Abstract
Objective:
Data are lacking regarding real-time prediction of post-operative complications following elective aneurysm repair. The neutrophil to lymphocyte ratio (NLR) has been evaluated as a predictor of outcomes following cardiac and infrapopliteal interventions, and is associated with poor outcomes for critical limb ischemia. We examined NLR and outcomes following abdominal aortic aneurysm (AAA) repair.
Methods:
Inpatients undergoing elective AAA repair (2008 to 2015) were selected from the Cerner Health Facts® database using ICD-9 procedure codes. Postoperative outcomes were identified using data from patient records within 1 week following surgery. NLR was calculated by dividing the absolute neutrophil count by the absolute lymphocyte count. The receiver operating characteristic (ROC) curve was analyzed to define low and high postoperative NLR groups. Chi-square analysis and multivariable logistic regression models were used to identify characteristics (demographics, diagnoses, postoperative NLR) associated with postoperative complications.
Results:
Elective AAA repair occurred in 5,655 patients. Of these, we could calculate postoperative NLR for 1908 (34%), with 1529 undergoing endovascular repair and 379 undergoing an open repair. Compared with patients with low postoperative NLR, patients with high postoperative NLR experienced longer hospital stays (5.7 vs. 2.6 days, p <.0001); higher rates of in-hospital death (2.9 vs. 1.4%, p = .002); higher rates of renal failure (11.6 vs. 3.9%, p <.0001), cardiac problems or myocardial infarction (3.8 vs. 1.2%, p = .0002), respiratory problems (13.3 vs. 5.8%, p <.0001), and infection (8.9 vs. 2.9%, p < .0001). The association between high postoperative NLR and adverse postoperative outcomes persisted on multivariable analysis. This included infection (OR 2.59, 95% CI 1.65–4.07), renal failure (OR 2.19, 95% CI 1.45–3.31), cardiac events (OR 2.41, 95% CI 1.21–4.77), and respiratory problems (OR 1.73, 95% CI 1.22–2.45).
Conclusions:
NLR was associated with adverse outcomes after elective endovascular and open AAA repair. An elevated NLR within 1 week after surgery was strongly associated with postoperative complications, and may identify at-risk patients who require closer follow-up. Given the perilous nature of vascular surgery and the risk-benefit ratio for prophylactic aneurysm repair, future study of postoperative outcome and preoperative NLR is needed to provide clinically important risk profiles prior to treatment decisions.
Keywords: abdominal aortic aneurysm repair, endovascular aneurysm repair, neutrophil-lymphocyte ratio
Introduction
Selecting patients for elective aneurysm repair has become a more complex clinical dilemma with the advent of endovascular aortic repair (EVAR). Elective open aortic aneurysm repair was historically reserved for patients who would experience long-term survival benefit and who were good-risk surgical candidates.1 Endovascular repair has expanded the pool of appropriate candidates due to reduced perioperative surgical stress, shorter recovery time, and early survival advantage.2,3 There are concerns, however, that the rate of rupture after EVAR and long-term survival may not be as favorable as for open repair, with mortality rates equalizing after 3 years.3–5
Appropriate selection of patients who will derive sustained benefit from elective aneurysm repair is of paramount importance in this endovascular era. Clinical frailty scores have shown good discrimination for mortality following open aneurysm repair and for life-threatening complications following both open and endovascular aneurysm repair.6–8 While biomarkers have been studied as prognostic indicators for outcomes in oncologic and cardiovascular disorders,9–12 they have not been as widely evaluated in vascular disorders. NLR has been reported as a strong marker for cancer-specific survival, overall survival and recurrence-free survival in urothelial cancer,13 and breast,14 colorectal,15 gastric16 and non-small-cell lung cancer17 outcomes now appear to be well-founded with a robust body of literature to support the association. The overarching mechanistic principle of using the NLR for cancer surveillance and progression of disease is based upon the imbalance of neutrophils and lymphocytes in patients with cancer and systemic inflammation that arises thereof.18 Systemic inflammation has been previously shown to play a key role in disease progression in cancer by promoting tumor angiogenesis, encouraging tumor metastasis and proliferation of cancer cells. Additionally a pro-inflammatory balance affects the response of tumors to different systemic chemotherapy agents.19 Neutrophils, specifically, have been shown to act as ‘tumor-promoting’ leukocytes with the ability to stimulate and suppress antitumor immune responses, promote tumor cell leakage and metastasis and secrete vascular endothelial growth factor (VEGF) that stimulates tumor angiogenesis.20
The neutrophil to lymphocyte ratio (NLR) and platelet to lymphocyte ratio (PLR) have been increasingly recognized as systemic markers of overall inflammation, and an association between atherosclerosis and NLR has been proposed.21–23 Despite mounting evidence associating elevated NLR with poor outcomes in these disease processes, the mechanism of action resulting in those observations remains unknown. The pathology of atherosclerosis is driven by inflammation, in turn regulated by white blood cells. Even when the white blood cell count is normal, a higher NLR has been associated with a larger atherosclerotic burden.24 No clear consensus exists, however, for what constitutes a normal NLR or PLR. The NLR is easily obtainable from the complete blood count with differential, and has been associated with adverse outcome in vascular disorders morbidity in a few preliminary studies of ruptured abdominal aortic aneurysm (AAA)25 and elective major vascular surgery.26 The understanding of the role of NLR in vascular disorders is still largely in a nascent stage and the potential of this novel biomarker has yet to be realized or studied.
AAA disease affects approximately 12.5% of men and 5.2% of women over the age of 74 and is responsible for over 10,000 deaths annually in the United States.27 As the size of an aneurysm increases, so does the rupture risk with mortality rates for ruptured AAA estimated to be approximately 90%.28 Due to the increasing perioperative benefits of endovascular aneurysm repair (EVAR) over traditional open aneurysm repair with a reduction in perioperative mortality of 1.4% after EVAR and 4.2% after open repair, over 80% of aneurysm repairs are now performed endovascularly.29
With this goal in mind, we undertook a national database study of the association of the NLR with adverse outcome following elective AAA repair.
Methods
Data Source
ICD-9-CM diagnosis and procedure codes were used to identify patients who underwent elective (non-ruptured) open or EVAR repair between September 2008 and October 2015 from the Cerner Health Facts® database.30 Health Facts is a proprietary database comprised of electronic medical records from hospitals and hospital systems that use Cerner Corporation’s electronic health record (EHR). It includes EHR data from over 400 acute-care hospitals and contains detailed information on diagnoses, procedures, laboratory studies, medications, patients, and encounters. Cerner applies rigorous validity checks to the data, removes identifiers, and standardizes data before including them in Health Facts using methods compliant with the Health Insurance Portability and Accountability Act (HIPAA).
We used these data to identify patients who had elective AAA procedures, determine surgical outcomes after each procedure, and evaluate patient and procedural characteristics that are associated with outcomes. We used the Agency for Healthcare Research and Quality’s (AHRQ) Clinical Classifications Software to group diagnosis codes into clinically relevant groups. Because Health Facts data are de-identified, informed patient consent was not needed. The Health Sciences Institutional Review Board at the University of Missouri deemed our study exempt.
Study Population
We included patients who underwent an open (procedure codes: 38.44 or 39.25) or EVAR (procedure codes: 39.71) procedure for a non-ruptured, infrarenal AAA (diagnosis codes: 441.02, 441.4, 447.72). The ICD-9 procedure codes for open and EVAR refer to discrete numeric codes that identify the procedure performed for a particular patient (e.g., 38.44 refers to: resection of vessel with replacement, aortic, abdominal). They were developed to identify procedures for billing, but are frequently used for research purposes.
To ensure we captured only infrarenal AAA procedures, we excluded ICD-9 codes that were related to thoracic aneurysm, thoracoabdominal aneurysm and other repairs of the suprarenal aorta. We also excluded patients who were less than 21 years old at admission; had an admission during which both EVAR and open procedures were performed; had admissions flagged as emergent or urgent; were discharged to hospice; had an adjacent encounter within 3 hours of admission or discharge of the encounter in which AAA repair occurred; were diagnosed with a ruptured aneurysm (diagnosis code 441.3); or had no laboratory data or diagnoses in the encounter record.
Analysis
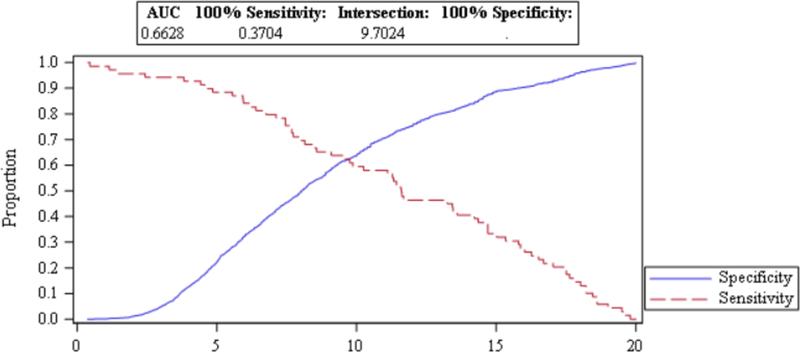
All analyses were performed in SAS version 9.4 (SAS Institute, Cary, NC). We calculated NLR by dividing the absolute neutrophil count by the absolute lymphocyte count. Post-operative NLR was divided into low (< 9.7024) and high (≥ 9.7024) values based on the results of a receiver operating characteristic (ROC) analysis that used a combined outcome of in-hospital mortality, cardiac problem, or myocardial infarction (see Figure 1).
FIGURE 1.
ROC CURVE ANALYSIS OF POSTOPERATIVE NLR
Chi-square analysis compared the characteristics of patients who had high vs. low post-operative NLR. Multivariable logistic regressions were examined to determine patient characteristics associated with post-operative outcomes. We used odds ratios (OR) and 95% confidence intervals (CI) to test for associations of covariates with outcomes. Because we excluded encounters with no laboratory data or diagnoses, we assumed that laboratory tests were not administered and diagnoses were not present when they were not found within the patient’s encounter record.
We developed separate multivariable logistic regression models for renal failure, in-hospital mortality, cardiac problems/myocardial infarction, respiratory problems, infection and length of stay > 10 days, using diagnoses, procedure type, patient demographic characteristics and NLR group as independent variables. We calculated the Charlson Index31 for each patient, based on the diagnoses (primary, secondary, discharge or billing) present during the encounter. We initially created a base model that included the procedure type (EVAR vs. open) and patient characteristics (age, sex, race/ethnicity, and Charlson Index). Comorbid diagnoses and encounter characteristics were allowed to enter into each base model using stepwise selection, using p = .10 as the significance level for variable entry and retention. Backwards elimination models were also run to determine whether the same variables were retained in the final models. Odds ratios (OR) and 95% confidence intervals (CI) were calculated. We assessed model discrimination with the c-statistic, where 1 indicates perfect fit and .5 is no better than a coin toss. Model calibration was assessed with the Hosmer-Lemeshow goodness-of-fit test, where p >.05 indicates adequate fit over the range of predicted outcome.
Results
Patient characteristics
There were 1,908 elective AAA procedures performed where post-operative NLR was able to be calculated. Endovascular repair was undertaken in 80% of patients (N = 1529; Table 1). Mean postoperative NLR was 9.0 with a range from 0.32–20. Postoperative NLR was split into groups low (< 9.7024) and high (≥ 9.7024) based upon the ROC analysis. Men comprised 76.4% of the sample, mean patient age was 72.2 years, and most (88.2%) were Caucasian. The mean Charlson Index was 2.1.
TABLE 1.
CHARACTERISTICS OF PARTICIPANTS (N=1908)a
| Low NLR | High NLR | ||||||
|---|---|---|---|---|---|---|---|
| Patient and Procedure Characteristics | N | (%) | N | (%) | N | (%) | p-value |
| Age (mean, sd) | 72.2 | (8.7) | 72.0 | (8.7) | 72.7 | (8.6) | .11 |
| Gender | .14 | ||||||
| Female | 451 | (23.6) | 264 | (22.5) | 187 | (25.4) | |
| Male | 1457 | (76.4) | 909 | (77.5) | 548 | (74.6) | |
| Race | .38 | ||||||
| Caucasian | 1682 | (88.2) | 1040 | (88.7) | 642 | (87.4) | |
| Other race | 226 | (11.8) | 133 | (11.3) | 93 | (12.6) | |
| Charlson Index (mean, sd) | 2.1 | (1.3) | 2.1 | (1.2) | 2.3 | (1.5) | .0004 |
| Procedure type | <.0001 | ||||||
| Endovascular | 1529 | (80.1) | 1040 | (88.7) | 489 | (66.5) | |
| Open | 379 | (19.9) | 133 | (11.3) | 246 | (33.5) | |
| NLR (mean, sd) | 9.0 | (4.6) | 5.93 | (2.1) | 13.8 | (2.9) | <.0001 |
| Low NLR | 1173 | (41.5) | -- | -- | -- | -- | |
| High NLR | 735 | (38.5) | -- | -- | -- | -- | |
| Pre-existing conditions | |||||||
| Chronic heart disease | 885 | (46.4) | 551 | (47.0) | 334 | (45.4) | .51 |
| Chronic kidney disease | 234 | (12.3) | 128 | (10.9) | 106 | (14.4) | .02 |
| Diabetes | 386 | (20.2) | 234 | (19.9) | 152 | (20.7) | .69 |
| Outcomes | |||||||
| Renal failure | 131 | (6.9) | 46 | (3.9) | 85 | (11.6) | <.0001 |
| Cardiac problems/myocardial infarction | 42 | (2.2) | 14 | (1.2) | 28 | (3.8) | .0002 |
| Respiratory problems | 166 | (8.7) | 68 | (5.8) | 98 | (13.3) | <.0001 |
| Infection | 100 | (5.2) | 34 | (2.9) | 66 | (8.9) | <.0001 |
| In-hospital death | 37 | (1.9) | 16 | (1.4) | 21 | (2.9) | .02 |
| Length of stay (mean, sd) | 3.8 | (5.8) | 2.6 | (3.8) | 5.7 | (7.6) | <.00001 |
| > 10 days | 135 | (7.1) | 29 | (2.5) | 106 | (14.4) | <.0001 |
NLR = neutrophil to lymphocyte ratio; sd = standard deviation
Low NLR < 9.7024, High NLR >= 9.7024
Number and (percentage) unless otherwise indicated
Chronic heart disease was present in 46% of patients, with chronic kidney disease present in 12% and diabetes in 20%. Regarding overall outcomes; in-hospital mortality was low (2%), with renal failure occurring in 7% of patients, cardiac/MI complications in 2.2%, respiratory problems in 8.7%, infection in 5% and a prolonged hospital stay greater than 10 days in 7% of all patients. Compared with patients with low postoperative NLR, patients with high postoperative NLR experienced (Table 1) longer hospital stays (5.7 vs. 2.6 days, p <.0001); higher rates of in-hospital death (2.9 vs. 1.4%, p = .002); and higher rates of renal failure (11.6 vs. 3.9%, p <.0001), cardiac problems/myocardial infarction (3.8 vs. 1.2%, p = .0002), respiratory problems (13.3 vs. 5.8%, p <.0001), or infection (8.9 vs. 2.9%, p < .0001).
Multivariable analysis
Analyses were performed for six post-operative outcomes (Table 2): renal failure, in-hospital mortality, cardiac/MI complications, respiratory problems, infection and length of stay > 10 days. The Hosmer-Lemeshow statistic demonstrated adequate fit for all models except the model for length of stay > 10 days (p = .001). Because model fit for this outcome was so poor, results for extended length of stay are not presented or discussed.
TABLE 2.
MULTIVARIABLE LOGISTIC REGRESSION MODELS
| OR (95% Cl) | p-value | |
|---|---|---|
| Renal Failure (n = 131) | ||
| NLR (high vs. low) | 2.19 (1.45–3.31) | .0002 |
| Age | 1.02 (0.99–1.04) | .16 |
| Gender (female vs. male) | 1.82 (1.19–2.77) | .005 |
| Race (Caucasian vs. other race) | 0.78 (0.44–1.39) | .39 |
| Procedure type (open vs. endovascular) | 4.30 (2.80–6.60) | <.0001 |
| Chronic heart disease | 1.13 (0.76–1.68) | .54 |
| Chronic kidney disease | 7.88 (5.18–12.0) | <.0001 |
| Diabetes | 1.50 (0.96–2.35) | .07 |
| In Hospital Mortality (n = 37) | ||
| NLR (high vs. low) | 0.96 (0.46–1.98) | .90 |
| Age | 1.06 (1.02–1.11) | .008 |
| Gender (female vs. male) | 3.11 (1.54–6.27) | .001 |
| Race (Caucasian vs. other race) | 1.80 (0.42–7.73) | .43 |
| Procedure type (open vs. endovascular) | 11.84 (5.26–26.6) | <.0001 |
| Chronic heart disease | 1.27 (0.62–2.59) | .51 |
| Chronic kidney disease | 2.74 (1.25–6.00) | .01 |
| Diabetes | 2.46 (1.16–5.24) | .01 |
| Cardiac Complication/MI (n = 42) | ||
| NLR (high vs. low) | 2.41 (1.21–4.77) | .01 |
| Age | 1.03 (0.99–1.08) | .09 |
| Gender (female vs. male) | 1.80 (0.93–3.48) | .07 |
| Race (Caucasian vs. other race) | 0.91 (0.35–2.39) | .84 |
| Procedure type (open vs. endovascular) | 2.49 (1.24–5.00) | .01 |
| Chronic heart disease | 1.39 (0.73–2.66) | .31 |
| Chronic kidney disease | 2.58 (1.27–5.25) | .009 |
| Diabetes | 1.39 (0.68–2.87) | .37 |
| Respiratory Problems (n = 166) | ||
| NLR (high vs. low) | 1.73 (1.22–2.45) | .003 |
| Age | 1.01 (0.99–1.03) | .63 |
| Gender (female vs. male) | 2.11 (1.48–3.00) | <.0001 |
| Race (Caucasian vs. other race) | 1.07 (0.63–1.81) | .80 |
| Procedure type (open vs. endovascular) | 4.03 (2.79–5.83) | <.0001 |
| Chronic heart disease | 0.99 (0.70–1.40) | .96 |
| Chronic kidney disease | 1.75 (1.13–2.71) | .0128 |
| Diabetes | 1.90 (1.30–2.79) | .001 |
| Infection (n = 100) | ||
| NLR (high vs. low) | 2.59 (1.65–4.07) | <.0001 |
| Age | 1.01 (0.99–1.04) | .37 |
| Gender (female vs. male) | 3.26 (2.13–5.00) | <.0001 |
| Race (Caucasian vs. other race) | 0.90 (0.48–1.68) | .73 |
| Procedure type (open vs. endovascular) | 2.64 (1.66–4.19) | <.0001 |
| Chronic heart disease | 0.90 (0.59–1.39) | .63 |
| Chronic kidney disease | 1.13 (0.62–2.06) | .70 |
| Diabetes | 2.11 (1.32–3.38) | .001 |
NLR = neutrophil to lymphocyte ratio; OR = odds ratio; CI = confidence interval
High post-operative NLR was associated with a 2.6 times increased odds of infection compared with low NLR (OR 2.59; 95% CI 1.65–4.07), a 2.4 times increased odds of cardiac complications/myocardial infarctions compared with low NLR (OR 2.41, 95% CI 1.21–4.77), a 2.2 times increased odds of renal failure compared with low NLR (OR 2.19, 95% CI 1.45–3.31), and a 1.7 times increased odds of respiratory failure compared with low NLR (OR 1.73; CI 1.22–2.45).
Renal failure after aneurysm repair was associated with chronic kidney disease (OR 7.88, 95% CI 5.18–12.0), having an open procedure performed (OR 4.30, 95% CI 2.80–6.60), and female sex (OR 1.82, 95% CI 1.19–2.77). Model fit was adequate (p = .49) and discrimination was moderate (c-statistic = .83).
In-hospital mortality was associated with open AAA repair (OR 11.8; 95% CI 5.26–26.6), female sex (OR 3.11; 1.54–6.27), chronic kidney disease (OR 2.74; 1.25–6.00), diabetes (OR 2.46; 95% CI 1.16–5.24) and increased age (OR 1.06; 95% CI 1.02–1.11). No association was found, however, between in-hospital mortality and post-operative NLR. Model discrimination was moderate (c-statistic = .86) and fit was adequate (p = .76).
Two covariates were associated with developing cardiac complications/myocardial infarctions following AAA repair: chronic kidney disease (OR 2.58, 95% CI 1.27–5.25) and having an open procedure performed (OR 2.49, 95% CI 1.24–5.00). Model fit was adequate (p = .12) and discrimination was modest (c-statistic = .77).
Respiratory problems were associated with open AAA repair (OR 4.03; 95% CI 2.79–5.83), female sex (OR 2.11; 95% CI 1.48–3.00), diabetes (OR 1.90; 95% CI 1.30–2.79), chronic kidney disease (OR 1.75, 95% CI 1.13–2.71). Similarly, infection in the post-operative period was associated with female sex (OR 3.26; 95% CI 2.13–5.00), diabetes (OR 2.11; 95% CI 1.32–3.38) and open AAA repair (OR 2.64; 95% CI 1.66–4.19). Model discrimination was modest and fit was adequate for both of these models as well.
Discussion
This retrospective large cohort study of outcomes following elective AAA repair identified common themes in terms of comorbidities and risk factors for complications. Broadly, open AAA repair, female gender, diabetes and chronic kidney disease were all associated with poor outcome following aneurysm repair. These risk factors were significantly associated for all six postoperative outcomes evaluated in this study; renal failure, in-hospital mortality, cardiac/MI, respiratory problems, infection and length of stay > 10 days, which were chosen as these represented clinically the most impactful complications following major vascular surgery.
The analysis was also focused on the strong association of elevated postoperative NLR with these six postoperative complications. An elevated NLR was independently associated with renal failure, cardiac/MI, respiratory problems, infection and length of stay > 10 days. This analysis highlights an important association between elevated NLR and poor outcome following AAA repair that has not been well-described previously.
The NLR has been studied in more detail in the cancer literature, with very little work described in the cardiovascular field, however, the association of inflammation and an elevated NLR has been recognized in the development of cardiovascular disorders and specifically atherosclerosis. Given the status as a biomarker for inflammation, as seen in the cancer literature, the role of NLR in atherosclerotic disorders has been more intensely studied recently. Balta et al. describe this relationship in a review describing the predictive effect of NLR on death, myocardial infarction, high-risk for coronary artery disease,32,33 as well as an association with classic atherosclerotic risk factors such as diabetes mellitus, hypertension, metabolic syndrome, obesity, hyperlipidemia and endothelial dysfunction.34 Animal models of atherosclerosis additionally demonstrate neutrophil invasion of atherosclerotic plaque and release of proteolytic enzymes by neutrophils may be responsible for plaque vulnerability – a known risk factor for myocardial infarction35 and postulated to be also involved in carotid thromboembolic processes.36
Moving from animal models and experimental evidence of the role of NLR in inflammation and atherosclerosis to the clinical realm; Bhutta et al. published their findings in a multi-institutional review of elective major vascular surgery evaluating the role of the NLR in predicting medium-term survival.26 The authors describe the two-year mortality associated with carotid endarterectomy, AAA repair, and lower limb revascularization and used a preoperative NLR cutoff of > 5 to categorize patients into low- and high-NLR groups. Multivariate analysis was used to evaluate all significant factors on univariate analysis and revealed that patients with a preoperative NLR of > 5 were more likely to die within two years following elective major vascular surgery. The clinical value of this information, despite the heterogeneity of the surgery and risk factors evaluated, should not be underestimated. The ability to predict poor outcomes following elective surgery, including death, is of paramount importance in a field such as vascular surgery, where many operations are performed ‘prophylactically’ to reduce the risk of a catastrophic event occurring. AAA surgery and carotid artery procedures exemplify vascular surgery procedures with a significant risk of complications that are performed on patients who are already at high risk to prevent a catastrophe such as aneurysm rupture or stroke.
Predicting outcome in vascular surgery has remained a fundamental principle when discussing operative or interventional treatment for any disorder due to the risks involved. Predictive tools and calculators of risks are sparse for vascular procedures, although these tools have been published and are used in clinical practice for specific cancer-related mortality decisions such as in prostate cancer37 or breast cancer.38 Vascular surgery risk calculators have focused primarily on adverse cardiac risk following common vascular surgery operations with the most commonly used and well-validated online risk calculators being the National Surgical Quality Improvement Program (NSQIP), which is a nationally validated, risk-adjusted, outcomes based program to measure and improve the quality of surgical care,39 the Revised Cardiac Risk Index (RCRI), which is a validated tool for estimating a patient’s risk of perioperative cardiac complications using patient-specific comorbidities40 and the Vascular Study Group of New England Cardiac Risk Index (VSG-CRI), which is a similar risk prediction tool based upon specific comorbidities in the vascular population.41 Although major adverse cardiac events (MACE) are seen commonly in vascular patients and are clinically important, there are presently no robust risk prediction tools for non-cardiac complications, such as stroke after carotid intervention, bypass thrombosis or restenosis following lower extremity revascularization. Furthermore, there is scant information regarding the risk of catastrophic events such as stroke or aneurysm rupture in patients who are being serially followed with these disorders although interest in the clinical value of NLR in vascular disorders is increasing.
A recent publication by Massiot et al. describes a moderate single-institution series of consecutive patients who underwent carotid endarterectomy for internal carotid artery (ICA) stenosis in the context of the NLR. The authors captured 270 patients and divided them into four groups based upon NLR quartiles. The proportion of patients with symptomatic ICA stenosis was significantly higher in the highest NLR quartile group than in the other groups. The authors conclude that a high preoperative NLR was significantly associated with symptomatic ICA stenosis and warrants further study as predictors of postoperative outcomes for carotid patients.42
Lareyre et al. similarly examine the association of high NLR with symptomatic and ruptured thoracic aortic aneurysm in a small single-institution series. The authors describe a significantly greater proportion of patients with pain or with ruptured thoracic aortic aneurysm (TAA) in the group with NLR > 3.5 compared with those < 3.5. Interestingly a higher preoperative NLR was not associated with 30-day overall mortality and morbidity. The authors conclude that circulating neutrophils and lymphocytes may be markers of aortic rupture and further studies are required to evaluate the potential predictive value of NLR on outcome and in the pathogenesis of TAA.43
In this present study, we demonstrated that a high postoperative NLR value was associated with significant complications following elective aneurysm repair. This was independent of the treatment modality and other comorbidities and risk factors of the patients. We did not find a significant correlation with preoperative NLR and postoperative outcome and this may have been due to the much lower numbers of patients with available preoperative data (470 patients) as opposed to those with postoperative values (1,536 patients).
This study has limitations secondary to using ICD-9 codes, as coding may vary between institutions. Because procedure type was not randomly assigned, selection bias could explain the differences between groups; we addressed this by controlling for potential confounders in multivariable regression models. Residual confounding is still possible despite controlling for multiple covariates.44 Cerner Corporations’ Health Facts is a proprietary database comprised of electronic clinical records from hospitals and hospital systems that use Cerner’s electronic health record, and the ethnicity and patient mix may not be representative of the wider US population. Proportions by ethnicity, however, are comparable to our previously published evaluation of carotid intervention using Medicare data, which is considered representative of the elderly US population.45
Conclusions
An elevated NLR < 1 week after surgery was strongly associated with postoperative complications after elective endovascular and open AAA repair. NLR has been identified to be associated with poor prognosis for cardiovascular and oncologic disorders and holds promise as a novel biomarker of outcome. Future study is planned to evaluate the role of preoperative NLR, in conjunction with other traditional predictors of outcome, as part of a multimodal risk prediction tool to stratify patients at risk for significant complications following vascular procedures.
Acknowledgments
Support from the Agency for Healthcare Research and Quality was used to fund the research reported in this publication (R24HS022140). The authors take sole responsibility in the content of this report, which does not necessarily represent the official views of the Agency for Healthcare Research and Quality.
Footnotes
Presented at the Midwestern Vascular Surgery Society 42nd Annual Meeting, September 13th–15th 2018, St. Louis, Missouri
The authors declare no conflicts of interest
References
- 1.Morisaki K, Matsumoto T, Matsubara Y, et al. Elective endovascular vs. open repair for abdominal aortic aneurysm in octogenarians. Vascular 2016;24:348–54. [DOI] [PubMed] [Google Scholar]
- 2.Patel R, Sweeting MJ, Powell JT, Greenhalgh RM, EVAR trial investigators. Endovascular versus open repair of abdominal aortic aneurysm in 15-years’ follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): A randomised controlled trial. Lancet 2016;388:2366–74. [DOI] [PubMed] [Google Scholar]
- 3.Schermerhorn ML, Buck DB, O’Malley AJ, et al. Long-term outcomes of abdominal aortic aneurysm in the Medicare population. N Engl J Med 2015;373:328–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.United Kingdom Evar Trial Investigators, Greenhalgh RM, Brown LC, et al. Endovascular versus open repair of abdominal aortic aneurysm. N Engl J Med 2010;362:1863–71. [DOI] [PubMed] [Google Scholar]
- 5.Lederle FA, Freischlag JA, Kyriakides TC, et al. Long-term comparison of endovascular and open repair of abdominal aortic aneurysm. N Engl J Med 2012;367:1988–97. [DOI] [PubMed] [Google Scholar]
- 6.Ehlert BA, Najafian A, Orion KC, Malas MB, Black JH, 3rd, Abularrage CJ Validation of a modified Frailty Index to predict mortality in vascular surgery patients. J Vasc Surg 2016;63:1595–601.e2. [DOI] [PubMed] [Google Scholar]
- 7.Arya S, Kim SI, Duwayri Y, et al. Frailty increases the risk of 30-day mortality, morbidity, and failure to rescue after elective abdominal aortic aneurysm repair independent of age and comorbidities. J Vasc Surg 2015;61:324–31. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Zou Y, Zhao J, et al. The impact of frailty on outcomes of elderly patients after major vascular surgery: A systematic review and meta-analysis. Eur J Vasc Endovasc Surg 2018;56:591–602. [DOI] [PubMed] [Google Scholar]
- 9.Zhang H, Zhang L, Zhu K, et al. Prognostic significance of combination of preoperative latelet count and Neutrophil-Lymphocyte Ratio (COP-NLR) in patients with non-small cell lung cancer: Based on a large cohort study. PLoS One 2015;10:e0126496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Ran Y. Prognostic role of elevated platelet count in patients with lung cancer: A systematic review and meta-analysis. Int J Clin Exp Med 2015;8:5379–87. [PMC free article] [PubMed] [Google Scholar]
- 11.He J, Li J, Wang Y, Hao P, Hua Q. Neutrophil-to-lymphocyte ratio (NLR) predicts mortality and adverse-outcomes after ST-segment elevation myocardial infarction in Chinese people. Int J Clin Exp Pathol 2014;7:4045–56. [PMC free article] [PubMed] [Google Scholar]
- 12.Bolca O, Gungor B, Ozcan KS, et al. The neutrophil-to-lymphocyte ratio is associated with bare-metal stent restenosis in STEMI patients treated with primary PCI. Coron Artery Dis 2015;26:402–8. [DOI] [PubMed] [Google Scholar]
- 13.Marchioni M, Primiceri G, Ingrosso M, et al. The Clinical Use of the Neutrophil to Lymphocyte Ratio (NLR) in Urothelial Cancer: A Systematic Review. Clin Genitourin Cancer 2016;14:473–84. [DOI] [PubMed] [Google Scholar]
- 14.Ethier JL, Desautels D, Templeton A, Shah PS, Amir E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res 2017;19:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haram A, Boland MR, Kelly ME, Bolger JC, Waldron RM, Kerin MJ. The prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic review. J Surg Oncol 2017;115:470–9. [DOI] [PubMed] [Google Scholar]
- 16.Miyamoto R, Inagawa S, Sano N, Tadano S, Adachi S, Yamamoto M. The neutrophil-to-lymphocyte ratio (NLR) predicts short-term and long-term outcomes in gastric cancer patients. Eur J Surg Oncol 2018;44:607–12. [DOI] [PubMed] [Google Scholar]
- 17.Diem S, Schmid S, Krapf M, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer 2017;111:176–81. [DOI] [PubMed] [Google Scholar]
- 18.Yin Y, Wang J, Wang X, et al. Prognostic value of the neutrophil to lymphocyte ratio in lung cancer: A meta-analysis. Clinics (Sao Paulo, Brazil) 2015;70:524–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature 2008;454:436–44. [DOI] [PubMed] [Google Scholar]
- 20.Faria SS, Fernandes PC Jr., Silva MJ, et al. The neutrophil-to-lymphocyte ratio: a narrative review. Ecancermedicalscience 2016;10:702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balta S, Celik T, Mikhailidis DP, et al. The relation between atherosclerosis and the Neutrophil-Lymphocyte Ratio. Clin Appl Thromb Hemost 2016;22:405–11. [DOI] [PubMed] [Google Scholar]
- 22.Afari ME, Bhat T. Neutrophil to lymphocyte ratio (NLR) and cardiovascular diseases: An update. Expert Rev Cardiovasc Ther 2016;14:573–7. [DOI] [PubMed] [Google Scholar]
- 23.Erturk M, Cakmak HA, Surgit O, et al. The predictive value of elevated neutrophil to lymphocyte ratio for long-term cardiovascular mortality in peripheral arterial occlusive disease. J Cardiol 2014;64:371–6. [DOI] [PubMed] [Google Scholar]
- 24.Balta S, Ozturk C, Balta I, et al. The Neutrophil-Lymphocyte Ratio and Inflammation. Angiology 2016;67:298–9. [DOI] [PubMed] [Google Scholar]
- 25.Kordzadeh A, Malietzis G, Browne T, Prionidis I, Panayiotopoulos YP. Neutrophil to lymphocyte ratio (NLR) of five predicts 30-day morbidity in ruptured abdominal aortic aneurysms (rAAA): A retrospective cohort study. International Journal of Surgery 2015;15:45–8. [DOI] [PubMed] [Google Scholar]
- 26.Bhutta H, Agha R, Wong J, Tang TY, Wilson YG, Walsh SR. Neutrophil-lymphocyte ratio predicts medium-term survival following elective major vascular surgery: A cross-sectional study. Vasc Endovascular Surg 2011;45:227–31. [DOI] [PubMed] [Google Scholar]
- 27.Keisler B, Carter C. Abdominal aortic aneurysm. Am Fam Physician 2015;91:538–43. [PubMed] [Google Scholar]
- 28.Scott RA, Wilson NM, Ashton HA, Kay DN. Influence of screening on the incidence of ruptured abdominal aortic aneurysm: 5-year results of a randomized controlled study. Br J Surg 1995;82:1066–70. [DOI] [PubMed] [Google Scholar]
- 29.Deery SE, Schermerhorn ML. Open versus endovascular abdominal aortic aneurysm repair in Medicare beneficiaries. Surgery 2017;162:721–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kosiborod M, Inzucchi SE, Goyal A, et al. Relationship between spontaneous and iatrogenic hypoglycemia and mortality in patients hospitalized with acute myocardial infarction. JAMA 2009;301:1556–64. [DOI] [PubMed] [Google Scholar]
- 31.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373–83. [DOI] [PubMed] [Google Scholar]
- 32.Horne BD, Anderson JL, John JM, et al. Which white blood cell subtypes predict increased cardiovascular risk? J Am Coll Cardiol 2005;45:1638–43. [DOI] [PubMed] [Google Scholar]
- 33.Papa A, Emdin M, Passino C, Michelassi C, Battaglia D, Cocci F. Predictive value of elevated neutrophil-lymphocyte ratio on cardiac mortality in patients with stable coronary artery disease. Clin Chim Acta 2008;395:27–31. [DOI] [PubMed] [Google Scholar]
- 34.Balta S, Kurtoglu E, Kucuk U, Demirkol S, Ozturk C. Neutrophil-lymphocyte ratio as an important assessment tool. Expert Rev Cardiovasc Ther 2014;12:537–8. [DOI] [PubMed] [Google Scholar]
- 35.Eriksson EE, Xie X, Werr J, Thoren P, Lindbom L. Direct viewing of atherosclerosis in vivo: Plaque invasion by leukocytes is initiated by the endothelial selectins. FASEB J 2001;15:1149–57. [DOI] [PubMed] [Google Scholar]
- 36.Kolodgie FD, Yahagi K, Mori H, et al. High-risk carotid plaque: lessons learned from histopathology. Semin Vasc Surg 2017;30:31–43. [DOI] [PubMed] [Google Scholar]
- 37.Latosinska A, Frantzi M, Merseburger AS, Mischak H. Promise and Implementation of Proteomic Prostate Cancer Biomarkers. Diagnostics 2018;8:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shachar SS, Muss HB. Internet tools to enhance breast cancer care. NPJ breast cancer 2016;2:16011-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yap MKC, Ang KF, Gonzales-Porciuncula LA, Esposo E. Validation of the American College of Surgeons Risk Calculator for preoperative risk stratification. Heart Asia 2018;10:e010993–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999;100:1043–9. [DOI] [PubMed] [Google Scholar]
- 41.Bertges DJ, Goodney PP, Zhao Y, et al. The Vascular Study Group of New England Cardiac Risk Index (VSG-CRI) predicts cardiac complications more accurately than the Revised Cardiac Risk Index in vascular surgery patients. J Vasc Surg 2010;52:674–83, 83.e1–83.e3. [DOI] [PubMed] [Google Scholar]
- 42.Massiot N, Lareyre F, Voury-Pons A, et al. High Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio are Associated with Symptomatic Internal Carotid Artery Stenosis. J Stroke Cerebrovasc Dis 2019;28:76–83. [DOI] [PubMed] [Google Scholar]
- 43.Lareyre F, Raffort J, Le D, et al. High Neutrophil to Lymphocyte Ratio Is Associated With Symptomatic and Ruptured Thoracic Aortic Aneurysm. Angiology 2018;69:686–91. [DOI] [PubMed] [Google Scholar]
- 44.Behrendt CA, Debus ES, Mani K, Sedrakyan A. The strengths and limitations of claims based research in countries with fee for service reimbursement. Eur J Vasc Endovasc Surg 2018;56:615–6. [DOI] [PubMed] [Google Scholar]
- 45.Galinanes EL, Dombroviskiy VY, Hupp CS, Kruse RL, Vogel TR. Evaluation of readmission rates for carotid endarterectomy versus carotid artery stenting in the US Medicare population. Vasc Endovascular Surg 2014;48:217–23. [DOI] [PubMed] [Google Scholar]