Abstract
Objective
Air pollution (AP) may affect neurodevelopment, but studies about the effects of AP on the growing human brain are still scarce.
We aimed to investigate the effects of prenatal exposure to AP on lateral ventricles (LV) and corpus callosum (CC) volumes in children and to determine whether the induced brain changes are associated with behavioral problems.
Methods
Among the children recruited through a set of representative schools of the city of Barcelona, (Spain) in the Brain Development and Air Pollution Ultrafine Particles in School Children (BREATHE) study, 186 typically developing participants aged 8–12 years underwent brain MRI on the same 1.5 T MR unit over a 1.5-year period (October 2012–April 2014). Brain volumes were derived from structural MRI scans using automated tissue segmentation. Behavioral problems were assessed using the Strengths and Difficulties Questionnaire (SDQ) and the criteria of the Attention Deficit Hyperactivity Disorder DSM-IV list. Prenatal fine particle (PM2.5) levels were retrospectively estimated at the mothers’ residential addresses during pregnancy with land use regression (LUR) models. To determine whether brain structures might be affected by prenatal PM2.5 exposure, linear regression models were run and adjusted for age, sex, intracranial volume (ICV), maternal education, home socioeconomic vulnerability index, birthweight and mothers’ smoking status during pregnancy. To test for associations between brain changes and behavioral outcomes, negative binomial regressions were performed and adjusted for age, sex, ICV.
Results
Prenatal PM2.5 levels ranged from 11.8 to 39.5 μg/m3 during the third trimester of pregnancy. An interquartile range increase in PM2.5 level (7 μg/m3) was significantly linked to a decrease in the body CC volume (mm3) (β = −53.7, 95%CI [-92.0, −15.5] corresponding to a 5% decrease of the mean body CC volume) independently of ICV, age, sex, maternal education, socioeconomic vulnerability index at home, birthweight and mothers’ smoking status during the third trimester of pregnancy. A 50 mm3 decrease in the body CC was associated with a significant higher hyperactivity subscore (Rate Ratio (RR) = 1.09, 95%CI [1.01, 1.17) independently of age, sex and ICV. The statistical significance of these results did not survive to False Discovery Rate correction for multiple comparisons.
Conclusions
Prenatal exposure to PM2.5 may be associated with CC volume decrease in children. The consequences might be an increase in behavioral problems.
Keywords: Neuroimaging, Corpus callosum, Lateral ventricle, Behavioral problems, Air pollution, Children
Highlights
-
•
We examined the effects of prenatal exposure to PM2.5 on the children's brain volumes.
-
•
Increase in PM2.5 levels was associated to a decrease in the corpus callosum volume.
-
•
This air pollution-related volume decrease may be associated to behavioral problems.
1. Introduction
Air pollution is a complex mixture of particulate matter (PM), gases, organic volatile compounds and metals produced by combustion of fossil fuels and industrial and agricultural processes.
Over the past decades, air pollution has emerged as a potential disruptor of neurodevelopment in children and has been associated with a higher prevalence of Attention Deficit Hyperactivity Disorder (ADHD) and autism spectrum disorder (ASD) (Suades-González et al., 2015). However, studies about the potential damages on the growing brain induced by chronic exposure to air pollution are still scarce. We recently showed in a large sample of schoolchildren (Sunyer et al., 2015) that higher traffic-related air pollution (TRAP) exposure during childhood was associated with lower functional integration in key brain network but not linked with any structural alteration (Pujol et al., 2016). Conversely, two neuroimaging studies assessing levels of prenatal exposure to air pollution observed that, in pre-adolescent children, such an exposure was associated with thinner cortex in several brain regions of both hemispheres (Guxens et al., 2018) and with substantial reductions in white matter (WM) surface, independently of levels of air pollution encountered during childhood (Peterson et al., 2015).
Prenatal exposure to high levels of air pollution may have more severe consequences for the developing brain than the exposure during childhood. Firstly because brain structures are forming during this period and then, because the effect of in utero exposure to environmental factors may cause permanent brain injury and predict cognitive impairment later in life (Lee et al., 2017). Supporting the importance of the prenatal period, a series of mice experiments consistently reported that pre- and neonatal exposure to fine PM and diesel exhaust particles induced inflammatory reactions in the corpus callosum (CC) (Allen et al., 2015; Klocke et al., 2017; Morris-Schaffer et al., 2019), the major WM tract in the brain, suggesting that the developing WM might be a target of PM toxicity (Klocke et al., 2018). Moreover, these inflammatory responses to PM exposure were accompanied by modifications in the CC size, aberrant myelination and enlargement of the cerebral lateral ventricles (LV) (Allen et al., 2015, 2014; Klocke et al., 2017). These observations in mice are of particular translational significance given that these brain structures alterations are morphological features commonly reported in neurodevelopmental disorders with behavioral manifestations such as ADHD and ASD (Cao et al., 2010; Dougherty et al., 2016; Lefebvre et al., 2015; Lyoo et al., 1996; Wolff et al., 2015). In addition, early-life exposure to PM has been found associated with an increased risk of ADHD (Aghaei et al., 2019) and ASD (Flores-Pajot et al., 2016).
However, whether the effects of prenatal exposure to air pollution on CC and LV observed in mice are similar in humans remains to determine. The main aim of this study was to investigate the effects of prenatal exposure to fine PM on WM, gray matter (GM), LV and CC in typically developing children from the general population, using a neuroimaging regions of interest (ROI) approach with the a priori hypothesis that higher prenatal exposure to fine PM would be associated with reduced CC volume, and higher LV volumes. Secondarily, we investigated whether reduced CC volume and higher LV volume were associated with behavioral problems in our population.
2. Methods
2.1. Study design and participants
This study was developed in the context of the Brain Development and Air Pollution Ultrafine Particles in School Children (BREATHE) project (Sunyer et al., 2015). Forty schools in Barcelona (Catalonia, Spain) were selected based on modelled traffic-related nitrogen dioxide (NO2) values. Low- and high -NO2 schools were paired by socioeconomic vulnerability index and type of school (i.e., public/private). Between 2012 and 2013, a total of 39 representative schools of the city of Barcelona and 2897 children agreed to participate in the whole survey.
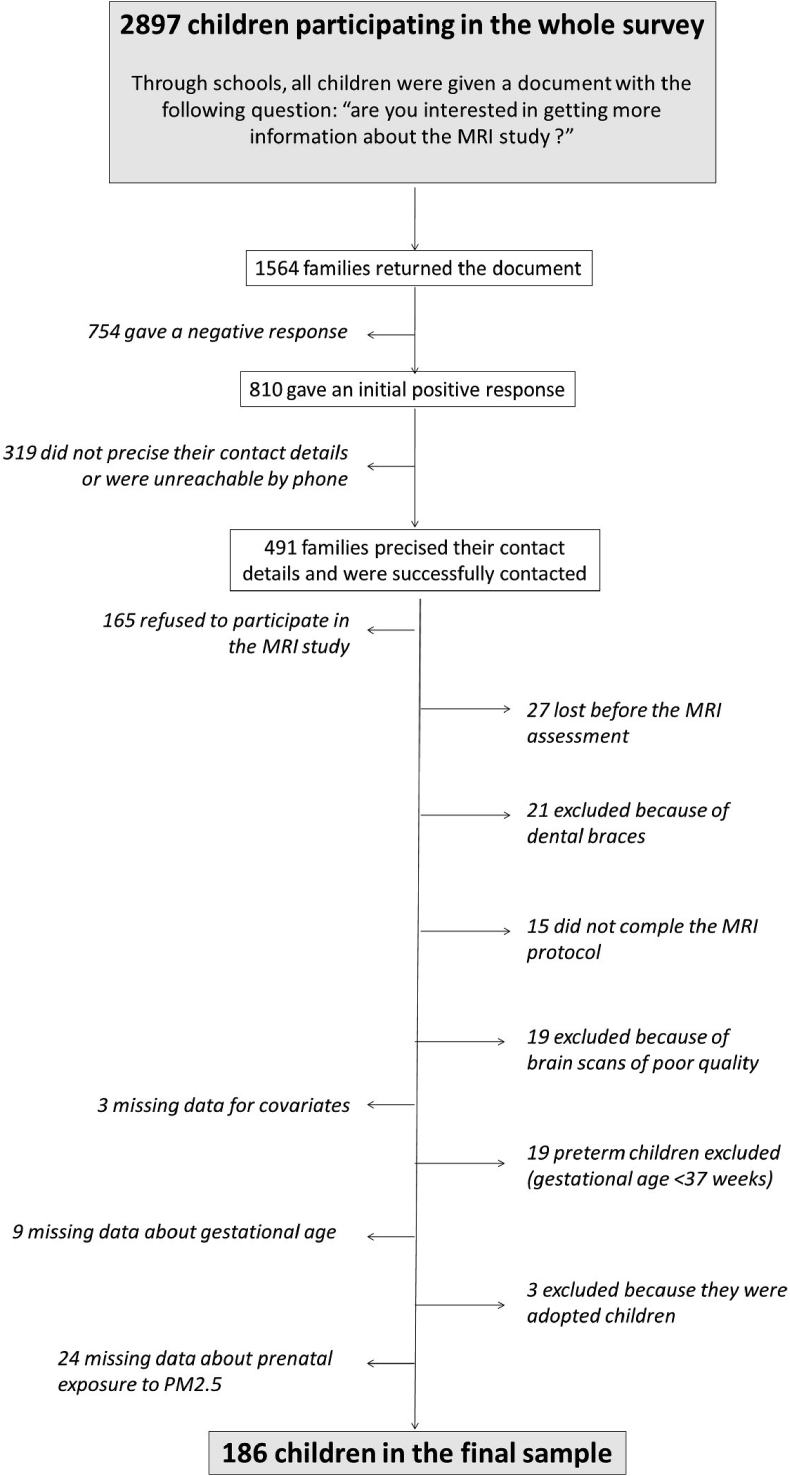
All participating children were given a document asking whether they were interested in the MRI study. The document was returned by 1564 families and among them, 810 gave an initial positive response. Parents of 491 children were successfully contacted. Consent to participate was not obtained in 165 cases, 27 children were lost before the MRI assessment, 21 were excluded because of dental braces, 34 had brain scans of poor quality and 58 had missing data (Fig. 1). The final MRI sample included 186 children (49% of girls, median age at MRI of 9.7 years).
Fig. 1.
Flow chart.
All parents or tutors signed the informed consent form approved by the Research Ethical Committee (No. 2010/41221/I) of the IMIM-Parc de Salut Mar., Barcelona, Spain and the FP7-ERC-2010-AdG Ethics Review Committee (268479–22022011).
2.2. Exposure to fine PM
From the prenatal period to the time of the MRI study, the history of exposures to PM with an aerodynamic diameter <2.5 μm (PM2.5) were estimated at the geocoded postal address of each participant using land use regression (LUR) models (Eeftens et al., 2012). The area-specific LUR models of annual PM2.5 used in this study were developed within the framework of the European Study of Cohorts for Air Pollution Effects (ESCAPE, www.escapeproject.eu). Briefly, PM were measured between October 2008 and April 2011 at twenty sampling sites in Barcelona. These measurements were then temporally corrected to estimate annual average concentrations in the Barcelona area (Eeftens et al., 2012). The Geographic information systems-derived variables which better predicted spatial variation of these annual average concentrations of PM in the Barcelona area were i) land-use (sum of Urban green and semi-natural and forested areas in a 100 m2 buffer size area), traffic-related (traffic intensity on nearest road in vehicules.day−1 x inverse squared distance to the nearest road, and total traffic load of all roads in a buffer of 100 m) for the PM2.5 LUR models, ii) traffic-related (traffic intensity on nearest road in vehicules.day−1 x inverse distance to the nearest road, and road length of all roads in a buffer of 25 m), and altitude variables for the PM10 LUR models.
Because the measurement periods in ESCAPE did not overlap with the time windows of exposure we wished to explore, residence-based exposures derived from the LUR models were then adjusted to the specific periods of time of interest in this study using daily PM records from an urban background station from the air quality national network (XVPCA, http://dtes.gencat.cat/icqa) covering the study area. PM2.5 was started to be monitored in Barcelona in 2008–2009, until then there are no records. The temporal modelling of PM2.5 for any time window prior to 2008 was conducted consequently with the PM10 routine monitoring data. A known ratio between PM10/PM2.5 was applied.
Parents reported the history of residence of the child via questionnaire where they indicated the periods that the family lived in each residence in case they moved. If they moved, we calculated the time-weighted average of the PM2.5 concentration estimated for each residence within the year. In cases of separated parents with shared custody, the participants were assigned the time averaged exposure according to the time that they spent in each home. We did not have the history of residences during the pregnancy period. We assumed the first residence of the child was the same during the pregnancy period.
Following this procedure, PM2.5 exposures were estimated i) for the whole pregnancy, ii) for the first, the second and the third trimesters of pregnancy, iii) for the 2 first years of life, iv) at the time of the MRI study. The pregnancy period, as well as the 3 trimesters of pregnancy were determined using the children's birth dates and gestational ages in weeks. Children for whom the gestational age was unknown (9 missing data, 3 adopted children, 19 preterm children with gestational age <37 weeks) were excluded from the analysis sample.
Prenatal PM2.5 levels were the exposure variables used in the main analyses. We also considered PM2.5 exposure during the 2 first years of life and PM2.5 exposure at the time of the MRI study in sensitive analyses.
2.3. MRI
2.3.1. MRI acquisition
Cerebral anatomical images of the entire surface of the brain were acquired between October 2012 and April 2014. In order to minimize movement in the scanner, children were asked to stand still once in the machine. A 1.5-T Signa Excite system (General Electric, Milwaukee, WI) equipped with an eight-channel phased-array head coil was used. Anatomical images were obtained using an axial T1-weighted three-dimensional (3D) fast spoiled gradient inversion recovery prepared sequence. A total of 134 contiguous slices were acquired with repetition time 11.9 msec, echo time 4.2 msec, flip angle 15°, field of view 30 cm, 256 × 256 pixel matrix, and slice thickness 1.2 mm. Total acquisition time was 5mn 40s for the 3D sequence.
2.3.2. Data preprocessing and segmentation
Cortical reconstruction and volumetric segmentation were performed with the version 5.3 of the Freesurfer image analysis suite (http://surfer.nmr.mgh.harvard.edu/). The technical details of these procedures are described in prior publications (Dale et al., 1999; Fischl et al., 2004, 2002; 1999a; Ségonne et al., 2004).
Briefly, this processing includes removal of non-brain tissue using a hybrid watershed/surface deformation procedure (Ségonne et al., 2004), automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures (including hippocampus, amygdala, caudate, putamen, ventricles) (Fischl et al., 2004, 2002) intensity normalization (Sled et al., 1998), tessellation of the gray matter white matter boundary, automated topology correction (Fischl et al., 2001; Ségonne et al., 2007), and surface deformation following intensity gradients to optimally place the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class (Dale et al., 1999).
Once the cortical models were complete, registration to a spherical atlas which is based on individual cortical folding patterns to match cortical geometry across subjects (Fischl et al., 1999b) was performed.
2.3.3. Quality control
All the anatomical images were visually inspected by a trained researcher before and after the pre-processing steps. Right after acquisition, cases were systematically excluded based on expert subjective criteria if the raw images showed obvious motion artefacts (ghost and blurring of the image), ringing or truncation artefacts, and susceptibility phenomena. No scan repetition was done. After preprocessing, cases were excluded if the images showed deformation of the three-dimensional brain anatomy and large truncated brain areas, non optimal removal of nonbrain tissue, and obvious tissue (Gray and White matter) misclassification.
2.3.4. Neuroimaging variables
Volumetric regions from both hemispheres were averaged. The segmentation of the CC provided the volume of several anatomic regions: CC posterior, CC mid-posterior, CC central, CC mid-anterior, CC anterior. CC mid-posterior, CC central and CC mid-anterior volumes were summed up to give the volume of the body CC. The volume of the total CC was obtained by summing up all the anatomical regions.
Intracranial volume (ICV) was also estimated and was further used as an adjustment factor in the statistical analyses to take brain size into account.
2.4. Behavioral outcomes
The Strengths and Difficulties Questionnaire (SDQ) is widely used as an international standardized instrument measuring child behavior (Goodman, 1997). The SDQ, filled out by parents in our study, comprises five separate subscales, each including 5 questions covering different behavioral aspects including emotional symptoms, conduct problems, hyperactivity/inattention, peer relationship problems, and prosocial behavior. Each question is rated on a 3-point scale [not true (0), somewhat true (1), and certainly true (2)]. A total difficulties score (ranging from 0 to 40) is generated by summing the scores for all subscales except the prosocial behavior scale (considered a behavioral strength). We analysed the total difficulties score as a continuous variable.
ADHD symptomatology was reported by teachers using the ADHD/Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) Scale, which consists of a list of 18 symptoms categorized under two separate symptom groups: inattention (nine symptoms) and hyperactivity/impulsivity (nine symptoms). Each ADHD symptom is rated on a 4-point scale (0 = never or rarely, 1 = sometimes, 2 = often, or 3 = very often).
ADHD scale and SDQ questionnaires were filled out by teachers and parents between September 2012 and June 2013. Higher SDQ total difficulties score and/or higher ADHD scores indicate more behavioral problems.
2.5. Contextual and individual covariates
Socio-demographic factors were measured using a neighborhood socio-economic vulnerability index (based on level of education, unemployment, and occupation in each census tract, the finest spatial census unit, with median area of 0.08 km2) (Ministry of Public Works, 2012) according to both school and home addresses, and maternal education (≤than primary, secondary or university) (Sunyer et al., 2015).
We also considered birthweight (<2.5 kg, 2.5–4 kg or >4 kg) and mothers’ smoking status during pregnancy (Smoking, No smoking).
2.6. Statistical analyses
To determine whether brain structures might be affected by prenatal PM2.5 exposure, we tested separate linear regression models for each neuroimaging variables as dependent variables (GM, WM, CC, and LV volumes) and prenatal (three trimesters and entire pregnancy) PM2.5 levels as independent variables. The analyses were adjusted for age, sex, ICV, maternal education, home socioeconomic vulnerability index, birthweight and mothers’ smoking status during pregnancy. Interaction effects between PM2.5 exposure levels and sex, and between PM2.5 exposure levels and age at the time of MRI, were examined including interaction terms in the regression models.
To test for associations between behavioral outcomes and brain volumes, we fit negative binomial regression in order to account for the overdispersion in the ADHD and SDQ scores (eFig. 1). Analyses were adjusted for age, sex, ICV.
To account for the fact that we examined multiple brain regions and multiple time windows of exposure, we adjusted significance levels using the false discovery rate (FDR) method (Benjamini and Hochberg, 1995). Analyses were run using Stata 14 (StataCorp). Statistical significance was set at p < 0.05.
2.6.1. Sensitivity analyses
Given that the participating children were recruited through schools, we ran linear mixed models with school as nested random effect to verify that the multilevel nature of the data did not influence the results.
We also ran regression linear models including exposures in all of the trimesters of pregnancy to determine which trimester may be the most predictive on the brain outcomes.
Then, we examined the relationships between prenatal PM2.5 exposure and brain structures with an additional adjustment for current PM2.5 exposure.
Finally, we investigated the relationship between PM2.5 exposure during the 2 first years of life and the brain volumes.
3. Results
Forty-nine percent of children were girls and age at MRI ranged between 8.0 and 11.7 years (Table 1). Characteristics of the MRI subsample were similar to those of the overall BREATHE sample, but for home socioeconomic index with less deprivation in the MRI subsample (eTable 1).
Table 1.
Children's characteristics, n = 186.
| Socio-demographic characteristics | |
| Age at MRI, yrs (median, range) | 9.7 (8.0, 11.7) |
| Sex (n, %) | |
| Girls | 91 (49%) |
| Boys | 95 (51%) |
| Home socioeconomic vulnerability index (median, range) | 0.39 (0.06, 0.90) |
| Maternal Education level (n, %) | |
| Primary or less than primary | 16 (9%) |
| Secondary | 50 (27%) |
| University | 120 (64%) |
| School attended (n, %) | |
| Private | 109 (59%) |
| Public | 77 (41%) |
| Birthweight (n, %) | |
| <2.5 kg | 7 (4%) |
| 2.5 – 4 kg | 164 (88%) |
| >4 kg | 15 (8%) |
| Mother's smoking status during pregnancy (n, %) | |
| Smoking | 13 (7%) |
| No smoking | 173 (93%) |
| Brain structures (median, range) | |
| ICV, cm3 | 1397 (1109, 1752) |
| White matter, mm3 | 404188 (314938, 549909) |
| Gray matter, mm3 | 693236 (541002, 845761) |
| Corpus callosum, mm3 | |
| Total | 2459 (1209, 3729) |
| Anterior | 703 (288, 1211) |
| Body | 1016 (606, 1490) |
| Posterior | 741 (316, 1112) |
| Lateral ventricles, mm3 | 7500 (2592, 32383) |
| Behavior (median, range) | |
| ADHD DSM-IV scale a | |
| ADHD total score (n = 181) | 4 (0, 42) |
| Inattention score (n = 182) | 2 (0, 24) |
| Hyperactivity score (n = 182) | 1 (0, 23) |
| SDQ | |
| Total difficulties score | 8 (0, 24) |
| PM2.5levels, μg/m3(median, range) | |
| 1st trimester of pregnancy | 24.1 (14.0, 49.0) |
| 2nd trimester of pregnancy | 23.7 (12.2, 47.5) |
| 3rd trimester of pregnancy | 22.8 (11.8, 39.5) |
| Whole pregnancy | 23.6 (12.7, 45.0) |
Abbreviations: ICV, intracranial volume; ADHD, Attention-Deficit Hyperactivity Disorder; SDQ, Strengths and Difficulties Questionnaire; PM2.5, Particle Matter with a diameter of 2.5 μm or less.
5 teachers gave back incomplete ADHD DSM-IV questionnaires.
Prenatal PM2.5 levels across the three trimesters of pregnancy had similar and normal distributions (eFig. 2) and were slightly variable (medians ranged from 22.8 to 24.1 μg/m3). Pearson's correlation coefficients between PM2.5 levels across the three trimesters of pregnancy ranged from 0.75 to 0.77 (eTable 2).
3.1. Prenatal exposure to PM2.5 and brain volumes
Table 2 presents the adjusted PM2.5 coefficients for the neuroimaging outcomes. Independently of ICV, age, sex, maternal education, socioeconomic vulnerability index at home, birthweight and mother's smoking status during pregnancy, an interquartile range increase in PM2.5 levels during the third trimester of pregnancy (7 μg/m3) was significantly linked to a decrease in the anterior and the body CC volumes (β = −24.8, 95%CI [-48.7, −0.95], β = −53.7, 95%CI [-92.0, −15.5] respectively). However, the statistical significance of these associations did not survived FDR correction for multiple comparisons (Benjamini-Hochberg correction (Benjamini and Hochberg, 1995)).
Table 2.
Difference (95%CI) in brain volumes by prenatal air pollution exposure to PM2.5 (interquartile range increase) in 186 children.
| Volumes, mm3 | PM2.5 exposure periods |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1st Trimester |
2nd Trimester |
3rd Trimester |
Whole pregnancy |
|||||||||
| β (95%CI) | pa | p FDRb | β (95%CI) | pa | p FDRb | β (95%CI) | pa | p FDRb | β (95%CI) | pa | p FDRb | |
| Gray Matter | 2890 (−1508, 7287) | 0.196 | 0.464 | 1835 (−3272, 6942) | 0.479 | 0.583 | 1124 (−4700, 6948) | 0.704 | 0.800 | 2094 (−2562, 6750) | 0.376 | 0.526 |
| White Matter | 215.7 (−3536, 3967) | 0.910 | 0.910 | −200.9 (−4543, 4141) | 0.859 | 0.891 | −2226 (−7162, 2710) | 0.375 | 0.526 | −582.6 (−4544, 3379) | 0.772 | 0.831 |
| Corpus callosum | ||||||||||||
| Total | −48.7 (−106.2, 8.7) | 0.096 | 0.361 | −35.6 (−102.4, 31.2) | 0.295 | 0.504 | −93.9 (−169.0, −18.8) | 0.015 | 0.210 | −57.2 (−117.7, 3.4) | 0.064 | 0.299 |
| Anterior | −9.5 (−27.7, 8.8) | 0.306 | 0.504 | −9.1 (−30.2, 12.0) | 0.397 | 0.529 | −24.8 (−48.7, −0.95) | 0.042 | 0.294 | −13.6 (−32.9, 5.6) | 0.164 | 0.464 |
| Body | −28.9 (−58.3, 0.36) | 0.053 | 0.297 | −22.3 (−56.4, 11.8) | 0.199 | 0.464 | −53.7 (−92.0, −15.5) | 0.006 | 0.168 | −33.8 (−64.7, −2.9) | 0.032 | 0.294 |
| Posterior | −10.3 (−29.6, 9.0) | 0.295 | 0.504 | −4.2 (−26.6, 18.2) | 0.714 | 0.800 | −15.4 (−40.8, 10.1) | 0.235 | 0.494 | −9.7 (−30.1, 10.7) | 0.348 | 0.526 |
| Lateral ventricles | 590.5 (−120.4, 1301) | 0.103 | 0.361 | 323.2 (−504.5, 1151) | 0.441 | 0.561 | 553.8 (−387.2, 1495) | 0.247 | 0.494 | 498.4 (−254.5, 1251) | 0.193 | 0.464 |
Difference (95% CI) in brain structures adjusted for age at MRI, sex, intracranial volume, maternal education, residential neighborhood socioeconomic status, birthweight and mother's smoking status during pregnancy.
Abbreviations:PM2.5, Particle Matter with a diameter of 2.5 μm or less.
Raw p values not adjusted for multiple comparisons.
False-discovery rate–corrected p values.
No associations were observed between PM2.5 and the other neuroimaging variables (LV, GM and WM).
No significant interaction between sex and prenatal exposure to PM2.5, nor between age and prenatal exposure to PM2.5, were observed (eTable 5).
3.2. Brain volumes and behavioral problems
Significant higher hyperactivity subscores were observed for a 50 mm3 decrease in the body CC (Rate Ratio (RR) = 1.09, 95%CI [1.01, 1.17], corresponding to a subscore 9% increase, independently of age, sex and ICV (Table 3). However, the statistical significance of these associations did not survive FDR correction.
Table 3.
Difference (95%CI) in behavioral problems by corpus callosum volumes in 186 children.
| Behavior | Corpus Callosum volumes, mm3 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total a |
Anteriorb |
Body c |
|||||||
| RR (95%CI) | pd | p FDRe | RR (95%CI) | pd | p FDRe | RR (95%CI) | pd | p FDRe | |
| ADHD score | |||||||||
| Total* | 1.03 (0.96, 1.10) | 0.382 | 0.946 | 1.00 (0.95, 1.06) | 0.890 | 0.961 | 1.04 (0.98, 1.11) | 0.171 | 0.684 |
| Inattention** | 1.01 (0.94, 1.08) | 0.812 | 0.961 | 1.00 (0.95, 1.05) | 0.917 | 0.961 | 1.02 (0.96, 1.08) | 0.588 | 0.961 |
| Hyperactivity ** | 1.07 (0.99, 1.16) | 0.085 | 0.510 | 1.02 (0.96, 1.09) | 0.472 | 0.946 | 1.09 (1.01, 1.17) | 0.023 | 0.276 |
| SDQ score | |||||||||
| Total difficulties score | 1.00 (0.97, 1.03) | 0.961 | 0.961 | 0.99 (0.97, 1.01) | 0.473 | 0.946 | 1.00 (0.98, 1.03) | 0.796 | 0.961 |
Negative binomial regression models adjusted for age, sex and intracranial volume.
Abbreviations: ADHD, attention deficit hyperactivity disorder; SDQ, Strengths and Difficulties Questionnaire; RR, Rate Ratio.
*n = 181, **n = 182.
By 100 mm3 decrease.
By 25 mm3 decrease.
By 50 mm3 decrease.
Raw p values not adjusted for multiple comparisons.
False-discovery rate–corrected p values.
3.3. Sensitivity analyses
Taking into account the multilevel nature of the data in linear mixed models with school as nested random effect did not influence the results (data not shown).
In the linear regression models including simultaneously exposures in all of the trimesters of pregnancy, higher exposure during the 3rd trimester remained significantly associated to decrease in CC volumes (eTable 4).
Additional adjustment for current PM2.5 exposure at home did not change the relationships between prenatal exposure to PM2.5 and CC volumes (eTable 6).
The investigation of the relationship between exposure to PM2.5 during the 2 first years of life and brain volumes did not reveal any significant impact of PM2.5 exposure on the CC and LV volumes during this period (eTable 7).
4. Discussion
We examined the relationship between prenatal exposure to PM2.5 and CC and LV volumes later in childhood. In our sample, we observed that prenatal exposure to PM2.5, particularly during the last trimester of pregnancy, may induce structural changes in the CC in children aged between 8 and 12 years from the general population. An increase of approximately 7 μg/m3 in PM2.5 level during the third trimester of pregnancy was indeed associated with a reduction corresponding to almost 5% of the mean volume of the body CC. Brain changes of this magnitude may result in an increase in ADHD symptoms (hyperactivity), as well as in more general behavior problems.
CC is composed of mostly myelinated axons, forming the largest WM bundle in the brain. It connects homologous areas of the two cerebral hemispheres and is essential for communication between them (Banich, 2003). Structural alterations of the CC are frequently observed in ADHD and ASD (Cao et al., 2010; Lefebvre et al., 2015; Lyoo et al., 1996; Wolff et al., 2015). The prenatal PM2.5 exposure-related reduction in CC volume observed in our study is possibly the consequence of changes in the number or size of axons connecting the two hemispheres, but may also reflect differences in the number of cortical neurons within homologous regions. Both neurons and oligodendrocytes (cells in charge of producing the myelin sheath in the central nervous system) proved to be vulnerable to TRAP exposure in experimental studies (Block and Calderón-Garcidueñas, 2009; Ejaz et al., 2014; Woodward et al., 2017). This toxicity to TRAP is thought to be mediated by microglia, the resident immune cells in the brain (Genc et al., 2012). Indeed, TRAP exposure appears to trigger an overactivation of microglia which then release factors (reactive oxygen species, cytokines and tumor necrosis factor) toxic for neurons and oligodendrocytes (Genc et al., 2012). Microglia's receptors are reachable by different ways. After inhalation and from the lungs, PM2.5 can directly translocate to the systematic circulation and/or provoke the release of circulating inflammatory agents. Both PM2.5 and inflammatory agents are then likely to cross the blood brain barrier. Regarding the prenatal period, TRAP may impair the placenta function (van den Hooven et al., 2012) which in turn could disrupt foetal neurodevelopment (Batalle et al., 2016; Egaña-Ugrinovic et al., 2015; Eixarch et al., 2016). In addition, experimental evidence for transplacental transfer of nano-size PM (Takeda et al., 2009) and maternal cytokines (Dahlgren et al., 2006) suggest that PM inhaled by the mother could also overactivate microglia in the foetus’ brain.
The study's volumetric ROI approach does not permit to determine the nature of the microstructural changes leading to the reduction of the CC volume. In order to investigate this question, we ran in parallel whole brain Voxel-Based Morphometry (VBM) and Diffusion Tensor Imaging (DTI) analyses in a naïve approach within the MRI subsample. However, at the voxel level, we did not observe any structural changes linked to prenatal exposure to PM2.5 during the 1st, the 2nd nor the 3rd trimester of pregnancy (Supplemental Material).
With the volumetric ROI approach, we nevertheless observed that the effects on the children's CC volume were the most severe during the third trimester of pregnancy. This observation is in coherence with results from animal studies which report reduction in CC size after exposure to particle during a developmental window of CNS development in mice equivalent to human 3rd trimester (Allen et al., 2015) (Clancy et al., 2007) but not for earlier exposures equivalent to human 1st and 2nd trimesters (Klocke et al., 2017). Moreover, besides a reduction of the CC area, mice presented hypomyelination and aberrant WM development (Allen et al., 2015). These observations, along with the fact that late gestation in humans is the seat of oligodendrocytes maturation (Dubois et al., 2014), suggest that the effects on CC reported in the context of our study are more likely to be the consequences of the PM2.5 toxicity towards oligodendrocytes rather than neurons. This hypothesis is supported by the absence of changes in GM volume, similarly to the findings from the Peterson's study showing that high levels of TRAP during the third trimester of pregnancy were associated with substantial reduction in WM surface but not with cortical thickness in 40 children 7–9 years of age (Peterson et al., 2015). Following the studies of Allen (Allen et al., 2015) and Peterson (Peterson et al., 2015) that point at late gestation as a particularly sensitive windows for the association between TRAP and WM development, we did not observe any significant impact on the CC for exposure to PM2.5 during the 2 first years of life and adjustment for current exposure to PM2.5 did not change the magnitude of the associations.
In addition, in the same participants and using VBM analyses, we recently reported no associations between TRAP exposure during childhood and brain anatomy (Pujol et al., 2016). In parallel to structural brain changes, a recent retrospective study on several cognitive functions in children suggests that the period from week 20 onwards of gestation could be the most vulnerable to TRAP exposure (Chiu et al., 2016). As well, a recent meta-analysis of studies investigating the associations between prenatal PM2.5 exposure and risk of ASD showed that the strength of the association increased in parallel with the pregnancy advancement (Flores-Pajot et al., 2016).
The magnitude of the effects, a 5% decrease of the mean body CC volume for an interquartile range increase in prenatal PM2.5 levels, might be of concern for several reasons. Firstly, because these effects were observed for a chronic prenatal exposure to PM2.5 at levels that did not exceed the limit target value of 25 μg/m3 established by the European Union for 2015 (Directive, 2008/50/EC). It is important to highlight that in the Allen's study (Allen et al., 2015), the deleterious effect of ultrafine particles on mice's CC were also reported for levels consistent with high traffic areas of major U.S. cities and consequently likely to be encountered by the general population. Secondly because, even though not specific to these neurodevelopmental disorders, CC reductions are commonly reported in ADHD and ASD (Dougherty et al., 2016). For example, the mean CC volume was reported to be 12% smaller in children with ASD compared with healthy controls (Frazier et al., 2012). Finally, higher hyperactivity score was observed for CC volume reduction estimated for a 7 μg/m³ increase in PM2.5 levels during the late prenatal life. These last findings provide additional information to the Allen's study which did not investigate the behavioral consequences of the reported air pollution-related reduction in CC area. However, in contrast to mice experiments, we did not observe significant increase of LV volume in children.
In this study, PM2.5 was targeted because, of all air pollutants, particles are thought to be the most important inhaled toxicants in urban air (Kaiser, 2005), particularly in relation to brain damage (González-Maciel et al., 2017). Besides, mice experiments reported effects of ultrafine PM on CC, possibly through iron toxicity (Klocke et al., 2017).
Several limitations in this study must be considered. Firstly, comparison of the characteristics of the MRI subsample population and the overall BREATHE population showed that home socioeconomic vulnerability index was significantly different between groups, with less deprivation in the MRI subsample. However, this probably does not affect the possibility of extrapolating the results to the overall BREATHE study since i) the distribution of the exposure and behavioral variables are not significantly different between the overall BREATHE cohort and the MRI subsample, ii) the index was not significantly associated with the neuroimaging variables and the behavior assessments.
Secondly, we cannot rule out that the findings presented here are false positive since their statistical significance did not survive to FDR correction for multiple testing. However, it might be just as troublesome, from a public health perspective, to conclude that there is no relationship between prenatal exposure to PM2.5 and CC volume because the Benjamini-Hochberg false discovery rate of 5% might be too high and not adequate in this context, since there is correlation among all the CC structures, as well as among the trimesters specific exposures to PM2.5, and for all of them the association was negative. In addition, whole brain VBM and DTI analyses did not support the volumetric findings. However, it is important to highlight that such analyses are also subject to correction for multiple comparisons rather restrictive given the number of voxels and consequently of tests performed.
Probably because of insufficient power, we did not observe any statistically significant associations between prenatal PM2.5 exposure and behavioral outcomes while we found such an association in previous work in the whole population of the BREATHE study (Forns et al., 2016). We were consequently not able to investigate whether the CC volume decrease was mediating any link between PM2.5 and behavioral problems.
Then, we only used ambient modelled PM2.5 estimates and did not have personal monitoring nor biomarkers, thus our PM2.5 levels only provide indirect estimates of the real exposure to PM2.5. Furthermore, we assumed that the first residence of the child reported in the residential history questionnaire was the same during the pregnancy period. These aspects of the exposure assessment method used in this study may have led to non-differential exposure misclassification and in turn to regression coefficients closer to the null value that they probably are.
Finally, TRAP is actually a mixture of environmental contaminants and the effects observed here might be not only attributable to PM2.5.
Even though the effects of PM2.5 we observed were more severe on brain structures for higher exposure during the third trimester of pregnancy, our study design does not permit to disentangle the effects of potential susceptibility windows. As well, we cannot neither rule out that this result was driven by a cumulative exposure effect throughout the 3 trimesters of pregnancy. Our findings only add up to an amount of observations suggesting that pregnancy is a particularly crucial period (Allen et al., 2015; Flores-Pajot et al., 2016).
Eventually, information about children's Intelligence Quotient and other deleterious prenatal exposures such as noise, that are both associated to PM2.5 levels and to neurobehavioral problems in children (Chiu et al., 2016; Forns et al., 2016; Sandtorv et al., 2018), were not available. We were consequently not able to consider these potential confounders in the analyses. It could have affected the accuracy of the regression parameters and rate ratios that we observed.
5. Conclusions
In conclusion, this study suggests that prenatal exposure to PM2.5 is associated with changes on the CC volume in pre-adolescent children, even for levels not exceeding the limit target values established in the European Union. We have replicated the experimental findings in mice using a neuroimaging Region of Interest approach, but we went beyond by showing that the consequences of this air pollution-related CC volume reduction might be an increase in behavioral problems. Future larger and prospective studies are needed to confirm these findings in children.
Conflicts of interest
The authors declare that they have no conflict of interest.
Funding
Marion Mortamais is supported by a Marie Skłodowska-Curie Individual Fellowship (H2020-MSCA-IF-2014; EU project 656294).
Natalia Vilor-Tejedor is funded by a pre-doctoral grant from the Agència de Gestió d’Ajuts Universitaris i de Recerca (2017 FI_B 00636). Silvia Alemany thanks the Institute of Health Carlos III for her Sara Borrell postdoctoral grant (CD14/00214).
This work was supported by the European Research Council under the ERC [grant number 268479]—the BREATHE project. The Agency of University and Research Funding Management of the Catalonia Government participated in the context of Research Group SGR2014-1673.
This project also received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement No 785994).
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964.
Helsinki declaration and its later amendments or comparable ethical standards.
Acknowledgments
We acknowledge Cecilia Persavento (Research Technician, ISGlobal), Judit González (ISGlobal), Laura Bouso (Research Technician, CREAL), Mónica López (Predoctoral Fellow, ISGlobal) and Pere Figueras (Research technician, CREAL) for their contribution to the field work. We also acknowledge all the families and schools participating in the study.
ISGlobal is a member of the CERCA Programme, Generalitat de Catalunya.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envres.2019.108734.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Aghaei M., Janjani H., Yousefian F., Jamal A., Yunesian M. Association between ambient gaseous and particulate air pollutants and attention deficit hyperactivity disorder (ADHD) in children; a systematic review. Environ. Res. 2019;173:135–156. doi: 10.1016/j.envres.2019.03.030. [DOI] [PubMed] [Google Scholar]
- Allen J.L., Liu X., Pelkowski S., Palmer B., Conrad K., Oberdörster G., Weston D., Mayer-Pröschel M., Cory-Slechta D.A. Early postnatal exposure to ultrafine particulate matter air pollution: persistent ventriculomegaly, neurochemical disruption, and glial activation preferentially in male mice. Environ. Health Perspect. 2014;122:939–945. doi: 10.1289/ehp.1307984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J.L., Oberdorster G., Morris-Schaffer K., Wong C., Klocke C., Sobolewski M., Conrad K., Mayer-Proschel M., Cory-Slechta D.A. Developmental neurotoxicity of inhaled ambient ultrafine particle air pollution: parallels with neuropathological and behavioral features of autism and other neurodevelopmental disorders. Neurotoxicology (Little Rock) 2015 doi: 10.1016/j.neuro.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich M.T. In: Interaction between the Hemispheres and its Implications for the Processing Capacity of the Brain. Hugdahl K., Davidson R.J., editors. 2003. [Google Scholar]
- Batalle D., Muñoz-Moreno E., Tornador C., Bargallo N., Deco G., Eixarch E., Gratacos E. Altered resting-state whole-brain functional networks of neonates with intrauterine growth restriction. Cortex J. Devoted Study Nerv. Syst. Behav. 2016;77:119–131. doi: 10.1016/j.cortex.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B Methodol. 1995;57:289–300. [Google Scholar]
- Block M.L., Calderón-Garcidueñas L. Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci. 2009;32:506–516. doi: 10.1016/j.tins.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Q., Sun L., Gong G., Lv Y., Cao X., Shuai L., Zhu C., Zang Y., Wang Y. The macrostructural and microstructural abnormalities of corpus callosum in children with attention deficit/hyperactivity disorder: a combined morphometric and diffusion tensor MRI study. Brain Res. 2010;1310:172–180. doi: 10.1016/j.brainres.2009.10.031. [DOI] [PubMed] [Google Scholar]
- Chiu Y.-H.M., Hsu H.-H.L., Coull B.A., Bellinger D.C., Kloog I., Schwartz J., Wright R.O., Wright R.J. Prenatal particulate air pollution and neurodevelopment in urban children: examining sensitive windows and sex-specific associations. Environ. Int. 2016;87:56–65. doi: 10.1016/j.envint.2015.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B., Finlay B.L., Darlington R.B., Anand K.J.S. Extrapolating brain development from experimental species to humans. Neurotoxicology (Little Rock) 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren J., Samuelsson A.-M., Jansson T., Holmäng A. Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr. Res. 2006;60:147–151. doi: 10.1203/01.pdr.0000230026.74139.18. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dougherty C.C., Evans D.W., Myers S.M., Moore G.J., Michael A.M. A comparison of structural brain imaging findings in autism spectrum disorder and attention-deficit hyperactivity disorder. Neuropsychol. Rev. 2016;26:25–43. doi: 10.1007/s11065-015-9300-2. [DOI] [PubMed] [Google Scholar]
- Dubois J., Dehaene-Lambertz G., Kulikova S., Poupon C., Hüppi P.S., Hertz-Pannier L. The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience. 2014;276:48–71. doi: 10.1016/j.neuroscience.2013.12.044. [DOI] [PubMed] [Google Scholar]
- Eeftens M., Beelen R., de Hoogh K., Bellander T., Cesaroni G., Cirach M., Declercq C., Dėdelė A., Dons E., de Nazelle A., Dimakopoulou K., Eriksen K., Falq G., Fischer P., Galassi C., Gražulevičienė R., Heinrich J., Hoffmann B., Jerrett M., Keidel D., Korek M., Lanki T., Lindley S., Madsen C., Mölter A., Nádor G., Nieuwenhuijsen M., Nonnemacher M., Pedeli X., Raaschou-Nielsen O., Patelarou E., Quass U., Ranzi A., Schindler C., Stempfelet M., Stephanou E., Sugiri D., Tsai M.-Y., Yli-Tuomi T., Varró M.J., Vienneau D., Klot S. von, Wolf K., Brunekreef B., Hoek G. Development of Land Use Regression models for PM(2.5), PM(2.5) absorbance, PM(10) and PM(coarse) in 20 European study areas; results of the ESCAPE project. Environ. Sci. Technol. 2012;46:11195–11205. doi: 10.1021/es301948k. [DOI] [PubMed] [Google Scholar]
- Egaña-Ugrinovic G., Savchev S., Bazán-Arcos C., Puerto B., Gratacós E., Sanz-Cortés M. Neurosonographic assessment of the corpus callosum as imaging biomarker of abnormal neurodevelopment in late-onset fetal growth restriction. Fetal Diagn. Ther. 2015;37:281–288. doi: 10.1159/000366160. [DOI] [PubMed] [Google Scholar]
- Eixarch E., Muñoz-Moreno E., Bargallo N., Batalle D., Gratacos E. Motor and cortico-striatal-thalamic connectivity alterations in intrauterine growth restriction. Am. J. Obstet. Gynecol. 2016;214 doi: 10.1016/j.ajog.2015.12.028. 725.e1–9. [DOI] [PubMed] [Google Scholar]
- Ejaz S., Anwar K., Ashraf M. MRI and neuropathological validations of the involvement of air pollutants in cortical selective neuronal loss. Environ. Sci. Pollut. Res. Int. 2014;21:3351–3362. doi: 10.1007/s11356-013-2294-5. [DOI] [PubMed] [Google Scholar]
- Fischl B., Liu A., Dale A.M. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans. Med. Imaging. 2001;20:70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., Busa E., Albert M., Dieterich M., Haselgrove C., van der Kouwe A., Killiany R., Kennedy D., Klaveness S., Montillo A., Makris N., Rosen B., Dale A.M. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B., Salat D.H., van der Kouwe A.J.W., Makris N., Ségonne F., Quinn B.T., Dale A.M. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl. 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Dale A.M. Cortical surface-based analysis. II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B., Sereno M.I., Tootell R.B., Dale A.M. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum. Brain Mapp. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Pajot M.-C., Ofner M., Do M.T., Lavigne E., Villeneuve P.J. Childhood autism spectrum disorders and exposure to nitrogen dioxide, and particulate matter air pollution: a review and meta-analysis. Environ. Res. 2016;151:763–776. doi: 10.1016/j.envres.2016.07.030. [DOI] [PubMed] [Google Scholar]
- Forns J., Dadvand P., Foraster M., Alvarez-Pedrerol M., Rivas I., López-Vicente M., Suades-Gonzalez E., Garcia-Esteban R., Esnaola M., Cirach M., Grellier J., Basagaña X., Querol X., Guxens M., Nieuwenhuijsen M.J., Sunyer J. Traffic-related air pollution, noise at school, and behavioral problems in Barcelona schoolchildren: a cross-sectional study. Environ. Health Perspect. 2016;124:529. doi: 10.1289/ehp.1409449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier T.W., Keshavan M.S., Minshew N.J., Hardan A.Y. A two-year longitudinal MRI study of the corpus callosum in autism. J. Autism Dev. Disord. 2012;42:2312–2322. doi: 10.1007/s10803-012-1478-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genc S., Zadeoglulari Z., Fuss S.H., Genc K. The adverse effects of air pollution on the nervous system. J. Toxicol. 2012. 2012:782462. doi: 10.1155/2012/782462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Maciel A., Reynoso-Robles R., Torres-Jardón R., Mukherjee P.S., Calderón-Garcidueñas L. Combustion-derived nanoparticles in key brain target cells and organelles in young urbanites: culprit hidden in plain sight in alzheimer's disease development. J. Alzheimers Dis. JAD. 2017;59:189–208. doi: 10.3233/JAD-170012. [DOI] [PubMed] [Google Scholar]
- Goodman R. The strengths and difficulties questionnaire: a research note. JCPP (J. Child Psychol. Psychiatry) 1997;38:581–586. doi: 10.1111/j.1469-7610.1997.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Guxens M., Lubczyńska M.J., Muetzel R.L., Dalmau-Bueno A., Jaddoe V.W.V., Hoek G., van der Lugt A., Verhulst F.C., White T., Brunekreef B., Tiemeier H., El Marroun H. Air pollution exposure during fetal life, brain morphology, and cognitive function in school-age children. Biol. Psychiatry. 2018;84:295–303. doi: 10.1016/j.biopsych.2018.01.016. [DOI] [PubMed] [Google Scholar]
- Kaiser J. Epidemiology. Mounting evidence indicts fine-particle pollution. Science. 2005;307:1858–1861. doi: 10.1126/science.307.5717.1858a. [DOI] [PubMed] [Google Scholar]
- Klocke C., Allen J.L., Sobolewski M., Blum J.L., Zelikoff J.T., Cory-Slechta D.A. Exposure to fine and ultrafine particulate matter during gestation alters postnatal oligodendrocyte maturation, proliferation capacity, and myelination. Neurotoxicology (Little Rock) 2018;65:196–206. doi: 10.1016/j.neuro.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klocke C., Allen J.L., Sobolewski M., Mayer-Pröschel M., Blum J.L., Lauterstein D., Zelikoff J.T., Cory-Slechta D.A. Neuropathological consequences of gestational exposure to concentrated ambient fine and ultrafine particles in the mouse. Toxicol. Sci. Off. J. Soc. Toxicol. 2017;156:492–508. doi: 10.1093/toxsci/kfx010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.J., Steiner R.J., Yu Y., Short S.J., Neale M.C., Styner M.A., Zhu H., Gilmore J.H. Common and heritable components of white matter microstructure predict cognitive function at 1 and 2 y. Proc. Natl. Acad. Sci. U.S.A. 2017;114:148–153. doi: 10.1073/pnas.1604658114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre A., Beggiato A., Bourgeron T., Toro R. Neuroanatomical diversity of corpus callosum and brain volume in autism: meta-analysis, analysis of the autism brain imaging data exchange project, and simulation. Biol. Psychiatry. 2015;78:126–134. doi: 10.1016/j.biopsych.2015.02.010. [DOI] [PubMed] [Google Scholar]
- Lyoo I.K., Noam G.G., Lee C.K., Lee H.K., Kennedy B.P., Renshaw P.F. The corpus callosum and lateral ventricles in children with attention-deficit hyperactivity disorder: a brain magnetic resonance imaging study. Biol. Psychiatry. 1996;40:1060–1063. doi: 10.1016/s0006-3223(96)00349-6. [DOI] [PubMed] [Google Scholar]
- Ministry of Public Works . 2012. Atlas of Urban Vulnerability in Spain: Methodology and Contents. [Google Scholar]
- Morris-Schaffer K., Merrill A.K., Wong C., Jew K., Sobolewski M., Cory-Slechta D.A. Limited developmental neurotoxicity from neonatal inhalation exposure to diesel exhaust particles in C57BL/6 mice. Part. Fibre Toxicol. 2019;16:1. doi: 10.1186/s12989-018-0287-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson B.S., Rauh V.A., Bansal R., Hao X., Toth Z., Nati G., Walsh K., Miller R.L., Arias F., Semanek D., Perera F. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatry. 2015;72:531–540. doi: 10.1001/jamapsychiatry.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J., Martínez-Vilavella G., Macià D., Fenoll R., Alvarez-Pedrerol M., Rivas I., Forns J., Blanco-Hinojo L., Capellades J., Querol X., Deus J., Sunyer J. Traffic pollution exposure is associated with altered brain connectivity in school children. Neuroimage. 2016;129:175–184. doi: 10.1016/j.neuroimage.2016.01.036. [DOI] [PubMed] [Google Scholar]
- Sandtorv L.B., Fevang S.K.E., Nilsen S.A., Bøe T., Gjestad R., Haugland S., Elgen I.B. Symptoms associated with attention deficit/hyperactivity disorder and autism spectrum disorders in school-aged children prenatally exposed to substances. Subst. Abus. Res. Treat. 2018;12 doi: 10.1177/1178221818765773. 1178221818765773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ségonne F., Dale A.M., Busa E., Glessner M., Salat D., Hahn H.K., Fischl B. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Ségonne F., Pacheco J., Fischl B. Geometrically accurate topology-correction of cortical surfaces using nonseparating loops. IEEE Trans. Med. Imaging. 2007;26:518–529. doi: 10.1109/TMI.2006.887364. [DOI] [PubMed] [Google Scholar]
- Sled J.G., Zijdenbos A.P., Evans A.C. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans. Med. Imaging. 1998;17:87–97. doi: 10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Suades-González E., Gascon M., Guxens M., Sunyer J. Air pollution and neuropsychological development: a review of the latest evidence. Endocrinology. 2015;156:3473–3482. doi: 10.1210/en.2015-1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunyer J., Esnaola M., Alvarez-Pedrerol M., Forns J., Rivas I., López-Vicente M., Suades-González E., Foraster M., Garcia-Esteban R., Basagaña X., Viana M., Cirach M., Moreno T., Alastuey A., Sebastian-Galles N., Nieuwenhuijsen M., Querol X. Association between traffic-related air pollution in schools and cognitive development in primary school children: a prospective cohort study. PLoS Med. 2015;12 doi: 10.1371/journal.pmed.1001792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Suzuki K., Ishihara A., Kubo-Irie M., Fujimoto R., Tabata M., Oshio S., Nihei Y., Ihara T., Sugamata M. Nanoparticles transferred from pregnant mice to their offspring can damage the genital and cranial nerve systems. J. Health Sci. 2009;55:95–102. doi: 10.1248/jhs.55.95. [DOI] [Google Scholar]
- van den Hooven E.H., Pierik F.H., de Kluizenaar Y., Hofman A., van Ratingen S.W., Zandveld P.Y.J., Russcher H., Lindemans J., Miedema H.M.E., Steegers E.A.P., Jaddoe V.W.V. Air pollution exposure and markers of placental growth and function: the generation R study. Environ. Health Perspect. 2012;120:1753–1759. doi: 10.1289/ehp.1204918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff J.J., Gerig G., Lewis J.D., Soda T., Styner M.A., Vachet C., Botteron K.N., Elison J.T., Dager S.R., Estes A.M., Hazlett H.C., Schultz R.T., Zwaigenbaum L., Piven J., IBIS Network Altered corpus callosum morphology associated with autism over the first 2 years of life. Brain J. Neurol. 2015;138:2046–2058. doi: 10.1093/brain/awv118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward N.C., Pakbin P., Saffari A., Shirmohammadi F., Haghani A., Sioutas C., Cacciottolo M., Morgan T.E., Finch C.E. Traffic-related air pollution impact on mouse brain accelerates myelin and neuritic aging changes with specificity for CA1 neurons. Neurobiol. Aging. 2017;53:48–58. doi: 10.1016/j.neurobiolaging.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.