Abstract
Background
The perioperative factors predicting or influencing early pancreatic ductal adenocarcinoma recurrence are unclear. This study attempted to identify the predictive factors for early pancreatic ductal adenocarcinoma recurrence post-pancreatectomy and the influence of pre- and post- operative adjuvant therapy.
Methods
One hundred and fifteen patients undergoing curative resection for pancreatic ductal adenocarcinoma between 2000 and 2016 at our institution were retrospectively analyzed. Patients were divided into two groups: those who did (n = 34) and did not (n = 81) experience a recurrence within 6 months postoperatively.
Results
Multivariate analyses demonstrated postoperative CA19–9 de-normalization, no postoperative adjuvant chemotherapy, and serosal invasion were independent risk factors for early recurrence (P < 0.001, P = 0.001, and P = 0.010, respectively). A subgroup analysis showed patients with (n = 51) and without (n = 64) preoperative chemoradiotherapy had different predictors. Although postoperative adjuvant chemotherapy was not a significant indicator in patients with preoperative chemoradiotherapy, CA19–9 de-normalization and no postoperative adjuvant chemotherapy were significant indicators in patients without preoperative chemotherapy. Preoperative chemotherapy strongly prevented early local recurrence while postoperative adjuvant chemotherapy prevented early distant recurrence.
Conclusions
CA19–9 de-normalization was an important predictor of early recurrence of pancreatic ductal adenocarcinoma. Although postoperative adjuvant chemotherapy was an important preventive measure against early recurrence, particularly for distant recurrence, preoperative chemoradiotherapy could strongly prevent the early local recurrence of pancreatic ductal adenocarcinoma. These perioperative adjuvant therapies could have a complementary relationship.
Keywords: Early recurrence, Predictor, Pattern, Preoperative chemoradiotherapy, Neoadjuvant therapy, Adjuvant therapy
Background
Pancreatic ductal adenocarcinoma (PDAC) has a poor prognosis; only 3% of patients survive at 5 years after diagnosis [1, 2]. Only 20% of patients with PDAC are eligible to undergo radical resection [3]. Although surgical resection provides the only chance for a cure, it is associated with a median overall survival (OS) period of 11 to 23 months, with a 5-year OS rate of about 20% [4, 5].
Wagner M et al. reported that R0 resection is the most important factor determining outcome in patients with PDAC [6], and Ihsan ED et al. reported that R1 was associated with a decreased OS and disease free survival (DFS) in PDAC when compared with R0 [7]. The early recurrence of PDAC postoperatively is a frequently observed, serious problem, even after microscopically curative resection is performed. La Torre et al. reported that 60% of patients experience local or systemic recurrence within the first 12 months after curative resection [4]. Some reports suggested that the preoperative factors that are associated with the survival time after surgery were tumor size [4], preoperative lymph node metastasis [4], the preoperative serum carbohydrate antigen 19–9 (CA19–9) level [3, 4, 8, 9], histological grades [4, 9], duration of symptoms [3], and the preoperative modified Glasgow Prognostic Score [10]. These might be predictors of the early recurrence of PDAC postoperatively.
Neoadjuvant therapy was not actually recommended for patients with resectable (R)-PDAC in the 2016 National Comprehensive Cancer Network (NCCN) guideline [11], but neoadjuvant chemotherapy or chemoradiotherapy (CRT) may reduce the early recurrence of PDAC. Upfront surgery might be a predictor of the early recurrence of PDAC, even for those with R-PDAC.
The aim of this study was to detect factors influencing on early recurrence and its patterns for the patients with PDAC including neoadjuvant CRT and adjuvant chemotherapy (ACT).
Methods
This study was approved by the institutional review board of Kagawa University. A total of 142 consecutive patients undergoing pancreatectomy for PDAC between January 2000 and May 2016 were retrospectively examined. Informed consent was obtained from all patients according to the institutional protocol of our hospital. All 142 patients had a PDAC that was histologically examined by at least two pathologists. Of the 142 patients, 27 patients were excluded. Ten patients were censored within 6 months, 10 were classified as unresectable category based on the 2016 NCCN guideline [11], 5 had unclear recurrence timing, 1 underwent R2 resection, and 1 had perioperative mortality. The data from the remaining 115 patients were retrospectively analyzed.
The patients were diagnosed with R (n = 86) or borderline resectable ([BR], n = 29) PDAC according to the 2016 NCCN guidelines [11]. All surgical procedures were divided into the following three types: classic, pylorus-preserving, or subtotal stomach-preserving pancreaticoduodenectomy (PD) in 75 patients (65%); distal pancreatectomy in 36 (31%); and total pancreatectomy in 4 (3%). Systematic lymph node dissection was performed in all operations. R0 resection was achieved in 104 patients (90%) and R1 was achieved in 11 (10%). R0 resection was defined as negative margin based on Union for International Cancer Control (UICC) definition. Preoperative CRT and postoperative ACT were given to 51 (44%) and 85 (74%) patients, respectively. Among the 115 patients, 34 (30%) experienced early recurrence within 6 months postoperatively (group E) and 81 (70%) did not (group NE).
Outcome measures
The variables included age; sex; body mass index (BMI); tumor location; resectability; serum C-reactive protein, serum albumin, hemoglobin, and serum CA19–9 levels; neutrophil/lymphocyte ratio (NLR); lymphocyte count; modified Glasgow Prognostic Score [10]; the standardized uptake value (SUV) seen on 18F-fluorodeoxy glucose positron emission tomography (FDG-PET); and presence or absence of preoperative CRT. The intraoperative data, including the operation time, estimated blood loss, blood transfusion, and portal vein resection; and postoperative data on morbidity according to the Clavien-Dindo classification [12], CA19–9 normalization status, and postoperative ACT induction, were reviewed and included. A receiver operating characteristic (ROC) curve was constructed to estimate the optimal cutoff value for the recurrence of PDAC within 6 months postoperatively, which was determined as the point closest to the upper left-hand corner of the graph. The ROC curves demonstrated that the cutoff points of the preoperative serum CA19–9 level, NLR, lymphocyte count, SUV on FDG-PET, and tumor size were 173, 4.65, 1648, 4.73, and 3.0, respectively; and the areas under the curve (AUC) were 0.637, 0.466, 0.556, 0.593, and 0.639, respectively.
Preoperative chemoradiotherapy
We introduced short-term neoadjuvant hypofractionated chemoradiotherapy with S1 in patients with R and BR PDAC between January 2009 and May 2016, and already reported the efficacy and safety [13]. Hypofractionated, external-beam radiotherapy (30 Gy in 10 fractions) with concurrent S1 (60 mg/m2) was delivered 5 days per week for 2 weeks prior to pancreatectomy. All of the patients with preoperative CRT (n = 51) in this study received radiotherapy in a similar way. Meanwhile, all patients before December 2008 underwent upfront surgery.
Adjuvant therapy and follow-up
ACT was applied postoperatively unless contraindicated by the patients’ conditions. The patients received gemcitabine, referring to the results of the CONKO-001 trial [14] between 2006 and 2012; or S-1, referring to the results of the JASPAC01 trial since 2013 [15], according to the recommended protocols. S-1 is an oral fluoropyrimidine consisting of tegafur, a prodrug of fluorouracil, and two biochemical modulators. It is characterized by the inhibition of dihydropyrimidine dehydrogenase activity by gimeracil, the maintenance of a high concentration of fluorouracil, and by the suppression of fluorouracil’s phosphorylation in the gastrointestinal tract by oteracil potassium, thereby reducing gastrointestinal toxicity. Gemcitabine at a dose of 1000 mg/m2 was administered weekly for 3 weeks, followed by 1 week of rest; oral S-1 (80 mg/m2/day) was administered from days 1 to 28, followed by a 2-week rest period or from days 1 to 14, followed by a 1-week rest period. Chemotherapy was initiated within 2 months postoperatively in all patients who were considered eligible for the treatment. The follow-up examinations were performed every 2–3 months for 1 year and every 6 months thereafter, until the disease progressed. Enhanced computed tomography was performed every 6 months. We moved the examination date forward or added magnetic resonance imaging or FDG-PET, if necessary.
Statistical analysis
The clinicopathological features of patients in the groups E and NE were compared. The categorical variables were compared between the groups using the chi-square test and Fisher’s exact test. Survival was calculated using the Kaplan–Meier method and was compared between the groups using the log-rank test. A multivariate analysis using the backward elimination method of the Cox proportional hazards model was undertaken, using variables from the univariate analysis that were considered to potentially affect survival (P < 0.10). P-values < 0.05 were considered statistically significant. All statistical analyses were performed using the SPSS Statistics 25.0 for Windows software program (SPSS, Inc., Chicago, IL, USA).
Results
The median follow-up period was 18 (range: 2–112) months. In the entire group of patients, the median OS period was 24 months, and the 3- and 5-year survival rates were 45.9 and 28.7%, respectively. The median OS times of the groups E (n = 34) and NE (n = 81) were 9 and 37 months, respectively (P < 0.001).
Table 1 shows the results of the univariate and multivariate analyses of the factors that possibly affected the early recurrence of PDAC among the 115 patients. Significant associations with early recurrence were observed for the serum CA19–9 level ≧173 (P = 0.026), NLR ≧4.65 (P = 0.020), lymphocyte count ≦1648 (P = 0.042), SUV on FDG-PET ≧4.73 (P = 0.043), postoperative serum CA19–9 de-normalization defined as no return into the normal range (≥37 U/ml) except in patients who had normal preoperative CA19–9 (P < 0.001), no postoperative ACT (P < 0.001), pathological tumor size ≧3.0 cm (P = 0.028), para-aortic lymph node metastases (P = 0.009), and positive serosal (S) and plexus (PL) invasion factors (P = 0.009 and P = 0.029, respectively). Preoperative CRT did not reach statistical significance in the univariate analysis (P = 0.093).
Table 1.
Univariate and multivariate analyses of the clinical factors affecting the recurrence of PDAC within 6 months after surgery
| Group E (n = 34)b | Group NE (n = 81)c | P-value | OR | 95% CI | P-value※ | ||
|---|---|---|---|---|---|---|---|
| Preoperative variables | |||||||
| Agea | ≧ 68 | 23 (68%) | 46 (57%) | 0.278 | |||
| Sex | Male | 19 (56%) | 43 (53%) | 0.784 | |||
| BMIa | ≦ 21.5 | 13 (59%) | 27 (44%) | 0.233 | |||
| Tumor location | head | 23 (68%) | 54 (67%) | 0.919 | |||
| Resectability | BR | 10 (29%) | 19 (23%) | 0.502 | |||
| CRPa | ≧ 0.18 | 17 (61%) | 32 (43%) | 0.103 | |||
| CA19–9a | ≧ 173 | 21 (62%) | 30 (39%) | 0.026 | |||
| NLRa | ≧4.65 | 8 (40%) | 10 (16%) | 0.020 | |||
| Lymphocyte counta | ≦1648 | 25 (96%) | 58 (79%) | 0.038 | |||
| mGPS | 1 or 2 | 7 (21%) | 13 (17%) | 0.382 | |||
| SUV in FDG-PETa | ≧ 4.73 | 18 (82%) | 37 (58%) | 0.043 | |||
| Preoperative CRT | No | 23 (68%) | 41 (51%) | 0.093 | |||
| Intraoperative variables | |||||||
| Operation timea | ≧ 609 | 11 (44%) | 39 (59%) | 0.197 | |||
| Blood lossa | ≧ 1767 | 5 (20%) | 20 (30%) | 0.326 | |||
| Transfusion | Yes | 5 (25%) | 15 (23%) | 0.860 | |||
| Portal vein resection | Yes | 11 (33%) | 26 (35%) | 0.893 | |||
| Postoperative variables | |||||||
| Morbidity | ≧CD3 | 5 (22%) | 13 (22%) | 0.948 | |||
| Postoperative CA19–9 normalization | No | 17 (81%) | 9 (20%) | < 0.001 | 23.10 | 4.21–126.86 | < 0.001 |
| Postoperative ACT | No | 18 (53%) | 11 (14%) | < 0.001 | 10.41 | 2.73–39.64 | 0.001 |
| Pathological variables | |||||||
| Tumor sizea | ≧ 3.0 | 22 (65%) | 32 (42%) | 0.028 | |||
| Histological grade | G2–4 | 14 (56%) | 36 (50%) | 0.564 | |||
| PALN metastasis | Positive | 4 (16%) | 1 (2%) | 0.009 | |||
| LN metastases | Positive | 20 (59%) | 40 (49%) | 0.355 | |||
| CY | Positive | 2 (9%) | 3 (5%) | 0.502 | |||
| ly | Positive | 28 (82%) | 57 (70%) | 0.182 | |||
| v | Positive | 32 (94%) | 73 (90%) | 0.488 | |||
| S | Positive | 25 (74%) | 38 (47%) | 0.009 | 4.942 | 1.47–16.62 | 0.010 |
| RP | Positive | 26 (76%) | 54 (67%) | 0.297 | |||
| A | Positive | 4 (11%) | 4 (5%) | 0.203 | |||
| PV | Positive | 10 (29%) | 21 (27%) | 0.757 | |||
| PL | Positive | 21 (62%) | 32 (40%) | 0.029 | |||
| Resection status | R1 | 6 (18%) | 5 (6%) | 0.056 | |||
※; logistic regression analysis
aThe cutoff values of age, BMI, CRP, Alb, Hb, CA19–9, NLR, lymphocyte count, SUV on FDG-PET, operation time, blood loss, and tumor size were set by drawing a receiver operating characteristic curve
bRecurrence within 6 months after surgery
cRecurrence at more than 6 months after surgery
In the multivariate analysis of factors that were found to affect the early recurrence of PDAC in the univariate analysis, postoperative serum CA19–9 de-normalization (odds ratio [OR], 23.10; 95% confidence interval [CI], 4.21–126.86; P < 0.001), no postoperative ACT (OR, 10.41; 95% CI, 2.73–39.64; P = 0.001) and positive S factor (OR, 4.94; 95% Cl, 1.47–16.62; P = 0.010) were independent risk factors for the early recurrence of PDAC. CA19–9 denormalization was seen in 17 of 21 patients (81%) who had preoperative CA19–9 elevation.
Table 2 shows the subgroup analysis results of the factors affecting the early recurrence of PDAC in the 51 patients with preoperative CRT and in the 64 patients with upfront surgery. The percentages of background factors such as resectability (P = 0.720), surgical procedure (P = 0.756) and Union for International Cancer Control stage (P = 0.808) were not significantly different between the E and NE groups (data not shown). Postoperative serum CA19–9 de-normalization (OR, 83.36; 95% CI, 3.32–2095.03; P = 0.007) and a positive S factor (OR, 13.08; 95% Cl, 1.25–137.46; P = 0.032) were independent risk factors for the early recurrence of PDAC in the multivariate analysis. Postoperative ACT did not significantly affect the early recurrence of PDAC in the multivariate analysis of this subgroup. On the other hand, the results of the univariate and multivariate analyses of the factors affecting the early recurrence of PDAC in the 64 patients who underwent upfront surgery revealed that postoperative serum CA19–9 de-normalization (OR, 39.26; 95% CI, 3.65–422.10; P = 0.002) and no postoperative ACT (OR, 15.53; 95% CI, 2.51–96.04; P = 0.003) were independent risk factors for early recurrence in this subgroup.
Table 2.
Subgroup analysis for the clinical factors affecting early recurrence in patients who received CRT preoperatively (n = 51) or upfront surgery (n = 64)
| Patients who received CRT preoperatively (n = 51) | Patients who received upfront surgery (n = 64) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group E (n = 11)b | Group NE (n = 40)c | P-value | HR | 95% CI | P-value※ | Group E (n = 23)b | Group NE (n = 41)c | P-value | HR | 95% CI | P-value※ | ||
| BMIa | < 21.5 | 7 (70%) | 15 (39%) | 0.085 | |||||||||
| CRPa | ≧ 0.19 | 13 (72%) | 18 (46%) | 0.066 | |||||||||
| CA19–9a | ≧ 173 | 14 (61%) | 15 (38%) | 0.088 | |||||||||
| NLRa | ≧ 4.65 | 6 (60%) | 9 (25%) | 0.037 | 2 (20%) | 1 (4%) | 0.098 | ||||||
| Lymphocyte counta | ≦ 1648 | 15 (94%) | 25 (66%) | 0.032 | |||||||||
| Postoperative CA19–9 normalization | No | 6 (86%) | 5 (22%) | 0.002 | 83.36 | 3.32–2095.0 | 0.007 | 11 (79%) | 4 (18%) | < 0.001 | 39.26 | 3.65–422.10 | 0.002 |
| Postoperative ACT | No | 5 (45%) | 7 (18%) | 0.053 | 13 (57%) | 5 (12%) | < 0.001 | 15.53 | 2.51–96.04 | 0.003 | |||
| Tumor sizea | ≧ 3.0 | 16 (70%) | 16 (43%) | 0.047 | |||||||||
| PALN metastasis | Positive | 1 (10%) | 0 (0%) | 0.052 | |||||||||
| LN metastasis | Positive | 8 (73%) | 17 (43%) | 0.076 | |||||||||
| ly | Positive | 9 (82%) | 21 (53%) | 0.080 | |||||||||
| S | Positive | 10 (91%) | 17 (43%) | 0.004 | 13.08 | 1.25–137.46 | 0.032 | ||||||
| A | Positive | 2 (18%) | 1 (3%) | 0.050 | |||||||||
| PL | Positive | 7 (64%) | 13 (33%) | 0.061 | |||||||||
| Resection status | R1 | 5 (21%) | 2 (5%) | 0.038 | 10.03 | 0.84–118.82 | 0.067 | ||||||
※; logistic regression analysis, Variables with P ≦ 0.10 are shown in this table
aThe cutoff values of BMI, CRP, NLR, lymphocyte count and tumor size were set by drawing a receiver operating characteristic curve
bRecurrence within 6 months after surgery
cRecurrence at more than 6 months after surgery
Discussion
Recently, the efficacy of preoperative neoadjuvant chemotherapy or CRT for PDAC has been reported, especially for patients with BR- or locally advanced unresectable-PDAC [16–18]. However, neoadjuvant therapy is not recommended for patients with R-PDAC in the NCCN guideline [11], presumably because of insufficient evidence. Preoperative therapy, however, might have a beneficial effect on patient survival in those with R-PDAC through preventing the early recurrence that is often seen even after curative resection.
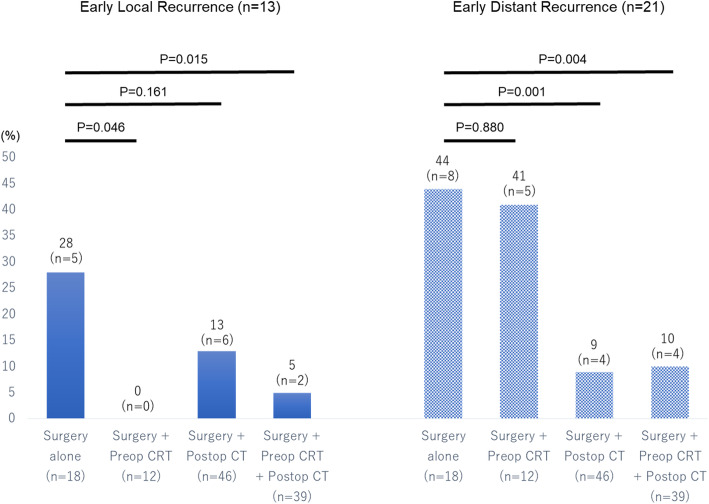
The postoperative recurrence patterns of PDAC in 34 patients in the group E regarding preoperative CRT and postoperative ACT are shown in Fig. 1. The recurrence patterns were defined as the location of the first recurrence. Local recurrence included regional lymph node and plexus nerve recurrence base on radiological findings. We made a final decision while taking account of comprehensive set of factors in terms of tumor marker, FDG-PET and MDCT. In addition, EUS-FNA was applied in several cases with difficult lesion for diagnosis. The recurrence patterns showed that 13 (38%) initially had local recurrence and 21 (62%) patients initially had distant metastasis. Early local recurrence occurred in only 2 patients among a total of 51 patients receiving CRT preoperatively. Even in a group with surgery plus preoperative CRT alone (n = 12), no early local recurrence occurred. In contrast, it was seen in 5 (28%) patients undergoing surgery alone (P = 0.046) and in 6 (13%) patients with surgery plus postoperative ACT alone. On the other hand, early distant recurrence developed in only 4 (9%) patients with surgery plus postoperative ACT alone. In sharp contrast, it occurred in 8 (44%) who underwent surgery alone (P = 0.004) and in 5 (41%) patients with surgery plus preoperative CRT alone. Furthermore, both early local recurrence and distant recurrence significantly decreased in patients who received combined preoperative CRT and postoperative ACT, compared with surgery alone (P = 0.015 and P = 0.004, respectively). These results clearly demonstrated that preoperative CRT strongly prevented local recurrence but not distant recurrence, and postoperative ACT prevented early distant recurrence but not local recurrence.
Fig. 1.
The postoperative early recurrence patterns of PDAC regarding preoperative CRT and postoperative a CRT and postoperative adjuvant chemotherapy. Early local recurrence significantly decreased in patients with preoperative CRT, while early distant recurrence significantly decreased in patients with postoperative chemotherapy
The potential advantages of the preoperative delivery of CRT include the ability to sterilize tissues at critical margins oncologically. In the current study, the exposure dose of preoperative CRT was relatively low, at a minimum value of 30 Gy. However, a report from the M.D. Anderson Cancer Center contended that hypofractionated CRT (30 Gy) was associated with margin-negative resection rates, treatment effects, local control, and OS, similar to those associated with standard fractionated CRT (50.4 Gy) [19]. Similar results were proven in our previous study [13] and the current study.
This study demonstrated that the lack of postoperative ACT was a significant predictor of early recurrence. As supported by the results from previous clinical trials [14, 15], ACT is one of the most important factors for preventing early recurrence postoperatively. Judging from the early recurrence pattern that was found in this study, postoperative ACT prevents the early distant recurrence of PDAC, but it might have almost no effect on early local recurrence. Interestingly, there was no significant difference between patients with and without postoperative ACT in those with preoperative CRT. We speculated that preoperative CRT might compensate for a lack of postoperative ACT.
Preoperative CRT itself was not found to be an independent preventive factor against the early recurrence of PDAC in the univariate and multivariate analyses of the entire series of patients. However, the current study demonstrated that preoperative CRT significantly prevented local recurrence. Early local recurrence occurred in only 2 patients among 51 patients receiving CRT preoperatively. Based on these results, preoperative CRT possesses strong efficacy regarding local control and might prevent the early local recurrence of PDAC. In this series, 39 patients were treated with both preoperative CRT and postoperative ACT. In this subgroup, the early recurrence of PDAC occurred in only 6 patients (15%).
The current study has several limitations. This was a retrospective study that was conducted at a single institution. Therefore, the sample size was small and a historical backdrop existed. The types of preoperative examinations such as FDG-PET and postoperative adjuvant therapy changed during the study period. Within the past several years, preoperative CRT has especially been performed for patients with R- and BR-PDAC. Furthermore, there are missing values for several factors in the tables, and the AUCs in the ROC were relatively low and might indicate inadequate cutoff points.
Conclusions
CA19–9 de-normalization was an important predictor of the early recurrence of PDAC within 6 months after pancreatic resection. Postoperative ACT was an important preventive measure for the early recurrence of PDAC, particularly for distant recurrence. Preoperative CRT had a strong potential to prevent the early local recurrence of PDAC. In addition, preoperative CRT might compensate for the lack of postoperative ACT. In patients who are not expected to be capable of receiving postoperative ACT, preoperative CRT should be considered.
Acknowledgements
This study was presented at the 13th World Congress of the International Hepato-Pancreato-Biliary Association, 4-7 September 2018, Geneva, Switzerland. The authors are grateful to all the patients who contributed data to this study. The authors would like to thank Editage (https://www.editage.jp) for the English language review.
Abbreviations
- A
Arterial invasion
- ACT
Adjuvant Chemotherapy
- Alb
Albumin
- BMI
Body mass index
- BR
Borderline resectable
- CA19–9
Carbohydrate antigen 19–9
- CD
Clavien-Dindo classification [10]
- CI
Confidence Interval
- CRP
C-reactive protein
- CRT
Chemoradiotherapy
- CY
Cytology
- FDG-PET
18F-fluorodeoxy Glucose Positron Emission Tomography
- Hb
Hemoglobin
- HR
Hazard Ratio
- LN
Lymph node
- ly
Lymph-vessel invasion
- mGPS
Modified Glasgow prognostic score [8]
- NLR
Neutrophil/Lymphocyte Ratio
- OR
Odds Ratio
- OS
Overall Survival
- PALN
Para-Aortic Lymph Node
- PD
Pancreaticoduodenectomy
- PDAC
Pancreatic Ductal Adenocarcinoma
- PL
Plexus invasion
- Postop
Postoperative
- Preop
Preoperative
- PV
Portal vein invasion
- R
Resectable
- ROC
Receiver Operating Characteristic
- RP
Retroperitoneal invasion
- S
Serosal invasion
- SUV
Standardized Uptake Value
- UICC
Union for International Cancer Control
- V
Venous invasion
Authors’ contributions
HS operated surgery and performed postoperative management of the patient and wrote the manuscript. MO and YA gathered operated on the patients, gave advice on surgery and revised the manuscript for intellectual content. ST, TS, HK1(Hideki Kamada), HK2(Hideki Kobara) and TM gave substantial contribution to the acquisition, analysis and interpretation of data. KO and YS operated on the patients, and supervised and contributed to the final version of the report. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant funding from agencies in the public, commercial, or not- for-profit sectors.
Availability of data and materials
The datasets used and/or analysed during the present study are available from the corresponding author (HS) on reasonable request.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This study was approved by the institutional review board of Kagawa University, and written informed consent was obtained from all individual participants before surgery for collection and analysis of the data. This article does not contain any studies with animals performed by any of the authors.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 2.Wray CJ, Ahmad SA, Matthews JB, Lowy AM. Surgery for pancreatic Cancer: recent controversies and current practice. Gastroenterology. 2005;128:1626–1641. doi: 10.1053/j.gastro.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 3.Barugola G, Partelli S, Marcucci S, Sartori N, Capelli P, Bassi C, Pederzoli P, Falconi M. Resectable pancreatic Cancer: who really benefits from resection? Ann Surg Oncol. 2009;16:3316–3322. doi: 10.1245/s10434-009-0670-7. [DOI] [PubMed] [Google Scholar]
- 4.La Torre M, Nigri G, Lo Conte A, Mazzuca F, Tierno SM, Salaj A, Marchetti P, Ziparo V, Ramacciato G. Is a preoperative assessment of the early recurrence of pancreatic Cancer possible after complete surgical resection? Gut Liver. 2014;8:102–108. doi: 10.5009/gnl.2014.8.1.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kimura K, Amano R, Nakata B, Yamazoe S, Hirata K, Murata A, Miura K, Nishio K, Hirakawa T, Ohira M, Hirakawa K. Clinical and pathological features of five-year survivors after Pancreatectomy for pancreatic adenocarcinoma. World J Surg Oncol. 2014;12:360. doi: 10.1186/1477-7819-12-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wagner M, Redaelli C, Lietz M, Seiler CA, Friess H, Büchler MW. Curative resection is the single most important factor determining outcome in patients with pancreatic adenocarcinoma. Br J Surg. 2004;915:536–594. doi: 10.1002/bjs.4484. [DOI] [PubMed] [Google Scholar]
- 7.Ihsan ED. Carsten J, A. Schlitter, Björn K, Lynne S, Stephan S, Elke T, Florian S, Lenika C, Rebekka S, Irene E, Wilko W, Helmut F, Güralp C. R0 versus R1 resection matters after Pancreaticoduodenectomy, and less after distal or Total Pancreatectomy for pancreatic Cancer. Ann Surg. 2018;268:1058–1068. doi: 10.1097/SLA.0000000000002345. [DOI] [PubMed] [Google Scholar]
- 8.Nakao A, Oshima K, Nomoto S, Takeda S, Kaneko T, Ichihara T, Kurokawa T, Nonami T, Takagi H. Clinical usefulness of CA-19-9 in pancreatic carcinoma. Semin Surg Oncol. 1998;15:15–22. doi: 10.1002/(SICI)1098-2388(199807/08)15:1<15::AID-SSU4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 9.Nishio K, Kimura K, Amano R, Yamazoe S, Ohrira G, Nakata B, Hirakawa K, Ohira M. Preoperative predictors for early recurrence of Resectable pancreatic Cancer. World J Surg Oncol. 2017;15:16. doi: 10.1186/s12957-016-1078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.La Torre M, Nigri G, Cavallini M, Mercantini P, Ziparo V, Ramacciato G. The Glasgow prognostic score as a predictor of survival in patients with potentially Resectable pancreatic adenocarcinoma. Ann Surg Oncol. 2012;19:2917–2923. doi: 10.1245/s10434-012-2348-9. [DOI] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network Clinical Practice guidelines in oncology pancreatic adenocarcinoma. Available from: http://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf. Accessed 31 October 2018.
- 12.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okano K, Suto H, Oshima M, Maeda E, Yamamoto N, Kakinoki K, Kamada H, Masaki T, Takahashi S, Shibata T, Suzuki Y. A prospective phase II trial of Neoadjuvant S-1 with concurrent Hypofractionated radiotherapy in patients with Resectable and borderline Resectable pancreatic ductal adenocarcinoma. Ann Surg Oncol. 2017;24:2777–2784. doi: 10.1245/s10434-017-5921-4. [DOI] [PubMed] [Google Scholar]
- 14.Oettle H, Neuhaus P, Hochhaus A, Hartmann JT, Gellert K, Ridwelski K, Niedergethmann M, Zülke C, Fahlke J, Arning MB, Sinn M, Hinke A, Riess H. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic Cancer: the CONKO-001 randomized trial. JAMA Surg. 2013;310:1473–1481. doi: 10.1001/jama.2013.279201. [DOI] [PubMed] [Google Scholar]
- 15.Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, Kaneoka Y, Shimizu Y, Nakamori S, Sakamoto H, Morinaga S, Kainuma O, Imai K, Sata N, Hishinuma S, Ojima H, Yamaguchi R, Hirano S, Sudo T, Ohashi Y. JASPAC 01 Study Group.Adjuvant Chemotherapy of S-1 Versus Gemcitabine for Resected Pancreatic Cancer: A Phase 3, Open-Label, Randomised, Non-Inferiority Trial (JASPAC 01) Lancet. 2016;388:248–257. doi: 10.1016/S0140-6736(16)30583-9. [DOI] [PubMed] [Google Scholar]
- 16.Katz MH, Pisters PW, Evans DB, Sun CC, Lee JE, Fleming JB, Vauthey JN, Abdalla EK, Crane CH, Wolff RA, Varadhachary GR, Hwang RF. Borderline Resectable pancreatic Cancer: the importance of this emerging stage of disease. J Am Coll Surg. 2008;206:833–846. doi: 10.1016/j.jamcollsurg.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chun YS, Milestone BN, Watson JC, Cohen SJ, Burtness B, Engstrom PF, Haluszka O, Tokar JL, Hall MJ, Denlinger CS, Astsaturov I, Hoffman JP. Defining venous involvement in borderline Resectable pancreatic Cancer. Ann Surg Oncol. 2010;17:2832–2838. doi: 10.1245/s10434-010-1284-9. [DOI] [PubMed] [Google Scholar]
- 18.Cloyd JM, Wang H, Egger ME, Tzeng CD, Prakash LR, Maitra A, Varadhachary GR, Shroff R, Javle M, Fogelman D, Wolff RA, Overman MJ, Koay EJ, Das P, Herman JM, Kim MP, Vauthey JN, Aloia TA, Fleming JB, Lee JE, Katz MHG. Association of Clinical Factors with a major pathologic response following preoperative therapy for pancreatic ductal adenocarcinoma. JAMA Surg. 2017;152:1048–1056. doi: 10.1001/jamasurg.2017.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cloyd JM, Crane CH, Koay EJ, Das P, Krishnan S, Prakash L, Snyder RA, Varadhachary GR, Wolff RA, Javle M, Shroff RT, Fogelman D, Overman M, Wang H, Maitra A, Lee JE, Fleming JB, Katz MH. Impact of Hypofractionated and standard fractionated Chemoradiation before Pancreatoduodenectomy for pancreatic ductal adenocarcinoma. Cancer. 2016;122:2671–2679. doi: 10.1002/cncr.30117. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the present study are available from the corresponding author (HS) on reasonable request.