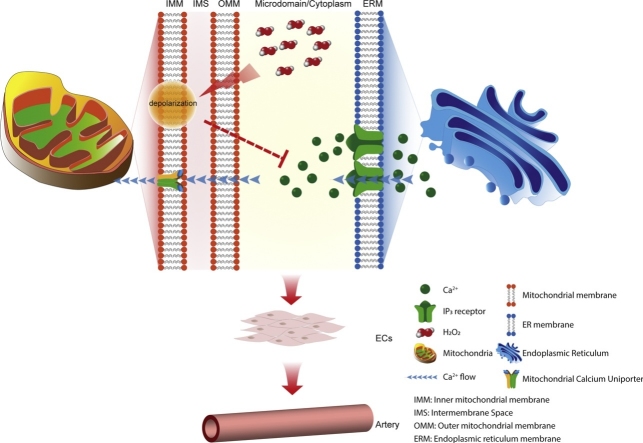
Graphical abstract
Keywords: Vascular; Endothelium; Calcium; Hydrogen peroxide; Free radical; Inositol 1,4,5‐trisphosphate
Highlights
-
•
H2O2 is produced by several cell processes including mitochondria and may act as an intracellular messenger and cell-cell signalling molecule.
-
•
Spontaneous local Ca2+ signals and IP3-evoked Ca2+ increases were inhibited by H2O2.
-
•
H2O2 suppression of IP3-evoked Ca2+ signalling may be mediated by mitochondria via a decrease in the mitochondrial membrane potential.
-
•
H2O2-induced mitochondrial depolarization and inhibition of IP3-evoked Ca2+ release, may protect mitochondria from Ca2+ overload during IP3-linked Ca2+ signals.
Abstract
Hydrogen peroxide (H2O2) is a mitochondrial-derived reactive oxygen species (ROS) that regulates vascular signalling transduction, vasocontraction and vasodilation. Although the physiological role of ROS in endothelial cells is acknowledged, the mechanisms underlying H2O2 regulation of signalling in native, fully-differentiated endothelial cells is unresolved. In the present study, the effects of H2O2 on Ca2+ signalling were investigated in the endothelium of intact rat mesenteric arteries. Spontaneous local Ca2+ signals and acetylcholine evoked Ca2+ increases were inhibited by H2O2. H2O2 inhibition of acetylcholine-evoked Ca2+ signals was reversed by catalase. H2O2 exerts its inhibition on the IP3 receptor as Ca2+ release evoked by photolysis of caged IP3 was supressed by H2O2. H2O2 suppression of IP3-evoked Ca2+ signalling may be mediated by mitochondria. H2O2 depolarized mitochondria membrane potential. Acetylcholine-evoked Ca2+ release was inhibited by depolarisation of the mitochondrial membrane potential by the uncoupler carbonyl cyanide 3-chlorophenylhydrazone (CCCP) or complex 1 inhibitor, rotenone. We propose that the suppression of IP3-evoked Ca2+ release by H2O2 arises from the decrease in mitochondrial membrane potential. These results suggest that mitochondria may protect themselves against Ca2+ overload during IP3-linked Ca2+ signals by a H2O2 mediated negative feedback depolarization of the organelle and inhibition of IP3-evoked Ca2+ release.
1. Introduction
The endothelium is the single layer of cells that lines the entire cardiovascular system and it is exposed constantly to a wide range of mechanical and chemical stimuli. The endothelium responds to these stimuli by releasing Ca2+-dependent vasoactive factors that include nitric oxide, prostacyclin, endothelium-derived contracting factors, von Willebrand factor, tissue plasminogen activator and endothelial derived hyperpolarising factor (1, 2). These vasoactive factors allow the endothelium to regulate almost all cardiovascular activities including vascular tone, immune responses, angiogenesis and vascular remodelling [1].
There is accumulating evidence that reactive oxygen species (ROS) also regulates endothelial function. ROS modulates endothelial cell growth, proliferation, endothelium-dependent relaxation, cytoskeletal reorganization, inflammatory responses and endothelium-regulated vascular remodelling. Among various ROS, hydrogen peroxide (H2O2) fulfils the prerequisites for serving as an intracellular messenger and acting as a cell-cell signalling molecule. H2O2 is a small and non-polar molecule produced by several cell processes that include mitochondria and NADPH oxidase [17,29,76]. During mitochondrial ATP production, the electron transport chain leaks electrons from complexes I and III resulting in the formation of superoxide anion radical () [11]. generates H2O2 spontaneously, or by the activity of superoxide dismutases. Although is not membrane permeable, H2O2 can diffuse across biological membranes or may cross membrane boundaries via channels like aquaporins [8] to regulate physiological and pathological cellular processes [3,62,75,85].
Many of the systems that produce H2O2, such as mitochondria, are modulated by the cytoplasmic Ca2+ concentration [6,24,36]. Ca2+ released from IP3Rs may result in Ca2+signals that propagate into the mitochondrial matrix [35,59]. Changes in mitochondrial Ca2+ may lead to enhanced ATP synthesis [74] and, as a result, increased ROS production [68]. Conversely, increased H2O2 generated by mitochondrial activity may modulate Ca2+ signalling to exert control on endothelial function. For example, in various cultured cell lines, H2O2 evokes Ca2+ release from the internal Ca2+ store [27,37,77].These observations raise the possibility that there may be feedback regulation of mitochondrial ATP production by changes in the cell activity mediated via the cytoplasmic Ca2+ concentration. To explore this possibility we measured the effects of H2O2 on Ca2+ signalling in the endothelium in large numbers of endothelial cells in intact blood vessels. We show that H2O2 depolarises mitochondria and suppresses IP3 evoked Ca2+ signalling.
2. Methods
2.1. Animals
All animal husbandry and euthanasia were carried out in accordance with the prior approval of the University of Strathclyde Animal Welfare and Ethical Review Body and under relevant UK Home Office Regulations, [Schedule 1 of the Animals (Scientific Procedures) Act 1986, UK]. Strathclyde BPU is a conventional unit which undertakes FELASA quarterly heath monitoring. Male Sprague-Dawley rats (10–12 weeks old), from an in-house colony, were used in the study. Animals were housed 3 per cage (RC2F cages, North Kent Plastics Company, UK), provided with enrichment (aspen wood chew sticks and hanging huts), nesting material (Sizzle nest, LBS Technology, UK), and fresh water and chow (RM1, Special Diet Services, UK) were available ad libitum. Room temperature was 19–23 °C (set point 21 °C), humidity was 45–65 %, and a 12 h light cycle was used. Rats were euthanatized by intraperitoneal injection of pentobarbital sodium (200 mg/kg, Pentaject, Merial Animal Health Ltd, UK).
2.2. Endothelial Ca2+ imaging
First order mesenteric arteries were isolated, placed into a physiological saline solution (PSS), cleaned of adherent fat and then used immediately. Each artery was then cut open and pinned flat on a sylgard block, with endothelial cells facing upward (en face preparation). The endothelium was then loaded with acetoxymethyl ester form of the Ca2+ indicator, Cal-520 (5 μM) and 0.02% pluronic F-127 in DMSO, for 30 min at 37 °C [55,[79], [80], [81]]. Following incubation, arteries were gently washed before the Sylgard block was inverted and placed in a custom-made bath chamber. The bottom of the chamber was a 0-thickness glass coverslip and two (0.2 μm diameter) steel pins were set between the coverslip and the block to prevent endothelial cells from contacting the coverslip, and to allow solutions to flow across the endothelium. Ca2+ images were acquired at 10 Hz on an inverted fluorescence microscope (TE300, Nikon, Japan) using a 40×, 1.4 NA oil immersion lens and a back-illuminated electron-multiplying charge-coupled device (EMCCD) camera (1024 × 1024 13 μm pixels; iXon 888; Andor, UK). Fluorescence excitation (488 nM wavelength) illumination was provided by a monochromator (Horiba, UK).
2.3. Localized flash photolysis
In some experiments, the endothelial Ca2+ response to local photolysis of caged IP3 was examined. In these experiments, the endothelium was loaded membrane permeant, caged IP3 (5 μM) for 30 min at 37 °C. A xenon flash lamp (Rapp Optoelecktronic, Germany) was used to uncage IP3 [13,47,80]. The output light was filtered using a UG-5 filter to select ultraviolet light. The light was focused and merged into the excitation light path via a fibre optic bundle and long pass dichroic mirror attached to the lens part of the microscope’s epi-illumination attachment [13,53,58]. The area of the photolysis site (∼80 μm diameter) resulted from the fiber optic diameter and the objective lens magnification (40x).
2.4. Imaging endothelial mitochondria
To assess mitochondrial membrane potential, arteries were pinned out in a Sylgard coated chamber designed for use on an upright microscope. Mitotracker Green FM (100 nM) was added to the PSS and the endothelium was incubated for 20 min followed by 20 min washing. Tetramethylrhodamine ethyl ester (TMRE) (60 nM) was added to the PSS and the endothelium was incubated 10 min [20,21,80]. TMRE (60 nm) was subsequently present in all perfusion solutions. Minimal photobleaching of TMRE was observed over the 5 min recording periods used. TMRE and Mitotracker Green images (10 Hz) were acquired on an upright microscope (Eclipse FN1; Nikon, Japan) equipped with a 60× water immersion objective (1.0 numerical aperture) and an EMCCD camera (iXon 888; Andor, UK).
2.5. Experimental protocols
The effect of H2O2 on basal endothelial Ca2+ activity was studied using a non-cumulative concentration response in the same preparation. In these experiments, a Ca2+-free PSS was used and H2O2 added to the Ca2+-free perfusate. To prevent depletion of internal Ca2+ stores, arteries were incubated in Ca2+-containing PSS between each exposure to H2O2.
The effect of H2O2 (with or without catalase, 1000 U ml−1) on evoked (acetylcholine, ACh; 100 nM) endothelial Ca2+ activity was studied in paired experiments. In these experiments, a control response (5-minute recording) to ACh (flowed rate: 1.5 ml min−1) was obtained before the tissue was washed for 5 min, and allowed to equilibrate for 10 min. ACh was then applied a second time, together with H2O2 (with or without catalase), and the responses compared. ACh and H2O2 (with or without catalase) were applied via separate syringe pumps each at 0.75 ml min−1. The effects of various pharmacological interventions on ACh-evoked Ca2+ signalling were also studied in paired experiments in which control responses were first obtained and then the endothelium was incubated with each antagonist for 20 min. Following the incubation period, ACh was applied a second time and responses compared to control. Each pharmacological agent was present throughout the second exposure to ACh.
Experiments utilising caged-IP3 also used a paired experimental design. An initial response to photolysis was recorded, and the tissue was then rested for 10 min. The endothelium was then incubated with H2O2 for 20 min before a second response, using the same photolysis location, was obtained.
2.6. Data analysis
Automated analysis of endothelial Ca2+ imaging recordings was carried out using custom-written Python routines [44,80,81]. In brief, average intensity projections were used to generate regions-of-interest (ROI) around each cell. The Ca2+ response of each endothelial cell was then extracted by averaging the fluorescence intensity within each ROI, for each cell and image in the dataset. Each ROI/cell was assigned an identification number so that the response of each cell could be compared within experimental series. Fluorescence signals are expressed as ratios (F/F0) of fluorescence counts (F) relative to baseline (control) values before stimulation (F0). The baseline (F0) was identified automatically as the 100 frame (10 s) period exhibiting the lowest noise prior to the introduction of any agonist. The total number of oscillations, and the amplitude of each oscillation were then extracted for each cell using a zero-crossing peak-detection algorithm [82] for signals exceeding 3 times the standard deviation of baseline noise.
2.7. Statistics
All data are presented as mean ± SEM of n biological replicates. Data were analysed using repeated measures one-way ANOVA with Geisser-Greenhouse correction and Dunnett’s multiple comparisons test, or paired t-test as appropriate. A p value less than 0.05 was considered statistically significant. All statistical analysis was performed using GraphPad Prism version 6.0 (GraphPad Software, USA) was used to run the statistical analysis.
2.8. Reagents and chemicals
The PSS consisted of (in mM):145 NaCl, 2.0 MOPS, 4.7 KCl, 1.3 NaH2PO4, 5.0 Glucose, 1.17, MgCl, 2.0 CaCl, 0.02 EDTA (pH adjusted to 7.4 with NaOH). In experiments using Ca2+ free PSS, CaCl2 was replaced with MgCl2 on an equimolar basis and EGTA (1 mM) was included. Caged-IP3 (caged-IP3 4,5-dimethoxy-2-nitrobenzyl) was obtained from Sichem (Germany). Cal-520 was obtained from Abcam (UK). Pluronic F-127 was obtained from Invitrogen (UK). Mitotracker Green FM was obtained from Invitrogen (UK). All other drugs and chemicals were obtained from Sigma (UK). Stock solutions of ACh, catalase-polyethylene glycol and H2O2 were prepared by dissolving each chemical in double-distilled, dionized water. 2-aminoethoxydiphenyl borate (2-APB), caged-IP3, Cal-520, carbonyl cyanide m-chlorophynyl hydrazine (CCCP), oligomycin, TMRE and Mitotracker Green FM were dissolved in DMSO.
2.9. Data availability
All data underpinning this study is available from the authors upon reasonable request.
3. Results
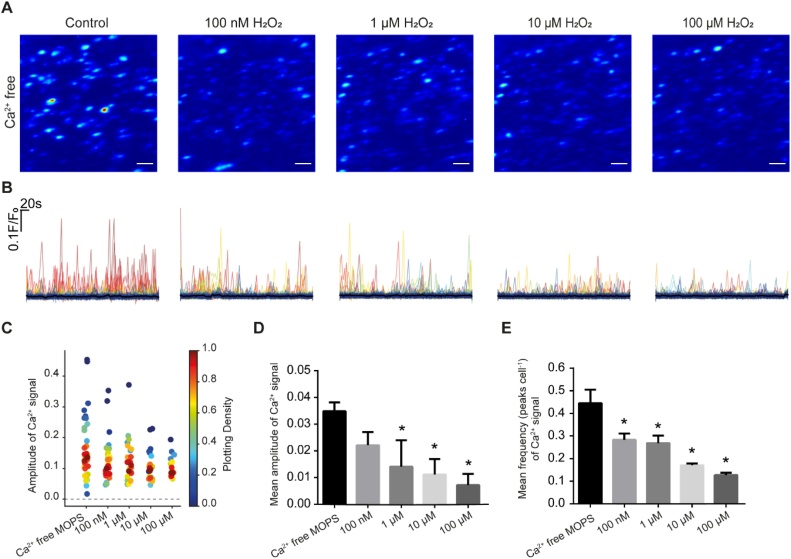
To determine if H2O2 alters spontaneous Ca2+ release from the internal store in intact mesenteric arteries, H2O2 (100 nM, 1 μM, 10 μM and 100 μM) was applied in a Ca2+ free PSS (Fig. 1). Between H2O2 applications arteries were washed in PSS (containing Ca2+) to allow the internal Ca2+ stores to refill. As the concentration of H2O2 increased, spontaneous Ca2+ release events decreased (Fig. 1). As spontaneous Ca2+ release arises from IP3-receptor activity [44,80,81], these results suggest that H2O2 may suppress Ca2+ release from the internal Ca2+ store.
Fig. 1.
Effect of H2O2 on spontaneous local Ca2+ signals arising from the internal Ca2+ stores. (A) Pseudo-colour images of spontaneous Ca2+ signals activity (red high, blue low amplitude) over a 5 min period in control (Ca2+-free PSS) and with H2O2 (100 nM to 100 μM). Between each recording, H2O2 was washed out and Ca2+ was restored to the bathing medium (10 min) to permit the store to refill. Scale bar: 20 μM. (B) Ca2+ signals measured in ∼200 cells shown in A. (C) Density plot of mean peak value of Ca2+ signalling in Ca2+ free MOPS and increasing concentrations of H2O2. Individual data points have been coloured (from blue, low to red, high) according to the density (i.e. occurrence) of particular values. (D) Summary of mean peak value of Ca2+ signalling in all cells. (E) Summary of number of Ca2+ signalling peaks in all cells n = 5, *p<0.05 vs Ca2+-free MOPS.
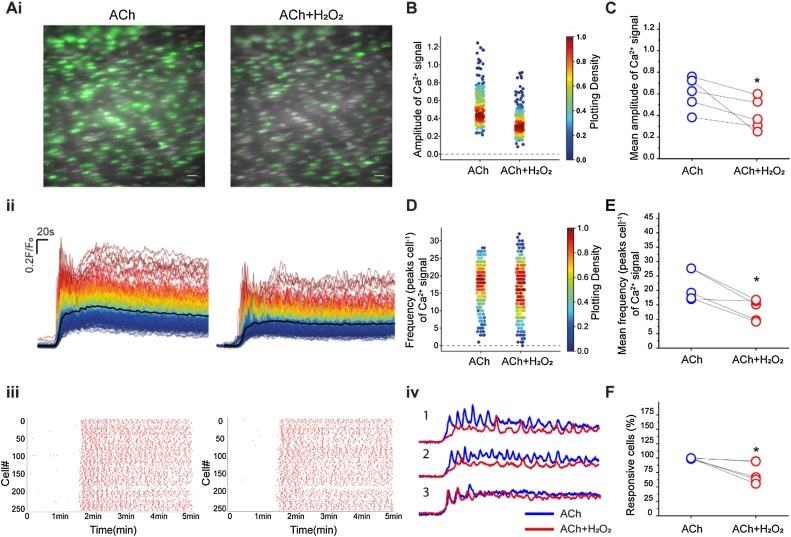
To further examine the effect of H2O2 on Ca2+ release from the store, the effects of the free radical were examined on ACh-evoked Ca2+ release. ACh (100 nM) evoked substantial Ca2+ signals that were heterogeneous across the endothelium and the amplitude and frequency of Ca2+ oscillations varied across cells. (Fig. 2A) (see also [2,38,44,49,52,55,81]). After washing out ACh, the endothelium was allowed to rest for 10 min and then challenged again with ACh (100 nM) and H2O2 (100 μM) applied simultaneously. H2O2 suppressed several aspects of ACh-evoked Ca2+ signalling. There was a reduction in the percentage of cells responding to ACh, a decrease in the amplitude, and a reduction frequency of oscillations in the presence of H2O2 when compared to controls (ACh alone; Fig. 2B-F). The effect of H2O2 on endothelial cells was also heterogeneous, and the free radical affected the Ca2+ response of some cells more than others’ (see Fig. 2Aiv). In the absence of H2O2, ACh (100 nM; 10 min. apart) evoked reproducible Ca2+ signals (Figure S1).
Fig. 2.
ACh-evoked Ca2+ release was suppressed by H2O2. (Ai) Pseudo-colour images (green) of Ca2+ signalling evoked by ACh (100 nM), and ACh (100 nM) in the presence of H2O2. (100 μM). Scale bar: 20 μM. (Aii) Overlaid Ca2+ signalling traces from ∼200 cells (shown in A) with the average shown as the black line in response to ACh and ACh+H2O2. Individual Ca2+ traces are coloured according to the magnitiude of the control (ACh) response. (Aiii) Rastergram plot of Ca2+ signals. Each red dot represents a Ca2+ peak in each cell (shown on the left axis) ACh (100 nM) left-side and ACh (100 nM) + H2O2 (100 μM) right-side. (Aiv) The effect of H2O2 varied on Ca2+ signals across cells. The panel shows traces of Ca2+ signalling from three typical cells from (Ai). The blue lines are the Ca2+ signals evoked by ACh (100 nM) and the red lines ACh (100 nM) + H2O2 (100 μM). Cell 3 was largely unaffected by H2O2. (B) Density plot of mean peak value of Ca2+ signalling from cells treated with ACh (100 nM) and ACh (100 nM) + H2O2 (100 μM). Individual data points have been coloured (from blue, low to red, high) according to the density (i.e. occurrence) of particular values (C) Summary of mean peak value of Ca2+ signalling in all cells. (D) Density plot of the frequency of Ca2+ signals. Individual data points have been coloured (from blue, low to red, high) according to the density (i.e. occurrence) of particular values (E) Summary of frequency of Ca2+ oscillations in all cells. (F) Summary of percentage of ACh-responsive cells. For all summary data (D–F) n = 6; *p < 0.05.
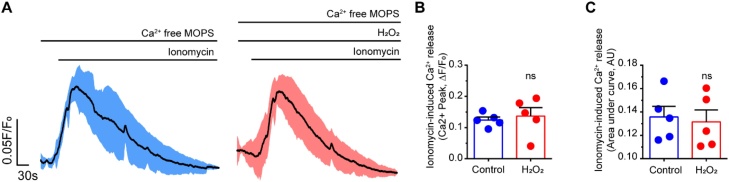
To determine if the internal Ca2+ store content was altered by H2O2, ionomycin (2 μM, in Ca2+ free PSS) was applied in the absence or presence of H2O2 (100 μM). Ionomycin-evoked Ca2+ signals were not significantly altered by H2O2 (Fig. 3A). Two measurements were used in this analysis; the amplitude of ionomycin-induced Ca2+ release and the area under the curve (Fig. 3B, C). Each measure was unchanged suggesting that H2O2 did not deplete the internal Ca2+ store.
Fig. 3.
Internal Ca2+ store content was unchanged in H2O2. (A) The Ca2+ store content was assessed using ionomycin (2 μM) applied in a Ca2+-free PSS. The left panel shows the averaged ionomycin-induced Ca2+ transient in the absence of H2O2 (100 μM) while the right panel is in the presence of H2O2. The black lines is the mean of five independent preparations blue and red lines show the standard error of the mean (SEM). (B–C) Summary data of mean peak response (B) and area under the curve (C) of ionomycin-induced Ca2+ increase (n = 5).
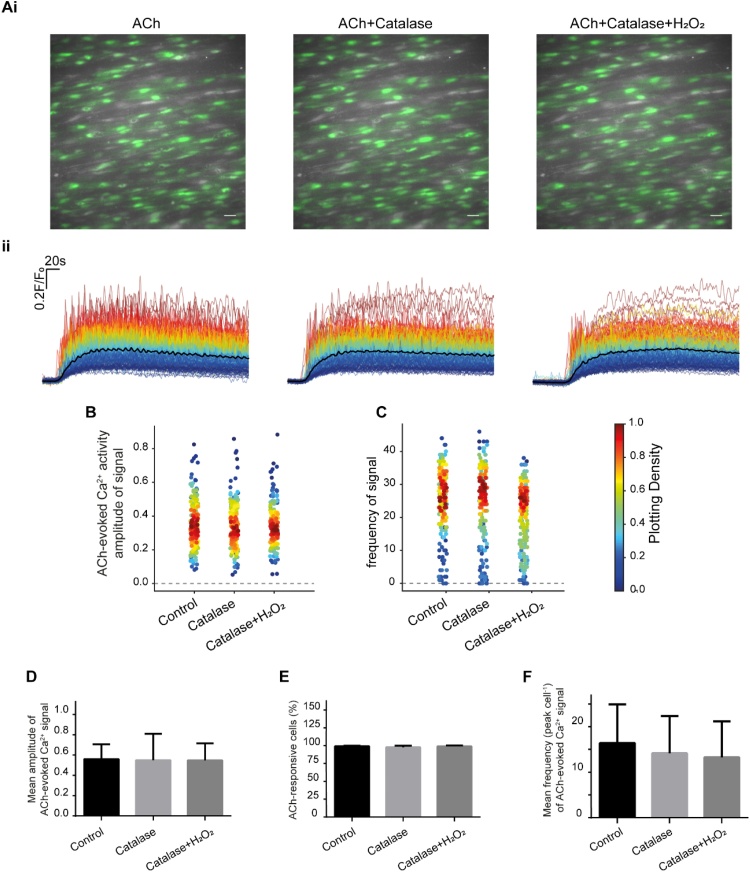
To confirm that the suppression of ACh-evoked Ca2+ signalling in endothelial cells arose from H2O2, catalase-peg (1000 U/ml) was used to breakdown H2O2. Catalase by itself did not alter the Ca2+ signal evoked by ACh (100 nM) when compared to controls (Fig. 4A-F). Furthermore, in the presence of catalase, H2O2 (100 μM) did not alter the amplitude (Fig. 4B, D), or the frequency (Fig. 4C,F) of the ACh-evoked Ca2+ signals, nor did it alter the percentage of cells activated by ACh (Fig. 4E). These data suggest that H2O2 supresses ACh-evoked Ca2+ signals in native endothelial cells.
Fig. 4.
Catalase eliminated of the effect of H2O2 on ACh-evoked Ca2+ signalling. (Ai) Pseudo colour (green) images of Ca2+ signalling evoked by ACh (100 nM), ACh (100 nM) + Catalase (1000 U/ml) and ACh (100 nM) + Catalase (1000 U/ml) + H2O2 (100 μM). Scale bar: 20 μM. (Aii) Overlaid Ca2+ signalling traces from each endothelial cell with treatment of ACh (100 nM), ACh (100 nM) + Catalase (1000 U/ml) and ACh (100 nM) + Catalase (1000 U/ml) + H2O2 (100 μM). Individual Ca2+ traces are coloured according to the magnitiude of the control (ACh) response. (B) Density plot of mean peak value of Ca2+ signalling. Individual data points have been coloured (from blue, low to red, high) according to the density (i.e. occurrence) of particular values (C) Density plot of number of the frequency Ca2+ signalling. (D) Summary data showing mean peak value of Ca2+ signalling in all cells. (E) Summary data showing the percentage of active cells. (F) Summary data showing the frequency of Ca2+ signals in all cells (n = 6 for all summary data).
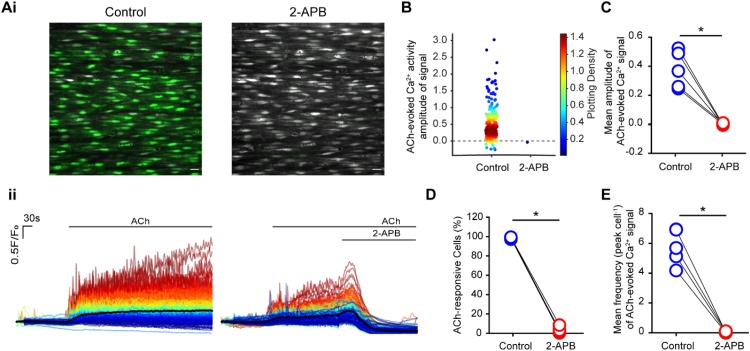
In native endothelial cells, ACh-evoked Ca2+ release requires activation of IP3Rs [1]. In support, ACh-evoked Ca2+ release was rapidly blocked by 2-APB (Fig. 5A-F). 2-APB significantly attenuated the amplitude (97% reduction; Fig. 5A, B, C) and frequency (99% reduction; Fig. 5E) of ACh-evoked Ca2+ signals, and the percentage of active cells activated by ACh (97% reduction; Fig. 5D).
Fig. 5.
2-APB inhibits ACh-evoked Ca2+ signalling. (Ai) Pseudo colour (green) images of Ca2+ signals evoked by ACh (100 nM) and ACh (100 nM) with 2-APB (100 μM). Scale bar: 20 μM. (Aii) Overlaid Ca2+ signalling traces of ACh (100 nM; left) and ACh (100 nM) with 2-APB (100 μM; right). Individual Ca2+ traces are coloured according to the magnitiude of the control (ACh) response. (B) Density plot of mean peak value of Ca2+ signalling. Individual data points have been coloured (from blue, low to red, high) according to the density (i.e. occurrence) of particular values. (C) Summary data of the mean peak value of Ca2+ signalling (E) percentage of active cells and (D) number of peaks of Ca2+ signalling. For all summary data (D–E), n = 5, * p<0.05.
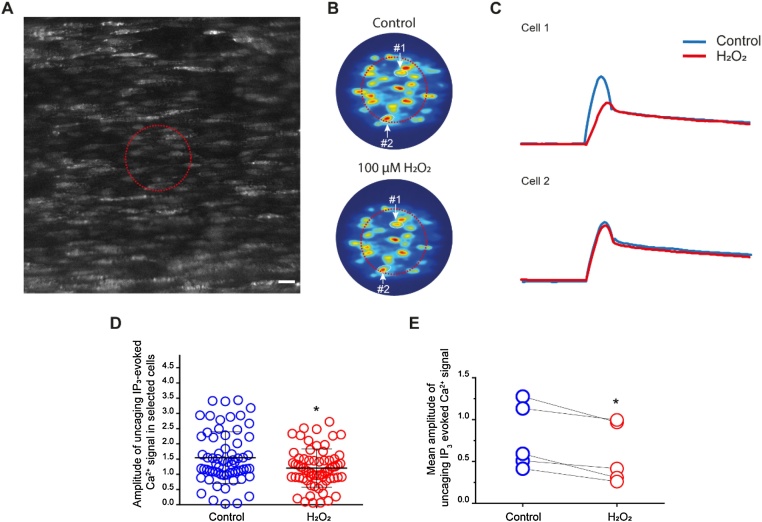
To determine which part of the IP3 pathway Ca2+ release was modified by H2O2, we performed experiments using the membrane-permeant, photoactivateable form of IP3 (caged-IP35 μM). IP3, released via the photolysis of caged-IP3, directly activates IP3Rs [13,51] and evoked a Ca2+ response (Fig. 6A-E). H2O2 (100 μM) significantly attenuated the Ca2+ response to photolysis of caged-IP3 (22% reduction; Fig. 6C-E). Again, there was heterogeneity in the sensitivity to H2O2 and some cells were less affected than others (Fig. 6C). These results demonstrate that H2O2 reduces ACh-evoked Ca2+ release by altering either the activity of IP3 receptors or the interaction between IP3 and IP3Rs.
Fig. 6.
H2O2 suppresses IP3 receptor activity. (A) Representative image showing endothelial cells of an intact artery. The red circle demarks the area that was selected for photolysis of caged IP3. Scale bar: 20 μM. (B) Heat map of Ca2+ signalling stimulated by photolysis of caged IP3 in the preselected area. (C) Uncaging IP3-evoked Ca2+ signalling traces of two representative single cells (cell 1 and cell 2 from B) before and after 20 min incubation of H2O2 (100 μM). As with ACh there was heterogeneity in the responses of cells to H2O2. The peak value of the Ca2+ signal was either unaltered (cell 2) or suppressed (cell 1) by H2O2. (D) Summary data showing the Ca2+ signal peak after uncaging. Control: blue; H2O2 (100 μM): red (n = 5). (E) Averaged peak value of Ca2+ signals from five different experiments. Control: blue; H2O2 treated: red, (n = 5; *p < 0.05).
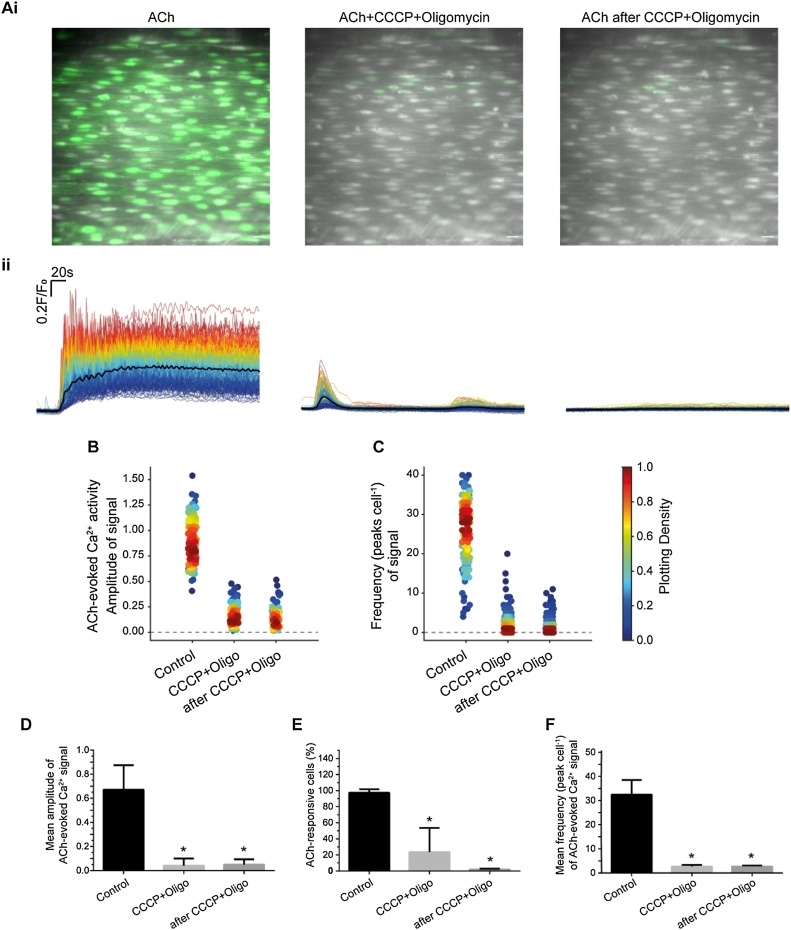
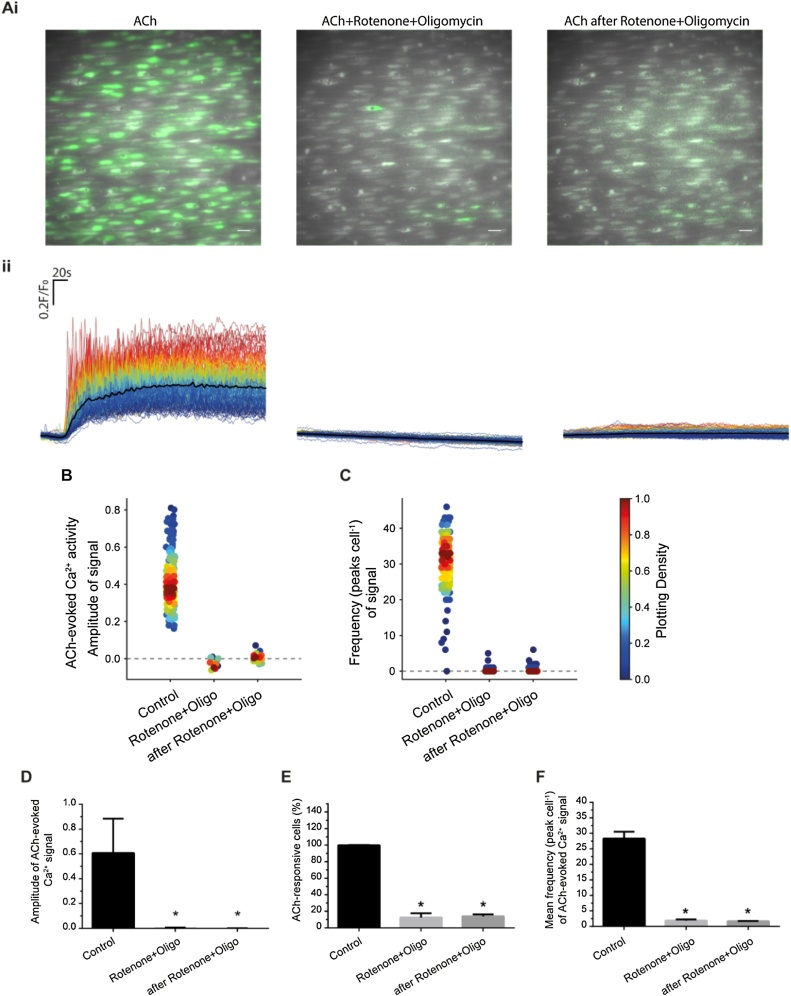
Since H2O2 is reported to increase the activity of IP3Rs [11], the question arises as to how H2O2 is able to decrease IP3-evoked Ca2+ release. Mitochondria exert profound control of IP3-evoked Ca2+ release [58,80] and H2O2 has been shown to alter mitochondrial function [56]. These observations raise the possibility that H2O2 may exert effects on IP3R indirectly. To determine if mitochondria mediate the effects of H2O2, we investigated the effect of uncoupling mitochondria on Ca2+ release from the internal Ca2+ store. To do this, the uncoupler, CCCP, and the complex I inhibitor, rotenone, were used in separate experiments. Each drug was used in combination with the ATP synthase inhibitor oligomycin, to prevent reversal of the ATP synthase. CCCP (5 μM) and oligomycin (6 μM) inhibited ACh-evoked Ca2+ signalling (Fig. 7A, B & Figure S2); the inhibition remained even after CCCP and oligomycin wash out (Fig. 7A, B). CCCP and oligomycin significantly reduced the amplitude and frequency of ACh-evoked Ca2+ signals, and the percentage of cells activated by ACh (Fig. 7C-F). Similarly, rotenone (2 μM) and oligomycin (6 μM) also inhibited ACh-evoked Ca2+ signaling (Fig. 8A-F). These results demonstrate that mitochondria regulate IP3-mediated Ca2+ release and that mitochondrial membrane potential depolarization inhibits IP3-evoked Ca2+ release.
Fig. 7.
The uncoupler, CCCP, and ATP synthase blocker, oligomycin, inhibited ACh-evoked Ca2+ signals. (Ai) Pseudo colour (green) images of Ca2+ activity evoked by ACh (100 nM; left), ACh (100 nM) + CCCP (5 μM) + oligomycin (6 μM; middle), and after washout of the CCCP and oligomycin (right). Scale bar: 20 μM. (Aii) Overlaid single cell Ca2+ traces from the endothelial cells shown in Aii. Individual Ca2+ traces are coloured according to the magnitiude of the control (ACh) response. (B) Density plot of mean peak value of the Ca2+ signal. Individual data points have been coloured (from blue, low to red, high) according to the density (i.e. occurrence) of particular values (C) Density plot of the frequency of Ca2+ signals. (D) Summary of the mean peak value of Ca2+ signals in all cells, (E) Percentage of active cells (F) and the frequency of signals in all cells. For all summary data (D–F), n = 3, *p<0.05.
Fig. 8.
Inhibition of complex I blocked ACh-evoked Ca2+ signalling. (Ai) Pseudo colour (green) images of Ca2+ signals evoked by ACh (100 nM), ACh (100 nM) + Rotenone (2 μM) + oligomycin (6 μM) and after washout of rotenone and oligomycin. Scale bar: 20 μM. (Aii) Overlaid Ca2+ signalling traces, from the cells shown in Ai, evoked by ACh (100 nM), ACh (100 nM) + Rotenone (2 μM) + oligomycin (6 μM) and after washout of rotenone and oligomycin. (B) Density plot of mean peak value of Ca2+ signalling. Individual data points have been coloured (from blue, low to red, high) according to the density (i.e. occurrence) of particular values (C) Density plot of number of peaks of Ca2+ signalling. (D) Summary of the mean peak value of Ca2+ signals in all cells, (E) Percentage of active cells (F) and the frequency of signals in all cells. For all summary data (D–F), n = 3, *p<0.05.
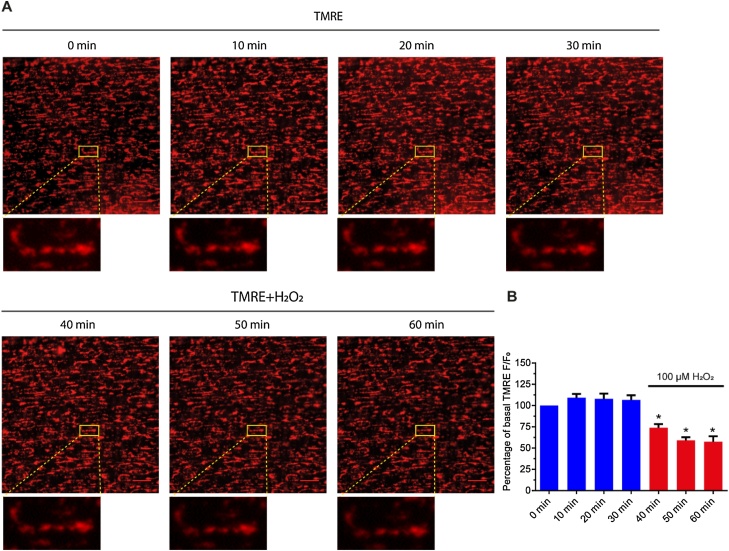
To explore the role H2O2 plays in mitochondria-regulated Ca2+ signaling, mitochondrial membrane potential was assessed using TMRE. TMRE is a lipophilic cation that is rapidly sequestered by the negatively-charged (∼−180 mV) mitochondrial membrane potential [21]. TMRE was imaged for 30 min to ensure the stability of the indicator. After 30 min, H2O2 (100 μM) was introduced and TMRE imaged for a further 30 min. H2O2 caused a significant decrease in TMRE fluorescence intensity (Fig. 9A, B). These findings suggest that H2O2 may suppress IP3-evoked Ca2+ release by depolarizing mitochondria. In a control experiments, to confirm mitochondrial localization, TMRE (60 nM) was loaded together with mitotracker green (100 nM). The two mitochondrial indicators largely overlapped in their localization (Figure S3). As expected from a mitochondrial localization of the dyes, mitochondrial membrane potential depolarization with CCCP (5 μM; applied with oligomycin (6 μM)) dispersed punctuate TMRE staining and reduced mitotracker green labelling (Figure S3).
Fig. 9.
Depolarisation of mitochondria by H2O2. (A) Pseudo colour images (red) of the mitochondrial membrane potential (reported by the membrane potential sensitive dye TMRE). In control (0 min ∼ 30 min) and after H2O2 (100 μM; 30 min–60 min). H2O2 decrease the mitochondrial membrane potential as revealed by the decrease in mitochondrial TMRE fluorescence intensity. Scale bar: 20 μM. (B) Quantification of the normalized intensity of TMRE (n = 6). *p < 0.05.
4. Discussion
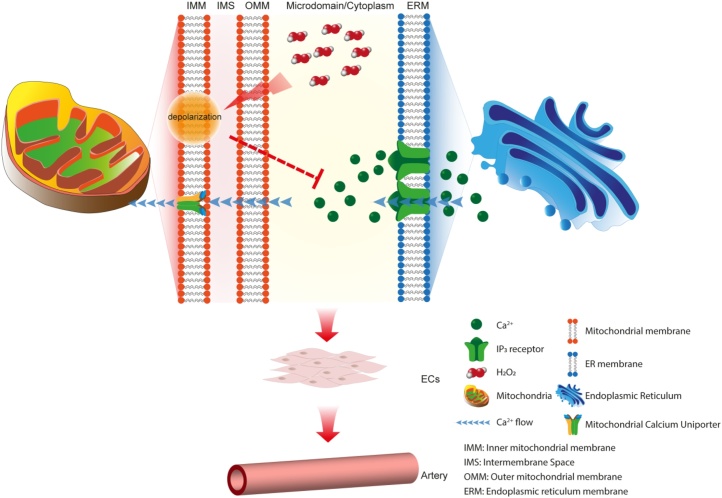
Interaction between the internal Ca2+ store and mitochondrial are critical in regulating cell signalling and cell performance. Several diffusible mediators communicate between the two organelles to control cell and tissue function. Of these, Ca2+ and H2O2 are of particular significance. Mitochondria are a major source, and the internal Ca2+ store a target, for H2O2. H2O2 modulates Ca2+ transport mechanisms on the internal Ca2+ store [5,12,24,60] and in turn, Ca2+ release from the store modulates mitochondrial function by regulating the enzymes of the Krebs cycle and oxidative phosphorylation [41]. Ca2+-induced changes in metabolic rate result in altered oxygen consumption, respiratory chain electron leakage and H2O2 levels [14]. Here, we have demonstrated H2O2 depolarises mitochondria and inhibits spontaneous and agonist-evoked IP3-induced Ca2+ signals. We suggest that suppression of IP3-evoked Ca2+ release arises from a H2O2-induced the decrease in mitochondrial membrane potential (Fig. 10).
Fig. 10.
Proposed mechanism for mitochondria regulation Ca2+ signalling in endothelial cells. The mitochondrial membrane potential is depolarized by H2O2. The depolarized membrane potential limits the driving force for Ca2+ uptake by mitochondria. As result, the concentration of Ca2+ in microdomain area between mitochondria and endoplasmic reticulum is increased and the ion suppresses Ca2+ release through IP3 receptor from the endoplasmic reticulum. Limited Ca2+ endothelial and artery function. On the other hand, reduced uptake of Ca2+ into mitochondria will lead to less production of ATP, therefore, reduced the metabolic production of H2O2. The negative feedback loop may help endothelial cells maintain normal metabolism and cell survival.
Mitochondria are potent modulators of IP3-evoked Ca2+ release. Ca2+ uptake by mitochondria may promote Ca2+ release from IP3Rs [18,19,23,43,54,65,72,73,78], limit IP3-evoked Ca2+ signals [4,34] or slow IP3-evoked Ca2+ wave progression [10,15,32,61,69,84]. At least two mechanisms have been proposed to account for mitochondrial control of IP3R activity. First, at sites of close contact between the internal Ca2+ store and mitochondria [26,48], channels on the internal Ca2+ store and mitochondrial channels (e.g. the uniporter and voltage-dependent anion-selective channel) may cluster, and Ca2+ uptake into mitochondria occurs at these sites [25,33,63,64]. Ca2+ uptake depends critically on the mitochondrial membrane potential. As the membrane potential decreases, so does mitochondrial Ca2+ uptake. Mitochondrial Ca2+ uptake limits a negative feedback process that operates at IP3 receptors to maintain Ca2+ release [19,54]. In smooth muscle, mitochondrial Ca2+ uptake is fast enough to regulate local spontaneous Ca2+ signals arising from IP3Rs (Ca2+ puffs) [57] and regulates store-operated Ca2+ entry [59], demonstrating tight functional coupling between IP3Rs and mitochondria. However, in other studies, close coupling between the internal store and mitochondria was not required for mitochondrial control of Ca2+ release to occur [80]. In the second mechanism proposed to account for mitochondrial control of the internal store, mitochondrial ATP production modulates Ca2+ release. When ATP production was restricted Ca2+ release was inhibited. This mechanism permits mitochondria to control Ca2+ release while being positioned far from the internal Ca2+ store [80]. Several studies show that ATP maintains IP3-mediated Ca2+ release. ATP potentiates IP3-induced Ca2+ release in permeabilized cells and from native endoplasmic reticulum vesicles, and it enhances activation of IP3-gated channels and purified, reconstituted IP3Rs [28,39,50,66] by increasing the open time of the channel s[7]. Thus factors provided by mitochondria may diffuse to IP3R to maintain IP3R activity.
The present results highlight another control mechanism which may operate between mitochondria and the internal Ca2+ store i.e. diffusion of H2O2. Our results suggest that the control of IP3 release by H2O2 is indirect and mediated by depolarization of mitochondria so combines aspects from both proposals (diffusible substance and mitochondrial depolarization). H2O2-mediated depolarization of the mitochondrial membrane potential will decrease the driving force for Ca2+ uptake by mitochondria and so limit negative feedback control of IP3-evoked Ca2+ release. Several proposals exist for the mechanisms by which H2O2 may depolarize mitochondria [30,31] and include inhibition of alpha-ketoglutarate dehydrogenase [22,56] or succinate dehydrogenase [56], or activation of the permeability transition pore [83]. Interestingly, the effect of H2O2 was not homogeneous across all cells and some were affected more than others. The reason for the heterogeneity is not clear, but perhaps differences in basal levels of H2O2, or metabolic state of the cells may contribute. Alternatively, antioxidant enzymes whose activities are directed at reducing hydrogen peroxide, such as catalase, glutathione peroxidase, and thioredoxin peroxidase, may vary across cells.
While the present results suggest that H2O2 inhibition of IP3R is indirect and mediated via mitochondria, ROS may also directly alter IP3 receptor activity. However, H2O2 is often reported to promote rather than inhibit IP3R activity. When H2O2 transients were prevented, Ca2+ oscillations were inhibited in some cells, implying that H2O2 makes subsequent Ca2+ release more likely to occur [11]. In support of these observations, in various cultured endothelial cell lines (HAECs, LECs, HUVECs and CVECs), H2O2 induces Ca2+ release from the internal store [27,37,77]. Various exogenously added oxidants stimulate rather than inhibit IP3R-mediated Ca2+release. Thimerosal [12,16,42], t-butylhydroperoxide [9], and diamide [45,46] each increase IP3-evoked Ca2+ release. In the case of thimerosal, the proposed mechanism involves an increased sensitivity of the receptor to [IP3] that results in Ca2+oscillations occurring at basal [IP3] in unstimulated cells [5,40]. Although sensitization to IP3may be a general mechanism responsible for the action of other oxidants, it has also been suggested that they (oxidants) may alter the sensitivity of IP3R to Ca2+ [9,45]. Our results suggest that the indirect effect of H2O2 on endothelial mitochondria may dominate control of IP3R. Since H2O2 may increase IP3R activity, these findings buttress the proposal that inhibition of Ca2+ release by H2O2 is indirect.
H2O2 regulates several key modulators of cell activity including cell proliferation, migration, and differentiation, and because H2O2 is membrane permeable, the ROS may exert widespread control across many endothelial cells to regulate cardiovascular function. H2O2 mediates at least part of its effects through changes in Ca2+ signalling. Ca2+ influx in native and in cultured endothelial cell lines is evoked by H2O2. In the native endothelium of superior epigastric arteries, H2O2 evoked Ca2+ increases result from Ca2+ influx via TRPV4 channels [67]. In mouse and human lung microvascular endothelial cell lines (MLMVEC, HPAE, H5V and HLMVEC) H2O2 evokes Ca2+ influx via TRPV4 or TRPM2 [70,71]. Our results reveal an additional complexity in the effects of H2O2. H2O2 may inhibit IP3-evoked Ca2+ release in native endothelial cells. The inhibition of IP3 evoked Ca2+ by H2O2 may be an indirect and mediated via depolarization of the mitochondrial membrane potential. This process may serve as a negative feedback modulation of mitochondrial function. Ca2+ increases associated with cell activity may initially stimulate mitochondrial ATP production. However, an increase in electron transport activity will result in increased ROS production, with a decrease in mitochondrial membrane potential and inhibition of IP3-evoked Ca2+ release as a result. H2O2 mediated mitochondrial depolarization may be a mechanism by which the ogranelles inhibit IP3-evoked Ca2+ signalling to protect themselves against Ca2+ overload .
Author contributions
XZ, MDL,CW & JGM developed the concept. XZ performed the experiments. JGM & XZ drafted the manuscript. JGM, ZX, CW & MDL edited and revised the manuscript. CW & JGM sourced funding. All authors approved the final version of the manuscript.
Declaration of Competing Interest
None.
Acknowledgments
This work was funded by the Wellcome Trust (202924/Z/16/Z; 204682/Z/16/Z) and the British Heart Foundation (PG/16/54/32230; PG16/82/32439), whose support is gratefully acknowledged. The authors would like to thank Margaret Macdonald for her excellent technical support.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ceca.2019.102108.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Adams D.J., Barakeh J., Laskey R., Van Breemen C. Ion channels and regulation of intracellular calcium in vascular endothelial cells. FASEB J. 1989;3:2389–2400. doi: 10.1096/fasebj.3.12.2477294. [DOI] [PubMed] [Google Scholar]
- 2.Aird W.C. Endothelial cell heterogeneity. Cold Spring Harb. Perspect. Med. 2012;2 doi: 10.1101/cshperspect.a006429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aon M.A., Cortassa S., Marban E., O’Rourke B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J. Biol. Chem. 2003;278:44735–44744. doi: 10.1074/jbc.M302673200. [DOI] [PubMed] [Google Scholar]
- 4.Arnaudeau S., Kelley W.L., Walsh J.V., Jr, Demaurex N. Mitochondria recycle Ca2+ to the endoplasmic reticulum and prevent the depletion of neighboring endoplasmic reticulum regions. J. Biol. Chem. 2001;276:29430. doi: 10.1074/jbc.M103274200. [DOI] [PubMed] [Google Scholar]
- 5.Bansaghi S., Golenar T., Madesh M., Csordas G., RamachandraRao S., Sharma K., Yule D.I., Joseph S.K., Hajnoczky G. Isoform-and species-specific control of inositol 1,4,5-trisphosphate (IP3) receptors by reactive oxygen species. J. Biol. Chem. 2014;289:8170–8181. doi: 10.1074/jbc.M113.504159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ben-Kasus Nissim T., Zhang X., Elazar A., Roy S., Stolwijk J.A., Zhou Y., Motiani R.K., Gueguinou M., Hempel N., Hershfinkel M., Gill D.L., Trebak M., Sekler I. Mitochondria control store-operated Ca(2+) entry through Na(+) and redox signals. EMBO J. 2017;36:797–815. doi: 10.15252/embj.201592481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bezprozvanny I., Ehrlich B.E. Atp modulates the function of inositol 1,4,5-trisphosphate-gated channels at 2 sites. Neuron. 1993;10:1175–1184. doi: 10.1016/0896-6273(93)90065-y. [DOI] [PubMed] [Google Scholar]
- 8.Bienert G.P., Chaumont F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta. 2014;1840:1596–1604. doi: 10.1016/j.bbagen.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Bird G.S., Burgess G.M., Putney J.W. Sulfhydryl-reagents and camp-dependent kinase increase the sensitivity of the inositol 1,4,5-trisphosphate receptor in hepatocytes. J. Biol. Chem. 1993;268:17917–17923. [PubMed] [Google Scholar]
- 10.Boitier E., Rea R., Duchen M.R. Mitochondria exert a negative feedback on the propagation of intracellular Ca2+ waves in rat cortical astrocytes. J. Cell. Biol. 1999;145:795–808. doi: 10.1083/jcb.145.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Booth D.M., Enyedi B., Geiszt M., Varnai P., Hajnoczky G. Redox nanodomains are induced by and control calcium signaling at the ER-mitochondrial interface. Mol. Cell. 2016;63:240–248. doi: 10.1016/j.molcel.2016.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bootman M.D., Taylor C.W., Berridge M.J. The thiol reagent, thimerosal, evokes Ca2+ spikes in hela-cells by sensitizing the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 1992;267:25113–25119. [PubMed] [Google Scholar]
- 13.Bradley K.N., Currie S., MacMillan D., Muir T.C., McCarron J.G. Cyclic ADP-ribose increases Ca2+ removal in smooth muscle. J. Cell. Sci. 2003;116:4291–4306. doi: 10.1242/jcs.00713. [DOI] [PubMed] [Google Scholar]
- 14.Brookes P.S., Yoon Y., Robotham J.L., Anders M.W., Sheu S.S. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am. J. Physiol. Cell Physiol. 2004;287:C817–33. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- 15.Bruce J.I.E., Giovannucci D.R., Blinder G., Shuttleworth T.J., Yule D.I. Modulation of [Ca2+]i signaling dynamics and metabolism by perinuclear mitochondria in mouse parotid acinar cells. J. Biol. Chem. 2004;279:12909. doi: 10.1074/jbc.M309070200. [DOI] [PubMed] [Google Scholar]
- 16.Bultynck G., Szlufcik K., Kasri N.N., Assefa Z., Callewaert G., Missiaen L., Parys J.B., De Smedt H. Thimerosal stimulates Ca2+ flux through inositol 1,4,5-trisphosphate receptor type 1, but not type 3, via modulation of an isoform-specific Ca2+-dependent intramolecular interaction. Biochem. J. 2004;381:87–96. doi: 10.1042/BJ20040072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burgoyne J.R., Oka S., Ale-Agha N., Eaton P. Hydrogen peroxide sensing and signaling by protein kinases in the cardiovascular system. Antioxid. Redox. Signal. 2013;18:1042–1052. doi: 10.1089/ars.2012.4817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chalmers S., McCarron J.G. Inhibition of mitochondrial calcium uptake rather than efflux impedes calcium release by inositol-1,4,5-trisphosphate-sensitive receptors. Cell Calcium. 2009;46:107–113. doi: 10.1016/j.ceca.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Chalmers S., McCarron J.G. The mitochondrial membrane potential and Ca2+ oscillations in smooth muscle. J. Cell. Sci. 2008;121:75–85. doi: 10.1242/jcs.014522. [DOI] [PubMed] [Google Scholar]
- 20.Chalmers S., Saunter C.D., Girkin J.M., McCarron J.G. Age decreases mitochondrial motility and increases mitochondrial size in vascular smooth muscle. J. Physiol. 2016;594:4283–4295. doi: 10.1113/JP271942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chalmers S., Saunter C.D., Girkin J.M., McCarron J.G. Flicker-assisted localization microscopy reveals altered mitochondrial architecture in hypertension. Nat. Sci. Rep. 2015;30:2000–2013. doi: 10.1038/srep16875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chinopoulos C., Adam-Vizi V. Depolarization of in situ mitochondria by hydrogen peroxide in nerve terminals. Ann. N. Y. Acad. Sci. 1999;893:269–272. doi: 10.1111/j.1749-6632.1999.tb07834.x. [DOI] [PubMed] [Google Scholar]
- 23.Collins T.J., Lipp P., Berridge M.J., Li W., Bootman M.D. Inositol 1,4,5-trisphosphate-induced Ca2+ release is inhibited by mitochondrial depolarization. Biochem. J. 2000;347:593. doi: 10.1042/0264-6021:3470593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Csordas G., Hajnoczky G. SR/ER-mitochondrial local communication: calcium and ROS. Biochim. Biophys. Acta. 2009;1787:1352–1362. doi: 10.1016/j.bbabio.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Csordas G., Renken C., Varnai P., Walter L., Weaver D., Buttle K.F., Balla T., Mannella C.A., Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell. Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Csordas G., Renken C., Varnai P., Walter L., Weaver D., Buttle K.F., Balla T., Mannella C.A., Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J. Cell. Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Doan T.N., Gentry D.L., Taylor A.A., Elliott S.J. Hydrogen peroxide activates agonist-sensitive Ca(2+)-flux pathways in canine venous endothelial cells. Biochem. J. 1994;297(Pt 1):209–215. doi: 10.1042/bj2970209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferris C.D., Huganir R.L., Snyder S.H. Calcium flux mediated by purified inositol 1,4,5-trisphosphate receptor in reconstituted lipid vesicles Is allosterically regulated by adenine-nucleotides. Proc. Natl. Acad. Sci. U. S. A. 1990;87:2147–2151. doi: 10.1073/pnas.87.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao L., Mann G.E. Vascular NAD(P)H oxidase activation in diabetes: a double-edged sword in redox signalling. Cardiovasc. Res. 2009;82:9–20. doi: 10.1093/cvr/cvp031. [DOI] [PubMed] [Google Scholar]
- 30.Garg T.K., Chang J.Y. 15-deoxy-delta 12, 14-prostaglandin J2 prevents reactive oxygen species generation and mitochondrial membrane depolarization induced by oxidative stress. BMC Pharmacol. 2004;4(6) doi: 10.1186/1471-2210-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gonzalez A., Granados M.P., Salido G.M., Pariente J.A. H2O2-induced changes in mitochondrial activity in isolated mouse pancreatic acinar cells. Mol. Cell Biochem. 2005;269:165–173. doi: 10.1007/s11010-005-3457-6. [DOI] [PubMed] [Google Scholar]
- 32.Haak L.L., Grimaldi M., Smaili S.S., Russell J.T. Mitochondria regulate Ca2+ wave initiation and inositol trisphosphate signal transduction in oligodendrocyte progenitors. J. Neurochem. 2002;80:405. doi: 10.1046/j.0022-3042.2001.00727.x. [DOI] [PubMed] [Google Scholar]
- 33.Hajnoczky G., Csordas G., Madesh M., Pacher P. The machinery of local Ca2+ signalling between sarco-endoplasmic reticulum and mitochondria. J. Physiol. 2000;529(Pt 1):69. doi: 10.1111/j.1469-7793.2000.00069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hajnoczky G., Hager R., Thomas A.P. Mitochondria suppress local feedback activation of inositol 1,4, 5-trisphosphate receptors by Ca2+ J. Biol. Chem. 1999;274:14157–14162. doi: 10.1074/jbc.274.20.14157. [DOI] [PubMed] [Google Scholar]
- 35.Hajnoczky G., Robb-Gaspers L.D., Seitz M.B., Thomas A.P. Decoding of cytosolic calcium oscillations in the mitochondria. Cell. 1995;82:415–424. doi: 10.1016/0092-8674(95)90430-1. [DOI] [PubMed] [Google Scholar]
- 36.Hempel N., Trebak M. Crosstalk between calcium and reactive oxygen species signaling in cancer. Cell Calcium. 2017;63:70–96. doi: 10.1016/j.ceca.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu Q., Corda S., Zweier J.L., Capogrossi M.C., Ziegelstein R.C. Hydrogen peroxide induces intracellular calcium oscillations in human aortic endothelial cells. Circulation. 1998;97:268–275. doi: 10.1161/01.cir.97.3.268. [DOI] [PubMed] [Google Scholar]
- 38.Huang T.Y., Chu T.F., Chen H.I., Jen C.J. Heterogeneity of [Ca2+]i signaling in intact rat aortic endothelium. FASEB J. 2000;14:797–804. doi: 10.1096/fasebj.14.5.797. [DOI] [PubMed] [Google Scholar]
- 39.Iino M. Effects of adenine-nucleotides on inositol 1,4,5-trisphosphate-induced calcium release in vascular smooth-muscle cells. J. Gen. Physiol. 1991;98:681–698. doi: 10.1085/jgp.98.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Joseph S.K. Role of thiols in the structure and function of inositol trisphosphate receptors structure and function of calcium release channels. Curr. Top. Membr. 2010;66:299–322. doi: 10.1016/S1063-5823(10)66013-9. [DOI] [PubMed] [Google Scholar]
- 41.Jouaville L.S., Pinton P., Bastianutto C., Rutter G.A., Rizzuto R. Regulation of mitochondrial ATP synthesis by calcium: evidence for a long-term metabolic priming. Proc. Natl. Acad. Sci. U. S. A. 1999;96:13807–13812. doi: 10.1073/pnas.96.24.13807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan S.A., Rossi A.M., Riley A.M., Potter B.V.L., Taylor C.W. Subtype-selective regulation of IP3receptors by thimerosal via cysteine residues within the 1P(3-binding core and suppressor domain. Biochem. J. 2013;451:177–184. doi: 10.1042/BJ20121600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Landolfi B., Curci S., Debellis L., Pozzan T., Hofer A.M. Ca2+ homeostasis in the agonist-sensitive internal store: functional interactions between mitochondria and the ER measured in situ in intact cells. J. Cell. Biol. 1998;142:1235. doi: 10.1083/jcb.142.5.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee M.D., Wilson C., Saunter C.D., Kennedy C., Girkin J.M., McCarron J.G. Spatially structured cell populations process multiple sensory signals in parallel in intact vascular endothelium. Sci. Signal. 2018;11 doi: 10.1126/scisignal.aar4411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lock J.T., Sinkins W.G., Schilling W.P. Effect of protein S-glutathionylation on Ca2+ homeostasis in cultured aortic endothelial cells. Am. J. Physiol.-Heart Circ. Physiol. 2011;300:H493–H506. doi: 10.1152/ajpheart.01073.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lock J.T., Sinkins W.G., Schilling W.P. Protein S-glutathionylation enhances Ca2+-induced Ca2+ release via the IP3 receptor in cultured aortic endothelial cells. J. Physiol. Lond. 2012;590:3431–3447. doi: 10.1113/jphysiol.2012.230656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Macmillan D., McCarron J.G. Regulation by FK506 and rapamycin of Ca2+ release from the sarcoplasmic reticulum in vascular smooth muscle: the role of FK506 binding proteins and mTOR. Br. J. Pharmacol. 2009;158:1112–1120. doi: 10.1111/j.1476-5381.2009.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mannella C. Origin of the tethers connecting mitochondria and endoplasmic reticulum: an electron tomographic study. Biophys. Soc. Proc. 2004 1852-Plat. [Google Scholar]
- 49.Marie I., Beny J.L. Calcium imaging of murine thoracic aorta endothelium by confocal microscopy reveals inhomogeneous distribution of endothelial cells responding to vasodilator agents. J. Vasc. Res. 2002;39:260–267. doi: 10.1159/000063691. [DOI] [PubMed] [Google Scholar]
- 50.Mayrleitner M., Chadwick C.C., Timerman A.P., Fleischer S., Schindler H. Purified IP3 receptor from smooth-muscle forms an IP3 gated and heparin sensitive Ca-2+ channel in planar Bilayers. Cell Calcium. 1991;12:505–514. doi: 10.1016/0143-4160(91)90032-a. [DOI] [PubMed] [Google Scholar]
- 51.McCarron J.G., Chalmers S., MacMillan D., Olson M.L. Agonist-evoked Ca2+ wave progression requires Ca2+ and IP3. J. Cell. Physiol. 2010;244:334–344. doi: 10.1002/jcp.22103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McCarron J.G., Lee M.D., Wilson C. The endothelium solves problems that endothelial cells Do not know exist. Trends Pharmacol. Sci. 2017;38:322–338. doi: 10.1016/j.tips.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McCarron J.G., MacMillan D., Bradley K.N., Chalmers S., Muir T.C. Origin and mechanisms of Ca2+ waves in smooth muscle as revealed by localized photolysis of caged inositol 1,4,5-trisphosphate. J. Biol. Chem. 2004;279:8417–8427. doi: 10.1074/jbc.M311797200. [DOI] [PubMed] [Google Scholar]
- 54.McCarron J.G., Muir T.C. Mitochondrial regulation of the cytosolic Ca2+ concentration and the InsP3-sensitive Ca2+ store in guinea-pig colonic smooth muscle. J. Physiol. 1999;516:149–161. doi: 10.1111/j.1469-7793.1999.149aa.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McCarron J.G., Wilson C., Heathcote H.R., Zhang X., Buckley C., Lee M.D. Heterogeneity and emergent behaviour in the vascular endothelium. Curr. Opin. Pharmacol. 2019;45:23–32. doi: 10.1016/j.coph.2019.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nulton-Persson A.C., Szweda L.I. Modulation of mitochondrial function by hydrogen peroxide. J. Biol. Chem. 2001;276:23357–23361. doi: 10.1074/jbc.M100320200. [DOI] [PubMed] [Google Scholar]
- 57.Olson M.L., Chalmers S., McCarron J.G. Mitochondrial Ca2+ uptake increases Ca2+ release from inositol 1,4,5-trisphosphate receptor clusters in smooth muscle cells. J. Biol. Chem. 2010;285:2040–2050. doi: 10.1074/jbc.M109.027094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olson M.L., Chalmers S., McCarron J.G. Mitochondrial organization and Ca2+ uptake. Biochem. Soc. Trans. 2012;40:158–167. doi: 10.1042/BST20110705. [DOI] [PubMed] [Google Scholar]
- 59.Pathak T., Trebak M. Mitochondrial Ca(2+) signaling. Pharmacol. Ther. 2018 doi: 10.1016/j.pharmthera.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Prosser B.L., Ward C.W., Lederer W.J. X-ROS signaling: rapid mechano-chemo transduction in heart. Science. 2011;333:1440–1445. doi: 10.1126/science.1202768. [DOI] [PubMed] [Google Scholar]
- 61.Rizzuto R., Pinton P., Carrington W., Fay F.S., Fogarty K.E., Lifshitz L.M., Tuft R.A., Pozzan T. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science. 1998;280:1763–1766. doi: 10.1126/science.280.5370.1763. [DOI] [PubMed] [Google Scholar]
- 62.Sena L.A., Chandel N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 2012;48:158–167. doi: 10.1016/j.molcel.2012.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shoshan-Barmatz V., Zalk R., Gincel D., Vardi N. Subcellular localization of VDAC in mitochondria and ER in the cerebellum. Biochim. Biophys. Acta. 2004;1657:105–114. doi: 10.1016/j.bbabio.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 64.Simpson P.B., Mehotra S., Lange G.D., Russell J.T. High density distribution of endoplasmic reticulum proteins and mitochondria at specialized Ca2+ release sites in oligodendrocyte processes. J. Biol. Chem. 1997;272:22654. doi: 10.1074/jbc.272.36.22654. [DOI] [PubMed] [Google Scholar]
- 65.Simpson P.B., Russell J.T. Mitochondria support inositol 1,4,5-trisphosphate-mediated Ca2+ waves in cultured oligodendrocytes. J. Biol. Chem. 1996;271:33493–33501. doi: 10.1074/jbc.271.52.33493. [DOI] [PubMed] [Google Scholar]
- 66.Smith J.B., Smith L., Higgins B.L. Temperature and nucleotide dependence of calcium release by myoinositol 1,4,5-trisphosphate in cultured vascular smooth-muscle cells. J. Biol. Chem. 1985;260:4413–4416. [PubMed] [Google Scholar]
- 67.Socha M.J., Boerman E.M., Behringer E.J., Shaw R.L., Domeier T.L., Segal S.S. Advanced age protects microvascular endothelium from aberrant Ca2+ influx and cell death induced by hydrogen peroxide. J. Physiol. 2015;593:2155–2169. doi: 10.1113/JP270169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sohal R.S., Allen R.G. Relationship between metabolic rate, free radicals, differentiation and aging: a unified theory. Basic Life Sci. 1985;35:75–104. doi: 10.1007/978-1-4899-2218-2_4. [DOI] [PubMed] [Google Scholar]
- 69.Straub S.V., Giovannucci D.R., Yule D.I. Calcium wave propagation in pancreatic acinar cells: functional interaction of inositol 1,4,5-trisphosphate receptors, ryanodine receptors, and mitochondria. J. Gen. Physiol. 2000;116:547–560. doi: 10.1085/jgp.116.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sun L., Yau H.Y., Wong W.Y., Li R.A., Huang Y., Yao X. Role of TRPM2 in H2O2-induced cell apoptosis in endothelial cells. PLoS One. 2012;7 doi: 10.1371/journal.pone.0043186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Suresh K., Servinsky L., Reyes J., Baksh S., Undem C., Caterina M., Pearse D.B., Shimoda L.A. Hydrogen peroxide-induced calcium influx in lung microvascular endothelial cells involves TRPV4. Am. J. Physiol. Lung Cell Mol. Physiol. 2015;309:L1467–77. doi: 10.1152/ajplung.00275.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sward K., Dreja K., Lindqvist A., Persson E., Hellstrand P. Influence of mitochondrial inhibition on global and local [Ca2+]i in rat tail artery. Circ. Res. 2002;90:792. doi: 10.1161/01.res.0000015214.40360.84. [DOI] [PubMed] [Google Scholar]
- 73.Szado T., Kuo K.H., Bernard-Helary K., Poburko D., Lee C.H., Seow C., Ruegg U.T., van Breemen C. Agonist-induced mitochondrial Ca2+ transients in smooth muscle. FASEB J. 2003;17:28–37. doi: 10.1096/fj.02-0334com. [DOI] [PubMed] [Google Scholar]
- 74.Szalai G., Krishnamurthy R., Hajnoczky G. Apoptosis driven by IP3-linked mitochondrial calcium signals. EMBO J. 1999;18:6349–6361. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Umanskaya A., Santulli G., Xie W., Andersson D.C., Reiken S.R., Marks A.R. Genetically enhancing mitochondrial antioxidant activity improves muscle function in aging. Proc. Natl. Acad. Sci. U. S. A. 2014;111:15250–15255. doi: 10.1073/pnas.1412754111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Veal E.A., Day A.M., Morgan B.A. Hydrogen peroxide sensing and signaling. Mol. Cell. 2007;26:1–14. doi: 10.1016/j.molcel.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 77.Volk T., Hensel M., Kox W.J. Transient Ca2+ changes in endothelial cells induced by low doses of reactive oxygen species: role of hydrogen peroxide. Mol. Cell. Biochem. 1997;171:11–21. doi: 10.1023/a:1006886215193. [DOI] [PubMed] [Google Scholar]
- 78.Wang Q., Downey G.P., Bajenova E., Abreu M., Kapus A., McCulloch C.A. Mitochondrial function is a critical determinant of IL-1-induced ERK activation. FASEB J. 2005;19:837. doi: 10.1096/fj.04-2657fje. [DOI] [PubMed] [Google Scholar]
- 79.Wilson C., Lee M., McCarron J.G. Acetylcholine released by endothelial cells facilitates flow-mediated dilatation. J. Physiol. 2016;594:7267–7307. doi: 10.1113/JP272927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wilson C., Lee M.D., Heathcote H.R., Zhang X., Buckley C., Girkin J.M., Saunter C.D., McCarron J.G. Mitochondrial ATP production provides long-range control of endothelial inositol trisphosphate-evoked calcium signaling. J. Biol. Chem. 2019;294:737–758. doi: 10.1074/jbc.RA118.005913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wilson C., Saunter C.D., Girkin J.M., McCarron J.G. Clusters of specialized detector cells provide sensitive and high fidelity receptor signaling in intact endothelium. FASEB J. 2016;30:2000–2013. doi: 10.1096/fj.201500090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wilson C., Saunter C.D., Girkin J.M., McCarron J.G. Pressure-dependent regulation of Ca2+ signaling in the vascular endothelium. J. Physiol. 2015;593:5231–5253. doi: 10.1113/JP271157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao R., Fang S.H., Lin K.N., Huang X.Q., Lu Y.B., Zhang W.P., Wei E.Q. Pranlukast attenuates hydrogen peroxide-induced necrosis in endothelial cells by inhibiting oxygen reactive species-mediated collapse of mitochondrial membrane potential. J. Cardiovasc. Pharmacol. 2011;57:479–488. doi: 10.1097/FJC.0b013e31821076d3. [DOI] [PubMed] [Google Scholar]
- 84.Zimmermann B. Control of InsP3-induced Ca2+ oscillations in permeabilized blowfly salivary gland cells: contribution of mitochondria. J. Physiol. 2000;525(Pt 3):707. doi: 10.1111/j.1469-7793.2000.t01-1-00707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zorov D.B., Filburn C.R., Klotz L.O., Zweier J.L., Sollott S.J. Reactive oxygen species (ROS)-induced ROS release: a new phenomenon accompanying induction of the mitochondrial permeability transition in cardiac myocytes. J. Exp. Med. 2000;192:1001–1014. doi: 10.1084/jem.192.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data underpinning this study is available from the authors upon reasonable request.