Abstract
We explored the mechanism of action of CD39 antibodies that inhibit ecto-enzyme CD39 conversion of extracellular ATP (eATP) to AMP and thus potentially augment eATP-P2-mediated pro-inflammatory responses. Using syngeneic and humanized tumor models, we contrast the potency and mechanism of anti-CD39 mAbs compared with other agents targeting the adenosinergic pathway. We demonstrate the critical importance of an eATP-P2X7-ASC-NALP3-inflammasome-IL-18 pathway in the anti-tumor activity mediated by CD39 enzyme blockade, rather than simply reducing adenosine as mechanism of action. Efficacy of anti-CD39 activity was underpinned by CD39 and P2X7 co-expression on intratumor myeloid subsets, an early signature of macrophage depletion, and active IL-18 release that facilitated the significant expansion of intratumor effector T cells. More importantly, anti-CD39 facilitated infiltration into T cell-poor tumors and rescued anti-PD1 resistance. Anti-human CD39 enhanced human T-cell proliferation and Th1 cytokine production and suppressed human B cell lymphoma in the context of autologous EBV-specific T cell transfer.
Introduction
Immune checkpoint blockade (ICB) using antagonistic antibodies to CTLA-4, PD1 and PD-L1 has revolutionized the cancer treatment paradigm (1). However, despite the unprecedented responses achieved among select “immunogenically hot” tumor types with these therapies, the majority of patients still fail to achieve clinically relevant responses in those indications and several tumor types show profound resistance to ICB (2). Additionally, a significant proportion of patients who initially demonstrate anti-tumor responses following ICB therapy eventually become refractory and experience tumor relapse (3). Taken together, these observations reveal the need for additional immunotherapeutics and suggest that additional immune escape mechanisms remain to be uncovered.
While a multitude of clinical agents have entered the clinic as single agents or combination therapies with established ICBs, the majority of these fall into two categories: antagonists of additional immune checkpoints (e.g. Lag-3, Tim-3, Tigit, etc.) or agonists of costimulatory molecules (e.g. GITR, OX-40, 4-1BB). Altering the tumor microenvironment (TME) by targeting tumor metabolic processes, such as the ATP-adenosine axis, is a new and promising avenue for therapeutic invention.
Purinergic signaling in the TME plays a key role in regulation of immune responses. In solid tumors, ATP is abundantly released in the extracellular space owing to cell death in the tumor core, metabolic and/or hypoxic stress and pro-inflammatory signals that stimulate active export of ATP, leading to an accumulation of eATP levels far in excess of that found in healthy tissues (4,5). eATP acts as a pro-inflammatory stimulus by agonizing P2 purinergic receptors (e.g. P2X7) on immune cells (6). However, tumors are proficient at scavenging eATP, converting it to immunosuppressive adenosine by means of two ectonucleotidases, CD39 and CD73, expressed on malignant cells, regulatory immune cells, and the vasculature (7). Adenosine exerts its suppressive function directly by binding to A2A receptors on multiple immune cells such as phagocytes, DC, NK cells, T cells and B cells (8-14). By controlling the initial steps in the phosphohydrolytic cascade, CD39 acts as the master regulator of this dynamic balance between pro-inflammatory eATP and immunosuppressive adenosine within the TME and thereby fosters a broadly immunosuppressive milieu (6).
In addition to elevated expression levels of CD39 in blood neoplasias and multiple solid tumor settings (15-17), CD39 is broadly expressed on the vasculature and specifically found on certain immune subsets, including B cells, natural killer (NK) cells, dendritic cells (DCs), monocytes, macrophages, and regulatory T cells (18). Within the TME, CD39 expression on Tregs (19,20) and MDSCs (21,22) has been shown to be directly correlated with the ability of these professional immunoregulatory cells to suppress T-cell function. CD8+ T cells, which show little detectable CD39 in peripheral blood, express significantly elevated CD39 levels across multiple human tumors types, including gastric, renal cell carcinoma (RCC), non-small cell lung carcinoma (NSCLC), head and neck squamous cell carcinoma (HNSCC), breast cancer and melanoma (23,24). This apparent upregulation is accompanied by reduced polyfunctionality and induction of T cell exhaustion signatures (24,25). Recent reports also suggest that CD39 is a marker of tumor reactive effector T cell subsets (25,26) and is increasingly appreciated as a regulatory marker (27).
The impact of CD39 on tumor growth and anti-tumor immunity has mostly been delineated using global CD39 gene-targeted mice; published data suggested that growth of multiple syngeneic tumors was reduced in these mice (28,29). Similarly, CD39-deficient mice display a resistance to the formation of metastasis in models of disseminated disease or spontaneous metastasis formation (30,31). In addition to genetic ablation, several reports from our laboratory and others have utilized the pharmacological blockade of CD39 activity with the broad ectonucleotidase inhibitor sodium polyoxotungstate (POM-1) to demonstrate improved anti-tumor immunity and decreased metastatic burden in pre-clinical models (30,31). Additionally, Bastid et al. (32) demonstrated that in vitro treatment with POM-1 reversed the suppression of T cells during co-culture with CD39+/CD73+ melanoma cell lines.
Agents targeting other players in the adenosine pathway are currently undergoing clinical testing, including small molecule inhibitors of A2AR and antagonistic antibodies of CD73. An outstanding question has been whether targeting CD39 offers any therapeutic advantage by targeting a different mechanism of action to these other approaches. Here we report the use of novel antibodies that selectively block the enzymatic function of CD39 in mouse and human. We use these to determine that targeting CD39 may be a more potent approach by virtue of the unique eATP-P2X7-inflammasome-IL-18 mechanism of action that reduces intratumor macrophages and enhances CD8+ T-cell effector function in the tumor microenvironment. In particular, we provide differentiation between therapeutic antibodies targeting CD39 and agents targeting other components of the adenosinergic system. Finally, we demonstrate the therapeutic potential of targeting CD39 in solid tumors, either as monotherapy or in potential ICB combinations, where anti-PD1 alone was not effective.
Results
Efficacy with anti-mouse CD39 mAb monotherapy
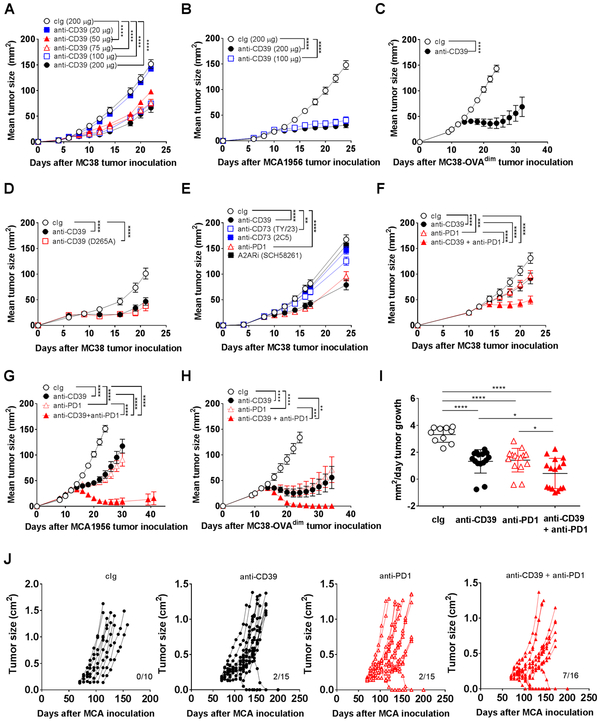
Despite the fact that pharmacological inhibitors of CD39, such as POM-1, have previously been described to have potent anti-metastatic activity (30,31), questions around specificity, therapeutic half-life, pharmacokinetics and toxicity have always left doubts about using this approach to target CD39. Effective anti-mouse CD39 mAb reagents have been lacking, in particular, a mAb that allosterically inhibits CD39. We created a novel anti-mouse CD39 antibody, which specifically binds to CD39-expressing cells (Supplementary Fig. S1A) and potently inhibits CD39 ATPase activity in vitro (Supplementary Fig. S1B). This antibody (designated B66) was first tested against subcutaneous MC38 colon adenocarcinoma tumors, since this model is considered a standard and demonstrated by many laboratories as anti-PD1 sensitive and immunogenic (33-35). Using a four-dose treatment schedule initiating once tumors were established, anti-CD39 was demonstrated to have potent single agent activity against MC38 with efficacy at 50-200 μg doses, but significantly reduced activity at 20 μg doses (Fig. 1A). Similarly, 100 and 200 μg dose schedules of anti-CD39 were equivalently effective against the anti-PD1 sensitive MCA1956 fibrosarcoma cell line (Fig. 1B), while the anti-CD39 monotherapy (200 μg schedule) was also very effective against MC38-OVAdim tumors (Fig. 1C). As determined by enzyme histochemistry, MC38 tumors displayed reduced ATPase activity two days after a single injection of mice with anti-CD39 compared with cIg (Supplementary Fig. S2A, B). To illustrate that the activity of anti-CD39 (B66) was mediated by CD39 ectoenzyme blockade, two additional IgG1 anti-mouse CD39 mAbs (designated Tz-617 and Tz-619) that bound CD39 with similar affinity but lacked CD39 ectoenzyme blocking activity, were shown to be ineffective against MC38 tumors (Supplementary Fig. S2C). To determine that the activity of anti-CD39 (B66) was mediated by CD39 binding rather than Fc activity, we mutated the FcR and compared mouse IgG1 and mouse IgG1 D265A variants of anti-CD39. Clearly, the Fc mutant anti-CD39 (B66) was as potent as the intact IgG1 (B66) in vivo against MC38 tumors (Fig. 1D).
Figure 1. Anti-CD39 suppresses established subcutaneous tumors.
(A) Anti-CD39 mAb suppresses MC38 tumor growth in a dose-dependent manner. Groups (n = 5/group) of WT mice were injected s.c. with MC38 (1 × 105) colon adenocarcinoma cells on day 0 and treated i.p with cIg (200 μg) or anti-CD39 mAb (200 μg, 100 μg, 75 μg, 50 μg, or 20 μg) on days 7, 10, 13, and 16. Tumor sizes were measured at indicated time points in the graph and data presented as mean ± SEM. This experiment is similar to two performed. (B) Anti-CD39 mAb suppresses MCA1956 tumor growth in a dose-dependent manner. Groups (n = 10/group) of WT mice were injected s.c. with MCA1956 (1 × 106) fibrosarcoma cells on day 0 and treated i.p. with cIg (200 μg) or anti-CD39 (200 μg, 100 μg) on days 10, 13, 16, and 19. (C) Anti-CD39 mAb suppresses MC38-ovadim tumor growth. Groups (n = 10/group) of WT mice were injected s.c. with MC38-ovadim (1 × 106) colon adenocarcinoma cells on day 0 and treated i.p. with cIg (200 μg) or anti-CD39 (200 μg) on days 12, 15, 18, and 21. (D) Fc receptor-independent efficacy of anti-CD39 mAb. Groups (n = 10/group) of WT mice were injected s.c. with MC38 (1 × 105) colon adenocarcinoma cells on day 0 and treated i.p with cIg (200 μg) or anti-CD39 (B66 or B66-D265A, 200 μg each) on days 6, 9, 12, and 15. (E) Anti-CD39 mAb compared to other adenosine pathway inhibitors. Groups (n = 7-10/group) of WT mice were injected s.c. with MC38 (1 × 105) colon adenocarcinoma cells on day 0 and treated i.p with cIg (200 μg), anti-CD39 (200 μg), anti-CD73 (2C5 or Ty/23, 200 μg), A2ARi (SCH58261, 10 mg/kg) or anti-PD1 (250 μg) on days 8, 11, 14, and 17. This experiment is representative of two performed. (F) Delayed sub-optimal anti-CD39 and anti-PD1 combination inhibited MC38 tumor growth. Groups (n = 10/group) of WT mice were injected s.c. with MC38 (1 × 105) colon adenocarcinoma cells on day 0 and treated i.p with cIg (200 μg), anti-CD39 (100 μg), anti-PD1 (100 μg) or combination of anti-CD39 and anti-PD1 (100 μg each) on days 12, 15, 18, and 21. (G) Sub-optimal anti-CD39 and anti-PD1 suppress MCA1956 tumor growth. Groups (n = 10/group) of WT mice were injected s.c. with MCA1956 (1 × 106) fibrosarcoma cells on day 0 and treated i.p. with cIg (100 μg I-536; 50 μg clone I-I), anti-CD39 (100 μg), anti-PD1 (50 μg), combination of anti-CD39 and anti-PD1 (100 μg; 50 μg) on days 12, 15, 18, and 21. (H) Sub-optimal anti-CD39 and anti-PD1 reject MC38-OVAdim tumors. Groups (n = 10/group) of WT mice were injected s.c. with MC38-OVAdim (1 × 106) colon adenocarcinoma cells on day 0 and treated i.p. with cIg (100 μg mIgG1; 50 μg clone I-I), anti-CD39 (100 μg), anti-PD1 (50 μg), combination of anti-CD39 and anti-PD1 (100 μg; 50 μg) on days 12, 16, 20, and 24. (I and J) Anti-CD39 and anti-PD1 combine to reject de novo MCA-induced fibrosarcomas. Groups of WT mice (n = 10-16/group) were inoculated s.c. in the hind flank with MCA (300 μg) in 0.1 mL of corn oil and treated i.p. with cIg (100 μg), anti-CD39 (100 μg), anti-PD1 (100 μg), or anti-CD39/anti-PD1 (100 μg; 100 μg) twice/week for 6 weeks from the second palpable tumor measurement. All the mice were monitored for fibrosarcoma development for 250 days and tumor growth rate (mm2/day) (I) or individual tumor growth curves (J) are presented. Numbers in parenthesis indicate the number of mice that rejected their tumors. Tumor sizes (mm2 or cm2) were measured at the indicated time points and presented as mean ± SEM. All experiments were performed once unless indicated. Significant differences among treatment groups were determined by a two-way ANOVA, followed by Tukey's multiple comparison test (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001).
Using the MC38 tumor model, we next compared the monotherapeutic activity of anti-CD39 versus other described mAb and inhibitors that block CD39 and other molecules in the adenosine pathway, including CD73 and adenosine A2A receptor (A2AR). Here we observed that anti-CD39 was as potent as anti-PD1 (RMP-14), while other inhibitors and antibodies to CD73, A2AR and CD39 were not nearly as effective as anti-CD39 monotherapy in this model (Fig. 1E, Supplementary Figure S3A). Activities of anti-CD73 (2C5) and A2ARi (SCH58261) were observed against MC38 when used in combination with anti-CD39, but the majority of anti-tumor activity in these combinations was anti-CD39-mediated (Supplementary Fig. S3B). Even when using a combination of two of anti-CD73, A2ARi (SCH58261) or A2BRi (PSB1115) in the appropriate Nt5e (Cd73)−/−, Adora2a−/− and Adora2b−/− strain of mice from the time of tumor inoculation, anti-CD39 retained significant anti-tumor activity in such treated mice (Supplementary Fig. S4A-D). Indeed, the anti-tumor effect of anti-CD39 was greater than complete blockade of adenosine generation by CD73 and adenosine signaling via A2AR and A2BR, in both the MC38 and MCA1956 tumor models, but greatest tumor growth inhibition was noted when all these molecules were collectively targeted.
Combination anti-PD1 and anti-CD39 mAb anti-tumor efficacy
We next evaluated the combination of suboptimal doses of anti-CD39 and anti-PD1 in a delayed treatment setting using a series of tumor models that were responsive to PD1 blockade (Fig. 1F-H). In each case, significant combination activity was observed between anti-CD39 and anti-PD1, with many combination-treated mice completely rejecting MCA1956 and MC38-OVAdim tumors (Supplementary Fig. S5A, B). Activity in immunogenic transplant models was encouraging, but it was not clear if anti-CD39 would be as effective in a de novo tumor setting where the tumor and immune system had co-evolved. We have previously published treatment in the MCA-induced fibrosarcoma model and these established tumors prove an interesting model of tumor immunity since a spectrum of immunotherapeutic responses can be demonstrated against these tumors – from almost no response to complete rejection (36-38). Historically, effective combinations have demonstrated activity in a fraction of mice. Consistent with these findings, we found anti-PD1 and anti-CD39 do have modest single agent anti-tumor activity, displaying some slowing of tumor growth post treatment compared with control Ig (cIg) and a low number of complete rejections (2/15 each) (Fig. 1I, J). Strikingly, the combination of anti-CD39 and anti-PD1 further slowed tumor growth and caused 7/16 complete rejections (Fig. 1I, J).
Mechanism of action of anti-CD39 mAb – role of T cells and IFNγ in vivo
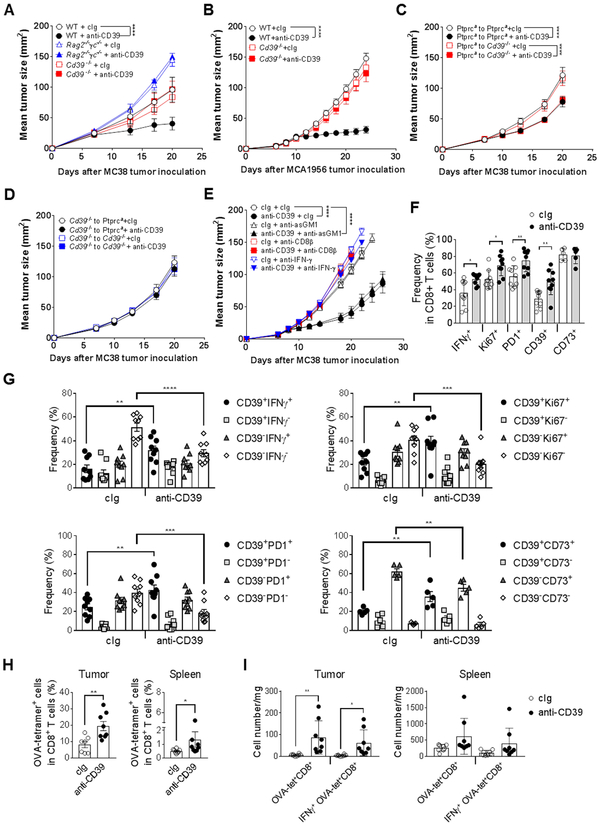
Given the single agent anti-tumor efficacy of the anti-mouse CD39 antibody B66 in the MC38 tumor model, we used this model and 200 μg dose schedule to evaluate the mechanism of action of this mAb in subsequent experiments. In a broad first evaluation, we determined that anti-CD39 anti-tumor efficacy was completely host lymphocyte- and CD39-dependent (Fig. 2A). Dependence on host CD39 was confirmed in a second MCA1956 tumor model (Fig. 2B). Bone marrow chimeras of congenic WT (Ptprca, CD45.1) and Cd39−/− mice, confirmed that anti-CD39 required hematopoietic CD39, but not non-hematopoietic CD39, for efficacy against MC38 tumors (Fig. 2C, D). Deeper exploration by depleting CD8+T cells or NK cells or neutralizing IFN-γ revealed that anti-CD39 efficacy was CD8+T cell- and IFN-γ-dependent, but independent of NK cells (Fig. 2E). Additional analysis using mice defective in IFN-γ (Supplementary Fig. S6A) or cytotoxic pathways (perforin, FasL) or neutralized for TRAIL (Supplementary Fig. S6B) or MC38 tumor cells overexpressing FLIP (to block all death receptor signaling) (Supplementary Fig. S6C) demonstrated that anti-CD39 efficacy was dependent on IFN-γ, but independent of the tested cytotoxic mechanisms.
Figure 2. Mechanism of action of anti-CD39 mAb – role of T cells.
(A) Host lymphocyte- and CD39-dependent tumor growth control by anti-CD39 mAb. Groups (n = 5-6/group) of WT, Rag2−/−γc−/− or Cd39−/− mice were injected s.c. with MC38 (1 × 105) colon adenocarcinoma cells on day 0 and treated i.p with cIg (200 μg) or anti-CD39 (200 μg) on days 7, 10, 13, and 16. (B) Anti-CD39 mAb efficacy against MCA1956 tumor growth is host CD39-dependent. Groups (n = 10/group) of WT and CD39−/− mice were injected s.c. with MCA1956 (1 × 106) fibrosarcoma cells on day 0 and treated i.p. with cIg (200 μg) or anti-CD39 (200 μg) on days 10, 13, 16, and 19. (C-D) Anti-CD39 mAb efficacy requires haematopoietic CD39. Groups (n = 5/group) of WT (Ptprca) and Cd39−/− bone marrow chimeric mice (generated 4 ways) were injected s.c. with MC38 (1 × 105) colon adenocarcinoma cells on day 0 and treated i.p. with cIg (200 μg) or anti-CD39 (200 μg) on days 7, 10, 13, and 16. (E) CD8+ T cell- and IFN-γ-dependent MC38 tumor growth control by anti-CD39 mAb. Groups (n = 7-10/group) of WT mice were injected s.c. with MC38 (1 × 105) colon adenocarcinoma cells on day 0 and treated i.p with either; cIg (200 μg) or anti-CD39 (200 μg) on days 8, 11 14, and 18 and cIg (100 μg), anti-asGM1 (50 μg), anti-CD8β (100 μg) or anti- mIFN-γ (250 μg) on days 7, 8, 14, and 21. Tumor sizes (mm2) were measured at the indicated time points and presented as mean ± SEM. All experiments were performed once unless indicated. Significant differences among treatment groups were determined by a two-way ANOVA, followed by Tukey's multiple comparison test (* P < 0.05, ** P < 0.01, **** P < 0.0001). (F-I) Groups (n = 4–8/group) of WT mice were injected s.c. with (F, G) MC38 (1 × 105) or (H, I) MC38-OVAdim (1 × 106) colon adenocarcinoma cells on day 0. Mice were treated i.p. with cIg (200 μg) or anti-CD39 (200 μg) on day 7. Samples of tumor and spleen were collected 48 h after mAb injection and processed for single cell suspensions then subjected to flow cytometry after ex-vivo stimulation for 4 h. F and G, Graphs showing frequencies of tumor infiltrating CD8+ T cells expressing the indicated surface and intracellular molecules (mean ± SEM with individual values). Data pooled from two experiments. H, Graphs showing frequencies of OVA-tetramer+ T cells as a proportion of total TCRβ+CD8+ T cells in the tumor and spleen. I, Graphs showing numbers of total OVA-tetramer+ T cells and those expressing IFNγ in the tumor and spleen. All experiments were performed once unless indicated. Significant differences between the indicated groups were determined by a two-way ANOVA, followed by Sidak's multiple comparison test (F, G, I) or Mann-Whitney test (H) (* P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001).
We next sought to examine various tumor and peripheral lymphoid organ immune cell subsets in the MC38 tumor model (gating shown in Supplementary Fig. S7, Supplementary Fig. S8A-E). Under anti-CD39 treatment conditions, within 48 h, the frequency of intratumor CD8+ T cells producing IFN-γ, staining for Ki67 and expressing PD1 increased and it was the CD39+ fraction of CD8+ T cells that increased in each case (Fig. 2F, G; Supplementary Fig. S8A, B). These changes occurred despite no obvious increase in CD45+ frequencies or subsets within them post anti-CD39 treatment at this early timepoint (Supplementary Fig. S8C). Similar changes in CD8+ T cells producing IFN-γ, staining for Ki67 and expressing PD1 were not detected in the periphery (spleen and dLN, Supplementary Fig. S8D). In the MC38-OVAdim model, OVA-specific T cells were detected using tetramers 48 h after anti-CD39 treatment. The percentage of ova tetramer+ T cells in tumor and spleen, increased with anti-CD39 treatment, however increases in the number of OVA-specific T cells and IFN-γ+ OVA-specific T cells was only observed in the tumor (Fig. 2H, I).
Mechanism of action of anti-CD39 mAb – myeloid changes in vivo.
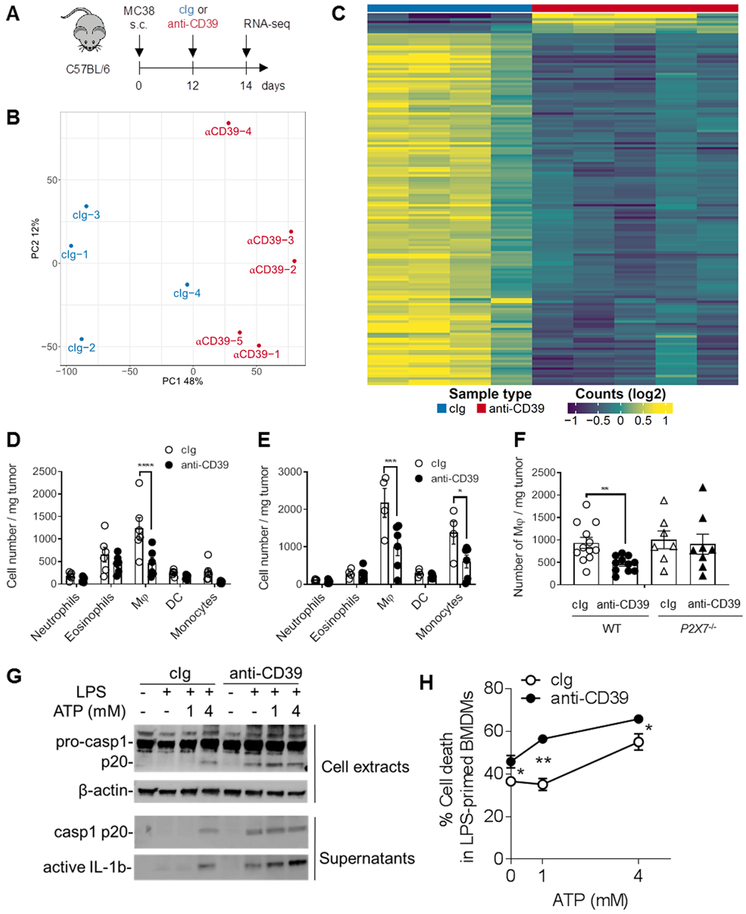
To assess global changes mediated by targeting CD39, we performed RNA-seq analysis. MC38-bearing WT mice were treated with anti-CD39 (B66) or cIg and whole tumor tissues were harvested 48 h later (Fig. 3A). Principal component and differential gene expression analysis revealed significant changes induced by blocking CD39 in the TME (Fig. 3B, C; Supplementary Fig. S9A-C). Interestingly, the top 150 differentially expressed genes in Figure 3C were downregulated in the anti-CD39 treatment group (Supplementary Table S1). Additionally, gene-set enrichment analysis (GSEA) revealed that anti-CD39-treatment induced a type I interferon response and led to the downregulation of gene signatures associated with immune suppression (Supplementary Fig. S9A, B). In line with our previous data, we observed downregulation of genes associated with immune suppressive myeloid cells (39) in anti-CD39-treated tumors. We next sought to examine myeloid cell subsets in the MC38 tumor model on day 9 (48 h after first injection of cIg or anti-CD39)(Fig. 3D) and 15 (48 h after third injection)(Fig. 3E). Under anti-CD39 treatment conditions, by day 9 the number of intratumor macrophages was reduced, and by day 15 both intratumor macrophages and monocytes were reduced. This reduction in intratumor macrophages did not occur in anti-CD39 treated P2X7−/− mice compared to WT mice at day 9 (Fig. 3F). Other intratumor myeloid cell (DC) and granulocyte (eosinophils and neutrophils) subset numbers were equivalent in cIg- and anti-CD39 treated mice (Fig. 3D, E). Macrophage and monocyte depletion were also not detected in the spleen of these same treated mice (Supplementary Fig. S10). These data were consistent with anti-CD39 liberated eATP triggering macrophage pyroptosis (40), thus resulting in inflammasome activation with pro-caspase-1 cleavage, IL-18/IL-1β release, and macrophage depletion. The death of LPS-primed macrophages in the presence or absence of exogenously added ATP was greatly increased by anti-mouse CD39 and this was accompanied by pro-caspase 1 cleavage and release of active IL-1β (Fig. 3G, H). Collectively this data possibly helps explain the marked early decrease in myeloid genes as determined by whole tumor RNA seq analyses (Fig. 3C) and the reduction in intratumor macrophage number post anti-CD39 (Fig. 3D-F). Interestingly, anti-CD39 anti-tumor activity was also compromised following myeloid cell depletion using clodronate liposomes or immobilization of myeloid cell movement using anti-CD11b (Supplementary Fig. S11A). By contrast additional experiments to deplete Ly-6G+ neutrophils did not affect anti-CD39 anti-tumor activity (Supplementary Fig. S11B). These data highlight the likelihood that anti-CD39 anti-tumor activity is regulated by both intratumor macrophage pyroptosis and inflammasome activation. We also noted increased T cell activation related transcripts 48 h post anti-CD39 compared with cIg treatment, indicating stronger TCR-signaling (Supplementary Fig. S9C). Overall, our in vivo, flow cytometry and gene expression data provide substantial evidence that anti-CD39 reduces intratumor macrophage number and enhances intratumor T cell activation.
Figure 3. Flow cytometry and transcriptional signature highlight macrophage reduction in tumors after anti-CD39 treatment.
(A) Schematic of experimental design. WT mice were injected s.c. with MC38 (1× 105) colon adenocarcinoma cells on day 0. On day 12, mice were treated i.p. with cIg or anti-CD39, and tumor samples were harvested 48 h post-treatment for RNA-sequencing. (B) Principal component analysis of TMM normalized RNA-seq data for protein-coding genes from control samples (cIg, n = 4, coloured blue) and treated samples (anti-CD39, n = 5, coloured red). (C) Heatmap of top 150 differentially expressed genes between control (cIg) and treated (anti-CD39) samples. The gene list is provided in Supplementary Table S1. (D, E) Groups (n = 4–6/group) of WT mice were injected s.c. with MC38 (1 × 105) colon adenocarcinoma cells on day 0 and treated i.p. with cIg (200 μg) or anti-CD39 (200 μg) on day 7, 10, and 13. Tumor samples were collected on day 9 (D) and 15 (E) and processed for single cell suspensions then subjected to flow cytometry. D and E, summary bar graphs of numbers of MC38 tumor infiltrating myeloid populations, as mean ± SEM with individual values shown. Data represent one experiment. (* P < 0.05, *** P < 0.001, **** P < 0.0001, determined by a two-way ANOVA, followed by Sidak's multiple comparison test). (F) Groups (n = 7-12/group) of WT or P2X7−/− mice were injected s.c. with MC38 (1 × 105) colon adenocarcinoma cells on day 0 and treated i.p. with cIg (200 μg) or anti-CD39 (200 μg) on day 7. Tumor samples were collected on day 9 and processed for single cell suspensions then subjected to flow cytometry. F, summary bar graphs of numbers MC38 tumor infiltrating macrophages, as mean ± SEM with individual values shown. Data is pooled from two experiments (** P < 0.01, determined by Mann-Whitney test). (G and H) LPS-primed (100 ng/ml for 3 h) bone marrow-derived macrophages (BMDM) were pre-treated with cIg or anti-CD39 (10 μg/ml), followed by stimulation with indicated concentrations of ATP for 90 min. (G) Immunoblots showing indicated proteins from cell lysates and culture supernatants. (H) Representative graphs showing the frequencies of propidium iodide-positive dead cells. Data are shown as mean ± SEM from triplicates from three experiments.
Mechanism of action of anti-CD39 mAb – critical role of myeloid CD39, P2X7, NALP3 inflammasome and IL-18
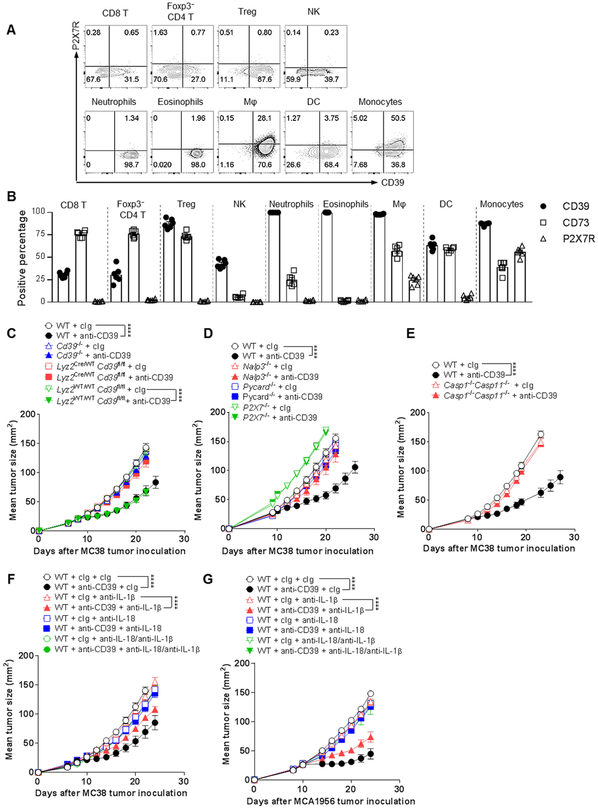
Our data indicated that inhibition of CD39 enzymatic function led to an accumulation of eATP, which could lead to an activation of myeloid cells via P2X7. Therefore, we sought to examine expression of P2XY receptors on various tumor and peripheral lymphoid organ immune cell subsets in the MC38 tumor model on day 8 after tumor inoculation. A number of intratumor leukocyte populations expressed CD39 including a proportion (~25-40%) of CD8+ T cells, FoxP3− and FoxP3+ CD4+ T cells, and most of the innate NK cells, neutrophils, eosinophils, macrophages, DC and monocytes (Fig. 4A). CD73 was expressed on a greater fraction of T cells, but was generally lower in the proportions of innate leukocytes (Fig. 4B). However, P2X7 receptor expression was far more restricted. P2X7 and CD39 co-expression was only notable in 25-50% of macrophages and monocytes amongst all the aforementioned leukocytes (Fig. 4A, B). When looking outside the tumor (spleen and tumor draining lymph node), CD39 expression was negligible in T cells, but still prevalent on innate leukocyte subsets (Supplementary Fig. S8E). P2X7 receptor was once again expressed by a proportion of macrophages and monocytes, but also by some DC in the spleen and tumor draining lymph node. To test the role of CD39 on myeloid cells, we created the Lyz2cre/wt CD39fl/fl and Lyz2wt/wt CD39fl/fl strains of mice and examined anti-CD39 anti-tumor activity in the MC38 model (Fig. 4C). Anti-CD39 was inactive in global Cd39−/− mice and conditional Lyz2cre/wt CD39fl/fl mice, but active in WT mice and Lyz2wt/wt CD39fl/fl mice.
Figure 4. Mechanism of action of anti-CD39 mAb – role of myeloid cells, P2X7 and NALP3 inflammasome.
(A, B) Groups (n = 4–8/group) of WT mice were injected s.c. with MC38 (1 × 105) colon adenocarcinoma cells on day 0. Samples of tumor and spleen were collected on day 8 and processed for single cell suspensions then subjected to flow cytometry. A, Representative FACS plots showing expression of CD39 and P2X7R on various tumor-infiltrating leukocytes (TILs) in MC38 tumors. B, Summary bar graphs of % CD39, CD73 and P2X7R expression on MC38 TIL subsets, as mean ± SEM. (C) Groups (n = 6-7/group) of WT, Cd39−/−, Lyz2Cre/WT CD39fl/fl, and Lyz2WT/WT CD39fl/fl mice were injected s.c. with MC38 (1 × 105) colon adenocarcinoma cells on day 0 and treated i.p with cIg (200 μg) or anti-CD39 (200 μg) on days 8, 11, 14, and 17. (D) Groups (n = 5/group) of WT, Cd39−/−, Pycard−/−, P2X7−/−, and Nalp3−/− mice were injected s.c. with MC38 (1 × 105) colon adenocarcinoma cells on day 0 and treated i.p with cIg (200 μg) or anti-CD39 (200 μg) on days 9, 12, 15, and 18. (E) Groups (n = 6/group) of WT, and caspase1−/− caspase11−/− mice were injected s.c. with MC38 (1 × 105) colon adenocarcinoma cells on day 0 and treated i.p with cIg (200 μg) or anti-CD39 (200 μg) on days 8, 11, 14, and 17. (C-E) Representative of two experiments performed. (F) Groups (n = 5/group) of WT mice were injected s.c. with MC38 (1 × 105) colon adenocarcinoma cells on day 0 and treated i.p with cIg (200 μg) or anti-CD39 (200 μg) on days 8, 11, 14, and 17. Mice additionally received i.p either cIg (250 μg), anti-IL-1β (250 μg), anti-IL-18 (250 μg) or anti-IL1β and anti-IL-18 (250 μg each) on days 7, 8, 15, and 22 after tumor inoculation. (G) Groups (n = 5/group) of WT mice were injected s.c. with MCA1956 (1 × 106) sarcoma cells on day 0 and treated i.p with cIg (200 μg) or anti-CD39 (200 μg) on days 10, 13, 16, and 19. Mice additionally received i.p either cIg (250 μg), anti-IL-1β (250 μg), anti-IL-18 (250 μg) or anti-IL1β and anti-IL-18 (250 μg each) on days 9, 10, 17, and 24 after tumor inoculation. (C-G) Tumor sizes (mm2) were measured at the indicated time points and presented as mean ± SEM. All experiments were performed once unless indicated. Significant differences among treatment groups were determined by a two-way ANOVA, followed by Tukey's multiple comparison test (**** P < 0.0001).
Positioned at the apex of the phosphohydrolytic cascade, CD39 is the pivotal regulator balancing pro-inflammatory eATP and immunosuppressive adenosine within the TME. P2X7 is the major functional eATP receptor on immune cells and is a critical factor in NALP3 inflammasome activity (41). Given this, we next examined whether host P2X7 receptor and downstream inflammasome components might be necessary for the anti-tumor activity of anti-CD39. MC38 tumors grew equivalently in WT, Pycard−/− and Nalp3−/− mice, but more aggressively in P2X7−/− mice. Importantly, anti-CD39 was effective against MC38 tumors in WT mice, but largely without activity in P2X7−/−, Pycard−/− or Nalp3−/− mice (Fig. 4D). Interestingly, anti-CD39 was ineffective in P2X7−/− and Nalp3−/− mice, but anti-PD1 was effective in the same setting, suggesting a very different mechanism of action (Supplementary Fig. S12A). Anti-PD1 and a combination of anti-PD1 and A2ARi were also equivalently effective in Pycard−/− or Nalp3−/− mice as WT mice (Supplementary Fig. S12B). The role of the inflammasome was further supported by the lack of anti-tumor activity of anti-CD39 in caspase 1−/−caspase 11−/− mice (Fig. 4E).
The ability of anti-CD39 to trigger the inflammasome was supported by increased IL-18 levels measured in MC38 tumor tissue lysates immediately post anti-CD39 therapy, in contrast to a lack of IL-18 in similar tumors derived from Nalp3−/− mice (Supplementary Fig. S13A). We next functionally tested the role of the inflammasome generated IL-1β and IL-18, both of which have been shown to promote downstream T cell anti-tumor function (42,43). In both the MC38 and MCA1956 tumor models, blockade of IL-18 over the course of anti-CD39 therapy, completely abrogated the anti-tumor activity of anti-CD39 (Fig. 4F, G). Anti-IL-1β displayed partial blockade of anti-CD39 anti-tumor activity. These data were supported by additional experiments comparing cIg and anti-CD39 against MC38 and MCA1956 in WT versus II18−/− or II1r−/− mice (Supplementary Fig. S13B, C). Overall, we hypothesize that anti-CD39 may bind CD39 on P2X7-expressing intratumor macrophages and monocytes to liberate eATP triggering the activation of the NALP3 inflammasome. The downstream activation release of IL-18 and IL-1β and probably other factors then promote CD8+ T cell proliferation and IFN-γ mediated effector function.
Anti-CD39 turns “cold” anti-PD1 resistant tumors “hot” and sensitive
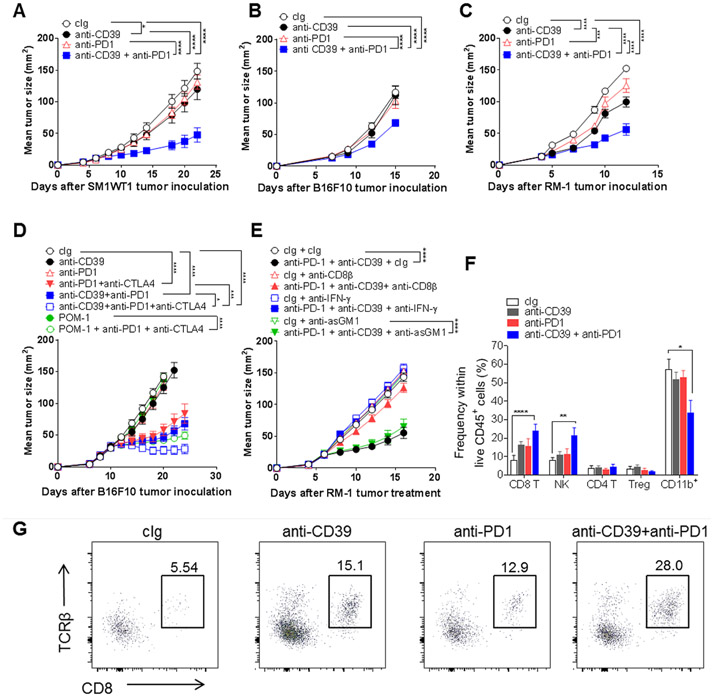
One of the most pressing issues in cancer immunotherapy is that several tumor types show profound innate resistance to ICB, including anti-PD1/anti-PDL1 (2). Thus, we employed the anti-CD39 and anti-PD1 combination in a series of anti-PD1 resistant and T cell poorly infiltrated tumor models, including the Braf mutant melanoma SM1WT1, the MHC class I low melanoma B16F10, and the MHC class I low prostate carcinoma RM1 (Fig. 5). In each model, combined treatment displayed anti-tumor activity where either monotherapy was largely ineffective. Early treatment with anti-CD39 did have a minor impact in these models (Fig. 5A-C). Similar data was obtained with the anti-PD1 refractory CT26 colon adenocarcinoma and Her2+ TUBO mammary cancers (Supplementary Fig. S14A, B). Consistent with our earlier report (44), in the latter setting, combinations of anti-PD1 were very effective with anti-Her2, but so was the combination of anti-CD39 and anti-Her2 (Supplementary Fig. S14B). Assessment of anti-CD39 in the context of contemporary anti-PD1 and anti-CTLA4 blockade in well-established B16F10 tumors again highlighted the additional benefit of anti-CD39 and its superiority to POM-1 (Fig. 5D). Preliminary assessment of mechanism of action of anti-PD1 and anti-CD39 in the RM-1 (Fig. 5E) and SM1WT1 (Supplementary Fig. S14C) tumor models revealed a CD8+ T cell- and IFN-γ-dependent effect. The RM-1 tumor model was interrogated in more depth and a significant increase in the frequency of intratumor CD45+ cells, intratumor CD8+ T cells and NK cells was detected two days after the third combination dose in the treatment schedule (day 14) where tumor mass had decreased (Fig. 5F, G; Supplementary Fig. S14D, E). CD8+ T cell number per mg tumor mass was also increased in the combination-treated group (Supplementary Fig. S14F).
Figure 5. Anti-CD39 mAb sensitizes anti-PD1-resistant tumors by increasing CD8+ T cell infiltration.
(A) SM1WT1 tumors. Groups (n = 6/group) of WT mice were injected s.c. with SM1WT1 melanoma (1 × 106) on day 0 and treated i.p with cIg (200 μg), anti-CD39 (200 μg), anti-PD1 (250 μg), or anti-CD39/anti-PD1 (200 μg;250 μg) on days 6, 9, 12, and 14. (B) B16F10 tumors. Groups (n = 7-9/group) of WT mice were injected s.c. with B16F10 melanoma (1 × 105) on day 0 and treated i.p with cIg (200 μg), anti-CD39 (200 μg), anti-PD1 (250 μg), anti-CD39/anti-PD1 (200 μg;250 μg) on days 7, 10, and 13. (C) RM-1 tumors. Groups (n = 7-8/group) of WT mice were injected s.c. with RM-1 prostate carcinoma cells (5 × 104) on day 0 and treated i.p with cIg (200 μg), anti-CD39 (200 μg), anti-PD1 (250 μg), anti-CD39/anti-PD1 (200 μg;250 μg) on days 3, 6, 9, and 12. (D) Combining anti-CD39, anti-PD1 and anti-CTLA4 improves therapeutic efficacy in resistant B16F10 tumors. Groups (n = 6-12/group) of WT mice were injected s.c. with B16F10 melanoma cells (1 × 105) on day 0 and treated i.p with cIg (200 μg), anti-CD39 (200 μg), anti-PD- (250 μg) anti-CTLA-4 (250 μg), POM-1 (250 μg) alone or in various combinations as indicated on days 10, 13, 16, and 19. (E) Combination efficacy is IFN-γ- and CD8+ T cell-dependent. Groups (n = 6/group) of WT mice were injected s.c. with RM-1 prostate carcinoma cells (5 × 104) on day 0 and treated i.p with cIg (200 μg), anti-CD39 (200 μg), anti-PD1 (250 μg) or anti-CD39/anti-PD1 (200 μg/250 μg) on days 6, 9, 12, and 15. Some groups of mice were also treated i.p. with either cIg (100 μg), anti-asGM1 (50 μg) anti-CD8β (100 μg) or anti-mIFN-γ (250 μg) on days 5, 6 and 13. Tumor sizes (mm2) were measured at the indicated time points and presented as mean ± SEM. Significant differences between the treatment groups were determined by a two-way ANOVA, followed by Tukey's multiple comparison test (* P < 0.05, *** P < 0.001, **** P < 0.0001). (F,G) Groups (n = 5-6/group) of WT mice were injected s.c. with RM-1 prostate carcinoma cells (5 × 104) on day 0 and treated i.p with cIg (200 μg), anti-CD39 (200 μg), anti-PD1 (250 μg), anti-CD39/anti-PD1 (200 μg;250 μg) on days 6, 9, and 12. The tumors were collected on day 14, 48 h after the third dose, for TIL analysis by flow cytometry. (F) Graphs showing the frequencies of immune cell subsets in RM-1 tumors 2 days post the third injection of the indicated mAbs. (G) Representative FACS plots showing increased CD8+ T cell infiltration in RM-1 tumors on day 14, 2 days post the third anti-CD39/anti-PD1 mAb treatment. All experiments were performed once unless indicated. Significant differences in percentage between the selected cell populations were determined by one-way ANOVA, followed by Tukey's multiple comparison test (* P < 0.05, ** P < 0.01, **** P < 0.0001).
To corroborate our findings, we also used a model of adoptive cell transfer (ACT) immunotherapy to treat immune cell poor melanoma (39). For this, WT mice were injected with HCmel12 melanoma expressing human gp100 under the control of the melanocytic lineage gene Tyrp1. Once tumors were established mice were treated with our ACT protocol using gp100-specific pmel-1 TCR transgenic T cells (39,45). Mice also received 5 doses of cIg or anti-CD39 (Supplementary Fig. S15A). Combination of ACT with anti-CD39 significantly inhibited tumor growth and improved survival (Supplementary Fig. S15B).
Anti-human CD39 mAb increases T cell proliferation and Th1 cytokine secretion
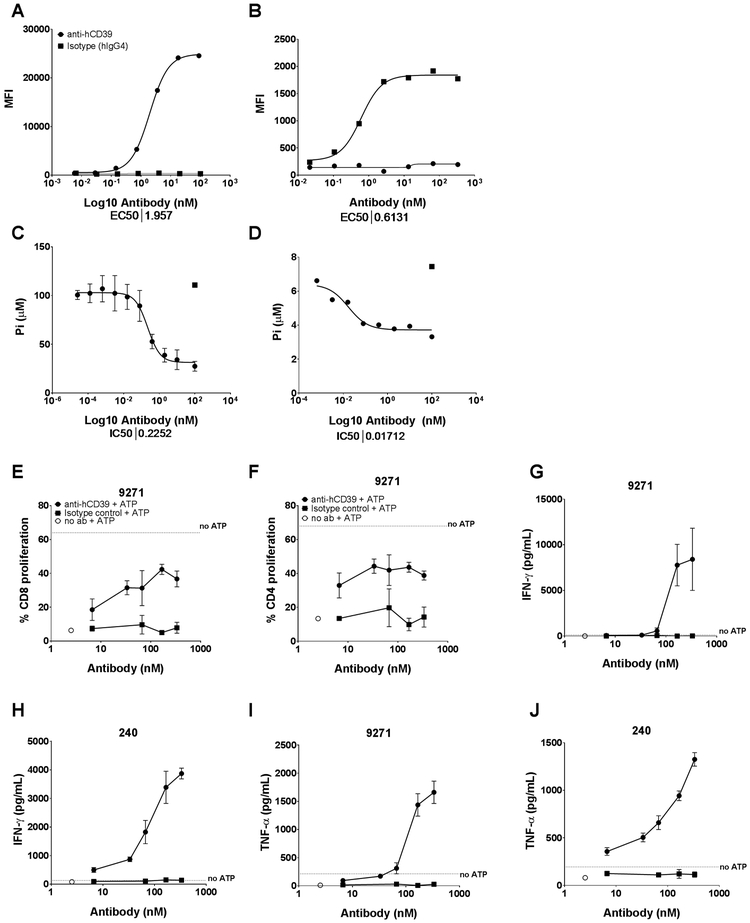
The monotherapy and combination anti-tumor activity of B66, anti-mouse CD39 mAb was very encouraging, but it was critical to produce an anti-human CD39 for clinical translation. Therefore, using yeast display of human V gene libraries, we produced a human IgG4 anti-human CD39 mAb, TTX-030 (46). This mAb bound human CD39 on 721.221 cells and human monocytes (Fig. 6A, B) and allosterically inhibited CD39 enzymatic activity on these cells (Fig. 6C, D; Supplementary Fig. S16A, B) at sub nM levels. Functional assessment of anti-human CD39 in vitro in anti-CD3/anti-CD28 stimulated PBMCs from various donors demonstrated the potent ability of this mAb to enhance CD4+ and CD8+ T cell proliferation (Fig. 6E, F; Supplementary Fig. S17A,B). Anti-human CD39 also increased Th1 cytokine production (IFN-γ, TNF-α and IL-2) in these cultures compared with a hIgG4 control mAb (Fig. 6G-J; Supplementary Fig. S17C-G).
Figure 6. Human anti-CD39 mAb blocks CD39 enzymatic activity and enhances T cell proliferation and effector function in the presence of ATP.
(A-D). Anti-hCD39 binds and inhibits CD39 enzymatic activity at sub nM potency. Anti-hCD39 mAb binds to the 721.221 cell line (A) and human monocytes (B) and inhibits CD39 enzymatic activity as measured by Pi release using the Malachite Green assay (C and D, respectively and Supplementary Fig. 14). (E-J). Anti-hCD39 enhances T cell proliferation and Th1 cytokine production. PBMCs from two different donors were stimulated with anti-CD3/anti-CD28 and either an isotype control or anti-human CD39, and assessed for T cell proliferation in the presence and absence of ATP by flow cytometry. The graphs represent anti-hCD39-induced increased CD8 (E) and CD4 (F) T cell proliferation in the presence of ATP (donor 9271), and increased IFN-γ (G, H), TNF-α (I, J) production in PBMCs (donors 240 and 9271), treated with anti-hCD39 in the presence of ATP. Human IgG4 isotype control was used for comparison.
Anti-human CD39 mAb activity with autologous EBV-specific human T cells
In the absence of having a fully humanized mouse model of cancer, we next decided to examine the anti-tumor activity of anti-human CD39 alone or in combination with autologous EBV-specific T cell transfer against LCL (EBV transformed B cells) derived from the same donors inoculated into immunodeficient NRG mice. These mice lack all lymphocytes and the only human CD39 expressing cells are the T cells and LCL injected. All LCL generated were highly CD39+ (Supplementary Fig. S18A), but we first selected donors based on the ability of their LCL to grow robustly in NRG mice. Two donors were chosen (LCL039 and LC043) and preliminary experiments with these CD39+ LCL and the CD39+ SK-MEL28 melanoma suggested that these were not sensitive to delayed anti-human CD39 treatment alone (Supplementary Fig. S18B, C).
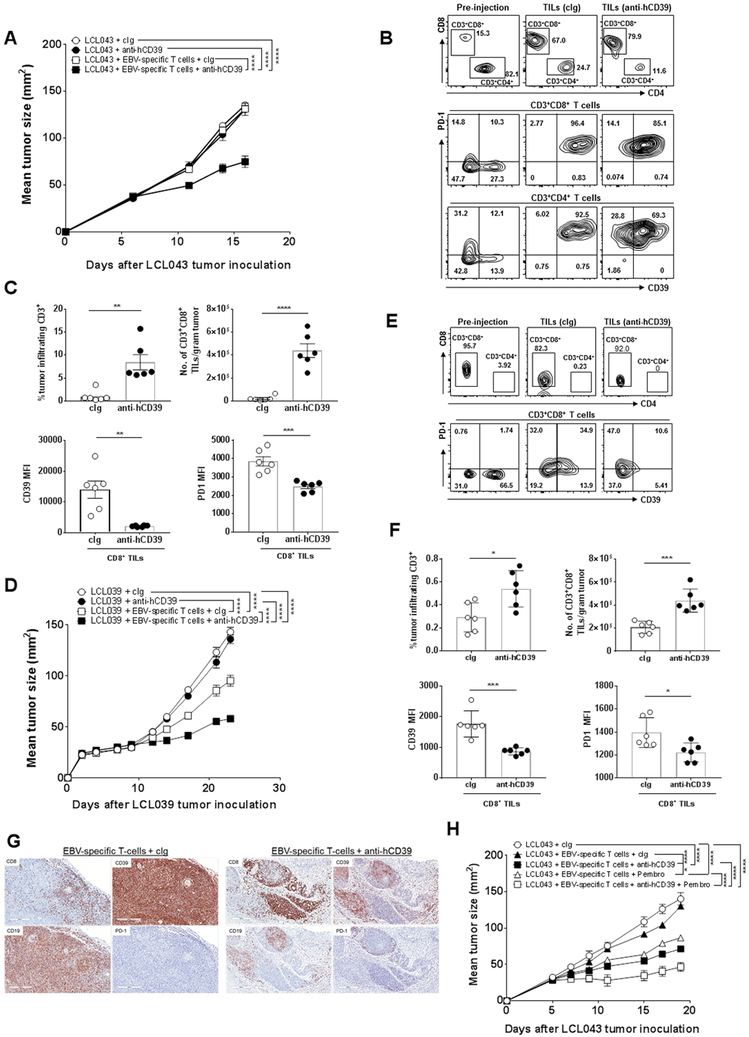
EBV-stimulated T cell cultures from different donors are distinct and those raised against LCL043 and LCL039 offered the opportunity to examine: a mixed but predominantly CD4+T cell culture with significant CD39 and PD1 co-expression on T cells (LCL043); and a predominantly CD8+T cell culture with a high proportion of CD8+ T cells expressing CD39, but largely lacking PD1 expression (LCL039). In the case of LCL043, either EBV-specific T cells alone or anti-CD39 did not suppress tumor growth compared with cIg, but the combination of T cells and anti-CD39 did significantly suppress tumor growth (Fig. 7A). Analysis of EBV-specific T cells pre-injection and in the LCL043 lymphoma post-injection (at end point day 17) revealed a significant increase in the CD8+T:CD4+ T cell ratio in the tumor regardless of whether cIg or anti-human CD39 was administered (Fig. 7B). Notably however, anti-human CD39 increased the percentage of tumor infiltrating CD3 cells and number of tumor infiltrating CD3+CD8+ T cells compared with cIg-treated mice (Fig. 7C). Anti-human CD39 therapy also dramatically decreased the CD39 expression levels on intratumor CD8+T cells compared with cIg-treatment (Fig. 7C). A more modest reduction in PD1 expression was also noted.
Figure 7. Anti-hCD39 mAb and EBV-specific T cells effectively control LCL tumor growth in NRG mice.
(A) Groups (n = 6/group) of NRG mice were injected s.c. with EBV-transformed lymphoblastoid cells LCL043 (1 × 107) on day 0. In some groups EBV-specific T cells (9 × 106) were administered i.v. on day 5 as indicated and all experimental groups were then treated i.p. with either human cIg (250 μg) or anti-hCD39 (250 μg) on days 5, 8, 11, and 14. Tumor sizes (mm2) were measured at the indicated time points and presented as mean ± SEM. This experiment is representative of two performed. Significant differences between the indicated groups were determined by a two-way ANOVA, followed by Tukey's multiple comparison test (**** P < 0.0001). (B-C). From (A), Tumors were collected, single cell suspensions were made and subjected to flow cytometry. (B) Representative FACS plots of pre-injection EBV-specific T cell cultures and tumors post cIg- or anti-CD39 (TTX-030) therapy (day 17 post tumor inoculation) and (C) summary bar graphs showing frequencies of tumor infiltrating CD3+ T cells, numbers of CD3+CD8+ T cells, and expression levels of CD39 and PD1 on CD8 T cells in LCL tumors post cIg- or anti-CD39 therapy. Significant differences were determined by Mann-Whitney U test (** P < 0.01, *** P < 0.001, **** P < 0.0001). (D) Groups (n= 6/group) of NRG mice were injected s.c. with donor-derived EBV-transformed lymphoblastoid cells LCL039 (1 × 107) on day 0. In some groups EBV-specific T cells (9 × 106) were administered i.v. on day 10 as indicated and all experimental groups were then treated i.p. with anti-hCD39 (250 μg) or human cIg (250 μg) on day 10, 13, 16, and 19. Tumor sizes (mm2) were measured at the indicated time points and presented as mean ± SEM. This experiment is representative of two performed. Significant differences between the indicated groups were determined by a two-way ANOVA, followed by Tukey's multiple comparison test (**** P < 0.0001). (E-F). From (D), tumors were collected, single cell suspensions were made and subjected to flow cytometry. (E) Representative FACS plots of pre-injection EBV-specific T cell cultures and tumors post cIg- or anti-human CD39 therapy (day 23 post tumor inoculation) and (F) summary bar graphs showing frequencies of tumor infiltrating CD3+ T cells, numbers of CD3+CD8+ T cells and expression levels of CD39 and PD1 on CD8 T cells in LCL tumors post cIg- or anti-CD39 therapy. Significant differences in percentages between the selected cell populations were determined by Mann-Whitney test (* P < 0.05, *** P < 0.001). (G) Representative immunohistochemical staining of CD8, CD39, PD1 and CD19 on day 23 (end point) LCL039 tumors showing increased CD8+ T cell infiltration and reduced CD39 expression in the EBV–specific T cell/anti-hCD39-treated tumors. (H) Anti-hCD39 and pembrolizumab combine with autologous EBV-specific T cells to suppress human B cell LCL043 lymphoma. Groups (n = 6/group) of NRG mice were injected s.c. with EBV transformed lymphoblastoid cells LCL043 (1 × 107) on day 0. In some groups EBV-specific T cells (9 × 106) were administered i.v. on day 5 as indicated and all experimental groups were then treated i.p. with human cIg (250 μg) or anti-hCD39 (250 μg) or pembrolizumab (anti-human PD1) (250 μg) or both (250 μg; 250 μg) on days 5, 8, 11, and 14. Tumor sizes (mm2) were measured at the indicated time points and presented as mean ± SEM. All experiments were performed once unless indicated. Significant differences between the indicated groups were determined by a two-way ANOVA, followed by Tukey's multiple comparison test (* P < 0.05, *** P < 0.001, **** P < 0.0001).
The LCL039 lymphoma had a lower tumorigenicity in NRG mice (than LCL043), but once again anti-CD39 alone was without effect. However, autologous EBV-specific T cells were more active alone in this setting (Fig. 7D) and again, following transfer, CD8+ T cells were enriched in the LCL tumor at endpoint (day 23) (Fig. 7E, F). Consistent with the LCL043 experiments, anti-human CD39 enhanced the anti-tumor activity of autologous EBV-specific T cells (Fig. 7D), increased the percentage of tumor infiltrating CD3 cells and number of tumor infiltrating CD3+CD8+ T cells compared with cIg-treated mice (Fig. 7F, G). Anti-human CD39 therapy again decreased the CD39 expression levels on intratumor CD8+ T cells compared with cIg-treatment (Fig. 7F, G). PD1 expression was increased on the CD8+ T cells post injection and again anti-CD39 therapy appeared to reduce the expression levels of PD1 on intratumor CD8+ T cells compared with cIg-treatment (Fig. 7E, F).
Given the lack of effect of autologous EBV-specific T cell transfer against established LCL043, and the limited anti-tumor activity of the anti-CD39 and T cell combination, we performed a second experiment to evaluate the addition of pembrolizumab (anti-human PD1). Here anti-CD39 and anti-PD1 displayed very similar anti-tumor activities when each administered with autologous EBV-specific T cells, but demonstrated an even greater effect on tumor growth when combined together with T cell transfer (Fig. 7H). In summary, the anti-human CD39 antibody enriches intratumor human CD8+ T cell number and suppresses human B-cell lymphoma following autologous EBV-specific T cell transfer.
Discussion
This study is the first to describe a mAb specifically targeting mouse CD39 and as such, we have been able to undertake comprehensive mechanism of action experiments in immunocompetent and gene-targeted mice. All prior assumptions regarding CD39 have been based on studies using; small molecule inhibitors of CD39 (31), Cd39−/− mice (30) and other genetic strategies to target CD39 (47), or anti-human CD39 mAbs in vitro or in immune compromised mice (17,32). The previous approaches have limitations with respect to translation and are inferior to assessing a potent anti-mouse CD39 mAb in syngeneic tumor models. Furthermore, our study has uncovered the critical importance of an eATP-P2X7-ASC-NALP3-inflammasome-IL-18 pathway in the anti-tumor activity mediated by CD39 enzyme blockade. This pathway has not previously been recognized to be critical for anti-CD39 anti-tumor activity. Rather, previous experimental evidence supported a dogma where blockade of CD39 primarily prevented CD73 from generating adenosine as mechanism of action. eATP liberation and signalling of immune stimulation has been postulated as a mechanism of action of CD39 blockade, but never demonstrated with any experimental evidence. Our data suggests that anti-CD39 activity is mediated by CD39 and P2X7 co-expression on intratumor myeloid subsets, an early signature of macrophage depletion, and active IL-18 release that facilitates subsequent T cell effector function. In syngeneic mice and autologous human T cell - tumor models, anti-CD39 effectively converted T cell poor tumors into T cell rich tumors and rescued anti-PD1 resistance.
Despite the impressive advances achieved by immunotherapies in the past few years, there is a significant proportion of patients whose cancer is either refractory to ICB or who develop adaptive resistance after achieving initial clinical responses. In either case, there is a growing body of evidence to suggest that adenosine-mediated immunosuppression is a major mechanism of immune evasion underlying these observations (48-50). These insights are supported by the increasing evidence gleaned from extensive immunophenotyping efforts. Dissection of the TME via flow cytometry, CyTOF, IHC or single-cell RNA-seq analysis have revealed that upregulation of CD39 expression on critical tumor infiltrating immune subsets is a common occurrence across multiple tumor types (23,24,51). The presence of high levels of CD39 on regulatory immune subsets (18-21) and on exhausted and tumor-reactive T cells (23-25) is directly correlated with immune dysfunction. Meanwhile, CD39 expression on the tumor itself appears to be restricted to a small subset of patients in most indications (32) or specific indication where increased prevalence has been reported (e.g. blood malignancies or melanoma (52)). Given these observations, our findings that inhibiting CD39 expression on the myeloid compartment is required for reversing immune suppression within the TME has implications for cancer patients that may benefit from anti-CD39 therapy.
First, while patients with immunologically active, ‘hot’ tumors are more likely to respond to existing immune checkpoint inhibitors, the majority still fail to achieve complete, durable responses, If adenosine-mediated immune suppression is indeed a major source of this innate or adaptive resistance, then disruption of the adenosinergic pathway might be effective in settings where immune infiltrates are present but immune checkpoint inhibitors either fail to elicit anti-tumor responses or where patients relapse as adaptive resistance develops. Secondly, we have demonstrated that inhibition of CD39 enzymatic function can sensitize intrinsically resistant, ‘cold’ tumor models to anti-PD1. Given that ICB has generally failed in settings where the pre-existing TME is T cell poor, this potentially opens up new indications for consideration. Our findings in both a poorly infiltrated syngeneic tumor model and in the humanized setting indicate that blocking the CD39 enzymatic activity is correlated with the increased infiltration of cytotoxic effector populations into the TME and a concomitant upregulation of activation markers on those infiltrates. While eATP has been reported as a chemotactic signal for attracting myeloid populations, especially neutrophils via P2Y2 (53,54), we see a decrease in intratumor macrophages.
One potential explanation is offered by the observation that, activation of the inflammasome appears necessary to achieve the full therapeutic benefit of CD39 enzymatic inhibition. In the context of the broader therapeutic landscape, this impact on innate immune function and antigen presenting subsets may be a key feature that distinguishes CD39 enzymatic inhibition from other modalities targeting the adenosinergic axis. Indeed, the NALP3 inflammasome was not required for the anti-tumor activity of anti-PD1 or anti-PD1 and A2ARi. In direct comparisons of anti-tumor activity, inhibition of CD39 enzymatic function was superior to antagonism of CD73 enzymatic function and/or downstream A2AR/A2BR signaling. Unlike therapeutic approaches that target the downstream production or function of adenosine, inhibition of CD39 not only limits the production of adenosine, but also prevents the rapid degradation of eATP in the TME. eATP is therefore made available to trigger purinergic receptors on both innate and adaptive immune cells (55,56). Also interesting was the finding that anti-CD39 was able to further suppress tumor immunity in mice blocked or deleted for CD73, A2AR and A2BR. This suggests that not all extracellular adenosine was inhibited by anti-CD39 and perhaps other adenosine generating pathways may contribute when CD39 is blocked. Whether similar combination effects can be achieved by locally inhibiting HIF-1/hypoxia as a major regulator of CD39 and CD73 remains to be determined (57). These data also confirm the distinct mechanism of action of anti-CD39. Further delineation of the adaptive versus innate contributions of eATP will require conditional P2X7 gene targeted mice to dissect the relative contributions of immune subsets, but is warranted in light of these findings.
Surprisingly global CD39 gene-targeted mice often did not display a tumor reduction phenotype where the anti-CD39 mAb treatment was very effective. This suggests either compensation in the CD39 gene-targeted strain involving other molecules (e.g. CD38) or different pro-tumor and anti-tumor roles of CD39 when expressed on different cell types. Lyz2Cre/WT CD39fl/fl mice did not display any profound tumor resistance phenotype, but clearly did not respond to tumor upon anti-CD39 treatment. Lyz2Cre/WT CD39fl/fl mice display a decrease in macrophage, monocyte and neutrophil CD39 expression, but only macrophages and monocytes are reduced in tumors post anti-CD39 treatment and only these myeloid cells co-express CD39 and P2X7 in the TME. Thus, it seems likely that intratumor myeloid cells expressing CD39 co-express P2X7 and respond to the eATP liberated by anti-CD39. This response includes NALP3 inflammasome activation, IL-18/IL-1β release and pyroptosis. Consistent with this mechanism, depletion or immobilization of myeloid cells or neutralization of IL-18/IL-1β completely abrogated the anti-tumor activity of anti-mouse CD39. It remains to be determined whether this mechanism of action applies in “cold” mouse tumors and human tumors. “Hot” and “cold” mouse tumors all have some level of immunosuppressive myeloid cells expressing CD39, but it is clearly more difficult to determine mechanism of action in “cold” tumors where anti-CD39 monotherapy is weak. Macrophages and monocytes were reduced in both “hot” MC38 (Figure 3D) and “cold” RM1 tumors 48 hours after anti-CD39 (Supplementary Fig. S19). Interestingly, in the EBV-specific T cell-LCL model, anti-human CD39 was effective only against established tumors when T cells were transferred despite the fact that the only human components were the human T cells and the human B cell lymphoma expressing CD39. Here it is possible that the transferred T cells or LCL tumors are the primary target of anti-human CD39. It is possible LCL may act as an antigen presenting cells in the autologous model and provide a source of IL-18 and hence in this setting, the need for myeloid cells might be bypassed. The contribution of mouse myeloid cells in this model has also not been assessed. Further experiments deleting CD39 in tumors and T cells will be required in mouse and humanized mouse tumor models to understand the importance of CD39 on these cells.
Our findings reveal insight into the pharmacologic inhibition of CD39 enzymatic activity on the TME. Previously, this has mostly been studied in the context of genetic deletion of CD39 in the host (28,30,31,58). While these studies provide valuable insights into the role of the adenosinergic system in cancer, genetic knockout can be subject to compensatory mechanisms, including desensitization of the purinergic receptors (59). POM-1 has also been used in mouse models to probe the importance of CD39 enzymatic function in cancer settings (28,31,51), but these studies are inherently limited by the lack of drug-like properties of this compound, including its poor serum half-life and broad ectonucleotidase specificity. Our studies suggest that enzymatic inhibition of CD39 by an antibody is in fact superior to both genetic deletion and POM-1-mediated inhibition. This is also true in metastatic models where NK cells are effectors (manuscript in preparation). Antibody binding of CD39 without enzyme inhibition was not effective. The ability of anti-CD39 to inhibit CD39 ATPase activity was demonstrated in vivo and suggests a potential biomarker strategy for efficacy in humans should fresh frozen tumor tissue be available.
In summary, our findings present compelling evidence for the impact of enzymatic inhibition of CD39 on the course of tumor progression. Translation of these pre-clinical findings into human trials will ultimately be required to determine the utility of this approach to treating human cancers, either as a monotherapy or in combination with other established or to-be-discovered agents. First in human trials of the anti-CD39 in advanced cancer patients have recently commenced (NCT03884556).
Materials and Methods
Mice
Wild-type (WT) C57BL/6 and BALB/c mice were purchased from the Walter and Eliza Hall Institute for Medical Research or bred in house. C57BL6 Ptprca (CD45.1+) mice, C57BL/6 CD39-deficient (Cd39−/−) mice (60), C57BL/6 NALP3-deficient (Nalp3−/−) mice, C57BL/6 P2X7-deficient (P2X7−/−) mice (61), C57BL/6 ASC-deficient (Pycard−/−) mice, and Nod-rag1-gamma c (NRG) mice were bred in-house and maintained at the QIMR Berghofer Medical Research Institute. C57BL/6 Rag2−/−γc−/− mice have been previously described (33). C57BL6 Lyz2Cre/WT CD39fl/fl and Lyz2WT/WT CD39fl/fl mice were generated by crossing Lyz2Cre mice (from Dr. Irmgard Foerster, University of Dusseldorf) (62) with CD39fl/fl mice (obtained from the EUCOMM Consortium). Mice greater than 6 weeks of age were sex-matched to the appropriate models. The number of mice in each group treatment or strain of mice for each experiment is indicated in the figure legends. In all studies, no mice were excluded based on pre-established criteria and randomization was only applied immediately pre-treatment in therapy experiments to ensure similar mean tumor size was the starting point. Experiments were conducted as approved by the QIMR Berghofer Medical Research Institute Animal Ethics Committee.
Human ethics
Healthy volunteers under written informed consent were recruited according to the principles of the Declaration of Helsinki and the National Statement on Ethical Conduct in Human Research in accordance with the National Health and Medical Research Council (Australia) Act. The Human Ethics Committee of QIMR Berghofer Medical Research Institute approved the protocol for the recruitment of healthy volunteers. Peripheral blood mononuclear cells (PBMC) from healthy volunteers were isolated using Ficoll-Paque gradients on SepMate columns (STEMCELL Technologies) and used to generate EBV-transformed lymphoblastoid cell lines (LCL) as described previously (63). Autologous LCL were used to generate EBV-specific T cells as outlined below. Alternatively, human PBMCs were isolated from a human peripheral blood leukopak purchased from STEMCELL Technologies. Using Institutional Review Board Approved consent forms and protocols, the material was obtained and distributed by STEMCELL for the purpose of in vitro research only.
Antibody generation
The B66 antibody to murine CD39 was discovered through immunization of wild type Sprague Dawley rats followed by hybridoma screening. Hits were produced as antibodies with Fc region derived from mouse IgG1 and were confirmed for binding to recombinant mCD39 extracellular domain (ECD; R&D Systems, CAT#4398-EN) or to mouse cells endogenously expressing CD39: BCL1, clone 5B1b (ATCC, CAT#TIB-197). The D265A variant was generated using QuickChange site directed mutagenesis (Agilent). Anti-human CD39 (TTX-030) was previously generated via phage display with a human Fab library and expressed by standard techniques (46).
Anti-CD39 efficacy in vitro – proliferation and cytokine effector function
PBMCs were labeled with Cell Trace Violet (ThermoFisher) to measure proliferation, and 50,000 cells/well were plated into a 96-well round bottom plate. Cells were pre-treated for 30 minutes with antibody in triplicates, then 2 μL Immunocult™ human CD3/CD28 T cell activator (STEMCELL) was added per well in addition to 50 μM final concentration of ATP or media alone (no ATP control). Cells were incubated for 96 hours, then spun 1700 rpm for 2 minutes. Supernatants were collected for evaluation of cytokines using Meso Scale Discovery (MSD). For flow cytometry, Human BD Fc Block™ was added to cells for 30 minutes before staining with CD4-PE/Cy7 (clone RPA-T4), CD8-APC (clone RPA-T8), CD3-Brilliant Violet 785™ (BV785, clone OKT3), CD14-Alexa Fluor® 488 (clone HCD14), eBioscience™ Fixable Viability dye eFluor™ 780 (e780) for 30 min. Cells were washed twice and then resuspended in staining buffer and run on BD Fortessa. Data were analyzed on FlowJo, and CD4 and CD8 T-cell proliferation was plotted as a histogram. Unstimulated cells were used to set a control peak for non-dividing cells, and remaining peaks were analyzed as % proliferation.
Cell culture
Mouse B16F10 (melanoma), MC38 (colon adenocarcinoma), RM-1 (prostate carcinoma) cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% Fetal Calf Serum (Bovogen), 1% Glutamine (Gibco), 1% HEPES (Gibco) and 1% Penicillin/Streptomycin (Gibco). Mouse SM1WT1 (melanoma), MCA1956 (fibrosarcoma) and 4T1.2 (mammary carcinoma) cells were cultured in RPMI 1640, supplemented with 10% Fetal Calf Serum (Bovogen), 1% Glutamine (Gibco), and 1% Penicillin-Streptomycin (Gibco)(complete RPMI). All the mouse tumors were CD39-negative in culture as previously demonstrated (31). Human EBV-transformed lymphoblastoid cell lines (LCL039 and LCL043) were established from healthy seropositive donors as previous described (64) and were routinely maintained in complete RPMI. Mouse bone marrow-derived macrophages were generated in complete RPMI supplemented with M-CSF (20 ng/ml, Peprotech) for 6 days. All cell lines were maintained at 37°C, 5% CO2, except MC38 which was cultured at 10% CO2. Cell injection and monitoring procedures were described in previous studies (64). All cell lines were routinely tested negative for Mycoplasma, but cell line authentication was not routinely performed.
Bone marrow chimera construction
As previously described, CD45.1+ Ptprca WT mice and CD45.2+ Cd39−/− mice as recipient mice (9-10 mice per group) were irradiated twice with a total dose of 1,050 cGy (33). Donor BM cells (1 × 107) from Ptprca mice or Cd39−/− mice were then i.v. injected into the irradiated mice to construct the BM chimeric mice. Neomycin containing water (1 mg/mL) was given to these mice for 3 weeks. After confirming the BM reconstruction by flow cytometry of peripheral blood 8 weeks after BM cell injection, MC38 cells (1 × 105) were s.c injected into the BM chimeric mice. Mice were then treated i.p. with cIg or anti-CD39 mAb as indicated and tumor size was measured at the time points indicated in the figure legends.
Subcutaneous tumor models
For primary tumor growth experiments, MC38 (1 × 105), B16F10 (1 × 105), MCA1956 (1 × 106), RM-1 (5 × 104), or SM1WT1 (1 × 106) cells were s.c. injected into mice in a final volume of 100-200 μl (day 0). Therapeutic antibody treatment commenced as indicated on day 3-10 after tumor inoculation and was given every 3 or 4 days up to a maximum of 4 doses. Digital callipers were used to measure the perpendicular diameters of the tumors. The tumor size was calculated and is presented as mean ± SEM.
MCA-induced fibrosarcoma
Groups of 10-16 male C57BL/6 WT mice were inoculated s.c. in the hind flank with 300 μg of MCA (Sigma-Aldrich) in 0.1 mL of corn oil. Mice were treated with mIgG1, anti-CD39 (B66), anti-PD1 (RMP1-14), or their combination (100 μg each i.p., twice/week) for 6 weeks from the second palpable tumor measurement (0.1–0.4 cm2, days 77–127 relative to MCA inoculation). Mice were then monitored for fibrosarcoma development for 250 days with measurements made with a caliper as the product of two perpendicular diameters (cm2). The number of mice that rejected tumors out of the total number of mice is shown. Growth rate of tumor was also measured from treatment start point to sacrifice or tumor rejection (as mm2/day).
Generation of CTL using LCL
To generate cytotoxic T lymphocytes (CTL) that predominantly recognize the immunodominant latent antigens of EBV (EBNA3-6), PBMCs were prepared prior to use and stored in liquid nitrogen. To prepare stimulator cells, 1-2 × 106 autologous LCL were harvested and γ-irradiated at 80 Gy. PBMCs were resuspended and cultured with stimulator cells in a final ratio of 30:1, with IL-2 in the medium (120 IU/ml). Additional IL-2 and medium were added every 3 to 4 days until the end of the culture period. On day 21, cells were removed from the incubator, and the number of viable cells were determined using trypan blue exclusion method. The expanded CTLs were stored for further experiments.
Adoptive transfer of EBV-specific T-cells
NRG mice were engrafted subcutaneously with 107 EBV-transformed lymphoblastoid cells (LCL039 or LCL043) per mouse. Tumor growth was monitored every 2–3 days using digital calipers. Five days (LCL043) or ten days (LCL039) after engraftment of lymphoblastoid cells, mice were either mock treated or infused with 9 × 106 EBV specific T-cells. Control lg (Palivizumab hG4, MedImmune) or anti-human CD39 (Tizona) were administrated i.p. on the same day of EBV-specific T-cell injection and every 3 days for 4 doses in total. At the end point, tumors were resected and either digested with a combination of collagenase type 4 (Worthington Biochemical Corp.) and DNase I (Roche) at 37°C or fixed with formalin. Tumor infiltrated CD8+ lymphocytes and expression of exhaustion markers were detected by flow cytometry and IHC.
In vivo treatments
For mouse tumor models, some groups of mice received either: anti-CD8β (53.5.8) (Bio X Cell) as indicated to deplete CD8+ T cells and anti-asialoGM1 (Wako) to deplete NK cells. Some groups of mice were neutralized for IFNγ (H22) using the scheduling and dosing as indicated. Some mice were treated with cIg (I-1 or I-536, Leinco), anti-mouse CD39 (B66, mIgG1, Tizona), anti-CD39 (B66, mIgG1 D265A, Tizona), anti-CD73 (2C5; mIgG1, Tizona), anti-PD1 (RMP1-14), A2AR inhibitor (SCH58261) (Sigma-Aldrich) with schedules and doses as indicated in the figure legends. For human tumor models, some groups of mice treated either: cIg (Palivizumab hG4, MedImmune), anti-human CD39 (TTX-030; Tizona), anti-PD1 (Pembrolizumab, Merck) with schedules and doses as indicated in the figure legends. No macroscopic signs of toxicity or weight change were detected post anti-CD39 therapy (data not shown).
Flow cytometry
Tumors, tumor draining lymph nodes, and spleens were harvested from mice untreated or treated with control or therapeutic antibodies as indicated in the figure legends. Tumors and lymph nodes were minced and digested with 1 mg/mL collagenase IV (Worthington Biochemical) and 0.02 mg/mL DNase I (Roche) and homogenized to prepare single cell suspensions. Single cell suspensions of spleens were depleted of erythrocytes. For surface staining, cells were stained in phosphate buffered saline (PBS) containing 2% (v/v) FBS with anti-CD45.2 (104; BD biosciences) or anti-CD45.2 (30-F11; Thermofisher), anti-CD4 (RM4-5; Biolegend) or anti-CD4 (GK1.5; eBioscience), anti-CD8a (53-6.7; Biolegend), anti-TCRβ (H57-597; Biolegend), anti-NK1.1 (PK136; BD biosciences), anti-CD39 (Duha59; BioLegend), anti-CD73 (TY/23; BD Bioscience), OVA-tetramer (assembled with biotinylated KbOVA monomer from Prof Andrew Brooks Lab, the University of Melbourne and streptavidin from BD biosciences), anti-CD279 (29F.1A12; BioLegend), anti-Ly6G (1A8; BioLegend), anti-CD11b (M1/70; BioLegend), anti-CD11c (N418; eBioscience), anti-CD64 (X54-5/7.1; BioLegend), anti-MHC II (M5/114.15.2; eBioscience), anti-CD274 (10F.9G2; BioLegend), anti-P2X7R (1F11; BioLegend). For intracellular staining, surface-stained cells were fixed and permeabilized using the Foxp3/Transcription Factor Staining Buffer Set (eBioscience) or BD Cytofix/Cytoperm (BD Biosciences) according to the manufacturer’s protocol and stained with anti-Foxp3 (FJK-16s, eBioscience), anti-IFNγ (XMG1.2; BioLegend), anti-Ki67 (16A8; BioLegend) or anti-Ki67 (B56; BD biosciences), and respective isotype antibodies. For intracellular staining of IFNγ, cells were stimulated ex vivo with eBioscience™ Cell Stimulation Cocktail (plus protein transport inhibitors) for 4 h before surface staining. Populations defined in CD45+ live cells: CD8+ T cells, TCRβ+CD8+; CD4+Foxp3− T cells, TCRβ+CD4+Foxp3−; regulatory T cells (Treg), TCRβ+CD4+Foxp3+; natural killer (NK) cells, NK1.1+TCRβ−; neutrophils, Ly6G+; eosinophils, Ly6G−CD64−MHCII−CD11b+SSChi; macrophages, Ly6G−CD64+MHCII+; dendritic cells (DC), Ly6G−CD64−MHCII+CD11c+; monocytes, Ly6G−CD64−/loMHCII−/loCD11bhiSSClo. Cells were acquired on the BD LSR Fortessa V (BD Biosciences) and analysis was carried out using FlowJo (Tree Star). Dead cells stained by 7-AAD (BioLegend) or Zombie Aqua (BioLegend) were excluded from analysis. Lymphoid and myeloid components were gated by markers indicated in Figure S7.
Immunohistochemistry
Immunohistochemistry (IHC) for CD8, CD39, PD1 and CD19 staining was performed on mouse tumors harvested at the end point (day 23), in 10% neutral buffered formalin (NBF). Tumor sections were cut at 3 μm onto superfrost+ glass slides and stored under vacuum until IHC was performed. Antibody specific IHC conditions are listed in Table S2. Briefly, antigen retrieval was done using a pressure decloaker (100°C; 20 minutes) and IHC was performed on a Dako Autostainer. Targets were visualized using the MACH3 HRP polymer detection system (Biocare; M3R531) and DAB Chromogen Kit (Biocare; BDB2004). Slides were counterstained with diluted hematoxylin and scanned using the 20x object lens on an Aperio AT system (Leica Biosystems). Representative images per tumor were captured using ImageScope software (Leica Biosystems).
Immunoblots
LPS-primed BMDM were stimulated with ATP for 90 min. Proteins in total cell lysates (prepared by RIPA buffer) and supernatants were detected by following mAbs: caspase-1 p20 (Casper-1, AdipoGen Life Science), anti-IL-1β (3A6, Cell signalling Technology), β-actin (13E5, Cell Signalling Technology).
RNA sequencing
WT mice were injected s.c with MC38 colon adenocarcinoma cells on day 0. Mice were treated i.p. with B66 or cIg (200 μg) on day 12 and tumor samples were harvested 48 h post-treatment, followed by snap-freezing by dry ice. RNA isolation was performed using RNeasy Mini kit (Qiagen) and RNA samples with RIN > 7 were selected for cDNA library preparation using TruSeq RNA sequencing (RNA-seq) using the Illumina (NextSeq 550) platform. A minimum of 27 million 76 bp paired-end reads were generated per sample. Sequence reads were trimmed for adapter sequences using Cutadapt (version 1.9; (65)) and aligned using STAR (version 2.5.2a; (66)) to the Mus musculus GRCm38 (MM10) reference genome assembly using the gene, transcript, and exon features model of Ensembl (release 70). Quality control metrics were computed using RNA-SeQC (version 1.1.8; (67)), and transcript abundances were quantified using RSEM (version 1.2.30; (68)). Further analysis of the RNA-seq data were carried out in R (version 3.5.1; URL https://www.R-project.org/). Protein-coding genes with < 3 counts per million in fewer than 5 samples were removed from down-stream analyses. Trimmed mean of M-values (TMM) normalization and differential gene expression analysis were performed using the edgeR package (69). The ‘prcomp’ function in R was used to perform principal component analysis on gene-wise centered and scaled values of TMM normalised expression data. Heatmaps were produced using ‘ComplexHeatmap’ R package (70) using gene-wise centered, scaled, log2 values of TMM normalised expression data, and “pearson” distance with “ward.D” criteria to cluster the rows. Gene set enrichment analysis (GSEA) was performed using the “fgsea” R package (71). RNA-seq data have been deposited in the European Nucleotide Archive (Accession Number: PRJEB32653).
Statistical analysis
Statistical analysis was determined with Graphpad Prism 7 (GraphPad Software). A 1-tailed Mann-Whitney U test was used for comparisons of 2 groups. Significance of differences was also calculated by log-rank t test for Kaplan-Meier survival analysis or 2-way ANOVA as necessary. Tukey’s multiple comparisons tests were utilized unless otherwise indicated. A Fisher’s exact test was also used to determine significance of proportion of tumor free mice. Differences between groups are shown as the mean ± SEM. P values of less than 0.05 were considered statistically significant.
Supplementary Material
Significance.
Overall, these data describe a potent and novel mechanism of action of antibodies that block mouse or human CD39, triggering an eATP-P2X7-inflammasome-IL-18 axis that reduces intratumor macrophage number, enhances intratumor T cell effector function, overcomes anti-PD1 resistance, and potentially enhances the efficacy of adoptive T cell transfer.
Acknowledgements
The authors wish to thank Liam Town and Brodie Quine for genotyping and maintenance and care of the mice used in this study. The authors wish to thank Andrew Wong for assistance with expression and purification of antibodies.
Financial support
M.J.S. was supported by a National Health and Medical Research Council (NH&MRC) Senior Principal Research Fellowship (1078671) and Program Grant (1132519) and a Melanoma Research Alliance grant. S.C.R. was supported by NIH R21CA221702, R01DK108894 and DoD W81XWH-15-PRMRP-FPA. M. W. L. T. was supported by a NH&MRC Career Development Fellowship (1159655) and Project Grant (1120887) and Prostate Cancer Foundation of Australia and It’s a Bloke Thing Foundation. Some of the work was supported by a scientific research agreement with Tizona Therapeutics. T.B. was supported by a National Health and Medical Research Council (NH&MRC) Early Career Research Fellowship (1124690) and Project Grant (1138757). K.N was supported by the Naito Foundation and a NHMRC Project Grant (1174363). M.H. was supported by DFG GRK2168 and M.E. by a scholarship within GRK2168.
Footnotes
Competing financial interests
M.J. Smyth has research agreements with Bristol Myers Squibb and Tizona Therapeutics and is a member of the Scientific Advisory Board (SAB) for Tizona Therapeutics and Compass Therapeutics. S.C. Robson has a research agreement with Tizona Therapeutics. C. Smith receives research and consultancy funding from Atara Biotherapeutics. A.C.A. is a member of the SAB for Tizona Therapeutics, Compass Therapeutics, Zumutor Biologics, and Astellas Pharma Global Development, Inc., which have interests in cancer immunotherapy. T.B. has research agreements with ENA Therapeutics and is a member of the SAB for Oncomyx.
References
- 1.Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol 2016;13(3):143–58 doi 10.1038/nrclinonc.2015.209. [DOI] [PubMed] [Google Scholar]
- 2.Siu LL, Ivy SP, Dixon EL, Gravell AE, Reeves SA, Rosner GL. Challenges and opportunities in adapting clinical trial design for immunotherapies. Clin Cancer Res 2017;23(17):4950–8 doi 10.1158/1078-0432.CCR-16-3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Restifo NP, Smyth MJ, Snyder A. Acquired resistance to immunotherapy and future challenges. Nat Rev Cancer 2016;16(2):121–6 doi 10.1038/nrc.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pellegatti P, Raffaghello L, Bianchi G, Piccardi F, Pistoia V, Di Virgilio F. Increased level of extracellular ATP at tumor sites: in vivo imaging with plasma membrane luciferase. PLoS One 2008;3(7):e2599 doi 10.1371/journal.pone.0002599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Di Virgilio F, Falzoni S, Giuliani AL, Adinolfi E. P2 receptors in cancer progression and metastatic spreading. Curr Opin Pharmacol 2016;29:17–25 doi 10.1016/j.coph.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 6.Takenaka MC, Robson S, Quintana FJ. Regulation of the T cell response by CD39. Trends Immunol 2016;37(7):427–39 doi 10.1016/j.it.2016.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Allard D, Turcotte M, Stagg J. Targeting A2 adenosine receptors in cancer. Immunol Cell Biol 2017;95(4):333–9 doi 10.1038/icb.2017.8. [DOI] [PubMed] [Google Scholar]
- 8.Eppell BA, Newell AM, Brown EJ. Adenosine receptors are expressed during differentiation of monocytes to macrophages in vitro. Implications for regulation of phagocytosis. J Immunol 1989;143(12):4141–5. [PubMed] [Google Scholar]
- 9.Hasko G, Szabo C, Nemeth ZH, Kvetan V, Pastores SM, Vizi ES. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol 1996;157(10):4634–40. [PubMed] [Google Scholar]
- 10.Hasko G, Kuhel DG, Chen JF, Schwarzschild MA, Deitch EA, Mabley JG, et al. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J 2000;14(13):2065–74 doi 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 11.Lappas CM, Rieger JM, Linden J. A2A adenosine receptor induction inhibits IFN-gamma production in murine CD4+ T cells. J Immunol 2005;174(2):1073–80. [DOI] [PubMed] [Google Scholar]
- 12.Minguet S, Huber M, Rosenkranz L, Schamel WW, Reth M, Brummer T. Adenosine and cAMP are potent inhibitors of the NF-kappa B pathway downstream of immunoreceptors. Eur J Immunol 2005;35(1):31–41 doi 10.1002/eji.200425524. [DOI] [PubMed] [Google Scholar]
- 13.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 2007;204(6):1257–65 doi 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ohta A, Ohta A, Madasu M, Kini R, Subramanian M, Goel N, et al. A2A adenosine receptor may allow expansion of T cells lacking effector functions in extracellular adenosine-rich microenvironments. J Immunol 2009;183(9):5487–93 doi 10.4049/jimmunol.0901247. [DOI] [PubMed] [Google Scholar]
- 15.Bastid J, Cottalorda-Regairaz A, Alberici G, Bonnefoy N, Eliaou JF, Bensussan A. ENTPD1/CD39 is a promising therapeutic target in oncology. Oncogene 2013;32(14):1743–51 doi 10.1038/onc.2012.269. [DOI] [PubMed] [Google Scholar]
- 16.Zhang B, Cheng B, Li FS, Ding JH, Feng YY, Zhuo GZ, et al. High expression of CD39/ENTPD1 in malignant epithelial cells of human rectal adenocarcinoma. Tumour Biol 2015;36(12):9411–9 doi 10.1007/s13277-015-3683-9. [DOI] [PubMed] [Google Scholar]
- 17.Hayes GM, Cairns B, Levashova Z, Chinn L, Perez M, Theunissen JW, et al. CD39 is a promising therapeutic antibody target for the treatment of soft tissue sarcoma. Am J Transl Res 2015;7(6):1181–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Koziak K, Sevigny J, Robson SC, Siegel JB, Kaczmarek E. Analysis of CD39/ATP diphosphohydrolase (ATPDase) expression in endothelial cells, platelets and leukocytes. Thromb Haemost 1999;82(5):1538–44. [PubMed] [Google Scholar]
- 19.Borsellino G, Kleinewietfeld M, Di Mitri D, Sternjak A, Diamantini A, Giometto R, et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 2007;110(4):1225–32 doi 10.1182/blood-2006-12-064527. [DOI] [PubMed] [Google Scholar]
- 20.Gu J, Ni X, Pan X, Lu H, Lu Y, Zhao J, et al. Human CD39(hi) regulatory T cells present stronger stability and function under inflammatory conditions. Cell Mol Immunol 2017;14(6):521–8 doi 10.1038/cmi.2016.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Limagne E, Euvrard R, Thibaudin M, Rebe C, Derangere V, Chevriaux A, et al. Accumulation of MDSC and Th17 cells in patients with metastatic colorectal cancer predicts the efficacy of a FOLFOX-bevacizumab drug treatment regimen. Cancer Res 2016;76(18):5241–52 doi 10.1158/0008-5472.CAN-15-3164. [DOI] [PubMed] [Google Scholar]
- 22.Montalban Del Barrio I, Penski C, Schlahsa L, Stein RG, Diessner J, Wockel A, et al. Adenosine-generating ovarian cancer cells attract myeloid cells which differentiate into adenosine-generating tumor associated macrophages - a self-amplifying, CD39- and CD73-dependent mechanism for tumor immune escape. J Immunother Cancer 2016;4:49 doi 10.1186/s40425-016-0154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thelen M, Lechner A, Wennhold K, von Bergwelt-Baildon M, Schlosser HA. CD39 expression defines cell exhaustion in tumor-infiltrating CD8(+) T cells-Letter. Cancer Res 2018;78(17):5173–4 doi 10.1158/0008-5472.CAN-18-0873. [DOI] [PubMed] [Google Scholar]
- 24.Canale FP, Ramello MC, Nunez N, Araujo Furlan CL, Bossio SN, Gorosito Serran M, et al. CD39 expression defines cell exhaustion in tumor-infiltrating CD8(+) T cells. Cancer Res 2018;78(1):115–28 doi 10.1158/0008-5472.CAN-16-2684. [DOI] [PubMed] [Google Scholar]
- 25.Simoni Y, Becht E, Fehlings M, Loh CY, Koo SL, Teng KWW, et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 2018;557(7706):575–9 doi 10.1038/s41586-018-0130-2. [DOI] [PubMed] [Google Scholar]
- 26.Duhen T, Duhen R, Montler R, Moses J, Moudgil T, de Miranda NF, et al. Co-expression of CD39 and CD103 identifies tumor-reactive CD8 T cells in human solid tumors. Nat Commun 2018;9(1):2724 doi 10.1038/s41467-018-05072-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao H, Bo C, Kang Y, Li H. What else can CD39 tell us? Front Immunol 2017;8:727 doi 10.3389/fimmu.2017.00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun X, Wu Y, Gao W, Enjyoji K, Csizmadia E, Muller CE, et al. CD39/ENTPD1 expression by CD4+Foxp3+ regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology 2010;139(3):1030–40 doi 10.1053/j.gastro.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng L, Sun X, Csizmadia E, Han L, Bian S, Murakami T, et al. Vascular CD39/ENTPD1 directly promotes tumor cell growth by scavenging extracellular adenosine triphosphate. Neoplasia 2011;13(3):206–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun X, Han L, Seth P, Bian S, Li L, Csizmadia E, et al. Disordered purinergic signaling and abnormal cellular metabolism are associated with development of liver cancer in Cd39/ENTPD1 null mice. Hepatology 2013;57(1):205–16 doi 10.1002/hep.25989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang H VD, Li XY, Robson SC, Geetha N, Teng MWL, et al. The role of NK cells and CD39 in the immunological control of tumor metastases. Oncoimmunology 2019; 8(6):e1593809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bastid J, Regairaz A, Bonnefoy N, Dejou C, Giustiniani J, Laheurte C, et al. Inhibition of CD39 enzymatic function at the surface of tumor cells alleviates their immunosuppressive activity. Cancer Immunol Res 2015;3(3):254–65 doi 10.1158/2326-6066.CIR-14-0018. [DOI] [PubMed] [Google Scholar]
- 33.Li XY, Das I, Lepletier A, Addala V, Bald T, Stannard K, et al. CD155 loss enhances tumor suppression via combined host and tumor-intrinsic mechanisms. J Clin Invest 2018;128(6):2613–25 doi 10.1172/JCI98769. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Ngiow SF, Young A, Jacquelot N, Yamazaki T, Enot D, Zitvogel L, et al. A threshold level of intratumor CD8+ T-cell PD1 expression dictates therapeutic response to anti-PD1. Cancer Res 2015;75(18):3800–11 doi 10.1158/0008-5472.CAN-15-1082. [DOI] [PubMed] [Google Scholar]
- 35.Selby MJ, Engelhardt JJ, Johnston RJ, Lu LS, Han M, Thudium K, et al. Preclinical development of ipilimumab and nivolumab combination immunotherapy: mouse tumor models, in vitro functional studies, and cynomolgus macaque toxicology. PLoS One 2016;11(9):e0161779 doi 10.1371/journal.pone.0161779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Teng MW, Ngiow SF, von Scheidt B, McLaughlin N, Sparwasser T, Smyth MJ. Conditional regulatory T-cell depletion releases adaptive immunity preventing carcinogenesis and suppressing established tumor growth. Cancer Res 2010;70(20):7800–9 doi 10.1158/0008-5472.CAN-10-1681. [DOI] [PubMed] [Google Scholar]
- 37.Uno T, Takeda K, Kojima Y, Yoshizawa H, Akiba H, Mittler RS, et al. Eradication of established tumors in mice by a combination antibody-based therapy. Nat Med 2006;12(6):693–8 doi 10.1038/nm1405. [DOI] [PubMed] [Google Scholar]
- 38.Ngiow SF, Loi S, Thomas D, Smyth MJ. Mouse models of tumor immunotherapy. Adv Immunol 2016;130:1–24 doi 10.1016/bs.ai.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 39.Glodde N, Bald T, van den Boorn-Konijnenberg D, Nakamura K, O’Donnell JS, Szczepanski S, et al. Reactive neutrophil responses dependent on the receptor tyrosine kinase c-MET limit cancer immunotherapy. Immunity 2017;47(4):789–802 e9 doi 10.1016/j.immuni.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, et al. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol 2006;176(7):3877–83 doi 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 41.Adinolfi E, Giuliani AL, De Marchi E, Pegoraro A, Orioli E, Di Virgilio F. The P2X7 receptor: A main player in inflammation. Biochem Pharmacol 2018;151:234–44 doi 10.1016/j.bcp.2017.12.021. [DOI] [PubMed] [Google Scholar]
- 42.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 2009;15(10):1170–8 doi 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 43.Hu B, Ren J, Luo Y, Keith B, Young RM, Scholler J, et al. Augmentation of Antitumor immunity by human and mouse CAR T cells secreting IL-18. Cell Rep 2017;20(13):3025–33 doi 10.1016/j.celrep.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stagg J, Loi S, Divisekera U, Ngiow SF, Duret H, Yagita H, et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci U S A 2011;108(17):7142–7 doi 10.1073/pnas.1016569108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Landsberg J, Kohlmeyer J, Renn M, Bald T, Rogava M, Cron M, et al. Melanomas resist T-cell therapy through inflammation-induced reversible dedifferentiation. Nature 2012;490(7420):412–6 doi 10.1038/nature11538. [DOI] [PubMed] [Google Scholar]
- 46.Lerner AG KM, Welch M, dela Cruz T, Jones J, Wong C, Spatola B, Eberhardt M, Wong A, Fung W, Lagpacan L, Losenkova K, Yegutkin G, Soros V, Corbin J, Beers C, Moesta AK. Targeting CD39 with a first-in-class inhibitory antibody prevents ATP processing and increases T-cell activation. [abstract] In: Proceedings of the American Association for Cancer Research, AACR Annual Meeting 2019; 2019 Mar 29 – Apr 3; Atlanta, GA Philadelphia (PA): AACR; 2019 Abstract nr 5012 2019. [Google Scholar]
- 47.Kashyap AS, Thelemann T, Klar R, Kallert SM, Festag J, Buchi M, et al. Antisense oligonucleotide targeting CD39 improves anti-tumor T cell immunity. J Immunother Cancer 2019;7(1):67 doi 10.1186/s40425-019-0545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vijayan D, Young A, Teng MWL, Smyth MJ. Targeting immunosuppressive adenosine in cancer. Nat Rev Cancer 2017;17(12):709–24 doi 10.1038/nrc.2017.86. [DOI] [PubMed] [Google Scholar]
- 49.Ohta A A metabolic immune checkpoint: adenosine in tumor microenvironment. Front Immunol 2016;7:109 doi 10.3389/fimmu.2016.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen L, Diao L, Yang Y, Yi X, Rodriguez BL, Li Y, et al. CD38-mediated immunosuppression as a mechanism of tumor cell escape from PD-1/PD-L1 blockade. Cancer Discov 2018;8(9):1156–75 doi 10.1158/2159-8290.CD-17-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sade-Feldman M, Yizhak K, Bjorgaard SL, Ray JP, de Boer CG, Jenkins RW, et al. Defining T cell states associated with response to checkpoint immunotherapy in melanoma. Cell 2018;175(4):998–1013 e20 doi 10.1016/j.cell.2018.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bonnefoy N, Bastid J, Alberici G, Bensussan A, Eliaou JF. CD39: A complementary target to immune checkpoints to counteract tumor-mediated immunosuppression. Oncoimmunology 2015;4(5):e1003015 doi 10.1080/2162402X.2014.1003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kronlage M, Song J, Sorokin L, Isfort K, Schwerdtle T, Leipziger J, et al. Autocrine purinergic receptor signaling is essential for macrophage chemotaxis. Sci Signal 2010;3(132):ra55 doi 10.1126/scisignal.2000588. [DOI] [PubMed] [Google Scholar]
- 54.Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 2006;314(5806):1792–5 doi 10.1126/science.1132559. [DOI] [PubMed] [Google Scholar]
- 55.Cekic C, Linden J. Purinergic regulation of the immune system. Nat Rev Immunol 2016;16(3):177–92 doi 10.1038/nri.2016.4. [DOI] [PubMed] [Google Scholar]
- 56.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol 2013;31:51–72 doi 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 57.Sitkovsky MV, Hatfield S, Abbott R, Belikoff B, Lukashev D, Ohta A. Hostile, hypoxia-A2-adenosinergic tumor biology as the next barrier to overcome for tumor immunologists. Cancer Immunol Res 2014;2(7):598–605 doi 10.1158/2326-6066.CIR-14-0075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jackson SW, Hoshi T, Wu Y, Sun X, Enjyoji K, Cszimadia E, et al. Disordered purinergic signaling inhibits pathological angiogenesis in cd39/Entpd1-null mice. Am J Pathol 2007;171(4):1395–404 doi 10.2353/ajpath.2007.070190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schaefer U, Machida T, Broekman MJ, Marcus AJ, Levi R. Targeted deletion of ectonucleoside triphosphate diphosphohydrolase 1/CD39 leads to desensitization of pre- and postsynaptic purinergic P2 receptors. J Pharmacol Exp Ther 2007;322(3):1269–77 doi 10.1124/jpet.107.125328. [DOI] [PubMed] [Google Scholar]
- 60.Enjyoji K, Sevigny J, Lin Y, Frenette PS, Christie PD, Esch JS 2nd, et al. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med 1999;5(9):1010–7 doi 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 61.Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, et al. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem 2001;276(1):125–32 doi 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 62.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Forster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res 1999;8(4):265–77. [DOI] [PubMed] [Google Scholar]
- 63.Smith C, Khanna R. Generation of cytotoxic T lymphocytes for immunotherapy of EBV-associated malignancies. Methods Mol Biol 2010;651:49–59 doi 10.1007/978-1-60761-786-0_3. [DOI] [PubMed] [Google Scholar]
- 64.Dasari V, Schuessler A, Smith C, Wong Y, Miles JJ, Smyth MJ, et al. Prophylactic and therapeutic adenoviral vector-based multivirus-specific T-cell immunotherapy for transplant patients. Mol Ther Methods Clin Dev 2016;3:16058 doi 10.1038/mtm.2016.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.M M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnetJournal 2011;17(1):10. [Google Scholar]
- 66.Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 2013;29(1):15–21 doi 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.DeLuca DS, Levin JZ, Sivachenko A, Fennell T, Nazaire MD, Williams C, et al. RNA-SeQC: RNA-seq metrics for quality control and process optimization. Bioinformatics 2012;28(11):1530–2 doi 10.1093/bioinformatics/bts196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li B, Dewey CN. RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinformatics 2011;12:323 doi 10.1186/1471-2105-12-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010;26(1):139–40 doi 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gu Z, Eils R, Schlesner M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016;32(18):2847–9 doi 10.1093/bioinformatics/btw313. [DOI] [PubMed] [Google Scholar]
- 71.Sergushichev AA, Loboda AA, Jha AK, Vincent EE, Driggers EM, Jones RG, et al. GAM: a web-service for integrated transcriptional and metabolic network analysis. Nucleic Acids Res 2016;44(W1):W194–200 doi 10.1093/nar/gkw266. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.