Abstract
Three new prenylated phenol derivatives, terreprenphenols A–C (1–3), along with four known related compounds (4–7), were isolated from Aspergillus terreus EN-539, an endophytic fungus obtained from the marine red alga Laurencia okamurai. The structures of these compounds were established by extensive analysis of 1D/2D NMR data, mass spectrometric data, and optical rotation (OR). The corresponding relationship between absolute configuration and optical rotation for known compounds anodendroic acid (4) and asperterreusine C (5) was ambiguous in literature, and their absolute configurations were therefore discussed and confirmed for the first time by time-dependent density functional (TDDFT) ECD and OR calculations. Compounds 1–7 inhibited some common aquatic bacteria with MIC values ranging from 2 to 64 μg/mL.
Keywords: Laurencia okamurai, Aspergillus terreus, endophytic fungus, prenylated phenol, benzofuran, antibacterial activity
1. Introduction
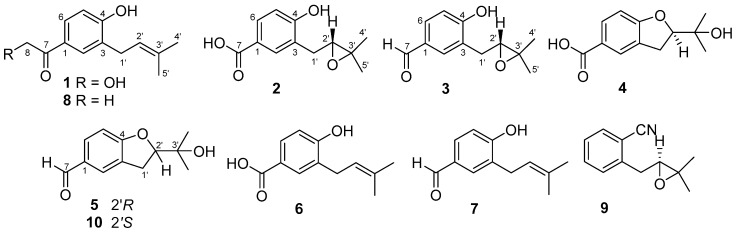
The growing trend in the discovery of new marine natural products from marine microorganisms has continued increasing over the last few years and represented 57% of the total new marine natural products reported in 2017 [1]. However, the rare occurrence of novel compounds and chemical rediscovery have been chronic problems in microbial secondary metabolites research [2,3]. Aspergillus terreus is a species complex currently comprised of 14 cryptic species, which is found worldwide in the environment [4]. In our previous research on new bioactive secondary metabolites from marine-derived fungi [5,6,7,8], a fungal strain Aspergillus terreus EN-539 was isolated from marine red alga Laurencia okamurai, which has been reported as a prolific producer of halogenated organic molecules, such as sesquiterpenes and nonterpenoid C15-acetogenins [9]. Chemical investigations were performed on the culture extracts of the marine fungus A. terreus EN-539. Two new meroterpenoids, aperterpenes N and O, along with related derivatives have been reported from A. terreus EN-539, which was cultured on rice-solid medium [7]. To enhance the chemical diversity of secondary metabolites, the fungal strain A. terreus EN-539 was further cultivated on MH2 medium, which resulted in the production of different metabolites compared with that culturing on rice-solid medium, as evidenced by HPLC analysis. As a result, three new prenylated phenol derivatives including terreprenphenol A (1), terreprenphenol B (2), and terreprenphenol C (3), along with four known related compounds (4–7), were isolated and identified. Moreover, as the corresponding relationship between stereochemistry and optical rotation (OR) for known compounds, anodendroic acid (4) and asperterreusine C (5), were ambiguous in literature, their absolute configurations were discussed and confirmed on the basis of time-dependent density functional (TDDFT)-ECD and OR calculations. The antimicrobial activities against some common aquatic bacteria, as well as antioxidative activity against DPPH (2,2-diphenyl-1-picrylhydrazyl) radical, were evaluated. This paper describes the isolation, characterization, and bioactivities of compounds 1–7 (Figure 1).
Figure 1.
Structures of the isolated compounds 1–7 and reference compounds 8–10.
2. Results and Discussion
2.1. Structure Elucidation of the New Compounds
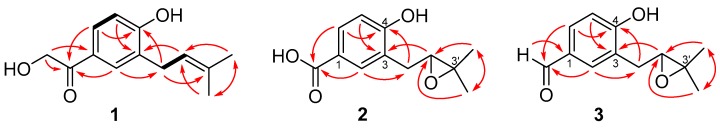
Terreprenphenol A (1) was obtained as a colourless solid, and its formula was determined as C13H16O3 on the basis of HRESIMS data (Figure S1), indicating six degrees of unsaturation. The 1H and 13C NMR spectroscopic data of 1 indicated the presence of a 1,3,4-trisubstituted benzene ring, two methyls, two methylenes (including one oxygenated), one olefinic methine, and two nonprotonated (including one ketone and one olefinic) carbon atoms. Detailed analysis of the 1H and 13C NMR data (Table 1) revealed that 1 is a prenylated phenol derivative similar to 4-hydroxy-3-(3-methyl-2-butenyl)acetophenone (HMBA, 8), which is the main secondary metabolite of Senecio nutans (Asteraceae), a medicinal plant of northwestern Argentina [10,11]. However, the resonances for one methyl at δH 2.58 and δC 26.1 in the 1H and 13C NMR spectra of HMBA were not present in those of 1. Instead, signals for an oxygenated methylene were observed at δH 4.63 (H-8) and δC 64.3 (C-8) in the 1H and 13C NMR spectra of 1. These spectroscopic features suggested that compound 1 was the 8-hydroxylated derivative of HMBA. The COSY and HMBC data (Figure 2) supported the above deduction. The structure of 1 was thus determined and it was named as terreprenphenol A.
Table 1.
1H (500 MHz) and 13C (125 MHz) NMR data of compounds 1–3.
| No. | 1 a | 2 b | 3 a | |||
|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 128.1, C | 129.1, C | 129.8, C | |||
| 2 | 128.9, CH | 7.59, s | 130.6, CH | 7.71, s | 133.2, CH | 8.50, s |
| 3 | 125.8, C | 118.2, C | 121.8, C | |||
| 4 | 160.8, C | 154.5, C | 159.2, C | |||
| 5 | 115.0, CH | 6.77, d (9.1) | 115.1, CH | 6.69, d (8.3) | 117.9, CH | 7.72, d (8.4) |
| 6 | 127.7, CH | 7.58, d (9.1) | 128.0, CH | 7.70, d (8.3) | 129.8, CH | 8.46, d (8.4) |
| 7 | 196.4, C | 173.4, C | 191.9, CH | 10.64, s | ||
| 8 | 64.3, CH2 | 4.63, s | ||||
| 1’ | 28.1, CH2 | 30.3, CH2 | 2.75, dd (7.4, 16.6) 3.03, dd (5.3, 16.6) |
31.4, CH2 | 3.54, dd (7.1, 16.8) 3.85, dd (4.9, 16.8) |
|
| 2’ | 122.7, CH | 3.22, d (7.3) | 68.6, CH | 3.77, dd (5.3, 7.4) | 68.0, CH | 4.54, dd (4.9, 7.1) |
| 3’ | 131.3, C | 5.29, t (7.3) | 76.6, C | 79.4, C | ||
| 4’ | 17.6, CH3 | 1.67, s | 19.2, CH3 | 1.26, s | 22.0, CH3 | 2.07, s |
| 5’ | 25.5, CH3 | 1.70, s | 24.1, CH3 | 1.33, s | 26.2, CH3 | 2.13, s |
a Measured in DMSO-d6. b Measured in CD3OD.
Figure 2.
Key 1H-1H COSY (bold lines) and HMBC (red arrows) correlations of compounds 1–3.
Terreprenphenol B (2) was obtained as a white, amorphous powder with the molecular formula C12H14O4 as established by HRESIMS data (Figure S7), indicating six degrees of unsaturation. Signals for a 1,3,4-trisubstituted benzene ring were also observed at δH 6.69 (d, J = 8.3 Hz, H-5), 7.70 (d, J = 8.3 Hz, H-6), and 7.71 (s, H-2) in the 1H NMR spectrum of 2, indicating that it could be another prenylated phenol derivative. The 1H and 13C NMR data (Table 1) of 2 were very similar to those of 4-hydroxy-3-prenybenzoic acid (6), a known benzoic acid derivative isolated from the dogwood anthracnose fungus Discula sp. by Venkatasubbaiah et al. [12]. However, the resonances of two olefinic carbons (δC 122.8, C-2’; 132.2, C-3’) in the 13C NMR spectrum of 4-hydroxy-3-prenybenzoic acid (6) were replaced by resonances of two oxygenated carbons (δC 68.6, C-2’; 76.6, C-3’) in that of 2. The HRESIMS as well as COSY and HMBC data of 2 suggested that the double bond at C-2’ and C-3’ in 6 was converted into an epoxide ring in 2.
Compound 2 only possessed one chiral center in epoxidized isopentyl group, similar to that of 2-{[(2R)-3,3-dimethyloxiran-2-yl]methyl}benzonitrile (9), an intermediate in a cascade approach to cyclic aminonitrones, which has been assigned as 2’R configuration on the basis of chemical synthesis and optical rotation [13]. The opposite signs of optical rotations of compound 2 +128.0 (c 0.54, CHCl3)] and 2-{[(2R)-3,3-dimethyloxiran-2-yl]methyl}benzonitrile −65.1 (c 1.4, CHCl3)] supported the assignement of the absolute configuration of 2 as 2’S.
Terreprenphenol C (3) was obtained as a colourless solid, and its formula was determined as C12H14O3, with one oxygen atom less than 2, on the basis of HRESIMS data (Figure S13). Comprehensive analysis of the 1H and 13C NMR data (Table 1) of 3 showed a structurally close relationship to that of 2, except that the carbonyl group at C-7 (δC 173.4) in 2 was replaced by an aldehyde group (δC 191.9; δH 10.64, s) in 3. The planar structure of 3 was further confirmed by the COSY and HMBC data (Figure 2). The absolute configuration of 3 was also determined as 2’S on the basis of its optical rotation value of +85.4 (c 0.43, CHCl3)], compared to that of 2-{[(2R)-3,3-dimethyloxiran-2-yl]methyl}benzonitrile (9) and 2. Moreover, it should be mentioned that the benzoate of 3, methyl 3-((3,3-dimethyloxiran-2-yl)methyl)-4-hydroxybenzoate, was reported as an intermediate in the syntheses of benzofuran derivatives hostmaniene, 5-formyl-2-(isopropyl-1’-ol)benzofuran, and anadenfroic acid, without purification and identification [14].
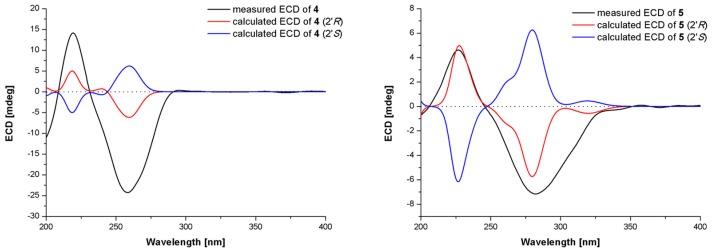
Anodendroic acid (4) and asperterreusine C (5) were isolated and identified by comparing their NMR spectroscopic data (Figures S19–S22) and optical rotations with those reported in the literatures [14,15,16,17]. However, the relationship between absolute configuration and optical rotation for the known compounds anodendroic acid (4) and asperterreusine C (5) was ambiguous in the literature, and their absolute configurations were therefore discussed and confirmed by TDDFT-ECD and OR calculations. Anodendroic acid (4) was first isolated from the higher plant Anodendron affine Durce with optical rotation of = −19 (c 0.7, EtOH) [15], and then its absolute configuration was determined as being R by chemical synthesis, with optical rotation of = −35.2 (c 0.682, EtOH) [18]. Recently, the (+)-S-anodendroic acid was isolated and identified from another higher plant Euodia lepta, with an optical rotation of = +42.0 (c 0.15, EtOH) [16]. Quadricinctafuran A, possessing the same planar structure as that of anodendroic acid, was established as having an R configuration on the basis of X-ray crystallographic analysis by Prompanya et al. [19]. However, the optical rotation of quadricinctafuran A was measured as = +74 (c 0.03, MeOH) (R configuration with positve OR and S configuration with negative OR), inconsistent with the corresponding relationship between sterechemistry and optical rotation (R configuration with negative OR, S configuration with positive OR) in other literature [14,15,16]. Considering the ambiguity, the absolute configuration of 4 was thus established by the TDDFT-ECD calculation in Gaussian 09 [20]. We obtained the minimum energy conformers by geometry optimization of S and R isomers of 4, and then employed the TDDFT method at the B3LYP/6-31G level to obtain a calculated ECD spectra of 4. The experimental ECD spectrum of 4 exhibited excellent accordance with that calculated for R isomer of 4 and was opposite to that calculated for an S isomer of 4, which allowed unambiguous assignment of its absolute configuration (Figure 3).
Figure 3.
Measured and calculated ECD spectra of compounds 4 and 5 at B3LYP/6-31G level.
Asperterreusine C (5) was ever isolated and identified from the marine-derived fungus Aspergillus terreus [CFCC 81836] [17] and its absolute configuration was determined as being R by comparing the optical rotation = −124 (c 1.20, MeOH) with that of its enantiomer, (S)-5-formyl-2-(isopropyl-1′-ol)-2,3-dihydrobenzofuran (10), isolated from the culture broth of Heterobasidion annosum = +109.1 (c 1.4, CHCl3)] [21]. The similar structural characterizations between 4 and 5 encouraged us to confirm the absolute configuration of 5 by using the TDDFT-ECD calculation. The measured ECD spectral behavior (Figure 3) of 5 assigned its absolute configuration as R unambiguously. The optical rotations of 4 and 5 were measured as = −95.0 (c 0.29, MeOH) and = −55.0 (c 0.51, MeOH), respectively, which satisfied the reported correspondances between sterechemistry and optical rotations (R configuration with negative OR and S configuration with positive OR) for anodendroic acid [18], (+)-S-anodendroic acid [16], asperterreusine C [17], and (S)-5-formyl-2-(isopropyl-1′-ol)-2,3-dihydrobenzofuran [21]. This corresponding relationship was further verified by OR calculation at the B3LYP/6-31G(d) level in Gaussian 09 [20]. The OR calculation for compounds 4 and 5 (Table 2) indicated that the R configuration was consistent with negative OR and the S configuration was featured with the opposite sign, which established the absulute configurations of 4 and 5 by comparing their measured OR values. This matched well with the results of the TDDFT-ECD calculation.
Table 2.
The calculated and measured optical rotations (ORs) of compounds 4 and 5 a.
| Compounds | 4 | 5 | ||
|---|---|---|---|---|
| 2’R | 2’S | 2’R | 2’S | |
| calculated OR | −60.9 | +61.0 | −91.3 | +95.5 |
| measured OR | −95.0 | −55.0 | ||
a The ORs were calculated and measured at 589.3 nm in MeOH.
In addition to compounds 1–5, the known prenylated phenol derivatives, 4-hydroxy-3-prenybenzoic acid (6) and 4-hydroxy-3-(3-methyl-but-2-enyl)-benzaldehyde (7), were isolated and identified from the MH2 culture extract of fungal strain A. terreus EN-539. Their structures were established by comparing their NMR data (Figures S23–S26) with those reported in the literature [12,22].
2.2. Biological Activities of the Isolated Compounds
Compounds 1–7 were assayed for their antioxidative activity against DPPH radical. The result showed that these prenylated phenol and benzofuran derivatives displayed no observed DPPH radical scavenging activity, except that compound 7 exhibited weak scavenging activity with IC50 value of 0.9 mM. Butylated hydroxytoluene (BHT) was measured as the positive control for DPPH radical scavenging activity (IC50, 72 μM).
Antimicrobial activities of compounds 1–7 were also evaluated against human pathogens (Escherichia coli and Staphylococcus aureus), aquatic bacteria (Aeromonas hydrophila, Edwardsiella tarda, Micrococcus luteus, Pseudomonas aeruginosa, V. harveyi, V. parahemolyticus, V. vulnificus), and plant-pathogenic fungi (Alternaria brassicae, Colletotrichum gloeosporioides, Fusarium oxysporum, Gaeumannomyces graminis, and Physalospora piricola). Compounds 1, 6, and 7 showed broad-spectrum inhibitory activity against the pathogenic bacteria in the assay with MIC values ranging from 2 to 64 μg/mL. Compound 1 in particular exhibited potent activity against the aquatic bacteria A. hydrophila, P. aeruginosa, and V. harveyi with MIC values of 9, 9, and 18 μM, respectively. These results (Table 3) indicated that the prenyl group in structures was indispensable for their antibacterial activity (1, 6, 7 vs. 2–5). Compounds 1–7 showed no inhibitory activity against the plant-pathogenic fungi in the assay.
Table 3.
The antimicrobial activities of compounds 1–7 (MIC, μg/mL) a.
| Compounds | Ah | Et | Ec | Ml | Pa | Sa | Vh | Vp | Vv |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 32 | 32 | 16 | 2 | 8 | 4 | 8 | 32 |
| 2 | 64 | n.a. | 32 | 32 | 64 | n.a. | n.a. | n.a. | n.a. |
| 3 | n.a. | n.a. | 64 | 32 | n.a. | n.a. | n.a. | n.a. | n.a. |
| 4 | n.a. | 32 | n.a. | n.a. | n.a. | 64 | n.a. | n.a. | n.a. |
| 5 | n.a. | 64 | n.a. | 32 | n.a. | 32 | n.a. | n.a. | n.a. |
| 6 | 8 | 16 | 16 | n.a. | n.a. | 64 | 32 | 8 | n.a. |
| 7 | 4 | 16 | 32 | 8 | 16 | 16 | 8 | 8 | 64 |
| Chloramphenicol | 1 | 0.5 | 2 | 2 | 1 | 2 | 2 | 4 | 4 |
aAh: Aeromonas hydrophila. Et: Edwardsiella tarda. Ec: Escherichia coli. Ml: Micrococcus luteus. Pa: Pseudomonas aeruginosa. Sa: Staphylococcus aureus. Vh: Vibrio harveyi. Vp: V. parahemolyticus. Vv: Vibrio vulnificus. n.a.: No activity, MIC > 64 μg/mL.
3. Experimental Section
3.1. General Experimental Procedures
Optical rotations were measured on an Optical Activity AA-55 polarimeter (Optical Activity Ltd., Cambridgeshire, UK). UV spectra were measured on a PuXi TU-1810 UV-visible spectrophotometer (Shanghai Lengguang Technology Co. Ltd., Shanghai, China). ECD spectra were acquired on a Chirascan spectropolarimeter (Applied Photophysics Ltd., Leatherhead, UK). The 1D and 2D NMR spectra were obtained at 500 and 125 MHz for 1H and 13C, respectively, on a Bruker Avance 500 MHz spectrometer (Bruker Biospin Group, Karlsruhe, Germany) with tetramethyl silane (TMS) as an internal standard. Mass spectra were obtained from an API QSTAR Pulsar 1 mass spectrometer (Applied Biosystems, Foster, Waltham, MA, USA). Analytical HPLC analyses were performed using a Dionex HPLC system (Dionex, Sunnyvale, CA, USA) equipped with P680 pump, ASI-100 automated sample injector, and UVD340U multiple wavelength detector controlled by Chromeleon software (version 6.80, Dionex, Sunnyvale, CA, USA). Column chromatography (CC) was performed with silica gel (200–300 mesh, Qingdao Haiyang Chemical Factory, Qingdao, China), Lobar LiChroprep RP-18 (40–60 μm, Merck, Darmstadt, Germany), and Sephadex LH-20 (18–110 μm, Merck). All solvents were distilled prior to use.
3.2. Fungal Material
The isolation and identification of the fungal material A. terreus EN-539 were the same as those reported in our previous publications [7]. The strain is preserved at the Key Laboratory of Experimental Marine Biology, Institute of Oceanology, Chinese Academy of Sciences (IOCAS).
3.3. Fermentation
For chemical investigations, the fresh mycelia of fungal strain A. terreus EN-539 was cultured on PDA (Potato Dextrose Agar) medium at 28 ℃ for seven days, and then inoculated into 1 L conical flasks containing 300 mL of MH2 broth medium (sucrose 2%, mannitol 2%, yeast extract 0.3%, peptone 0.5%, K2HPO4 0.05%, and MgSO4·7H2O 0.03% in seawater, which was naturally sourced and filtered from the Huiquan Gulf of the Yellow Sea near the campus of IOCAS, pH 6.5–7.0) for 30 days at room temperature.
3.4. Extraction and Isolation
The whole fermented cultures (100 flasks, 30 L) were filtered to separate the broth from the mycelia. The former was extracted four times with ethyl acetate (EtOAc), while the mycelia was extracted four times with a mixture of 80% acetone and 20% H2O. The acetone solution was evaporated under reduced pressure to develop an aqueous solution, which was then extracted four times with EtOAc. The two EtOAc solutions were combined and concentrated under reduced pressure to give an extract (48 g), on the basis of TLC and HPLC analysis, which was fractionated by silica gel vacuum liquid chromatography (VLC) using different solvents of increasing polarity from petroleum ether (PE) to MeOH to yield 9 fractions (Frs. 1–9). Fr. 4 (4.5 g), eluted with PE-EtOAc (2:1), was further purified by reversed-phase column chromatography (CC) over Lobar LiChroprep RP-18 with a MeOH-H2O gradient (from 10:90 to 100, v/v) to yield 10 subfractions. Fr 4.2 (MeOH-H2O 20:80, 220 mg) was subjected to CC on silica gel (CH2Cl2-MeOH, from 150:1 to 10:1, v/v), and then purified by Sephadex LH-20 (MeOH) and preparative TLC (plate: 20 × 20 cm, developing solvents: PE-acetone, 3:1) to obtain compounds 2 (35.6 mg) and 5 (29.1 mg). Fr 4.3 (MeOH-H2O 30:70, 330 mg) was fractionated by CC on silica gel eluting with CH2Cl2-acetone (from 15:1 to 2:1, v/v) to afford 4 subfractions (Frs. 4.3.1–4.3.4). Fr 4.3.2 was further purified by preparative TLC (plate: 20 × 20 cm, developing solvents: CH2Cl2-MeOH, 20:1) and CC on Sephadex LH-20 (MeOH) to yield compound 4 (74.0 mg), while Fr 4.3.3 was directly purified by CC on Sephadex LH-20 (MeOH) to yield compound 3 (6.2 mg). Fr. 5 (6.9 g), eluted with PE-EtOAc (1:1), was further purified by reversed-phase CC over Lobar LiChroprep RP-18 with a MeOH-H2O gradient (from 10:90 to 100, v/v) to yield 10 subfractions. Fr 5.5 (MeOH-H2O 50:50, 520 mg) was subjected to CC on silica gel (CH2Cl2-MeOH, from 100:1 to 10:1, v/v), and then purified by Sephadex LH-20 (MeOH) and preparative TLC (plate: 20 × 20 cm, developing solvents: PE- EtOAc, 1:1) to obtain compound 1 (8.1 mg). Fr 5.5 (MeOH-H2O 80:20, 320 mg) was purified by CC on Sephadex LH-20 (MeOH) and then on silica gel (CH2Cl2-acetone, from 20:1 to 5:1, v/v) to yield compounds 6 (22.0 mg) and 7 (42.5 mg).
Terreprenphenol A (1): Colourless solid; UV (MeOH) λmax (log ε) 230 (2.32), 297 (2.85) nm; 1H and 13C NMR data, see Table 1; HRESIMS m/z 243.0997 [M + Na]+ (calcd. for C13H16NaO3, 243.0992).
Terreprenphenol B (2): White, amorphous powder; +128.0 (c 0.54, CHCl3); UV (MeOH) λmax (log ε) 204 (2.57), 250 (2.16) nm; ECD (5.40 mM, MeOH) λmax (Δ ε) 205 (–1.63), 214 (+1.28), 228 (–0.29), 246 (+0.68) nm; 1H and 13C NMR data, see Table 1; HRESIMS m/z 245.0786 [M + Na]+ (calcd. for C12H14NaO4, 245.0784).
Terreprenphenol C (3): Colourless solid; +85.4 (c 0.43, CHCl3); UV (MeOH) λmax (log ε) 202 (2.74), 228 (2.56), 287 (2.61) nm; ECD (4.85 mM, MeOH) λmax (Δ ε) 206 (–1.56), 216 (+0.94), 230 (–0.25), 244 (+0.62) nm;; 1H and 13C NMR data, see Table 1; HRESIMS m/z 205.0872 [M – H]– (calcd. for C12H13O3, 205.0870).
3.5. Antioxidant and Antimicrobial Assays
Evaluation of compounds 1–7 for antioxidative activity against DPPH free radical was carried out using the method described previously [23]. BHT was used as positive control against the DPPH free radical. Antimicrobial activity against human pathogens (E. coli EMBLC-1 and S. aureus EMBLC-2), aquatic bacteria (A. hydrophila QDIO-1, E. tarda QDIO-2, M. luteus QDIO-3, P. aeruginosa QDIO-4, V. harveyi QDIO-7, V. parahemolyticus QDIO-8, V. vulnificus QDIO-10), and plant-pathogenic fungi (A. brassicae QDAU-1, C. gloeosporioides QDAU-2, F. oxysporum QDAU-5, G. graminis QDAU-3, and P. piricolav QDAU-6), was carried out by the 96-well microtiter plates assay [24]. The pathogens were obtained from the Institute of Oceanology, Chinese Academy of Sciences. Chloramphenicol and amphotericin were used as positive controls for bacteria and fungi, respectively.
3.6. Computational Section
Conformational searches were performed via molecular mechanics using the MM+ method in HyperChem software (Version 8.0, Hypercube, Inc., Gainesville, FL, USA), and the geometries were further optimized at the B3LYP/6-31G(d) level via Gaussian 09 software (Version D.01; Gaussian, Inc.:Wallingford, CT, USA) [20] to give the energy-minimized conformers. Then, the optimized conformers were subjected to the calculations of ECD and OR by using TDDFT at B3LYP/6-31G level. Solvent effects of the MeOH solution were evaluated at the same DFT level using the SCRF/PCM method.
4. Conclusions
In summary, chemical investigations were performed on the marine fungus A. terreus EN-539. Two new meroterpenoids, aperterpenes N and O, along with related derivatives have been reported from A. terreus EN-539, which was cultured on rice-solid medium [7]. The coculture of A. terreus EN-539 and the symbiotic fungus Paecilomyces lilacinus EN-531 induced the production of a new terrein derivative, namely asperterrein and a known dihydroterrein, which were not detected in the axenic cultures of both strains [25]. To enhance the chemical diversity of secondary metabolites, A. terreus EN-539 was further cultivated on MH2 medium, which resulted in the production of three new prenylated phenol derivatives including terreprenphenol A (1), terreprenphenol B (2), and terreprenphenol C (3), along with four known related compounds (4–7). The absolute configurations of benzofuran derivatives (4 and 5) were discussed and confirmed on the basis of TDDFT-ECD and OR calculations. Compounds 1, 6, and 7 showed broad-spectrum inhibitory activity against the pathogenic bacteria in the assay with MIC values ranging from 2 to 64 μg/mL, which might be used as potential molecules in the development of drug leads, or modified to find more active derivatives for the treatment of microbial infection in aquaculture.
Acknowledgments
H.-L.L. acknowledges the Young Scientists Partner Program of Shenyang Branch of Chinese Academy of Sciences-Shandong Academy of Sciences for project supporting. B.-G.W. appreciates the support of Taishan Scholar Program from Shandong Province.
Supplementary Materials
The following are available online at https://www.mdpi.com/1660-3397/17/11/605/s1: The HRESIMS, 1D and 2D NMR spectra of compounds 1–3, as well as the 1H and 13C NMR spectra of compounds 4–7.
Author Contributions
H.-L.L. performed the experiments for the isolation, structure elucidation, antioxidative and antimicrobial evaluation, ECD calculations, and prepared the manuscript; X.-M.L. performed the 1D and 2D NMR experiments; S.-Q.Y. participated the collection of marine red alga Laurencia okamurai. L.-H.M. and X.L. supervised the ECD and OR calculations; B.-G.W. supervised the research work and revised the manuscript.
Funding
This work was financially supported by the National Natural Science Foundation of China (31330009), the Natural Science Foundation of Shandong Province (ZR2018BD031), and the China Postdoctoral Science Foundation (2018M632732).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Carroll A.R., Copp B.R., Davis R.A., Keyzers R.A., Prinsep M.R. Marine natural products. Nat. Prod. Rep. 2019;36:122–173. doi: 10.1039/C8NP00092A. [DOI] [PubMed] [Google Scholar]
- 2.Pettit R.K. Mixed fermentation for natural product drug discovery. Appl. Microbiol. Biot. 2009;83:19–25. doi: 10.1007/s00253-009-1916-9. [DOI] [PubMed] [Google Scholar]
- 3.Strobel G.A. Endophytes as sources of bioactive products. Microbes Infect. 2003;5:535–544. doi: 10.1016/S1286-4579(03)00073-X. [DOI] [PubMed] [Google Scholar]
- 4.Samson R.A., Peterson S.W., Frisvad J.C., Varga J. New species in Aspergillus section Terrei. Stud. Mycol. 2011;69:39–55. doi: 10.3114/sim.2011.69.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H.L., Xu R., Li X.M., Yang S.Q., Meng L.H., Wang B.G. Simpterpenoid A, a meroterpenoid with a highly functionalized cyclohexadiene moiety featuring gem-propane-1,2-dione and methylformate groups, from the mangrove-derived Penicillium simplicissimum MA-332. Org. Lett. 2018;20:1465–1468. doi: 10.1021/acs.orglett.8b00327. [DOI] [PubMed] [Google Scholar]
- 6.Li H.L., Li X.M., Mándi A., Antus S., Li X., Zhang P., Liu Y., Kurtán T., Wang B.G. Characterization of cladosporols from the marine algal-derived endophytic fungus Cladosporium cladosporioides EN-399 and configurational revision of the previously reported cladosporol derivatives. J. Org. Chem. 2017;82:9946–9954. doi: 10.1021/acs.joc.7b01277. [DOI] [PubMed] [Google Scholar]
- 7.Li H.L., Li X.M., Li X., Yang S.Q., Wang B.G. Structure, absolute configuration and biological evaluation of polyoxygenated meroterpenoids from the marine algal-derived Aspergillus terreus EN-539. Phytochem. Lett. 2019;32:138–142. doi: 10.1016/j.phytol.2019.05.017. [DOI] [Google Scholar]
- 8.Cao J., Li X.M., Meng L.H., Konuklugil B., Li X., Li H.L., Wang B.G. Isolation and characterization of three pairs of indolediketopiperazine enantiomers containing infrequent N-methoxy substitution from the marine algal-derived endophytic fungus Acrostalagmus luteoalbus TK-43. Bioorg. Chem. 2019;90:103030. doi: 10.1016/j.bioorg.2019.103030. [DOI] [PubMed] [Google Scholar]
- 9.Wang B.G., Gloer J.B., Ji N.Y., Zhao J.C. Halogenated organic molecules of Rhodomelaceae origin: Chemistry and biology. Chem. Rev. 2013;113:3632–3685. doi: 10.1021/cr9002215. [DOI] [PubMed] [Google Scholar]
- 10.Gil D.M., Lizárraga E., Echeverría G.A., Piro O.E., Catalán C.A.N., Altabef A.B. A combined experimental and theoretical study of the supramolecular self-assembly of the natural benzopyran 2,2-dimethyl-3-hydroxy-6-acetyl-chromane and its isomeric benzofuran 10,11-dihydro-10-hydroxytremetone. J. Mol. Struct. 2017;1146:164–178. doi: 10.1016/j.molstruc.2017.05.137. [DOI] [Google Scholar]
- 11.Lizárraga E., Romano E., Rudyk R.A., Catalán C.A.N., Brandán S.A. Structural study, coordinated normal analysis and vibrational spectra of 4-hydroxy-3-(3-methyl-2-butenyl) acetophenone. Spectrochim. Acta A. 2012;97:202–208. doi: 10.1016/j.saa.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Venkatasubbaiah P., Chilton W.S. Toxins produced by the dogwood anthracnose fungus Discula SP. J. Nat. Prod. 1991;54:1293–1297. doi: 10.1021/np50077a009. [DOI] [Google Scholar]
- 13.Sharma R., Bulger P.G., McNevin M., Dormer P.G., Ball R.G., Streckfuss E., Cuff J.F., Yin J.J., Chen C.Y. A cascade approach to cyclic aminonitrones: Reaction discovery, mechanism, and scope. Org. Lett. 2009;11:3194–3197. doi: 10.1021/ol9010147. [DOI] [PubMed] [Google Scholar]
- 14.Mohammat M.F., Osman N.S., Shaameri Z., Hamzah A.S. First consecutive linear synthesis of hostmaniene, 5-formyl-2-(isopropyl-1′-ol) benzofuran and anadendroic acid using prenylated phenol. Org. Commun. 2017;10:130–134. doi: 10.25135/acg.oc.17.17.03.011. [DOI] [Google Scholar]
- 15.Shima K., Hisada S., Inagaki I. Studies on the constituents of Anodendron affine Durce. V. Isolation and structure of two new constituents. Yakugaku Zasshi. 1972;92:1410–1414. doi: 10.1248/yakushi1947.92.11_1410. [DOI] [PubMed] [Google Scholar]
- 16.Zhao M.B., Zhou S.X., Zhang Q.Y., Wei W.F., Li M.H., Xing J.Y., Jiang Y., Tu P.F. Prenylated benzoic acid derivatives from the stem of Euodia lepta. Nat. Prod. Res. 2017;31:1589–1593. doi: 10.1080/14786419.2017.1283493. [DOI] [PubMed] [Google Scholar]
- 17.Wu Z.D., Li D.Y., Zeng F.R., Tong Q.Y., Zheng Y.Y., Liu J.J., Zhou Q., Li X.N., Chen C.M., Lai Y.J., et al. Brasilane sesquiterpenoids and dihydrobenzofuran derivatives from Aspergillus terreus [CFCC 81836] Phytochemistry. 2018;156:159–166. doi: 10.1016/j.phytochem.2018.10.006. [DOI] [PubMed] [Google Scholar]
- 18.Kawase Y., Yamaguchi S., Inoue O., Sannomiya M., Kawabe K. The syntheses and absolute configurations of fomannoxin, (−)-5-acetyl-2-(1-hydroxymethylvinyl)-2,3-dihydrobenzofuran, and anodendroic acid. Chem. Lett. 1980;12:1581–1584. doi: 10.1246/cl.1980.1581. [DOI] [Google Scholar]
- 19.Prompanya C., Dethoup T., Gales L., Lee M., Pereira J.A., Silva A.M.S., Pinto M.M.M., Kijjoa A. New polyketides and new benzoic Aacid derivatives from the marine sponge-associated fungus Neosartorya quadricincta KUFA 0081. Mar. Drug. 2016;14:134. doi: 10.3390/md14070134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Mennucci B., Petersson G.A., et al. Gaussian 09. Gaussian Inc.; Wallingford, CT, USA: 2013. [Google Scholar]
- 21.Donnelly D.M.X., Fukuda N., Kouno I., Martin M., O’Reilly J. Dihydrobenzofurans from Heterobasidion annosum. Phytochemistry. 1988;27:2709–2713. doi: 10.1016/0031-9422(88)87048-1. [DOI] [Google Scholar]
- 22.Moriarty R.M., Grubjesic S., Surve B.C., Chandersekera S.N., Prakash O., Naithani R. Synthesis of Abyssinone II and related compounds as potential chemopreventive agents. Eur. J. Med. Chem. 2006;41:263–267. doi: 10.1016/j.ejmech.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 23.Zhang P., Meng L.H., Mándi A., Li X.M., Kurtán T., Wang B.G. Structure, absolute configuration, and conformational study of resorcylic acid derivatives and related congeners from the fungus Penicillium brocae. RSC Adv. 2015;5:39870–39877. doi: 10.1039/C5RA02203G. [DOI] [Google Scholar]
- 24.Pierce C.G., Uppuluri P., Tristan A.R., Wormley F.L., Jr., Mowat E., Ramage G., Lopez-Ribot J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008;3:1494–1500. doi: 10.1038/nprot.2008.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li H.L., Li X.M., Yang S.Q., Cao J., Li Y.H., Wang B.G. Induced terreins production from marine red algal-derived endophytic fungus Aspergillus terreus EN-539 co-cultured with symbiotic fungus Paecilomyces lilacinus EN-531. J. Antibiot. 2019 doi: 10.1038/s41429-019-0242-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.