Abstract
Objective
To evaluate the associations between maternal diabetes diagnosed before or during pregnancy and early onset cardiovascular disease (CVD) in offspring during their first four decades of life.
Design
Population based cohort study.
Setting
Danish national health registries.
Participants
All 2 432 000 liveborn children without congenital heart disease in Denmark during 1977-2016. Follow-up began at birth and continued until first time diagnosis of CVD, death, emigration, or 31 December 2016, whichever came first.
Exposures for observational studies
Pregestational diabetes, including type 1 diabetes (n=22 055) and type 2 diabetes (n=6537), and gestational diabetes (n=26 272).
Main outcome measures
The primary outcome was early onset CVD (excluding congenital heart diseases) defined by hospital diagnosis. Associations between maternal diabetes and risks of early onset CVD in offspring were studied. Cox regression was used to assess whether a maternal history of CVD or maternal diabetic complications affected these associations. Adjustments were made for calendar year, sex, singleton status, maternal factors (parity, age, smoking, education, cohabitation, residence at childbirth, history of CVD before childbirth), and paternal history of CVD before childbirth. The cumulative incidence was averaged across all individuals, and factors were adjusted while treating deaths from causes other than CVD as competing events.
Results
During up to 40 years of follow-up, 1153 offspring of mothers with diabetes and 91 311 offspring of mothers who did not have diabetes were diagnosed with CVD. Offspring of mothers with diabetes had a 29% increased overall rate of early onset CVD (hazard ratio 1.29 (95% confidence interval 1.21 to 1.37); cumulative incidence among offspring unexposed to maternal diabetes at 40 years of age 13.07% (12.92% to 13.21%), difference in cumulative incidence between exposed and unexposed offspring 4.72% (2.37% to 7.06%)). The sibship design yielded results similar to those of the unpaired design based on the whole cohort. Both pregestational diabetes (1.34 (1.25 to 1.43)) and gestational diabetes (1.19 (1.07 to 1.32)) were associated with increased rates of CVD in offspring. We also observed varied increased rates of specific early onset CVDs, particularly heart failure (1.45 (0.89 to 2.35)), hypertensive disease (1.78 (1.50 to 2.11)), deep vein thrombosis (1.82 (1.38 to 2.41)), and pulmonary embolism (1.91 (1.31 to 2.80)). Increased rates of CVD were seen in different age groups from childhood to early adulthood until age 40 years. The increased rates were more pronounced among offspring of mothers with diabetic complications (1.60 (1.25 to 2.05)). A higher incidence of early onset CVD in offspring of mothers with diabetes and comorbid CVD (1.73 (1.36 to 2.20)) was associated with the added influence of comorbid CVD but not due to the interaction between diabetes and CVD on the multiplicative scale (P value for interaction 0.94).
Conclusions
Children of mothers with diabetes, especially those mothers with a history of CVD or diabetic complications, have increased rates of early onset CVD from childhood to early adulthood. If maternal diabetes does have a causal association with increased CVD rate in offspring, the prevention, screening, and treatment of diabetes in women of childbearing age could help to reduce the risk of CVD in the next generation.
Introduction
Cardiovascular disease (CVD) remains the leading cause of mortality and morbidity worldwide.1 2 Incidence and mortality rates of CVD have decreased in some countries,1 2 3 4 but the prevalence of CVD has increased in children and young adults in recent decades.5 6 The risk factors for CVD change over the course of a lifetime,7 and early onset CVD could have a different cause than CVD diagnosed in later adulthood. Limited but growing evidence suggests intergenerational links between maternal health during or before pregnancy and risk factors for CVD among offspring.8 9 10 11 A better understanding of such a link is essential to prevent and manage CVD among children and young adults.8 12 13 14
The prevalence of pregestational and gestational diabetes has been increasing globally.15 16 In pregnancies complicated by diabetes, the diabetic intrauterine environment could cause placental dysfunction and hormonal alterations, leading to disease development.15 Prenatal exposure to maternal diabetes has also been associated with congenital heart disease, obesity, and diabetes in offspring. These diseases could, in turn, lead to an increased risk of CVD in later life.15 17 18 19 20 The offspring of mothers with diabetes also have a higher prevalence of metabolic syndrome and other risk factors for future CVD.9 21 22 It is unclear, however, whether or to what extent prenatal exposure to maternal diabetes increases the risk of CVD in offspring over a lifetime.
In this Danish population based cohort study, we hypothesised that intrauterine exposure to maternal diabetes could lead to an excess risk of early onset CVD in offspring from childhood to early adulthood (up to 40 years). Using data from Danish registers, we examined associations between types of maternal diabetes and early onset of CVD in offspring, the combined effect of maternal diabetes and maternal history of CVD on the rate of early onset CVD in offspring, and whether pregestational complications of diabetes further increased the risk of CVD in offspring.
Methods
Study population
The unique personal identification number assigned to all Danish residents allows accurate linkage of individual level information from all national registries (a detailed description of registers is provided in supplementary appendix 1).23 We conducted a population based cohort study including all live births in Denmark during 1977-2016 (n=2 475 209). Our final cohort comprised 2 432 000 births after excluding 43 209 babies with congenital heart disease identified at birth or diagnosed later. A total of 27 046 (62.6%) of these babies with congenital heart disease were diagnosed up to 1 year, 8923 (20.7%) were diagnosed between 1 and 4 years of age, 3670 (8.5%) between 5 and 9 years, 2750 (6.4%) between 10 and 19 years, and 820 (1.9%) between 20 and 40 years. Follow-up started at birth and ended at the first diagnosis of a CVD, death, emigration, or 31 December 2016, whichever came first. People who emigrated or died from a cause other than CVD during follow-up were censored at the time of emigration or death.
Exposure
Offspring born to mothers diagnosed with diabetes before childbirth were considered to have been prenatally exposed to maternal diabetes mellitus.23 Maternal diabetes was categorised as gestational diabetes or pregestational diabetes. Pregestational diabetes was further defined as type 1 or type 2 diabetes before childbirth (a detailed description of the methods used to identify diabetes is provided in supplementary appendix 2). If a mother was diagnosed with more than one type of diabetes during one pregnancy, possibly owing to misclassification, she was classified in the main analysis in accordance with the first type diagnosed. We also used two other approaches in sensitivity analyses: hierarchical ordering of diabetes types (type 1, type 2, and then gestational diabetes)20; and restriction to offspring of mothers with only one type of diabetes. Information on the diagnosis of diabetes was retrieved from the Danish National Diabetes Register, the Danish National Patient Registry (DNPR), and the Danish National Prescription Registry (supplementary appendix 1).
Because pregestational diabetic complications could reflect diabetes severity due to poor glycaemic control, we identified mothers with such complications—namely, diabetic coma; ketoacidosis; diabetes with kidney disease; and ophthalmic, neurological, circulatory, unspecified, or multiple complications. Women with pregestational diabetic complications were classified into two groups: those with one complication and those with multiple complications.
Outcome of interest
The outcome of interest was early onset CVD in offspring, defined as the first occurrence of CVD in the DNPR or the Danish Register of Cause of Death.23 Diagnoses of congenital heart disease were excluded. We identified the outcome by using ICD-8 and ICD-10 (international classification of diseases, 8th and 10th revisions) codes for CVD (ICD-8: 390-444.1, 444.3-458, 782.4; ICD-10: I00-I99), or surgery codes for coronary artery bypass graft surgery and percutaneous coronary interventions (supplementary table S1). We also investigated the following types of CVD: ischaemic heart disease, cerebrovascular disease, stroke, heart failure, atrial fibrillation, hypertensive disease, deep vein thrombosis, pulmonary embolism, and other types of CVD (table S1).
Covariates
Potential confounders were selected based on directed acyclic graphs (fig S1) depicting the best known relations between the variables in this study. Confounders included maternal age (<20, 20-24, 25-29, 30-34, or ≥35 years), parity (1, 2, or ≥3 children), maternal cohabitation (single or cohabitating), maternal education (0-9, 10-14, or ≥15 years), maternal residence (Copenhagen, cities with 100 000 or more inhabitants, or other), maternal smoking during pregnancy (yes or no), maternal and paternal CVD history before the birth of their child (yes or no). We also included singleton delivery, sex of offspring, and calendar period of delivery (before 1980, five year intervals during 1981-2010, and the interval 2011-16).
To deal with the problem of missing data on covariates, we used the SAS multiple imputation procedure with fully conditional specification to impute five replications (chosen to be greater than the percentage of data missing as advised by White et al24 and to make the computation tractable for our large dataset with millions of observations). The covariates and multiple imputations are described in detail in supplementary appendix 3. Missing indicator method and complete case analysis were also performed for comparison.
Statistical analysis
Cox regression was used to estimate hazard ratio with 95% confidence intervals to assess the association between exposure to maternal diabetes and overall CVD/specific types of CVD in offspring, with offspring’s age as the time scale. Evaluation of log-minus-log survival curves showed that the curves were roughly parallel (fig S2). Although they appeared to be converging slightly, no obvious evidence was seen to support the interaction between hazard ratio and time (P value for interaction 0.70). Thus, we find it reasonable to assume that proportional hazard assumption was not violated. Treating deaths from causes other than CVD as competing events, we performed competing risk analysis to estimate the cumulative incidence and cumulative incidence difference between exposed and unexposed offspring averaged across the distribution of the covariates, described above using the inverse probability of treatment weighting approach.25 26 27 We evaluated the joint effect of maternal diabetes and maternal history of CVD on early onset CVD in offspring by including the interaction term between maternal diabetes and maternal history of CVD. Furthermore, we examined the association between maternal diabetes and CVD in offspring stratified by maternal diabetic complications and the age group of the offspring (0-9, 10-14, 15-19, 20-24, and 25-40 years).
In sensitivity analyses, we evaluated risks of CVD in offspring in relation to the timing of diagnosis of maternal type 1 diabetes with respect to childbirth (pregestational diagnoses and diagnoses ≤2, 2-5, and >5 years after childbirth). Corresponding risks were also evaluated in relation to the timing of diagnosis of pregestational (type 1 and type 2) diabetes. Furthermore, we restricted analyses to offspring born at term (≥37 completed gestational weeks).
We used a sibship design to evaluate the influence of uncontrolled confounding due to shared genetic or familial characteristics by analysing the data of sibling pairs and comparing the outcome of each sibling exposed to maternal diabetes with the outcome of their unexposed sibling.28 We performed conditional Poisson regression for the sibling subcohort analysis. Similar to the stratified Cox regression,29 the conditional Poisson regression included a separate stratum for each family identified by the mother’s unique civil registration number, in which only sibling pairs discordant for both maternal diabetes and offspring CVD were informative and contributed to the effect estimate. Thus, each family had its own baseline rate function reflecting the family’s shared genetic or familial characteristics. The association between maternal diabetes during pregnancy and early onset CVD in offspring was analysed only among siblings. Thus, the analysis controlled for genetic or familial characteristics shared among family members. As type 1 and type 2 diabetes would not change between pregnancies, and gestational diabetes was more common in older women with higher parity, the sibship design was appropriate for gestational diabetes in a second pregnancy or later pregnancies. We further analysed the data to assess the influence of possible uncontrolled confounding. We evaluated also whether parental factors, including maternal country of origin, maternal body mass index before pregnancy, and paternal diabetes before the child’s birth, affected the observed associations. In addition, we used paternal diabetes as an exposure to explore potential confounding by genetic and familial factors. We used inverse-probability-of-selection weighting to evaluate possible live birth bias due to maternal diabetes and other (uncontrolled or unmeasured) risk factors for CVD in offspring.30 These risk factors could lead to fetal loss such that naive analysis of data on live births could be misleading.30 31
We performed subanalyses stratified by sex of offspring, and restricted to primiparous women, and singleton offspring. Owing to ICD code changes (ICD-10 was adopted in 1994) and the availability of data on confounders (maternal smoking and maternal pregnancy body mass index became available since 1991 and 2004, respectively), we restricted subanalyses to offspring born after 1991, 1994, and 2004. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC) and Stata 14 (StatCorp, College Station, TX).
Patient and public involvement
No patients were involved in setting the research question or the outcome measures, nor were they involved in developing plans for design or implementation of the study. No patients were asked to advise on interpretation or writing up of results. There are no plans to disseminate the results of the research to study patients or the relevant patient community.
Results
Of 2 432 000 liveborn offspring without congenital heart disease, 54 864 (2.3%) were exposed to maternal pregestational diabetes (type 1: 0.9%, type 2: 0.3%) or gestational diabetes (1.1%). The proportion of offspring born to mothers with diabetes increased over time (fig S3). A total of 93 881 offspring (3.9%) were censored at the end of follow-up owing to emigration (n=74 377) or non-cardiovascular death (n=19 504). Compared with mothers who did not have diabetes, mothers with diabetes were more likely to be older, to have had higher education, to have higher parity, to live alone, and to smoke less during pregnancy. Compared with unexposed offspring, offspring exposed to maternal diabetes were more likely to have a parental history of CVD and to have a higher rate of developing diabetes, obesity, hypertension, hypercholesterolaemia, and chronic kidney diseases (tables S2-3).
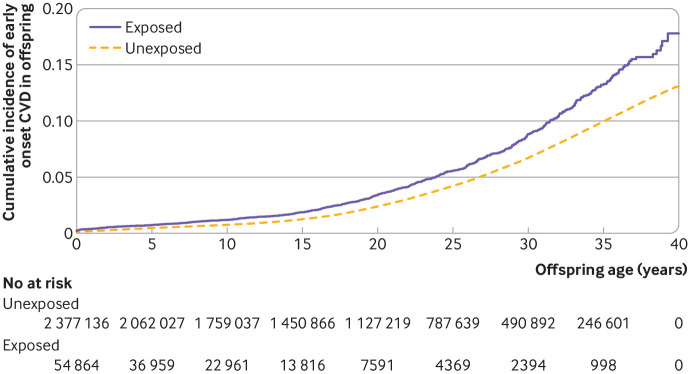
During up to 40 years of follow-up, 1153 offspring of mothers with diabetes and 91 311 offspring of mothers who did not have diabetes were diagnosed with CVD. Exposed offspring had a higher rate of overall CVDs than unexposed offspring (hazard ratio 1.29 (95% confidence interval 1.21 to 1.37); cumulative incidence among unexposed offspring at 40 years of age (13.07% (12.92% to 13.21%), cumulative incidence difference between the exposed and the unexposed offspring (4.72% (2.37% to 7.06%); table 1, fig 1, and fig S4). An increased rate of early onset CVD was seen in offspring exposed to pregestational diabetes (1.34 (1.25 to 1.43)) or gestational diabetes (1.19 (1.07 to 1.32)). Early onset of CVD in offspring was of similar magnitude in maternal type 1 and type 2 diabetes. The rates for most specific types of CVD were also increased in offspring exposed to maternal diabetes, with higher rates seen for heart failure (1.45 (0.89 to 2.35)), hypertensive disease (1.78 (1.50 to 2.11)), deep vein thrombosis (1.82 (1.38 to 2.41)), and pulmonary embolism (1.91 (1.31 to 2.80); table 1). We generally observed increased hazard ratios for offspring exposed to maternal diabetes in each age group in childhood (before 20 years of age) and early adulthood (from 20 to 40 years of age), regardless of the type of maternal diabetes (fig 2).
Table 1.
Associations between maternal diabetes and early onset of overall CVD and specific types of CVD in offspring
| Outcome* and exposure | No of offspring with CVD | Rate per 1000 person years | Hazard ratio (95% CI), model 1 | Hazard ratio (95% CI), model 2 |
|---|---|---|---|---|
| Overall CVD | ||||
| No diabetes | 91 311 | 2.01 | 1.0 (ref) | 1.0 (ref) |
| Maternal diabetes: | 1153 | 2.02 | 1.47 (1.39 to 1.56) | 1.29 (1.21 to 1.37) |
| Pregestational diabetes | 792 | 2.28 | 1.45 (1.35 to 1.56) | 1.34 (1.25 to 1.43) |
| Pregestational diabetes, type 1 | 531 | 2.13 | 1.46 (1.34 to 1.59) | 1.31 (1.20 to 1.43) |
| Pregestational diabetes, type 2 | 261 | 2.68 | 1.44 (1.27 to 1.62) | 1.39 (1.23 to 1.57) |
| Gestational diabetes | 361 | 1.60 | 1.51 (1.36 to 1.67) | 1.19 (1.07 to 1.32) |
| Ischaemic heart disease | ||||
| No diabetes | 3753 | 0.08 | 1.0 (ref) | 1.0 (ref) |
| Maternal diabetes: | 28 | 0.05 | 1.21 (0.83 to 1.75) | 1.18 (0.82 to 1.72) |
| Pregestational diabetes | 26 | 0.07 | 1.38 (0.94 to 2.03) | 1.37 (0.93 to 2.01) |
| Pregestational diabetes, type 1 | 16 | 0.06 | 1.35 (0.83 to 2.21) | 1.35 (0.83 to 2.21) |
| Pregestational diabetes, type 2 | 10 | 0.10 | 1.42 (0.76 to 2.64) | 1.40 (0.75 to 2.61) |
| Gestational diabetes | <3 | 0.01 | 0.46 (0.11 to 1.83) | 0.43 (0.11 to 1.71) |
| Cerebrovascular disease | ||||
| No diabetes | 7113 | 0.15 | 1.0 (ref) | 1.0 (ref) |
| Maternal diabetes: | 102 | 0.18 | 1.49 (1.22 to 1.81) | 1.38 (1.13 to 1.68) |
| Pregestational diabetes | 67 | 0.19 | 1.46 (1.15 to 1.86) | 1.39 (1.10 to 1.77) |
| Pregestational diabetes, type 1 | 48 | 0.19 | 1.54 (1.16 to 2.05) | 1.46 (1.10 to 1.94) |
| Pregestational diabetes, type 2 | 19 | 0.19 | 1.30 (0.83 to 2.03) | 1.25 (0.79 to 1.96) |
| Gestational diabetes | 35 | 0.15 | 1.54 (1.10 to 2.14) | 1.36 (0.97 to 1.90) |
| Stroke | ||||
| No diabetes | 4678 | 0.10 | 1.0 (ref) | 1.0 (ref) |
| Maternal diabetes: | 67 | 0.12 | 1.39 (1.09 to 1.77) | 1.27 (1.00 to 1.62) |
| Pregestational diabetes | 43 | 0.12 | 1.37 (1.01 to 1.85) | 1.29 (0.96 to 1.74) |
| Pregestational diabetes, type 1 | 30 | 0.12 | 1.38 (0.97 to 1.98) | 1.29 (0.90 to 1.85) |
| Pregestational diabetes, type 2 | 13 | 0.13 | 1.34 (0.77 to 2.30) | 1.28 (0.74 to 2.21) |
| Gestational diabetes | 24 | 0.11 | 1.43 (0.96 to 2.13) | 1.23 (0.82 to 1.85) |
| Heart failure | ||||
| No diabetes | 1222 | 0.03 | 1.0 (ref) | 1.0 (ref) |
| Maternal diabetes: | 17 | 0.03 | 1.48 (0.92 to 2.40) | 1.45 (0.89 to 2.35) |
| Pregestational diabetes | 11 | 0.03 | 1.42 (0.78 to 2.58) | 1.40 (0.77 to 2.54) |
| Pregestational diabetes, type 1 | 5 | 0.02 | 0.95 (0.40 to 2.29) | 0.95 (0.39 to 2.28) |
| Pregestational diabetes, type 2 | 6 | 0.06 | 2.41 (1.08 to 5.38) | 2.32 (1.04 to 5.19) |
| Gestational diabetes | 6 | 0.03 | 1.61 (0.72 to 3.60) | 1.54 (0.68 to 3.47) |
| Atrial fibrillation | ||||
| No diabetes | 2339 | 0.05 | 1.0 (ref) | 1.0 (ref) |
| Maternal diabetes: | 17 | 0.03 | 1.17 (0.73 to 1.89) | 1.12 (0.69 to 1.81) |
| Pregestational diabetes | 16 | 0.05 | 1.36 (0.83 to 2.22) | 1.33 (0.81 to 2.18) |
| Pregestational diabetes, type 1 | 11 | 0.04 | 1.51 (0.83 to 2.73) | 1.48 (0.82 to 2.67) |
| Pregestational diabetes, type 2 | 5 | 0.05 | 1.12 (0.47 to 2.69) | 1.10 (0.46 to 2.64) |
| Gestational diabetes | <3 | 0.00 | 0.36 (0.05 to 2.56) | 0.31 (0.04 to 2.23) |
| Hypertensive disease | ||||
| No diabetes | 9615 | 0.21 | 1.0 (ref) | 1.0 (ref) |
| Maternal diabetes: | 135 | 0.23 | 2.10 (1.77 to 2.49) | 1.78 (1.50 to 2.11) |
| Pregestational diabetes | 100 | 0.28 | 1.99 (1.63 to 2.42) | 1.78 (1.46 to 2.17) |
| Pregestational diabetes, type 1 | 58 | 0.23 | 1.81 (1.40 to 2.35) | 1.57 (1.22 to 2.04) |
| Pregestational diabetes, type 2 | 42 | 0.42 | 2.29 (1.69 to 3.10) | 2.18 (1.61 to 2.95) |
| Gestational diabetes | 35 | 0.15 | 2.50 (1.79 to 3.48) | 1.77 (1.27 to 2.48) |
| Deep vein thrombosis | ||||
| No diabetes | 4514 | 0.10 | 1.0 (ref) | 1.0 (ref) |
| Maternal diabetes: | 50 | 0.09 | 1.86 (1.41 to 2.46) | 1.82 (1.38 to 2.41) |
| Pregestational diabetes | 44 | 0.13 | 1.99 (1.48 to 2.68) | 1.97 (1.47 to 2.66) |
| Pregestational diabetes, type 1 | 22 | 0.09 | 1.62 (1.07 to 2.47) | 1.60 (1.05 to 2.43) |
| Pregestational diabetes, type 2 | 22 | 0.22 | 2.56 (1.68 to 3.89) | 2.58 (1.70 to 3.93) |
| Gestational diabetes | 6 | 0.03 | 1.26 (0.57 to 2.82) | 1.17 (0.53 to 2.62) |
| Pulmonary embolism | ||||
| No diabetes | 2246 | 0.05 | 1.0 (ref) | 1.0 (ref) |
| Maternal diabetes: | 27 | 0.05 | 1.85 (1.26 to 2.70) | 1.91 (1.31 to 2.80) |
| Pregestational diabetes | 21 | 0.06 | 1.82 (1.18 to 2.80) | 1.86 (1.21 to 2.85) |
| Pregestational diabetes, type 1 | 7 | 0.03 | 0.97 (0.46 to 2.04) | 1.01 (0.48 to 2.12) |
| Pregestational diabetes, type 2 | 14 | 0.14 | 3.22 (1.90 to 5.44) | 3.22 (1.90 to 5.45) |
| Gestational diabetes | 6 | 0.03 | 1.95 (0.87 to 4.36) | 2.14 (0.96 to 4.79) |
| Other CVDs† | ||||
| No diabetes | 69 915 | 1.53 | 1.0 (ref) | 1.0 (ref) |
| Maternal diabetes: | 876 | 1.53 | 1.40 (1.31 to 1.50) | 1.22 (1.14 to 1.30) |
| Pregestational diabetes | 582 | 1.67 | 1.36 (1.26 to 1.47) | 1.25 (1.15 to 1.35) |
| Pregestational diabetes, type 1 | 397 | 1.59 | 1.38 (1.26 to 1.53) | 1.23 (1.12 to 1.36) |
| Pregestational diabetes, type 2 | 185 | 1.89 | 1.31 (1.14 to 1.51) | 1.28 (1.11 to 1.47) |
| Gestational diabetes | 294 | 1.30 | 1.50 (1.34 to 1.68) | 1.16 (1.04 to 1.30) |
CVD=cardiovascular disease; ICD-8, ICD-10=international classification of disease, 8th revision, 10th revision; ref=reference; model 1=offspring’s age as time scale; model 2=offspring’s age as time scale, and controlled for calendar year, sex, singleton status, parity, maternal smoking, maternal education, maternal cohabitation, maternal residence at birth, maternal history of CVD before childbirth, paternal history of CVD before birth of the child, and maternal age (restricted cubic spline with five knots at five evenly spaced centiles).
Ischaemic heart disease (ICD-8 codes: 410-414; ICD-10 codes: I20-I25), cerebrovascular disease (ICD-8 codes: 430-438; ICD-10 codes: I60-I69), stroke (ICD-8 codes: 430-436; ICD-10 codes: I61-I64), heart failure (ICD-8 codes: 427.0, 427.1,782.4; ICD-10 codes: I110, I130, I132, I50), atrial fibrillation (ICD-8 codes: 427.93, 427.94; ICD-10 code: I48), hypertensive disease (ICD-8 codes: 400-404; ICD-10 codes: I10-I15), deep vein thrombosis (ICD-8 codes: 451.00; ICD-10 codes: I80.1-I80.3), pulmonary embolism (ICD-8 codes: 450.99; ICD-10 codes: I26), other types of CVD (the remainder of the codes included under CVD overall (ICD-8 codes: 390-444.1, 444.3-458, 782.4; ICD-10 codes: I00-I99)).
The most important diagnostic entities contributing to this category: cardiomyopathy, cardiac arrest, paroxysmal tachycardia, complications and ill-defined descriptions of heart disease, diseases of capillaries, varicose veins of other sites, non-specific lymphadenitis, and hypotension.
Fig 1.
Adjusted cumulative incidence of early cardiovascular disease (CVD) onset among offspring exposed versus unexposed to maternal diabetes. The adjusted cumulative incidence was averaged across the distribution of the covariates—calendar year, sex, singleton status, parity, age, smoking, education, cohabitation, residence at childbirth, history of CVD before childbirth, and paternal history of CVD before birth of the child—using the inverse probability of treatment weighting approach
Fig 2.
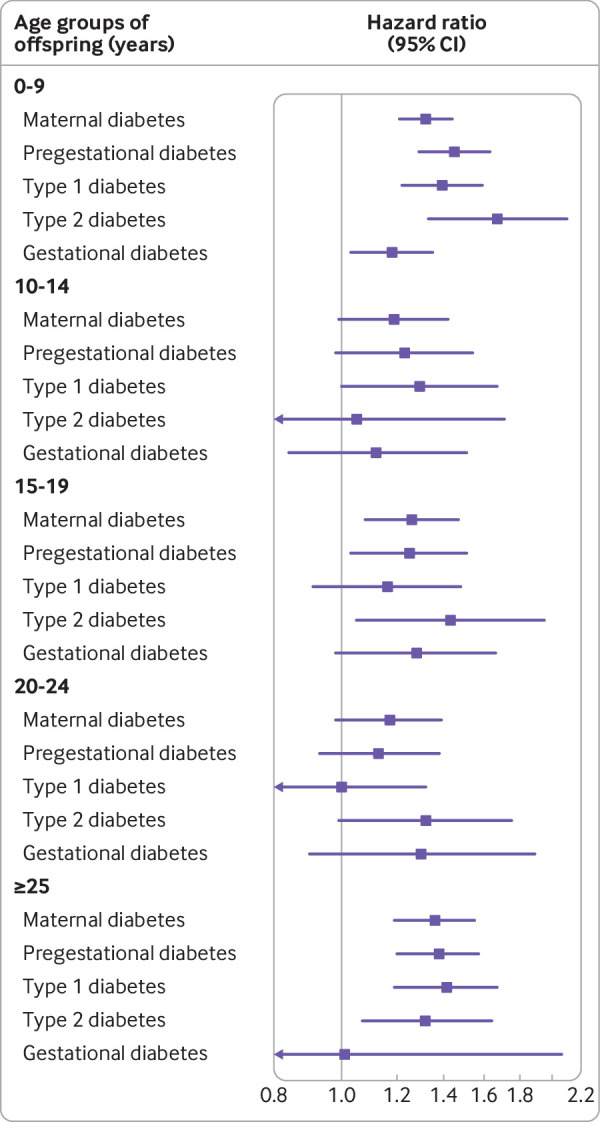
Associations between maternal diabetes and early onset of cardiovascular disease (CVD) in offspring by offspring age and diabetes type.Maternal diabetes included gestational diabetes and pregestational diabetes. Pregestational diabetes included type 1 and type 2 diabetes. Associations were controlled for calendar year, sex, singleton status, parity, maternal smoking, maternal education, maternal cohabitation, maternal residence at birth, maternal history of CVD before childbirth, paternal history of CVD before birth of the child, and maternal age (restricted cubic spline with five knots at five evenly spaced centiles). Arrows indicate a lower limit of <0.8
We found a higher incidence of CVD in offspring of mothers with both diabetes and comorbid CVD (1.73 (1.36 to 2.20)) than in offspring of mothers with diabetes only (1.29 (1.21 to 1.37)). This increased incidence was due to the added influence of comorbid CVD but not their interaction on the multiplicative scale (P value for interaction 0.94; table 2). Offspring of mothers with pregestational diabetes and diabetic complications had a higher incidence of CVD (1.60 (1.25 to 2.05)) than the offspring of mothers with pregestational diabetes but no diabetic complications (1.31 (1.16 to 1.48); table 3), representing a 22% increased rate (1.22 (0.92 to 1.62)) due to diabetic complications. Our study population for this analysis was young (up to 21 years of age with a median age of 11), and only a limited number of CVD events (n=62) in offspring of mothers with diabetes with complications were available for this analysis.
Table 2.
Joint effect of maternal diabetes and maternal CVD history before childbirth on early CVD onset in offspring
| Exposure | No of offspring with CVD | Rate per 1000 person years | Hazard ratio (95% CI), model 1 | Hazard ratio (95% CI), model 2 |
|---|---|---|---|---|
| No maternal diabetes and CVD | 89 372 | 2.00 | 1.0 (ref) | 1.0 (ref) |
| Maternal diabetes only | 1086 | 1.99 | 1.45 (1.37 to 1.54) | 1.29 (1.21 to 1.37) |
| Maternal CVD only | 1939 | 2.23 | 1.41 (1.35 to 1.48) | 1.33 (1.27 to 1.39) |
| Joint effect of maternal diabetes and CVD | 67 | 2.46 | 2.10 (1.65 to 2.67) | 1.73 (1.36 to 2.20) |
| Maternal diabetes×CVD | — | — | 1.02 (0.80 to 1.31) | 1.01 (0.79 to 1.30) |
| P value for interaction (maternal diabetes×CVD) | — | — | 0.86 | 0.94 |
CVD=cardiovascular disease; model 1=offspring’s age as time scale; model 2=offspring’s age as time scale, and controlled for calendar year, sex, singleton status, parity, maternal smoking, maternal education, maternal cohabitation, maternal residence at birth, maternal history of CVD before childbirth, paternal history of CVD before birth of the child, and maternal age (restricted cubic spline with five knots at five evenly spaced centiles).
Table 3.
Associations between maternal pregestational diabetes with or without diabetic complications and early onset of CVD in offspring born during 1996-2016*
| Exposure | No of offspring with CVD | Rate per 1000 person years | Hazard ratio (95% CI), model 1 | Hazard ratio (95% CI), model 2 |
|---|---|---|---|---|
| No pregestational diabetes | 16 251 | 1.22 | 1.0 (ref) | 1.0 (ref) |
| Pregestational diabetes without diabetic complications | 266 | 1.64 | 1.38 (1.23 to 1.56) | 1.31 (1.16 to 1.48) |
| Pregestational diabetes with diabetic complications | 62 | 1.99 | 1.67 (1.30 to 2.14) | 1.60 (1.25 to 2.05) |
| 1 complication | 24 | 1.90 | 1.59 (1.07 to 2.37) | 1.55 (1.04 to 2.32) |
| ≥2 complications | 38 | 2.05 | 1.73 (1.25 to 2.37) | 1.63 (1.18 to 2.24) |
| Pregestational diabetes with diabetic complication v without diabetic complication | — | — | 1.21 (0.91 to 1.59) | 1.22 (0.92 to 1.62) |
CVD, cardiovascular disease; HR, hazard ratio; ICD-10=international classification of disease, 10th revision; model 1=offspring’s age as time scale; model 2=offspring’s age as time scale, and controlled for calendar year, sex, singleton status, parity, maternal smoking, maternal education, maternal cohabitation, maternal residence at birth, maternal history of CVD before childbirth, paternal history of CVD before birth of the child, and maternal age (restricted cubic spline with five knots at five evenly spaced centiles).
Pregestational diabetic complications: ICD-10 codes (E10.0–E10.8, E11.0–E11.8, and H36.0). Women with pregestational diabetic complications were classified as having one or multiple complications (ICD-10: E10.7, E11.7; ≥2 complications as provided above)
Sensitivity analysis yielded similar findings to those of the main analyses (tables S4-6), including use of hierarchical ordering of diabetes types (type 1, type 2, and gestational diabetes), and restriction to offspring of mothers diagnosed with only one diabetes type during their pregnancy; or offspring born at term. The results from sibship design in the sibling cohort (1.26 (1.18 to 1.35)) were similar to those of the main analysis in the unmatched whole population cohort. Moreover, for the timing of the diagnosis of maternal type 1 diabetes, the association between maternal type 1 diabetes and CVD was strongest when mothers were diagnosed before childbirth (1.35 (1.23 to 1.48)). The association was attenuated with greater time to diagnosis after birth (table S7). The pattern was similar when type 1 and type 2 diabetes were combined. The results were essentially unchanged when we controlled for maternal country of origin, maternal body mass index before pregnancy, and paternal diabetes before the birth of the child, either separately or together in the model (table S8). In addition, paternal diabetes remained weakly associated with early onset CVD in offspring (table S9).
In our evaluation of live birth bias, the inverse-probability-weighting approach yielded results similar to those of the primary analyses, showing that maternal diabetes increased the rate of early onset CVD in offspring (table S10). The analysis stratified by sex of the offspring showed similar results. Results from separate analyses restricted to singletons, offspring of primiparous women, individuals with complete data, and offspring born after 1991, 1994, or 2004, and using the missing indicator method, were similar to those obtained in the primary analyses (table S11).
Discussion
In this large population based cohort study, we found that maternal diabetes before or during pregnancy was associated with increased rates of early onset CVD in general, and with most specific types of early onset CVD in offspring, persisting from childhood through early adulthood. The strongest associations were found among offspring of mothers with diabetic complications or with diabetes and a history of CVD.
Comparisons with other studies
During pregnancies complicated by diabetes, large amounts of maternal glucose freely cross the placenta, which could lead to increased secretion of fetal insulin.15 This increase would result in a state of hyperinsulinaemia and hyperglycaemia in the fetal circulation.15 Exposure to hyperinsulinaemia and hyperglycaemia in utero could have longlasting effects on fetal vascular gene expression and result in changes in vascular function, thereby contributing to higher CVD risks in offspring.15 32 The results of animal studies33 34 indicated abnormalities in vascular reactivity and increased risks of hypertension and cardiovascular dysfunction in offspring of diabetic rats. Several human studies have shown that the diabetic intrauterine environment could have a programming effect on fetal vascular dysfunction, leading to a poor CVD risk profile after birth.15 32 This profile would include increased concentrations of markers of endothelial dysfunction, macrosomia, increased aortic intima-media thickness, and arterial stiffening.15 32 Poor glycaemic control in pregnant women could result in more unhealthy fetuses with more adverse birth outcomes,15 35 36 37 which are risk factors for future CVD.38 In addition, offspring of mothers with diabetes tend to be at increased risk of exposure to many classic cardiovascular risk factors, such as hypercholesterolaemia, hypertension, obesity, diabetes, chronic kidney disease, and metabolic syndrome.9 10 15 32 37 39 40 41 Moreover, factors such as genetic susceptibility, family environment variables, or lifestyle characteristics might also contribute to the increased risk of CVD in children born to mother with diabetes.15 37
Empirical evidence is lacking for an association of maternal diabetes during pregnancy with the overall risk of early onset CVD in offspring, with the exception of an increased risk of congenital heart diseases.18 42 One study reported that the risk of congenital heart disease was higher among offspring exposed to pregestational diabetes than among those exposed to gestational diabetes.18 A Swedish study found that pregestational and gestational diabetes were associated with congenital heart defects.42 In our large study, we found increased rates of early onset CVD in offspring prenatally exposed to three types of maternal diabetes (pregestational type 1 and type 2, and gestational diabetes). The variation in the magnitudes of the estimated effect size might be due to the different mechanisms underlying different types of diabetes.15 43 The similarity in disease risk due to maternal type 1 versus type 2 diabetes for early onset CVD and congenital heart disease, respectively, suggests a shared pathological process between both types of diabetes in fetal heart development.18 43 The higher CVD rate in offspring of those with pregestational diabetes, compared with offspring of mothers with gestational diabetes, suggests that a high glucose level in early pregnancy has a major role in CVD development in offspring.18 44 45 The difference in associations found between maternal diabetes and specific types of CVD could stem from different underlying mechanisms for CVD.46 47
Notably, an almost twofold risk of higher early onset of CVD was seen in offspring of mothers with diabetes and a history of CVD, compared with the offspring of mothers with no such history. The greater effect of coexisting maternal CVD and maternal diabetes on CVD risk in offspring requires additional research to examine the burden of multimorbidity during pregnancy.
Higher insulin resistance is also associated with an increased risk of pregestational diabetic complications.48 Therefore, diabetic complications due to poor glycaemic control might reflect the severity of pregestational diabetes. Our observation suggests that children of mothers with diabetic complications have a higher risk of very early onset CVD. This result is consistent with earlier studies that found a higher risk of genital anomalies and congenital heart diseases in offspring of mothers with diabetic complications.18 20 Since the diagnostic code for diabetic complications was available only in ICD-10 (since 1994), our study population was relatively too young for enough CVD events to have occurred for this analysis. Further follow-up and investigation in other study settings would be valuable to confirm these findings.
Strengths and limitations of this study
The main strength of our study is the high quality, prospectively collected data covering all Danish residents with nearly complete follow-up, thus minimising the possibility of recall or selection bias. The large sample size and long follow-up of up to 40 years provide an opportunity to investigate specific types of CVD and to evaluate the long term consequences of maternal diabetes for offspring of different ages. In addition, we used a sibship design to evaluate the influence of uncontrolled confounding due to shared familial (genetic or environmental) characteristics. Such (unmeasured) characteristics are often difficult to control in a conventional cohort study. We could also adjust for a wide range of covariates, such as maternal sociodemographic and lifestyle variables, and history of CVD. Previous studies have reported that maternal diabetes could increase the risks of preterm birth, pre-eclampsia, and macrosomia15 35 36 37; therefore, these factors could lie in the pathway from maternal diabetes during pregnancy to early onset CVD in offspring. Thus, we considered these variables to be primarily potential mediators, and did not adjust for these factors in the main analysis, as reported in the main text. Nevertheless, for explorative purposes, in an additional analysis, we did adjust for maternal pre-eclampsia, preterm birth, and large for gestational age, and found that only slightly attenuated associations were seen, compared with the main analysis (table S11).
Several limitations must also be noted. Firstly, although we adjusted for a wide range of confounders, we could not rule out the possibility of residual confounding by some uncontrolled genetic or familial environment or lifestyle characteristics, such as maternal alcohol consumption, glycaemic control, folic acid supplementation, prenatal care, physical inactivity, psychological stress, offspring smoking, and genetic variants, in fetal susceptibility to the diabetic intrauterine environment. Residual confounding of unmeasured factors may partially account for the association between maternal diabetes and early onset CVD in offspring. However, our sibship design yielded results similar to those of the unpaired design based on the whole cohort. We have to acknowledge that, although the sibship design has a number of methodological strengths, it also has several drawbacks.49 50 51 For example, compared with an unpaired design (which was also used in our study), the sibship design may lead to bias in estimating exposure effects if carryover or spillover effects are present.50 51 In addition, a sibship design cannot adequately control for unmeasured confounders that change across pregnancies. Moreover, the sibship design restricts the analysis to women who have had at least two pregnancies, leading to loss of generalisability to the target population. Thus, the sibship design could not fully rule out the possibility of uncontrolled confounding, possibly requiring bias analysis.49 50 51 52 The strongest associations were for offspring born to mothers with a diagnosis of diabetes before childbirth. This association was attenuated when maternal diabetes was diagnosed after childbirth. These findings suggest that non-genetic effects are important determinants of early onset CVD in offspring, and that the observed associations are not entirely attributable to confounding by genetics and familial environment. In addition, the considerably greater influence of maternal diabetes, compared with paternal diabetes, on the risk of CVD in offspring, further suggests that our findings are unlikely to be attributable entirely to uncontrolled confounding.
Secondly, there is a possibility of live birth selection bias, which we assessed using information available on stillbirths. Our sensitivity analyses provided reassurance that restricting our sample to live births was unlikely to affect the observed associations appreciably.
Thirdly, potential misclassification bias might remain, because the same code (ICD-8:250) was used to record type 1 and type 2 diabetes before 1986.18 However, the ascertainment and verification of diabetes in Denmark are considered highly reliable, implying that misclassification of maternal diabetes overall is unlikely.53 A validation study that examined cardiovascular diagnoses in the DNPR in 2010-2012 indicated that cardiovascular diagnoses were of overall high quality and adequate for epidemiological research.54 Although we could not rule out misclassification bias, we think that it would probably be non-differential, would tend to attenuate our estimates, and would not change our conclusion substantially.
Finally, our study could not examine the risk of CVD offspring in late adulthood because the longest follow-up period so far is 40 years. The maximum follow-up period for CVD events in offspring of mothers with diabetic complications was only 21 years, which might prevent us from obtaining stable risk estimates for some subgroup analyses. However, this is the best available evidence and we expect the associations to be even stronger when older offspring can be studied. Future studies with longer follow-up are well warranted.
Conclusion and policy implications
The diabetic intrauterine environment could have a programming effect on the development of CVD in offspring. Our study provides evidence that children of mothers with diabetes, especially those with a history of CVD or with diabetic complications, had increased rates of early onset CVD throughout the early decades of life. These findings highlight the importance of effective strategies for screening and preventing diabetes in women of childbearing age. We need to monitor CVD risks in offspring of mothers with diabetes and investigate possible life course interventions that could reduce the occurrence of CVD. A history of CVD or diabetic complications in women with diabetes should be taken into account in designing public health strategies that target offspring at increased risk of early onset CVD. Future research should examine the degree of glycaemic control during pregnancy that would minimise the risk of CVD in offspring throughout their life.
What is already known on this topic
The prevalence of cardiovascular disease (CVD) has increased in children and young adults in recent decades
Maternal diabetes before or during pregnancy is associated with increased risks of metabolic syndrome and congenital heart disease in offspring
Whether prenatal exposure to maternal diabetes affects early onset CVD in offspring during their early decades of life is not known
What this study adds
Maternal diabetes during pregnancy was found to be associated with an increased rate of early onset CVD among offspring across the first four decades of life, especially for the offspring of those mothers with a history of CVD or diabetic complications
Preventing, screening, and treating diabetes in women of childbearing age could be important not only for improving the health of the women but also for reducing long term risks of CVD in their offspring
Web extra.
Extra material supplied by authors
Web appendix: Supplementary material
Contributors: GQ (gyqin@fudan.edu.cn) and JL (jl@clin.au.dk) are corresponding authors and senior authors who contributed equally to this study. YY, GQ, and JL have full access to all the data in this study and take full responsibility as guarantors for the integrity of the data and the accuracy of the data analysis. YY, OAA, GQ, and JL conceived and designed the study. YY, OAA, and GQ undertook the statistical analysis. YY drafted the manuscript. All authors provided critical input to the analyses, interpreted the data, and revised the manuscript critically. The corresponding author confirms that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was supported by unrestricted grants from the Lundbeck Foundation (R232-2016-2462 and R265-2017-4069) to YY; grants from the Danish Council for Independent Research and Independent Research Fund Denmark (DFF-6110-00019 and 9039-00010) to JL; grants from the Nordic Cancer Union (176673, 186200, and R217-A13234-18-S65) to JL; a grant from the Karen Elise Jensens Fond (2016) to JL; a grant from Novo Nordisk Foundation (NNF18OC0052029) to JL; a grant from the Danish Centre for Strategic Research in Type 2 Diabetes Project (DD2) and the Program for Clinical Research Infrastructure (PROCRIN) established by the Lundbeck Foundation and the Novo Nordisk Foundation to HTS, a grant from the National Natural Science Foundation of China (11871164) to GQ, an NIH grant from the National Center for Advancing Translational Science (UL1TR001881) to OAA, and a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD072296) to OAA. The investigators conducted the research independently. The funders had no role in study design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: support from the Lundbeck Foundation, the Danish Council for Independent Research and Independent Research Fund Denmark, the Nordic Cancer Union, the Karen Elise Jensens Fond, Novo Nordisk Foundation, the Danish Centre for Strategic Research in Type 2 Diabetes Project (DD2) and the Program for Clinical Research Infrastructure (PROCRIN) established by the Lundbeck Foundation and the Novo Nordisk Foundation, the National Natural Science Foundation of China, NIH grant from the National Center for Advancing Translational Science, the Eunice Kennedy Shriver National Institute of Child Health and Human Development for the submitted work; no financial relationships with any organisations that might have an interest in the submitted work in the previous three years, no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: The study was approved by the Data Protection Agency (record number 2013-41-2569). By Danish law, no informed consent is required for a register based study of anonymised data.
Data sharing: No additional data available.
The lead authors (the manuscript’s guarantors) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
References
- 1. McClellan M, Brown N, Califf RM, Warner JJ. Call to action: urgent challenges in cardiovascular disease: a presidential advisory from the American Heart Association. Circulation 2019;139:e44-54. 10.1161/CIR.0000000000000652. [DOI] [PubMed] [Google Scholar]
- 2. Timmis A, Townsend N, Gale C, et al. ESC Scientific Document Group European Society of Cardiology: cardiovascular disease statistics 2017. Eur Heart J 2018;39:508-79. 10.1093/eurheartj/ehx628. [DOI] [PubMed] [Google Scholar]
- 3. Schmidt M, Jacobsen JB, Lash TL, Bøtker HE, Sørensen HT. 25 year trends in first time hospitalisation for acute myocardial infarction, subsequent short and long term mortality, and the prognostic impact of sex and comorbidity: a Danish nationwide cohort study. BMJ 2012;344:e356. 10.1136/bmj.e356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ford ES, Ajani UA, Croft JB, et al. Explaining the decrease in U.S. deaths from coronary disease, 1980-2000. N Engl J Med 2007;356:2388-98. 10.1056/NEJMsa053935. [DOI] [PubMed] [Google Scholar]
- 5. George MG, Tong X, Kuklina EV, Labarthe DR. Trends in stroke hospitalizations and associated risk factors among children and young adults, 1995-2008. Ann Neurol 2011;70:713-21. 10.1002/ana.22539. [DOI] [PubMed] [Google Scholar]
- 6. George MG, Tong X, Bowman BA. Prevalence of cardiovascular risk factors and strokes in younger adults. JAMA Neurol 2017;74:695-703. 10.1001/jamaneurol.2017.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jousilahti P, Vartiainen E, Tuomilehto J, Puska P. Sex, age, cardiovascular risk factors, and coronary heart disease: a prospective follow-up study of 14 786 middle-aged men and women in Finland. Circulation 1999;99:1165-72. 10.1161/01.CIR.99.9.1165. [DOI] [PubMed] [Google Scholar]
- 8. Barker DJ. Fetal origins of coronary heart disease. BMJ 1995;311:171-4. 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. West NA, Crume TL, Maligie MA, Dabelea D. Cardiovascular risk factors in children exposed to maternal diabetes in utero. Diabetologia 2011;54:504-7. 10.1007/s00125-010-2008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lawlor DA, Lichtenstein P, Långström N. Association of maternal diabetes mellitus in pregnancy with offspring adiposity into early adulthood: sibling study in a prospective cohort of 280,866 men from 248,293 families. Circulation 2011;123:258-65. 10.1161/CIRCULATIONAHA.110.980169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reynolds RM, Allan KM, Raja EA, et al. Maternal obesity during pregnancy and premature mortality from cardiovascular event in adult offspring: follow-up of 1 323 275 person years. BMJ 2013;347:f4539. 10.1136/bmj.f4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bateson P, Barker D, Clutton-Brock T, et al. Developmental plasticity and human health. Nature 2004;430:419-21. 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- 13. Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 2008;359:61-73. 10.1056/NEJMra0708473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marmot M, Allen J, Bell R, Bloomer E, Goldblatt P, Consortium for the European Review of Social Determinants of Health and the Health Divide WHO European review of social determinants of health and the health divide. Lancet 2012;380:1011-29. 10.1016/S0140-6736(12)61228-8. [DOI] [PubMed] [Google Scholar]
- 15. McCance D, Maresh M, Sacks DA. A practical manual of diabetes in pregnancy. John Wiley & Sons, 2017. 10.1002/9781119043805. [DOI] [Google Scholar]
- 16. Ferrara A. Increasing prevalence of gestational diabetes mellitus: a public health perspective. Diabetes Care 2007;30(Suppl 2):S141-6. 10.2337/dc07-s206. [DOI] [PubMed] [Google Scholar]
- 17. Liu X, Agerbo E, Li J, et al. Maternal pregestational or gestational diabetes and childhood wheezing: a population-based cohort study. Allergy 2018;73:2247-50. 10.1111/all.13551. [DOI] [PubMed] [Google Scholar]
- 18. Øyen N, Diaz LJ, Leirgul E, et al. Prepregnancy diabetes and offspring risk of congenital heart disease: a nationwide cohort study. Circulation 2016;133:2243-53. 10.1161/CIRCULATIONAHA.115.017465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wicklow BA, Sellers EAC, Sharma AK, et al. Association of gestational diabetes and type 2 diabetes exposure in utero with the development of type 2 diabetes in first nations and non-first nations offspring. JAMA Pediatr 2018;172:724-31. 10.1001/jamapediatrics.2018.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Arendt LH, Lindhard MS, Henriksen TB, et al. Maternal diabetes mellitus and genital anomalies in male offspring: a nationwide cohort study in 2 Nordic countries. Epidemiology 2018;29:280-9. 10.1097/EDE.0000000000000781. [DOI] [PubMed] [Google Scholar]
- 21. Bunt JC, Tataranni PA, Salbe AD. Intrauterine exposure to diabetes is a determinant of hemoglobin A(1)c and systolic blood pressure in pima Indian children. J Clin Endocrinol Metab 2005;90:3225-9. 10.1210/jc.2005-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics 2005;115:e290-6. 10.1542/peds.2004-1808. [DOI] [PubMed] [Google Scholar]
- 23. Schmidt M, Schmidt SAJ, Adelborg K, et al. The Danish health care system and epidemiological research: from health care contacts to database records. Clin Epidemiol 2019;11:563-91. 10.2147/CLEP.S179083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377-99. 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 25. Austin PC, Lee DS, Fine JP. Introduction to the analysis of survival data in the presence of competing risks. Circulation 2016;133:601-9. 10.1161/CIRCULATIONAHA.115.017719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Coviello V, Boggess M. Cumulative incidence estimation in the presence of competing risks. Stata J 2004;4:103-12 10.1177/1536867X0400400201. [DOI] [Google Scholar]
- 27. Sato T, Matsuyama Y. Marginal structural models as a tool for standardization. Epidemiology 2003;14:680-6. 10.1097/01.EDE.0000081989.82616.7d. [DOI] [PubMed] [Google Scholar]
- 28. Yu Y, Cnattingius S, Olsen J, et al. Prenatal maternal bereavement and mortality in the first decades of life: a nationwide cohort study from Denmark and Sweden. Psychol Med 2017;47:389-400. 10.1017/S003329171600266X. [DOI] [PubMed] [Google Scholar]
- 29. Cnattingius S, Kramer MS, Norman M, Ludvigsson JF, Fang F, Lu D. Keep it in the family: comparing perinatal risks in small-for-gestational-age infants based on population vs within-sibling designs. Int J Epidemiol 2019;48:297-306. 10.1093/ije/dyy196. [DOI] [PubMed] [Google Scholar]
- 30. Thompson CA, Arah OA. Selection bias modeling using observed data augmented with imputed record-level probabilities. Ann Epidemiol 2014;24:747-53. 10.1016/j.annepidem.2014.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liew Z, Olsen J, Cui X, Ritz B, Arah OA. Bias from conditioning on live birth in pregnancy cohorts: an illustration based on neurodevelopment in children after prenatal exposure to organic pollutants. Int J Epidemiol 2015;44:345-54. 10.1093/ije/dyu249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sallam NA, Palmgren VAC, Singh RD, John CM, Thompson JA. Programming of vascular dysfunction in the intrauterine milieu of diabetic pregnancies. Int J Mol Sci 2018;19:E3665. 10.3390/ijms19113665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Holemans K, Gerber RT, Meurrens K, De Clerck F, Poston L, Van Assche FA. Streptozotocin diabetes in the pregnant rat induces cardiovascular dysfunction in adult offspring. Diabetologia 1999;42:81-9. 10.1007/s001250051117. [DOI] [PubMed] [Google Scholar]
- 34. Dib A, Payen C, Bourreau J, et al. In utero exposure to maternal diabetes is associated with early abnormal vascular structure in offspring. Front Physiol 2018;9:350. 10.3389/fphys.2018.00350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Metzger BE, Lowe LP, Dyer AR, et al. HAPO Study Cooperative Research Group Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991-2002. 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 36. Catalano PM, McIntyre HD, Cruickshank JK, et al. HAPO Study Cooperative Research Group The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care 2012;35:780-6. 10.2337/dc11-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen L, Wang WJ, Auger N, et al. Diabetes in pregnancy in associations with perinatal and postneonatal mortality in First Nations and non-indigenous populations in Quebec, Canada: population-based linked birth cohort study. BMJ Open 2019;9:e025084. 10.1136/bmjopen-2018-025084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barker DJ. Fetal programming of coronary heart disease. Trends Endocrinol Metab 2002;13:364-8. 10.1016/S1043-2760(02)00689-6 [DOI] [PubMed] [Google Scholar]
- 39. Clausen TD, Mathiesen ER, Hansen T, et al. Overweight and the metabolic syndrome in adult offspring of women with diet-treated gestational diabetes mellitus or type 1 diabetes. J Clin Endocrinol Metab 2009;94:2464-70. 10.1210/jc.2009-0305. [DOI] [PubMed] [Google Scholar]
- 40. Nilsson P. Increased weight and blood pressure in adolescent male offspring to mothers with pre-pregnancy diabetes—a genetic link? Nature Publishing Group, 1999. 10.1038/sj.jhh.1000819. [DOI] [PubMed] [Google Scholar]
- 41. Krishnaveni GV, Veena SR, Jones A, et al. Exposure to maternal gestational diabetes is associated with higher cardiovascular responses to stress in adolescent indians. J Clin Endocrinol Metab 2015;100:986-93. 10.1210/jc.2014-3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Persson M, Razaz N, Edstedt Bonamy AK, Villamor E, Cnattingius S. Maternal overweight and obesity and risk of congenital heart defects. J Am Coll Cardiol 2019;73:44-53. 10.1016/j.jacc.2018.10.050. [DOI] [PubMed] [Google Scholar]
- 43. American Diabetes Association Diagnosis and classification of diabetes mellitus. Diabetes Care 2014;37(Suppl 1):S81-90. 10.2337/dc14-S081 [DOI] [PubMed] [Google Scholar]
- 44. Nakano H, Minami I, Braas D, et al. Glucose inhibits cardiac muscle maturation through nucleotide biosynthesis. Elife 2017;6:e29330. 10.7554/eLife.29330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Moazzen H, Lu X, Ma NL, et al. N-Acetylcysteine prevents congenital heart defects induced by pregestational diabetes. Cardiovasc Diabetol 2014;13:46. 10.1186/1475-2840-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Soler EP, Ruiz VC. Epidemiology and risk factors of cerebral ischemia and ischemic heart diseases: similarities and differences. Curr Cardiol Rev 2010;6:138-49. 10.2174/157340310791658785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mendis S, Puska P, Norrving B, et al. Global atlas on cardiovascular disease prevention and control. World Health Organization, 2011. [Google Scholar]
- 48. Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: “double diabetes” in the Diabetes Control and Complications Trial. Diabetes Care 2007;30:707-12. 10.2337/dc06-1982. [DOI] [PubMed] [Google Scholar]
- 49. Hutcheon JA, Harper S. Invited commentary: promise and pitfalls of the sibling comparison design in studies of optimal birth spacing. Am J Epidemiol 2019;188:17-21. 10.1093/aje/kwy195. [DOI] [PubMed] [Google Scholar]
- 50. Frisell T, Öberg S, Kuja-Halkola R, Sjölander A. Sibling comparison designs: bias from non-shared confounders and measurement error. Epidemiology 2012;23:713-20. 10.1097/EDE.0b013e31825fa230. [DOI] [PubMed] [Google Scholar]
- 51. Sjölander A, Frisell T, Kuja-Halkola R, Öberg S, Zetterqvist J. Carryover effects in sibling comparison designs. Epidemiology 2016;27:852-8. 10.1097/EDE.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 52. Arah OA. Bias analysis for uncontrolled confounding in the health sciences. Annu Rev Public Health 2017;38:23-38. 10.1146/annurev-publhealth-032315-021644. [DOI] [PubMed] [Google Scholar]
- 53. Green A, Sortsø C, Jensen PB, Emneus M. Validation of the Danish National Diabetes Register. Clin Epidemiol 2014;7:5-15. 10.2147/CLEP.S72768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sundbøll J, Adelborg K, Munch T, et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open 2016;6:e012832. 10.1136/bmjopen-2016-012832. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix: Supplementary material