Abstract
Immune checkpoints and agonists modulate ongoing, antigen-specific immune responses. Therapeutic blockade of CTLA-4, PD-1, and PD-L1 has proven to be an effective treatment approach for a subset of patients with a variety of cancers of epithelial, mesenchymal, or hematologic origin. In multiple myeloma, a B-cell lymphoid malignancy of terminally differentiated plasma cells, PD-1 pathway blockade is ineffective as a single agent. The initial promise in combination approaches utilizing anti–PD-1 with the immunomodulatory drugs, lenalidomide or pomalidomide, was not confirmed in randomized trials. Here, we explore available data for and against manipulation of the PD-1 pathway and other immune checkpoints in myeloma and highlight several promising concepts and challenges that face ongoing development of immunotherapeutics for this disease.
Keywords: Multiple Myeloma, Checkpoint Blockade, Immunotherapy, PD-1, CTLA-4
Introduction
A broad range of infectious challenges can be eradicated by the immune system and elicit personalized long-term memory for future protection of the individual from re-challenge. Tumor immunologists have sought to translate observations from anti-microbial immune responses into therapies that elicit similar responses against self-derived cancer cells and that lead to long-lasting disease control. A recent focus on mechanisms of tolerance mediated by immune checkpoints has transformed cancer immunotherapy. The 2018 Nobel Prize in medicine was awarded to James Allison for his discovery of the mechanism of action of CTLA-4 (the prototypical immune checkpoint) and to Tasuku Honjo for his discovery of the PD-1 receptor. Rapid clinical investigation focused on targeting immune checkpoints, especially with PD-1 pathway inhibitors, has revealed significant clinical activity in patients with various cancer types (1). Further combinatorial approaches built on knowledge of the human immune response, that have been derived from basic research, will continue to expand the benefits of immunotherapy to a growing number of cancer patients. However, experience with checkpoint blockade in multiple myeloma (MM) has revealed some of the challenges of investigating immune modulators in a cancer with immune cell origin. Here, lessons from combination therapy approaches utilizing immune checkpoint blockade (ICB), immunomodulatory drugs (IMiDs), and monoclonal antibodies will be reviewed.
Immune checkpoints and cancer therapy
T-cell receptor (TCR) engagement with a specific peptide presented by MHC on antigen presenting cells (APCs) licenses T-cell activation. Interactions with additional signals, known as costimulation, shape the resulting effector and memory response. The classic costimulatory receptor on T cells is CD28, which engages B7 family members on APCs. This results in activation, proliferation, and secretion of cytokines. Activating costimulatory receptors on the T-cell surface such as ICOS, 4-1BB, or OX40 and inhibitory receptors such as CTLA-4, PD-1, or LAG-3 act to further augment or diminish T-cell responses (2). The inhibitory receptors, also known as immune checkpoints, are thought to mediate peripheral tolerance to antigens.
Initial studies of anti–CTLA4 revealed unique toxicities and novel response kinetics, including durable responses in sensitive patients. Key biomarker studies in solid tumor malignancies treated with ICB have lent mechanistic insight into how these agents work in the clinic. Analysis of baseline tumor-intrinsic factors (tumor mutation burden, PD-L1 expression, tumor antigen presentation machinery, and IFNγ responsiveness), immune microenvironment factors (tumor immune infiltrate – “cold” vs. “hot” tumors), and host factors (microbiome, FCγR polymorphisms, and HLA haplotype) can predict sensitivity to ICB (extensively reviewed in (3)). However, it remains to be seen if these factors can be evaluated in a coordinated fashion to guide treatment decisions in patients.
ICB therapies in hematologic malignancies have also shown promise, especially in classical Hodgkin lymphoma (cHL). PD-1 blockade with nivolumab or pembrolizumab has been approved by the FDA for treatment of relapsed cHL based on high response rates(4). Tumor intrinsic PD-L1/L2 upregulation via gene amplification of the 9p24.1 locus in cHL may be a potential driver of sensitivity to PD-1 pathway blockade. MHC II expression on Reed-Sternberg (RS) cells also correlates with survival in cHL suggesting CD4+ T-cell activity is important (5). However, antigen presentation is often absent on RS cells due to mutations in β2-microglobulin (β2M) or CIITA translocations, which limit expression of MHC I or II, respectively (4). Other non-Hodgkin’s lymphoma subtypes, such as primary mediastinal B-cell lymphoma (PMBL), testicular lymphoma, and CNS lymphoma, have demonstrated evidence of PD-L1/L2 expression driven by 9p24.1 gene amplification and appear to also be sensitive to PD-1 blockade (4). Hints of clinical activity for single-agent PD-1 blockade in several non-Hodgkin’s lymphoma subtypes and in a few combinatorial strategies in acute myeloid leukemia have been seen, but limited activity has been a more common observation, and in some T-cell malignancies, increased disease progression raises the possibility that PD-1 blockade may be harmful (6-8). These clinical and correlative observations highlight major differences in the cell of origin of the tumor and the tumor microenvironment that distinguish treatment response in hematologic cancers from solid tumors.
The current challenge is how to incorporate ICB into immunologically sound combination therapies in a disease- and patient-specific manner. There are currently more than 1000 trials evaluating ICB in combination with traditional chemotherapy, tumor microenvironment–directed agents, or cellular therapies across all cancers. A variety of combination approaches in MM have also been pursued (Table 1, Supplementary Table S1).
Table 1:
Selected Ongoing Studies Utilizing Immune Checkpoint Blockade
| NCI Identifier | Trial title | Ph | Status | Patient cohort |
|---|---|---|---|---|
| An Investigational Immuno-Therapy Study to Determine the Safety and Effectiveness of Nivolumab and Daratumumab in Patients With Multiple Myeloma | I | Open | relapsed | |
| A Study of Atezolizumab (Anti-Programmed Death-Ligand 1 [PD-L1] Antibody) Alone or in Combination With an Immunomodulatory Drug and/or Daratumumab in Participants With Multiple Myeloma (MM) | Ib | Open | relapsed | |
| Pembrolizumab and Radiation Therapy in Patients With Relapsed or Refractory Multiple Myeloma | I | Open | relapsed | |
| NY-ESO-1c259T Alone and in Combination With Pembrolizumab for Multiple Myeloma | II | Open | relapsed | |
| ASCT With Nivolumab in Patients With Multiple Myeloma | I/II | Open | newly diagnosed or relapsed | |
| Dexamethasone, Carfilzomib, & Nivolumab With Reovirus for Relapsed/Refractory Multiple Myeloma | I | Open | relapsed | |
| Nivolumab Combined With Daratumumab With or Without Low-dose Cyclophosphamide | II | Open | relapsed | |
| Radiotherapy With Immunotherapy for Systemic Effect in Myeloma (RISE-M) (RISE-M) | II | Open | relapsed | |
| Check Point Inhibition After Autologous Stem Cell Transplantation in Patients at High Risk of Post Transplant Recurrence (CPIT001) | I/II | Open | relapsed | |
| A Study of Cobimetinib Administered as Single Agent and in Combination With Venetoclax, With or Without Atezolizumab, in Participants With Relapsed and Refractory Multiple Myeloma | I/II | Open | relapsed | |
| Study of Single Agent CJM112, and PDR001 in Combination With LCL161 or CJM112 in Patients With Multiple Myeloma | I | Open | relapsed | |
| Isatuximab in Combination With Cemiplimab in Relapsed/Refractory Multiple Myeloma (RRMM) Patients | I/II | Open | relapsed | |
| Lenalidomide and Nivolumab in Treating Patients With Relapsed or Refractory Multiple Myeloma | II | Open | relapsed | |
| Dendritic Cell (DC)/Myeloma Fusions in Combination With Nivolumab in Patients With Relapsed Multiple Myeloma | II | Pending | relapsed |
Immune pathogenesis and immune surveillance in myeloma
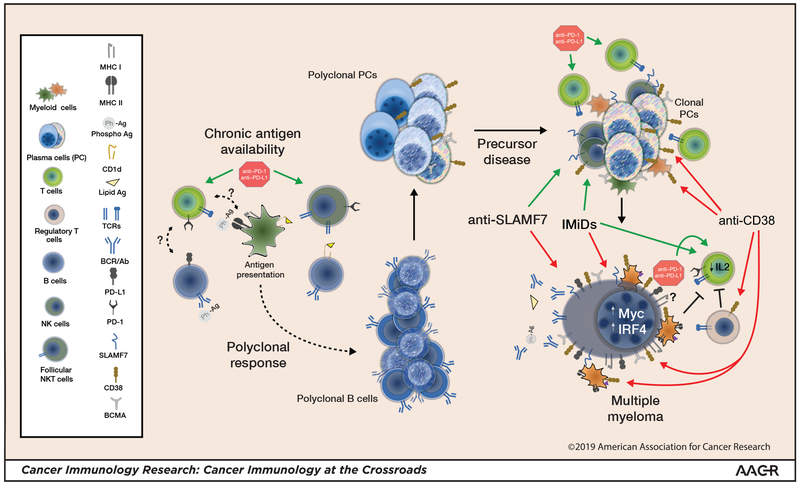
Chronic immune stimuli can potentially be a contributing driver of MM pathogenesis (Fig. 1). Development of plasma cell disorders has been linked with autoimmune disorders (9), obesity (10), and antibody response against phosphorylated self-antigens. Analysis of paraprotein reactivity in patients with monoclonal gammopathy of unknown significance(MGUS)/MM identified that 4%, 15%, and 37% of individuals of Japanese, European, and African American descent, respectively, had reactivity to a hyper-phosphorylated version of SLP-2, the most common of these phospho-antigens (11). A group of investigators also found that long-term stimulation of lipid-reactive NKT cells may be involved in the pathogenesis of one-third of sporadic MGUS and MM (12).
Figure 1: Myelomagenesis, myeloma, and modifying the immune response.
Lipid antigen or phosphoantigen (Ph-Ag)–reactive B cells give rise to phosphoantigen- and/or lipid antigen–reactive plasma cells. Follicular NKT cells, which are typically PD-1high , and possibly other helper T cells play a role. Ongoing chronic stimulation increases the potential for an emerging clonal myeloma precursor. T cell- and NK cell–mediated immune surveillance may limit myeloma progression. Frank progression to myeloma is associated with diminished T-cell and NK cell function and expansion of Tregs and myeloid-derived suppressor cells (MDSCs). IMiDs and mAbs lead to malignant plasma cell killing, which releases antigen and concurrently diminishes myeloma-driven suppressive mechanisms. Antigen release in the proper inflammatory milieu may elicit or expand an effector immune response. Anti–PD-1 could have pleiotropic effects, affecting both the effector response and follicular helper cell–mediated B-cell maturation. Combination strategies to shift the immune response toward an effector response could yield greatest clinical benefit. Green arrows: activation; Red arrows: inhibition; Blunted black lines: inhibition; (?): unknown effect.
The malignant plasma cells found in MM and MGUS are genetically similar, supporting the notion that evolution to MM may require escape from immunosurveillance. Robust T cell immune responses to MM antigens are detectable in the bone marrow of MGUS, but not MM patients (13). Loss of immunity to the embryonic stem cell antigen SOX2 correlates with progression of MGUS or smoldering MM (SMM) to MM (14). Malignant plasma cells also secrete larger amounts of soluble (s)MICA, a ligand for NKG2D, with progression of MGUS to MM, which is associated with decreased natural killer cell and CD8+ T-cell function (15). These observations suggest that restoring CD8+ T-cell and NK cell immunity against malignant plasma cells maybe therapeutically beneficial.
Immune checkpoints in myeloma: pre-clinical studies
In mouse models, adoptive transfer of expanded PD-1+ T cells can eliminate established MM, suggesting these are antigen exposed (16). A combination of transplantation, whole-cell vaccination, and PD-L1 inhibition has also demonstrated pre-clinical efficacy(17). However, PD-1 blockade alone is not efficacious. CD137 agonists and other checkpoint inhibitors, such as anti-TIGIT, do appear to have efficacy in certain models (18,19). Patients with MM are older and have had a lifetime of antigen exposure thus, PD-1 expression may indicate myeloma antigen–exposed T cells, viral antigen–specific memory T cells, or immune senescent cells (20). PD-1 upregulation is observed on CD8+ T cells, CD4+ T cells, NK cells and bone marrow phospho-antigen–reactive Vγ9Vδ2 T cells in MM (21-23). Co-expression of other immune checkpoints, such as LAG-3 and TIGIT, on patient T cells suggests multiple pathways limiting T-cell responses. Blocking PD-1 in vitro can partially restore T-cell and NK cell function (21-23). Collectively, these observations suggest that PD-1 blockade is more likely to be effective in combinatorial approaches that focus on MM antigen-specific T cells.
PD-L1, the major ligand of PD-1, is upregulated on malignant plasma cells and can be upregulated by inflammatory cytokines or transcriptionally upregulated by both MYC and downstream effects of MAPK activation, both relevant genetic pathways in MM (24). Studies in vitro suggest that PD-L1+ plasma cells are more proliferative, resistant to T cell–mediated killing, and also resistant to traditional myeloma drugs (25). PD-L1 expression appears to be highest among drug-resistant cells present in the minimal residual disease state (26). Similarly, patients with SMM, whose plasma cells express PD-L1, progress more quickly to symptomatic MM, suggesting PD-L1 may be a marker of a proliferative clone, as well as a potential mediator of immune evasion (14).
Combination approaches specific to myeloma
Thalidomide, lenalidomide, or pomalidomide (IMiDs) function by enforcing ubiquitination and proteasomal degradation of Ikaros family proteins (IKZF1/IKZF3) (27), which leads to plasma cell apoptosis. In immune cells, IKZF3 represses IL2 production, and thus, IMiDs enhance secretion of IL2, leading to increased proliferation of T cells and NK cells, but diminished proliferation of regulatory T cells (Tregs) (28). IMiDs also enhance antigen presentation by releasing antigen through plasma cell death, upregulation of B7 expression, and enhancing immune synapse formation between T cells and APCs (29,30). Pleiotropic effects of IMiDs on immune cells support the notion that greater anti-MM effect could be obtained through combination strategies with ICB independent of plasma cell specific IMiD resistance.
Monoclonal antibodies (mAbs) targeting CD38 and SLAMF7 proteins also have both direct anti-MM effects and immune-modulating effects. The CD38 mAb daratumumab has been shown to induce depletion of MDSCs, Tregs, and regulatory B-cell subsets and increase CD8+ T-cell number and clonality among responding MM patients (31). However, CD38 is expressed on T-cells that are the targets for reinvigoration by anti–PD-1 in solid tumors (32). Thus, although the former observations provide support for combination with ICB in MM, whether this can be generalized to other cancers is not clear. Recently, a study () combining daratumumab with PD-1 pathway blockade in lung cancer was halted due to lack of benefit. The anti-SLAMF7 antibody elotuzumab can also modulate the immune response via downstream activation of EAT2 on NK cells, suggesting a potential rationale for combinations that enhance NK cell function. Early studies evaluating combinations of elotuzumab with anti-KIR and 4-1BB agonists remain to reported ().
In addition to IMiD- and mAb-based combinations, numerous bi-specific T-cell engagers (BiTEs), chimeric antigen T-cell receptor (CAR) therapies, and antibody drug conjugates directed at the B-cell maturation antigen (BCMA) or other targets are showing promise in MM (33). Although many of these therapies are in early stages of clinical development, it is apparent that lack of persistent anti-MM T cells may be a contributing factor in disease relapse. Combination strategies incorporating ICB may be beneficial.
Immune checkpoints in myeloma: clinical studies
Initial studies of single-agent PD-1 blockade did not result in a clinical benefit. A phase 1b study of lenalidomide, dexamethasone, and pembrolizumab demonstrated a 50% overall response rate (ORR) and a 38% response rate in lenalidomide-refractory patients (34). Pembrolizumab in combination with pomalidomide and dexamethasone also appeared promising, demonstrating a 60% ORR (35). These results prompted investigation of these combinations in randomized phase III trials as follows: (i) lenalidomide and dexamethasone ± pembrolizumab (P-Ld vs. Ld) in newly diagnosed myeloma (Keynote-185); (ii) pomalidomide and dexamethasone +/− pembrolizumab (P-Pd vs. Pd) in relapsed myeloma (Keynote-183); and (iii) pomalidomide and dexamethasone +/− nivolumab in relapsed myeloma (Checkmate 602). A pre-planned interim analysis in June 2017 revealed an imbalance in deaths in the two pembrolizumab-containing arms of the Keynote trials and the FDA issued a full clinical hold in September 2017 and also paused or halted multiple studies of PD-1 pathway blockade, including Checkmate 602 (36).
The Keynote-185 study targeted previously untreated, transplant-ineligible patients with myeloma. The study had a median follow-up time of 6.6 months and accrued 301 of 640 planned patients. Similar response rates of 64% vs 62% in the P-Ld (n=151) vs Ld (n=150) arms, respectively, and hazard ratio (HR) for 6-month progression-free survival (PFS) of 1.22 (95% CI: 0.67-2.22) and overall survival (OS) of 2.06 (95% CI: 0.93-4.55) were observed. The Keynote-183 study enrolled patients who had relapsed following two or more prior therapies. The study had a median f/u of 8.1 months and accrued 249 of 300 planned patients. Response rates of 34% vs 40% in the P-Pd (n=125) vs Pd (n=124) arms, respectively, and HR for PFS of 1.53 (95% CI: 1.05-2.22) and OS of 1.61 (95% CI: 0.91-2.85) were observed. Checkmate 602 had similar enrollment criteria to Keynote-183 and re-opened for enrollment in May 2018 while a concurrent early futility analysis based on 170 of subjects enrolled was performed at the request of the FDA. Enrollment in Checkmate 602 was stopped in September 2018 based on the results of this analysis, with a median f/u of 9.3 months and HR for PFS of 1.08 (95% CI: 0.68-1.70) and OS of 1.19 (95% CI: 0.64-2.2) were observed with nivolumab-Pd (NPd) when compared to Pd alone. ICB has been associated with novel immune-mediated side effects that typically can be managed with appropriate vigilance and intervention (37). Adverse event frequency was similar between the anti–PD-1–containing cohorts and the IMiD/dexamethasone cohorts. However, in all three trials serious adverse events were observed at higher frequency in the anti–PD-1–containing arms, including absolute frequency of fatalities. Immune-mediated causes of death, such as myocarditis and pneumonitis, were observed, but overall, a unifying cause for the imbalance in deaths as well as the imbalance in high grade toxicities was not apparent.
Single-arm trials evaluating PD-1 pathway inhibition alone or in combinations with IMiDs in SMM, newly diagnosed, and some refractory MM patient settings have stopped accrual, have been re-designed, or were withdrawn in response to these findings (Supplementary Table S1). Intriguing results have been reported at conference proceedings, including a complete response in a SMM patient with pembrolizumab and encouraging durability for the combination anti–PD-L1 (atezolizumab) with daratumumab for a small number of patients treated prior to these FDA-mandated actions. Current studies evaluating IMiDs or anti-CD38–targeting agents in combination with PD-1 pathway blockade are exploring these agents in more advanced disease settings or in the context of randomization +/− an anti–PD-1 agent (Table 1). Additional data on safety and efficacy will determine not only if these combinations continue to be explored, but also the development paths for newer combinations of PD-1 blockade that seek to enhance the efficacy of vaccination, radiation, engineered CARs or BiTEs.
Summary and future directions
Phase III clinical trials evaluating the combination of IMiD, dexamethasone, and anti–PD-1 did not demonstrate improvement in disease response for patients with MM. The results also raise questions regarding a potential synergy in toxicities from this combination. Given the nature of the studies, it has not been possible to discern the specific contribution of each individual drug or patient characteristics to define either a toxicity risk or potential for benefit in a specific patient subset. For example, although IMiDs may promote an immune response, the degree to which dexamethasone may blunt this effect has not been assessed. Additional biomarker exploration from the phase Ib, II, and III trials of these combinations could be informative in this regard, if tissue samples are available. Drug exposure and overall follow-up on each of the studies is short in an era when MM patients are living many years, such that potential exploration of a hypothetical survival plateau was not possible. Taken together the disparity in the results of the phase I/II and III trials of PD-1 blockade with IMiD and dexamethasone in MM suggests that a more deliberate mechanism- or biomarker-driven approach may have identified a patient population with potential to derive clinical benefit from this treatment approach.
Obtaining adequate tissue samples for correlative studies to more fully understand how the endogenous immune response is altered is critically important as the field explores targets such as LAG-3, TIGIT, and others. Studies linking myelomagenesis with inflammation suggest a cautionary note regarding a theoretic possibility of promoting disease progression. IMiDs, proteasome inhibitors, mAbs, steroids, and traditional chemotherapeutics are all critical components of MM therapy in today’s age. PD-1 blockade demonstrates clearly that safe and effective combinations of current MM treatment strategies with modalities that promote antigen-specific immune responses require a greater understanding of how the T cell–antigen interface impacts MM development and is, itself, impacted by current MM therapeutics (Fig. 1).
Exploration of combinations with the potential to change the MM treatment landscape is ongoing (Table 1). The relative paucity of mutated antigens, immune senescence, and lower relative frequency of bone marrow T cells means modalities to direct endogenous, adoptive, or engineered T cells against MM tumor–specific targets will be necessary. However, current data suggests that none of these alone are likely to result in durable remissions. Immune checkpoint blockade or immune agonist stimulation is a potent way to encourage an ongoing antigen-specific immune response toward greater clinical efficacy. Thus, despite initial setbacks, we should remain undaunted in our efforts to explore all these available tools to realize the full potential of immunotherapy for the benefit of patients.
Supplementary Material
Acknowledgements
A.M.L. gratefully acknowledges support of the MSK Cancer Center Core Grant (P30 CA008748), the MSK Sawiris Foundation, and the Parker Institute for Cancer Immunotherapy at MSKCC.
References
- 1.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science 2018;359(6382):1350–5 doi 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lesokhin AM, Callahan MK, Postow MA, Wolchok JD. On being less tolerant: Enhanced cancer immunosurveillance enabled by targeting checkpoints and agonists of T cell activation. Sci Transl Med 2015;7(280):280sr1 doi 10.1126/scitranslmed.3010274. [DOI] [PubMed] [Google Scholar]
- 3.Zappasodi R, Wolchok JD, Merghoub T. Strategies for Predicting Response to Checkpoint Inhibitors. Curr Hematol Malig Rep 2018;13(5):383–95 doi 10.1007/s11899-018-0471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pianko MJ, Moskowitz AJ, Lesokhin AM. Immunotherapy of Lymphoma and Myeloma: Facts and Hopes. Clin Cancer Res 2018;24(5):1002–10 doi 10.1158/1078-0432.CCR-17-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roemer MGM, Redd RA, Cader FZ, Pak CJ, Abdelrahman S, Ouyang J, et al. Major Histocompatibility Complex Class II and Programmed Death Ligand 1 Expression Predict Outcome After Programmed Death 1 Blockade in Classic Hodgkin Lymphoma. J Clin Oncol 2018;36(10):942–50 doi 10.1200/JCO.2017.77.3994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang H, Kaur G, Sankin AI, Chen F, Guan F, Zang X. Immune checkpoint blockade and CAR-T cell therapy in hematologic malignancies. J Hematol Oncol 2019;12(1):59 doi 10.1186/s13045-019-0746-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lesokhin AM, Ansell SM, Armand P, Scott EC, Halwani A, Gutierrez M, et al. Nivolumab in Patients With Relapsed or Refractory Hematologic Malignancy: Preliminary Results of a Phase Ib Study. J Clin Oncol 2016;34(23):2698–704 doi 10.1200/JCO.2015.65.9789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ratner L, Waldmann TA, Janakiram M, Brammer JE. Rapid Progression of Adult T-Cell Leukemia-Lymphoma after PD-1 Inhibitor Therapy. N Engl J Med 2018;378(20):1947–8 doi 10.1056/NEJMc1803181. [DOI] [PubMed] [Google Scholar]
- 9.Hemminki K, Forsti A, Sundquist K, Sundquist J, Li X. Familial associations of monoclonal gammopathy of unknown significance with autoimmune diseases. Leukemia 2016;30(8):1766–9 doi 10.1038/leu.2016.43. [DOI] [PubMed] [Google Scholar]
- 10.Landgren O, Rajkumar SV, Pfeiffer RM, Kyle RA, Katzmann JA, Dispenzieri A, et al. Obesity is associated with an increased risk of monoclonal gammopathy of undetermined significance among black and white women. Blood 2010;116(7):1056–9 doi 10.1182/blood-2010-01-262394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Preuss KD, Fadle N, Regitz E, Held G, Pfreundschuh M. Inactivation of protein-phosphatase 2A causing hyperphosphorylation of autoantigenic paraprotein targets in MGUS/MM is due to an exchange of its regulatory subunits. Int J Cancer 2014;135(9):2046–53 doi 10.1002/ijc.28864. [DOI] [PubMed] [Google Scholar]
- 12.Nair S, Branagan AR, Liu J, Boddupalli CS, Mistry PK, Dhodapkar MV. Clonal Immunoglobulin against Lysolipids in the Origin of Myeloma. N Engl J Med 2016;374(6):555–61 doi 10.1056/NEJMoa1508808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhodapkar MV, Krasovsky J, Osman K, Geller MD. Vigorous premalignancy-specific effector T cell response in the bone marrow of patients with monoclonal gammopathy. J Exp Med 2003;198(11):1753–7 doi 10.1084/jem.20031030 jem.20031030 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhodapkar MV, Sexton R, Das R, Dhodapkar KM, Zhang L, Sundaram R, et al. Prospective analysis of antigen-specific immunity, stem-cell antigens, and immune checkpoints in monoclonal gammopathy. Blood 2015;126(22):2475–8 doi 10.1182/blood-2015-03-632919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jinushi M, Vanneman M, Munshi NC, Tai YT, Prabhala RH, Ritz J, et al. MHC class I chain-related protein A antibodies and shedding are associated with the progression of multiple myeloma. Proc Natl Acad Sci U S A 2008;105(4):1285–90 doi 0711293105 [pii] 10.1073/pnas.0711293105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jing W, Gershan JA, Blitzer GC, Palen K, Weber J, McOlash L, et al. Adoptive cell therapy using PD-1(+) myeloma-reactive T cells eliminates established myeloma in mice. Journal for immunotherapy of cancer 2017;5:51 doi 10.1186/s40425-017-0256-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hallett WH, Jing W, Drobyski WR, Johnson BD. Immunosuppressive effects of multiple myeloma are overcome by PD-L1 blockade. Biol Blood Marrow Transplant 2011;17(8):1133–45 doi 10.1016/j.bbmt.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Guillerey C, Ferrari de Andrade L, Vuckovic S, Miles K, Ngiow SF, Yong MC, et al. Immunosurveillance and therapy of multiple myeloma are CD226 dependent. J Clin Invest 2015;125(5):2077–89 doi 10.1172/JCI77181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minnie SA, Kuns RD, Gartlan KH, Zhang P, Wilkinson AN, Samson L, et al. Myeloma escape after stem cell transplantation is a consequence of T-cell exhaustion and is prevented by TIGIT blockade. Blood 2018;132(16):1675–88 doi 10.1182/blood-2018-01-825240. [DOI] [PubMed] [Google Scholar]
- 20.Sponaas AM, Yang R, Rustad EH, Standal T, Thoresen AS, Dao Vo C, et al. PD1 is expressed on exhausted T cells as well as virus specific memory CD8+ T cells in the bone marrow of myeloma patients. Oncotarget 2018;9(62):32024–35 doi 10.18632/oncotarget.25882.` [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung DJ, Pronschinske KB, Shyer JA, Sharma S, Leung S, Curran SA, et al. T-cell Exhaustion in Multiple Myeloma Relapse after Autotransplant: Optimal Timing of Immunotherapy. Cancer immunology research 2016;4(1):61–71 doi 10.1158/2326-6066.CIR-15-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benson DM Jr., Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 2010;116(13):2286–94 doi blood-2010-02-271874 [pii] 10.1182/blood-2010-02-271874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castella B, Foglietta M, Sciancalepore P, Rigoni M, Coscia M, Griggio V, et al. Anergic bone marrow Vgamma9Vdelta2 T cells as early and long-lasting markers of PD-1-targetable microenvironment-induced immune suppression in human myeloma. Oncoimmunology 2015;4(11):e1047580 doi 10.1080/2162402X.2015.1047580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Hamrouni A, Wolowiec D, Coiteux V, Kuliczkowski K, Hetuin D, et al. Plasma cells from multiple myeloma patients express B7-H1 (PD-L1) and increase expression after stimulation with IFN-{gamma} and TLR ligands via a MyD88-, TRAF6-, and MEK-dependent pathway. Blood 2007;110(1):296–304 doi blood-2006-10-051482 [pii] 10.1182/blood-2006-10-051482. [DOI] [PubMed] [Google Scholar]
- 25.Ishibashi M, Tamura H, Sunakawa M, Kondo-Onodera A, Okuyama N, Hamada Y, et al. Myeloma Drug Resistance Induced by Binding of Myeloma B7-H1 (PD-L1) to PD-1. Cancer immunology research 2016;4(9):779–88 doi 10.1158/2326-6066.CIR-15-0296. [DOI] [PubMed] [Google Scholar]
- 26.Paiva B, Azpilikueta A, Puig N, Ocio EM, Sharma R, Oyajobi BO, et al. PD-L1/PD-1 presence in the tumor microenvironment and activity of PD-1 blockade in multiple myeloma. Leukemia 2015. doi 10.1038/leu.2015.79. [DOI] [PubMed] [Google Scholar]
- 27.Kronke J, Udeshi ND, Narla A, Grauman P, Hurst SN, McConkey M, et al. Lenalidomide causes selective degradation of IKZF1 and IKZF3 in multiple myeloma cells. Science 2014;343(6168):301–5 doi 10.1126/science.1244851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gandhi AK, Kang J, Havens CG, Conklin T, Ning Y, Wu L, et al. Immunomodulatory agents lenalidomide and pomalidomide co-stimulate T cells by inducing degradation of T cell repressors Ikaros and Aiolos via modulation of the E3 ubiquitin ligase complex CRL4(CRBN.). Br J Haematol 2014;164(6):811–21 doi 10.1111/bjh.12708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vallet S, Palumbo A, Raje N, Boccadoro M, Anderson KC. Thalidomide and lenalidomide: Mechanism-based potential drug combinations. Leuk Lymphoma 2008;49(7):1238–45 doi 792758569 [pii] 10.1080/10428190802005191. [DOI] [PubMed] [Google Scholar]
- 30.Ramsay AG, Johnson AJ, Lee AM, Gorgun G, Le Dieu R, Blum W, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest 2008;118(7):2427–37 doi 10.1172/JCI35017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krejcik J, Casneuf T, Nijhof IS, Verbist B, Bald J, Plesner T, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016;128(3):384–94 doi 10.1182/blood-2015-12-687749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017;545(7652):60–5 doi 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh A, Mailankody S, Giralt SA, Landgren CO, Smith EL, Brentjens RJ. CAR T cell therapy for multiple myeloma: where are we now and where are we headed? Leuk Lymphoma 2017:1–12 doi 10.1080/10428194.2017.1393668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mateos M-V, Orlowski RZ, Siegel DSD, Reece DE, Moreau P, Ocio EM, et al. Pembrolizumab in combination with lenalidomide and low-dose dexamethasone for relapsed/refractory multiple myeloma (RRMM): Final efficacy and safety analysis. Journal of Clinical Oncology 2016;34(15_suppl):8010- doi 10.1200/JCO.2016.34.15_suppl.8010. [DOI] [Google Scholar]
- 35.Badros A, Hyjek E, Ma N, Lesokhin A, Dogan A, Rapoport AP, et al. Pembrolizumab, pomalidomide, and low-dose dexamethasone for relapsed/refractory multiple myeloma. Blood 2017;130(10):1189–97 doi 10.1182/blood-2017-03-775122. [DOI] [PubMed] [Google Scholar]
- 36.FDA Alerts Healthcare Professionals and Oncology Clinical Investigators about Two Clinical Trials on Hold Evaluating KEYTRUDA® (pembrolizumab) in Patients with Multiple Myeloma. https://www.fda.gov/Drugs/DrugSafety/ucm574305.htm?platform=hootsuite; 2017.
- 37.Postow MA, Sidlow R, Hellmann MD. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N Engl J Med 2018;378(2):158–68 doi 10.1056/NEJMra1703481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.