SUMMARY
Inconsistent activity limits use of CRISPR/Cas9 in zebrafish. We show supernumerary guanine nucleotides at the 5’ ends of sgRNAs account for diminished CRISPR/Cas9 activity in zebrafish embryos. Genomic sequences can be targeted consistently with extremely high efficiency using Cas9 RNPs containing either a sgRNA molecule or a synthetic crRNA:tracrRNA duplex that perfectly matches the protospacer target site. Following injection of zebrafish eggs with such RNPs, virtually every copy of a targeted locus harbors an induced indel mutation. Loss of gene function is often complete, as F0 embryos closely resemble true null mutants without detectable non-specific effects. Mosaicism is sufficiently low in F0 embryos that cell non-autonomous gene functions can be probed effectively and redundant activities of genes can be uncovered when two genes are targeted simultaneously. Finally, heritable deletion mutations of at least 50 kbp can be readily induced using pairs of duplex guide RNPs targeted to a single chromosome.
IN BRIEF (eTOC)
Cas9 RNP complexes consisting of synthetic crRNA:tracrRNA duplexes consistently induce mutations in virtually all copies of a targeted gene in zebrafish embryos. Hoshijima et al show these tools allow effective screening of individual or combinations of gene function in F0 embryos and the facile induction of designed deletion mutations.
INTRODUCTION
Identification and understanding of the phenotype associated with complete loss of gene function is key to gaining insight into its normal role. Hence isolation and characterization of the null mutant has become central to the analysis of gene activity (Muller, 1932). Here we describe methods in zebrafish that significantly advance both the recapitulation of the null state in F0 embryos for the purpose of initial screening of gene function and the generation of heritable gene deletions for the production of null mutants.
In the absence of a true mutant, methods that allow effective sampling of the null condition of genes have great utility: 1) as a first step in assessing a gene’s function (Klatt Shaw et al., 2018), 2) for screening among a large collection of candidate genes to identify those that contribute to a biological process of interest (Boutros and Ahringer, 2008; Housden et al., 2017), or 3) for testing potential gene interactions and assaying the combined functions of genes (Arduini et al., 2009; Dorsky et al., 2003). Unfortunately, the inconsistent efficacy of RNAi, antisense, and gene silencing methods has limited their value for screening purposes in many model organisms (Boutros and Ahringer, 2008; Dorsett and Tuschl, 2004; Housden et al., 2017). Furthermore, gene expression knockdown methods, such as the use of antisense morpholino oligonucleotides, often trigger unwanted global physiological and developmental perturbations and are sometimes associated with gene-specific off-target effects (Gentsch et al., 2018; Kok et al., 2015; Robu et al., 2007; Stainier et al., 2017; Tsai et al., 2015). The frequent failure of current methods to interfere completely with gene expression in F0 embryos, combined with their off-target effects, cloud the interpretation of results and make it cumbersome to apply these methods in well-controlled experiments (Stainier et al., 2017).
The ease with which CRISPR/Cas systems can be applied to induce mutations at virtually any locus makes them particularly attractive as a tool for screening gene functions in zebrafish (Varshney et al., 2015). RNA-guided CRISPR/Cas9 nucleases can be targeted with precision to induce a double strand break (DSB) at a specific locus in a genome, triggering repair mechanisms that can lead to the generation of indel mutations at the site of the lesion (Doudna and Charpentier, 2014; Hsu et al., 2014; Sander and Joung, 2014). DSB repair-mediated mutations generated in zebrafish often reduce or eliminate gene function (Bedell et al., 2012; Burger et al., 2016; Dahlem et al., 2012; Doyon et al., 2008; Gagnon et al., 2014; Jao et al., 2013; Meng et al., 2008). Hence, introduction of CRISPR/Cas9 targeting activity into the zebrafish egg has been used to screen gene functions in developing embryos (Shah et al., 2015). As currently applied, the efficiency with which target sites are mutagenized with a CRISPR/Cas9 complex rarely approaches 100%, and thus mutant cells are often generated in a mosaic manner among the cells of a growing zebrafish embryo (Burger et al., 2016; Gagnon et al., 2014; Jao et al., 2013). As a result CRISPR/Cas screens to detect the consequences of loss of gene function have been designed most often to detect cell autonomous phenotypes that can be detected amongst a heterogeneous pool of mutagenized cells, such as altered growth properties of cells in tissue culture (Shalem et al., 2014; Wang et al., 2015; Wang et al., 2014) or loss of specific synaptic connections in zebrafish embryos (Shah et al., 2015).
To screen effectively for gene functions whose loss may lead to complex or subtle developmental phenotypes in the zebrafish, a method is needed that consistently produces bi-allelic mutations in almost all cells of the growing embryo. One approach to improving the efficiency of mutagenesis with standard sgRNA/Cas9 complexes has called for use of a mixture of multiple sgRNPs, each targeting a different site within a gene (Wu et al., 2018). Nevertheless, inefficient nuclease activity still limits its applicability for many purposes, such as the simultaneous inactivation of two genes in an F0 embryo or the induction of deletion mutations. Indeed deletion mutations are a needed resource for defining the null state, as emphasized by an increasing number of findings indicating that mutations that conceptually result in premature termination codons may sometimes not produce null mutations. Studies in mammalian cells and zebrafish revealed that mutations altering coding sequences can promote skipping of the mutant exon (Anderson et al., 2017; Lalonde et al., 2017; Mou et al., 2017; Prykhozhij et al., 2017), at times resulting in production of a partially functional protein (Flanigan et al., 2011). Screens of human populations whose genomes harbor large autozygous regions have revealed healthy individuals who are homozygous for apparent premature stop mutations in genes known to be associated with disease (Narasimhan et al., 2016). Finally, some mutant alleles that generate transcripts that undergo nonsense-mediated decay can trigger compensatory transcription of related genes, effectively masking the consequences of loss of the mutant gene (El-Brolosy et al., 2019; Ma et al., 2019; Rossi et al., 2015). Deletion alleles that remove transcribed sequences can escape the functional rescue accomplished by alternative splicing or compensatory transcription. As Drosophila researchers have long realized, deletion mutations are effective tools for revealing the null state (Muller, 1932).
Here we demonstrate that the versatility and consistency of CRISPR/Cas9-mediated mutagenesis in zebrafish is greatly improved by use of a two-RNA component (crRNA:tracrRNA) version of the CRISPR system (Jacobi et al., 2017). In this system, RNA duplexes of chemically synthesized crRNA and tracrRNA molecules are complexed with Cas9 protein to form ‘duplex guide RNPs’ (dgRNPs). Duplex guide RNPs had been shown previously to be effective at inducing mutations in zebrafish, but their remarkable utility had not been explored (Kotani et al., 2015).
We find that duplex guide RNA-Cas9 RNPs are highly effective at stimulating DSB repair-induced mutations in the zebrafish, even at target sites that appear relatively resistant to the activity of canonical sgRNA-Cas9 RNPs. Following delivery of dgRNPs into zebrafish eggs, almost all cells present in developing embryos harbor bi-allelic indel mutations, while the embryos themselves generally develop without unrelated abnormalities or elevated levels of cell death. As a result, mutagenized F0 embryos often resemble true protein null mutants in remarkable detail. Furthermore, using pairs of dgRNPs targeting different sites on a single chromosome, germline-transmissible deletion mutations of at least 50 kbp can be readily generated and recovered in subsequent generations.
DESIGN
Because CRISPR/Cas9 methods can at times stimulate high levels of DSB-repair mediated mutagenesis in zebrafish embryos (Burger et al., 2016; Gagnon et al., 2014; Hwang et al., 2013; Jao et al., 2013; Varshney et al., 2015), we reasoned improving the consistency of CRISPR/Cas9-mediated mutagenesis could permit both routine screening for the null phenotype in F0 embryos and generation of heritable deletion mutations. At present, highest levels of mutagenesis are achieved by injection of eggs with in vitro-assembled ribonucleoproteins composed of a single guide RNA (sgRNA) complexed with Cas9 protein (sgRNPs) (Burger et al., 2016; Gagnon et al., 2014). We investigated whether use of alternate forms of guide RNAs might improve the consistency of Cas9 RNP activity in vivo.
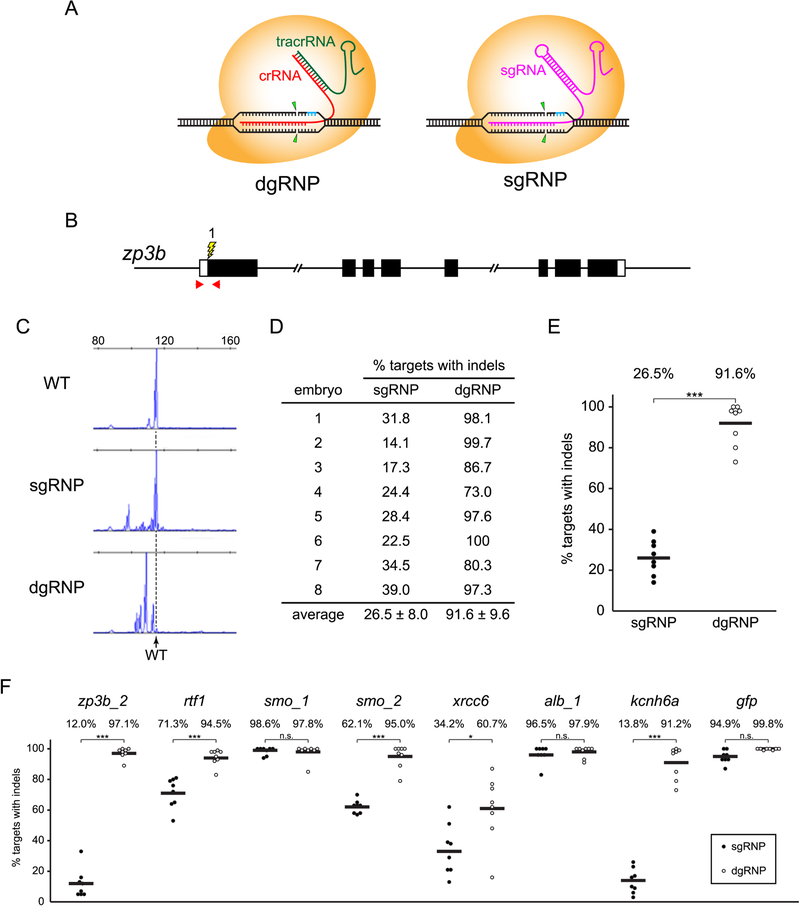
Figure 1A illustrates the differences between the two-RNA component (crRNA:tracrRNA) dgRNA and the more common sgRNA CRISPR/Cas9 RNP complexes. crRNA:tracrRNA RNP complexes are composed of the Cas9 protein and two separate chemically synthesized RNAs designed to mimic the crRNA and tracrRNA molecules that comprise the native form of the CRISPR/Cas9 system of S. pyogenes (Jinek et al., 2012). The synthetic RNAs can be chemically modified (eg. Alt-R, IDT) to enhance resistance to degradation by nucleases and evade stimulating innate immunity responses in some cells (Hendel et al., 2015; Schubert et al., 2018). In both the S. pyogenes and the synthetic crRNA:tracrRNA systems, the short crRNA perfectly complements the target site, providing target specificity, whereas the tracrRNA forms a duplex structure with the crRNA to provide a scaffold for RNP formation with Cas9 (Jiang and Doudna, 2017). The commonly used sgRNA developed by Jinek and colleagues (Jinek et al., 2012) derives from a fusion of the crRNA and tracrRNA sequences. As RNPs composed of Cas9 and either a sgRNA or a crRNA:tracrRNA duplex can be generated to target an identical genomic sequence, we compared the ability of each type of RNP complex to induce mutations in the zebrafish genome. Further, as sgRNA molecules are typically generated from in vitro transcription reactions that obligate the presence of Guanine nucleotides at the 5’ end of the transcripts, we tested whether the supernumerary G’s in some sgRNAs affect Cas9 RNP activity.
Figure 1.
Experimental approach to compare the mutagenesis activities of sgRNP and dgRNP complexes in zebrafish. (A) Schematic illustration of the CRISPR/Cas9 complexes used in this study. dgRNPs, designed to mimic the native form of the CRISPR/Cas9 complex found in S. pyogenes, consist of the Cas9 protein (orange) with two RNA molecules (crRNA, red, and tracrRNA, green) that form a duplexRNA. The crRNA provides target site specificity. crRNA and tracrRNA molecules were chemically synthesized, often with Alt-R (IDT) modifications to enhance resistance to host cell degradation. sgRNPs consist of the Cas9 protein (orange) with a single gRNA (magenta), designed as a fusion of the crRNA and tracrRNA sequences. Unless otherwise specified, sgRNAs used in this study were generated by in vitro transcription. Both gRNPs require PAM sequences (blue) adjacent to the target site and cleave target sequences similarly (arrowheads), allowing direct comparison of their activities. (B) Target zpb3b_1 (lightning bolt) lies in exon 1, close to the translation initiation site of the gene (boxes indicate exons, filled regions indicate coding sequences). Positions of primer pairs used to amplify the target sequence are indicated (red arrows). (C) Examples of capillary electrophoresis traces of the population of amplicons generated from the genomes of a 24 hpf uninjected WT embryo or embryos that had been injected with gRNPs targeting zpb3b_1. Dashed vertical line indicates mobility of the predominant WT peak. (D, E) Following injection of sgRNPs or dgRNPs into WT eggs, the percent loci with indel mutations was calculated for each embryo (D) and plotted (E). Independent injections yielded highly reproducible results. Statistical differences were calculated using an unpaired Student’s t-test (***p<0.001). (F) dgRNPs are consistently highly mutagenic and their activity exceeds that of sgRNPs at many sites. Fertilized eggs were injected with 1 nl 5μM sgRNP (filled circles) or 5μM dgRNP (open circles) (2.5μM gRNP was injected to target gfp), and the induction of indel mutations was measured by capillary electrophoresis as in (C). Statistical differences were calculated using an unpaired Student’s t-test (*, p<0.05; ***, p<0.001; n.s., not significant). See also Figures S1 and S2.
RESULTS
Determining the mutagenesis efficiency of sgRNA- and dgRNA-Cas9 RNP complexes
Initially the efficiency of mutation induction by RNPs generated with Alt-R-modified dgRNAs was compared with that of RNPs containing in vitro transcribed sgRNAs by targeting the first exon of zp3b (Figure 1B), a genomic site at which we had found sgRNPs induced an intermediate level of mutations. The cytoplasm of fertilized eggs was injected with 5μM sgRNP or 5μM dgRNP complexes targeting zp3b site 1 (zp3b_1, Table S1 lists the sequences of all sites targeted in this work). As zp3b is expressed specifically in developing oocytes (Onichtchouk et al., 2003), newly induced mutations were not expected to affect embryonic development. The extent of targeted mutagenesis at the zp3b locus was determined at 24 hours post fertilization (hpf). To estimate the fraction of targeted loci that harbored mutations in each embryo, genomic DNA was isolated from individual 24 hpf embryos, the targeted site was amplified using fluorescently labeled primers, amplicons were resolved by capillary electrophoresis (Figure 1C), and the fraction of amplicons larger or smaller than (WT), reflecting the presence of indel mutations at the targeted site in the genome, was determined (Carrington et al., 2015). This determination is likely to underestimate mutagenesis, as indel mutations may not alter the number of nucleotides at the locus and some indels may remove primer binding sites and thus avoid amplification. The percentage of zp3b loci that harbored indel mutations in individual embryos injected with either sgRNPs or dgRNPs is presented in Figures 1D and 1E. These results illustrate: i) for each gRNP type, there is remarkable consistency in the degree to which independently injected embryos are mutagenized; ii) dgRNPs induce mutations at almost all copies of a targeted locus in an injected embryo; and iii) dgRNPs can be more effective than sgRNPs.
Optimized conditions for mutagenesis with sgRNPs or duplexRNPs
Functional CRISPR/Cas9 RNP complexes were generated by incubating Cas9 protein with an equal molar amount of sgRNA or crRNA:tracrRNA duplex. As sgRNAs are derived from in vitro transcription reactions, a particular sgRNP might appear to have diminished activity as a consequence of incorrect estimation of sgRNA concentration or the inefficient formation of sgRNA-Cas9 RNPs. We tested whether insufficient RNA might have limited the activity of the sgRNPs in our experiments. RNP complexes targeting gfp sequences were generated by incubating sgRNA or dgRNA with Cas9 protein at molar ratios of 0.4:1, 1:1, or 2:1. Complexes were injected into eggs heterozygous for a single copy gfp reporter gene (see STAR Methods), and the percentage of mutagenized targets in the genomes of individual embryos was determined (Figure S1A). RNPs generated from 1:1 mixtures of sgRNA or dgRNA and Cas9 protein exhibited maximal activity; RNP activity was not enhanced nor diminished by incubation with a molar excess of RNA. Thus Cas9 is the factor limiting gRNP activity in our experiments, allowing valid comparison of the activities of similar amounts of sgRNPs and dgRNPs targeted to identical genomic sequences.
Experiments targeting the gfp reporter indicated the induction of mutations is dependent on the amount of active CRISPR/Cas9 RNP delivered to a zebrafish egg: injection of 2.5μM sgRNP or dgRNP complex yielded significantly more indel mutations than did solutions of 1μM gRNPs (Figure S1A). To determine the amount of injected gRNP required to obtain maximal mutagenesis, embryos were injected with approximately 1 nl solution containing 2.5, 5, or 10μM gRNP targeting the kcnh6a or albino locus (Figures S1B and S1C). Little if any difference was observed in the degree of mutagenesis attained at each concentration. We adopted standard conditions in which 5μM gRNP is injected.
We tested whether embryos injected with 5μM sgRNP or dgRNP exhibited off-target toxic consequences of the CRISPR/Cas9 complexes. Injection of RNPs targeting two different coding sequences of the zp3b gene or intron 1 of the kcnh6a locus did not affect survival to the free-swimming swimbladder stage (Figure S2). Further, injection of sgRNPs or dgRNPs targeting kcnh6a did not induce elevated levels of apoptosis as measured by immunohistochemical staining for activated Caspase 3 (Figures S2B – S2E). In sum, under standard injection conditions, gRNPs can stimulate production of mutations at almost all copies of a targeted locus without causing abnormal cell death or affecting viability. These conditions were employed in all experiments described below, except where specifically noted.
dgRNPs are consistently highly mutagenic; 5’ supernumerary guanines can interfere with sgRNP activity
As the dgRNP targeting site 1 in the zp3b gene appeared significantly more effective at inducing indel mutations than its sgRNP counterpart (Figure 1E), we examined the relative mutagenesis activity of the two gRNP types at additional loci (Figure 1F). sgRNPs generated with in vitro-transcribed sgRNAs can be highly effective at inducing indel mutations at many but not all sites of the zebrafish genome (Burger et al., 2016; Gagnon et al., 2014; Jao et al., 2013; Varshney et al., 2015). Injection of fertilized eggs with sgRNPs produced developing embryos in which approximately 90% of the copies of targeted smo_1, alb_1, or gfp sequences harbored indel mutations. dgRNPs targeted to these sites were equally effective at inducing mutations. However at other sites, dgRNPs were far more effective than the sgRNPs. Consistent with previous reports indicating that in vitro-transcribed sgRNA cannot effectively mutagenize some targets in zebrafish (Thyme et al., 2016), we found sgRNPs directed at numerous target sequences (rtf_1, xrcc6, zp3b_2, smo_2, and kcnh6a) induced indel mutations at poor (<20% copies with mutations) or intermediate (<70% copies with mutations) levels. At each of these targets, dgRNPs were significantly more effective, inducing mutations in ~90% of the targeted loci at all but the xrcc6 locus.
The inefficiency of the sgRNPs did not reflect errors in design or production: the sequences of the zp3b_2 and kcnh6a genome targets and the sgRNA transcription templates were reconfirmed by Sanger sequencing, and independently generated sgRNPs once again had poor activity (data not shown). Moreover, injecting increased amounts of kcnh6a sgRNP did not significantly increase the induction of mutations (Figure S1B).
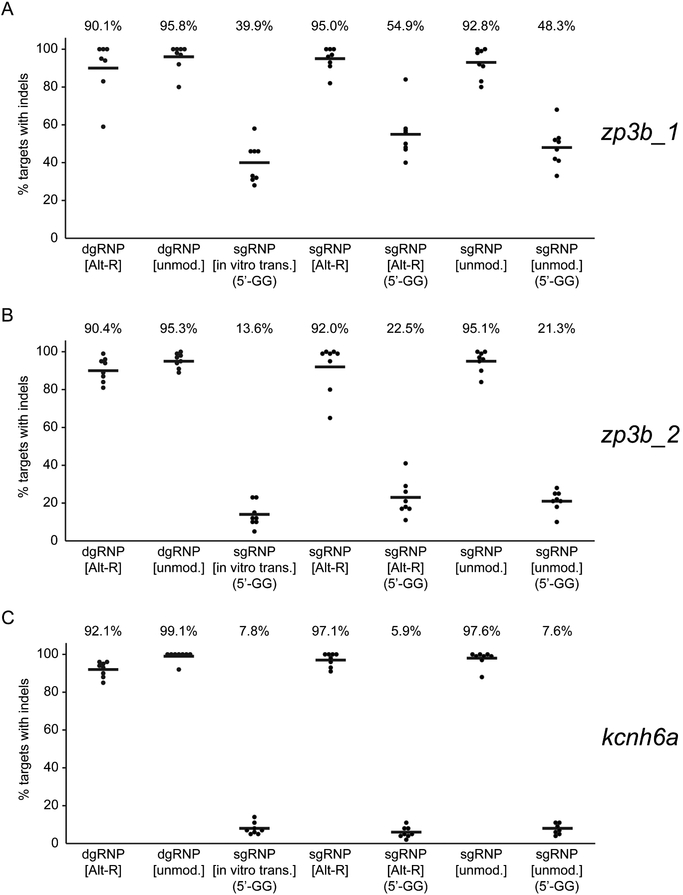
We considered several differences that might have accounted for the different behaviors of the two types of RNPs, noting i) the dgRNPs we used employed Alt-R modifications not present in the sgRNAs and ii) some of the sgRNAs contained extra 5’ end guanine nucleotides, which did not match the genome target, necessitated by use of a T7 promoter to initiate their transcription. Chemical modifications of gRNAs had been shown to enhance CRISPR/Cas9 activity in some systems (Hendel et al., 2015; Schubert et al., 2018), and the presence of extra guanine nucleotides at the 5’ ends of sgRNAs had been found clearly to correlate with reduced mutagenic activity in zebrafish (Varshney et al., 2015). We therefore compared the efficiency of mutagenesis of RNPs composed of i) standard in vitro transcribed sgRNAs, ii) chemically synthesized sgRNAs identical in sequence to the in vitro synthesized sgRNAs, with or without Alt-R modification, iii) chemically synthesized “ideal” (without supernumerary G’s) sgRNAs, with or without the Alt-R modificiation, and iv) dgRNAs with or without Alt-R modifications. The results at three different targets were wholly consistent (Figure 2): presence of extra guanines at the 5’ ends of gRNAs was the single predominant factor that diminished the mutagenesis activity of an RNP. All single and duplex guide RNPs with gRNAs whose 5’ ends were perfectly complementary to the protospacer target sequence were uniformly effective at inducing mutations. In contrast, the presence of supernumerary 5’ G’s in chemically synthesized or in vitro transcribed sgRNAs was detrimental to activity. Under conditions used here, chemical modification of gRNAs does not measurably enhance CRISPR/Cas9 mutagenicity in zebrafish.
Figure 2.
Supernumerary guanines can reduce sgRNP activity. To identify features of gRNA design and composition that affect gRNP activity, the mutagenic activities of a series of gRNPs targeting (A) zp3b_1, (B) zp3b_2, or (C) kcnh6a were compared. Chemically synthesized duplex guide RNAs were generated with unmodified (unmod.) or Alt-R-modified nucleotides. Single guide RNAs generated by in vitro transcription (in vitro trans.) contained two supernumerary guanine nucleotides (5’-GG) at the 5’ end of the transcript that were not complementary to the target site. Chemically synthesized sgRNAs that perfectly matched the protospacer target sequence or that contained two supernumerary guanine nucleotides (5’-GG) at their 5’ ends were generated with unmodified (unmod.) or Alt-R-modified nucleotides. One-cell embryos were injected with about 1 nl 5μM gRNPs and the induction of indel mutations was measured as in Figure 1. ‘Ideal’ gRNPs composed of gRNAs whose 5’ ends were perfectly complementary to the target site were uniformly highly mutagenic. Each type of gRNP that contained a gRNA with extra 5’ guanine nucleotides was significantly less active than the group of ‘ideal’ gRNPs (p<0.001, One-way ANOVA test).
Because shorter RNAs are easier to synthesize with fidelity, we have employed Alt-R duplex guide RNPs as standard in our experiments, except where explicitly noted. dgRNP activity stimulated production of mutations in almost all copies of a targeted locus at all but one (xrcc6) of the 23 different sites targeted in this study (Table S1).
Mutagenesis with duplexRNPs produces phenocopies of null mutants in F0 embryos
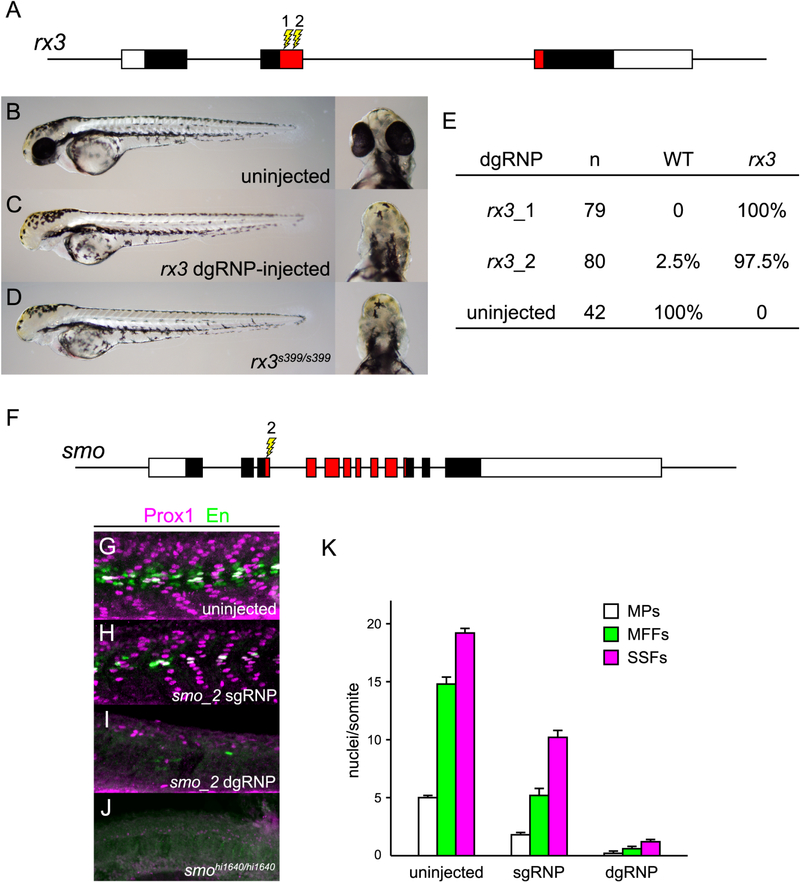
As dgRNP-injected embryos do not appear to exhibit non-specific developmental defects even though nearly all copies of targeted loci suffer mutations, we analyzed the extent to which injected F0 embryos resemble true mutants. rx3 encodes a paired-like homeodomain-containing transcription factor that functions cell autonomously in the developing anterior forebrain to specify eye fate (Stigloher et al., 2006). dgRNPs were designed to target sequences in exon 2 that encode the homeodomain (Figure 3A). Two day post fertilization (dpf) null mutant embryos exhibit a remarkably normal body plan but lack eyes (Figures 3B and 3D). All scorable eggs injected with dgRNPs targeting rx3_1 and 98% of those injected with dgRNPs targeting rx3_2 developed without eyes (Figures 3C and 3E). Targeting other loci also produced phenocopies of mutants. For example 80% of embryos injected with dgRNP targeting exon 1 of albino/slc45a2 (alb_1) lacked all evidence of pigmentation at 2 dpf (Figure S3).
Figure 3.
dgRNPs can produce accurate phenocopies of null mutants. (A–E) F0 embryos that develop from WT eggs injected with rx3 dgRNPs phenotypically resemble null rx3 mutants. (A) Two dgRNPs were designed to target sites (lightning bolts) within the region of rx3 exon 2 that encodes the DNA binding domain of the Rx3 transcription factor (red-shaded exon sequences). (B–E) Nearly all embryos injected with dgRNP complexes targeting either sites rx3_1 or rx3_2 lacked eyes and were indistinguishable from homozygous null mutants. (F–K) Injection of dgRNP complexes targeting smoothened (smo) produces F0 embryos that phenotypically resemble null smo mutants. (F) gRNPs were designed to target site smo_2 (lightning bolt) within the 5’ portion of the region that encodes the ‘Frizzled-domain’ of Smoothened (red-shaded exon sequences). (G–K) Development of three distinguishable types of muscle fibers is dependent on Sonic hedgehog signaling and Smoothened activity. Nuclei of Slow Muscle Pioneer Cells (MPs) express both the Prox1 and Engrailed (En) transcription factors, whereas Medial Fast Fibers (MFFs) only express En, and Superficial Slow Fibers (SSFs) only express Prox1. Shh-dependent muscle fiber nuclei, marked by Prox1 (magenta) and/or En (green) expression, were visualized following immunostaining of (G) uninjected, (H) smo_2 sgRNP-injected, or (I) smo_2 dgRNP-injected WT embryos, or (J) smohi1640 null mutant embryos. (K) The presence of Shh-dependent muscle fibers was determined by analysis of the nuclear staining in a standard set of five trunk somites in each embryo. The average numbers of Shh-dependent muscle fiber nuclei per somite for each embryo is plotted (uninjected (n=6), smo_2 sgRNP-injected (n=5), smo_2 dgRNP-injected (n=6)). See also Figure S3.
Patterning defects associated with some developmental mutants can be obscured if loss of gene function is induced in a mosaic fashion. To determine whether dgRNPs could be used to generate an accurate phenocopy of a complex developmental mutant, we targeted smoothened (smo), whose product is a cell-autonomous activator of Sonic hedgehog (Shh) signaling (Lee et al., 2016) (Figure 3). The specification and development of three types of somitic muscle cells, slow muscle pioneer cells, superficial slow fibers, and medial fast fibers, are dependent on different levels of Shh signaling (Figures 3G and 3K) (Wolff et al., 2003); smo null mutants lack all three types of cells (Figure 3J). Mosaic loss of smo function can be detected readily, as illustrated by incomplete loss of the three cell types present in F0 embryos injected with sgRNPs targeting smo exon 3 (site 2) (Figures 3H and 3K). In contrast, targeting the smo_2 site with dgRNPs produced embryos that uniformly resembled true null smo mutants and were nearly devoid of Shh-dependent muscle cells (Figures 3I, 3K).
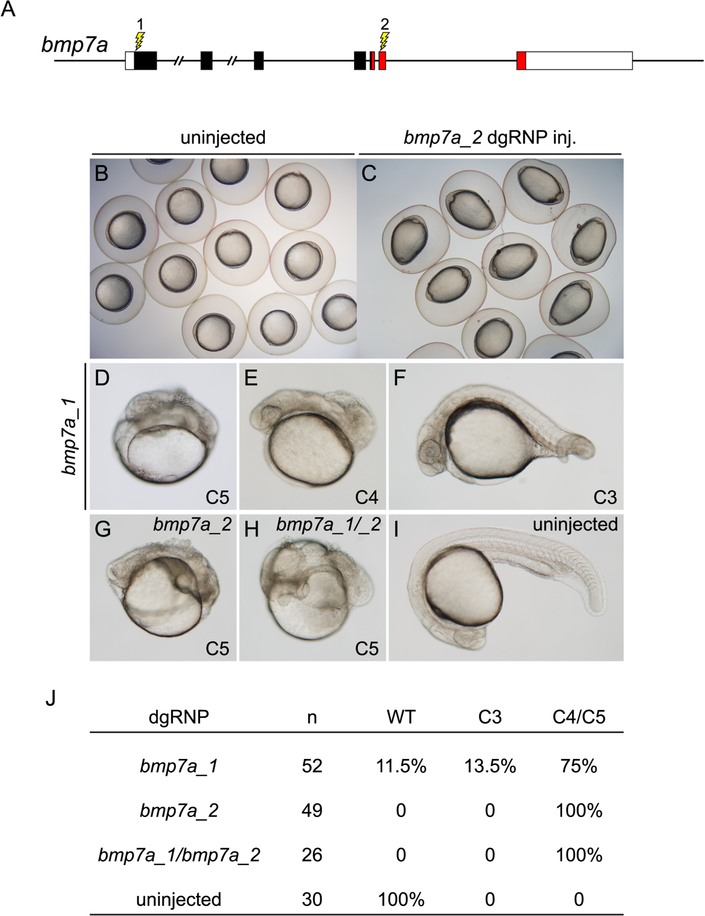
Detection of a cell non-autonomous patterning phenotype is particularly challenging in embryos that are mosaic for loss of a diffusible signaling factor. BMPs are diffusible factors that pattern dorsoventral tissue development in the early zebrafish (Hammerschmidt and Mullins, 2002; Kondo, 2007; Zinski et al., 2017). As even intermediate levels of exposure to ligand promote tissue differentiation (Schumacher et al., 2011), partial depletion of BMP production in the embryo results in embryos with intermediate patterning defects. dgRNPs were used to target sequences encoding the N-terminal signal sequence (bmp7a_1) or the diffusible ligand portion (bmp7a_2) of BMP7a (Figure 4A), whose loss of function results in severely dorsalized embryos (Dick et al., 2000; Schmid et al., 2000). Tailbud stage embryos injected with either RNP exhibited an abnormal ovoid shape distinctive of BMP signaling mutants (Mullins et al., 1996; Schmid et al., 2000) (Figures 4B and 4C). Among embryos that survived to 24 hpf, the vast majority appeared very strongly dorsalized (Figures 4D – 4J), closely resembling true bmp7a mutants (Dick et al., 2000; Schmid et al., 2000). The severity of dorsalization indicates that few WT BMP-secreting cells remained in the F0 embryos.
Figure 4.
Cell non-autonomous phenotypes can be recapitulated accurately in F0 embryos. (A) dgRNP complexes were designed to target sequences in bmp7a that either encoded the N-terminal signal sequence (bmp7a_1) or the mature ligand domain (red sequences, bmp7a_2) of Bmp7a. (B, C) At the tailbud stage, all embryos injected with bmp7a_2 dgRNP targeting the ligand domain displayed a distinctive ovoid morphology typical of severely dorsalized embryos (C) that is not seen in WT embryos (B). (D – J) Targeting of bmp7a produced dorsalized 24 hpf F0 embryos. Embryos were scored for dorsalized phenotypes as in (Mullins et al., 1996), where C3 indicates intermediate dorsalization and C4 and C5 are considered severe phenotypes. Targeting the sequences encoding the N-terminus of Bmp7a generated dorsalized embryos of varying severity (D – F, J). Targeting sequences encoding the ligand domain, or targeting both sequences simultaneously (bmp7a_1/bmp7a_2) yielded only severely dorsalized embryos (G, H, and J).
The usefulness of dgRNPs as reagents to screen gene functions in F0 embryos requires that their injection causes minimal disruption to normal development. Embryos injected with dgRNPs were inspected for evidence of developmental anomalies that were not associated with the function of the targeted gene. Embryos injected with a range of concentrations of dgRNPs targeting 13 different sites were examined at 24 hpf for expression of wildtype or expected mutant phenotypes and for expression of any evidence of unrelated aberrant development, necrosis, or inviability (Table S2). Under conditions in which virtually all copies of targeted genes were mutagenized and the vast majority of injected embryos exhibited anticipated mutant phenotypes, on average only 7% of the injected embryos failed to develop or exhibited some unexpected morphological defect at 24 hpf. A consistent pattern of defects was never observed. It appears that dgRNPs rarely have off-target effects with phenotypic consequences in the zebrafish, and any of the dgRNPs used in this study can serve as a control in future screens for background developmental effects due simply to the injection of dgRNPs.
Detection of redundant gene interactions in F0 embryos
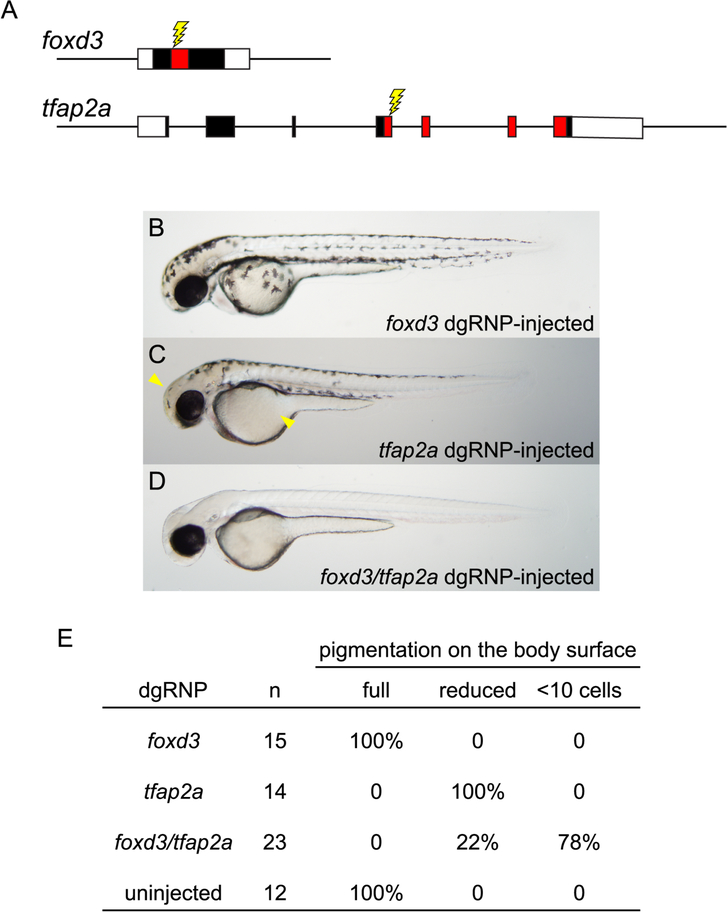
Given that dgRNPs have very high mutagenic activity in the absence of discernible non-specific effects, we reasoned they could be used effectively to target multiple genes in an embryo. As most cells of dgRNP-injected embryos were bi-allelic for induced mutations, we tested whether these reagents could be used to inactivate two genes simultaneously, allowing us to uncover in F0 embryos developmental phenotypes that result only from the combined loss of both genes. foxd3 and tfap2a encode transcription factors that contribute to the specification of the neural crest (Simoes-Costa and Bronner, 2015). Loss of either gene partially compromises neural crest development; only upon simultaneous loss of both gene functions are neural crest-derived pigmented melanophores completely eliminated (Arduini et al., 2009). As reported in previous loss-of-function studies, we found that targeting of foxd3 had no discernible effect on pigment cell development, and targeting of tfap2a resulted in only a mild reduction of melanophores (Figures 5A – 5C, and 5E). In stark contrast, 78% of the F0 embryos that were injected with dgRNPs targeting the two genes simultaneously developed completely devoid of pigmented melanophores (Figures 5D and 5E). As melanophores arise from a large number of neural crest progenitors (Pavan and Raible, 2012), complete loss of pigment in doubly targeted embryos indicates the vast majority of cells in F0 embryos had bi-allelic mutations inactivating both foxd3 and tfap2a.
Figure 5.
Redundant gene functions can be uncovered by targeting two genes simultaneously with dgRNPs. (A) dgRNPs were designed to target sequences (lightning bolts) encoding the DNA binding domains (red-shaded exon sequences) of the transcription factor products of the foxd3 and tfap2a genes. (B – E) Embryos targeted with either foxd3 dgRNPs or tfap2a dgRNPs resemble single mutants: embryos targeted with foxd3 dgRNPs appear morphologically near-normal at 2 dpf, and embryos targeted with tfap2a dgRNPs have moderately reduced pigmentation most notable on the head and yolk (arrowheads). Three-fourths of the doubly injected embryos were devoid of melanophores at 2 dpf, resembling true foxd3;tfap2a double mutants (Arduini et al., 2009). The remaining doubly-injected embryos have markedly reduced pigmentation, consistent with incomplete loss of function of the targeted genes.
Efficient generation of deletion mutations with dgRNPs
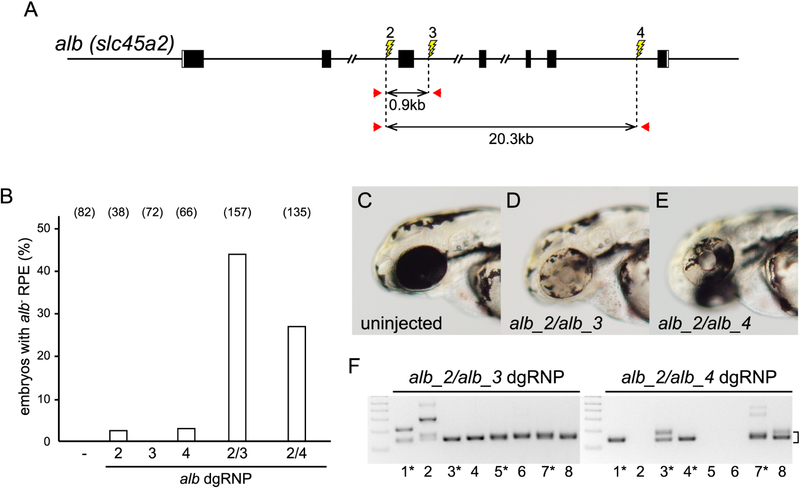
Targeting of two linked sites with TALENs or CRISPR/Cas9 complexes had been shown previously to be capable of inducing deletion mutations, but the recovery of such lesions can be inefficient (Gupta et al., 2013; Lim et al., 2013; Ota et al., 2014; Varshney et al., 2015). Given the very high frequency with which dgRNPs induced mutations, we reasoned pairs of dgRNPs targeting a single chromosome might simultaneously generate DSBs that would be repaired to generate deletion mutations. We developed a test to determine whether deletion mutations could be induced readily in somatic tissue. Combinations of albino introns were targeted (Figure 6A) with the expectation that small indel mutations resulting from the activities of individual RNPs would likely be confined to an individual intron, not affect gene function, and not produce cells with pigmentless phenotype. In contrast, deletion mutations that removed exons would abolish gene function. The induction of mutant albino cells can be readily detected in the retinal pigment epithelium (RPE) by their lack of pigmentation in 2 dpf embryos. However, as loss-of-function albino mutations are recessive, only cells with bi-allelic deletions would be recognized phenotypically. About 2% of the embryos injected with a single dgRNP targeting intron 2 (site 2), intron 3 (site 3), or intron 6 (site 4) appeared to have a small clone of pigmentless cells, indicating that targeting a single site does not generally lead to production of large deletion mutations (Figure 6B). In contrast, mutant clones in the RPE were observed in about 40% of the embryos injected with the pair of dgRNPs targeting sites 1 kbp apart in introns 2 and 3 and 25% of embryos injected with the dgRNPs targeting sites in introns 2 and 6 separated by 21 kbp (Figure 6B). The vast majority of these doubly targeted embryos contained patches of pigmentless tissue representing over half the RPE (Figures 6C – 6E), a result most consistent with the induction of mutations at early cleavage stages. Using primer pairs that bordered the targeted sites, deletion mutations of the expected size could be detected only in the genomes of embryos that had been injected with pairs of dgRNPs (Figures 6A, 6F, and data not shown). Sequence analysis of the deletion mutations present in the soma of 4 dpf embryos injected with pairs of dgRNPs targeting introns revealed that mutations were near-perfect joinings of sequences distal to the targeted sites (Figure S4). Each mutation contained a distinct deletion junction, consistent with the interpretation that generation of small indel mutations accompanied the joining of the chromosome regions flanking the induced DSBs.
Figure 6.
Deletion mutations are readily generated by simultaneously targeting two sites on a chromosome. (A) dgRNPs were designed to target sites (lightning bolts) in intron 2 (alb_2), intron 3 (alb_3), or intron 6 (alb_4). The approximate distances between target sites, and the locations of primer pairs (red arrows) used to detect deletion mutations that result in joining of target sites, are indicated. (B) Uninjected embryos (−) or embryos injected with individual or pairs of dgRNPs targeting alb locus introns were inspected for pigmentless patches of tissue in the retinal pigmented epithelium (RPE) at 2–3 dpf. The incidence of embryos with alb mutant tissue is plotted (numbers of scored embryos are indicated). (C – E) Embryos injected with dgRNP pairs (D, E) often exhibited large clones of pigmentless mutant tissue not seen in the eyes of uninjected embryos (C). (F) Deletion mutations were detected in the genomes of F0 embryos injected with pairs of dgRNPs by PCR-amplification with primer pairs flanking the targeted sites. Junction fragment amplicons of the expected size (bracket) were detected in eight of eight 4 dpf embryos that had been injected simultaneously with alb_2 and alb_3 dgRNPs and in five of eight 4 dpf embryos injected with alb_2 and alb_4 dgRNPs. Junction fragments recovered from starred embryos were sequenced and reported in Figure S4. See also Figures S4 – S7.
Deletion mutations of 0.5 to 50 kbp can be readily induced at an array of loci, including alb, smo, bmp7a, egfl7, zp3b, and megf6b (Figures 6 and 7, and Figures S5 – S7). Analysis of DNA from embryos injected with dgRNPs targeting two linked sites indicated presence of junction fragments that brought together sequences distal to each target site and that also contained small indel mutations at the joining site (Figure S5). Deletions could be induced using any type of RNP that contained gRNA sequences that perfectly matched the protospacer target sequence (Figure S6). To determine whether large deletions could be induced and stably transmitted through the germ line, we targeted sites 52 kbp apart in exons 2 and 31 of megf6b (Figure S7). Analysis of genomic DNA of embryos injected with the dgRNP pair indicated each embryo harbored deletion mutations that joined exons 2 and 31. Injected embryos were raised to adulthood and the germlines of 16 randomly selected F0 male adults were analyzed for transmission of newly induced megf6b deletion mutations. Four of the F0 adults transmitted a total of five distinct mutations. The heritable deletion mutations appeared to result from the simple joining of sequences distal to the targeted sites, accompanied by small indel sequence changes affecting 5 to 33 bases. Two of the deletion mutations were selected at random and further propagated. Each mutation was found to segregate as a simple loss-of-function mutation affecting a single locus, indicating simple deficiencies can be readily recovered.
Figure 7.
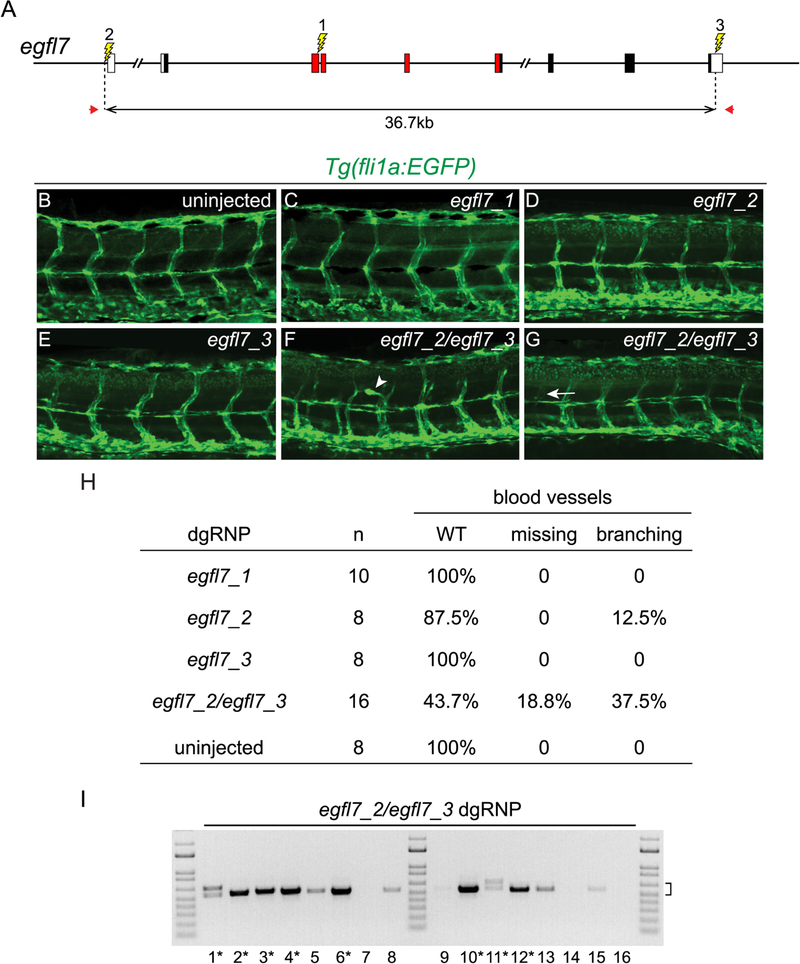
Deletion mutations uncover gene functions in F0 embryos. (A) The gene structure of egfl7 is indicated. Red regions encode conserved domains of EGFL7, including an EMI (EMILIN) domain, an EGF domain, and a Ca-binding EGF-like domain. dgRNPs designed to target sites (lightning bolts) in exon 3 (egfl7_1), just upstream of the transcription start site (egfl7_2), or in the 3’UTR (egfl7_3) of egfl7. (B – H) Embryo carrying the fli1a:EGFP reporter transgene were scored for morphology of intersegmental vessels after injection with individual egfl7 dgRNPs or a combination of egfl7_2 and egfl7_3 dgRNPs. Embryos injected with individual dgRNPs (C – E) exhibited WT morphology (B), with the exception of a single embryo in which the promoter region had been targeted (H). Embryos targeted simultaneously at the 5’ and 3’ ends of egfl7 often had vasculature defects (F – H). (I) Genomic DNA from embryos targeted simultaneously at the 5’ and 3’ ends of egfl7 was amplified with primers (A, red arrows) flanking the egfl7_2 and egfl7_3 sites, revealing junction fragments whose size (bracket) was consistent with the presence of induced deletion mutations. Junction fragments recovered from starred embryos were sequenced and reported in Figure S5.
Intragenic deletion mutations are useful for uncovering gene function
In some instances the phenotype associated with loss-of-function of a gene is best revealed by examining cells that are bi-allelic for deletion mutations that remove the gene. The presence of premature termination codon (PTC) mutations in some genes, as might be generated upon repair of DSBs, can lead to the nonsense-mediated decay-dependent compensatory transcription of related genes (El-Brolosy et al., 2019; Ma et al., 2019). Upregulated expression of the related genes can mask the functional consequence of loss of the mutant gene. As RNA degradation products of the mutant mRNA stimulate the compensatory transcription, mutations that block transcript production fail to promote the compensatory response.
Whereas embryos that fail to produce egfl7 transcripts have vasculature defects, embryos with PTC mutations in egfl7 have WT vasculature as a consequence of upregulated expression of emilin genes (Rossi et al., 2015). We asked whether the induction of deletion mutations in F0 embryos could be used to reveal the phenotype associated with loss of egfl7. To measure effects on vasculature development, transgenic embryos expressing the fli1a:EGFP reporter (Lawson and Weinstein, 2002) were targeted, and their intersegmental vessels were analyzed at 2 dpf. Three sites within the egfl7 locus were targeted with dgRNPs: essential coding sequences in exon 3 (egfl7_1), presumptive promoter sequences lying just upstream of the transcription start site (TSS) (egfl7_2), or sequences in the non-coding 3’UTR (egfl7_3) (Figure 7). Each dgRNP effectively mutagenized its target in vivo (data not shown). Consistent with previous findings that frame-shift mutations in exon 3 stimulated transcription compensation so that embryos homozygous for such mutations appeared WT (Rossi et al., 2015), we found that targeting exon 3 with dgRNPs had no discernible phenotypic effect (Figures 7C and 7H). Targeting just upstream of the TSS occasionally produced embryos with aberrant vessels (Figures 7D and 7H) and targeting 3’ UTR sequences did not appear to affect vasculature (Figures 7E and 7H). In contrast, simultaneous targeting of the 5’ and 3’ ends of the gene produced egfl7 deletion mutations in the developing embryos (Figure 7I), over half of which had missing or aberrantly branched vessels (Figures 7F – 7H, and Figure S5). We conclude the generation of deletion mutations with dgRNPs is sufficiently efficient that cell-autonomous functions of genes linked to compensatory networks can some times be probed in F0 embryos. The efficiency with which induced deletions can be used to screen gene functions in F0 embryos will depend on the fraction of tissue that harbors bi-allelic deletion mutations and the ease with which the relevant mutant phenotype can be detected in subsets of cells.
DISCUSSION
Work described here significantly expands the versatility of the CRISPR/Cas9 system for analyses of gene function in the zebrafish. Previous studies have shown RNPs composed of Cas9 and canonical single guide RNAs generated by in vitro transcription are generally effective at inducing mutations in the zebrafish, but their efficiency is highly variable, and some sgRNPs have been found to lack mutagenic activity (Burger et al., 2016; Gagnon et al., 2014; Thyme et al., 2016). These features have limited their application as a tool for conducting rapid preliminary screens of gene function in F0 embryos and for generating heritable deletion mutations needed to ascertain the null state (Wu et al., 2018). In contrast, RNPs composed of Cas9 protein complexed with a duplex guide RNA generated from chemically synthesized crRNA and tracrRNA molecules can be used to initiate targeted double strand breaks (DSBs) and subsequent repair events with extremely high efficiency and consistency. Duplex guide RNPs are uniformly highly mutagenic, such that following injection of dgRNPs into the cytoplasm of a fertilized egg, almost every cell of the developing embryo is bi-allelic for newly induced indel mutations. We suspect no locus will be resistant to the activity of dgRNPs during the early cleavage stages of zebrafish development.
Our work demonstrates the key to generating an effective gRNP in zebrafish is that the 5’ end of its gRNA must provide an exact match to the protospacer target sequence. A previous study that analyzed the efficiency of a very large number of sgRNAs had noted a highly significant correlation between the presence of supernumerary 5’ guanines and reduced mutagenic efficiency (Varshney et al., 2015). In our studies, the presence of one or two unpaired supernumerary guanines at the 5’ end of sgRNAs, as are often introduced when generating sgRNAs by in vitro transcription, significantly diminished gRNP activity in all cases (Figures 1 and 2 and Table S1). We found that ideally designed synthetic single or duplex guide RNAs, whether Alt-R chemically modified to enhance RNase-resistance or not, were similarly very active at inducing mutations. Because of the ease of synthesizing the short crRNA required for dgRNAs, we adopted use of the Alt-R dgRNPs as standard conditions. dgRNPs equaled or surpassed the activity of standard in vitro transcribed sgRNPs at every site, and dgRNPs were mutagenic at sites that were only ineffectively targeted by cognate sgRNPs. The efficacy of the dgRNPs makes them exceptionally valuable as a tool that can applied in response to two emerging needs in the zebrafish field: i) rapid functional testing of candidate genes as might be identified in RNA sequencing analyses, and ii) definition of the null state.
Recent advances in transcriptome analyses have identified genes whose expression patterns correlate intriguingly with cell differentiation events and thus warrant functional analyses (Lukoseviciute et al., 2018; Lush et al., 2019; Raj et al., 2018). Studies presented here demonstrate that dgRNPs can be used to screen rapidly and accurately for and uncover the effects of complete ablation of gene function. In general, we targeted sequences encoding known functional or highly conserved domains of proteins, with the expectation that even in-frame mutations might interfere frequently with WT function. Injection of WT fertilized eggs with dgRNPs targeting a gene of interest routinely produced F0 embryos whose development recapitulated that of true null mutants with remarkable fidelity. Complex patterning phenotypes, reflecting the cumulative gene function in a large population of precursor cells, such as seen in smoothened mutants, can be readily mimicked with dgRNPs in F0 embryos. Mosaicism is sufficiently low that targeting of bmp7a, which encodes a signaling ligand that patterns the early embryo, yielded F0 embryos with severe phenotypes that mimicked true null bmp7a mutants with remarkable fidelity. Mutant cells are induced very early in development so that loss-of-function defects can be detected even in gastrula stage embryos. The efficiency of mutagenesis is sufficient to enable informative probing of gene interactions in F0 embryos, as indicated by our analyses of the consequences of simultaneously targeting tfap2a and foxd3.
Finally, there is a compelling need for deletion mutations to help determine the consequences associated with simple loss of function of a gene of interest. Genome sequence analyses of human populations (Amorim et al., 2017; Flanigan et al., 2011; Narasimhan et al., 2016) and study of the effects of sequence variants induced with programmable nucleases (Amorim et al., 2017; Anderson et al., 2017; El-Brolosy and Stainier, 2017; Lalonde et al., 2017; Rossi et al., 2015) have together revealed that we have an incomplete understanding of the genome sequence changes that produce the null condition. What appear to be premature translation termination signals in coding sequences expected to produce simple loss-of-function alleles (Popp and Maquat, 2016), instead can trigger novel transcript splicing events or compensatory expression of related genes that obscure the null phenotype (Anderson et al., 2017; El-Brolosy et al., 2019; Lalonde et al., 2017; Ma et al., 2019; Mou et al., 2017). Deletion alleles can likely circumvent the problems that are sometimes associated with simple point mutations.
The methods described here provide a simple and efficient method for inducing deletion mutations. Injection of zebrafish eggs with pairs of dgRNPs can be used to produce F0 embryos with substantial amounts of tissue composed of cells bi-allelic for newly induced deletions. These genetically mosaic F0 embryos can be useful, especially as part of an initial screen, for detecting the phenotypic consequences of complete loss of function of genes in the absence of transcription compensation. Heritable deletion mutations of at least 50 kbp can be readily recovered from the germlines of mutagenized fish. We surmise the activity of individual dgRNPs is sufficiently high that frequently two sites on a shared chromosome can be cleaved simultaneously, stimulating repair events that join regions distal to the targeted sites. We predict, but have not determined, that additional chromosome rearrangements such as inversions and translocations can be similarly induced.
Limitations
Because the mutation rate of dgRNPs is so high, targeting of essential genes will often yield inviable embryos, thus making it difficult to recover and propagate induced mutations. As the induction of mutations is sensitive to the amount of dgRNPs that is injected (Figure S1), injection of reduced concentrations of dgRNPs or injection of a combination of mRNA encoding Cas9 along with duplex guide RNAs can be used to introduce mutations in a mosaic fashion allowing their recovery in the F1 generation.
Because it is so easy to induce heritable mutations, the transient elimination of gene function in F0 embryos should not be used for definitive characterizations of gene function. F0 screens are most appropriate for generating hypotheses regarding the potential function of a gene of interest. Analysis of mutants that harbor heritable null mutations will be needed to test and confirm the gene function postulated on the basis of the F0 screen.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, David Grunwald (grunwald@genetics.utah.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Zebrafish Strains
Zebrafish Danio rerio were maintained in accordance with approved institutional protocols at the University of Utah. Adult zebrafish were maintained under standard conditions (Westerfield, 2000) and kept on a light-dark cycle of 14 hours in light and 10 hours in dark at 27°C. AB and Tu strains were used as WT zebrafish. Zebrafish carrying the rx3s399 (Loosli et al., 2003) or smohi1640 (Chen et al., 2001) null mutations were obtained from Kristen Kwan, U of Utah. Zebrafish used in this study that carried a single copy of gfp were heterozygous for the kcnh6az46 allele. The kcnh6az46 allele, called kcnh6aloxP in (Hoshijima et al., 2016), contains an α-crystallin::Venus reporter gene embedded in intron 6 of kcnh6a. The reporter gene drives expression of GFP in the lens.
Zebrafish Embryo Culture
Embryos from natural spawnings were generated and collected as described (Westerfield, 2000). Live embryos were maintained at 28°C. Devel opmental staging was based on (Kimmel et al., 1995). Reporting of sex is not included as dgRNPs were injected into eggs and consequences were analyzed in embryos prior to the time sex can be distinguished. Uninjected clutch mates were used as controls.
METHOD DETAILS
gRNA target site design
Potential gRNA target sites were identified using the web programs CRISPR Design (http://CRISPR.mit.edu) or CHOPCHOP (http://chopchop.cbu.uib.no/index.php). gRNA target sequences are listed in Table S1. Genomic DNA sequences retrieved from Ensembl GRCz10 or z11 (http://uswest.ensembl.org/Danio_rerio/Info/Index) were used for the target site searches. Target sequences were chosen that had no related potential off-target sites in the genome with fewer than 3bp mismatches. To generate loss-of-function mutations, sequences that encoded conserved domains of known function were selected as targets. Loci to be targeted were amplified from genomic DNA prepared from fin clips and then sequenced. Only adults carrying verified target sequences were mated to produce eggs for genome editing experiments.
Generation of gRNAs
sgRNA was prepared by in vitro transcription of double-stranded deoxyoligonucleotide templates as described in (Gagnon et al., 2014) with modifications. Target-specific forward oligonucleotides, GAAATTAATACGACTCACTATA(G/G)N20GTTTTAGAGCTAGAAATAGC, (N20: gRNA target sequence, (G/G): added one or two G bases in cases where N20 gRNA target sequences lack GG at the 5’-end,), were synthesized, column-purified, and dissolved in distilled water as 100 μM stock solution. The common reverse oligonucleotide AAAAGCACCGACTCGGTGCCACTTTTTCAAGTTGATAACGGACTAGCCTTATTTTAACTTGC TATTTCTAGCTCTAAAAC was synthesized, purified by HPLC, and dissolved in distilled water as 100 μM stock solution. Ten microliters each of the forward and reverse oligonucleotides were mixed in NEB buffer #2.1 (New England Biolabs (NEB): 10 mM Tris-HCl (pH 7.9), 50 mM NaCl, 10 mM MgCl2, 0.1 mg/ml BSA) and annealed by heating followed by gradual cooling: 95°C, 5 min; cool to 85°C at 2°C/sec; 85°C, 1 min; cool to 25°C at 0.1°C/sec; 25°C, 5 min; cool to 4°C. The 3’-ends of the annealed double-stranded oligonucleotides were filled in by incubation at 12°C, 20 min in the presence of 1.25 mM each dNTP a nd 0.15 U/ul T4 DNA polymerase (NEB). Oligonucleotides were then purified using E.Z.N.A. Cycle Pure Kit (Omega Bio-Tek) and recovered in distilled water. sgRNA was transcribed from the annealed double-stranded oligonucleotide template using MEGAshortscript™ T7 transcription Kit (Invitrogen) according to the manufacturer’s instructions: 1× T7 reaction buffer, 1× T7 enzyme mix, 7.5 mM each dNTP, and 1.5 – 2.5 ug template oligonucleotide in 40 ul reaction volume incubated at 37°C, 20 h, followed by template digestion with 0.2 U/ul TURBO DNase at 37°C, 20 min. Transcribed sgRNA was purified using RNA clean & concentrator-25 (ZYMO Research) and recovered in distilled water.
Chemically synthesized Alt-R®-modified or unmodified crRNAs, tracrRNAs, or sgRNAs were obtained from Integrated DNA Technologies (IDT).
crRNA:tracrRNA duplex preparation
Target-specific Alt-R® crRNA and common Alt-R® tracrRNA were synthesized by IDT and each RNA was dissolved in duplex buffer (IDT) as 100μM stock solution. Stock solutions were stored at −20°C. To prepare the crRNA:tracrRNA duplex, equ al volumes of 100μM Alt-R® crRNA and 100μM Alt-R® tracrRNA stock solutions were mixed together and annealed by heating followed by gradual cooling in a PCR machine: 95°C, 5 min; c ool at 0.1°C/sec to 25°C; 25°C, 5 min; cool to 4°C rapidly. The 50 μM crRNA:tracrRNA duplex stock solution was stored at −20°C.
Preparation of gRNA:Cas9 RNP complexes
Cas9 protein (Alt-R® S.p. Cas9 nuclease, v.3, IDT) was adjusted to 25μM stock solution in 20mM HEPES-NaOH (pH 7.5), 350mM KCl, 20% glycerol, dispensed as 8 ul aliquots, and stored at −80°C. 25 μM crRNA:tracrRNA duplex was produced by mixing equal volumes of 50μM crRNA:tracrRNA duplex stock and duplex buffer (IDT). We typically use 5μM RNP complex. To generate 5μM crRNA:tracrRNA:Cas9 RNP or sgRNA:Cas9 RNP complexes: 1 ul 25μM crRNA:tracrRNA duplex or 1 ul 25μM sgRNA was gently mixed with 1 ul 25μM Cas9 stock, 2 ul H2O, and 1ul 0.25% phenol red solution. Prior to microinjection, the RNP complex solution was incubated at 37°C, 5 min and then placed at room temperature. Approximately one nanoliter of 5μM gRNA:Cas9 RNP complex was injected into the cytoplasm of one-cell stage embryos.
Detection of indel mutations
Genomic DNA was extracted from individual embryos at 1 dpf, 2 dpf or 4 dpf: dechorionated embryos were incubated in 30 ul (1 dpf- or 2 dpf-embryo) or 50 ul (4 dpf-embryo) 50 mM NaOH at 95°C, 20 min. After cooling to 4°C, 1/10 volume of 1 M Tris-HCl (pH 8.0) was added to neutralize. Genome sequences containing CRISPR/Cas9 target sites were amplified with pairs of primers listed in Table S3. For fragment analysis by capillary electrophoresis, each forward primer was labeled with 6-carboxyfluorescein (6-FAM, IDT) at the 5’ end. The reaction mixture was assembled in 10 ul using KAPA2G Fast HotStart PCR Kit (KAPA Biosystems): 1× KAPA2G buffer A, 200 μM each dNTP, 500 nM each of forward and reverse primers, 0.05 U/ul KAPA2G Fast HotStart DNA polymerase, and 1ul genomic DNA. Amplification reaction consisted of initial denaturation at 95°C, 3 min, followed by 35 cycles (95°C, 10 s; 66 – 68°C, 10 s; 72°C, 1 s) and final extension at 72°C, 5min. The amplified fragme nts were diluted 20-fold with distilled water. Two microliters diluted amplicons was mixed with 5 volumes Hi-Di™ formamide (ThermoFisher) and 1/4 volume of GeneScan™ 500 LIZ™ dye size standard (ThermoFisher), heat-denatured at 95°C, 5 min followed by chilling on ice, 5 min, and run on Applied Biosystems® 3730 DNA analyzer (Applied Biosystems). Collected data were analyzed with GeneMapper Software v4.0 (Applied Biosystems), which digitized fluorescent intensities to 0 ~ 30,000 at each fragment size.
Detection of deletion mutations
To detect deletions that removed exon 3 of the albino locus and brought together target sites alb_2 and alb_3, genomic DNA of each embryo was digested with AluI, which cleaves exon 3, and then PCR-amplified with primers that flanked target sites 2 and 3 (Figures 6 and S4). Deletions that brought target sites alb_2 and alb_4 close together were detected following PCR-amplification with primers that flanked those sites (Figures 6 and S4). Similarly, at all other loci, deletion mutations were detected by PCR-amplification with primers that flanked distal sites, as indicated in Figures 7, S5, S6, and S7. Primer sequences are listed in Table S4.
Visualizing vasculature in injected embryos
egfl7 dgRNPs were injected into Tg(fli1a:EGFP) (Lawson and Weinstein, 2002) one-cell embryos. At 48 hpf, embryos were fixed lightly in 4% paraformaldehyde in the dark for 1 hour, washed in PTw (PBS with 0.1% Tween-20), and transferred to 75% glycerol in PTw. Heads and yolks were removed, and the remaining trunks with tails were mounted on slides. Confocal z-stacks were acquired at 4 μm intervals for a total of ~40 μm.
Immunohistochemistry and in situ hybridization
Embryos were fixed with fresh 4% paraformaldehyde in PBS at room temperature for 2 hours. 26 hpf embryos were analyzed to detect muscle antigens; 24 hpf embryos were analyzed to detect activated Caspase 3. Fixed embryos were dehydrated in methanol and stored at −20°C until processing for immunohistochemistry according to standard procedures (Westerfield, 2000). In brief, embryos were rehydrated into PTw, incubated 7 minutes in acetone at −20°C, washed in water, then PTw, and then incubated in blocking agent (10% heat-inactivated sheep serum, 2mg/mL BSA, 1% DMSO, 0.1% TritonX-100 in PBS) for at least 1 hour at room temperature. Acetone incubation was omitted in the processing of embryos to detect activated Caspase 3. Embryos were incubated in primary antibodies diluted in blocking agent overnight at 4°C. Primary antibodies were removed and embryos we re washed extensively with PBDT (2 mg/mL BSA, 1% DMSO, 0.1% TritonX-100 in PBS). Embryos were next incubated with appropriate secondary antibodies in the dark for either 2 hours at room temperature or overnight at 4°C followed by extensive washes in PBDT. Prima ry antibodies used were: 4D9 at 1:5 (anti-En; Monoclonal Antibody Facility, Institute of Neuroscience, University of Oregon), anti-Prox1 at 1:1000 (Prox1; AngioBio), and anti-activated Caspase 3 at 1:500 (BD Biosciences). Goat anti-rabbit IgG-594 (Jackson Labs) was used at 1:500 as a secondary antibody to detect Prox-1 staining. Goat anti-mouse IgG-HRP (Jackson Labs) was used at 1:250, followed by tyramide amplification (ThermoFisher TSA-488 amplification kit) to detect 4D9 staining. Goat anti-rabbit IgG-488 (Jackson Labs) was used at 1:500 to detect anti-activated Caspase 3 staining. Embryos were taken stepwise through a glycerol series into 75% glycerol. Heads and yolks were removed and trunks were mounted prior to image acquisition.
QUANTIFICATION AND STATISTICAL ANALYSIS
All sample sizes (n) are indicated for the number of embryos used in each experiment. Quantifications were completed blind to condition.
An unpaired Student’s t-test was performed to compare paired sets of data in which a common site was targeted by gRNPs. When comparing more than two groups, a One-way ANOVA test was completed followed by the appropriate correction for multiple pairwise comparisons among the groups. For all plots: *p<0.05, **p<0.01, ***p<0.001, n.s. not significant.
To quantify capillary electrophoresis data (Figures 1D–1F, 2, and S1), the proportion of targeted sites that acquired indel mutations in control or CRISPR/Cas9-injected embryos was calculated as follows: 1) Fluorescence intensity signals were collected corresponding to DNA fragments larger than 60bp, however signals of less than 150 units were considered as background and omitted. Fluorescence intensity signals were summed to obtain “Total fluorescence”. 2) “WT peak fluorescence” was determined as the fluorescence intensity signals corresponding to the WT peaks. 3) For each condition, uninjected or gRNP-injected, the “Fraction targeted loci without indels” was calculated as WT peak fluorescence / Total Fluorescence. The “Average fraction loci without indels, uninjected” was determined by averaging the “Fraction targeted loci without indels” obtained from analyses of 8 uninjected samples. 4) For each injected sample, the “Normalized fraction loci without indels” was determined as “Fraction loci without indels, injected” / “Average Fraction without indels, uninjected”. 5) The “% targeted loci with indel mutations” was calculated as (1 - “Normalized fraction loci without indels”) × 100.
To calculate the number of muscle fiber nuclei per somite (Figure 4), cells in the last 5 somites over the yolk extension (somites 11 through 15) were counted, and an average value of cells/nuclei per somite was determined for each embryo blind to condition. The average values per embryo (of nuclei/somite) were used as individual data points in all statistical analyses. In other words, only one measure per embryo was included in statistical analyses. When counting muscle nuclei: MPs were defined as nuclei having any level (even low intensity) of both Prox1 and En staining, MFFs were defined as nuclei labeled by En and not by Prox1 staining, and SSFs were defined as nuclei labeled by Prox1 and not by En staining.
To score vasculature in Tg(fli1a:EGFP) embryos injected with dgRNPs targeting egfl7 (Figure 7), the vessels present in six to seven segments at the posterior end of the yolk extension were analyzed in each embryo. Z-stacks were scored blind to condition. Embryos that possessed at least one extra GFP+ vessel in addition to the stereotypical vessels present vertically between somites and horizontally at the horizontal myoseptum were scored as having “additional branches”. Embryos with a unilateral vessel were scored as having a “missing vessel”. Because of the typical disorganization of the ventral hematopoietic niche adjacent to the yolk and yolk extension, this area was not scored for vasculature defects.
GraphPad Prism was used for statistical analysis. Image processing was completed using Adobe Photoshop and Fiji. Figures were assembled using Adobe Illustrator.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Anti-activated Caspase3 | BD Biosciences | RRID: AB_397274 |
| 4D9 (Mouse anti-Engrailed) | Monoclonal Antibody Facility, Institute of Neuroscience, University of Oregon | RRID: AB_528224 |
| Rabbit polyclonal anti-Prox1 | AngioBio | RRID: AB_10013720 |
| Goat anti-rabbit lgG-488 | Jackson Labs | Cat# 111-545-144 |
| Goat anti-rabbit lgG-594 | Jackson Labs | Cat# 711-585-152 |
| Goat anti-mouse IgG-HRP | Jackson Labs | Cat# 115-035-003 |
| Chemicals, Peptides, and Recombinant Proteins | ||
| NEBufferIM2.1 | NEB | Cat# B7202S |
| Duplex buffer | IDT | Cat# 11-05-01-03 |
| Alt-R® S.p. Cas9 nuclease, v.3 | IDT | Cat# 1081058 |
| Hi-DiIM formamide | ThermoFisher | Cat# 4401457 |
| Critical Commercial Assays | ||
| E.Z.N.A. Cycle Pure Kit | Omega | Cat# D6492-01 |
| MEGAshortscriptIM T7 transcription kit | Invitrogen | Cat# AM 1354 |
| RNA clean and concentrator-25 | Zymo Research | Cat# R1017 |
| KAPA2G Fast HotStart PCR Kit | KAPA Biosystems | Cat# KK5008 |
| GeneScanIM 500 LIZIM dye size standard | ThermoFisher | Cat# 4322682 |
| TSA-488 Amplification Kit | ThermoFisher | Cat# T20912 |
| Experimental Models: Organisms/Strains | ||
| Zebrafish Danio rerio AB wildtype | Zebrafish International Resource Center, Eugene, OR | N/A |
| Zebrafish Danio rerio Tubingen wildtype | Zebrafish International Resource Center, Eugene, OR | N/A |
| rx3s99/s99 | Loosli et al., 2003 | N/A |
| smohl1640/hl1640 | Chen et al., 2001 | N/A |
| kcnh6z46 | Hoshijima et al., 2016 | N/A |
| Tg(fli1a:EGFP) | Lawson and Weinstein, 2002 | N/A |
| Oligonucleotides | ||
| Common reverse oligonucleotide AAAAGCACCGACTCGGTGCCACTTTTTCAAGTTGATAACG GACTAGCCTTATTTTAACTTGCTATTTCTAGCTCTAAAAC | Gagnon et al., 2014 | N/A |
| Target specific forward oligonucleotides, see Table S1 | This study | N/A |
| Chemically synthesized Alt-R®-modified or unmodified crRNAs, tracrRNAs, or sgRNAs; see Table S1 | IDT | N/A |
| 6-FAM labeled primers, See Table S3 | IDT | N/A |
| Primers to detect deletion mutations, See Table S4. | This Study | N/A |
| Software and Algorithms | ||
| GeneMapper Software v4.0 | Applied Biosystems | Cat# 4440915 |
| Fiji | N/A | https://fiji.sc |
| GraphPad Prism version 6 | GraphPad Software | https://www.graphpad.com/scientific-software/prism/ |
| Adobe Photoshop | Adobe Systems | http://www.adobe.com/products/photoshop.html |
| Adobe Illustrator | Adobe Systems | http://www.adobe.com/products/illustrator.html |
| Other | ||
| T4 DNA polymerase | NEB | Cat# M0203S |
| TURBO DNase | Invitrogen | Cat# AM2238 |
HIGHLIGHTS.
Cas9 RNPs with synthetic crRNA:tracrRNAs are highly mutagenic in zebrafish embryos
Mutagenized F0 embryos mimic null mutants and lack confounding non-specific traits
Redundant gene activity can be uncovered and heritable deletions can be induced
5’ guanines of gRNAs that do not complement the protospacer diminish Cas9 RNP activity
ACKNOWLEDGMENTS
We thank colleagues at U of Utah for critical feedback. This work was supported by a grant to D.J.G. from the NIH (1R01HD081950). We also received financial support for the research from the Huntsman Cancer Foundation and the Nuclear Control of Cell Growth and Differentiation Program at Huntsman Cancer Institute; we also acknowledge support by NCI under Award Number P30CA042014. D.K.S. was supported by a predoctoral fellowship from the Developmental Biology Training Grant (NIH T32HD007491). We thank the U of U Health Sciences Core Facilities for DNA sequencing, oligonucleotide synthesis, imaging support, and zebrafish husbandry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DECLARATION OF INTERESTS
Authors K.H., M.J.J., D.K.S., and D.J.G. declare no competing financial interests. Authors A.M.J. and M.A.B. are employed by Integrated DNA Technologies, Inc. (IDT), which manufactures reagents similar to some described in the manuscript. M.A.B. owns equity in DHR, the parent company of IDT.
REFERENCES
- Amorim CEG, Gao Z, Baker Z, Diesel JF, Simons YB, Haque IS, Pickrell J, and Przeworski M (2017). The population genetics of human disease: The case of recessive, lethal mutations. PLoS genetics 13, e1006915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JL, Mulligan TS, Shen MC, Wang H, Scahill CM, Tan FJ, Du SJ, Busch-Nentwich EM, and Farber SA (2017). mRNA processing in mutant zebrafish lines generated by chemical and CRISPR-mediated mutagenesis produces unexpected transcripts that escape nonsense-mediated decay. PLoS genetics 13, e1007105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arduini BL, Bosse KM, and Henion PD (2009). Genetic ablation of neural crest cell diversification. Development 136, 1987–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedell VM, Wang Y, Campbell JM, Poshusta TL, Starker CG, Krug RG 2nd, Tan W, Penheiter SG, Ma AC, Leung AY, et al. (2012). In vivo genome editing using a high-efficiency TALEN system. Nature 491, 114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutros M, and Ahringer J (2008). The art and design of genetic screens: RNA interference. Nature reviews Genetics 9, 554–566. [DOI] [PubMed] [Google Scholar]
- Burger A, Lindsay H, Felker A, Hess C, Anders C, Chiavacci E, Zaugg J, Weber LM, Catena R, Jinek M, et al. (2016). Maximizing mutagenesis with solubilized CRISPR-Cas9 ribonucleoprotein complexes. Development 143, 2025–2037. [DOI] [PubMed] [Google Scholar]
- Carrington B, Varshney GK, Burgess SM, and Sood R (2015). CRISPR-STAT: an easy and reliable PCR-based method to evaluate target-specific sgRNA activity. Nucleic acids research 43, e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Burgess S, and Hopkins N (2001). Analysis of the zebrafish smoothened mutant reveals conserved and divergent functions of hedgehog activity. Development 128, 2385–2396. [DOI] [PubMed] [Google Scholar]
- Dahlem TJ, Hoshijima K, Jurynec MJ, Gunther D, Starker CG, Locke AS, Weis AM, Voytas DF, and Grunwald DJ (2012). Simple methods for generating and detecting locus-specific mutations induced with TALENs in the zebrafish genome. PLoS genetics 8, e1002861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick A, Hild M, Bauer H, Imai Y, Maifeld H, Schier AF, Talbot WS, Bouwmeester T, and Hammerschmidt M (2000). Essential role of Bmp7 (snailhouse) and its prodomain in dorsoventral patterning of the zebrafish embryo. Development 127, 343–354. [DOI] [PubMed] [Google Scholar]
- Dorsett Y, and Tuschl T (2004). siRNAs: applications in functional genomics and potential as therapeutics. Nature reviews Drug discovery 3, 318–329. [DOI] [PubMed] [Google Scholar]
- Dorsky RI, Itoh M, Moon RT, and Chitnis A (2003). Two tcf3 genes cooperate to pattern the zebrafish brain. Development 130, 1937–1947. [DOI] [PubMed] [Google Scholar]
- Doudna JA, and Charpentier E (2014). Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1258096. [DOI] [PubMed] [Google Scholar]
- Doyon Y, McCammon JM, Miller JC, Faraji F, Ngo C, Katibah GE, Amora R, Hocking TD, Zhang L, Rebar EJ, et al. (2008). Heritable targeted gene disruption in zebrafish using designed zinc-finger nucleases. Nature biotechnology 26, 702–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Brolosy MA, Kontarakis Z, Rossi A, Kuenne C, Gunther S, Fukuda N, Kikhi K, Boezio GLM, Takacs CM, Lai SL, et al. (2019). Genetic compensation triggered by mutant mRNA degradation. Nature 568, 193–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Brolosy MA, and Stainier DYR (2017). Genetic compensation: A phenomenon in search of mechanisms. PLoS genetics 13, e1006780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanigan KM, Dunn DM, von Niederhausern A, Soltanzadeh P, Howard MT, Sampson JB, Swoboda KJ, Bromberg MB, Mendell JR, Taylor LE, et al. (2011). Nonsense mutation-associated Becker muscular dystrophy: interplay between exon definition and splicing regulatory elements within the DMD gene. Human mutation 32, 299–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon JA, Valen E, Thyme SB, Huang P, Akhmetova L, Pauli A, Montague TG, Zimmerman S, Richter C, and Schier AF (2014). Efficient mutagenesis by Cas9 protein-mediated oligonucleotide insertion and large-scale assessment of single-guide RNAs. PloS one 9, e98186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch GE, Spruce T, Monteiro RS, Owens NDL, Martin SR, and Smith JC (2018). Innate Immune Response and Off-Target Mis-splicing Are Common Morpholino-Induced Side Effects in Xenopus. Developmental cell 44, 597–610 e510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Hall VL, Kok FO, Shin M, McNulty JC, Lawson ND, and Wolfe SA (2013). Targeted chromosomal deletions and inversions in zebrafish. Genome research 23, 1008–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammerschmidt M, and Mullins MC (2002). Dorsoventral patterning in the zebrafish: bone morphogenetic proteins and beyond. Results and problems in cell differentiation 40, 72–95. [DOI] [PubMed] [Google Scholar]
- Hendel A, Bak RO, Clark JT, Kennedy AB, Ryan DE, Roy S, Steinfeld I, Lunstad BD, Kaiser RJ, Wilkens AB, et al. (2015). Chemically modified guide RNAs enhance CRISPR-Cas genome editing in human primary cells. Nature biotechnology 33, 985–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshijima K, Jurynec MJ, and Grunwald DJ (2016). Precise Editing of the Zebrafish Genome Made Simple and Efficient. Developmental cell 36, 654–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housden BE, Muhar M, Gemberling M, Gersbach CA, Stainier DY, Seydoux G, Mohr SE, Zuber J, and Perrimon N (2017). Loss-of-function genetic tools for animal models: cross-species and cross-platform differences. Nature reviews Genetics 18, 24–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PD, Lander ES, and Zhang F (2014). Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang WY, Fu Y, Reyon D, Maeder ML, Kaini P, Sander JD, Joung JK, Peterson RT, and Yeh JR (2013). Heritable and precise zebrafish genome editing using a CRISPR-Cas system. PloS one 8, e68708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi AM, Rettig GR, Turk R, Collingwood MA, Zeiner SA, Quadros RM, Harms DW, Bonthuis PJ, Gregg C, Ohtsuka M, et al. (2017). Simplified CRISPR tools for efficient genome editing and streamlined protocols for their delivery into mammalian cells and mouse zygotes. Methods 121–122, 16–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jao LE, Wente SR, and Chen W (2013). Efficient multiplex bi-allelic zebrafish genome editing using a CRISPR nuclease system. Proceedings of the National Academy of Sciences of the United States of America 110, 13904–13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F, and Doudna JA (2017). CRISPR-Cas9 Structures and Mechanisms. Annual review of biophysics 46, 505–529. [DOI] [PubMed] [Google Scholar]
- Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, and Charpentier E (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, and Schilling TF (1995). Stages of embryonic development of the zebrafish. Developmental dynamics : an official publication of the American Association of Anatomists 203, 253–310. [DOI] [PubMed] [Google Scholar]
- Klatt Shaw D, Gunther D, Jurynec MJ, Chagovetz AA, Ritchie E, and Grunwald DJ (2018). Intracellular Calcium Mobilization Is Required for Sonic Hedgehog Signaling. Developmental cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok FO, Shin M, Ni CW, Gupta A, Grosse AS, van Impel A, Kirchmaier BC, Peterson-Maduro J, Kourkoulis G, Male I, et al. (2015). Reverse genetic screening reveals poor correlation between morpholino-induced and mutant phenotypes in zebrafish. Developmental cell 32, 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo M (2007). Bone morphogenetic proteins in the early development of zebrafish. The FEBS journal 274, 2960–2967. [DOI] [PubMed] [Google Scholar]
- Kotani H, Taimatsu K, Ohga R, Ota S, and Kawahara A (2015). Efficient Multiple Genome Modifications Induced by the crRNAs, tracrRNA and Cas9 Protein Complex in Zebrafish. PloS one 10, e0128319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalonde S, Stone OA, Lessard S, Lavertu A, Desjardins J, Beaudoin M, Rivas M, Stainier DYR, and Lettre G (2017). Frameshift indels introduced by genome editing can lead to in-frame exon skipping. PloS one 12, e0178700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson ND, and Weinstein BM (2002). In vivo imaging of embryonic vascular development using transgenic zebrafish. Developmental biology 248, 307–318. [DOI] [PubMed] [Google Scholar]
- Lee RT, Zhao Z, and Ingham PW (2016). Hedgehog signalling. Development 143, 367–372. [DOI] [PubMed] [Google Scholar]
- Lim S, Wang Y, Yu X, Huang Y, Featherstone MS, and Sampath K (2013). A simple strategy for heritable chromosomal deletions in zebrafish via the combinatorial action of targeting nucleases. Genome biology 14, R69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loosli F, Staub W, Finger-Baier KC, Ober EA, Verkade H, Wittbrodt J, and Baier H (2003). Loss of eyes in zebrafish caused by mutation of chokh/rx3. EMBO reports 4, 894–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukoseviciute M, Gavriouchkina D, Williams RM, Hochgreb-Hagele T, Senanayake U, Chong-Morrison V, Thongjuea S, Repapi E, Mead A, and Sauka-Spengler T (2018). From Pioneer to Repressor: Bimodal foxd3 Activity Dynamically Remodels Neural Crest Regulatory Landscape In Vivo. Developmental cell 47, 608–628 e606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lush ME, Diaz DC, Koenecke N, Baek S, Boldt H, St Peter MK, Gaitan-Escudero T, Romero-Carvajal A, Busch-Nentwich EM, Perera AG, et al. (2019). scRNA-Seq reveals distinct stem cell populations that drive hair cell regeneration after loss of Fgf and Notch signaling. eLife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, Zhu P, Shi H, Guo L, Zhang Q, Chen Y, Chen S, Zhang Z, Peng J, and Chen J (2019). PTC-bearing mRNA elicits a genetic compensation response via Upf3a and COMPASS components. Nature 568, 259–263. [DOI] [PubMed] [Google Scholar]
- Meng X, Noyes MB, Zhu LJ, Lawson ND, and Wolfe SA (2008). Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nature biotechnology 26, 695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou H, Smith JL, Peng L, Yin H, Moore J, Zhang XO, Song CQ, Sheel A, Wu Q, Ozata DM, et al. (2017). CRISPR/Cas9-mediated genome editing induces exon skipping by alternative splicing or exon deletion. Genome biology 18, 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HJ (1932). Further studies on the nature and causes of gene mutations. Proceedings of the Sixth International Congress of Genetics 1, 213–255. [Google Scholar]
- Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Haffter P, Heisenberg CP, et al. (1996). Genes establishing dorsoventral pattern formation in the zebrafish embryo: the ventral specifying genes. Development 123, 81–93. [DOI] [PubMed] [Google Scholar]
- Narasimhan VM, Hunt KA, Mason D, Baker CL, Karczewski KJ, Barnes MR, Barnett AH, Bates C, Bellary S, Bockett NA, et al. (2016). Health and population effects of rare gene knockouts in adult humans with related parents. Science 352, 474–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onichtchouk D, Aduroja K, Belting HG, Gnugge L, and Driever W (2003). Transgene driving GFP expression from the promoter of the zona pellucida gene zpc is expressed in oocytes and provides an early marker for gonad differentiation in zebrafish. Developmental dynamics : an official publication of the American Association of Anatomists 228, 393–404. [DOI] [PubMed] [Google Scholar]
- Ota S, Hisano Y, Ikawa Y, and Kawahara A (2014). Multiple genome modifications by the CRISPR/Cas9 system in zebrafish. Genes to cells : devoted to molecular & cellular mechanisms 19, 555–564. [DOI] [PubMed] [Google Scholar]
- Pavan WJ, and Raible DW (2012). Specification of neural crest into sensory neuron and melanocyte lineages. Developmental biology 366, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp MW, and Maquat LE (2016). Leveraging Rules of Nonsense-Mediated mRNA Decay for Genome Engineering and Personalized Medicine. Cell 165, 1319–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prykhozhij SV, Steele SL, Razaghi B, and Berman JN (2017). A rapid and effective method for screening, sequencing and reporter verification of engineered frameshift mutations in zebrafish. Disease models & mechanisms 10, 811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj B, Gagnon JA, and Schier AF (2018). Large-scale reconstruction of cell lineages using single-cell readout of transcriptomes and CRISPR-Cas9 barcodes by scGESTALT. Nature protocols 13, 2685–2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robu ME, Larson JD, Nasevicius A, Beiraghi S, Brenner C, Farber SA, and Ekker SC (2007). p53 activation by knockdown technologies. PLoS genetics 3, e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi A, Kontarakis Z, Gerri C, Nolte H, Holper S, Kruger M, and Stainier DY (2015). Genetic compensation induced by deleterious mutations but not gene knockdowns. Nature 524, 230–233. [DOI] [PubMed] [Google Scholar]
- Sander JD, and Joung JK (2014). CRISPR-Cas systems for editing, regulating and targeting genomes. Nature biotechnology 32, 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid B, Furthauer M, Connors SA, Trout J, Thisse B, Thisse C, and Mullins MC (2000). Equivalent genetic roles for bmp7/snailhouse and bmp2b/swirl in dorsoventral pattern formation. Development 127, 957–967. [DOI] [PubMed] [Google Scholar]
- Schubert MS, Cedrone E, Neun B, Behlke MA, and Dobrovolskaia MA (2018). Chemical Modification of CRISPR gRNAs Eliminate type I Interferon Responses in Human Peripheral Blood Mononuclear Cells. Journal of cytokine biology 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher JA, Hashiguchi M, Nguyen VH, and Mullins MC (2011). An intermediate level of BMP signaling directly specifies cranial neural crest progenitor cells in zebrafish. PloS one 6, e27403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AN, Davey CF, Whitebirch AC, Miller AC, and Moens CB (2015). Rapid reverse genetic screening using CRISPR in zebrafish. Nature methods 12, 535–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelson T, Heckl D, Ebert BL, Root DE, Doench JG, et al. (2014). Genome-scale CRISPR-Cas9 knockout screening in human cells. Science 343, 84–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes-Costa M, and Bronner ME (2015). Establishing neural crest identity: a gene regulatory recipe. Development 142, 242–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainier DYR, Raz E, Lawson ND, Ekker SC, Burdine RD, Eisen JS, Ingham PW, Schulte-Merker S, Yelon D, Weinstein BM, et al. (2017). Guidelines for morpholino use in zebrafish. PLoS genetics 13, e1007000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stigloher C, Ninkovic J, Laplante M, Geling A, Tannhauser B, Topp S, Kikuta H, Becker TS, Houart C, and Bally-Cuif L (2006). Segregation of telencephalic and eye-field identities inside the zebrafish forebrain territory is controlled by Rx3. Development 133, 2925–2935. [DOI] [PubMed] [Google Scholar]
- Thyme SB, Akhmetova L, Montague TG, Valen E, and Schier AF (2016). Internal guide RNA interactions interfere with Cas9-mediated cleavage. Nature communications 7, 11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai SQ, Zheng Z, Nguyen NT, Liebers M, Topkar VV, Thapar V, Wyvekens N, Khayter C, Iafrate AJ, Le LP, et al. (2015). GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nature biotechnology 33, 187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney GK, Pei W, LaFave MC, Idol J, Xu L, Gallardo V, Carrington B, Bishop K, Jones M, Li M, et al. (2015). High-throughput gene targeting and phenotyping in zebrafish using CRISPR/Cas9. Genome research 25, 1030–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Birsoy K, Hughes NW, Krupczak KM, Post Y, Wei JJ, Lander ES, and Sabatini DM (2015). Identification and characterization of essential genes in the human genome. Science 350, 1096–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Wei JJ, Sabatini DM, and Lander ES (2014). Genetic screens in human cells using the CRISPR-Cas9 system. Science 343, 80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerfield M (2000). The Zebrafish Book (Univ. of Oregon Press, Eugene: ). [Google Scholar]
- Wolff C, Roy S, and Ingham PW (2003). Multiple muscle cell identities induced by distinct levels and timing of hedgehog activity in the zebrafish embryo. Curr Biol 13, 1169–1181. [DOI] [PubMed] [Google Scholar]
- Wu RS, Lam II, Clay H, Duong DN, Deo RC, and Coughlin SR (2018). A Rapid Method for Directed Gene Knockout for Screening in G0 Zebrafish. Developmental cell 46, 112–125 e114. [DOI] [PubMed] [Google Scholar]
- Zinski J, Bu Y, Wang X, Dou W, Umulis D, and Mullins MC (2017). Systems biology derived source-sink mechanism of BMP gradient formation. eLife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.