Abstract
Adipocyte-tumor cell crosstalk is one of the critical mediators of tumor progression and an emerging facilitator of therapy evasion. Tumor cells that metastasize to adipocyte-rich bone marrow take advantage of the interplay between metabolic and inflammatory pathways to activate pro-survival mechanisms that allow them to thrive and escape therapy. Using in vitro and in vivo models of marrow adiposity, we demonstrate that metastatic prostate carcinoma (PCa) cells engage bone marrow adipocytes in a functional crosstalk that promotes IL-1β expression in tumor cells. Tumor-supplied IL-1β contributes to adipocyte lipolysis and regulates a pro-inflammatory phenotype in adipocytes via upregulation of COX-2 and MCP-1. We further show that the enhanced activity of the IL-1β/COX-2/MCP-1 axis and a resulting increase in PGE2 production by adipocytes coincide with augmented hypoxia signaling and activation of pro-survival pathways in tumor cells, revealing a potential mechanism of chemoresistance. The major consequence of this interplay is the reduced response of PCa cells to docetaxel, a phenomenon sensitive to the inhibition of lipolysis.
Keywords: bone marrow adipocyte, bone metastasis, inflammation, lipolysis, docetaxel, IL-1β, PGE2, COX-2, MCP-1
INTRODUCTION
Growing evidence suggests that tumor cells can be protected from therapy-induced cell death via signaling events driven by neighboring cells. Specifically, the aggressive and often lethal phenotype of bone trophic cancers, such as tumors of the prostate and breast, acute myeloid leukemia, or multiple myeloma, is increasingly being attributed to adipocytes, a significant component of adult bone marrow (1–3). However, understanding the molecular mechanisms driving adipocyte-tumor cell crosstalk towards progression and therapy evasion has been very limited and remains a critical gap in designing effective therapies for metastatic disease. For a patient with advanced prostate cancer (PCa), current therapeutic approaches include androgen deprivation therapy (ADT) in combination with microtubule-targeting docetaxel, a first life-prolonging drug for metastatic PCa, and a standard of care treatment since 2004 (4). Unfortunately, the majority of patients eventually progress and succumb to the disease, and overcoming chemotherapy resistance remains an unmet clinical need (5). Whether marrow adiposity is a contributing factor to this limited chemotherapy response is not well-understood.
It is becoming increasingly evident that the metastatic tumor cells colonizing bone marrow modulate the metabolic phenotype of marrow fat cells, thereby supporting tumor progression (6–8). Specifically, prostate carcinoma cells can stimulate triglyceride hydrolysis (lipolysis) in marrow adipocytes (7), a phenomenon also observed in adipocytes interacting with breast, ovarian and colon cancer cells, as well as leukemic blasts (1). In return, adipocyte-supplied fatty acids have been shown to fuel the metabolism of the cancer cell and ultimately promote tumor growth and survival (1,7,9,10). In addition to fatty acids, the repertoire of factors supplied by marrow fat cells include hormones, adipokines, cytokines, incretins, growth factors and bioactive lipids mediators (2,11,12). These molecules contribute to a number of key processes including regulation of inflammation, insulin sensitivity and redox metabolism, and are subject to modulation by the metabolic events within the fat cell as well as cues from the microenvironment (2,11).
Activation of lipolysis (13) or exposure to a hypoxic microenvironment (14) are examples of metabolic and environmental cues associated with adipose tissue inflammation, increased expression of cyclooxygenase-2 (COX-2), and augmented biosynthesis of prostaglandins by white adipose tissue. Both hypoxia and lipolysis also contribute to the increased production of pro-inflammatory factors such as macrophage chemoattractant protein (MCP-1; CCL2) or interleukin-6 (IL-6) (13,15). COX-2 and MCP-1 play critical functions in the regulation of bone homeostasis and prostate cancer progression (2,16), yet their involvement in tumor cell-adipocyte crosstalk in metastatic PCa has not been investigated.
In addition to hypoxia or lipolytic stimulation, expression of pro-inflammatory genes such as COX-2 or MCP-1 can be modulated by other pro-inflammatory molecules supplied by neighboring cells, including tumor necrosis factor α (TNFα) or interleukin 1β (IL-1β) (17–19). Specifically, tumor cell-derived IL-1β has been shown to induce COX-2 levels in tumor-associated mesenchymal stem cells leading to skeletal progression of prostate PC3-ML tumors (20). This is of significance, as we have previously shown that IL-1β expression is highly induced in experimental PCa bone tumors from mice with diet-induced marrow adiposity, and its secretion is augmented in tumor cells exposed to adipocyte-conditioned media (21). Other studies have reported augmented IL-1β expression in metastatic PCa as compared to primary tumors (20) and IL-1β presence in the tumor was demonstrated to be important for seeding in the skeletal niche (22). The potential role of tumor-supplied IL-1β in regulating inflammatory pathways in bone marrow adipose tissue and its effects on response to chemotherapy have not been previously addressed.
The goal of the present study was to investigate the molecular mechanisms driving a crosstalk between prostate cancer cells and bone marrow adipocytes in the context of tumor survival and therapeutic response. Using three different in vivo models of marrow adiposity, as well as in vitro co-culture systems, we demonstrate that exposure to marrow adipocytes significantly augments IL-1β levels in metastatic tumor cells. We also show that tumor cell-derived IL-1β induces the adipocyte expression of COX-2 and microsomal prostaglandin E synthase (mPGES), two enzymes involved in the biosynthesis of prostaglandin E2 (PGE2). This apparent tumor-induced adipocyte inflammation is further exhibited by augmented expression of MCP-1. We show that both tumor IL-1β levels and adipocyte COX-2/MCP-1 expression are induced by the stimulation of lipolysis. We also demonstrate that sensitivity of PCa cells to docetaxel treatment is enhanced both by siRNA-mediated silencing of IL-1β and pharmacological inhibition of lipolysis. Our studies point to PGE2 supplied by adipocytes as a potential regulator of pro-survival pathways in the tumor. These findings are first to demonstrate the interaction between tumor-supplied IL-1β and marrow adipocyte COX-2/MCP-1 pathways, and offer important insight into the potential involvement of this crosstalk in therapeutic response in metastatic disease.
MATERIALS AND METHODS
Materials
DMEM, RPMI-1640, insulin, and Isoproterenol were obtained from Sigma-Aldrich (St. Louis, MO). HyClone FBS, Trizol, TaqMan reagents, and RNAiMAX were from ThermoFisher Scientific (Waltham, MA). Trypsin-EDTA and collagenase were from Invitrogen (Carlsbad, CA). PureCol® collagen type I was from Advanced Biomatrix (San Diego, CA). Transwell cell-support systems were from Corning (Corning, NY). Z-fix was from Anatech LTD (Battle Creek, MI). StemXVivo Adipogenic Supplement, Cultrex™, recombinant IL-1β, and recombinant IL-1RA were from R&D Systems (Minneapolis, MN). β-tubulin (#E7-C) antibody was from Developmental Studies Hybridoma Bank (Iowa City, IA). β-actin antibody (#NB600–501) was from Novus Biologicals (Littleton, CO). Antibodies to IL-1β (#12703), Cyclin D (#2978), p-GSK-3β (#12456), GSK-3β (#5558), and p-β-Catenin (#9561) were from Cell Signaling Technology (Danvers, MA). Cyclooxygenase 2 (COX-2; #ab15191) antibody was from Abcam (Cambridge, MA). β-Catenin antibody (#610153) was from BD Transduction Laboratories (Lexington, KY). RNeasy Mini Kits were from Qiagen (Germantown, MD). Immunoblotting Luminata Forte Western HRP substrate was from EMD Millipore (Billerica, MA). Rosiglitazone, CAY10585, BAY 11–7082, and Forskolin were from Cayman Chemical (Ann Arbor, MI), BAY59–9435 was a kind gift from Dr. Young-Hoon Ahn (WSU). ImmPACT NovaRED Peroxidase Substrate and ImmPRESS Anti-Rabbit Peroxidase Reagent kit were from Vector Laboratories (Burlingame, CA).
Cell Lines
PC3 cells were purchased from ATCC (Manassas, VA). ARCaP(M) cells were purchased from Novicure Biotechnology (Birmingham, AL). Murine RM-1 cell line was a kind gift from Dr. Timothy Thompson (MD Anderson, Houston, TX). PC3 and RM-1 cells were cultured in DMEM with 10% FBS and ARCaP(M) cells were cultured in RPMI-1640 with 5% FBS. All media were supplemented with 25mM HEPES, and 100U/ml penicillin-streptomycin. Primary mouse bone marrow stromal cells (mBMSC) were isolated from tibiae and femurs of 6- to 8-week old FVB/N mice. To induce bone marrow adipocyte differentiation, mBMSCs were treated with adipogenic cocktail (30% StemXVivo Adipogenic Supplement, 1μM insulin, 2μM Rosiglitazone) for 8–10 days as previously described (21). Human cell lines used in this study have been authenticated by the WSU Genomics facility. All cell lines are routinely tested for mycoplasma using MycoFluor Mycoplasma Detection Kit (Thermo Fisher) and LookOut Mycoplasma PCR Detection Kit (Sigma). Cells are used within 10–12 passages from thawing. All cells are maintained in a 37°C humidified incubator ventilated with 5% CO2.
Clinical specimens
Bone biopsy tissue specimens were obtained from prostate cancer patients enrolled in human protocol #2011–185 and approved by Karmanos Cancer Institute and Wayne State University Institutional Review Board. Written informed consent was obtained from all patients participating in the study and all immunohistochemical analyses were performed according to procedures approved by the protocol and in agreement with protocol guidelines and regulations.
Animals
All experiments involving mice were performed in accordance with the protocol approved by the institutional Animal Investigational Committee of Wayne State University and NIH guidelines. In vivo xenograft studies and subcutaneous tumors using either low-fat (LFD), high-fat (HFD), or Rosiglitazone (ROSI) diet were performed in 8- to 10-week old male mice in the FVB/N background with homozygous null mutation in the Rag-1 gene (FVB/N/Rag-1−/−), bred in house. In vivo syngeneic studies in genetically obese mice were performed in 3-month old male mice with the homozygous Lepob mutation (ob/ob) in C57BL/6J background (Jackson Laboratory; Bar Harbor, ME). C57BL/6J mice were used as control group.
Diets
At 5 weeks of age, FVB/N/Rag-1−/− mice were started on LFD (10% calories from fat; Research Diets D12450Ji), HFD (60% calories from fat; Research Diets D12492i), or 20mg/kg ROSI diet (Research Diets D15022201i; 10% calories from fat supplemented with Rosiglitazone from Cayman Chemical by Research Diets). D12450Ji is a standard matched control diet used for both D12492i and D15022201i (as recommended by Research Diets). Mice were maintained on diets for 8 weeks (LFD/HFD) or 14 weeks (LFD/ROSI) prior to tumor implantation and continued on the respective diets after implantation. Diet and water were available ad libitum.
Intratibial and subcutaneous injection of prostate cancer cells
Intratibial and subcutaneous tumor injections were performed under isoflurane inhalation anesthesia according to our published procedures (7,21). Mice were euthanized two weeks (RM-1 cells), six weeks (PC3 cells), or eight weeks (ARCaP(M) cells) post-injection. X-ray images of tumor-bearing and control bones were obtained using a Carestream In Vivo Xtreme Imager (Carestream, Rochester, NY). Tibiae samples and subcutaneous tumors were either fixed in Z-fix, decalcified, and embedded in paraffin for tissue staining, or snap-frozen in liquid nitrogen, powderized, and stored at −80°C for RNA and lipidomic analyses. RNA was extracted using Trizol, chloroform, and alcohol, followed by the protocol from RNeasy Mini Kit.
Quantification of adipocyte numbers and immunohistochemistry
Longitudinal sections (5μm thick) from the control and tumor-bearing tibiae were deparaffinized and stained with H&E as described previously (21). Digital images were captured under 4x magnification using an Olympus BX43 upright light microscope with UC50 (CCD chip) camera (Olympus Scientific Solutions, Waltham, MA). The entire area of each tibia was reconstructed from the 4x images. To quantify adipocytes, the marrow of the bone from the growth plate to the tibiofibular junction was outlined in ImageJ and the adipocytes within the region were manually counted using ImageJ Cell Counter function. For immunohistochemical analyses of IL-1β expression, ImmPRESS Anti-Goat Peroxidase Polymer Detection systems along with a NovaRED kit as a substrate were used for the peroxidase-mediated immunostaining reaction.
Transwell co-cultures and conditioned media treatments
Two-dimensional (2D) Transwell co-cultures with adipocytes were performed according to our established protocols (7,23). All experiments were performed either at normoxic (21% O2; 5% CO2,) or hypoxic (1% O2; 5% CO2,) conditions as specified. HIF-1α inhibitor CAY10585 (5μM) treatments were applied overnight prior to sample collection. Forskolin (20μM), Isoproterenol (10μM), BAY59–9435 (5μM), recombinant human IL-1β (5ng/ml), and recombinant human IL-1RA (200ng/ml) treatments were applied upon seeding. Conditioned media from PC3 and ARCaP(M) cells were generated from 48h in 100mm and used to treat adipocytes at 1:1 with serum-free media (SFM) for 48h hours. RNA from tumor cells and adipocytes was extracted using RNeasy Plus Mini Kit. For protein collection, cells were washed with PBS and collected using SME (PCa cells) or RIPA buffer (adipocyte cells) containing protease (MBL International, Woburn, MA) and phosphatase (ThermoFisher Scientific) inhibitors. For ELISA assays, Transwell cultures were washed with PBS and changed to SFM overnight. Media were collected, spun down, snap frozen and stored at −80°C. ELISA assay was performed according to manufacturer’s protocol (R&D Systems).
For three-dimensional (3D) Transwell co-cultures, adipocytes were prepared in collagen gels as described for the 2D system (7,21,23). 3D cultures of ARCaP(M) and PC3 cells were established on coverslips as described previously (24). Briefly, single cell suspensions containing 10,000 cells were plated on top of coverslips coated with Cultrex. One 3D coverslip was then placed on each Transwell membrane positioned above differentiated adipocyte culture and overlayed with 2% Cultrex in growth media allowing for free exchange of nutrients between the compartments. Cultures were established for 48 hours and exposed to siRNA approaches and docetaxel treatment as indicated.
Lipidomic analyses
PC3 and ARCaP(M) cells were grown alone or in Transwell co-culture with marrow adipocytes for 48 hours, washed with PBS and changed to SFM overnight. Culture supernatants and tumor cell pellets were collected separately, snap-frozen and stored at −80°C. Control and tumor-bearing tibiae from LFD and HFD mice were snap-frozen, powderized, resuspended in methanol at a protein concentration of 200mg/ml, and stored at −80°C until use.
Fatty acyl lipidomic analysis of media samples and bone extracts was performed by LC-MS as described earlier with minor modifications (13,25). Briefly, the samples were spiked with a mixture of deuterated internal standards (PGE1-d4, RvD2-d5, LTB4-d4, 15-HETE-d8, and 14(15)-EpETrE-d11) for quantitation and purified by solid phase extraction using C18 cartridges (StrataX C18, 30mg, Phenomenex). The extracts were directly analyzed by LC-MS using optimized Multiple Reaction Monitoring (MRM) methods and each peak detected was further analyzed by Enhanced Product Ion mass spectrum for confirmation (QTRAP5500, Sciex). LC-MS data were analyzed by MultiQuant (Sciex) and relative quantitation performed against internal standards. MarkerView (a multivariate analysis software to analyze the mass spectral data by ABSCIEX) was performed to identify compounds that significantly differ between samples. For lipids extracted from media samples, changes in eicosanoid levels between the sum (T+A) of single cultures of tumor cells (T) and adipocytes (A) and the Transwell co-culture (AT) were determined. For the in vivo samples, changes in eicosanoid levels between control and tumor-bearing bone for each diet were determined. Significantly changed lipids were found using volcano plots based on unadjusted p-value ≤ 0.05 and fold change ≥ 1.5.
Immunoblot analyses
Lysate samples were loaded based on DNA concentrations and proteins were electrophoresed on 12% or 15% SDS-PAGE gels, transferred to PVDF membranes (Bio-Rad, Hercules, CA) and immunoblotted for indicated proteins, using peroxidase-labeled secondary antibodies. All images comply with the digital image and integrity policies. Densitometry using FujiFilm’s (Minato, Tokyo, Japan) Multi Gauge software was used to verify the fold change in protein levels between conditions.
TaqMan RT-PCR
The cDNA from cells and in vivo samples was prepared using High-Capacity cDNA Reverse Transcription kit (ThermoFisher Scientific). The analyses of genes were performed using TaqMan® Individual Gene Expression assays for Human IL-1β (Hs00174097), COX-2 (PTGS2; Hs00153133), mPGES (PTGES; Hs00610420), MCP-1 (CCL2; Hs00234140), Murine IL-1β (Mm00434228), COX-2 (Mm03294838), mPGES (PTGES; Mm00452105), GLUT1 (SLC2A1; Mm00441480), HIF-1α (Mm00468869), and MCP-1 (CCL2; Mm00441242). Assays were done on three biological replicates using TaqMan® Fast Universal PCR Master Mix and 50ng of cDNA/well. All reactions were run on an Applied Biosystems StepOnePlus™ system and data were normalized to hypoxanthine phosphoribosyltransferase (HPRT1; Hs02800695), 18S (Hs03003631), or adiponectin (Adipoq; Mm00456425). DataAssist™ Software (ThermoFisher Scientific) was used for all analyses.
NFkB/p65 immunocytochemistry
Adipocytes were cultured alone, in the presence of recombinant human IL-1β (for 4 hours) or in Transwell co-culture with ARCaP(M) cells (for 24 hrs). Where specified, NFκB inhibitor (BAY 11–7082) was added at plating. Adipocyte cultures were fixed with 3.7% formaldehyde, stained with NFκB (p65) antibody and imaged on a Zeiss LSM 780 confocal microscope with a 40x water immersion objective.
siRNA approaches
For gene expression analyses, PC3 or ARCaP(M) cells were plated in 6-well plates or on Transwell filters and grown overnight, then a unique 27mer siRNA duplex targeting IL-1β transcripts (OriGene, Rockville, MD: SR302365, Locus ID 3553) or Trilencer-27 Universal scrambled negative control (Origene: SR30004) was added using RNAiMAX transfection reagent at a final concentration of 20μM (based on manufacturer’s protocol). After 6 hours to overnight, cells were moved into Transwell co-culture with adipocytes or grown alone. After 48 hours, cells were collected and processed for RNA analyses as described above. For Live/Dead assays, 3D cultures were treated with IL-1β siRNA duplex or scrambled control and exposed to docetaxel 24 hours later as described below.
Live/Dead assays in 3D cultures
Assays were performed on live PC3 and ARCaP(M) cells using Molecular Probes™ Live/Dead Viability/Cytotoxicity Kit (Invitrogen). Clinical grade Docetaxel (DCTx) was a kind gift from Karmanos Cancer Institute Pharmacy. Established 3D spheroids were treated with either DCTx (10nM) or vehicle (1% ETOH) for 72 hours and re-treated after 48 hours. For IL-1β siRNA experiments, DCTx or vehicle treatments were performed for 96 hours without re-treatment. For all experiments, coverslips were stained with 2μM Calcein AM and 5μM Ethidium homodimer-1 (Live/Dead Viability/Cytotoxicity Kit) for 30 minutes at room temperature, placed in PBS and immediately imaged by capturing z-stacks through the depth of structures using a Zeiss LSM 780 confocal microscope with a 40x water immersion objective. Tile images were obtained using a 10x water immersion objective. Live cells (green; Calcein AM) were captured using excitation at 488nm and emission at 507nm. Dead cells (red; Ethidium homodimer-1) were recorded using excitation at 488nm, emission at 730nm. 3D reconstruction and the sum of channel intensity were quantified using Volocity Software (Perkin Elmer, Waltham, MA). For each spheroid, the volume of live signal over total signal, and dead signal over total signal, was obtained and shown as percent control of untreated cells.
In Silico Oncomine Analyses
The Oncomine database (Oncomine™ v4.5: 729 datasets, 91,866 samples) was used for the analysis of primary (P) vs. metastatic (M) tumors by employing filters for selection of conditions and genes of interest (prostate cancer; metastasis vs. primary; genes). Data were ordered by ‘overexpression’ and the threshold was adjusted to P-value <1E-4; fold change, 2; and gene rank, top 10%. For each database, only genes that met the criteria for significance were reported.
Statistical analyses
An unpaired two-sided t-test was used to compare between two groups and, for three or more groups, a one-way analysis of variance (ANOVA) was used at a 5% significance level. Data were presented as mean ± standard deviation (SD).
RESULTS
Exposure to adipocytes reduces sensitivity of PCa cells to Docetaxel treatment
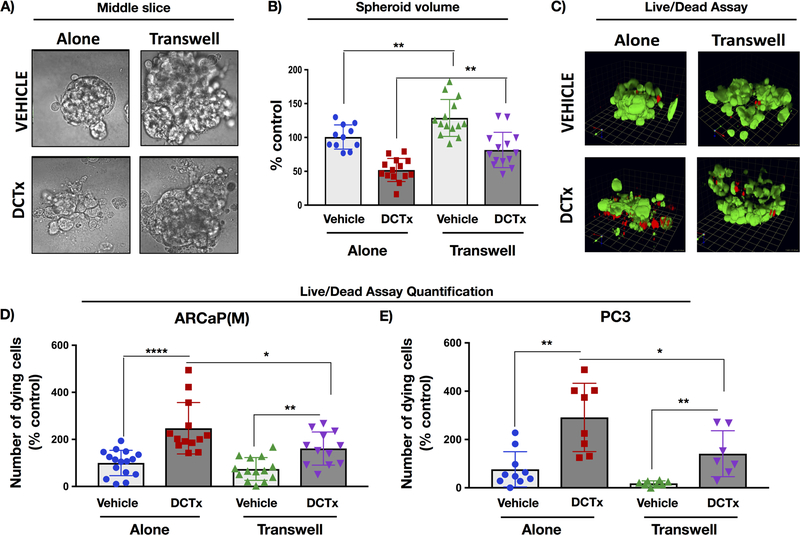
Previous findings from our laboratory and data by others have shown that tumor cells interacting with adipocytes are capable of taking up and utilizing adipocyte-derived lipids (1,8,10,21). Specifically, transfer of lipids between fat cells and tumor cells coincides with increased tumor cell proliferation and invasiveness (10,21), accelerated progression in bone (21), as well as changes in tumor metabolism and survival (1,7,8,23). To determine whether these adipocyte-driven events might affect tumor response to therapy, we cultured ARCaP(M) 3D spheroids alone or in Transwell with adipocytes in the absence or presence of 10nM DCTx (Fig.1). The volume of the spheroids cultured in the presence of adipocytes was visibly larger than the spheroids cultured alone, a result in agreement with our previous reports of growth-promoting effects of adipocytes (24) (Fig.1A,B vehicle). Strikingly, this difference in spheroid size between Transwell and control cultures persisted throughout the 5-day treatment with 10nM DCTx (Fig.1A,B DCTx). Furthermore, ethidium homodimer-1 staining (Live/dead assay) demonstrated that the number of dying ARCaP(M) cells (Fig.1C,D) and PC3 cells (Fig.1E) upon DCTx treatment was reduced under Transwell conditions, indicating potential chemoprotective effects of adipocytes.
Figure 1. Interaction with adipocytes reduces sensitivity of 3D spheroids from ARCaP(M) and PC3 cells to docetaxel (DCTx).
3D cultures of ARCaP(M) grown in Transwell co-culture with bone marrow adipocytes in the absence or presence of 10nM DCTx. A: DIC images of a slice through the middle of 3D spheroid; B: Quantification of total spheroid volume; C: 3D reconstruction of Live/Dead assay results from ARCaP(M) spheroids grown alone or in Transwell with marrow adipocytes and treated with vehicle (EtOH) or 10nM DCTx; green: Calcein AM-positive live cells; red: ethidium homodimer–positive dead cells; Quantification of ethidium–positive (dead) ARCaP(M) (D) and PC3 (E) cells per total spheroid volume shown as percent control; * p<0.05; **p<0.01; ****p<0.0001.
Prostaglandin synthesis is increased in marrow adipocytes interacting with PCa cells in vitro and in vivo.
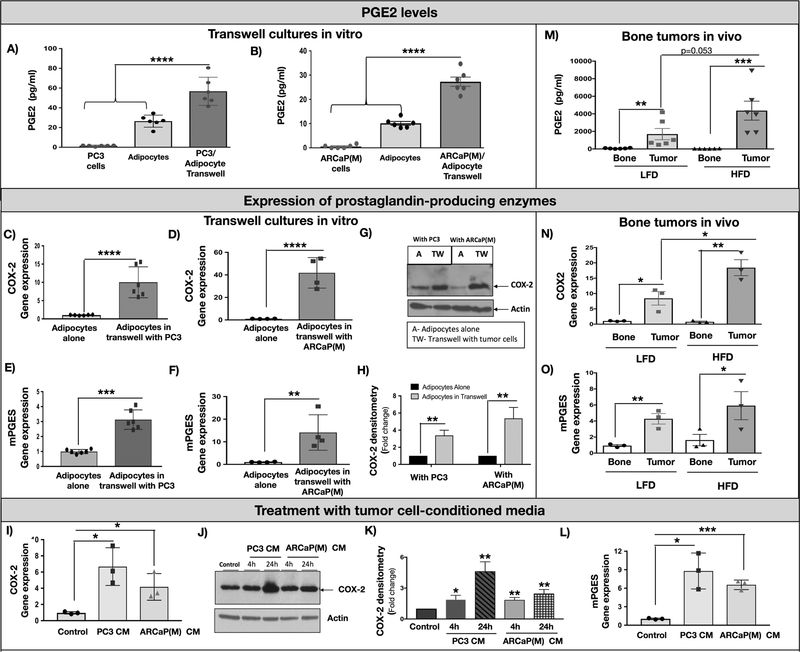
To determine if there are specific lipid mediators responsible for pro-tumor effects of adipocytes, we performed analyses of fatty acyl lipids present in the supernatants from marrow adipocytes and PCa cells interacting via Transwell co-culture in vitro. LC-MS/MS of nearly 200 species generated by three major pathways (cyclooxygenase, lipoxygenase and epoxygenase) revealed that prostaglandins, products of cyclooxygenases, are the predominant lipid mediators induced in Transwell co-cultures, with PGE2, an important regulator of pro-survival pathways in the tumor (26), being the most highly augmented species (Fig.2A,B and Fig.S1A). This increase in prostaglandin levels was accompanied by an induced expression of prostaglandin-producing enzymes, COX-2 and mPGES in adipocytes (Fig.2C–H), but not the tumor cells (Fig.S1B,C). In addition, the relative levels of the primary producer of PGE2, COX-2 enzyme, were significantly lower in tumor cells as compared to adipocytes (Fig.S1D), suggesting that PCa cells may not be a significant supplier of PGE2. Notably, COX-2 and mPGES expression in marrow adipocytes was not only induced by Transwell co-culture with PCa cells but also by tumor cell-conditioned media (Fig.2I–L), suggesting involvement of tumor-supplied factor in this process.
Figure 2: Interaction with PCa cells induces COX-2 signaling in adipocytes.
PGE2 levels in adipocyte cultures with PC3 (A) and ARCaP(M) cells (B) as determined by MS/MS analysis of cell culture supernatants from tumor cells alone, adipocytes alone and Transwell co-cultures. Data are from 6 replicate wells and expressed in pg/ml. Taqman RT PCR results showing augmented COX-2 (C,D) and mPGES (E,F) levels in bone marrow adipocytes cultured in Transwell with PC3 and ARCaP(M) cells; G: Immunoblot analysis of COX-2 protein expression levels in adipocytes cultured alone or in Transwell with PC3 and ARCaP(M) cells. H: Densitometric analysis of COX-2 bands normalized to Actin. Data are mean of three separate experiments. I: COX-2 gene expression in adipocytes cultured alone (control) or in the presence of media conditioned by PC3 or ARCaP(M) cells (PC3 CM and ARCaP(M) CM). J: Immunoblot analysis of COX-2 (1:500) expression in adipocytes treated with PC3 CM and ARCaP(M) CM for 24 and 48 hours. K: Densitometric analysis of COX-2 bands normalized to Actin (1:1000). Data are mean of three separate experiments. L: mPGES gene expression in adipocytes cultured alone (control) or in the presence of PC3 CM and ARCaP(M) CM. M: PGE2 levels in control and tumor-bearing tibiae from LFD and HFD mice. mRNA levels of COX-2 (N) and mPGES (O) in control and tumor-bearing bones as determined by Taqman RT-PCR. * p<0.05; ** p < 0.01; ***p < 0.001; **** p < 0.0001.
To determine whether similar effects on prostaglandin production can be driven by increased marrow adiposity in vivo, we examined the fatty acyl lipidome of control and tumor-bearing tibiae from mice fed LFD vs. HFD, which we and others have shown to augment marrow adiposity (2,21,27). Mirroring our in vitro findings, our results revealed that PGE2 and several other metabolites of the cyclooxygenase pathway are significantly induced in tumor-bearing bones as compared to control bones, especially in mice fed HFD (Fig.2M and Fig.S1F). Using human (tumor) and mouse (host)-specific Taqman probes, we determined that augmented PGE2 levels coincide with increased expression of host (mouse) COX-2 (Fig.2N) and mPGES (Fig.2O) in tumor-bearing tibiae relative to control bones. Notably, the increase in the expression of host COX-2, the major PGE2-producing enzyme, was significantly higher in tumor-bearing tibiae of HFD mice as compared to LFD mice, whereas human COX-2 and mPGES levels were not increased (Fig.S1G,H). There was no cross-reactivity between the human and mouse Taqman probes (Fig.S1I), which further indicated that diet-induced adiposity might be increasing PGE2 production by the host microenvironment in response to tumor. To determine if these effects are mediated by marrow adiposity rather than HFD, we evaluated the host expression of COX-2 and mPGES in two other models with augmented levels of marrow fat cells: mice fed ROSI diet (28,29) and genetically obese (ob/ob) mice (30). Both ROSI and ob/ob mice exhibited high levels of bone marrow adiposity compared to control mice (Fig.S2A,B,F,G) and showed increases in COX-2 and mPGES levels in tumor-bearing tibiae (Fig.S2D,E,I,J), suggesting adipocyte- rather than diet-mediated effects. In both ROSI and ob/ob models, bone tumor burden was increased with augmented marrow adiposity (Fig.S2C,H), similar to results previously observed with HFD model (21). Furthermore, in line with previous reports linking COX-2 activity with adipocyte inflammation (13), we observed highly augmented levels of CCL2/MCP-1 cytokine in adipocytes interacting with PCa cells in vitro and in vivo (Fig.S3). Notably, in contrast to robust effects of marrow adiposity on COX-2, mPGES and MCP-1 levels in bone tumors, no significant differences were observed in subcutaneous tumors with diet or Rosiglitazone-mediated adiposity, suggesting this might be a bone tumor-specific phenotype (Fig.S4).
COX-2/MCP-1 expression in marrow adipocytes is mediated by tumor cell-supplied IL-1β
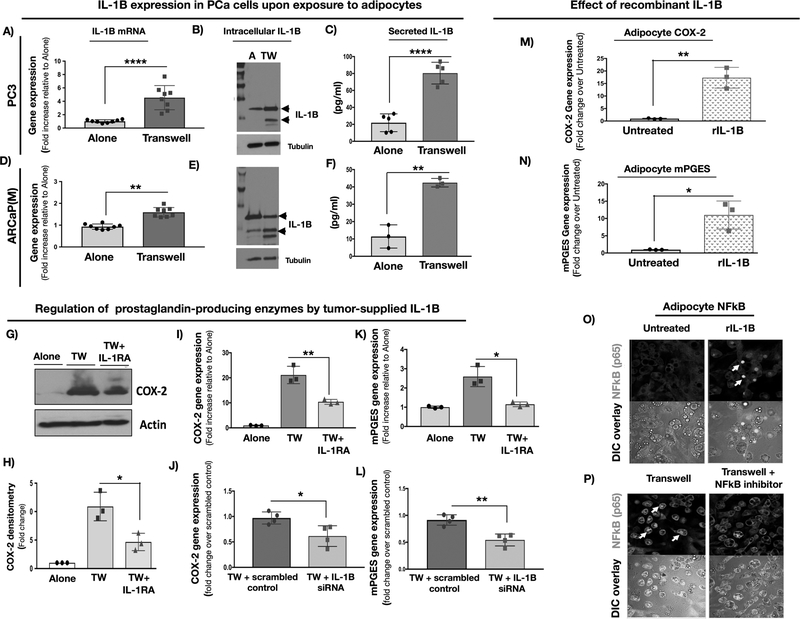
Both MCP-1 and COX-2 levels and activity can be regulated via pro-inflammatory processes, including IL-1β-mediated activation of the IL-1 receptor (IL-1R) (17,31,32). Notably, our previous studies reported IL-1β as one of the top genes upregulated in prostate bone tumors from HFD mice (21). To examine whether this is also true for IL-1β protein, we performed immunohistochemical analyses of ARCaP(M) bone tumors from LFD and HFD mice and observed strong IL-1β localization to the tumor cells, along with augmented expression in tumors from mice with HFD-induced marrow adiposity (Fig.S5A,B). Importantly, IL-1β presence at the protein (Fig.S5C) and mRNA (Fig.S5D) levels was also revealed in bone lesions from metastatic prostate cancer patients. This is in line with previous reports of IL-1β expression in metastatic PCa (20) and its importance for seeding in the skeletal niche (22).
To directly examine the effects of tumor cell-supplied IL-1β on marrow adipocytes, we first established that expression levels and secretion of IL-1β by PC3 and ARCaP(M) cells are indeed augmented at the mRNA and protein levels upon Transwell co-cultures with fat cells (Fig.3A–F). We then observed that exposure of Transwell cultures to IL-1 receptor antagonist (IL-1RA), or co-culture with tumor cells in which IL-1β expression was silenced by three non-overlapping siRNAs, partially reduces expression of COX-2 (Fig.3G–J), as well as mPGES (Fig.3K–L) and MCP-1 (Fig.S6A). Reciprocally, adipocyte treatment with recombinant IL-1β strongly induced COX-2 (Fig.3M) as well as mPGES (Fig.3N) and MCP-1 levels (Fig.S6B), indicating IL-1β-mediated effects. This IL-1β-induced inflammatory phenotype appeared to be NFkB-driven, as demonstrated by the nuclear localization of p65 upon IL-1β treatment or exposure to Transwell adipocyte co-cultures, a phenomenon reversed by the treatment with NFkB inhibitor BAY 11–0782 (Fig.3O,P).
Figure 3: Adipocyte-tumor cell crosstalk: IL-1β expression and secretion by prostate carcinoma cells augments COX-2 signaling in adipocytes.
PC3 (A-C) and ARCaP(M) (D-F) cells were grown alone or in Transwell co-culture with bone marrow adipocytes. Taqman RT-PCR results show highly induced mRNA levels of IL-1β in PC3 (A) and ARCaP(M) cells (D); graph representative of multiple experiments. Immunoblot analyses depicting increased levels of IL-1β (1:1000) protein in PC3 (B) and ARCaP(M) cells (E). Tubulin (1:2000) shown for equal loading control. C,F: ELISA assay results depicting levels of IL-1β secreted by PC3 cells (C) or ARCaP(M) cells (F) grown alone or in Transwell with adipocytes. G: COX-2 protein levels in the absence or presence of recombinant IL-1RA (200 ng/ml). H: Densitometry of COX-2 bands normalized to actin bands; data represent the mean of three experiments I: COX-2 gene expression in bone marrow adipocytes grown alone or in Transwell with ARCaP(M) cells in the absence or presence of IL-1RA; J: siRNA-mediated IL-1β knockdown in ARCaP(M) cells reduces COX-2 gene expression levels in marrow adipocytes grown in Transwell cultures with ARCaP(M) cells as compared to cells transfected with scrambled control; K: mPGES mRNA levels in marrow adipocytes treated with IL-1RA; L: mPGES gene expression in adipocytes upon siRNA-mediated knockdown of IL-1β in tumor cells; mRNA levels of COX-2 (M) and mPEGS (N) in marrow adipocytes treated with recombinant IL-1β. O: p65(NFκB) immunofluorescence in bone marrow adipocytes grown under control conditions or treated with recombinant IL-1β. NFκB activation is demonstrated by the nuclear p65 staining in response to IL-1β. P: Marrow adipocytes grown in Transwell with ARCaP(M) cells. Reduced nuclear p65 upon treatment with NFκB inhibitor BAY 11–0782. * p < 0.05; **p < 0.01. **** p < 0.0001.
IL-1β/COX-2/MCP-1 crosstalk is stimulated by lipolysis
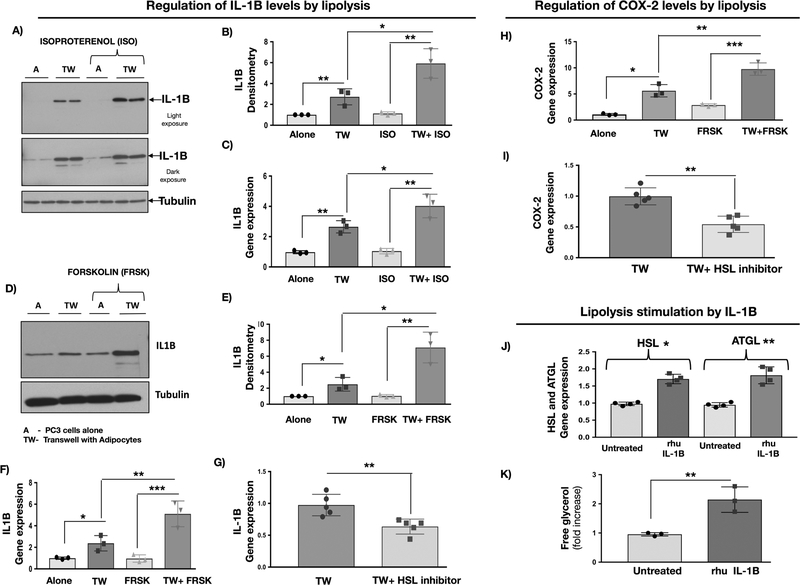
Previous studies in white adipose tissue showed that COX-2 and MCP-1 expression in fat cells is sensitive to stimulation of lipolysis (13). Our previous work has also shown that Transwell co-culture of adipocytes with PCa cells leads to the release of free glycerol, indicating activation of lipolysis. This process was reversed by the inhibition of adipocyte triglyceride lipase (ATGL), suggesting tumor contribution to the regulation of lipolytic machinery in fat cells (7). Intriguingly, treatment with lipolysis-promoting agents, such as Isoproterenol (Fig.4A–C) or Forskolin (Fig.4D–F), significantly increased IL-1β expression already augmented by adipocytes in Transwell cultures. Reciprocally, treatment with BAY59–9435, an inhibitor of hormone sensitive lipase (HSL), significantly reduced IL-1β levels (Fig.4G). Similar modulatory effects of lipolysis were observed for COX-2, mPGES and MCP-1 whose levels were significantly increased by Forskolin (Fig.4H and Fig.S6C,D, left panels) and reduced by BAY59–9435 (Fig.4I and Fig.S6C,D, right panels). Interestingly, treatment of adipocytes with recombinant IL-1β induced the expression of HSL and ATGL, as well as the release of free glycerol (Fig.4J,K), underscoring the potential contribution of tumor-supplied IL-1β to the inflammatory phenotype in adipocytes and suggesting lipolysis might be involved in regulation of tumor cell-adipocyte crosstalk.
Figure 4. IL-1β expression in PC3 cells and COX-2 levels in adipocytes are modulated by lipolysis.
A: Protein levels of IL-1β in PC3 cells cultured alone or in Transwell with adipocytes in the absence or presence of lipolysis-inducing agent Isoproterenol; representative blot is shown; B: IL-1β densitometry normalized to β-actin; data represent the mean from 3 experiments; C: IL-1β gene expression in PC3 cells upon treatment with Isoproterenol; D: Protein levels of IL-1β in PC3 cells cultured alone or in Transwell with adipocytes in the absence or presence of lipolysis-inducing agent Forskolin; representative blot is shown; E: IL-1β densitometry normalized to tubulin; data represent the mean from 3 experiments; F: IL-1β gene expression in PC3 cells upon treatment with Forskolin; G: IL-1β gene expression in PC3 cells grown in Transwell co-cultures with adipocytes in the absence or presence of 5μM inhibitor of hormone sensitive lipase BAY59–9435 (BAY). H: COX-2 mRNA levels in marrow adipocytes cultured alone or in Transwell with PC3 cells in the absence or presence of Forskolin; three individual experiments are shown. I: COX-2 mRNA levels in marrow adipocytes grown in Transwell with PC3 cells and in the absence or presence of 5μM BAY. J: Gene expression of HSL and ATGL and free glycerol release (K) by adipocytes upon treatment with recombinant IL-1β. * p < 0.05; ** p < 0.01; ***p < 0.001.
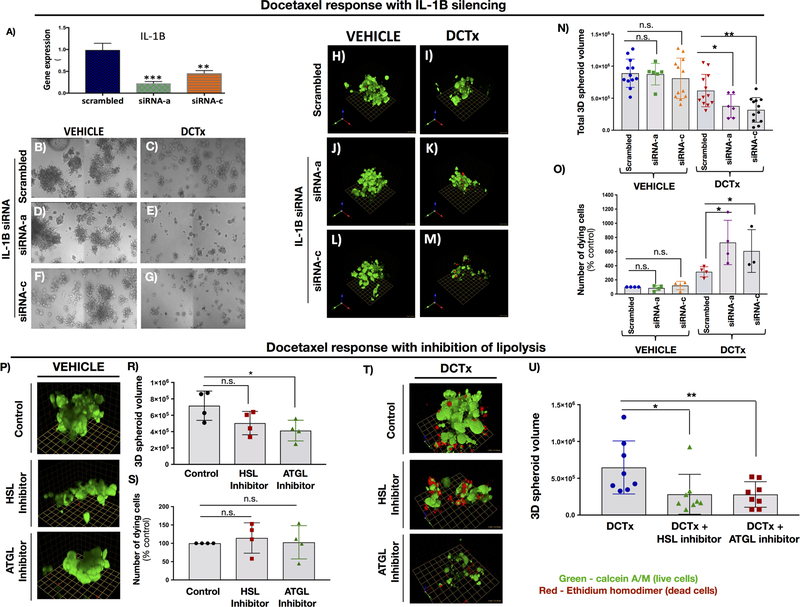
Sensitivity of prostate carcinoma cells to DCTx treatment is regulated by IL-1β and lipolysis
Given the strong IL-1β presence in metastatic tissues from PCa patients, and its regulation by marrow adipocytes, we wondered whether the reduced sensitivity to DCTx in the presence of adipocytes (Fig.1) can be overcome by reducing the levels of IL-1β. Indeed, siRNA-mediated knockdown of IL-1β in ARCaP(M) cells with two non-overlapping siRNAs (Fig.5A) reduced the size of tumor spheroids exposed to the DCTx treatment (Fig.5B–G). Further examination by Live/Dead assay (Fig.5H–M) confirmed reduced spheroid size (Fig.5N) and revealed an augmented number of cells dying in response to DCTx upon IL-1β knockdown (Fig.5O). Since adipocyte-mediated IL-1β expression in tumor cells appears to be modulated by lipolysis, we next determined whether sensitivity to DCTx treatment can be further increased by using inhibitors of lipases HSL and ATGL. Under 2D conditions, HSL and ATGL inhibitors had minimal effects on tumor cell proliferation and viability. Cellular DNA concentrations in lysates were 106.9 ± 3.4% of vehicle control for BAY59–9435 and 108.4± 5.7% for Atglistatin-treated cells in the absence of adipocytes. In Transwell co-culture with adipocytes, DNA concentrations were 95.5 ± 3.1% of vehicle control for BAY59–9435 and 84.1 ± 6.4% for Atglistatin. In 3D assays in the presence of adipocytes (Fig.5P), however, the volume of ARCaP(M) spheroids was moderately reduced in the presence of ATGL inhibitor, possibly due to a decreased supply of lipids for tumor growth (Fig.5R). At the same time, neither HSL nor ATGL inhibitors alone showed any detectable toxicity towards the PCa cells as demonstrated by the unchanged number of dying cells with treatment (Fig.5S). On the other hand, the combined use of Docetaxel and the inhibitors of lipolysis resulted in significant enhancement of sensitivity to DCTx as evident by significant reduction in spheroid volume, particularly in the presence of ATGL inhibitor (Fig.5T,U). Severe disintegration of the spheroid upon combined DCTx/ATGL inhibitor treatment and the resulting loss of the majority of ethidium homodimer-labeled red cells did not allow for accurate quantification of dying cells under these conditions.
Fig 5: IL-1β silencing and inhibition of lipolysis sensitize ARCaP(M) spheroids to docetaxel treatment.
A: IL-1β mRNA levels in ARCaP(M) cells upon treatment with two non-overlapping siRNAs. B-G: DIC tile images of 3D ARCaP(M) spheroids treated with scrambled control or IL-1β siRNA A and C and grown in the absence (B,D,F) or presence (C,E,G) of 10nM DCTx. IL-1β siRNA A and C have a visibly significant effect on spheroid size in the presence of DCTx. H-M: Live/Dead assay of control and IL-1β silenced 3D cultures treated with vehicle (H,J,L) or 10nM DCTx (I,K,M). Green: Calcein AM-positive live cells; Red: ethidium homodimer–positive dead cells; N: Quantification of spheroid volume in ARCaP(M) cells treated with scrambled control or IL-1β siRNA in the absence or presence of DCTx O: Quantification of ethidium–positive (dead) cells/total spheroid volume shown as percent control; P: 3D images from Live/Dead assay on 3D cultures of ARCaP(M) cells grown in culture with adipocytes and exposed to HSL and ATGL inhibitors; R: Quantification of spheroid volume upon treatment with HSL inhibitor BAY59–9435 and ATGL inhibitor, Atglistatin; S: Quantification of ethidium–positive (dead) cells/total spheroid volume shown as percent control; T: 3D images from Live/Dead assay on 3D cultures of ARCaP(M) cells grown in culture with adipocytes and exposed to 10nM DCTx in the absence or presence of HSL and ATGL inhibitors; U: Quantification of total 3D spheroid volume in response to treatment. * p<0.05; **p<0.01; ***p<0.001.
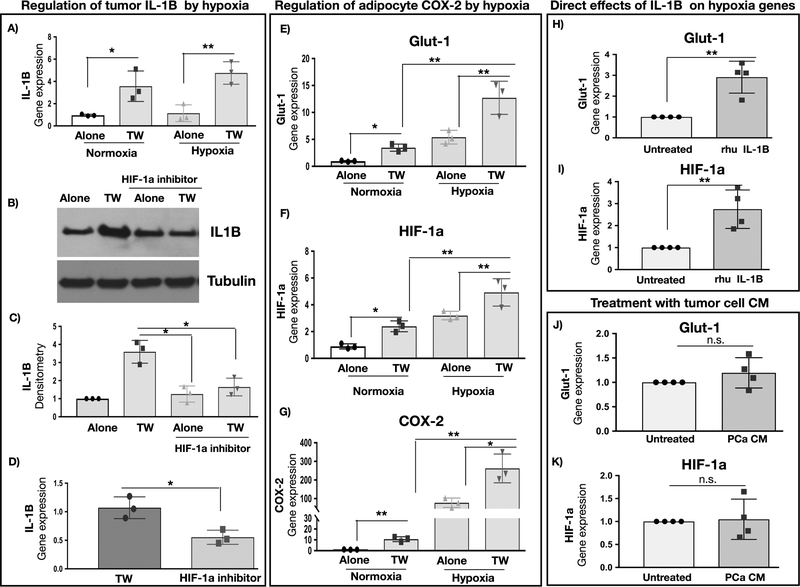
Contribution of hypoxia to IL-1β/COX-2/MCP-1 crosstalk
One known mechanism of IL-1β regulation is via HIF-1α-mediated transcription (33,34). Indeed, our previous data have shown that adipocytes induce HIF-1α signaling in PCa cells (7). We also observe that, although IL-1β levels induced by Transwell co-culture are not further augmented under hypoxia (Fig.6A), treatment with an inhibitor of HIF-1α transcription (CAY10585) significantly reduces IL-1β levels that were increased upon interaction with adipocytes (Fig.6B–D). In addition, both hypoxia and Transwell co-culture with tumor cells promote HIF-1α signaling in adipocytes, as indicated by significantly augmented mRNA levels of GLUT1 and HIF-1α (Fig.6E,F). This induction of a hypoxic phenotype parallels the robust increases in levels of COX-2, mPGES and MCP-1 (Fig.6G and Fig.S6E,F), suggesting the link between HIF-1α signaling and inflammatory phenotype in adipocytes. Notably, GLUT1 and HIF-1α expression in adipocytes can be directly induced by exposure to recombinant IL-1β (Fig.6H,I) but not upon treatment with tumor cell conditioned media (Fig. 6J,K). This indicates that the presence of both cell types in Transwell co-culture might be needed for the effective activation of HIF-1α signaling in adipocytes.
Figure 6. COX-2 levels in adipocytes and IL-1β levels in PCa cells are sensitive to hypoxia.
A: Gene expression of IL-1β in PC3 cells cultured alone or in Transwell with adipocytes under normoxic or hypoxic conditions; B: IL-1β protein levels in PC3 cells cultured alone or in Transwell and in the absence or presence of 5μM HIF-1α inhibitor CAY10585; representative blot is shown; C: IL-1β densitometry normalized to tubulin; data represent the mean+/− SD from 3 separate experiments; D: IL-1β gene expression in PC3 cells grown in Transwell cultures in the absence or presence of 5μM CAY10585. Gene expression of GLUT1 (E), HIF-1α (F) and COX-2 (G) in bone marrow adipocytes cultured alone or in Transwell with PC3 cells under normoxic (21% O2) or hypoxic (1% O2) conditions. Data are shown as the mean of 3 biological replicate experiments. Gene expression of GLUT1 (H) and HIF-1α (I) of adipocytes upon treatment with recombinant IL-1β. Gene expression of GLUT1 (J) and HIF-1α (K) of adipocytes upon treatment with media conditioned by PCa cells; +/− SD. * p < 0.05; ** p < 0.01; n.s. -not significant.
Adipocyte-supplied PGE2 contributes to pro-survival signaling in tumor cells
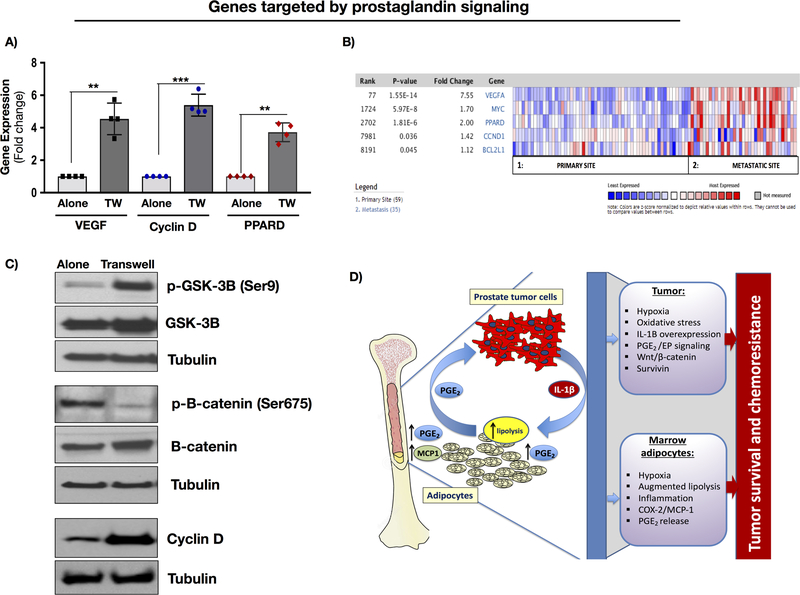
Both hypoxia and PGE2 signaling have been linked to pro-survival signaling in the tumor. Our previous studies have shown that exposure to adipocytes promotes clonogenic growth and significantly augments the expression of Survivin and Bcl-xl (23), which have been shown to be regulated by PGE2 via EP receptor-mediated signaling (17,35). Other targets of the PGE2/EP axis, such as VEGF, cyclin D, and PPARD are also highly induced upon Transwell culture with adipocytes (Fig.7A). Notably, these gene targets are also augmented in metastatic vs. primary PCa tumors (Fig.7B), indicating EP receptor signaling might be important in metastatic disease. Further evidence of adipocyte-mediated effects on pro-survival pathways in PCa cells is the observed increase in phosphorylation of glycogen synthase kinase 3 (GSK-3β) at Ser9 and a coincident decrease in phosphorylation of β-catenin in PCa cells grown in Transwell with adipocytes (Fig.7C). This is in line with reduced kinase activity of GSK-3β and impeded targeting of β-catenin to the proteasome for degradation (36). Increased levels of cyclin D1 upon Transwell co-culture further suggest that exposure to marrow adipocytes activates Wnt/β-catenin pathway in tumor cells. Together, these results underscore the involvement of tumor-derived IL-1β in regulating PGE2 production by marrow adipocytes in collaborative effort to promote pro-survival signaling in tumor cells (Fig.7D).
Figure 7. Downstream targets of PGE2 signaling are induced upon PCa-marrow adipocyte crosstalk.
A: Gene expression analysis of VEGF, Cyclin D and PPARD in PC3 cells cultured alone or in Transwell co-culture with adipocytes; B: Oncomine gene analysis comparing the expression of prostaglandin/EP receptor target genes (VEGF, MYC, PPARD, and CCND1) in patient samples collected from metastatic or primary sites. Data were ordered by “overexpression” and the threshold was adjusted to P-value < 1E-4; fold change, 2 and gene rank, top 10%. C: Immunoblot analysis of p-GSK-3β (1:1000), GSK-3β (1:1000), p-β-catenin (1:1000), β-catenin (1:1000) and Cyclin D (1:1000) proteins in PC3 cells grown alone or in Transwell with adipocytes. D: Proposed mechanism of adipocyte-tumor cell crosstalk involving IL-1β/COX-2/MCP-1 axis. Tumor cells stimulate adipocyte lipolysis. Lipolysis-mediated increase in IL-1β and COX-2 levels and augmented production of PGE2 lead to pro-survival effects on the tumor and therapy resistance.
DISCUSSION
Metastatic progression in the skeleton is a collaborative process between the tumor cells and bone microenvironment. As a major component of the adult bone marrow, adipocytes actively impact the metastatic niche via secretion of lipids, growth factors, adipokines and inflammatory mediators (1,2,37). Inflammatory pathways, which under normal physiological conditions protect and maintain normal bone homeostasis, are drastically altered under conditions of increased marrow adiposity (2). This is further complicated by the presence of metastatic tumor cells, capable of engaging marrow adipocytes in a crosstalk that ultimately supports tumor progression and survival. The present study focused on the adipocyte-tumor cell crosstalk involving tumor-supplied IL-1β and adipocyte-derived COX-2 and MCP-1 in the context of DCTx response in prostate cancer. Our data revealed that IL-1β expression in metastatic tumor cells is significantly induced by the exposure to marrow adipocytes in vitro and in vivo. Reciprocally, adipocyte expression of COX-2 and MCP-1, and the consequent production of prostaglandins, are driven at least partially by tumor-supplied IL-1β. Notably, this bi-directional adipocyte-tumor cell crosstalk appears to reduce sensitivity of prostate carcinoma cells to DCTx, a phenomenon muted by IL-1β knockdown. Strikingly, inhibition of adipocyte lipolysis, a process that appears to regulate tumor IL-1β and adipocyte COX-2/MCP-1 levels, leads to significant improvement in tumor cell response to DCTx. These findings suggest that activation of the inflammatory IL-1β/COX-2/MCP-1 axis in the bone-tumor microenvironment might be a significant contributor to limited chemotherapy response in metastatic disease.
Traditionally, the main suppliers of IL-1β in the tumor microenvironment are mononuclear phagocytes, fibroblasts, and lymphocytes, and the main functions of this cytokine are thought to involve the regulation of chemotaxis, cellular stress responses, and apoptosis (38). IL-1β is mostly known as a component of an assembly complex of intracellular proteins termed the inflammasome, which is typically associated with immune cells (39). However, growing evidence begins to suggest this pro-inflammatory cytokine might play important functions in tumor cells. One potential mechanism of how cancer cell-supplied IL-1β might be contributing to tumor progression is through a constitutively activated tumor-derived inflammasome as reported in late-stage melanoma (40). In prostate cancer specifically, several recent studies linked IL-1β expression in prostate carcinoma cells with metastatic potential and successful colonization in bone (20,22,41). Recent studies also identified IL-1β among cytokines whose circulating levels are increased in prostate cancer patients with progressive disease after DCTx treatment (42) and whose expression is augmented post-treatment, as compared to matched pretreatment tumor cells microdissected from biopsy samples (43). How tumor-supplied IL-1β modulates bone-tumor microenvironment to evade therapy has not been previously studied.
Adipose tissue inflammation is tightly linked to adipocyte metabolism and inflammatory factors such as COX-2 and MCP-1, which have been shown to be sensitive to lipolytic stimulation (13). Our previous studies demonstrated that PCa cells stimulate lipolysis in bone marrow adipocytes when grown under Transwell conditions (7). In the present study, we further show that IL-1β expression by tumor cells and COX-2/MCP-1 levels in marrow adipocytes are sensitive to pharmacological inducers and inhibitors of lipolysis, suggesting triglyceride hydrolysis might be a vital regulator of the inflammatory IL-1β/COX-2/MCP-1 axis. More importantly, sensitivity of PCa cells to DCTx treatment is significantly improved by IL-1β knockdown and inhibition of lipolysis, linking triglyceride hydrolysis with IL-1β-mediated pro-survival signaling in the tumor. IL-1β has been previously reported to regulate lipid storage capacity in adipose tissue and to promote lipolysis by downregulating PPARγ in adipocytes (44). The mechanisms behind its direct contribution to marrow adipocyte lipolysis remain to be established.
The fact that adipocyte-derived COX-2 and MCP-1 appear to be regulated by tumor cell-derived IL-1β has important implications in the context of metastatic progression. Both COX-2 and MCP-1 have been previously implicated in tumor-associated bone disease and reduced chemotherapy response via mechanisms attributed to their expression by the tumor cells (2,45,46). Importantly, prominent effects of host-derived MCP-1 on reduced DCTx response have been reported (47), underscoring the importance of bone tumor microenvironment in chemotherapy resistance. Studies in prostate cancer suggested that COX-2 involvement in skeletal tumor growth is mediated via PGE2 action on osteoblasts and activation of RANKL-mediated osteoclastogenesis and bone degradation (48). This is of importance as our lipidomics and gene expression analyses indicate that adipocytes might be the predominant source of PGE2 in adipocyte-tumor cell co-cultures. Our data also show that adipocyte-supplied PGE2 might be contributing to the pro-survival signaling in the tumor. We recognize that our study has limitations. Although we have utilized three independent in vivo models of marrow adiposity, all of which have shown that the production of PGE2-producing enzymes in the host bone marrow is induced with tumor burden and correlates with adiposity, we cannot unequivocally link PGE2 production in the bone tumor microenvironment to marrow adipocytes. However, our data do collectively implicate adiposity-mediated IL-1β/PGE2 signaling in tumor-induced bone disease. Further investigations utilizing adipocytes isolated from control and tumor-bearing mice will deepen our understanding of this axis in metastatic disease.
The rate-limiting step in the production of IL-1β is its transcription, and one of the candidate transcriptional regulators of IL-1β, previously demonstrated in macrophages and astrocytes, is HIF-1α (33,34). Indeed, our previous studies have shown that HIF-1α signaling is activated in PCa upon adipocyte exposure, and it correlates with transformation to a glycolytic phenotype and enhanced lipid uptake by tumor cells (7). Studies presented herein further demonstrate that enhanced hypoxia signaling in both PCa cells and the fat cells perpetuates the activation of the inflammatory IL-1β/COX-2/MCP-1 axis in the adipocyte-tumor cell crosstalk. Previous studies in lung epithelial cells demonstrated that IL-1β-mediated NFkB activation and subsequent increases in COX-2 expression and PGE2 production lead to stabilization of HIF-1α (49). Given our results demonstrating activation of NFkB in marrow adipocytes by tumor cell-supplied IL-1β, it is plausible that this pro-inflammatory interplay contributes to stabilization of HIF-1α and subsequent activation of pro-survival signaling. Our results demonstrating augmented expression of PGE2/EP and Wnt/β-catenin target genes in tumor cells interacting with adipocytes further support this idea and call for further investigations.
Bone marrow niche is dynamic and complex, posing a major challenge to designing therapies that effectively target metastatic lesions within bone. Metastatic tumor cells engage specific components of the bone-tumor microenvironment to help them thrive in the marrow space and evade therapy. Stromal components of the bone were recently suggested to evoke chemoprotective effects on metastatic tumor cells via downregulation of latexin (50). Studies presented herein are the first to demonstrate that the interaction of prostate cancer cells with another component of bone marrow stroma, adipocytes, results in a functional crosstalk that involves tumor-supplied IL-1β and adipocyte COX-2/MCP-1 pathways. Specifically, our data reveal for the first time that the hyperactivation of IL-1β and COX-2/MCP-1 due to this bi-directional crosstalk promotes reduced sensitivity of tumor cells to docetaxel. Our studies also point to the hypoxic microenvironment within bone, as well as tumor-induced lipolysis in adipocytes, as the microenvironmental modulators of IL-1β/COX-2/MCP-1 axis that contribute to tumor aggressiveness (Fig.7D). Although COX-2 and MCP-1 have been previously suggested as targets for therapy, there has not been much success with blocking these pathways in metastatic disease. Results from this study underscore the significance of marrow adipose tissue inflammation in metastatic prostate cancer and demonstrate the need to study the mechanisms of its regulation during metastatic progression. Understanding the molecular mechanisms of hypoxia and lipolysis involvement in the regulation of IL-1β/COX-2/MCP-1 axis is critical towards identifying novel therapeutic approaches for cancers that thrive in adipocyte-rich bone marrow.
Supplementary Material
Implications.
Studies presented herein highlight adipocyte lipolysis as a tumor-regulated metabolic event that engages pro-inflammatory crosstalk in the microenvironment to promote PCa progression in bone. Understanding the impact of bone marrow adipose tissue on tumor adaptation, survival and chemotherapy response is fundamentally important, as current treatment options for metastatic PCa are palliative.
ACKNOWLEDGEMENTS
We thank Dr. Kamiar Moin and the Microscopy, Imaging and Cytometry Resources Core (MICR) for assistance with confocal microscopy analyses. Grant support was provided by NIH/NCI 1 R01 CA181189 (I. Podgorski, PI), DOD W81XWH-14-1-0036 (IP), NIH 5T32CA009531 (JD), NIH/NCI 1F31CA203036 (JD), Wayne State University Research Stimulus funds (IP), and P30 CA 22453 (MICR).
Footnotes
The authors disclose no potential conflicts of interest
REFERENCES
- 1.Diedrich JD, Herroon MK, Rajagurubandara E, Podgorski I. The Lipid Side of Bone Marrow Adipocytes: How Tumor Cells Adapt and Survive in Bone. Curr Osteoporos Rep 2018;16(4):443–57 doi 10.1007/s11914-018-0453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardaway AL, Herroon MK, Rajagurubandara E, Podgorski I. Bone marrow fat: linking adipocyte-induced inflammation with skeletal metastases. Cancer Metastasis Rev 2014;33(2–3):527–43 doi 10.1007/s10555-013-9484-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reagan MR, Rosen CJ. Navigating the bone marrow niche: translational insights and cancer-driven dysfunction. Nat Rev Rheumatol 2016;12(3):154–68 doi 10.1038/nrrheum.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tannock IF, de Wit R, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004;351(15):1502–12 doi 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 5.Nuhn P, De Bono JS, Fizazi K, Freedland SJ, Grilli M, Kantoff PW, et al. Update on Systemic Prostate Cancer Therapies: Management of Metastatic Castration-resistant Prostate Cancer in the Era of Precision Oncology. Eur Urol 2019;75(1):88–99 doi 10.1016/j.eururo.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 6.Dirat B, Bochet L, Dabek M, Daviaud D, Dauvillier S, Majed B, et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res 2011;71(7):2455–65 doi 10.1158/0008-5472.CAN-10-3323. [DOI] [PubMed] [Google Scholar]
- 7.Diedrich JD, Rajagurubandara E, Herroon MK, Mahapatra G, Huttemann M, Podgorski I. Bone marrow adipocytes promote the Warburg phenotype in metastatic prostate tumors via HIF-1alpha activation. Oncotarget 2016;7(40):64854–77 doi 10.18632/oncotarget.11712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shafat MS, Oellerich T, Mohr S, Robinson SD, Edwards DR, Marlein CR, et al. Leukemic blasts program bone marrow adipocytes to generate a protumoral microenvironment. Blood 2017;129(10):1320–32 doi 10.1182/blood-2016-08-734798. [DOI] [PubMed] [Google Scholar]
- 9.Balaban S, Shearer RF, Lee LS, van Geldermalsen M, Schreuder M, Shtein HC, et al. Adipocyte lipolysis links obesity to breast cancer growth: adipocyte-derived fatty acids drive breast cancer cell proliferation and migration. Cancer Metab 2017;5:1 doi 10.1186/s40170-016-0163-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nieman K, Kenny H, Penicka C, Ladanyi A, Buell-Gutbrod R, Zillhardt M, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nature medicine 2011;17(11):1498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Paula FJA, Rosen CJ. Structure and Function of Bone Marrow Adipocytes. Compr Physiol 2017;8(1):315–49 doi 10.1002/cphy.c170010. [DOI] [PubMed] [Google Scholar]
- 12.Falank C, Fairfield H, Reagan MR. Signaling Interplay between Bone Marrow Adipose Tissue and Multiple Myeloma cells. Frontiers in endocrinology 2016;7:67 doi 10.3389/fendo.2016.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gartung A, Zhao J, Chen S, Mottillo E, VanHecke GC, Ahn YH, et al. Characterization of Eicosanoids Produced by Adipocyte Lipolysis: IMPLICATION OF CYCLOOXYGENASE-2 IN ADIPOSE INFLAMMATION. J Biol Chem 2016;291(31):16001–10 doi 10.1074/jbc.M116.725937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan PC, Hsiao FC, Chang HM, Wabitsch M, Hsieh PS. Importance of adipocyte cyclooxygenase-2 and prostaglandin E2-prostaglandin E receptor 3 signaling in the development of obesity-induced adipose tissue inflammation and insulin resistance. FASEB J 2016;30(6):2282–97 doi 10.1096/fj.201500127. [DOI] [PubMed] [Google Scholar]
- 15.Mottillo EP, Shen XJ, Granneman JG. beta3-adrenergic receptor induction of adipocyte inflammation requires lipolytic activation of stress kinases p38 and JNK. Biochim Biophys Acta 2010;1801(9):1048–55 doi S1388–1981(10)00091–0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Craig MJ, Loberg RD. CCL2 (Monocyte Chemoattractant Protein-1) in cancer bone metastases. Cancer Metastasis Rev 2006;25(4):611–9 doi 10.1007/s10555-006-9027-x. [DOI] [PubMed] [Google Scholar]
- 17.Molina-Holgado E, Ortiz S, Molina-Holgado F, Guaza C. Induction of COX-2 and PGE(2) biosynthesis by IL-1beta is mediated by PKC and mitogen-activated protein kinases in murine astrocytes. British journal of pharmacology 2000;131(1):152–9 doi 10.1038/sj.bjp.0703557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozaki K, Hanazawa S, Takeshita A, Chen Y, Watanabe A, Nishida K, et al. Interleukin-1 beta and tumor necrosis factor-alpha stimulate synergistically the expression of monocyte chemoattractant protein-1 in fibroblastic cells derived from human periodontal ligament. Oral Microbiol Immunol 1996;11(2):109–14. [DOI] [PubMed] [Google Scholar]
- 19.Vila-del Sol V, Fresno M. Involvement of TNF and NF-kappa B in the transcriptional control of cyclooxygenase-2 expression by IFN-gamma in macrophages. J Immunol 2005;174(5):2825–33. [DOI] [PubMed] [Google Scholar]
- 20.Liu Q, Russell MR, Shahriari K, Jernigan DL, Lioni MI, Garcia FU, et al. Interleukin-1beta promotes skeletal colonization and progression of metastatic prostate cancer cells with neuroendocrine features. Cancer Res 2013;73(11):3297–305 doi 10.1158/0008-5472.CAN-12-3970. [DOI] [PubMed] [Google Scholar]
- 21.Herroon MK, Rajagurubandara E, Hardaway AL, Powell K, Turchick A, Feldmann D, et al. Bone marrow adipocytes promote tumor growth in bone via FABP4-dependent mechanisms. Oncotarget 2013;4(11):2108–23 doi 10.18632/oncotarget.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shahriari K, Shen F, Worrede-Mahdi A, Liu Q, Gong Y, Garcia FU, et al. Cooperation among heterogeneous prostate cancer cells in the bone metastatic niche. Oncogene 2017;36(20):2846–56 doi 10.1038/onc.2016.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Herroon MK, Rajagurubandara E, Diedrich JD, Heath EI, Podgorski I. Adipocyte-activated oxidative and ER stress pathways promote tumor survival in bone via upregulation of Heme Oxygenase 1 and Survivin. Scientific reports 2018;8(1):40 doi 10.1038/s41598-017-17800-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Herroon MK, Diedrich JD, Podgorski I. New 3D-Culture Approaches to Study Interactions of Bone Marrow Adipocytes with Metastatic Prostate Cancer Cells. Frontiers in endocrinology 2016;7:84 doi 10.3389/fendo.2016.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maddipati KR, Zhou SL. Stability and analysis of eicosanoids and docosanoids in tissue culture media. Prostaglandins Other Lipid Mediat 2011;94(1–2):59–72 doi S1098–8823(11)00007–4 [pii] [DOI] [PubMed] [Google Scholar]
- 26.Nakanishi M, Rosenberg DW. Multifaceted roles of PGE2 in inflammation and cancer. Semin Immunopathol 2013;35(2):123–37 doi 10.1007/s00281-012-0342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosen CJ, Ackert-Bicknell C, Rodriguez JP, Pino AM. Marrow fat and the bone microenvironment: developmental, functional, and pathological implications. Crit Rev Eukaryot Gene Expr 2009;19(2):109–24 doi 32dcc8826e8b77f6,4bae1a4b22a0281c [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ackert-Bicknell CL, Shockley KR, Horton LG, Lecka-Czernik B, Churchill GA, Rosen CJ. Strain-specific effects of rosiglitazone on bone mass, body composition, and serum insulin-like growth factor-I. Endocrinology 2009;150(3):1330–40 doi en.2008–0936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ali AA, Weinstein RS, Stewart SA, Parfitt AM, Manolagas SC, Jilka RL. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology 2005;146(3):1226–35 doi en.2004–0735 [DOI] [PubMed] [Google Scholar]
- 30.Reed MJ, Scribner KA. In-vivo and in-vitro models of type 2 diabetes in pharmaceutical drug discovery. Diabetes Obes Metab 1999;1(2):75–86. [DOI] [PubMed] [Google Scholar]
- 31.Bing C Is interleukin-1beta a culprit in macrophage-adipocyte crosstalk in obesity? Adipocyte 2015;4(2):149–52 doi 10.4161/21623945.2014.979661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu W, Reinmuth N, Stoeltzing O, Parikh AA, Tellez C, Williams S, et al. Cyclooxygenase-2 is up-regulated by interleukin-1 beta in human colorectal cancer cells via multiple signaling pathways. Cancer Res 2003;63(13):3632–6. [PubMed] [Google Scholar]
- 33.Zhang W, Petrovic JM, Callaghan D, Jones A, Cui H, Howlett C, et al. Evidence that hypoxia-inducible factor-1 (HIF-1) mediates transcriptional activation of interleukin-1beta (IL-1beta) in astrocyte cultures. J Neuroimmunol 2006;174(1–2):63–73 doi 10.1016/j.jneuroim.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Tannahill GM, Curtis AM, Adamik J, Palsson-McDermott EM, McGettrick AF, Goel G, et al. Succinate is an inflammatory signal that induces IL-1beta through HIF-1alpha. Nature 2013;496(7444):238–42 doi 10.1038/nature11986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greenhough A, Smartt HJ, Moore AE, Roberts HR, Williams AC, Paraskeva C, et al. The COX-2/PGE2 pathway: key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009;30(3):377–86 doi 10.1093/carcin/bgp014. [DOI] [PubMed] [Google Scholar]
- 36.Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci 2010;35(3):161–8 doi 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Veldhuis-Vlug AG, Rosen CJ. Clinical implications of bone marrow adiposity. J Intern Med 2018;283(2):121–39 doi 10.1111/joim.12718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van de Veerdonk FL, Netea MG, Dinarello CA, Joosten LA. Inflammasome activation and IL-1beta and IL-18 processing during infection. Trends Immunol 2011;32(3):110–6 doi S1471–4906(11)00004–4 [pii] [DOI] [PubMed] [Google Scholar]
- 39.Petrilli V The multifaceted roles of inflammasome proteins in cancer. Current opinion in oncology 2017;29(1):35–40 doi 10.1097/CCO.0000000000000346. [DOI] [PubMed] [Google Scholar]
- 40.Okamoto M, Liu W, Luo Y, Tanaka A, Cai X, Norris DA, et al. Constitutively active inflammasome in human melanoma cells mediating autoinflammation via caspase-1 processing and secretion of interleukin-1beta. J Biol Chem 2010;285(9):6477–88 doi M109.064907 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schulze J, Weber K, Baranowsky A, Streichert T, Lange T, Spiro A, et al. p65-Dependent production of interleukin-1β by osteolytic prostate cancer cells causes an induction of chemokine expression in osteoblasts. Cancer Letters 2012;317(1):106–13. [DOI] [PubMed] [Google Scholar]
- 42.Mahon KL, Lin HM, Castillo L, Lee BY, Lee-Ng M, Chatfield MD, et al. Cytokine profiling of docetaxel-resistant castration-resistant prostate cancer. British journal of cancer 2015;112(8):1340–8 doi 10.1038/bjc.2015.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang CY, Beer TM, Higano CS, True LD, Vessella R, Lange PH, et al. Molecular alterations in prostate carcinomas that associate with in vivo exposure to chemotherapy: identification of a cytoprotective mechanism involving growth differentiation factor 15. Clin Cancer Res 2007;13(19):5825–33 doi 10.1158/1078-0432.CCR-07-1037. [DOI] [PubMed] [Google Scholar]
- 44.Nov O, Shapiro H, Ovadia H, Tarnovscki T, Dvir I, Shemesh E, et al. Interleukin-1beta regulates fat-liver crosstalk in obesity by auto-paracrine modulation of adipose tissue inflammation and expandability. PLoS ONE 2013;8(1):e53626 doi 10.1371/journal.pone.0053626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qian DZ, Rademacher BL, Pittsenbarger J, Huang CY, Myrthue A, Higano CS, et al. CCL2 is induced by chemotherapy and protects prostate cancer cells from docetaxel-induced cytotoxicity. Prostate 2010;70(4):433–42 doi 10.1002/pros.21077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rozel S, Galban CJ, Nicolay K, Lee KC, Sud S, Neeley C, et al. Synergy between anti-CCL2 and docetaxel as determined by DW-MRI in a metastatic bone cancer model. J Cell Biochem 2009;107(1):58–64 doi 10.1002/jcb.22056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Loberg RD, Ying C, Craig M, Day LL, Sargent E, Neeley C, et al. Targeting CCL2 with systemic delivery of neutralizing antibodies induces prostate cancer tumor regression in vivo. Cancer Res 2007;67(19):9417–24 doi 10.1158/0008-5472.CAN-07-1286. [DOI] [PubMed] [Google Scholar]
- 48.Takahashi T, Uehara H, Bando Y, Izumi K. Soluble EP2 neutralizes prostaglandin E2-induced cell signaling and inhibits osteolytic tumor growth. Mol Cancer Ther 2008;7(9):2807–16 doi 10.1158/1535-7163.MCT-08-0153. [DOI] [PubMed] [Google Scholar]
- 49.Jung YJ, Isaacs JS, Lee S, Trepel J, Neckers L. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J 2003;17(14):2115–7 doi 10.1096/fj.03-0329fje. [DOI] [PubMed] [Google Scholar]
- 50.Zhang M, Osisami M, Dai J, Keller JM, Escara-Wilke J, Mizokami A, et al. Bone Microenvironment Changes in Latexin Expression Promote Chemoresistance. Mol Cancer Res 2017;15(4):457–66 doi 10.1158/1541-7786.MCR-16-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.