Abstract
Previous studies have identified reduced heart rate variability (HRV) in posttraumatic stress disorder (PTSD), which may temporally precede the onset of the disorder. A separate line of functional neuroimaging research in PTSD has consistently demonstrated hypoactivation of the ventromedial prefrontal cortex (vmPFC), a key aspect of a descending neuromodulatory system that exerts inhibitory control over heart rate. No research to date, however, has simultaneously investigated whether altered vmPFC activation is associated with reduced HRV and elevated PTSD symptoms in the same individuals. Here, we collected fMRI data during alternating conditions of threat of shock and safety from shock in 51 male, combat-exposed veterans with either high or low levels of PTSD symptoms. Pulse rate variability (PRV) - an HRV surrogate calculated from pulse oximetry - was assessed during a subsequent resting scan. Correlational analyses tested for hypothesized relationships between reduced vmPFC activation, lower PRV, and elevated PTSD symptomatology. We found that PTSD re-experiencing symptoms were inversely associated with high-frequency (HF)-PRV, thought to primarily reflect parasympathetic control of heart rate, in veterans with elevated PTSD symptoms. Reduced vmPFC activation for the contrast of safety-threat was associated both with lower HF-PRV and elevated PTSD re-experiencing symptoms. These results tie together previous observations of reduced HRV/PRV and impaired vmPFC function in PTSD and call for further research on reciprocal brain-body relationships in understanding PTSD pathophysiology.
1. Introduction
Trauma is an embodied experience. In addition to the psychological consequences of trauma, somatic symptoms cause extreme distress in posttraumatic stress disorder (PTSD) and pose a substantial hurdle in the treatment of and recovery from trauma (van der Kolk, 2014). In spite of this, etiological theories of PTSD – such as dominant fear learning perspectives – typically emphasize neural mechanisms and treat somatic symptoms as epiphenomenal. The investigation of relationships between the brain and periphery, and how these relationships are altered in trauma-exposed individuals, may help elucidate novel candidate mechanisms of PTSD etiology.
One way to learn more about such mechanisms is by assessing peripheral psychophysiology in conjunction with brain imaging techniques. A peripheral index of particular interest in this regard is resting heart rate variability (HRV), a reliable measure (Guijt, Sluiter, & Frings-Dresen, 2007) thought to reflect a trait-like index of adaptive regulatory control of autonomic function by central mechanisms (Thayer & Lane, 2000). Of particular interest is high-frequency (HF)-HRV, the parasympathetically dominated component of HRV tied to the respiration cycle (typically in the frequency of 0.12–0.40 or 0.15–0.40 Hz; Allen, Chambers, & Towers, 2007). Consistent with a role for HRV in flexible regulatory control, reduced HRV is seen in psychiatric disorders marked by deficient inhibitory control of emotional and physiological responding, including depression (Kemp et al., 2010) and anxiety disorders (Chalmers, Quintana, Abbott, & Kemp, 2014). A meta-analysis of 19 studies comparing PTSD patients to controls demonstrated reduced HRV in PTSD, particularly for the parasympathetically dominant HF-HRV (Nagpal, Gleichauf, & Ginsberg, 2013). Further, two large studies in pre-deployment soldiers found that reduced HF-HRV prior to combat exposure (or, similarly, a smaller HF/low-frequency ratio) predicted post-deployment PTSD symptoms (Minassian et al., 2015; Pyne et al., 2016).
While brainstem regions are directly involved in regulating heart rate and other autonomic processes, translational evidence points to a critical role for the medial prefrontal cortex (mPFC), and the ventromedial prefrontal cortex (vmPFC) in particular, in higher-level autonomic control triggered by motivational and contextual demands. Rat infralimbic cortex, which may be functionally analogous to primate vmPFC, has dense projections to regions directly involved in autonomic control including the nucleus tract of the solitarius, a critical brainstem region for vagal output (Gabbott, Warner, Jays, Salway, & Busby, 2005). Lesion and electrical stimulation studies provide further evidence for a causal influence of rodent mPFC, particularly vmPFC, in parasympathetic control of heart rate (McKlveen, Myers, & Herman, 2015; Resstel, Fernandes, & Correa, 2004). Lesion studies in marmosets highlight a specific role for vmPFC in regulating vagally mediated HRV (Wallis, Cardinal, Alexander, Roberts, & Clarke, 2017), and a study in humans found reduced HRV with lesions to the mPFC (Buchanan et al., 2010). In addition to this translational evidence for a causal link between vmPFC and HRV/parasympathetic control, a meta-analysis of correlational human neuroimaging data found greater vmPFC activation to be consistently associated with increased heart rate variability (Thayer et al., 2012). The authors suggested that descending projections from the vmPFC and other aspects of a descending “visceromotor system” have a critical regulatory role on the autonomic nervous system and support context-appropriate threat responding, in part through greater HRV.
Notably, the vmPFC has also been extensively implicated in the pathophysiology of PTSD, as underscored by quantitative meta-analyses of functional neuroimaging research comparing patients and controls (Hayes, Hayes, & Mikedis, 2012; Stark et al., 2015). In particular, reduced activation of the vmPFC during extinction recall or in response to cues representing safety has been noted in PTSD (Garfinkel et al., 2014; Grupe, Wielgosz, Davidson, & Nitschke, 2016; Milad et al., 2009). Collectively, these data suggest that diminished vmPFC activity may lead both to reduced HRV and elevated PTSD symptoms in trauma-exposed individuals. Because HRV is proposed to index physiological flexibility in response to changing environmental demands, vmPFC dysfunction and consequently reduced HRV could compromise traumatized individuals’ ability to respond adaptively across dynamic contexts, resulting in contextually inappropriate and overgeneralized threat responding best encapsulated by hypervigilance and other symptoms of hyperarousal. Alternatively, reduced regulatory control reflected in lower levels of vmPFC activity and reduced HRV may allow unwanted traumatic memories or flashbacks to emerge (Gillie & Thayer, 2014), consistent with a broader proposed role linking lower HRV to reduced inhibitory control of thoughts, emotions, and physiology in a broad array of anxiety disorders (Chambers et al., 2014). However, the hypothesis that compromised vmPFC function and reduced HRV reflect a common mechanism in the pathology of PTSD remains somewhat theoretical, as few if any studies have simultaneously explored relationships among PTSD symptoms, vmPFC function, and HRV in the same participants.
In the current study, we investigated each of these factors in a functional MRI study of combat trauma-exposed veterans. Participants took part in an fMRI task involving alternating conditions of unpredictable threat and safety. During a subsequent resting-state scan, we collected pulse-rate data using pulse oximetry (owing to the difficulty of collecting electrocardiography data in the MRI environment) and analyzed high-frequency pulse rate variability, or HF-PRV. Importantly, PRV and HRV, which capture different physiological readouts of cardiac variability, are highly correlated during resting conditions (Hayano, Barros, Kamiya, Ohte, & Yasuma, 2005; Schäfer & Vagedes, 2013). We hypothesized that elevated PTSD symptoms would be associated with reduced resting HF-PRV (Nagpal et al., 2013). Based on the perspective that resting HRV/PRV provides an index of emotion regulatory flexibility (Porges, 1995; Thayer & Lane, 2000), and evidence linking greater HRV to increased vmPFC activation during emotional challenges (Thayer et al., 2012), we also hypothesized that greater differences in vmPFC activation between safety and threat-of-shock would be associated with greater resting HF-PRV. Finally, we examined the spatial similarity of the vmPFC region showing a relationship with HF-PRV to a vmPFC region we previously found to be associated with PTSD symptoms in the same sample (Grupe et al., 2016).
2. Method
2.1. Participants
Participants for this study were veterans of Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) who were exposed to one or more life-threatening war zone trauma events during deployment. These individuals were recruited through online and community advertisements and in collaboration with the Madison VA Hospital, the Madison Veterans’ Center, the Wisconsin National Guard, and other veterans’ organizations. We previously reported on relationships between individual PTSD symptom clusters and brain responses to unpredictable threat anticipation in this sample (Grupe et al., 2016).
Following written informed consent, a team of graduate-level trainees in clinical psychology, counseling psychology, or social work administered the Clinician-Administered PTSD Scale (CAPS; Blake et al., 1990) and Structured Clinical Interview for DSM-IV (SCID; First, Spitzer, Gibbon, & Williams, 2002) under the supervision of a licensed clinical psychologist (JBN). Exclusionary conditions included substance dependence within the past 3 months and current or past bipolar, psychotic, or cognitive disorders. Based on CAPS scores, individuals were enrolled into a combat-exposed control (CEC) group or a posttraumatic stress symptoms (PTSS) group. Members of the CEC group were free of current Axis I disorders and had CAPS scores < 10, and members of the PTSS group had PTSD symptoms occurring at least monthly with moderate intensity and CAPS scores ≥ 20. Current major depression or dysthymia was not exclusionary in the PTSS group. Current treatment with psychotropic medications (other than benzodiazepines or beta-blockers) or maintenance psychotherapy was permitted if treatment was stable for 8 weeks prior to the beginning of the study.
Although 58 participants were enrolled, we only analyzed data from male participants as the sample included only 4 women. In addition, 2 participants could not tolerate the shock and 1 exceeded the motion threshold during fMRI scanning. The final sample of 51 male veterans consisted of 17 in the CEC group and 34 in the PTSS group, 16 of whom met full PTSD diagnostic criteria and 18 of whom met criteria for only 1 or 2 of the symptom clusters (Table 1).
Table 1: Sample characteristics.
Demographic and clinical variables for all participants and results of independent samples t tests comparing participants in the posttraumatic stress symptoms (PTSS) and combat exposed control (CEC) groups.
| PTSS Mean (N=34) | SD | CEC Mean (N=17) | SD | t(49) | |
|---|---|---|---|---|---|
| Age | 30.6 | 6.8 | 31.0 | 6.4 | −0.21 |
| ln(High-Frequency PRV) | 6.9 | 1.1 | 6.5 | 1.0 | 1.29 |
| Mean Respiration Rate1 | 15.5 | 3.8 | 15.0 | 2.6 | 0.47 |
| Years since deployment | 4.7 | 2.7 | 5.6 | 2.9 | −1.02 |
| Combat Exposure Scale | 19.9 | 2.7 | 17.0 | 2.9 | 1.04 |
| CAPS | 48.4 | 11.2 | 3.9 | 2.6 | 13.69 |
| CAPS B (re-experiencing) | 11.2 | 6.8 | 0.4 | 1.2 | 8.99 |
| CAPS C (avoidance/numbing) | 17.1 | 10.1 | 0.0 | 0.0 | 9.86 |
| CAPS D (hyperarousal) | 20.1 | 7.0 | 3.5 | 2.6 | 12.25 |
Notes: bolded values = group differences at p < 0.05. PRV = pulse rate variability; CAPS = Clinician Administered PTSD Scale.
Only 44 participants had valid respiration data.
Our inclusion criteria for the PTSS group initially required more severe PTSD symptoms (CAPS ≥ 40), but difficulty meeting recruitment targets led us to relax this criterion. This resulted in a non-continuous and non-normal distribution of PTSD symptoms, with a large cluster of participants at the very low end, no participants with CAPS between 10–19, and a relatively normal distribution of participants in the PTSS group. Notably, whereas symptoms in the CEC group were almost entirely restricted to the hyperarousal cluster, participants in the PTSS group had on average moderate-to-high levels of each of the 3 DSM-IV symptom clusters. Based on these symptom distributions, we elected to conduct analyses in a dimensional manner with regard to PTSD symptoms both within the entire sample and within the PTSS group alone.
2.2. fMRI task
We previously published complete details of the fMRI task in this sample (Grupe et al., 2016). Briefly, during a baseline visit within 2 weeks of the MRI scan, participants took part in a shock calibration procedure to determine a level of shock, delivered to the right ventral wrist, that was perceived as “very unpleasant, but not painful”, after which they practiced the task described below.
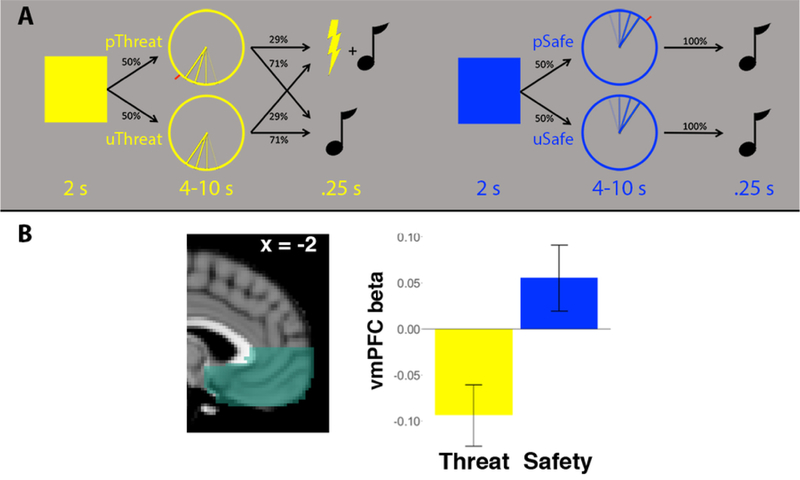
On the day of the MRI scan a single shock was delivered to confirm the shock calibration procedure, and participants took part in an instructed threat anticipation task (Figure 1A). Each trial began with a 2-s presentation of a blue or yellow square, which participants were explicitly instructed indicated threat of shock or safety from shock (counterbalanced). Next, the same color clock appeared for 4–10 s (mean duration = 7.67 s). On predictable trials, a red mark appeared in a random location and the anticipation period ended when a slowly rotating hand reached this mark. On unpredictable trials, no red mark appeared and participants could not predict the end of the anticipation period. On 12/42 threat trials, a 200-ms electric shock was delivered concurrently with a neutral tone. On all other trials, the anticipation period concluded with a 200-ms tone only. All trials were followed by a 5–9 s inter-trial interval. The scan session included 42 threat trials and 30 safe trials, split evenly between predictable/unpredictable conditions. The relatively sparse reinforcement schedule (29% of trials), comparable to previous studies examining neural responses to threat of shock (e.g., Schiller, Levy, LeDoux, Niv, & Phelps, 2008), was chosen to ensure a sufficient number of trials uncontaminated by shock, and allowed us to analyze an equivalent of non-reinforced threat and safe trials.
Figure 1. fMRI task.
(A) During the threat anticipation task, the color of a 2s square indicated threat of shock or safety from shock (counterbalanced). A subsequent 4–10s anticipation period terminated in shock on 29% of trials in the threat condition. Analyses focused on unpredictable trials (bottom row), where participants had no visual cue to indicate the termination of the anticipation period. (B) Across the anatomically defined ventromedial prefrontal cortex (vmPFC) ROI, the group as a whole showed greater activation during safe vs. threat trials (t(50) = 6.29, p < 0.001).
Given the central role of uncertainty in anxiety and trauma-related disorders, we expected more robust relationships with PTSD symptoms for unpredictable trials and focused on this condition for a priori analyses, as in Grupe et al., (2016). We contrasted parameter estimates for to the 4–10s anticipation epoch between unpredictable threat (uThreat) and unpredictable safe (uSafe) trials. In secondary analyses we ran analogous analyses for the contrast of predictable threat vs. predictable safe trials.
2.3. MRI data collection
MRI data were collected on a 3T X750 GE Discovery scanner using an 8-channel head coil and ASSET parallel imaging with an acceleration factor of 2. Data collected included 3 sets of echo planar images (EPIs) during the threat anticipation task (240 volumes/8:00, TR = 2000, TE = 20, flip angle = 60°, field of view = 220 mm, 96 × 64 matrix, 3-mm slice thickness with 1-mm gap, 40 interleaved sagittal slices), 1 set of EPIs during a subsequent resting-state scan (210 volumes/7:00) and a T1-weighted anatomical image for functional data registration. Visual stimuli were presented using Avotec fiberoptic goggles, auditory stimuli were presented binaurally using Avotec headphones, and behavioral responses were recorded using a Current Designs button box.
2.4. Psychophysiology data collection and processing
Peripheral physiological data were acquired during each of the 3 task runs and the subsequent resting-state scan; analyses here utilized data from the 7-minute resting-state scan. Pulse rate data were acquired using a pulse oximeter on the second finger of the left hand (contralateral to shock delivery), and respiration data were acquired using a belt placed at the bottom of the ribcage. All peripheral physiological data were amplified using a BIOPAC MP-150 system and digitized at 1000 Hz.
Pulse rate data were preprocessed using in-house Matlab software that automatically detected heartbeats, after which missing or extra beats were manually identified. The resulting time series of interbeat intervals (IBIs) was analyzed in CMetX (Allen et al., 2007). Detected artifacts (>300 ms difference between consecutive IBIs) were manually reviewed and rejected if determined to be artifactual. The primary outcome of interest was high-frequency pulse rate variability (HF-PRV; Schäfer & Vagedes, 2013), defined as the natural log of the band-pass filtered IBI time series between 0.12–0.40 Hz, or the typical frequency range for the respiratory cycle.
Mean respiration rate was calculated using in-house Matlab scripts. We visually inspected the respiration signal and filtered out distorted or artifactual data before extracting trough-to-trough intervals from useable data and calculating mean respiration rate (breaths/minute). We excluded participants from respiration analyses if they had less than 60s of clean data from which we could estimate respiration rate.
2.5. Data analysis
Statistical analyses were conducted in R version 3.2.2. All fMRI processing and analysis was conducted using FEAT (FMRI Expert Analysis Tool) Version 6.00, part of FSL (FMRIB’s Software Library, www.fmrib.ox.ac.uk/fsl), as fully described in our previous publication (Grupe et al., 2016).
To test hypothesis 1 (inverse relationship between PRV and PTSD symptoms), we first conducted an independent samples t test to compare HF-PRV between PTSS and CEC groups. We also calculated Pearson correlations between HF-PRV and total CAPS scores, both in the full sample (N = 51) and the PTSS group (N = 34). In follow-up analyses we tested whether a significant inverse correlation identified in the PTSS group was specific to individual symptom clusters (re-experiencing, avoidance/numbing, and hyperarousal), first by calculating Pearson correlations between each of the 3 symptom clusters, and second by simultaneous regression of HF-PRV on these 3 clusters. Because HF-PRV is coupled with respiration, we re-ran these analyses using residualized HF-PRV after regressing out resting respiration rate (due to poor respiration data quality in some participants, we could only estimate respiration rate in 29/34 PTSS and 15/17 CEC participants).
To test hypothesis 2, we conducted voxelwise correlation analysis of HF-PRV and uSafe-uThreat contrast estimates constrained to the anatomically defined vmPFC, in the full sample and the PTSS group alone. We again ran follow-up analyses using respiration-residualized HF-PRV. Repeating an analysis from a previous report in this sample linking vmPFC activation to re-experiencing symptoms of PTSD (Grupe et al., 2016), we also conducted simultaneous voxelwise regression of vmPFC contrast estimates on each of the 3 PTSD symptom clusters. The vmPFC mask consisted of medial portions of Brodmann Areas 10, 11, 12, 24, 25, and 32 ventral to the genu of the corpus callosum (generated using the Wake Forest University PickAtlas; Maldjian, Laurienti, Kraft, & Burdette, 2003). Exploratory regression analyses were conducted across the entire brain. Cluster threshold correction was applied to the vmPFC and across the whole brain using a voxelwise threshold of p < 0.001, resulting in corrected significance of p < 0.05. The cluster-forming threshold was 31 voxels for small-volume-corrected vmPFC analyses and 94–96 voxels for whole-brain analyses, depending on the specific analysis.
Non-thresholded, voxelwise statistical maps for all fMRI analyses are provided online at https://neurovault.org/collections/4544/.
3. Results
3.1. Pulse rate variability is inversely associated with PTSD re-experiencing symptoms
There was no significant difference in HF-PRV between participants in the PTSS and CEC groups (t(49) = 1.29, p = 0.20, d = 0.38), nor was there a relationship between HF-PRV and PTSD CAPS symptoms measured continuously within the full sample (r(49) = −0.05, 95% CI = [−0.32, 0.23], p = 0.72).
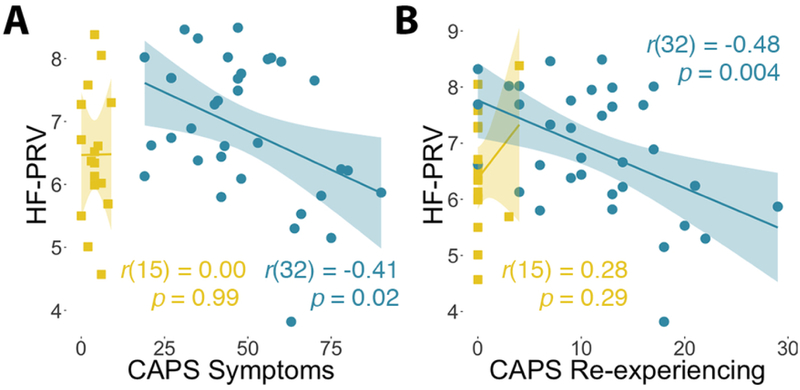
Within the PTSS group alone, there was a significant inverse relationship between HF-PRV and total CAPS symptoms (r(32) = −0.41, 95% CI = [−0.66, −0.08], p = 0.015; Figure 2a). This relationship was strongest for the re-experiencing cluster (r(32) = −0.48, 95% CI = [−0.70, −0.17], p = 0.004; Figure 2b), and was not significant for avoidance/numbing (r(32) = −0.31, 95% CI = [−0.59, 0.03], p = 0.08) or hyperarousal symptoms (r(32) = −0.19, 95% CI = [−0.50, 0.16], p = 0.28). To test the specificity of this relationship to re-experiencing symptoms, simultaneous regression of HF-PRV on these 3 symptom clusters was conducted. This revealed a significant relationship between re-experiencing symptoms and HF-PRV (t(30) = −2.39, p = 0.023), and no relationships for avoidance/numbing or hyperarousal symptoms (ts < 1, ps > 0.4).
Figure 2. PTSD symptoms and pulse rate variability.
(A) Across the entire sample, there was no correlation between total PTSD symptom severity on the Clinician-Administered PTSD Scale (CAPS) and high-frequency pulse rate variability (HF-PRV; r(49) = −0.04, p = 0.72). There was also no correlation in the control group (yellow), but there was a significant inverse correlation in the posttraumatic stress symptoms (PTSS) group (blue). (B) This inverse relationship in the PTSS group was strongest for re-experiencing CAPS symptoms.
Although our sample was enrolled on the basis of PTSD symptoms, we conducted follow-up analyses with anxiety and depression symptoms, owing to comorbidity of these conditions with PTSD and observations of reduced HRV in mood and anxiety disorders (Chalmers et al., 2014; Kemp et al., 2010). Beck Anxiety Inventory scores showed a non-significant, inverse correlation with HF-PRV in the PTSS group (r(32) = −0.27, p = 0.11) and no relationship with HF-PRV in the full sample (r(49) = −0.05, p = 0.69). Beck Depression Inventory scores were not associated with HF-PRV in the PTSS group (r(32) = −0.08, p = 0.65) or in the full sample (r(49) = 0.12, p = 0.40). Notably, greater CAPS re-experiencing symptoms were still associated with reduced HF-PRV within the PTSS group in a model including other CAPS symptoms as well as anxiety and depression symptoms, although this relationship did not meet statistical significance (t(28) = − 2.02, p = 0.053; all other ts < 1.3, ps > 0.2).
We conducted additional analyses to ensure that individual differences in respiration rate were not driving relationships between HF-PRV and re-experiencing symptoms. Of the 44 participants with valid respiration data, 43 had respiration frequencies within the 0.12–0.40 Hz window used to band-pass filter the IBI time series (the 44th participant fell just outside this window, frequency=0.44 Hz). There was a non-significant inverse relationship between respiration rate and HF-PRV, such that faster breathing tended to be associated with lower HF-PRV (full sample r(42) = 0.24, 95% CI = [−0.06, 0.50], p = 0.12; PTSS r(27) = 0.29, 95% CI = [−0.09, 0.59], p = 0.12). Importantly, however, respiration rate was unrelated to overall CAPS symptoms (full sample r(42) = 0.19, 95% CI = [−0.11, 0.46], p = 0.22; PTSS r(27) = 0.24, 95% CI = [−0.14, 0.56], p = 0.20) or re-experiencing symptoms (full sample r(42) = 0.17, 95% CI = [−0.13, 0.44], p = 0.27; PTSS r(27) = 0.17, 95% CI = [−0.21, 0.51], p = 0.37). Additionally, we also observed a significant correlation in the PTSS group between respiration-adjusted HF-PRV and re-experiencing symptoms (r(27) = −0.43, 95% CI = [−0.69, −0.08], p = 0.02). In the simultaneous regression model with all 3 symptom clusters, re-experiencing symptoms no longer accounted for significant unique variance in respiration-adjusted HF-PRV (t(25) = −1.89, p = 0.07).
3.2. PRV is positively associated with anticipatory safe-threat vmPFC activation
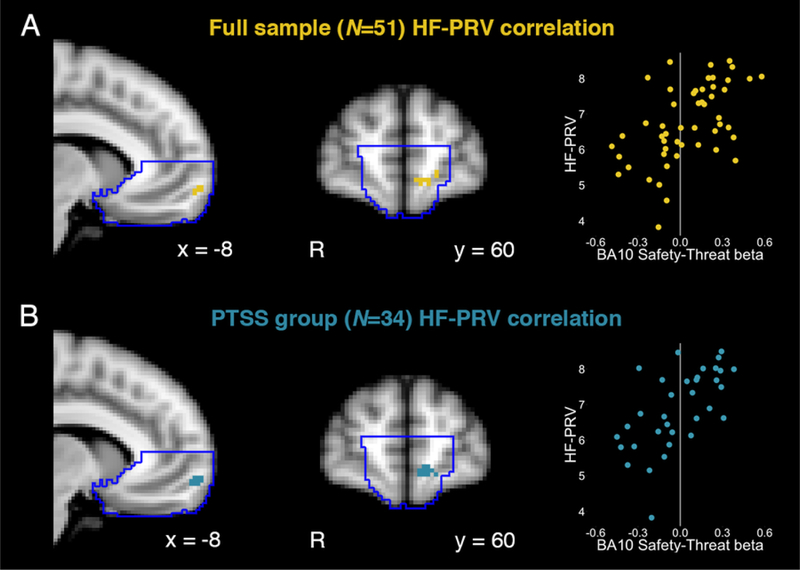
Voxelwise regression of uSafe-uThreat contrast estimates revealed small volume-corrected clusters within the vmPFC that showed a positive relationship with HF-PRV for the full sample (Figure 3a) and the PTSS group (Figure 3b). Participants with greater anticipatory vmPFC activation for safety vs. threat had relatively higher HF-PRV (greater safe vs. threat activation being the normative pattern across the vmPFC; Figure 1b). Each of these clusters was localized to BA10, or the medial frontopolar aspect of vmPFC. Similar small volume-corrected clusters were observed for respiration-adjusted HF-PRV in participants with valid respiration data. No clusters survived whole-brain significance for the PTSS group or the full sample. For the contrast of predictable safe - predictable threat trials (pSafe-pThreat), there were no significant whole-brain or vmPFC clusters that showed an association with HF-PRV in the PTSS group or the full sample.
Figure 3. Pulse rate variability and brain responses to safety.
(A) Across the entire sample, there was a positive correlation between high-frequency pulse rate variability (HF-PRV) and BOLD responses to safety vs. threat anticipation within the small-volume corrected (SVC) ventromedial prefrontal cortex (vmPFC), corresponding to BA10. (B) This relationship was also observed in the posttraumatic stress symptoms (PTSS) group alone. Scatter plots of beta weights from functionally defined clusters are presented for illustrative and not inferential purposes.
Extraction of uSafe and uThreat betas from these functional clusters showed that greater HF-PRV was associated with greater vmPFC activation to safety (full sample r = 0.54; PTSS r = 0.53) and lower vmPFC activation (or greater vmPFC deactivation) to threat (full sample r = −0.26; PTSS r = −0.38; valid statistical inferences cannot be made regarding the relative magnitude of parameter estimates from this functionally defined cluster (Kriegeskorte, Simmons, Bellgowan, & Baker, 2009)).
Most of the literature on PTSD and HRV has investigated resting HRV, thought to reflect a stable (Guijt et al., 2007), trait-like index of the capacity for flexible autonomic responding to emotional challenge. For completeness, we also ran analyses relating task-related HF-PRV (averaged across Runs 1–3) to PTSD symptoms and vmPFC activation during the task (one participant from the PTSS group did not have valid task PRV data). Task-related HF-PRV was highly correlated with resting HF-PRV (full sample r(48) = 0.87, p < 0.001; PTSS r(31) = 0.85, p < 0.001). Task-related HF-PRV showed a similar negative relationship with total CAPS scores in the PTSS group (r(31) = −0.39, p = 0.025) that was significant for the re-experiencing cluster only (r(31) = −0.43, p = 0.013). Task-related HF-PRV was positively correlated with uSafe-uThreat activation in a similar aspect of vmPFC that did not meet small-volume-corrected significance (full sample: 17 voxels at p < 0.001, uncorrected; PTSS: 26 voxels at p < 0.001, uncorrected; voxel-forming threshold is 31 voxels). An exploratory whole-brain regression in the PTSS group revealed a positive correlation between left ventrolateral prefrontal cortex activation for uSafe-uThreat and task-related HF-PRV (peak coordinate: −50, 24, −10).
3.3. PTSD re-experiencing symptoms are negatively associated with activation of adjacent vmFPC
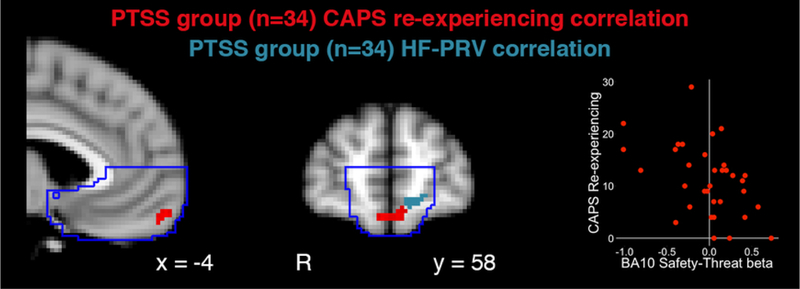
In our previous report on this sample, we identified an inverse relationship in a similar aspect of BA10 between uSafe-uThreat activation and PTSD re-experiencing symptoms (Grupe et al., 2016, Figure S5b). Overlaying statistical maps from the HF-PRV and re-experiencing regression analyses, we observed that the HF-PRV cluster was immediately adjacent to a small-volume-corrected cluster in which greater uSafe-uThreat activation was correlated with lower re-experiencing symptoms (Figure 4).
Figure 4. Adjacent vmPFC regions associated with pulse rate variability and re-experiencing symptoms.
Within the posttraumatic stress symptoms (PTSS) group, BOLD responses to safety vs. threat were negatively correlated with re-experiencing symptoms on the Clinician-Administered PTSD Scale (CAPS; red) in the BA10, immediately adjacent to the cluster in which activation was positively associated with high-frequency pulse rate variability (HF-PRV; blue). The scatter plots of beta weights from the functionally defined CAPS re-experiencing cluster is presented for illustrative and not inferential purposes
The regression of pSafe-pThreat on CAPS re-experiencing symptoms revealed a left vmPFC cluster just lateral to that for the uSafe-uThreat contrast at p < 0.001 (uncorrected) that, at 28 voxels, failed to meet small volume-corrected significance.
Non-thresholded statistical maps for each of these regression analyses are available at https://neurovault.org/collections/4544/.
4. Discussion
In a study of combat-exposed male veterans, we simultaneously investigated relationships between vmPFC responses to safety vs. threat, resting high-frequency pulse rate variability, and PTSD re-experiencing symptoms. Three key findings emerged from this investigation that shed light on potential brain-body mechanisms of PTSD.
First, in veterans with elevated symptoms of PTSD (CAPS > 20), we identified a specific relationship between greater re-experiencing symptoms of PTSD and reduced PRV, a surrogate measure for HRV that can be obtained in the MRI environment. Relationships between re-experiencing symptoms in PTSD and executive control deficits (Aupperle, Melrose, Stein, & Paulus, 2012; Bomyea, Amir, & Lang, 2012; Vasterling, Brailey, Constans, & Sutker, 1998) suggest that reduced inhibitory control compromises the ability to prevent unwanted traumatic memories from rising into awareness in PTSD. Further, it has been proposed that HRV indexes one’s ability to implement flexible regulatory control in the face of distractors or challenges to one’s well-being (Thayer et al., 2012), and HRV has been linked to difficulties suppressing unwanted memories in healthy college students (Gillie, Vasey, & Thayer, 2014). Gillie & Thayer (Gillie & Thayer, 2014) proposed that reduced HRV contributes to the inability to control unwanted memories or thoughts related to trauma and as such represents a physiological mechanism of re-experiencing symptoms, such as flashbacks and nightmares. Our results provide empirical support for this theoretical model, although replication of these results in a larger sample that also incorporates behavioral indices of cognitive control is an important next step.
The second key finding was that reduced PRV was associated with reduced vmPFC recruitment under conditions of safety vs. threat. Convergent clinical observations and neuroscientific evidence suggests that PTSD is not associated with exaggerated responses to threat per se, but rather contextually inappropriate, inflexible, and overgeneralized threat responding (Garfinkel et al., 2014; Kaczkurkin et al., 2017; Levy-Gigi, Richter-Levin, Szabo, & Keri, 2015; Morey et al., 2015). An inability of the vmPFC to differentially respond to threat vs. safety, and corresponding autonomic inflexibility reflected in reduced PRV, may contribute to elevated threat responding in an objectively safe context. The region of vmPFC identified here corresponds to frontopolar cortex or medial BA10, a region that has expanded considerably in humans relative to non-human primates and which is theorized to be important for shifting attentional or executive resources away from current goals to other potential goals in the environment (Mansouri, Koechlin, Rosa, & Buckley, 2017). This perspective on the broad function of BA10 is not inconsistent with the flexible, context-specific, inhibitory control function ascribed by Thayer and colleagues to HRV (Thayer et al., 2012). Notably, a previous study identified a positive correlation between HRV and activation in a similar vmPFC region during self-control challenges (Maier & Hare, 2017), a finding consistent with the current findings in light of the above framework linking re-experiencing symptoms to deficient cognitive control.
Third, the vmPFC cluster related to reduced HF-PRV was immediately adjacent to a BA10 region in which we previously identified a relationship with re-experiencing symptoms in these same participants. This anatomical similarity suggests that a common deficit in vmPFC function may have negative consequences both for PRV/HRV and PTSD re-experiencing symptoms, although strong evidence for the specific mechanistic relationship linking these processes would require alternative designs to the current correlational study. For example, it would be informative to test whether the effectiveness of somatic interventions targeting PTSD symptoms - for example, aerobic exercise (Fetzner & Asmundson, 2015), meditation (Polusny et al., 2015), yoga (Gallegos, Crean, Pigeon, & Heffner, 2017), or HRV feedback training (Tan, Dao, Farmer, Sutherland, & Gevirtz, 2011) – is predicted by normalized vmPFC function and corresponding increases in HRV.
One question raised by our results is why control participants with the lowest HF-PRV levels had few or no re-experiencing symptoms. HF-PRV was, on average, no different between CEC and PTSS groups and was related to re-experiencing symptoms only in the PTSS group (Figure 2a). These observations underscore that although reduced HRV/PRV may contribute to (or be exacerbated by) PTSD intrusions, it is by no means a sufficient condition. Control participants with low levels of HRV/PRV may possess compensatory mechanisms that enhance resilience against psychopathology. For example, these individuals may be better able to leverage explicit, cognitive emotion regulation resources - supported by dorsal and lateral PFC (Buhle et al., 2014) – to compensate for deficient autonomic regulatory control. Alternatively, they may exhibit enhanced hippocampal function that allows them to better differentiate between safe and threatening contexts (Anacker & Hen, 2017). The concurrent investigation in traumatized individuals of HRV/PRV and neuroimaging investigations of explicit emotion regulation or hippocampal-dependent tasks (e.g., pattern separation behavior) would allow for a test of these speculative hypotheses.
One important limitation of these results is that PRV and HRV, while measuring the same underlying signal, are not fully equivalent measures. We assessed PRV using pulse oximetry due to the difficulty of collecting electrocardiography data in the MRI environment. Although PRV and HRV are highly correlated, particularly at rest (Hayano et al., 2005; Schäfer & Vagedes, 2013), the two measures rely on distinct physiological readouts of cardiac function and PRV may be less accurate for the measurement of high-frequency variability in particular (Wong et al., 2012). Much of the extant literature on PTSD and cardiac autonomic control assesses HRV and not PRV, (although some published “HRV” studies in fact utilize photoplethysmography; e.g., (Minassian et al., 2015), and caution is warranted in generalizing the current results to the HRV literature until PRV is better established as an index of regulatory control and psychopathology. An additional important limitation is that we studied a relatively homogeneous sample (male OEF/OIF veterans who experienced combat trauma), which while reducing potential sources of variability also limits generalizability of findings to female veterans or individuals exposed to non-combat trauma. Finally, although we have emphasized the trait-like nature of resting PRV/HRV and interpreted results accordingly, the resting data were acquired immediately after the threat-of-shock task. We cannot be certain that similar relationships would be observed if resting PRV were assessed during an independent session, and this limitation should be addressed in future studies.
In summary, we have presented evidence that reduced functional activation in spatially overlapping voxels of the vmPFC is associated with both reduced HF-PRV and increased re-experiencing symptoms of PTSD. These data provide preliminary support for a mechanistic link between previous observations of reduced HRV and impaired vmPFC function in PTSD. More broadly, these findings underscore the potential of research on reciprocal brain-body relationships that may enhance our understanding of the pathophysiology of PTSD and suggest novel treatment targets for somatically focused therapeutic approaches.
Acknowledgments
The authors thank the participants for their military service and their involvement in this study, as well as the Wisconsin National Guard, the Madison Veterans’ Center, the Madison VA Hospital, and other veterans’ community organizations for their assistance in recruitment. The authors thank Kate Rifken, Andrea Hayes, Emma Seppala, Michael Anderle, Lisa Angelos, Isa Dolski, Ron Fisher, and Nate Vack for their help with study planning, execution, and analysis, and Jared Martin for thoughtful feedback on this manuscript.
This work was supported by the Dana Foundation to JBN; the University of Wisconsin Institute for Clinical and Translational Research to Emma Seppala; and a core grant to the Waisman Center from the National Institute of Child Health and Human Development (P30-HD003352). DWG was supported by a Graduate Research Fellowship from the National Science Foundation.
Portions of this work were previously presented at the 72nd Annual Scientific Convention of the Society of Biological Psychiatry, San Diego, May 18, 2017.
Dr. Davidson is the founder and president, and serves on the board of directors, for the non-profit organization Healthy Minds Innovations, Inc.
Footnotes
The other authors report no conflicts of interest or financial disclosures.
References
- Allen JJB, Chambers AS, & Towers DN (2007). The many metrics of cardiac chronotropy: A pragmatic primer and a brief comparison of metrics. Biological Psychology, 74(2), 243–262. 10.1016/j.biopsycho.2006.08.005 [DOI] [PubMed] [Google Scholar]
- Anacker C, & Hen R. (2017). Adult hippocampal neurogenesis and cognitive flexibility — linking memory and mood. Nature Reviews Neuroscience, 18(6), 335–346. 10.1038/nrn.2017.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aupperle RL, Melrose AJ, Stein MB, & Paulus MP (2012). Executive function and PTSD: Disengaging from trauma. Neuropharmacology, 62(2), 686–694. 10.1016/j.neuropharm.2011.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DD, Weathers RW, Namy LM, Kaloupek DG, Klauminzer G, Charnet DS, & Keane TM (1990). A clinician rating scale for assessing current and lifetime PTSD: The CAPS-1. Behavior Therapist, 13, 187–188. [Google Scholar]
- Bomyea J, Amir N, & Lang AJ (2012). The relationship between cognitive control and posttraumatic stress symptoms. Journal of Behavior Therapy and Experimental Psychiatry, 43(2), 844–848. 10.1016/j.jbtep.2011.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TW, Driscoll D, Mowrer SM, Sollers JJ, Thayer JF, Kirschbaum C, & Tranel D. (2010). Medial prefrontal cortex damage affects physiological and psychological stress responses differently in men and women. Psychoneuroendocrinology, 35(1), 56–66. https://doi.org/10.1016Zj.psyneuen.2009.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, … Ochsner KN (2014). Cognitive reappraisal of emotion: A meta-analysis of human neuroimaging studies. Cerebral Cortex, 24(11), 2981–2990. 10.1093/cercor/bht154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers JA, Quintana DS, Abbott MJ-A, & Kemp AH (2014). Anxiety disorders are associated with reduced heart rate variability: A meta-analysis. Frontiers in Psychiatry, 5 10.3389/fpsyt.2014.00080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fetzner MG, & Asmundson GJG (2015). Aerobic Exercise Reduces Symptoms of Posttraumatic Stress Disorder: A Randomized Controlled Trial. Cognitive Behaviour Therapy, 44(4), 301–313. 10.1080/16506073.2014.916745 [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JBW (2002). Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/P). New York: Biometrics Research, New York State Psychiatric Institute. [Google Scholar]
- Gabbott PLA, Warner TA, Jays PRL, Salway P, & Busby SJ (2005). Prefrontal cortex in the rat: Projections to subcortical autonomic, motor, and limbic centers. Journal of Comparative Neurology, 492(2), 145–177. 10.1002/cne.20738 [DOI] [PubMed] [Google Scholar]
- Gallegos AM, Crean HF, Pigeon WR, & Heffner KL (2017). Meditation and yoga for posttraumatic stress disorder: A meta-analytic review of randomized controlled trials. Clinical Psychology Review, 58(November 2016), 115–124. 10.1016/j.cpr.2017.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, Abelson JL, King AP, Sripada RK, Wang X, Gaines LM, & Liberzon I (2014). Impaired contextual modulation of memories in PTSD: An fMRI and psychophysiological study of extinction retention and fear renewal. Journal of Neuroscience, 34(40), 13435–13443. 10.1523/JNEUR0SCI.4287-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillie BL, & Thayer JF (2014). Individual differences in resting heart rate variability and cognitive control in posttraumatic stress disorder. Frontiers in Psychology, 5 10.3389/fpsyg.2014.00758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillie BL, Vasey MW, & Thayer JF (2014). Heart Rate Variability Predicts Control Over Memory Retrieval. Psychological Science, 25(2), 458–465. 10.1177/0956797613508789 [DOI] [PubMed] [Google Scholar]
- Grupe DW, Wielgosz J, Davidson RJRJ, & Nitschke JBJB (2016). Neurobiological correlates of distinct post-traumatic stress disorder symptom profiles during threat anticipation in combat veterans. Psychological Medicine, 46(9), 1885–1895. 10.1017/S0033291716000374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guijt AM, Sluiter JK, & Frings-Dresen MHW (2007). Test-Retest Reliability of Heart Rate Variability and Respiration Rate at Rest and during Light Physical Activity in Normal Subjects. Archives of Medical Research, 38(1), 113–120. 10.1016/j.arcmed.2006.07.009 [DOI] [PubMed] [Google Scholar]
- Hayano J, Barros AK, Kamiya A, Ohte N, & Yasuma F. (2005). Assessment of pulse rate variability by the method of pulse frequency demodulation. BioMedical Engineering OnLine, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Hayes SM, & Mikedis AM (2012). Quantitative meta-analysis of neural activity in posttraumatic stress disorder. Biology of Mood {&} Anxiety Disorders, 2(1), 9 10.1186/2045-5380-2-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczkurkin AN, Burton PC, Chazin SM, Manbeck AB, Espensen-Sturges T, Cooper SE, … Lissek S. (2017). Neural substrates of overgeneralized conditioned fear in PTSD. American Journal of Psychiatry, 174(2), 125–134. 10.1176/appi.ajp.2016.15121549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, & Gatt JM (2010). Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biological Psychiatry, 67(11), 1067–1074. 10.1016/j.biopsych.2009.12.012 [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PSF, & Baker CI (2009). Circular analysis in systems neuroscience: The dangers of double dipping. Nature Neuroscience, 12(5), 535–540. 10.1038/nn.2303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy-Gigi E, Richter-Levin G, Szabo C, & Keri S. (2015). Reduced Hippocampal Volume Is Associated With Overgeneralization of Negative Context in Individuals With PTSD. Neuropsychology, 29(1), 151–161. 10.1037/neu0000131 [DOI] [PubMed] [Google Scholar]
- Maier SU, & Hare TA (2017). Higher Heart-Rate Variability Is Associated with Ventromedial Prefrontal Cortex Activity and Increased Resistance to Temptation in Dietary Self-Control Challenges. The Journal of Neuroscience, 37(2), 446–455. 10.1523/JNEUR0SCI.2815-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, & Burdette JH (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. NeuroImage, 19(3), 1233–1239. 10.1016/S1053-8119(03)00169-1 [DOI] [PubMed] [Google Scholar]
- Mansouri FA, Koechlin E, Rosa MGP, & Buckley MJ (2017). Managing competing goals — a key role for the frontopolar cortex. Nature Reviews Neuroscience, 18(11), 645–657. 10.1038/nrn.2017.111 [DOI] [PubMed] [Google Scholar]
- McKlveen JM, Myers B, & Herman JP (2015). The Medial Prefrontal Cortex: Coordinator of Autonomic, Neuroendocrine and Behavioural Responses to Stress. Journal of Neuroendocrinology, 27(6), 446–456. 10.1111/jne.12272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, … Rauch SL (2009). Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biological Psychiatry, 66(12), 1075–1082. https://doi.org/10.1016Zj.biopsych.2009.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian A, Maihofer AX, Baker DG, Nievergelt CM, Geyer M. a., & Risbrough VB (2015). Association of predeployment heart rate variability with risk of postdeployment posttraumatic stress disorder in active-duty marines. JAMA Psychiatry, 92103, 1 10.1001/jamapsychiatry.2015.0922 [DOI] [PubMed] [Google Scholar]
- Morey RA, Dunsmoor JE, Haswell CC, Brown VM, Vora A, Weiner J, … LaBar KS (2015). Fear learning circuitry is biased toward generalization of fear associations in posttraumatic stress disorder. Translational Psychiatry, 5(12), e700. 10.1038/tp.2015.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagpal ML, Gleichauf K, & Ginsberg JP (2013). Meta-analysis of heart rate variability as a psychophysiological indicator of posttraumatic stress disorder. Trauma {&} Treatment, 03(01), 1–8. 10.4172/2167-1222.1000182 [DOI] [Google Scholar]
- Polusny MA, Erbes CR, Thuras P, Moran A, Lamberty GJ, Collins RC, … Lim KO (2015). Mindfulness-based stress reduction for posttraumatic stress disorder among veterans a randomized clinical trial. JAMA - Journal of the American Medical Association, 314(5), 456–465. 10.1001/jama.2015.8361 [DOI] [PubMed] [Google Scholar]
- Porges SW (1995). Cardiac vagal tone: A physiological index of stress. Neuroscience and Biobehavioral Reviews, 19(2), 225–233. 10.1016/0149-7634(94)00066-A [DOI] [PubMed] [Google Scholar]
- Pyne JM, Constans JI, Wiederhold MD, Gibson DP, Kimbrell T, Kramer TL, … McCune TR (2016). Heart rate variability: Pre-deployment predictor of post-deployment PTSD symptoms. Biological Psychology, 121, 91–98. https://doi.org/10.1016Zj.biopsycho.2016.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resstel LBM, Fernandes KBP, & Corrêa FMA (2004). Medial prefrontal cortex modulation of the baroreflex parasympathetic component in the rat. Brain Research, 1015(1–2), 136–144. 10.1016/j.brainres.2004.04.065 [DOI] [PubMed] [Google Scholar]
- Schäfer A, & Vagedes J. (2013). How accurate is pulse rate variability as an estimate of heart rate variability? International Journal of Cardiology, 166(1), 15–29. 10.1016/j.ijcard.2012.03.119 [DOI] [PubMed] [Google Scholar]
- Schiller D, Levy I, LeDoux JE, Niv Y, & Phelps EA (2008). From fear to safety and back: Reversal of fear in the human brain. The Journal of Neuroscience, 28(45), 11517–11525. 10.1523/jneurosci.2265-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark EA, Parsons CE, Van Hartevelt TJ, Charquero-Ballester M, McManners H, Ehlers A, … Kringelbach ML (2015). Post-traumatic stress influences the brain even in the absence of symptoms: A systematic, quantitative meta-analysis of neuroimaging studies. Neuroscience and Biobehavioral Reviews, 56, 207–221. https://doi.org/10.1016Zj.neubiorev.2015.07.007 [DOI] [PubMed] [Google Scholar]
- Tan G, Dao TK, Farmer L, Sutherland RJ, & Gevirtz R. (2011). Heart rate variability (HRV) and posttraumatic stress disorder (PTSD): a pilot study. Applied Psychophysiology and Biofeedback, 36(1), 27–35. 10.1007/s10484-010-9141-y [DOI] [PubMed] [Google Scholar]
- Thayer JF, Åhs F, Fredrikson M, Sollers JJ, Wager TD, Ahs F, … Wager TD (2012). A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neuroscience and Biobehavioral Reviews, 36(2), 747–756. 10.1016/j.neubiorev.2011.11.009 [DOI] [PubMed] [Google Scholar]
- Thayer JF, & Lane RD (2000). A model of neurovisceral integration in emotion regulation and dysregulation. Journal of Affective Disorders, 61(3), 201–216. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11163422 [DOI] [PubMed] [Google Scholar]
- van der Kolk BA (2014). The Body Keeps the Score (1st ed). New York: Viking. [Google Scholar]
- Vasterling JJ, Brailey K, Constans JI, & Sutker PB (1998). Attention and memory dysfunction in posttraumatic stress disorder. Neuropsychology, 12(1), 125–133. [DOI] [PubMed] [Google Scholar]
- Wallis CU, Cardinal RN, Alexander L, Roberts AC, & Clarke HF (2017). Opposing roles of primate areas 25 and 32 and their putative rodent homologs in the regulation of negative emotion. Proceedings of the National Academy of Sciences, 114(20), E4075–E4084. 10.1073/pnas.1620115114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J-S, Lu W-A, Wu K-T, Liu M, Chen G-Y, & Kuo C-D (2012). A comparative study of pulse rate variability and heart rate variability in healthy subjects. Journal of Clinical Monitoring and Computing, 26(2), 107–114. 10.1007/s10877-012-9340-6 [DOI] [PubMed] [Google Scholar]