Abstract
Lineage plasticity has emerged as an important mechanism of treatment resistance in prostate cancer (PC). Treatment refractory PCs are increasingly associated with loss of luminal prostate markers, and in many cases induction of developmental programs, stem cell-like phenotypes, and neuroendocrine/neuronal features. Clinically, lineage plasticity may manifest as low prostate specific antigen (PSA) progression, resistance to AR pathway inhibitors, and sometimes small cell/neuroendocrine pathologic features observed on metastatic biopsy. This mechanism is not restricted to prostate cancer as other malignancies also demonstrate lineage plasticity during resistance to targeted therapies. At present, there is no established therapeutic approach for patients with advanced prostate cancer developing lineage plasticity or small cell neuroendocrine prostate cancer (NEPC) due to knowledge gaps in the underlying biology, few clinical trials address questions in this space, and the outlook for patients remains poor. To move forward, urgently needed are: (i) a fundamental understanding of how lineage plasticity occurs and how it can best be defined; (ii) the temporal contribution and cooperation of emerging drivers; (iii) preclinical models that recapitulate biology of the disease and the recognized phenotypes; (iv) identification of therapeutic targets; and (v) novel trial designs dedicated to the entity as it is defined. This Perspective represents a consensus arising from the National Cancer Institute (NCI) Workshop on Lineage Plasticity and Androgen Receptor-Independent Prostate Cancer. We focus on the critical questions underlying lineage plasticity and AR-independent prostate cancer, outline knowledge and resource gaps, and identify strategies to facilitate future collaborative clinical translational and basic studies in this space.
Introduction
Lineage plasticity is a biologic process that occurs during normal development and later as a mechanism that promotes cell survival when adapting to their environment, evading stress, or repairing tissues. Plasticity may manifest as reversible or irreversible changes in cellular ‘identity’, whereby cells take on an alternative morphologic, phenotypic, or epigenetic state(1). In cancer, lineage plasticity facilitates carcinogenesis, metastasis, and treatment resistance(2). During therapy-related lineage plasticity, differentiated tumor cells acquire new phenotypes, in some cases reverting back to a more ‘stem-like’ state followed by re-differentiating towards an alternative ‘cell fate’ in order to bypass therapeutic pressure. This versality of cellular state is particularly prominent in cancer types with effective therapies that target major growth programs and lineage directing factors (eg., BRAF-mutant melanoma, EGFR-mutant lung cancer, AR-driven prostate cancer). In these cases, early ‘targetable’ genomic alterations are often retained, but expression of the pressured target is suppressed. Despite preserving a molecular memory of their differentiated cancer cell precursor, alternative lineage programs facilitate subsequent tumor progression.
Prostate cancer is a malignancy driven by androgen receptor (AR) signaling, and AR-targeted therapies are commonly used to treat patients at all stages of the disease. Prostate tumors most frequently display an adenocarcinoma morphology reminiscent of normal luminal prostate architecture, with higher levels of disorganization associated with advanced tumor grade. Downstream markers of canonical AR activity, such as prostate specific antigen (PSA), are used as clinical biomarkers to confirm a diagnosis of prostate cancer and for disease monitoring. Although several prostate cancer drugs are effective at lowering androgen levels and/or blocking the AR directly, metastatic prostate cancers universally develop treatment resistance(3). Acquired resistance is typically due to re-activation of AR signaling mediated, in part, by genomic mutation, amplification, or structural rearrangement of the AR gene itself. However, loss of AR expression and/or downstream signaling occurs in an estimated 15–20% of castration resistant tumors(4–6). In extreme cases, tumors may reprogram towards alternative pathways adopting features of neuroendocrine, neuronal, or other lineages. Clinically, these cancers are notable for attenuated AR signaling and a range of histologies of which the most common exhibit small cell neuroendocrine carcinoma characteristics. At present, the definition and mechanistic underpinnings of this subset remain ill-defined and there are no established therapeutic approaches for small cell/NEPC or other phenotypes. Few clinical trials address lineage plasticity, and the outlook for patients remains poor.
To address the challenges resulting from lineage plasticity, the NCI organized a Workshop focused on Lineage Plasticity and Androgen Receptor-Independent Prostate Cancer. Five working groups were assembled prior to the workshop to formulate questions underlying basic, translational, and clinical knowledge gaps and to develop approaches to address them (Supplementary Information). This Perspective generated as a result of the workshop summarizes concepts, data, deficiencies, and opportunities that drive critical questions underlying lineage plasticity and AR-independent prostate cancer.
Gap 1: A fundamental understanding of how lineage plasticity occurs
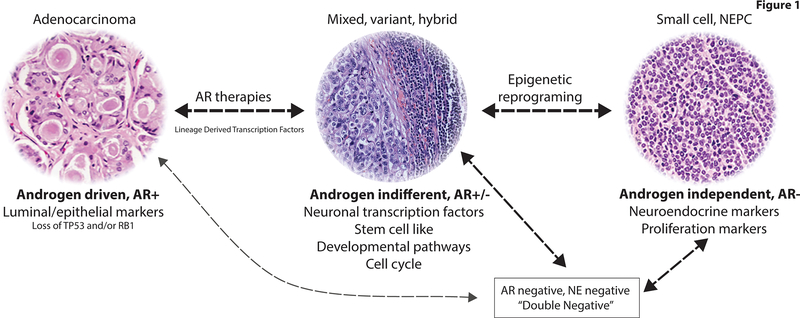
Lineage plasticity is a term that implies that cells are capable of reprogramming their identity by acquiring an alternative lineage state and that this process is at some point plastic, or reversible. Whether AR-independent treatment resistance is mediated through an intermediate stem-like state, an ‘epithelial mesenchymal transition’, or through direct transdifferentiation to acquire new characteristics and the extent of the reversibility of these processes, are not well understood. Tumors with mixed or overlapping features expressing both AR and AR pathway genes as well as neuroendocrine markers (‘hybrid’, or ‘amphicrine’ tumors), or those lacking both AR and neuroendocrine markers (‘double negative’ tumors) may further represent distinct disease states or a continuum (Figure 1). Lineage programs such as gastrointestinal, squamous, and others have also been described(7). Identifying subsets within AR independent disease provides expanding insights into the unique biology of AR independent therapy resistance as well as potentially distinct therapeutics, such as the preferential activation of fibroblast growth factor signaling in ‘double negative’ CRPC(4).
Figure 1.
Schematic of the proposed molecular events and transition states underlying lineage plasticity that occurs during CRPC progression from an AR-positive, AR-driven prostate adenocarcinoma (luminal phenotype) towards an AR-negative, AR-independent cellular state (e.g., small cell/neuroendocrine).
The transition of prostate cancer away from a luminal epithelial phenotype is facilitated by genomic loss of the tumor suppressors RB1 and TP53, leading to changes in stem cell, developmental, and EMT programs, mediated in part by the lineage pluripotency transcription factor SOX2(8–10). Down-regulation of REST (a transcription factor that normally silences the expression of neuron-specific genes in non-neuronal cells) through splicing regulated by SRRM4(11,12), as well as activation of lineage associated transcription factors such as N-myc(13,14), Onecut2(15,16), and BRN2(17) and de-repression of developmental genes such as PEG10(18) also occur. These changes in cellular programs associates with an increase in cellular proliferation and amplification or overexpression of cell cycle regulators (eg., aurora kinase, polo-like kinase)(13,19), leading to aggressive tumor growth and often visceral metastatic spread. Lineage tracing studies (20) have supported a luminal cell of origin of neuroendocrine prostate cancer cells that arise upon potent androgen blockade, supporting a ‘transdifferentiation’ process. Important questions concerning the timing, cooperation, and feedback of the drivers of transdifferentiation processes and their relationships to oncogenic programs and AR silencing remain to be addressed. Further, identification of noncanonical AR programs associated with AR-positive, PSA low tumors as well as genes de-repressed by AR inhibition could provide biologic insights into other potential early mediators of lineage plasticity. Epigenetic modifications including changes in DNA methylation and upregulation of the polycomb complex gene enhancer of zeste 2 (EZH2) also play a role in silencing the luminal program and in reprogramming lineage(8,10). Targeting epigenetic programs may result in some degree of reversibility or reversion back to a more luminal state. Metabolic shifts mediated in part by loss of PKCλ/ι(21), which support proliferation and epigenetic changes, as well as changes in tumor hypoxic state(15) are also observed during NEPC progression. A better understanding of the fundamental cellular and molecular interactions that drive lineage plasticity and the role of the tumor microenvironment, metabolic alterations, and changes in epigenetic state require further focus and study. This will help to further refine the working definition of this cellular state in prostate cancer.
Working Definition of Lineage Plasticity in Prostate Cancer
A progressive state of castration resistant prostate cancer associated with the loss of AR-regulated lineage characteristics and in some situations the acquisition of new phenotypes (e.g. neuroendocrine features). Plasticity is driven by intrinsic and/or acquired alterations in the biological activities of tumor cells and the tumor microenvironment that involve metabolic, genetic and epigenetic changes. The consequences of these changes comprise a gene expression profile/phenotype consistent with AR/androgen independence and sustained proliferation.
Open questions
Is loss of canonical AR signaling equivalent to AR independence? For instance, could the presence of AR in cases with low AR signaling still drive a non-canonical program to sustain tumor growth?
How many lineages are there and how do we best recognize and define them?
What degree of reversibility underlies lineage plasticity? Could targeting epigenetic alterations completely revert back toward a luminal state, and/or do genetic factors ‘fix’ tumors in an alternate state?
What are the mechanisms and mediators of tumor microenvironment regulation that underlie lineage plasticity–aging, senescence, inflammation, metabolism, oncogenic and non-oncogenic biological processes; and the cellular dynamics of immune cells, fibroblasts, adipocytes, endothelial, and neuronal cells that effect this process?
How do lineage determining transcription factors interact or cooperate during prostate cancer progression?
What extent do factors related to the metastatic environment (e.g., liver, bone), and/or oncogene-associated or therapy-induced senescence contribute to lineage plasticity?
Gap 2: Determining the temporal contribution and cooperation of emerging drivers
How AR-directed therapies impact clonal evolution, clonal selection, and the evolutionary bottlenecks that shape lineage plasticity is not well established. Mapping these processes have important clinical implications for biomarkers and therapeutics. For instance, hormone-naive high-grade localized prostate cancers with low PSA levels may express neuroendocrine genes and harbor lower AR signaling(22), which may represent a high risk patient population for developing lineage plasticity when later treated with AR pathway inhibitors. RB1 loss, with or without TP53 mutation or deletion, also occurs in a subset of prostate adenocarcinomas along the disease spectrum(23,24), and may identify patients for aggressive surveillance and/or early intervention therapeutic strategies. The timing and cooperation of lineage derived transcription factors further shapes downstream programs as well as the epigenetic landscape. Whether intermittent AR inhibition, potentially alternating with therapies that target these alterations to induce differentiation, could delay the onset of lineage plasticity or prevent its emergence due to reconstitution of the tumor ecosystem with luminal lineage cells is intriguing and requires further study.
Understanding how mixed CRPC tumors that express both AR and neuroendocrine (or other) markers respond to AR therapies, and whether double negative and small cell NEPC share treatment vulnerabilities such as sensitivity to platinum chemotherapy, have important clinical implications for therapy selection. Assessment of the impact of emerging therapies (and combination approaches) on the prevention or reversal of phenotype or for their impact on tumor kill will help refine treatment goals; the readouts from targeting epigenetic alterations or alternative splicing, for instance, may differ from the goals of targeting proliferation or downstream effects of fixed genomic events. Serial metastatic biopsies or liquid biopsies will be useful for understanding the sequence of aberrations in patients, and when combined with clinical features such as PSA trends and sites of radiologic progression, have the potential to improve the diagnosis of patients progressing through the various biologic disease states and to help refine clinical endpoints. One proposed translational research strategy for identifying the context and impact of RB1 alterations is highlighted in Box 1.
Box 1. One of the proposed translational research strategies developed at the workshop to address the role and cooperation of RB1 and TP53 in driving lineage plasticity in prostate cancer and other Rb1 deficient tumors (e.g., SCLC) and an approach towards the development of novel biomarker-driven therapeutics.
What are the vulnerabilities in NEPC vs. SCLC and other RB deficient cancers?
Aim 1: Develop a series of collaborating institutions with large clinical volumes of CRPC patients and clinical databases to determine clinical parameters associated with RB loss +/− TP53 loss and type of alteration (mutation; copy loss). Compare to other dual RB/p53 loss cancer types (SCLC).
Aim 2: Develop tissue and blood based markers (IHC/ISH/transcriptional/proteomic/metabolic signatures) that detect/identify and associate with specific RB1 +/− TP53 aberrations.
Aim 3: Develop isogenic models of RB +/−TP53 deficiency in PC backgrounds (cell lines, PDX, organoids) to assess: i) molecular consequence for E2F and TP53 signaling, ii) biological impact and iii) relevance for reflecting clinical observations as per Aim 1.
Aim 4: Utilize models developed in Aim 3 to screen for vulnerabilities/synthetic lethality using high throughput strategies (e.g. CRISPR screening, FDA approved compound screening) with the goal of establishing the foundation for the next phase of clinical testing
Aim 5: Refine strategies for accurately assessing RB and TP53 status in and liquid biopsy, and develop longitudinal studies to determine the impact on progression from PC to CRPC to NEPC.
Open questions
What are the relationships between lineage-drivers and oncogenic programs?
Does the presence of RB1 and/or TP53 aberrations in prostate adenocarcinoma predispose patients toward developing lineage plasticity and small cell/neuroendocrine features?
Is there an intermediate clinical state where intervention may be effective in preventing or reversing lineage plasticity?
Do PSA dynamics adequately reflect AR signaling in patients?
How and when is the AR lost in the context of disease progression and how do specific therapeutics influence resistance pathways?
Are there strategies to maintain cell differentiation and AR dependency while still restraining tumor growth?
Do increased DNA damage response gene activities, or other specific transcriptional signatures contribute to the lineage plasticity program, and can these activities be exploited therapeutically?
Are there synthetic lethal therapeutic approaches that may be exploited to target lineage plasticity and AR independent prostate cancer?
What degree of intra-tumoral heterogeneity is seen within and across metastases in individual patients?
Gap 3: Preclinical models that recapitulate biology of the disease and the recognized phenotypes
Preclinical models that represent the prostate cancer disease spectrum are essential for understanding biology and for the development of novel therapeutics. Currently, the number and types of cell lines that recapitulate lineage plasticity are limited, though there are patient derived xenografts and organoid models of AR negative and neuroendocrine prostate cancer that have been described and characterized(25–29) (Table 1). Dynamic in vivo models, such as a PDX model that changes phenotype from an AR-positive adenocarcinoma to an AR-negative NEPC(30) and xenografts that lose AR signaling dependence during enzalutamide resistance(17) have been used to capture the lineage plasticity process. Genetically engineered mouse models such as the TRAMP model and others designed to alter key molecular events, including loss of PTEN, TP53, and RB1, and/or gain of MYCN, are also useful tools to study the cooperation and timing of emerging tumor suppressors and oncogenes(9,10,14,20,21,31). Optimizing and sharing protocols for model development and optimization was discussed as an unmet need, especially as fresh tumor biopsies are more commonly performed clinically and may be used for patient derived model generation.
Table 1: Preclinical models.
Table of preclinical models that display lineage plasticity, small cell /NEPC histologic or molecular features, and/or AR-signaling indifference.
| Model | Source | Pathologic and molecular features |
|---|---|---|
| NCI-H660 cell line | Lymph node metastasis | Small cell carcinoma; AR negative; PSA negative; TMPRSS2-ERG gene fusion positive; synaptophysin, CD56, NSE positive |
| LuCAP 49, 93, 145.1, 145.2 patient derived xenograft (PDX) and PDX-organoid models | Omental metastasis (LuCAP 49), TURP (LuCAP 93), liver (LuCAP 145.1), lymph node (LuCAP 145.2) | Neuroendocrine histology; AR negative; synaptophysin positive |
| MDA PCa 144–4, 144–13, 155–2, MDA PCa 177–0, MDA PCa 189–1 PDX models | Salvage pelvic exenteration (MDA 144, MDA 155–2), prostate (MDA 177, MDA 189–1) | Small cell and large cell carcinoma; Aggressive variant clinical features; TMPRSS2-ERG gene fusion positive (MDA 144) |
| LTL352 and LTL370 PDX models | Urethral (LTL352) and penile (LTL370) metastasis | Small cell carcinoma; AR negative; PSA negative; synaptophysin, NSE positive |
| LTL331 transdifferentiation PDX model | Primary prostate cancer (LTL331) PDX model that develops castration resistant NEPC in vivo (LTL331R) | Primary high-grade adenocarcinoma (LTR331) and neuroendocrine prostate cancer histology (LTL331R); TMPRSS2-ERG fusion positive; LTR331: AR positive; LTL331R: AR negative, PSA negative, synaptophysin positive, chromogranin positive, CD56 positive |
| Enzalutamide resistant LNCaP (42D, 42F) | Cell lines developed from enzalutamide resistant LNCaP xenograft tumors | AR positive; PSA low; chromogranin, synaptophysin positive |
| MSK-PCA4 Patient Derived Organoids | Pleural effusion | Neuroendocrine features; AR low; synaptophysin positive |
| WCM Patient Derived Organoids | Metastatic lesions liver (155), bone (154), lymph node (1078), soft tissue (1262) | Small cell carcinoma; AR negative; PSA negative; synaptophysin, NSE, chromogranin positive |
| TRAMP mouse | C57BL/6 mice expressing the rat probasin driving expression of SV40 large and small T antigens in prostatic epithelial cells | Adenocarcinoma to Small cell carcinoma; Rb1 and Tp53 loss; Visceral metastases |
| p53PE−/−RbPE −/− mouse | Conditional knockout of p53 and Rb (p53PE−/−; RbPE−/−) from the epithelium of all lobes of the mouse prostate | Small cell carcinoma; Rb1 and Tp53 loss |
| N-Myc-myrAKT1 mouse | Human Prostate Basal Cells overexpressing NMYC and AKT1 implanted subcutaneously in NOD-SCID-IL2Rγnull (NSG) mice | Neuroendocrine features; AR low; chromogranin, synaptophysin positive |
| Ptenf/f;LSL-MYCN+/+ mouse | GEMM mice carrying MYCN gene integrated into the ROSA26 (LSL-MYCN) locus, a Tmprss2-driven tamoxifen-activated Cre recombinase and a Pten conditional knockout allele | Divergent differentiation Neuroendocrine features; AR low/AR negative; chromogranin, synaptophysin positive; visceral metastases with castration |
| PBCre4:Ptenf/f:Rb1f/f(DKO), PBCre4:Ptenf/f: Rb1f/f:Trp53f/f (TKO) | GEMM mice, PBCre4 transgene is used to delete floxed alleles specifically in prostate epithelium | Neuroendocrine features with metastases; AR positive/ AR low; synaptophysin positive |
| NPp53 mice | GEMM mice, inducible Nkx3.1CreERT2 driver to delete PTEN and TP53 genes in adult prostate epithelium | Abiraterone resistant; neuroendocrine features- transdifferentiation (lineage tracing); AR low/AR negative; synaptophysin positive |
| Ptenf/f-Prkcif/f-PbCre4+ mice (DKO) | GEMM mice, Ptenf/f-PbCre4+ mouse line (PTEN KO) with PTEN specifically deleted in the prostate epithelium, crossed with Prkcif/f mice | Neuroendocrine features; AR low/AR negative; chromogranin, synaptophysin positive |
Open questions
What are the preclinical models that can be manipulated in such a way to reflect transition from androgen-dependent to independent states and further transitions that encompass new differentiation programs such as neuroendocrine phenotypes?
Are the genomics, transcriptomics, epigenetics, metabolomics of these model systems representative of advanced human prostate cancer? Do they change over time or with conditions?
Can we develop of a series of paired PDXs-organoids with morphological and molecular information of organoids, PDXs and human tumor of origin representing the complex molecular landscapes of prostate cancer?
How do we use model systems to assess reversibility and thereby understand the degree of plasticity?
What are “the human models” that can be utilized to compare and contrast with the animal models (e.g. rapid autopsies, CTCs, cfDNA etc)?
Gaps 4 and 5: Identification of therapeutic targets and novel trial designs dedicated to the entity as it is defined
Given the relatively high reported frequency of small cell NEPC post AR-directed therapy, the NCCN guidelines now recommend consideration of metastatic biopsy for any CRPC patient to look for small cell transformation. If found, patients could be considered for platinum-based chemotherapy based on extrapolation of clinical data for small cell lung cancer and supported by recent platinum-chemotherapy studies in aggressive variant prostate cancer(32). Although these practice guidelines have recently changed, the diagnosis of lineage plasticity remains a clinical challenge due to a lack of standardized or widely accepted clinical or pathologic criteria. While pure small cell carcinoma defined by morphology is most often congruent between pathologists, those with mixed/hybrid or with varied degrees of neuroendocrine differentiation are often subject to inter-observer variability(33).
The diagnosis of other phenotypes may require more detailed studies that involve immunohistochemistry or other measures of gene expression. There are no standard criteria for when to perform ancillary studies such as immunohistochemistry for classical neuroendocrine markers (e.g., chromogranin, synaptophysin), AR protein, PSA, or other markers(34,35). There are also times when AR is expressed in NEPC but the canonical AR transcriptome (including downstream targets like PSA) is low; identification of downstream genes that may be activated by AR in this setting would be informative and potentially help refine definitions. The incidence of AR-negative or AR-low CRPC without NEPC features or alternative lineage CRPC, and the degree of heterogeneity within this spectrum, are also not known. The workshop discussed a path toward the standardization of tumor morphology nomenclature and systematic prioritization of ancillary testing in metastatic CRPC (Table 2).
Table 2: Pathology morphology and available ancillary testing.
Prioritization of pathology assessment when lineage plasticity is suspected. Tissue should be evaluated for morphologic characteristics that may support small cell carcinoma, neuroendocrine differentiation, or other histologies. Possible ancillary protein studies to consider performing by immunohistochemistry (IHC) are listed, with markers in red indicating existing CLIA grade tests.
| Morphology |
| %tumor |
| %glandular |
| %small cell |
| Protein studies (red indicates existing CLIA-validated biomarkers) |
| Molecular/Cell cycle |
| • Rb (Rb function: cyclin D1/p16) |
| • P53 (p53 function: p21) |
| • Ki-67 (discovery for threshold) |
| Lineage markers |
| • Chg (Canonical) |
| • Syp (Canonical) |
| • CD56 |
| • FOXA2 |
| • INSM1 |
| • ASCL |
| • Cytokeratin—Cam5.2 |
| • Neurofilament |
| Androgen signaling |
| • AR (canonical) |
| • AR-v7 |
| • PSA |
Given the current challenges of performing tumor biopsies and the variability in morphologic features that occur post-therapy, platinum-based chemotherapy trials have been conducted for patient with aggressive clinical features even the absence of tumor biopsy (32). Notably, these patients defined by clinical features suggestive of AR independence often harbor combined somatic tumor alterations involving PTEN, TP53, and/or RB1, similar to what has been observed in small cell NEPC(29).
With a loss of AR expression, tumors may also lose other related proteins including prostate specific membrane antigen (PSMA). This manifests clinically as PSMA-negative lesions on PSMA PET imaging. Low or heterogeneous PSMA PET-CT combined with FDG positivity identifies patients with poor prognosis(36), therefore representing a potential non-invasive means to identify AR-independent resistance. However, there are still many unknowns regarding PSMA regulation and PSMA imaging characteristics along prostate cancer progression and therapy resistance. Multi-institutional collaborative studies combining PSMA with FDG PET imaging, in combination with tumor biopsies of discordant lesions and liquid/tissue molecular assessment, were designed at the workshop.
There is no known effective next line therapy for patients with small cell NEPC especially after platinum chemotherapy. Although second line small cell lung cancer regimens may be considered, their data in prostate cancer is scarce. Notably, while loss of AR activity eliminates the AR pathway as a therapeutic target, the acquisition of new characteristics that associate with NEPC and other phenotypes exposes new targets and vulnerabilities. Available drugs targeting the AURKA/MYCN or AURK/RB1 axis, LSD1, EZH2, DLL3, and approaches to target the immune landscape (eg., vaccine, TGF-βi, IL8i and immune checkpoint inhibitors) are in development for NEPC, as well as drugs targeting FGF/MAPK for double negative CRPC. The design of rational combination or co-targeting strategies, such as EZH2 inhibition in combination with AR pathway inhibitors or immunotherapy, may also have value. Ultimately, defining the appropriate inclusion criteria and endpoints for trials focused on lineage plasticity will be critical. Trials will require multicenter collaboration and molecularly-based biomarker inclusion and careful patient selection. Incorporation of emerging molecular biomarkers in combination with clinical features may help distinguish sub-categories of AR-independent CRPC and inform the development of liquid and imaging biomarker approaches for clinical trial design.
Open questions
What are the clinical and pathologic differences between small cell carcinoma and CRPC with neuroendocrine differentiation? Are IHC markers required and do they capture AR activity?
Is the increased detection of small cell /NEPC due to increased awareness or is this due to more potent AR directed therapies? Will the incidence of NEPC increase with recent approvals of potent AR-targeted drugs earlier in the disease (ie., metastatic castrate sensitive prostate cancer and non-metastatic castration resistant prostate cancer)?
How well does loss of PSMA or PSMA heterogeneity on imaging in combination with FDG PET-CT non-invasively identify patients developing lineage plasticity? What other radiologic or radiomic (i.e., subvisual tools) may be applied for the early detection of lineage plasticity?
Platinum sensitivity is also mediated by germline or somatic alterations involving DNA repair genes, which occurs in approximately 20% of CRPC independent of histology. What is the degree of overlap between DNA repair deficiency and lineage plasticity?
Is reversal of phenotype or tumor kill the primary goal of therapy? What clinical endpoints may be used to assess these outcomes?
What would a multi-arm clinical trial design look like for patients developing lineage plasticity and AR independent prostate cancer? Which clinical or molecular features are ready to use as inclusion criteria?
Lessons from other cancer types (the scope of the problem)
Similar to prostate cancer, malignancies arising from other anatomic sites, tissues and cell types also develop lineage plasticity as a mechanism of therapy resistance. For instance, 5–15% of EGFR-mutant lung adenocarcinomas transform to small cell lung cancer (SCLC) histology during acquired resistance to EGFR targeted therapies with retention of the original EGFR mutation(37). Cases of small cell transformation in lung cancer have also been reported after immunotherapy(38). RB1 and TP53 genomic alterations are universally present in both de novo and transformed SCLC and rarely present in unselected lung adenocarcinomas other than those that later develop histologic transdifferentiation(39–41). Whether pre-existing RB1 and TP53 loss may be used as biomarkers to identify high risk lung adenocarcinoma patients for alternative treatments is yet to be determined. Understanding whether this predisposition for lineage plasticity is also the case for the subset of castration resistant prostate adenocarcinomas and localized prostate cancers that harbor TP53/RB1 loss has important implications for the early detection and management. In melanoma, phenotypic switching with distinct cellular populations (e.g., invasive versus proliferative) and developmental cell states (i.e., MITF low or high) frequently co-existing and dynamically regulated plays a central role in metastasis and therapy resistance(42–44). This switching is hypothesized to be largely regulated by epigenetic modifiers, hypoxia, and the tumor microenvironment, but specific factors remain to be fully elucidated. Similar to prostate cancer, much is still to be learned in melanoma plasticity regarding the number and types of phenotypic states that co-exist and their interface with the genetics of the tumor (i.e. do some genomic lesions make the cells more or less responsive to plasticity signals?). In pancreatic ductal adenocarcinoma, lineage heterogeneity associates with poor outcomes; lineage tracing studies have pointed to an acinar cell of origin of neuroendocrine cells and this is regulated by MYC and epigenomic changes in response to environmental signals(45). A subset of breast cancers also develop lineage plasticity and in some cases the luminal phenotype and/or estrogen receptor expression are lost with disease progression; this process may be mediated in part by subpopulations of cells with stem-like properties(46). Given these disease parallels, emerging mechanisms underlying plasticity, stemness, and cellular reprogramming during therapy resistance are pointing to shared mechanisms and potential targets across malignancies.
Summary
It is increasingly recognized that a subset of prostate cancers evades AR-targeted therapies through the development of lineage plasticity. This is associated with loss of AR or AR signaling, frequent RB1/TP53 loss of function, and activation of alternative lineage programs including neuronal, neuroendocrine, stem-like and developmental pathways. In some instances, lineage plasticity occurs directly through trans-differentiation processes whereas in other situations tumor cells de-differentiate to a stem-like state followed by reprogramming to a new phenotype. Continued tumor evolution under treatment pressures may occur through clonal selection. Although there are many open questions, recent studies have identified newly relevant biologic pathways and actionable targets. Addressing the outlined gaps in knowledge will ultimately accelerate the translation of new biologic discoveries into the clinic.
Supplementary Material
Box 2: Glossary.
AR -signaling (canonical): androgen receptor signaling that occurs through ligand (androgen)-mediated or ligand-independent means, resulting in activation of downstream programs critical for prostate growth, development, and function
AR -signaling independent or AR indifferent: sustained growth of prostate tumor cells that is not driven by or reliant on downstream canonical AR signaling
Cellular identity or differentiation: a means of classifying cells based on their phenotype or physiologic function
Cellular determination: the process by which a cell becomes specialized to perform a specific function
Clonality: cells that share a common ancestry
Differentiation: the process by which a stem cell or progenitor cell matures into a cell with a specific identity. De-differentiation is when a differentiated cell loses its matured cellular identity to become less mature and more stem-like
Double negative prostate cancer: a subset of castration resistant prostate cancer that does not express the AR and also does not express neuroendocrine markers
Epigenome/epigenetics: Changes in gene expression and cellular phenotype caused by mechanisms other than changes in DNA
Epithelial Mesenchymal Transition: a process by which epithelial cells lose their cell-cell adhesion and gain migratory and invasive properties to become more like mesenchymal cells, which may facilitate cancer growth and metastatic spread
Embryonic stem cells: pluripotent stem cells that have the potential to differentiate into multiple cell types and are capable of self-renewal
Lineage determining transcription factors: proteins that bind to DNA, either alone or in cooperation with other partners, to control transcriptional output of a cell type to regulate its phenotype and functional characteristics
Lineage reprogramming: the conversion of a mature, terminally differentiated cell type into another mature cell type with or without undergoing de-differentiation
Lineage tracing: A method that delineates all progeny produced by a single cell or a group of cells
Multipotent: Potential of a cell to form different lineages
Plasticity: Ability of a cell to convert from one cell type to another. This may refer to the potential of a differentiated cell to de-differentiate and then re-differentiate into a new state
Progenitor: ancestor cell that gives rise to a specific type of differentiated cell
Neuroendocrine: cells that are neural and endocrine in structure or function. In the pathologic classification of neoplasms, this designation is typically based on tumor morphology rather than function. Well-differentiated neuroendocrine tumors (eg., carcinoids) and poorly- differentiated neoplasms (eg., small cell carcinoma) have distinct biology and may arise in various anatomic sites
Pluripotent: A cell that capable of developing into any cell type
Small cell carcinoma: morphologic definition based on pathologic review of tumor hematoxylin and eosin stained slides demonstrating small blue cells, high grade features, scant cytoplasm, distinct nuclear features (i.e., fine chromatin, lacking prominent nucleoli). Protein expression of classical neuroendocrine markers such as chromogranin or NSE may be present but are not required
Stem-like: cells that possess characteristics similar to normal stem cells, such as the ability to give rise to other cell types within a tumor
Transdifferentiation: reprogramming of a differentiated cell of one lineage into a differentiated cell of another lineage
Statement of Translational Relevance.
Lineage plasticity associated with loss of AR signaling dependence and the acquisition of alternative lineage programs occurs in up to 20% of advanced prostate cancer patients as a mechanism of treatment resistance, with important clinical and therapeutic implications. We discuss our current understanding of mechanisms underlying this process and outline a path towards the development of novel biomarkers and trials for this molecularly distinct subset of patients.
Acknowledgements
This manuscript represents a consensus perspective arising from the National Cancer Institute (NCI) Workshop on Lineage Plasticity and Androgen Receptor-Independent Prostate Cancer (held December 2018), a joint effort by the NCI’s Division of Cancer Treatment and Diagnosis, Division of Cancer Biology, Center for Cancer Research, Coordinating Center for Clinical Trials, together with investigators of the NCI SPOREs in Prostate Cancer and the Prostate Cancer Task Force of the NCI Genitourinary Steering Committee. The was co-chaired by H.B., W.D., and P.S.N. The goal was to assemble leaders in the field to share scientific insights and identify emerging opportunities in basic, translational and clinical research. We thank Tamara Walton with her help in the planning and coordination of the workshop. This perspective is dedicated to Dr. Andrew Hruszkewycz, recently deceased, whose scientific expertise, dedication and genuine passion were instrumental in the success of this workshop.
Conflicts of Interest Statement. H.B. has received research funding from AbbVie, Astellas, Janssen and has consulted with Janssen, Astellas, Sanofi Genzyme. H.I.S has received research funding from Janssen, Epic Sciences, and has consulted with Amgen and Janssen. E.Y. has received research funding from Agensys, Bayer, Dendreon, Merck, Seattle Genetics, and fees and honoraria from Amgen, Bayer, Dendreon, Janssen, Merck, Pharmacyclics, QED, and Seattle Genetics. A.D. has consulted with Roche, EMD Serono, Celldex, Janssen, Cybrexa, Self Care Catalyst. J.G. research has been supported by NCI collaborative research and development agreements (CRADAs) with a number of companies including Bavarian Nordic, EMD Serono, Pfizer and Etubics. C.L.S. serves on the board of directors of Novartis; is a cofounder of ORIC Pharmaceuticals and coinventor of enzalutamide and apalutamide; is a science advisor to Agios, Beigene, Blueprint, Column Group, Foghorn, Housey Pharma, Nextech, KSQ, Petra, and PMV; and is a cofounder of Seragon, purchased by Genentech/Roche in 2014. C.M.R. has consulted with AbbVie, Amgen, Ascentage, Astra Zeneca, BMS, Celgene, Daiichi Sankyo, Genentech/Roche, Ipsen, Loxo, and Pharmar, and is on the scientific advisory boards of Bridge, Elucida, and Harpoon.
Footnotes
Supplementary Information
- Pre-meeting document with Working Group summaries and questions
References
- 1.Graf T, Enver T. Forcing cells to change lineages. Nature 2009;462(7273):587–94 doi 10.1038/nature08533. [DOI] [PubMed] [Google Scholar]
- 2.Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature 2013;501(7467):328–37 doi 10.1038/nature12624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watson PA, Arora VK, Sawyers CL. Emerging mechanisms of resistance to androgen receptor inhibitors in prostate cancer. Nat Rev Cancer 2015;15(12):701–11 doi 10.1038/nrc4016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bluemn EG, Coleman IM, Lucas JM, Coleman RT, Hernandez-Lopez S, Tharakan R, et al. Androgen Receptor Pathway-Independent Prostate Cancer Is Sustained through FGF Signaling. Cancer Cell 2017;32(4):474–89.e6 doi 10.1016/j.ccell.2017.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal R, Huang J, Alumkal JJ, Zhang L, Feng FY, Thomas GV, et al. Clinical and Genomic Characterization of Treatment-Emergent Small-Cell Neuroendocrine Prostate Cancer: A Multi-institutional Prospective Study. J Clin Oncol 2018:JCO2017776880 doi 10.1200/JCO.2017.77.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abida W, Cyrta J, Heller G, Prandi D, Armenia J, Coleman I, et al. Genomic correlates of clinical outcome in advanced prostate cancer. Proc Natl Acad Sci U S A 2019. doi 10.1073/pnas.1902651116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shukla S, Cyrta J, Murphy DA, Walczak EG, Ran L, Agrawal P, et al. Aberrant Activation of a Gastrointestinal Transcriptional Circuit in Prostate Cancer Mediates Castration Resistance. Cancer Cell 2017;32(6):792–806.e7 doi 10.1016/j.ccell.2017.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med 2016;22(3):298–305 doi 10.1038/nm.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mu P, Zhang Z, Benelli M, Karthaus WR, Hoover E, Chen CC, et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science 2017;355(6320):84–8 doi 10.1126/science.aah4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, Goodrich ZW, et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science 2017;355(6320):78–83 doi 10.1126/science.aah4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lapuk AV, Wu C, Wyatt AW, McPherson A, McConeghy BJ, Brahmbhatt S, et al. From sequence to molecular pathology, and a mechanism driving the neuroendocrine phenotype in prostate cancer. J Pathol 2012;227(3):286–97 doi 10.1002/path.4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Coleman IM, Brown LG, True LD, Kollath L, Lucas JM, et al. SRRM4 Expression and the Loss of REST Activity May Promote the Emergence of the Neuroendocrine Phenotype in Castration-Resistant Prostate Cancer. Clin Cancer Res 2015;21(20):4698–708 doi 10.1158/1078-0432.CCR-15-0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beltran H, Rickman DS, Park K, Chae SS, Sboner A, MacDonald TY, et al. Molecular characterization of neuroendocrine prostate cancer and identification of new drug targets. Cancer Discov 2011;1(6):487–95 doi 10.1158/2159-8290.CD-11-0130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dardenne E, Beltran H, Benelli M, Gayvert K, Berger A, Puca L, et al. N-Myc Induces an EZH2-Mediated Transcriptional Program Driving Neuroendocrine Prostate Cancer. Cancer Cell 2016;30(4):563–77 doi 10.1016/j.ccell.2016.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo H, Ci X, Ahmed M, Hua JT, Soares F, Lin D, et al. ONECUT2 is a driver of neuroendocrine prostate cancer. Nat Commun 2019;10(1):278 doi 10.1038/s41467-018-08133-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rotinen M, You S, Yang J, Coetzee SG, Reis-Sobreiro M, Huang WC, et al. ONECUT2 is a targetable master regulator of lethal prostate cancer that suppresses the androgen axis. Nat Med 2018;24(12):1887–98 doi 10.1038/s41591-018-0241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bishop JL, Thaper D, Vahid S, Davies A, Ketola K, Kuruma H, et al. The Master Neural Transcription Factor BRN2 Is an Androgen Receptor-Suppressed Driver of Neuroendocrine Differentiation in Prostate Cancer. Cancer Discov 2017;7(1):54–71 doi 10.1158/2159-8290.CD-15-1263. [DOI] [PubMed] [Google Scholar]
- 18.Akamatsu S, Wyatt AW, Lin D, Lysakowski S, Zhang F, Kim S, et al. The Placental Gene PEG10 Promotes Progression of Neuroendocrine Prostate Cancer. Cell Rep 2015;12(6):922–36 doi 10.1016/j.celrep.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 19.Beltran H, Oromendia C, Danila DC, Montgomery B, Hoimes C, Szmulewitz RZ, et al. A phase II trial of the aurora kinase A inhibitor alisertib for patients with castration resistant and neuroendocrine prostate cancer: efficacy and biomarkers. Clin Cancer Res 2018. doi 10.1158/1078-0432.CCR-18-1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zou M, Toivanen R, Mitrofanova A, Floch N, Hayati S, Sun Y, et al. Transdifferentiation as a Mechanism of Treatment Resistance in a Mouse Model of Castration-Resistant Prostate Cancer. Cancer Discov 2017;7(7):736–49 doi 10.1158/2159-8290.CD-16-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reina-Campos M, Linares JF, Duran A, Cordes T, L’Hermitte A, Badur MG, et al. Increased Serine and One-Carbon Pathway Metabolism by PKCλ/ι Deficiency Promotes Neuroendocrine Prostate Cancer. Cancer Cell 2019;35(3):385–400.e9 doi 10.1016/j.ccell.2019.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahal BA, Yang DD, Wang NQ, Alshalalfa M, Davicioni E, Choeurng V, et al. Clinical and Genomic Characterization of Low-Prostate-specific Antigen, High-grade Prostate Cancer. Eur Urol 2018;74(2):146–54 doi 10.1016/j.eururo.2018.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robinson D, Van Allen EM, Wu YM, Schultz N, Lonigro RJ, Mosquera JM, et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015;161(5):1215–28 doi 10.1016/j.cell.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaur HB, Lu J, Guedes LB, Maldonado L, Reitz L, Barber JR, et al. TP53 missense mutation is associated with increased tumor-infiltrating T-cells in primary prostate Cancer. Hum Pathol 2019. doi 10.1016/j.humpath.2019.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nguyen HM, Vessella RL, Morrissey C, Brown LG, Coleman IM, Higano CS, et al. LuCaP Prostate Cancer Patient-Derived Xenografts Reflect the Molecular Heterogeneity of Advanced Disease an--d Serve as Models for Evaluating Cancer Therapeutics. Prostate 2017;77(6):654–71 doi 10.1002/pros.23313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao D, Vela I, Sboner A, Iaquinta PJ, Karthaus WR, Gopalan A, et al. Organoid cultures derived from patients with advanced prostate cancer. Cell 2014;159(1):176–87 doi 10.1016/j.cell.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Puca L, Bareja R, Prandi D, Shaw R, Benelli M, Karthaus WR, et al. Patient derived organoids to model rare prostate cancer phenotypes. Nat Commun 2018;9(1):2404 doi 10.1038/s41467-018-04495-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beshiri ML, Tice CM, Tran C, Nguyen HM, Sowalsky AG, Agarwal S, et al. A PDX/Organoid Biobank of Advanced Prostate Cancers Captures Genomic and Phenotypic Heterogeneity for Disease Modeling and Therapeutic Screening. Clin Cancer Res 2018;24(17):4332–45 doi 10.1158/1078-0432.CCR-18-0409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aparicio AM, Shen L, Tapia EL, Lu JF, Chen HC, Zhang J, et al. Combined Tumor Suppressor Defects Characterize Clinically Defined Aggressive Variant Prostate Cancers. Clin Cancer Res 2016;22(6):1520–30 doi 10.1158/1078-0432.CCR-15-1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin D, Wyatt AW, Xue H, Wang Y, Dong X, Haegert A, et al. High fidelity patient-derived xenografts for accelerating prostate cancer discovery and drug development. Cancer Res 2014;74(4):1272–83 doi 10.1158/0008-5472.CAN-13-2921-T. [DOI] [PubMed] [Google Scholar]
- 31.Lee JK, Phillips JW, Smith BA, Park JW, Stoyanova T, McCaffrey EF, et al. N-Myc Drives Neuroendocrine Prostate Cancer Initiated from Human Prostate Epithelial Cells. Cancer Cell 2016;29(4):536–47 doi 10.1016/j.ccell.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aparicio AM, Harzstark AL, Corn PG, Wen S, Araujo JC, Tu SM, et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin Cancer Res 2013;19(13):3621–30 doi 10.1158/1078-0432.CCR-12-3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Epstein JI, Amin MB, Beltran H, Lotan TL, Mosquera JM, Reuter VE, et al. Proposed morphologic classification of prostate cancer with neuroendocrine differentiation. Am J Surg Pathol 2014;38(6):756–67 doi 10.1097/PAS.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai H, Morais CL, Alshalalfa M, Tan HL, Haddad Z, Hicks J, et al. Cyclin D1 Loss Distinguishes Prostatic Small-Cell Carcinoma from Most Prostatic Adenocarcinomas. Clin Cancer Res 2015;21(24):5619–29 doi 10.1158/1078-0432.CCR-15-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan HL, Sood A, Rahimi HA, Wang W, Gupta N, Hicks J, et al. Rb loss is characteristic of prostatic small cell neuroendocrine carcinoma. Clin Cancer Res 2014;20(4):890–903 doi 10.1158/1078-0432.CCR-13-1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sue Ping Thang a b, JVc, SSd e, AIa, TAa e, GKa e, et al. Poor Outcomes for Patients with Metastatic Castration-resistant Prostate Cancer with Low Prostate-specific Membrane Antigen (PSMA) Expression Deemed Ineligible for 177 Lu-labelled PSMA Radioligand Therapy. 2018. [DOI] [PubMed] [Google Scholar]
- 37.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011;3(75):75ra26 doi 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imakita T, Fujita K, Kanai O, Terashima T, Mio T. Small cell lung cancer transformation during immunotherapy with nivolumab: A case report. Respir Med Case Rep 2017;21:52–5 doi 10.1016/j.rmcr.2017.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.George J, Lim JS, Jang SJ, Cun Y, Ozretić L, Kong G, et al. Comprehensive genomic profiles of small cell lung cancer. Nature 2015;524(7563):47–53 doi 10.1038/nature14664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niederst MJ, Sequist LV, Poirier JT, Mermel CH, Lockerman EL, Garcia AR, et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat Commun 2015;6:6377 doi 10.1038/ncomms7377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee JK, Lee J, Kim S, Youk J, Park S, An Y, et al. Clonal History and Genetic Predictors of Transformation Into Small-Cell Carcinomas From Lung Adenocarcinomas. J Clin Oncol 2017;35(26):3065–74 doi 10.1200/JCO.2016.71.9096. [DOI] [PubMed] [Google Scholar]
- 42.Tirosh I, Izar B, Prakadan SM, Wadsworth MH, Treacy D, Trombetta JJ, et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 2016;352(6282):189–96 doi 10.1126/science.aad0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim IS, Heilmann S, Kansler ER, Zhang Y, Zimmer M, Ratnakumar K, et al. Microenvironment-derived factors driving metastatic plasticity in melanoma. Nat Commun 2017;8:14343 doi 10.1038/ncomms14343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rambow F, Rogiers A, Marin-Bejar O, Aibar S, Femel J, Dewaele M, et al. Toward Minimal Residual Disease-Directed Therapy in Melanoma. Cell 2018;174(4):843–55.e19 doi 10.1016/j.cell.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 45.Farrell AS, Joly MM, Allen-Petersen BL, Worth PJ, Lanciault C, Sauer D, et al. MYC regulates ductal-neuroendocrine lineage plasticity in pancreatic ductal adenocarcinoma associated with poor outcome and chemoresistance. Nat Commun 2017;8(1):1728 doi 10.1038/s41467-017-01967-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Korkaya H, Liu S, Wicha MS. Breast cancer stem cells, cytokine networks, and the tumor microenvironment. J Clin Invest 2011;121(10):3804–9 doi 10.1172/JCI57099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.