Abstract
Background:
Sleep disturbance and genetic profile are risks for cognitive decline in non-cancer populations, yet their role in cancer-related cognitive problems remains understudied.
Objective:
This study examined whether sleep disturbance were associated with worse neurocognitive outcomes in breast cancer survivors, and if sleep effects on cognition varied by genotype.
Methods:
Newly diagnosed female patients (n=344) ages 60+ with stage 0–3 breast cancer were recruited from August 2010-December 2015. Assessments were done before systemic therapy and 12 and 24 months later. Neuropsychological testing measured attention, processing speed, executive function, learning and memory; self-perceived cognitive functioning was also assessed. Sleep disturbance was defined by self-report of routine poor or restless sleep. Genotyping included APOE, BDNF, and COMT polymorphisms. Random effects fluctuation models tested associations of between-person and within-person differences in sleep, genotype, and sleep-genotype interactions and cognition, controlling for age, reading level, race, site, and treatment.
Results:
One third of patients reported sleep disturbance at each time point. There was a sleep x APOE4 interaction (P=0.002), in which patients with the APOE4 allele and sleep disturbance had significantly lower learning and memory scores vs. those who were APOE4 negative and without sleep disturbance. There was also a sleep disturbance-COMT genotype interaction (P=0.02), where COMT-Val carriers with sleep disturbance had lower perceived cognition than non-carriers.
Conclusion:
Sleep disturbance was common and associated with worse cognitive performance in older breast cancer survivors, especially those with a genetic risk for cognitive decline. Survivorship care should include sleep assessment and intervention to address sleep problems.
Keywords: Sleep, older, cognition, breast cancer, genotype, BDNF, APOE, COMT
Precis:
This study reports sleep disturbance in breast cancer survivors to relate to worse self-perceived cognitive functioning and learning and memory performance, especially those with a genetic risk (e.g., APOE4) for cognitive decline.
The protocol for this study is listed on clinicaltrials.gov as .
Introduction
Shifting demographics, increasing life expectancies, increasing rates of cancer with advancing age, and better cancer survival have led to an increasing number of older cancer survivors, with those over age 65 expected to represent three-quarters of US survivors in the next two decades.1 These older survivors often have additional health concerns and long-term changes to quality of life related to their cancer and its treatment.1–3 Of particular concern for older survivors is the potentially elevated risk for cognitive decline.4–6
Sleep disturbance has been identified as a risk factor for the development of cognitive decline and Alzheimer’s disease in non-cancer populations.7–10 Likewise, a number of genetic vulnerabilities for cognitive decline in later life have been identified, including variants in the APOE,11,12 COMT,13,14 and BDNF15–17 genes. Prior research has proposed that these genetic variants increase risk for cognitive decline in general populations and in cancer survivors post-treatment, and that specific vulnerability factors may interact in accelerating this risk (i.e., a gene by environment interaction).12,14 One salient factor that could influence this risk is poor sleep.15,18,19 Despite these links, limited research has tested the interaction of genotype with sleep disturbance in predicting risk for cognitive decline,18,19 with no research to date testing these relationships in cancer survivors. Indeed, the contribution of sleep to cognitive declines and genetic vulnerability among cancer survivors remains relatively unexplored, despite the observation that sleep disturbance is highly prevalent among cancer survivors, with incidence of insomnia in cancer survivors two- to three-fold greater than rates among similarly aged controls.20,21 Further, over half of survivors report sustained difficulties in sleeping,20 and a similar prevalence of genetic risk alleles for cognitive decline has been observed in cancer survivors.23 Moreover, the majority of sleep disturbances are treatable, with evidence that a number of behavioral treatments are efficacious in cancer patient populations.22,23 Thus, knowledge about the relationship of sleep disturbance, genotype, and cognitive function among cancer survivors could inform clinical care of this growing segment of the survivor population.
In the present analyses, we used data from the Thinking and Living with Cancer (TLC) Study,24 a multi-site cohort study of older breast cancer survivors followed prospectively for two years, to fill these clinical gaps. We tested the hypothesis that sleep disturbance would be associated with lower objective neurocognitive performance and subjective reports of decreased cognitive functioning. We further tested whether the effects of sleep disturbance on cognition varied by polymorphisms in genes associated with vulnerability for cognitive decline (APOE, BDNF, and COMT). The results are intended to inform survivorship care and suggest future avenues for mechanistic research and interventions to improve cognitive outcomes among cancer survivors.
Methods
This study was conducted at Georgetown University and its affiliated practices, Memorial Sloan Kettering Cancer Center, Moffitt Cancer Center, City of Hope Comprehensive Cancer Center, Hackensack University Medical Center, Indiana University (IU) School of Medicine, and University of California at Los Angeles (UCLA). IU (participant recruitment and laboratory support) and UCLA (laboratory support) joined the study in 2016, so data in this report are from the five other sites. All Institutional Review Boards approved the protocol ().
Population
Newly diagnosed English-speaking female breast cancer patients ages 60+ with Stage 0–3 cancers were recruited to participate and are followed prospectively with subsequent visits occurring 12 and 24 later. We included 345 women recruited between August 1, 2010 and December 31, 2015. The study is ongoing, and has been described elsewhere.24 Briefly, women with a history of stroke, head injury, major Axis I psychiatric disorder, or neurodegenerative disorder were ineligible. Those with a history of other cancers were excluded if they received active treatment <5 years prior to enrollment or ever had chemotherapy or hormonal therapy. Among eligible cases, 355 consented. Additional screening of eligible participants included the Mini-Mental State Examination (MMSE) and the Wide Range Achievement Test, 4th edition (WRAT-4) Reading subtest. Those with MMSE <24 or WRAT-4 Reading <3rd grade level were deemed ineligible (n=1). An additional 5 withdrew consent, 1 had a recurrence. We did not include non-cancer controls in this investigation, since we were interested in the experiences of survivors, and identification of remediable risks for cognitive problems that could be addressed in survivorship care.
Data Collection
Neuropsychological testing and interviews were completed at the baseline visit and 12 and 24 months later. Biosamples for genotype determination were obtained at baseline or in some cases on follow-up visits. The baseline assessment was completed after cancer-related surgery, but before initiation of systemic chemotherapy, radiation, or hormone treatments.
A total of thirteen neuropsychological tests were administered to measure two domains of cognitive function: attention, processing speed, and executive function and learning and memory.25 Raw neuropsychological test scores were converted to z-scores by standardizing to age- and education-group-matched non-cancer control means and standard deviations. Standardized z-scores were then calculated for each domain.25
Perceived cognitive function was assessed using the Functional Assessment of Cancer Therapy: Cognitive Function (FACT-Cog) test.26 The presence of a sleep disturbance (yes/no) was determined from endorsement of one or both of two questions: “During the last 7 days, I have been sleeping well”, with subjects reporting “not at all” and “a little bit” coded as having a sleep disturbance; “During the past week my sleep was restless”, with subjects reporting “occasionally or moderate amount of time” or “most or all the time” coded as having a sleep disturbance. We used data from a separate sample of women from the same study who were enrolled after 2016 when the Pittsburgh Sleep Quality Index (PSQI)27 was added to the protocol to validate the two-item assessment of sleep disturbance against the PSQI. In analyses of 663 data points derived from this sample of women enrolled after the PSQI was added to the protocol we found good concordance between these measures of sleep, with median PSQI global scores of 9 in those categorized as having a sleep disturbance using the two-item measure, and a median score of 4 for those without a sleep disturbance. The PSQI was not administered to the current cohort of women, and was therefore not available for our analyses of sleep and cognitive function.
DNA from blood or saliva (Oragene kit; DNA Genotek, Kanata, Ontario, Canada) was tested to determine genotypes. Biospecimens were processed centrally and tested in batches; 100% of the specimens had sufficient DNA for analysis. DNA was assayed with genome-wide association study (GWAS) Affymetrix Axiom Precision Medicine arrays at the Children’s Hospital of Philadelphia, Center for Applied Genomics. Data from the arrays were converted to PLINK format and cleaned, and quality control was performed. Genetic sex was confirmed as female for all samples, and subjects with <95% call rate were removed (N=1). For this analysis, candidate SNP genotypes were obtained for COMT (rs4680 c.472G>A Val158Met) and BDNF (rs6265 C.196G>A Val66Met). APOE genotypes (rs429358 determining whether subject is an epsilon 4 carrier) were also obtained from DNA for all samples via independent SNP testing. Further detail on assays have been described previously.24
Statistical Analysis
T-tests and chi-square tests were used to compare characteristics of those with sleep disturbance vs. not and evaluate potential confounders of associations between sleep and cognition.
We used random effects fluctuation models to test the hypothesis that sleep disturbance would be associated with lower objective neurocognitive performance and subjective report of decreased cognitive functioning, and to further test whether the effects of sleep disturbance on cognition varied by polymorphisms in genes associated with vulnerability for cognitive decline. Genotype polymorphisms were analyzed as having the allele (vs. not) previously reported to be associated with cognitive decline, including APOE epsilon 4 carrier vs non-carrier, BDNF Met allele carrier vs. Val/Val homozygote, or COMT Val allele carrier vs Met/Met homozygote.
The random effects fluctuation models tested the effect of sleep disturbance on cognitive domain scores and perceived cognitive functioning using data from the three observation points (baseline, 12, and 24 months). In each model, sleep disturbance was included as a between-person predictor and a within-person predictor.28 The between-person predictor was defined as a participant’s average sleep disturbance over the three time points relative to the average of other participants. For between-person differences, sleep disturbance was compared across participants depending upon whether it was present on none, one, two or all three of the measurement points. The within-person predictor was a time-varying predictor that indexed whether a participant had a sleep disturbance at a given time point compared to their average. For the within-person comparisons, the estimates were based upon whether a sleep disturbance was present or absent at any of the measurement points for each individual. To adjust for multiple comparisons, we applied the Benjamini-Hochberg29 method to estimate the adjusted P value using a False Discovery Rate, q=.05.
All models controlled for age, WRAT-4 Reading score, race, site, and treatment (chemotherapy+/−hormonal therapy vs. hormonal therapy); treatment-sleep interactions were not significant, so were not included in the models. Main effect of genotype and genotype-sleep disturbance interaction were tested using separate models for each genotype. We used the coefficients from the regression models to calculate the adjusted mean cognitive score and graphically depict significant genotype-sleep interactions.
In sensitivity analyses, we tested whether conclusions varied if we used the Likert responses to the two-sleep items as a continuous scale score. Since results were unchanged, we present results for the categorical sleep disturbance measure for ease of interpretation of interactions. We also evaluated other covariates for their effects on the relationship between sleep variables on cognition and sleep-genotype interactions, including frailty, comorbidities, anxiety, depressive symptoms (CES-D), and sleep medications. All analyses were done using SAS version 9.4.
Results
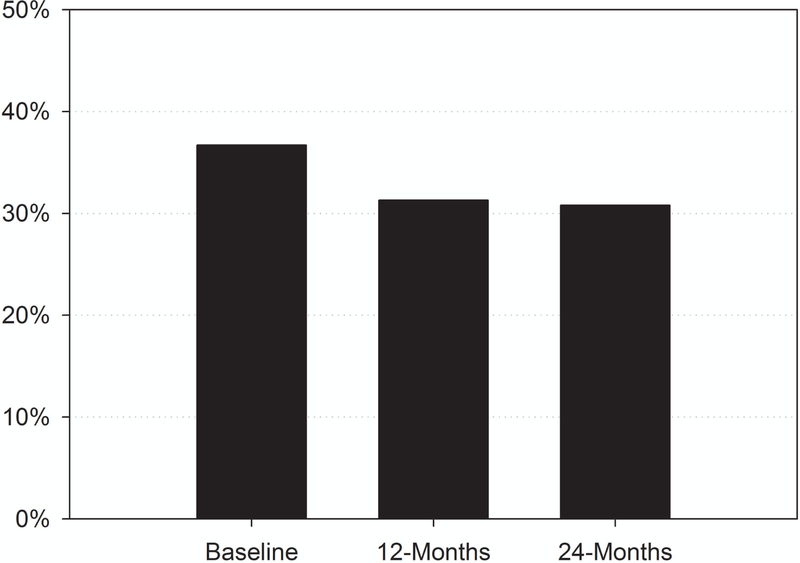
At any one time-point, one third of this sample of older breast cancer survivors reported having a sleep disturbance (Figure 1; baseline, 12, and 24 months), and many took sleep medications at baseline (Table 1). However, only 5.4% of survivors reported sleep disturbance at all 3 time points, 13% reported at two of the three, and 33% reported at only one of the time points. Sleep disturbance did not differ by treatment. The prevalence of the different genotypes did not vary by presence of sleep disturbance.
Figure 1.
Prevalence of sleep disturbances among TLC breast cancer survivors.
Table 1.
Characteristics of TLC Breast Cancer Participants
| All Mean (SD) [Range] or N (%) |
Sleep Disturbance |
No Sleep Disturbance |
P Value | ||
|---|---|---|---|---|---|
| Sociodemographic | |||||
| Age | 68.1 (6.1) [60–98] | 67.2 (5.4) [60–83] | 68.8 (6.5) [60–98] | 0.03 | |
| Race | 0.73 | ||||
| Other | 73 (21.2) | 23 (19.7) | 43 (21.3) | ||
| White (Non-hispanic) | 271 (78.8) | 94 (80.3) | 159 (78.7) | ||
| Education (years) | 15.1 (2.2) [9–18] | 15.1 (2.0) [10–18] | 15.2 (2.3) [9–18] | 0.75 | |
| WRAT 4 Standardized Score | 110.9 (15.4) | 109.3 (15.6) | 112.3 (15.2) | 0.10 | |
| Clinical | |||||
| AJCC stage | Stage 0 | 41 (12.0) | 12 (10.3) | 23 (11.4) | 0.66 |
| Stage 1 | 190 (55.4) | 64 (55.2) | 114 (56.4) | ||
| Stage 2 | 94 (27.4) | 36 (31.0) | 53 (26.2) | ||
| Stage 3 | 18 (5.2) | 4 (3.4) | 12 (5.9) | ||
| ER Status | Positive | 301(87.5) | 98 (83.8) | 181 (89.6) | 0.13 |
| Negative | 43(12.5) | 19 (16.2) | 21 (10.4) | ||
| HER2 Status | Positive | 39(13.4) | 15 (15.2) | 22 (12.8) | 0.59 |
| Negative | 251(86.6) | 84 (84.8) | 150 (87.2) | ||
| Surgery | Lumpectomy Only | 44 (12.9) | 13 (11.1) | 29 (14.5) | 0.67 |
| Lumpectomy with Radiation | 145 (42.5) | 50 (42.7) | 85 (42.5) | ||
| Mastectomy | 152 (44.6) | 54 (46.2) | 86 (43.0) | ||
| Comorbidity Low/High [Baseline] | <=2 Comorbidities | 168 (52.7) | 57 (48.7) | 111 (55.0) | 0.28 |
| >2 Comorbidities | 151 (47.3) | 60 (51.3) | 91 (45.0) | ||
| Frailty (Robust, Pre-frail, Frail) [Baseline] | Frail | 13 (4.1) | 9 (7.7) | 4 (2.0) | 0.04 |
| Pre-frail | 68 (21.5) | 26 (22.2) | 42 (21.0) | ||
| Robust | 236 (74.4) | 82 (70.1) | 154 (77.0) | ||
| Sleep Medication Use | 62 (19.8) | 39 (33.3) | 23 (11.7) | <.0001 | |
| Genotypes | |||||
| APOE e4+ ● | 63 (20.3) | 26 (23.9) | 31 (16.8) | 0.14 | |
| APOE e4 Negative | 248 (79.9) | 83 (76.1) | 153 (83.2) | ||
| BDNF Val/Val | 191 (64.7) | 70 (68.8) | 109 (60.9) | 0.19 | |
| BDNF Met/Val or Met/Met● | 104 (35.3) | 32 (31.4) | 70 (39.1) | ||
| COMT Met/Met | 58 (20.2) | 22 (22.0) | 33 (19.1) | 0.56 | |
| COMT Val/Met or Val/Val● | 229 (29.8) | 78 (78.0) | 140 (80.9) | ||
Note: Risk genotypes indicated with ●
Attention, Processing Speed, and Executive Function
Attention, processing speed, and executive function scores were not significantly associated with sleep disturbance or genetic polymorphisms (p <.10, >.025) (Table 2).
Table 2.
Adjusted associations of fluctuations in sleep disturbances and cognitive performance in older breast cancer patients over time and interactions of sleep effects with genotype
| Sleep Disturbance Predicting Attention, Processing, Executive Function | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N=319 | N=292 +APOE |
N=277 +BDNF |
N=269 +COMT |
|||||||||
| Variable | Est | SE | p | Est | SE | p | Est | SE | p | Est | SE | p |
| Between Person Sleep | 0.10 | 0.09 | 0.268 | 0.11 | 0.11 | 0.292 | 0.02 | 0.12 | 0.846 | 0.41 | 0.22 | 0.059 |
| Within Person Sleep | −0.07 | 0.04 | 0.071 | −0.07 | 0.04 | 0.087 | −0.08 | 0.05 | 0.083 | −0.13 | 0.09 | 0.115 |
| Gene | −0.07 | 0.09 | 0.435 | −0.05 | 0.08 | 0.495 | 0.14 | 0.09 | 0.123 | |||
| Gene X BP Sleep | −0.02 | 0.23 | 0.915 | 0.21 | 0.21 | 0.302 | −0.39 | 0.24 | 0.105 | |||
| Gene X WP Sleep | 0.07 | 0.10 | 0.473 | 0.09 | 0.09 | 0.297 | 0.09 | 0.09 | 0.337 | |||
| Sleep Disturbance Predicting Learning and Memory | ||||||||||||
| N=319 | N=292 +APOE |
N=277 +BDNF |
N=269 +COMT |
|||||||||
| Variable | Est | SE | p | Est | SE | p | Est | SE | p | Est | SE | p |
| Between Person Sleep | −0.06 | 0.11 | 0.614 | −0.28 | 0.13 | 0.034 | −0.22 | 0.15 | 0.133 | 0.21 | 0.26 | 0.429 |
| Within Person Sleep | −0.01 | 0.05 | 0.846 | 0.02 | 0.06 | 0.685 | 0.07 | 0.07 | 0.256 | 0.03 | 0.12 | 0.829 |
| Gene | −0.14 | 0.11 | 0.201 | 0.03 | 0.09 | 0.712 | −0.10 | 0.11 | 0.356 | |||
| Gene X BP Sleep | 0.91 | 0.28 | 0.001a | 0.38 | 0.25 | 0.135 | −0.35 | 0.30 | 0.239 | |||
| Gene X WP Sleep | −0.14 | 0.15 | 0.324 | −0.11 | 0.12 | 0.366 | 0.01 | 0.13 | 0.934 | |||
| Sleep disturbance predicting self-reported perceived cognitive function | ||||||||||||
| N=319 | N=292 +APOE |
N=277 +BDNF |
N=269 +COMT |
|||||||||
| Variable | Est | SE | p | Est | SE | p | Est | SE | p | Est | SE | p |
| Between Person Sleep | −7.26 | 2.73 | 0.008c | −6.89 | 3.18 | 0.036 | −9.82 | 3.57 | 0.006 | −10.11 | 6.47 | 0.119 |
| Within Person Sleep | −4.43 | 1.41 | 0.002b | −4.03 | 1.59 | 0.012 | −5.21 | 1.73 | 0.003 | 1.26 | 3.18 | 0.693 |
| Gene | −0.47 | 2.64 | 0.858 | −0.69 | 2.19 | 0.753 | 2.59 | 2.66 | 0.330 | |||
| Gene X BP Sleep | 1.97 | 6.88 | 0.775 | 6.54 | 6.23 | 0.295 | 4.71 | 7.24 | 0.515 | |||
| Gene X WP Sleep | −3.67 | 3.87 | 0.344 | 0.82 | 3.31 | 0.803 | −8.20 | 3.62 | 0.024d | |||
Note: Models adjusting for age, reading score, race, site, and treatment.
Notes: All models control for age, race, WRAT score, site, chemotherapy/hormone treatment group. Additional models adjusted for frailty, anxiety, depressive symptoms, or sleep medication, with no appreciable modifications to the effect of sleep disturbance.
FDR adjusted
p=.0125;
p=.017,
p=.025,
p=.05.
Learning and Memory
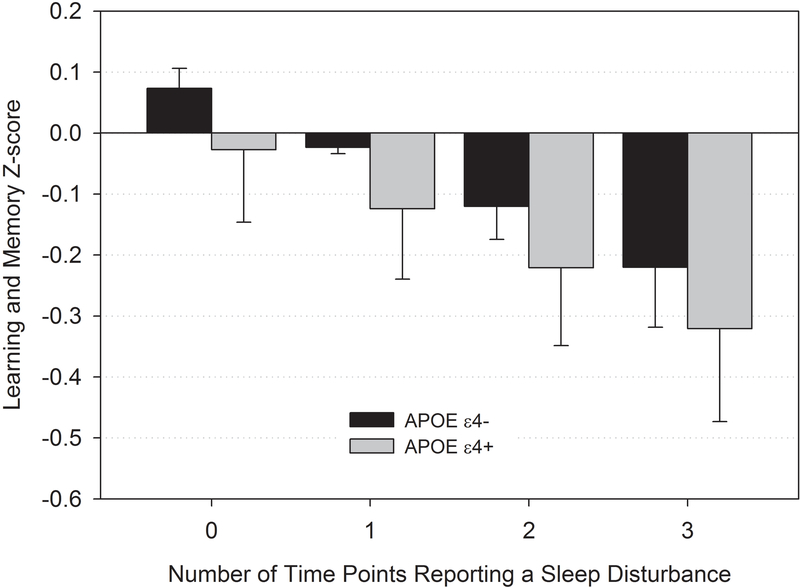
There was an interaction effect of between-person sleep disturbance and APOE genotype on the learning and memory domain (p<0.01), where those who were APOE4 carriers exhibited progressively worse learning and memory performance with greater sleep disturbance than non-carriers (Figure 2 and Table 2). Analyses of genotype-sleep interactions for BDNF and COMT were not significant (Table 2). When frailty, comorbidities, anxiety, depressive symptoms, and sleep medications were each included in the model, these variables did not change the effects of sleep disturbance on learning and memory scores or the interaction of sleep with APOE.
Figure 2.
Sleep disturbance by APOE genotype predicting learning and memory scores from pre-systemic therapy to 24 months among older breast cancer survivors.
Self-Perceived Cognition
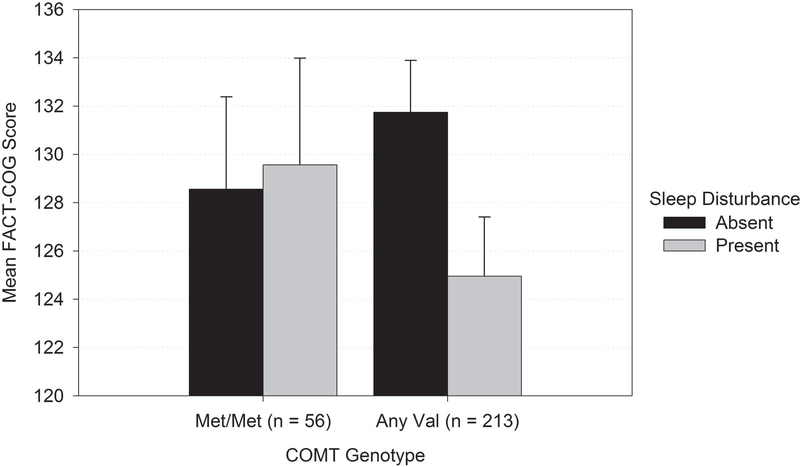
Self-reported perceived cognitive function scores were lower with both between-person and within-person sleep disturbance (p <0.01; Table 2). There was also a significant interaction of within-person sleep disturbance with having a COMT Val allele in the prediction of self-reported cognitive scores (p<0.025; Figure 3), but there were no interactions with other genotypes. Among those with a COMT Val allele, at times when the patient reported a sleep disturbance, their self-reported perceived cognitive function score was almost 7 points lower (M = 6.98, p < 0.001) than when they did not report a sleep disturbance. For persons who were homozygous for the Met allele, there was no difference in self-reported cognition as a function of within-person sleep disturbance. These relationships did not change when comorbidity, frailty, anxiety, depressive symptoms, or sleep medications were considered.
Figure 3.
Sleep disturbance by COMT genotype interaction predicting perceived cognition among older breast cancer survivors.
Discussion
This is the first large prospective cohort study to examine the relationship among sleep, genotype, and cognitive performance in older breast cancer survivors. There were important effects of sleep on cognition; these effects were most pronounced among survivors that had a genetic polymorphism associated with increased risk of neurodegenerative disease. Specifically, we found that sleep disturbance was prevalent in older breast cancer survivors and was associated with worse performance on learning and memory, particularly among carriers of the APOE4 gene. We also found that self-report of having worse than one’s usual sleep or more sleep problems than other participants was associated with lower self-reported cognitive function, and this effect interacted with the COMT gene. These results were robust after considering adjuvant systemic treatment, affective symptoms, sleep medications, and other factors.
Our findings extend the existing evidence that has linked sleep disturbance with cognitive decline and dementia risk in non-cancer populations.7–10 This prior research points to a two-hit effect of APOE4 carrier status and sleep disruption on neurofibrillary tangles and incidence of Alzheimer’s disease (AD) non-cancer populations.18 APOE4 has also been reported in association with cognitive declines in cancer patient populations.24,30,31 Our findings support a sleep-by-APOE4 carrier status interaction effect on learning and memory in older breast cancer survivors and suggests potential parallels between cancer-related cognitive decline and AD.
Polymorphisms in the COMT gene have been associated with AD and with cancer-related cognitive declines.13,14 Our findings extend this research to show a COMT-by-sleep interaction in self-reported cognition, with a clinically meaningful difference32 of 7 points in Fact-Cog scores. The COMT gene regulates the production of an enzyme that catabolizes catecholamine, with Val carriers having increased production of this enzyme.33 Sleep deprivation is associated with increased catecholamine release,34,35 which may be exacerbated in COMT Val carriers. One hypothesis is that sleep disturbance in Val carriers may deplete catecholamine reserves, resulting in an altered perception of cognitive function with a perceived inability to perform. While we did not find any interactions of sleep with BDNF genotype in this cancer survivor cohort, this genotype has been shown to interact with sleep in effects on cognition in non-cancer patients. Taken together, our results suggest that sleep may be particularly important for memory among cancer survivors with polymorphisms that are known to increase risk of dementias.19 Future exploration of a wider array of genotypes could advance our knowledge about mechanisms of cancer-related cognitive decline.
Sleep may impact cognitive decline of cancer survivors through a number of pathways. Mechanistically, decline in cognitive function is thought to be the result of years of cumulative damage, including oxidative stress, overproduction of proteinaceous debris, and failure to clear waste products within the brain, with significant variability in susceptibility.36,37 In the case of cancer survivors, the additional exposure to chemotherapy and hormonal therapy may accelerate the accumulation of waste within the brain and drive declines in cognitive function.37,38
A critical missing piece to understanding the development of cognitive decline in both older adults and cancer survivors may be what happens when the person is not awake. Sleep appears to be critical in maintaining healthy brain function.8,9,39,40 Recent evidence has demonstrated that the sleeping brain actively clears metabolic debris and neurotoxic waste, including amyloid β, through the glymphatic system (the brain-wide system that exchanges cerebrospinal fluid and interstitial fluid), providing a mechanistic pathway through which disturbance in sleep could result in lasting “aging” effects on the brain and declines in cognition.41,42 Future work should consider sleep among cancer survivors, as it may be an important behavioral target that might protect from cognitive decline by enabling waste clearance.
There are several potential implications of our results for clinical care. First, sleep disturbance is treatable, and common behavioral treatments have established efficacy in cancer patient populations.22 Although our findings are correlative, not causal, additional data in non-cancer populations supports the possibility of causal relationships.7–10 Failure to assess and treat patients for sleep disturbance could have potential long-term impact on their risk for later cognitive difficulties. Sleep medications may be insufficient to address these concerns. In our patient sample who reported a sleep disturbance, roughly a third also took sleep medications, and adjustment in our analyses for sleep medication did not alter the findings. The decrements in perceived cognition seen in association with sleep disturbance in the present study were clinically meaningful, and indicated small to moderate effects, which parallel similar findings in patients with insomnia.43 Sleep also has a significant impact on other important patient outcomes, including mood, fatigue, quality of life, risk for secondary cancers, cardiovascular disease, metabolic syndrome, diabetes, and biological aging.23,44–53 While the findings in the current study are preliminary, the results support screening for and addressing patient complaints of sleep disturbance as a typical clinical and preventative behavioral health target, similar to diet, exercise, and smoking.
Several caveats of this work should be recognized. First, these analyses were planned after data collection had commenced for the larger cohort. As a consequence, the measurement of sleep disturbance was determined using available questionnaire items. Here we report that 30% of our sample complained of disturbed sleep at baseline before systemic therapy initiation and up to 2 years after diagnosis. These estimates are slightly lower than prior reports of insomnia symptoms,20 suggesting there may be some underreporting using our measure. However, in our analyses of data in a new cohort of women with both PSQI sleep data and our currently used two-item measure of sleep disturbance we found good concordance, with differences in median global sleep scores on the PSQI in those categorized as sleep disturbed vs. not. Future work should consider better characterization of sleep in cancer survivors using validated objective and subjective methods for assessing the presence of sleep disturbance. Likewise, we cannot rule out the possibility that sleep apnea contributed to the estimates of sleep disturbance and cognitive decline, as we did not have a measure of sleep apnea. An alternative explanation for the results is also possible, that neurological functional declines impact the ability of the brain to maintain a sleeping state, and self-reported sleep disturbance may serve as an early symptom of subsequent cognitive deficits. Future longitudinal research is needed to disentangle such sequential effects. Last, statistical power was limited for detection of genotype interaction effects and these should be investigated in larger samples.
In summary, we report that sleep disturbance was common and related to worse self-perceived cognitive functioning and learning and memory performance in older breast cancer survivors. APOE4 carriers with sleep disturbance were particularly vulnerable to decrements in learning and memory, while COMT Val carriers were more likely to perceive cognitive difficulties when sleep disturbance was present. Assessment of sleep may be important in survivorship care and as an intervention target. Future models for survivorship6 care should include sleep disturbance as part of behavioral assessments and as a treatment target in cancer survivors.
Acknowledgments
This work was conducted while Dr. Jacobsen was Associate Center Director, Division of Population Science, Moffitt Cancer Center, Tampa, FL.
This research was supported by the National Cancer Institute at the National Institutes of Health grants #R01CA129769 and R35 CA197289 to JM. This study was also supported in part by the National Cancer Institute at the National Institutes of Health grant #P30CA51008 to Lombardi Comprehensive Cancer Center for support of the Biostatistics and Bioinformatics Shared Resources at Lombardi Comprehensive Cancer Center.
The work of AJS and BCM was supported in part by the National Institutes of Health grants P30AG10133, R01AG19771 and #R01LM01136. The work of TAA was supported in part by NIH grants R01CA172119 and a Cancer Center Core grant (P30 CA008748). The work of DT was supported by the National Cancer Institute at the National Institutes of Health grant #T32CA117865. Dr. Carroll is supported by an American Cancer Society Research Scholars grant 128660-RSG-15–187-01-PCSM. DT is supported by grant F31CA220964.
Dilawari: Cardinal Health (Advisory board)
Extermann: GTx (research support)
Hurria: Seattle Genetics, Amgen Pharmaceuticals, and Genentech (consultation);
Glaxo Smith Kline, Abraxis Bioscience, and Celgene (research support)
Isaacs: Genentech (consulting and speakers bureau), Pfizer (consulting and speakers bureau), AstraZeneca (consulting and speakers bureau), Novartis (consulting), Nanostring (consulting)
Stern: Athena Diagnostics (consultant); Psychological Assessment Resources, Inc. (royalties received for tests, including the Neuropsychological Assessment Battery)
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of Interest:
All remaining authors have declared no conflicts of interest.
References
- 1.Bluethmann SM, Mariotto AB, Rowland JH. Anticipating the ‘Silver Tsunami’: Prevalence Trajectories and Comorbidity Burden among Older Cancer Survivors in the United States. Cancer Epidemiol Biomarkers Prev 2016; 25: 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin; 62: 220–41. [DOI] [PubMed] [Google Scholar]
- 3.Runowicz CD, Leach CR, Henry NL, Henry KS, Mackey HT, Cowens-Alvarado RL et al. American Cancer Society/American Society of Clinical Oncology Breast Cancer Survivorship Care Guideline. J Clin Oncol 2016; 34: 611–635. [DOI] [PubMed] [Google Scholar]
- 4.Mandelblatt JS, Hurria A, McDonald BC, Saykin AJ, Stern RA, VanMeter JW et al. Cognitive Effects of Cancer and Its Treatments at the Intersection of Aging: What Do We Know; What Do We Need to Know? Semin Oncol 2013; 40: 709–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganz PA, Kwan L, Castellon SA, Oppenheim A, Bower JE, Silverman DHS et al. Cognitive complaints after breast cancer treatments: examining the relationship with neuropsychological test performance. J Natl Cancer Inst 2013; 105: 791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment-associated cognitive change: an update on the state of the science. J Clin Oncol 2012; 30: 3675–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bubu OM, Brannick M, Mortimer J, et al. Sleep, cognitive impairment, and Alzheimer’s disease: a systematic review and meta-analysis.. Sleep 2017; 40 zsw032. doi: 10.1093/sleep/zsw032. [DOI] [PubMed] [Google Scholar]
- 8.Van Someren EJW, Cirelli C, Dijk D-J, Van Cauter E, Schwartz S, Chee MWL. Disrupted Sleep: From Molecules to Cognition. J Neurosci 2015; 35: 13889–13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lo JC, Groeger JA, Cheng GH, Dijk D-J, Chee MWL. Self-reported sleep duration and cognitive performance in older adults: a systematic review and meta-analysis. Sleep Med 2016; 17: 87–98. [DOI] [PubMed] [Google Scholar]
- 10.Kang DW, Lee CU, Lim HK. Role of Sleep Disturbance in the Trajectory of Alzheimer’s Disease. Clin Psychopharmacol Neurosci 2017; 15: 89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonomini F, Filippini F, Hayek T, Aviram M, Keidar S, Rodella LF et al. Apolipoprotein E and its role in aging and survival. Exp Gerontol 2010; 45: 149–157. [DOI] [PubMed] [Google Scholar]
- 12.Small BJ, Rosnick CB, Fratiglioni L, Bäckman L. Apolipoprotein E and Cognitive Performance: A Meta-Analysis. Psychol Aging 2004; 19: 592–600. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg TE, Egan MF, Gscheidle T, Coppola R, Weickert T, Kolachana BS et al. Executive Subprocesses in Working Memory. Arch Gen Psychiatry 2003; 60: 889. [DOI] [PubMed] [Google Scholar]
- 14.Small BJ, Rawson KS, Walsh E, Jim HSL, Hughes TF, Iser L et al. Catechol-O-methyltransferase genotype modulates cancer treatment-related cognitive deficits in breast cancer survivors. Cancer 2011; 117: 1369–76. [DOI] [PubMed] [Google Scholar]
- 15.Gosselin N, De Beaumont L, Gagnon K, Baril A-A, Mongrain V, Blais H et al. BDNF Val66Met Polymorphism Interacts with Sleep Consolidation to Predict Ability to Create New Declarative Memories. J Neurosci 2016; 36: 8390–8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaieb L, Antal A, Ambrus GG, Paulus W. Brain-derived neurotrophic factor: its impact upon neuroplasticity and neuroplasticity inducing transcranial brain stimulation protocols. Neurogenetics 2014; 15: 1–11. [DOI] [PubMed] [Google Scholar]
- 17.Voineskos AN, Lerch JP, Felsky D, Shaikh S, Rajji TK, Miranda D et al. The Brain-Derived Neurotrophic Factor Val66Met Polymorphism and Prediction of Neural Risk for Alzheimer Disease. Arch Gen Psychiatry 2011; 68: 198. [DOI] [PubMed] [Google Scholar]
- 18.Lim ASP, Yu L, Kowgier M, Schneider JA, Buchman AS, Bennett DA. Modification of the relationship of the apolipoprotein E ε4 allele to the risk of Alzheimer disease and neurofibrillary tangle density by sleep. JAMA Neurol 2013; 70: 1544–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gosselin N, De Beaumont L, Gagnon K, Baril A-A, Mongrain V, Blais H et al. BDNF Val66Met Polymorphism Interacts with Sleep Consolidation to Predict Ability to Create New Declarative Memories. J Neurosci 2016; 36: 8390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol 2001; 19: 895–908. [DOI] [PubMed] [Google Scholar]
- 21.Liou KT, Ahles TA, Garland SN, Li QS, Bao T, Li Y et al. The relationship between insomnia and cognitive impairment in breast cancer survivors. JNCI Cancer Spectr [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson JA, Rash JA, Campbell TS, Savard J, Gehrman PR, Perlis M et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev 2016; 27: 20–28. [DOI] [PubMed] [Google Scholar]
- 23.Garland SN, Johnson JA, Savard J, Gehrman P, Perlis M, Carlson L et al. Sleeping well with cancer: a systematic review of cognitive behavioral therapy for insomnia in cancer patients. Neuropsychiatr Dis Treat 2014; 10: 1113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mandelblatt JS, Small BJ, Luta G, Hurria A, Jim H, McDonald BC et al. Cancer-Related Cognitive Outcomes Among Older Breast Cancer Survivors in the Thinking and Living With Cancer Study. J Clin Oncol 2018; : JCO1800140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clapp JD, Luta G, Small BJ, Ahles TA, Root JC, Graham D et al. The Impact of Using Different Reference Populations on Measurement of Breast Cancer-Related Cognitive Impairment Rates. Arch Clin Neuropsychol 2018. doi: 10.1093/arclin/acx142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wagner LI, Sweet J, Butt Z, Lai J, Cella D. Measuring Patient Self-Reported Cognitive Function: Development of the Functional Assessment of Cancer Therapy-Cognitive Function Instrument. J Support Oncol 2009; 7: W32–39. [Google Scholar]
- 27.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 1989; 28: 193–213. [DOI] [PubMed] [Google Scholar]
- 28.Hoffman L. Longitudinal analysis: Modeling within-person fluctuation and change. Routledge: New York, 2015. [Google Scholar]
- 29.Benjamini Y, Hochberg Y. On the Adaptive Control of the False Discovery Rate in Multiple Testing With Independent Statistics. J Educ Behav Stat Spring 2000; 25: 60–83. [Google Scholar]
- 30.Ahles TA, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, Cole B et al. The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology 2003; 12: 612–619. [DOI] [PubMed] [Google Scholar]
- 31.Ahles TA, Li Y, McDonald BC, Schwartz GN, Kaufman PA, Tsongalis GJ et al. Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: the impact of APOE and smoking. Psychooncology 2014; 23: 1382–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheung YT, Foo YL, Shwe M, Tan YP, Fan G, Yong WS et al. Minimal clinically important difference (MCID) for the functional assessment of cancer therapy: Cognitive function (FACT-Cog) in breast cancer patients. J Clin Epidemiol 2014; 67: 811–820. [DOI] [PubMed] [Google Scholar]
- 33.Dauvilliers Y, Tafti M, Landolt HP. Catechol-O-methyltransferase, dopamine, and sleep-wake regulation. Sleep Med Rev 2015; 22: 47–53. [DOI] [PubMed] [Google Scholar]
- 34.Faraut B, Nakib S, Drogou C, Elbaz M, Sauvet F, De Bandt J-P et al. Napping Reverses the Salivary Interleukin-6 and Urinary Norepinephrine Changes Induced by Sleep Restriction. J Clin Endocrinol Metab 2015; 100: E416–E426. [DOI] [PubMed] [Google Scholar]
- 35.Irwin MR, Ziegler M. Sleep deprivation potentiates activation of cardiovascular and catecholamine responses in abstinent alcoholics. Hypertension 2005; 45: 252–257. [DOI] [PubMed] [Google Scholar]
- 36.Chinta SJ, Woods G, Rane A, Demaria M, Campisi J, Andersen JK. Cellular senescence and the aging brain. Exp Gerontol 2015; 68: 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat Rev Cancer 2007; 7: 192–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scuric Z, Carroll JE, Bower JE, Ramos-Perlberg S, Petersen L, Esquivel S et al. Biomarkers of aging associated with past treatments in breast cancer survivors. npj Breast Cancer 2017; 3: 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Santhi N, Lazar AS, McCabe PJ, Lo JC, Groeger JA, Dijk D-J. Sex differences in the circadian regulation of sleep and waking cognition in humans. Proc Natl Acad Sci 2016; 113: E2730–E2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raven F, Van der Zee EA, Meerlo P, Havekes R. The role of sleep in regulating structural plasticity and synaptic strength: Implications for memory and cognitive function. Sleep Med Rev 2017. doi: 10.1016/j.smrv.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 41.Iliff JJ, Lee H, Yu M, Feng T, Logan J, Nedergaard M et al. Brain-wide pathway for waste clearance captured by contrast-enhanced MRI. J Clin Invest 2013; 123: 1299–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iliff JJ, Nedergaard M. Is there a cerebral lymphatic system? Stroke 2013; 44: S93–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cole SW. Elevating the perspective on human stress genomics. Psychoneuroendocrinology 2010; 35: 955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palesh O, Aldridge-Gerry A, Zeitzer JM, Koopman C, Neri E, Giese-Davis J et al. Actigraphy-measured sleep disruption as a predictor of survival among women with advanced breast cancer. Sleep 2014; 37: 837–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Irwin MR, Olmstead RE, Ganz PA, Haque R. Sleep disturbance, inflammation and depression risk in cancer survivors. Brain Behav Immun 2013; 30 Suppl: S58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cappuccio FP, Cooper D, D’Elia L, Strazzullo P, Miller MA. Sleep duration predicts cardiovascular outcomes: a systematic review and meta-analysis of prospective studies. Eur Heart J 2011; 32: 1484–92. [DOI] [PubMed] [Google Scholar]
- 47.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep 2010; 33: 585–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ju S-Y, Choi W-S. Sleep duration and metabolic syndrome in adult populations: a meta-analysis of observational studies. Nutr Diabetes 2013; 3: e65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Quantity and quality of sleep and incidence of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 2010; 33: 414–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carroll JE, Irwin MR, Levine M, Seeman TE, Absher D, Assimes T et al. Epigenetic Aging and Immune Senescence in Women With Insomnia Symptoms: Findings From the Women’s Health Initiative Study. Biol Psychiatry 2017; 81: 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carroll JE, Esquivel S, Goldberg A, Seeman TE, Effros RB, Dock J et al. Insomnia and Telomere Length in Older Adults. Sleep 2016; 39: 559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carroll JE, Seeman TE, Olmstead R, Melendez G, Sadakane R, Bootzin R et al. Improved sleep quality in older adults with insomnia reduces biomarkers of disease risk: Pilot results from a randomized controlled comparative efficacy trial. Psychoneuroendocrinology 2015; 55: 184–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Savard J, Simard S, Ivers H, Morin CM. Randomized Study on the Efficacy of Cognitive-Behavioral Therapy for Insomnia Secondary to Breast Cancer, Part I: Sleep and Psychological Effects. J Clin Oncol 2005; 23: 6083–6096. [DOI] [PubMed] [Google Scholar]