapeutic efficacy of NDRs in other forms of non-communicable diseases, including pulmonary hypertension, cerebrovascular disease and dementia, chronic inflammatory and autoimmune diseases, and cancer where activation of Nox plays a central role [11, 23, 43, 120].
Role of funding sources
Supported by the Swiss National Science Foundation (grants 108258 & 122504 to M.B and 135874 & 141501 to M.R.M.) and the National Institutes of Health (R01 CA163890 and CA194496 to E.R.P.). E.R.P is also supported by a grant from Dialysis Clinic Inc., the Center of Biomedical Research Excellence in Autophagy, Inflammation and Metabolism (P20 GM121176) and the UNM Comprehensive Cancer Center (P30 CA118100).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Competing interests
M.B., M.R.M., and E.R.P. are inventors on U.S. patent No. 10,251,870, owned by the University of New Mexico, for the therapeutic use of Nox1-downregulators. E.R.P. is also an inventor on U.S. patent No. 7,875,721 for GPER-selective ligands, including G-1, G15, and G36,
References
- [1].GBD Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017, Lancet 393(10184) (2019) 1958–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cao B, Bray F, Ilbawi A, Soerjomataram I, Effect on longevity of one-third reduction in premature mortality from non-communicable diseases by 2030: a global analysis of the Sustainable Development Goal health target, Lancet Glob Health 6(12) (2018) e1288–e1296. [DOI] [PubMed] [Google Scholar]
- [3].Adair T, Progress towards reducing premature NCD mortality, Lancet Glob Health 6(12) (2018) e1254–e1255. [DOI] [PubMed] [Google Scholar]
- [4].Dzau V, Fuster V, Frazer J, Snair M, Investing in Global Health for Our Future, N Engl J Med 377(13) (2017) 1292–1296. [DOI] [PubMed] [Google Scholar]
- [5].Fuster V, Frazer J, Snair M, Vedanthan R, Dzau V, Committee on Global E. the Future of the United States: A Report of the National Academies of Sciences, Medicine, The Future Role of the United States in Global Health: Emphasis on Cardiovascular Disease, J Am Coll Cardiol 70(25) (2017) 3140–3156. [DOI] [PubMed] [Google Scholar]
- [6].Yusuf S, Ounpuu S, Anand S, The global epidemic of atherosclerotic cardiovascular disease, Med Princ Pract 11(Suppl 2) (2002) 3–8. [DOI] [PubMed] [Google Scholar]
- [7].Barton M, Husmann M, Meyer MR, Accelerated vascular aging as a paradigm for hypertensive vascular disease: prevention and therapy, Can J Cardiol 32(5) (2016) 680–686 e4. [DOI] [PubMed] [Google Scholar]
- [8].Brandes RP, Weissmann N, Schroder K, NADPH oxidases in cardiovascular disease, Free Radic Biol Med 49(5) (2010) 687–706. [DOI] [PubMed] [Google Scholar]
- [9].Förstermann U, Münzel T, Endothelial nitric oxide synthase in vascular disease: from marvel to menace, Circulation 113(13) (2006) 1708–1714. [DOI] [PubMed] [Google Scholar]
- [10].Spychalowicz A, Wilk G, Sliwa T, Ludew D, Guzik TJ, Novel therapeutic approaches in limiting oxidative stress and inflammation, Curr Pharm Biotechnol 13(13) (2012) 2456–2466. [PubMed] [Google Scholar]
- [11].Schramm A, Matusik P, Osmenda G, Guzik TJ, Targeting NADPH oxidases in vascular pharmacology, Vascular Pharmacology 56(5–6) (2012) 216–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vivekananthan DP, Penn MS, Sapp SK, Hsu A, Topol EJ, Use of antioxidant vitamins for the prevention of cardiovascular disease: meta-analysis of randomised trials, Lancet 361(9374) (2003) 2017–2023. [DOI] [PubMed] [Google Scholar]
- [13].Bleys J, Miller ER 3rd, Pastor-Barriuso R, Appel LJ, Guallar E, Vitamin-mineral supplementation and the progression of atherosclerosis: a meta-analysis of randomized controlled trials, Am J Clin Nutr 84(4) (2006) 880–887; quiz 954–5. [DOI] [PubMed] [Google Scholar]
- [14].Montezano AC, Touyz RM, Molecular mechanisms of hypertension--reactive oxygen species and antioxidants: a basic science update for the clinician, Can J Cardiol 28(3) (2012) 288–295. [DOI] [PubMed] [Google Scholar]
- [15].Lassegue B, San Martin A, Griendling KK, Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system, Circ Res 110(10) (2012) 1364–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, Mitch WE, Harrison DG, Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension, J Clin Invest 111(8) (2003) 1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vanhoutte PM, Nitric oxide: from good to bad, Ann Vasc Dis 11(1) (2018) 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Laufs U, Adam O, Strehlow K, Wassmann S, Konkol C, Laufs K, Schmidt W, Bohm M, Nickenig G, Down-regulation of rac-1 GTPase by estrogen, J Biol Chem 278(8) (2003) 5956–5962. [DOI] [PubMed] [Google Scholar]
- [19].Barton M, Primum non nocere: why calcitriol (“vitamin” D) hormone therapy is not a magic bullet, Arterioscler Thromb Vasc Biol 39(2) (2019) 117–120. [DOI] [PubMed] [Google Scholar]
- [20].Meyer MR, Barton M, GPER blockers as Nox downregulators: A new drug class to target chronic non-communicable diseases, J Steroid Biochem Mol Biol 176 (2018) 82–87. [DOI] [PubMed] [Google Scholar]
- [21].Altenhöfer S, Radermacher KA, Kleikers PW, Wingler K, Schmidt HH, Evolution of NADPH oxidase inhibitors: selectivity and mechanisms for target engagement, Antioxid Redox Signal 23(5) (2015) 406–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Buvelot H, Jaquet V, Krause KH, Mammalian NADPH Oxidases, Methods Mol Biol 1982 (2019) 17–36. [DOI] [PubMed] [Google Scholar]
- [23].Konior A, Schramm A, Czesnikiewicz-Guzik M, Guzik TJ, NADPH oxidases in vascular pathology, Antioxid Redox Signal 20(17) (2014) 2794–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Schröder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Luedike P, Michaelis UR, Weissmann N, Dimmeler S, Shah AM, Brandes RP, Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase, Circ Res 110(9) (2012) 1217–1225. [DOI] [PubMed] [Google Scholar]
- [25].Meyer MR, Fredette NC, Daniel C, Sharma G, Amann K, Arterburn JB, Barton M, Prossnitz ER, Obligatory role for GPER in cardiovascular aging and disease, Sci Signal 9(452) (2016) ra105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Morimoto H, Iwata K, Ogonuki N, Inoue K, Atsuo O, Kanatsu-Shinohara M, Morimoto T, Yabe-Nishimura C, Shinohara T, ROS are required for mouse spermatogonial stem cell self-renewal, Cell Stem Cell 12(6) (2013) 774–786. [DOI] [PubMed] [Google Scholar]
- [27].Morimoto H, Kanastu-Shinohara M, Ogonuki N, Kamimura S, Ogura A, Yabe-Nishimura C, Mori Y, Morimoto T, Watanabe S, Otsu K, Yamamoto T, Shinohara T, ROS amplification drives mouse spermatogonial stem cell self-renewal, Life Sci Alliance 2 (2) (2019) e201900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wang C, Wang Z, Liu W, Ai Z, ROS-generating oxidase NOX1 promotes the self-renewal activity of CD133+ thyroid cancer cells through activation of the Akt signaling, Cancer Lett 447 (2019) 154–163. [DOI] [PubMed] [Google Scholar]
- [29].Briones AM, Tabet F, Callera GE, Montezano AC, Yogi A, He Y, Quinn MT, Salaices M, Touyz RM, Differential regulation of Nox1, Nox2 and Nox4 in vascular smooth muscle cells from WKY and SHR, J Am Soc Hypertens 5(3) (2011) 137–153. [DOI] [PubMed] [Google Scholar]
- [30].Aguado A, Fischer T, Rodriguez C, Manea A, Martinez-Gonzalez J, Touyz RM, Hernanz R, Alonso MJ, Dixon DA, Briones AM, Salaices M, Hu antigen R is required for NOX-1 but not NOX-4 regulation by inflammatory stimuli in vascular smooth muscle cells, J Hypertens 34(2) (2016) 253–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, Prossnitz ER, Virtual and biomolecular screening converge on a selective agonist for GPR30, Nat Chem Biol 2(4) (2006) 207–212. [DOI] [PubMed] [Google Scholar]
- [32].Barton M, Meyer MR, HuR-ry up: how hydrogen sulfide protects against atherosclerosis, Circulation 139(1) (2019) 115–118. [DOI] [PubMed] [Google Scholar]
- [33].Lassegue B, Sorescu D, Szocs K, Yin Q, Akers M, Zhang Y, Grant SL, Lambeth JD, Griendling KK, Novel gp91(phox) homologues in vascular smooth muscle cells: nox1 mediates angiotensin II-induced superoxide formation and redox-sensitive signaling pathways, Circ Res 88(9) (2001) 888–894. [DOI] [PubMed] [Google Scholar]
- [34].Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD, Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5, Gene 269(1–2) (2001) 131–140. [DOI] [PubMed] [Google Scholar]
- [35].Schürmann C, Rezende F, Kruse C, Yasar Y, Lowe O, Fork C, van de Sluis B, Bremer R, Weissmann N, Shah AM, Jo H, Brandes RP, Schröder K, The NADPH oxidase Nox4 has anti-atherosclerotic functions, Eur Heart J 36(48) (2015) 3447–3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Brandes RP, Takac I, Schroder K, No superoxide--no stress?: Nox4, the good NADPH oxidase!, Arterioscler Thromb Vasc Biol 31(6) (2011) 1255–1257. [DOI] [PubMed] [Google Scholar]
- [37].Rajaram RD, Dissard R, Jaquet V, de Seigneux S, Potential benefits and harms of NADPH oxidase type 4 in the kidneys and cardiovascular system, Nephrol Dial Transplant 34(4) (2019) 567–576. [DOI] [PubMed] [Google Scholar]
- [38].Ray R, Murdoch CE, Wang M, Santos CX, Zhang M, Alom-Ruiz S, Anilkumar N, Ouattara A, Cave AC, Walker SJ, Grieve DJ, Charles RL, Eaton P, Brewer AC, Shah AM, Endothelial Nox4 NADPH oxidase enhances vasodilatation and reduces blood pressure in vivo, Arterioscler Thromb Vasc Biol 31(6) (2011) 1368–1376. [DOI] [PubMed] [Google Scholar]
- [39].Hancock M, Hafstad AD, Nabeebaccus AA, Catibog N, Logan A, Smyrnias I, Hansen SS, Lanner J, Schroder K, Murphy MP, Shah AM, Zhang M, Myocardial NADPH oxidase-4 regulates the physiological response to acute exercise, Elife 7 (2018). e410044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Touyz RM, Anagnostopoulou A, Rios F, Montezano AC, Camargo LL, NOX5: Molecular biology and pathophysiology, Exp Physiol 104(5) (2019) 605–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jha JC, Banal C, Okabe J, Gray SP, Hettige T, Chow BSM, Thallas-Bonke V, De Vos L, Holterman CE, Coughlan MT, Power DA, Skene A, Ekinci EI, Cooper ME, Touyz RM, Kennedy CR, Jandeleit-Dahm K, NADPH Oxidase Nox5 Accelerates Renal Injury in Diabetic Nephropathy, Diabetes 66(10) (2017) 2691–2703. [DOI] [PubMed] [Google Scholar]
- [42].Jha JC, Dai A, Holterman CE, Cooper ME, Touyz RM, Kennedy CR, Jandeleit-Dahm KAM, Endothelial or vascular smooth muscle cell-specific expression of human NOX5 exacerbates renal inflammation, fibrosis and albuminuria in the Akita mouse, Diabetologia 62(9) (2019) 1712–1726. [DOI] [PubMed] [Google Scholar]
- [43].Guzik TJ, Harrison DG, Vascular NADPH oxidases as drug targets for novel antioxidant strategies, Drug Discov Today 11(11–12) (2006) 524–533. [DOI] [PubMed] [Google Scholar]
- [44].Kramer C, Sunkomat J, Witte J, Luchtefeld M, Walden M, Schmidt B, Tsikas D, Boger RH, Forssmann WG, Drexler H, Schieffer B, Angiotensin II receptor-independent antiinflammatory and antiaggregatory properties of losartan: role of the active metabolite EXP3179, Circ Res 90(7) (2002) 770–776. [DOI] [PubMed] [Google Scholar]
- [45].Li P, Ferrario CM, Brosnihan KB, Nonpeptide angiotensin II antagonist losartan inhibits thromboxane A2-induced contractions in canine coronary arteries, J Pharmacol Exp Ther 281(3) (1997) 1065–1070. [PubMed] [Google Scholar]
- [46].Barton M, Carmona R, Krieger JE, Goettsch W, Morawietz H, d’Uscio LV, Lattmann T, Luscher TF, Shaw S, Endothelin regulates angiotensin-converting enzyme in the mouse kidney, J Cardiovasc Pharmacol 36(5 Suppl 1) (2000) S244–S247. [DOI] [PubMed] [Google Scholar]
- [47].Barton M, Carmona R, Morawietz H, d’Uscio LV, Goettsch W, Hillen H, Haudenschild CC, Krieger JE, Munter K, Lattmann T, Luscher TF, Shaw S, Obesity is associated with tissue-specific activation of renal angiotensin-converting enzyme in vivo: evidence for a regulatory role of endothelin, Hypertension 35(1 Pt 2) (2000) 329–336. [DOI] [PubMed] [Google Scholar]
- [48].McDonnell DP, Wardell SE, Norris JD, Oral selective estrogen receptor downregulators (SERDs), a breakthrough endocrine therapy for breast cancer, J Med Chem 58(12) (2015) 4883–4837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Prossnitz ER, Barton M, The G-protein-coupled estrogen receptor GPER in health and disease, Nat Rev Endocrinol 7(12) (2011) 715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Faull AW, Brewster AG, Brown GR, Smithers MJ, Jackson R, Dual-acting thromboxane receptor antagonist/synthase inhibitors: synthesis and biological properties of [2-substituted-4-(3-pyridyl)-1,3-dioxan-5-yl] alkenoic acids, J Med Chem 38(4) (1995) 686–694. [DOI] [PubMed] [Google Scholar]
- [51].Ackerley N, Brewster AG, Brown GR, Clarke DS, Foubister AJ, Griffin SJ, Hudson JA, Smithers MJ, Whittamore PR, A novel approach to dual-acting thromboxane receptor antagonist/synthase inhibitors based on the link of 1,3-dioxane-thromboxane receptor antagonists and -thromboxane synthase inhibitors, J Med Chem 38(10) (1995) 1608–1628. [DOI] [PubMed] [Google Scholar]
- [52].Grychowska K, Chaumont-Dubel S, Kurczab R, Koczurkiewicz P, Deville C, Krawczyk M, Pietrus W, Satala G, Buda S, Piska K, Drop M, Bantreil X, Lamaty F, Pekala E, Bojarski AJ, Popik P, Marin P, Zajdel P, Dual 5-HT6 and D3 receptor antagonists in a group of 1 H-pyrrolo[3,2- c]quinolines with neuroprotective and procognitive activity, ACS Chem Neurosci 10 (7) (2019) 3183–3191. [DOI] [PubMed] [Google Scholar]
- [53].Kudelka MR, Grossniklaus HE, Mandell KJ, Emergence of dual VEGF and PDGF antagonists in the treatment of exudative age-related macular degeneration, Expert Rev Ophthalmol 8(5) (2013) 475–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Kowala MC, Murugesan N, Tellew J, Carlson K, Monshizadegan H, Ryan C, Gu Z, Kane B, Fadnis L, Baska RA, Beyer S, Arthur S, Dickinson K, Zhang D, Perrone M, Ferrer P, Giancarli M, Baumann J, Bird E, Panchal B, Yang Y, Trippodo N, Barrish J, Macor JE, Novel dual action AT1 and ETA receptor antagonists reduce blood pressure in experimental hypertension, J Pharmacol Exp Ther 309(1) (2004) 275–284. [DOI] [PubMed] [Google Scholar]
- [55].Agrawal N, Machhi J, Rathwa V, Kanhed AM, Patel S, Murumkar P, Gandhi H, Yadav MR, Exploration of 6,7-dimethoxyquinazoline derivatives as dual acting α1- and AT1-receptor antagonists: synthesis, evaluation, pharmacophore & 3D-QSAR modeling and receptor docking studies, RSC Adv 6 (2016) 30661–30682. [Google Scholar]
- [56].Norman P, New dual-acting bronchodilator treatments for COPD, muscarinic antagonists and beta2 agonists in combination or combined into a single molecule, Expert Opin Investig Drugs 22(12) (2013) 1569–1580. [DOI] [PubMed] [Google Scholar]
- [57].Wielders PL, Ludwig-Sengpiel A, Locantore N, Baggen S, Chan R, Riley JH, A new class of bronchodilator improves lung function in COPD: a trial with GSK961081, Eur Respir J 42(4) (2013) 972–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Heffernan GD, Coghlan RD, Manas ES, McDevitt RE, Li Y, Mahaney PE, Robichaud AJ, Huselton C, Alfinito P, Bray JA, Cosmi SA, Johnston GH, Kenney T, Koury E, Winneker RC, Deecher DC, Trybulski EJ, Dual acting norepinephrine reuptake inhibitors and 5-HT(2A) receptor antagonists: Identification, synthesis and activity of novel 4-aminoethyl-3-(phenylsulfonyl)-1H-indoles, Bioorg Med Chem 17(22) (2009) 7802–7815. [DOI] [PubMed] [Google Scholar]
- [59].AlSiraj Y, Chen X, Thatcher SE, Temel RE, Cai L, Blalock E, Katz W, Ali HM, Petriello M, Deng P, Morris AJ, Wang X, Lusis AJ, Arnold AP, Reue K, Thompson K, Tso P, Cassis LA, XX sex chromosome complement promotes atherosclerosis in mice, Nat Commun 10(1) (2019) 2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Suh YA, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD, Cell transformation by the superoxide-generating oxidase Mox1, Nature 401(6748) (1999) 79–82. [DOI] [PubMed] [Google Scholar]
- [61].Gimenez M, Schickling BM, Lopes LR, Miller FJ Jr., Nox1 in cardiovascular diseases: regulation and pathophysiology, Clin Sci (Lond) 130(3) (2016) 151–165. [DOI] [PubMed] [Google Scholar]
- [62].Meijles DN, Sahoo S, Al Ghouleh I, Amaral JH, Bienes-Martinez R, Knupp HE, Attaran S, Sembrat JC, Nouraie SM, Rojas MM, Novelli EM, Gladwin MT, Isenberg JS, Cifuentes-Pagano E, Pagano PJ, The matricellular protein TSP1 promotes human and mouse endothelial cell senescence through CD47 and Nox1, Sci Signal 10 (501) (2017) eaaj1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ranayhossaini DJ, Rodriguez AI, Sahoo S, Chen BB, Mallampalli RK, Kelley EE, Csanyi G, Gladwin MT, Romero G, Pagano PJ, Selective recapitulation of conserved and nonconserved regions of putative NOXA1 protein activation domain confers isoform-specific inhibition of Nox1 oxidase and attenuation of endothelial cell migration, J Biol Chem 288(51) (2013) 36437–36450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Costa T, Herz A, Antagonists with negative intrinsic activity at delta opioid receptors coupled to GTP-binding proteins, Proc Natl Acad Sci U S A 86(19) (1989) 7321–7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Bond RA, Ijzerman AP, Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery, Trends Pharmacol Sci 27(2) (2006) 92–96. [DOI] [PubMed] [Google Scholar]
- [66].Wacker D, Stevens RC, Roth BL, How ligands illuminate GPCR molecular pharmacology, Cell 170(3) (2017) 414–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Bennesch MA, Picard D, Minireview: Tipping the balance: ligand-independent activation of steroid receptors, Mol Endocrinol 29(3) (2015) 349–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Barton M, Meyer MR, Prossnitz ER, Estrogen-independent activation of estrogen receptors, Hypertension 57(6) (2011) 1056–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Shimada K, Kitazato KT, Kinouchi T, Yagi K, Tada Y, Satomi J, Kageji T, Nagahiro S, Activation of estrogen receptor-alpha and of angiotensin-converting enzyme 2 suppresses ischemic brain damage in oophorectomized rats, Hypertension 57(6) (2011) 1161–1166. [DOI] [PubMed] [Google Scholar]
- [70].Lu Q, Schnitzler GR, Vallaster CS, Ueda K, Erdkamp S, Briggs CE, Iyer LK, Jaffe IZ, Karas RH, Unliganded estrogen receptor alpha regulates vascular cell function and gene expression, Mol Cell Endocrinol 442 (2017) 12–23. [DOI] [PubMed] [Google Scholar]
- [71].Barton M, Filardo EJ, Lolait SJ, Thomas P, Maggiolini M, Prossnitz ER, Twenty years of the G protein-coupled estrogen receptor GPER: Historical and personal perspectives, J Steroid Biochem Mol Biol 176 (2018) 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Filardo EJ, Quinn JA, Bland KI, Frackelton AR Jr., Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF, Mol Endocrinol 14(10) (2000) 1649–1660. [DOI] [PubMed] [Google Scholar]
- [73].Filardo EJ, Quinn JA, Frackelton AR Jr., Bland KI, Estrogen action via the G protein-coupled receptor, GPR30: stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis, Mol Endocrinol 16(1) (2002) 70–84. [DOI] [PubMed] [Google Scholar]
- [74].Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER, A transmembrane intracellular estrogen receptor mediates rapid cell signaling, Science 307(5715) (2005) 1625–1630. [DOI] [PubMed] [Google Scholar]
- [75].Thomas P, Pang Y, Filardo EJ, Dong J, Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells, Endocrinology 146(2) (2005) 624–632. [DOI] [PubMed] [Google Scholar]
- [76].Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, Prossnitz ER, In vivo effects of a GPR30 antagonist, Nat Chem Biol 5(6) (2009) 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Dennis MK, Field AS, Burai R, Ramesh C, Petrie WK, Bologa CG, Oprea TI, Yamaguchi Y, Hayashi SI, Sklar LA, Hathaway HJ, Arterburn JB, Prossnitz ER, Identification of a GPER/GPR30 antagonist with improved estrogen receptor counterselectivity, J Steroid Biochem Mol Biol 127 (2011) 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Wong W, Keeping hearts and blood vessels young, Science 354(6312) (2016) 593. [Google Scholar]
- [79].Meyer MR, Fredette NC, Sharma G, Barton M, Prossnitz ER, GPER is required for the age-dependent upregulation of the myocardial endothelin system, Life Sci 159 (2016) 61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Fredette NC, Meyer MR, Prossnitz ER, Role of GPER in estrogen-dependent nitric oxide formation and vasodilation, J Steroid Biochem Mol Biol 176 (2018) 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Broughton BR, Miller AA, Sobey CG, Endothelium-dependent relaxation by G protein-coupled receptor 30 agonists in rat carotid arteries, Am J Physiol Heart Circ Physiol 298(3) (2010) H1055–H1061. [DOI] [PubMed] [Google Scholar]
- [82].Meyer MR, Fredette NC, Howard TA, Hu C, Ramesh C, Daniel C, Amann K, Arterburn JB, Barton M, Prossnitz ER , G protein-coupled estrogen receptor protects from atherosclerosis, Sci Reports 4 (2014) 7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Prossnitz ER, Barton M, Estrogen biology: new insights into GPER function and clinical opportunities, Mol Cell Endocrinol 389(1–2) (2014) 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Petrie WK, Dennis MK, Hu C, Dai D, Arterburn JB, Smith HO, Hathaway HJ, Prossnitz ER, G protein-coupled estrogen receptor-selective ligands modulate endometrial tumor growth, Obstet Gynecol Int 2013 (2013) 472720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Dauvois S, Danielian PS, White R, Parker MG, Antiestrogen ICI 164,384 reduces cellular estrogen receptor content by increasing its turnover, Proc Natl Acad Sci U S A 89(9) (1992) 4037–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Valente AJ, Yoshida T, Murthy SN, Sakamuri SS, Katsuyama M, Clark RA, Delafontaine P, Chandrasekar B, Angiotensin II enhances AT1-Nox1 binding and stimulates arterial smooth muscle cell migration and proliferation through AT1, Nox1, and interleukin-18, Am J Physiol Heart Circ Physiol 303(3) (2012) H282–H296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hashad AM, Sancho M, Brett SE, Welsh DG, Reactive oxygen species mediate the suppression of arterial smooth muscle T-type Ca(2+) channels by angiotensin II, Sci Rep 8(1) (2018) 3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Rey FE, Cifuentes ME, Kiarash A, Quinn MT, Pagano PJ, Novel competitive inhibitor of NAD(P)H oxidase assembly attenuates vascular O(2)(−) and systolic blood pressure in mice, Circ Res 89(5) (2001) 408–414. [DOI] [PubMed] [Google Scholar]
- [89].Schächinger V, Britten MB, Zeiher AM, Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease, Circulation 101(16) (2000) 1899–1906. [DOI] [PubMed] [Google Scholar]
- [90].Gavazzi G, Banfi B, Deffert C, Fiette L, Schappi M, Herrmann F, Krause KH, Decreased blood pressure in NOX1-deficient mice, FEBS Lett 580(2) (2006) 497–504. [DOI] [PubMed] [Google Scholar]
- [91].Aono J, Suzuki J, Iwai M, Horiuchi M, Nagai T, Nishimura K, Inoue K, Ogimoto A, Okayama H, Higaki J, Deletion of the angiotensin II type 1a receptor prevents atherosclerotic plaque rupture in apolipoprotein E−/− mice, Arterioscler Thromb Vasc Biol 32(6) (2012) 1453–1459. [DOI] [PubMed] [Google Scholar]
- [92].Granger DN, Kvietys PR, Reperfusion injury and reactive oxygen species: The evolution of a concept, Redox Biol 6 (2015) 524–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Münzel T, Camici GG, Maack C, Bonetti NR, Fuster V, Kovacic JC, Impact of oxidative stress on the heart and vasculature: part 2 of a 3-part series, J Am Coll Cardiol 70(2) (2017) 212–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Schiattarella GG, Altamirano F, Tong D, French KM, Villalobos E, Kim SY, Luo X, Jiang N, May HI, Wang ZV, Hill TM, Mammen PPA, Huang J, Lee DI, Hahn VS, Sharma K, Kass DA, Lavandero S, Gillette TG, Hill JA, Nitrosative stress drives heart failure with preserved ejection fraction, Nature 568(7752) (2019) 351–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Triplett KD, Pokhrel S, Castleman MJ, Daly SM, Elmore BO, Joyner JA, Sharma G, Herbert G, Campen MJ, Hathaway HJ, Prossnitz ER, Hall PR, GPER activation protects against epithelial barrier disruption by Staphylococcus aureus alpha-toxin, Sci Rep 9(1) (2019) 1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Nilsson ME, Vandenput L, Tivesten A, Norlen AK, Lagerquist MK, Windahl SH, Borjesson AE, Farman HH, Poutanen M, Benrick A, Maliqueo M, Stener-Victorin E, Ryberg H, Ohlsson C, Measurement of a comprehensive sex steroid profile in rodent serum by high-sensitive gas chromatography-tandem mass spectrometry, Endocrinology 156(7) (2015) 2492–2502. [DOI] [PubMed] [Google Scholar]
- [97].Meyer MR, Haas E, Barton M, Need for research on estrogen receptor function: importance for postmenopausal hormone therapy and atherosclerosis, Gend Med 5 Suppl A (2008) S19–S33. [DOI] [PubMed] [Google Scholar]
- [98].Meyer MR, Barton M, Estrogens and coronary artery disease: new clinical perspectives, Adv Pharmacol 77 (2016) 307–360. [DOI] [PubMed] [Google Scholar]
- [99].Upadhya B, Pisani B, Kitzman DW, Evolution of a geriatric syndrome: pathophysiology and treatment of heart failure with preserved ejection fraction, J Am Geriatr Soc 65(11) (2017) 2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Amgalan D, Kitsis RN, A mouse model for the most common form of heart failure, Nature 568(7752) (2019) 324–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Olver TD, Edwards JC, Jurrissen TJ, Veteto AB, Jones JL, Gao C, Rau C, Warren CM, Klutho PJ, Alex L, Ferreira-Nichols SC, Ivey JR, Thorne PK, McDonald KS, Krenz M, Baines CP, Solaro RJ, Wang X, Ford DA, Domeier TL, Padilla J, Rector RS, Emter CA, Western diet-fed, aortic-banded Ossabaw swine. A preclinical model of cardio-metabolic heart failure, J Am Coll Cardiol Basic Trans Science 4 (2019) 404–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Rajamohan SB, Raghuraman G, Prabhakar NR, Kumar GK, NADPH oxidase-derived H(2)O(2) contributes to angiotensin II-induced aldosterone synthesis in human and rat adrenal cortical cells, Antioxid Redox Signal 17(3) (2012) 445–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Barton M, Shaw S, d’Uscio LV, Moreau P, Luscher TF, Angiotensin II increases vascular and renal endothelin-1 and functional endothelin converting enzyme activity in vivo: role of ETA receptors for endothelin regulation, Biochem Biophys Res Commun 238(3) (1997) 861–865. [DOI] [PubMed] [Google Scholar]
- [104].Ortmann J, Amann K, Brandes RP, Kretzler M, Munter K, Parekh N, Traupe T, Lange M, Lattmann T, Barton M, Role of podocytes for reversal of glomerulosclerosis and proteinuria in the aging kidney after endothelin inhibition, Hypertension 44(6) (2004) 974–981. [DOI] [PubMed] [Google Scholar]
- [105].Barton M, Reversal of proteinuric renal disease and the emerging role of endothelin, Nat Clin Pract Nephrol 4(9) (2008) 490–501. [DOI] [PubMed] [Google Scholar]
- [106].Adamczak M, Gross ML, Krtil J, Koch A, Tyralla K, Amann K, Ritz E, Reversal of glomerulosclerosis after high-dose enalapril treatment in subtotally nephrectomized rats, J Am Soc Nephrol 14(11) (2003) 2833–2842. [DOI] [PubMed] [Google Scholar]
- [107].Remuzzi A, Sangalli F, Macconi D, Tomasoni S, Cattaneo I, Rizzo P, Bonandrini B, Bresciani E, Longaretti L, Gagliardini E, Conti S, Benigni A, Remuzzi G, Regression of renal disease by angiotensin II antagonism Is caused by regeneration of kidney vasculature, J Am Soc Nephrol 27(3) (2016) 699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Zhu K, Kakehi T, Matsumoto M, Iwata K, Ibi M, Ohshima Y, Zhang J, Liu J, Wen X, Taye A, Fan C, Katsuyama M, Sharma K, Yabe-Nishimura C, NADPH oxidase NOX1 is involved in activation of protein kinase C and premature senescence in early stage diabetic kidney, Free Radic Biol Med 83 (2015) 21–30. [DOI] [PubMed] [Google Scholar]
- [109].Cha JJ, Min HS, Kim KT, Kim JE, Ghee JY, Kim HW, Lee JE, Han JY, Lee G, Ha HJ, Bae YS, Lee SR, Moon SH, Lee SC, Kim G, Kang YS, Cha DR, APX-115, a first-in-class pan-NADPH oxidase (Nox) inhibitor, protects db/db mice from renal injury, Lab Invest 97(4) (2017) 419–431. [DOI] [PubMed] [Google Scholar]
- [110].Barton M, Tharaux PL, Endothelin and the podocyte, Clin Kidney J 5(1) (2012) 17–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Meyer MR, Daniel C, Woods CD, Sharma G, Fredette NC, Amann K, Barton M, Prossnitz ER, GPER is required for age-dependent albuminuria and glomerulosclerosis: evidence for its role in podocyte injury and mesangial Nox1 regulation, Hypertension 72(Suppl.) (2018) AP261. [Google Scholar]
- [112].Meyer MR, Rosemann T, Barton M, Prossnitz ER, GPER mediates functional endothelial aging in renal rteries, Pharmacology 100(3–4) (2017) 188–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Jiang JX, Chen X, Serizawa N, Szyndralewiez C, Page P, Schroder K, Brandes RP, Devaraj S, Torok NJ, Liver fibrosis and hepatocyte apoptosis are attenuated by GKT137831, a novel NOX4/NOX1 inhibitor in vivo, Free Radic Biol Med 53(2) (2012) 289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Choi SH, Kim M, Lee HJ, Kim EH, Kim CH, Lee YJ, Effects of NOX1 on fibroblastic changes of endothelial cells in radiationinduced pulmonary fibrosis, Mol Med Rep 13(5) (2016) 4135–4142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Gorin Y, Cavaglieri RC, Khazim K, Lee DY, Bruno F, Thakur S, Fanti P, Szyndralewiez C, Barnes JL, Block K, Abboud HE, Targeting NADPH oxidase with a novel dual Nox1/Nox4 inhibitor attenuates renal pathology in type 1 diabetes, Am J Physiol Renal Physiol 308(11) (2015) F1276–F1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Fischer MT, Sharma R, Lim JL, Haider L, Frischer JM, Drexhage J, Mahad D, Bradl M, van Horssen J, Lassmann H, NADPH oxidase expression in active multiple sclerosis lesions in relation to oxidative tissue damage and mitochondrial injury, Brain 135(Pt 3) (2012) 886–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Skurlova M, Stofkova A, Jurcovicova J, Anxiety-like behavior in the elevated-plus maze tests and enhanced IL-1beta, IL-6, NADPH oxidase-1, and iNOS mRNAs in the hippocampus during early stage of adjuvant arthritis in rats, Neurosci Lett 487(2) (2011) 250–254. [DOI] [PubMed] [Google Scholar]
- [118].Nortley R, Korte N, Izquierdo P, Hirunpattarasilp C, Mishra A, Jaunmuktane Z, Kyrargyri V, Pfeiffer T, Khennouf L, Madry C, Gong H, Richard-Loendt A, Huang W, Saito T, Saido TC, Brandner S, Sethi H, Attwell D, Amyloid beta oligomers constrict human capillaries in Alzheimer’s disease via signaling to pericytes, Science (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Barton M, Meyer MR, Prossnitz ER, Potential role for Nox1 for endothelin-induced brain capillary vasoconstriction in Alzheimer’s disease and dementia, Science 365 (6450) (2019) eaav9518.31221773 [Google Scholar]
- [120].Fan LM, Geng L, Cahill-Smith S, Liu F, Douglas G, McKenzie CA, Smith C, Brooks G, Channon KM, Li JM, Nox2 contributes to age-related oxidative damage to neurons and the cerebral vasculature, J Clin Invest 130 (2019) 3374–3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Weaver JR, Grzesik W, Taylor-Fishwick DA, Inhibition of NADPH oxidase-1 preserves beta cell function, Diabetologia 58(1) (2015) 113–121. [DOI] [PubMed] [Google Scholar]
- [122].Vara D, Campanella M, Pula G, The novel NOX inhibitor 2-acetylphenothiazine impairs collagen-dependent thrombus formation in a GPVI-dependent manner, Br J Pharmacol 168(1) (2013) 212–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Chocry M, Leloup L, Kovacic H, Reversion of resistance to oxaliplatin by inhibition of p38 MAPK in colorectal cancer cell lines: involvement of the calpain / Nox1 pathway, Oncotarget 8(61) (2017) 103710–103730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Echizen K, Horiuchi K, Aoki Y, Yamada Y, Minamoto T, Oshima H, Oshima M, NF-kappaB-induced NOX1 activation promotes gastric tumorigenesis through the expansion of SOX2-positive epithelial cells, Oncogene 38(22) (2019) 4250–4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Gianni D, Taulet N, Zhang H, DerMardirossian C, Kister J, Martinez L, Roush WR, Brown SJ, Bokoch GM, Rosen H, A novel and specific NADPH oxidase-1 (Nox1) small-molecule inhibitor blocks the formation of functional invadopodia in human colon cancer cells, ACS Chem Biol 5(10) (2010) 981–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Liang S, Ma HY, Zhong Z, Dhar D, Liu X, Xu J, Koyama Y, Nishio T, Karin D, Karin G, McCubbin R, Zhang C, Hu R, Yang G, Chen L, Ganguly S, Lan T, Karin M, Kisseleva T, Brenner DA, NADPH oxidase 1 in liver macrophages promotes inflammation and tumor development in mice, Gastroenterology 156(4) (2019) 1156–1172 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Shen K, Lu F, Xie J, Wu M, Cai B, Liu Y, Zhang H, Tan H, Pan Y, Xu H, Cambogin exerts anti-proliferative and pro-apoptotic effects on breast adenocarcinoma through the induction of NADPH oxidase 1 and the alteration of mitochondrial morphology and dynamics, Oncotarget 7(31) (2016) 50596–50611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Stalin J, Garrido-Urbani S, Heitz F, Szyndralewiez C, Jemelin S, Coquoz O, Ruegg C, Imhof BA, Inhibition of host NOX1 blocks tumor growth and enhances checkpoint inhibitor-based immunotherapy, Life Sci Alliance 2(4) (2019) e201800265. [DOI] [PMC free article] [PubMed] [Google Scholar]