Abstract
The chemokine system mediates acute inflammation by driving leukocyte migration to damaged or infected tissues. However, elevated expression of chemokines and their receptors can contribute to chronic inflammation and malignancy. Thus, great effort has been taken to target these molecules. The first hint of the druggability of the chemokine system was derived from the role of chemokine receptors in HIV infection. CCR5 and CXCR4 function as essential co-receptors for HIV entry, with the former accounting for most new HIV infections worldwide. Not by chance, an anti-CCR5 compound, maraviroc, was the first FDA-approved chemokine receptor-targeting drug. CCR5, by directing leukocytes to sites of inflammation and regulating their activation, also represents an important player in the inflammatory response. This function is shared with CCR2 and its selective ligand CCL2, which constitute the primary chemokine axis driving the recruitment of monocytes/macrophages to inflammatory sites. Both receptors are indeed involved in the pathogenesis of several immune-mediated diseases, and dual CCR5/CCR2 targeting is emerging as a more efficacious strategy than targeting either receptor alone in the treatment of complex human disorders. In this review, we focus on the distinctive and complementary contributions of CCR5 and CCR2/CCL2 in HIV infection, multiple sclerosis, liver fibrosis and associated hepatocellular carcinoma. The emerging therapeutic approaches based on the inhibition of these chemokine axes are highlighted.
Keywords: AIDS, Autoimmunity, Liver disease, Neuroinflammation, Therapeutic antibody, Chemokine receptor antagonist
Introduction
The chemokine system is a key regulator of leukocyte trafficking during immune and inflammatory responses. It also controls survival and effector functions of immune cells as well as processes such as organogenesis, angiogenesis, haematopoiesis, fibrosis and tissue remodeling [1]. Alterations of chemokines and their receptors have been described in pathological processes, including inflammatory and autoimmune diseases, transplant rejection, tumor growth, and metastasis. In addition, some pathogens can interfere with the host chemokines/chemokine receptors network either for host cell entry or for promoting their own survival [2]. Not surprising, these molecules represent the largest target family in modern pharmacology [3].
CCR5 and CCR2 are structurally related chemokine receptors whose genes share significant sequence homology (73%), probably arising from a gene duplication event. CCR5 is expressed on a broad range of cells, including T lymphocytes, macrophages, granulocytes, dendritic cells (DC), microglia, astrocytes, neurons, fibroblasts, and also on epithelium, endothelium, and vascular smooth muscle [4]. Conversely, CCR2 expression is relatively restricted to certain cell types, mainly monocytes, NK and T lymphocytes, though it can be induced in other cells under inflammatory conditions. CCR2 is mainly considered pro-inflammatory, but anti-inflammatory roles have been described in particular cell types such as regulatory T lymphocytes [5].
CCR5 binds with high affinity to CCL3, CCL4, CCL5, CCL3L1, CCL8, CCL11, CCL13, CCL14, CCL16 (agonists), and CCL7 (antagonist) [4]. CCR2 also binds to several chemokines (i.e., CCL2, CCL7, CCL8, CCL12, and CCL13), but CCL2 is the most potent and the only selective ligand. Monocytes/macrophages are the major source of CCL2. Other cells, including DCs, endothelial, epithelial, fibroblast, smooth muscle, astrocytic, microglial and mesangial cells may produce CCL2 either constitutively or in response to several mediators [5–7].
CCR2 can form homo- or heterodimers with other chemokine receptors. Homodimerization may be necessary for CCR2 chemotactic activity and occurs in the absence of the ligand, although it is favored by CCL2 dimers. CCR2/CCR5 heterocomplexes activate calcium response and support cell adhesion rather than chemotaxis, whereas CCR2/CXCR4 heterodimers have an allosteric trans-inhibitory effect on CCL2 binding. Heterodimerization may influence drug selectivity, because the specific antagonist of one receptor may cross-inhibit the other one [8].
CCR5 and CCR2 are key players in the trafficking of lymphocytes and monocytes/macrophages and have been implicated in the pathophysiology of a number of diseases, including viral infections and complex disorders with an inflammatory component. Accordingly, genetic polymorphisms in their coding or regulatory regions, which affect receptor expression or function, have been shown to influence the incidence and/or the course of HIV infection and inflammatory diseases [5, 9–11]. While recent reviews have described the role of CCR5 or CCR2 in human pathological conditions [4, 5, 9, 12], this review is focused on the involvement of both receptors in HIV infection, multiple sclerosis (MS), liver fibrosis and associated hepatocellular carcinoma (HCC), in which these chemokine axes both represent essential component of the pathological processes. We highlight the differences/similarities in their role, discuss their simultaneous blockade as a comprehensive therapeutic perspective for at least some of these multifaceted disorders, and propose new avenues for the future development of more successful therapies.
CCR5 and CCR2/CCL2 in the pathogenesis of HIV infection
CCR5 as a gateway for HIV infection of target cells
CCR5 is the main co-receptor used by HIV to infect new target cells (Fig. 1a) and it plays a crucial role in HIV mucosal transmission, which accounts for more than 95% of new infections worldwide [13]. The female reproductive tract (FRT), which accounts for around 40% of all HIV transmissions [14], has been largely studied over the past years to better understand sexual HIV transmission and to develop strategies to prevent mucosal HIV acquisition. Compared to heterosexual men, women have bigger mucosal surfaces, a very high level of CCR5 expression on their genital cells and specific pro-inflammatory immune environment; all these factors make FRT more susceptible to HIV infection [15]. The selective mucosal transmission of CCR5-dependent (R5) HIV strains is due to several “gatekeepers” which protect against CXCR4-dependent (X4) (the other main co-receptor) HIV transmission. These include: mucus in the endocervix may trap X4 viruses through various mechanisms (e.g., production of the CXCR4 binding chemokine SDF-1), epithelial cells express CCR5 but not CXCR4, Langerhans cells can be infected by R5 viruses, HIV passes easily through mucosal epithelium if genital ulcerative disease or abrasion is present in genital ulcerative disease or abrasion, macrophages can be preferentially infected by R5 viruses, DCs can be infected by R5 viruses and can trap HIV via DC-SIGN [16].
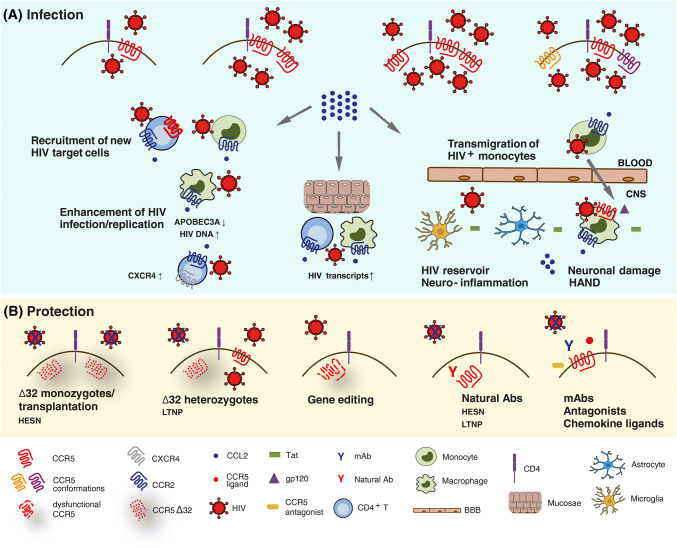
Fig. 1.
Schematic view of the role of CCR5 and CCR2/CCL2 in the pathogenesis of HIV infection. a Infection by HIV occurs when the virus attaches to a susceptible cell and fuses with the cell membrane. The players in this process are the CD4 receptor and a co-receptor, mainly CCR5. The level of viral infection correlates with the number of CCR5 molecules expressed on cell surface, independently from their conformation. HIV-infected cells then release CCL2, which enhances HIV replication with cell type-dependent mechanisms, recruits new HIV target cells and mediates the transmigration of HIV-infected monocytes into the central nervous system (CNS) across the blood–brain barrier (BBB), thus contributing to neuroinflammation, neuronal damage and HIV-associated neurocognitive disorders (HAND). b Individuals homozygous for the CCR5Δ32 allele are protected against HIV infection, whereas those heterozygous for CCR5Δ32 progress slowly toward the disease. Natural Abs to CCR5 also confer protection against HIV infection, as they have been found in HIV-exposed seronegative (HESN) subjects and long-term non-progressors (LTNP). Pharmacological blockade of CCR5 through chemical antagonists, chemokine ligands, mAbs, and gene editing may as well protect from HIV infection
The evidence of the crucial role of CCR5 in HIV pathogenesis came from the discovery of the Δ32 allele, a 32 base pair deletion in the coding region of the CCR5 gene, which results in functional or non-functional phenotypes depending on its allelic status. Individuals homozygous for this mutation lack functional CCR5 on the cell surface, and are almost completely resistant to HIV infection (Fig. 1b). Homozygosis for CCR5Δ32 was indeed found to be associated with the resistance to infection of HIV-exposed seronegative (HESN) subjects [17]. Conversely, heterozygous individuals, which display decreased cell surface CCR5, are not completely protected, but progress slowly toward the disease [18]. Another CCR5 polymorphism (the so-called -2459 A/G or -59029 A/G), which is located within the gene promoter region, was linked to the level of CCR5 expression and the magnitude of HIV replication in vitro and was associated with a more rapid disease progression in vivo [19, 20]. Notably, CCR5-depleted cell therapy was exploited as functional cure in the “Berlin patient”, who received allogeneic bone marrow transplantation from a CCR5Δ32 homozygous subject and did not have detectable viremia since then [21]. He represented the only subject with a documented HIV cure until the very recent report of the so-called “London patient”, a second case of sustained HIV remission following CCR5Δ32/Δ32 haematopoietic stem-cell transplantation [22]. These observations are good examples of the beneficial effect of CCR5-based approaches for the long-term control of HIV in the absence of antiretroviral therapy.
The number of CCR5 molecules expressed on the membrane of different T-cell subsets is crucial for the susceptibility to HIV infection. CCR5 is expressed at very low level on naïve T cells and at high level on central memory (CM), transitional memory and effector memory (EM) CD4+ T lymphocytes [23]. However, a subset of EM cells is relatively resistant to HIV due to post-entry block mechanisms [24]. In HIV-infected CD8+ T cells, CCR5 expression is increased by immune activation. Accumulation and increased proliferation of CCR5+CD8+ EM cells in the inflamed tissues are due to chemokines, such as RANTES, thus CCR5 may drive the migration of such T-cell subset to inflammatory and secondary lymphoid tissues where HIV replicates [25]. CCR5 expression is also dependent of T-cell differentiation and activation. Its levels progressively decrease from T helper (Th) 1, Th17, Th2 and T-follicular helper (Tfh) cells, with the two latter expressing very low levels. Despite Tfh cells almost being CCR5−, they represent a relevant T-cell compartment of both latent and replicative virus [26], possibly due to the contribution of CCR5+ Tfh precursors to the pool of infected cells. Finally, environmental conditions may influence CCR5 expression as well. Indeed, it was shown that in Africa, parasitic infections elicit immune activation with increased CCR5 expression, which could be responsible of the high HIV infection rate [27].
HIV activates CCR5 in a similar way to chemokines, although it targets a different set of CCR5 conformations. In particular, chemokines bind CCR5 with high or low affinity when it is, respectively, associated to G proteins or not activated. Conversely, HIV recognizes CCR5 with similar affinity either when CCR5 is or is not activated [28]. Moreover, CCR5 regulation is cell type specific and this could explain why chemokines are weak HIV entry inhibitors in macrophages compared with T lymphocytes. This finding may account for CCR5 conformational heterogeneity and explain ligand- and cell type-specific sensitivity of receptor down-regulation [29].
Interestingly, natural antibodies (Abs) to CCR5 were found in several cohorts of HESN subjects [30]. These Abs recognize the first extracellular loop of CCR5 and do not interfere with HIV binding, which takes place in the N-terminus and the second extracellular loop. However, they elicit a long-lasting CCR5 internalization, resulting in a deep block of HIV infection in either CD4+ T lymphocytes or T cell lines [31–34]. The natural occurrence of mucosal immunoglobulin (Ig) A and systemic IgG to CCR5 in some HESN [35, 36] suggests that, similarly to the CCR5Δ32 mutation, these Abs may play a role in the protection against HIV infection/transmission (Fig. 1b). CCR5 down-regulating Igs are also found in a subset of long-term non-progressors (LTNP) [37], suggesting a role for such Abs in controlling viral replication in vivo. The mechanism induced by exposure to natural CCR5 Abs could be useful to design molecules eliciting more stable receptor degradation, thus resulting in a deep block of HIV infection for a long time.
Of note, natural IgA to CCR5, but not commercial Abs to CCR5 such as 2D7 (an HIV blocking monoclonal Ab recognizing the second extracellular loop of CCR5), specifically block HIV transcytosis (i.e., HIV transfer across mucosal membranes) in several epithelial cell lines. Thus, the blocking mechanism of anti-CCR5 Abs at mucosal membranes may differ from that exerted on CD4+ T lymphocytes, since HIV would bind CCR5 intracellularly in the endosomes during transcytosis. We hypothesize that natural anti-CCR5 Abs present at mucosal sites bind CCR5 and are then internalized with it, thus preventing interaction with HIV and subsequent transcytosis [38].
The CCR2/CCL2 chemokine axis as a key driver of HIV-associated chronic inflammation
CCR2-mediated monocyte recruitment is crucial for the resistance to several viral infections [39], but it is deleterious and enhances pathology during influenza virus and HIV infections. Although CCR2 was reported to act as a co-receptor by rare HIV strains [40, 41], it is not used for cell entry in vivo and its function in the pathogenesis of HIV infection is mostly linked to the role played in leukocyte movement and inflammation, leading to the recruitment of new targets for infection in a favorable environment for viral replication.
CCR2 and CCL2 polymorphisms have been shown to affect susceptibility to HIV infection, disease progression and HIV-associated morbidities, although many works were conflicting and the mechanisms were not clear [5]. The most studied were the single nucleotide polymorphisms CCR2-V64I and CCL2-2518 A/G (alternatively designed -2578). The former was associated with a slower disease progression in some studies [42, 43]. This may be linked to the ability to heterodimerize with CCR5 and/or CXCR4 and reduce their expression. Indeed, CXCR4 can dimerize with the CCR2-V64I mutant, but not with wild-type CCR2 [44]. However, this polymorphism was not associated with altered CCR5 expression or co-receptor function in HIV-infected individuals [45]. Since the CCR2-V64I mutation tracks, through linkage disequilibrium, with mutations in the promoter region of CCR5, population-specific patterns of CCR2 and CCR5 haplotypes may also explain disparities in infection or disease progression [43, 46]. Homozygosity for the CCL2-2518 G allele, which leads to increased CCL2 expression, was associated with a 50% reduction of the risk of acquiring HIV, although after infection this genotype enhanced disease progression and the risk of HIV-associated neurocognitive disorders (HAND) [47]. CCL2 may thus partially protect from infection, but it may accelerate disease progression and increase the risk of HAND once infection is established, through its pro-inflammatory properties and ability to stimulate HIV replication. Interestingly, individuals with the -2578G allele showed higher cerebrospinal fluid (CSF) CCL2 levels, increased CSF pro-inflammatory markers and worse neurocognitive functions [48]. This allele also conferred an increased risk for atherosclerosis to HIV-infected subjects [49].
Increased CCL2 levels in the blood and CSF of HIV-infected individuals were found to correlate with viral load [50–52]. Either HIV infection or exposure to viral proteins (i.e., gp120, Nef, Tat, p17) induced CCL2 and/or CCR2 expression in different cell types [i.e., monocytes/macrophages, peripheral blood mononuclear cells (PBMCs), hepatic stellate cells (HSCs), astrocytes, microglia, endothelial cells] [5]. Interestingly, activation of CCR5 signaling by gp120 mediated CCL2 up-regulation in both macrophages and HSCs [53–55]. High CCL2/CCR2 levels in HIV+ subjects are tightly linked to increased inflammation/immune activation and development of co-morbidities through leukocyte recruitment and maintenance of the inflammatory status that represents a hallmark of HIV infection also in the post-HAART era [5]. These mechanisms have been extensively studied in the central nervous system (CNS) (Fig. 1a). The transmigration of HIV-infected monocytes into the CNS, mainly mediated by CCL2, transports the virus into the CNS, resulting in infection of macrophages and microglia thus contributing to the establishment and maintenance of the CNS viral reservoir and to HAND [56, 57]. The CNS infected cells release soluble factors and viral proteins, leading to additional monocytes recruitment, neuroinflammation, and neuronal damage. CCL2 levels are highly increased in brain tissues and CSF of people with HAND [52, 58] and remain elevated even with successful antiretroviral therapy [59], resulting in ongoing monocyte transmigration into the brain and chronic, low-level neuroinflammation. A mature CD14+CD16+ monocyte subset expressing high CCR2 levels was detected in individuals with HAND and proposed as a key player of HIV entry into the CNS and a peripheral blood biomarker of HAND [60, 61]. Furthermore, a higher frequency of CCR2+CCR5+ monocytes was found in symptomatic cognitively impaired HIV+ subjects [62].
The CCL2/CCR2 axis is also implicated in the inflammatory processes that facilitate HIV replication in mucosal tissues. The immune system of the FRT is regulated by the variations during the menstrual cycle of the sex hormones estradiol and progesterone [63]. Studies with nonhuman primates and human explant cultures have suggested the existence of a window of susceptibility for HIV infection during the luteal phase of the menstrual cycle, characterized by high progesterone levels. Very recently, an elevated cervicovaginal lavage concentration of CCL2 was found during the follicular phase of the hormonal/menstrual cycle, associated with an increase in the proportion of CCR5+CD69+CD4+ T lymphocyte [64]. This suggests that these highly susceptible cells may be infected during the follicular phase and their recirculation during the luteal phase may disseminate the virus, thus explaining the association of productive infection with this latter phase. Low estradiol levels in post-menopausal women may be linked to an inflammatory status that increases HIV transmission and replication. Indeed, higher levels of CCL2, associated with enhanced HIV p24 Gag release and viral transcription were found in ex vivo explants of ectocervical tissue from post- compared to pre-menopausal women, and blocking of this chemochine was shown to decrease HIV transcription [65]. Cervicovaginal lavage of women with detectable genital tract viral load contained higher CCL2 concentrations compared to those with undetectable viral load [66], further suggesting that CCL2 is a key player of HIV infection in the FRT.
CCL2 also has direct, cell type-dependent effects on viral replication (Fig. 1a). In resting CD4+ T cells, CCL2 exposure led to CXCR4 up-regulation, making these cells more permissive to X4 HIV infection and increasing their migration in response to gp120 [67]. This phenomenon might be particularly relevant in late disease stages when both high CCL2 levels and X4 viruses are present. In HIV+ CD8+ T cell-depleted PBMCs treated with mitogen plus IL-2 or co-cultivated with allogeneic T cell blasts of uninfected subjects, CCL2 addition stimulated HIV production in most patient’s cultures and co-cultures secreting low CCL2 levels (< 20 ng/mL). A positive correlation between the enhancement of HIV replication and CCL2 levels was observed in co-cultures. Depletion of CD14+ monocyte from allogeneic T blasts and addition of CCL2, respectively, down- and up-regulated virus replication during co-cultivation with CD8-depleted PBMC of HIV+ subjects [68]. Overall, these findings suggest that CCL2 may represent a key factor enhancing HIV spreading, particularly in anatomical sites where infection of macrophages plays a prevalent role. In these latter cells, CCL2 is produced at high levels either constitutively or following HIV infection. Blocking CCL2 activity by neutralizing Abs determined a potent restriction of HIV replication, associated with up-modulation of innate immune genes involved in the defense response to viruses, such as APOBEC3A [69, 70].
Interestingly, double CCR2+/CCR5+ immune cell populations may play key roles in HIV pathogenesis. In particular, CCR7+CD62L+ T CM lymphocytes, which represent major HIV reservoirs, were found to co-express CCR5 and CCR2 [71]. These lymphoid cells migrate in response to CCL2 and are susceptible to HIV infection. Thus, CCL2 produced early in HIV infection may recruit CCR2+ CM T cells to the site of inflammation, where these cells can become productively infected and produce virus, or become latently infected and contribute to the stable reservoir. A reduced susceptibility to HIV infection due to low transcriptional levels of both CCR5 and CCR2 was very recently found in a small population of HIV-infected subjects who maintained a very low level of viremia in the absence of combined antiretroviral therapy (cART) [72]. These findings support the contribution of these two chemokine receptors to seeding of the latent reservoir and suggest that antagonizing CCR2/CCL2 and CCR5 may represent an innovative effective strategy to fight latency [73].
Targeting CCR5 and CCR2/CCL2 in HIV infection
Since CCR5-defective individuals have normal inflammatory and immune reactions, CCR5 was interpreted as a redundant and therefore dispensable molecule in adults, thus becoming a potential preventive and therapeutic target for blocking HIV entry and for immune modulation. Though several strategies to prevent CCR5 function in HIV entry were developed and tested (Fig. 1b), the small molecule antagonist maraviroc (MRV) is right now the only CCR5 targeting drug approved for clinical use [13]. Yet, as MRV did not showed better antiviral activity compared to conventional drugs, it is employed only in treatment-multiexperienced patients [74]. Moreover, HIV escape mutants to this drug were described and it was demonstrated that HIV can infect target cells by utilizing drug-bound CCR5 as well [75, 76]. A recent study demonstrated that MRV induces latent HIV transcription in resting CD4+ T cells from subjects on cART by activating NF-kB, thus acting as a weak receptor agonist [77]. Thus, CCR5 targeting may also represent a new latency-reversing approach to interfere with HIV persistence during antiretroviral therapy.
In recent years, monoclonal Abs (mAbs) were developed as a distinct class of CCR5 inhibitors (Fig. 1b). They potently inhibit R5 HIV in vitro, and have strong antiviral activity in HIV+ subjects. CCR5 mAbs offer several potential advantages over existing drugs in terms of less frequent dosing, tolerability and limited interactions with other drugs or food [78]. Two human mAbs to CCR5 (Mab004 and PRO140) are currently in clinical development in HIV infection [79]. MAb004 appeared safe and significantly reduced viral load in HIV-infected patients. PRO140, which blocks HIV entry and replication at concentrations that do not affect receptor activity, is currently in phase III clinical trial. It determined a potent short-term dose-dependent HIV RNA suppression without significant adverse events in patients. Both mAbs bind CCR5 in the HIV-binding site, thus acting with a competitive rather than allosteric mechanism, as MRV, and they did not affect lymphocyte activity [80, 81]. The use of Abs might solve the limitations of currently available therapies for HIV-infected patients, such as the complication associated with multidrug resistant viruses, drug–drug interactions and also the potential interactions with redundant chemokine receptors. Another mAb, ST6, is a CCR5 intrabody (it binds CCR5 intracellularly), which recognizes the N-terminus of CCR5 and efficiently down-regulates the receptor from the cell membrane, thus preventing HIV entry and replication [82].
Mixtures of HIV-entry inhibitors or “super Abs” binding different epitopes are emerging as more potent strategies with respect to a single inhibitor or an Ab recognizing only one epitope [80]. Furthermore, viral strains resistant to CCR5 inhibitors were more susceptible to be neutralized by cross-neutralizing mAbs compared with the parental virus, probably because drug-bound CCR5 induces virus adaptation to the new receptor conformation, thus rendering the envelope more susceptible to the neutralizing mAbs [75].
Modified chemokines, such as CCL5/RANTES analogs, represent another class of CCR5 inhibitors. In particular, PSC-RANTES, an N-terminally modified analog of RANTES, showed strong HIV inhibition, although a high concentration was needed to block viral replication in animal models and the drug-induced signaling that could increase inflammation [83]. 6P4-, 5P12-, 5P14-RANTES are modified PSC-RANTES molecules. 6P4-RANTES makes the intracellular sequestration of CCR5 longer, whereas 5P12- and 5P14-RANTES induce CCR5 internalization without signaling activity [84]. AOP-RANTES internalizes CCR5, blocks HIV infection in macrophages and inhibits CCR5 re-expression on cell membrane [85, 86].
RNA-based methods and gene editing to induce a CCR5− phenotype are other approaches under clinical evaluation [87]. The more promising RNA-based strategies are RNA silencing and antisense RNAs delivered by pseudotyped lentiviral or adenoviral vectors. Three main technologies have been used to edit CCR5. Zinc finger and transcription activator-like nucleases recognize their target DNA sequence and induce a double-stranded break through Fok1 endonucleases. The clustered regularly interspaced short palindromic repeats/Cas9 system works through a guide RNA sequence that forms a complex with the Cas9 endonuclease [88]. Some of these approaches have been or are being tested in clinical trials in HIV+ individuals [89].
Targeting CCR2 and CCL2 is a very active area of drug development with potential application in several important acute and chronic human diseases, and it has been the subject of several reviews in recent years [5, 8, 90]. Two main strategies have been used to block CCR2/CCL2 function, namely small molecule CCR2 antagonists and CCR2 or CCL2 specific neutralizing Abs.
Cross-reactivity with the highly homologous CCR5 was the major challenge in the development of small molecule CCR2 antagonists. Indeed, one of the early CCR5 antagonists developed, TAK779, was found to be a potent antagonist of both CCR5 and CCR2 [91] and cross-reactivity with CCR5 is a common property to many CCR2 antagonists. Since this feature may be therapeutically advantageous in the treatment of complex diseases such as HIV infection and a number of inflammatory conditions, some companies have developed compounds that inhibit both receptors, such as cenicriviroc (CVC) [92].
CVC is a first-in-class dual CCR2/CCR5 antagonist that was initially developed for the treatment of HIV infection. By blocking CCR5, it inhibits HIV entry into host cells, but has also potential anti-inflammatory effect due to CCR2 inhibition. Thus, unlike MRV, in addition to the direct anti-HIV effects, CVC could be effective in treating the chronic immune activation associated with suppressed HIV infection, thus improving the management of HIV-infected patients. CVC completed phase II clinical development in HIV-infected subjects, showing favorable safety and efficacy [93]. Notably, the analysis of immune and inflammatory biomarkers revealed a decrease in sCD14 and sCD163 levels, as well as up-regulation of the defense response gene APOBEC3A, in CVC-treated individuals [93–95]. Although data from clinical trials supported its further evaluation as a backbone for new multi-drug combination therapy for HIV in phase III registration studies, CVC is not longer being developed in HIV infection.
CCR5 and CCR2/CCL2 in the pathogenesis of MS
Complex roles of CCR5 and CCR2/CCL2 in inflammation, axonal damage and repair in MS
MS is a heterogeneous, multifactorial disease of the CNS, and broadly comprises two main clinical stages: a relapsing–remitting (RRMS) stage, characterized by discrete episodes of neurologic dysfunction followed by clinical remission, and a progressive stage with steadily worsening disability. Progressive MS usually evolves from RRMS, although some patients may have progressive disease from onset [96, 97].
The pathologic hallmark of MS is the inflammatory lesion, characterized by leukocyte infiltration, demyelination, oligodendrocyte loss, axonal damage, and ultimately leading to the neurologic dysfunction associated with clinical relapses. To limit inflammation and initiate repair, immune-modulatory networks are triggered, which result in at least partial remyelination associated with clinical remission. The “classical active lesion” with profound inflammatory leukocyte infiltration (CD4+ and CD8+ T lymphocytes, B and B cell-derived plasma cells, macrophages) predominates in RRMS. In progressive disease, lesions tend to have an inactive core surrounded by a narrow rim of activated microglia, astrocytes and macrophages [96].
Of note, CNS invading Th1 cells and monocytes, as well as CNS resident innate immune cells such as microglia and astrocytes, co-express CCR2 and CCR5 [98] and several lines of evidence point to a complex role of these receptors in MS pathophysiology (Fig. 2). The disease course has been reported to be less severe in carriers of the CCR5Δ32 allele, although contrasting results have been reported on the association between CCR5Δ32 and MS risk [99–101]. Both CCR2 and CCR5 are highly expressed within and around active MS lesions, mainly in infiltrating T cells and macrophages, and in resident microglia [99, 102]. Their ligands CCL2, CCL3, CCL4 and CCL5 are also up-regulated. CCL2 immunoreactivity was mainly associated with astrocytes and macrophages within the lesion, and with hypertrophic astrocytes in the surrounding parenchyma. CCL2 expression was found to correlate with lesion activity and appears mainly restricted to white matter lesions, which contain a more massive leukocyte infiltrate than gray matter lesions [103]. In addition to leukocyte recruitment into the CNS in RRMS, CCL2 has been proposed to promote activation of microglia/macrophages and expansion of demyelinating lesions in secondary progressive MS [104].
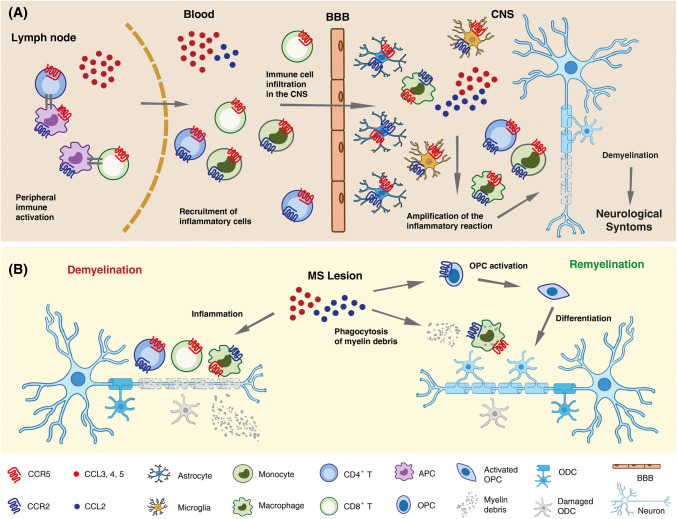
Fig. 2.
Schematic representation of the multiple roles of CCR5 and CCR2/CCL2 in inflammation, axonal damage and repair in MS. a In the lymph node, CCR5 ligands secreted by activated antigen-presenting cell (APCs) and CD4+ T helper cells mediate CD8+ T cells recruitment and T cell activation. CCR5 ligands also promote trans-endothelial migration and crossing of the blood–brain barrier (BBB) by circulating CCR5+ effector T cells and phagocytes. CNS invading Th1 cells and monocytes, as well as CNS resident innate immune cells such as microglia and astrocytes, co-express CCR2 and CCR5. Their ligands CCL2, CCL3, CCL4 and CCL5 are also up-regulated in MS lesions where they can be produced by activated microglia, astrocytes and neurons. CCL2 and CCR5 ligands secreted in situ amplify local autoimmune/inflammatory responses ultimately leading to demyelination and axonal damage. b Besides mediating demyelination and neuronal damage (left), CCL2 and CCR5 ligands contribute to remyelination and damage repair (right). CCR5+CCR2+ macrophages are essential for the clearance of myelin debris. CCL2 also enhances oligodendrocyte precursor (OPC) mobility, enabling them to populate demyelinated lesions, where they differentiate into myelin sheath-forming oligodendrocytes (ODC)
CCR5 ligands are also present within active demyelinating plaques, although with a different spatial distribution compared to CCL2, being mainly expressed in the blood vessel endothelium, perivascular cells and surrounding astrocytes [105, 106]. CCR5 is expressed on most CD8+ T cells, monocytes and macrophages within inflammatory MS lesions; it could, therefore, contribute to recruitment of these cells to the inflamed tissue, their activation and/or their survival [99]. Importantly, both chemokine/receptor axes may also contribute to remyelination (Fig. 2b). Remyelinating lesions display significantly more abundant CCR5+ cells than demyelinating lesions, suggesting a role of these cells in the repair process [107]. Macrophages recruited via CCL2/CCR2 play essential roles in the phagocytosis of myelin debris and promote the regenerative response [108]. Furthermore, CCL2 up-regulation was described in activated oligodendrocyte progenitor cells nearby demyelinated areas, and CCL2 proved to enhance in vitro chemotaxis of these cells. It has thus been suggested that CCL2, by enhancing oligodendrocyte progenitor mobility, enable them to populate demyelinated lesions. Once within the lesion, progenitors may differentiate into mature myelin sheath-forming oligodendrocytes, thus allowing remyelination [109].
Along with their role in inflammation, both CCL2 and CCL5 have been shown, at least in mice, to regulate neuronal excitability and synaptic transmission [110–112]. Hence, they likely play an important role in the neuroimmune crosstalk, which contributes to neuronal damage associated with acute inflammation, but it is crucial for the development of compensatory neuronal plasticity as well [110].
While generally co-expressed in MS lesions, CCR2 and CCR5 ligands in body fluids of MS patients seem to follow an opposite trend of regulation. Almost unique among inflammatory chemokines, generally up-regulated, CCL2 is consistently present at lower levels in the CSF (and often in the blood) of MS patients compared to healthy controls (HC) or patients with other non-inflammatory diseases. The more active/inflammatory the disease stage is, the lower the CCL2 concentration will be (i.e., CCL2 levels in relapse < remission < progressive MS < HC or non-inflammatory diseases) [113]. CCL2 concentration in the CSF is higher than in serum, indicating an intrathecal production, and CSF CCL2 inversely correlated with other markers of CNS inflammation as well as with the level of the neurofilament light protein, a marker for axonal damage [114, 115]. Conversely, CCL5 concentration in the blood and in the CNS of MS patients directly correlates with disease activity and inflammation, with the highest levels of CCL5 found during relapses. A corresponding enrichment in the intrathecal compartment (CSF and brain lesions), of CCR5+ cells, particularly CD8+ T lymphocytes, DCs and monocytes was observed [99, 116]. Interestingly, a unique population of CCR2+CCR5+ T cells was found selectively enriched in the CSF of MS patients during relapse but not in patients with other neurologic diseases and proposed as a therapeutic target. This Th1 subset produced high levels of two proteins involved in the CNS pathology, matrix metalloproteinase-9 and osteopontin, which showed high invasive potential across an in vitro blood–brain barrier model and was reactive to myelin basic protein, one of the putative MS autoantigens [117]. Disease-modifying therapies for MS affect the endogenous availability of CCR2 and CCR5 ligands in CNS. Following treatment with methylprednisolone, widely used to accelerate relapse recovery, blood CCL2 increased and CCL5 decreased in parallel with normalization of inflammation markers [102]. A decrease of CCR5 expression in CD4+ cells in the CSF was also reported [118]. CCL2 is strongly inducible by IFN and serum CCL2 levels are consistently higher in MS patients undergoing IFN therapy compared to untreated patients [119–122]. It was hypothesized that IFN-induced chemokine up-regulation in the periphery could desensitize chemokine receptors on leukocytes or cause a “reverse-gradient” which neutralize leukocyte recruitment into the CNS. However, CCL2 plasma levels during long-term IFN treatment of MS patients did not predict therapeutic response at 1 or 2 years of therapy, thus questioning the relevance of CCL2 induction as a main mediator of the therapeutic action of IFN [122].
In conclusion, although a pathogenic role of CCL2/CCR2 as well as of CCR5 and its ligands in MS is widely recognized, mainly due to their role in leukocyte recruitment to active lesions and amplification of the local inflammatory response, the role of these chemokine/receptors axes in MS is likely much more complicated.
Targeting CCR5 and CCR2/CCL2 in MS
In recent decades, better understanding of mechanisms underlying RRMS has led to the development of immunosuppressive and immune-modulating therapies that reduce both severity and frequency of new relapses. Many of the current therapies actually target inflammation and leukocyte trafficking in the CNS. In this context, some CCR2 targeting drugs have entered in clinical trials for MS in past years. They generally showed a favorable safety profile, but failed from a therapeutic point of view and are not currently involved in clinical studies [5, 123]. A potent CCR2 antagonist, MK-018, showed good pharmacokinetic profiles in preclinical studies and demonstrated efficacy in animal models. This drug entered in phase II clinical trials for MS, but no significant improvement compared with placebo was reported [124].
The humanized anti-CCR2 mAb MLN1202 was also tested in MS patients and reported to be effective in reducing the number of lesions in the brain [125]. However, the compound was no longer developed for such indication, suggesting that the activity may be insufficient to compete with current therapies.
The use of MRV was proposed to manage the progressive multifocal leukoencephalopathy (PML) and subsequent immune reconstitution inflammatory syndrome (IRIS) that are among the most concerning side effects associated with MS immunotherapies, in particular with natalizumab. The rationale is the presence of CD8+ T cells expressing high CCR5 levels in the CNS inflammatory infiltrate that results from PML Furthermore, MRV is assumed to readily penetrate the blood–CSF barrier [99]. Although initial case reports suggested that MRV could be beneficial in PML–IRIS management, a subsequent report of three cases with no clear clinical effect of the drug questioned its use in MS [99, 126, 127].
The past 20 years have witnessed remarkable and unprecedented advances in the treatment of RRMS, However, most of them are not effective for progressive MS, whose treatment remains a major challenge in MS research. The pleiotropic effects of chemokines, including CCL2 and CCR5 ligands, in the neuro-immune crosstalk and in neurodegeneration remains to be fully elucidated and may constitute an avenue for future research to address this challenge.
CCR5 and CCR2/CCL2 in the pathogenesis of liver disease
CCR2/CCL2 and CCR5 as drivers of hepatic fibrosis and HCC development
Liver fibrosis is a response to hepatic insults that occurs in most types of chronic liver diseases, most importantly alcoholic and non-alcoholic fatty liver diseases (ALD and NAFLD) as well as viral hepatitis [128]. NAFLD is the most common liver disease in industrialized countries, and especially its progressive inflammatory form, non-alcoholic steatohepatitis (NASH) predisposes to cirrhosis and HCC (Fig. 3a) [129]. NAFLD and NASH are commonly associated with obesity-related disorders (e.g., type 2 diabetes mellitus and metabolic syndrome), and due to the rapidly increasing frequencies of these conditions, they are projected to become an enormous clinical and economic burden [130]. Hepatic fibrosis, characterized by excessive deposition of extracellular matrix (ECM) proteins, is the major feature predicting liver-related and overall mortality in patients with NASH. The accumulation of fibrogenic macrophages in the liver is the major central pathway driving fibrosis progression identified in patients and mouse models of liver fibrosis [131]. Hepatic macrophages are heterogeneous cell populations, consisting of liver resident phagocytes, termed Kupffer cells (KCs), and monocyte-derived macrophages, which represent the dominant macrophage population. During acute or chronic liver injury, circulating monocytes are massively attracted to sites of hepatic injury, where they differentiate into liver macrophages that promote the activation of HSCs to become myofibroblasts, the main source of ECM, especially collagen types 1 and 3, in the chronically inflamed liver. Hepatocytes, KCs and infiltrating monocytes/macrophages are major producers of TGF-β, a key fibrogenic cytokine promoting collagen production by activated HSCs [132]. In addition, HSCs secrete several cytokines and chemokines that amplify and maintain the inflammatory response.
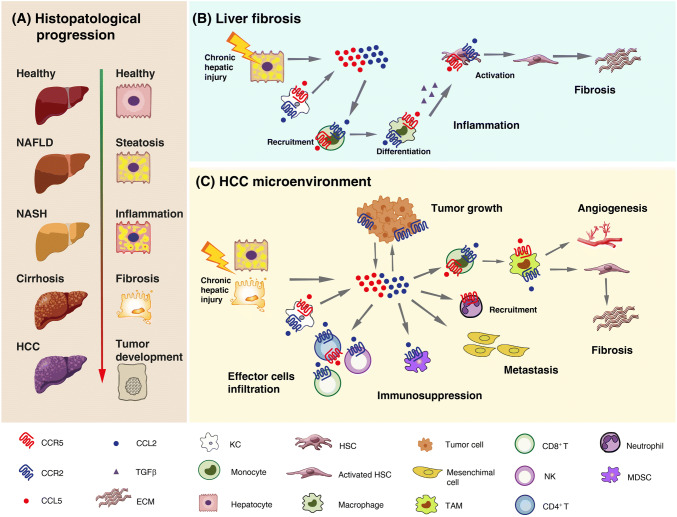
Fig. 3.
Schematic model of the role of CCR5 and CCR2/CCL2 in liver fibrosis and tumor development. a Hepatocyte histological changes during the progression from a healthy liver to non-alcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), cirrhosis and hepatocellular carcinoma (HCC). b Contribution of CCR5 and CCR2/CCL2 to the major pathogenic events leading to liver inflammation and fibrosis. Activated Kupffer cells (KCs) and damaged hepatocytes secrete CCR2 and CCR5 ligands, which mediate monocyte recruitment, their differentiation into macrophages, and activation of hepatic stellate cells (HSCs), thus contributing to inflammation and extracellular matrix (ECM) deposition. c Contribution of CCR5 and CCR2/CCL2 to HCC progression. In the tumor microenvironment, CCR2 and CCR5 ligands secreted by KCs, damaged hepatocytes and tumor cells promote tumor growth and mediates monocyte recruitment and their maturation into tumor-associated macrophages (TAMs) with pro-angiogenic and pro-fibrotic features; the infiltration of myeloid-derived suppressor cells (MDSCs), thus contributing to immunosuppression; the progression of primary tumors towards metastases by promoting the migration, invasion and epithelial–mesenchymal transition of HCC cells. On the other hand, these chemokine axes also promote the infiltration of effectors cells, which contributes to tumor eradication
Recruitment of extra-hepatic inflammatory cells to the site of hepatic injury is largely mediated by chemokines and their receptors [133]. Monocytes, KCs, HSCs and damaged hepatocytes express CCR2 and CCR5 on their surface. Increasing evidence implicates these receptors and their ligands CCL2 and CCL5, secreted by various liver cells like activated KCs or damaged hepatocytes, in the pathogenesis of liver fibrosis through promotion of monocyte/macrophage recruitment and tissue infiltration, as well as HSC activation following liver injury (Fig. 3b). In mouse models of hepatic fibrosis, either targeted deletion or pharmacological inhibition of CCR2 or CCR5 resulted in lower immune-cell activation and reduced liver fibrosis [134, 135]. Although many of the mechanistic concepts are derived from animal models of NASH and liver fibrosis, the same pathways were shown to be active in human disease. For instance, CCR2/CCL2 is up-regulated in fibrotic livers from patients, alongside an accumulation of inflammation-polarized monocyte-derived phagocytes that activate HSCs [136]. Patients with NAFLD have increased levels of the macrophage activation marker sCD163, underlining the importance of macrophages in chronic liver injury [137]. Furthermore, increased proportions of CCR2+ macrophages in visceral adipose tissue are associated with histological disease severity of NASH in obese patients [138].
Interestingly, NAFLD frequency is higher in HIV-infected patients (30–40%) than in the general population (14–31%) [139]. Although the definition of NAFLD excludes viral hepatitis, hepatic steatosis is a common condition in HCV-infected subjects and its prevalence is even higher in HIV/HCV-coinfected individuals (30–70%). In these patients, the interactions among HIV infection, viral hepatitis and steatosis accelerate the progression to hepatic fibrosis, resulting in an earlier appearance of end-stage liver disease. HIV gp120 was shown to modulate different aspects of HSC biology, including stimulation of migration and increased expression of type I procollagen and pro-inflammatory cytokines such as CCL2. These actions are mediated via activation of CCR5 and are blocked by a CCR5 antagonist [55]. These data suggest a direct role of HIV in the process of hepatic fibrogenesis.
Chronic liver disease and cirrhosis associated with viral hepatitis and excessive alcohol intake are the most important risk factors for HCC development. However, NAFLD- and NASH-related HCC greatly increased in recent years and it is anticipated to rise exponentially due to the growing epidemic of obesity and diabetes [140]. HCC is among the most lethal and prevalent cancers in the human population. Its development is closely related to the presence of chronic liver disease and is a complex multistep process that involves sustained inflammatory damage, hepatocyte necrosis and regeneration, fibrotic deposition, and genomic alterations [141].
The chemokine system plays a fundamental role in hepatocarcinogenesis by modulating the immune response in the tumor microenvironment (TME) and directly affecting HCC cell growth, invasion and migration properties [133, 142]. As discussed previously in this paragraph, CCR2, CCR5 and their ligands are key players in orchestrating the interaction among parenchymal liver cells, KCs, HSCs, and infiltrating immune cells during liver inflammation (Fig. 3b). These cellular interactions result in the remodeling of the hepatic microenvironment toward a pro-inflammatory, pro-fibrotic, pro-angiogenic and thus pre-neoplastic milieu. Once developed, liver neoplasms provoke pro- and anti-tumor immune responses that are also critically regulated through differential activation of chemokine networks.
In HCC tissues, CCR2, CCR5 and their ligands are expressed by tumor as well as non-tumor cells and are modulated by inflammatory cytokines. Enhanced CCL2 expression in human liver cancer was reported to represent an independent prognostic indicator in patients with HCC and was linked to a decreased survival rate [143]. In addition, the CCR2-64I gene polymorphism was shown to be an important factor for HCC susceptibility, but it did not influence the clinical progression of HCC [144].
An increased presence within the HCC TME of tumor-associated macrophages (TAMs), which mediate fibrogenesis and angiogenesis, has been consistently associated with poor patient prognosis. The CCL2/CCR2 axis plays a fundamental role in monocyte recruitment and their maturation into TAMs (Fig. 3c). Bartneck et al. dissected the TAM subtypes, particularly those mobilized by CCL2/CCR2, involved in fibrogenesis-driven hepatocarcinogenesis. They found a specific accumulation of CCR2+ TAMs at the stroma/tumor interface in resected human HCCs, where they co-localize with endothelial cells in areas of intense vascularization. These TAMs did not belong to the suppressive M2-like population, but to an M1 population showing an inflammatory and pro-angiogenic polarization. In a mouse model of liver fibrosis and hepatocarcinogenesis, CCL2 inhibition by an RNA aptamer resulted in reduced TAM1 liver infiltrate and pathogenic angiogenesis, improvement of tissue fibrosis, and a significant inhibition of tumor progression [145]. In addition to TAMs, the CCL2/CCR2 axis can drive the infiltration of myeloid-derived suppressor cells, thus contributing to increased immunosuppression in the TME [146, 147]. Finally, CCL2 was demonstrated to promote the migration, invasion and epithelial–mesenchymal transition (EMT) of HCC cells, which is implicated in the progression of primary tumors towards metastases [148, 149]. On the other hand, CCL2 expression in human HCC directly correlates with the infiltration of effectors cells, such as CD4+ Th1, CD8+ and NK cells, which contributes to tumor eradication [150]. These observations confirm that chemokines can have a dual role (suppression and activation) on immune cells in the HCC microenvironment. CCL2 also influences the growth of HCC cells. In particular, CCL2/CCR2 over-expression promoted the proliferation of HCC cells in response to apigenin or co-culture with cancer-associated fibroblasts [151, 152].
CCR5-mediated inflammation is as well important in hepatocarcinogenesis. The CCL5–CCR5 axis participates in the development of HCC, and CCL3 and CCL4 show a definitive role in accelerating the course of HCC [153]. CCL3, which is remarkably increased in different HCC cell lines when stimulated with IL-1α or IL-1β, may attract a large amount of macrophages and neutrophils into the inflammation sites. CCR5 has been demonstrated to play a critical role in both the development and progression of liver cancer. Indeed, in a mouse model of diet-induced HCC, treatment with MRV determined significantly higher survival rates, less liver fibrosis, lower levels of liver injury markers and chemokines, less apoptosis and proliferation, as well as a lower tumor burden compared to controls [154]. Likewise, in the same mouse model, CCR5 knockout was shown to significantly reduce macrophage recruitment and trafficking to the liver, inflammation, and periductal accumulation of oval cells, which are the putative liver progenitor cells that proliferate and differentiate in response to liver damage, thus resulting in abrogation of fibrosis and significant decrease in tumor incidence and size [155].
Targeting CCR2/CCL2 and CCR5 in NASH and HCC
Despite its rising prevalence, there are currently no approved treatments for NASH. Targeting central pathways driving fibrosis progression, such as CCR2/CCR5-mediated accumulation of fibrogenic macrophages in the liver, might provide therapeutic opportunities for the therapy of NAFLD/NASH. Thus, CCR2 and CCR5 have become promising targets for anti-fibrotic therapy.
A number of studies demonstrated that CVC displayed anti-inflammatory and anti-fibrotic effects across a range of in vivo animal models, including liver fibrosis, NASH, ALD and kidney fibrosis [156, 157]. Therefore, the drug was investigated in a phase II clinical trial (CENTAUR; NCT02217475) and is currently being evaluated in a phase III study in patients with NASH and fibrosis (NCT03028740). Data from CENTAUR suggested that NASH patients who received CVC have a greater likelihood of a sustained reduction in liver fibrosis over 2 years compared with those who received placebo [158]. Other agents with different mechanisms of action are under investigation in NASH. Combination therapies targeting both inflammatory and metabolic pathways in NASH might represent valuable therapeutic options to further improve treatment outcomes, and are under evaluation in a phase II study (NCT03517540).
HCC is a chemotherapy-resistant tumor that is most frequently diagnosed at advanced stages with limited treatment options and is thus associated with a high mortality rate. Therapies for HCC are dependent on disease stage. In early stages, surgery represents the standard treatment with a 5-year survival rate in 70% of treated patients [159]. When surgery or liver transplantation is not applicable, second-line loco-regional therapies have highly variable 3–5-year survival rates [160]. In advanced unresectable HCC, the inhibitor of tyrosine protein kinases, sorafenib is the only approved systemic therapy, providing a very limited survival benefit [161]. Interestingly, CCL2/CCR2 targeting by CCR2 knockdown, CCR2 antagonists, neutralizing Abs, or RNA aptamers has been shown to inhibit malignant growth and metastasis, reduce postsurgical recurrence and enhance survival in different HCC models [143, 156, 162, 163]. In such a scenario, immunotherapeutic strategies targeting the chemokine system, alone or in combination with standard chemotherapy, may improve clinical outcome in HCC patients [164–166]. In this regard, a pre-clinical study demonstrated that a CCR2 antagonist shows anti-cancer effects increasing CD8+ T cells via blocking tumor-infiltrating macrophage-mediated immunosuppression, and that the anti-tumor effect was improved by combining the antagonist with low-dose sorafenib [167, 168].
Conclusions and future perspectives
CCR5 and CCR2 are important mediators of leukocyte trafficking in inflammatory processes. The emerging evidence of their role not only in HIV infection, but also in several human inflammatory diseases and cancers, led to a growing interest in CCR5 and CCR2 therapeutic targeting. Specific small-molecule antagonists and mAbs are feasible therapeutic options to interfere with these chemokine axes, and phase I–II trials have demonstrated their safety in humans. Since in some complex disorders either CCR5 or CCR2 have emerged as key drivers of disease pathophysiology, drugs targeting both receptors represent a novel and promising way to generate effective therapeutics. The simultaneous targeting of two different disease molecules with one drug makes development less complex from a technological and regulatory perspective, because manufacturing, preclinical and clinical testing are reduced to a single, bispecific compound. Dual receptor chemical antagonists are currently the only available tools to simultaneously interfere with CCR5 and CCR2, and one of these compounds is in active clinical trials in NASH patients.
During the past decade, dual targeting with bispecific Abs has emerged as an alternative to monospecific Ab combination therapy. A substantial breakthrough was made thanks to promising clinical trial results of some bispecific Abs and development of new formats, which largely ease manufacturing and physicochemical property challenges encountered by early formats. Bispecific Abs targeting CCR5 and CCR2 may have several advantages over chemical antagonists. In particular, they can be engineered to be specific and with similar strong neutralizing activity for both receptors, and to extend their half-life even to months. This latter is an important tool to develop long-acting therapies that could be taken once a month or even less, thus improving patients’ quality of life and adherence. Abs may also elicit additional strong immune responses through Fc-mediated complement-dependent cytotoxicity or Ab-dependent cellular cytotoxicity, thus enhancing in vivo efficacy at least in some pathological conditions. Compared to the monospecific mAbs already in clinical development, bispecific mAbs would preferentially target cells co-expressing both receptors. Interestingly, double CCR5+CCR2+ immune cell populations were described in both HIV and MS patients, and they were proposed as relevant drivers of disease pathogenesis. In HIV infection, the simultaneous inhibition of CCR5 and CCR2 may offer a new chance to tackle viral persistence and inflammation and could be exploited as the component of complex therapeutic approaches aimed at a functional cure. Conversely, although a pathogenic role of CCR2 and CCR5 networks in MS is widely recognized, a deeper mechanistic understanding of their functions is still needed before implementing novel anti-chemokine strategies in patients. Targeting CCR5/CCR2 pathways might be also promising to complement conventional surgery-based and chemotherapeutic approaches in HCC. Since these chemokine networks mediate hepatic inflammation, fibrosis, angiogenesis, and EMT of hepatic tumor cells, their targeting might be useful in the prevention of cancer in patients with chronic liver diseases. In the forthcoming years, results from the undergoing phase III clinical trial with CVC in NASH patients will hopefully confirm the therapeutic efficacy observed in phase II studies on a large number of patients and will also demonstrate the true impact of CCR5/CCR2 inhibition on HCC development.
Acknowledgements
We thank Stefano Bonifazi for excellent assistance with figure preparation. As a result of space limitations, we apologize for being unable to cite many primary references relevant to the topic of this Review. Our work on this topic was in part supported by a grant from the Italian Ministry of Health, Ricerca Finalizzata to LF (RF-2011-02347224).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Laura Fantuzzi, Phone: +390649903194, Email: laura.fantuzzi@iss.it.
Lucia Lopalco, Phone: +390226437936, Email: lopalco.lucia@hsr.it.
References
- 1.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. 2014;32:659–702. doi: 10.1146/annurev-immunol-032713-120145. [DOI] [PubMed] [Google Scholar]
- 2.Szpakowska M, Fievez V, Arumugan K, van Nuland N, Schmit JC, Chevigne A. Function, diversity and therapeutic potential of the N-terminal domain of human chemokine receptors. Biochem Pharmacol. 2012;84:1366–1380. doi: 10.1016/j.bcp.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 3.Lu M, Wu B. Structural studies of G protein-coupled receptors. IUBMB Life. 2016;68:894–903. doi: 10.1002/iub.1578. [DOI] [PubMed] [Google Scholar]
- 4.Brelot A, Chakrabarti LA. CCR4 revisited: how mechanisms of HIV entry govern AIDS pathogenesis. J Mol Biol. 2018;430:2557–2589. doi: 10.1016/j.jmb.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 5.Covino DA, Sabbatucci M, Fantuzzi L. The CCL2/CCR2 axis in the pathogenesis of HIV-1 infection: a new cellular target for therapy? Curr Drug Targets. 2016;17:76–110. doi: 10.2174/138945011701151217110917. [DOI] [PubMed] [Google Scholar]
- 6.Sanseverino I, Rinaldi AO, Purificato C, Cortese A, Millefiorini E, Gessani S, Gauzzi MC. CCL2 induction by 1,25(OH)2D3 in dendritic cells from healthy donors and multiple sclerosis patients. J Steroid Biochem Mol Biol. 2014;144 Pt A:102–105. doi: 10.1016/j.jsbmb.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 7.Fantuzzi L, Canini I, Belardelli F, Gessani S. IFN-beta stimulates the production of beta-chemokines in human peripheral blood monocytes. Importance of macrophage differentiation. Eur Cytokine Netw. 2001;12:597–603. [PubMed] [Google Scholar]
- 8.Bachelerie F, Ben-Baruch A, Burkhardt AM, Combadiere C, Farber JM, Graham GJ, Horuk R, Sparre-Ulrich AH, Locati M, Luster AD, Mantovani A, Matsushima K, Murphy PM, Nibbs R, Nomiyama H, Power CA, Proudfoot AE, Rosenkilde MM, Rot A, Sozzani S, Thelen M, Yoshie O, Zlotnik A. International Union of Basic and Clinical Pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev. 2013;66:1–79. doi: 10.1124/pr.113.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vangelista L, Vento S. The expanding therapeutic perspective of CCR5 blockade. Front Immunol. 2018;8:1981. doi: 10.3389/fimmu.2017.01981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stone MJ, Hayward JA, Huang C, Huma EZ, Sanchez J. Mechanisms of regulation of the chemokine-receptor network. Int J Mol Sci. 2017 doi: 10.3390/ijms18020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naranbhai V, Carrington M. Host genetic variation and HIV disease: from mapping to mechanism. Immunogenetics. 2017;69:489–498. doi: 10.1007/s00251-017-1000-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O’Connor T, Borsig L, Heikenwalder M. CCL2-CCR2 signaling in disease pathogenesis. Endocr Metab Immune Disord Drug Targets. 2015;15:105–118. doi: 10.2174/1871530315666150316120920. [DOI] [PubMed] [Google Scholar]
- 13.Hartley O, Martins E, Scurci I. Preventing HIV transmission through blockade of CCR5: rationale, progress and perspectives. Swiss Med Wkly. 2018;148:w14580. doi: 10.4414/smw.2018.14580. [DOI] [PubMed] [Google Scholar]
- 14.Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nat Rev Immunol. 2008;8:447–457. doi: 10.1038/nri2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yi TJ, Shannon B, Prodger J, McKinnon L, Kaul R. Genital immunology and HIV susceptibility in young women. Am J Reprod Immunol. 2013;69(Suppl 1):74–79. doi: 10.1111/aji.12035. [DOI] [PubMed] [Google Scholar]
- 16.Grivel JC, Shattock RJ, Margolis LB. Selective transmission of R5 HIV-1 variants: where is the gatekeeper? J Transl Med. 2011;9(Suppl 1):S6. doi: 10.1186/1479-5876-9-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu R, Paxton WA, Choe S, Ceradini D, Martin SR, Horuk R, MacDonald ME, Stuhlmann H, Koup RA, Landau NR. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/s0092-8674(00)80110-5. [DOI] [PubMed] [Google Scholar]
- 18.Stewart GJ, Ashton LJ, Biti RA, Ffrench RA, Bennetts BH, Newcombe NR, Benson EM, Carr A, Cooper DA, Kaldor JM, The Australian Long-Term Non-Progressor Study Group Increased frequency of CCR-5 delta 32 heterozygotes among long-term non-progressors with HIV-1 infection. AIDS. 1997;11:1833–1838. doi: 10.1097/00002030-199715000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Martin MP, Dean M, Smith MW, Winkler C, Gerrard B, Michael NL, Lee B, Doms RW, Margolick J, Buchbinder S, Goedert JJ, O’Brien TR, Hilgartner MW, Vlahov D, O’Brien SJ, Carrington M. Genetic acceleration of AIDS progression by a promoter variant of CCR5. Science. 1998;282:1907–1911. doi: 10.1126/science.282.5395.1907. [DOI] [PubMed] [Google Scholar]
- 20.Salkowitz JR, Bruse SE, Meyerson H, Valdez H, Mosier DE, Harding CV, Zimmerman PA, Lederman MM. CCR5 promoter polymorphism determines macrophage CCR5 density and magnitude of HIV-1 propagation in vitro. Clin Immunol. 2003;108:234–240. doi: 10.1016/s1521-6616(03)00147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutter G, Nowak D, Mossner M, Ganepola S, Mussig A, Allers K, Schneider T, Hofmann J, Kucherer C, Blau O, Blau IW, Hofmann WK, Thiel E. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med. 2009;360:692–698. doi: 10.1056/NEJMoa0802905. [DOI] [PubMed] [Google Scholar]
- 22.Gupta RK, Abdul-Jawad S, McCoy LE, Mok HP, Peppa D, Salgado M, Martinez-Picado J, Nijhuis M, Wensing AMJ, Lee H, Grant P, Nastouli E, Lambert J, Pace M, Salasc F, Monit C, Innes A, Muir L, Waters L, Frater J, Lever AML, Edwards SG, Gabriel IH, Olavarria E. HIV-1 remission following CCR5Delta32/Delta32 haematopoietic stem-cell transplantation. Nature. 2019;568:244–248. doi: 10.1038/s41586-019-1027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J Exp Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oswald-Richter K, Grill SM, Leelawong M, Tseng M, Kalams SA, Hulgan T, Haas DW, Unutmaz D. Identification of a CCR5-expressing T cell subset that is resistant to R5-tropic HIV infection. PLoS Pathog. 2007;3:e58. doi: 10.1371/journal.ppat.0030058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasi A, Chiodi F. Mechanisms regulating expansion of CD8 + T cells during HIV-1 infection. J Intern Med. 2018;283:257–267. doi: 10.1111/joim.12722. [DOI] [PubMed] [Google Scholar]
- 26.Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, De Leval L, Graziosi C, Pantaleo G. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Clerici M, Butto S, Lukwiya M, Saresella M, Declich S, Trabattoni D, Pastori C, Piconi S, Fracasso C, Fabiani M, Ferrante P, Rizzardini G, Lopalco L. Immune activation in Africa is environmentally-driven and is associated with upregulation of CCR5. Italian-Ugandan AIDS project. AIDS. 2000;14:2083–2092. doi: 10.1097/00002030-200009290-00003. [DOI] [PubMed] [Google Scholar]
- 28.Colin P, Benureau Y, Staropoli I, Wang Y, Gonzalez N, Alcami J, Hartley O, Brelot A, Arenzana-Seisdedos F, Lagane B. HIV-1 exploits CCR5 conformational heterogeneity to escape inhibition by chemokines. Proc Natl Acad Sci USA. 2013;110:9475–9480. doi: 10.1073/pnas.1222205110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fox JM, Kasprowicz R, Hartley O, Signoret N. CCR5 susceptibility to ligand-mediated down-modulation differs between human T lymphocytes and myeloid cells. J Leukoc Biol. 2015;98:59–71. doi: 10.1189/jlb.2A0414-193RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lopalco L, Barassi C, Paolucci C, Breda D, Brunelli D, Nguyen M, Nouhin J, Luong TT, Truong LX, Clerici M, Calori G, Lazzarin A, Pancino G, Burastero SE. Predictive value of anti-cell and anti-human immunodeficiency virus (HIV) humoral responses in HIV-1-exposed seronegative cohorts of European and Asian origin. J Gen Virol. 2005;86:339–348. doi: 10.1099/vir.0.80585-0. [DOI] [PubMed] [Google Scholar]
- 31.Venuti A, Pastori C, Siracusano G, Riva A, Sciortino MT, Lopalco L. ERK1-based pathway as a new selective mechanism to modulate CCR5 with natural antibodies. J Immunol. 2015;195:3045–3057. doi: 10.4049/jimmunol.1500708. [DOI] [PubMed] [Google Scholar]
- 32.Venuti A, Pastori C, Pennisi R, Riva A, Sciortino MT, Lopalco L. Class B beta-arrestin2-dependent CCR5 signalosome retention with natural antibodies to CCR5. Sci Rep. 2016;6:39382. doi: 10.1038/srep39382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Venuti A, Pastori C, Siracusano G, Pennisi R, Riva A, Tommasino M, Sciortino MT, Lopalco L. The abrogation of phosphorylation plays a relevant role in the CCR5 signalosome formation with natural antibodies to CCR5. Viruses. 2017 doi: 10.3390/v10010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Venuti A, Pastori C, Lopalco L. The role of natural antibodies to CC chemokine receptor 5 in HIV infection. Front Immunol. 2017;8:1358. doi: 10.3389/fimmu.2017.01358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopalco L, Barassi C, Pastori C, Longhi R, Burastero SE, Tambussi G, Mazzotta F, Lazzarin A, Clerici M, Siccardi AG. CCR5-reactive antibodies in seronegative partners of HIV-seropositive individuals down-modulate surface CCR5 in vivo and neutralize the infectivity of R5 strains of HIV-1 In vitro. J Immunol. 2000;164:3426–3433. doi: 10.4049/jimmunol.164.6.3426. [DOI] [PubMed] [Google Scholar]
- 36.Barassi C, Lazzarin A, Lopalco L. CCR5-specific mucosal IgA in saliva and genital fluids of HIV-exposed seronegative subjects. Blood. 2004;104:2205–2206. doi: 10.1182/blood-2004-06-2134. [DOI] [PubMed] [Google Scholar]
- 37.Pastori C, Weiser B, Barassi C, Uberti-Foppa C, Ghezzi S, Longhi R, Calori G, Burger H, Kemal K, Poli G, Lazzarin A, Lopalco L. Long-lasting CCR5 internalization by antibodies in a subset of long-term nonprogressors: a possible protective effect against disease progression. Blood. 2006;107:4825–4833. doi: 10.1182/blood-2005-06-2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bomsel M, Pastori C, Tudor D, Alberti C, Garcia S, Ferrari D, Lazzarin A, Lopalco L. Natural mucosal antibodies reactive with first extracellular loop of CCR5 inhibit HIV-1 transport across human epithelial cells. AIDS. 2007;21:13–22. doi: 10.1097/QAD.0b013e328011049b. [DOI] [PubMed] [Google Scholar]
- 39.Shi C, Pamer EG. Monocyte recruitment during infection and inflammation. Nat Rev Immunol. 2011;11:762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Doranz BJ, Rucker J, Yi Y, Smyth RJ, Samson M, Peiper SC, Parmentier M, Collman RG, Doms RW. A dual-tropic primary HIV-1 isolate that uses fusin and the beta-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 41.Frade JM, Llorente M, Mellado M, Alcami J, Gutierrez-Ramos JC, Zaballos A, Real G, Martinez-A C. The amino-terminal domain of the CCR2 chemokine receptor acts as coreceptor for HIV-1 infection. J Clin Invest. 1997;100:497–502. doi: 10.1172/JCI119558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith MW, Dean M, Carrington M, Winkler C, Huttley GA, Lomb DA, Goedert JJ, O’Brien TR, Jacobson LP, Kaslow R, Buchbinder S, Vittinghoff E, Vlahov D, Hoots K, Hilgartner MW, O’Brien SJ. Contrasting genetic influence of CCR2 and CCR5 variants on HIV-1 infection and disease progression. Hemophilia growth and development study (HGDS), multicenter AIDS Cohort study (MACS), multicenter hemophilia Cohort study (MHCS), San Francisco City Cohort (SFCC) ALIVE Study. Science. 1997;277:959–965. doi: 10.1126/science.277.5328.959. [DOI] [PubMed] [Google Scholar]
- 43.Kostrikis LG, Huang Y, Moore JP, Wolinsky SM, Zhang L, Guo Y, Deutsch L, Phair J, Neumann AU, Ho DD. A chemokine receptor CCR43 allele delays HIV-1 disease progression and is associated with a CCR43 promoter mutation. Nat Med. 1998;4:350–353. doi: 10.1038/nm0398-350. [DOI] [PubMed] [Google Scholar]
- 44.Mellado M, Rodriguez-Frade JM, Vila-Coro AJ, de Ana AM, Martinez-A C. Chemokine control of HIV-1 infection. Nature. 1999;400:723–724. doi: 10.1038/23382. [DOI] [PubMed] [Google Scholar]
- 45.Mariani R, Wong S, Mulder LC, Wilkinson DA, Reinhart AL, LaRosa G, Nibbs R, O’Brien TR, Michael NL, Connor RI, Macdonald M, Busch M, Koup RA, Landau NR. CCR2-64I polymorphism is not associated with altered CCR2 expression or coreceptor function. J Virol. 1999;73:2450–2459. doi: 10.1128/jvi.73.3.2450-2459.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lawhorn C, Yuferov V, Randesi M, Ho A, Morgello S, Kreek MJ, Levran O. Genetic diversity and linkage disequilibrium in the chemokine receptor CCR2-CCR5 region among individuals and populations. Cytokine. 2013;64:571–576. doi: 10.1016/j.cyto.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gonzalez E, Rovin BH, Sen L, Cooke G, Dhanda R, Mummidi S, Kulkarni H, Bamshad MJ, Telles V, Anderson SA, Walter EA, Stephan KT, Deucher M, Mangano A, Bologna R, Ahuja SS, Dolan MJ, Ahuja SK. HIV-1 infection and AIDS dementia are influenced by a mutant MCP-1 allele linked to increased monocyte infiltration of tissues and MCP-1 levels. Proc Natl Acad Sci U S A. 2002;99:13795–13800. doi: 10.1073/pnas.202357499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thames AD, Briones MS, Magpantay LI, Martinez-Maza O, Singer EJ, Hinkin CH, Morgello S, Gelman BB, Moore DJ, Heizerling K, Levine AJ. The role of chemokine C-C motif ligand 2 genotype and cerebrospinal fluid chemokine C-C motif ligand 2 in neurocognition among HIV-infected patients. AIDS. 2015;29:1483–1491. doi: 10.1097/QAD.0000000000000706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alonso-Villaverde C, Coll B, Parra S, Montero M, Calvo N, Tous M, Joven J, Masana L. Atherosclerosis in patients infected with HIV is influenced by a mutant monocyte chemoattractant protein-1 allele. Circulation. 2004;110:2204–2209. doi: 10.1161/01.CIR.0000143835.95029.7D. [DOI] [PubMed] [Google Scholar]
- 50.Floris-Moore M, Fayad ZA, Berman JW, Mani V, Schoenbaum EE, Klein RS, Weinshelbaum KB, Fuster V, Howard AA, Lo Y, Schecter AD. Association of HIV viral load with monocyte chemoattractant protein-1 and atherosclerosis burden measured by magnetic resonance imaging. AIDS. 2009;23:941–949. doi: 10.1097/QAD.0b013e328329c76b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weiss L, Si-Mohamed A, Giral P, Castiel P, Ledur A, Blondin C, Kazatchkine MD, Haeffner-Cavaillon N. Plasma levels of monocyte chemoattractant protein-1 but not those of macrophage inhibitory protein-1alpha and RANTES correlate with virus load in human immunodeficiency virus infection. J Infect Dis. 1997;176:1621–1624. doi: 10.1086/517341. [DOI] [PubMed] [Google Scholar]
- 52.Cinque P, Vago L, Mengozzi M, Torri V, Ceresa D, Vicenzi E, Transidico P, Vagani A, Sozzani S, Mantovani A, Lazzarin A, Poli G. Elevated cerebrospinal fluid levels of monocyte chemotactic protein-1 correlate with HIV-1 encephalitis and local viral replication. AIDS. 1998;12:1327–1332. doi: 10.1097/00002030-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 53.Fantuzzi L, Spadaro F, Purificato C, Cecchetti S, Podo F, Belardelli F, Gessani S, Ramoni C. Phosphatidylcholine-specific phospholipase C activation is required for CCR5-dependent, NF-kB-driven CCL2 secretion elicited in response to HIV-1 gp120 in human primary macrophages. Blood. 2008;111:3355–3363. doi: 10.1182/blood-2007-08-104901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spadaro F, Cecchetti S, Purificato C, Sabbatucci M, Podo F, Ramoni C, Gessani S, Fantuzzi L. Nuclear phosphoinositide-specific phospholipase C beta1 controls cytoplasmic CCL2 mRNA levels in HIV-1 gp120-stimulated primary human macrophages. PLoS One. 2013;8:e59705. doi: 10.1371/journal.pone.0059705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bruno R, Galastri S, Sacchi P, Cima S, Caligiuri A, DeFranco R, Milani S, Gessani S, Fantuzzi L, Liotta F, Frosali F, Antonucci G, Pinzani M, Marra F. gp120 modulates the biology of human hepatic stellate cells: a link between HIV infection and liver fibrogenesis. Gut. 2010;59:513–520. doi: 10.1136/gut.2008.163287. [DOI] [PubMed] [Google Scholar]
- 56.Farhadian S, Patel P, Spudich S. Neurological complications of HIV infection. Curr Infect Dis Rep. 2017;19:50. doi: 10.1007/s11908-017-0606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Williams DW, Veenstra M, Gaskill PJ, Morgello S, Calderon TM, Berman JW. Monocytes mediate HIV neuropathogenesis: mechanisms that contribute to HIV associated neurocognitive disorders. Curr HIV Res. 2014;12:85–96. doi: 10.2174/1570162x12666140526114526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Conant K, Garzino-Demo A, Nath A, McArthur JC, Halliday W, Power C, Gallo RC, Major EO. Induction of monocyte chemoattractant protein-1 in HIV-1 Tat-stimulated astrocytes and elevation in AIDS dementia. Proc Natl Acad Sci USA. 1998;95:3117–3121. doi: 10.1073/pnas.95.6.3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kamat A, Lyons JL, Misra V, Uno H, Morgello S, Singer EJ, Gabuzda D. Monocyte activation markers in cerebrospinal fluid associated with impaired neurocognitive testing in advanced HIV infection. J Acquir Immune Defic Syndr. 2012;60:234–243. doi: 10.1097/QAI.0b013e318256f3bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Veenstra M, Leon-Rivera R, Li M, Gama L, Clements JE, Berman JW. Mechanisms of CNS viral seeding by HIV(+) CD14(+) CD16(+) monocytes: establishment and reseeding of viral reservoirs contributing to HIV-associated neurocognitive disorders. MBio. 2017 doi: 10.1128/mbio.01280-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Veenstra M, Byrd DA, Inglese M, Buyukturkoglu K, Williams DW, Fleysher L, Li M, Gama L, Leon-Rivera R, Calderon TM, Clements JE, Morgello S, Berman JW. CCR2 on peripheral blood CD14(+)CD16(+) monocytes correlates with neuronal damage, HIV-associated neurocognitive disorders, and peripheral HIV DNA: reseeding of CNS reservoirs? J Neuroimmune Pharmacol. 2018;14:120–133. doi: 10.1007/s11481-018-9792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Byron MM, Valcour V, Ananworanich J, Agsalda M, Chalermchai T, Tipsuk S, Sithinamsuwan P, Schuetz A, Hutchings N, Barbour J, Phanuphak N, Shikuma C, Shiramizu B, Ndhlovu L. CD16+ expressing monocyte subsets and CD14+ HIV DNA levels predict HIV-associatedneurocognitive disorders (HAND) in treatment-naïve HIV infected Thai subjects (P3043) J Immunol. 2013;190:55. [Google Scholar]
- 63.Wira CR, Rodriguez-Garcia M, Patel MV. The role of sex hormones in immune protection of the female reproductive tract. Nat Rev Immunol. 2015;15:217–230. doi: 10.1038/nri3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Boily-Larouche G, Lajoie J, Dufault B, Omollo K, Cheruiyot J, Njoki J, Kowatsch M, Kimani M, Kimani J, Oyugi J, Fowke KR. Characterization of the genital mucosa immune profile to distinguish phases of the menstrual cycle: implications for HIV susceptibility. J Infect Dis. 2019;219:856–866. doi: 10.1093/infdis/jiy585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rollenhagen C, Asin SN. Enhanced HIV-1 replication in ex vivo ectocervical tissues from post-menopausal women correlates with increased inflammatory responses. Mucosal Immunol. 2011;4:671–681. doi: 10.1038/mi.2011.34. [DOI] [PubMed] [Google Scholar]
- 66.Mukura LR, Ghosh M, Fahey JV, Cu-Uvin S, Wira CR. Genital tract viral load in HIV Type 1-positive women correlates with specific cytokine levels in cervical-vaginal secretions but is not a determinant of infectious virus or anti-HIV activity. AIDS Res Hum Retroviruses. 2012;28:1533–1539. doi: 10.1089/aid.2011.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Campbell GR, Spector SA. CCL2 increases X4-tropic HIV-1 entry into resting CD4 + T cells. J Biol Chem. 2008;283:30745–30753. doi: 10.1074/jbc.M804112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vicenzi E, Alfano M, Ghezzi S, Gatti A, Veglia F, Lazzarin A, Sozzani S, Mantovani A, Poli G. Divergent regulation of HIV-1 replication in PBMC of infected individuals by CC chemokines: suppression by RANTES, MIP-1alpha, and MCP-3, and enhancement by MCP-1. J Leukoc Biol. 2000;68:405–412. [PubMed] [Google Scholar]
- 69.Fantuzzi L, Spadaro F, Vallanti G, Canini I, Ramoni C, Vicenzi E, Belardelli F, Poli G, Gessani S. Endogenous CCL2 (monocyte chemotactic protein-1) modulates human immunodeficiency virus type-1 replication and affects cytoskeleton organization in human monocyte-derived macrophages. Blood. 2003;102:2334–2337. doi: 10.1182/blood-2002-10-3275. [DOI] [PubMed] [Google Scholar]
- 70.Sabbatucci M, Covino DA, Purificato C, Mallano A, Federico M, Lu J, Rinaldi AO, Pellegrini M, Bona R, Michelini Z, Cara A, Vella S, Gessani S, Andreotti M, Fantuzzi L. Endogenous CCL2 neutralization restricts HIV-1 replication in primary human macrophages by inhibiting viral DNA accumulation. Retrovirology. 2015;12:4. doi: 10.1186/s12977-014-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Packard TA, Herzig E, Roan NR, Greene WC. Establishing the HIV reservoir: HIV-susceptible cells and the signals that recruit them. J Immunol. 2017;198(Suppl 1):125.1. [Google Scholar]
- 72.Gonzalo-Gil E, Rapuano PB, Ikediobi U, Leibowitz R, Mehta S, Coskun AK, Porterfield JZ, Lampkin TD, Marconi VC, Rimland D, Walker BD, Deeks S, Sutton RE. Transcriptional down-regulation of ccr5 in a subset of HIV + controllers and their family members. Elife. 2019 doi: 10.7554/elife.44360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Packard TA, Luo X, Grimmett ZW, Herzig E, Roan NR, Greene WC. Establishing the HIV reservoir: HIV-susceptible cells and the signals that recruit them. J Immunol. 2018;200(Suppl 1):182.4. [Google Scholar]
- 74.Van Der Ryst E. Maraviroc - A CCR5 antagonist for the treatment of HIV-1 infection. Front Immunol. 2015;6:277. doi: 10.3389/fimmu.2015.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Harada S, Yoshimura K. Driving HIV-1 into a vulnerable corner by taking advantage of viral adaptation and evolution. Front Microbiol. 2017;8:390. doi: 10.3389/fmicb.2017.00390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Roche M, Jakobsen, Ellett A, Salimiseyedabad H, Jubb B, Westby M, Lee, Lewin SR, Churchill MJ, Gorry PR. HIV-1 predisposed to acquiring resistance to maraviroc (MVC) and other CCR5 antagonists in vitro has an inherent, low-level ability to utilize MVC-bound CCR5 for entry. Retrovirology. 2011;8:89. doi: 10.1186/1742-4690-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Madrid-Elena N, García-Bermejo ML, Serrano-Villar S, Díaz-de Santiago A, Sastre B, Gutiérrez C, Dronda F, Coronel Díaz M, Domínguez E, López-Huertas MR, Hernández-Novoa B, Moreno S. Maraviroc is associated with latent HIV-1 reactivation through NF-κB activation in resting CD4+ T cells from HIV-infected individuals on suppressive antiretroviral therapy. J Virol. 2018;92:e01931-17. doi: 10.1128/JVI.01931-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Olson WC, Jacobson JM. CCR5 monoclonal antibodies for HIV-1 therapy. Curr Opin HIV AIDS. 2009;4:104–111. doi: 10.1097/COH.0b013e3283224015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Flego M, Ascione A, Cianfriglia M, Vella S. Clinical development of monoclonal antibody-based drugs in HIV and HCV diseases. BMC Med. 2013;11:4–21. doi: 10.1186/1741-7015-11-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Walker LM, Burton DR. Passive immunotherapy of viral infections: ‘super-antibodies’ enter the fray. Nat Rev Immunol. 2018;18:297–308. doi: 10.1038/nri.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Li L, Tian JH, Yang K, Zhang P, Jia WQ. Humanized PA14 (a monoclonal CCR5 antibody) for treatment of people with HIV infection. Cochrane Database Syst Rev. 2014;7:CD008439. doi: 10.1002/14651858.CD008439.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Steinberger P, Sutton JK, Rader C, Elia M, Barbas CF. Generation and characterization of a recombinant human CCR5-specific antibody. A phage display approach for rabbit antibody humanization. J Biol Chem. 2000;275:36073–36078. doi: 10.1074/jbc.M002765200. [DOI] [PubMed] [Google Scholar]
- 83.Kuhmann SE, Hartley O. Targeting chemokine receptors in HIV: a status report. Annu Rev Pharmacol Toxicol. 2008;48:425–461. doi: 10.1146/annurev.pharmtox.48.113006.094847. [DOI] [PubMed] [Google Scholar]
- 84.Gaertner H, Cerini F, Escola JM, Kuenzi G, Melotti A, Offord R, Rossitto-Borlat I, Nedellec R, Salkowitz J, Gorochov G, Mosier D, Hartley O. Highly potent, fully recombinant anti-HIV chemokines: reengineering a low-cost microbicide. Proc Natl Acad Sci USA. 2008;105:17706–17711. doi: 10.1073/pnas.0805098105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Simmons G, Clapham PR, Picard L, Offord RE, Rosenkilde MM, Schwartz TW, Buser R, Wells TN, Proudfoot AE. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science. 1997;276:276–279. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- 86.Mack M, Luckow B, Nelson PJ, Cihak J, Simmons G, Clapham PR, Signoret N, Marsh M, Stangassinger M, Borlat F, Wells TN, Schlondorff D, Proudfoot AE. Aminooxypentane-RANTES induces CCR5 internalization but inhibits recycling: a novel inhibitory mechanism of HIV infectivity. J Exp Med. 1998;187:1215–1224. doi: 10.1084/jem.187.8.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Venuti A, Lopalco L. Tackling HIV: Genetic vs Immune CCR5 targeting. Journal of AIDS & clinical research. 2014;5:344–353. [Google Scholar]
- 88.Allen AG, Chung CH, Atkins A, Dampier W, Khalili K, Nonnemacher MR, Wigdahl B. Gene editing of HIV-1 Co-receptors to prevent and/or cure virus infection. Front Microbiol. 2018;9:2940. doi: 10.3389/fmicb.2018.02940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Haworth KG, Peterson CW, Kiem HP. CCR5-edited gene therapies for HIV cure: closing the door to viral entry. Cytotherapy. 2017;19:1325–1338. doi: 10.1016/j.jcyt.2017.05.013. [DOI] [PubMed] [Google Scholar]
- 90.Struthers M, Pasternak A. CCR2 antagonists. Curr Top Med Chem. 2010;10:1278–1298. doi: 10.2174/156802610791561255. [DOI] [PubMed] [Google Scholar]
- 91.Baba M, Nishimura O, Kanzaki N, Okamoto M, Sawada H, Iizawa Y, Shiraishi M, Aramaki Y, Okonogi K, Ogawa Y, Meguro K, Fujino M. A small-molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc Natl Acad Sci USA. 1999;96:5698–5703. doi: 10.1073/pnas.96.10.5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Baba M, Takashima K, Miyake H, Kanzaki N, Teshima K, Wang X, Shiraishi M, Iizawa Y. TAK-652 inhibits CCR5-mediated human immunodeficiency virus type 1 infection in vitro and has favorable pharmacokinetics in humans. Antimicrob Agents Chemother. 2005;49:4584–4591. doi: 10.1128/AAC.49.11.4584-4591.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Thompson M, Saag M, DeJesus E, Gathe J, Lalezari J, Landay AL, Cade J, Enejosa J, Lefebvre E, Feinberg J. A 48-week randomized phase 2b study evaluating cenicriviroc versus efavirenz in treatment-naive HIV-infected adults with C-C chemokine receptor type 5-tropic virus. AIDS. 2016;30:869–878. doi: 10.1097/QAD.0000000000000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.D’Antoni ML, Paul RH, Mitchell BI, Kohorn L, Fischer L, Lefebvre E, Seyedkazemi S, Nakamoto BK, Walker M, Kallianpur KJ, Ogata-Arakaki D, Ndhlovu LC, Shikuma C. Improved cognitive performance and reduced monocyte activation in virally suppressed chronic HIV after dual CCR2 and CCR5 antagonism. J Acquir Immune Defic Syndr. 2018;79:108–116. doi: 10.1097/QAI.0000000000001752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Covino DA, Purificato C, Catapano L, Galluzzo CM, Gauzzi MC, Vella S, Lefebvre E, Seyedkazemi S, Andreotti M, Fantuzzi L. APOBEC3G/3A expression in human immunodeficiency virus type 1-infected individuals following initiation of antiretroviral therapy containing cenicriviroc or efavirenz. Front Immunol. 2018;9:1839. doi: 10.3389/fimmu.2018.01839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dobson R, Giovannoni G. Multiple sclerosis - a review. Eur J Neurol. 2019;26:27–40. doi: 10.1111/ene.13819. [DOI] [PubMed] [Google Scholar]
- 97.Baecher-Allan C, Kaskow BJ, Weiner HL. Multiple sclerosis: mechanisms and immunotherapy. Neuron. 2018;97:742–768. doi: 10.1016/j.neuron.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 98.Pranzatelli MR. Advances in biomarker-guided therapy for pediatric- and adult-onset neuroinflammatory disorders: targeting chemokines/cytokines. Front Immunol. 2018;9:557. doi: 10.3389/fimmu.2018.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Martin-Blondel G, Brassat D, Bauer J, Lassmann H, Liblau RS. CCR5 blockade for neuroinflammatory diseases–beyond control of HIV. Nat Rev Neurol. 2016;12:95–105. doi: 10.1038/nrneurol.2015.248. [DOI] [PubMed] [Google Scholar]
- 100.Lee YH, Bae SC. Monocyte chemoattractant protein-1 promoter -2518 polymorphism and susceptibility to vasculitis, rheumatoid arthritis, and multiple sclerosis: a meta-analysis. Cell Mol Biol (Noisy-le-grand) 2016;62:65–71. [PubMed] [Google Scholar]
- 101.Troncoso LL, Pontillo A, Oliveira EML, Finkelszteijn A, Schneider S, Chies JAB. CCR5Delta32—A piece of protection in the inflammatory puzzle of multiple sclerosis susceptibility. Hum Immunol. 2018;79:621–626. doi: 10.1016/j.humimm.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 102.Szczucinski A, Losy J. Chemokines and chemokine receptors in multiple sclerosis. Potential targets for new therapies. Acta Neurol Scand. 2007;115:137–146. doi: 10.1111/j.1600-0404.2006.00749.x. [DOI] [PubMed] [Google Scholar]
- 103.Prins M, Dutta R, Baselmans B, Breve JJ, Bol JG, Deckard SA, van der Valk P, Amor S, Trapp BD, de Vries HE, Drukarch B, van Dam AM. Discrepancy in CCL2 and CCR2 expression in white versus grey matter hippocampal lesions of Multiple Sclerosis patients. Acta Neuropathol Commun. 2014;2:98. doi: 10.1186/s40478-014-0098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tanuma N, Sakuma H, Sasaki A, Matsumoto Y. Chemokine expression by astrocytes plays a role in microglia/macrophage activation and subsequent neurodegeneration in secondary progressive multiple sclerosis. Acta Neuropathol. 2006;112:195–204. doi: 10.1007/s00401-006-0083-7. [DOI] [PubMed] [Google Scholar]
- 105.Simpson JE, Newcombe J, Cuzner ML, Woodroofe MN. Expression of monocyte chemoattractant protein-1 and other beta-chemokines by resident glia and inflammatory cells in multiple sclerosis lesions. J Neuroimmunol. 1998;84:238–249. doi: 10.1016/s0165-5728(97)00208-7. [DOI] [PubMed] [Google Scholar]
- 106.Boven LA, Montagne L, Nottet HS, De Groot CJ. Macrophage inflammatory protein-1alpha (MIP-1alpha), MIP-1beta, and RANTES mRNA semiquantification and protein expression in active demyelinating multiple sclerosis (MS) lesions. Clin Exp Immunol. 2000;122:257–263. doi: 10.1046/j.1365-2249.2000.01334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Trebst C, Konig F, Ransohoff R, Bruck W, Stangel M. CCR5 expression on macrophages/microglia is associated with early remyelination in multiple sclerosis lesions. Mult Scler. 2008;14:728–733. doi: 10.1177/1352458508089359. [DOI] [PubMed] [Google Scholar]
- 108.McMurran CE, Jones CA, Fitzgerald DC, Franklin RJ. CNS Remyelination and the Innate Immune System. Front Cell Dev Biol. 2016;4:38. doi: 10.3389/fcell.2016.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Moyon S, Dubessy AL, Aigrot MS, Trotter M, Huang JK, Dauphinot L, Potier MC, Kerninon C, Melik Parsadaniantz S, Franklin RJ, Lubetzki C. Demyelination causes adult CNS progenitors to revert to an immature state and express immune cues that support their migration. J Neurosci. 2015;35:4–20. doi: 10.1523/JNEUROSCI.0849-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mori F, Nistico R, Nicoletti CG, Zagaglia S, Mandolesi G, Piccinin S, Martino G, Finardi A, Rossini PM, Marfia GA, Furlan R, Centonze D. RANTES correlates with inflammatory activity and synaptic excitability in multiple sclerosis. Mult Scler. 2016;22:1405–1412. doi: 10.1177/1352458515621796. [DOI] [PubMed] [Google Scholar]
- 111.Pittaluga A. CCL5-glutamate cross-talk in astrocyte-neuron communication in multiple sclerosis. Front Immunol. 2017;8:1079. doi: 10.3389/fimmu.2017.01079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou Y, Tang H, Liu J, Dong J, Xiong H. Chemokine CCL2 modulation of neuronal excitability and synaptic transmission in rat hippocampal slices. J Neurochem. 2011;116:406–414. doi: 10.1111/j.1471-4159.2010.07121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Scarpini E, Galimberti D, Baron P, Clerici R, Ronzoni M, Conti G, Scarlato G. IP-10 and MCP-1 levels in CSF and serum from multiple sclerosis patients with different clinical subtypes of the disease. J Neurol Sci. 2002;195:41–46. doi: 10.1016/s0022-510x(01)00680-3. [DOI] [PubMed] [Google Scholar]
- 114.Sorensen TL, Sellebjerg F, Jensen CV, Strieter RM, Ransohoff RM. Chemokines CXCL10 and CCL2: differential involvement in intrathecal inflammation in multiple sclerosis. Eur J Neurol. 2001;8:665–672. doi: 10.1046/j.1468-1331.2001.00327.x. [DOI] [PubMed] [Google Scholar]
- 115.Sorensen TL, Ransohoff RM, Strieter RM, Sellebjerg F. Chemokine CCL2 and chemokine receptor CCR2 in early active multiple sclerosis. Eur J Neurol. 2004;11:445–449. doi: 10.1111/j.1468-1331.2004.00796.x. [DOI] [PubMed] [Google Scholar]
- 116.De Laere M, Berneman ZN, Cools N. To the brain and back: migratory paths of dendritic cells in multiple sclerosis. J Neuropathol Exp Neurol. 2018;77:178–192. doi: 10.1093/jnen/nlx114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sato W, Tomita A, Ichikawa D, Lin Y, Kishida H, Miyake S, Ogawa M, Okamoto T, Murata M, Kuroiwa Y, Aranami T, Yamamura T. CCR2(+)CCR5(+) T cells produce matrix metalloproteinase-9 and osteopontin in the pathogenesis of multiple sclerosis. J Immunol. 2012;189:5057–5065. doi: 10.4049/jimmunol.1202026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Misu T, Onodera H, Fujihara K, Matsushima K, Yoshie O, Okita N, Takase S, Itoyama Y. Chemokine receptor expression on T cells in blood and cerebrospinal fluid at relapse and remission of multiple sclerosis: imbalance of Th1/Th2-associated chemokine signaling. J Neuroimmunol. 2001;114:207–212. doi: 10.1016/s0165-5728(00)00456-2. [DOI] [PubMed] [Google Scholar]
- 119.Szczucinski A, Losy J. Long-term effect of IFN-beta 1a therapy on CCL2 (MCP-1) chemokine in patients with multiple sclerosis. Folia Neuropathol. 2004;42:15–18. [PubMed] [Google Scholar]
- 120.Sellebjerg F, Krakauer M, Hesse D, Ryder LP, Alsing I, Jensen PE, Koch-Henriksen N, Svejgaard A, Soelberg Sorensen P. Identification of new sensitive biomarkers for the in vivo response to interferon-beta treatment in multiple sclerosis using DNA-array evaluation. Eur J Neurol. 2009;16:1291–1298. doi: 10.1111/j.1468-1331.2009.02716.x. [DOI] [PubMed] [Google Scholar]
- 121.Cepok S, Schreiber H, Hoffmann S, Zhou D, Neuhaus O, von Geldern G, Hochgesand S, Nessler S, Rothhammer V, Lang M, Hartung HP, Hemmer B. Enhancement of chemokine expression by interferon beta therapy in patients with multiple sclerosis. Arch Neurol. 2009;66:1216–1223. doi: 10.1001/archneurol.2009.138. [DOI] [PubMed] [Google Scholar]
- 122.Buttmann M, Merzyn C, Hofstetter HH, Rieckmann P. TRAIL, CXCL10 and CCL2 plasma levels during long-term Interferon-beta treatment of patients with multiple sclerosis correlate with flu-like adverse effects but do not predict therapeutic response. J Neuroimmunol. 2007;190:170–176. doi: 10.1016/j.jneuroim.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 123.Pease J, Horuk R. Chemokine receptor antagonists. J Med Chem. 2012;55:9363–9392. doi: 10.1021/jm300682j. [DOI] [PubMed] [Google Scholar]
- 124.Horuk R. Chemokine receptor antagonists: overcoming developmental hurdles. Nat Rev Drug Discov. 2009;8:23–33. doi: 10.1038/nrd2734. [DOI] [PubMed] [Google Scholar]
- 125.Sharrack B, Leach T, Jacobson E, Donaldson DD, Xu X, Hu M. Frequent MRI study of a novel CCR2 antagonist in relapsing-remitting multiple sclerosis. Annal Neurol. 2007;62:S74–S75. [Google Scholar]
- 126.Scarpazza C, Prosperini L, Mancinelli CR, De Rossi N, Lugaresi A, Capobianco M, Moiola L, Naldi P, Imberti L, Gerevini S, Capra R. Is maraviroc useful in multiple sclerosis patients with natalizumab-related progressive multifocal leukoencephalopathy? J Neurol Sci. 2017;378:233–237. doi: 10.1016/j.jns.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 127.Hodecker SC, Sturner KH, Becker V, Elias-Hamp B, Holst B, Friese MA, Heesen C. Maraviroc as possible treatment for PML-IRIS in natalizumab-treated patients with MS. Neurol Neuroimmunol Neuroinflamm. 2017;4:e325. doi: 10.1212/NXI.0000000000000325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lee YA, Wallace MC, Friedman SL. Pathobiology of liver fibrosis: a translational success story. Gut. 2015;64:830–841. doi: 10.1136/gutjnl-2014-306842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat Med. 2018;24:908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fazel Y, Koenig AB, Sayiner M, Goodman ZD, Younossi ZM. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism. 2016;65:1017–1025. doi: 10.1016/j.metabol.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 131.Tacke F. Targeting hepatic macrophages to treat liver diseases. J Hepatol. 2017;66:1300–1312. doi: 10.1016/j.jhep.2017.02.026. [DOI] [PubMed] [Google Scholar]
- 132.Fabregat I, Caballero-Diaz D. Transforming growth factor-beta-Induced cell plasticity in liver fibrosis and hepatocarcinogenesis. Front Oncol. 2018;8:357. doi: 10.3389/fonc.2018.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014;147:577–594.e1. doi: 10.1053/j.gastro.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 134.Seki E, De Minicis S, Gwak GY, Kluwe J, Inokuchi S, Bursill CA, Llovet JM, Brenner DA, Schwabe RF. CCR1 and CCR5 promote hepatic fibrosis in mice. J Clin Invest. 2009;119:1858–1870. doi: 10.1172/JCI37444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Seki E, de Minicis S, Inokuchi S, Taura K, Miyai K, van Rooijen N, Schwabe RF, Brenner DA. CCR2 promotes hepatic fibrosis in mice. Hepatology. 2009;50:185–197. doi: 10.1002/hep.22952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zimmermann HW, Seidler S, Nattermann J, Gassler N, Hellerbrand C, Zernecke A, Tischendorf JJ, Luedde T, Weiskirchen R, Trautwein C, Tacke F. Functional contribution of elevated circulating and hepatic non-classical CD14CD16 monocytes to inflammation and human liver fibrosis. PLoS One. 2010;5:e11049. doi: 10.1371/journal.pone.0011049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kazankov K, Barrera F, Moller HJ, Rosso C, Bugianesi E, David E, Younes R, Esmaili S, Eslam M, McLeod D, Bibby BM, Vilstrup H, George J, Gronbaek H. The macrophage activation marker sCD163 is associated with morphological disease stages in patients with non-alcoholic fatty liver disease. Liver Int. 2016;36:1549–1557. doi: 10.1111/liv.13150. [DOI] [PubMed] [Google Scholar]
- 138.du Plessis J, van Pelt J, Korf H, Mathieu C, van der Schueren B, Lannoo M, Oyen T, Topal B, Fetter G, Nayler S, van der Merwe T, Windmolders P, Van Gaal L, Verrijken A, Hubens G, Gericke M, Cassiman D, Francque S, Nevens F, van der Merwe S. Association of adipose tissue inflammation with histologic severity of nonalcoholic fatty liver disease. Gastroenterology. 2015;149:635–48.e14. doi: 10.1053/j.gastro.2015.05.044. [DOI] [PubMed] [Google Scholar]
- 139.Verna EC. Non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in patients with HIV. Lancet Gastroenterol Hepatol. 2017;2:211–223. doi: 10.1016/S2468-1253(16)30120-0. [DOI] [PubMed] [Google Scholar]
- 140.Kutlu O, Kaleli HN, Ozer E. Molecular pathogenesis of nonalcoholic steatohepatitis- (NASH-) related hepatocellular carcinoma. Can J Gastroenterol Hepatol. 2018;2018:8543763. doi: 10.1155/2018/8543763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. doi: 10.1016/S0140-6736(18)30010-2. [DOI] [PubMed] [Google Scholar]
- 142.Ehling J, Tacke F. Role of chemokine pathways in hepatobiliary cancer. Cancer Lett. 2016;379:173–183. doi: 10.1016/j.canlet.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 143.Li X, Yao W, Yuan Y, Chen P, Li B, Li J, Chu R, Song H, Xie D, Jiang X, Wang H. Targeting of tumour-infiltrating macrophages via CCL2/CCR2 signalling as a therapeutic strategy against hepatocellular carcinoma. Gut. 2017;66:157–167. doi: 10.1136/gutjnl-2015-310514. [DOI] [PubMed] [Google Scholar]
- 144.Yeh CB, Tsai HT, Chen YC, Kuo WH, Chen TY, Hsieh YH, Chou MC, Yang SF. Genetic polymorphism of CCR2-64I increased the susceptibility of hepatocellular carcinoma. J Surg Oncol. 2010;102:264–270. doi: 10.1002/jso.21623. [DOI] [PubMed] [Google Scholar]
- 145.Bartneck M, Schrammen PL, Möckel D, Govaere O, Liepelt A, Krenkel O, Ergen C, McCain MV, Eulberg D, Luedde T. The CCR2 macrophage subset promotes pathogenic angiogenesis for tumor vascularization in fibrotic livers. Cell Mol Gastroenterol Hepatol. 2018;7:371–390. doi: 10.1016/j.jcmgh.2018.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Huang B, Lei Z, Zhao J, Gong W, Liu J, Chen Z, Liu Y, Li D, Yuan Y, Zhang GM, Feng ZH. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 2007;252:86–92. doi: 10.1016/j.canlet.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 147.Fujisaka Y, Iwata T, Tamai K, Nakamura M, Mochizuki M, Shibuya R, Yamaguchi K, Shimosegawa T, Satoh K. Long non-coding RNA HOTAIR up-regulates chemokine (C-C motif) ligand 2 and promotes proliferation of macrophages and myeloid-derived suppressor cells in hepatocellular carcinoma cell lines. Oncol Lett. 2018;15:509–514. doi: 10.3892/ol.2017.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhuang H, Cao G, Kou C, Liu T. CCL2/CCR2 axis induces hepatocellular carcinoma invasion and epithelial-mesenchymal transition in vitro through activation of the Hedgehog pathway. Oncol Rep. 2018;39:21–30. doi: 10.3892/or.2017.6069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shih YT, Wang MC, Zhou J, Peng HH, Lee DY, Chiu JJ. Endothelial progenitors promote hepatocarcinoma intrahepatic metastasis through monocyte chemotactic protein-1 induction of microRNA-21. Gut. 2015;64:1132–1147. doi: 10.1136/gutjnl-2013-306302. [DOI] [PubMed] [Google Scholar]
- 150.Chew V, Chen J, Lee D, Loh E, Lee J, Lim KH, Weber A, Slankamenac K, Poon RT, Yang H, Ooi LL, Toh HC, Heikenwalder M, Ng IO, Nardin A, Abastado JP. Chemokine-driven lymphocyte infiltration: an early intratumoural event determining long-term survival in resectable hepatocellular carcinoma. Gut. 2012;61:427–438. doi: 10.1136/gutjnl-2011-300509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cai J, Zhao XL, Liu AW, Nian H, Zhang SH. Apigenin inhibits hepatoma cell growth through alteration of gene expression patterns. Phytomedicine. 2011;18:366–373. doi: 10.1016/j.phymed.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 152.Lin ZY, Chuang YH, Chuang WL. Cancer-associated fibroblasts up-regulate CCL2, CCL26, IL6 and LOXL2 genes related to promotion of cancer progression in hepatocellular carcinoma cells. Biomed Pharmacother. 2012;66:525–529. doi: 10.1016/j.biopha.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 153.Liang CM, Chen L, Hu H, Ma HY, Gao LL, Qin J, Zhong CP. Chemokines and their receptors play important roles in the development of hepatocellular carcinoma. World J Hepatol. 2015;7:1390–1402. doi: 10.4254/wjh.v7.i10.1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ochoa-Callejero L, Perez-Martinez L, Rubio-Mediavilla S, Oteo JA, Martinez A, Blanco JR. Maraviroc, a CCR5 antagonist, prevents development of hepatocellular carcinoma in a mouse model. PLoS One. 2013;8:e53992. doi: 10.1371/journal.pone.0053992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Barashi N, Weiss ID, Wald O, Wald H, Beider K, Abraham M, Klein S, Goldenberg D, Axelrod J, Pikarsky E, Abramovitch R, Zeira E, Galun E, Peled A. Inflammation-induced hepatocellular carcinoma is dependent on CCR5 in mice. Hepatology. 2013;58:1021–1030. doi: 10.1002/hep.26403. [DOI] [PubMed] [Google Scholar]
- 156.Tacke F. Cenicriviroc for the treatment of non-alcoholic steatohepatitis and liver fibrosis. Expert Opin Investig Drugs. 2018;27:301–311. doi: 10.1080/13543784.2018.1442436. [DOI] [PubMed] [Google Scholar]
- 157.Ambade A, Lowe P, Kodys K, Catalano D, Gyongyosi B, Cho Y, Iracheta Vellve A, Adejumo A, Saha B, Calenda C, Mehta J, Lefebvre E, Vig P, Szabo G. Pharmacological inhibition of CCR2/5 signaling prevents and reverses alcohol-induced liver damage, steatosis and inflammation in mice. Hepatology. 2018;69:1105–1121. doi: 10.1002/hep.30249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Friedman S, Sanyal A, Goodman Z, Lefebvre E, Gottwald M, Fischer L, Ratziu V. Efficacy and safety study of cenicriviroc for the treatment of non-alcoholic steatohepatitis in adult subjects with liver fibrosis: cENTAUR Phase 2b study design. Contemp Clin Trials. 2016;47:356–365. doi: 10.1016/j.cct.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 159.Fong ZV, Tanabe KK. The clinical management of hepatocellular carcinoma in the United States, Europe, and Asia: a comprehensive and evidence-based comparison and review. Cancer. 2014;120:2824–2838. doi: 10.1002/cncr.28730. [DOI] [PubMed] [Google Scholar]
- 160.Lencioni R, Crocetti L. Local-regional treatment of hepatocellular carcinoma. Radiology. 2012;262:43–58. doi: 10.1148/radiol.11110144. [DOI] [PubMed] [Google Scholar]
- 161.Siegel AB, Olsen SK, Magun A, Brown RS., Jr Sorafenib: where do we go from here? Hepatology. 2010;52:360–369. doi: 10.1002/hep.23633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Teng KY, Han J, Zhang X, Hsu SH, He S, Wani NA, Barajas JM, Snyder LA, Frankel WL, Caligiuri MA, Jacob ST, Yu J, Ghoshal K. Blocking the CCL2-CCR2 axis using CCL2-neutralizing antibody is an effective therapy for hepatocellular cancer in a mouse model. Mol Cancer Ther. 2017;16:312–322. doi: 10.1158/1535-7163.MCT-16-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Ehling J, Bartneck M, Wei X, Gremse F, Fech V, Mockel D, Baeck C, Hittatiya K, Eulberg D, Luedde T, Kiessling F, Trautwein C, Lammers T, Tacke F. CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis. Gut. 2014;63:1960–1971. doi: 10.1136/gutjnl-2013-306294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tagliamonte M, Petrizzo A, Tornesello ML, Ciliberto G, Buonaguro FM, Buonaguro L. Combinatorial immunotherapy strategies for hepatocellular carcinoma. Curr Opin Immunol. 2016;39:103–113. doi: 10.1016/j.coi.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 165.Tagliamonte M, Petrizzo A, Mauriello A, Tornesello ML, Buonaguro FM, Buonaguro L. Potentiating cancer vaccine efficacy in liver cancer. Oncoimmunology. 2018;7:e1488564. doi: 10.1080/2162402X.2018.1488564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Buonaguro L, Petrizzo A, Tagliamonte M, Tornesello ML, Buonaguro FM. Challenges in cancer vaccine development for hepatocellular carcinoma. J Hepatol. 2013;59:897–903. doi: 10.1016/j.jhep.2013.05.031. [DOI] [PubMed] [Google Scholar]
- 167.Marabelle A, Kohrt H, Caux C, Levy R. Intratumoral immunization: a new paradigm for cancer therapy. Clin Cancer Res. 2014;20:1747–1756. doi: 10.1158/1078-0432.CCR-13-2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yao W, Ba Q, Li X, Li H, Zhang S, Yuan Y, Wang F, Duan X, Li J, Zhang W, Wang H. A Natural CCR168 antagonist relieves tumor-associated macrophage-mediated immunosuppression to produce a therapeutic effect for liver cancer. EBioMedicine. 2017;22:58–67. doi: 10.1016/j.ebiom.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]