Abstract
BACKGROUND
Evaluation of stable symptomatic outpatients with suspected coronary artery disease (CAD) may be challenging because they have a wide range of cardiovascular risk. The role of troponin testing to assist clinical decision making in this setting is unexplored.
OBJECTIVES
This study sought to evaluate the prognostic meaning of single-molecule counting high-sensitivity troponin I (hsTnI) (normal range <6 ng/l) among outpatients with stable chest symptoms and suspected CAD.
METHODS
Participants with available blood samples in PROMISE (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) were studied, and hsTnI results were analyzed relative to the primary outcome of death, acute myocardial infarction (MI), or hospitalization for unstable angina by 1 year. The secondary outcome was the composite of cardiovascular death or acute MI.
RESULTS
The study sample consisted of 4,021 participants; 98.6% had measurable hsTnI concentrations. The median hsTnI value was 1.6 ng/l. In upper hsTnI quartiles, patients had higher-risk clinical profiles. Higher hsTnI concentrations were associated with greater event probabilities for death, acute MI, or hospitalization for unstable angina. In multivariable models, hsTnI concentrations independently predicted death, acute MI, or hospitalization for unstable angina (hazard ratio: 1.54 per increase in log-hsTnI interquartile range; p < 0.001) and cardiovascular death or acute MI (hazard ratio: 1.52 per increase in log-hsTnI interquartile range; p < 0.001) and were particularly associated with near-term events, compared with longer follow-up.
CONCLUSIONS
In symptomatic outpatients with suspected CAD, higher concentrations of hsTnI within the normal range were associated with heightened near-term risk for death, acute MI, or hospitalization. (Prospective Multicenter Imaging Study for Evaluation of Chest Pain [PROMISE]; )
Keywords: chest pain, stable angina, troponin
It is estimated that 3.4 million adult ambulatory patients are currently affected by stable symptoms suggestive of coronary ischemia, with rates rising with age; >10% of men and women older than age 80 years have angina (1). At first presentation, evaluation and management of patients with stable symptoms and suspected coronary artery disease (CAD) may be challenging. In contrast to those with acute coronary syndromes, patients with more stable presentations have a broader range of risk for progression to complications such as acute myocardial infarction (MI) or death. The ability to recognize the uncommon, higher-risk patient within a generally low-risk population is difficult on clinical grounds alone, and currently recommended risk scores substantially overestimate hazard (2,3). Beyond clinical history and physical examination, adjunctive testing is widely used to assist in detection of obstructive CAD. Such testing may include stress modalities with or without imaging, as well as coronary computed tomography angiography (CTA). Although useful, these imaging modalities may have limitations, including availability, cost, need for specialized interpretation, and exposure to ionizing radiation. Further, in such patients, stress testing is rarely abnormal, and among those referred to invasive coronary angiography, obstructive CAD is uncommon (3).
Use of high-sensitivity troponin (hsTn) for diagnostic and prognostic evaluation of patients with suspected acute MI is well established. In groups of patients with stable ischemic heart disease, hsTn is prognostic for incident MI or death (4–7). However, such analyses were performed on complete groups of patients with established CAD. In contrast, the role of hsTn testing for prognostic evaluation of patients presenting with stable symptoms possibly indicative of coronary ischemia in the outpatient setting remains uncertain. In theory, such testing could augment the ability to properly triage patients who are more likely to have CAD while avoiding needless evaluation of those without the diagnosis. Recent development of refined hsTn assays providing ability to measure minute concentrations of the biomarker may allow more robust evaluation of such patients, and it prompts consideration of how hsTn may be used in groups of patients other than those with suspected acute MI. We recently measured concentrations of high-sensitivity troponin I (hsTnI) in symptomatic outpatients in PROMISE (Prospective Multicenter Imaging Study for Evaluation of Chest Pain) (3,8) who were randomized to CTA imaging, using a highly sensitive method that “counts” individual molecules of troponin. In this analysis, we found concentrations of the biomarker associated with the presence and severity of obstructive CAD in this group (9). Given such an association, in the present study (performed in an even larger cross section of the PROMISE participants) we hypothesized that concentrations of hsTnI would predict risk for major adverse cardiovascular events, particularly in proximity to index presentation.
METHODS
All study procedures were approved by appropriate local or central Institutional Review Boards. Patients in this analysis gave consent for their blood samples to be analyzed in future studies.
STUDY DESIGN AND STUDY GROUP
Design and primary results of PROMISE have been previously published (3,8). In brief, PROMISE was a pragmatic comparative effectiveness trial enrolling 10,003 participants at 193 sites in North America. Stable symptomatic outpatients without known CAD who were judged to require further evaluation with nonurgent, noninvasive cardiovascular testing were randomly allocated to receive either functional testing (exercise electrocardiography or exercise or pharmacological nuclear stress testing or stress echocardiography) or coronary CTA. Study participants were approached for consent for baseline blood collection to the PROMISE Study Biorepository; of these participants, 4,031 agreed. No major differences in baseline variables were found between those participating in the biorepository and those who did not (Online Table 1).
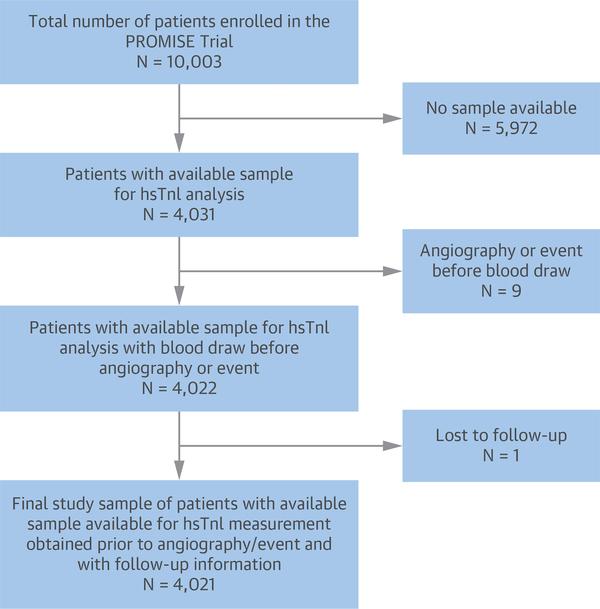
A study flow diagram is detailed in Figure 1. For the purposes of this analysis, we focused on those subjects who had available blood samples drawn before invasive angiography (if performed) or any acute coronary event and who had available information regarding vital status at follow-up. Thus, the total study sample for this analysis was 4,021 subjects. Patients were equally distributed between the 2 study arms of the trial. Members of an independent clinical events committee adjudicated all endpoints in a blinded fashion using established definitions (8). Median (interquartile range [IQR]) follow-up for death, MI, or hospitalization for unstable angina was 735 days (524 to 984 days).
FIGURE 1. Study Flow for the Present Analysis.
Of an original 10,003 participants, 4,021 were included. hsTnI = high-sensitivity troponin I; PROMISE = Prospective Multicenter Imaging Study for Evaluation of Chest Pain.
hsTnI MEASUREMENT
Concentrations of hsTnI were quantified using a single-molecule counting method (SMC TnI, Singulex, Alameda, California) on an Erenna platform in a Clinical Laboratory Improvement Amendments (CLIA)-licensed, College of American Pathologists (CAP)-accredited clinical laboratory. Samples and controls were added to a 96-well assay plate with an automated EVO 150 robotic system (TECAN Group Ltd., Männedorf, Switzerland). Standards, capture reagent, and detection reagent were added to the assay plate. During incubation, the TnI in the specimen bound to capture antibodies biotinylated to microparticles and to fluorescently conjugated detection antibodies. After the unbound fluorescent detection antibody was removed by a wash procedure, an elution buffer was added to dissociate bead-bound antibody sandwiches, thus releasing fluorescent detection antibody into the eluent. The eluate was automatically transferred into a new microwell plate, which was then manually loaded onto the Erenna system; to quantify concentrations of TnI, fluorescent TnI-antibody complexes are “counted” as they pass through an aperture. This very highly sensitive TnI assay has a limit of detection of 0.5 ng/l and a 99th percentile reference limit of 6 ng/l in apparently healthy individuals (10). For this study, the assay controls provided inter-run imprecision of 10% at 1.5 ng/l and 6% at 13 ng/l.
STATISTICAL METHODS
Baseline characteristics across hsTnl quartiles were compared using a chi-square test for categorical variables and the Kruskal-Wallis test for continuous variables. Rates of missingness were low; missing values were imputed with medians for continuous variables and the most frequent category for dichotomous variables. Only 1.4% of subjects had concentrations of hsTnI below the limit of detection; these values were imputed to one-half of the limit (0.25 ng/l).
The time horizon for all outcome analyses was 1 year from randomization. Event probabilities for incident death, acute MI, or hospitalization for unstable angina by 1 year were examined as a function of hsTnI quartiles; log-rank testing was used to detect statistically significant differences in event rates across quartiles. These analyses were repeated for the composite of cardiovascular death or acute MI. Multivariable Cox proportional hazards analyses were performed for the primary endpoint of death, acute MI, or hospitalization for unstable angina by 1 year, adjusting models for traditional variables predictive of cardiovascular risk: age, sex, race (modeled as white, black, or other), history of diabetes mellitus, tobacco use, or antihypertensive use, and systolic blood pressure. Concentrations of hsTnI were entered as a log-transformed variable. Nonlinearity of continuous predictors was assessed using restricted cubic splines, and a linear functional form was found to be adequate. Hazard ratios (HRs), expressed per increase in log hsTnI IQR, were generated along with 95% confidence intervals (CIs). The 25th and 75th quantiles for log-transformed hsTnI, 0 and 0.92, respectively, correspond to 1.0 and 2.5 ng/l on the untransformed scale. These results were repeated for the endpoint of cardiovascular death or acute MI. The proportional hazards assumption was assessed using scaled Schoenfeld residuals and calculating interval-specific HRs. This enabled assessing the time-dependent change in the HR. Interval-specific HRs were estimated by partitioning time, excluding subjects whose event or censoring time occurred before the start of the interval, and censoring events occurring after the interval (11). For very short-term follow-up intervals (e.g., 30 days), models were adjusted only for age and sex because of low event rates.
Incremental value of adding hsTnI to a base model of all pre-specified risk factors was assessed by change in the Harrell C-statistic and tested using a likelihood ratio test. The optimism bootstrap was used to correct for bias. Calibration was assessed by calibration plots and tested using the method of Demler et al. (12). Finally, time-to-event analyses for death, acute MI, or hospitalization for unstable angina as a function of hsTnI quartiles were performed using Kaplan-Meier curves with log-rank testing; similar curves were generated for the outcome of cardiovascular death or acute MI.
All p values are 2-sided, with values ≤0.05 considered significant. Analyses were performed in the R environment for statistical computing (R Foundation for Statistical Computing, Vienna, Austria) (13).
RESULTS
A histogram of hsTnI concentrations in the study participants is displayed in Online Figure 1. Among study participants, concentrations of hsTnI ranged from below the limit of detection of 0.5 ng/l to a maximum of 3,468.7 ng/l; 98.6% had measurable hsTnI concentrations. The median hsTnI value for the whole group was 1.6 ng/l, with a highest quartile >2.6 ng/l; 7.1% of study participants were ≥6 ng/l, the assay’s 99th percentile for patients free of risk factors for CAD or prevalent atherosclerosis, heart failure, or kidney disease (10). The 99th percentile hsTnI concentration in this cohort was 44.5 ng/l.
BASELINE CLINICAL CHARACTERISTICS
Clinical characteristics as a function of hsTnI quartiles are detailed in Table 1. In higher hsTnI quartiles, we observed increasing prevalence and number of CAD risk factors and/or preventive treatment for CAD. Patients in higher hsTnI quartiles were also more likely to have typical angina symptoms. Moreover, the Framingham Risk Score was greatest in the highest hsTnI quartile.
TABLE 1.
Baseline Clinical Characteristics as a Function of hsTnI Concentration
| hsTnI Quartile |
|||||
|---|---|---|---|---|---|
| Q1 (≤ ng/l) (n = 1,105) | Q2 (1.1-1.6 ng/l) (n = 1,021) | Q3 (1.7-2.5 ng/l) (n = 913) | Q4 (≥2.6 ng/l) (n = 982) | p Value | |
| Age, yrs | 57.5 ± 6.7 | 60.0 ± 7.6 | 60.9 ± 8.6 | 62.0 ± 9.0 | <0.001 |
| Male | 32.9 (364) | 44.1 (450) | 54.7 (499) | 57.3 (563) | <0.001 |
| Race | <0.001 | ||||
| Black | 6.5 (72) | 7.9 (81) | 9.6 (88) | 11.9 (117) | |
| Other | 2.8 (31) | 3.0 (31) | 3.7 (34) | 1.9 (19) | |
| White | 90.1 (996) | 88.8 (907) | 86.0 (785) | 85.7 (842) | |
| Cardiac risk factors | |||||
| Hypertension | 54.6 (603) | 67.7 (691) | 68.8 (628) | 72.4 (711) | <0.001 |
| Dyslipidemia | 70.5 (779) | 68.2 (696) | 66.4 (606) | 63.3 (622) | 0.005 |
| Peripheral or cerebrovascular disease | 5.1 (56) | 5.6 (57) | 6.9 (63) | 6.0 (59) | 0.36 |
| Diabetes | 19.7 (218) | 21.7 (222) | 20.6 (188) | 25.8 (253) | 0.006 |
| Smoker | |||||
| Never | 50.0 (552) | 49.2 (502) | 47.0 (429) | 49.4 (485) | 0.67 |
| Current | 18.2 (201) | 17.0 (174) | 17.4 (159) | 18.3 (180) | |
| Former | 31.9 (352) | 33.8 (345) | 35.5 (324) | 32.3 (317) | |
| Family history of premature CAD | 38.7 (428) | 30.8 (314) | 31.8 (290) | 28.4 (279) | <0.001 |
| Depression | 28.0 (309) | 25.4 (259) | 22.5 (205) | 21.7 (213) | 0.003 |
| Sedentary lifestyle | 47.3 (523) | 48.3 (493) | 46.2 (422) | 44.6 (438) | 0.38 |
| Framingham Risk Score | 15.8 ± 11.3 | 20.1 ± 13.5 | 23.7 ± 15.2 | 26.4 ± 16.4 | <0.001 |
| Type of angina | 0.05 | ||||
| Typical | 10.8 (119) | 11.4 (116) | 12.8 (117) | 15.5 (152) | |
| Atypical | 80.1 (885) | 79.4 (811) | 78.5 (717) | 76.2 (748) | |
| Noncardiac | 9.1 (101) | 9.2 (94) | 8.7 (79) | 8.4 (82) | |
| Medication use | |||||
| Aspirin | 40.0 (442) | 47.0 (480) | 46.0 (420) | 48.2 (473) | 0.002 |
| Statin | 43.9 (485) | 44.8 (457) | 44.2 (404) | 44.3 (435) | 0.99 |
| Beta-blocker | 20.2 (223) | 23.6 (241) | 26.9 (246) | 30.5 (300) | <0.001 |
| ACEI or ARB | 33.0 (365) | 42.5 (434) | 42.8 (391) | 50.0 (491) | <0.001 |
| Antihypertensive | 51.1 (565) | 59.1 (603) | 63.7 (582) | 69.8 (685) | <0.001 |
| Systolic blood pressure, mm Hg | 126.9 ± 15.2 | 130.6 ± 16.3 | 132.7 ± 16.9 | 135.5 ± 17.4 | <0.001 |
| Diastolic blood pressure, mm Hg | 78.1 ± 9.6 | 78.8 ± 10.0 | 78.8 ± 10.3 | 79.6 ± 10.5 | <0.001 |
| BMI, kg/m2 | 30.0 ± 5.9 | 30.5 ± 5.9 | 30.9 ± 5.8 | 31.3 ± 5.7 | <0.001 |
Values are mean ± SD or % (n).
ACEI = angiotensin-converting enzyme inhibitor; ARB = quartile = angiotensin II receptor blocker; BMI = body mass index; CAD = coronary artery disease; hsTnI = high-sensitivity troponin I; Q = quartile.
OUTCOMES
By 1 year, 74 study participants experienced the primary endpoint of death, acute MI, or hospitalization for unstable angina, whereas 28 participants experienced the composite outcome of cardiovascular death or acute MI. Among patients who died or who had acute MI or hospitalization for unstable angina by 1 year, median hsTnI concentrations were higher at enrollment, compared with patients who did not experience these events (2.1 ng/l vs. 1.6 ng/l; p < 0.001). In a similar fashion, patients who experienced incident cardiovascular death or acute MI by 1 year had higher hsTnI concentrations at enrollment than those who did not (2.4 ng/l vs. 1.6 ng/l; p = 0.02).
We observed a stepwise increase in probability for death, acute MI, or hospitalization for unstable angina by 1 year from 0.8% to 3.1% across hsTnI quartiles at enrollment (Table 2). Although numerically higher (from 0.4% to 1.2%), the event probability for the composite of cardiovascular death or acute MI across hsTnI quartiles was not significant (Table 2). In multivariable Cox proportional hazards models for prediction of death, acute MI, or hospitalization for unstable angina at 1 year, concentrations of hsTnI were an independent predictor of events (HR: 1.54 per increase in log hsTnI IQR; 95% CI: 1.33 to 1.78; p <0.001). Addition of hsTnI results to the model resulted in increase in the Harrell’s C-statistic from 0.68 (0.61 to 0.74) to 0.70 (0.65 to 0.77), with a bias-corrected change of 0.65 to 0.68. The likelihood ratio test comparing models with and without hsTnI was also significant (p < 0.001), indicating value beyond traditional risk factors. There was no evidence of miscalibration (p = 0.85). (Full 1-year model results are given in Online Table 2.) Of all covariates tested, hsTnI results most strongly explained the variation in the primary outcome (Online Figure 2). In age-adjusted Cox proportional hazards models, concentrations of hsTnI provided similar predictive value for the primary endpoint in women (HR: 1.65 per increase in log hsTnI IQR; 95% CI: 1.31 to 2.07; p < 0.001) versus men (HR: 1.50 per increase in log hsTnI IQR; 95% CI: 1.26 to 1.77; p < 0.001).
TABLE 2.
Estimated Event Probability for Cardiovascular Events at 1 Year as a Function of hsTnI Concentration at the Time of Presentation
| hsTnI Quartile |
|||||
|---|---|---|---|---|---|
| Q1 (≤1 ng/l) (n = 1,105) | Q2 (1.1–1.6 ng/l) (n = 1,021) | Q3 (1.7–2.5 ng/l) (n = 913 | Q4 (≥2.6 ng/l) (n = 982) | p Value* | |
| Estimated event probability for death, acute MI, or hospitalization for unstable angina | 0.8 (9) | 1.5 (15) | 2.3 (20) | 3.1 (30) | 0.001 |
| Estimated event probability for cardiovascular death or acute MI | 0.4 (4) | 0.6 (6) | 0.8 (7) | 1.2 (11) | 0.20 |
Values are % (n).
The p value is generated using the Kaplan-Meier method. hsTnI = high-sensitivity troponin I; MI = myocardial infarction; Q = quartile.
Examining the composite of cardiovascular death or acute MI by 1 year, in Cox proportional hazards, concentrations of hsTnI were significantly predictive of events in a similar magnitude (HR: 1.52 per increase in log hsTnI IQR; 95% CI: 1.19 to 1.94; p < 0.001); again, the likelihood ratio test was significant in this model from addition of hsTnI results (p = 0.005). (Full 1-year model results are given in Online Table 2.) In age-adjusted Cox proportional hazards models, concentrations of hsTnI provided similar predictive value for the endpoint of cardiovascular death or acute MI by 1 year, with comparable predictive value in women (HR: 1.63 per increase in log hsTnI IQR; 95% CI: 1.13 to 2.36; p = 0.01) and men (HR: 1.47 per increase in log hsTnI IQR; 95% CI: 1.12 to 1.93; p = 0.006). No interaction between hsTnI and 1-year prognosis as a function of male or female sex was found (p = 0.54).
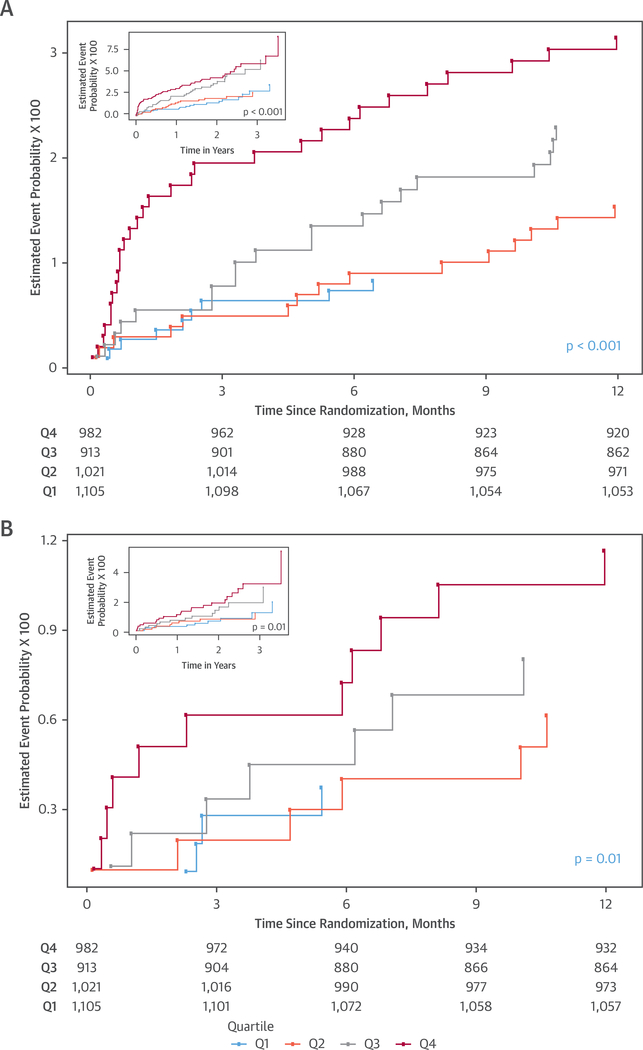
Studying model fit, we detected a decrease in the HR of hsTnI over time, as shown in Online Figure 3. To understand the time-varying effect of hsTnI for predicting events, we first examined shorter followup time horizons and found that hsTnI strongly predicted events by 30 days (primary endpoint HR: 1.83; 95% CI: 1.52 to 2.20; p < 0.001; cardiovascular death or acute MI HR: 2.18; 95% CI: 1.63 to 2.91; p < 0.001). Predictive value of hsTnI for events persisted to 90 days (primary endpoint HR: 1.71; 95% CI: 1.45 to 2.03; p < 0.001; cardiovascular death or acute MI HR: 1.75; 95% CI: 1.37 to 2.25; p < 0.001). Regarding the 90-day time point, adding hsTnI to the model increased the C-statistic for the primary endpoint from 0.70 to 0.74. Beyond 90 days of follow-up, however, a further decrease of HR is noted (Online Figure 4). In an analysis of patients reaching 90 days without an event, concentrations of hsTnI did not predict either the primary endpoint (HR: 1.18; 95% CI: 0.97 to 1.45; p = 0.10) or the composite of cardiovascular death or acute MI (HR: 1.23; 95% CI: 0.94 to 1.61; p = 0.12) for the rest of follow-up. These results are thus reflected in rapid divergence of event curves in Kaplan-Meier time-to-event analyses, suggesting that those patients with higher concentrations of hsTnI at enrollment had a shorter time to death, acute MI, or hospitalization for unstable angina (Figure 2A), with similar findings for cardiovascular death or acute MI (Figure 2B).
FIGURE 2. Cumulative Hazard Curves Depicting Time-to-Events as a Function of hsTnI Quartiles at Presentation.
(A) Death, acute myocardial infarction, or hospitalization for unstable angina at 1 year. (B) Cardiovascular death or acute myocardial infarction at 1 year. The insets detail outcomes to 3 years of follow-up. Outcomes are depicted to 12 months; insets demonstrate outcomes through complete follow-up of the PROMISE trial. Q = quartile; other abbreviations as in Figure 1.
DISCUSSION
The symptom of stable chest discomfort is commonly encountered in modern medical practice and may be challenging to assess and manage accurately. In contrast to the paradigm for evaluating acute coronary presentations (14), no established role exists for any biomarker assay (including troponin testing) for short-term decision making in patients with stable coronary syndromes (15). However, with emergence of hsTn methods able to detect minute quantities of myocardial injury or necrosis with tests that are more sensitive than most commercially available troponin assays, we hypothesized potential value of such testing for stratifying risk in patients presenting with chest discomfort thought to be potentially of a cardiovascular nature; as a part of a comprehensive evaluation strategy, such knowledge could help to support clinical decision making. To be explicitly clear, although previous studies have examined the role of hsTn for long-term risk prediction in entire groups of patients with stable CAD (6,7), such analyses provide no guidance regarding how hsTnI could be used to evaluate patients with symptoms of chest discomfort who have not yet been diagnosed with CAD. Indeed, in the PROMISE trial, many patients had symptoms that were not related to cardiac disease, and many did not have anatomically significant CAD. Accordingly, this study differs considerably: in this analysis of patients with stable chest pain–many of whom were without angina or other cardiovascular cause of their symptoms–we hypothesized that hsTnI not only would provide useful prognostic information, but also would do so for events with a short time horizon. In essence, this analysis addresses a commonly asked question–specifically, whether hsTn testing could have a role for assessment of stable patients in the office- based setting for short-term decision making.
Among 4,021 study participants enrolled in the PROMISE trial who had stable chest discomfort and possible CAD, we found that concentrations of an extremely sensitive “single-molecule counting” hsTnI assay were prognostic for cardiovascular events, including major complications such as death, acute MI, or hospitalization for unstable angina. Concentrations of hsTnI were detectable in nearly all study participants, in contrast to other hsTn methods, which detect considerably less biomarker in such low-risk groups (16). Concentrations of hsTnI appeared similarly useful in men and women and were particularly prognostic for near-term events. We thus not only have linked hsTnI concentrations to the presence and severity of CAD in patients with stable chest symptoms, but also now extend these results to show that hsTnI values in these subjects are prognostic for near-term events. Taken together, these results suggest a potential utility for blood-based biomarkers in general, and for more highly sensitive troponin methods in particular, for assessment of stable chest pain syndromes (Central Illustration).
CENTRAL ILLUSTRATION. Previous Data Suggest Concentrations of hsTnI Predict Presence and Severity of Underlying Coronary Artery Disease.
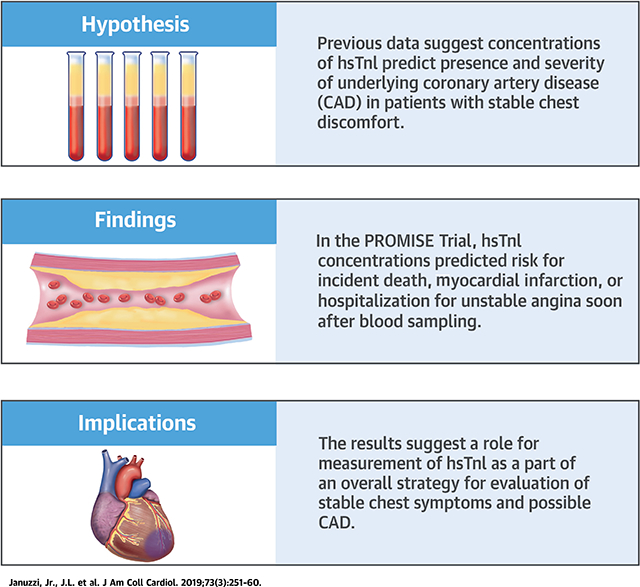
In the present analysis, we found that high-sensitivity troponin I (hsTnI) concentrations predicted risk for incident death, myocardial infarction (MI), or hospitalization for unstable angina in a graded fashion. In adjusted analyses, high-sensitivity troponin I represented a strong independent predictor of these outcomes, and did so by predicting earlier rather than later events. These results suggest a possible role for measurement of troponin I using a very highly sensitive assay as a part of an overall strategy for evaluation of stable chest symptoms and possible coronary artery disease.
Beyond standard clinical evaluation, for appropriate patients with stable chest discomfort, stress testing (with or without imaging) or CTA is often used for both diagnosis and risk stratification. Although useful, such approaches have limitations, including cost and availability. A broadly available, inexpensive, and easily interpretable tool to support clinical judgment such as a blood test would be an attractive option for evaluating stable patients with chest discomfort. Given its association with prevalent CAD in this cohort (9), we hypothesized that concentrations of hsTnI–drawn before any imaging studies in the PROMISE trial–could predict short-term complications such as death, acute MI, or more urgent treatment for unstable angina. Our data suggest that concentrations of hsTnI may identify those patients at highest risk for impending near-term major cardiovascular events. Although hsTn concentrations in stable patients may be driven by several factors, including heart muscle disease, taken together, our data suggest at least plausible links between severity of underlying CAD and the hsTn concentrations in our subjects. In statistical analyses, as a continuous variable, higher concentrations of hsTnI identify a heightened risk for adverse outcome within a short period following sampling. Conceptually, clinicians could measure hsTnI when encountering a patient with symptoms thought to result from stable angina. Higher concentrations could trigger a more direct means for their evaluation (e.g., coronary angiography rather than functional testing). Although it appears that an association between higher hsTnI and short-term risk is present, it remains unclear how individual results (e.g., 7 ng/l vs. 8 ng/l) could be interpreted on a clinical level. Evaluating cost-effectiveness of an hsTnI-leveraged clinical approach, including development of concentration-specific strategies for interpretation, is worth exploration.
In comparison with other biomarkers for which refinements in assay technology revealed an ability to stratify risk even in what was perceived as the “normal range” (17), it is noteworthy to point out that most subjects in our study had hsTnI concentrations within the normal range for the assay and below where many hsTn assays can accurately measure (16,18); for example, in a low-risk group such as this, 53% and 75% of subjects had detectable hsTnT and hsTnI concentrations, respectively (16). With such greater sensitivity, we were able to detect hsTnI in nearly 99% of study participants and could show heightened risk across greater hsTnI concentrations even within a troponin range considered absolutely normal. Although previous studies showed hsTn to be predictive of adverse outcomes, including acute MI or death in patients with stable CAD (6,7), such studies focused on entire groups of subjects with CAD, rather than specifically on the utility of using hsTnI to examine patients presenting with the diagnostic challenge of a new symptom consistent with stable angina. Further, previous studies have used hsTn methods with less sensitivity than the assay in this analysis. Thus, our data are unique.
STUDY STRENGTHS AND LIMITATIONS
PROMISE was a pragmatic study of a diverse, demographically balanced patient group representative of those presenting with suspected CAD, and our data suggest potential value of hsTnI testing as an adjunct to clinical decision making in patients with stable symptoms. However, although hsTnI appeared modestly associated with typical symptoms, risk factor burden, and short-term outcomes, more data are needed to understand better how such highly sensitive troponin methods perform in different patient groups. The low event rates in PROMISE are also noteworthy; such rates could increase the potential for type 1 or 2 statistical errors. Nonetheless, our findings are consistent and robust. Moreover, our findings illustrate the potential for severe complications even within a group of patients often thought of as relatively lower risk. Given the design of the PROMISE trial, most patients in this analysis had relatively well preserved kidney function. Thus, we cannot comment on how hsTnI would perform for prognosis in patients with renal insufficiency; to the extent that worse kidney function is a risk factor for CAD but also could lead to elevation in hsTnI absent CAD, this is an area worthy of further exploration. The hsTnI method that was used in this analysis is considerably more sensitive than conventionally used assays and does not have regulatory approval for this indication. Thus, our results cannot be necessarily extrapolated to other hsTn tests; when reaching higher concentrations of hsTnI used in this study, clinically used assays may be able to provide similar results, but we lack head-to-head comparisons with these assays. Clinicians should not assume that our results hold for commercial hsTnT or other hsTnI methods. Although hsTnI was prognostic for near-term events, identifying a single cutoff that provides stand-alone utility could be challenging. Future analyses should examine this question, addressing whether sex-based cutoffs are needed for this application. We do not have serial blood measures, either sampled short term or longer term. Beyond serial sampling (as has been done in acute chest pain situations), other approaches may be reasonable to refine risk prediction further. For example, we suggest that integration of hsTnI concentrations as a continuous variable–providing linear risk for events–together with other variables and analyzed holistically to provide a probability for adverse near-term events may be a future means by which such results could be leveraged for superior evaluation of patients with stable chest pain. More data in this regard are clearly needed.
CONCLUSIONS
Precision medicine techniques may reveal important subgroups within a large diagnostic category that may merit specific management strategies to improve their outcomes. It is possible that those patients identified with hsTnI at impending risk for complications in PROMISE represent a unique subset of “high-risk stable angina” within an overall group of patients with more modest risk. It is reasonable to hypothesize that patients with stable chest pain syndromes with relatively higher hsTnI could merit more aggressive diagnostic or therapeutic management. Besides typical recommendations for higher-risk patients, such as avoidance of tobacco use, exercise prescription, or more aggressive medical management of risk factors, proceeding directly to coronary CTA imaging or invasive angiography may be an option for such patients, although it is premature to make such a recommendation. Given the ongoing controversy regarding optimal management strategies for those with stable coronary syndromes (19), equipoise is present to explore this hypothesis to inform the most cost-effective diagnostic and therapeutic approaches for this important group of patients.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN PATIENT CARE
Elevated serum concentrations of hsTn are associated with higher shortterm risk of adverse cardiovascular events in a stable group of patients with chest pain.
TRANSLATIONAL OUTLOOK
Further studies are needed to clarify the role of hsTnI measurement to guide clinical decisions for patients with this common presentation.
Acknowledgments
This project was also supported by grants R01HL098237, R01HL098236, and R01HL98305 from the National Heart, Lung, and Blood Institute (NHLBI). Dr. Januzzi is supported in part by the Hutter Family Professorship in Cardiology; has received grant support from Singulex, Abbott, and Prevencio; has received consulting income from Roche Diagnostics, Critical Diagnostics, Philips, Abbott, Prevencio, and Novartis; and participates in clinical endpoint committees or data safety monitoring boards for Siemens, Abbvie, Pfizer, Amgen, Janssen, and Boehringer Ingelheim. Dr. Hoffmann has received institutional research grants from Abbott, HeartFlow, Kowa Pharmaceuticals, and Medimmune. Dr. Patel has received research grants from AstraZeneca, Janssen, Bayer, NHLBI, Philips, and Heartflow; and serves on advisory boards for Janssen, AstraZeneca, and Bayer. Dr. Ferencik was supported in part by American Heart Association Fellow to Faculty Award 13FTFI6450001. Dr. Tardif holds the Canada Research Chair in personalized and translational medicine; has received research funding from Amarin, AstraZeneca, DalCor, Esperion, Ionis, Merck, Pfizer, Sanofi, and Servier; has received honoraria from DalCor, Pfizer, Sanofi, and Servier; and holds a minor equity interest in DalCor. Dr. Ginsburg has served as a consultant or advisory board member for CardioDx, Interleukin Genetics, Pappas Ventures, Fabric Genomics, Genome Magazine, Exploragen, Origin Commercial Advisors, and Dr. Footprint; has stock options from CardioDx, Alere, Fabric Genomics, Predigen, Exploragen, Dr. Footprint, and Origin Commercial Advisors; has served on the board of directors for Alere; has received royalties from Elsevier; has received research funding to his institution from Singulex, Abbott, and 23andMe; and is a founder of Predigen. Dr. Douglas has received research grants from GE Healthcare and HeartFlow. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. Allan S. Jaffee, MD, served as Guest Editor for this paper.
ABBREVIATIONS AND ACRONYMS
- CAD
coronary artery disease
- CI
confidence interval
- CTA
computed tomography angiography
- HR
hazard ratio
- hsTn
high-sensitivity troponin
- hsTnI
high-sensitivity troponin I
- IQR
interquartile range
- MI
myocardial infarction
Footnotes
APPENDIX For supplemental tables and figures, please see the online version of this paper.
REFERENCES
- 1.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation 2017;135:e146–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng VY, Berman DS, Rozanski A, et al. Performance of the traditional age, sex, and angina typicality-based approach for estimating pretest probability of angiographically significant coronary artery disease in patients undergoing coronary computed tomographic angiography: results from the multinational coronary CT angiography evaluation for clinical outcomes: an international multicenter registry (CONFIRM). Circulation 2011; 124:2423–32. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med 2015;372: 1291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonaca MP, O’Malley RG, Jarolim P, et al. Serial cardiac troponin measured using a high-sensitivity assay in stable patients with ischemic heart disease. J Am Coll Cardiol 2016;68:322–3. [DOI] [PubMed] [Google Scholar]
- 5.Eisen A, Bonaca MP, Jarolim P, et al. High-sensitivity troponin I in stable patients with atherosclerotic disease in the TRA 2 degrees P - TIMI 50 trial. Clin Chem 2017;63:307–15. [DOI] [PubMed] [Google Scholar]
- 6.Everett BM, Brooks MM, Vlachos HE, et al. Troponin and cardiac events in stable ischemic heart disease and diabetes. N Engl J Med 2015; 373:610–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Omland T, Pfeffer MA, Solomon SD, et al. Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J Am Coll Cardiol 2013;61: 1240–9. [DOI] [PubMed] [Google Scholar]
- 8.Douglas PS, Hoffmann U, Lee KL, et al. PRO-spective Multicenter Imaging Study for Evaluation of chest pain: rationale and design of the PROMISE trial. Am Heart J 2014;167:796–803.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Januzzi JL, Suchindran S, Coles A, et al. , PROMISE Investigators. High-sensitivity troponin I and coronary computed tomography in symptomatic outpatients with suspected coronary artery disease: insights from the PROMISE trial. J Am Coll Cardiol Img 2018. March 18 [E-pub ahead of print]. [Google Scholar]
- 10.Estis J, Wu AHB, Todd J, Bishop J, Sandlund J, Kavsak PA. Comprehensive age and sex 99th percentiles for a high-sensitivity cardiac troponin I assay. Clin Chem 2018;64:398–9. [DOI] [PubMed] [Google Scholar]
- 11.Harrell F Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression and Survival Analysis. 2nd edition. New York, NY: Springer, 2015. [Google Scholar]
- 12.Demler OV, Paynter NP, Cook NR. Tests of calibration and goodness-of-fit in the survival setting. Stat Med 2015;34:1659–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrell F Regression Modeling Strategies. R Foundation for Statistical Computing, 2017. Available at: https://CRAN.R-project.org/package=rms. Accessed November 20, 2018. [Google Scholar]
- 14.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60:1581–98. [DOI] [PubMed] [Google Scholar]
- 15.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/ American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol 2012;60: e44–164. [DOI] [PubMed] [Google Scholar]
- 16.Welsh P, Preiss D, Shah ASV, et al. Comparison between high-sensitivity cardiac troponin T and cardiac troponin I in a large general population cohort. Clin Chem 2018;64:1607–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ridker PM. A test in context: high-sensitivity C-reactive protein. J Am Coll Cardiol 2016;67: 712–23. [DOI] [PubMed] [Google Scholar]
- 18.Apple FS. A new season for cardiac troponin assays: it’s time to keep a scorecard. Clin Chem 2009;55:1303–6. [DOI] [PubMed] [Google Scholar]
- 19.Al-Lamee R, Thompson D, Dehbi HM, et al. Percutaneous coronary intervention in stable angina (ORBITA): a double-blind, randomised controlled trial. Lancet 2018;391:31–40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.