Abstract
OBJECTIVE:
The inhibitory potential and percent inhibition of Syzygium aromaticum oil and fresh juice of Ocimum sanctum leaves on beta-lactamase enzyme of cecal samples of healthy broilers were studied on samples phenotypically positive for extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli.
MATERIALS AND METHODS:
Four hundred cecal samples screened for ESBL-producing E. coli were collected from 38 poultry sale outlets located in Jabalpur. The effect of S. aromaticum oil and O. sanctum leaves was seen by colorimetric assay with CENTA and Nitrocefin as chromogenic substrate.
RESULTS:
Mean absorbance value was inversely propotional to the inhibitory potential. Syzigium aromaticum exhibited 0.4±0.02 and 0.41±0.03 mean absorbance value, 28 per cent and 27 per cent of inhibition with CENTA and Nitrocefin respectively. Ocimum sanctum mean absorbance value and per cent inhibition with CENTA and Nitrocefin was 2.03±0.02 and 10.0 ; 1.97±0.06 and 10.0 respectively (p>0.05) showing non- significant difference in CENTA and Nitrocefin activity. Tazobactum (100 μM) as standard control exhibited a mean absorbance value of 0.12 ± 0.01 and 0.13 ± 0.01 and percent inhibition of 99.88 and 98 against CENTA and Nitrocefin, respectively. Combination of Ocimum sanctum and Syzigium aromaticum showed range of 1.69±0.05 to 1.90±0.08 1.61±0.06 to 1.92±0.08 of absorbance value with per cent inhibition of 14 to 15.9 with CENTA and Nitrocefin respectively.
CONCLUSION:
The results depicted that the inhibition of beta-lactamase enzyme activity with S. aromaticum oil was higher than that of O. sanctum leaf juice, and combination of both the herbs showed not much difference in activity.
Keywords: CENTA, colorimetric assay, Escherichia coli, extended-spectrum beta-lactamase, nitrocefin, Ocimum sanctum, Syzygium aromaticum
Introduction
Poultry farmers are facing a tremendous problem of antimicrobial resistance. Intensive rearing of poultry is very common nowadays. The usage of antibiotics at low doses as growth promoters to the entire flock by poultry farmers leads to high antibiotic selection pressure. It further results in increased percentage of resistant bacteria in fecal flora of poultry. Escherichia coli in poultry population mostly contains resistant phenotypes and hence, commensal E. coli acts as an indicator bacterium for the Gram-negative group of species.[1] In 1989, the first experimental sample holding extended-spectrum beta-lactamase (ESBL) E. coli property was obtained from ear discharge of a very young child from Munich (Germany) aging 4 months. It was known CTX-M-1; here, CTX means cefotaximase and M means Munich. Another cefotaximase designated as MEN-1 was obtained in France from an Italian patient, identical to CTX-M-1.[2,3] In Argentina, CTX-M-2 was obtained from Salmonella typhimurium isolated from meningitis, septicemia, and enteritis patients. Kumar et al., in 2012, reported 55.55% ESBL, including 66.67% from blood, 65% from aspirate, 57.14% from human feces, 55% from wound, and 54.67% from urine at various hospitals. In the USA, ESBL–producing E. coli ranged from 0% to 25%. Japan observed 0.1% prevalence of ESBL-producing E. coli.[4,5] The percentage of ESBL producers reported in India ranged 22%–75%.[2,3,4]
Shailesh et al. in 2013 also reported the occurrence of TEM, SHV, and OXA genes isolated from ulcerative foot of diabetic patients. These researches further proved the clinical relevance of ESBL enzyme-producing bacteria in humans as well.[6]
As indicator bacteria are present in the animal population, the incidence of resistance is better studied in E. coli which is an indicator bacterium.[7] In the period of 2 years, Gram-negative bacilli-producing ESBL enzyme has seemed as a major threat due to the clonal expansion of producer bacteria. The main reason behind such expansion is the lateral transfer of ESBL genes on plasmids.[5,8,9]
Antimicrobial resistance has made scientists to think for the use of plants as an alternative to antibiotics. Zaichang et al.[10] screened many ethanol extracts of Chinese herbs and medicines for beta-lactamase inhibitor activity through enzyme assay colorimetric method. Solanki and Selvanayagam[11] have also reported the inhibitory potential of Ocimum sanctum, Punica granatum, Syzygium aromaticum, Glycyrrhiza glabra, Piper longum, Zingiber officinalis, and 15 other plant extracts against ESBL enzyme using chromogenic substrate CENTA. The nitrocefin assay method[12] was used for screening inhibitors of beta-lactamase enzymes from the extracts of herbs from China. Reasonable judgment of these herbs was, however, hampered due to brown or yellow color of the extract solution.
Materials and Methods
Sample collection
In the present study, 400 cecal swab samples from freshly slaughtered broilers of 38 poultry sale outlets of Jabalpur were collected and screened for the presence of ESBL-producing E. coli.
Phenotypic screening of samples
After selective enrichment of samples in buffered peptone water and Mueller–Hinton broth (cefotaxime 2 μg/ml and aztreonam 4 μg/ml), phenotypic screening by double-disc synergy test method, combined disc test method (cefotaxime [30 μg] and cefotaxime + clavulanic acid [30 μg + 10 μg]), and enzyme minimum inhibitory concentration (MIC) strip were undertaken [Table 1].
Table 1.
Phenotypic screening of extended-spectrum beta-lactamase Escherichia coli
| Type of samples | CDDT method | DDST method | Ezy MIC strip |
|---|---|---|---|
| Positive samples | 135 | 115 | 84 |
| Negative samples | 0 | 20 | 51 |
| Total number of samples | 135 | 135 | 135 |
| Percentage sensitivity | 100 | 85 | 62 |
| χ2 | 67.7** | ||
**P<0.01 results are highly significant.DDST: Double-disc synergy test, CDDT: Combined disc diffusion test, MIC: Minimum inhibitory concentration
Isolation of beta-lactamase enzyme
Beta-lactamase enzyme was isolated from positive samples and was further subjected to the study of inhibitory potential of the two herbs. Overnight cultures of bacteria were freshly inoculated for 2-h growth in a rotary shaker, maintaining a temperature of 35°C. Inducer (penicillin G 400 μg/ml) was supplemented, with additional incubation of 4 h. The pellets of cells were poised by centrifugation, again suspended, and eroded with potassium phosphate buffer (0.05 M, pH 7.0) at 4°C. The bacteria were again centrifuged and suspended in the phosphate buffer that is ten-fold concentrated. Later, sonication of the sample was done for 5 min in an ice bath sonicator. Cellular debris was discarded by centrifugation at a speed of 40,000 rpm for 20 min at 4°C. The resulting supernatants were stored at −20°C till further use.
Grounding of the herbs
Oil of S. aromaticum – Commercial product of clove oil was used in the volume of 50 μl/well.
Fresh leaf juice of O. sanctum – Fresh leaves of O. sanctum were obtained from the Centre for Medicinal and Aromatic Plants, JNKVV, Jabalpur; after grinding the leaves in water, they were lyophilized to obtain dry powder which was later used at a concentration of 10 mg/ml (volume of 50 μl/well).
Colorimetric assay
CENTA and Nitrocefin-(Nitrocefin- Calbiochem make 5 mg) (CENTA – Calbiochem make 25 mg) were used as chromogenic substrates for the present assay. Nitrocefin (98% pure) was dissolved in dimethyl sulfoxide at a final concentration of 0.4 mmol/L. CENTA was dissolved in distilled water at a final concentration of 0.4 mmol/L. Colorimetric assay was carried out in a Thermo Scientific Multiskan EX 200-240V,50/60Hz, wavelength range 400-750nm, with (Ascent software, Bengaluru, Karnataka, India). The explicit activity of the enzyme extract was read in a 100-μl reaction mixture. Briefly, 8 μl of the enzyme was initially stabilized with the potassium phosphate buffer (100 mmol/l) with pH 7.0 for 10–15 min at 25°C. To each respective wells 50 micro litres of Tazobactum (Sigma Aldrich make) as standard beta-lactamase inhibitor in 100micromolar concentration, S. aromaticum oil and fresh leaves of O. sanctum (concentration10 mg/ml ) were added. After 25 min, 5 μl of the substrate was put into the wells and again incubated for 20 min at 25°C; potassium phosphate buffer was added to make the final volume of 100 μl. The plate was again incubated for 20–25 min at 25°C after desired incubation period color development was analyzed at 405 nm and 486 nm wavelength for CENTA and Nitrocefin, respectively, in the form of absorbance. In blank 95 micro litre potassium phosphate buffer was used along with the substrate.[13,14]
Preparation of standard curve
Stock solution of CENTA and nitrocefin was taken; nitrocefin was dissolved in Dimethylsulfoxide, making a final concentration of 4 μmol/ml, and this was further diluted to make a two-fold dilution of 2, 4, 8, 12, 16, 20, and 24 nmol/ml to prepare a standard curve. CENTA was dissolved in distilled water, and like nitrocefin, it was further diluted to make a two-fold dilution of 2, 4, 8, 16, 20, and 24 nmol/ml to prepare a standard curve from the stock solution of 4 μmol/ml [Tables 2 and 3].
Table 2.
Standard curve of Nitrocefin in distilled water
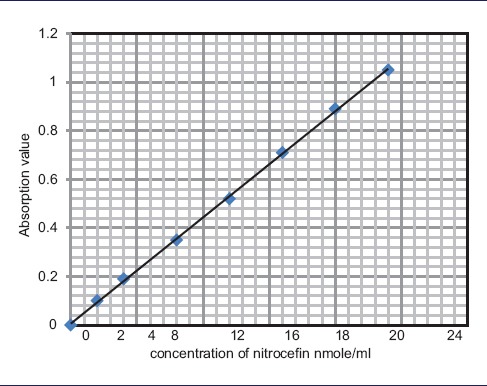 |
Table 3.
Standard curve of CENTA in distilled water
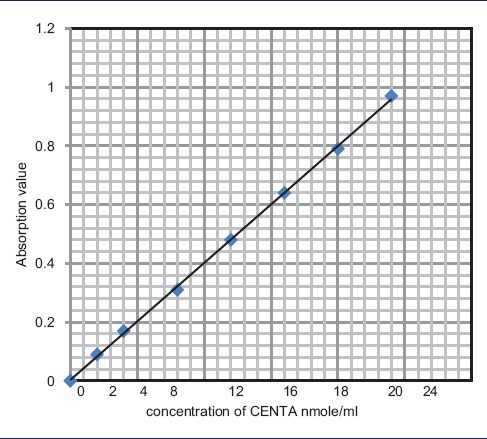 |
Results and Discussion
CENTA and Nitrocefin, chromogenic substrate analyzed the inhibitory potential and percent inhibition of the S. aromaticum oil and O. sanctum leaves on the beta-lactamase enzyme. S. aromaticum oil gave the percent inhibition in the range of 21%–32% with CENTA and 25%–35% of inhibition with nitrocefin and the inhibitory potential exhibited in the form of absorption value of 0.4 ± 0.01–0.5 ± 0.02 and 0.35 ± 0.04–0.43 ± 0.03 with CENTA and nitrocefin, respectively, giving a nonsignificant difference in the activity of the two substrates. Absorption value 2.00 ± 0.01–2.06 ± 0.03 and 9.6%–10.3% inhibition with CENTA and 1.75 ± 0.23–2.05 ± 0.02 and 9.3%–10.3% inhibition of enzyme activity with nitrocefin was observed with O. sanctum leaves [Tables 4 and 5]. Higher absorption value with O. sanctum leaves indicates its weak inhibitory potential and percent inhibition activity against β-lactamase enzyme as compared to S. aromaticum. Combination of two herbs gave absorption value in the lower range of 1.69 ± 0.05–1.90 ± 0.08 and percent inhibition of 12%–14% with CENTA and 1.61 ± 0.06–1.92 ± 0.08 and percent inhibition of 13.5%–15.9% with nitrocefin, respectively [Table 6]. Tazobactum (100 μM) (standard control) exhibited 0.12 ± 0.01 and 0.13 ± 0.01 (mean ± standard error) of absorption value and 99.88% and 98% inhibition of enzyme against CENTA and Nitrocefin, respectively [Table 7]. Statistically, no significant difference was found between the values of inhibitory potential and percent inhibition as shown by the combination of S. aromaticum and O. sanctum (1.80 ± 0.05 and 14.5% and 1.76 ± 0.06 and 14%) with CENTA and Nitrocefin, respectively, which further states that the chromogenic properties of Nitrocefin and CENTA can be used in simple and rapid assays for the detection of β-lactamases.
Table 4.
Comparative study of inhibitory potential and percent inhibition of Syzygium aromaticum oil by colorimetric method using (CENTA and Nitrocefin)
| Sample | Based on absorbance value |
|||
|---|---|---|---|---|
| Mean±SE CENTA (405 nm) (n=3) | Percent Inhibition CENTA | Mean±SE. Nitrocefin (486 nm) (n=3) | Percent Inhibition Nitrocefin | |
| 1 | 0.4±0.01 | 30 | 0.41±0.01 | 32 |
| 2 | 0.4±0.03 | 31 | 0.43±0.03 | 28 |
| 3 | 0.5±0.02 | 27 | 0.42±0.02 | 30 |
| 4 | 0.5±0.01 | 24 | 0.47±0.02 | 25 |
| 5 | 0.4±0.02 | 32 | 0.4±0.04 | 32 |
| 6 | 0.4±0.05 | 26 | 0.35±0.04 | 34 |
| Mean±SE (n=18) | 0.4±0.02 | 28 (n=6) | 0.41±0.03 | 27 (n=6) |
| t | 0.343 (NS) | |||
NS=Nonsignificant (P>0.05), SE=Standard error
Table 5.
Comparative study of inhibitory potential and percent inhibition of fresh leaf juice of Ocimum sanctum by colorimetric method using CENTA and Nitrocefin
| Sample number | Based on Absorbance value |
|||
|---|---|---|---|---|
| Mean±SE CENTA (405 nm) (n=3) | Percent Inhibition CENTA | Mean±SE Nitrocefin (486 nm) (n=3) | Percent Inhibition Nitrocefin | |
| 1 | 2.06±0.03 | 9.6 | 1.75±0.23 | 9.3 |
| 2 | 2.04±0.03 | 10.3 | 2.05±0.02 | 10.3 |
| 3 | 2.00±0.01 | 9.6 | 2.00±0.01 | 9.6 |
| 4 | 2.01±0.01 | 10.2 | 2.02±0.02 | 10.2 |
| 5 | 2.05±0.03 | 10.3 | 2.01±0.04 | 10.2 |
| 6 | 2.01±0.01 | 10.2 | 2.01±0.01 | 10.2 |
| Mean±SE (n=18) | 2.03±0.02 | 10.0 (n=6) | 1.97±0.06 | 10.0 (n=6) |
| t | 0.91 (NS) | |||
NS=Nonsignificant (P>0.05), SE=Standard error
Table 6.
Comparative study of inhibitory potential and percent inhibition of combination of Syzygium aromaticum oil and fresh leaves juice of Ocimum sanctum by colorimetric method using CENTA and Nitrocefin
| Sample | Based on absorbance value |
|||
|---|---|---|---|---|
| Mean±SE CENTA (405 nm) (n=3) | Percent Inhibition CENTA | Mean±SE Nitrocefin (486 nm) (n=3) | Percent Inhibition Nitrocefin | |
| 1 | 1.84±0.06 | 14.0 | 1.77±0.05 | 13.5 |
| 2 | 1.78±0.03 | 14.69 | 1.74±0.04 | 14.4 |
| 3 | 1.79±0.09 | 13.66 | 1.78±0.11 | 13.6 |
| 4 | 1.90±0.08 | 15.74 | 1.92±0.08 | 15.9 |
| 5 | 1.69±0.05 | 14 | 1.61±0.06 | 13.3 |
| 6 | 1.80±0.01 | 14.85 | 1.76±0.03 | 14.5 |
| Mean±SE (n=18) | 1.80±0.05 | 14.5 (n=6) | 1.76±0.06 | 14.2 (n=6) |
| t | 0.68 (NS) | |||
NS=Nonsignificant (P>0.05), SE=Standard error
Table 7.
Comparative study of inhibitory potential of tazobactum as standard control by colorimetric method using CENTA and Nitrocefin
| Sample | Based on absorbance value |
|||
|---|---|---|---|---|
| Mean±SE CENTA (405 nm) (n=3) | Percent Inhibition CENTA | Mean±SE Nitrocefin (486 nm) (n=3) | Percent Inhibition Nitrocefin | |
| 1 | 0.13±0.002 | 99.87 | 0.12±0.00 | 98.8 |
| 2 | 0.12±0.00 | 99.88 | 0.13±0.001 | 98.7 |
| 3 | 0.13±0.00 | 99.87 | 0.13±0.00 | 99.6 |
| 4 | 0.12±0.00 | 99.88 | 0.13±0.00 | 98.9 |
| 5 | 0.13±0.001 | 92.18 | 0.12±0.001 | 98.8 |
| 6 | 0.12±0.001 | 99.88 | 0.13±0.00 | 97.9 |
| Mean±SE (n=18) | 0.12±0.01 | 99.88 (n=6) | 0.13±0.01 | 98 (n=6) |
| t | 0.79 (NS) | |||
NS=Non-significant (P>0.05), SE=Standard error
Another study conducted on clove oil and rosemary essential oil exhibited antibacterial activity against multidrug-resistant bacteria.[8,15] Both these substrates being chromogenic in nature show change in color when they come in contact with β-lactamase enzyme; the color change is read as the absorption value in the microtiter plate assay. When the β-lactamase enzyme activity is inhibited, there is less color change, which, in turn, gives higher absorption value. In the present study, S. aromaticum oil yielded lower absorption value in comparison to the control; this indicates a higher percent inhibition activity of the β-lactamase enzyme by the S. aromaticum oil.
Solanki and Selvanayagam[11] have also reported the inhibitory potential of O. sanctum, P. granatum, S. aromaticum, G. glabra, P. longum, Z. officinalis, and 15 other plant extracts against ESBL enzyme by β-lactamase enzyme inhibition assay method using chromogenic substrate CENTA, and percent inhibition of β-lactamase activity was seen on the basis of optical density (OD) values. Rawat also gave similar reports of antibacterial activity of Tulsi, Neem, and Amla.[16] Previously, Nitrocefin was used as a testing substrate for observing interactions between β-lactamase enzyme and its inhibitors.[17,18,19] In the past, various concentrations of inhibitors were used to analyze a relative substrate affinity index on the basis of 5-min reaction between β-lactamase enzyme, its inhibitor, and the substrate (Nitrocefin). CENTA is a chromogenic cephalosporin reagent β-lactamase variable. It turns into chrome yellow from light yellow, which is interrelated to the chemical breakdown of the β-lactam ring in the presence of water. In order to detect isolates producing β-lactamase enzyme, CENTA can be used as an indicator component similar to other chromogens such as PADAC and Nitrocefin. It also shows some antimicrobial effect against E. coli, Klebsiella spp., Proteus mirabilis, Staphylococcus aureus, and nonenterococcal Streptococcus spp. Variation in the results of percent inhibition of herbs was also reported earlier with the Nitrocefin competition assay, and the use of β-lactamase inhibitors screened from the extracts of traditional Chinese herbs has also been reported by some workers.[12] Comparing the properties of both the substrate CENTA and Nitrocefin is quite unpretentious by frequently used microbiological media; this conclusion simulates the previous observation where CENTA was added to regularly used microbiological broth media (Mueller–Hinton, brain–heart infusion, Schaedler, and Trypticase soy) and no noteworthy general color change happened as observed by microplate reader in β-lactamase-producing isolates.[14]
Statistical analysis was done using standard t-test within two groups.[20]
Conclusion
E. coli-producing ESBL enzymes were found to be prevalent in healthy broilers of Jabalpur, with a prevalence percentage of 33.5, contributing to a major cause of antibiotic resistance. S. aromaticum oil showed maximum and O. sanctum fresh leaf juice showed minimum inhibitory potential and percent inhibition against ESBL enzymes. The combination of both herbs showed better activity than that of individual herbs. The difference in the observations could be due to the color of the herbs, which may have caused the hindrance in the absorbance value, giving a low percentage of inhibition rate. A crucial advantage of a colorimetric method is that change in color can be directly detected. Herbs alone or in combination can be an improved substitute to the antibiotics for the poultry and other livestock sectors. Phenotypic and genotypic characteristics of such resistant genes are same in humans and animals; hence inhibitory effect of the herbs used in the present study could be equally beneficial to humans as well.[11]
Financial support and sponsorship
The present study was the PhD research findings of the corresponding author. Financial support was given by the Director, Research Services, NDVSU, Jabalpur, and Dean, College of Veterinary Science and Animal Husbandry, Jabalpur.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The author is thankful to Director, Research Services, Nanaji Deshmukh Veterinary Science University, Jabalpur, and Dean, Veterinary College, Jabalpur, for providing monetary support.
References
- 1.Dierikx CM, van der Goot JA, Smith HE, Kant A, Mevius DJ. Presence of ESBL/AmpC-producing Escherichia coli in the broiler production pyramid: A descriptive study. PLoS One. 2013;8:e79005. doi: 10.1371/journal.pone.0079005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abhilash KP, Veeraraghavan B, Abraham OC. Epidemiology and outcome of bacteremia caused by extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli and Klebsiella spp. In a tertiary care teaching hospital in South India. J Assoc Physicians India. 2010;58(Suppl):13–7. [PubMed] [Google Scholar]
- 3.Aruna K, Mobashshera T. Prevalence of extended-spectrum beta-lactamase production among uropathogens in South Mumbai and its antibiogram pattern. EXCLI J. 2012;11:363–72. [PMC free article] [PubMed] [Google Scholar]
- 4.Dalela G. Prevalence of extended-spectrum beta-lactamase (ESBL) producers among gram-negative bacilli from various clinical isolates in a tertiary care hospital at Jhalawar, Rajasthan, India. J Clin Diagn Res. 2012;6:182–7. [Google Scholar]
- 5.National Nosocomial Infections Surveillance System. National nosocomial infections surveillance (NNIS) system report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–85. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 6.Shailesh K, Shahi Vinay K, Kumar SA. Detection of Escherichia coli and associated b-lactamases genes from diabetic foot ulcers by multiplex PCR and molecular modeling and docking of SHV-1, TEM-1, and OXA-1 b-Lactamases with clindamycin and piperacillin-tazobactam. Plos One. 2013;8:1–3. doi: 10.1371/journal.pone.0068234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.European Food Safety Authority (EFSA): Technical specifications for the analysis and reporting of data on antimicrobial resistance in the European union summary report. Eur Food Saf Authority J. 2012;10:2587, 53. [Google Scholar]
- 8.Joshi B, Sah GP, Basnet BB, Bhatt MR, Sharma D, Subedi K, et al. Phytochemical extraction and antimicrobial properties of different medicinal plants: Ocimum sanctum (Tulsi), Eugenia caryophyllata (Clove), Achyranthes bidentata (Datiwan) and Azadirachta indica (Neem) J Microbio Antimicrob. 2011;3:1–7. [Google Scholar]
- 9.Paterson DL, Hujer KM, Hujer AM, Yeiser B, Bonomo MD, Rice LB. Extended-spectrum beta-lactamases in Klebsiella pneumoniae bloodstream isolates from seven countries: Dominance and widespread prevalence of SHV- and CTX-M-type beta-lactamases. Antimicrob Agents Chemother. 2003;47:3554–60. doi: 10.1128/AAC.47.11.3554-3560.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zaichang Y, Xiaosheng Y, Yule N. A novel and rapid method to screen for β-lactamase inhibitors from extracts of herbs. Int J Integr Biol. 2009;6:62–4. [Google Scholar]
- 11.Solanki SS, Selvanayagam M. Beta-lactamase inhibition potential and antibacterial potentiation of certain medicinal plants and extracts against ESBL producers. Adv Biotechnol. 2013;12:6–10. [Google Scholar]
- 12.Papanicolaou GA, Medeiros AA. Discrimination of extended-spectrum beta-lactamases by a novel nitrocefin competition assay. Antimicrob Agents Chemother. 1990;34:2184–92. doi: 10.1128/aac.34.11.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dai X, Xiang S, Li J, Gao Q, Yang K. Development of a colorimetric assay for rapid quantitative measurement of clavulanic acid in microbial samples. Sci China Life Sci. 2012;55:158–63. doi: 10.1007/s11427-012-4287-x. [DOI] [PubMed] [Google Scholar]
- 14.Jones RN, Barry AL, Thornsberry C, Wilson HW. In vitro antimicrobial activity evaluation of cefodizime (HR221), a new semisynthetic cephalosporin. Antimicrob Agents Chemother. 1981;20:760–8. doi: 10.1128/aac.20.6.760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abdullah BH, Faisal S, Jumaa HW. A comparative study of the antibacterial activity of clove and rosemary essential oils on multidrug resistant bacteria. UK J Pharm Biosci. 2015;3:18–22. [Google Scholar]
- 16.Rawat S. Antimicrobial activity of Neem, Tulsi, henna and Amla against pathogenic bacteria. J Chem Pharm Res. 2015;7:1056–9. [Google Scholar]
- 17.Gutmann L, Ferré B, Goldstein FW, Rizk N, Pinto-Schuster E, Acar JF, et al. SHV-5, a novel SHV-type beta-lactamase that hydrolyzes broad-spectrum cephalosporins and monobactams. Antimicrob Agents Chemother. 1989;33:951–6. doi: 10.1128/aac.33.6.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hedges RW, Datta N, Kontomichalou P, Smith JT. Molecular specificities of R factor-determined beta-lactamases: Correlation with plasmid compatibility. J Bacteriol. 1974;117:56–62. doi: 10.1128/jb.117.1.56-62.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.James R. Relative substrate affinity index values: A method for identification of beta-lactamase enzymes and prediction of successful beta-lactam therapy. J Clin Microbiol. 1983;17:791–8. doi: 10.1128/jcm.17.5.791-798.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snedecor GW, Cochran WG. Statistical Methods. 8th ed. Calcutta, India: Oxford & IBH Publishing Co; 1994. pp. 243–657. [Google Scholar]


