Abstract
Hydrolyzed collagen (HC) is a group of peptides with low molecular weight (3–6 KDa) that can be obtained by enzymatic action in acid or alkaline media at a specific incubation temperature. HC can be extracted from different sources such as bovine or porcine. These sources have presented health limitations in the last years. Recently research has shown good properties of the HC found in skin, scale, and bones from marine sources. Type and source of extraction are the main factors that affect HC properties, such as molecular weight of the peptide chain, solubility, and functional activity. HC is widely used in several industries including food, pharmaceutical, cosmetic, biomedical, and leather industries. The present review presents the different types of HC, sources of extraction, and their applications as a biomaterial.
Keywords: hydrolyzed collagen, peptide, antioxidant activity, denaturation, hydrolysates
1. Introduction
Collagen is the most important protein produced by the human body, it is mainly formed by the amino acid glycine (33%), proline and hydroxyproline (22%) (primary structure) in a triplex helix which is formed by three α chains. Each alpha chain is composed for 1014 amino acids approximately with a molecular weight around 100 kDa. These chains are coiled into a left-handed helix with three amino acids per turn (secondary structure). The chains are twisted around each other into a triple helix to form a rigid structure (tertiary structure). The super helix represents the basic collagen structure (quaternary structure). This collagen structure is very stable because of the intramolecular hydrogen bonds between glycine in adjacent chains. The collagen molecule is formed for a triple helical region and two nonhelical regions at either end of the helix structure with ≈300 kDa molecular weight, 280 nm in length, and 1.4 nm in diameter [1,2,3].
Nearly 28 types of collagen have been identified, but collagen type I is the most common in skin, bone, teeth, tendon, ligaments, vascular ligature, and organs. Collagen type II is present in the cartilages. For collagen type III, the skin, muscle, and blood vessels are the most common sources of this protein. Type IV has been reported in the epithelium-secreted layer of the basement membrane and the basal lamina. Collagen type V is one of the principal components of cell surfaces and placenta [4,5,6,7,8,9]. Collagens are different according to their α-chain composition, depending on the repeat and length of the Gly–X–Y amino acid repetition, with and without interruptions, also the occupation of the X and Y positions by proline and its hydroxylated form, hydroxyproline, respectively [1,10].
Collagen has been classified into different families such as fibrillar and network-forming collagens, the FACITs (fibril-associated collagens with interrupted triple helices), MACITs (membrane-associated collagens with interrupted triple helices), and MULTIPLEXINs (multiple triple-helix domains and interruptions) [11,12]. Fibrillar collagen is the most abundant collagen in vertebrates and it plays a structural role by contributing to the molecular architecture, shape, and mechanical properties of tissues such as tensile strength in skin and the resistance to traction in ligaments (collagens type I, II, III, V, XI, XXIV, and XXVII) [13,14,15].
FACITs (fibril-associated collagens with interrupted triple helices) do not form fibrils by themselves, but they are associated with the surface of collagen fibrils. FACITs collagens have their triple helix interrupted by non-collagenous domains which can act as joints. These interruptions are useful because they allow proteolytic cleavage of the structure overcoming the resistance to proteases of native triple helices [2,16]. The MULTIPLEXIN collagen family includes types XV and XVIII. Collagen types XV and XVIII represent a molecule of the basement membranes. Collagen XV is found in skeletal and cardiac muscle, and collagen type XVIII is a component in the liver. The MACIT collagen family has numerous interruptions in the triple helix, does not self-assemble into fibrils, and has roles in cell adhesion and signaling. Other collagen types are in very low concentration and in specific organs in the body [14,17].
Native collagen type I can be extracted from different sources, however, the main source of extraction is bovine because of its availability as well as its biocompatibility. Collagen extraction can be carried out from different tissues such as bones, tendons, lung tissue, or even connective tissue [18,19,20,21].
Another common source is porcine byproducts. This source has high resemblance to human collagen. There are no allergenic limitations to its usage because it has been widely used as a tendon reinforcement [22,23], hernia repairment [24], and for skin and wound healing as a plastic and reconstructive surgery material [25].
Alternative sources for native collagen extraction that are not of bovine or porcine origin have been developed from ovine tendon and skin [26,27]; fish tissue such as bones, skin, and scales or waste fish byproducts, or others sources such as chicken, duck, and rabbit skin [28,29,30,31,32].
Extraction can be carried out by an acid or alkaline treatment [33]. Extraction under acid treatment is usually applied for extraction of collagen type I from tissues of porcine or fish skin origin [34]. Acetic acid is the most common reagent for collagen extraction. The concentration of this acid will affect the final pH value changing the electrostatic interaction and structure. It also determines the solubility and extraction capacity from animal tissue [35]. A combination of both acidic and enzymatic treatment produces a higher and more efficient collagen extraction process [26]. Pepsin can be obtained from porcine gastric mucosa. This enzyme affects the telopeptidic region in the collagen molecule increasing its solubility in an acidic medium [36,37]. The use of ultrasound as an alternative method for collagen extraction does not change the molecule and facilitates the enzymatic action. This technology can be applied in different tissues such as fish skin and bovine tendons in order to produce higher collagen concentrations in shorter extraction times [38,39,40].
Pre-treatment conditions, dialysis, and source of extraction are the main factors that determine final collagen characteristics such as molecular weight, amino acid composition, and molecular structure [41,42].
2. Hydrolyzed Collagen: Extraction and Properties
2.1. Extraction and Structure of Hydrolyzed Collagen
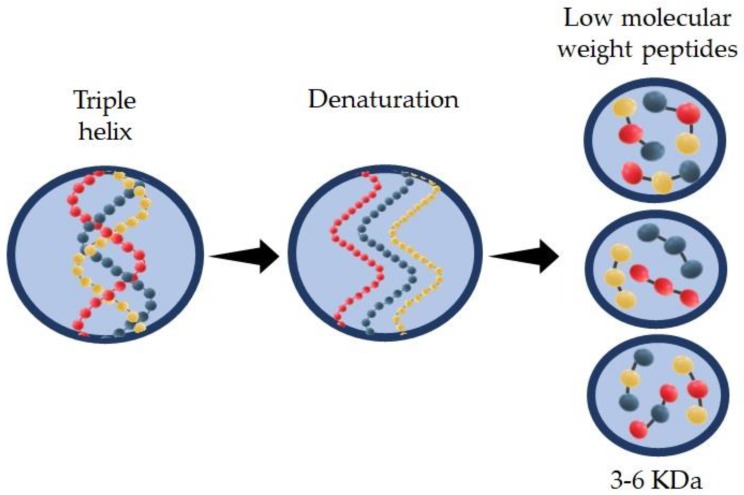
From Figure 1, it can be seen that denaturation of native collagen produces three α chains in their random coiled form. It can be observed by thermal treatment of collagen above 40 °C. Once the chains are separated, the hydrolysis is carried out by the action of proteolytic enzymes (alcalase, papain, pepsin, and others). The resulting product is commonly called hydrolyzed collagen (HC). It is composed of small peptides with low molecular weight 3–6 KDa [43,44,45,46]. Its solubility and functional activity (antioxidant, antimicrobial) are related to the type and degree of hydrolysis as well as the type of enzyme used in the process [47,48,49,50]. Another type of hydrolysis is by use of chemical products in acidic [45,51,52,53] (acetic acid, hydrochloric acid, and phosphoric acid) or alkaline media [27,52]. These two types of extraction are strongly corrosive and produce a high salt concentration in the final product after neutralization [54]. Alternative methods of extraction consist in thermal treatment [55] or applying high temperature and pressure to the protein. It includes subcritical water level (SCW) that exists at a temperature between 100 and 374 °C and a pressure of less than 22 MPa [56,57].
Figure 1.
Denaturation of native collagen into small low-molecular-weight peptides.
2.2. Techniques for HC Molecular Weight Measurements
The determination of HC molecular weight is a difficult task because of its low molecular weight (Mw) which ranges between 3 and 6 KDa. The most common technique used is SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis). It can separate proteins in the mass range of 1–100 KDa. The molecules are separated according to their charge, the moving speed is related to the charge of the molecule. This method uses polyacrylamide gels (PAGE—polyacrylamide gel electrophoresis) in the presence of the anionic detergent sodium dodecyl sulfate (SDS). The gel polymerization of acrylamide monomers produces linear chains. By including bisacrylamide, this formed a three-dimensional matrix of the gel. The size of the pores formed depends on the concentration of acrylamide and the degree of crosslinking. The first gel is the staking gel, it is a low-concentration gel (4%), and the second gel called resolving gel usually has a 10–12.5% concentration and is used to separate proteins in the range of 1–100 KDa. Thus, varying the concentration of acrylamide and bisacrylamide in the gel preparation results in different degrees of porosity and therefore different protein separation intervals [58,59,60].
Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) is another technique that helps to detect molecules in a large range of molecular weights. It is a technique where peptides are first mixed with a large molar excess of a matrix compound such as DHB (2,5-dihydroxybenzoic acid) to ionize low-molecular-weight peptides, next the matrix that carries the peptides is vaporized by laser radiation, and finally the mass of vaporized peptides is determined from the ionic time-of-flight. However, the limitation of this technique is that some peptide peaks fail to resolve in a single matrix [61,62,63].
HPLC-MS/MS is a powerful tool not only for the identification, but also for quantification of peptides and proteins. It is rather limited to the quantification of selected peptides of biological importance such as the quantification of collagen. The quantification of collagen types is usually carried out by amino acid analysis [64,65,66].
2.3. Hydrolyzed Collagen Properties
Native collagen properties are very different to those of hydrolyzed collagen as illustrated in Table 1. After denaturation, the triple-helix structure of native collagen changes to a random coil form due to the dissociation of the hydrogen bonds when collagen suffers hydrolysis. This treatment can break the bonds in the polypeptide chain to obtain a large number of peptides. The molecular weight of collagen peptides obtained from hydrolysis is very low (3–6 KDa) compared to that of its precursor native collagen (285–300 KDa). Enzymatic hydrolysis affects not only the size of the peptides but also physicochemical and biological properties [67,68]. Viscosity is one of the physicochemical properties of collagen; the native form shows higher values due to stronger electrostatic repulsion among the molecular chains even at low concentrations of collagen solution. However, its hydrolyzed form shows very low viscosity no matter the concentration because of the low molecular weight of the small chain segments [69]. Electrostatic properties of proteins such as the isoelectric point (pI) are important parameters which are related to the proportion of acid amino residues and base amino residues in protein. Collagen is an amphoteric macromolecule that possesses a pI value between 7 and 8. On the hydrolysis process, the pI value is shifted to lower values between 3.68 and 5.7. This change will depend on the amino acid sequences and distribution of amino acid residues according to the type or time of hydrolysis [65,70,71,72]. The composition and degree of hydrolysis of collagen are factors that increase functional properties such as antioxidant capacity, antimicrobial activity, and higher bioavailability. These properties are related mainly to the molecular weight value. It makes HC to act as an electron donor to produce more stable products reacting with free radicals [73,74].
Table 1.
Properties of native and hydrolyzed collagen.
Native collagen is commonly used in different industries because it has excellent biocompatibility and biodegradability, low immunogenicity, and high versatility to fabricate films. However, HC is highly soluble in water but not able to form films by itself. It is necessary to combine it with other biopolymers [26,75]. HC presents several advantages compared to native collagen. Some of them consist in higher therapeutic loading, cost-effectiveness and not requiring a multistep extraction procedure, highly digestible and is easily absorbed and distributed in the human body [75,76]. Furthermore, it exhibits lower viscosity in aqueous solution, neutral odor, colorlessness, transparency, emulsification and stabilization, foam forming, film forming, wettability, solubility, dispersibility, powder compressibility, carrier substance and low allergenicity [65,77].
Collagen peptides can be used as an ingredient for functional food supplements because they present antioxidant and antimicrobial activity [6,78,79] and its quality depends on the methodology used to extract it [76]. These peptides have shown capacity to bind Ca+ ions promoting the bioavailability and making it more compatible to the human body [80,81]. HC also helps to improve memory health shown through in vivo studies, it could be a candidate ingredient of drugs used to manage and improve health [82]. In food science, HC helps to minimize or prevent damage in cells and tissues during freezer storage, therefore, it is an option to use in food that requires low-temperature storage [83].
3. Hydrolyzed Collagen: Sources and Applications
3.1. Sources
3.1.1. Bovine
HC can be extracted from different sources and tissues [71], it can be extracted from bovine Achilles tendon by using different enzymes such as alcalase, pepsin, trypsin, and collagenase produced by Penicillium aurantiogriseum. It shows antihypertensive, antioxidant, and antimicrobial activity [85,86]. HC from bovine lung showed antioxidant and anti-inflammatory activity [87]. HC from the nuchal ligament of bovine by papain action can be used as a promising precursor of angiotensin-I-converting enzyme (ACE)-inhibitory peptides [88].
3.1.2. Porcine
Another traditional source of HC is porcine skin. It presents a low molecular weight around 1–10 KDa. It is produced by a hydrothermal process and fractionated by ultra-filtration membranes; showing antioxidant, anti-aging, skin permeation properties [89], and ACE-inhibitory potency [90]. HC from porcine skin contains functional peptides commonly used in dietary supplements [91]. HC porcine extraction can be carried out by treatments that include high temperature (150–250 °C) and pressure (350–3900 KPa). These parameters of extraction generate peptides with lower molecular weight than 15 KDa [92].
3.1.3. Marine
HC extraction from traditional sources such as porcine and bovine involves some limitations due to health problems such as swine flu [93] and bovine spongiform encephalopathy [94]. Moreover, religious issues must be included [95]. Researchers have been focused on the development of a new source of extraction. Alternative sources have been investigated from marine sources such as fish and other invertebrates such as jellyfishes or sponges [25,96,97]. HC from Prionace glauca extracted with alcalase enzyme hydrolysis reported peptides with molecular weight lower than 20 KDa and nutraceutical effects [98]. Tilapia scales (Oreochromis niloticus) have been used to produce HC with high quality and low Mw [99]. HC extracted from pacu and rohu waste by using collagenase Type I from Clostridium, showed molecular weight hydrolysates of around 5 KDa. It was used as a peroxide inhibitor in lipid-based food and cytoprotective agent in cell culture [100]. Marine byproducts such as fish viscera also represent a good source for extraction of HC. This waste material has been used to produce HC with functional bioactive properties [101]. However, by changing the extraction parameters from different temperatures (150–300 °C), pressure (50–100 bar), and reaction time (5 min), it is possible to obtain HC from tuna skin with low molecular weight (<600 Da) and antioxidant and antimicrobial activity [102].
Other marine sources for preparation of HC were obtained from cod protein hydrolysate (Gadus morhua) [103], Alaska pollock [104], and cartilage of spotless smooth hound [105]. The extracted biomaterials results were lower molecular weights (3–5 KDa) and antioxidative activity.
3.1.4. Alternative Sources
Some alternative sources present great functionality properties. HC extracted from chicken legs by enzymatic action (proteases) [106] and skin of Rana chensinensis by acid hydrolysis [107] exhibited high solubility, angiotensin-converting enzyme inhibition, and antioxidant activity. Chicken feet treated with papain enzymes at different temperatures (4, 30, and 56 °C) and different extraction times (20, 24, and 28 h) showed functional properties such as water and oil retention capacity as well as emulsifying and foaming properties [108,109].
3.2. Applications
The molecular weight and functional properties of HC depended on the source, type of extraction, and type of enzyme used during extraction. These properties could help determine the applications of HC in cosmetic, pharmaceutical, biomaterials, food, and nutraceutical industries [101,110,111].
3.2.1. Oral Collagen Supplementation
Collagen loss in the body starts at 18–29 years of age, after 40 years the human body can lose around 1% per year, and at around 80 years collagen production in the body can decrease 75% overall in comparison to that of young adults [112,113]. There are other factors contributing to this such as free radicals in the organism, deficient diet, smoking, alcoholism, and disease. The role of collagen in the body is very important because it helps the development of the organs; wound and tissue healing; cornea, gums, and scalp repair. Collagen helps in bone and blood vessel reparation. In the cornea, collagen tissue gets mechanical and optical properties. It is present in biological functions of the cell such as proliferation, cell survival, and differentiation; so collagen is present in the human body as a whole in bones, tendons, ligament, hair, skin, and muscles [2,114,115].
The skin is the largest organ in the human body, collagen elastic fibers and hyaluronic acid are its major structural constituents. Skin protects the organism from external damages, regulates temperature, and performs other bodily functions [116,117,118]. Aging is a natural process which involves changes in the human body; the skin suffers morphologic, structural, and functional deterioration; collagen reduces and elastin fibers promote the formation of lines and wrinkles. Skin aging control is a challenge in the cosmetic industry, but HC has proved to be an alternative solution in slowing down the effects of aging [74,110,119,120]. HC from milkfish scales exhibited excellent water-holding capacity, moisture absorption, and retention as well as anti-skin aging, and anti-melanogenic capacities, proving a potential active ingredient in skin care products [121].
Hydrolyzed collagen acts in two different forms in the dermis; in the first action, the free amino acids provide building blocks for the formation of collagen and elastin fibers. In the second action, collagen oligopeptides act as ligands, binding to receptors on the fibroblasts’ membrane and stimulating the production of new collagen, elastin, and hyaluronic acid [76].
In recent years, oral collagen supplementation has become popular as it has been increasingly marketed to consumers as an anti-aging product, because HC oral supplementation reaches the deeper layers of the skin and improves skin physiology and appearance increasing hydration, elasticity, firmness, wrinkle reduction, and skin rejuvenation [122,123] (Table 2).
Table 2.
Hydrolyzed collagen (HC) applications as functional supplementation food with positive effect on the skin.
| Product | Source | Functionality | Reference |
|---|---|---|---|
| HC from fish | Milkfish scales | Excellent water-holding capacity, moisture absorption, and retention and anti-skin aging, and anti-melanogenic capacities. | [121] |
| HC oral supplementation | Sutchi catfish skin | Improved skin hydration, wrinkling, and elasticity in women aged 40–60 years for 12 weeks. | [117] |
| HC oral nutrient supplement | Delphynol® | Improved dermal thickness, skin firmness, and elasticity in women between 35–65 years old for three months. | [124] |
| Fish HC oral supplementation | Fish | Improved skin texture and elasticity and in addition a protective effect on joint health in 120 subjects for 90 days. | [119] |
| HC supplementation | Fish | Increased skin hydration and collagen density in the dermis in women between 40 and 59 years old. | [125] |
| HC oral supplementation with vit A, C, E and zinc. | Not defined by the author | Improved the hydration, texture, elasticity, and firmness of the skin in women between 40 and 50 years after 28 days of treatment. | [126] |
| HC beverage | Nitta Gelatin Inc ® | Changes in periorbital wrinkles, facial skin hydration, and skin elasticity in healthy women aged 30–60 years. | [127] |
| HC drinking ampoules with acerola fruit extract, vitamin C,E zinc, and biotin | ELASTEN® | 12 weeks oral supplementation improved the skin properties such as: hydration, elasticity, roughness, and density. | [123] |
Studies in vivo in woman between 40 and 60 years of age with HC oral supplementation during 12 weeks, showed a significant improvement in skin hydration, wrinkling, and elasticity [117]. HC as an oral nutrient supplement in women between 35 and 65 years of age proved after three months enhancement in dermal thickness, skin firmness, and elasticity after treatment [124]. Another study with 120 subjects during 90 days, where they consumed daily oral supplementation of a liquid nutraceutical containing fish HC, resulted in improvement of skin texture and elasticity. Additionally, a protective effect on joint health was observed [119]. Oral supplementation of HC in women between 40 and 59 years of age revealed a significant increment in skin hydration and collagen density at dermis level. However, fragmentation of the dermal collagen network significantly decreased after four weeks of supplementation and these effects persisted after 12 weeks [125].
A study of sixty healthy female subjects, aged between 40 and 50 years, after 28 days of oral supplementation showed that this acted on skin elasticity and presented a more pronounced effect on dermis echogenicity, reducing skin pores, improving hydration, texture, elasticity, and firmness of the skin. The oral supplementation product was composed of HC with a mix of vitamins A, C, E, and zinc [126]. HC from tilapia fish scale (Oreochromis mosambicus) in the form of 20 mL beverage (Nitta Gelatin Inc®., India) was consumed by healthy women aged 30–60 years over 12 weeks, after this time the result was enhanced facial skin moisture [127]. HC drinking ampoules with fruit extract, Vit C, E, zinc, and biotin ELASTEN® were supplied to 36 healthy women aged 35 years or older for 12 weeks and their skin showed higher hydration, elasticity, smoothness and density [123].
3.2.2. Food Industry
HC presents antioxidant and antimicrobial activity, so it can be used as a functional ingredient in food supplements as well [6,78,79]. Collagen hydrolysates can attach calcium ions, improving its bioavailability, therefore, HC can be used in functional food ingredients in the management of mineral deficiencies [80,81]. HC acts as an anticoagulant because it helps to decrease the damage in cells and tissues originated by low temperatures, therefore, it could be useful in foods that require storage in cold or freezing temperatures [128]. HC has been used in the preparation of different products such as meat products, beverages, soups, and others. It helps increase and maintain their sensorial, chemical, and physical properties.
HC has been used in processed foods such as sausages to replace pork fat at 50% level of replacement. The final product results had greater water holding capacity, better stability after cooking, and improved texture such as hardness and chewiness [129]. The use of fish HC in meat products such as buffalo patties, resulted in higher protein content, lower fat content, similar sensory acceptability, and better texture as compared to the buffalo patties without HC [130]. HC from bovine skin was used in combination with modified starch and guar gum in ham elaboration. Lower syneresis with 2.0% of HC final concentration in the product was reported as the best treatment [131].
HC from fish can be added in beverages such as orange juice (2.5%), and the product showed an improvement in nutritional and functional properties with a higher protein content, bioavailability, and low viscosity as well as high solubility in water [48]. The development of a fermented dairy drink using ricotta cheese whey with HC added as a functional ingredient presented low syneresis and sedimentation, with good physical–chemical and microbiological properties [132]. Dairy beverages using HC, açaí pulp, and cheese showed higher sensorial acceptability, affecting positively the viscosity and presenting adequate physicochemical and microbiological parameters after 28 days of storage [133].
HC can be added in soup as well, it has an effect in its viscosity and the functional properties. It presented high 2,2′-azino-bis-3-ethylbenzothiazoline-6-sulphonic acid (ABTS) and 2,2-diphenyl-1-picryl-hydrazyl (DPPH) radical scavenging activities [134]. Addition of HC from pigskin shavings (collagen waste) in a chrysanthemum beverage, showed an excellent clarification effect, better sensorial quality, and storage stability. However, the amount of HC added as a clarifier was lower compared to that of other commercial clarifiers [135] (Table 3). HC from pig skin extracted by enzymatic action exhibited high flocculation capability under acidic and neutral conditions, this property could be caused by the synergistic effect of optimal molecular weight distribution and electric charge [136]. Additionally, HC has been used in different food products to develop their physicochemical and functional properties. This makes HC one of the most promising functional ingredients because it does not affect sensorial properties.
Table 3.
Hydrolyzed collagen in the food industry.
| Product | Source | Functionality | Reference |
|---|---|---|---|
| HC in sausages to replace pork backfat | Germina® | Sausages with greater water holding capacity and improved texture | [129] |
| Fish HC in buffalo patties | Az-Zahrah Sdn. Bhd® | Product with higher protein content, lower fat content | [130] |
| Ham with bovine HC-modified starch and guar gum | Bovine skin | Ham with lower syneresis | [131] |
| Fish HC in orange juice | Fish | Higher protein and high solubility in water, low viscosity | [48] |
| Fermented dairy drink | Luchebras® | Low syneresis and sedimentation, with good physical–chemical and microbiological properties | [132] |
| Dairy beverage with HC, açaí pulp, and cheese | Tovani Benzaquen Ingredients® | High sensory acceptability, good physicochemical and microbiological parameters | [133] |
| HC in herbal soup | Seabass skin | High antioxidant activity by radicals ABTS and DPPH inhibition | [134] |
| Chrysanthemum beverage | Pigskin | Excellent clarification effect and better sensory quality and storage stability | [135] |
3.2.3. Biomaterials
Collagen presents good biocompatibility and biodegradability, hence, it is safe and effective as a biomaterial, it has been used in the last years as a safe and effective biomaterial in tissue engineering and clinical applications [75,137,138].
Compared to native collagen, HC has a main advantage—it presents higher solubility; moreover, HC extraction is simple and does not require a multistep extraction procedure [75,139,140]. However, HC cannot form scaffolds by itself because of the low molecular weight of the peptides, but it can be mixed with other biopolymers such as cellulose and chitosan.
Films prepared with a blend of cellulose–HC exhibited good transparence, good ultraviolet radiation absorption, and excellent support for cell adhesion and proliferation. High biocompatibility dictates that the films would have promising applications in the biomaterial field [139]. Collagen–HC films developed from leather waste were very transparent and had excellent barrier properties against UV light and studies such as FTIR and differential scanning calorimetry (DSC) showed total miscibility between both polymers [141].
The development of a HC collagen biomaterial could be beneficial for the management of bone and joint disorders because of HC’s low molecular weight and amino acid composition. It is more bioavailable and induces a better osteointegration by promoting collagen synthesis [140].
Alternative biomaterials with HC are chitosan sponges. They are prepared by sol–gel transition methodology showing porous morphology, improved biostability, good water uptake capacity, excellent biocompatibility, and antimicrobial activity by the presence of HC [75].
HC has been used in hydrogel development for the delivery of drugs such as insulin and methylene blue, showing lower water absorbency. The hydrogel delivery was faster at pH 2. It is useful for drugs susceptible to degradation under the acidic and proteolytic environment of gastric fluids [142]. Hydrogels prepared with chitosan and fish HC showed antibacterial activity against Escherichia coli, Staphylococcus aureus, pro-cell proliferation and migration, and wound healing efficiency [143]. Nanofibrous scaffolds can be functionalized with HC as a regenerative component using electrospinning methodology. It presented porous morphology, adequate mechanical strength, excellent biocompatibility, and antimicrobial properties against E. coli and Pseudomonas aeruginosa [144] (Table 4).
Table 4.
Hydrolyzed collagen biomaterial applications.
| Product | Source | Functionality | Reference |
|---|---|---|---|
| Cellulose–HC films | Jinjian Gelatin Co. Ltd.® | Capacity for ultraviolet radiation absorption and good support fir cell adhesion and proliferation, showing good biocompatibility | [139] |
| Collagen–HC films | Wastes by leather industries | Excellent barrier properties against UV light | [141] |
| HC biomaterial | Calf pelt | Beneficial for management of bone and joint disorders | [140] |
| HC-chitosan sponges | Bovine Achilles tendon | Good water uptake capacity and excellent biocompatibility and antimicrobial activity | [75] |
| HC hydrogels | Udomkorn Engineering Co., Ltd.® | Low water absorbency and faster drug release in acid pH | [142] |
| HC-chitosan hydrogels | Tilapia fish | Antibacterial activity against Escherichia coli and Staphylococcus aureus | [143] |
| Nanofibrous scaffold functionalized with HC | Tilapia fish | Antimicrobial property against E. coli and Pseudomonas aeruginosa | [144] |
4. Future Trends
Hydrolyzed collagen can be obtained from agro-food waste (bones, tendons, and skin). The recycling of these byproducts can help reduce the pollution generated by these types of wastes, transforming them into a new product with a high functional value. Traditionally, denaturation of collagen has been carried out with acids or alkaline products; however, the application of emergent technologies such as the combination of high temperatures and pressures as well as high intensity ultrasound have been investigated in order to decrease the disposal of chemical products. Low-molecular-weight peptides could be applied into food systems, for example, beverages with HC show a great advantage of easy digestion, high assimilation (about 80%), and good absorption at the intestinal level. It would be important to develop drinks with HC considering they will have functional properties for consumption in most people due to its null toxicity and zero allergenicity [111,145].
5. Conclusions
HC has a wide range of applications due to its properties such as: low viscosity in aqueous solutions, neutral odor, colorlessness, transparency, emulsification and stabilization, foam forming, film forming, wettability, solubility, dispersibility, powder compressibility, substance carrier of low allergenicity as well as antioxidant and antimicrobial activity. For cosmetic applications, some studies have shown that HC has good biological functions such as increment of cell proliferation, water-holding capacity, moisture absorption, retention, and anti-aging in skin. HC is widely used as a functional ingredient in the food industry because of its properties to increase water holding in meat products, sensorial development, and improvement of chemical and physical properties in beverages and dairy products. Within the biomedical industry, the application of HC blended with cellulose or chitosan for the preparation of scaffolds has helped with promotion of collagen synthesis, management of bone and joint disorders, wound treatment, excellent biocompatibility, and antimicrobial properties.
Author Contributions
A.L.-L., A.M.-P., V.M.M.-J., and A.V.-T. developed the concept for the review and co-wrote the manuscript. D.I.Z. and G.A.-Á. revised and edited the manuscript
Funding
This research was funded by National Council of Science and Technology (CONACYT), grant number 621400.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Sorushanova A., Delgado L.M., Wu Z., Shologu N., Kshirsagar A., Raghunath R., Mullen A.M., Bayon Y., Pandit A., Raghunath M.J.A.M. The collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019;31:1801651. doi: 10.1002/adma.201801651. [DOI] [PubMed] [Google Scholar]
- 2.Gelse K., Pöschl E., Aigner T. Collagens—structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003;55:1531–1546. doi: 10.1016/j.addr.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Schrieber R., Gareis H. Gelatine Handbook. Theory and Industrial Practice. WILEY-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2007. pp. 45–117. [Google Scholar]
- 4.Asghar A., Henrickson R.L. Chemical, Biochemical, Functional, and Nutritional Characteristics of Collagen in Food Systems. In: Chichester C.O., Mrak E.M., Stewart G.F., editors. Advances in Food Research. Vol. 28. Academic Press; Cambridge, MA, USA: 1982. pp. 231–372. [DOI] [PubMed] [Google Scholar]
- 5.Bateman J.F., Lamande S.R., Ramshaw J.A. Collagen superfamily. Extracell. Matrix. 1996;2:22–67. [Google Scholar]
- 6.Gómez-Guillén M.C., Giménez B., López-Caballero M.E., Montero M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011;25:1813–1827. doi: 10.1016/j.foodhyd.2011.02.007. [DOI] [Google Scholar]
- 7.Lin K., Zhang D., Macedo M.H., Cui W., Sarmento B., Shen G. Advanced collagen-based biomaterials for regenerative biomedicine. Adv. Funct. Mater. 2018;29:1804943. doi: 10.1002/adfm.201804943. [DOI] [Google Scholar]
- 8.Liu D., Nikoo M., Boran G., Zhou P., Regenstein J.M. Collagen and gelatin. Annu. Rev. Food Sci. Technol. 2015;6:527–557. doi: 10.1146/annurev-food-031414-111800. [DOI] [PubMed] [Google Scholar]
- 9.Nimni M.E. Collagen. Volume 1. CRC Press; Boca Raton, FL, USA: 2018. Biochemistry; pp. 23–35. [Google Scholar]
- 10.Muiznieks L.D., Keeley F.W. Molecular assembly and mechanical properties of the extracellular matrix: A fibrous protein perspective. Biochim. Et Biophys. Acta (Bba) - Mol. Basis Dis. 2013;1832:866–875. doi: 10.1016/j.bbadis.2012.11.022. [DOI] [PubMed] [Google Scholar]
- 11.Haq F., Ahmed N., Qasim M. Comparative genomic analysis of collagen gene diversity. 3 Biotech. 2019;9:83. doi: 10.1007/s13205-019-1616-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shoulders M.D., Raines R.T. Collagen structure and stability. Annu. Rev. 2009;78:929–958. doi: 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ricard-Blum S. The collagen family. Cold Spring Harb. Perspect. Biol. 2011;3:a004978. doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Purcel G., Meliţă D., Andronescu E., Grumezescu A.M. Collagen-based nanobiomaterials: Challenges in soft tissue engineering. In: Grumezescu A.M., editor. Nanobiomaterials in Soft Tissue Engineering. Applications of Nanobiomaterials. Volume 5. William Andrew Publishing; Oxford, UK: 2016. pp. 173–200. [Google Scholar]
- 15.Bella J., Hulmes D.J.S. Fibrillar Collagens. In: Parry D.A.D., Squire J.M., editors. Fibrous Proteins: Structures and Mechanisms. Volume 82. Springer International Publishing; Cham, Switzerland: 2017. pp. 457–490. [DOI] [PubMed] [Google Scholar]
- 16.Gordon M.K., Hahn R.A. Collagens. Cell Tissue Res. 2009;339:247. doi: 10.1007/s00441-009-0844-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang M., Yuan J., Peng C., Li Y. Collagen as a double-edged sword in tumor progression. Tumor Biol. 2014;35:2871–2882. doi: 10.1007/s13277-013-1511-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ferraro V., Gaillard-Martinie B., Sayd T., Chambon C., Anton M., Santé-Lhoutellier V. Collagen type I from bovine bone. Effect of animal age, bone anatomy and drying methodology on extraction yield, self-assembly, thermal behaviour and electrokinetic potential. Int. J. Biol. Macromol. 2017;97:55–66. doi: 10.1016/j.ijbiomac.2016.12.068. [DOI] [PubMed] [Google Scholar]
- 19.Darine S., Christophe V., Gholamreza D. Production and functional properties of beef lung protein concentrates. Meat Sci. 2010;84:315–322. doi: 10.1016/j.meatsci.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 20.Santos M.H., Silva R.M., Dumont V.C., Neves J.S., Mansur H.S., Heneine L.G.D. Extraction and characterization of highly purified collagen from bovine pericardium for potential bioengineering applications. Mater. Sci. Eng. C. 2013;33:790–800. doi: 10.1016/j.msec.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 21.Paschou A.M., Katsikini M., Christofilos D., Arvanitidis J., Ves S. High pressure Raman study of type-I collagen. Febs J. 2018;285:2641–2653. doi: 10.1111/febs.14506. [DOI] [PubMed] [Google Scholar]
- 22.Lin Y.-K., Lin T.-Y., Su H.-P. Extraction and characterisation of telopeptide-poor collagen from porcine lung. Food Chem. 2011;124:1583–1588. doi: 10.1016/j.foodchem.2010.08.018. [DOI] [Google Scholar]
- 23.Rieu C., Picaut L., Mosser G., Trichet L. From tendon injury to collagen-based tendon regeneration: Overview and recent advances. Curr. Pharm. Des. 2017;23:3483–3506. doi: 10.2174/1381612823666170516130515. [DOI] [PubMed] [Google Scholar]
- 24.Abraham G.A., Murray J., Billiar K., Sullivan S.J. Evaluation of the porcine intestinal collagen layer as a biomaterial. J. Biomed. Mater. Res. 2000;51:442–452. doi: 10.1002/1097-4636(20000905)51:3<442::AID-JBM19>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 25.Silvipriya K., Kumar K.K., Bhat A., Kumar B.D., John A., Lakshmanan P. Collagen: Animal sources and biomedical application. J. Appl. Pharm. Sci. 2015;5:123–127. doi: 10.7324/JAPS.2015.50322. [DOI] [Google Scholar]
- 26.Fauzi M.B., Lokanathan Y., Aminuddin B.S., Ruszymah B.H.I., Chowdhury S.R. Ovine tendon collagen: Extraction, characterisation and fabrication of thin films for tissue engineering applications. Mater. Sci. Eng. C. 2016;68:163–171. doi: 10.1016/j.msec.2016.05.109. [DOI] [PubMed] [Google Scholar]
- 27.León-López A., Fuentes-Jiménez L., Hernández-Fuentes A.D., Campos-Montiel R.G., Aguirre-Álvarez G. Hydrolysed collagen from sheepskins as a source of functional peptides with antioxidant activity. Int. J. Mol. Sci. 2019;20:3931. doi: 10.3390/ijms20163931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhagwat P.K., Dandge P.B. Isolation, characterization and valorizable applications of fish scale collagen in food and agriculture industries. Biocatal. Agric. Biotechnol. 2016;7:234–240. doi: 10.1016/j.bcab.2016.06.010. [DOI] [Google Scholar]
- 29.Oechsle A.M., Akgün D., Krause F., Maier C., Gibis M., Kohlus R., Weiss J. Microstructure and physical–chemical properties of chicken collagen. Food Struct. 2016;7:29–37. doi: 10.1016/j.foostr.2016.02.001. [DOI] [Google Scholar]
- 30.Kim H.-W., Yeo I.-J., Hwang K.-E., Song D.-H., Kim Y.-J., Ham Y.-K., Jeong T.-J., Choi Y.-S., Kim C.-J. Isolation and characterization of pepsin-soluble collagens from bones, skins, and tendons in duck feet. Korean J. Food Sci. Anim. Resour. 2016;36:665. doi: 10.5851/kosfa.2016.36.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martínez-Ortiz M.A., Hernández-Fuentes A.D., Pimentel-González D.J., Campos-Montiel R.G., Vargas-Torres A., Aguirre-Álvarez G. Extraction and characterization of collagen from rabbit skin: Partial characterization. Cyta-J. Food. 2015;13:53–258. [Google Scholar]
- 32.Zou Y., Wang L., Cai P., Li P., Zhang M., Sun Z., Sun C., Xu W., Wang D. Effect of ultrasound assisted extraction on the physicochemical and functional properties of collagen from soft-shelled turtle calipash. Int. J. Biol. Macromol. 2017;105:1602–1610. doi: 10.1016/j.ijbiomac.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Schmidt M., Dornelles R., Mello R., Kubota E., Mazutti M., Kempka A., Demiate I. Collagen extraction process. Int. Food Res. J. 2016;23:913–922. [Google Scholar]
- 34.Almeida A.J., Fernandes A.I. Exploring a new jellyfish collagen in the production of microparticles for protein delivery AU - Calejo, M. Teresa. J. Microencapsul. 2012;29:520–531. doi: 10.3109/02652048.2012.665089. [DOI] [PubMed] [Google Scholar]
- 35.Liu D., Wei G., Li T., Hu J., Lu N., Regenstein J.M., Zhou P. Effects of alkaline pretreatments and acid extraction conditions on the acid-soluble collagen from grass carp (Ctenopharyngodon idella) skin. Food Chem. 2015;172:836–843. doi: 10.1016/j.foodchem.2014.09.147. [DOI] [PubMed] [Google Scholar]
- 36.Li Z.-R., Wang B., Chi C.-f., Zhang Q.-H., Gong Y.-d., Tang J.-J., Luo H.-y., Ding G.-f. Isolation and characterization of acid soluble collagens and pepsin soluble collagens from the skin and bone of Spanish mackerel (Scomberomorous niphonius) Food Hydrocoll. 2013;31:103–113. doi: 10.1016/j.foodhyd.2012.10.001. [DOI] [Google Scholar]
- 37.Yu D., Chi C.-F., Wang B., Ding G.-F., Li Z.-R. Characterization of acid-and pepsin-soluble collagens from spines and skulls of skipjack tuna (Katsuwonus pelamis) Chin. J. Nat. Med. 2014;12:712–720. doi: 10.1016/S1875-5364(14)60110-2. [DOI] [PubMed] [Google Scholar]
- 38.Kim H.K., Kim Y.H., Kim Y.J., Park H.J., Lee N.H. Effects of ultrasonic treatment on collagen extraction from skins of the sea bass Lateolabrax japonicus. Fish. Sci. 2012;78:485–490. doi: 10.1007/s12562-012-0472-x. [DOI] [Google Scholar]
- 39.Li D., Mu C., Cai S., Lin W. Ultrasonic irradiation in the enzymatic extraction of collagen. Ultrason. Sonochemistry. 2009;16:605–609. doi: 10.1016/j.ultsonch.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 40.Ran X.G., Wang L.Y. Use of ultrasonic and pepsin treatment in tandem for collagen extraction from meat industry by-products. J. Sci. Food Agric. 2014;94:585–590. doi: 10.1002/jsfa.6299. [DOI] [PubMed] [Google Scholar]
- 41.Prestes R. Collagen and its derivatives: Characteristics and applications in meat products. Rev. Unopar Cient. Ciên. Biol. Saúde. 2013;15:65–74. [Google Scholar]
- 42.Skopinska-Wisniewska J., Olszewski K., Bajek A., Rynkiewicz A., Sionkowska A. Dialysis as a method of obtaining neutral collagen gels. Mater. Sci. Eng. C. 2014;40:65–70. doi: 10.1016/j.msec.2014.03.029. [DOI] [PubMed] [Google Scholar]
- 43.Ketnawa S., Benjakul S., Martínez-Alvarez O., Rawdkuen S. Fish skin gelatin hydrolysates produced by visceral peptidase and bovine trypsin: Bioactivity and stability. Food Chem. 2017;215:383–390. doi: 10.1016/j.foodchem.2016.07.145. [DOI] [PubMed] [Google Scholar]
- 44.Thuanthong M., De Gobba C., Sirinupong N., Youravong W., Otte J. Purification and characterization of angiotensin-converting enzyme-inhibitory peptides from Nile tilapia (Oreochromis niloticus) skin gelatine produced by an enzymatic membrane reactor. J. Funct. Foods. 2017;36:243–254. doi: 10.1016/j.jff.2017.07.011. [DOI] [Google Scholar]
- 45.Hong H., Chaplot S., Chalamaiah M., Roy B.C., Bruce H.L., Wu J. Removing cross-linked telopeptides enhances the production of low-molecular-weight collagen peptides from spent hens. J. Agric. Food Chem. 2017;65:7491–7499. doi: 10.1021/acs.jafc.7b02319. [DOI] [PubMed] [Google Scholar]
- 46.Cheung I.W.Y., Li-Chan E.C.Y. Enzymatic production of protein hydrolysates from steelhead (Oncorhynchus mykiss) skin gelatin as inhibitors of dipeptidyl-peptidase IV and angiotensin-I converting enzyme. J. Funct. Foods. 2017;28:254–264. doi: 10.1016/j.jff.2016.10.030. [DOI] [Google Scholar]
- 47.Barzideh Z., Latiff A.A., Gan C.-Y., Abedin M.Z., Alias A.K. ACE inhibitory and antioxidant activities of collagen hydrolysates from the ribbon jellyfish (Chrysaora sp.) Food Technol. Biotechnol. 2014;52:495–504. doi: 10.17113/ftb.52.04.14.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bilek S.E., Bayram S.K. Fruit juice drink production containing hydrolyzed collagen. J. Funct. Foods. 2015;14:562–569. doi: 10.1016/j.jff.2015.02.024. [DOI] [Google Scholar]
- 49.Offengenden M., Chakrabarti S., Wu J. Chicken collagen hydrolysates differentially mediate anti-inflammatory activity and type I collagen synthesis on human dermal fibroblasts. Food Sci. Hum. Wellness. 2018;7:138–147. doi: 10.1016/j.fshw.2018.02.002. [DOI] [Google Scholar]
- 50.Masuda R., Dazai Y., Mima T., Koide T. Structure-activity relationships and action mechanisms of collagen-like antimicrobial peptides. Pept. Sci. 2017;108:e22931. doi: 10.1002/bip.22931. [DOI] [PubMed] [Google Scholar]
- 51.Chi C.-F., Cao Z.-H., Wang B., Hu F.-Y., Li Z.-R., Zhang B. Antioxidant and functional properties of collagen hydrolysates from spanish mackerel skin as influenced by average molecular weight. Molecules. 2014;19:11211–11230. doi: 10.3390/molecules190811211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Onuh J.O., Girgih A.T., Aluko R.E., Aliani M. In vitro antioxidant properties of chicken skin enzymatic protein hydrolysates and membrane fractions. Food Chem. 2014;150:366–373. doi: 10.1016/j.foodchem.2013.10.107. [DOI] [PubMed] [Google Scholar]
- 53.Lin Y.-J., Le G.-W., Wang J.-Y., Li Y.-X., Shi Y.-H., Sun J. Antioxidative peptides derived from enzyme hydrolysis of bone collagen after microwave assisted acid pre-treatment and nitrogen protection. Int. J. Mol. Sci. 2010;11:4297–4308. doi: 10.3390/ijms11114297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hong H., Fan H., Chalamaiah M., Wu J. Preparation of low-molecular-weight, collagen hydrolysates (peptides): Current progress, challenges, and future perspectives. Food Chem. 2019;301:125222. doi: 10.1016/j.foodchem.2019.125222. [DOI] [PubMed] [Google Scholar]
- 55.Elavarasan K., Shamasundar B., Badii F., Howell N. Angiotensin I-converting enzyme (ACE) inhibitory activity and structural properties of oven-and freeze-dried protein hydrolysate from fresh water fish (Cirrhinus mrigala) Food Chem. 2016;206:210–216. doi: 10.1016/j.foodchem.2016.03.047. [DOI] [PubMed] [Google Scholar]
- 56.Powell T., Bowra S., Cooper H.J. Subcritical water hydrolysis of peptides: Amino acid side-chain modifications. J. Am. Soc. Mass Spectrom. 2017;28:1775–1786. doi: 10.1007/s13361-017-1676-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jo Y.-J., Kim J.-H., Jung K.-H., Min S.-G., Chun J.-Y. Effect of sub-and super-critical water treatment on physicochemical properties of porcine skin. Korean J. Food Sci. Anim. Resour. 2015;35:35. doi: 10.5851/kosfa.2015.35.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Laemmli U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 59.Schägger H. Tricine–sds-page. Nat. Protoc. 2006;1:16. doi: 10.1038/nprot.2006.4. [DOI] [PubMed] [Google Scholar]
- 60.Haider S.R., Reid H.J., Sharp B.L. Tricine-sds-page. In: Kurien B.T., Scofield R.H., editors. Protein Electrophoresis: Methods and Protocols. Volume 869. Humana Press; New York, NY, USA: 2012. pp. 81–91. [DOI] [PubMed] [Google Scholar]
- 61.Karas M., Hillenkamp F. Laser desorption ionization of proteins with molecular masses exceeding 10,000 daltons. Anal. Chem. 1988;60:2299–2301. doi: 10.1021/ac00171a028. [DOI] [PubMed] [Google Scholar]
- 62.Hema G., Joshy C., Shyni K., Chatterjee N.S., Ninan G., Mathew S. Optimization of process parameters for the production of collagen peptides from fish skin (Epinephelus malabaricus) using response surface methodology and its characterization. J. Food Sci. Technol. 2017;54:488–496. doi: 10.1007/s13197-017-2490-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lecchi P., Olson M., Brancia F.L. The role of esterification on detection of protonated and deprotonated peptide ions in matrix assisted laser desorption/ionization (MALDI) mass spectrometry (MS) J. Am. Soc. Mass Spectrom. 2005;16:1269–1274. doi: 10.1016/j.jasms.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 64.Pataridis S., Eckhardt A., Mikulikova K., Sedlakova P., Mikšík I. Identification of collagen types in tissues using HPLC-MS/MS. J. Sep. Sci. 2008;31:3483–3488. doi: 10.1002/jssc.200800351. [DOI] [PubMed] [Google Scholar]
- 65.Zhang G., Sun A., Li W., Liu T., Su Z. Mass spectrometric analysis of enzymatic digestion of denatured collagen for identification of collagen type. J. Chromatogr. A. 2006;1114:274–277. doi: 10.1016/j.chroma.2006.03.039. [DOI] [PubMed] [Google Scholar]
- 66.Mikulíková K., Eckhardt A., Pataridis S., Mikšík I. Study of posttranslational non-enzymatic modifications of collagen using capillary electrophoresis/mass spectrometry and high performance liquid chromatography/mass spectrometry. J. Chromatogr. A. 2007;1155:125–133. doi: 10.1016/j.chroma.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 67.Zhang Y., Zhang Y., Liu X., Huang L., Chen Z., Cheng J. Influence of hydrolysis behaviour and microfluidisation on the functionality and structural properties of collagen hydrolysates. Food Chem. 2017;227:211–218. doi: 10.1016/j.foodchem.2017.01.049. [DOI] [PubMed] [Google Scholar]
- 68.Li Z., Wang B., Chi C., Gong Y., Luo H., Ding G. Influence of average molecular weight on antioxidant and functional properties of cartilage collagen hydrolysates from Sphyrna lewini, Dasyatis akjei and Raja porosa. Food Res. Int. 2013;51:283–293. doi: 10.1016/j.foodres.2012.12.031. [DOI] [Google Scholar]
- 69.Sun Pan B., En Chen H.O.A., Sung W.C. Molecular and thermal characteristics of acid-soluble collagen from orbicular batfish: Effects of deep-sea water culturing. Int. J. Food Prop. 2018;21:1080–1090. doi: 10.1080/10942912.2018.1476873. [DOI] [Google Scholar]
- 70.Chen J., Li L., Yi R., Xu N., Gao R., Hong B. Extraction and characterization of acid-soluble collagen from scales and skin of tilapia (Oreochromis niloticus) Lwt-Food Sci. Technol. 2016;66:453–459. doi: 10.1016/j.lwt.2015.10.070. [DOI] [Google Scholar]
- 71.Abdollahi M., Rezaei M., Jafarpour A., Undeland I. Sequential extraction of gel-forming proteins, collagen and collagen hydrolysate from gutted silver carp (Hypophthalmichthys molitrix), a biorefinery approach. Food Chem. 2018;242:568–578. doi: 10.1016/j.foodchem.2017.09.045. [DOI] [PubMed] [Google Scholar]
- 72.Kezwoń A., Chromińska I., Frączyk T., Wojciechowski K. Effect of enzymatic hydrolysis on surface activity and surface rheology of type I collagen. Colloids Surf. B: Biointerfaces. 2016;137:60–69. doi: 10.1016/j.colsurfb.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 73.Wang J., Luo D., Liang M., Zhang T., Yin X., Zhang Y., Yang X., Liu W. Spectrum-effect relationships between high-performance liquid chromatography (HPLC) fingerprints and the antioxidant and anti-inflammatory activities of collagen peptides. Molecules. 2018;23:3257. doi: 10.3390/molecules23123257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang L., Jiang Y., Wang X., Zhou J., Cui H., Xu W., He Y., Ma H., Gao R. Effect of oral administration of collagen hydrolysates from Nile tilapia on the chronologically aged skin. J. Funct. Foods. 2018;44:112–117. doi: 10.1016/j.jff.2018.03.005. [DOI] [Google Scholar]
- 75.Ramadass S.K., Perumal S., Gopinath A., Nisal A., Subramanian S., Madhan B. Sol–gel assisted fabrication of collagen hydrolysate composite scaffold: A novel therapeutic alternative to the traditional collagen scaffold. Acs Appl. Mater. Interfaces. 2014;6:15015–15025. doi: 10.1021/am502948g. [DOI] [PubMed] [Google Scholar]
- 76.Sibilla S., Godfrey M., Brewer S., Budh-Raja A., Genovese L. An overview of the beneficial effects of hydrolysed collagen as a nutraceutical on skin properties: Scientific background and clinical studies. Open Nutraceuticals J. 2015;8:29–42. doi: 10.2174/1876396001508010029. [DOI] [Google Scholar]
- 77.Denis A., Brambati N., Dessauvages B., Guedj S., Ridoux C., Meffre N., Autier C. Molecular weight determination of hydrolyzed collagens. Food Hydrocoll. 2008;22:989–994. doi: 10.1016/j.foodhyd.2007.05.016. [DOI] [Google Scholar]
- 78.Najafian L., Babji A.S. A review of fish-derived antioxidant and antimicrobial peptides: Their production, assessment, and applications. Peptides. 2012;33:178–185. doi: 10.1016/j.peptides.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 79.Santana R.C., Perrechil F.A., Sato A.C.K., Cunha R.L. Emulsifying properties of collagen fibers: Effect of pH, protein concentration and homogenization pressure. Food Hydrocoll. 2011;25:604–612. doi: 10.1016/j.foodhyd.2010.07.018. [DOI] [Google Scholar]
- 80.Guo L., Harnedy P.A., Zhang L., Li B., Zhang Z., Hou H., Zhao X., FitzGerald R.J. In vitro assessment of the multifunctional bioactive potential of Alaska pollock skin collagen following simulated gastrointestinal digestion. J. Sci. Food Agric. 2015;95:1514–1520. doi: 10.1002/jsfa.6854. [DOI] [PubMed] [Google Scholar]
- 81.Pal G.K., Suresh P.V. Sustainable valorisation of seafood by-products: Recovery of collagen and development of collagen-based novel functional food ingredients. Innov. Food Sci. Emerg. Technol. 2016;37:201–215. doi: 10.1016/j.ifset.2016.03.015. [DOI] [Google Scholar]
- 82.Pei X., Yang R., Zhang Z., Gao L., Wang J., Xu Y., Zhao M., Han X., Liu Z., Li Y. Marine collagen peptide isolated from Chum Salmon (Oncorhynchus keta) skin facilitates learning and memory in aged C57BL/6J mice. Food Chem. 2010;118:333–340. doi: 10.1016/j.foodchem.2009.04.120. [DOI] [Google Scholar]
- 83.Wang J., Pei X., Liu H., Zhou D. Extraction and characterization of acid-soluble and pepsin-soluble collagen from skin of loach (Misgurnus anguillicaudatus) Int. J. Biol. Macromol. 2018;106:544–550. doi: 10.1016/j.ijbiomac.2017.08.046. [DOI] [PubMed] [Google Scholar]
- 84.Chi C., Hu F., Li Z., Wang B., Luo H. Influence of different hydrolysis processes by trypsin on the physicochemical, antioxidant, and functional properties of collagen hydrolysates from Sphyrna lewini, Dasyatis akjei, and Raja porosa. J. Aquat. Food Prod. Technol. 2016;25:616–632. doi: 10.1080/10498850.2014.898004. [DOI] [Google Scholar]
- 85.Zhang Y., Olsen K., Grossi A., Otte J. Effect of pretreatment on enzymatic hydrolysis of bovine collagen and formation of ACE-inhibitory peptides. Food Chem. 2013;141:2343–2354. doi: 10.1016/j.foodchem.2013.05.058. [DOI] [PubMed] [Google Scholar]
- 86.Lima C.A., Campos J.F., Lima Filho J.L., Converti A., da Cunha M.G.C., Porto A.L. Antimicrobial and radical scavenging properties of bovine collagen hydrolysates produced by Penicillium aurantiogriseum URM 4622 collagenase. J. Food Sci. Technol. 2015;52:4459–4466. doi: 10.1007/s13197-014-1463-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.O’Sullivan S.M., Lafarga T., Hayes M., O’Brien N.M. Bioactivity of bovine lung hydrolysates prepared using papain, pepsin, and Alcalase. J. Food Biochem. 2017;41:e12406. doi: 10.1111/jfbc.12406. [DOI] [Google Scholar]
- 88.Fu Y., Young J.F., Løkke M.M., Lametsch R., Aluko R.E., Therkildsen M. Revalorisation of bovine collagen as a potential precursor of angiotensin I-converting enzyme (ACE) inhibitory peptides based on in silico and in vitro protein digestions. J. Funct. Foods. 2016;24:196–206. doi: 10.1016/j.jff.2016.03.026. [DOI] [Google Scholar]
- 89.Choi D., Min S.G., Jo Y.J. Functionality of porcine skin hydrolysates produced by hydrothermal processing for liposomal delivery system. J. Food Biochem. 2018;42:e12464. doi: 10.1111/jfbc.12464. [DOI] [Google Scholar]
- 90.O’Keeffe M.B., Norris R., Alashi M.A., Aluko R.E., FitzGerald R.J. Peptide identification in a porcine gelatin prolyl endoproteinase hydrolysate with angiotensin converting enzyme (ACE) inhibitory and hypotensive activity. J. Funct. Foods. 2017;34:77–88. doi: 10.1016/j.jff.2017.04.018. [DOI] [Google Scholar]
- 91.Yazaki M., Ito Y., Yamada M., Goulas S., Teramoto S., Nakaya M.-a., Ohno S., Yamaguchi K. Oral ingestion of collagen hydrolysate leads to the transportation of highly concentrated Gly-Pro-Hyp and its hydrolyzed form of Pro-Hyp into the bloodstream and skin. J. Agric. Food Chem. 2017;65:2315–2322. doi: 10.1021/acs.jafc.6b05679. [DOI] [PubMed] [Google Scholar]
- 92.Min S.-G., Jo Y.-J., Park S.H. Potential application of static hydrothermal processing to produce the protein hydrolysates from porcine skin by-products. Lwt-Food Sci. Technol. 2017;83:18–25. doi: 10.1016/j.lwt.2017.04.073. [DOI] [Google Scholar]
- 93.Dandagi G.L., Byahatti S.M. An insight into the swine-influenza A (H1N1) virus infection in humans. Lung India. 2011;28:34–38. doi: 10.4103/0970-2113.76299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bradley R. Bovine spongiform encephalopathy (BSE): The current situation and research. Eur. J. Epidemiol. 1991;7:532–544. doi: 10.1007/BF00143136. [DOI] [PubMed] [Google Scholar]
- 95.Regenstein J.M., Chaudry M.M., Regenstein C.E. The kosher and halal food laws. Compr. Rev. Food Sci. Food Saf. 2003;2:111–127. doi: 10.1111/j.1541-4337.2003.tb00018.x. [DOI] [PubMed] [Google Scholar]
- 96.Felician F.F., Xia C., Qi W., Xu H. Collagen from marine biological sources and medical applications. Chem. Biodivers. 2018;15:e1700557. doi: 10.1002/cbdv.201700557. [DOI] [PubMed] [Google Scholar]
- 97.Pati F., Adhikari B., Dhara S. Isolation and characterization of fish scale collagen of higher thermal stability. Bioresour. Technol. 2010;101:3737–3742. doi: 10.1016/j.biortech.2009.12.133. [DOI] [PubMed] [Google Scholar]
- 98.Sanchez A., Blanco M., Correa B., Perez-Martin R., Sotelo C. Effect of fish collagen hydrolysates on type I collagen mRNA levels of human dermal fibroblast culture. Mar. Drugs. 2018;16:144. doi: 10.3390/md16050144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chen J., Li L., Yi R., Gao R., He J. Release kinetics of Tilapia scale collagen I peptides during tryptic hydrolysis. Food Hydrocoll. 2018;77:931–936. doi: 10.1016/j.foodhyd.2017.11.040. [DOI] [Google Scholar]
- 100.Das J., Dey P., Chakraborty T., Saleem K., Nagendra R., Banerjee P. Utilization of marine industry waste derived collagen hydrolysate as peroxide inhibition agents in lipid-based food. J. Food Process. Preserv. 2018;42:e13430. doi: 10.1111/jfpp.13430. [DOI] [Google Scholar]
- 101.Villamil O., Váquiro H., Solanilla J.F. Fish viscera protein hydrolysates: Production, potential applications and functional and bioactive properties. Food Chem. 2017;224:160–171. doi: 10.1016/j.foodchem.2016.12.057. [DOI] [PubMed] [Google Scholar]
- 102.Ahmed R., Chun B.-S. Subcritical water hydrolysis for the production of bioactive peptides from tuna skin collagen. J. Supercrit. Fluids. 2018;141:88–96. doi: 10.1016/j.supflu.2018.03.006. [DOI] [Google Scholar]
- 103.Sabeena Farvin K.H., Andersen L.L., Otte J., Nielsen H.H., Jessen F., Jacobsen C. Antioxidant activity of cod (Gadus morhua) protein hydrolysates: Fractionation and characterisation of peptide fractions. Food Chem. 2016;204:409–419. doi: 10.1016/j.foodchem.2016.02.145. [DOI] [PubMed] [Google Scholar]
- 104.Liu C., Ma X., Che S., Wang C., Li B. The effect of hydrolysis with neutrase on molecular weight, functional properties, and antioxidant activities of Alaska pollock protein isolate. J. Ocean. Univ. China. 2018;17:1423–1431. doi: 10.1007/s11802-018-3649-9. [DOI] [Google Scholar]
- 105.Tao J., Zhao Y.-Q., Chi C.-F., Wang B. Bioactive peptides from cartilage protein hydrolysate of spotless smoothhound and their antioxidant activity in vitro. Mar. Drugs. 2018;16:100. doi: 10.3390/md16040100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Saiga A., Iwai K., Hayakawa T., Takahata Y., Kitamura S., Nishimura T., Morimatsu F. Angiotensin I-converting enzyme-inhibitory peptides obtained from chicken collagen hydrolysate. J. Agric. Food Chem. 2008;56:9586–9591. doi: 10.1021/jf072669w. [DOI] [PubMed] [Google Scholar]
- 107.Zhao Y., Wang Z., Zhang J., Su T. Extraction and characterization of collagen hydrolysates from the skin of Rana chensinensis. 3 Biotech. 2018;8:181. doi: 10.1007/s13205-018-1198-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dhakal D., Koomsap P., Lamichhane A., Sadiq M.B., Anal A.K. Optimization of collagen extraction from chicken feet by papain hydrolysis and synthesis of chicken feet collagen based biopolymeric fibres. Food Biosci. 2018;23:23–30. doi: 10.1016/j.fbio.2018.03.003. [DOI] [Google Scholar]
- 109.Soladoye O.P., Saldo J., Peiro L., Rovira A., Mor-Mur M. Antioxidant and angiotensin 1 converting enzyme inhibitory functions from chicken collagen hydrolysates. J. Nutr. Food Sci. 2015;5:1–9. doi: 10.4172/2155-9600.1000369. [DOI] [Google Scholar]
- 110.Venkatesan J., Anil S., Kim S.-K., Shim M. Marine fish proteins and peptides for cosmeceuticals: A review. Mar. Drugs. 2017;15:143. doi: 10.3390/md15050143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hashim P., Sofberi M., Ridzwan M., Bakar J., Mat Hashim D. Collagen in food and beverage industries. Int. Food Res. J. 2015;22:1–8. [Google Scholar]
- 112.Varani J., Dame M.K., Rittie L., Fligiel S.E.G., Kang S., Fisher G.J., Voorhees J.J. Decreased collagen production in chronologically aged skin: Roles of age-dependent alteration in fibroblast function and defective mechanical stimulation. Am. J. Pathol. 2006;168:1861–1868. doi: 10.2353/ajpath.2006.051302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Baumann L. Skin ageing and its treatment. J. Pathol. 2007;211:241–251. doi: 10.1002/path.2098. [DOI] [PubMed] [Google Scholar]
- 114.Hays N.P., Kim H., Wells A.M., Kajkenova O., Evans W.J. Effects of whey and fortified collagen hydrolysate protein supplements on nitrogen balance and body composition in older women. J. Am. Diet. Assoc. 2009;109:1082–1087. doi: 10.1016/j.jada.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 115.Zorrilla García A.E. El envejecimiento y el estrés oxidativo. Rev. Cuba. De Investig. Biomédicas. 2002;21:178–185. [Google Scholar]
- 116.Zouboulis C.C., Makrantonaki E. Clinical aspects and molecular diagnostics of skin aging. Clin. Dermatol. 2011;29:3–14. doi: 10.1016/j.clindermatol.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 117.Kim D.-U., Chung H.-C., Choi J., Sakai Y., Lee B.-Y. Oral intake of low-molecular-weight collagen peptide improves hydration, elasticity, and wrinkling in human skin: A randomized, double-blind, placebo-controlled study. Nutrients. 2018;10:826. doi: 10.3390/nu10070826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tobin D.J. Introduction to skin aging. J. Tissue Viability. 2017;26:37–46. doi: 10.1016/j.jtv.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 119.Czajka A., Kania E.M., Genovese L., Corbo A., Merone G., Luci C., Sibilla S. Daily oral supplementation with collagen peptides combined with vitamins and other bioactive compounds improves skin elasticity and has a beneficial effect on joint and general wellbeing. Nutr. Res. 2018;57:97–108. doi: 10.1016/j.nutres.2018.06.001. [DOI] [PubMed] [Google Scholar]
- 120.Haydont V., Bernard B.A., Fortunel N.O. Age-related evolutions of the dermis: Clinical signs, fibroblast and extracellular matrix dynamics. Mech. Ageing Dev. 2019;177:150–156. doi: 10.1016/j.mad.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 121.Chen Y.-P., Wu H.-T., Wang G.-H., Liang C.-H. Improvement of Skin Condition on Skin Moisture and Anti-Melanogenesis by Collagen Peptides from Milkfish (Chanos chanos) Scales. Materials Science and Engineering; Nanjing, China: 2018. pp. 1–7. (IOP Conference Series). [Google Scholar]
- 122.Jhawar N., Wang J.V., Saedi N. Oral collagen supplementation for skin aging: A fad or the future? J. Cosmet. Dermatol. 2019 doi: 10.1111/jocd.13096. [DOI] [PubMed] [Google Scholar]
- 123.Bolke L., Schlippe G., Gerß J., Voss W. A collagen supplement improves skin hydration, elasticity, roughness, and density: Results of a randomized, placebo-controlled, blind study. Nutrients. 2019;11:2494. doi: 10.3390/nu11102494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Addor F.A.S., Cotta Vieira J., Abreu Melo C.S. Improvement of dermal parameters in aged skin after oral use of a nutrient supplement. Clin. Cosmet. Investig. Dermatol. 2018;11:195–201. doi: 10.2147/CCID.S150269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Asserin J., Lati E., Shioya T., Prawitt J. The effect of oral collagen peptide supplementation on skin moisture and the dermal collagen network: Evidence from an ex vivo model and randomized, placebo-controlled clinical trials. J. Cosmet. Dermatol. 2015;14:291–301. doi: 10.1111/jocd.12174. [DOI] [PubMed] [Google Scholar]
- 126.Maia Campos P.M., Melo M.O., Siqueira César F.C. Topical application and oral supplementation of peptides in the improvement of skin viscoelasticity and density. J. Cosmet. Dermatol. 2019 doi: 10.1111/jocd.12893. [DOI] [PubMed] [Google Scholar]
- 127.Koizumi S., Inoue N., Shimizu M., Kwon C.-j., Kim H.-y., Park K.S. Effects of dietary supplementation with fish scales-derived collagen peptides on skin parameters and condition: A randomized, placebo-controlled, double-blind study. Int. J. Pept. Res. Ther. 2018;24:397–402. doi: 10.1007/s10989-017-9626-0. [DOI] [Google Scholar]
- 128.Wang W., Chen M., Wu J., Wang S. Hypothermia protection effect of antifreeze peptides from pigskin collagen on freeze-dried Streptococcus thermophiles and its possible action mechanism. Lwt-Food Sci. Technol. 2015;63:878–885. doi: 10.1016/j.lwt.2015.04.007. [DOI] [Google Scholar]
- 129.Sousa S.C., Fragoso S.P., Penna C.R.A., Arcanjo N.M.O., Silva F.A.P., Ferreira V.C.S., Barreto M.D.S., Araújo Í.B.S. Quality parameters of frankfurter-type sausages with partial replacement of fat by hydrolyzed collagen. Lwt-Food Sci. Technol. 2017;76:320–325. doi: 10.1016/j.lwt.2016.06.034. [DOI] [Google Scholar]
- 130.Ibrahim F.N., Ismail-Fitry M.R., Yusoff M.M., Shukri R. Effects of Fish Collagen Hydrolysate (FCH) as fat replacer in the production of buffalo patties. J. Adv. Res. Appl. Sci. Eng. Technol. 2018;11:108–117. [Google Scholar]
- 131.Prestes R.C., Carneiro E.B.B., Demiate I.M. Hydrolyzed collagen, modified starch and guar gum addition in turkey ham. Ciência Rural. 2012;42:1307–1313. doi: 10.1590/S0103-84782012005000037. [DOI] [Google Scholar]
- 132.Gerhardt Â., Monteiro B.W., Gennari A., Lehn D.N., Souza C.F.V.d. Características físico-químicas e sensoriais de bebidas lácteas fermentadas utilizando soro de ricota e colágeno hidrolisado. Physicochemical and sensory characteristics of fermented dairy drink using ricotta cheese whey and hydrolyzed collagen. Rev. Do Inst. De Laticínios Cândido Tostes. 2013;68:41–50. doi: 10.5935/2238-6416.20130007. [DOI] [Google Scholar]
- 133.Da Mata Rigoto J., Ribeiro T.H.S., Stevanato N., Sampaio A.R., Ruiz S.P., Bolanho B.C. Effect of açaí pulp, cheese whey, and hydrolysate collagen on the characteristics of dairy beverages containing probiotic bacteria. J. Food Process. Eng. 2019;42:e12953. doi: 10.1111/jfpe.12953. [DOI] [Google Scholar]
- 134.Benjakul S., Chantakun K., Karnjanapratum S. Impact of retort process on characteristics and bioactivities of herbal soup based on hydrolyzed collagen from seabass skin. J. Food Sci. Technol. 2018;55:3779–3791. doi: 10.1007/s13197-018-3310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhang Q.-X., Fu R.-J., Yao K., Jia D.-Y., He Q., Chi Y.-L. Clarification effect of collagen hydrolysate clarifier on chrysanthemum beverage. LWT. 2018;91:70–76. doi: 10.1016/j.lwt.2018.01.041. [DOI] [Google Scholar]
- 136.Fu R., Yao K., Zhang Q., Jia D., Zhao J., Chi Y. Collagen hydrolysates of skin shavings prepared by enzymatic hydrolysis as a natural flocculant and their flocculating property. Appl. Biochem. Biotechnol. 2017;182:55–66. doi: 10.1007/s12010-016-2310-6. [DOI] [PubMed] [Google Scholar]
- 137.Ramshaw J.A. Biomedical applications of collagens. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2016;104:665–675. doi: 10.1002/jbm.b.33541. [DOI] [PubMed] [Google Scholar]
- 138.Zeugolis D.I., Paul R.G., Attenburrow G. Factors influencing the properties of reconstituted collagen fibers prior to self-assembly: Animal species and collagen extraction method. J. Biomed. Mater. Res. Part. A. 2008;86:892–904. doi: 10.1002/jbm.a.31694. [DOI] [PubMed] [Google Scholar]
- 139.Pei Y., Yang J., Liu P., Xu M., Zhang X., Zhang L. Fabrication, properties and bioapplications of cellulose/collagen hydrolysate composite films. Carbohydr. Polym. 2013;92:1752–1760. doi: 10.1016/j.carbpol.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 140.Ficai A., Albu M.G., Birsan M., Sonmez M., Ficai D., Trandafir V., Andronescu E. Collagen hydrolysate based collagen/hydroxyapatite composite materials. J. Mol. Struct. 2013;1037:154–159. doi: 10.1016/j.molstruc.2012.12.052. [DOI] [Google Scholar]
- 141.Ocak B. Film-forming ability of collagen hydrolysate extracted from leather solid wastes with chitosan. Environ. Sci. Pollut. Res. 2018;25:4643–4655. doi: 10.1007/s11356-017-0843-z. [DOI] [PubMed] [Google Scholar]
- 142.Noppakundilograt S., Choopromkaw S., Kiatkamjornwong S. Hydrolyzed collagen-grafted-poly [(acrylic acid)-co-(methacrylic acid)] hydrogel for drug delivery. J. Appl. Polym. Sci. 2018;135:45654. doi: 10.1002/app.45654. [DOI] [Google Scholar]
- 143.Ouyang Q.-Q., Hu Z., Lin Z.-P., Quan W.-Y., Deng Y.-F., Li S.-D., Li P.-W., Chen Y. Chitosan hydrogel in combination with marine peptides from tilapia for burns healing. Int. J. Biol. Macromol. 2018;112:1191–1198. doi: 10.1016/j.ijbiomac.2018.01.217. [DOI] [PubMed] [Google Scholar]
- 144.Ramadass S.K., Nazir L.S., Thangam R., Perumal R.K., Manjubala I., Madhan B., Seetharaman S. Type I collagen peptides and nitric oxide releasing electrospun silk fibroin scaffold: A multifunctional approach for the treatment of ischemic chronic wounds. Colloids Surf. B: Biointerfaces. 2019;175:636–643. doi: 10.1016/j.colsurfb.2018.12.025. [DOI] [PubMed] [Google Scholar]
- 145.Sontakke S.B., Jung J.-h., Piao Z., Chung H.J. Orally available collagen tripeptide: Enzymatic stability, intestinal permeability, and absorption of Gly-Pro-Hyp and Pro-Hyp. J. Agric. Food Chem. 2016;64:7127–7133. doi: 10.1021/acs.jafc.6b02955. [DOI] [PubMed] [Google Scholar]