Abstract
Nicorandil is a well-known antianginal agent, which has been recommended as one of the second-line treatments for chronic stable angina as justified by the European guidelines. It shows an efficacy equivalent to that of classic antianginal agents. Nicorandil has also been applied clinically in various cardiovascular diseases such as variant or unstable angina and reperfusion-induced damage following coronary angioplasty or thrombolysis. Different mechanisms have been involved in the protective effects of nicorandil in various diseases through either opening of adenosine triphosphate-sensitive potassium (KATP) channel or donation of nitric oxide (NO). The predominance or participation of any of these proposed mechanisms depends on the dose of nicorandil used, the location of diseased conditions, and if this mechanism is still functioning or not. The protection afforded by nicorandil has been shown to be mainly attributed to KATP channel opening in experimental models of myocardial and pulmonary fibrosis as well as renal injury or glomerulonephritis, whereas NO donation predominates as a mechanism of protection in hepatic fibrosis and inflammatory bowel diseases. Therefore, in different diseased conditions, it is important to know which mechanism plays the major role in nicorandil-induced curative or protective effects. This can bring new insights into the proper use of selected medication and its recommended dose for targeting certain disease.
Keywords: Nicorandil, nitric oxide, potassium channel
Introduction
Nicorandil is a safe, well-known antianginal agent that has been approved as a long-term therapy for chronic stable angina in Japan and Europe.[1] The Japanese Coronary Artery Disease (CAD) and the Impact of Nicorandil in Angina studies have revealed a beneficial impact for nicorandil on mortality and morbidity in patients with CAD.[2,3] The use of nicorandil has been recommended by the European Society of Cardiology as one of the second-line treatments for chronic stable angina.[4] Overall, comparative clinical trials have shown an equivalent efficacy of nicorandil in improving effort angina and ischemic symptoms compared to β blockers and calcium antagonists with minimal hemodynamic disturbance.[5] Moreover, nicorandil intake has not been associated with significant drop in blood pressure (BP) when used with calcium antagonists or β receptor blockers.[6] Importantly, nicorandil acts as an efficient anti-ischemic agent in patients with contraindications to use of beta blockers, such as bradycardia or exacerbated pulmonary disease.[7] According to its pharmacokinetic profile, nicorandil use appears to be safe for patients using anticoagulant therapies or those with renal or hepatic impairments.[8] In addition, its side effects are minimal, including headache as a common side effect in addition to less common side effects such as dizziness, gastrointestinal upset, flushing, and malaise. Nicorandil is contraindicated to be used in the setting of hypotension or concomitantly with other vasodilators.[9]
Nicorandil has been applied clinically in various cardiovascular diseases such as variant angina (coronary vasospasm), unstable angina, and reperfusion-induced damage following coronary angioplasty or thrombolysis.[6,10] Nicorandil has shown to be effective as a therapy for refractory angina in a clinical study, resulting in great improvements in its frequency or duration, electrocardiographic perturbations, and patients' adverse reactions.[11] Interestingly, a meta-analysis from 17 clinical trials has demonstrated that nicorandil treatment ameliorated left ventricular ejection fraction and microvascular function when used in patients with acute myocardial infarction (AMI) in conjugation with coronary reperfusion therapy.[12] Long-term therapy of nicorandil also showed beneficial effects on left ventricular remodeling and sympathetic nerve activity of myocardium in patients with AMI when used after reperfusion therapy.[13] In addition, nicorandil treatment when used as adjunctive to coronary angioplasty was accompanied by better clinical and functional outcomes in patients with anterior AMI compared to angioplasty alone. This was mainly attributed to a decrease in myocardial injury and improvement in microvascular function and rate of no-reflow.[14] Another study revealed that intravenous nicorandil reduced QT dispersion and ventricular fibrillation in patients after successful coronary angioplasty.[15] On the other hand, several reports demonstrated that nicorandil may cause severe vasodilation and fall in BP when used preoperatively before coronary artery bypass graft, which could be related to several potentiating factors acting during surgery. This necessitates the discontinuation of nicorandil therapy 3 days at least before admission for operation.[6]
Pharmacological Actions of Nicorandil
Nicorandil is an opener for adenosine triphosphate-sensitive potassium (KATP) channel and a donor for nitric oxide (NO). Several mechanisms have been proposed for its cardioprotective effects, including improvement of myocardial blood perfusion; reduction in preload and afterload; protection against ischemic damage; anti-arrhythmic effects; prevention of calcium overload; energy-modulating actions; and anti-inflammatory, antiapoptotic, and antiproliferative effects.[16,17] Interestingly, nicorandil improves cardiac function without affecting BP as well as cardiac conduction or contraction. Nicorandil produces its antianginal or anti-ischemic effect by dilatation of coronary arteries and by reduction of myocardial oxygen demand mainly by affecting afterload and to a lesser extent, preload. Nicorandil is considered a balanced vasodilator which affects both arterial and venous blood vessels. The effect of nicorandil on preload may be less than that observed with nitrates due to the significant decrease in systemic vascular resistance which tends to increase venous return.[6,16,18]
Nitric oxide donation
Nicorandil administration increases the level of NO through reaction of its nitrate group with sulfhydryl group in cells of vascular smooth muscle. This, in turn, either activates guanylate cyclase or facilitates the release of NO, leading to increased cGMP levels with a reduction in intracellular calcium and vascular smooth muscle cell relaxation.[18]
KATP channel opening
Concerning its action as an opener of KATP channel, this channel is known to be sensitive for the adenosine triphosphate/adenosine diphosphate ratio, which reflects the condition of the cell and its energy status. Depending on the type of potassium channel and its tissue specificity, opening of these channels shows diverse actions. Such channels have been described at both sarcolemmal and mitochondrial levels in myocardial cells in addition to cells of vascular smooth muscle.[17,19,20] Nicorandil is known to activate the receptors, Kir6.2/sulfonylurea receptor 2A (SUR2A) and Kir6.2/SUR2B, which confirms its specificity for KATP channels of cardiac and smooth muscles. In addition, nicorandil shows no observable effect on insulin secretion, which is consistent with lack of its effect on Kir6.2/SUR1 currents, revealing its good tolerability in diabetic patients.[21]
The sarcolemmal KATP channels of cardiomyocytes give a mean of linking the electrical activity to the metabolic and energy states. Specifically, this type of channel modulates the action potential duration where its opening during ischemic status results in action potential shortening and reduction in myocardial work.[22]
On the other hand, when the sarcolemmal KATP channels in vascular smooth muscle are opened, this produces hyperpolarization with subsequent close of the voltage-sensitive calcium channels and decrease in calcium influx and intracellular calcium, resulting in myosin light chain dephosphorylation and vascular smooth muscle relaxation.[6,23] This type of sarcolemmal KATP channels is also involved in the basal vascular tone maintenance both in mesenteric and coronary arteries.[24] Thus, nicorandil's NO donation and KATP channel opening contribute to its vasodilatory properties. Notably, the opening of KATP channels mainly dilates peripheral and coronary resistance arterioles using low doses of nicorandil, whereas its NO donating property mainly dilates epicardial coronary arteries and veins using high doses.[25,26,27] The lack of tolerance with nicorandil administration compared to nitrates is likely due to its effect as an opener of KATP channel and not due to its nitrate activity.[17]
Mitochondrial KATP channel opening and its related cardioprotection
Nicorandil exhibits cardioprotective effects which are mostly attributed to mitochondrial KATP opening. Nonhypotensive dose of nicorandil was reported to be a selective mitochondrial KATP channel opener.[28] Its exact mechanism on mitochondria is still debated where several hypotheses have been proposed. The well-accepted hypothesis includes an increase in potassium uptake in the mitochondrial matrix by mitochondrial KATP channel opening. This may prevent the accumulation of mitochondrial calcium through depolarization of mitochondrial membrane and reduction of the electrochemical gradient for calcium entry via its uniporter.[29,30] The prevention of calcium overload protects the heart through either inhibition of mitochondrial permeability transition pore and/or cardiomyocyte hypercontracture.[31] Mitochondrial KATP channel opening could also trigger the generation of low levels of reactive oxygen species (ROS), thereby amplifying the signaling pathway, stimulating the antioxidative action in mitochondria (manganese superoxide dismutase; MnSOD) and inhibiting mitochondrial nicotinamide adenine dinucleotide phosphate oxidase, which is the main source of ROS generation in cardiomyocytes.[32] The activation of this protective signaling pathway also preserves the capacity of oxidative phosphorylation and energy production in addition to maintenance of mitochondrial membrane integrity and inhibition of apoptotic signaling pathway, mitochondrial ultrastructural changes, and DNA fragmentation.[33]
There may be certain interactions between the mechanisms by which nicorandil provides cardioprotection through mitochondrial KATP channel opening. First, NO released from nicorandil can itself activate the mitochondrial KATP channels.[34] Second, protein kinase C (PKC) represents a key-signaling molecule, which by phosphorylation mediates the cardioprotection triggered by both NO and mitochondrial KATP channels.[35] Therefore, the cardioprotection provided by nicorandil may not be related just due to mitochondrial KATP channels opening, but may involve complex interactions between NO, KATP channel, and PKC.[36] Nicorandil can also exert anti-free radical properties that are independent from its KATP channel opening. This could be related to its nicotinamide moiety, which acts as a hydroxyl radical scavenger.[37]
Different Mechanisms Involved in Different Diseased Conditions
Controversy mechanisms have been involved in the protective effects of nicorandil. The predominance or participation of any of proposed mechanisms (KATP channel opening or NO donation) depends on the dose of nicorandil used, the location of diseased conditions, and if this mechanism is still functioning or not [Figure 1].
Figure 1.
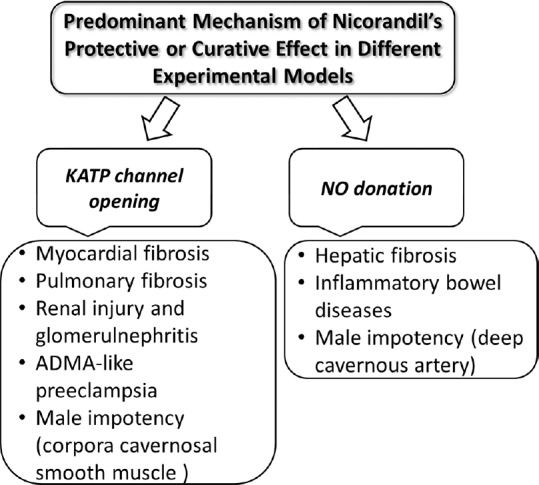
Predominant mechanism of action of nicorandil in different experimental models
Cardiovascular diseases
Nicorandil exerted its beneficial effect on stunned myocardium of anesthetized dogs by directly activating KATP channels where its afforded cardioprotection was blocked by pretreatment with glibenclamide (a KATP channel blocker).[38] Moreover, KATP channel opening may have important biological actions that prevent cardiac fibrosis. Nicorandil attenuated MI-induced cardiac fibrosis in rats, and its beneficial actions on differentiations of fibroblast were blocked by adding glibenclamide.[39] Glibenclamide is both a blocker for KATP channel and a vasorelaxant through NO generation.[40] This study suggested the predominant role of KATP channels and excluded the role of NO donation by nicorandil when coadministered with glibenclamide.
Nicorandil has been revealed to provide its cardioprotection through mitochondrial KATP channels opening in various experimental models of myocardial injury as ischemia reperfusion (IR) through a phenomenon known as pharmacological preconditioning.[36,41] Pharmacological preconditioning with nicorandil was found to attenuate myocardial IR injury in rats through selective mitochondrial KATP channel opening by the lower oral dose of nicorandil (3 not 6 mg/kg/day), which offered more cardioprotection against biochemical changes and ventricular arrhythmias induced by IR.[42] Other experiments on ventricular myocytes showed that the protective effect of nicorandil was abolished using selective blocker of mitochondrial KATP channel (5-hydroxydecanoate), confirming the role of mitochondrial KATP and not its sarcolemmal counterpart as a target for nicorandil's cardioprotective action.[43] Nicorandil also ameliorated the dysfunction of mitochondria and its downstream pathways in experimentally induced heart failure via amelioration of mitochondrial oxidative stress status and its energy production capacity as well as inhibition of mitochondrial ultrastructural changes, apoptotic signaling pathway, and DNA fragmentation.[33] In addition, the cardioprotection afforded by nicorandil against doxorubicin-induced ROS in HL-1 cardiomyocyte cell line was not related to its NO donation, but to its mitochondrial KATP opening.[32]
Importantly, pharmacological preconditioning with nicorandil showed promising results when coadministered with stem cells where it improved the efficacy of bone marrow-derived mesenchymal stem cell (BM-MSC) transplantation after isoproterenol-induced myocardial damage through establishment of a supportive environment for BM-MSC and improvement of its survival and homing by reduction of factors of inflammation, fibrogenesis, and apoptosis that might interfere with the efficiency of cell-based therapy.[44]
Pulmonary diseases
Nicorandil was previously reported to attenuate monocrotaline-induced endothelial damage and pulmonary arterial hypertension in rats, which was mainly attributed to KATP channel opening with an adjunctive effect to its NO-releasing property. This was confirmed by blockade of its beneficial effects using glibenclamide and N omega-nitro-L-arginine methyl ester (L-NAME) (an inhibitor of NO synthase).[45] On the other hand, activation of KATP channel mainly contributes to the beneficial effect of nicorandil against pulmonary fibrosis induced by cyclophosphamide in rats where concomitant administration of glibenclamide completely blocked the effects provided by nicorandil.[46]
Renal diseases
Nicorandil was demonstrated to have beneficial effects in several models of experimental renal diseases. Nicorandil potently reduced renal injury and urinary albumin excretion in diabetic eNOS-deficient mice excluding the role of NO donation in its mediated protection. The protective mechanism was shown to involve the reduction of oxidative stress, likely stimulated via KATP channel opening where it was diminished by the use of glibenclamide.[47] Nicorandil also protected podocytes in kidney from hyperglycemia-induced oxidative stress by activating KATP channels and stimulation of MnSOD expression in the mitochondria.[48] On the other side, nicorandil ameliorated renal injury induced by unilateral ureteral obstruction in rats through an increase in renal NO and a reduction of transforming growth factor-beta where these renoprotective effects were blunted by L-NAME codministration.[49]
Hepatic diseases
Experimentally, nicorandil has been demonstrated to be an adequate therapy against the induction of liver fibrosis by bile duct ligation where its protective effects on biochemical and histological changes were completely reversed by the coadministration of L-NAME, whereas glibenclamide coadministration showed less protection compared to that provided by nicorandil alone. These data suggested that the protection revealed by nicorandil against hepatic fibrosis was related mainly to its action as a donor for NO and to a smaller extent to its KATP channel opening.[50]
Bowel diseases
Nicorandil ameliorated experimentally-induced inflammatory bowel disease (IBD) using a dose with no significant effect on BP and a mechanism, which is partially or completely independent of KATP channels as observed on coadministration of glibenclamide. It seems that upregulation of eNOS, production of NO as well as its antioxidant potential though its nicotinamide moiety could be mainly responsible for its effects in remission of IBD.[37] Nicorandil can also exert an anti-inflammatory effect through inhibition of inflammatory mediators release such as tumor necrosis factor-alpha mainly via donation of NO and to a smaller extent through opening of KATP channel.[51]
Male and female reproductive diseases
Nicorandil showed significant improvement of functional disorders in animals with asymmetric dimethylarginine-induced preeclampsia. Activation of KATP channels seems to play a predominant role in these effects where glibenclamide reduced significantly but not completely the effect of nicorandil.[52] In addition, the capability of nicorandil to relax the uterine muscle, which was mainly achieved through KATP channels, can improve the placental microcirculation.[53]
Moreover, nicorandil may have remarkable effectiveness in the treatment of male impotency. Nicorandil relaxed in an in vitro experiment the corpora cavernosal smooth muscle mainly through its KATP channel opening and to a lesser extent through its NO donation. On the other hand, its vasodilative action on the deep cavernous artery was mediated mainly through guanylate cyclase stimulation.[54,55]
Conclusion
Therefore, in various diseased or clinical conditions, it is important to know which mechanism plays the major role in nicorandil-induced curative or protective effects. This can bring new insights into the proper use of the selected medication and its recommended dose for targeting certain disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Roland E. Safety profile of an anti-anginal agent with potassium channel opening activity: An overview. Eur Heart J. 1993;14(Suppl B):48–52. doi: 10.1093/eurheartj/14.suppl_b.48. [DOI] [PubMed] [Google Scholar]
- 2.Walker A, McMurray J, Stewart S, Berger W, McMahon AD, Dargie H, et al. Economic evaluation of the impact of nicorandil in angina (IONA) trial. Heart. 2006;92:619–24. doi: 10.1136/hrt.2003.026385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horinaka S, Yabe A, Yagi H, Ishimitsu T, Yamazaki T, Suzuki S, et al. Effects of nicorandil on cardiovascular events in patients with coronary artery disease in the Japanese coronary artery disease (JCAD) study. Circ J. 2010;74:503–9. doi: 10.1253/circj.cj-09-0649. [DOI] [PubMed] [Google Scholar]
- 4.Task Force Members. Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, et al. 2013 ESC guidelines on the management of stable coronary artery disease: The task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 5.Ferrari R, Pavasini R, Camici PG, Crea F, Danchin N, Pinto F, et al. Anti-anginal drugs-beliefs and evidence: Systematic review covering 50 years of medical treatment. Eur Heart J. 2019;40:190–4. doi: 10.1093/eurheartj/ehy504. [DOI] [PubMed] [Google Scholar]
- 6.Falase B, Easaw J, Youhana A. The role of nicorandil in the treatment of myocardial ischaemia. Expert Opin Pharmacother. 2001;2:845–56. doi: 10.1517/14656566.2.5.845. [DOI] [PubMed] [Google Scholar]
- 7.Morishita S, Maeba H, Takehana K, Shiojima I. Nicorandil was an effective treatment option for a patient with bland-white-garland syndrome. Intern Med. 2017;56:2295–9. doi: 10.2169/internalmedicine.8516-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frydman A. Pharmacokinetic profile of nicorandil in humans: An overview. J Cardiovasc Pharmacol. 1992;20(Suppl 3):S34–44. doi: 10.1097/00005344-199206203-00008. [DOI] [PubMed] [Google Scholar]
- 9.Dunn N, Freemantle S, Pearce G, Wilton LV, Mann RD. Safety profile of nicorandil – Prescription-event monitoring (PEM) study. Pharmacoepidemiol Drug Saf. 1999;8:197–205. doi: 10.1002/(SICI)1099-1557(199905/06)8:3<197::AID-PDS422>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 10.Krumenacker M, Roland E. Clinical profile of nicorandil: An overview of its hemodynamic properties and therapeutic efficacy. J Cardiovasc Pharmacol. 1992;20(Suppl 3):S93–102. [PubMed] [Google Scholar]
- 11.Li Y, Liu Y, Peng W, Wang B, Geng T, Xu Z. Therapeutic effect and safety of nicorandil in treatment of refractory angina pectoris. Int J Clin Exp Med. 2018;11:6993–8. [Google Scholar]
- 12.Iwakura K, Ito H, Okamura A, Koyama Y, Date M, Higuchi Y, et al. Nicorandil treatment in patients with acute myocardial infarction: A meta-analysis. Circ J. 2009;73:925–31. doi: 10.1253/circj.cj-08-1059. [DOI] [PubMed] [Google Scholar]
- 13.Kasama S, Toyama T, Sumino H, Kumakura H, Takayama Y, Ichikawa S, et al. Long-term nicorandil therapy improves cardiac sympathetic nerve activity after reperfusion therapy in patients with first acute myocardial infarction. J Nucl Med. 2007;48:1676–82. doi: 10.2967/jnumed.107.043075. [DOI] [PubMed] [Google Scholar]
- 14.Ito H, Taniyama Y, Iwakura K, Nishikawa N, Masuyama T, Kuzuya T, et al. Intravenous nicorandil can preserve microvascular integrity and myocardial viability in patients with reperfused anterior wall myocardial infarction. J Am Coll Cardiol. 1999;33:654–60. doi: 10.1016/s0735-1097(98)00604-4. [DOI] [PubMed] [Google Scholar]
- 15.Ueda H, Nakayama Y, Tsumura K, Yoshimaru K, Hayashi T, Yoshikawa J. Intravenous nicorandil can reduce the occurrence of ventricular fibrillation and QT dispersion in patients with successful coronary angioplasty in acute myocardial infarction. Can J Cardiol. 2004;20:625–9. [PubMed] [Google Scholar]
- 16.Kinoshita M, Sakai K. Pharmacology and therapeutic effects of nicorandil. Cardiovasc Drugs Ther. 1990;4:1075–88. doi: 10.1007/BF01856503. [DOI] [PubMed] [Google Scholar]
- 17.Schmid JP, Schroeder V. Nicorandil-review of pharmacological properties and clinical applications. Heart Drug. 2005;5:220–9. [Google Scholar]
- 18.Taira N. Similarity and dissimilarity in the mode and mechanism of action between nicorandil and classical nitrates: An overview. J Cardiovasc Pharmacol. 1987;10(Suppl 8):S1–9. [PubMed] [Google Scholar]
- 19.Escande D, Henry P. Potassium channels as pharmacological targets in cardiovascular medicine. Eur Heart J. 1993;14(Suppl B):2–9. doi: 10.1093/eurheartj/14.suppl_b.2. [DOI] [PubMed] [Google Scholar]
- 20.Tamargo J, Caballero R, Gómez R, Valenzuela C, Delpón E. Pharmacology of cardiac potassium channels. Cardiovasc Res. 2004;62:9–33. doi: 10.1016/j.cardiores.2003.12.026. [DOI] [PubMed] [Google Scholar]
- 21.Zhang M, Zhang H, Liu C, Li X, Ling M, Wang Z, et al. Myocardial protective effects of nicorandil on rats with type 2 diabetic cardiomyopathy. Med Sci Monit Basic Res. 2018;24:141–5. doi: 10.12659/MSMBR.910974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nichols CG, Lederer WJ. Adenosine triphosphate-sensitive potassium channels in the cardiovascular system. Am J Physiol. 1991;261:H1675–86. doi: 10.1152/ajpheart.1991.261.6.H1675. [DOI] [PubMed] [Google Scholar]
- 23.Kreye VA, Lenz T, Theiss U. The dualistic mode of action of the vasodilator drug, nicorandil, differentiated by glibenclamide in 86Rb flux studies in rabbit isolated vascular smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1991;343:70–5. doi: 10.1007/BF00180679. [DOI] [PubMed] [Google Scholar]
- 24.Daut J, Klieber HG, Cyrys S, Noack T. KATP channels and basal coronary vascular tone. Cardiovasc Res. 1994;28:811–7. doi: 10.1093/cvr/28.6.811. [DOI] [PubMed] [Google Scholar]
- 25.Holzmann S. Cyclic GMP as possible mediator of coronary arterial relaxation by nicorandil (SG-75) J Cardiovasc Pharmacol. 1983;5:364–70. doi: 10.1097/00005344-198305000-00004. [DOI] [PubMed] [Google Scholar]
- 26.Lefer DJ, Lefer AM. Studies on the mechanism of the vasodilator action of nicorandil. Life Sci. 1988;42:1907–14. doi: 10.1016/0024-3205(88)90031-8. [DOI] [PubMed] [Google Scholar]
- 27.Holzmann S, Kukovetz WR, Braida C, Pöch G. Pharmacological interaction experiments differentiate between glibenclamide-sensitive K+ channels and cyclic GMP as components of vasodilation by nicorandil. Eur J Pharmacol. 1992;215:1–7. doi: 10.1016/0014-2999(92)90600-9. [DOI] [PubMed] [Google Scholar]
- 28.Das B, Sarkar C, Karanth KS. Effects of administration of nicorandil or bimakalim prior to and during ischemia or reperfusion on survival rate, ischemia/reperfusion-induced arrhythmias and infarct size in anesthetized rabbits. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:383–96. doi: 10.1007/s002100100457. [DOI] [PubMed] [Google Scholar]
- 29.Holmuhamedov EL, Wang L, Terzic A. ATP-sensitive K+ channel openers prevent Ca2+ overload in rat cardiac mitochondria. J Physiol. 1999;519:347–60. doi: 10.1111/j.1469-7793.1999.0347m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crestanello JA, Doliba NM, Babsky AM, Doliba NM, Niibori K, Whitman GJ, et al. Ischemic preconditioning improves mitochondrial tolerance to experimental calcium overload. J Surg Res. 2002;103:243–51. doi: 10.1006/jsre.2001.6361. [DOI] [PubMed] [Google Scholar]
- 31.Kim JS, Jin Y, Lemasters JJ. Reactive oxygen species, but not Ca2+ overloading, trigger pH- and mitochondrial permeability transition-dependent death of adult rat myocytes after ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2006;290:H2024–34. doi: 10.1152/ajpheart.00683.2005. [DOI] [PubMed] [Google Scholar]
- 32.Asensio-López MC, Soler F, Pascual-Figal D, Fernández-Belda F, Lax A. Doxorubicin-induced oxidative stress: The protective effect of nicorandil on HL-1 cardiomyocytes. PLoS One. 2017;12:e0172803. doi: 10.1371/journal.pone.0172803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed LA, El-Maraghy SA. Nicorandil ameliorates mitochondrial dysfunction in doxorubicin-induced heart failure in rats: Possible mechanism of cardioprotection. Biochem Pharmacol. 2013;86:1301–10. doi: 10.1016/j.bcp.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Sasaki N, Sato T, Ohler A, O'Rourke B, Marbán E. Activation of mitochondrial ATP-dependent potassium channels by nitric oxide. Circulation. 2000;101:439–45. doi: 10.1161/01.cir.101.4.439. [DOI] [PubMed] [Google Scholar]
- 35.Nakano A, Liu GS, Heusch G, Downey JM, Cohen MV. Exogenous nitric oxide can trigger a preconditioned state through a free radical mechanism, but endogenous nitric oxide is not a trigger of classical ischemic preconditioning. J Mol Cell Cardiol. 2000;32:1159–67. doi: 10.1006/jmcc.2000.1152. [DOI] [PubMed] [Google Scholar]
- 36.Tsuchida A, Miura T, Tanno M, Sakamoto J, Miki T, Kuno A, et al. Infarct size limitation by nicorandil: Roles of mitochondrial K(ATP) channels, sarcolemmal K(ATP) channels, and protein kinase C. J Am Coll Cardiol. 2002;40:1523–30. doi: 10.1016/s0735-1097(02)02268-4. [DOI] [PubMed] [Google Scholar]
- 37.Hosseini-Tabatabaei A, Esmaily H, Rahimian R, Khorasani R, Baeeri M, Barazesh-Morgani A, et al. Benefit of nicorandil using an immunologic murine model of experimental colitis. Cent Eur J Biol. 2009;4:74–85. [Google Scholar]
- 38.Auchampach JA, Cavero I, Gross GJ. Nicorandil attenuates myocardial dysfunction associated with transient ischemia by opening ATP-dependent potassium channels. J Cardiovasc Pharmacol. 1992;20:765–71. [PubMed] [Google Scholar]
- 39.Lee TM, Lin SZ, Chang NC. Nicorandil regulates the macrophage skewing and ameliorates myofibroblasts by inhibition of RhoA/Rho-kinase signalling in infarcted rats. J Cell Mol Med. 2018;22:1056–69. doi: 10.1111/jcmm.13130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chan W, Yao X, Ko W, Huang Y. Nitric oxide mediated endothelium-dependent relaxation induced by glibenclamide in rat isolated aorta. Cardiovasc Res. 2000;46:180–7. doi: 10.1016/s0008-6363(99)00423-x. [DOI] [PubMed] [Google Scholar]
- 41.Mizumura T, Nithipatikom K, Gross GJ. Infarct size-reducing effect of nicorandil is mediated by the KATP channel but not by its nitrate-like properties in dogs. Cardiovasc Res. 1996;32:274–85. doi: 10.1016/0008-6363(96)00061-2. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed LA, Salem HA, Attia AS, Agha AM. Pharmacological preconditioning with nicorandil and pioglitazone attenuates myocardial ischemia/reperfusion injury in rats. Eur J Pharmacol. 2011;663:51–8. doi: 10.1016/j.ejphar.2011.04.038. [DOI] [PubMed] [Google Scholar]
- 43.Sato T, Sasaki N, O'Rourke B, Marbán E. Nicorandil, a potent cardioprotective agent, acts by opening mitochondrial ATP-dependent potassium channels. J Am Coll Cardiol. 2000;35:514–8. doi: 10.1016/s0735-1097(99)00552-5. [DOI] [PubMed] [Google Scholar]
- 44.Mohamed SS, Ahmed LA, Attia WA, Khattab MM. Nicorandil enhances the efficacy of mesenchymal stem cell therapy in isoproterenol-induced heart failure in rats. Biochem Pharmacol. 2015;98:403–11. doi: 10.1016/j.bcp.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 45.Sahara M, Sata M, Morita T, Hirata Y, Nagai R. Nicorandil attenuates monocrotaline-induced vascular endothelial damage and pulmonary arterial hypertension. PLoS One. 2012;7:e33367. doi: 10.1371/journal.pone.0033367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ahmed LA, El-Maraghy SA, Rizk SM. Role of the KATP channel in the protective effect of nicorandil on cyclophosphamide-induced lung and testicular toxicity in rats. Sci Rep. 2015;5:14043. doi: 10.1038/srep14043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tanabe K, Lanaspa MA, Kitagawa W, Rivard CJ, Miyazaki M, Klawitter J, et al. Nicorandil as a novel therapy for advanced diabetic nephropathy in the eNOS-deficient mouse. Am J Physiol Renal Physiol. 2012;302:F1151–60. doi: 10.1152/ajprenal.00596.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tamura Y, Tanabe K, Kitagawa W, Uchida S, Schreiner GF, Johnson RJ, et al. Nicorandil, a K(atp) channel opener, alleviates chronic renal injury by targeting podocytes and macrophages. Am J Physiol Renal Physiol. 2012;303:F339–49. doi: 10.1152/ajprenal.00158.2012. [DOI] [PubMed] [Google Scholar]
- 49.Masunaga A, Ito K, Asano T, Tsuda H, Asano T. Nicorandil increases renal nitric oxide (no), decreases trasforming growth factor (TGF)-β, and ameliorates renal injury in unilateral ureteral obstruction (uuo) in rats. J Urol. 2018;199:e115. [Google Scholar]
- 50.Mohamed YS, Ahmed LA, Salem HA, Agha AM. Role of nitric oxide and KATP channel in the protective effect mediated by nicorandil in bile duct ligation-induced liver fibrosis in rats. Biochem Pharmacol. 2018;151:135–42. doi: 10.1016/j.bcp.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 51.Hosseini-Tabatabaei A, Abdollahi M. Potassium channel openers and improvement of toxic stress: Do they have role in the management of inflammatory bowel disease? Inflamm Allergy Drug Targets. 2008;7:129–35. doi: 10.2174/187152808785748164. [DOI] [PubMed] [Google Scholar]
- 52.Stupakova EG, Lazareva GA, Gureev VV. Correction of morphofunctional disturbances arising when modeling preeclampsia with resveratrol and nicorandil. Res Results Pharmacol. 2018;4:59–71. [Google Scholar]
- 53.Hong SH, Kyeong KS, Kim CH, Kim YC, Choi W, Yoo RY, et al. Regulation of myometrial contraction by ATP-sensitive potassium (KATP) channel via activation of SUR2B and kir 6.2 in mouse. J Vet Med Sci. 2016;78:1153–9. doi: 10.1292/jvms.15-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hedlund P, Holmquist F, Hedlund H, Andersson KE. Effects of nicorandil on human isolated corpus cavernosum and cavernous artery. J Urol. 1994;151:1107–13. doi: 10.1016/s0022-5347(17)35193-5. [DOI] [PubMed] [Google Scholar]
- 55.Lee SW, Wang HZ, Christ GJ. Characterization of ATP-sensitive potassium channels in human corporal smooth muscle cells. Int J Impot Res. 1999;11:179–88. doi: 10.1038/sj.ijir.3900398. [DOI] [PubMed] [Google Scholar]


