Abstract
Inflammation is a generalized, nonspecific, and beneficial host response of foreign challenge or tissue injury. However, prolonged inflammation is undesirable. It will cause loss function of involve organs, such as heat, pain redness, and swelling. Marine natural products have gained more and more attention due to their unique mechanism of anti-inflammatory action, and have considered a hotspot for anti-inflammatory drug development. Marine-derived fungi are promising sources of structurally unprecedented bioactive natural products. So far, a plethora of new secondary metabolites with anti-inflammatory activities from marine-derived fungi had been widely reported. This review covers 133 fungal metabolites described in the period of 2000 to 2018, including the structures and origins of these secondary metabolites.
Keywords: marine-derived fungi, marine natural products, anti-inflammatory
1. Introduction
Inflammation has been described as the general, complex, and beneficial immune system in response to external challenges or tissue damage [1]. It can ultimately restore tissue structure and function. Without inflammation, wounds would never be healed. However, if inflammation is not controlled for a long time, genetic mutations caused by immune cell-derived reactive oxygen species and numerous pathogenesis involved in the inflammatory response might contribute to many diseases, for example, cancer, multiple sclerosis, atherosclerosis, arthritis, heart disease, insulin resistance, and others [2,3]. At the same time, it will cause excessive expression of various inflammatory media to produce conditions conducive to many chronic diseases occurrence such as cancer, neurodegenerative disorders, diabetes, and cardiovascular diseases [4,5].
During the inflammatory process, the stimulated immune monocytes and macrophages trigger the transactivation of several important transcription factors. The well-known inflammatory signal pathway is NF-κB signal pathway called a canonical pathway [6]. NF-κB located in the cytoplasm is composed of two subunits (p50 and p65) as an inactive heterodimer bond to IκB-α, which is an inhibitory protein. In the stimulated condition, the phosphorylation and proteolytically degradation of IκB-α allows translocation of NF-κB into nuclear to regulate target gene transcription by binding to the κB site in the DNA’s structure [7]. The NF-κB transactivation will increase activities of the downstream responses such as pro-inflammatory cytokines (such as IL-1β IL-6, and TNF-α) [8], the important pro-inflammatory enzymes (such as iNOS and COX-2) and their derived production NO and PGE2, respectively [9]. In addition to NF-κB activation, another important pathway, MAPK signal pathway such as extracellular signal-regulated kinases (ERK), p38 MAPK, and cJun NH2-terminal kinases (JNK) [7], also can be activated by inflammation and regulates the transcription of various inflammatory-related genes then overexpress the downstream inflammatory response [10]. Amounts of inflammatory mediators and factors are involved in cell damage and inflammatory such as redness, pain, fever, and swelling [11,12]. Therefore, inhibition of the overproduction of these is an important target in the treatment of inflammatory disease [13]. Researchers usually evaluated the anti-inflammatory activity by the suppressed expression of pro-inflammatory cytokines, the pro-inflammatory enzyme of COX-2, iNOS and their derived production, and the various inflammatory-related protein in NF-κB and MAPK signal pathways in immune monocytes and macrophages (BV2 cells, RAW264.7 cells and more) stimulated by LPS in vitro [14], or by the inhibited swelling rate in mouse ear edema model induced by phorbol myristate acetate (PMA) in vivo [15].
Toward the aim of discovering new natural products with anti-inflammatory activities, researchers spend a lot of time and energy to discover novel sources in different environment. The oceans with their unique aquatic environment and plentiful biodiversity has drawn attention for the rich source of diverse secondary metabolites with significantly anti-inflammatory, antitumor, antimicrobial, antiviral, antimalarial, and anti-oxidant activities [16,17]. According to the MarinLit database (http://pubs.rsc.org/marinlit), annually more than 1200 novel natural products are reported from a variety of marine sources, such as algae, ascidians, bryozoan, corals, microorganisms, sea hares, sea squirts, sponges, and so on [18,19]. Since Alexander Fleming discovered penicillin in 1928 from Penicillium [20], people have never stopped discovering new drugs from fungi. Fungi is a crucial source as lead structures for novel pharmaceuticals [21]. Fungi also act as an important ecological role in the marine environment, such as pathogens of marine invertebrates, primary decomposers, and obligate symbionts [22]. Especially, marine-derived fungi play a vital role in the discovery of new anti-inflammatory drugs. Many novel secondary metabolites showing potent anti-inflammatory activities have been discovered from fungi residing in or on algae, sediments, water, and corals. Due to its unique mechanism of action, marine fungal compounds have received more and more attention and become one of the hotspot area for the development of anti-inflammatory drugs.
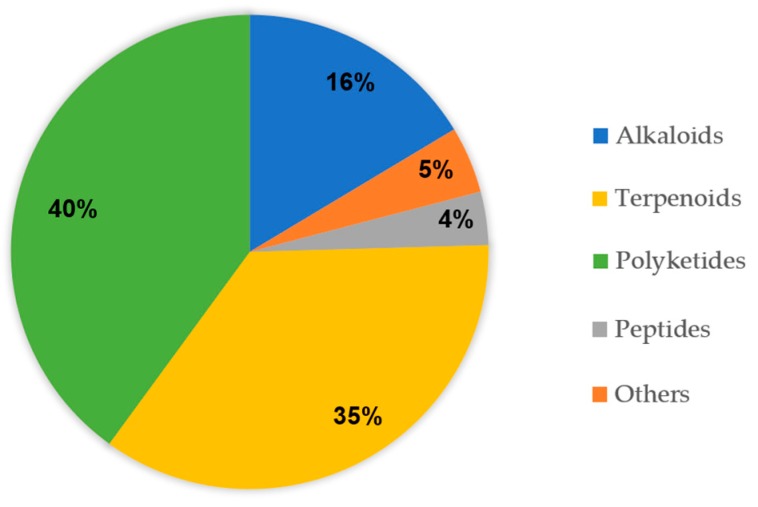
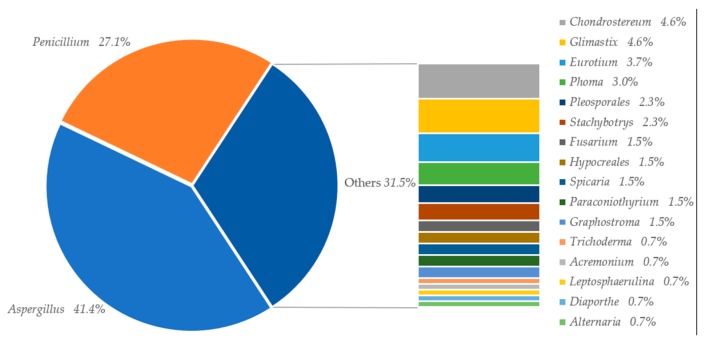
This review provides a comprehensive overview of 133 marine fungi-derived anti-inflammatory compounds assorted into five structure types, including alkaloids (Table 1), terpenoids (Table 2), polyketides (Table 3), peptides (Table 4), and others (Table 5), which show the proportion of structure types, 16%, 35%, 40%, 5%, and 4%, respectively (Figure 1). A large proportion of the secondary metabolites produced by Aspergillus (41.4%), and Penicillium (27.1%; Figure 2). Some of these natural products, such as preussin G (5) and preussin I (7), were shown to have remarkable anti-inflammatory activities even stronger than these of the positive control [23]. Therefore, these compounds will emerge as new lead structures for potential anti-inflammatory drugs.
Table 1.
Anti-inflammatory alkaloids from marine fungi.
| Metabolites | Species | Activities | Reference |
|---|---|---|---|
| Preussins C–K (1–9) | A. flocculosus 16D-1 | against IL–6 with IC50 values of 0.11–22 μM in LPS-activated THP-1 | [23] |
| Asperversiamides B, C, F, G (10–13) | A. versicolor | against iNOS with IC50 values of 5.39–16.58 μM in LPS-activated RAW264.7 cells | [24] |
| Luteoride E (14) | A. terreus | against NO with IC50 value of 24.65 μM in LPS-activated RAW264.7 cells | [25] |
| Chrysamide C (15) | P. chrysogenum SCSIO41001 | against IL–6 with 40.06% inhibitory at 1.0 μM | [26] |
| Viridicaol (16) | Penicillium sp. SF-5295 | against NO and PGE2 with IC50 values of 46.03 and 30.37 μM in LPS-activated RAW264.7 and 43.03 and 34.20 μM in LPS-activated BV2 cells | [27] |
| Brevicompanines E, H (17, 18) | Penicillium sp. | against NO with IC50 values of 27 and 45 μg/mL in LPS-activated RAW264.7 cells | [28] |
| Methylpenicinoline (19) | Penicillin sp. SF-5995 | against NO, PGE2, iNOS, and COX-2 with IC50 values from 34 to 49 μM | [29] |
| Neocechinulin A (20) | Eurotium sp. SF-5989 | significantly affection at concentrations exceeding 25 µM | [30] |
Table 2.
Anti-inflammatory terpenoids from marine fungi.
| Metabolites | Species | Activities | Reference |
|---|---|---|---|
| Brasilanones A and E (21, 22) | A. terreus CFCC 81836 | against NO with 47.7% and 37.3% inhibition rates at 40 μM in LPS-activated RAW264.7 cells | [31] |
| Dihydrobipolaroxins B−D (23−25) Dihydrobipolaroxin (26) |
Aspergillus sp. SCSIOW2 | against NO with moderate anti-inflammatory effects | [32] |
| Thomimarine E (27) | P. thomii KMM 4667 | against NO with 22.5% inhibition rate at 10.0 μM in LPS-activated RAW264.7 cells | [33] |
| Graphostromane F (28) | Graphostroma sp. MCCC 3A00421 | against NO with IC50 value of 14.2 μM in LPS-activated RAW264.7 cells | [34] |
| Khusinol B (29) | Graphostroma sp. MCCC 3A00421 | against NO with IC50 values of 17 μM in LPS-activated RAW264.7 cells | [35] |
| 1R,6R,7R,10S-10-hydroxy-4(5)-cadinen-3-one (30) | Hypocreales sp. HLS-104 | against NO with Emax values of 10.22% at 1 μM in LPS-activated RAW264.7 cells | [36] |
| Mangicols A and B (31, 32) | F. heterosporum CNC-477 | 81% and 57% inhibition rate at 50 μg per ear in PMA-induced mouse ear edema assay | [37] |
| Chondroterpenes A, B, H (33–35) Hirsutanol A (36) Chondrosterins A, B (37, 38) |
Chondrostereum sp. NTOU4196 | against NO with considerable inhibitory effects at 20 μM in LPS-activated BV-2 cells | [38] |
| Lovastatin (39) | A. terreus | against NO with IC50 value of 17.45 μM in LPS-activated RAW264.7 cells | [25] |
| Aspertetranones A−D (40−43) | Aspergillus sp. ZL0-1b14 | against IL-6 with 43% and 69% inhibition rates at 40 μM in LPS-activated RAW264.7 cells | [39] |
| Pleosporallins A−C (44−46) | Phoma sp. NTOU4195 | against IL-6 with about 30.0% inhibition rate at 5–20 μg/mL in LPS-activated RAW264.7 cells | [40] |
| 7-acetoxydehydroaustinol (47) Austinolide (48) 7-acetoxydehydroaustin (49) 11-hydroxyisoaustinone (50) 11-acetoxyisoaustinone (51) |
Penicillium sp. SF-5497 | against NO with IC50 values of 61.0, 30.1, 58.3, 37.6, and 40.2 μM in LPS-activated BV-2 cells | [41] |
| Citreohybridonol (52) | P. atrovenetum | anti-neuroinflammatory activity | [42] |
| Tanzawaic acid Q (53) Tanzawaic acids A (54), C (55), D (56), and K (57) |
P. steckii 108YD142 | against NO with considerably anti-inflammatory activity in LPS-activated RAW264.7 cells | [43] |
| 2E,4Z-tanzawaic acid D (58) Tanzawaicacids A (54), E (59) | Penicillium sp. SF-6013 | against NO with IC50 values of 37.8, 7.1, and 42.5 μM in LPS-activated RAW264.7 cells | [44] |
| Stachybotrysin C (60), Stachybonoid F (61), Stachybotylactone (62) | S. chartarum 952 | against NO with IC50 values of 27.2, 52.5, and 17.9 μM in LPS-activated RAW264.7 cells | [45] |
Table 3.
Anti-inflammatory polyketides from marine fungi.
| Metabolites | Species | Activities | Reference |
|---|---|---|---|
| Versicolactone G (63) Territrem A (64) |
A. terreus | against NO with IC50 values of 15.72 and 29.34 μM in LPS-activated RAW264.7 cells | [25] |
| Eurobenzophenone B (65) Canthone A (66) 3-de-O-methylsulochrin (67) Yicathin B (68) Dermolutein (69) Methylemodin (70) |
A. europaeus WZXY-SX-4-1 | 66 against NF-κB with significant inhibition in LPS-activated SW480 cells 65, 67, 68, 69, 70 against NF-κB with inhibition and against NO with weak inhibition in LPS-activated SW480 cells |
[46] |
| Violaceol II (71) Cordyol E (72) |
A. sydowii J05B-7F-4 | against NO with weak inhibition in LPS-activated RAW264.7 cells | [47] |
| TMC-256C1 (73) | Aspergillus sp. SF-6354 | against NO and PGE2 with considerable anti-neuroinflammatory activity in LPS-activated BV2 cells | [48] |
| Aurasperone F (74) Aurasperone C (75) Asperpyrone A (76) |
A. niger SCSIO Jcsw6F30 | against COX-2 with IC50 values of 11.1, 4.2, and 6.4 μM in LPS-activated RAW264.7 cells | [49] |
| Diorcinol (77) Cordyol C (78) 3,7-dihydroxy-1,9-Dimethyldibenzofuran (79) |
Aspergillus sp. SCSIO Ind09F01 | against the COX-2 expression with IC50 values of 2.4−10.6 μM | [50] |
| Cladosporin 8-O-α-ribofuranoside (80) Cladosporin (81) Asperentin 6-O-methyl ether (82) Cladosporin 8-O-methyl ether (83) 4′-hydroxyasperentin (84) 5′-hydroxyasperentin (85) |
Aspergillus sp. SF-5974 and Aspergillus sp. SF-5976 | against NO and PGE2 with IC50 values of 20−65 μM in LPS-activated microglial cells | [51] |
| Asperlin (86) | Aspergillus sp. SF-5044 | against NO and PGE2 in LPS-activated murine macrophages | [52] |
| Guaiadiol A (87) 4,10,11-trihydroxyguaiane (88) | P. thomii KMM 4667 | against NO with 24.1% and 36.6% inhibition at 10.0 μM in LPS-activated murine macrophages | [33] |
| Citrinin H1 (89) | Penicillium sp. SF-5629 | against NO with IC50 values of 8.1 and 8.0 μM in LPS-activated BV2 cells | [53] |
| Penicillospirone (90) | Penicillium sp. SF-5292 | against NO and PGE2 with IC50 values of 21.9–27.6 μM in LPS-activated RAW264.7 and BV2 cells | [27] |
| Penicillinolide A (91) | Penicillium sp. SF-5292 | against NO, PGE2, TNF-α, IL-1β and IL-6 with IC50 values of 20.47, 17.54, 8.63, 11.32, and 20.92 μM in LPS-activated RAW264.7 and BV2 cells | [54] |
| Penstyrylpyrone (92) | Penicillium sp. JF-55 | against NO, PGE2, TNF-α, IL-1β with IC50 values of 12.32, 9.35, 13.54, and 18.32 μM in LPS-activated murine peritoneal macrophages | [55] |
| Curvularin (93), (11R,15S)-11-hydroxycurvularin (94) (11S,15S)-11-hydroxycurvularin (95) (11R,15S)-11-methoxycurvularin (96) (11S,15S)-11-methoxycurvularin (97) (10E,15S)-10,11-dehydrocurvularin (98) (10Z,15S)-10,11-dehydrocurvularin (99) | Penicillium sp. SF-5859 | against NO and PGE2 with IC50 values of 1.9–18.1, and 2.8–18.7 µM in LPS-activated RAW264.7 cells | [56] |
| Pyrenocine A (100) | P. paxilli | against TNF-α and PGE2 in LPS-activated macrophages | [57] |
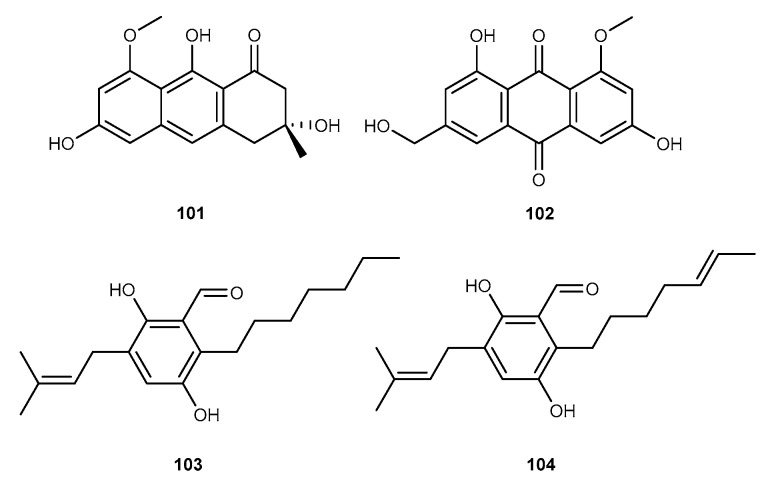
| Asperflavin (101) | E. amstelodami | against NO and PGE2 with 4.6% and 55.9% inhibition rates to NO and PGE2 at 200 μM in LPS-activated RAW264.7 cells | [58] |
| Questinol (102) | E. amstelodami | against NO and PGE2 with 73.0% and 43.5% inhibition rates at 200 μM against NO and PGE2 | [59] |
| Flavoglaucin (103) Isotecrahydro-auroglaucin (104) | Eurotium sp. SF-5989 | against NO and PGE2 in LPS-activated RAW264.7 cells | [60] |
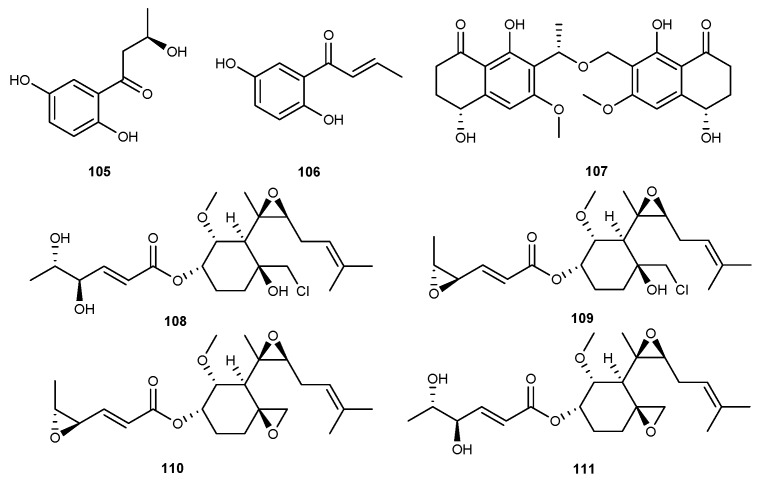
| 1-(2,5-dihydroxyphenyl)-3-hydroxybutan-1-one (105) 1-(2,5-dihydroxyphenyl)-2-buten-1-one (106) |
Paraconiothyrium sp. VK-13 | against NO and PGE2 with IC50 values of 3.9–12.5 µM in LPS-activated RAW264.7 cells | [61] |
| (4R,10S,4’S)-leptothalenone B (107) | L. chartarum 3608 | against NO with IC50 value of 44.5 µM in LPS-activated RAW264.7 cells | [62] |
| Phomaketides A−C (108−110) FR-111142 (111) |
Phoma sp. NTOU4195 | against NO with E max and IC50 value of 100% and 8.8 μM in LPS-activated RAW264.7 cells | [63] |
| Expansols A−F (112−117) | Glimastix sp. ZSDS1-F11 | against expression of COX-2 with IC50 values of 3.1, 5.6, 3.0, 5.1, 3.2, and 3.7 µM against expression of COX-1 with 5.3, 16.2, 30.2, 41.0, and 56.8 µM |
[64] |
| Spicarins C (118) and D (119) | S. elegans KLA03 | against NO with IC50 values of 30 and 75 µM in LPS-activated BV2 cells | [65] |
| (R)-5,6-dihydro-6-pentyl-2H-pyran-2-one (120) | Hypocreales sp. strain HLS-104 | against NO with Emax value of 26.46% at 1 μM in LPS-activated RAW264.7 cells | [36] |
| Mycoepoxydiene (121) | Diaporthe sp. HLY-1 | against NO and TNF-α, IL-6, and IL-1β in LPS-activated macrophages | [66] |
Table 4.
Anti-inflammatory peptides from marine fungi.
| Metabolites | Species | Activities | Reference |
|---|---|---|---|
| Methyl 3,4,5-trimethoxy-2-(2-(nicotinamido)benzamido)benzoate (122) | A. terreus | against NO with IC50 value of 5.48 μM in LPS-activated RAW264.7 cells | [25] |
| Violaceotide A (123) Diketopiperazine dimer (124) |
A. violaceofuscus | against IL-10 expression with inhibitory rate of 84.3% and 78.1% at 10 μM in LPS-activated THP-1 cells | [67] |
| Aurantiamide acetate (125) | Aspergillus sp. | against NO and PGE2 with IC50 values of 49.70 and 51.3 μM in LPS-activated BV2 cells | [68] |
| (S)-2-(2-hydroxypropanamido) Benzoic Acid (126) | P. citrinum SYP-F-2720 | with the swelling rate of 191% at 100 mg/kg | [69] |
| Oxepinamide A (127) | Acremonium sp. | inhibition rate of 82% at 50 μg per ear in RTX-activated mouse ear edema assay | [70] |
| Alternaramide (128) | Alternaria sp. SF-5016 | against NO and PGE2 with IC50 values ranging from 27.63 to 40.52 μM in LPS-activated RAW264.7 and BV2 cells | [71] |
Table 5.
Anti-inflammatory other compounds from marine fungi.
| Metabolites | Species | Activities | Reference |
|---|---|---|---|
| (3E,7E)-4,8-di-methyl-undecane-3,7-diene-1,11-diol (129) 14α-hydroxyergosta-4,7,22-triene-3,6-dione (130) | A. terreus | against NO with IC50 values of 17.45 and 29.34 μM in LPS-activated RAW264.7 cells | [25] |
| Methyl 8–hydroxy–3-methoxycarbonyl-2-methylenenonanoate (131) (3S)-Methyl 9-hydroxy-3-methoxycarbonyl-2-methylenenonanoate (132) | Penicillium sp. (J05B-3-F-1) | against IL-1β with weakly inhibition at 200 μM | [72] |
| Trichodermanone C (133) | T. citrinoviride | strong inhibitory effect on nitrite levels in LPS-activated J774A.1 macrophages | [73] |
Figure 1.
Anti-inflammatory compounds isolated from marine fungi according to structure types.
Figure 2.
The sources of marine fungal compounds with anti-inflammatory activities.
2. Alkaloids
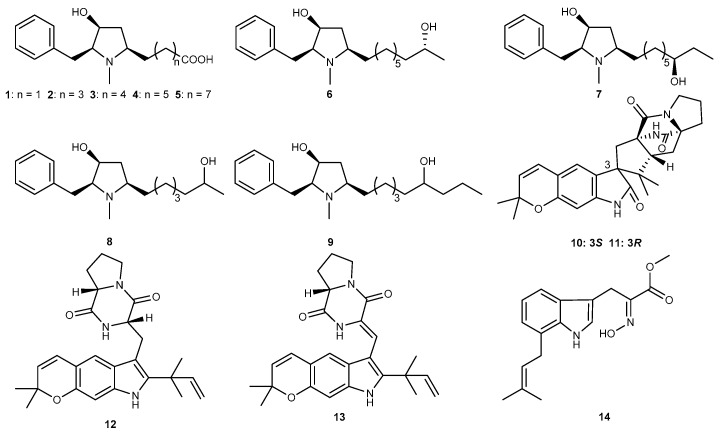
The fungus Aspergillus flocculosus 16D-1 was associated with the inner tissue of the sponge Phakellia fusca colonizing in Yongxing Island, China, and produced new pyrrolidine alkaloids, preussins C–I (1–7, Figure 3) and (11R)/(11S)–preussins J and K (8 and 9, Figure 3) [23]. Compounds 5 and 7 showed remarkable anti-inflammatory activity toward interleukin (IL)–6 production in lipopolysaccharide (LPS)–activated THP-1 cells with IC50 values of 0.11 μM and 0.19 μM, which was stronger than that of corylifol A, a positive control with the IC50 value of 0.67 μM, while other compounds possessed moderate inhibitory effects, with IC50 values in the range of 2.3 to 22 μM [23]. Chemical examination of the cultured mycelium of the fungus Aspergillus versicolor collected from the mud of the South China Sea led to the isolation of some novel linearly fused prenylated indole alkaloids: asperversiamides B, C, F, and G (10–13, Figure 3). These compounds exerted potential inducible nitric oxide synthase (iNOS) inhibitory effects and suppressed the release of nitric oxide (NO) in LPS-induced RAW264.7 cells. And of these compounds, asperversiamide G showed a potent inhibitory effect against iNOS with the IC50 value of 5.39 μM, while others exhibited weak activities with IC50 values ranging from 9.95 to 16.58 μM. Considering the significant inhibitory activity of asperversiamide B, it can synthesize the potential derivatives in the development of new anti-inflammatory drugs for the treatment of various related disorders [24]. A prenylated tryptophan derivative, luteoride E (14, Figure 3) was purified from the coral-associated fungus Aspergillus terreus associated with the coral Sarcophyton subviride, which was gathered from the coast of Xisha Island in the South China Sea. This compound exhibited inhibitory activity against NO production with IC50 value of 24.65 μM in LPS-stimulated RAW264.7 cells [25].
Figure 3.
Chemical structures of compounds 1–14.
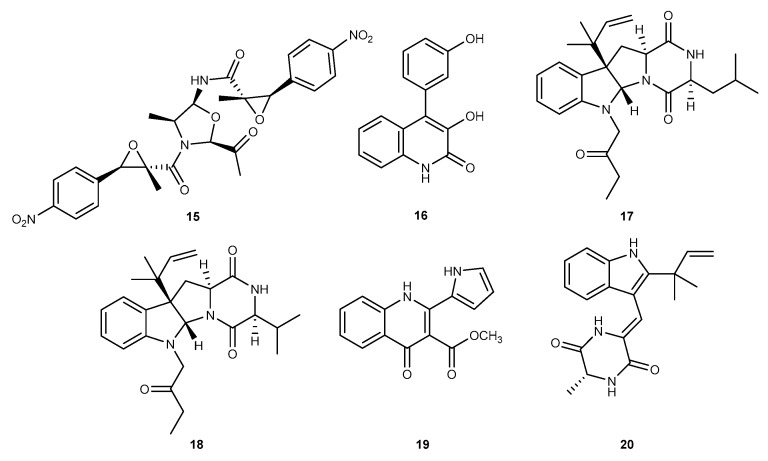
Chrysamide C (15, Figure 4), a new dimeric nitrophenyl trans-epoxyamides, was obtained from the marine-derived fungus Penicillium chrysogenum SCSIO41001, collected from deep sea sediment in the Indian Ocean [26]. Chrysamide C was observed to be most active on inhibitory activity toward the proinflammatory cytokine IL-17 production, while inhibitory rate of chrysamide C was found to 40.06% at 1.0 μM [26]. A new quinolone alkaloid, viridicatol (16, Figure 4), was discovered in the marine-derived fungus Penicillium sp. SF-5295 [27]. Compound 16 displayed anti-inflammatory potency in LPS-stimulated RAW264.7 cells and BV2 cells. Viridicatol inhibited the production of iNOS-derived NO in RAW264.7 cells with IC50 values of 46.03 μM in RAW264.7 cells and 43.03 μM in BV2 cells and suppressed the production of cyclooxygenase-2 (COX-2)-derived prostaglandin E2 (PGE2) with an IC50 value of 30.37 μM in RAW264.7 cells and 34.20 μM in BV2 cells. Compound 16 also inhibited the mRNA expression of IL-1β, IL-6, and tumor necrosis factor-α (TNF-α), which were pro-inflammatory cytokines [27]. In the further evaluation, compound 16 exerted anti-inflammatory activity through suppressing the NF-κB pathway by blocking the phosphorylation of inhibitor kappa B (IκB)-α, and suppressing the translocation of NF-κB dimers, namely p50 and p65 in RAW264.7 macrophages and BV2 microglia induced by LPS [27]. Another study on the Penicillium sp. derived from a deep ocean sediment resulted in the discovery of two novel diketopiperazine alkaloids, brevicompanines E and H (17 and 18, Figure 4) [28]. These compounds were shown to have the moderate anti-inflammatory activity to inhibit NO production in LPS-induced BV2 microglial cells, with IC50 values of 27 and 45 μg/mL, respectively [28]. In addition, these compounds displayed no cytotoxic effect at these concentrations. Some evidence indicate that substituents at the N-6 position were significant for inhibitory activity of NO production [28]. These compounds may be a potential for finding a chemotherapeutic candidate that has anti-inflammatory with no cytotoxic effects [28]. A soft coral samples collected at Terra Nova bay, Antaratica, resulted in the isolation of Penicillium sp. SF-5995, which led to the isolation of a pyrrolyl 4-quinoline alkaloid, methylpenicinoline (19, Figure 4) [29]. Compound 19 suppressed the NO and PGE2 production by attenuating iNOS and COX-2 expression, respectively, in LPS-stimulated RAW264.7 macrophages and BV2 microglia with the IC50 values of ranging from 34–49 μM [29]. Furthermore, compound 19 inhibited the pro-inflammatory cytokine IL-1β production [29]. In the further study, compound 19 suppressed the expression of pro-inflammatory cytokines through the NF-κB and mitogen-activated protein kinase (MAPK) pathway in LPS-induced RAW264.7 macrophages and BV2 cells [29]. Another marine fungus Eurotium sp. SF-5989 was also isolated from a soft coral collected at Terra Nova bay, Antarctica. Chemical investigation of the fungus Eurotium sp. SF-5989 afforded a diketopiperazine-type indole alkaloid, neoechinulin A (20, Figure 4) [30]. Compound 20 suppressed the production of pro-inflammatory mediators, NO and PGE2, and these inhibitory activities were mediated by inhibiting the expression of COX-2 and iNOS in RAW264.7 macrophages stimulated by LPS. The anti-inflammatory mechanism of compound 20 was due to attenuation of two major signaling pathways, NF-κB pathway and MAPK signaling pathway in LPS-stimulated RAW264.7 macrophages and BV2 microglia [30].
Figure 4.
Chemical structures of compounds 15–20.
3. Terpenoids
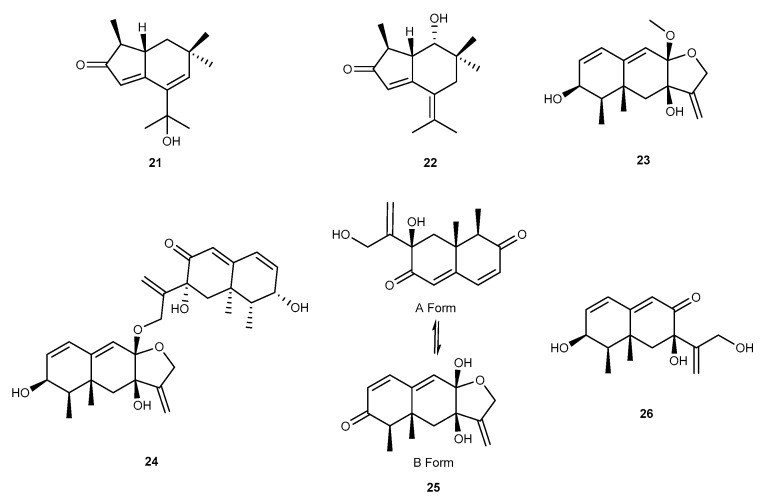
Two novel brasilane sesquiterpenoids, brasilanones A and E (21, 22, Figure 5), were separated from the extract of the marine-derived fungus A. terreus CFCC 81836, which displayed moderate inhibitory activities against NO production with inhibition rates of 47.7% and 37.3% at 40 μM in RAW264.7 mouse macrophages induced by LPS [31]. Liyan Wang et al. firstly reported three new eremophilane-type sesquiterpenoids of dihydrobipolaroxin B–D (23–25, Figure 5) and a known sesquiterpene of dihydrobipolaroxin (26, Figure 5). These compounds were isolated from a deep sea-derived fungus, Aspergillus sp. SCSIOW2, from a deep marine sediment sample gathered from the South China Sea at a depth of 2439 m [32]. All of these compounds were shown to have moderate anti-inflammatory effects to inhibit NO induced by LPS/INF-γ. Meanwhile, all four compounds exhibited no cytotoxic effects [32].
Figure 5.
Chemical structures of compounds 21–26.
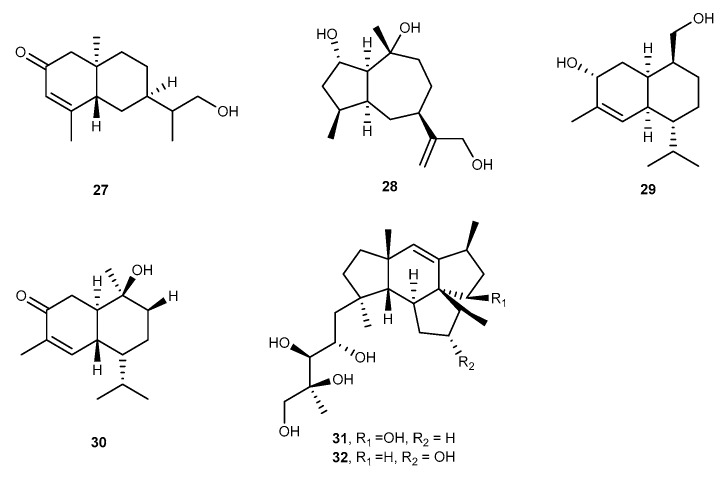
Thomimarine E (27, Figure 6) was a new eudesmane-type sesquiterpene that was obtained from marine fungus Penicillium thomii KMM 4667 [33]. Thomimarine E (27) exhibited anti-inflammatory effect and inhibited the production of NO in LPS-stimulated RAW264.7 cells with inhibition rate of 22.5% ± 5.1% at the concentration of 10.0 μM [33]. Graphostroma sp. MCCC 3A00421 isolated from Atlantic Ocean hydrothermal sulfide deposit at a depth of 2721 m produced a new guaiane, graphostromane F (28, Figure 6) [34]. Graphostromane F (28) exhibited considerable inhibitory activity by inhibiting the release of NO in RAW264.7 macrophages induced by LPS with an IC50 value of 14.2 μM, which was even lower than the aminoguanidine as positive control with an IC50 value of 23.4 μM [34]. Another study on the same Graphostroma sp. MCCC 3A00421 resulted in the discovery of a novel fungal sesquiterpene, khusinol B (29, Figure 6) [35]. Khusinol B (29) was found considerable anti-inflammatory activity in LPS-induced RAW264.7 cells against NO production with IC50 value of 17 μM, which was even stronger than that of the positive control with the IC50 value was 23 μM [35]. Chemical study of the sea-derived fungus Hypocreales sp. strain HLS-104, which was isolated from a sponge Gelliodes carnosa colonizing in the South China Sea afforded a derivative, 1R,6R,7R,10S-10-hydroxy-4(5)-cadinen-3-one (30, Figure 6) with moderate anti-inflammatory activity [36]. The average maximum inhibition (Emax) values of this molecule against the production of the NO in LPS-treated RAW264.7 cells was 10.22% at the concentration of 1 μM [36]. William Fenical et al., isolated mangicols A and B (31 and 32, Figure 6) from a marine fungus, Fusarium heterosporum CNC-477, which was separated from a driftwood sample collected from Sweetings Cay mangrove habitat, Bahamas [37]. Mangicols A and B were novel sesterterpene polyols that exhibited considerable anti-inflammatory effects in the phorbol myristate acetate (PMA)-induced mouse ear edema assay with the reduction of 81% and 57%, respectively, at the standard of 50 μg per ear which were similar to those of indomethacin, the positive control, with the reduction of 71% [37].
Figure 6.
Chemical structures of compounds 27–32.
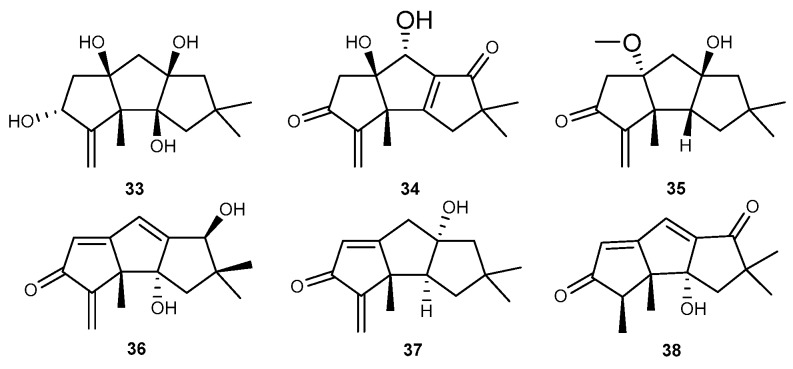
George Hsiao et al. reported the isolation of eight novel hirsutane-type sesquiterpenoids along with seven known derivatives from the EtOAc extract of the fermented broth of Chondrostereum sp. NTOU4196, a fungal strain isolated from the marine red alga Pterocladiella capillacea, collected from the northeast and north intertidal zone of Taiwan [38]. Among them, chondroterpenes A, B, H (33–35, Figure 7) and hirsutanol A (36, Figure 7), chondrosterins A and B (37 and 38, Figure 7) showed strong anti-inflammatory effects and possessed the expression of NO in murine BV-2 microglial cells stimulated by LPS at a concentration of 20 μM [38].
Figure 7.
Chemical structures of compounds 33–38.
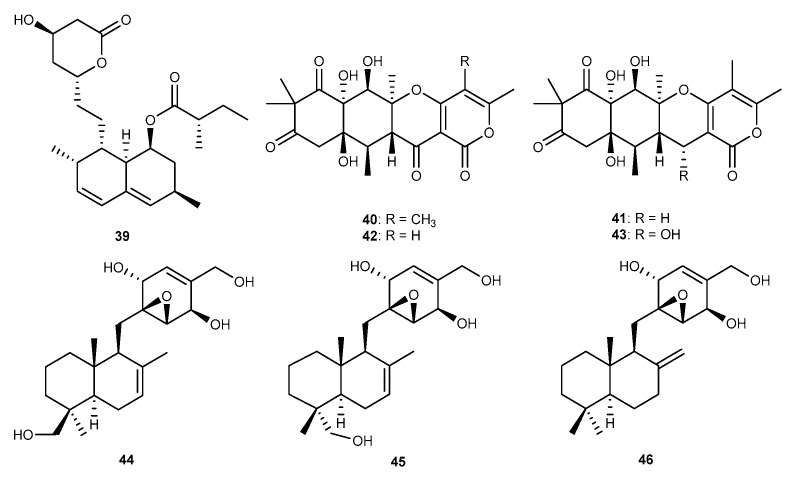
Lovastatin (39, Figure 8) was purified from the coral-associated fungus A. terreus associated with the coral S. subviride, collected from the coast of Xisha Island in the South China Sea [25]. This compound showed inhibitory activity on the NO production with IC50 value of 17.45 μM in RAW264.7 cells stimulated by LPS [25]. A sea green algal species Enteromorpha collected in Dongshi salt pan, Fujian Province, China, resulted in the isolation of a fungus Aspergillus sp. ZL0-1b14 [39]. The fungus extracts displaying anti-inflammatory activities was chemically analyzed, which led to the isolation of a family group of new triketide-sesquiterpenoid meroterpenoids, aspertetranones A−D (40−43, Figure 8) [39]. Aspertetranones A−D showed different anti-inflammatory activities. Notably, aspertetranones A and D exhibited the suppress potency against the production of IL-6 in LPS-stimulated RAW264.7 macrophages with 43% and 69% inhibition at 40 μM [39]. Chemical investigation of a marine-derived fungus, Pleosporales sp. strain derived from a marine alga Enteromorpha clathrate collected from the South China Sea in Hainan Province, yielded three new compounds, pleosporallins A−C (44−46, Figure 8) [40]. They possessed moderate inhibitory activities against the production of proinflammatory cytokine IL-6 in LPS-stimulated RAW264.7 macrophages cells with the inhibition rate about 30.0% compared to control at the concentration of 5−20 μg/mL [40].
Figure 8.
Chemical structures of compounds 39−46.
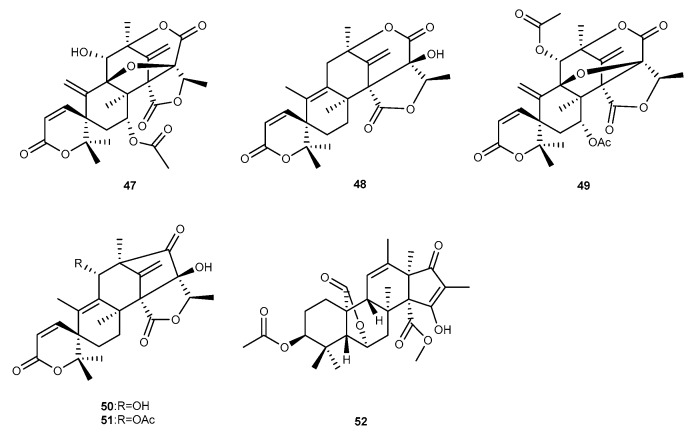
Jin-Soo Park et al. separated two novel meroterpenoid-type metabolites along with eight known analogs from the ethyl acetate extract of a marine-derived fungal strain Penicillium sp. SF-5497, which was isolated from a sample of sea sand collected from Gijiang-gun, Busan [41]. All the isolated metabolites were evaluated for anti-inflammatory activities against NO production in microglial BV-2 cells challenged by LPS, only 7-acetoxydehydroaustinol (47, Figure 9), and four other known analogs austinolide (48, Figure 9), 7-acetoxydehydroaustin (49, Figure 9), 11-hydroxyisoaustinone (50, Figure 9), and 11-acetoxyisoaustinone (51, Figure 9), were shown to have weak inhibitory effects with IC50 values of 61.0, 30.1, 58.3, 37.6, and 40.2 μM, respectively [41]. The marine fungus Penicillium atrovenetum was shown to produce an undescribed meroterpenoid, citreohybridonol (52, Figure 9) [42]. This compound was found to have anti-neuroinflammatory activity [42].
Figure 9.
Chemical structures of compounds 47−52.
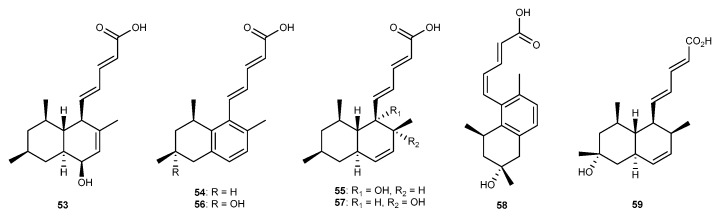
A new tanzawaic acid derivative, tanzawaic acid Q (53, Figure 10), together with four known analogues, tanzawaic acids A (54, Figure 10), C (55, Figure 10), D (56, Figure 10), and K (57, Figure 10), have been isolated from a marine-derived fungus, Penicillium steckii 108YD142, residing in a marine sponge sample collected at Wangdolcho, in the Republic of Korea’s Eastern reef [43]. These compounds considerably inhibited LPS-stimulated NO production in RAW264.7 macrophages cells. Moreover, tanzawaic acid Q reduced the expression of pro-inflammatory mediators such as COX-2 and iNOS and also possessed the production of PGE2, TNF-α, and IL-1β mRNA protein [43]. Marine-derived fungus Penicillium sp. SF-6013 derived from the sea urchin Brisaster latifrons collected from the Sea of Okhotsk, was shown to produce a new tanzawaic acid derivative, 2E,4Z-tanzawaic acid D (58, Figure 10), along with two known analogues, tanzawaic acids A (54) and E (59, Figure 10). These three tanzawaic acids inhibited the overproduction of NO in BV-2 microglial cells activated by LPS with IC50 values of 37.8, 7.1, and 42.5 μM, respectively [44]. Furthermore, tanzawaic acid A also inhibited the NO production and reduced the expression of iNOS and COX-2 in RAW264.7 and BV2 cells stimulated by LPS [44].
Figure 10.
Chemical structures of compounds 53−59.
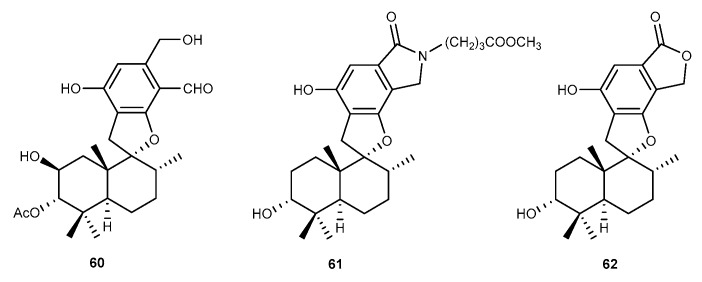
Three meroterpenoids, named as stachybotrysin C (60, Figure 11), stachybonoid F (61, Figure 11), and stachybotylactone (62, Figure 11) were obtained from Stachybotrys chartarum 952 isolated from a marine crinoid (Himerometra magnipinna) [45]. Compounds 60, 61, and 62 moderately suppressed the production of NO (the pro-inflammatory mediator) with IC50 values of 27.2, 52.5, and 17.9 μM in RAW264.7 macrophages stimulated by LPS [45].
Figure 11.
Chemical structures of compounds 60−62.
4. Polyketides
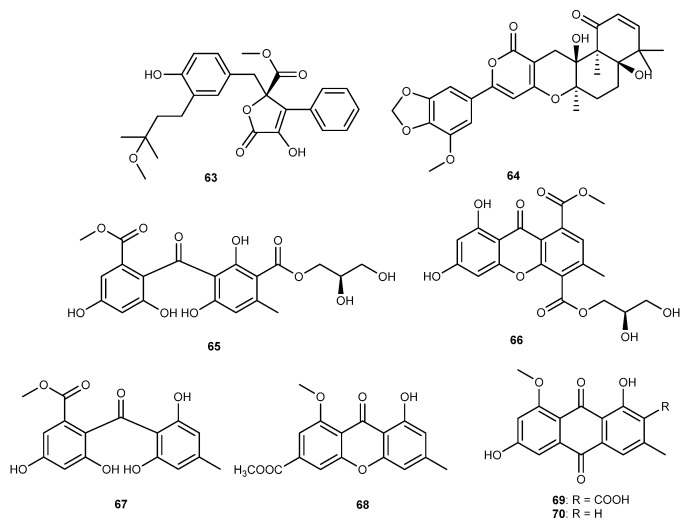
A detailed chemical investigation of a coral-associated fungus A. terreus, cultured from the coral S. subviride collected from the coast of Xisha Island in the South China Sea, resulted in the isolation of one unusual metabolite, versicolactone G (63, Figure 12), along with a known analog, territrem A (64, Figure 12) [25]. They were shown to potent anti-inflammatory activity with IC50 values of 15.72 and 29.34 μM, respectively, against LPS-induced NO production [25]. Aspergillus europaeus WZXY-SX-4-1 was found in the marine sponge Xestospongia testudinaria, and produced two new polyketide derivatives, eurobenzophenone B (65, Figure 12), xanthone A (66, Figure 12), along with four known compounds, 3-de-O-methylsulochrin (67, Figure 12), yicathin B (68, Figure 12), dermolutein (69, Figure 12), and methylemodin (70, Figure 12) [46]. 3-de-O-methylsulochrin showed the significant inhibition against NF-κB pathway in LPS-stimulated SW480 cells [46]. Eurobenzophenone B, xanthone A, yicathin B, dermolutein, and methylemodin showed to inhibit NF-κB pathway and weakly suppressed the expression of NO in LPS-stimulated SW480 cells [46].
Figure 12.
Chemical structures of compounds 63−70.
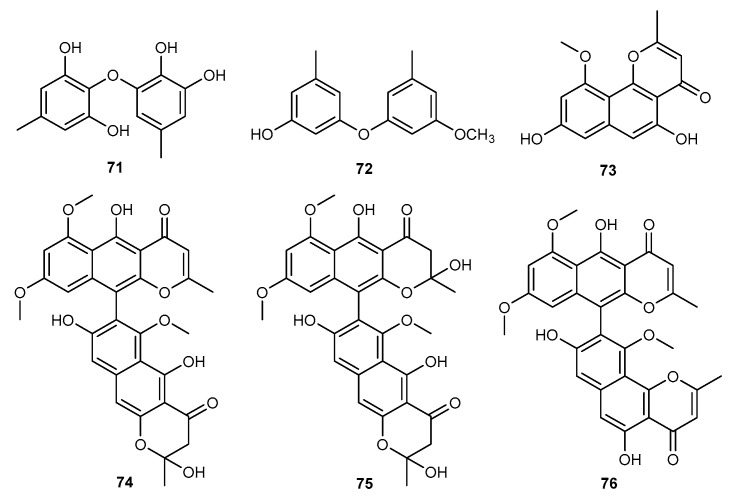
In order to search for bioactive secondary metabolites from marine fungi, Sen Liu et al. isolated two new metabolites together with six diphenylethers, a diketopiperazine, a chromone, and a xanthone from an EtOAc extract of the fungus Aspergillus sydowii J05B-7F-4 associated with the marine sponge Stelletta sp. [47]. Among them, only violaceol II (71, Figure 13) and cordyol E (72, Figure 13) displayed weak inhibitory effect against LPS-induced NO production in RAW264.7 cells [47]. A marine fungus, identified as Aspergillus sp. SF-6354, was found to produce TMC-256C1 (73, Figure 10) [48]. TMC-256C1 showed considerable anti-neuroinflammatory activity toward the mRNA expression of TNF-α, IL-6 and IL-12 production in LPS-activated BV2 cells. This compound also suppressed NO and PGE2 production in LPS-activated BV2 cells by the suppression of iNOS and COX-2 protein expression [48]. The surface of a marine algae Sargassum sp. from the Yongxing Island, South China Sea, provided Aspergillus niger SCSIO Jcsw6F30, which produced three asperpyrone-type bis-naphtho-γ-pyrones (BNPs): aurasperone F (74, Figure 13), aurasperone C (75, Figure 13), and asperpyrone A (76, Figure 13) [49]. These compounds possessed significant anti-inflammatory potency through down-regulate the expression of the COX-2 protein in LPS-activated RAW264.7 macrophages with IC50 values of 11.1, 4.2, and 6.4 μM, respectively [49].
Figure 13.
Chemical structures of compounds 71−76.
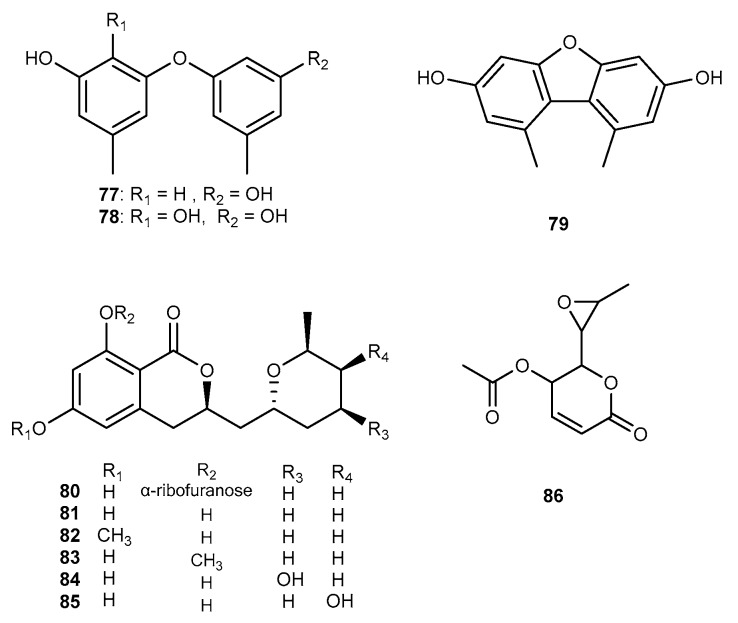
Two new compounds together with 10 known compounds were detected in the EtOAc extract of the fungal strain Aspergillus sp. SCSIO Ind09F01, which was isolated from the deep-sea sediment sample of Indian Ocean [50]. Among them, only three known compounds, diorcinol (77, Figure 14), cordyol C (78, Figure 14), and 3,7-dihydroxy-1,9-dimethyldibenzofuran (79, Figure 14) possessed the inhibitory effects on the expression of COX-2 with the IC50 values from 2.4 to 10.6 μM [50]. Dong-Cheol Kim et al. isolated a new dihydroisocoumarin derivative, cladosporin 8-O-α-ribofuranoside (80, Figure 14), along with five known metabolites, cladosporin (81, Figure 14), asperentin 6-O-methyl ether (82, Figure 14), cladosporin 8-O-methyl ether (83, Figure 14), 4′-hydroxyasperentin (84, Figure 14), and 5′-hydroxyasperentin (85, Figure 14) from the EtOAc extracts of marine-derived fungus Aspergillus sp. SF-5974 and Aspergillus sp. SF-5976, obtained from an unidentified red macroalgae collected using a dredge at a depth of 300 m at the Ross Sea [51]. These compounds showed to inhibit the production of NO and PGE2 in LPS-stimulated microglial cells with IC50 values ranging from 20 to 65 μM due to suppressing the expression of iNOS and COX-2, respectively [51]. Furthermore, cladosporin 8-O-α-ribofuranoside exhibited the suppression of the phosphorylation and degradation of IκB-α and NF-κB, and also reduced the activation of p38 mitogen-activated protein kinase (MAPK) [51]. The marine-derived Aspergillus sp. SF-5044 produced a crystalline metabolite, asperlin (86, Figure 14) [52]. The isolated compound 86 was evaluated for its anti-inflammatory potency. It suppressed the expression of the iNOS protein and reduced iNOS-derived NO, inhibited the expression of the COX-2 protein and reduced the COX-derived PGE2 in murine peritoneal macrophages and RAW264.7 caused by activated of LPS [52]. Compound 86 also can reduce the production of pro-inflammatory cytokines including TNF-α and IL-1β. In addition, it suppressed the phosphorylation of IκB-α and the p65 nuclear translocation [52]. Further, compound 86 reduced the expression of pro-inflammatory cytokines and mediators in LPS-activated RAW264.7 cells by increasing HO activity [52].
Figure 14.
Chemical structures of compounds 77−86.
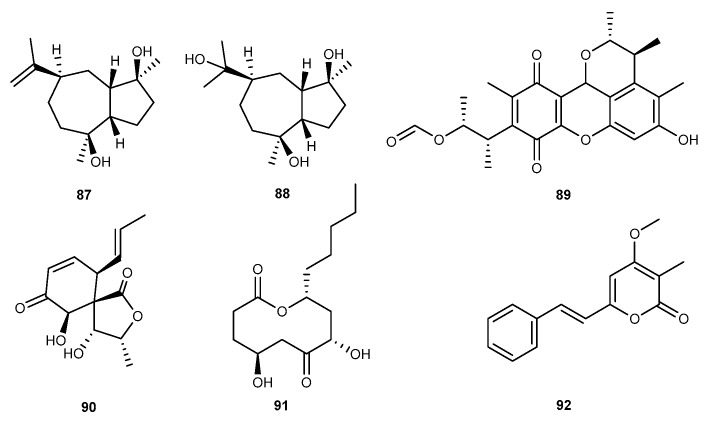
Guaiadiol A (87, Figure 15) and 4,10,11-trihydroxyguaiane (88, Figure 15) were obtained from marine fungus P. thomii KMM 4667 [33]. These compounds exhibited anti-inflammatory effects against NO production in LPS-stimulated murine macrophages by 24.1% ± 2.7%, and 36.6% ± 6.4%, respectively, at the concentration of 10.0 μM [33]. Nguyen Thi Thanh Ngan et al., isolated citrinin H1 (89, Figure 15) from the marine-derived fungal strain Penicillium sp. SF-5629 [53]. Citrinin H1 was found to be active on inhibitory effects on the production of NO and PGE2 in LPS-activated BV2 microglia, with IC50 values of 8.1 ± 1.9 and 8.0 ± 2.8 μM [53]. Penicillospirone (90, Figure 15), a new polyketide-type metabolite, was isolated from an EtOAc extract of the sea-derived fungal Penicillium sp. SF-5292 [27]. Penicillospirone exerted the anti-inflammatory effect on iNOS derived NO and COX-2 derived PGE2 production with IC50 values from 21.9 to 27.6 μM in RAW264.7 macrophages and BV2 microglia stimulated by LPS [27]. Furthermore, penicillospirone also suppressed the mRNA expression of proinflammatory cytokines, including TNF-α, IL-1β, IL-6, and IL-12. In the further evaluation, penicillospirone was shown to inhibit NF-κB pathway in RAW264.7 and BV2 cells stimulated by LPS [27]. Chemical study was applied to the EtOAc extract of marine Penicillium sp. SF-5292, resulting in the discovery of a new 10-membered lactone, penicillinolide A (91, Figure 15) [54]. Penicillinolide A inhibited the NO and PGE2 production by suppressing the expression of iNOS and COX-2 in LPS-stimulated macrophages, with IC50 values of 20.47 and 17.54 μM [54]. Penicillinolide A also inhibited the mRNA expression of TNF-α, IL-1β, and IL-6 with IC50 values of 8.63, 11.32, and 20.92 μM due to the degradation of IκB-α, NF-κB nuclear translocation, and NF-κB DNA binding activity [54]. Dong-Sung Lee et al., successfully purified penstyrylpyrone (92, Figure 15), a styrylpyrone-type metabolite from the methylethylketone extract of sea-derived fungus Penicillium sp. JF-55 colonizing in an unidentified sponge gathered from the shores of Jeju Island [55]. Penstyrylpyrone inhibited the overproduction of NO and PGE2 with IC50 values of 12.32 and 9.35 μM in LPS-stimulated murine peritoneal macrophages and these inhibitory activities were correlated with the overexpressions of iNOS and COX-2, respectively. Penstyrylpyrone also inhibited the mRNA expression of pro-inflammatory cytokines such as TNF-α, IL-1β with IC50 values of 13.54 and18.32 μM [55]. In addition, penstyrylpyrone was shown to inhibit IκB-α pathway and the NF-κB DNA-binding activity in LPS-stimulated murine peritoneal macrophages [55].
Figure 15.
Chemical structures of compounds 87−92.
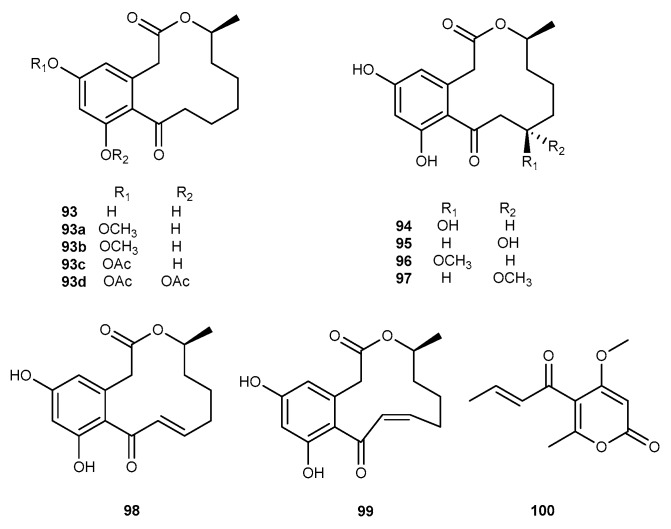
Chemical study on a marine-derived fungal strain Penicillium sp. SF-5859 resulted in the discovery of seven compounds, namely curvularin (93, Figure 16), (11R,15S)-11-hydroxycurvularin (94, Figure 16), (11S,15S)-11-hydroxycurvularin (95, Figure 16), (11R,15S)-11-methoxycurvularin (96, Figure 16), (11S,15S)-11-methoxycurvularin (97, Figure 16), (10E,15S)-10,11-dehydrocurvularin (98, Figure 16), and (10Z,15S)-10,11-dehydrocurvularin (99, Figure 16) [56]. These analogs exhibited strong inhibitory effects on NO and PGE2 with IC50 values ranging from 1.9 to 18.1 µM, and from 2.8 to 18.7 µM, respectively, in RAW264.7 cells induced by LPS [56]. Compound 99 also suppressed the production of iNOS and COX-2. Furthermore, (10E,15S)-10,11-dehydrocurvularin exhibited to inhibit the NF-κB pathway [56]. The fungus Penicillium paxilli Ma(G)K isolated from a sponge sample Mycale angulosa, produced a novel compound pyrenocine A (100, Figure 16), which possessed considerable anti-inflammatory effect against TNF-α and PGE2 in LPS-stimulated macrophages [57].
Figure 16.
Chemical structures of compounds 93−100.
Asperflavin (101, Figure 17) was isolated from the sea-derived fungus Eurotium amstelodami [58]. Asperflavin displayed the overproduction of proinflammatory mediators NO and PGE2 in LPS-stimulated RAW264.7 cells by 4.6% and 55.9% at the concentration of 200 μM. Additionally, asperflavin possessed the expression of mRNA proinflammatory cytokines, including TNF-α, IL-1β, IL-6, and IL-12 [58]. Chemical study of the marine-derived fungus E. amstelodami separated from an unidentified marine animal collected from the Sungsan coast in Jeju Island, Korea, have been found an anthraquinone analog, questinol (102, Figure 17) [59]. Questinol showed considerable inhibitory effect on NO and PGE2 production in LPS-stimulated RAW264.7 cells with the inhibition rates of 73.0% and 43.5% at the concentrations of 200 μM and also displayed to inhibit the production of pro-inflammatory cytokines such as TNF-α, IL-1β, and IL-6 [59]. Furthermore, questinol also suppressed the protein expression of iNOS but weak inhibited the protein expression of COX-2 at the concentration of 200 μM [59]. Two benzaldehyde-type fungal analogs, flavoglaucin (103, Figure 17) and isotecrahydro-auroglaucin (104, Figure 17) were extracted in culture extracts of Eurotium sp. SF-5989 [60]. Compounds 103 and 104 can suppress the production of pro-inflammatory mediators, NO and PGE2, and these inhibitory activities were mediated by inhibiting the expression of COX-2 and iNOS in RAW264.7 macrophages stimulated by LPS. The anti-inflammatory activities of compounds 103 and 104 were due to attenuation of major signaling pathways, NF-κB pathway in LPS-stimulated RAW264.7 macrophages. Furthermore, the anti-inflammatory effects of these were observed through reduction of HO-1 expression regulated by nuclear transcription factorE2-related factor 2 (Nrf2) [60].
Figure 17.
Chemical structures of compounds 101−104.
One new phenolic metabolite, 1-(2,5-dihydroxyphenyl)-3-hydroxybutan-1-one (105, Figure 18), along with 1-(2,5-dihydroxyphenyl)-2-buten-1-one (106, Figure 18), were found in the EtOAc extract of the marine endophytic fungus Paraconiothyrium sp. VK-13 [61]. 1-(2,5-dihydroxyphenyl)-3- hydroxybutan-1-one and 1-(2,5-dihydroxyphenyl)-2-buten-1-one displayed inhibitory effect against iNOS derived NO and COX-2 derived PGE2 in LPS-stimulated RAW264.7 cells, with IC50 values from 3.9 and 12.5 µM [61]. The anti-inflammatory effects of these compounds were attributed to the significant inhibition of the expression of iNOS and COX-2 proteins and the inhibition of mRNA expression of anti-pressure cytokines including TNF-α, IL-1β, IL-6, and IL-12 [61]. From a marine crinoid collected in Xuwen, Zhanjing City, Guangdong Province, China, fungal strain Leptosphaerulina chartarum 3608, was selected for chemical study [62]. One new secondary metabolite, (4R,10S,4’S)-leptothalenone B (107, Figure 18), was discovered in the fungus [62]. It was observed to suppress NO production in LPS-induced RAW264.7 cells, with an IC50 value of 44.5 µM [62]. A detailed chemical investigation of an in-house marine-derived fungi Phoma sp. NTOU4195 associated with the marine red alga P. capillacea resulted in the isolation of three novel polyketides with anti-inflammatory activity, phomaketides A−C (108−110, Figure 18), along with a known analog, FR-111142 (111, Figure 18) [63]. Phomaketides A−C and FR-111142 exhibited strong inhibitory effect on NO production murine macrophage RAW264.7 cells induced by LPS [63]. Phomaketides C exerted the most significant inhibition activity with Emax and IC50 value of 100% and 8.8 μM, respectively [63].
Figure 18.
Chemical structures of compounds 105−111.
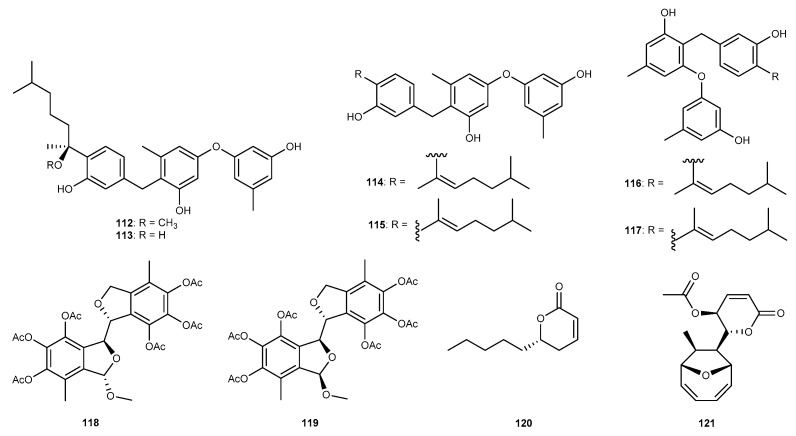
Expansols A−F (112−117, Figure 19) were polyphenols that were isolated from the marine-derived fungus Glimastix sp. ZSDS1-F11 associated with marine sponge samples, P. fusca gathered from the Yongxing island of Xisha [64]. All these compounds strongly suppressed the protein expression of COX-2 with IC50 values of 3.1, 5.6, 3.0, 5.1, 3.2, and 3.7 µM, respectively [64]. Furthermore, expansols A−F also exhibited strong COX-1 inhibitory activity with IC50 values of 5.3, 16.2, 30.2, 41.0 and 56.8 µM, respectively [64]. Two isobenzofuran dimers, spicarins C (118, Figure 19) and D (119, Figure 19), were purified from the marine-derived fungus Spicaria elegans KLA03 collected from the marine sediments in Jiaozhou Bay, China [65]. These compounds inhibited the overproduction of NO in BV2 microglial cells induced with LPS with IC50 values of 30 and 75 µM, respectively [65]. Chemical study of the sea-derived fungus Hypocreales sp. strain HLS-104 isolated from a sponge G. carnosa colonizing in the South China Sea afforded two derivatives, (R)-5,6-dihydro-6-pentyl-2H-pyran-2-one (120, Figure 19) [36]. They showed moderate anti-inflammatory activity against the production of the NO in LPS-treated RAW264.7 cells with average maximum inhibition (Emax) values of 26.46% at 1 μM [36]. The polyketide mycoepoxydiene (121, Figure 19) was discovered from Diaporthe sp. HLY-1, which was isolated from submerged rotten leaves of Kandelia candel collected in a mangrove forest in Fujian Province, China [66]. Compound 121 markedly suppressed the LPS-stimulated production of pro-inflammatory mediators and cytokines such as NO, TNF-α, IL-6, and IL-1β in macrophages [66]. Furthermore, the effect of compound 121 on LPS-stimulated activation were due to block the NF-κB pathway and MAPK signaling pathway [66].
Figure 19.
Chemical structures of compounds 112−121.
5. Peptides
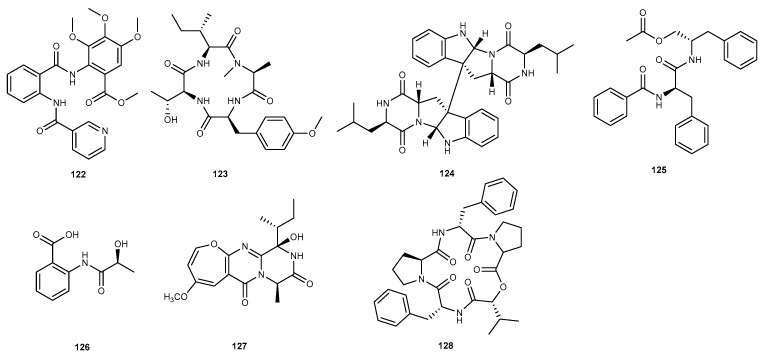
Methyl 3,4,5-trimethoxy-2-(2-(nicotinamido) benzamido) benzoate (122, Figure 20), was isolated from a coral-associated fungus A. terreus associated with the coral S subviride, which was gathered from Xisha Island in the South China Sea [25]. This compound showed a considerable anti-inflammatory activity with an IC50 value of 5.48 μM [25]. Bioassay-guided investigation of the EtOAc extract of marine sponge-derived fungus Aspergillus violaceofuscus afforded new anti-inflammatory activity metabolites named violaceotide A (123, Figure 20) and diketopiperazine dimer (124, Figure 20) [67]. The fungus A. violaceofuscus was isolated from the inner part of the marine sponge Reniochalina sp. collected from the Xisha Islands in the South China Sea. Violaceotide A and diketopiperazine dimer reduced IL-10 expression in THP-1 cells stimulated by LPS with inhibitory rate of 84.3% and 78.1% at concentration of 10 μM, respectively [67]. Investigation of biologically active peptides from the marine fungus Aspergillus sp. SF-5921 (from an unidentified sponge, Sea of Ross) resulted in isolation of aurantiamide acetate (125, Figure 20) [68]. Compound 125 showed inhibitory potency against the LPS-stimulated production of NO and PGE2 with IC50 values of 49.70 and 51.3 μM in BV2 microglia cells [68]. In addition, it has anti-neuron-flammatory effects through its inhibition of the NF-κB, c-Jun N-terminal kinases (JNK), and p38 pathways [68]. (S)-2-(2-hydroxypropanamido) benzoic acid (126, Figure 20), a novel benzoic acid, was isolated as natural product from a sponge-derived marine fungus P. chrysogenum SYP-F-2720 [69]. Compound 126 exhibited stronger anti-inflammatory activity than aspirin (swelling rate of 193%) with the swelling rate of 191% in the mouse ear edema model induced by xylene when administered at 100 mg/kg [69]. Chemical investigation of a marine-derived fungus Acremonium sp. from the surface of the Caribbean tunicate Ecteinascidia turbinata. yielded a new peptide derivative, oxepinamide A (127, Figure 20) [70]. Oxepinamide A showed potent anti-inflammatory effect in a topical resiniferatoxin (RTX)-induced mouse ear edema assay, with the inhibition rate of 82% at the standard testing dose of 50 μg per ear [70]. Alternaramide (128, Figure 20), a marine Alternaria sp. SF-5016 metabolite, was interesting in that it contained unusual hydrophobic D-amino acid residues [71]. Compound 128 suppressed the production of PGE2 and NO, and these inhibitory effects were correlated with down-regulation of iNOS and COX-2 expression in LPS-induced RAW264.7 and BV2 macroglia cells with IC50 values ranging from 27.63 to 40.52 μM [71]. It also inhibited pro-inflammatory cytokines, such as TNF-α, IL-1β, IL-6, and IL-12 in LPS-induced RAW264.7 and BV2 macroglia cells. In addition, the compound 128 suppressed the NF-κB and MAPK signaling pathway. Furthermore, compound 128 significantly reduced the Toll-like receptor 4 (TLR4) and myeloid differentiation primary response gene 88 (MyD88) in LPS-induced RAW264.7 and BV2 macroglia cells at the mRNA and protein levels [71].
Figure 20.
Chemical structures of compounds 122−128.
6. Others
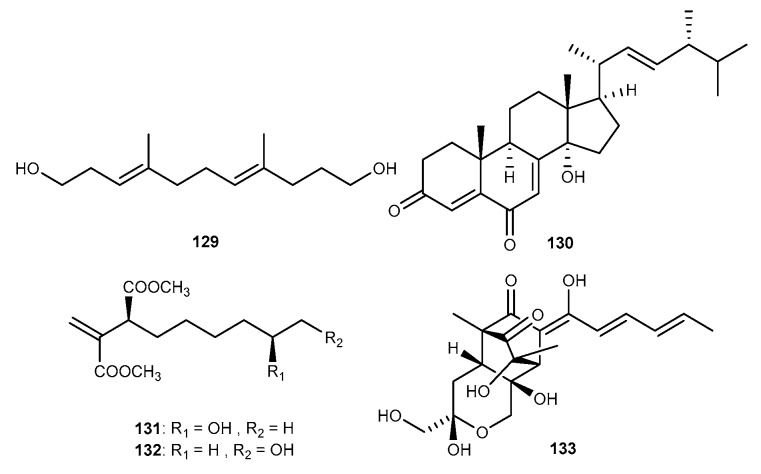
A novel linear aliphatic alcohol, (3E,7E)-4,8-di-methyl-undecane-3,7-diene-1,11-diol (129, Figure 21), together with three known compounds, 14α-hydroxyergosta-4,7,22-triene-3,6-dione (130, Figure 21) were purified from the coral-associated fungus A. terreus associated with the coral S. subviride, which was collected from the coast of Xisha Island in the South China Sea [25]. These compounds exhibited considerable inhibitory activity against NO production with IC50 values ranging from 17.45 to 29.34 μM [25]. Two hexylitaconic acid derivatives, methyl8-hydroxy- 3-methoxycarbonyl-2-methylenenonanoate (131, Figure 21) and (3S)-methyl9-hydroxy-3- methoxycarbonyl-2-methylenenonanoate (132, Figure 21), were separated from the EtOAc extract of the fungal strain Penicillium sp. (J05B-3-F-1) colonizing in a sponge Stelletta sp. collected from the coast of Jeju island, Korea [72]. The two isolates weakly inhibited the production of IL-1β at the concentration of 200 μM [72]. Trichodermanone C (133, Figure 21) was isolated from the marine fungal strain A12 of Trichoderma citrinoviride associated with the green alga Cladophora sp. collected in Italy [73]. Trichodermanone C was evaluated to show strong inhibitory effect on nitrite levels in LPS-stimulated J774A.1 macrophages [73].
Figure 21.
Chemical structures of compounds 129−133.
7. Conclusions
The inflammatory disease is one of the most common diseases around the world [74]. Recent literatures showed that the prevalence, severity, and complexity of the disease were rising rapidly and adding to the healthcare costs considerably [75]. What is more, the inflammatory disease operates by an advanced system and has a broad influence on physiological aspects and human pathology. Currently, with the development of the synthetic drug formulation, some classes of anti-inflammatory drugs such as aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), and corticosteroids are used in the clinic [76]. However, all the therapeutics can cause quite harmful side effects to human beings after long-term and high-dose medication. Marine fungi have the potential ability to produce diverse chemical structures with anti-inflammatory activities. During 2000–2018, about 133 anti-inflammatory compounds in 52 references belonging to five diverse chemical classes were reported, including alkaloids, terpenoids, polyketides, peptides, and others. Over 50 compounds were found to display significantly anti-inflammatory activities. For example, preussions G (5) and I (7), graphostromanes F (28), khusinol B (29), and mangicol A (31), which IC50 values or reduction were even stronger than that positive control. From distribution point of view, 75% of all anti-inflammatory structures were polyketides and terpenoids indicating that polyketides and terpenoids have great potential in the development of anti-inflammatory drugs. This review provided a lot of potential lead compounds for finding novel anti-inflammatory agents from marine-derived fungi, especially, Aspergillus (41.4%), and Penicillium (27.1%). Lots of potential agents derived from marine fungi were found to have significant effects against inflammation. Therefore, it could be suggested that marine fungi-derived natural products will play a vital role in developing novel drugs against inflammation with satisfactory tolerability for long-term use [77,78].
Author Contributions
J.X., M.Y. and L.D. collected the references; J.X. and S.H. analyzed the data; J.X. and L.D. wrote the paper.
Funding
This research was funded by Natural Science Foundation of China (41776168, 41706167), the National College Students’ Innovation and Entrepreneurship Training Program, the Program of “Xinmiao” Talents in Zhejiang Province, Ningbo Public Service Platform for High-Value Utilization of Marine Biological Resources (NBHY-2017-P2), the National 111 Project of China (D16013), the Li Dak Sum Yip Yio Chin Kenneth Li Marine Biopharmaceutical Development Fund, and the K.C. Wong Magna Fund in Ningbo University.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Coussens L.M., Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhong Y., Chiou Y.S., Pan M.H., Shahidia F. Anti-inflammatory activity of lipophilic epigallocatechin gallate (EGCG) derivatives in LPS-stimulated murine macrophages. Food Chem. 2012;134:742–748. doi: 10.1016/j.foodchem.2012.02.172. [DOI] [PubMed] [Google Scholar]
- 3.Hunter P. The inflammation theory of disease. EMBO Rep. 2012;13:968–970. doi: 10.1038/embor.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schetter A.J., Heegaard N.H., Harris C.C. Inflammation and cancer: Interweaving microRNA, free radical, cytokine and p53 pathways. Carcinogenesis. 2010;31:37–49. doi: 10.1093/carcin/bgp272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotamisligil G.S. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 6.Gilmore T.D. The Rel/NF-κB signal transduction pathway: Introduction. Oncogene. 1999;18:6842–6844. doi: 10.1038/sj.onc.1203237. [DOI] [PubMed] [Google Scholar]
- 7.Dray A. Inflammatory mediators of pain. Br. J. Anaesth. 1995;75:125–131. doi: 10.1093/bja/75.2.125. [DOI] [PubMed] [Google Scholar]
- 8.Zhang J., An J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007;45:27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vo T., Ngo D., Kim S. Potential targets for anti-inflammatory and anti-allergic activities of marine algae: an overview. Inflamm. Allergy—Drug Targets. 2012;11:90–101. doi: 10.2174/187152812800392797. [DOI] [PubMed] [Google Scholar]
- 10.Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 11.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 12.Kotas M.E., Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell. 2015;160:816–827. doi: 10.1016/j.cell.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernando I.P.S., Nah J., Jeon Y. Potential anti-inflammatory natural products from marine algae. Environ. Toxicol. Pharmacol. 2016;48:22–30. doi: 10.1016/j.etap.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 14.Niu X., Wang Y., Li W., Zhang H., Wang X., Mu Q., He Z., Yao H. Esculin exhibited anti-inflammatory activities in vivo and regulated TNF-α and IL-6 production in LPS-stimulated mouse peritoneal macrophages in vitro through MAPK pathway. Int. Immunopharmacol. 2015;29:779–786. doi: 10.1016/j.intimp.2015.08.041. [DOI] [PubMed] [Google Scholar]
- 15.Yasukawa K., Takido M., Takeuchi M., Nakagawa S. Effect of chemical constituents from plants on 12-O-tetradecanoylphorbol-13-acetate-induced inflammation in mice. Chem. Pharm. Bull. 1989;37:1071–1073. doi: 10.1248/cpb.37.1071. [DOI] [PubMed] [Google Scholar]
- 16.Blunt J.W., Carroll A.R., Copp B.R., Davis R.A., Keyzers R.A., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2018;35:8–53. doi: 10.1039/C7NP00052A. [DOI] [PubMed] [Google Scholar]
- 17.Ramos A.A., Pratasena M., Castrocarvalho B., Dethoup T., Buttachon S., Kijjoa A., Rocha E. Potential of four marine-derived fungi extracts as anti-proliferative and cell death-inducing agents in seven human cancer cell lines. Asian Pac. J. Trop. Med. 2015;8:798–806. doi: 10.1016/j.apjtm.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 18.Blunt J.W., Copp B.R., Keyzers R.A., Munro M.H.G., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2009;26:170–244. doi: 10.1039/b805113p. [DOI] [PubMed] [Google Scholar]
- 19.Blunt J.W., Copp B.R., Munro M.H.G., Northcote P.T., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2011;28:196–268. doi: 10.1039/C005001F. [DOI] [PubMed] [Google Scholar]
- 20.Fleming A. On the antibacterial action of cultures of a Penicillium, with Special Reference to their use in the isolation of B. influenzæ. Br. J. Exp. Pathol. 1929;10:226–236. doi: 10.1093/clinids/2.1.129. [DOI] [Google Scholar]
- 21.Antia B.S., Aree T., Kasettrathat C., Wiyakrutta S., Ekpa O.D., Ekpe U.J., Mahidol C., Ruchirawat S., Kittakoop P. Itaconic acid derivatives and diketopiperazine from the marine-derived fungus Aspergillus aculeatus CRI322-03. Phytochemistry. 2011;72:816–820. doi: 10.1016/j.phytochem.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 22.Ducklow H.W., Carlson C.A. Oceanic bacterial production. Adv. Microb. Ecol. 1992;12:113–181. [Google Scholar]
- 23.Gu B., Jiao F., Wu W., Jiao W., Li L., Sun F., Wang S., Yang F., Lin H. Preussins with inhibition of IL-6 expression from Aspergillus flocculosus 16D-1, a fungus isolated from the marine sponge Phakellia fusca. J. Nat. Prod. 2018;81:2275–2281. doi: 10.1021/acs.jnatprod.8b00662. [DOI] [PubMed] [Google Scholar]
- 24.Li H., Sun W., Deng M., Zhou Q., Wang J., Liu J., Chen C., Qi C., Luo Z., Xue Y. Asperversiamides, linearly fused prenylated indole alkaloids from the marine-derived fungus Aspergillus versicolor. J. Org. Chem. 2018;83:8483–8492. doi: 10.1021/acs.joc.8b01087. [DOI] [PubMed] [Google Scholar]
- 25.Liu M., Sun W., Wang J., He Y., Zhang J., Li F., Qi C., Zhu H., Xue Y., Hu Z., et al. Bioactive secondary metabolites from the marine-associated fungus Aspergillus terreus. Bioorg. Chem. 2018;80:525–530. doi: 10.1016/j.bioorg.2018.06.029. [DOI] [PubMed] [Google Scholar]
- 26.Chen S., Wang J., Lin X., Zhao B., Wei X., Li G., Kaliaperumal K., Liao S., Yang B., Zhou X., et al. Chrysamides A-C, three dimeric nitrophenyl trans-epoxyamides produced by the deep-sea-derived fungus Penicillium chrysogenum SCSIO41001. Cheminform. 2016;18:3650–3653. doi: 10.1021/acs.orglett.6b01699. [DOI] [PubMed] [Google Scholar]
- 27.Ko W., Sohn J.H., Kim Y.-C., Oh H. Viridicatol from marine-derived fungal strain Penicillium sp. SF-5295 exerts anti-inflammatory effects through inhibiting NF-κB signaling pathway on lipopolysaccharide-induced RAW264. 7 and BV2 cells. Nat. Prod. Sci. 2015;21:240–247. doi: 10.20307/nps.2015.21.4.240. [DOI] [Google Scholar]
- 28.Du L., Yang X., Zhu T., Wang F., Xiao X., Park H., Gu Q. Diketopiperazine alkaloids from a deep ocean sediment derived fungus Penicillium sp. Chem. Pharm. Bull. 2009;57:873–876. doi: 10.1248/cpb.57.873. [DOI] [PubMed] [Google Scholar]
- 29.Dong-Cheol K., Hee-Suk L., Wonmin K., Dong-Sung L., Jae S., Joung Y., Youn-Chul K., Hyuncheol O. Anti-Inflammatory effect of methylpenicinoline from a marine isolate of Penicillium sp. (SF-5995): inhibition of NF-κB and MAPK pathways in lipopolysaccharide-induced RAW264.7 macrophages and BV2 microglia. Molecules. 2014;19:18073. doi: 10.3390/molecules191118073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kyoung-Su K., Xiang C., Dong-Sung L., Jae S., Joung Y., Youn-Chul K., Hyuncheol O. Anti-inflammatory effect of neoechinulin A from the marine fungus Eurotium sp. SF-5989 through the suppression of NF-кB and p38 MAPK pathways in lipopolysaccharide-stimulated RAW264.7 macrophages. Molecules. 2013;18:13245–13259. doi: 10.3390/molecules181113245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu Z., Li D., Zeng F., Tong Q., Zheng Y., Liu J., Zhou Q., Li X.N., Chen C., Lai Y. Brasilane sesquiterpenoids and dihydrobenzofuran derivatives from Aspergillus terreus [CFCC 81836] Phytochemistry. 2018;156:159–166. doi: 10.1016/j.phytochem.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 32.Wang L., Li M., Tang J., Li X. Eremophilane sesquiterpenes from a deep marine-derived fungus, Aspergillus sp. SCSIOW2, cultivated in the presence of epigenetic modifying agents. Molecules. 2016;21:473. doi: 10.3390/molecules21040473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Afiyatullov S.S., Leshchenko E.V., Sobolevskaya M.P., Antonov A.S., Denisenko V.A., Popov R.S., Khudyakova Y.V., Kirichuk N.N., Kuz’Mich A.S., Pislyagin E.A. New thomimarine E from marine isolate of the fungus Penicillium thomii. Chem. Nat. Compd. 2017;53:290–294. doi: 10.1007/s10600-017-1972-9. [DOI] [Google Scholar]
- 34.Niu S., Xie C., Xia J., Luo Z., Shao Z., Yang X. New anti-inflammatory guaianes from the Atlantic hydrotherm-derived fungus Graphostroma sp. MCCC 3A00421. Sci. Rep. 2018;8:530. doi: 10.1038/s41598-017-18841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niu S., Xie C., Zhong T., Xu W., Luo Z., Shao Z., Yang X. Sesquiterpenes from a deep-sea-derived fungus Graphostroma sp. MCCC 3A00421. Tetrahedron. 2017;73:7267–7273. doi: 10.1016/j.tet.2017.11.013. [DOI] [Google Scholar]
- 36.Hong Z., Hua X.X., Gong T., Jie P., Qi H., Ping Z. Hypocreaterpenes A and B, cadinane-type sesquiterpenes from a marine-derived fungus, Hypocreales sp. Phytochem. Lett. 2013;6:392–396. [Google Scholar]
- 37.Renner M.K., Jensen P.R., Fenical W. Mangicols: Structures and biosynthesis of a new class of sesterterpene polyols from a marine fungus of the genus Fusarium. J. Org. Chem. 2000;31:4843–4852. doi: 10.1021/jo000081h. [DOI] [PubMed] [Google Scholar]
- 38.Hsiao G., Chi W.C., Pang K.L., Chen J.J., Kuo Y.H., Wang Y.K., Cha H.J., Chou S.C., Lee T.H. Hirsutane-type sesquiterpenes with Inhibitory Activity of Microglial Nitric Oxide Production from the Red Alga-Derived Fungus Chondrostereum sp. NTOU4196. J. Nat. Prod. 2017;80:1615–1622. doi: 10.1021/acs.jnatprod.7b00196. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y., Qi S., Zhan Y., Zhang N., Wu A.A., Gui F., Guo K., Yang Y., Cao S., Hu Z., et al. Aspertetranones A-D, putative meroterpenoids from the marine algal-associated fungus Aspergillus sp. ZL0-1b14. J. Nat. Prod. 2015;78:2405–2410. doi: 10.1021/acs.jnatprod.5b00487. [DOI] [PubMed] [Google Scholar]
- 40.Chen C.J., Zhou Y.Q., Liu X.X., Zhang W.J., Hu S.S., Lin L.P., Huo G.M., Jiao R.H., Tan R., Ge H.M. Antimicrobial and anti-inflammatory compounds from a marine fungus Pleosporales sp. Tetrahedron Lett. 2015;56:6183–6189. doi: 10.1016/j.tetlet.2015.09.079. [DOI] [Google Scholar]
- 41.Park J.S., Quang T.H., Yoon C.S., Kim H.J., Sohn J.H., Oh H. Furanoaustinol and 7-acetoxydehydroaustinol: New meroterpenoids from a marine-derived fungal strain Penicillium sp. SF-5497. J. Antibiot. 2018;71:557–563. doi: 10.1038/s41429-018-0034-2. [DOI] [PubMed] [Google Scholar]
- 42.Özkaya F.C., Ebrahim W., Klopotowski M., Liu Z., Janiak C., Proksch P. Isolation and X-ray structure analysis of citreohybridonol from marine-derived Penicillium atrovenetum. Nat. Prod. Res. 2017;32:840–843. doi: 10.1080/14786419.2017.1311893. [DOI] [PubMed] [Google Scholar]
- 43.Shin H.J., Pil G.B., Heo S.J., Lee H.S., Lee J.S., Lee Y.J., Lee J., Won H.S. Anti-inflammatory activity of Tanzawaic acid derivatives from a marine-derived fungus Penicillium steckii 108YD142. Mar. Drugs. 2016;14:14. doi: 10.3390/md14010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quang T.H., Ngan N.T.T., Ko W., Kim D., Yoon C., Sohn J.H., Yim J.H., Kim Y., Oh H. Tanzawaic acid derivatives from a marine isolate of Penicillium sp. (SF-6013) with anti-inflammatory and PTP1B inhibitory activities. Bioorganic Med. Chem. Lett. 2014;24:5787–5791. doi: 10.1016/j.bmcl.2014.10.035. [DOI] [PubMed] [Google Scholar]
- 45.Zhang P., Li Y., Jia C., Lang J., Niaz S., Li J., Yuan J., Yu J., Chen S., Liu L. Antiviral and anti-inflammatory meroterpenoids: Stachybonoids A–F from the crinoid-derived fungus Stachybotrys chartarum 952. RSC Adv. 2017;7:49910–49916. doi: 10.1039/C7RA09859F. [DOI] [Google Scholar]
- 46.Du X., Liu D., Huang J., Zhang C., Proksch P., Lin W. Polyketide derivatives from the sponge associated fungus Aspergillus europaeus with antioxidant and NO inhibitory activities. Fitoterapia. 2018;130:190–197. doi: 10.1016/j.fitote.2018.08.030. [DOI] [PubMed] [Google Scholar]
- 47.Liu S., Wang H., Su M., Hwang G.J., Hong J., Jung J.H. New metabolites from the sponge-derived fungus Aspergillus sydowii J05B-7F-4. Nat. Prod. Res. 2017;31:1682–1686. doi: 10.1080/14786419.2017.1289205. [DOI] [PubMed] [Google Scholar]
- 48.Kim D.C., Cho K.H., Ko W., Yoon C.S., Sohn J.H., Yim J.H., Kim Y.C., Oh H. Anti-inflammatory and cytoprotective effects of TMC-256C1 from marine-derived fungus Aspergillus sp. SF-6354 via up-regulation of heme oxygenase-1 in murine hippocampal and microglial cell lines. Int. J. Mol. Sci. 2016;17:529. doi: 10.3390/ijms17040529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fang W., Lin X., Wang J., Liu Y., Tao H., Zhou X. Asperpyrone-type bis-naphtho-γ-pyrones with COX-2–inhibitory activities from marine-derived fungus Aspergillus niger. Molecules. 2016;21:941. doi: 10.3390/molecules21070941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tian Y., Qin X., Lin X., Kaliyaperumal K., Zhou X., Liu J., Ju Z., Tu Z., Liu Y. Sydoxanthone C and acremolin B produced by deep-sea-derived fungus Aspergillus sp. SCSIO Ind09F01. J. Antibiot. 2015;68:703–706. doi: 10.1038/ja.2015.55. [DOI] [PubMed] [Google Scholar]
- 51.Kim D., Quang T.H., Ngan N.T.T., Yoon C., Sohn J.H., Yim J.H., Feng Y., Che Y., Kim Y., Oh H. Dihydroisocoumarin derivatives from marine-derived fungal isolates and their anti-inflammatory effects in lipopolysaccharide-induced BV2 microglia. J. Nat. Prod. 2015;78:2948–2955. doi: 10.1021/acs.jnatprod.5b00614. [DOI] [PubMed] [Google Scholar]
- 52.Lee D.-S., Jeong G.-S., Li B., Lee S.U., Oh H., Kim Y.-C. Asperlin from the marine-derived fungus Aspergillus sp. SF-5044 exerts anti-inflammatory effects through heme oxygenase-1 expression in murine macrophages. J. Pharmacol. Sci. 2011;116:283. doi: 10.1254/jphs.10219FP. [DOI] [PubMed] [Google Scholar]
- 53.Ngan N.T.T., Quang T.H., Kim K., Kim H.J., Sohn J.H., Kang D.G., Lee H.S., Kim Y., Oh H. Anti-inflammatory effects of secondary metabolites isolated from the marine-derived fungal strain Penicillium sp. SF-5629. Arch. Pharm. Res. 2017;40:328–337. doi: 10.1007/s12272-017-0890-5. [DOI] [PubMed] [Google Scholar]
- 54.Lee D., Ko W., Quang T.H., Kim K.S., Sohn J.H., Jang J., Ahn J.S., Kim Y., Oh H. Penicillinolide A: a new anti-inflammatory metabolite from the marine fungus Penicillium sp. SF-5292. Mar. Drugs. 2013;11:4510–4526. doi: 10.3390/md11114510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee D., Jang J., Ko W., Kim K.S., Sohn J.H., Kang M., Ahn J.S., Kim Y., Oh H. PTP1B inhibitory and anti-inflammatory effects of secondary metabolites isolated from the marine-derived fungus Penicillium sp. JF-55. Mar. Drugs. 2013;11:1409–1426. doi: 10.3390/md11041409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ha T.M., Ko W., Lee S.J., Kim Y.C., Son J.Y., Sohn J.H., Yim J.H., Oh H. Anti-inflammatory effects of curvularin-type metabolites from a marine-derived fungal strain Penicillium sp. SF-5859 in lipopolysaccharide-induced RAW264.7 macrophages. Mar. Drugs. 2017;15:282. doi: 10.3390/md15090282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Toledo T.R., Dejani N.N., Monnazzi L.G.S., Kossuga M.H., Berlinck R.G.S., Sette L.D., Medeiros A.I. Potent anti-inflammatory activity of Pyrenocine A isolated from the marine-derived fungus Penicillium paxilli Ma(G)K. Mediat. Inflamm. 2014;2014:767061. doi: 10.1155/2014/767061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang X., Kang M.C., Li Y., Kim E.A., Kang S.M., Jeon Y.J. Asperflavin, an anti-inflammatory compound produced by a marine-derived fungus, Eurotium amstelodami. Molecules. 2017;22:1823. doi: 10.3390/molecules22111823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang X., Kang M., Li Y., Kim E., Kang S., Jeon Y. Anti-inflammatory activity of questinol isolated from marine-derived fungus Eurotium amstelodami in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Microbiol. Biotechnol. 2014;24:1346–1353. doi: 10.4014/jmb.1405.05035. [DOI] [PubMed] [Google Scholar]
- 60.Kim K.S., Cui X., Lee D.S., Ko W., Sohn J.H., Yim J.H., An R.B., Kim Y.C., Oh H. Inhibitory effects of Benzaldehyde derivatives from the marine fungus Eurotium sp. SF-5989 on inflammatory mediators via the induction of heme oxygenase-1 in lipopolysaccharide-stimulated RAW264.7 macrophages. Int. J. Mol. Sci. 2014;15:23749–23765. doi: 10.3390/ijms151223749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Quang T.H., Kim D.C., Van Kiem P., Van Minh C., Nhiem N.X., Tai B.H., Yen P.H., Ngan N.T.T., Kim H.J., Oh H. Macrolide and phenolic metabolites from the marine-derived fungus Paraconiothyrium sp. VK-13 with anti-inflammatory activity. J. Antibiot. 2018;71:826–830. doi: 10.1038/s41429-018-0073-8. [DOI] [PubMed] [Google Scholar]
- 62.Zhang P., Jia C., Lang J., Li J., Luo G., Chen S., Yan S., Liu L. Mono- and dimeric naphthalenones from the marine-derived fungus Leptosphaerulina chartarum 3608. Mar. Drugs. 2018;16:173. doi: 10.3390/md16050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee M.S., Wang S., Wang G.J., Pang K., Lee C.K., Kuo Y., Cha H.J., Lin R.K., Lee T. Angiogenesis inhibitors and anti-inflammatory agents from Phoma sp. NTOU4195. J. Nat. Prod. 2016;79:2983–2990. doi: 10.1021/acs.jnatprod.6b00407. [DOI] [PubMed] [Google Scholar]
- 64.Wang J., Qin X., Xu F., Zhang T., Liao S., Lin X., Yang B., Liu J., Wang L., Tu Z. Tetramic acid derivatives and polyphenols from sponge-derived fungus and their biological evaluation. Nat. Prod. Res. 2015;29:1761–1765. doi: 10.1080/14786419.2014.999061. [DOI] [PubMed] [Google Scholar]
- 65.Lin Z., Ma X., Wei H., Li D., Gu Q., Zhu T. Spicarins A–D from acetylated extract of fungus Spicaria elegans KLA03. RSC Adv. 2015;5:35262–35266. doi: 10.1039/C5RA01923K. [DOI] [Google Scholar]
- 66.Chen Q., Chen T., Li W., Zhang W., Zhu J., Li Y., Huang Y., Shen Y., Yu C. Correction: Mycoepoxydiene inhibits lipopolysaccharide-induced inflammatory responses through the suppression of TRAF6 polyubiquitination. PLoS ONE. 2012;7:e44890. doi: 10.1371/annotation/9a2fb76a-b2c3-43b4-a0b1-e8ae773378b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu J., Gu B., Yang L., Yang F., Lin H. New Anti-inflammatory cyclopeptides from a sponge-derived fungus Aspergillus violaceofuscus. Front. Chem. 2018;6:226. doi: 10.3389/fchem.2018.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yoon C.S., Kim D.-C., Lee D.-S., Kim K.-S., Ko W., Sohn J.H., Yim J.H., Kim Y.-C., Oh H. Anti-neuroinflammatory effect of aurantiamide acetate from the marine fungus Aspergillus sp. SF-5921: Inhibition of NF-κB and MAPK pathways in lipopolysaccharide-induced mouse BV2 microglial cells. Int. Immunopharmacol. 2014;23:568–574. doi: 10.1016/j.intimp.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 69.Li L., Zhang Y., Li Z., Yu Z., Sun T. Stereochemical investigation of a novel biological active substance from the secondary metabolites of marine fungus Penicillium chrysogenum SYP-F-2720. Rev. De La Soc. Química De Mex. 2017;59:53–58. [Google Scholar]
- 70.Belofsky G.N., Anguera M., Jensen P.R., Fenical W., Kock M. Oxepinamides A-C and Fumiquinazolines H-I: Bioactive metabolites from a marine isolate of a fungus of the genus Acremonium. Chem. A Eur. J. 2000;6:1355–1360. doi: 10.1002/(SICI)1521-3765(20000417)6:8<1355::AID-CHEM1355>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 71.Ko W., Sohn J.H., Jang J.H., Ahn J.S., Kang D.G., Lee H.S., Kim J.S., Kim Y.C., Oh H. Inhibitory effects of alternaramide on inflammatory mediator expression through TLR4-MyD88-mediated inhibition of NF-кB and MAPK pathway signaling in lipopolysaccharide-stimulated RAW264.7 and BV2 cells. Chem.—Biol. Interact. 2016;244:16–26. doi: 10.1016/j.cbi.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 72.Li J.L., Zhang P., Lee Y.M., Hong J., Yoo E.S., Bae K.S., Jung J.H. Oxygenated hexylitaconates from a marine sponge-derived fungus Penicillium sp. Chem. Pharm. Bull. 2011;59:120–123. doi: 10.1248/cpb.59.120. [DOI] [PubMed] [Google Scholar]
- 73.Marra R., Nicoletti R., Pagano E., Dellagreca M., Salvatore M.M., Borrelli F., Lombardi N., Vinale F., Woo S.L., Andolfi A. Inhibitory effect of trichodermanone C, a sorbicillinoid produced by Trichoderma citrinoviride associated to the green alga Cladophora sp., on nitrite production in LPS-stimulated macrophages. Nat. Prod. Res. 2018;33:3389–3397. doi: 10.1080/14786419.2018.1479702. [DOI] [PubMed] [Google Scholar]
- 74.Galli S.J., Tsai M., Piliponsky A.M. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gordon S. The role of the macrophage in immune regulation. Res. Immunol. 1998;149:685–688. doi: 10.1016/S0923-2494(99)80039-X. [DOI] [PubMed] [Google Scholar]
- 76.Fawthrop F. Inflammation: Basic principles and clinical correlates. Ann. Rheum. Dis. 1993;52:701. doi: 10.1136/ard.52.10.701-b. [DOI] [Google Scholar]
- 77.Gautam R., Jachak S.M. Recent Developments in antiinflammatory natural products. ChemInform. 2009;29:767–820. doi: 10.1002/chin.200947240. [DOI] [PubMed] [Google Scholar]
- 78.Abad M., Bedoya L., Bermejo P. Natural marine anti-inflammatory products. Mini Rev. Med. Chem. 2008;8:740–754. doi: 10.2174/138955708784912148. [DOI] [PubMed] [Google Scholar]