Abstract
Purpose:
Vα24-invariant natural killer T cells (NKTs) are attractive carriers for chimeric antigen receptors (CARs) due to their inherent antitumor properties and preferential localization to tumor sites. However, limited persistence of CAR-NKTs in tumor-bearing mice is associated with tumor recurrence. Here, we evaluated whether co-expression of the NKT homeostatic cytokine IL-15 with a CAR enhances the in vivo persistence and therapeutic efficacy of CAR-NKTs.
Experimental design:
Human primary NKTs were ex vivo expanded and transduced with CAR constructs containing an optimized GD2-specific single-chain variable fragment and either the CD28 or 4–1BB costimulatory endodomain, each with or without IL-15 (GD2.CAR or GD2.CAR.15). Constructs that mediated robust CAR-NKT cell expansion were selected for further functional evaluation in vitro and in xenogeneic mouse models of neuroblastoma (NB).
Results:
Co-expression of IL-15 with either costimulatory domain increased CAR-NKT absolute numbers. However, constructs containing 4–1BB induced excessive activation-induced cell death and reduced numeric expansion of NKTs compared with respective CD28-based constructs. Further evaluation of CD28-based GD2.CAR and GD2.CAR.15 showed that co-expression of IL-15 led to reduced expression levels of exhaustion markers in NKTs and increased multi-round in vitro tumor cell killing. Following transfer into mice bearing NB xenografts, GD2.CAR.15 NKTs demonstrated enhanced in vivo persistence, increased localization to tumor sites, and improved tumor control compared to GD2.CAR NKTs. Importantly, GD2.CAR.15 NKTs did not produce significant toxicity as determined by histopathological analysis.
Conclusions:
Our results informed selection of the CD28-based GD2.CAR.15 construct for clinical testing and led to initiation of a first-in-human CAR-NKT cell clinical trial ().
Introduction
Recent clinical trials have demonstrated that T cells expressing CD19-specific chimeric antigen receptors (CARs) induce sustained complete responses in patients with B cell malignancies, leading to recent FDA approval of CD19-specific CAR T cell therapies (1-3). However, clinical results obtained using CAR-redirected immunotherapy for solid tumors have been largely disappointing (4,5). Hence, there is an urgent need for alternative strategies that improve the efficacy of CAR-mediated cancer immunotherapy in a wider range of cancers.
CARs can be expressed in T cell subsets with defined functions. For instance, CARs have been expressed in cytotoxic T lymphocytes (CTLs) specific for viral antigens such as those derived from the Epstein Barr Virus (6). Infusion of CTLs expressing a GD2-specific CAR (GD2.CAR) derived from the 14G2a monoclonal antibody to children with neuroblastoma (NB) was proven safe and achieved complete tumor responses in three of 11 patients with refractory/relapsed disease evaluated in one study (7,8). However, CAR-CTLs did not effectively infiltrate tumors or persist in vivo, observations that were associated with tumor recurrence or lack of objective responses in the majority of patients. An attempt to increase affinity of GD2.CAR via mutation of 14G2a single chain variable fragment (scFv) enhanced antitumor activity of CAR T cells against NB xenografts, but also produced lethal central nervous system toxicity due to recognition of low GD2-expressing normal brain cells in mice (9). New lines of research are examining CAR-based therapies for NB and other solid tumors using innate and innate-like lymphocytes with natural tumor-trafficking and tumor-targeting properties including NK (10), γδ T (11), and Vα24-invariant natural killer T (NKT) cells (12).
NKTs are an evolutionarily conserved sub-lineage of innate-like T cells that express the Vα24-Jα18 invariant TCR α-chain and are characterized by reactivity to self- and microbial-derived glycolipids presented by the monomorphic HLA class-I-like molecule CD1d (13). Although the majority of solid tumors are CD1d-negative, the antitumor potential of NKTs has been demonstrated in numerous cancer models (13). We have reported that NKTs actively localize to tumors in response to tumor-derived chemokines CCL2 and CCL20 and that the presence of NKTs within the primary tumor site is associated with improved outcomes in children with NB (14,15). We have also shown that NKTs can be isolated from the peripheral blood, transduced with CARs, and expanded to clinical scale for adoptive cell therapy applications (12,16).
CD19-specific CAR T cells with either CD28 or 4–1BB endodomains have been shown to be effective in patients with B cell malignancies (2). In NB patients, a GD2-specific CAR containing both CD28 and OX40 endodomains enhanced CAR T cell expansion in the peripheral blood compared to a CAR without costimulation, but the magnitude of expansion was still limited (17). Furthermore, Long et al. reported that, unlike CD19-specific CARs, GD2.CARs undergo tonic signaling resulting from spontaneous dimerization caused specifically by the 14G2a scFv. This unintended signaling, which led to early exhaustion of GD2.CAR T cells, was exacerbated in CARs containing the CD28 endodomain and attenuated in CARs containing 4–1BB (18).
We recently described a modified GD2.CAR containing a truncated form of the 14G2a VH that despite retaining CD28 induces minimal tonic signaling and mediates enhanced antitumor activity in transduced T cells compared to the original GD2.CAR (19). In the same study, we also demonstrated that inclusion of IL-15 in the optimized construct further enhanced the in vitro functionality and in vivo therapeutic potency of CAR.GD2 T cells in NB models.
Mouse studies and in vitro human experimental models have both highlighted the central role of IL-15 in NKT cell development and homeostatic maintenance (20,21). Importantly, IL-15 protects human NKTs from hypoxia, and transgenic expression of IL-15 in adoptively transferred NKTs significantly enhances their antitumor activity (15). Therefore, we hypothesized that co-expressing IL-15 with an optimized GD2.CAR would enhance the survival and antitumor effector functions of NKTs within NB tissues, leading to sustained tumor control. Our results reveal that GD2.CAR constructs encoding 4–1BB, but not CD28, costimulation triggered excessive activation-induced cell death (AICD) in NKTs during in vitro expansion. Importantly, co-expression of IL-15 with the GD2.CAR containing the CD28 costimulatory endodomain increased the in vivo persistence and antitumor efficacy of CAR-NKTs in metastatic NB models without causing evident toxicity. These preclinical assessments were instrumental in the implementation of first-in-human CAR-NKT clinical testing ().
Materials and Methods
Cell lines and culture conditions
CHLA-255, CHLA-136, LA-N-1, and LA-N-6 cell lines were established and maintained as previously described (15,22,23) and as detailed in Supplemental Methods. 293T cells were obtained from the American Type Culture Collection (ATCC). All cell lines were STR fingerprinted at MD Anderson Cancer Center within one year of use and regularly validated to be free of mycoplasma.
NKT cell isolation, expansion, and transduction
NKT cells were isolated from healthy human donor peripheral blood, ex vivo expanded, and transduced with retroviral vectors encoding CAR constructs as previously described (24).
CAR constructs and retroviral vector production
SFG retroviral vectors were constructed as previously described (19) and used to produce retroviral supernatants. Retroviral supernatants were produced by transfecting 293T cells with a combination of an anti-GD2 CAR-containing plasmid, the RDF plasmid encoding the RD114 envelope, and the PeqPam3 plasmid encoding the MoMLV gag-pol as previously described (25). GeneJuice® reagent (Novagen) was used to assist with transfection. Viral supernatants were collected after 48 and 72 hours, filtered with 0.45 μM filters, and frozen.
Flow cytometry
Immunophenotyping was performed using the monoclonal antibodies (mAbs) and reagents detailed in Supplemental Methods. GD2.CARs were detected with the 14G2a anti-idiotype 1A7 mAb. Analysis was performed on an LSR-II 5-laser flow cytometer (BD Biosciences) using BD FACSDiva software version 6.0 and FlowJo 10.1 (Tree Star, Ashland, OR). SPICE software was used to evaluate expression of exhaustion markers (26).
Cytotoxicity assays
Short-term cytotoxicity of parental and GD2.CAR NKTs against CHLA-255, CHLA-136, LA-N-1, and LA-N-6 cells was evaluated using a four-hour luciferase assay as previously described (16). The calcein-AM M2 macrophage cytotoxicity assay was performed as previously described (27) and detailed in the Supplemental Methods. For the long-term cytotoxicity assay, GFP-tagged CHLA-255, CHLA-136 and LA-N-1 cells were plated in a 24-well plate one day prior to adding NKT cells at a 1:1 effector-to-target ratio. 50 U/mL IL-2 were added every other day. Cells were quantified by flow cytometry at day 7 based on NKT invariant TCR and GFP expression, respectively, with non-transduced NKTs as negative control.
Serial Tumor Challenge Assay
CHLA-255, LA-N-1, CHLA-136 cells (0.5 × 106) and CAR NKT cells (0.5 × 106) transduced with GD2.28z or GD2.28z.15 CAR were co-cultured in a 24-well plate using fresh culture medium with 50 U/mL IL-2, respectively. Five days later, cells were harvested, quantified by trypan-blue exclusion method, and analyzed for NKT and NB cell markers by flow cytometry. CAR NKT cells were then replated at a 1:1 E:T ratio with fresh NB cells in fresh cell culture medium to start the next round tumor co-cultures. At the conclusion of the 4th cycle, NKT cells were counted, and the co-culture was analyzed by flow cytometry.
Multiplex cytokine quantification assay and ELISA
Culture supernatants of NKTs alone or after 48 hours of co-culture with CHLA-255 were collected to measure IL-15 release using the Human IL-15 ELISA Kit (R&D Systems). Supernatants from in vitro cultures of NKTs with and without CHLA-255 cells were collected after 24 hours and analyzed for expression of IFNγ, IL-4, GM-CSF, TNFα, and IL-10 using the MILLIPLEX MAP Human Cytokine/Chemokine Immunoassay kit (Millipore) for Luminex® analysis. Mouse IL-6 levels in the plasma were determined using the mouse IL-6 ELISA kit (R&D Systems).
In vivo studies
NSG mice were obtained from The Jackson Laboratory and maintained at the BCM animal care facility. For in vivo persistence experiments, mice were injected intravenously (IV) with 1×106 CHLA-255 cells. NKTs were co-transduced with a GD2.CAR and firefly luciferase using retroviral constructs. Seven days after tumor engraftment, approximately 5×106 CAR-NKTs or control NKTs were injected IV into tumor-bearing mice and monitored using bioluminescent imaging every two days (Small Animal Imaging core facility, Texas Children’s Hospital). At the time of euthanasia, blood, spleen, liver, lung, and bone marrow were collected and cells were processed for flow cytometry analysis. For in vivo therapeutic experiments, mice were injected IV with 1×106 firefly luciferase-labeled CHLA-255, CHLA-136, or 2×106 LA-N-1 NB cells. On day seven, mice were treated with 5×106 CAR-NKTs followed by intraperitoneal (IP) injection of IL-2 (2000 U/mouse) every other day for two weeks. Tumor growth was assessed twice weekly by bioluminescent imaging. Animal experiments were performed according to IACUC-approved protocols.
Immunofluorescent microscopy analyses
Livers of non-transduced, IL-15, GD2.28z, and GD2.28z.15 mice were cryosectioned, fixed, and immunostained with anti-human Vα24Jα18 TCR Biotin (clone 6B11, eBioscience), streptavidin Alexa Fluor® 488 conjugate (Thermofisher), and anti-tyrosine hydroxylase Alexa Fluor® 555 conjugate (clone LNC1, Millipore). Slides were co-stained with ProLong Gold Antifade Reagent with DAPI (Thermofisher) and images were obtained using an LSM 780 Confocal Microscope (Zeiss).
Statistics
For in vitro and in vivo experiments, two-sided unpaired or paired Student’s t tests were used to evaluate differences in continuous variables between two groups. One-way ANOVA with post-test Bonferroni correction was used for continuous variables among more than two groups, and two-way ANOVA was used when two independent variables were considered. Survival was analyzed using the Kaplan-Meier method and the log-rank (Mantel-Cox) test to compare two groups. Statistics were computed using GraphPad Prism 7.0 (GraphPad Software). Differences were considered significant when the P value was less than 0.05.
Results
Generation and functional testing of NKTs expressing CAR constructs with or without IL-15
We generated retroviral constructs incorporating an optimized 14G2a scFv, human CD8α-derived hinge and transmembrane domains, a human CD28 (GD2.28z) or 4–1BB (GD2.BBz) costimulatory endodomain, and a human CD3ζ signaling domain ((19) and Supplementary Fig. S1A). The cDNA sequence encoding human IL-15 was added to GD2.28z (GD2.28z.15) or GD2.BBz (GD2.BBz.15) CAR constructs following a self-cleaving viral 2A peptide sequence, allowing production of separate peptide fragments from a single mRNA. Primary human NKTs were expanded ex vivo following the procedure outlined in Supplementary Fig. S1B and stably transduced with each of the four constructs. CAR expression in NKTs was similar for all constructs as assessed three days post-transduction (Fig. 1A). NKTs expressing GD2.28z.15 and GD2.BBz.15 secreted IL-15 following a 48-hour culture without or with GD2-positive CHLA-255 NB cells, with higher levels observed in the latter condition following NKT activation by CAR engagement (Supplementary Fig. S1C,D).
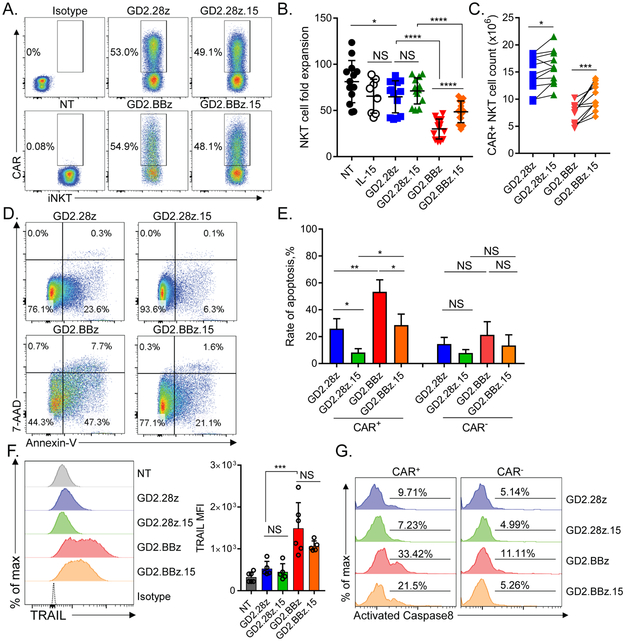
Figure 1. GD2.CAR constructs containing CD28 costimulatory endodomain enable superior ex vivo expansion of CAR-NKTs.
(A) Following a 10-day primary stimulation with α-GalCer-pulsed autologous PBMCs, NKTs were re-stimulated and transduced 2 days later with the indicated CAR constructs or no construct (NT: non-transduced). Representative flow cytometry analysis of indicated GD2.CAR construct expression 3 days after transduction by gating on 1A7+ (14G2a anti-idiotype)/ Vα24-Jα18+ NKT cells (n=13). (B) Quantification of total NKT cell fold expansion, mean ± SD following 10 days of secondary expansion (n=13). NKT cell number was determined using the Cellometer Auto Cell Viability Counter with AOPI staining. *p < 0.05, ****p < 0.0001, NS: not significant, one-way ANOVA. (C) Quantification of CAR+ NKT cell expansion calculated following 10 days of secondary expansion. *p < 0.05, *** p < 0.001, Student’s paired t-test. (D) NKTs transduced with the indicated GD2.CAR constructs were stained with annexin-V and 7-AAD 5 days after transduction. Shown are representative results from one of four donors. (E) Quantification of annexin-V +/7-AAD- NKTs ± SD in both CAR+ gated and CAR- gated populations for indicated GD2.CAR NKT groups (n=4). *p < 0.05, **p < 0.01, NS: not significant, one-way ANOVA. (F) TRAIL expression in CAR+ gated populations for the indicated GD2.CAR NKTs 7 days post-transduction. Shown are TRAIL median fluorescence intensity (MFI) results from a representative donor and mean ± SD of TRAIL expression MFI from six donors. ***p < 0.001, NS: not significant, one-way ANOVA. (G) Levels of activated caspase 8 were evaluated in the indicated CAR+ and CAR- NKT populations using FAM-LETD-FMK labeling 7 days post-transduction. Shown are representative results from one of three donors.
GD2.28z and GD2.28z.15 NKTs had on average 1.25- and 1.14-fold lower expansion rates than non-transduced (NT) NKTs, respectively at day 10 after secondary stimulation (Fig. 1B). GD2.BBz and GD2.BBz.15 NKT expansion rates dropped to 2.71- and 1.67-fold lower on average than those of NT NKTs, respectively, and were also significantly lower than those of their respective CD28-based counterparts (p < 0.0001). When only CAR-positive cells were considered, IL-15 expression increased absolute numbers of NKTs expressing both CD28- and 4–1BB-based constructs (Fig. 1C). Flow cytometry analysis of CAR-NKTs five days post-transduction revealed a significantly higher proportion of early apoptotic cells (annexin-V-positive/7-AAD-negative) in GD2.BBz NKTs versus GD2.28z NKTs (53.4% ± 8.8% vs. 25.9% ± 7.45%, p < 0.01) and in GD2.BBz.15 NKTs vs. GD2.28z.15 NKTs (28.7% ± 8.14% vs. 8.2% ± 2.88%, p < 0.05) (Fig. 1D,E). Furthermore, 4–1BB-CAR-NKTs expressed markedly elevated levels of TNF-related apoptosis-inducing ligand (TRAIL) (Fig. 1F) and FAS (Supplementary Fig. S2A) compared to CD28-CAR-NKTs. We also found that all CAR-NKT groups expressed death receptor TRAIL-R1 (DR4) and to a lesser extent FASL (Supplementary Fig. S2A,B), which together with TRAIL and FAS respectively could lead to self-initiated death receptor signaling in these cells. Indeed, we found higher levels of death receptor-dependent caspase-8 activity in 4–1BB-CAR-NKTs than CD28-CAR-NKTs (Fig. 1G). Thus, the 4–1BB costimulatory endodomain induces AICD, leading to loss of CAR-NKTs during ex vivo expansion. Instead, NKTs transduced with CD28-containing GD2.CAR constructs—particularly with IL-15— undergo relatively low levels of AICD and are capable of extensive numeric expansion, making them a superior option for generating clinical-scale CAR-NKT cell products. Therefore, we selected the GD2.28z and GD2.28z.15 constructs for further pre-clinical evaluation.
Co-expression of IL-15 enhances survival and reduces exhaustion-associated phenotype in GD2.CAR NKTs
Co-expression of IL-15 with a CAR construct should provide a survival advantage for effector cells in the immunosuppressive tumor microenvironment. To determine whether transgenic IL-15 co-expressed with the GD2.CAR mediates this intended effect, we cultured GD2.28z and GD2.28z.15 NKTs in cytokine-deprived serum-free medium and evaluated key molecules of the IL-15-mediated pro-survival signaling pathway. We found that GD2.28z.15, but not GD2.28z NKTs robustly phosphorylated Stat5 (Fig. 2A) and upregulated expression of Bcl-2 family members Bcl-2 (Fig. 2B) and Bcl-xL (Fig. 2C), indicating that transgenic IL-15 recapitulates the pro-survival functionality of the natural cytokine.
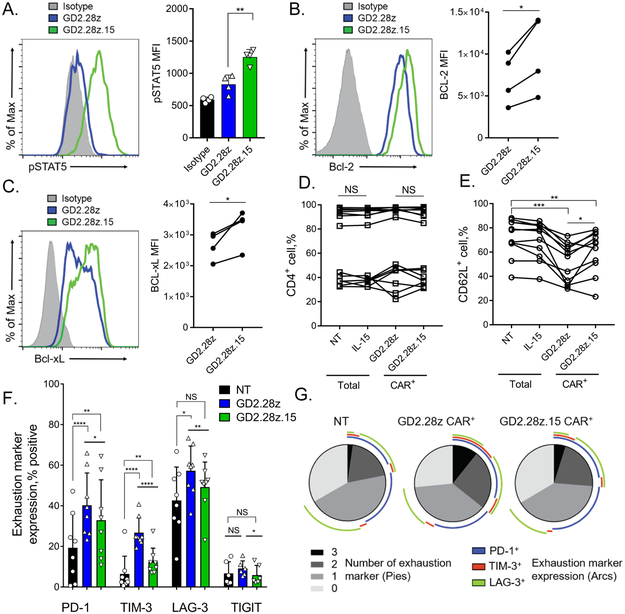
Figure 2. Co-expression of IL-15 enhances survival and reduces exhaustion-associated phenotype in GD2.CAR NKTs.
(A) Expression of phosphorylated STAT5 (pSTAT5) was evaluated in NKTs expressing the GD2.28z or GD2.28z.15 CAR 7 days post-transduction following 12 hours of culture in serum- and cytokine-starved conditions. Shown is a representative histogram from one of four donors (left) and mean ± SD of MFI for all donors (n=4). **p < 0.01, one-way ANOVA. (B) Expression of intracellular Bcl-2 was evaluated in NKTs expressing the GD2.28z or GD2.28z.15 CAR 7 days post-transduction following 12 hours of culture in serum- and cytokine-starved conditions. Shown is a representative histogram from one of four donors (left) and Bcl-2 expression MFI for each individual donor expressing the GD2.28z versus the GD2.28z.15 CAR (n=4). *p < 0.05, Student’s paired t-test. (C) Expression of intracellular Bcl-xL was evaluated in NKTs expressing the GD2.28z or GD2.28z.15 CAR 7 days post-transduction following 12 hours of culture in serum- and cytokine-starved conditions. Shown is a representative histogram from one of four donors (left) and Bcl-xL expression MFI for each individual donor expressing the GD2.28z versus the GD2.28z.15 CAR (n=4). *p < 0.05, Student’s paired t-test. (D) Following 21-day in vitro expansion, NKTs from 14 individual donors transduced with the indicated constructs or non-transduced (NT) NKTs were evaluated for CD4 expression and (E) 11 of them were also examined for CD62L expression by flow cytometry. NS: not significant, *p < 0.05, **p < 0.01, ***p < 0.001, Student’s paired t-test. (F) NKTs transduced with the GD2.28z or GD2.28z.15 CAR or NT were evaluated for expression of the indicated exhaustion markers 7 days after transduction (n=8). *p < 0.05, **p < 0.01, ****p < 0.0001, NS: not significant, one-way ANOVA. (G) SPICE analysis of exhaustion marker expression from three of the donors shown in F. Pie charts reflect proportions of indicated NKT groups expressing indicated numbers (0-3) of exhaustion markers. Colored arcs indicate the specific combinations of exhaustion markers expressed.
Next, we examined expression trends for major human NKT functional markers CD4 and CD62L in GD2.28z and GD2.28z.15 NKTs at the end of in vitro expansion. We confirmed previously reported inter-individual variability in CD4 expression across all donors tested (28). NKTs from donors with high frequency of CD4+ cells (80 – 100%) retained high proportion of CD4+ NKTs after CAR transduction (Fig. 2D). In contrast, NKTs from donors with low to intermediate levels of CD4 expression (20 – 50%) either decreased or increased the proportion of CD4+ cells after CAR transduction. We detected a consistent decrease in the proportion of CD62L+ NKTs in CAR-transduced versus NT cells, which is likely due to a residual tonic signaling induced by GD2.CAR, a common phenomenon in the currently used CARs (29). However, GD2.28z.15 NKTs retained a significantly higher proportion of CD62L+ cells compared to GD2.28z NKTs in 9 of 11 donors (Fig. 2E). Evaluation of exhaustion marker levels revealed that CAR expression led to significant upregulation of PD-1, TIM-3, and LAG-3 in NKTs (Fig. 2F). However, GD2.28z.15 NKTs expressed significantly lower levels of PD-1, TIM-3, LAG-3, and TIGIT (Fig. 2F) and were characterized by significantly fewer cells positive for three of the exhaustion-associated markers than GD2.28z NKTs (Fig. 2G). There was no significant difference in the level of CAR expression observed between the GD2.28z and GD2.28z.15 NKTs (p > 0.05, paired t-test, n=6). The addition of soluble IL-15 to NKT cell culture medium for 7 days after TCR stimulation resulted in a similar PD-1 downregulation in non-transduced and GD2.28z NKTs (Supplementary Fig. S3E). Therefore, co-expression of IL-15 with the GD2.CAR enhances NKT survival and alleviates the exhaustion phenotype.
Co-expression of IL-15 enhances in vitro functional fitness of GD2.CAR NKTs
To determine the efficacy of CAR-mediated killing by NKT cells, we co-cultured GD2.CAR NKTs with firefly luciferase (Ffluc)-expressing CD1d-negative and GD2-high (CHLA-255 and LA-N-1), GD2-medium (CHLA-136), or GD2-negative (LA-N-6) NB cells (Fig. 3A) for four hours. NKTs expressing either GD2.28z or GD2.28z.15 did not kill GD2-negative LA-N-6 cells, but mediated equally potent short-term cytotoxicity against GD2-high NB cell lines (Fig. 3B). GD2-medium CHLA-136 cells were killed less effectively than GD2-high lines in four hours, but were eliminated by either GD2.28z or GD2.28z.15 NKTs within 24 hours (Fig. 3C). While parental NKTs did not kill CD1d-negative NB cells, GD2.CAR NKTs retained their native CD1d-restricted reactivity, as they killed α-galactosylceramide-pulsed M2-polarized macrophages (GD2-negative/CD1d-positive) as effectively as parental NKTs (Fig. 3D). Next, we examined changes in GD2.CAR NKT numbers and phenotype after challenge with NB cells. Seven days after adding any of three NB cell lines, GD2.28z.15 mediated expansion/survival of significantly higher numbers of NKTs compared with GD2.28z. However, challenge with CHLA-136 cells resulted in fewer GD2.CAR NKTs compared to CHLA-255 or LA-N-1 cells (Fig. 3E).
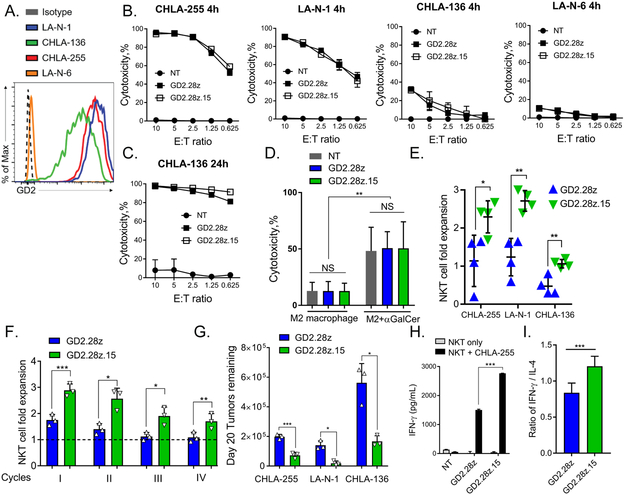
Figure 3. Co-expression of IL-15 enhances the in vitro functional fitness of GD2.CAR NKTs.
(A) Flow cytometry analysis of GD2 expression in the indicated NB cell lines. (B) The indicated luciferase-transduced NB cell lines were co-cultured with GD2.CAR NKTs for 4 or (C) 24 hours. Cytotoxicity was calculated by measuring luminescence intensity of lysed target cells with a plate reader. Shown are results from a representative of four experiments (each with a separate NKT donor) with three technical replicates. (D) GD2.CAR NKTs were co-cultured with calcein-AM-labeled M2-polarized macrophages with or without α-GalCer for 4 hours. Cytotoxicity was assessed by flow cytometry. Shown are mean ± SD from four donors. **p < 0.01, NS: not significant, two-way ANOVA. (E) NKT cell fold expansion measured after 1:1 co-culture with NB cells for 7 days with 50 U/mL IL-2. NKT cell number was determined using the cell counter and flow cytometry. Shown are mean ± SD from four donors. *p < 0.05, **p < 0.01, Student’s unpaired t-test. (F) NB cells expressing GFP were plated at a 1:1 effector-to-target (E:T) ratio with GD2.CAR NKTs every 5 days for four cycles. NKT cells were harvested from the previous co-culture and then re-plated in new culture medium with fresh tumor cells at the same 1:1 E:T ratio. At each cycle, cells were counted and analyzed by flow cytometry. Shown are results of a representative cell line, CHLA-255, from one of two independent experiments performed with three NKT donors per experiment. *p < 0.05, **p < 0.01, ***p < 0.001, Student’s paired t-test. (G) Tumor cells remaining after cycle 4 following the experiment described in F. *p < 0.05, ***p < 0.001, Student’s paired t-test. (H) GD2.CAR NKTs were stimulated with CHLA-255 cells or left unstimulated and supernatants were collected at 24 hours. IFNγ concentrations were measured by Luminex assay. Shown are mean ± SD from a representative of three experiments with three technical replicates. ***p < 0.001, two-way ANOVA. (I) Ratio of IFNγ-to-IL-4 production by GD2.CAR NKTs following 24 hour co-culture with CHLA-255 cells. Shown are mean ± SD for three donors with three replicates each. ***p < 0.001, Student’s unpaired t-test.
To directly test functional fitness, we co-cultured GD2.CAR NKTs with GFP-transduced NB cells for five days, then added equal numbers of the effector cells to fresh tumor cells for another five days and repeated such settings in four rounds. At the end of each round, viable NKT and NB cells were analyzed by flow cytometry (Supplementary Fig. S3A,B). We found that GD2.28z.15 mediated greater NKT cell numeric expansion after each round compared with GD2.28z (Fig. 3F). While NKTs expressing either CAR construct effectively eliminated NB cells in the first three rounds, GD2.28z.15 was more effective in controlling tumor cell numbers compared with GD2.28z at the end of the fourth round (Fig. 3G). The flow cytometry analysis of NKTs after the fourth round revealed higher levels of PD-1 and TIGIT expression in NKTs transduced with GD2.28z compared to GD2.28z.15 (Supplementary Fig. S3C), suggesting that the presence of IL-15 in CAR construct attenuates NKT-cell exhaustion that may enhance their functionality. Similar results were obtained in a repeat challenge experiment using an IncuCyte instrument which allows continuous measurement of viable tumor cells in co-culture with CAR NKTs (Supplementary Fig. S3D).
To further evaluate the functional potential of GD2.CAR NKTs, we determined their cytokine production profiles in response to stimulation with CHLA-255 NB cells. GD2.28z and GD2.28z.15 NKTs both produced high levels of TH1 cytokine interferon-γ (IFNγ) in the presence CHLA-255 cells, with levels approximately two-fold higher in GD2.28z.15 NKTs (Fig. 3H). Both groups produced similar levels of TH2 cytokine IL-4, resulting in an overall higher IFNγ-to-IL-4 ratio in GD2.CAR NKTs with IL-15 (Fig. 3I). Similar trends were observed with TH1 cytokines GM-CSF and TNFα, while IL-10 was not detected from either GD2.CAR NKT group (Supplementary Fig. S4).
GD2.28z.15 construct supports superior NKT cell in vivo persistence
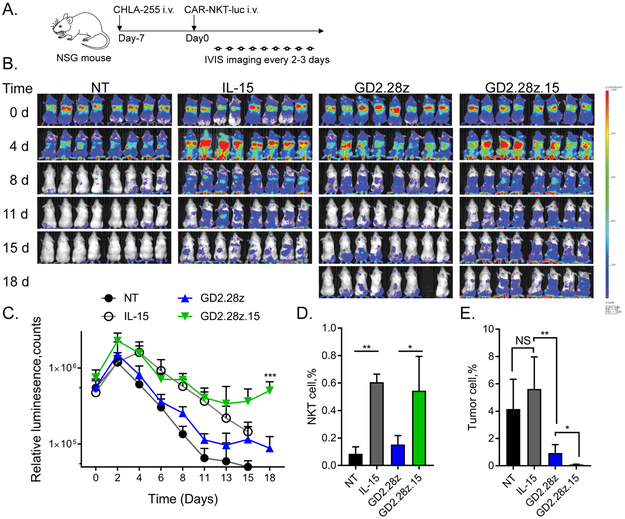
Long-term persistence of CAR-effector cells is critical to the success of adoptive cell therapies (30-33). We examined the in vivo persistence of GD2.28z and GD2.28z.15 NKTs co-transduced with high-intensity Ffluc cDNA following transfer into NSG mice with 7-day established NB xenografts (Fig. 4A). Mice injected with Ffluc-only and IL-15/Ffluc NKTs served as control groups. Transgenic expression of IL-15 on its own improved NKT persistence compared to NT/Ffluc NKTs while GD2.28z only marginally boosted persistence above NT/Ffluc control (Fig. 4B,C). Importantly, addition of IL-15 to the GD2.28z construct significantly enhanced the ability of infused NKTs to expand and persist beyond levels seen in GD2.28z or IL-15 NKTs. We checked the bone marrow, the primary site of NB metastases in humans and our mouse model, for the presence of tumor and NKTs 20 days after NKT cell transfer. Both IL-15/Ffluc and GD2.28z.15 NKT cells were present at approximately six times higher frequency in the bone marrow than NT/Ffluc NKTs, while GD2.28z NKT frequency did not exceed that of control NKTs (Fig. 4D). Accordingly, mice treated with GD2.28z.15 NKTs had significantly fewer NB cells in the bone marrow than mice treated with GD2.28z NKTs (Fig. 4E).
Figure 4. The GD2.28z.15 construct supports superior NKT cell in vivo persistence.
(A) Overview of in vivo NKT persistence experiments. NSG mice were injected intravenously with CHLA-255 neuroblastoma cells followed 7 days later by luciferase-labeled CAR.GD2 or control NKTs. NKTs were tracked by bioluminescence imaging every two-to-three days. (B) Bioluminescent monitoring of NT, IL-15, GD2.28z, and GD2.28z.15 NKTs injected into groups of mice (n=8/group) carrying CHLA-255 xenografts. Shown are representative results from one of three experiments (each with a separate NKT donor). (C) Quantification of bioluminescence images in B. ***p < 0.001, GD2.28z versus GD2.28z.15, Student’s unpaired t-test. (D) Quantification of NKT cells (human CD45+/NKT+) in total bone marrow cells at day 20 as determined by flow cytometry. *p < 0.05, **p < 0.01, one-way ANOVA. (E) Quantification of neuroblastoma tumor cells (human CD56+/GD2+) in total bone marrow cells at day 20 as determined by flow cytometry. Five mice in both NT and IL-15 groups were granted exceptions to delay euthanasia until day 20, but could not be imaged on day 18. *p < 0.05, **p < 0.01, NS: not significant, Student’s unpaired t-test.
GD2.28z.15 construct mediates effective infiltration of NKTs into solid tumor tissues
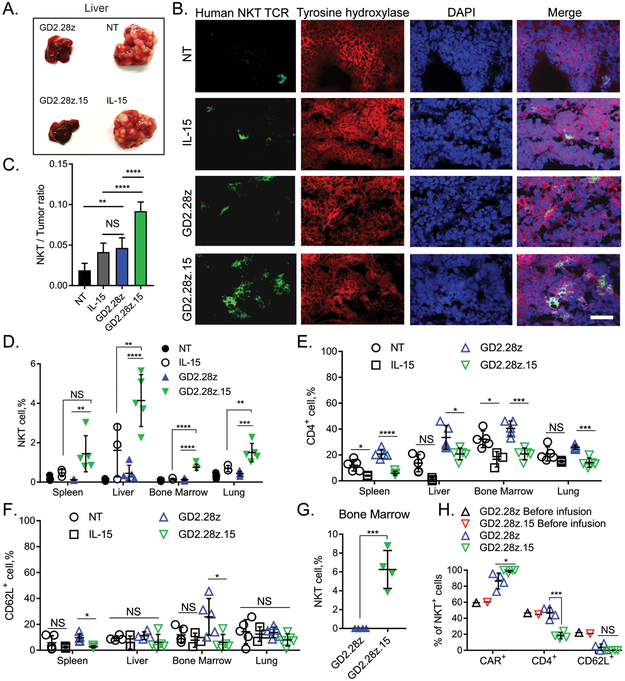
To control tumor growth, therapeutic effector cells must effectively traffic to the tumor site. Using tumor tissues from NB nodules in the livers of NSG mice (Fig. 5A), we performed confocal microscopy to evaluate the ability of GD2.CAR NKTs to infiltrate the tumor 13 days after treatment. Consistent with previous observations (12,15), we found that transgenic expression of either IL-15 alone or a GD2.CAR significantly increased the frequency of NKTs in the tumor tissue as compared to control NKTs (Fig. 5B,C). GD2.28z.15 NKTs were present at significantly higher numbers within NB nodules than both GD2.28z NKTs and IL-15-only NKTs (Fig. 5B,C). In addition to the tumor, GD2.28z and GD2.28z.15 CAR-NKTs were detected in the spleen, liver, bone marrow, and lung (Fig. 5D). Within these tissues, GD2.28z.15 NKTs were predominantly CD4- compared to GD2.28z NKTs (Fig. 5E), indicating that local IL-15 production preferentially benefits survival and/or expansion of CD4- over CD4+ NKTs. CD62L expression was generally low in GD2.CAR NKTs infiltrating the tissues regardless of transgenic IL-15 (Fig. 5F). At the later time point (8 weeks) after infusion, CAR-NKTs could still be found in bone marrow at the frequencies of 6.26%±1.006% and 0.026%±0.003% for GD2.28z.15 and GD2.28z, respectively (Fig. 5G, p < 0.001). Again, these long-term persisting GD2.28z.15 NKTs were preferentially CD4- cells in three experiments from NKT-cell donors with 20 – 50% CD4+ NKT frequency in peripheral blood (Fig. 5H). However, GD2.28z.15 NKTs from one donor with high proportion of CD4+ subset at baseline still exhibited increased persistence in bone marrow while retaining CD4 expression (Supplementary Fig. S5A,B). Therefore, GD2.28z.15 NKTs effectively home to and persist in tumor tissues, in particular due to increased survival and/or expansion of the CD4- subset with a possible exception for donors with high proportion of CD4+ NKTs.
Figure 5. GD2.28z.15 mediates effective infiltration of NKTs into solid tumor tissues and expansion of CD4-negative NKTs.
(A) CHLA-255-bearing NSG mice were injected with luciferase-labeled GD2.CAR or control NKTs in a similar experimental setup as shown in Figure 4A. Thirteen days after injection of NKTs, resected livers were photographed. Shown are representative images from 5 mice per group, one of three experiments. (B) Liver metastases from “A” were fixed and processed for cryosectioning. Sections were probed with antibodies against the Vα24-Jα18 NKT TCR, neuroblastoma tumor marker tyrosine hydroxylase, and DAPI. Images were visualized and quantified by confocal microscopy. (C) Absolute numbers of NKT cells and tumor cells were counted in 10 fields per mouse using images in “B”, 5 mice per group. **p < 0.01, ****p < 0.0001, NS: not significant, one-way ANOVA. (D) Quantification of human NKT cells (human CD45+/NKT+) in total cell populations collected from indicated organs (n=5), one of three experiments. **p < 0.01, ***p < 0.001, ****p < 0.0001, NS: not significant, one-way ANOVA. (E) Quantification of CD4+ NKT cells in total cell populations collected from indicated organs (n=5). *p < 0.05, ***p < 0.001, ****p < 0.0001, NS: not significant, one-way ANOVA. (F) Quantification of CD62L+ NKT cells in total cell populations collected from indicated organs (n=5). *p < 0.05, NS: not significant, one-way ANOVA. (G) At week 8 after injection, human NKT cells were quantified in total cell populations collected from bone marrow (n=4). ***p < 0.001, Student’s unpaired t-test. (H) Quantification of CAR+,CD4+ or CD62L+ subsets of NKT cells in bone marrow as in G (n=4). Data from a representative of three independent experiments. *p < 0.05, ***p < 0.001, NS: not significant, Student’s unpaired t-test.
GD2.28z.15 construct enables superior therapeutic activity of GD2.CAR NKTs in mice without significant toxicity
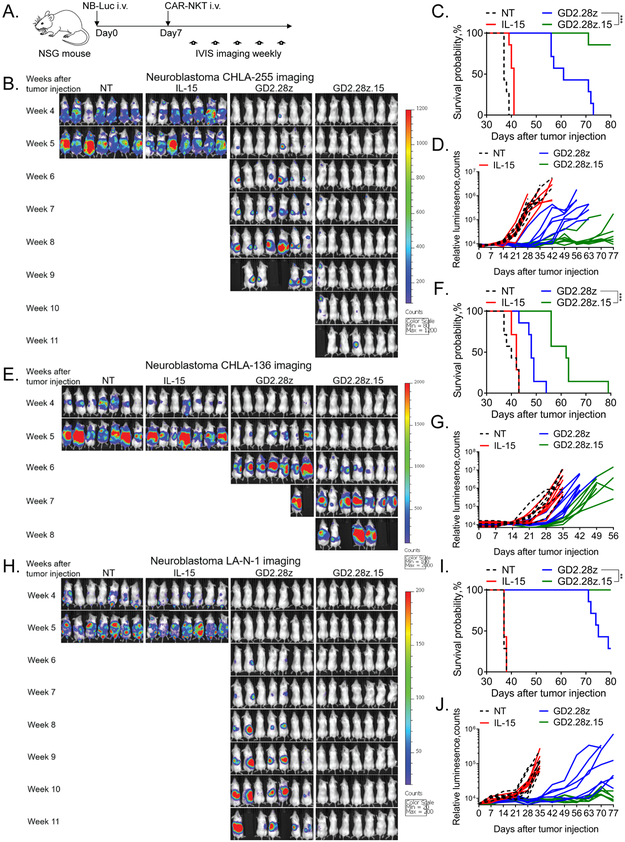
We next evaluated the ability of GD2.CAR NKTs to control tumor growth in three xenogeneic NB models. NSG mice were intravenously injected with CHLA-255, LA-N-1, or CHLA-136 NB cells stably expressing Ffluc, and seven days later they received 5 × 106 of either GD2.28z or GD2.28z.15 NKTs intravenously (Fig. 6A). Mice treated with NT or IL-15-transduced NKTs were used as control groups. We found that GD2.28z.15 NKTs controlled tumor growth significantly longer than GD2.28z NKTs with all three cell lines, (p < 0.001) (Figs. 6B-J). The GD2.28z.15 NKT cell therapy was particularly effective against xenografts of two NB cell lines with high level GD2 expression, achieving a prolonged survival without evidence of disease in the majority of mice at the time of sacrifice at 3 months. We also treated CHLA-255 tumor-bearing mice with a lower (2 × 106) or a higher (107) dose of CAR NKTs. Supplementary Fig. S6 demonstrates that NKTs expressing IL-15-containing CAR retain superior antitumor activity at a low and a high dose. Moreover, only GD2.28z.15 NKT therapy achieved a long-term disease free survival in the majority of mice at the high dose. Thus, consistent with its ability to mediate the longest duration of in vivo persistence and most effective tumor infiltration, GD2.28z.15 also imparted NKTs with superior dose-dependent antitumor activity, especially against tumors with high level GD2 expression.
Figure 6. GD2.28z.15 enables superior in vivo therapeutic activity of GD2.CAR NKTs without causing significant toxicity.
(A) Schematic representation of in vivo tumor challenge experiments. NSG mice were injected intravenously with luciferase-labeled NB cell lines followed 7 days later by CAR.GD2 NKTs. Tumor growth was assessed weekly by bioluminescence imaging. (B) Weekly bioluminescent monitoring of CHLA-255 tumor growth in mice (n=7/group). (C) Kaplan-Meier curve generated from survival of animals in B. ***p < 0.001 Log-rank (Mantel-Cox). (D) Quantification of bioluminescence in B. (E) Weekly bioluminescent monitoring of CHLA-136 tumor growth. (F) Kaplan-Meier curve generated from survival of animals in E. ***p < 0.001 Log-rank (Mantel-Cox). (G) Quantification of bioluminescence in E. (H) Weekly bioluminescent monitoring of LA-N-1 tumor growth. (I) Kaplan-Meier curve generated from survival of animals in H. **p < 0.01 Log-rank (Mantel-Cox). (J) Quantification of bioluminescence in H. Shown are representative results from one of four independent experiments with the CHLA-255 model and one experiment each for the CHLA-136 and LA-N-1 models.
We observed mild hunched mouse stature and ruffled fur twelve days post-injection of GD2.28z.15 NKTs that completely resolved within two-to-three days. At approximately the same time, transient weight loss not exceeding 10% of total body weight also occurred (Supplementary Fig. S7A). Beyond day 14, animals maintained or increased weight. Serum collected on days 12 and 15 was analyzed by ELISA for the presence of human IL-15 and murine IL-6, the latter of which is produced predominantly by murine macrophages in a manner that parallels development of cytokine release syndrome in humans (34). Murine IL-6 levels were not significantly different between GD2.28z.15 NKT-treated and untreated control mice (Supplementary Fig. S7B), while human IL-15 remained undetectable in both groups. Detailed pathological examination was also performed by an experienced clinical pathologist in mice sacrificed at day 12 or 15 post-NKT injection. Lung, heart, liver, spleen, kidney, adrenals, stomach, intestine, pancreas, spine, brain (cerebrum, hippocampus, and cerebellum), skin, bone with bone marrow, and skeletal muscle revealed minimally detectable tumor cells in two mice. Additionally, no pathologic abnormalities were detected in any organ or tissue with the exception of the lungs, where mice in both groups exhibited signs of focal minimal-to-mild interstitial inflammation. An additional long-term tissue evaluation performed between 45 and 60 days post-injection of GD2.28z.15 NKTs also showed complete absence of toxicity or features of graft-versus-mouse disease (Supplementary Fig. S7C). There was also no tissue toxicity observed including that in brain in mice treated with 107 GD2.28z.15 NKTs (Supplementary Fig. S8). Thus, GD2.28z.15 NKTs exert potent anti-NB activity without causing significant off-target toxicity in mice.
Discussion
NKTs are a promising cellular platform for CAR-redirected cancer immunotherapy. Our previous studies have demonstrated that NKTs naturally traffic to NB in xenograft models in response to tumor-derived chemokines and that their ability to infiltrate tumors is further enhanced by expression of a tumor-specific GD2.CAR (12,15).
We have also found that NKT-cell functionality is inhibited in the hypoxic tumor microenvironment and at least in part rescued by the transgenic expression of IL-15 (15). Therefore, we sought to test the hypothesis that co-expression of IL-15 with the GD2.CAR would enhance the ability of NKTs to traffic to and attack NB tumors. Our results demonstrate that NKTs expressing either GD2.28z or GD2.28z.15, but not GD2.BBz or GD2.BBz.15 CAR constructs can be expanded ex vivo to clinical scale and mediate potent CAR-dependent killing of NB cells in vitro. Compared to GD2.28z, GD2.28z.15 CAR NKTs demonstrated superior functional fitness, prolonged in vivo persistence, and superior tumor control in mice without causing significant toxicity.
When comparing GD2.CAR constructs incorporating the two most commonly used costimulatory endodomains, CD28 and 4–1BB, we found that 4–1BB-containing constructs induced higher levels of AICD in NKTs than CD28-containing constructs, regardless of IL-15 co-expression. This AICD was associated with increased TRAIL and FAS expression, activation of caspase-8, and induction of apoptosis, resulting in lower overall NKT cell numeric expansion. In line with our findings in NKTs, Mamonkin et al. observed that 4–1BB-containing CARs expressed from a retroviral vector with strong LTR promoter activity enhanced AICD levels in transduced T cells (35). Specifically, 4–1BB-mediated TRAF-dependent NFκB activation led to upregulation of death receptors and an increased rate of AICD in CAR T cells.
Although GD2.28z and GD2.28z.15 NKTs were equally effective in short-term cytotoxicity assays against two GD2-high (CHLA-255 and LA-N-1) NB cell lines, both mediated reduced cytotoxicity against GD2-medium (CHLA-136) NB cells. CHLA-255 is a MYCN-non-amplified line while LA-N-1 and CHLA-136 are MYCN-amplified lines, suggesting that the efficacy of CAR.GD2 NKT cell therapy depends on the level of GD2 expression on NB cells rather than their MYCN status. GD2.28z.15 NKTs demonstrated significantly more effective tumor control in a repeat challenge assay. This activity was associated with expansion of GD2.28z.15 but not GD2.28z NKTs. We recently showed parallel results with T cells expressing the same two constructs; GD2.28z.15 T cells demonstrated superior expansion and antitumor activity after numerous tumor cell exposures compared to GD2.28z T cells (19). Additionally, we have found that expression of IL-15-containing CARs is associated with reduced exhaustion marker expression in both T and NKT cells, suggesting that IL-15 prevents NKT-cell exhaustion in the same manner as it does in T cells.
Co-expression of IL-15 with the GD2.CAR enriched for T cells with memory and stem-cell-like phenotypes (19). While the mechanisms governing memory differentiation of human NKTs remain less explored than those of T cells, we recently demonstrated that CD62L+ central memory-like NKTs can expand in culture and are largely responsible for the in vivo persistence and therapeutic potential of CAR-NKTs (16). The addition of IL-15 to the CAR increased the frequency of CD62L+ NKTs in 9 of 11 donors examined, suggesting that IL-15 protects NKTs from cell death and exhaustion, and may provide a selective advantage for central memory-like NKTs.
After adoptive transfer to tumor-bearing mice, GD2.28z.15 NKTs demonstrated remarkable persistence especially in the bone marrow and liver (primary sites of NB metastasis) despite the highly hypoxic microenvironment of NB liver metastases that hampers NKT survival and function (15). We previously reported that transgenic IL-15 protects NKTs from hypoxia (15), and here we further observed that IL-15 promotes survival of CAR-NKTs in NB xenografts in the liver. Furthermore, phenotypic analysis of infiltrated CAR-NKTs revealed enrichment of NKTs expressing low levels of CD62L, suggesting that persistent CAR stimulation promotes effector-memory-like differentiation of NKTs as occurs in T cells.
NKTs produce numerous cytokines, which individually may exert either pro-inflammatory or inhibitory effects. Our in vitro testing showed that NKTs co-expressing IL-15 and a GD2.CAR predominantly secrete a TH1-like profile following CAR engagement as evidenced by increased production of IFN-γ, GM-CSF, and TNFα as well as an increased IFN-γ to IL-4 ratio. This observation is consistent with our previous findings showing higher expression of the IL-2Rβ/IL-15Rβ subunit (CD122) in CD4- NKTs (21), which produce more TH1 and less TH2 cytokines than CD4+ NKTs (28). Moreover, in murine models, CD4- NKTs have been reported to have better antitumor functionality in vitro and in vivo compared to their CD4+ counterparts (36). Despite increased sensitivity of CD4- NKTs to IL-15, we did not observe consistent changes in CD4+/CD4- proportion during in vitro expansion of GD2.28z and GD2.28z.15 NKTs, likely due to the presence of IL-2 in culture medium. In contrast, after adoptive transfer to tumor-bearing mice, GD2.28z.15 NKTs derived from 3 donors with low to intermediate levels of CD4+ cells persisted predominantly as CD4- cells within the tumor. We did observe, however, that GD2.28z.15 NKTs derived from a donor with high proportion of CD4+ NKTs retained CD4 expression in vivo, a phenomenon that needs to be further explored.
We found that the potent antitumor effect of GD2.28z.15 NKTs occurred without significant toxicity. The pathological analysis of mouse tissues including brain did not reveal signs of inflammation. In contrast, Richman et al. observed lethal CNS toxicity when testing T cells expressing a mutated CAR.GD2 with an increased affinity to GD2 (9), indicating that increasing CAR affinity to GD2 may not be a viable strategy for increasing therapeutic efficacy of GD2-targetting immunotherapy. Instead, our approach is based on the enhancing functionality of the CAR-expressing effector cells that leads to enhanced antitumor activity without evident toxicity to normal tissues. In contrast, systemic administration of IL-15 in patients with cancer has been associated with dose-limiting toxicities (37), although IL-15/IL15Ra superagonists such as ALT-803 show an increased therapeutic index in the initial clinical testing (38). IL-15 remained undetectable in the serum of mice treated with GD2.28z.15 NKTs, indicating that the therapeutic effects of IL-15 are likely associated with local release of the cytokine at the tumor site.
Based on results of a randomized phase III clinical trial (39), FDA approved anti-GD2 monoclonal antibody ch14.18 (dinutuximab) in combination with GM-CSF, IL-2, and 13-cis-retinoic acid for maintenance therapy of high-risk NB. The antitumor activity of dinutuximab largely depends on ADCC, which is mediated by NK and myeloid cells and augmented by IL-2 and GM-CSF, respectively (40,41). Ch14.18 given as a single agent is ineffective (42). While the role of individual cytokines in this combination therapy is still under investigation, the current regimen remains toxic and not curative for a third of the high-risk patients (43). GD2.28z.15 NKT cell therapy is designed to maximize direct neuroblastoma cell killing without dependence on ADCC or systemic cytokine administration that may lead to a more effective and safe immunotherapy of high-risk and relapsed NB.
Supplementary Material
Translational Relevance.
CAR-redirected T cell adoptive immunotherapy remains largely ineffective in solid tumors due in part to the limited ability of conventional T cells to localize to and survive in tumor tissues. In this work, we exploit the intrinsic tumor-trafficking properties of natural killer T (NKT) cells to home to neuroblastoma tumors and target them with an engineered GD2-specific CAR co-expressing human IL-15 to enhance CAR-NKT survival. We expressed a set of optimized GD2-based CAR constructs with either the human CD28 or 4-1BB costimulatory endodomain—each with or without IL-15—in primary human NKTs and compared the therapeutic potential and toxicity of resultant CAR-NKTs. The results of this study informed the design of an ongoing first-in-human clinical evaluation of CAR-NKT cell therapy in children with neuroblastoma ().
Acknowledgments
The authors are grateful for the excellent technical assistance provided by the staff at Flow Cytometry Core Laboratory of the Texas Children’s Cancer and Hematology Center, and Small Animal Imaging core facility at Texas Children’s Hospital. This work was supported by grants from the National Institutes of Health (RO1 CA116548 and S10 OD020066 to L.S.M.), Cell Medica, Ltd (to L.S.M.), Alex’s Lemonade Stand Foundation for Childhood Cancer (to L.S.M. and A.H.), and Cookies for Kid’s Cancer Foundation (to L.S.M.).
Abbreviations list:
- AICD
activation-induced cell death
- CAR
Chimeric antigen receptor
- DR4
death receptor TRAIL-R1
- Ffluc
firefly luciferase
- GD2.CAR
anti-GD2 CAR
- GD2.CAR.15
anti-GD2 CAR with IL-15
- GD2.28z
anti-GD2 CAR with CD28 co-stimulatory endodomain
- GD2.BBz
anti-GD2 CAR with 4–1BB co-stimulatory endodomain
- GD2.28z.15
GD2.28z with IL-15
- GD2.BBz.15
GD2.BBz with IL-15
- IL-15
interleukin 15
- IP
intraperitoneal
- IV
intravenous
- LAG-3
Lymphocyte-activation gene 3
- mAbs
monoclonal antibodies
- NB
neuroblastoma
- NKT
Vα24-invariant natural killer T
- NSG
NOD/SCID/IL-2Rγnull
- PD-1
Programmed cell death protein 1
- scFv
single chain variable fragment
- TIGIT
T-cell immunoreceptor with Ig and ITIM domains
- TIM-3
T-cell immunoglobulin and mucin-domain containing-3
- TRAIL
TNF-related apoptosis-inducing ligand
Footnotes
Conflict of interest disclosure statement: A.H., D.L., A.N.C., G.D., and L.S.M are co-inventors on pending patent applications that relate to the use of NKTs in cancer immunotherapy and have been licensed by BCM to Cell Medica, Ltd. for commercial development. Cell Medica, Ltd. provided research support for this project (to L.S.M.) via a sponsored research agreement with BCM.
X.X., W.H., L.G., M.W., J.J., B.L., E.J.D.P., J.H., G.A.B, H.N., Y.C., and B.S. declare no competing financial interests.
References
- 1.Lim WA, June CH. The principles of engineering immune cells to treat cancer. Cell 2017;168(4):724–40 doi 10.1016/j.cell.2017.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brudno JN, Kochenderfer JN. Chimeric antigen receptor T-cell therapies for lymphoma. Nat Rev Clin Oncol 2018;15(1):31–46 doi 10.1038/nrclinonc.2017.128. [DOI] [PubMed] [Google Scholar]
- 3.Smith M, Zakrzewski J, James S, Sadelain M. Posttransplant chimeric antigen receptor therapy. Blood 2018;131(10):1045–52 doi 10.1182/blood-2017-08-752121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dotti G, Gottschalk S, Savoldo B, Brenner MK. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev 2014;257(1):107–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Watanabe K, Kuramitsu S, Posey AD Jr., June CH. Expanding the Therapeutic Window for CAR T Cell Therapy in Solid Tumors: The Knowns and Unknowns of CAR T Cell Biology. Front Immunol 2018;9:2486 doi 10.3389/fimmu.2018.02486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manzo T, Heslop HE, Rooney CM. Antigen-specific T cell therapies for cancer. Hum Mol Genet 2015;24(R1):R67–73 doi 10.1093/hmg/ddv270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pule MA, Savoldo B, Myers GD, Rossig C, Russell hv, Dotti G, et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med 2008;14(11):1264–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Louis CU, Savoldo B, Dotti G, Pule M, Yvon E, Myers GD, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 2011;118(23):6050–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richman SA, Nunez-Cruz S, Moghimi B, Li LZ, Gershenson ZT, Mourelatos Z, et al. High-affinity GD2-specific CAR T cells induce fatal encephalitis in a preclinical neuroblastoma model. Cancer Immunol Res 2018;6(1):36–46 doi 10.1158/2326-6066.CIR-17-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rezvani K, Rouce R, Liu E, Shpall E. Engineering natural killer cells for cancer immunotherapy. Mol Ther 2017;25(8):1769–81 doi 10.1016/j.ymthe.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrer DC, Simon B, Fujii SI, Shimizu K, Uslu U, Schuler G, et al. RNA-transfection of gamma/delta T cells with a chimeric antigen receptor or an alpha/beta T-cell receptor: a safer alternative to genetically engineered alpha/beta T cells for the immunotherapy of melanoma. BMC Cancer 2017;17(1):551 doi 10.1186/s12885-017-3539-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heczey A, Liu D, Tian G, Courtney AN, Wei J, Marinova E, et al. Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood 2014;124(18):2824–33 doi blood-2013–11-541235 [pii]; 10.1182/blood-2013-11-541235 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Metelitsa LS. Anti-tumor potential of type-I NKT cells against CD1d-positive and CD1d-negative tumors in humans. Clin Immunol 2011;140(2):119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Metelitsa LS, Wu HW, Wang H, Yang Y, Warsi Z, Asgharzadeh S, et al. Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. J Exp Med 2004;199(9):1213–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu D, Song L, Wei J, Courtney AN, Gao X, Marinova E, et al. IL-15 protects NKT cells from inhibition by tumor-associated macrophages and enhances antimetastatic activity. J Clin Invest 2012;122:2221–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tian G, Courtney AN, Jena B, Heczey A, Liu D, Marinova E, et al. CD62L+ NKT cells have prolonged persistence and antitumor activity in vivo. J Clin Invest 2016;126(6):2341–55 doi 10.1172/JCI83476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heczey A, Louis CU, Savoldo B, Dakhova O, Durett A, Grilley B, et al. CAR T cells administered in combination with lymphodepletion and PD-1 inhibition to patients with neuroblastoma. Mol Ther 2017;25(9):2214–24 doi 10.1016/j.ymthe.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, et al. 4–1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med 2015;21(6):581–90 doi nm.3838 [pii]; 10.1038/nm.3838 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y, Sun C, Landoni E, Metelitsa LS, Dotti G, Savoldo B. Eradication of neuroblastoma by T cells redirected with an optimized GD2-specific chimeric antigen receptor and interleukin-15. Clin Cancer Res 2019. doi 10.1158/1078-0432.CCR-18-1811. [DOI] [PubMed] [Google Scholar]
- 20.Matsuda JL, Gapin L, Sidobre S, Kieper WC, Tan JT, Ceredig R, et al. Homeostasis of V alpha 14i NKT cells. Nat Immunol 2002;3(10):966–74. [DOI] [PubMed] [Google Scholar]
- 21.Baev DV, Peng XH, Song L, Barnhart JR, Crooks GM, Weinberg KI, et al. Distinct homeostatic requirements of CD4+ and CD4- subsets of Valpha24-invariant natural killer T cells in humans. Blood 2004;104(13):4150–6. [DOI] [PubMed] [Google Scholar]
- 22.Keshelava N, Zuo JJ, Chen P, Waidyaratne SN, Luna MC, Gomer CJ, et al. Loss of p53 function confers high-level multidrug resistance in neuroblastoma cell lines. Cancer Res 2001;61(16):6185–93. [PubMed] [Google Scholar]
- 23.Seeger RC, Rayner SA, Banerjee A, Chung H, Laug WE, Neustein HB, et al. Morphology, growth, chromosomal pattern and fibrinolytic activity of two new human neuroblastoma cell lines. Cancer Res 1977;37(5):1364–71. [PubMed] [Google Scholar]
- 24.Ngai H, Tian G, Courtney AN, Ravari SB, Guo L, Liu B, et al. IL-21 Selectively Protects CD62L(+) NKT cells and enhances their effector functions for adoptive immunotherapy. J Immunol 2018;201(7):2141–53 doi 10.4049/jimmunol.1800429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vera J, Savoldo B, Vigouroux S, Biagi E, Pule M, Rossig C, et al. T lymphocytes redirected against the kappa light chain of human immunoglobulin efficiently kill mature B lymphocyte-derived malignant cells. Blood 2006;108(12):3890–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roederer M, Nozzi JL, Nason MC. SPICE: exploration and analysis of post-cytometric complex multivariate datasets. Cytometry A 2011;79(2):167–74 doi 10.1002/cyto.a.21015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song L, Asgharzadeh S, Salo J, Engell K, Wu HW, Sposto R, et al. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest 2009;119(6):1524–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee PT, Benlagha K, Teyton L, Bendelac A. Distinct functional lineages of human V(alpha)24 natural killer T cells. J Exp Med 2002;195(5):637–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ajina A, Maher J. Strategies to address chimeric antigen receptor tonic signaling. Mol Cancer Ther 2018;17(9):1795–815 doi 10.1158/1535-7163.MCT-17-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371(16):1507–17 doi 10.1056/NEJMoa1407222 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turtle CJ, Hanafi LA, Berger C, Gooley TA, Cherian S, Hudecek M, et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J Clin Invest 2016;126(6):2123–38 doi 10.1172/JCI85309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 2012;119(12):2709–20 doi 10.1182/blood-2011-10-384388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet 385(9967):517–28 doi 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teachey DT, Rheingold SR, Maude SL, Zugmaier G, Barrett DM, Seif AE, et al. Cytokine release syndrome after blinatumomab treatment related to abnormal macrophage activation and ameliorated with cytokine-directed therapy. Blood 2013;121(26):5154–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gomes-Silva D, Mukherjee M, Srinivasan M, Krenciute G, Dakhova O, Zheng Y, et al. Tonic 4–1BB costimulation in chimeric antigen receptors impedes T cell survival and is vector-dependent. Cell Rep 2017;21(1):17–26 doi 10.1016/j.celrep.2017.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R, Pellicci DG, et al. Differential antitumor immunity mediated by NKT cell subsets in vivo. J Exp Med 2005;202(9):1279–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol 2015;33(1):74–82 doi 10.1200/JCO.2014.57.3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Robinson TO, Schluns KS. The potential and promise of IL-15 in immuno-oncogenic therapies. Immunol Lett 2017;190:159–68 doi 10.1016/j.imlet.2017.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 2010;363(14):1324–34 doi 10.1056/NEJMoa0911123 [doi]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sondel PM, Hank JA. Combination therapy with interleukin-2 and antitumor monoclonal antibodies. Cancer J Sci Am 1997;3 Suppl 1:S121–S7. [PubMed] [Google Scholar]
- 41.Metelitsa LS, Gillies SD, Super M, Shimada H, Reynolds CP, Seeger RC. Antidisialoganglioside/granulocyte macrophage-colony-stimulating factor fusion protein facilitates neutrophil antibody-dependent cellular cytotoxicity and depends on FcgammaRII (CD32) and Mac-1 (CD11b/CD18) for enhanced effector cell adhesion and azurophil granule exocytosis. Blood 2002;99(11):4166–73. [DOI] [PubMed] [Google Scholar]
- 42.Simon T, Hero B, Faldum A, Handgretinger R, Schrappe M, Niethammer D, et al. Consolidation treatment with chimeric anti-GD2-antibody ch14.18 in children older than 1 year with metastatic neuroblastoma. J Clin Oncol 2004;22(17):3549–57 doi 10.1200/JCO.2004.08.143. [DOI] [PubMed] [Google Scholar]
- 43.Ozkaynak MF, Gilman AL, London WB, Naranjo A, Diccianni MB, Tenney SC, et al. A comprehensive safety trial of chimeric antibody 14.18 with GM-CSF, IL-2, and isotretinoin in high-risk neuroblastoma patients following myeloablative therapy: children’s oncology group study ANBL0931. Front Immunol 2018;9:1355 doi 10.3389/fimmu.2018.01355. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.