Abstract
Background:
Among older adults (age ≥75) hospitalized for acute myocardial infarction, acute kidney injury after coronary angiography is common. Aging-related conditions may independently predict acute kidney injury, but have not yet been analyzed in large acute myocardial infarction cohorts.
Methods:
We analyzed data from 2212 participants age ≥75 in the SILVER-AMI study who underwent coronary angiography. Acute kidney injury was defined using KDIGO criteria (serum Cr increase ≥0.3 mg/dL from baseline or ≥1.5 times baseline). We analyzed the associations of traditional acute kidney injury risk factors and aging-related conditions (ADL impairment, prior falls, cachexia, low physical activity) with acute kidney injury, and then performed logistic regression to identify independent predictors.
Results:
Participants’ mean age was 81.3 years, 45.2% were female, and 9.5% were nonwhite; 421 (19.0%) experienced acute kidney injury. Comorbid diseases and aging-related conditions were both more common among individuals experiencing acute kidney injury. However, after multivariable adjustment, no aging-related conditions were retained. There were 11 risk factors in the final model; the strongest were heart failure on presentation (OR 1.91, 95% CI 1.41-2.59), BMI >30 (vs. BMI 18-25: OR 1.75, 95% CI 1.27-2.42), and nonwhite race (OR 1.65, 95% CI 1.16-2.33). The final model achieved an AUC of 0.72 and was well calibrated (Hosmer-Lemeshow P=0.50). Acute kidney injury was independently associated with 6 month mortality (OR 1.98, 95% CI 1.36-2.88) but not readmission (OR 1.26, 95% CI 0.98-1.61).
Conclusions:
Acute kidney injury is common among older adults with acute myocardial infarction undergoing coronary angiography. Predictors largely mirrored those in previous studies of younger individuals, which suggests that geriatric conditions mediate their influence through other risk factors.
Keywords: Acute myocardial infarction, older adults, acute kidney injury, risk prediction
INTRODUCTION:
Acute kidney injury is common among patients hospitalized with acute myocardial infarction undergoing invasive coronary angiography. Older adults (age ≥75) are at increased risk of acute kidney injury due to a variety of aging-related factors, including nephrosclerosis, vascular changes, inflammation, and reduced autoregulatory capacity.1–3 Due to changing demographics, older adults also represent a growing proportion of the population hospitalized for acute myocardial infarction in developed countries.4 These demographic changes, coupled with increasing rates of coronary angiography over time, heighten the potential for incident acute kidney injury cases among the acute myocardial infarction population.
As development of acute kidney injury after coronary angiography is associated with adverse outcomes, including increased length of stay, excess costs, progression to end-stage renal disease, and mortality,5–7 accurate prediction of acute kidney injury risk at the time of acute myocardial infarction hospitalization is critical to both prevention efforts and informed decision making (e.g. regarding invasive management). While prior reports have identified several factors that predict acute kidney injury among acute myocardial infarction patients, including baseline renal function, anemia, cardiogenic shock, and heart failure,7 few studies have focused on exclusively older adult populations where aging-related renovascular changes are common. While these changes are difficult to measure directly, they may share a common pathway with other aging-related conditions such as frailty and sarcopenia, and several small investigations have shown that these conditions independently predict acute kidney injury.8,9 However, these findings have not yet been confirmed in larger acute myocardial infarction cohorts.
To address this gap in knowledge, we analyzed the incidence and determinants of acute kidney injury among participants in the Comprehensive Evaluation of Risk Factors in Older Patients with AMI (SILVER-AMI) cohort study. SILVER-AMI enrolled 3041 participants age ≥75 hospitalized with acute myocardial infarction at 94 U.S. medical centers, and collected data on aging-related conditions as well as traditional risk factors. We hypothesized that aging-related conditions present at the time of admission (including pre-hospital activity of daily living impairment, multiple falls within the past year, cachexia, and low physical activity) would independently predict the development of acute kidney injury after invasive coronary angiography. Further, we hypothesized that acute kidney injury would be associated with poor intermediate-term outcomes (mortality and hospital readmission) within 6 months of hospitalization.
METHODS
Study Participants
The design of SILVER-AMI has been described previously.10 Briefly, patients age ≥75 were enrolled if they met criteria for the Third Universal Definition of acute myocardial infarction,11 as verified by physician investigators at the Yale Coordinating Center. Patients were excluded from enrollment in SILVER-AMI if they were not initially hospitalized for acute myocardial infarction (e.g. experienced acute myocardial infarction secondary to an inpatient procedure or surgery), if they were transferred to the study hospital from another hospital after >24 hours, if they were incarcerated prior to hospital admission, or if they were unable to provide informed consent with no proxy available.
Assessments
At the time of hospitalization, participants underwent a comprehensive baseline assessment including demographics, symptoms, and aging-related conditions. This assessment was complemented by an in-depth medical record review performed by a local research coordinator which included details of initial presentation (blood pressure, heart rate), comorbidities, and laboratory results. Medical records were also provided to the Yale Coordinating Center where a research nurse performed a review to collect information about medications and cardiac procedures. Institutional review board approval was obtained at all participating centers. There were 3041 participants in SILVER-AMI, who were enrolled at 94 U.S. study hospitals from 1/11/2013-10/28/2016 (with the last follow-up assessment completed on 6/14/17). As our interest was in predicting risk of acute kidney injury after invasive coronary angiography, we restricted our analysis to the 2212 participants in SILVER-AMI who underwent this procedure.
Outcomes
The primary outcome was in-hospital acute kidney injury, defined based on the Kidney Disease Improving Global Outcomes (KDIGO) criteria: increase in peak serum creatinine of ≥0.3 mg/dL from baseline or ≥1.5 times baseline.12 The reference (baseline) creatinine value was defined as admission creatinine, since outpatient values were not universally available in all study participants. For descriptive purposes, we separated severity of acute kidney injury into stage 1/mild (increase in creatinine from 1.5-1.9 times baseline), stage 2/moderate (increase in creatinine from ≥2.0-2.9 times baseline), and stage 3/severe (increase in creatinine ≥3.0 times baseline, or need for dialysis).
In order to assess the effect of acute kidney injury on post-discharge outcomes, we also examined 6-month mortality and 6-month hospital readmission, which were obtained in all study participants. Mortality was ascertained through review of medical records (in-hospital death), death certificates (out-of-hospital death), or obituaries (if out-of-hospital death occurred and death certificate was not provided due to state restrictions on release [GA, NY, NJ, PA, MO, LA]). When these records were not available (fewer than 5% of cases), mortality information was obtained through secondary report such as a family member. All deaths were double-adjudicated by physician investigators who reviewed source documents when available to determine cause of death. Readmissions were identified through a two-stage process. During enrollment, the participant identified the hospitals he or she utilized for medical care and signed the appropriate medical release forms. When the 6-month follow-up window closed, the research coordinator contacted the hospitals that were identified at the time of enrollment to assess and collect readmission records. Separately, as part of the 6-month follow-up interview, the participant also reported hospital readmissions to the Yale Coordinating Center. The Yale Coordinating Center then reconciled the hospital records collected by the coordinator against self-reported events to ensure that no readmissions were missing from the assessment. If necessary, additional records were collected to capture all events (e.g. a readmission occurring at an out-of-area hospital during travel).
Statistical Analysis
We compared characteristics of participants who experienced acute kidney injury versus no acute kidney injury, using means for continuous variables and percentages for categorical variables. For categorical variables, we chose thresholds based on clinical relevance and distributions. We then applied backward selection (threshold of p<0.05) to a logistic regression model to determine the independent risk factors for acute kidney injury, selecting candidate variables based on both elements in previous acute kidney injury risk models7,13 and clinical plausibility based on judgment of the study investigators. These included the following elements: demographics (age , sex, race), acute myocardial infarction subtype (ST elevation acute myocardial infarction vs. non ST elevation acute myocardial infarction), admission characteristics (heart rate, heart failure on presentation, atrial fibrillation, medical history (prior coronary disease, prior myocardial infarction, heart failure, hypertension, diabetes, cardiac arrhythmia, chronic lung disease, peripheral vascular disease, body mass index), admission laboratory values (creatinine clearance, WBC count, hemoglobin), left ventricular ejection fraction (LVEF), and selected aging-related conditions that were present at baseline (pre-hospital activity of daily living impairment, ≥2 falls within past year, cachexia [≥10 pound weight loss in past year], low physical activity) (eTable 1). We excluded assessments and events that occurred after the first 24 hours (e.g. in-hospital complications such as bleeding) as their temporality with development of acute kidney injury was unclear, as well as variables with >90% or <5% prevalence (intra-aortic balloon pump use, hypotension [systolic blood pressure <90 mmHg]). Missing data, present in <1% of the sample for most variables (with the exception of LVEF, missing in 7.9%), were multiply imputed.
We evaluated discrimination and calibration of a model using our final set of predictors with the C-statistic and the Hosmer-Lemeshow goodness of fit statistic, respectively. We generated a calibration plot (by quintile) for observed versus expected risk of acute kidney injury.
For 6 month outcomes (mortality and readmission), we used the Kaplan-Meier method to describe event-free survival over time separately in participants who did and did not develop acute kidney injury. These two groups were compared using crude and multivariable logistic regression to adjust for potential confounders, including known prior predictors of these outcomes in this patient population.14,15 We chose logistic regression (analyzing mortality and readmission as binary outcomes at 6 months) over Cox proportional hazards regression, despite having longitudinal data on event timing, given that several variables (including acute kidney injury) violated the proportional hazards assumption. A P value of <0.05 was considered statistically significant for all tests. Analyses were performed in STATA Version 15.
RESULTS
Baseline Characteristics
Among the study cohort, mean age was 81.3 years, 45.2% were female, 9.5% were nonwhite (among nonwhite: 76.7% Black, 12.4% Asian, 10.9% Other), and slightly more than half (53.1%) had prior known coronary disease. One-third (33.0%) presented with STEMI. On coronary angiography, 37.7% had 3-vessel or left main disease. 77.2% of participants underwent PCI, most of which involved a single vessel.
In-Hospital Acute Kidney Injury
Acute kidney injury occurred among 421 participants (19.0%). Among those who developed, 88.4% was Stage 1, 7.6% was Stage 2, and 4.0% was Stage 3 (Figure 1). Participants who developed acute kidney injury generally had a higher burden of comorbid disease, including heart failure (30.2% vs. 13.6%, P<0.001), diabetes (48.0% vs. 33.3%, P<0.001), atrial fibrillation (15.2% vs. 9.1%, P<0.001), and chronic lung disease (17.8% vs. 13.5%, P=0.023) (Table 1). Mean baseline creatinine clearance was lower among patients who developed acute kidney injury (51.4 vs. 59.6, P<0.001), and mean hemoglobin was lower (12.3 mg/dL vs. 13.2 mg/dL, P<0.001). Baseline ADL impairment, unintentional weight loss, and low self-reported physical activity were all more common among participants who developed acute kidney injury (ADL impairment: 17.1% vs. 10.9%, P<0.001; weight loss: 26.7% vs. 20.8%, P=0.009; low activity: 22.3% vs. 14.0%, P<0.001). Mean length of stay was higher among patients who developed acute kidney injury versus no acute kidney injury (8.1 days vs. 4.0 days, P=0.003).
Figure 1. Distribution of AKI severity.
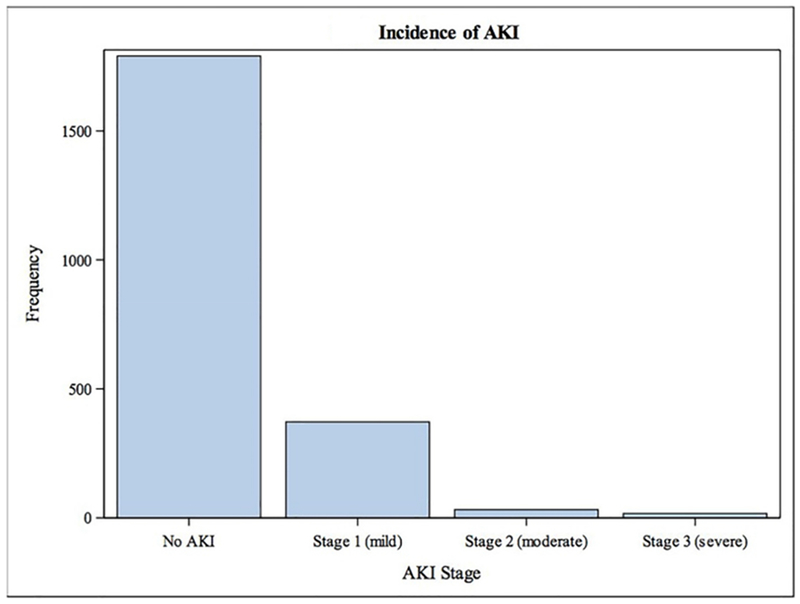
AKI occurred among 421 participants (19.0%); most cases (88.4%) were mild (KDIGO Stage 1).
Table 1:
Participant characteristics
| In-Hospital AKI (n=421) Mean (SD) or N (%) |
No In-Hospital AKI (n=1791) Mean (SD) or N (%) |
P value | |
|---|---|---|---|
| Demographics | |||
| Age, mean ± SD | 81.5 (4.8) | 81.3 (4.7) | 0.32 |
| Male sex (%) | 224 (53.2) | 989 (55.2) | 0.45 |
| Nonwhite race (%) | 63 (15.4) | 147 (8.3) | <0.001 |
| Medical History | |||
| Arrhythmia (any) (%) | 124 (29.5) | 427 (23.8) | 0.017 |
| Heart failure (%) | 127 (30.2) | 244 (13.6) | <0.001 |
| Peripheral arterial disease (%) | 62 (14.7) | 199 (11.1) | 0.039 |
| Diabetes mellitus (%) | 202 (48.0) | 596 (33.3) | <0.001 |
| COPD (%) | 75 (17.8) | 242 (13.5) | 0.023 |
| Prior myocardial infarction (%) | 122 (29.0) | 469 (26.2) | 0.24 |
| Presentation Characteristics | |||
| ST elevation MI (%) Body mass index, mean ± SD |
127 (30.2) | 603 (33.7) | 0.17 |
| 28.1 (5.7) | 27.4 (5.0) | 0.015 | |
| Heart failure on presentation (%) | 96 (22.8) | 164 (9.2) | <0.001 |
| First systolic BP, mean ± SD (mmHg) | 144.4 (32.5) | 146.1 (30.4) | 0.31 |
| First diastolic BP, mean ± SD (mmHg) | 77.2 (18.3) | 78.5 (17.5) | 0.19 |
| First heart rate, mean ± SD (BPM) | 86.5 (21.7) | 81.3 (22.7) | <0.001 |
| Initial hemoglobin, mean ± SD | 12.3 (2.1) | 13.2 (1.9) | <0.001 |
| Initial WBC count, mean ± SD | 10.2 (5.6) | 9.4 (5.0) | 0.005 |
| Atrial fibrillation at admission | 64 (15.2) | 163 (9.1) | <0.001 |
| Estimated GFR, mL/min/1.73m2 | 51.4 (25.3) | 59.6 (21.2) | <0.001 |
| In-hospital diagnostics | |||
| Left ventricular ejection fraction* | <0.001 | ||
| Normal (≥50%) | 168 (43.1) | 954 (57.9) | |
| Mildly reduced (40-50%) | 84 (21.5) | 387 (23.5) | |
| Moderately reduced (30-40%) | 83 (21.3) | 202 (12.3) | |
| Severely reduced (<30%) | 55 (14.1) | 104 (6.3) | |
| Missing | 31 (7.4) | 144 (8.0) | |
| 3-vessel or left main disease | 185 (43.9) | 648 (36.2) | 0.003 |
| Revascularization status | <.001 | ||
| No PCI | 141 (34.8) | 425 (25.0) | |
| Single-vessel PCI | 237 (58.5) | 1125 (66.1) | |
| Multi-vessel PCI | 27 (6.7) | 152 (8.9) | |
| Functional impairments | |||
| Unintentional weight loss (>10 lbs. in 1 year) | 112 (26.7) | 371 (20.8) | 0.009 |
| Activity of daily living disability (any) | 72 (17.1) | 196 (10.9) | <0.001 |
| Multiple falls (≥2 falls within past year) | 90 (21.5) | 341 (19.1) | 0.26 |
| Low physical activity | 93 (22.3) | 250 (14.0) | <0.001 |
eGFR = estimated glomerular filtration rate
Multivariable results
After multivariable adjustment, there were 11 risk factors independently associated with acute kidney injury (Table 2). The strongest categorical risk factors were heart failure on presentation (odds ratio [OR] 1.91, 95% confidence interval [CI] 1.41-2.59), BMI (BMI ≥30 vs. BMI 18-25: OR 1.75, 95% CI 1.27-2.42), and nonwhite race (nonwhite vs. white race: OR 1.65, 95% CI 1.16-2.33). Lower initial hemoglobin, which was analyzed continuously, was a risk factor for acute kidney injury (per mg/dl decrease: OR 1.16, 95% CI 1.10-1.23). None of the aging-related conditions were retained after multivariable adjustment. The final model using the 11 risk factors achieved an area under the receiver operating characteristic curve (AUC) of 0.72. Across quintiles of observed risk, the model was well calibrated (Hosmer-Lemeshow P=0.50) (Figure 2).
Table 2.
Predictors of AKI after Multivariable Adjustment
| Association with Risk of AKI Odds Ratio (95% Confidence Interval) | ||
|---|---|---|
| Unadjusted | Adjusted | |
| Previous acute myocardial infarction | 1.15 (0.91-1.46) | 0.77 (0.59-1.00) |
| Initial hemoglobin (per mg/dL decrease) | 1.25 (1.19-1.33) | 1.16 (1.10-1.23) |
| Creatinine clearance (per mL/min decrease) | 1.02 (1.01-1.02) | 1.01 (1.01-1.02) |
| Initial heart rate (per category increase)* | 1.16 (1.10-1.23) | 1.07 (1.01-1.14) |
| Body mass index (reference: 18-25) | ||
| Overweight (>25 to <30) | 0.90 (0.69-1.15) | 1.12 (0.85-1.49) |
| Obese (>30) | 1.23 (0.95-1.61) | 1.75 (1.27-2.42) |
| History of diabetes | 1.85 (1.49-2.29) | 1.33 (1.04-1.69) |
| Left ventricular ejection fraction (per category worsening)* | 1.45 (1.31-1.61) | 1.33 (1.18-1.49) |
| Atrial fibrillation at admission | 1.79 (1.31-2.44) | 1.54 (1.09-2.17) |
| History of heart failure | 2.74 (2.14-3.51) | 1.54 (1.16-2.06) |
| Non-white race | 2.02 (1.47-2.77) | 1.65 (1.17-2.33) |
| Heart failure on presentation | 2.93 (2.22-3.87) | 1.91 (1.41-2.59) |
C-statistic over 20 imputed datasets: 0.73
Level variable definitions: heart rate: <50 (reference), 50-69. 70-79. 80-89. 90-99.100-109.110-129.130-149. ≥150; Ejection Fraction: ≥50% (reference), 40-49%, 30-39%, <30%
Figure 2. Model calibration.
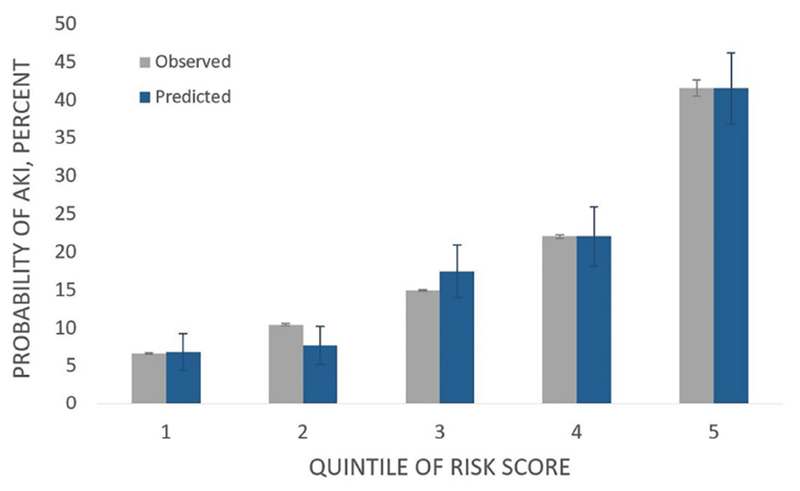
Across quintiles of risk, observed and expected rates of AKI were well calibrated (Hosmer-Lemeshow P=0.50).
Acute kidney injury and 6-month outcomes
At 6 months, acute kidney injury was associated with both mortality (14.5% vs. 5.4%, P<0.001) and hospital readmission (53.3% vs. 36.1%, P<0.001) (Figures 3 and 4). After adjusting for age, sex, comorbid diseases, presentation characteristics, revascularization status, and length of stay, the association with mortality remained statistically significant (adjusted odds ratio [OR] mortality = 1.98, 95% CI 1.36-2.88) but readmission was no longer significant (adjusted OR readmission = 1.26, 95% CI 0.98-1.61).
Figure 3. Mortality within 6 months of AMI hospitalization.
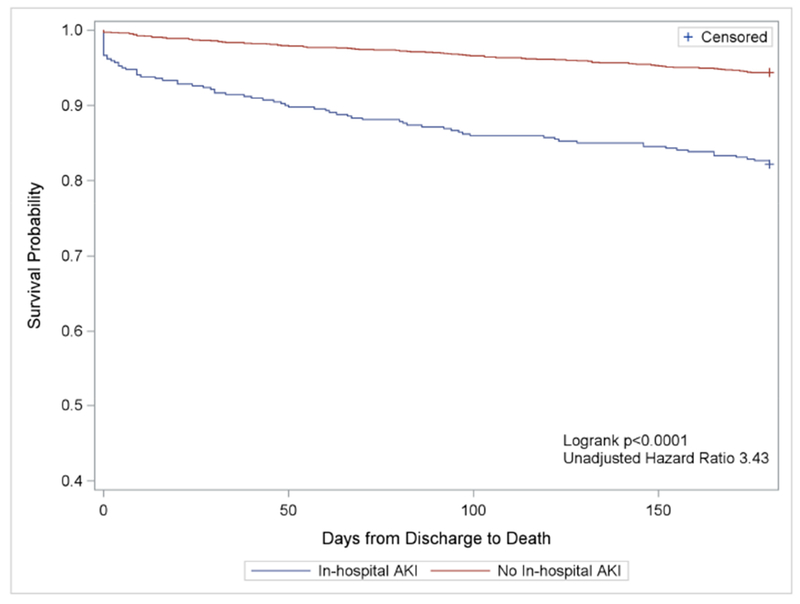
6-month survival curves (mortality) are shown for participants who developed AKI (blue) versus no AKI (red). Participants who experienced AKI were significantly more likely to experience mortality (log-rank P < 0.0001).
Figure 4. Hospital readmission within 6 months of AMI hospitalization.

6-month survival curves (readmission) are shown for participants who developed AKI (blue) versus no AKI (red). Participants who experienced AKI were significantly more likely to experience readmission (log-rank P < 0.0001).
DISCUSSION
Our study had several key findings: first, acute kidney injury was relatively common among our cohort, occurring in nearly one in five participants undergoing invasive coronary angiography during an admission for acute myocardial infarction. Second, the factors associated with acute kidney injury in these individuals (who were all ≥75 years of age) were largely similar to those described in younger populations;7,16,17 aging-related functional impairments were not independently predictive of acute kidney injury after adjusting for other clinically-relevant factors. Third, while most cases of acute kidney injury were mild (nearly 90% were KDIGO Stage I), acute kidney injury was nonetheless associated with both mortality and hospital readmission at 6 months. This highlights the prognostic importance of acute kidney injury in older adults, which has been previously shown by others in longitudinal studies.18,19
Our factors were similar to those reported in other risk acute kidney injury models developed for patients undergoing coronary angiography. 7,16,17,20 For example, Huang et al. used data from 947,091 patients in the NCDR Cath-PCI registry (mean age 64.8 years) and found that history of heart failure, creatinine clearance, diabetes, recent heart failure episode, hemoglobin, BMI, and ejection fraction (all identified as risk factors in SILVER-AMI) were independent predictors of acute kidney injury.20 Notably, this model (and most acute kidney injury risk models) were developed for all patients undergoing invasive coronary angiography, while our sample was restricted to patients with acute myocardial infarction. Nonetheless there are clear mechanistic links between these factors and acute kidney injury after coronary angiography: for example, cardiogenic shock leads to renal hypoperfusion, resulting in ischemic injury.21 Diabetes is associated with impaired endothelial function that can increase the potential for injury from intravenous contrast.22 Several other factors reported in prior models were not included in our analysis, either because they were extremely rare in SILVER-AMI (e.g. intra-aortic balloon pump) or unavailable (e.g. volume of contrast given).
A strength of SILVER-AMI over prior acute myocardial infarction studies was the capture of aging-related conditions at the time of hospitalization. We hypothesized that aging-related conditions ascertained from participant self-report (pre-hospital ADL impairment, multiple falls within the past year, cachexia, and low physical activity) would be associated with acute kidney injury, even after adjusting for traditional risk factors, given that they convey information about unique vulnerabilities in older patients. While all of these elements except for multiple falls were more common among participants who experienced acute kidney injury, none were retained after multivariable adjustment. Notably, we excluded several aging-related conditions from consideration (even though they were obtained as part of the SILVER-AMI study) because they were obtained during hospitalization, rather than present at baseline. These included functional mobility (using the Timed Up and Go [TUG]) and cognitive impairment (using the Telephone interview for Cognitive Status). Our rationale was that we were unable to precisely determine the temporality of these impairments relative to the onset of acute kidney injury. We have previously reported in SILVER-AMI that impaired functional mobility (based on TUG) predicts 30-day hospital readmission,23 underscoring that aging-related conditions are important for selected outcomes. Whether baseline functional impairment (for example, obtained in the outpatient setting) predicts acute kidney injury at the time of incident acute myocardial infarction is a worthy question for future studies but beyond the scope of our data. Another notable finding in our study is that nonwhite race (the majority of whom [76.7%] were Black) was associated with a 65% higher odds of developing acute kidney injury, after multivariable adjustment. Previous reports have found similar associations;24,25 for example, a study from Grams et al. analyzed data from 10,588 participants enrolled in the Atherosclerosis Risk in Communities (ARIC) study and found that Black participants experienced a 30% greater risk of acute kidney injury over a 13-year time period.24 Reasons for such associations are unclear; while Grams et al. hypothesized that disparities in socioeconomic status (including lack of health insurance among Black patients, leading to poor quality outpatient care) may have played a role, our sample by definition was restricted to hospitalized inpatients (rather than community-dwelling outpatients) where these factors likely played less of an immediate role. The observed differences may be due to genetic or protein variants (e.g. apolipoprotein L[ISP]I) or differences in comorbidity burden not captured by our dataset (e.g. severity of diabetes measured by hemoglobin A[ISP]l[ISP]e). Future studies that incorporate genetic and/or biomarker analyses may help to further elucidate mechanisms.
Our study has several strengths that enhance the external validity of our findings, including a relatively high proportion of women compared with prior acute myocardial infarction studies, central adjudication of events, and broad representation of hospitals across the U.S. including a mix of academic, community, and regional referral centers. However, there are also several limitations to our study that warrant consideration. First, we were unable to analyze volume of contrast administered as a risk factor for acute kidney injury, as this information was unavailable in the SILVER-AMI dataset. However, given previous reports of a graded association between contrast volume and acute kidney injury risk,26 it seems prudent to pursue a “least is best” approach in older adults. Second, participants were excluded from enrollment in SILVER-AMI if they had severe cognitive impairment with no proxy available, as these individuals were unlikely to be able to complete the baseline assessment. We are therefore unable to comment on acute kidney injury risk in these patients. Third, we were unable to attribute a specific cause of acute kidney injury (e.g. intravenous contrast agents, hypotension, atheroembolism, or medication-related toxicities), although this is also often difficult to determine in clinical practice.
In conclusion, acute kidney injury was a relatively common occurrence in our cohort of acute myocardial infarction patients age ≥75 who underwent invasive coronary angiography, with predictors that largely mirrored those described in previous studies of younger individuals. Acute kidney injury was associated with poor 6-month outcomes (mortality and hospital readmission), which underscores the importance of both prevention efforts and informed decision-making.
Supplementary Material
Clinical significance.
Nearly one in five older adults undergoing cardiac catheterization for acute myocardial infarction experiences acute kidney injury.
While prior studies suggested that aging-related functional impairments (e.g. activities of daily living impairment, cachexia) predict acute kidney injury, we found they were not significant after also considering traditional comorbidities (e.g. diabetes, heart failure).
Acute kidney injury is associated with both mortality and hospital readmission within 6 months; particular attention is therefore warranted for these individuals in outpatient follow-up.
ACKNOWLEDGMENTS
We would like to thank Jenny Summapund for assistance with the preparation of this manuscript for submission.
This research was supported by the National Heart, Lung, And Blood Institute (NHLBI) of the National Institutes of Health (NIH) (R01HL115295). This work was conducted at the Yale Program on Aging/Claude D. Pepper Older Americans Independence Center (P30AG21342). The project described used REDCap which is supported by the National Center for Advancing Translational Sciences (NCATS), NIH, through grant UL1 TR00000. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Dr. Dodson is the recipient of Patient Oriented Career Development Award (K23 AG052463) from the NIH/National Institute on Aging. Dr. Hajduk is supported by a training grant from the National Institute on Aging (T32 AG19134).
Funding Source: National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH) (R01HL115295).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: No authors have a conflict of interest.
Access and Roles: All authors had access to the data and a role in the production of this manuscript.
DISCLOSURES
None.
REFERENCES
- 1.Gussenhoven MJE, Ravensbergen J, Van Bockel JH, et al. Renal dysfunction after angiography; a risk factor analysis in patients with peripheral vascular disease. J Cardiovasc Surg (Torino). 1991;32(1):81–86. doi: 10.1099/00221287-102-2-255. [DOI] [PubMed] [Google Scholar]
- 2.Coca SG. Acute Kidney Injury in Elderly Persons. Am J Kidney Dis. 2010;56(1):122–131.doi: 10.1053/j.ajkd.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson S, Eldadah B, Halter JB, et al. Acute Kidney Injury in Older Adults. J Am Soc Nephrol. 2011;22(1):28–38. doi: 10.1681/ASN.2010090934. [DOI] [PubMed] [Google Scholar]
- 4.Yeh RW, Sidney S, Chandra M, et al. Population trends in the incidence and outcomes of acute myocardial infarction. N Engl J Med. 2010;362(23):2155–2165. doi:362/23/2155[pii]10.1056/NEJMoa0908610. [DOI] [PubMed] [Google Scholar]
- 5.Parikh CR, Coca SG, Wang Y, et al. Long-term prognosis of acute kidney injury after acute myocardial infarction. Arch Intern Med. 2008;168(9):987–995. doi: 10.1001/archinte.168.9.987. [DOI] [PubMed] [Google Scholar]
- 6.Wi J, Ko YG, Kim JS, et al. Impact of contrast-induced acute kidney injury with transient or persistent renal dysfunction on long-term outcomes of patients with acute myocardial infarction undergoing percutaneous coronary intervention. Heart. 2011;97(21):1753 LP–1757. http://heart.bmj.com/content/97/21/1753.abstract. [DOI] [PubMed] [Google Scholar]
- 7.Tsai TT, Patel UD, Chang TI, et al. Contemporary Incidence, Predictors, and Outcomes of Acute Kidney Injury in Patients Undergoing Percutaneous Coronary Interventions: Insights From the NCDR Cath-PCI Registry. JACC Cardiovasc Interv. 2014;7(1):1–9. doi: 10.1016/j.jcin.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baek SH, Lee SW, Kim SW, et al. Frailty as a predictor of acute kidney injury in hospitalized elderly patients: A single center, retrospective cohort study. PLoS One. 2016;11(6). doi: 10.1371/journal.pone.0156444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morton S, Isted A, Avery P, Wang J. Is Frailty a Predictor of Outcomes in Elderly Inpatients with Acute Kidney Injury? A Prospective Cohort Study. Am J Med. 2018;131(10):1251–1256. e2. doi: 10.1016/j.amjmed.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 10.Dodson JA, Geda M, Krumholz HM, et al. Design and rationale of the comprehensive evaluation of risk factors in older patients with AMI (SILVER-AMI) study. BMC Health Serv Res. 2014;14(1). doi: 10.1186/s12913-014-0506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Eur Heart J. 2012;33(20):2551–2567. doi: 10.1093/eurheartj/ehs184. [DOI] [PubMed] [Google Scholar]
- 12.Kellum JA, Lameire N, Aspelin P, et al. Diagnosis, evaluation, and management of acute kidney injury: A KDIGO summary (Part 1). Crit Care. 2013;17(1). doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen DW, Ma B, Leung KC, et al. Risk Prediction Models for Contrast-Induced Acute Kidney Injury Accompanying Cardiac Catheterization: Systematic Review and Meta-analysis. Can J Cardiol. 2017;33(6):724–736. doi: 10.1016/j.cjca.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 14.Smith LN, Makam AN, Darden D, et al. Acute myocardial infarction readmission risk prediction models. Circ Cardiovasc Qual Outcomes. 2018;11(1). http://circoutcomes.ahajournals.org/content/11/1/e003885.abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eagle KA, Lim MJ, Dabbous OH, et al. A validated prediction model for all forms of acute coronary syndrome estimating the risk of 6-month postdischarge death in an international registry. J Am Med Assoc. 2004;291(22):2727–2733. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 16.Sreenivasan J, Zhuo M, Khan MS, et al. Anemia (Hemoglobin ≤ 13 g/dL) as a Risk Factor for Contrast-Induced Acute Kidney Injury Following Coronary Angiography. Am J Cardiol. 2018;122(6):961–965. doi: 10.1016/j.amjcard.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 17.Mehran R, Aymong ED, Nikolsky E, et al. A simple risk score for prediction of contrast-induced nephropathy after percutaneous coronary intervention: Development and initial validation. J Am Coll Cardiol. 2004. doi: 10.1016/j.jacc.2004.06.068. [DOI] [PubMed] [Google Scholar]
- 18.Parikh CR, Coca SG, Wang Y, et al. Long-term prognosis of acute kidney injury after acute myocardial infarction. Arch Intern Med. 2008;168(9):987–995 10.1001/archinte.168.9.987. [DOI] [PubMed] [Google Scholar]
- 19.Sun G, Chen P, Wang K, et al. Contrast-Induced Nephropathy and Long-Term Mortality After Percutaneous Coronary Intervention in Patients With Acute Myocardial Infarction. Angiology. 2018. doi: 10.1177/0003319718803677. [DOI] [PubMed] [Google Scholar]
- 20.Huang C, Murugiah K, Mahajan S, et al. Enhancing the prediction of acute kidney injury risk after percutaneous coronary intervention using machine learning techniques: A retrospective cohort study. PLoS Med. 2018. doi: 10.1371/journal.pmed.1002703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koreny M, Karth GD, Geppert A, et al. Prognosis of patients who develop acute renal failure during the first 24 hours of cardiogenic shock after myocardial infarction. Am J Med. 2002;112(2):115–119. doi: 10.1016/S0002-9343(01)01070-1. [DOI] [PubMed] [Google Scholar]
- 22.Toprak O, Cirit M, Yesil M, et al. Impact of diabetic and pre-diabetic state on development of contrast-induced nephropathy in patients with chronic kidney disease. Nephrol Dial Transplant. 2006;22(3):819–826. doi: 10.1093/ndt/gfl636. [DOI] [PubMed] [Google Scholar]
- 23.Dodson JA, Hajduk AM, Murphy TE, et al. Thirty-Day Readmission Risk Model for Older Adults Hospitalized With Acute Myocardial Infarction. Circ Cardiovasc Qual Outcomes. 2019;12(5):e005320. doi: 10.1161/CIRCOUTCOMES.118.005320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grams ME, Matsushita K, Sang Y, et al. Explaining the Racial Difference in AKI Incidence. J Am Soc Nephrol. 2014;25(8):1834 LP–1841. doi: 10.1681/ASN.2013080867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shashaty MGS, Meyer NJ, Localio AR, et al. African American race, obesity, and blood product transfusion are risk factors for acute kidney injury in critically ill trauma patients. J Crit Care. 2012;27(5):496–504. doi: 10.1016/j.jcrc.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McCullough PA. Contrast-Induced Acute Kidney Injury. J Am Coll Cardiol. 2008;51(15):1419 LP–1428. doi: 10.1016/j.jacc.2007.12.035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


