Abstract
Background:
This study uses a novel geographic approach to summarize the distribution of breast cancer in San Francisco and aims to identify the neighborhoods and racial/ethnic groups that are disproportionately affected by this disease.
Methods:
Nine geographic groupings were newly defined based on racial/ethnic composition and neighborhood socioeconomic status. Distribution of breast cancer cases from the Greater Bay Area Cancer Registry in these zones were examined. Multivariable logistic regression models were used to determine neighborhood associations with stage IIB+ breast cancer at diagnosis. Cox proportional hazards regression was used to estimate the hazard ratios for all-cause and breast cancer specific mortality.
Results:
A total of 5,595 invasive primary breast cancers were diagnosed between January 1, 2006 and December 31, 2015. We found neighborhood and racial/ethnic differences in stage of diagnosis, molecular subtype, survival, and mortality. Patients in the Southeast (Bayview/Hunter’s Point) and Northeast (Downtown, Civic Center, Chinatown, Nob Hill, Western Addition) areas were more likely to have stage IIB+ breast cancer at diagnosis, and those in the East (North Beach, Financial District, South of Market, Mission Bay, Potrero Hill) and Southeast were more likely to be diagnosed with triple negative breast cancers (TNBC). Compared to other racial/ethnic groups, Blacks/African Americans (B/AA) experienced the greatest disparities in breast cancer related outcomes across geographic areas.
Conclusion:
San Francisco neighborhoods with lower socioeconomic status and larger minority populations experience worse breast cancer outcomes.
Impact:
Our findings, which reveal breast cancer disparities at sub-county geographic levels, have implications for population level health interventions.
Keywords: Breast cancer, health disparities, race/ethnicity, place and health
INTRODUCTION
Breast cancer is one of the most commonly diagnosed cancers among women in the United States, accounting for one in three cancer diagnoses. It is also the second leading cause of cancer death among women, following lung cancer (1). From 2005–2015, new breast cancer cases in the United States increased an average of 0.3% per year (2).
Racial/ethnic disparities in breast cancer risk and survival at the national and regional (e.g., states, counties) level have been well documented (3,4). However, researchers have recently begun to examine this phenomenon at sub-county geographic levels (5,6). More granular geographic level studies offer a unique opportunity to understand the local impacts of disease and to inform the targeted development of programs and policies to address them.
Studies on breast cancer at sub-county (e.g., city) levels have revealed trends in disparities across racial/ethnic categories that are similar to those at the national level. Black or African American (B/AA) vs. Non-Hispanic White (NHW) differences in breast cancer incidence and mortality have not improved over time in Chicago, Illinois (7), Memphis, Tennessee (8), and across many of the most populous cities in the United States.
To our knowledge, no recent studies have described the state of breast cancer in San Francisco, California. San Francisco, which is both a city and county with a population of approximately 880,000 (9), is unique in having one of the highest socioeconomic profiles in the U.S., and through established mechanisms, among the highest breast cancer incidence rates in California (10). In 2017, the population of San Francisco had an average income of $96,265, which is the highest among the 25 largest metropolitan areas in the country (11). San Francisco is also characterized by a high degree of racial/ethnic diversity, which is particularly relevant for breast cancer burden. A plurality of the city’s population is NHW (40.5% of the population), followed by Asian American, Native Hawaiian, or Pacific Islanders (AANHPI, 36.3% of the population), Hispanics/Latinos (H/L, 15.2% of the population), B/AA (5.5% of the population) and other/mixed (5.0% of the population) (12).
Building upon the work being done by the San Francisco Cancer Initiative (13), this paper aims to (1) introduce a novel approach for describing meaningful disease burden in the city of San Francisco by establishing new geographic groupings based on racial/ethnic composition and neighborhood SES, and (2) provide an update on the burden of breast cancer in the city, including identifying specific geographic regions that might experience disparate rates of disease.
MATERIALS AND METHODS
Neighborhood definition
In order to investigate breast cancer disparities in San Francisco, it was necessary to define new geographic grouping of neighborhoods because most traditionally defined neighborhoods are too small to yield stable estimates of disease distribution. We opted to combine contiguous clusters of block groups based on attribute similarity on the basis of racial/ethnic composition and neighborhood SES (nSES). A series of racial/ethnic composition variables were created based on the block group population being above or below the San Francisco median population proportion for each of the main racial/ethnic non-NHW groups (B/AA, H/L, and AANHPI). These variables were combined into 8 mutually exclusive categories as follows: 1) below median for all 3 groups (predominantly NHW), 2) above median for AANHPI only, 3) above median for B/AA only, 4) above median for H/L only, 5) above AANHPI and B/AA median only, 6) above AANHPI and H/L median only, 7) above B/AA and H/L median only, and 8) above median for all 3 groups (predominantly minority neighborhoods). We used a multi-component measure of nSES at the census block group level (14,15). This measure incorporated the 2010 U.S. Census and the 2007–2011 American Community Survey data on education, occupation, unemployment, household income, poverty, rent, and house values. Each block group was assigned a neighborhood SES (nSES) quintile, which was then categorized into a low/high level (low is quintile 1–3 and high is quintile 4–5), based on the distribution of SES across census block groups in San Francisco.
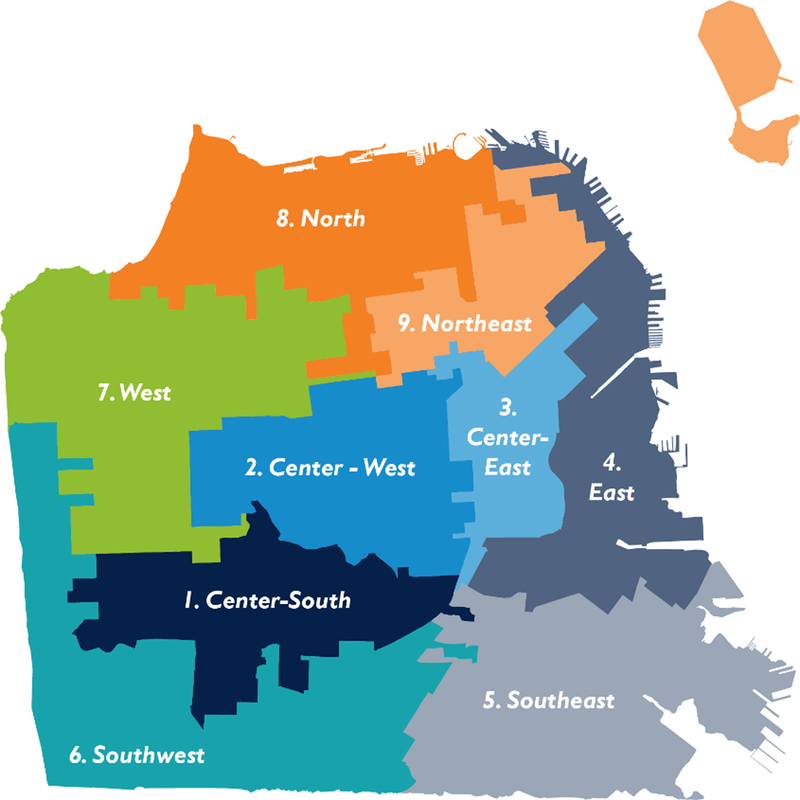
A 16-category combined race/ethnicity-nSES variable was developed and assigned to each of the 579 inhabited block groups in San Francisco (Supplementary Table S1). The variable is developed as: 1) low nSES/below median for all three race-ethnicity groups, 2) high nSES/below median for all three race-ethnicity groups, 3) low nSES/above AANHPI median only, 4) high nSES/above AANHPI median only, 5) low nSES/above B/AA only, 6) high nSES/above B/AA only, 7) low nSES/above H/L median only, 8) high nSES/above H/L median only, 9) low nSES/above AANHPI and B/AA median only, 10) high nSES/above AANHPI and B/AA median only, 11) low nSES/above AANHPI and H/L median only, 12) high nSES/above AANHPI and H/L median only, 13) low nSES/above B/AA and H/L median only, 14) high nSES/above B/AA and H/L median only, 15) low nSES/above median for all 3 groups, and 16) high nSES/above median for all 3 groups (Supplementary Table S1). We mapped the San Francisco block groups using the combined race/ethnicity-nSES variable, and visually grouped contiguous block units to create 9 newly defined areas in San Francisco (Figure 1). This 16-category classification was used to combine contiguous block groups into neighborhoods. For block groups with unclear neighborhood classification, adjudication was conducted via discussion and community feedback. Thus, the process was both data- and community- driven.
Figure 1.
Newly defined areas based on neighborhood SES and racial/ethnic composition in San Francisco.
SF CAN Areas:
1. Center-South: West Portal, Diamond Heights, Glen Park
2. Center-West: Inner Sunset, Haight Ashbury, Castro
3. Center-East: Mission, Bernal Heights
4. East: North Beach, Financial District, South of Market, Mission Bay, Potrero Hill
5. Southeast: Bayview/Hunter’s Point
6. Southwest: Lakeshore, Excelsior
7. West: Sunset, Richmond
8. North: Presidio, Marina, Pacific Heights
9. Northeast: Downtown, Civic Center, Chinatown, Nob Hill, Western Addition.
Breast Cancer Case Population
Information about all breast cancers [defined by SEER Site Recode 26000] diagnosed among residents of San Francisco from 1/1/2006 to 12/31/2015 was obtained from the population-based Greater Bay Area Cancer Registry (GBACR). Available information routinely abstracted from the medical record included age at diagnosis, race/ethnicity (grouped into NHW, B/AA, H/L, AANHPI, or other/unknown), marital status, residential address at diagnosis, stage at diagnosis, tumor size (in centimeters [cm]), lymph node involvement, histology, grade (I, II, III/IV, or unknown), primary source of payment (private, any public/Medicaid/military, Medicare only/Medicare + private, no insurance, and unknown), tumor marker expression status [estrogen receptor (ER) and progesterone receptor (PR)-together referred as hormone receptor (HR), and human epidermal growth factor receptor 2 (HER2)], as well as initial treatment modalities [surgery, radiation, and chemotherapy (endocrine therapy is under-captured in cancer registry data)]. We classified breast cancers into four mutually exclusive subtype categories: HR+/HER2- (defined as ER and/or PR positive and HER2 negative), HR+/HER2+ (ER and/or PR positive and HER2 positive), HR-/HER2+ (ER and PR negative and HER2 positive), and triple-negative breast cancer (TNBC, ER, PR, and HER2 negative). The residential address at diagnosis was geocoded and assigned to a block group and one of the nine newly-designed neighborhood areas. Forty-four males, 4 cases with sex coded other than male or female, 5 cases with invasive behavior but coded to in situ stage, and 7 cases with unknown address at diagnosis were excluded, resulting in a total of 5,622 cases for analysis.
Statistical Analysis
Distributions of breast cancer cases by key patient and tumor characteristics within each of the newly defined areas were examined. We estimated the association between the newly defined areas and odds of stage IIB+ breast cancer at diagnosis using sequential multivariable logistic regression. We chose stage IIB+ as our outcome of interest because of the comparatively more burdensome treatment implications of IIB+ (larger tumor and lymph node compromise) compared to cancers diagnosed at stage I or IIA. Covariates included age, race/ethnicity, nSES, insurance status, marital status, and molecular subtype, and were selected a priori. Survival analysis was limited to the first breast cancer diagnosis per patient. Cases that were diagnosed on death certificate or autopsy only (n=25) or not microscopically confirmed (N=53) were excluded from survival analysis. Patients with missing/unknown tumor size, diagnosis by mammography only, tumor not found, diffuse tumor, and macroscopic focus only (N=234) were additionally excluded, for a final sample size of 5,363 for the survival analysis. Cox proportional hazards regression was used to estimate hazard ratios and corresponding associated 95% confidence intervals (CI). The multivariable model included year of diagnosis, age at diagnosis, marital status, molecular subtypes, race/ethnicity, insurance status, nSES block group quintile (specific to San Francisco), tumor size, lymph node involvement, tumor grade, and histological subtype; AJCC stage was included as underlying stratifying variable given lack of proportionality of hazards by stage, and we additionally adjusted for clustering by block group. For deceased patients, survival time was measured in days from the date of diagnosis to the date of death. For cause-specific survival analysis, patients who died of a cause other than breast cancer (ICD-10 = C50) were censored on the date of death. Patients were followed for vital status by linkage with vital records as of December 31st, 2015. Patients alive at the study end date (12/31/2015) were censored at this time or at the date of last follow-up (i.e., last known contact). All statistical tests were carried out using SAS software version 9.4 (SAS Institute).
Mammography prediction surfaces were created using an optimized ordinary kriging model in ArcGIS (16) based on the census tract level mammogram values from the 500 Cities Project (17). Neighborhood level estimates were derived by extracting the values from the prediction surface for 1000 randomly placed points in each neighborhood polygon and calculating the mean. The interpolation of mammography prediction surfaces allowed for the assignment of predicted values across our newly defined geographic units.
RESULTS
A total of 5,622 invasive primary breast cancers were diagnosed in San Francisco female residents between January 1, 2006 and December 31, 2015. Figure 1 shows the newly defined areas based on neighborhood SES and race/ethnicity composition in San Francisco. Characteristics and distribution of the breast cancer cases within the newly defined areas are shown on Table 1. There are substantial variations in the racial/ethnic distribution of breast cancer cases within specific areas compared to San Francisco overall. NHW and AANHPI made up the greatest proportion of patients in San Francisco overall (47.5% and 36.3%, respectively), but the racial/ethnic distribution of breast cancer cases varied across areas. While 7.2% of the breast cancer cases in San Francisco were B/AA, 25.5% of the cases in the Southeast (Bayview/Hunter’s Point) were B/AA. Similarly, while 8.4% of the breast cancer cases in San Francisco were H/L, 24.8% of the cases in the Center-East (Mission and Bernal Heights) were H/L.
Table 1.
Distribution of race/ethnicity, nSES, stage at diagnosis and molecular subtype by newly defined areas for female invasive breast cancer cases diagnosed in San Francisco, 2006–2015
| All | 1. Center-South | 2. Center-West | 3. Center-East | 4. East | 5. Southeast | 6. Southwest | 7. West | 8. North | 9. Northeast | |
|---|---|---|---|---|---|---|---|---|---|---|
| All | 5622 | 511 (9.1%) | 614 (10.9%) | 330 (5.9%) | 348 (6.2%) | 588 (10.5%) | 810 (14.4%) | 871 (15.5%) | 671 (11.9%) | 879 (15.6%) |
| Race/ethnicity | ||||||||||
| NHW | 2,673 (47.5%) | 290 (56.8%) | 424 (69.1%) | 121 (36.7%) | 219 (62.9%) | 116 (19.7%) | 236 (29.1%) | 369 (42.4%) | 519 (77.3%) | 379 (43.1%) |
| B/AA | 404 (7.2%) | 17 (3.3%) | 23 (3.7%) | 15 (4.5%) | 18 (5.2%) | 150 (25.5%) | 57 (7.0%) | 12 (1.4%) | 18 (2.7%) | 94 (10.7%) |
| H/L | 475 (8.4%) | 31 (6.1%) | 41 (6.7%) | 82 (24.8%) | 34 (9.8%) | 80 (13.6%) | 102 (12.6%) | 30 (3.4%) | 28 (4.2%) | 47 (5.3%) |
| AANHPI | 2,043 (36.3%) | 171 (33.5%) | 121 (19.7%) | 110 (33.3%) | 74 (21.3%) | 239 (40.6%) | 411 (50.7%) | 458 (52.6%) | 104 (15.5%) | 355 (40.4%) |
| Other/Unknown | 27 (0.5%) | * | * | * | * | * | * | * | * | * |
| nSES | ||||||||||
| 1 | 1,133 (20.2%) | * | * | 71 (21.5%) | 12 (3.4%) | 381 (64.8%) | 224 (27.7%) | * | * | 445 (50.6%) |
| 2 | 1,224 (21.8%) | * | <5 (0.5%) | 169 (51.2%) | 13 (3.7%) | 122 (20.7%) | 448 (55.3%) | 222 (25.5%) | 12 (1.8%) | 235 (26.7%) |
| 3 | 1,127 (20.0%) | 59 (11.5%) | 81 (13.2%) | 84 (25.5%) | * | 85 (14.5%) | 133 (16.4%) | 481 (55.2%) | 34 (5.1%) | 170 (19.3%) |
| 4 | 1,053 (18.7%) | 234 (45.8%) | 237 (38.6%) | * | 152 (43.7%) | * | 5 (0.6%) | 148 (17.0%) | 261 (38.9%) | 16 (1.8%) |
| 5 | 1,085 (19.3%) | 218 (42.7%) | 293 (47.7%) | 6 (1.8%) | 171 (49.1%) | * | * | 20 (2.3%) | 364 (54.2%) | 13 (1.5%) |
| Age at Diagnosis (m, SD) | 61.07 (14.81) | 63.53 (15.15) | 59.64 (14.78) | 57.87 (15.63) | 56.56 (14.1) | 59.81 (13.99) | 61.2 (13.8) | 61.54 (14.92) | 61.82 (15.37) | 63.32 (14.76) |
| Stage at Diagnosis | ||||||||||
| Stage I-IIa | 4,022 (71.5%) | 382 (74.8%) | 433 (70.5%) | 232 (70.3%) | 254 (73.0%) | 387 (65.8%) | 598 (73.8%) | 632 (72.6%) | 518 (77.2%) | 586 (66.7%) |
| Stage IIb and higher | 1,430 (25.4%) | 116 (22.7%) | 165 (26.9%) | 87 (26.4%) | 88 (25.3%) | 178 (30.3%) | 193 (23.8%) | 210 (24.1%) | 138 (20.6%) | 255 (29.0%) |
| Stage unknown | 170 (3.0%) | 13 (2.5%) | 16 (2.6%) | 11 (3.3%) | 6 (1.7%) | 23 (3.9%) | 19 (2.3%) | 29 (3.3%) | 15 (2.2%) | 38 (4.3%) |
| Molecular Subtype | ||||||||||
| HR+/HER2- | 3,785 (67.3%) | 364 (71.2%) | 438 (71.3%) | 221 (67.0%) | 237 (68.1%) | 381 (64.8%) | 521 (64.3%) | 607 (69.7%) | 474 (70.6%) | 542 (61.7%) |
| HR+/HER2+ | 565 (10.0%) | 40 (7.8%) | 56 (9.1%) | 40 (12.1%) | 37 (10.6%) | 59 (10.0%) | 91 (11.2%) | 73 (8.4%) | 64 (9.5%) | 105 (11.9%) |
| HR-/HER2+ | 296 (5.3%) | 21 (4.1%) | 28 (4.6%) | 15 (4.5%) | 15 (4.3%) | 36 (6.1%) | 43 (5.3%) | 51 (5.9%) | 26 (3.9%) | 61 (6.9%) |
| TNBC | 540 (9.6%) | 42 (8.2%) | 52 (8.5%) | 35 (10.6%) | 43 (12.4%) | 70 (11.9%) | 86 (10.6%) | 81 (9.3%) | 54 (8.0%) | 77 (8.8%) |
| Unclassified | 436 (7.8%) | 44 (8.6%) | 40 (6.5%) | 19 (5.8%) | 16 (4.6%) | 42 (7.1%) | 69 (8.5%) | 59 (6.8%) | 53 (7.9%) | 94 (10.7%) |
Suppressed due to n<5
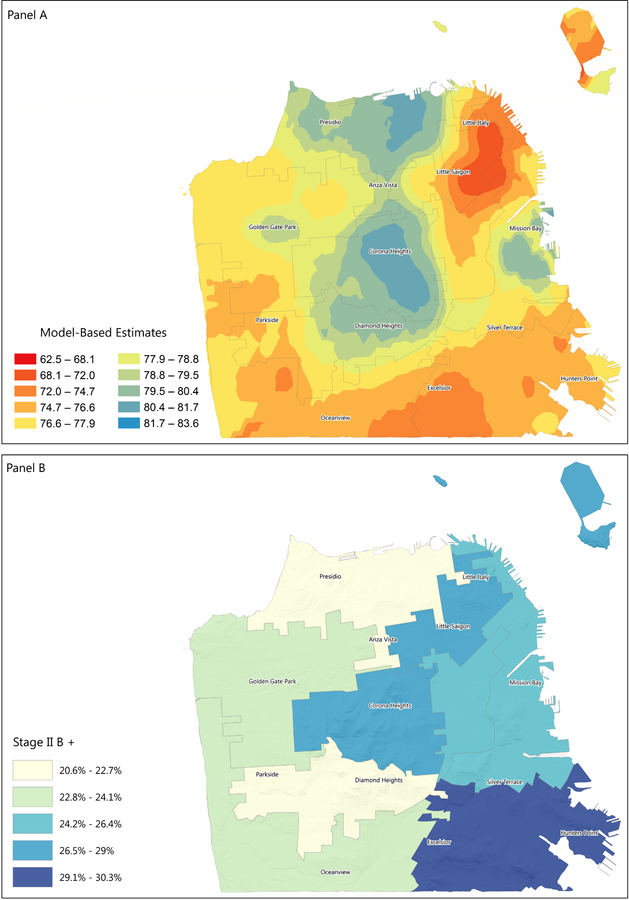
Compared to other areas, more cases in the East (12.4%, including North Beach, Financial District, South of Market, Mission Bay, Potrero Hill) and Southeast (11.9%) were diagnosed with a triple-negative breast cancer (TNBC, a more aggressive molecular subtype of breast cancer). The Southeast and Northeast (Downtown, Civic Center, Chinatown, Nob Hill, Western Addition) areas had greater proportions of stage IIB+ breast cancer at diagnosis (, 30.3% and 29.0%, respectively), as well as unknown stage at diagnosis (3.9% and 4.3%, respectively). The Northeast area also had the highest proportion of unclassified molecular subtype (10.7%). (Table 1). This is consistent with the model-based estimates for mammography use obtained from the 500 cities data, which show that the Southeast and Northeast areas have the lowest screening rates (Figure 1).
B/AA breast cancer patients have the highest proportion of TNBC (20.0%, compared to 8.2%, 11.6%, and 9% in NHW, H/L, and AANHPI) (Supplementary Figure S1). The proportion of B/AA patients diagnosed with TNBC is high across all San Francisco areas, even those with low proportions of B/AA residents (33.3%, 19.3% and 21.3% in the East, Southwest, and Northeast areas) (Supplementary Table S2).
The survival analyses were limited to microscopically confirmed first primary breast tumors not reported on death certificate only, resulting in 5,363 patients with breast cancer. The lowest 5-year breast cancer specific survival rates were observed in the Northeast (rate 88.5, 95% CI 85.6–90.9), Center-South (rate 89.7, 95% CI 85.9–92.5), and Southeast (rate 89.7, 95% CI 86.1–92.4). Compared to other racial ethnic groups, B/AA had worse 5-year overall (rate 71.1, 95% CI 65.4–76.1) and breast cancer specific survival (rate 81.8, 95% CI 76.5–86.0) (Table 2).
Table 2.
Overall and breast cancer specific survival and 95% confidence interval for female invasive breast cancer cases diagnosed in San Francisco by neighborhood and race-ethnicity, San Francisco, 2006–2015
| 5-year | ||
|---|---|---|
| Area | Overall | BCa-specific |
| 1. Center-South | 83.2 (78.8–86.8) | 89.7 (85.9–92.5) |
| 2. Center-West | 86.0 (82.1–89.1) | 91.5 (88.2–93.9) |
| 3. Center-East | 85.4 (79.9–89.5) | 89.8 (84.8–93.2) |
| 4. East | 88.3 (82.9–92.1) | 94.1 (89.9–96.6) |
| 5. Southeast | 82.7 (78.6–86.1) | 89.7 (86.1–92.4) |
| 6. Southwest | 85.2 (82.0–88.0) | 91.9 (89.3–93.9) |
| 7. West | 85.6 (82.5–88.1) | 92.1 (89.7–94.0) |
| 8. North | 88.3 (84.9–91.0) | 94.0 (91.3–95.8) |
| 9. Northeast | 80.5 (77.1–83.5) | 88.5 (85.6–90.9) |
| Race/ethnicity | ||
| NHW | 84.9 (83.1–86.5) | 92.0 (90.6–93.2) |
| B/AA | 71.1 (65.4–76.1) | 81.8 (76.5–86.0) |
| H/L | 84.2 (79.6–87.8) | 90.0 (86.1–92.8) |
| AANHPI | 87.7 (85.8–89.3) | 92.3 (90.8–93.6) |
| Stage | ||
| Stage I-IIa | 91.3 (90.2–92.3) | 97.3 (96.6–97.9) |
| Stage IIb and higher | 69.0 (66.0–71.9) | 75.2 (72.3–77.9) |
| Stage unknown | 56.5 (46.5–65.4) | 73.9 (63.5–81.7) |
| Molecular subtype | ||
| HR+/HER2+ | 87.3 (83.6–90.2) | 93.0 (89.8–95.2) |
| HR+/HER2- | 87.9 (86.5–89.2) | 94.2 (93.1–95.0) |
| HR-/HER2+ | 82.3 (76.3–86.9) | 86.5 (80.8–90.6) |
| TNBC | 71.6 (66.8–75.8) | 77.8 (73.2–81.7) |
| Unclassified | 73.0 (67.8–77.4) | 83.9 (79.3–87.6) |
Results from sequential multivariable-adjusted analysis examining the association between neighborhood and stage IIB+ cancer at diagnosis are shown in Table 3. Results from the univariable analysis show that, compared to the North, which comprises a greater proportion of wealthy NHW residents, those living in the Northeast (OR 1.64, 95% CI 1.28–2.11), Center-East (1.41, 95% CI 1.02–1.95), Southeast (1.77, 95% CI 1.35–2.32) and Center-West (1.48, 95% CI 1.13–1.94) had greater odds of being diagnosed at a higher stage. Observed disparities associated with the Center-East area could be mostly explained by the area-specific age distribution, race/ethnicity and nSES composition. In both the Northeast and Southeast, the biggest change on the coefficient occurred with the incorporation of nSES, although there also appears to be a smaller change when adding race/ethnicity to the model. After adding all relevant covariates, women in the Center-West area still have 1.53-fold increased odds (95% CI 1.16–2.02) of having a stage IIB+ diagnosis (Table 3 and Supplementary Table S5).
Table 3:
Sequential logistic models for the association between neighborhood area and diagnosis at stage IIB+, San Francisco, 2006–2015
| Model 1 (Univariable) |
Model 2 (+Age) |
Model 3 (+Race/Ethnicity) |
Model 4 (+SES Quintile) |
Model 5 (+Insurance) |
Model 6 (+Marital Status) |
Model 7 (+Subtype) |
|
|---|---|---|---|---|---|---|---|
| Area | |||||||
| 1. Center-South | 1.17 ( 0.87– 1.56) | 1.20 ( 0.89– 1.60) | 1.18 ( 0.88– 1.59) | 1.19 ( 0.89– 1.60) | 1.20 ( 0.89– 1.61) | 1.24 ( 0.92– 1.67) | 1.27 ( 0.94– 1.71) |
| 2. Center-West | 1.48 ( 1.13– 1.94) | 1.46 ( 1.11– 1.92) | 1.45 ( 1.10– 1.91) | 1.46 ( 1.11– 1.92) | 1.48 ( 1.12– 1.95) | 1.50 ( 1.13– 1.97) | 1.53 ( 1.16– 2.02) |
| 3. Center-East | 1.41 ( 1.02– 1.95) | 1.37 ( 0.99– 1.90) | 1.26 ( 0.90– 1.75) | 1.05 ( 0.70– 1.59) | 1.03 ( 0.68– 1.56) | 1.00 ( 0.66– 1.51) | 0.99 ( 0.65– 1.50) |
| 4. East | 1.30 ( 0.95– 1.79) | 1.25 ( 0.91– 1.72) | 1.22 ( 0.88– 1.68) | 1.19 ( 0.86– 1.64) | 1.18 ( 0.85– 1.63) | 1.16 ( 0.84– 1.60) | 1.17 ( 0.84– 1.62) |
| 5. Southeast | 1.77 ( 1.35– 2.32) | 1.76 ( 1.35– 2.31) | 1.57 ( 1.18– 2.09) | 1.21 ( 0.82– 1.78) | 1.23 ( 0.84– 1.82) | 1.27 ( 0.86– 1.87) | 1.25 ( 0.84– 1.86) |
| 6. Southwest | 1.21 ( 0.94– 1.57) | 1.23 ( 0.95– 1.60) | 1.17 ( 0.90– 1.53) | 0.94 ( 0.65– 1.36) | 0.98 ( 0.68– 1.42) | 0.99 ( 0.69– 1.44) | 0.96 ( 0.66– 1.40) |
| 7. West | 1.24 ( 0.96– 1.61) | 1.26 ( 0.97– 1.62) | 1.26 ( 0.97– 1.64) | 1.21 ( 0.88– 1.68) | 1.24 ( 0.90– 1.72) | 1.27 ( 0.91– 1.75) | 1.27 ( 0.91– 1.76) |
| 8. North | Ref | Ref | Ref | Ref | Ref | Ref | Ref |
| 9. Northeast | 1.64 ( 1.28– 2.11) | 1.70 ( 1.32– 2.18) | 1.64 ( 1.27– 2.12) | 1.30 ( 0.91– 1.86) | 1.26 ( 0.88– 1.80) | 1.24 ( 0.86– 1.77) | 1.19 ( 0.83– 1.71) |
Hazard ratios for breast cancer specific mortality are presented in Table 4. In univariable analysis, the Northeast (HR 1.82, 95% CI 1.21–2.74) and Southeast (HR 1.70, 95% CI 1.09–2.64) had higher overall mortality compared to the North. These estimates were largely diminished upon inclusion of age, race/ethnicity, and nSES in the model, though the effect of race/ethnicity appeared to be more substantial in the Southeast (Table 4 and Supplementary Tables S3 & S4).
Table 4.
Hazard ratios and 95% confidence intervals for female invasive breast cancer cases living in San Francisco, 2006–2015
| Breast cancer specific mortality HR (95% CI) | ||||||
|---|---|---|---|---|---|---|
| Area | N deaths | Model 1 (Univariable) |
Model 2 (+Age) |
Model 3 (+nSES) |
Model 4 (+ Race/Ethnicity) |
Full Model† |
| 1. Center-South | 38 | 1.55 (0.97–2.47) | 1.52 (0.96–2.43) | 1.55 (0.97–2.47) | 1.53 (0.95–2.44) | 1.23 (0.76–2.01) |
| 2. Center-West | 40 | 1.37 (0.86–2.17) | 1.43 (0.90–2.26) | 1.48 (0.93–2.34) | 1.46 (0.92–2.31) | 1.04 (0.65–1.67) |
| 3. Center-East | 18 | 1.15 (0.65–2.04) | 1.22 (0.69–2.16) | 1.33 (0.66–2.69) | 1.32 (0.65–2.68) | 1.29 (0.63–2.64) |
| 4. East | 17 | 1.00 (0.56–1.79) | 1.11 (0.62–2.00) | 1.09 (0.60–1.95) | 1.04 (0.58–1.88) | 0.77 (0.42–1.41) |
| 5. Southeast | 49 | 1.70 (1.09–2.64) | 1.78 (1.14–2.77) | 1.67 (0.89–3.14) | 1.33 (0.70–2.53) | 1.00 (0.52–1.90) |
| 6. Southwest | 49 | 1.15 (0.74–1.78) | 1.16 (0.75–1.80) | 1.24 (0.67–2.29) | 1.17 (0.63–2.17) | 0.94 (0.50–1.74) |
| 7. West | 56 | 1.26 (0.82–1.94) | 1.25 (0.82–1.93) | 1.61 (0.96–2.72) | 1.64 (0.97–2.79) | 1.16 (0.67–1.99) |
| 8. North | 33 | Ref | Ref | Ref | Ref | Ref |
| 9. Northeast | 75 | 1.82 (1.21–2.74) | 1.74 (1.15–2.62) | 1.74 (0.97–3.12) | 1.69 (0.94–3.04) | 1.22 (0.68–2.19) |
Full model includes area, age, nSES, race/ethnicity, marital status, insurance, molecular subtype, stage (as a stratification variable), grade, surgery, radiation, and chemotherapy
DISCUSSION
Our study introduces an innovative approach to describe the burden of cancer at a sub-county level. Our findings reveal that, similar to other regions across the United States, women that reside in low SES areas with larger representation of minority populations are diagnosed with more advanced and aggressive breast cancer and have lower survival than women who reside in high SES NHW neighborhoods (18). In particular, although B/AA make up only 7.2% of all breast cancer cases between 2006–2015, they experienced the greatest proportion of TNBC diagnosis across all neighborhoods, as well as the worst 5-year overall and breast cancer specific survival. H/L and AANHPI communities experience disparities as well, but differences are less marked than those between B/AA and NHW.
Ongoing research suggests that disparities observed in tumor subtype distribution between B/AA and NHW could be due, in part, to genetics (19). There is also evidence that some lifestyle factors, such as number of full-term pregnancies and breastfeeding are associated with risk of TNBC and could also explain the higher incidence of TNBC in B/AA women (20,21). The particular tumor subtype distribution in H/L and AANHPI could also be partly due to differences in genetics and environmental/lifestyle exposures that impact tumor biology (22,23). However, the overlap between stage at diagnosis and screening rates in the different areas of San Francisco (Figure 2) strongly suggest that the observed disparity in stage at diagnosis and its impact on breast cancer survival and quality of life could be addressed, at least in part, by closing the gap in screening rates between women in different areas of the city.
Figure 2:
Distribution of mammography and stage IIB+ cancer at diagnosis in San Francisco
Panel A: Model-based estimates for mammography use among women aged 50–74 years, 2016
Panel B: Proportion of cases with stage IIB+ cancer at diagnosis
While several studies have suggested that improving access to high quality care and follow-up in patients from low SES areas is likely to reduce survival disparities (24,25), simply increasing access may not be sufficient for eliminating racial differences (18,26,27). In fact, although health care for all has been available in San Francisco since 2007 (28), in meetings of the SF CAN Breast Cancer Task Force, community representatives from underserved communities report that they do not generally know about this. The disproportional burden of unknown stage at diagnosis and unclassified molecular subtype may reflect the quality of care that individuals in certain SF areas receive. Similar to other metropolitan cities in the United States (29,30), structural racism could be a contributing factor to disparities among B/AA women in San Francisco.
Even after accounting for the effect of age, race/ethnicity, nSES, insurance type, marital status, and clinical features, living in the Center-East area is still associated with increased odds of stage IIB+ cancer at diagnosis. The Center-East comprises the Castro district, which has historically served as a safe haven for sexual and gender minority (SGM) populations. There is some evidence suggesting higher risk of breast cancer and mortality among lesbian and bisexual women (31,32), but results are inconsistent, in large part due to lack of data (e.g., no data on sexual gender minority status in cancer registries). Considering the large population and diversity of SGM status within the city, San Francisco could be an ideal location to further investigate the relationship between gender identity, sexual orientation, and breast cancer risk and mortality, and to tailor interventions toward non-heterosexual women. Incorporation of sexual gender minority data into population-based cancer registries will be crucial to better document the burden of cancer in this underserved population (33,34).
One of the limitations of our current study is the inability to produce neighborhood level incidence estimates. Using block groups as a building block provided the fine granularity to define areas with meaningful specificity. However, as population estimates required to compute incidence and mortality rates are available at the census tract level, not at the block group level, we were unable to calculate incidence rates for the 9 areas. Our recommendation for future studies is to use census tracts as the building blocks if the intent is to calculate disease rates requiring population denominators. An additional limitation in our study is the small number of breast cancer specific deaths, which could have contributed to the lack of significant associations for breast cancer specific mortality across neighborhoods. Additionally, since the San Francisco population is rapidly changing, our description based on most recently available cancer registry data may not be an accurate picture of the current burden of breast cancer in the city. Specifically, as the economic and technological landscape continues to expand, it will be critical to continue monitoring breast cancer disparities due to both racial/ethnic and socioeconomic inequity. Finally, although data are available on neighborhood-level attributes that may potentially account for some of the observed neighborhood-level disparities, the intent of this analysis was to provide a descriptive examination of the burden of breast cancer across the city. We recognize, however, the importance of providing neighborhood-level data to stakeholders and, as such, have extended this work to develop a statewide tool that allows interactive query and mapping of cancer incidence rates alongside population-level sociodemographic and behavioral risk factors (www.californiahealthmaps.org).
Given what our research has revealed regarding breast cancer health disparities in San Francisco, our present challenge will be to design programs and interventions that could more effectively promote breast cancer preventive behaviors and access to appropriate care among the city’s most impacted racial/ethnic groups. This work is being undertaken by the San Francisco Cancer Initiative’s Breast Cancer Task Force. Specifically, the Task Force will be focusing on the design and implementation of programs tailored toward AA/B, H/L and AANHPI populations in San Francisco, with the long-term goal of reducing observed disparities in stage at diagnosis and survival. Ongoing programs, based on community feedback and evidence of efficacy, include the compilation and distribution of information about resources and services that are already available to support breast health related practices among women in San Francisco (35–37), and the implementation of a high school student-based breast cancer awareness and education program to promote screening and health behavior change in their communities (38–41). We plan to monitor changes in geographic burden over time and document potential impacts of these and future programs.
Supplementary Material
ACKNOWLEDGEMENTS
The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP006344; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute, Cancer Registry of Greater California. The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors. The authors acknowledge the following members of the SF CAN Breast Cancer Task Force: Becky Mark, Carol Simmons, Catherine Thomsen, Cecilia Thomas, Fern Orenstein, Galen Joseph, Judith Biletnikoff Harkins, Alyssa Nickel, Niharika Dixit, Olivia Fe, Kylie Cooper, Vivian Lee, Lei-Chun Fung, Janice Barlow, Laura van ‘t Veer, Jeffrey A. Tice, Roberto Vargas, Carmen Ortiz, and Kaya Balke.
Funding Statement: This research was funded by the Helen Diller Comprehensive Cancer Center at the University of California, San Francisco through the San Francisco Cancer Initiative. The collection of cancer incidence data used in this study was supported by the California Department of Public Health pursuant to California Health and Safety Code Section 103885; Centers for Disease Control and Prevention’s (CDC) National Program of Cancer Registries, under cooperative agreement 5NU58DP006344; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract HHSN261201800032I awarded to the University of California, San Francisco, contract HHSN261201800015I awarded to the University of Southern California, and contract HHSN261201800009I awarded to the Public Health Institute, Cancer Registry of Greater California. The ideas and opinions expressed herein are those of the author(s) and do not necessarily reflect the opinions of the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their Contractors and Subcontractors.
Abbreviations list:
- B/AA
Black or African American
- NHW
Non-Hispanic White
- AANHPI
Asian American, Native Hawaiian, or Pacific Islanders
- H/L
Hispanic/Latino
- nSES
Neighborhood socioeconomic status
- TNBC
Triple Negative Breast Cancer
- HER2
Human epidermal growth factor receptor 2
Footnotes
Conflict of Interest:
The authors have no conflicts of interest to report.
REFERENCES
- 1.American Cancer Society. Cancer Facts and Statistics [Internet]. American Cancer Society; [cited 2018 Sep 23]. Available from: https://www.cancer.org/content/cancer/en/research/cancer-facts-statistics.html [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results Program. Cancer Stat Facts: Female Breast Cancer [Internet]. 2018. [cited 2018 Sep 16]. Available from: https://seer.cancer.gov/statfacts/html/breast.html [Google Scholar]
- 3.DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state: Breast Cancer Statistics, 2017. CA Cancer J Clin. 2017. November;67(6):439–48. [DOI] [PubMed] [Google Scholar]
- 4.Davis Lynn BC, Rosenberg PS, Anderson WF, Gierach GL. Black–White Breast Cancer Incidence Trends: Effects of Ethnicity. JNCI J Natl Cancer Inst. 2018. November 1;110(11):1270–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Highfield L Spatial Patterns of Breast Cancer Incidence and Uninsured Women of Mammography Screening Age. Breast J. 2013. May;19(3):293–301. [DOI] [PubMed] [Google Scholar]
- 6.Williams F, Thompson E. Disparity in Breast Cancer Late Stage at Diagnosis in Missouri: Does Rural Versus Urban Residence Matter? J Racial Ethn Health Disparities. 2016. June;3(2):233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirschman J, Whitman S, Ansell D. The black:white disparity in breast cancer mortality: the example of Chicago. Cancer Causes Control. 2007. April;18(3):323–33. [DOI] [PubMed] [Google Scholar]
- 8.Lamb EP, Pritchard FE, Nouer SS, Tolley EA, Boyd BS. Understanding Disparities in Breast Cancer Care in Memphis, Tennessee. The American Surgeon. 2018;84(5):620–7. [PubMed] [Google Scholar]
- 9.U.S. Census Bureau, Population Division. Annual Estimates of the Resident Population, April 1, 2010 to July 1, 2018 [Internet]. 2019. Available from: https://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?src=bkmk
- 10.Cancer Prevention Institute of California. The Greater Bay Area Cancer Registry Annual Report: Incidence and Mortality Review, 1988–2015 [Internet]. Cancer Prevention Institute of California; [cited 2018 Dec 19]. Available from: https://cancerregistry.ucsf.edu/sites/cancerregistry.ucsf.edu/files/wysiwyg/GBACR_Annual_Incidence_and_Mortality_Review_2018.pdf [Google Scholar]
- 11.Guzman GG. Household Income: 2016. [Internet]. 2017. September Available from: https://www.census.gov/content/dam/Census/library/publications/2017/acs/acsbr16-02.pdf [Google Scholar]
- 12.U.S. Census Bureau. San Francisco County, California: [Internet]. [cited 2017 Jul 1]. (Quick Facts). Available from: https://www.census.gov/quickfacts/sanfranciscocountycalifornia [Google Scholar]
- 13.Hiatt RA, Sibley A, Fejerman L, Glantz S, Nguyen T, Pasick R, et al. The San Francisco Cancer Initiative: A Community Effort To Reduce The Population Burden Of Cancer. Health Aff (Millwood). 2018. January;37(1):54–61. [DOI] [PubMed] [Google Scholar]
- 14.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. 2011. October;12(8):703–11. [DOI] [PubMed] [Google Scholar]
- 15.Yang J, Schupp CW, Harrati A, Clarke C, Keegan THM, Gomez SL. Developing an area-based socioeconomic measure from American Community Survey data [Internet] Fremont, CA: Cancer Prevention Institute of California; 2014. Available from: https://cancerregistry.ucsf.edu/sites/cancerregistry.ucsf.edu/files/wysiwyg/Yang%20et%20al.%202014_CPIC_ACS_SES_Index_Documentation_3-10-2014.pdf [Google Scholar]
- 16.Oliver MA, Webster R. Kriging: a method of interpolation for geographical information systems. Int J Geogr Inf Syst. 1990. July;4(3):313–32. [Google Scholar]
- 17.Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Division of Population Health. 500 Cities Project Data [online] [Internet]. 2018. Available from: https://www.cdc.gov/500cities
- 18.Keegan THM, Kurian AW, Gali K, Tao L, Lichtensztajn DY, Hershman DL, et al. Racial/Ethnic and Socioeconomic Differences in Short-Term Breast Cancer Survival Among Women in an Integrated Health System. Am J Public Health. 2015. May;105(5):938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gong Z, Wang J, Wang D, Buas MF, Ren X, Freudenheim JL, et al. Differences in microRNA expression in breast cancer between women of African and European ancestry. Carcinogenesis [Internet] 2018. October 13 [cited 2018 Dec 18]; Available from: https://academic.oup.com/carcin/advance-article/doi/10.1093/carcin/bgy134/5128950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anstey EH, Shoemaker ML, Barrera CM, O’Neil ME, Verma AB, Holman DM. Breastfeeding and Breast Cancer Risk Reduction: Implications for Black Mothers. Am J Prev Med. 2017. September;53(3):S40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.John EM, Hines LM, Phipps AI, Koo J, Longacre TA, Ingles SA, et al. Reproductive history, breast-feeding and risk of triple negative breast cancer: The Breast Cancer Etiology in Minorities (BEM) study: Reproductive factors and triple negative breast cancer. Int J Cancer. 2018. June 1;142(11):2273–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Banegas MP, Tao L, Altekruse S, Anderson WF, John EM, Clarke CA, et al. Heterogeneity of breast cancer subtypes and survival among Hispanic women with invasive breast cancer in California. Breast Cancer Res Treat. 2014. April;144(3):625–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mavaddat N, Antoniou AC, Easton DF, Garcia-Closas M. Genetic susceptibility to breast cancer. Mol Oncol. 2010. June;4(3):174–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Engmann NJ, Ergas IJ, Yao S, Kwan ML, Roh JM, Ambrosone CB, et al. Genetic Ancestry Is not Associated with Breast Cancer Recurrence or Survival in U.S. Latina Women Enrolled in the Kaiser Permanente Pathways Study. Cancer Epidemiol Biomarkers Prev. 2017. September;26(9):1466–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reeder-Hayes KE, Anderson BO. Breast Cancer Disparities at Home and Abroad: A Review of the Challenges and Opportunities for System-Level Change. Clin Cancer Res. 2017. June 1;23(11):2655–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurian AW, Lichtensztajn DY, Keegan THM, Leung RW, Shema SJ, Hershman DL, et al. Patterns and predictors of breast cancer chemotherapy use in Kaiser Permanente Northern California, 2004–2007. Breast Cancer Res Treat. 2013. January;137(1):247–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livaudais JC, Hershman DL, Habel L, Kushi L, Gomez SL, Li CI, et al. Racial/ethnic differences in initiation of adjuvant hormonal therapy among women with hormone receptor-positive breast cancer. Breast Cancer Res Treat. 2012. January;131(2):607–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katz MH, Brigham TM. Transforming A Traditional Safety Net Into A Coordinated Care System: Lessons From Healthy San Francisco. Health Aff (Millwood). 2011. February;30(2):237–45. [DOI] [PubMed] [Google Scholar]
- 29.Beyer KMM, Zhou Y, Matthews K, Bemanian A, Laud PW, Nattinger AB. New spatially continuous indices of redlining and racial bias in mortgage lending: links to survival after breast cancer diagnosis and implications for health disparities research. Health Place. 2016. July;40:34–43. [DOI] [PubMed] [Google Scholar]
- 30.Dai D Black residential segregation, disparities in spatial access to health care facilities, and late-stage breast cancer diagnosis in metropolitan Detroit. Health Place. 2010. September;16(5):1038–52. [DOI] [PubMed] [Google Scholar]
- 31.Dibble SL, Roberts SA, Nussey B. Comparing breast cancer risk between lesbians and their heterosexual sisters. Womens Health Issues. 2004. March;14(2):60–8. [DOI] [PubMed] [Google Scholar]
- 32.Cochran SD, Mays VM. Risk of Breast Cancer Mortality Among Women Cohabiting with Same Sex Partners: Findings from the National Health Interview Survey, 1997–2003. J Womens Health. 2012. May;21(5):528–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez SL, Duffy C, Griggs J, John EM. Surveillance of cancer among sexual and gender minority populations: where are we and where do we need to go? Cancer Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Malone J, Snguon S, Dean LT, Adams MA, Poteat T. Breast Cancer Screening and Care Among Black Sexual Minority Women: A Scoping Review of the Literature from 1990 to 2017. J Womens Health. 2019. March 18;jwh.2018.7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark CR, Baril N, Kunicki M, Johnson N, Soukup J, Ferguson K, et al. Addressing Social Determinants of Health to Improve Access to Early Breast Cancer Detection: Results of the Boston REACH 2010 Breast and Cervical Cancer Coalition Women’s Health Demonstration Project. J Womens Health. 2009. May;18(5):677–90. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez M Evaluation of ENCOREplus A community-based breast and cervical cancer screening program. Am J Prev Med. 1999. January;16(1):35–49. [DOI] [PubMed] [Google Scholar]
- 37.Rapkin BD, Massie MJ, Jansky EJ, Lounsbury DW, Murphy PD, Powell S. Developing a Partnership Model for Cancer Screening with Community-Based Organizations: The ACCESS Breast Cancer Education and Outreach Project. Am J Community Psychol. 2006. December;38(3–4):287–97. [DOI] [PubMed] [Google Scholar]
- 38.Jourdan D, Christensen JH, Darlington E, Bonde AH, Bloch P, Jensen BB, et al. The involvement of young people in school- and community-based noncommunicable disease prevention interventions: a scoping review of designs and outcomes. BMC Public Health. 2016. December;16(1):1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morse LL, Allensworth DD. Placing Students at the Center: The Whole School, Whole Community, Whole Child Model. J Sch Health. 2015. November;85(11):785–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beck AJ, Reilly SM. What Can Secondary School Students Teach Educators and School Nurses About Student Engagement in Health Promotion? A Scoping Review. J Sch Nurs. 2017. February;33(1):30–42. [DOI] [PubMed] [Google Scholar]
- 41.Soto‐Perez‐de‐Celis E, Smith DD, Rojo‐Castillo MP, Hurria A, Pavas‐Vivas AM, Gitler‐Weingarten R, et al. Implementation of a School‐Based Educational Program to Increase Breast Cancer Awareness and Promote Intergenerational Transmission of Knowledge in a Rural Mexican Community. The Oncologist. 2017. October;22(10):1249–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.