Abstract
The bark of Rhus verniciflua Stokes (RVS) has been used to treat cancer in Korean herbal medicine. When we screened for PD-1 and CTLA-4 immune checkpoint inhibitors (PD-1/PD-L1 CTLA-4/CD80) from around 800 herbal extracts using competitive Enzyme-Linked Immunosorbent Assay (ELISA), we found that RVS blocked both the PD-1/PD-L1 and the CTLA-4/CD80 interactions. To identify the active compounds from RVS, we performed bioactivity-guided fractionation, and the ethyl acetate (EtOAc) fraction of RVS proved to be the most effective at blocking the PD-1/PD-L1 and CTLA-4/CD80 interactions. In addition, we isolated and identified 20 major compounds in the EtOAc fraction of RVS and then examined the blocking effects of these 20 compounds on PD-1/PD-L1 and CTLA-4/CD80. Among them, four compounds [eriodictyol (7) > fisetin (9) > quercetin (18) > liquiritigenin (13)] blocked the interaction of PD-1/PD-L1 on competitive ELISA. In addition, four different compounds [protocatechuic acid (2) > caffeic acid (19) > taxifolin (5) > butin (6)] blocked the interaction of CTLA-4/CD80. Our findings suggest that RVS and its components could be used as a potential immune checkpoint inhibitor blockade and could be developed for immuno-oncological therapeutics.
Keywords: Rhus verniciflua Stokes, immune checkpoints, PD-1, CTLA-4, flavonoid, polyphenol
1. Introduction
Rhus verniciflua Stokes (RVS) (Anacardiaceae), commonly known as Chinese lacquer tree, is distributed in Korea, Japan, and China [1]. RVS tissues, particularly the bark, have been shown to contain a large number of bioactive phytochemical constituents, including alkaloids, polyphenols, and flavonoids [2,3]. Since ancient times, RVS have been used as herbal medicinal plant to treat various conditions, such as gastroenteritis, arthritis, hypertension, diabetes, stroke, and chronic fatigue disease [3]. However, the blocking effects of this plant on the immune checkpoint inhibitors, such as PD-1/PD-L1 and CTLA-4/CD80, are not currently understood. In the present study, as part of an investigation of novel bioactive constituents in RVS, bioactivity-guided fractionation, and isolation from RVS bark revealed 20 secondary metabolites (1–20).
Immune checkpoints, which can stimulate or inhibit T cell responses, were well known, as a result of the award of the Nobel Prize in Physiology or Medicine in 2018 to James Allison and Tasuku Honjo for their discovery of CTLA-4 and PD-1, respectively. When CD80 molecules on antigen-presenting cells (APC) interact with CD28 on T cells, T cell activities are stimulated and sustained, whereas when CD80 molecules bind with CTLA-4, a negative signal is sent to activated T cells [4]. Similarly, T cell proliferation and cytokine production were inhibited when PD-1 on T cells interacted with PD-L1 or PD-L2 on APC or tumor cells [5]. Blocking monoclonal antibodies for PD-1 (Pembrolizumab, Nivolumab, and Cemiplimab), PD-L1 (Atezolizumab, Avelumab, and Durvalumab), and CTLA-4 (Ipilimumab) have been approved by the US Food and Drug Administration and have been used for treatment of metastatic melanoma and non-small lung cancer [6]. However, there have been many cases of immune-related adverse events such as colitis, thyroiditis and type 1 diabetes in response to these monoclonal antibodies [7]. In addition, these monoclonal antibodies are expensive and show limited impact on solid tumors because antibodies are large molecules cannot easily penetrate such a tumor. Various studies using small molecules to overcome the limitation of monoclonal antibody therapy have been conducted recently [8,9], but most of these studies have not succeeded because of low effectiveness as well as toxicities associated with these drugs. However, oriental herbal medicines, which have a long anecdotal history of safe use, are promising anticancer drug candidates because their toxicities and side effects are well known. In the present study, we screened approximately 800 herbal medicines for their potential blocking effects on PD-1/PD-L1 and CTLA-4/CD80, and discovered that RVS blocked both the immune checkpoint inhibitors PD-1/PD-L1 and CTLA-4/CD80 in competitive Enzyme-Linked Immunosorbent Assay (ELISA) studies.
2. Results
2.1. RVS Blocks the PD-1/PD-L1 Interaction
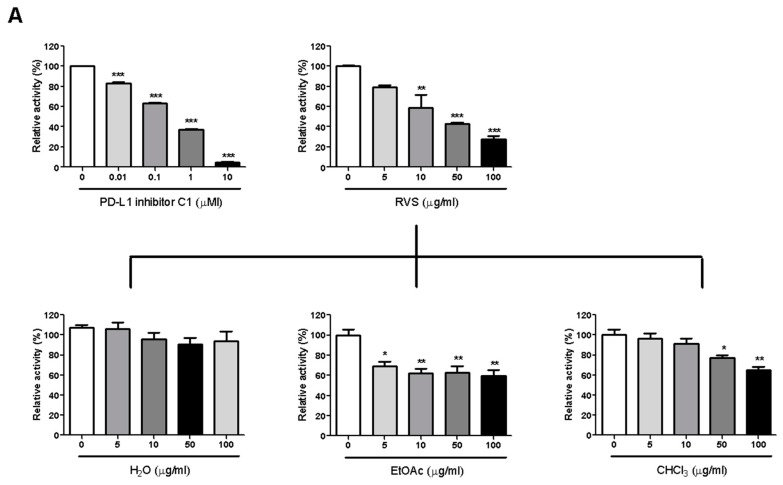
We investigated PD-1/PD-L1 blocking effect by RVS using competition ELISA. RVS blocked the PD-1/PD-L1 interaction in a dose-dependent manner, with a half-maximal inhibitory concentration (IC50) at 26.22 μg/mL. To identify the main constituents of RVS that blocked activity against PD-1/PD-L1 binding, we partitioned the RVS extract with ethyl acetate (EtOAc), chloroform (CHCl3) and water (H2O). The EtOAc fraction of the extract showed more effective blocking efficacy than did other fractions. This observation indicates that the blocking effect of RVS on the PD-1/PD-L1 interaction was attributable to constituents enriched in the EtOAc fraction (Figure 1A).
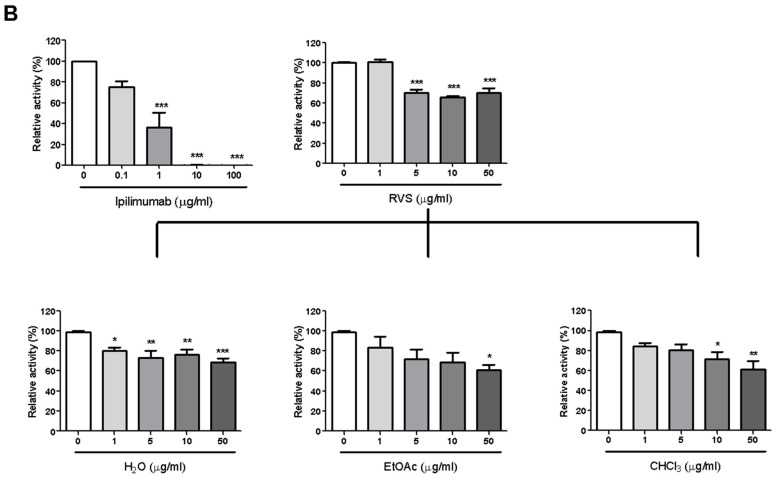
Figure 1.
Immune checkpoint blocking effects of Rhus verniciflua Stokes (RVS) extract and fractions tested by competitive Enzyme-Linked Immunosorbent Assay (ELISA). Effect of PD-L1 inhibitor C1, RVS extract and fractions on PD-1/PD-L1 binding activity (A); Effect of anti-CTLA-4 antibody, RVS extract and fractions on CTLA-4/CD80 binding activity (B). The relative binding activity was normalized to the relative percentage of the vehicle control group. Half-maximal inhibitory concentration (IC50) was calculated using Prism “log[inhibitor] vs. normalized response” equation. All results are presented as the mean value of three independent biological replicates. * p < 0.05, ** p < 0.01, *** p < 0.001, compared with the vehicle control group.
2.2. RVS Blocks the CTLA-4/CD80 Interaction
The CTLA-4/CD80 blocking activity of RVS was examined via competition ELISA as described before. Similar to the results with respect to the PD-1/PD-L1 blockade, RVS blocked 29.9% of CTLA-4/CD80 interaction at 5μg/mL and the EtOAc fraction exhibited the most effective blocking activity on CTLA-4/CD80 binding (Figure 1B).
2.3. Structural Elucidation of Compounds 1–20
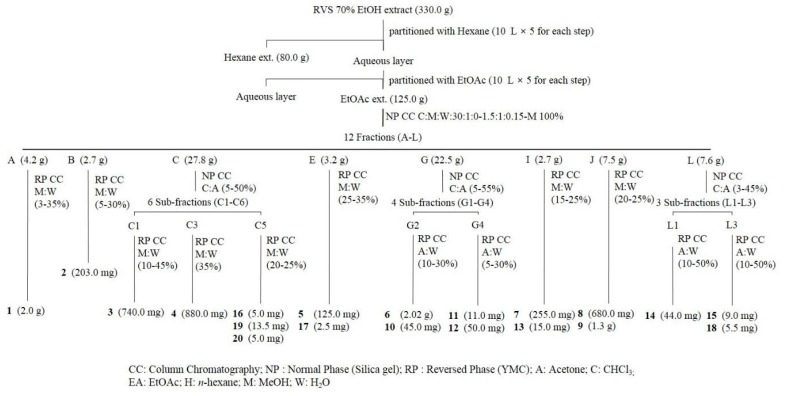
Using combined chromatographic separations (Figure 2), 20 secondary metabolites were isolated from RVS, including four benzoic acids (1–3 and 19), 11 flavonoids (4–13 and 18), and five polyphenols (14–17 and 20). Their structures were identified as gallic acid (1), protocatechuic acid (2), gallic acid cetyl ester (3), fustin (4) [10], taxifolin (5) [11], butin (6) [11], eriodictyol (7) [12], 5-deoxyluteolin (8) [3], fisetin (9) [13], garbanzol (10) [13], aromadendrin (11) [14], naringenin (12) [14], liquiritigenin (13) [14], sulfuretin (14) [13], rhusopolyphenol F (15) [10], rhusopolyphenol B (16) [10], rhusopolyphenol A (17) [10], quercetin (18) [11], caffeic acid (19), and 2-benzyl-2,3′,4′,6-tetrahydroxybenzo[b]furan-3(2H)-one (20) [10] (Figure 3). Their structures were elucidated by 1-D and 2-D nuclear magnetic resonance (NMR), mass spectrometry, and compared with those reported in the literature (Figures S2–S21). In this study, to the best of our knowledge, compounds 11–13 were isolated from R. verniciflua for the first time.
Figure 2.
Isolation scheme of compounds 1–20 from Rhus verniciflua Stokes (RVS).
Figure 3.
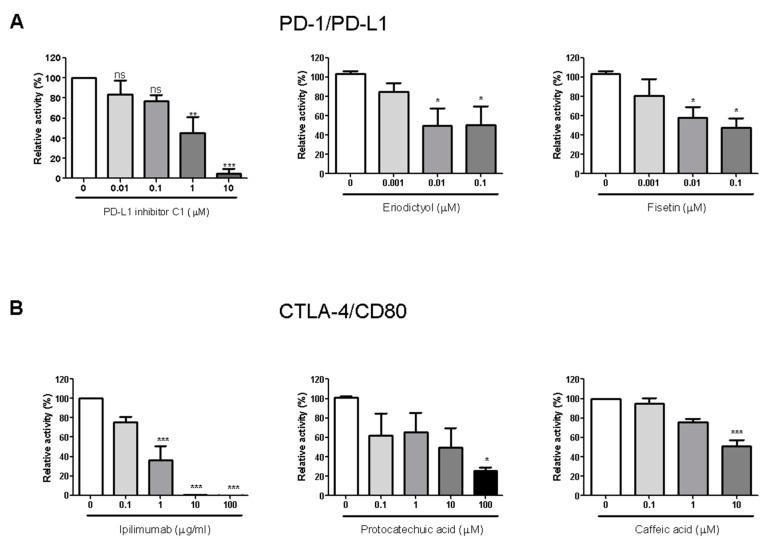
Immune checkpoint blocking effects of isolated compounds by competitive Enzyme-Linked Immunosorbent Assay (ELISA). Effect of PD-L1 inhibitor C1, compounds eriodictyol (7) and fisetin on PD-1/PD-L1 binding activity (9) (A); effect of anti-CTLA-4 antibody (Ipilimumab), compounds protocatechuic acid (2) and caffeic acid (19) on CTLA-4/CD80 binding activity (B). The relative binding activity was normalized to the relative percentage of the vehicle control group. Half-maximal inhibitory concentration (IC50) was calculated using Prism “log[inhibitor] vs. normalized response” equation. All results are presented as the mean of three independent biological replicates. * p < 0.05, ** p < 0.01, *** p < 0.001, compared with the vehicle control group.
2.4. PD-1/PD-L1 Blocking Effect of Isolated Compounds
The 20 compounds isolated from the EtOAc fraction of RVS were tested for their PD-1/PD-L1 blocking effects (Figure S22). Small molecule PD-L1 inhibitor C1 was used as a positive control (IC50 value 0.55 μM). Compounds 7, 9, 13, and 18 showed blocking effects, with IC50 values of 0.04, 0.04, 11.85, and 5.71 μM, respectively. Of these, compounds 7 and 9 in particular exhibited strong blocking effects, with IC50 values below 1 μM (Figure 3A).
2.5. CTLA-4/CD80 Blocking Effect of Isolated Compounds
The 20 compounds isolated from the EtOAc fraction were tested for CTLA-4/CD80 blocking activity (Figure S23). Anti-CTLA-4 antibody was used as a positive control (IC50 0.43 μg/mL). Compounds 2, 5, 6, and 19 showed blocking effect, with IC50 values of 4.51, 35.45, 134.10, and 10.04 μM, respectively (Figure 3B).
3. Discussion
Many preclinical studies have been carried out on the anticancer effects of RVS [15,16,17,18,19,20,21], including, in two cases, clinical reports of treatment for metastatic renal cell carcinoma [22]. To our knowledge, there has been no report of an immuno-oncological study on RVS. Most of the anticancer studies on RVS have described the apoptotic effects of RVS [23,24,25] and its mechanism of action [26].
Competitive ELISA results showed that RVS markedly blocked both the PD-1/PD-L1 and CTLA-4/CD80 interactions. Further, we identified eriodictyol (7) and fisetin (9) as being active compounds for blocking the PD-1/PD-L1 interaction, and protocatechuic acid (2) and caffeic acid (19) as being active compounds for blocking the CTLA-4/CD80 interaction. According to previous studies, some natural compounds, known as Pan-assay interference compounds (PAINS), have nonspecific activity [27,28]. Toxoflavin, isothiazolones, hydroxyphenyl hydrazones, curcumin, phenol-sulfonamides, rhodanines, enones, quianones, and catechols have all been reported as common PAINS [29]. If the RVS compounds active with respect to the blocking of PD-1/PD-L1 are PAINS that react nonspecifically with biological targets, they should nonspecifically block CTLA-4/CD80 as well. But the RVS compounds that actively blocked PD-1/PD-L1 did not block CTLA-4/CD80, and vice versa. Based on these observed results, therefore, we cautiously speculate that the active compounds from RVS may not be PAINS [30].
Recent studies have shown that immune checkpoint blockades have synergistic effects with conventional cancer therapies, such as chemotherapy and radiation therapy [31]. Immune checkpoint blockades are known to stimulate T cell activation, whereas the conventional cancer therapies promote antigen release and presentation [31]. In this respect, RVS, which is known for its anticancer effects like chemotherapy, would kill tumor cells and then promote antigen presentation. In addition, RVS, which can block PD-1/PD-L1 and CTLA-4/CD80 checkpoints, would stimulate the sustained activation of T cells. For the future study, we will examine the immuno-oncological activity of RVS in cell model and animal model systems.
4. Materials and Methods
4.1. General Experimental Procedures
The NMR spectra were recorded using a Bruker Avance III 600 NMR spectrometer (1H, 600 MHz; 13C, 150 MHz) (Bruker Biospin GmbH, Karlsruhe, Germany), with tetramethylsilane (TMS) as an internal standard. Heteronuclear multiple quantum correlation, heteronuclear multiple bond correlation, rotating frame nuclear overhauser effect spectroscopy (ROESY), and 1H–1H correlation spectroscopy (COSY) spectra were recorded using a pulsed-field gradient. The high-resolution–electrospray ionization–mass spectrometry (HR-ESI-MS) spectra were obtained using an Agilent 1200 Liquid Chromatography/Mass Selective Detector (LC/MSD) Trap spectrometer (Agilent, Santa Clara, CA, USA). Preparative-HPLC was performed using a Gilson 321 pump, a 151 UV/VIS detector (Gilson SAS, Villiers-le-Bel, France), and a RS Tech HECTOR-M C18 column (5-µm particle size, 250 × 21.2 mm) (RS Tech Corp, Chungju, South Korea). Column chromatography was performed using silica gel (Kieselgel 60, 70–230, and 230–400 mesh; Merck, Darmstadt, Germany), and YMC C18 resin. Thin-layer chromatography was performed using pre-coated silica gel 60 F254 and RP-18 F254S plates (both 0.25-mm thickness; Merck, Darmstadt, Germany), the spots being detected under UV light and using 10% H2SO4.
4.2. Plant Material
Dried bark of RVS was kindly provided from Bomyeong Herbal Market, Seoul in 2018. Its identity was confirmed by one of the authors (Dr. Wei Li). A voucher specimen (IC-180018) was deposited at the Herbarium of Korean Medicine (KM)-Application Center, Korea Institute of Oriental Medicine, Republic of Korea.
4.3. Extraction and Isolation of Chemicals
The dried bark of RVS (8.0 kg) was exhaustively extracted under reflux with 70% EtOH three times, each time with 50 L solvent. The total 70% EtOH extract (330.0 g) was suspended in deionized water and partitioned with CHCl3 (80.0 g), with the water fraction being partitioned sequentially with EtOAc (125.0 g). The EtOAc fraction was subjected to silica gel column chromatography with a gradient of CHCl3-MeOH-H2O (30:1:0, 15:1:0, 10:1:0, 6:1:0.1, 5:1:0.1, 4:1:0.1, 3:1:0.1, 1.5:1:0.15, MeOH; 8.0 L for each step) to give 12 fractions (fractions A-L). Fraction A was isolated with a gradient of MeOH-H2O (3–35%) by middle-pressure liquid chromatography (MPLC) using a YMC C18 column to give compound 1 (2.0 g). Fraction B was isolated with a gradient of MeOH-H2O (5–30%) by MPLC using YMC C18 column to give compound 2 (203.0 mg). Fraction C was separated on a silica gel column (2.5 × 80 cm) with a gradient of CHCl3-acetone (5–50%) to give six sub-fractions (C1–C6). Fraction C1 was isolated by preparative-HPLC (MeOH-H2O: 5–30%) to give compound 3 (740.0 mg). Fraction C3 was isolated by preparative-HPLC (MeOH-H2O: 35%) to give compound 4 (880.0 mg). Fraction C5 was isolated by preparative-HPLC (MeOH-H2O: 20–25%) to give compounds 16 (5.0 mg), 19 (13.5 mg), and 20 (5.0 mg). Fraction E was isolated with a gradient of MeOH-H2O (5–30%) by MPLC using a YMC C18 column to give compounds 5 (125.0 mg) and 17 (2.5 mg). Fraction G was separated on a silica gel column (3.0 × 80 cm) with a gradient of CHCl3-acetone (5–55%) to give four sub-fractions (G1–G4). Fraction G2 was isolated by preparative-HPLC (acetone-H2O: 10–30%) to give compounds 6 (2.02 g) and 10 (45.0 mg). Fraction G4 was isolated by preparative-HPLC (acetone-H2O: 5–30%) to give compounds 11 (11.0 mg) and 12 (50.0 mg). Fraction I was isolated with a gradient of MeOH-H2O (15–25%) by MPLC using a YMC C18 column to give compounds 7 (255.0 mg) and 13 (15.0 mg). Fraction J was isolated with a gradient of MeOH-H2O (20–25%) by MPLC using a YMC C18 column to give compounds 8 (680.0 mg) and 9 (1.3 g). Fraction L was separated on a silica gel column (1.5 × 80 cm) with a gradient of CHCl3-acetone (3–45%) to give three sub-fractions (L1-L3). Fraction L1 was isolated by preparative-HPLC (acetone-H2O: 10F50%) to give compound 14 (44.0 mg). Fraction L3 was isolated by preparative-HPLC (acetone-H2O: 10–50%) to give compounds 15 (9.0 mg) and 18 (5.5 mg).
4.4. Chemicals and Antibodies
PD–1/PD-L1 Inhibitor Screening Assay Kit (#72005), anti-PD–1 Neutralizing Antibody (#71120), and CTLA-4/CD80 Inhibitor Screening Assay Kit (#72009) were purchased from BPS Bioscience (San Diego, CA, USA). Anti-CTLA-4 antibody (#A2001) was purchased from Selleck (Houston, TX, USA).
4.5. Competitive ELISA
Competitive ELSIA was performed using a PD–1/PD-L1 or CTLA-4/CD80 Inhibitor Screening Assay Kit, according to the supplier’s instructions. An aliquot of 1 μg/mL recombinant human PD-L1 (BPS Bioscience, #71104) or CTLA-4 (BPS Bioscience, #71149) were coated onto a 96-well plate overnight. Plates were washed with phosphate-buffered saline (PBS) containing 0.1% Tween (PBS-T), blocked for 1 h at room temperature with PBS-T containing 2% (w/v) bovine serum albumen, and washed again. After washing, vehicle or test samples were added, and the reaction continued for 1 h. As a positive control, PD-L1 inhibitor C1 or anti-CTLA-4 neutralizing antibody were used. Biotinylated hPD–1 (BPS Bioscience, #71109) or biotinylated hCD80 (BPS Bioscience, #71114) was added to each well, and plates were incubated for 2 h at room temperature. After three washes with PBS-T, diluted streptavidin-horseradish peroxidase (HRP) was added to each well, and plates were reacted for 1 h while shaking at low speed. After the reaction, plates were washed three times with PBS-T, and HRP substrates A and B were added. Relative chemiluminescence was measured on a SpectraMax L Luminometer (Molecular Devices, San Jose, California, USA) and expressed as the relative binding activity. The results were normalized to the relative percentage of the vehicle control group. Half-maximal inhibitory concentration (IC50) was calculated using Prism “log[inhibitor] vs. normalized response” equation. All results are presented as the mean of three independent biological replicates. * p < 0.05, ** p < 0.01, *** p < 0.001, compared with the vehicle control group [32,33,34,35,36,37].
4.6. Statistical Analysis
All values are expressed as means ± standard error of the mean. The statistical significance threshold (p < 0.05 for all analyses) was assessed by one-way ANOVA followed by Tukey’s post-hoc test for multiple comparisons using Prism 5.01 software (GraphPad Software Inc., San Diego, CA, USA).
5. Conclusions
We screened diverse medicinal plants to discover new candidates for immune checkpoint inhibitors. Among them, RVS showed remarkable blocking effect against both PD-1/PD-L1 and CTLA-4/CD80 interaction. To determine active constituents of RVS, we conducted bioactivity-guided fractionation and repeated column chromatography. Also, 20 compounds were isolated from EtOAc fraction of RVS and active compounds such as eriodictyol (7) and fisetin (9) were identified as inhibitors of PD-1/PD-L1 and protocatechuic acid (2) for CTLA-4/CD80 inhibitors. Our findings show that RVS and its active compounds could be applied as candidates of small-molecule inhibitors PD-1 and CTLA-4 immune checkpoint.
Supplementary Materials
Supplementary materials are available online.
Author Contributions
The list authors contributed to this work as follows: W.L. performed the isolation, structure elucidation of the constituents. T.I.K. conducted the bioassay experiments and prepared the manuscript. J.H.K. assisted the experiments; The whole research was performed based on the planning of H.-S.C. All authors approved the final version of the manuscript.
Funding
This work has been supported by the Grant KSN1812150 awarded to Korea Institute of Oriental Medicine (KIOM) from Ministry of Science, ICT and Future Planning (MSIP), Republic of Korea and by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2017R1D1A1B03033510).
Conflicts of Interest
The authors declare no competing financial interest.
Footnotes
Sample Availability: Samples of the compounds are available from the authors.
References
- 1.Niimura N. Determination of the type of lacquer on East Asian lacquer ware. Int. J. Mass Spectrom. 2009;284:93–97. doi: 10.1016/j.ijms.2009.03.004. [DOI] [Google Scholar]
- 2.Lee J.D., Huh J.E., Jeon G.S. Flavonol-rich RVHxR from Rhus verniciflua Stokes and its major compound fisetin inhibits inflammation-related cytokines and angiogenic factor in rheumatoid arthritic fibroblast-like synovial cells and in vivo models. Int. Immunopharmacol. 2009;9:2068–2276. doi: 10.1016/j.intimp.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 3.Kim J.S., Kwon Y.S., Chun W.J., Kim T.Y., Sun J., Yu C.Y., Kim M.J. Rhus verniciflua Stokes flavonoid extracts have anti-oxidant, anti-microbial and α-glucosidase inhibitory effect. Food Chem. 2010;120:539–543. doi: 10.1016/j.foodchem.2009.10.051. [DOI] [Google Scholar]
- 4.Thompson C.B., Allison J.P. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–450. doi: 10.1016/S1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 5.Latchman Y., Wood C.R., Chernova T., Chaudhary D., Borde M., Chernova I., Iwai Y., Long A.J., Brown J.A., Nunes R., et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001;2:261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 6.Dholaria B., Hammond W., Shreders A., Lou Y. Emerging therapeutic agents for lung cancer. J. Hematol. Oncol. 2016;9:138. doi: 10.1186/s13045-016-0365-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.June C.H., Warshauer J.T., Bluestone J.A. Is autoimmunity the Achilles’ heel of cancer immunotherapy? Nat. Med. 2017;23:540–547. doi: 10.1038/nm.4321. [DOI] [PubMed] [Google Scholar]
- 8.Musielak B., Kocik J., Skalniak L., Magiera-Mularz K., Sala D., Czub M., Stec M., Siedlar M., Holak T.A., Plewka J. CA-170—A Potent Small-Molecule PD-L1 Inhibitor or Not? Molecules. 2019;24:2804. doi: 10.3390/molecules24152804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Skalniak L., Zak K.M., Guzik K., Magiera K., Musielak B., Pachota M., Szelazek B., Kocik J., Grudnik P., Tomala M., et al. Small-molecule inhibitors of PD-1/PD-L1 immune checkpoint alleviate the PD-L1-induced exhaustion of T-cells. Oncotarget. 2017;8:72167–72181. doi: 10.18632/oncotarget.20050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim K.H., Moon E., Choi S.U., Pang C., Kim S.Y., Lee K.R. Identification of cytotoxic and anti-inflammatory constituents from the bark of Toxicodendron vernicifluum (Stokes) F.A. Barkley. J. Ethnopharmacol. 2015;162:231–237. doi: 10.1016/j.jep.2014.12.071. [DOI] [PubMed] [Google Scholar]
- 11.Park M.H., Kim I.S., Kim S.A., Na C.S., Hong C.Y., Dong M.S., Yoo H.H. Inhibitory effect of Rhus verniciflua Stokes extract on human aromatase activity; butin is its major bioactive component. Bioorg. Med. Chem. Lett. 2014;24:1730–1733. doi: 10.1016/j.bmcl.2014.02.039. [DOI] [PubMed] [Google Scholar]
- 12.Cho N., Choi J.H., Yang H., Jeong E.J., Lee K.Y., Kim Y.C., Sung S.H. Neuroprotective and anti-inflammatory effects of flavonoids isolated from Rhus verniciflua in neuronal HT22 and microglial BV2 cell lines. Food Chem. Toxicol. 2012;50:1940–1945. doi: 10.1016/j.fct.2012.03.052. [DOI] [PubMed] [Google Scholar]
- 13.Jeong S.J., Park J.G., Kim S., Kweon H.Y., Seo S., Na D.S., Lee D., Hong C.Y., Na C.S., Dong M.S., et al. Extract of Rhus verniciflua stokes protects the diet-induced hyperlipidemia in mice. Arch. Pharm. Res. 2015;38:2049–2058. doi: 10.1007/s12272-015-0579-6. [DOI] [PubMed] [Google Scholar]
- 14.Huang A.C., Wilde A., Ebmeyer J., Skouroumounis G.K., Taylor D. Examination of the phenolic profile and antioxidant activity of the leaves of the Australian native plant Smilax glyciphylla. J. Nat. Prod. 2013;76:1930–1936. doi: 10.1021/np4005163. [DOI] [PubMed] [Google Scholar]
- 15.Kim M.S., Lee C.W., Kim J.H., Lee J.C., An W.G. Extract of Rhus verniciflua Stokes Induces p53-Mediated Apoptosis in MCF-7 Breast Cancer Cells. Evid. Based Complement Alternat Med. 2019:9407340. doi: 10.1155/2019/9407340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang Y., Yoon S.W., Park B. Allergen-removed Rhus verniciflua Stokes suppresses invasion and migration of pancreatic cancer cells through downregulation of the JAK/STAT and Src/FAK signaling pathways. Oncol. Rep. 2018;40:3060–3068. doi: 10.3892/or.2018.6699. [DOI] [PubMed] [Google Scholar]
- 17.Chae J., Lee S., Lee S. Potential Efficacy of Allergen Removed Rhus Verniciflua Stokes Extract to Maintain Progression-Free Survival of Patients With Advanced Hepatobiliary Cancer. Explore. 2018;14:300–304. doi: 10.1016/j.explore.2017.10.013. [DOI] [PubMed] [Google Scholar]
- 18.Lee K.W., Um E.S., Jung B.B., Choi E.S., Kim E.Y., Lee S., Jang E., Lee J.H., Kim Y. Rhus verniciflua Stokes extract induces inhibition of cell growth and apoptosis in human chronic myelogenous leukemia K562 cells. Oncol. Rep. 2018;39:1141–1147. doi: 10.3892/or.2018.6179. [DOI] [PubMed] [Google Scholar]
- 19.Kang S.H., Hwang I.H., Son E., Cho C.K., Choi J.S., Park S.J., Jang B.C., Lee K.B., Lee Z.W., Lee J.H., et al. Allergen-removed Rhus verniciflua extract induces ovarian cancer cell death via JNK activation. Am. J. Chin. Med. 2016;44:1719–1735. doi: 10.1142/S0192415X16500968. [DOI] [PubMed] [Google Scholar]
- 20.Jang I.S., Park J.W., Jo E.B., Cho C.K., Lee Y.W., Yoo H.S., Park J., Kim J., Jang B.C., Choi J.S. Growth inhibitory and apoptosis-inducing effects of allergen-free Rhus verniciflua Stokes extract on A549 human lung cancer cells. Oncol. Rep. 2016;36:3037–3043. doi: 10.3892/or.2016.5131. [DOI] [PubMed] [Google Scholar]
- 21.Suruga T., Hiruma W., Kadokura K., Tomita T., Miyata A., Sekino Y., Kimura M., Yamaguchi N., Murai T., Komatsu Y., et al. Antitumor and apoptopic effects of a plant exact mixture containing Rhus verniciflua and other herbs in human leukemia cells. Drug Res. 2017;67:127–130. doi: 10.1055/s-0042-113458. [DOI] [PubMed] [Google Scholar]
- 22.Lee S.K., Jung H.S., Eo W.K., Lee S.Y., Kim S.H., Shim B.S. Rhus verniciflua Stokes extract as a potential option for treatment of metastatic renal cell carcinoma: Report of two cases. Ann. Oncol. 2010;21:1383–1385. doi: 10.1093/annonc/mdq154. [DOI] [PubMed] [Google Scholar]
- 23.Lee J.O., Moon J.W., Lee S.K., Kim S.M., Kim N., Ko S.G., Kim H.S., Park S.H. Rhus verniciflua extract modulates survival of MCF-7 breast cancer cells through the modulation of AMPK-pathway. Biol. Pharm. Bull. 2014;37:794–801. doi: 10.1248/bpb.b13-00893. [DOI] [PubMed] [Google Scholar]
- 24.Lee K.W., Chung K.S., Seo J.H., Yim S.V., Park H.J., Choi J.H., Lee K.T. Sulfuretin from heartwood of Rhus verniciflua triggers apoptosis through activation of Fas, Caspase-8, and the mitochondrial death pathway in HL-60 human leukemia cells. J. Cell. Biochem. 2012;113:2835–2844. doi: 10.1002/jcb.24158. [DOI] [PubMed] [Google Scholar]
- 25.Kim J.H., Jung C.H., Jang B.H., Go H.Y., Park J.H., Choi Y.K., Hong S., Shin Y.C., Ko S.G. Selective cytotoxic effects on human cancer cell lines of phenolic-rich ethyl-acetate fraction from Rhus verniciflua Stokes. Am. J. Chin. Med. 2009;37:609–620. doi: 10.1142/S0192415X09007090. [DOI] [PubMed] [Google Scholar]
- 26.Kim J.H., Kim H.P., Jung C.H., Hong M.H., Hong M.C., Bae H.S., Lee S.D., Park S.Y., Park J.H., Ko S.G. Inhibition of cell cycle progression via p27Kip1 upregulation and apoptosis induction by an ethanol extract of Rhus verniciflua Stokes in AGS gastric cancer cells. Int. J. Mol. Med. 2006;18:201–208. doi: 10.3892/ijmm.18.1.201. [DOI] [PubMed] [Google Scholar]
- 27.Baell J.B. Feeling Nature’s PAINS: Natural Products, Natural Product Drugs, and Pan Assay Interference Compounds (PAINS) J. Nat. Prod. 2016;79:616–628. doi: 10.1021/acs.jnatprod.5b00947. [DOI] [PubMed] [Google Scholar]
- 28.Baell J.B., Nissink J.W.M. Seven Year Itch: Pan-Assay Interference Compounds (PAINS) in 2017-Utility and Limitations. ACS Chem. Biol. 2018;13:36–44. doi: 10.1021/acschembio.7b00903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baell J., Walters M.A. Chemistry: Chemical con artists foil drug discovery. Nature. 2014;513:481–483. doi: 10.1038/513481a. [DOI] [PubMed] [Google Scholar]
- 30.Jesus A.R., Vila-Viçosa D., Machuqueiro M., Marques A.P., Dore T.M., Rauter A.P. Targeting Type 2 Diabetes with C-Glucosyl Dihydrochalcones as Selective Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: Synthesis and Biological Evaluation. J. Med. Chem. 2017;60:568–579. doi: 10.1021/acs.jmedchem.6b01134. [DOI] [PubMed] [Google Scholar]
- 31.Swart M., Verbrugge I., Beltman J.B. Combination Approaches with Immune-Checkpoint Blockade in Cancer Therapy. Front Oncol. 2016;6:233. doi: 10.3389/fonc.2016.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li D., Xu J., Wang Z., Gong Z., Liu J., Zheng Y., Li J., Li J. Epitope mapping reveals the binding mechanism of a functional antibody cross-reactive to both human and murine programmed death 1. MAbs. 2017;9:628–637. doi: 10.1080/19420862.2017.1296612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin D.Y., Tanaka Y., Iwasaki M., Gittis A.G., Su H.P., Mikami B., Okazaki T., Honjo T., Minato N., Garboczi D.N. The PD-1/PD-L1 complex resembles the antigen-binding Fv domains of antibodies and T cell receptors. Proc. Natl. Acad. Sci. USA. 2008;105:3011–3016. doi: 10.1073/pnas.0712278105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stewart R., Morrow M., Hammond S.A., Mulgrew K., Marcus D., Poon E., Watkins A., Mullins S., Chodorge M., Andrews J., et al. Identification and Characterization of MEDI4736, an Antagonistic Anti-PD-L1 Monoclonal Antibody. Cancer Immunol. Res. 2015;3:1052–1062. doi: 10.1158/2326-6066.CIR-14-0191. [DOI] [PubMed] [Google Scholar]
- 35.Wang S., Bajorath J., Flies D.B., Dong H., Honjo T., Chen L. Molecular modeling and functional mapping of B7-H1 and B7-DC uncouple costimulatory function from PD-1 interaction. J. Exp. Med. 2003;197:1083–1091. doi: 10.1084/jem.20021752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Linsley P.S., Brady W., Urnes M., Grosmaire L.S., Damle N.K., Ledbetter J.A. CTLA-4 is a second receptor for the B cell activation antigen B7. J. Exp. Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hurwitz A.A., Foster B.A., Kwon E.D., Truong T., Choi E.M., Greenberg N.M., Burg M.B., Allison J.P. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 2000;60:2444–2448. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.