Abstract
Objective:
Altered activity within reward-related neural regions, including the ventral striatum (VS) and medial prefrontal cortex (mPFC), is associated with concurrent problematic substance use. The aims of the present study were to (a) identify patterns of reward-related neural activity that prospectively predict changes in alcohol use two years after magnetic resonance imaging (MRI) scanning in a sample of adolescents, and (b) examine whether these patterns differ by sex. We also tested whether depression symptoms or impulsivity mediated associations between neural activity and future alcohol use.
Method:
Participants were 262 Mexican-origin adolescents (129 male) who completed the Monetary Incentive Delay task during an fMRI scan at age 16. Participants reported on their alcohol use at ages 16 and 18.
Results:
Results indicated that different patterns of reward-related neural activity predicted future increases in alcohol use for male and female adolescents. In boys, higher VS activity during reward anticipation and average ventral mPFC activity during reward feedback predicted increases in alcohol use from age 16 to 18; in girls, higher dorsal mPFC activity and blunted VS activity during reward anticipation predicted increases in alcohol use from age 16 to 18. Depression symptoms or impulsivity did not mediate these associations.
Conclusion:
The results suggest that different pathways of risk may lead to problematic alcohol use for adolescent boys and girls. These sex differences in neural risk pathways have important implications for prevention and intervention approaches targeting Mexican-origin youth.
Keywords: fMRI, reward, alcohol, adolescence, sex
Introduction
By the time they reach 18 years old, most American adolescents will have tried alcohol and a quarter will have engaged in binge drinking.1–3 Identifying patterns of neural activity that predict increases in alcohol use during this period will provide an important foundation for identifying adolescents at risk for alcohol use problems and designing targeted preventions and interventions informed by neurobiological pathways of risk. Moreover, focusing on adolescents will provide critical information about the patterns of neural activity associated with the early stages of alcohol use. The goal of the present study is to identify patterns of neural activity that prospectively predict increases in alcohol use during late adolescence in a sample of Mexican- origin adolescents, a population that generally evidences earlier onset of alcohol use relative to other racial/ethnic groups.3
Individual differences in ventral striatum (VS) and medial prefrontal cortex (mPFC) response to reward have been linked to problematic alcohol use in prior research.4–7 The VS, which includes the nucleus accumbens, is involved in processing reward anticipation and receipt.8, 9 The VS is functionally connected to the mPFC, which is involved in evaluating reward cues and regulating responses to reward.8 Reward-related neural activity has been examined in relation to many different sub-processes; two of the most commonly examined in this context are reward anticipation (i.e., neural activity to cues signifying potential for reward) and reward feedback (i.e., neural activity to cues indicating receipt of reward, such as money), although some task designs (e.g., blocked) do not allow for the separation of BOLD signal between the reward anticipation and feedback stages.
Altered reward processing within the VS and mPFC has been associated with alcohol use problems,6, 10–14 and findings from neuroimaging and behavioral studies have suggested two potential routes to problematic alcohol use.15 One potential pathway involves a relatively high VS response while receiving rewards and when response to anticipating and receiving rewards is assessed together (e.g., as with a blocked task paradigm).4, 6, 12 According to the impulsivity theory of addiction,7 higher VS response to reward cues may lead to problematic alcohol use through a pathway of higher sensation-seeking and impulsivity.14, 16, 17 Relatedly, blunted mPFC response during reward anticipation and reward feedback has been associated with more problematic alcohol use,4, 5, 10 which may reflect reduced prefrontal regulation of reward processing. Thus, a relatively heightened VS response to reward or a blunted mPFC response to reward could lead to more problematic alcohol use through increased impulsivity and reduced regulation of reward-related behavior, though notably these effects do not always replicate.18
The second potential neural pathway is a blunted VS response when anticipating rewards or, again, when response to anticipating and receiving rewards is measured together.6, 7, 10 In line with the reward deficiency syndrome theory,7, 19 individuals with a blunted VS response to reward may consume substances as a means of compensating for this blunted response to ordinary rewards such as money.6, 11, 20 Because a blunted VS response to reward cues is observed in individuals who are depressed and associated with anhedonia,21–24 this pattern of brain activity may also predict using alcohol to cope with internalizing symptoms.11, 15 Importantly, while the majority of this research has been cross-sectional, some studies have shown that these patterns of brain activity prospectively predict future alcohol use at longitudinal assessments.6, 14, 25
There are two potential, not mutually exclusive, explanations for these distinct patterns of VS and mPFC activity observed in association with problematic alcohol use. First, these patterns may depend on whether neural activity was measured during anticipation or feedback of reward. For example, a meta-analysis suggested that individuals with substance use disorders evidence a blunted VS response during reward anticipation but heightened VS response during reward outcome.7 In contrast, research examining mPFC activity suggests that effects are similar for the reward anticipation and feedback phases.4, 5, 7 In other studies, reward paradigms use designs that place both stages of reward processing within blocks,6, 10–12 precluding the ability to examine responses to anticipation and feedback separately. Thus, there is a need to examine VS and mPFC activity separately during the reward anticipation and feedback phases.
The second potential explanation is that these patterns may reflect different pathways of risk in different individuals. For example, one study of a large sample of college students found two separate patterns of brain activity associated with alcohol use problems in different individuals: heightened VS activity to reward and blunted amygdala activity to threat in one group, and blunted VS activity to reward and heightened amygdala activity to threat in a second group.6 The authors suggested the former group may exhibit more problematic alcohol use due to an impulsive/sensation-seeking risk pathway whereas the latter group may exhibit problematic alcohol use due to an internalizing pathway.
The explanations offered above may further depend on the sex of the participants, given there are sex differences in the prevalence of problematic alcohol use. Specifically, though sex differences in alcohol use disorders tend to be small during adolescence, they begin to emerge in late adolescence and young adulthood, with alcohol use disorders becoming more prevalent in men.3, 26, 27 There are also sex differences in motives for alcohol use. Male adolescents are more likely to endorse enhancement motives (i.e., drinking for fun and sensation-seeking) compared to female adolescents.28 Moreover, there are stronger associations between depressive symptoms, coping motives (i.e., drinking to cope with negative emotions), and heavy alcohol use in female participants compared to male participants.29 Relatedly, rates of depression are higher in female adolescents compared to male adolescents,30, 31 possibly rendering female adolescents more likely to follow an internalizing risk pathway. Sex differences have also been observed in the development of reward-related brain structure and function across adolescence,32 including in the developmental timing of striatal volume changes in humans33 and in neuronal pruning of the mPFC in rats post-puberty.34 Thus, there may be sex differences in the patterns of reward-related neural activity that prospectively predict future increases in alcohol use during late adolescence.
In the present study, we tested whether reward-related neural activity measured at age 16 prospectively predicts increases in alcohol use from age 16 to 18. Importantly, all participants were adolescents from Mexican-origin families, a growing U. S. demographic underrepresented in neuroscience-based research who experience high levels of substance use problems.3, 35, 36 We hypothesized two potential patterns of brain activity would predict future increases in alcohol use: a) heightened VS response or blunted mPFC response during reward anticipation and feedback (consistent with an impulsive, sensation-seeking pathway to alcohol use) or b) blunted VS response during reward anticipation (consistent with an anhedonic pathway to alcohol use). Additionally, based on sex differences in drinking motives and internalizing disorder prevalence, we predicted the heightened VS/blunted mPFC (sensation-seeking/impulsive) pattern would be more predictive of future alcohol use in male adolescents whereas the blunted VS (anhedonic/internalizing) pattern would be more predictive of future alcohol use in female adolescents. We also tested these proposed pathways by examining whether impulsivity or depression symptoms mediated the association between neural activity and increases in alcohol use.
Method
Participants
Participants included a subsample drawn from a 10-year longitudinal parent study of 674 Mexican-origin families.35, 37 The first assessment occurred when the participants were in 5th grade (mean age = 10.8 years); subsequent follow-up assessments were conducted annually. When participants were between 15–17 years old, they were selected for two neuroimaging sub-studies (one focused on substance use and one focused on depression risk) that both included completing the Monetary Incentive Delay task during an fMRI scan. See Supplement 1, available online, for further participant selection details. Neuroimaging data from these participants has been published in previous research.36, 38, 39 All procedures were approved by the University of California, Davis IRB; parents provided informed consent and adolescent participants provided informed assent before beginning study procedures; participants received monetary compensation for their study participation. The final sample reported on in this paper includes 197 participants from the depression sub-study and 89 participants from the substance use sub- study, yielding 286 participants. After excluding 24 participants for not meeting quality control criteria (see Supplement 1, available online), valid fMRI data were available for 262 participants (51% female; Table 1).
Table 1.
Sample Characteristics for N=262 Participants With Valid Functional Magnetic Resonance Imaging Data (fMRI)
| Male Participants (n=129) | Female Participants (n=133) | ||||||
|---|---|---|---|---|---|---|---|
| Mean | Standard Deviation | Min-Max | Mean | Standard Deviation | Min-Max | Group Difference | |
| Age at fMRI scan | 16.88 | .60 | 15.37–17.98 | 16.85 | .58 | 15.47–17.97 | t(260)=.44, p=.66 |
| Frequency of alcohol use at age 16 | .53 | 1.33 | 0–8 | .51 | 1.01 | 0–5 | t(260)=.11, p=.91 |
| Frequency of alcohol use at age 18 | .87 | 1.58 | 0–7 | .86 | 1.45 | 0–8 | t(255)=.08, p=.93 |
| Any alcohol use at age 16 (%) | 20.2 | 26.3 | χ2(1)=1.39, p=.24 | ||||
| Any alcohol use at age 18 (%) | 34.4 | 38.6 | χ2(1)=.50, p=.48 | ||||
| Depression symptoms at age 16 | 2.31 | 2.78 | 0–14 | 4.52 | 4.05 | 0–17 | t(260)=-5.14, p<.001 |
| Depression symptoms at age 17 | 2.05 | 2.60 | 0–12 | 3.96 | 3.58 | 0–17 | t(259)=-4.91, p=.001 |
| Impulsivity at age 16 | 2.45 | .62 | 1.13–4.13 | 2.26 | .67 | 1.00–4.00 | t(260)=2.37, p=.019 |
| Impulsivity at age 18 | 2.09 | .62 | 1.00–4.00 | 1.99 | .65 | 1.00–4.75 | t(255)=1.19, p=.24 |
| Reaction time for MID (ms) | 124.60 | 24.91 | 44.28–189.24 | 123.11 | 29.42 | 13.63–177.59 | t(260)=.44, p=.66 |
| Accuracy for MID (%) | 62.81 | 9.76 | 39–87 | 61.91 | 9.92 | 24–89 | t(260)=.74, p=.46 |
Note: Frequency of alcohol use was determined based on responses on the Alcohol, Tobacco, and Other Drugs survey. Depression symptoms were assessed with the National Institute of Mental Health Diagnostic Interview Schedule for Children Version 4. Impulsivity was assessed with the Weinberger Adjustment Inventory. Five participants were missing data on alcohol use at age 18; 1 participant was missing data on depression symptoms at age 17; 5 participants were missing data on impulsivity at age 18. Reaction time and accuracy for the MID are reported for all trials (gain, loss, and neutral). MID = Monetary Incentive Delay task performed during fMRI scanning.
Monetary Incentive Delay (MID) Task
The MID9 has been used extensively in prior research on adolescent samples.40–42 The MID was presented with E-Prime computer software (PST, Inc., Pittsburgh, PA). The version of the MID used in this study consisted of two runs (10.44 minutes each) of 70 contiguous 6-s trials. Participants viewed a cue indicating potential gain or loss of one of three magnitudes of money, or no change in outcome (a neutral condition). Participants then had to press a button in response to a target as quickly as possible to gain money on gain trials or avoid losing money on loss trials. See Supplement 1, available online, for further details.
BOLD fMRI data acquisition, preprocessing, and analysis
Participants were scanned on a research-dedicated 3T Siemens Tim Trio whole-body MRI system (Siemens Medical Solutions, Erlangen, Germany) with a 32-channel head coil. See Supplement 1, available online, for details regarding pre-processing, first-level models, group- level analysis, and definitions of regions of interest. The regions of interest (ROIs) included the left and right ventral striatum and the medial PFC. Functional clusters that were significantly activated by each contrast were identified at a small-volume family-wise error corrected p<.05 threshold. Next, mean contrast values were extracted from these functional clusters for use in further statistical analyses. Because contrast values for the left and right VS were highly correlated (r=.94, p<.001 for reward anticipation and r=.90, p<.001 for reward feedback), these were averaged to obtain mean measures of VS activity during reward anticipation and reward feedback. Outliers for VS and mPFC activity were winsorized to within 3 SD of the mean.
Self-report of alcohol use
Participants reported their history of substance use on the Alcohol, Tobacco, and Other Drugs (ATOD)43 survey at each wave of the study. This survey included questions asking participants how often they had used or tried beer, wine, and hard liquor in the past 3 months. The questions on beer and wine use specified “more than just a few sips.” The response options were: Never (0), Less than once per week (1), About once per week (2), Two to three times per week (3), and Almost every day (4). Frequency of alcohol use at each age was determined by summing these scores for the three types of alcohol, resulting in a total frequency score ranging from 0 to 12. Age 18 alcohol use was missing for 5 participants.
Covariates
Covariates included the number of years since initiation of substance use, mean life stress between age 10 to 16, alcohol use at age 16, and age at the fMRI scan (see Supplement 1, available online). Following the recommendations of Keller,44 we tested predictor x covariate interactions for each predictor (VS activity, mPFC activity, sex) and covariate in the models one at a time. We retained interactions that were significant in the final model.
Mediators of the association between reward-related brain function and alcohol use
To test our hypotheses that different patterns of brain activity predict future alcohol use through an impulsive/sensation-seeking pathway (for boys) and an internalizing/depression pathway (for girls), we examined two variables available in the dataset as potential mediators of this association. For the sensation-seeking/impulsive pathway, we used scores on the Impulsivity subscale of the Weinberger Adjustment Inventory.45 This scale contains 8 items, with response options ranging from 1 (“Not at all true”) to 5 (“Very true”). The items were averaged to obtain a mean measure of impulsivity. For the internalizing/depression pathway, depression symptoms were assessed using the Major Depression/Dysthymic Disorder module of the National Institute of Mental Health Diagnostic Interview Schedule for Children Version 4 (DISC-IV).46 For this measure, participants reported on depression symptoms experienced in the past year with a yes/no response. Responses were summed to obtain a continuous measure of symptoms. Outliers were winsorized to within 3 SD of the mean for both mediators.
Statistical analysis
After extracting contrast values for VS and mPFC activity during reward anticipation and feedback, all self-report data and contrast values were imported into Mplus v7 software for further statistical analysis and were mean-centered. To test our hypotheses that differences in reward-related brain function would be associated with future alcohol use, we created two separate models. In the first, we modeled paths from VS and mPFC activity during reward anticipation (measured at age 16) as a predictor of alcohol use measured at age 18. We also included predictors for sex, sex x VS and sex x mPFC interactions, as well as quadratic terms for VS and mPFC activity and interactions with sex. Quadratic terms were included for the brain variables given evidence for potential quadratic effects from prior research (e.g., both blunted and heightened VS activity to reward predict problematic alcohol use).4, 6, 7, 10, 12 Because the alcohol use variable had a zero-inflated count distribution, we used a zero-inflated Poisson regression model. This method uses a logit model to examine predictors of binary alcohol use (0=No, 1=Yes) and a Poisson regression to examine predictors of the frequency count of alcohol use. We entered the same predictors for each part of the model. As described above, we also tested predictor x covariate interactions and included these when significant. In the second model, we conducted a similar zero-inflated Poisson regression using the same regressors and covariates, but used VS and mPFC contrast values for the reward feedback contrast.
To test the proposed mediators, we examined with linear regression whether VS and mPFC activity (at age 16) predicted depression symptoms (at age 17) or impulsivity (at age 18), controlling for age 16 depression symptoms or impulsivity, respectively (assessing the mediators at age 17 is ideal for establishing the temporal order of effects, but impulsivity was not assessed at age 17 so we used the age 18 measure). In the final regression, we used zero-inflated Poisson regression to test whether depression symptoms (age 17) or impulsivity (age 18) predicted alcohol use (age 18), and modeled interactions with sex. Because only brain activity for the reward anticipation contrasts showed any associations with the mediators, we only controlled for these contrasts in this final regression.
Overall, we conducted 48 statistical tests examining neural predictors of alcohol use, impulsivity, and depression, so we used the Benjamini-Hochberg false-discovery rate approach47 to control for multiple comparisons. All statistical tests were two-tailed and were conducted with MPlus v7 software using full information maximum likelihood (FIML) estimation with robust standard errors.
Results
Main effects of reward anticipation in the VS and mPFC
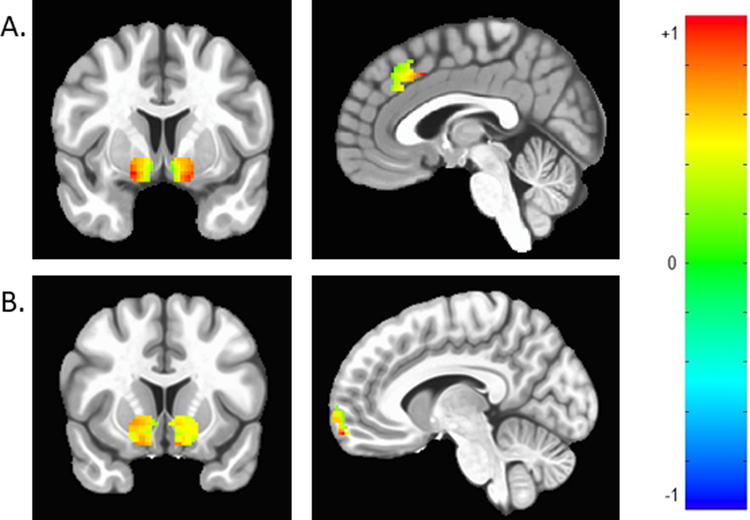
As illustrated in Figure 1, the contrast of gain > neutral cues resulted in significant activation within the left VS: t(262)=9.47 (average), p-corrected <.05, peak coordinates = (−16, 4, −14), and the right VS: t(262)=9.54 (average), p-corrected <.05, peak coordinates = (18, 6, −12). There was also significant activation to gain > neutral cues within the dorsal mPFC (dmPFC): t(262)=4.95 (average), p-corrected <.05, peak coordinates = (2, 16, 42). Mean contrast values were extracted from these functionally activated clusters and contrast values for the left and right VS were averaged. This resulted in two variables for the reward anticipation phase: bilateral VS activity and dmPFC activity. Table S1, available online, displays bivariate correlations between brain activity and other key variables.
Figure 1. Main Effects of the Task.
Note: A. Bilateral ventral striatum (VS) and dorsal medial prefrontal cortex (mPFC) activity for the contrast of gain cues > neutral cues, minimum threshold t = 2.575, p<.05 corrected for each search region. B. Bilateral VS and ventral mPFC activity for the contrast of gain feedback > neutral feedback, minimum threshold t = 2.575, p<.05 corrected for each search region.
Main effects of reward feedback in the VS and mPFC
The contrast of gain > neutral feedback elicited significant activation in the left VS: t(261)=3.20 (average), p-corrected < .05, peak coordinates = (−12, 8, −18) and the right VS: t(261)=3.19 (average), p-corrected<.05, peak coordinates = (8, 8, −18) (see Figure 1). Significant activation occurred in one ventral cluster of the mPFC (vmPFC): t(261)=3.29 (average), p-corrected < .05, peak coordinates = (8, 64, −14). Contrast values for the left and right VS were averaged to create a single bilateral VS variable, resulting in two variables for the reward feedback contrast: bilateral VS activity and vmPFC activity.
Brain activity related to reward anticipation and future alcohol use
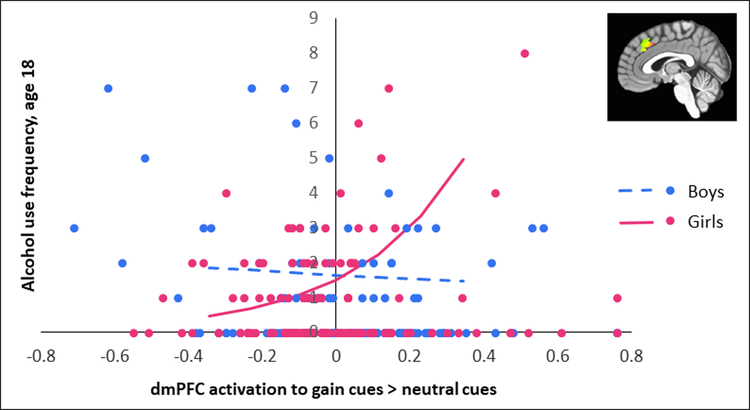
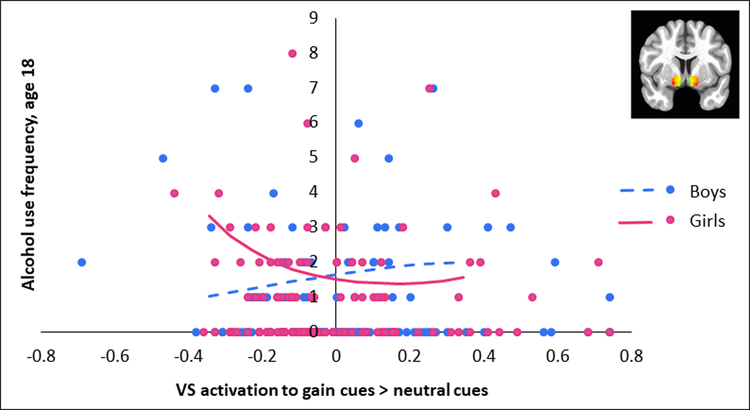
We next examined whether VS or dmPFC response to gain > neutral cues at age 16 predicted future alcohol use approximately two years later, at age 18, controlling for age 16 alcohol use. Two interactions were significant for the Poisson model (see Table S2, available online). First, there was a significant interaction between dmPFC activity and sex, B=3.80, SE=.80, p<.001. This interaction was driven by a (non-significant) negative association between dmPFC activity and future alcohol use in boys, B=−.65, SE=.75, p=.384, but a positive association between dmPFC activity and future alcohol use in girls, B=4.43, SE=.76, p<.001 (Figure 2). The quadratic term for dmPFC activity was not significant. Second, there was a significant interaction between the quadratic VS term and sex, B=4.69, SE=1.56, p=.003. This was driven by a negative quadratic effect of VS activity on future alcohol use in boys, B=-1.72, SE=.79, p=.029, but a positive quadratic effect of VS activity on future alcohol use in girls, B=2.53, SE=1.13, p=.025 (Figure 3). In boys, the negative quadratic effect suggests lower levels of VS activity are associated with lower alcohol use frequency, but this effect plateaus at higher levels of VS activity and turns slightly downward. In girls, the positive quadratic effect of VS activity suggests there is a sharper increase in alcohol use frequency at lower levels of VS activity, but this effect plateaus until higher positive values of VS activity, when the pattern turns slightly upward.
Figure 2. Association Between Dorsal Medial Prefrontal Cortex (dmPFC) Activation to Gain Cues > Neutral Cues and Future Alcohol Use in Male and Female Adolescents.
Note: Lines are plotted for dorsal mPFC activity (mean-centered) from 1.5 standard deviations below the mean to 1.5 standard deviations above the mean using coefficients from the Poisson model (see Table S2, available online). Points on the y-axis represent age 18 alcohol use (see methods for description of how alcohol use was calculated).
Figure 3. Association Between Bilateral Ventral Striatum (VS) Activation to Gain Cues > Neutral Cues and Future Alcohol Use in Male and Female Adolescents.
Note: Lines are plotted for VS activity (mean-centered) from 1.5 standard deviations below the mean to 1.5 standard deviations above the mean using coefficients from the Poisson model (see Table S2, available online). Points on the y-axis represent age 18 alcohol use.
The interaction of VS activity and sex, B=-2.10, SE=.84, p=.012, was significant at an uncorrected threshold, but was not significant when applying an FDR threshold. There was also a significant interaction of dmPFC activity x stress, B=-.29, SE=.13, p=.026, but as this was intended as a covariate and not a predictor of interest, this was not included in the FDR correction and not explored further. Likewise, for the logit model, there was a significant interaction of dmPFC activity x stress, B=-.93, SE=.34, p=.006, but we did not include this in the FDR correction or explore it further. No other effects were significant for the logit model.
Brain activity related to reward feedback and future alcohol use
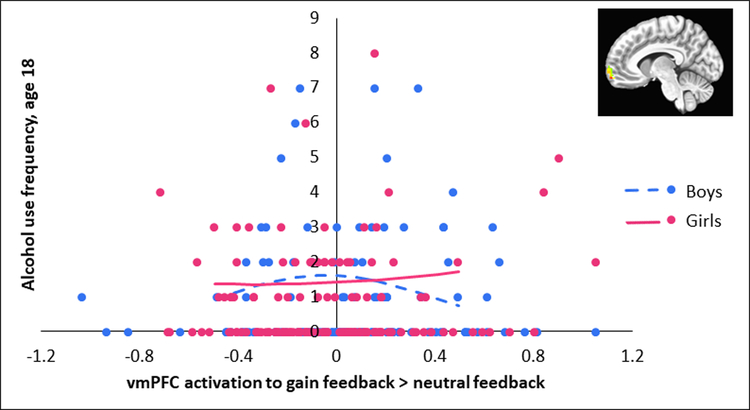
We used a similar approach to examine whether VS or vmPFC response to gain > neutral feedback at age 16 predicted future alcohol use at age 18. In the Poisson model, there were two significant effects meeting the FDR corrected p-threshold: a quadratic effect of vmPFC activity, B=-2.72, SE=.84, p=.001, and an interaction between the quadratic vmPFC effect and sex, B=2.99, SE=1.02, p=.003 (see Table S3, available online). As shown in Figure 4, this was due to a significant negative quadratic effect of vmPFC activity in boys, B=-2.86, SE=1.24, p=.021, and a (non-significant) positive quadratic effect in girls, B=.68, SE=.48, p=.158. The negative quadratic effect in boys suggests that average vmPFC activity during reward feedback is associated with higher alcohol use, but that this effect turns downward at low or high levels of vmPFC activity. There was also an effect of VS activity on future alcohol use, B=-3.15, SE=1.39, p=.024, although it did not survive correction for multiple comparisons. For the logit model, no effects were significant even at an uncorrected p-threshold.
Figure 4. Association Between Ventral Medial Prefrontal Cortex (vmPFC) Activation to Gain Feedback > Neutral Feedback and Future Alcohol Use in Male and Female Adolescents.
Note: Lines are plotted for ventral mPFC activity (mean-centered) from 1.5 standard deviations below the mean to 1.5 standard deviations above the mean using coefficients from the Poisson model (see Table S3, available online). Points on the y-axis represent age 18 alcohol use.
Mediators of the association between reward-related brain function and alcohol use
We first tested whether neural activity predicted depression at age 17 or impulsivity at age 18. There was a quadratic association between VS activity during reward anticipation and depression symptoms, B=4.17, SE=1.64, p=.011 (see Table S4, available online), although this did not survive correction for multiple comparisons. No other effects of brain activity on depression symptoms or impulsivity were significant (see Tables S4–S7, available online). Finally, we examined whether depression or impulsivity predicted alcohol use at age 18. In the Poisson model, there was a significant association between impulsivity at age 18 and alcohol use at age 18, B=.58, SE=.26, p=.026 (see Table S8, available online).
Discussion
The aim of the present study was to examine whether reward-related neural activity at age 16 prospectively predicted alcohol use at age 18, controlling for age 16 alcohol use, in a sample of Mexican-origin adolescents and to test two proposed mediating pathways. We found that the patterns of neural activity predictive of future alcohol use were different for male and female adolescents. For boys, higher VS activity during reward anticipation and average vmPFC activity during reward feedback predicted greater increases in alcohol use from age 16 to 18. For girls, blunted VS activity and higher dmPFC activity during reward anticipation predicted greater increases in alcohol use from age 16 to 18. These results indicate that different neural risk pathways are associated with increased alcohol use for male and female adolescents. However, neither impulsivity nor depression symptoms mediated these effects.
In boys, the pattern of higher VS activity during reward anticipation could indicate a sensation-seeking/impulsive pathway to alcohol use. Higher VS activity during reward anticipation may reflect a motivated drive towards rewarding stimuli. The quadratic effect of vmPFC activity on future alcohol use was not hypothesized and should be interpreted with caution until replicated. It is possible that high and low levels of vmPFC activity may indicate different psychological processes that may reduce risk through different mediating pathways, and this possibility should be tested in future research; we suggest some potential mediators below.
In contrast, in girls, a pattern of higher dmPFC activity and blunted VS activity during reward anticipation predicted future increases in alcohol use. Blunted VS activity during reward anticipation has previously been associated with problematic alcohol use, and is consistent with an internalizing/anhedonic pathway to alcohol use. It is possible this blunted VS response while anticipating potential reward in girls reflects low levels of motivated behavior for obtaining ordinary rewards such as money, which could lead to alcohol use as a means to compensate for a blunted response to ordinary reward cues. Additionally, in girls, the positive quadratic effect indicates that, while there was a steeper association between blunted VS activity and increased frequency of alcohol use, there was also a modest association between higher VS activity and alcohol use, suggesting that an average level of VS activity may be associated with the lowest risk for increased alcohol use. Though we did not directly hypothesize that higher dmPFC activity during reward anticipation would predict increased alcohol use in girls, this pattern of brain activity has been associated with higher depression symptoms in other samples,48, 49 and could be consistent with an internalizing pathway of risk. However, in this sample the bivariate correlations (see Table S1, available online) suggest that higher dmPFC activity is associated with higher impulsivity in girls, which was counter to our predictions. Thus, further research is needed to identify the psychological processes underlying this effect. Overall, the separate patterns of results observed for male and female adolescents and the two contrasts suggest that differences in prior research findings may reflect the stage of reward processing examined as well as different risk processes across individuals.
Another key finding is that reward-related brain activity predicted increased alcohol use at age 18 in the Poisson model (count outcome) testing increases in the frequency of alcohol use. However, these markers do not predict the initiation of new alcohol use (the binary outcome) during this time span. This indicates that different psychological mechanisms and different indices of brain function may be useful for differentiating risk for the onset of substance use vs. the acceleration of substance use, with the current indices of brain function predicting the latter.
It is important to note, however, that despite our theoretical framework for the two proposed risk pathways mediated by depression and impulsivity, these mediators were generally not supported in the present analyses, although impulsivity was associated with frequency of alcohol use at age 18. It is perhaps surprising that impulsivity predicted the frequency but not the onset of alcohol use, but this may be because we measured these processes in late adolescence. Impulsivity may predict the onset of alcohol use earlier in adolescence, and as we observed, increases in frequency of alcohol use later in adolescence. Additionally, these may not have been the ideal measures for the proposed mediators. For example, a specific measure of drinking motives may have been a stronger predictor of alcohol use than depression symptoms.29 Alternatively, there may be different mediators than the ones proposed in the theoretical framework. Identifying the psychological and behavioral mechanisms that explain these associations will be an important direction for future research. This can be accomplished both by using imaging paradigms that can better parse or constrain the psychological processes engaged during reward processing as well as measuring additional mediators (e.g., drinking motives, sensation seeking, delay discounting) that may link these patterns of brain activity to alcohol use.
The current study has some limitations. First, data on neural function were not collected before age 16, precluding our ability to determine whether alcohol use prior to age 16 preceded development of these patterns of brain activity. However, we controlled for years since initiation of substance use in our analyses to control for potential effects of prior substance use. Second, the MID task may not have been ideal for measuring the reward feedback stage of processing because, although difficulty level was set to result in ~66% accuracy, participants varied somewhat in their accuracy levels during the task, which led to a variable number of reward feedback trials available for each participant. Third, further research is necessary to establish the reliability of neural activity measured with the MID. Research conducted to date has generally focused on adults and suggests variable test-retest reliability for this or similar tasks, with ICCs ranging between .43 to .81.50, 51 It will be critical for future research to establish the reliability of this measure in adolescents before applying it clinically. Fourth, not all participants in the study had initiated alcohol use by age 18, resulting in a zero-inflated count distribution. As a result, we used a zero-inflated Poisson regression to test hypotheses. Thus, the frequency count portion of the model was effectively limited to the participants who reported some amount of alcohol use at age 18. Fifth, the alcohol use measure used in these analyses asked about the frequency of alcohol use in the past 3 months but did not assess differences in the quantity of alcohol use. Sixth, although we had a strong rationale for modeling quadratic effects of VS activity and the effects survived a conservative Bonferroni correction, it is important to acknowledge that some of the quadratic effects may reflect overfitting of the data (particularly the unexpected quadratic effect of the vmPFC in boys). Seventh, this sample was selected based on risk for either substance use or depression. Although this approach helped ensure observable variability in both potential pathways of risk (impulsive and internalizing), generalizability of these results may be limited to adolescents who are already exhibiting risk for either substance use or internalizing problems. Finally, because the sample was composed entirely of Mexican-origin adolescents, it is unknown whether these results will generalize to other populations.
Despite these limitations, several strengths of our study allowed us to extend prior research findings. Notably, our sample was large, comprised of both boys and girls, and consisted of a population (Mexican-origin adolescents) under-represented in prior clinical neuroscience research but with high risk for substance use. Our study also used a prospective longitudinal design to predict change in later markers of risk at age 18 from imaging assessments collected at age 16. Replication of the current findings could aid in tailoring interventions designed to reduce adolescent alcohol use based on factors such as sex. Further research of this type holds promise for delineating the neural pathways associated with the development of future psychopathology, which could inform the prevention and treatment of substance use problems.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health grants R01MH098370 (A.E.G., P.D.H.) and R01DA017902 (R.W.R.), a William T. Grant Foundation Scholars Award 180021 (A.E.G.), a William T. Grant Foundation Mentoring Award 182606 (A.E.G.), and the University of California, Davis–Imaging Research Center Pilot Program (A.E.G.). J.R.S. was supported by Prop. 63, the Mental Health Services Act and the Behavioral Health Center of Excellence at UC Davis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data from this paper were presented as an oral presentation at the Association for Psychological Science 30th Annual Convention; May 24–27, 2018; San Francisco, California.
Disclosure: Drs. Swartz, Weissman, Ferrer, Fassbender, Robins, Hastings, and Guyer and Ms. Beard report no biomedical financial interests or potential conflicts of interest.
References
- 1.Kosterman R, Hawkins JD, Guo J, Catalano RF, Abbott RD. The dynamics of alcohol and marijuana initiation: Patterns and predictors of first use in adolescence. American Journal of Public Health. 2000;90(3):360–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adger JH, Saha S Alcohol use disorders in adolescents. Pediatr Rev. 2013;34(3):103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patrick ME, Schulenberg JE. Prevalence and predictors of adolescent alcohol use and binge drinking in the United States. Alcohol Res. 2013;35(2):193–200. [PMC free article] [PubMed] [Google Scholar]
- 4.Hasler BP, Sitnick SL, Shaw DS, Forbes EE. An altered neural response to reward may contribute to alcohol problems among late adolescents with an evening chronotype. Psychiatry Research: Neuroimaging. 2013;214:357–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casement MD, Shaw DS, Sitnick SL, Musselman S, Forbes EE. Life stress in adolescence predicts early adult reward-related brain function and alcohol dependence. SCAN. 2015;10(3):416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikolova YS, Knodt AR, Radtke SR, Hariri AR. Divergent responses of the amygdala and ventral striatum predict stress-related problem drinking in young adults: Possible differential markers of affective and impulsive pathways of risk for alcohol use disorder. Molecular Psychiatry. 2016;21:348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luijten M, Schellekens AF, Kuhn S, Machielse MWJ, Sescousse G. Disruption of reward processing in addiction: An image-based meta-analysis of functional magnetic resonance imaging studies. JAMA Psychiatry. 2017;74(4):387–398. [DOI] [PubMed] [Google Scholar]
- 8.Haber SN, Knutson B. The reward circuit: Linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. J Neurosci. 2001;21(16):RC159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Forbes EE, Rodriguez EE, Musselman S, Narendran R. Prefrontal response and frontostriatal functional connectivity to monetary reward in abstinent alcohol-dependent young adults. PLoS One. 2014;9:e94640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corral-Frias NS, Nikolova YS, Michalski LJ, Baranger DAA, Hariri AR, Bogdan R. Stress-related anhedonia is associated with ventral striatum reactivity to reward and transdiagnostic psychiatric symptomatology. Psychol Med. 2015;45(12):2605–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nikolova YS, Singhi EK, Drabant EM, Hariri AR. Reward-related ventral striatum reactivity mediates gender-specific effects of a galanin remote enhancer haplotype on problem drinking. Genes, Brain and Behavior. 2013;12:516–524. [DOI] [PubMed] [Google Scholar]
- 13.Hardin MG, Ernst M. Functional brain imaging of development-related risk and vulnerability for substance use in adolescents J Addict Med. 2009;3(2):47–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carey CE, Knodt AR, Conley ED, Hariri AR, Bogdan R. Reward-related ventral striatum activity links polygenic risk for attention-deficit/hyperactivity disorder to problematic alcohol use in young adulthood. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 2017;2:180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hussong AM, Jones DJ, Stein GL, Buacom DH, Boeding S. An internalizing pathway to alcohol use and disorder. Psychology of Addictive Behaviors. 2011;25(3):390–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forbes EE, Brown SM, Kimak M, Ferrell RE, Manuck SB, Hariri AR. Genetic variation in components of dopamine neurotransmission impacts ventral striatal reactivity associated with impulsivity. Molecular Psychiatry. 2009;14:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bjork JM, Knutson B, Hommer DW. Incentive-elicited striatal activation in adolescent children of alcoholics. Addiction. 2008;103(8):1308–1319. [DOI] [PubMed] [Google Scholar]
- 18.Elsayed NM, Kim MJ, Fields KM, Olvera RL, Hariri AR, Williamson DE. Trajectories of alcohol initiation and use during adolescence: The role of stress and amygdala reactivity. J Am Acad Child Adolesc Psychiatry. 2018;57(8):550–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blum K, Cull JG, Braverman RR, Comings DE. Reward deficiency syndrome. American Scientist. 1996;84(2):132–145. [Google Scholar]
- 20.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stringaris A, Vidal-Ribas Belil P, Artiges E, et al. The brain’s response to reward anticipation and depression in adolescence: Dimensionality, specificity, and longitudinal predictions in a community-based sample. Am J Psychiatry. 2015;172(12):1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nikolova YS, Bogdan R, Brigidi BD, Hariri AR. Ventral striatum reactivity to reward and recent life stress interact to predict positive affect. Biol Psychiatry. 2012;72(2):157–163. [DOI] [PubMed] [Google Scholar]
- 23.Wacker J, Dillon DG, Pizzagalli DA. The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: Integration of resting EEG, fMRI, and volumetric techniques. NeuroImage. 2009;46(1):327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Epstein J, Pan H, Kocsis JH, et al. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. American Journal of Psychiatry. 2006;163(10):1784–1790. [DOI] [PubMed] [Google Scholar]
- 25.Heitzeg MM, Villafuerte S, Weiland BJ, et al. Effect of GABRA2 genotype on development of incentive-motivation circuitry in a sample enriched for alcoholism risk. Neuropsychopharmacology. 2014;39:3077–3086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schulte MT, Ramo D, Brown SA. Gender differences in factors influencing alcohol use and drinking progression among adolescents. Clin Psychol Rev. 2009;29(6):535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young SE, Corley RP, Stallings MC, Rhee SH, Crowley TJ, Hewitt JK. Substance use, abuse and dependence in adolescence: Prevalence, symptom profiles and correlates. Drug and Alcohol Dependence. 2002;68:309–322. [DOI] [PubMed] [Google Scholar]
- 28.Kuntsche E, Knibbe R, Gmel G, Engels R. Who drinks and why? A review of socio- demographic, personality, and contextual issues behind the drinking motives in young people. Addictive Behaviors. 2006;31(10):1844–1857. [DOI] [PubMed] [Google Scholar]
- 29.Pedrelli P, Collado A, Shapero BG, Brill C, MacPherson L. Different pathways explain alcohol-related problems in female and male college students. Journal of American College Health. 2016;64(7):535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Merikangas KR, He J, Burstein M, et al. Lifetime prevalence of mental disorders in U.S. adolescents: Results from the National Comorbidity Survey Replication-Adolescent Supplement (NCS-A). J Am Acad Child Adolesc Psychiatry. 2010;49(10):980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mojtabai R, Olfson M, Han B. National trends in the prevalence and treatment of depression in adolescents and young adults. Pediatrics. 2016;138(6):e20161878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker DM, Bell MR, Flores C, Gulley JM, Willing J, Paul MJ. Adolescence and reward: Making sense of neural and behavioral changes amid the chaos. The Journal of Neuroscience. 2017;37(45):10855–10866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raznahan A, Shaw PW, Lerch JP, et al. Longitudinal four-dimensional mapping of subcortical anatomy in human development. PNAS. 2014;111(4):1592–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willing J, Juraska JM. The timing of neuronal loss across adolescence in the medial prefrontal cortex of male and female rats. Neuroscience. 2015;301:268–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Atherton OE, Conger RD, Ferrer E, Robins RW. Risk and protective factors for early substance use initiation: A longitudinal study of Mexican-origin youth. Journal of Research on Adolescence. 2016;26(4):864–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weissman DG, Schriber RA, Fassbender C, et al. Earlier adolescent substance use onset predicts stronger connectivity between reward and cognitive control brain networks. Dev Cogn Neurosci. 2015;16:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin MJ, Conger RD, Robins RW. Family stress, familism, and substance use by Mexican-American adolescents. Developmental Psychology. 2018;Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schriber RA, Anbari Z, Robins RW, Conger RD, Hastings PD, Guyer AE. Hippocampal volume as an amplifier of the effect of social context on adolescent depression. Clin Psychol Sci. 2017;5(4):632–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weissman DG, Conger RD, Robins RW, Hastings PD, Guyer AE. Income change alters default mode network connectivity for adolescents in poverty. Developmental Cognitive Neuroscience. 2018;30:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guyer AE, Nelson EE, Perez-Edgar K, et al. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. J Neurosci. 2006;26(24):6399–6405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lamm C, Benson BE, Guyer AE, et al. Longitudinal study of striatal activation to reward and loss anticipation from mid-adolescence into late adolescence/early adulthood. Brain Cogn. 2014;89:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cho YT, Fromm S, Guyer AE, et al. Nucleus accumbens, thalamus and insula connectivity during incentive anticipation in typical adults and adolescents. NeuroImage. 2013;66:508–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elliott DS, Ageton SS, Huizinga D. Explaining delinquency and drug use. Boulder, CO: Behavioral Research Institute;1982. [Google Scholar]
- 44.Keller MC. Gene x environment interaction studies have not properly controlled for potential confounders: The problem and the (simple) solution. Biol Psychiatry. 2014;75(1):18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinberger DA, Schwartz GE. Distress and restraint as superordinate dimensions of self- reported adjustment: A typological perspective. Journal of Personality. 1990;58(2):381–417. [DOI] [PubMed] [Google Scholar]
- 46.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39(1):28–38. [DOI] [PubMed] [Google Scholar]
- 47.Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. 1995;57:289–300. [Google Scholar]
- 48.Romens SE, Casement MD, McAloon R, et al. Adolescent girls’ neural response to reward mediates the relation between childhood financial disadvantage and depression. J Child Psychol Psychiatry. 2015;56(11):1177–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Casement MD, Keenan KE, Hipwell AE, Guyer AE, Forbes EE. Neural reward processing mediates the relationship between insomnia symptoms and depression in adolescence. Sleep. 2016;39(2):439–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Plichta MM, Schwarz AJ, Grimm O, et al. Test-retest reliability of evoked BOLD signals from a cognitive-emotive fMRI test battery. NeuroImage. 2012;60:1746–1758. [DOI] [PubMed] [Google Scholar]
- 51.Wu CC, Samanez-Larkin GR, Katovich K, Knutson B. Affective traits link to reliable neural markers of incentive anticipation. NeuroImage. 2014;84:279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.