Abstract
Background
Cerebrotendinous xanthomatosis (CTX) is a rare inborn lipid-storage disease caused by mutations in the sterol 27-hydroxylase (CYP27A1) gene with an autosomal recessive pattern of inheritance. To date, only 19 CTX patients from 16 families have been reported in the Chinese population.
Results
Three novel likely pathogenic mutations (c.368_374delCCAGTAC, c.389 T > A and c.571C > T) and 7 previously reported pathogenic mutations (c.379C > T, c.435G > T, c.1016C > T, c.1214G > A, c.1263 + 1G > A, c.1420C > T and c.1435C > T) were identified. In addition, we summarized the genotypes and phenotypes of reported Chinese CTX patients. The most predominant mutations in CYP27A1 were c.410G > A and c.379C > T, and the most common clinical manifestations were pyramidal signs, xanthomatosis, cerebellar ataxia, and cognitive impairment.
Conclusion
Our study broadens the genetic and clinical spectrum of CTX and provides insightful information to help better diagnose and understand the disease.
Keywords: Cerebrotendinous xanthomatosis, CYP27A1, Mutation, Clinical feature
Introduction
Cerebrotendinous xanthomatosis (CTX) (OMIM: 213700) is a rare inborn lipid-storage disease, characterized by accumulation of cholestanol-containing xanthomas predominantly in tendons and the brain [1]. CTX is caused by mutations in the sterol 27-hydroxylase gene (CYP27A1) [2]. The human CYP27A1 gene is located on chromosome 2 and contains 9 exons and encodes sterol 27-hydroxylase. Sterol 27-hydroxylase is a mitochondrial cytochrome P450 enzyme that plays a critical role in side-chain oxidation of cholesterol necessary for the synthesis of the bile acid [3–5]. The ability to convert cholesterol to bile acids is impaired in CTX patients, leading to the elevations of cholestanol and accumulation of cholesterol and cholestanol in multiple tissues, such as tendons, the central nervous system and lungs [6–8]. The common clinical presentations include infantile-onset chronic diarrhea, juvenile cataracts, progressive cognitive dysfunction and dementia, cerebellar ataxia, spasticity, osteoporosis, peripheral polyneuropathy and other atypical neurological symptoms [9–12]. However, the clinical manifestations of CTX can vary significantly even within the same family [13].
To date, over 100 variants in the CYP27A1 gene and more than 300 CTX patients have been identified worldwide [14, 15]. In the Chinese population, only 19 patients from 16 families have been reported [16–27]. Here, we reported the genetic features and clinical findings of 6 unrelated Chinese patients with CTX and summarized the genotypes and phenotypes of all Chinese patients with CTX.
Methods
Subjects and clinical evaluation
Six pedigrees of CTX, including 6 patients and 12 family members, were collected from July 2015 to December 2018. The clinical evaluations and neurological examinations were performed by two senior neurologists. This study was approved by the Ethics Committee of Second Affiliated Hospital, Zhejiang University School of Medicine. Written informed consents were obtained from all the participants.
Genetic testing of CYP27A1
Genomic DNA was extracted from peripheral blood samples using a commercial blood genomic extraction kit (Qiagen, Hilden, Germany). Polymerase chain reaction (PCR) was carried out to amplify all exons and flanking regions of CYP27A1. Direct Sanger sequencing was performed on an ABI 3500xl Dx Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) as described previously [28]. The primers for CYP27A1 were listed in Additional file 1: Table S1. The 1000 Genomes Project (https://www.ncbi.nlm.nih. gov/variation/tools/1000 genomes/) and the ExAC database (https://exac.broadinstitute.org/) were used to check the frequency of variants in the general population. Three software programs, including SIFT (http://sift.jcvi.org/), PolyPhen-2 (http://genetics.bwh.harvard.edu/pph2/) and Mutation Taster (http://www.mutationtaster.org/) were used to predict the possible protein functional changes caused by the variants.
Literature review
We reviewed all of the CTX patients reported in the Chinese population from 1992 to April 31, 2019. Nineteen patients with integrated clinical information in 13 studies were included in our study [8, 14–18, 20–27]. The genotypes and phenotypes of Chinese CTX patients were summarized.
Results
Mutations identified in CYP27A1
Three novel variants including c.368_374delCCAGTAC, c.389 T > A (p.M130K) and c.571C > T (p.Q191*), and 7 previously reported pathogenic mutations (c.379C > T, c.435G > T, c.1016C > T, c.1214G > A, c.1263 + 1G > A, c.1420C > T and c.1435C > T) in CYP27A1 (ClinVar database: https://www.ncbi.nlm.Nih.gov/clinvar/) were identified in 6 CTX families. The 3 novel variants were not found in the 1000 Genomes Project and the ExAC databases. Additionally, they were not found in our targeted next-generation sequencing (NGS) database that covered CYP27A1, which contained 800 Chinese subjects without CTX. According to the guidelines provided by the American College of Medical Genetics (ACMG), c.368_374delCCAGTAC (1 piece of very strong pathogenic evidence and 3 pieces of moderate pathogenic evidence), c.389 T > A and c.571C > T (3 pieces of moderate pathogenic evidence and 2 pieces of supporting pathogenic evidence) were classified as likely pathogenic mutations [29].
Clinical features of six CTX patients
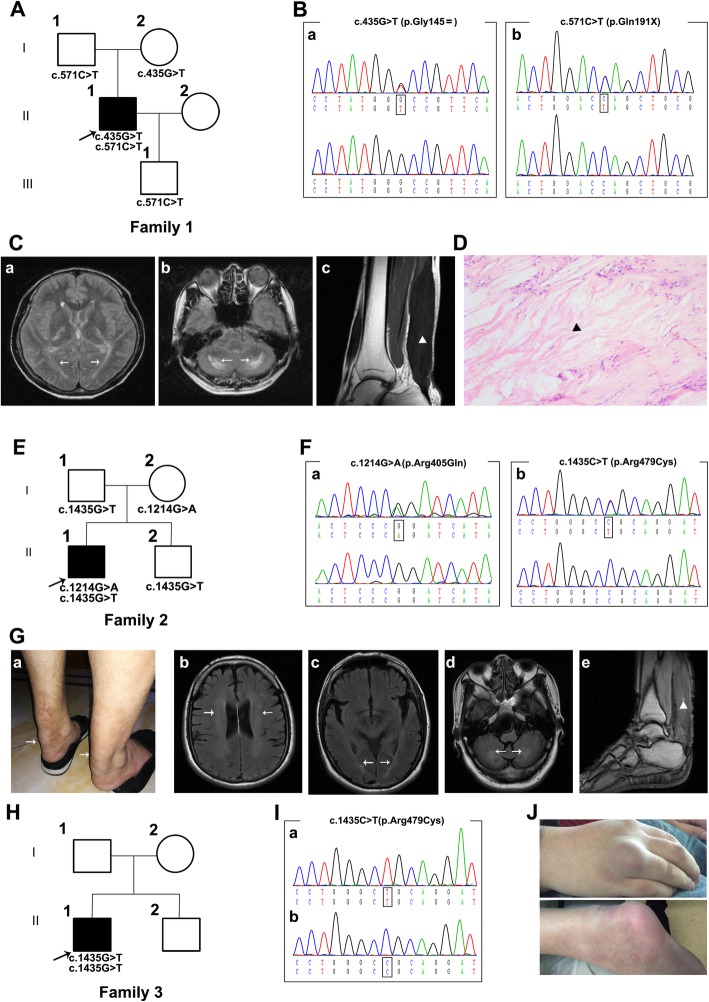
The proband in Family 1 (Fig. 1A) was found to carry one novel likely pathogenic mutation (c.571C > T, p.Q191*) and one previously recognized mutation (c.435G > T, p.G145=) (Fig. 1B). It is worth mentioning that the synonymous mutation c.435G > T (p.G145=) was previously reported as a pathogenic mutation that causes alternative pre-mRNA splicing of CYP27A1 [30]. The proband in Family 1 was a 45-year-old male presenting with a 7-year history of slowly progressive gait disturbance and clumsy movement. He noticed xanthomas in bilateral Achilles tendons at 36 years old, and a surgical operation was performed to remove the xanthomas two years later. He was diagnosed with CTX and received simvastatin (20 mg per day) treatment for approximately one year. However, the above symptoms gradually worsened. Symptoms originated with mild stiffness in the neck and right upper extremity two years ago, followed by slurred speech and occasional depression. In addition, gait disturbance became more serious with significant unsteadiness when walking downstairs. The above symptoms developed gradually during the next two years, and now the patient cannot walk without auxiliary equipment. On examination, he had bilateral enlargement of the Achilles tendons and subcutaneous masses. Neurologic examinations revealed dysarthria and gait ataxia. Cognitive function was normal with a Mini-Mental State Examination (MMSE) score of 28. The muscle strength of the limbs was 5/5. Increased tendon reflexes were observed. Bilateral Hoffman signs and Babinski signs were positive. He was unable to touch the tip of his nose with his index finger, wipe one palm alternately with the palm and dorsum of the other hand, and slide the heel of one foot down the shin of the other leg. The plasma cholestanol concentration was not tested because there is a lack of proper test methods for plasma cholestanol levels in most hospitals in China. Electromyography (EMG) showed multiple motor sensory demyelinating peripheral neuropathies. Brain magnetic resonance imaging (MRI) demonstrated hyperintense signals in the bilateral cerebella and posterior cerebral white matter fibers (Fig. 1C). Histological examination of the paraffin section of the tendon showed lipid crystal clefts in hematoxylin-eosin (H-E) staining (Fig. 1D).
Fig. 1.
Pedigree charts and clinical findings of Family 1–3. A, E, H. Pedigree charts of 3 Chinese CTX families, Squares indicate males; circles indicate females; black symbols indicate affected individuals; the arrow indicates the proband. B. The chromatogram of the CYP27A1 variants (a.435G > T and b.c.571C > T) identified in Family 1. C. Hyperintense signals in bilateral cerebella and posterior cerebral white matter fibers of proband in Family 1 (a and b); Sagittal proton density-weighted image shows fusiform thickening of the Achilles tendon (c) (marked with arrow). D. HE staining of the tendon masses reveals dispersed lipid crystal clefts. 100×. F. The chromatogram of CYP27A1 variants (c.1214G > A and c.1435C > T) identified in Family 2 (a and b) (marked with triangle). G. Enlargement of the Achilles tendons of proband in Family 2 (a); Hyperintense signals in bilateral cerebella, lateral ventricle and posterior cerebral white matter fibers of proband in Family 2 (b, c and d); Hyperintense signal on T1-weighted images of proband in Family 2 (e) (marked with arrow and triangle). I. The chromatogram of CYP27A1 variant (c.1435G > T) identified in Family 3. J. Subcutaneous masses of proband in Family 3
The proband from Family 2 (Fig. 1E) carried two reported pathogenic missense mutations, c.1214G > A (p.R405Q) and c.1435C > T (p.R479C) (Fig. 1F). He was a 40-year-old male admitted to our hospital with a chief complaint of a 3-year history of slowly progressive gait disturbance. He noticed xanthomas in his bilateral Achilles tendons one year ago, and a surgical operation was performed to remove the xanthomas in a local hospital. In the last four months, his gait disturbance developed gradually. He denied symptoms of cognitive impairment, sight loss or numbness. Physical examination showed bilateral mild swelling of the Achilles tendons. Neurological examinations showed that the muscle strength of the right limbs was 4/5 and 5/5 in the left limbs. Bilateral Babinski signs were positive. She swayed slightly when she touched the tip of her nose with index finger. It is difficult for her to wipe her palm quickly and place the heel on the knee. Brain MRI indicated cerebellar atrophy and hyperintense signals in the bilateral cerebella and posterior cerebral white matter fibers (Fig. 1G). An MRI scan of the ankle showed hyperintense and hypertrophy of the gastrocnemius and peroneus longus (Fig. 1G).
The proband from Family 3 (Fig. 1H) carried a pathogenic homozygous mutation of c.1435C > T (p.R479C) (Fig. 1I). He was a 30-year-old male admitted to our hospital presenting with a 24-year history of cognitive impairment and a 15-year history of gait disturbance. He developed transient loss of consciousness and epileptic seizure attack at 6 years old and presented cognitive impairment in the next year. At 15 years old, progressively unsteady gait developed, causing falls, especially when running, followed by gradual weakness and progressive spasm and paresis in legs. At 22 years old, he developed bilateral blurred vision and was diagnosed with cataracts. Vision was recovered after the surgical operation was performed four years later. He had enlargement of tendons and subcutaneous masses in hands (Fig. 1J). Neurological examinations showed mild cognitive impairment with an MMSE score of 21. The muscle strength of the upper limbs was normal, while it was 4/5 in the lower limbs. Bilateral Hoffman signs and Babinski signs were positive. Bilateral tendon reflexes increased in the lower limbs.
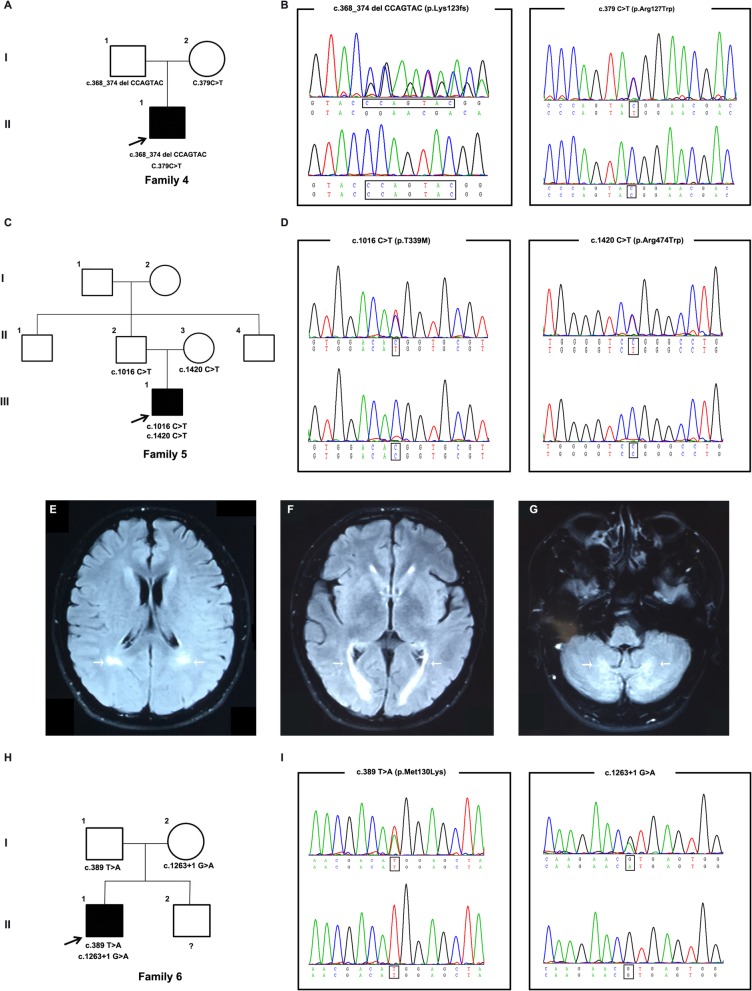
The proband from Family 4 (Fig. 2a) was identified to have one novel likely pathogenic mutation (c.368_374delCCAGTAC, p.L123 fs) and one previously reported pathogenic mutation (c.379C > T, p.R127W) (Fig. 2b). He was a 32-year-old male admitted to our hospital with a chief complaint of a 20-year history of gait disturbance. He developed progressively unsteady gait at 12 years old, followed by gradually spasm and paresis in legs. On examination, he had a short stature and bilateral pes cavus deformity. No enlargement of tendons was noticed. Neurological examinations showed that the muscle strength of the lower limbs was 4/5. He was unable to touch the tip of his nose with his index finger. Bilateral quadriceps and gastrocnemius muscle atrophy were found. Bilateral Hoffman signs and Babinski signs were positive. Bilateral tendon reflexes increased in all limbs (4+).
Fig. 2.
Pedigree charts and clinical findings of Family 4–6. a, c, h Pedigree charts of 3 Chinese CTX families, Squares indicate males; circles indicate females; black symbols indicate affected individuals; the arrow indicates the proband. b The chromatogram of the CYP27A1 variants (c.368_374delCCAGTAC and c.379C > T) identified in Family 4. d The chromatogram of CYP27A1 variants (c.1016C > T and c.1420C > T) identified in Family 2. e-g Hyperintense signals in bilateral cerebella and posterior cerebral white matter fibers of proband in Family 5 (marked with arrow). i The chromatogram of CYP27A1 variant (c.389 T > A and c.1263 + 1G > A) identified in Family 6
The proband from Family 5 (Fig. 2c) was found to carry two reported pathogenic missense mutations, c.1016C > T (p.T339 M) and c.1420C > T (p.R474W) (Fig. 2d). He was a 24-year-old male who presented with a 20-year history of slowly progressive gait disturbance. Unsteady gait first appeared at 4 years old. One year ago, his gait disturbance became worse. On examination, he had scoliosis and bilateral pes cavus deformity. Neurological examinations showed mild cognitive impairment with an MMSE score of 22. Mild atrophy of the thenar and interosseous muscles was found in the right hand. Bilateral Hoffman signs and Babinski signs were positive. The tendon reflexes of the extremities were symmetrically increased (4+). Brain MRI demonstrated hyperintense signals in the bilateral periventricular white matter (Fig. 2e-g).
The proband in Family 6 (Fig. 2h) carried one novel likely pathogenic mutation (c.389 T > A, p.M130K) and one previously reported pathogenic mutation (c.1263 + 1G > A) (Fig. 2i). He was a 27-year-old male who came to our clinic with a chief complaint of a half-year history of gait disturbance. He denied sight loss or numbness. No tendons enlargement was noticed. Neurological examinations showed bilateral tendon reflexes increased in all four limbs (3+). Bilateral Babinski signs were positive. The main clinical findings of these 6 patients are summarized in Table 1.
Table 1.
Clinical features of six patients with cerebrotendinous xanthomatosis
| Variable | Patient1 | Patient2 | Patient3 | Patient4 | Patient5 | Patient6 |
|---|---|---|---|---|---|---|
| CYP27A1 mutation | c.435G > T | c.1214G > A | c.1435C > T | c.368_374delCCAGTAC | c.1016C > T | c.389 T > A |
| c.571C > T | c.1435C > T | c.1435C > T | c.379C > T | c.1420C > T | c.1263 + 1G > A | |
| Gender | Male | Male | Male | Male | Male | Male |
| Age | 45 | 40 | 30 | 32 | 24 | 27 |
| Age of diagnosis | 38 | 40 | 30 | 32 | 24 | 27 |
| Diarrhoea | – | – | + | – | – | – |
| Tendon xanthomas | + | + | + | – | – | – |
| Cataracts | – | – | + | – | – | – |
| Epilepsy | – | – | + | – | – | – |
| Cognitive impairment | – | – | + | – | + | – |
| Pyramidal signs | + | + | + | + | + | + |
| Cerebellar signs | + | + | + | – | + | – |
| EEG abnormality | + | + | NP | + | + | + |
| Dentate nuclei lesions | + | + | NP | – | + | – |
NP: Not present
Genotypes and phenotypes of Chinese CTX patients
We reviewed all of the previous CTX patients reported in the Chinese population and found that the most frequent mutations in CYP27A1 were c.410G > A (p.R137Q, 22.7%), c.379C > T (p.R127W, 18.2%), c.1435C > T (p.R479C, 9%) and c.305delC (p.P102Lfs, 9%) (Table 2). Combined with our study and a previous study16, the most frequent clinical manifestations of CTX patients in the Chinese population were pyramidal signs (88.5%), xanthomatosis (84.6%), cerebellar ataxia (57.7%), cognitive impairment (57.7%), cataracts (38.5%), and peripheral neuropathy (30.8%), which were quite different from those in the Caucasian population (Table 3). Moreover, the spectrum of CYP27A1 mutations in the Caucasian population differed from that in the Chinese Han population (Fig. 3).
Table 2.
Clinical and genetic features of patients with cerebrotendinous xanthomatosis in the Chinese population
| Family | Case | Geographical distribution | Mutation 1 | Mutation 2 | AAO | AAE | Clinical symptoms and signs | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | 1 | Mainland SE | p.Q191X | p.G145= | 36 | 38 | Xanthoma; Cerebellar ataxia; Pyramidal signs; Peripheral neuropathy | This study |
| 2 | 2 | Mainland SE | p.R405Q | p.R479C | 37 | 40 | Xanthoma; Cerebellar ataxia; Pyramidal signs; Peripheral neuropathy | This study |
| 3 | 3 | Mainland SE | p.R479C | p.R479C | 6 | 30 | Xanthoma; Cognitive impairment; Pyramidal signs; Cataracts; Chronic diarrhea; Epilepsy | This study |
| 4 | 4 | Mainland SE | p.K123 fs | p.R127W | 12 | 32 | Pyramidal signs;Peripheral neuropathy | This study |
| 5 | 5 | Mainland SE | p.T339 M | p.R474W | 4 | 24 | Pyramidal signs;Cerebellar ataxia; Cognitive impairment; Peripheral neuropathy | This study |
| 6 | 6 | Mainland SE | p.M130K | c.1263 + 1G > A | 26 | 27 | Pyramidal signs;Peripheral neuropathy | This study |
| 7 | 7 | Mainland SE | p.R513C | c.1477-2A > C | 33 | 48 | Xanthoma; Cerebellar ataxia; Pyramidal signs; Cognitive impairment; Peripheral neuropathy | Chen et al.2017 [16] |
| 7 | 8 | Mainland SE | p.R513C | c.1477-2A > C | NG | 43 | Xanthoma; Pyramidal signs; Cognitive impairment | Chen et al.2017 [16] |
| 8 | 9 | Mainland SE | p.R137Q | p.R137Q | 30 | 35 | Xanthoma; Pyramidal signs | Chen et al.2017 [16] |
| 9 | 10 | Mainland SE | p.R137Q | p.R127W | 42 | 44 | Xanthoma; Pyramidal signs | Chen et al.2017 [16] |
| 10 | 11 | Mainland SE | p.R188X | p.R405Q | 33 | 37 | Xanthoma; Cerebellar ataxia; Pyramidal signs; Cognitive impairment | Chen et al.2017 [16] |
| 11 | 12 | Mainland SE | p.R127W | p.E392K | 8 | 27 | Xanthoma; Cerebellar ataxia; Pyramidal signs; Cognitive impairment; Cataracts | Zhang et al.2016 [22] |
| 12 | 13 | Mainland S | c.446 + 1G > T | p.T339 M | 14 | 14 | Xanthoma; Cerebellar ataxia; Cognitive impairment; Cataracts | Zhong et al.2014 [26] |
| 13 | 14 | Mainland SE | p.T339 M | p.T339 M | NG | 36 | Xanthoma; Cerebellar ataxia; Pyramidal signs; Cognitive impairment; | Wei et al.2012 [25] |
| 14 | 15 | Mainland N | c.73-74delG | c.369-375delGTACCCA | 7 | 36 | Xanthoma; Cerebellar ataxia; Pyramidal signs; Cataracts | Tian et al.2011 [18] |
| 15 | 16 | Taiwan | c.205-206delC | p.R104W | NG | 42 | Xanthoma; Cerebellar ataxia; Pyramidal signs; Cognitive impairment; Peripheral neuropathy | Chen et al.2011 [20] |
| 16 | 17 | Mainland N | p.R127W | p.R474W | 20 | 42 | Xanthoma | Wang et al.2007 [19] |
| 17 | 18 | Taiwan | p.P102Lfs | p.P102Lfs | 6 | 42 | Xanthoma; Pyramidal signs | Wang et al.2006 [21] |
| 18 | 19 | Hong kong | c.1185-1G > T | p.R372Q | 7 | 48 | Xanthoma; Cerebellar ataxia; Pyramidal signs; Cognitive impairment; Cataracts | Mak et al. 2004 [23] |
| 18 | 20 | Hong kong | c.1185-1G > T | p.R372Q | NG | 44 | Xanthoma; Pyramidal signs | Mak et al. 2004 [23] |
| 18 | 21 | Hong kong | c.1185-1G > T | p.R372Q | NG | 50 | Xanthoma; Pyramidal signs; Cognitive impairment; Cataracts | Mak et al. 2004 [23] |
| 19 | 22 | Taiwan | c.1263 + 1G > A | p.R127W | NG | NG |
Xanthoma; Cerebellar ataxia; Pyramidal signs; Cognitive impairment; Cataracts; Peripheral neuropathy |
Lee et al.2002 [27] |
| 20 | 23 | Hong kong | Unknown | Unknown | 16 | 34 | Xanthoma; Cerebellar ataxia; Pyramidal signs; Cognitive impairment; Cataracts | Ko et al.2001 [24] |
| 21 | 24 | NG | p.G472A | p.G472A | NG | NG |
Xanthoma; Cerebellar ataxia; Pyramidal signs; Cognitive impairment; Cataracts; Peripheral neuropathy |
Verrips et al. 2000 [14] |
| 22 | 25 | Taiwan | Unknown | Unknown | NG | 31 | Xanthoma; Cerebellar ataxia; Pyramidal signs; Cognitive impairment; Cataracts | Chang et al.1992 [17] |
SE Southeast, S South, N North, NG Not given, AAO Age at onset, AAE Age at examination
Table 3.
Clinical features of patients with CTX in different populations
| Clinical phenotypes | Chinese population (n = 25) | Caucasian population (Spanish n = 25) | p-value |
|---|---|---|---|
| Pyramidal signs | 88.5% | 92.0% | P = 0.157 |
| Xanthomatosis | 84.6% | 56.0% | P < 0.01 |
| Cerebellar ataxia | 57.7% | 76.0% | P < 0.05 |
| Cognitive impairment | 57.7% | Not given | |
| Cataracts | 38.5% | 92.0% | P < 0.01 |
| Peripheral neuropathy | 30.8% | 64.0% | P < 0.05 |
| Chronic diarrhea | 3.8% | 92.0% | P < 0.01 |
| Epilepsy | 3.8% | 32.0% | P < 0.01 |
Fig. 3.
The spectrum of CYP27A1 pathogenic mutations in Chinese and Caucasian populations was depicted according to ClinVar database (https://www.ncbi.nlm.nih.gov/clinvar/) a CYP27A1 pathogenic mutations in the Chinese population. b CYP27A1 pathogenic mutations in the Caucasian population
Discussion
CTX is a rare sterol storage disease caused by mutations in CYP27A1 with an autosomal recessive pattern of inheritance [31]. Since the first CTX patient was reported in 1937, more than 300 patients have been reported worldwide [32], and 19 patients have been reported in the Chinese Han population [16]. There is no consensus on the prevalence of CTX, with an estimated rate of < 5/100,000 worldwide [33]. Currently, 108 variants of the CYP27A1 gene have been reported, and over 50 variants were considered pathogenic or likely pathogenic according to the Human Gene Mutation Database (HGMD). As CTX is a potentially treatable disease, early diagnosis and treatment are critical to improve the prognosis of CTX. However, diagnosis usually has a delay of several years [34]. Summarizing the genetic and clinical characteristics to help early diagnosis and treatment from a clinical perspective has great significance.
In our study, we reported 6 Chinese families with CTX. The diagnosis of CTX was confirmed by genetic sequencing of the CYP27A1 gene. Three novel likely pathogenic mutations (c.368_374delCCAGTAC, c.389 T > A, c.571C > T) and 7 previously reported pathogenic mutations (c.379C > T, c.435G > T, c.1016C > T, c.1214G > A, c.1263 + 1G > A, c.1420C > T and c.1435C > T) in CYP27A1 were identified in our study. According to a recent nationwide survey on CTX in Japan, the most frequent mutations in the CYP27A1 gene were c.1214G > A (p.R405Q, 31.6%), c.1421G > A (p.R474Q, 26.3%), and c.435G > T (p.Gly145=, 15.8%) [35]. In the Chinese population, we found that the most frequent mutations in the CYP27A1 gene were c.410G > A (p.R137Q, 22.7%), c.379C > T (p.R127W, 18.2%), and c.1435C > T (p.R479C, 9%). The most frequent mutations reported in the Japanese population, such as c.1214G > A (p.R405Q, 31.6%) and c.435G > T (p.G145=, 15.8%) were also found in the Chinese population. Many more CTX patients have been reported in the Caucasian population than in the Chinese Han population. However, the spectrum of CYP27A1 mutations in the Caucasian population differed from that in the Chinese Han population. The most frequent mutations in CYP27A1 were located in exon 2 (50%) in the Chinese Han population and in the region from exon 4 to exon 8 (75%) in the Caucasian population.
Combined with our study and a previously reported study [16], the most frequent clinical manifestations of CTX patients in the Chinese population were pyramidal signs, xanthomatosis, cerebellar ataxia, cognitive impairment, cataracts, and peripheral neuropathy. In our study, we first reported that a CTX patient had initial symptoms of epileptic seizure attack, expanding the clinical spectrum of CTX in the Chinese population. The most common CTX symptoms in the Japanese population were tendon xanthoma, followed by spastic paraplegia, cognitive dysfunction, cataract, ataxia, and epilepsy [35]. In a study performed in the Spanish population containing 25 CTX patients, the most common clinical manifestations were chronic diarrhea, cataracts, pyramidal signs, cerebellar ataxia, peripheral neuropathy and xanthomatosis [36].
The genetic and clinical characteristics differed greatly between the Chinese and Caucasian populations. Several reasons need to be considered. First, as CTX is a rare disease, the sample size is relatively small in most studies in the Chinese population, multicenter studies with large samples may help to clearly identify the characteristics of CTX in the population. Second, genetic background may be one of the major reasons for the differences in CTX genotypes and phenotypes between the Chinese and Caucasian populations. In addition, most of the hospitals in China have no proper test methods for plasma cholestanol level, leading to most of the CTX patients not being diagnosed until tendon xanthomas were observed. However, the emerging development of target next-generation sequencing will help better diagnose the disease.
Conclusions
In conclusion, we reported 6 CTX families of Chinese Han origin. Three novel likely pathogenic mutations including c.368_374delCCAGTAC, c.389 T > A, c.571C > T in CYP27A1 were identified. In addition, we compared the genetic and clinical features of CTX between Chinese and Caucasian population. In the Chinese population, the most predominant mutations in the CYP27A1 gene were c.410G > A (p.R137Q) and c.379C > T (p.R127W), the most frequent clinical manifestations were pyramidal signs, xanthomatosis, cerebellar ataxia, and cognitive impairment.
Supplementary information
Additional file 1 Table S1. Primer sequences of CYP27A1. (DOCX 14 kb)
Acknowledgements
We would like to thank all of the participants for their supports and willingness to participate in this study.
Abbreviations
- CTX
Cerebrotendinous xanthomatosis
- LDL
Low-density lipoprotein
- MMSE
Mini-Mental State Examination
- MRI
Magnetic resonance imaging
- NGS
Next-generation sequencing
- PCR
Polymerase chain reaction;
Authors’ contributions
Q-QT: data acquisition, analysis and interpretation, and manuscript preparation; YZ: data acquisition, analysis and interpretation; H-XL: data acquisition; H-LD: data acquisition; WN: data acquisition; Z-YW: study design and conceptualization, data acquisition, analysis and interpretation, critical revision of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by the research foundation for distinguished scholar of Zhejiang University to Zhi-Ying Wu (188020–193810101/ 089, Hangzhou).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Ethics approval and consent to participate
The study protocol was in accordance with the tenets of the Declaration of Helsinki and was approved by the Ethics Committee of Second Affiliated Hospital, Zhejiang University School of Medicine. Written informed consents were obtained from all the participants.
Consent for publication
Written informed consents for publication were obtained from all the participants.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Qing-Qing Tao and Yun Zhang contributed equally to this work.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s13023-019-1252-9.
References
- 1.Nie S, Chen G, Cao X, Zhang Y. Cerebrotendinous xanthomatosis: a comprehensive review of pathogenesis, clinical manifestations, diagnosis, and management. Orphanet Journal of Rare Diseases. 2014;9:179. doi: 10.1186/s13023-014-0179-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bjorkhem I, Hansson M. Cerebrotendinous xanthomatosis: an inborn error in bile acid synthesis with defined mutations but still a challenge. Biochem Biophys Res Commun. 2010;396:46–49. doi: 10.1016/j.bbrc.2010.02.140. [DOI] [PubMed] [Google Scholar]
- 3.Preiss Y, Santos JL, Smalley SV, Maiz A. Cerebrotendinous xanthomatosis: physiopathology, clinical manifestations and genetics. Rev Med Chil. 2014;142:616–622. doi: 10.4067/S0034-98872014000500010. [DOI] [PubMed] [Google Scholar]
- 4.Lorbek G, Lewinska M, Rozman D. Cytochrome P450s in the synthesis of cholesterol and bile acids--from mouse models to human diseases. FEBS J. 2012;279:1516–1533. doi: 10.1111/j.1742-4658.2011.08432.x. [DOI] [PubMed] [Google Scholar]
- 5.Bjorkhem I, Leoni V, Meaney S. Genetic connections between neurological disorders and cholesterol metabolism. J Lipid Res. 2010;51:2489–2503. doi: 10.1194/jlr.R006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chales G, Coiffier G, Guggenbuhl P. Miscellaneous non-inflammatory musculoskeletal conditions. Rare thesaurismosis and xanthomatosis. Best Pract Res Clin Rheumatol. 2011;25:683–701. doi: 10.1016/j.berh.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Vogeli I, Jung HH, Dick B, et al. Evidence for a role of sterol 27-hydroxylase in glucocorticoid metabolism in vivo. J Endocrinol. 2013;219:119–129. doi: 10.1530/JOE-13-0141. [DOI] [PubMed] [Google Scholar]
- 8.Verrips A, Nijeholt GJ, Barkhof F, et al. Spinal xanthomatosis: a variant of cerebrotendinous xanthomatosis. Brain : a journal of neurology. 1999;122(Pt 8):1589–1595. doi: 10.1093/brain/122.8.1589. [DOI] [PubMed] [Google Scholar]
- 9.Federico A, Dotti MT. Cerebrotendinous xanthomatosis: clinical manifestations, diagnostic criteria, pathogenesis, and therapy. J Child Neurol. 2003;18:633–638. doi: 10.1177/08830738030180091001. [DOI] [PubMed] [Google Scholar]
- 10.Di Taranto MD, Gelzo M, Giacobbe C, et al. Cerebrotendinous xanthomatosis, a metabolic disease with different neurological signs: two case reports. Metab Brain Dis. 2016;31:1185–1188. doi: 10.1007/s11011-016-9841-y. [DOI] [PubMed] [Google Scholar]
- 11.Rubio-Agusti I, Kojovic M, Edwards MJ, et al. Atypical parkinsonism and cerebrotendinous xanthomatosis: report of a family with corticobasal syndrome and a literature review. Mov Disord. 2012;27:1769–1774. doi: 10.1002/mds.25229. [DOI] [PubMed] [Google Scholar]
- 12.Demily C, Sedel F. Psychiatric manifestations of treatable hereditary metabolic disorders in adults. Ann General Psychiatry. 2014;13:27. doi: 10.1186/s12991-014-0027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saxena V, Pradhan P. Cerebrotendinous xanthomatosis; a genetic condition: clinical profile of three patients from a rural Indian family and review of literature. J Clin Orthop Trauma. 2016;7:122–126. doi: 10.1016/j.jcot.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verrips A, Hoefsloot LH, Steenbergen GC, et al. Clinical and molecular genetic characteristics of patients with cerebrotendinous xanthomatosis. Brain. 2000;123(Pt 5):908–919. doi: 10.1093/brain/123.5.908. [DOI] [PubMed] [Google Scholar]
- 15.Lee MH, Hazard S, Carpten JD, et al. Fine-mapping, mutation analyses, and structural mapping of cerebrotendinous xanthomatosis in U.S. pedigrees. J Lipid Res. 2001;42:159–169. [PMC free article] [PubMed] [Google Scholar]
- 16.Chen C, Zhang Y, Wu H, et al. Clinical and molecular genetic features of cerebrotendinous xanthomatosis patients in Chinese families. Metab Brain Dis. 2017;32:1609–1618. doi: 10.1007/s11011-017-0047-8. [DOI] [PubMed] [Google Scholar]
- 17.Chang WN, Kuriyama M, Lui CC, Jeng SF, Chen WJ, Chee EC. Cerebrotendinous xanthomatosis in three siblings from a Taiwanese family. J Formos Med Assoc. 1992;91:1190–1194. [PubMed] [Google Scholar]
- 18.Tian D, Zhang ZQ. 2 novel deletions of the sterol 27-hydroxylase gene in a Chinese family with Cerebrotendinous Xanthomatosis. BMC Neurol. 2011;11:130. doi: 10.1186/1471-2377-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Z, Yuan Y, Zhang W, Zhang Y, Feng L. Cerebrotendinous xanthomatosis with a compound heterozygote mutation and severe polyneuropathy. Neuropathology. 2007;27:62–66. doi: 10.1111/j.1440-1789.2006.00739.x. [DOI] [PubMed] [Google Scholar]
- 20.Chen WC, Wu KC, Hu CH, Chern TC, Jou IM. A compound heterozygous mutation of CYP27A1 gene in a Taiwanese patient with cerebrotendinous xanthomatosis. J Orthop Sci. 2011;16:825–827. doi: 10.1007/s00776-011-0072-0. [DOI] [PubMed] [Google Scholar]
- 21.Wang PW, Chang WN, Lu CH, Chao D, Schrag C, Pan TL. New insights into the pathological mechanisms of cerebrotendinous xanthomatosis in the Taiwanese using genomic and proteomic tools. Proteomics. 2006;6:1029–1037. doi: 10.1002/pmic.200500159. [DOI] [PubMed] [Google Scholar]
- 22.Zhang L, Zhang L, Nian N, et al. Analysis of a cerebrotendinous xanthomatosis case with mental retardation as the initial symptom. Chinese journal of medical genetics. 2016;33:476–480. doi: 10.3760/cma.j.issn.1003-9406.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 23.Mak CM, Lam KS, Tan KC, Ma OC, Tam S. Cerebrotendinous xanthomatosis in a Hong Kong Chinese kinship with a novel splicing site mutation IVS6-1G>T in the sterol 27-hydroxylase gene. Mol Genet Metab. 2004;81:144–146. doi: 10.1016/j.ymgme.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 24.Ko KF, Lee KW. Cerebrotendinous xanthomatosis in three siblings from a Chinese family. Singap Med J. 2001;42:30–32. [PubMed] [Google Scholar]
- 25.Wei B, Mao SY, Liu ZR, Ding MP. Clinical characteristics and gene mutation analysis of cerebrotendinous xanthomatosis. Chin J Neurol. 2012;45:41–42. [Google Scholar]
- 26.Zhong C, Zhao Q, Deng J, Wang YM, Hu B, Li XH. Mutation analysis of CYP27A1 gene in a patient with cerebrotendinous xanthomatosis. Chin Journal Nerv Mental Dis. 2014;1:65–66. [Google Scholar]
- 27.Lee MJ, Huang YC, Sweeney MG, Wood NW, Reilly MM, Yip PK. Mutation of the sterol 27-hydroxylase gene ( CYP27A1) in a Taiwanese family with cerebrotendinous xanthomatosis. J Neurol. 2002;249(9):1311–2. doi: 10.1007/s00415-002-0762-9. [DOI] [PubMed] [Google Scholar]
- 28.Dong Yi, Ni Wang, Chen Wan-Jin, Wan Bo, Zhao Gui-Xian, Shi Zhu-Qing, Zhang Yue, Wang Ning, Yu Long, Xu Jian-Feng, Wu Zhi-Ying. Spectrum and Classification of ATP7B Variants in a Large Cohort of Chinese Patients with Wilson's Disease Guides Genetic Diagnosis. Theranostics. 2016;6(5):638–649. doi: 10.7150/thno.14596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richards Sue, Aziz Nazneen, Bale Sherri, Bick David, Das Soma, Gastier-Foster Julie, Grody Wayne W., Hegde Madhuri, Lyon Elaine, Spector Elaine, Voelkerding Karl, Rehm Heidi L. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genetics in Medicine. 2015;17(5):405–423. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen W, Kubota S, Teramoto T, et al. Silent Nucleotide Substitution in the Sterol 27-Hydroxylase Gene (27) Leads to Alternative Pre-mRNA Splicing by Activating a Cryptic 5‘ Splice Site at the Mutant Codon in Cerebrotendinous Xanthomatosis Patients. Biochem. 1998;37(13):4420–28. doi: 10.1021/bi972940a. [DOI] [PubMed] [Google Scholar]
- 31.Salen G, Steiner RD. Epidemiology, diagnosis, and treatment of cerebrotendinous xanthomatosis (CTX) J Inherit Metab Dis. 2017;40:771–781. doi: 10.1007/s10545-017-0093-8. [DOI] [PubMed] [Google Scholar]
- 32.Gallus GN, Dotti MT, Federico A. Clinical and molecular diagnosis of cerebrotendinous xanthomatosis with a review of the mutations in the CYP27A1 gene. Neurol Sci. 2006;27:143–149. doi: 10.1007/s10072-006-0618-7. [DOI] [PubMed] [Google Scholar]
- 33.Lorincz MT, Rainier S, Thomas D, Fink JK. Cerebrotendinous xanthomatosis: possible higher prevalence than previously recognized. Arch Neurol. 2005;62:1459–1463. doi: 10.1001/archneur.62.9.1459. [DOI] [PubMed] [Google Scholar]
- 34.Tibrewal S, Duell PB, DeBarber AE, Loh AR. Cerebrotendinous xanthomatosis: early diagnosis on the basis of juvenile cataracts. J AAPOS. 2017;21:505–507. doi: 10.1016/j.jaapos.2017.07.211. [DOI] [PubMed] [Google Scholar]
- 35.Sekijima Y, Koyama S, Yoshinaga T, Koinuma M, Inaba Y. Nationwide survey on cerebrotendinous xanthomatosis in Japan. J Hum Genet. 2018;63:271–280. doi: 10.1038/s10038-017-0389-4. [DOI] [PubMed] [Google Scholar]
- 36.Pilo-de-la-Fuente B, Jimenez-Escrig A, Lorenzo JR, et al. Cerebrotendinous xanthomatosis in Spain: clinical, prognostic, and genetic survey. Eur J Neurol. 2011;18:1203–1211. doi: 10.1111/j.1468-1331.2011.03439.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1 Table S1. Primer sequences of CYP27A1. (DOCX 14 kb)
Data Availability Statement
All data generated or analyzed during this study are included in this published article.