Abstract
Purpose
To investigate the association between serum 25-hydroxyvitamin D (25[OH]D) concentrations at visit 2 (1990–1992) and the 18-year incidence of age-related macular degeneration (AMD) between visit 3 (1993–1995) and visit 5 (2011–2013).
Methods
This prospective analysis was conducted in a subset of participants (n = 1225) from the Atherosclerosis Risk in Communities Study. We evaluated the incidence of any, early, and late AMD from visit 3 to 5. The 25(OH)D concentrations were assessed in 2012–2013 by using stored serum from visit 2. Retinal fundus photographs taken at both visits were graded side by side to determine the incidence of AMD. Logistic regression was used to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) for incident AMD outcomes during 18 years of follow-up (1993–1995 to 2011–2013) by tertile of 25(OH)D adjusted for age, race, and smoking status. P for linear trend was estimated by using continuous 25(OH)D concentrations. Sensitivity analyses applied inverse probability weights to account for selection to have eye photographs, death, and loss to follow-up.
Results
There was a decreased odds of any incident AMD (n = 139) and large, soft drusen (n = 80) in 25(OH)D tertile 3 versus 1, with OR (95% CI) = 0.57 (0.36–0.90), P trend = 0.11 and with 0.52 (0.28–0.93), P trend = 0.18, respectively. Applying sampling weights attenuated these results to 0.66 (0.38–1.16), P trend = 0.32 (any incident AMD) and 0.54 (0.27–1.09), P trend = 0.36 (large, soft drusen), respectively, suggesting these associations may be biased by loss to follow-up and sampling for retinal photographs at visit 5. No statistically significant results were observed with pigmentary abnormalities (n = 46) or incident late AMD (n = 26).
Conclusions
High 25(OH)D concentrations, approximately >70 nM, may be associated with decreased odds of incident early AMD.
Keywords: vitamin D, 25-hydroxyvitamin D, macular degeneration, retinal diseases, epidemiology, cohort studies
For the past decade, vitamin D has been proposed as a novel, modifiable risk factor for age-related macular degeneration (AMD) owing to its immune-modulating and antiangiogenic properties.1 This area of inquiry has produced many cross-sectional and case-control studies examining the associations between prevalent AMD and the biomarker of 25-hydroxyvitamn D (25[OH]D)1–12; however, such study designs cannot determine the temporality of the observed association. Reverse causality could explain the protective associations observed, especially those examining vision-threatening late-stage disease.8,12 Vision-impaired individuals are more likely to have mobility limitations13,14 and may be less likely to go outside and be exposed to sunlight for dermal production of vitamin D.
To the best of our knowledge, only two studies of vitamin D status and incident AMD have been published.15,16 More prospective studies of this association with careful phenotyping of vitamin D and AMD, as well as studies examining the risk of development of early AMD, are needed. Further, only two previous studies on vitamin D and AMD have been conducted in racially diverse samples,1,15 limiting our understanding of this association in racial groups such as African Americans who have one of the highest burdens of vitamin D deficiency.17
We examined the association between 25(OH)D concentrations and the 18-year progression of AMD, using data from the Atherosclerosis Risk in Communities (ARIC) Study, a well-characterized epidemiologic cohort.18 We hypothesized that ARIC participants with higher 25(OH)D concentrations will have a decreased risk of incident AMD than those with lower 25(OH)D concentrations. We also explored how the observed association varied by age, race, sex, and high-risk AMD genotype.
Methods
Study Design
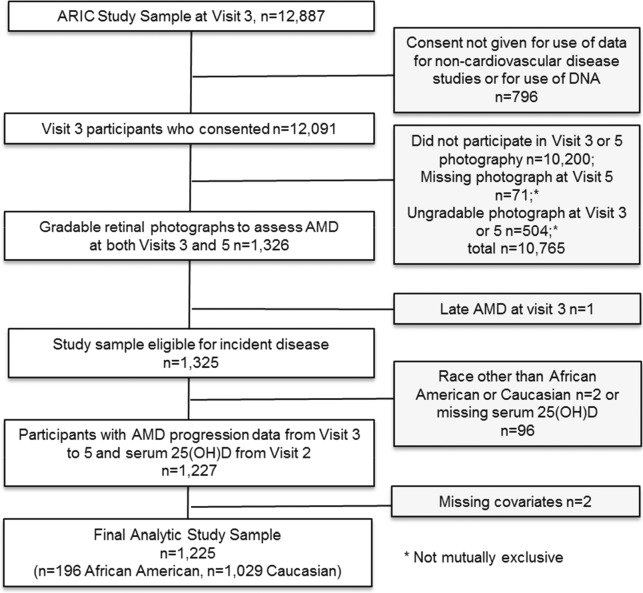
The ARIC Study is a population-based, prospective study of atherosclerosis with five visits since 1987–1989.18 Participants were 45 to 65 years of age at visit 1 and recruited from four centers: Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis, Minnesota; and Washington County, Maryland. For the present analyses, participants needed to have available serum 25(OH)D concentrations from blood drawn at visit 2 (1990–1992) and gradable retinal fundus photographs taken at visits 3 (1993–1995) and 5 (2011–2013) (Fig.). There were 11,863 participants for whom retinal photographs of one randomly chosen eye were taken at visit 3.
Figure.
Study sample selection of ARIC participants with gradable, retinal photographs from visit 3 to visit 5.
Retinal photographs were taken in one or both eyes of a subset (n = 2629) of 6538 participants at visit 5.19 Of these, approximately a third were recruited from a random sample of participants without any dementia or mild cognitive impairment.20 Participants with low neurocognitive test scores suggesting dementia or mild cognitive impairment made up the additional sample. Of these, 2124 had gradable images in at least one of two eyes from visit 5, and of these, 1326 also have gradable photographs from a matching eye at visit 3 among the previously noted 11,863 persons with retinal photos at visit 3. Participants were further excluded if they had late AMD at visit 3 (n = 1), were not Caucasian or African American (n = 2), or were missing data on 25(OH)D (n = 96). Two additional participants were excluded owing to missing data on smoking status, leaving an analytic sample of 1225 participants (1029 Caucasians, 196 African Americans). Because of the targeted recruitment of participants with low neurocognitive scores for retinal photographs, our sample included 89 participants with dementia, 440 with mild cognitive impairment, 694 with normal cognitive function, and 2 with unknown cognitive function status.
Participants of this study provided signed informed consent.21 For those determined to have mild cognitive impairment or dementia, consent to participate was given by a designated proxy and agreement to participate was also obtained from the participant. The study protocol was approved by the institutional review boards at each ARIC study site and complies with the Declaration of Helsinki as revised in 1983.
Data on Participant Characteristics
At all study visits, participants answered questionnaires on their lifestyle habits, medical history,18 had a physical exam and a blood draw.18,22 Before the visit, participants were asked to fast for 12 hours and to bring with them any medications or supplements they had taken within the past 2 weeks.18
At visits 1 and 3, physical activity was assessed by using a modified version of the previously validated23,24 Baecke questionnaire.25 We averaged questionnaire scores obtained at both visits to create a composite physical activity index score ranging from 0 (low overall physical activity) to 6.
Data on the genotypes of two high-risk AMD single nucleotide polymorphisms (SNPs) shown to be associated with increased risk of early AMD26 were used. ARMS2 A69S (rs10490924) was genotyped by using the Affymetrix Genome-Wide Human SNP Array 6.0 (Affymetrix, Inc., Santa Clara, CA, USA).27 CFH Y402H (rs1061170) was imputed by using the HapMap and 1000 Genomes reference panels (appropriate for race). For ARMS2 rs10490924, the minor allele frequency and Hardy-Weinberg equilibrium was met. For CFH rs1061170, the imputation quality score was >0.8.
Incidence of Early and Late AMD
At visit 3, nonmydriatic retinal film photographs were taken of one randomly chosen eye by using a photograph centered between the disc and fovea. Digital photographs without mydriatics were taken of both eyes (fields 1 [optic nerve] and 2 [macula])28,29 at visit 5 follow-up. Research shows film and digital grading of AMD to be comparable.30 Photographs were graded independently by using the Wisconsin Age-Related Maculopathy Grading System,31 with graders masked to grading from previous visits. Eyes that changed across visits within the three-step ARM severity scale (none, early, late) underwent a side-by-side review to confirm that the change was real and not an error related to image quality/differences or grader (BEKK, RK) error.
Participants were considered to have early AMD if they had any of the following: (1) soft drusen (≥63-μm circle) present with a grid area >500-μm circle or (2) any soft drusen ≥125-μm circle (either distinct of indistinct) present in the grid and any pigmentary abnormality present (increased retinal pigment or depigmentation in the grid) or (3) large (≥125-μm circle) soft indistinct drusen present. Participants were considered to have late AMD if they had any evidence of the following lesions: geographic atrophy, retinal pigment epithelial detachments/retinal detachments, subretinal hemorrhage, subretinal fibrous scar, subretinal new vessels, or history of treatment (laser, photodynamic therapy of intravitreal injections).
Early AMD cases were further subdivided by presence of large, soft drusen (≥1 soft drusen with a diameter ≥125 μm and a grid area >500 μm) or pigmentary abnormalities (presence of increased pigmentation or retinal pigment epithelial depigmentation).
Serum 25-Hydroxyvitamin D
As previously described by Lutsey et al.,32 serum obtained at visit 2, and stored at −80°C until measurement in 2012–2013, has been assessed for concentrations of 25(OH)D, both D2 and D3 forms, with liquid chromatography–tandem-mass spectrometry (Waters Alliance e2795; Waters, Milford, MA, USA) at the University of Minnesota Molecular Epidemiology and Biomarker Research Laboratory (Minneapolis, MN, USA). Quality control measures were applied to minimize laboratory variation across batches of samples sent for analysis. Adjustment of 25(OH)D for season of blood draw was conducted as previously described,33 and adjusted values were used in all subsequent analyses.
Statistical Analysis
Participants were categorized into tertiles of serum 25(OH)D, and clinically used cut points34,35 for exploratory analyses; however, these cut points are for bone health and may not apply to ocular health. We compared characteristics of our participants by tertile of 25(OH)D and AMD. Logistic regression was used to relate the log odds of incident AMD by tertile of 25(OH)D with the lowest tertile as the referent category. P for linear trend was computed by using continuous 25(OH)D concentrations. We assessed addition of potential confounders (age, race, sex, education, income, health insurance, smoking status, drinking status, ethanol intake, body weight, waist circumference, waist to hip ratio, body mass index [BMI], physical activity, measures of fasting serum total cholesterol, serum high density lipoprotein, serum triglycerides, and use of hormone therapy [in females]) to an age-adjusted model in a stepwise fashion with covariates that changed the OR 10% or more considered for inclusion. A priori we decided to adjust for, at minimum, age, race, and smoking status. Blood pressure, hypertension status, blood glucose concentration, and diabetes status were examined as potential pathway (intermediary) variables. In secondary analyses, we examined the association between 25(OH)D and incident early AMD and incident late AMD, as well as large, soft drusen and pigmentary abnormalities among early incident cases.
In exploratory analyses, we stratified our risk estimates by age, sex, and race. Tertiles 2 and 3 were collapsed for analyses within African Americans as no cases of AMD existed in tertile 3. We examined effect modification of our primary association by high-risk AMD genotype. Genetic analyses were limited to Caucasians owing to an inadequate number of cases of AMD in African Americans. We present P values for the interaction terms (e.g., vitamin D * sex) in the logistic regression models, with a P value of <0.10 considered statistically significant.
Because of the concern for potential bias resulting from loss to follow-up and selection at visit 5, we compared characteristics of participants who attended visit 3 and were included to those who attended visit 3 and were excluded from these analyses. We also present results with and without the inclusion of inverse probability weights to account for loss to follow-up as previously done,36 and selection into visit 5, stage 2/3, for retinal photography.37 We applied weights provided by the ARIC Coordinating Center, specifically S2SAMWT51, from the derived dataset for visit 5,37 by using PROC SURVEYLOGISTIC.38 Two participants were missing weights and not included in weighted analyses. The mean weight value was 2.2 with a standard deviation (SD) ± 1.7 in all participants, with 1.9 ± 2.4 (range, 1.0–8.7) in African Americans and 2.3 ± 1.5 (1.1–6.8) in Caucasians. We also compared our primary analysis with and without inclusion of participants with low neurocognitive scores because visit-5 participants with low neurocognitive scores were oversampled for retinal photography. Finally, we conducted a bias analysis where we recategorized some participants considered to not have developed incident AMD to incident outcomes. If a participant's eye was considered to have early or late AMD at visit 5, but not photographed at visit 3, that individual was recategorized as an incident AMD case (n = 33). The primary analysis was repeated by considering these individuals as additional cases of incident AMD.
Results
At visit 3, the 1225 participants were on average 58.6 ± 5 (mean ± SD) years old, 58% female, and 16% African American. Follow-up from visits 3 to 5 spanned a mean of 18 years. At visit 3, there were 19 cases of prevalent, early AMD, with 5 developing incident, late AMD. Of participants with no AMD at visit 3, a total of 113 developed early AMD and 21 developed late AMD by visit 5. The incidence of early AMD was 9% (n = 113 of 1206 at risk) and 2% for late AMD (n = 26 of 1225 at risk). Those who developed incident disease were more likely to be older, Caucasian, current or former smokers, those with greater waist to hip ratios, and those less physically active (Table 1).
Table 1.
Characteristics* by Incident AMD Status and Tertiles of 25(OH)D Concentrations (N = 1225): the ARIC Study
|
Characteristics |
N |
AMD Incidence From Visit 3 to Visit 5 |
Tertiles of 25(OH)D |
R
(P
Value)† |
|||
|
No (n
= 1086) |
Yes (n
= 139) |
Tertile 1 (10.8–53.6) |
Tertile 2 (53.7–69.3) |
Tertile 3 (69.4–173) |
|||
| Season-adjusted serum 25(OH)D, nM, mean (SD) | 1225 | 63 (20.3) | 64 (18.7) | 43 (8.7) | 62 (4.6) | 85 (14.2)‡ | NA |
| Prevalent early AMD at visit 3, n (%) | 19 | 14 (1) | 5 (4)‡ | 5 (1) | 8 (2) | 6 (2) | – |
| Incident AMD, early or late, n (%) | 139 | 0 (0) | 139 (100)‡ | 47 (12) | 48 (12) | 44 (11) | – |
| Demographics | |||||||
| Age, y, mean (SD) | 1225 | 55 (5.0) | 58 (4.9)‡ | 55 (4.9) | 56 (4.9) | 56 (5.3)‡ | 0.07 (0.016) |
| Sex, n (% women) | 711 | 633 (58) | 78 (56) | 274 (67) | 233 (57) | 204 (50)‡ | – |
| Race, n (% Caucasian) | 1029 | 896 (83) | 133 (96)‡ | 272 (67) | 366 (90) | 391 (96)‡ | – |
| Field center, n (%) | – | ||||||
| Forsyth County, NC | 135 | 116 (11) | 19 (14)‡ | 39 (10) | 43 (11) | 53 (13)‡ | |
| Jackson, MS | 185 | 179 (17) | 6 (4) | 127 (31) | 41 (10) | 17 (4) | |
| Minneapolis, MN | 430 | 384 (35) | 46 (33) | 117 (29) | 153 (37) | 160 (39) | |
| Washington County, MD | 475 | 407 (37) | 68 (49) | 125 (30) | 172 (42) | 178 (44) | |
| Education, visit 1§, n (%) | – | ||||||
| Basic or 0 years | 144 | 128 (12) | 16 (12) | 55 (14) | 51 (12) | 38 (9) | |
| Intermediate | 545 | 482 (44) | 63 (45) | 168 (41) | 178 (44) | 199 (49) | |
| Advanced | 536 | 476 (44) | 60 (43) | 185 (45) | 180 (44) | 171 (42) | |
| Health and lifestyle characteristics | |||||||
| Health insurance, n (% yes) | 1161 | 1031 (95) | 130 (94) | 377 (92) | 393 (96) | 391 (96)‡ | – |
| Smoking status, n (%) | – | ||||||
| Current | 178 | 160 (15) | 18 (13)‡ | 67 (16) | 54 (13) | 57 (14)‡ | |
| Former | 463 | 396 (36) | 67 (48) | 123 (30) | 154 (38) | 186 (46) | |
| Never | 584 | 530 (49) | 54 (39) | 218 (54) | 201 (49) | 165 (40) | |
| Drinking status, n (%) | – | ||||||
| Current | 814 | 716 (66) | 98 (71) | 239 (59) | 280 (68) | 295 (72)‡ | |
| Former | 178 | 157 (14) | 21 (15) | 65 (16) | 52 (13) | 61 (15) | |
| Never | 233 | 213 (20) | 20 (14) | 104 (25) | 77 (19) | 52 (13) | |
| Waist circumference, cm, mean (SD) | 1225 | 96 (13.7) | 98 (11.8) | 99 (15.4) | 97 (12.4) | 93 (12.0)‡ | −0.14 (<0.001) |
| Waist to hip ratio, mean (SD) | 1225 | 0.91 (0.08) | 0.93 (0.07)‡ | 0.91 (0.08) | 0.92 (0.08) | 0.91 (0.08)‡ | −0.01 (0.713) |
| BMI category, kg/m2, n (%) | −0.20 (<0.001) | ||||||
| Under/normal weight (<25 kg/m2) | 378 | 341 (31) | 37 (27) | 108 (27) | 108 (26) | 162 (40)‡ | |
| Overweight (25–30 kg/m2) | 536 | 468 (43) | 68 (49) | 164 (40) | 192 (47) | 180 (44) | |
| Obese (≥30 kg/m2) | 311 | 277 (26) | 34 (24) | 136 (33) | 109 (27) | 66 (16) | |
| Physical activity index visits 1 and 3, mean (SD) | 1179 | 3.1 (1.3) | 2.8 (1.4)‡ | 2.8 (1.3) | 3.0 (1.3) | 3.3 (1.2)‡ | 0.15 (<0.001) |
| Diastolic blood pressure, mm Hg, mean (SD) | 1225 | 72 (9.5) | 72 (9.4) | 72 (9.3) | 72 (9.7) | 72 (9.6) | 0.004 (0.887) |
| Systolic blood pressure, mm Hg, mean (SD) | 1225 | 118 (16.1) | 118 (14.7) | 118 (16.0) | 118 (15.0) | 118 (16.9) | −0.02 (0.497) |
| Hypertension, n (% yes)‖ | 305 | 273 (25) | 32 (23) | 118 (29) | 101 (25) | 86 (21)‡ | – |
| Total cholesterol, mg/dL, mean (SD) | 1222 | 208 (37.4) | 212 (38.1) | 208 (37.5) | 212 (40.3) | 206 (34.2) | 0.01 (0.780) |
| HDL, mg/dL, mean (SD) | 1218 | 51 (16.6) | 50 (14.8) | 51 (15.9) | 50 (16.1) | 51 (17.1) | −0.01 (0.786) |
| LDL, mg/dL, mean (SD) | 1198 | 131 (33.9) | 136 (35.1) | 132 (34.7) | 134 (36.0) | 129 (31.1) | −0.02 (0.511) |
| Triglycerides, mg/dL, mean (SD) | 1222 | 133 (84.2) | 132 (61.0) | 125 (76.5) | 140 (91.2) | 132 (76.6)‡ | 0.07 (0.019) |
| Glucose, mg/dL, mean (SD) | 1225 | 106 (27.0) | 104 (15.5) | 107 (28.4) | 107 (31.9) | 102 (13.8)‡ | −0.12 (<0.001) |
| Hormone use, among females, n (%) | – | ||||||
| Current estrogen user | 132 | 120 (22) | 12 (18) | 47 (20) | 33 (17) | 52 (28)‡ | |
| Current estrogen and progestin user | 93 | 82 (15) | 11 (16) | 23 (10) | 27 (14) | 43 (24) | |
| Never used hormones | 363 | 322 (59) | 41 (60) | 161 (67) | 125 (64) | 77 (42) | |
| Former hormone user | 29 | 25 (4) | 4 (6) | 9 (3) | 9 (5) | 11 (6) | |
Bolded values represent statistically significant results at a P value of <0.05 or smaller. NA, not applicable.
Characteristics assessed at visit 2 unless otherwise noted.
Spearman correlation coefficient and associated P value for the correlation between season-adjusted serum 25(OH)D and the respective continuous variable. Values are not shown (–) for categorical variables.
P value < 0.05 For continuous variables, t-tests or ANOVAs were used to compare means of characteristics between those with and without any incident AMD or across tertiles of 25(OH)D, respectively. For categorical variables, χ2 tests were used to compare proportions of characteristics between those with and without any incident AMD or across tertiles of 25(OH)D.
Education defined as basic or 0 years (≤11 years or less, i.e., high school with no degree or less), intermediate (12–16 years, i.e., high-school graduate or vocational school), or advanced (17–21 years, i.e., college or higher).
Average systolic blood pressure ≥140 mm Hg, or diastolic ≥90 mm Hg, or high blood pressure medication use in the past 2 weeks.
Characteristics of individuals with high (tertile 3) compared to low (tertile 1) 25(OH)D were more likely to be older, men, Caucasian, those with health insurance, former smokers, current drinkers, those with smaller waist circumferences and BMIs, those more physically active, those with less hypertension, higher triglycerides, lower blood glucose, and those more likely to be current hormone therapy users (Table 1). Individuals excluded, as compared to included, for this analysis had lower 25(OH)D and did not differ by prevalence of any AMD at baseline33 (Supplementary Table S1). Participants excluded were older, more likely to be men, African American, hypertensive, to have greater waist circumferences, waist to hip ratios, BMI, systolic blood pressure, LDL, and blood glucose. They were less likely to have advanced education, health insurance, and to have never smoked, drank, or taken hormones; and their HDL was lower.
Only adjustment for race and the physical activity index changed the age-adjusted OR for any incident AMD among those in tertile 3 compared to tertile 1 by 10% or more. Therefore, model 1 is adjusted for covariates chosen a priori (age, race, and smoking status). There was a significant decreased odds of any incident AMD (early and late combined) in tertile 3 compared to 1 for 25(OH)D, with a P trend = 0.11 (Table 2). After adjustment for sampling weights, this OR was no longer statistically significant. Further adjustment for physical activity (model 2) attenuated the ORs for tertile 3 compared to 1 and the P trend, but did not remove the statistical significance of the third tertile OR until after weights were applied. Further adjustment for sex and prevalent, early AMD at visit 3, in addition to age, race, and smoking status, also did not substantially change these results (unweighted OR = 0.58 [0.36–0.92], P trend = 0.14 and weighted OR = 0.64 [0.36–1.13], P trend = 0.28). When clinical cut points of 25(OH)D were used, an inverse, but not statistically significant, OR for those with 25(OH)D ≥75 compared to <50 nM was observed in unweighted, but not weighted, analyses.
Table 2.
ORs and 95% CIs for AMD Incidence by 25(OH)D Concentrations Defined by Using Both Tertiles and Clinical Outpoints of 25(OH)D (N = 1225): the ARIC Study
| Model |
Tertiles of 25(OH)D, nM, Range |
P
Value for Trend* |
||
|
Tertile 1 (10.8–53.6) |
Tertile 2 (53.7–69.3) |
Tertile 3 (69.4–173.0) |
||
| No. outcome/total No. | 47/408 | 48/409 | 44/408 | |
| Age-adjusted | 1 | 0.92 (0.59–1.42) | 0.83 (0.53–1.30) | 0.96 |
| Weighted† | 1 | 1.11 (0.65–1.90) | 0.89 (0.51–1.54) | 0.88 |
| Model 1‡ | 1 | 0.70 (0.45–1.10) | 0.57 (0.36–0.90) | 0.11 |
| Weighted | 1 | 0.88 (0.53–1.48) | 0.66 (0.38–1.16) | 0.32 |
| Model 2§ | 1 | 0.79 (0.50–1.26) | 0.61 (0.38–0.99) | 0.37 |
| Weighted | 1 | 0.97 (0.57–1.64) | 0.73 (0.41–1.31) | 0.63 |
| Model |
Clinical Cutpoints Defined by 25(OH)D, nM |
P
Value for Trend* |
||
|
<50 Deficient/Inadequate |
50 to <75 Adequate |
≥75 Adequate |
||
| No. outcome/total No. | 33/316 | 69/599 | 37/310 | |
| Age-adjusted | 1 | 1.02 (0.65–1.60) | 1.04 (0.62–1.72) | 0.96 |
| Weighted | 1 | 1.52 (0.87–2.66) | 1.33 (0.71–2.50) | 0.88 |
| Model 1‡ | 1 | 0.75 (0.47–1.19) | 0.68 (0.40–1.15) | 0.11 |
| Weighted | 1 | 1.20 (0.61–2.34) | 0.99 (0.48–2.05) | 0.32 |
| Model 2§ | 1 | 0.87 (0.54–1.42) | 0.79 (0.45–1.38) | 0.37 |
| Weighted | 1 | 1.38 (0.69–2.77) | 1.17 (0.56–2.47) | 0.63 |
Bolded values represent statistically significant results at a P value of < 0.05 or smaller.
P for trend calculated by using season-adjusted serum 25(OH)D as a continuous variable.
Inverse probability weights applied; n = 2 individuals missing weights.
Model 1: adjusted for age, race, and smoking status.
Model 2: adjusted for age, race, and smoking status and composite physical activity index averaging visits 1 and 3 data (n = 46 participants were missing this variable).
The inverse association was stronger in those >55 years and women, but no statistically significant age or sex interactions were observed (Table 3). Results in Caucasians paralleled findings in the overall sample. Findings in African Americans showed an inverse association. However, the P trend was only significant in weighted analyses and appeared to be driven by one case with a high weight value. No significant interaction was observed with the CFH or ARMS2 genotypes.
Table 3.
Adjusted ORs and 95% CIs for Incident AMD From Visit 3 (1993–1995) to Visit 5 (2011–2013) by Tertiles of 25(OH)D Stratified by Race, Age, Sex, Smoking Status, and BMI: ARIC Study Participants With Gradable Eye Photos at Visit 3 and Visit 5 and Available Serum 25(OH)D at Visit 2 (1990–1992) (N = 1225)
| Model |
Tertiles of 25(OH)D, nM, Range |
P
Trend* |
||
|
Tertile 1 (10.8–53.6) |
Tertile 2 (53.7–69.3) |
Tertile 3 (69.4–173.0) |
||
| Age group, y | ||||
| ≤ Median of 55 years, n = 642 | ||||
| No. with outcome/No. total | 10/223 | 14/199 | 16/220 | |
| Model 1† | 1 | 1.20 (0.50–2.86) | 1.17 (0.50–2.77) | 0.85 |
| Weighted‡ | 1 | 1.36 (0.52–3.55) | 1.31 (0.49–3.47) | 0.62 |
| > Median of 55 years, n = 583 | ||||
| No. with outcome/No. total | 37/185 | 34/210 | 28/188 | |
| Model 1† | 1 | 0.56 (0.33–0.97) | 0.42 (0.24–0.75) | 0.10 |
| Weighted‡ | 1 | 0.70 (0.37–1.31) | 0.44 (0.22–0.89) | 0.12 |
| P for interaction§ = 0.74 (weighted‡ = 0.93) | ||||
| Sex | ||||
| Men, n = 514 | ||||
| No. with outcome/No. total | 13/134 | 22/176 | 26/204 | |
| Model 1† | 1 | 1.04 (0.49–2.22) | 0.90 (0.43–1.91) | 0.70 |
| Weighted‡ | 1 | 1.16 (0.51–2.66) | 0.75 (0.32–1.76) | 0.94 |
| Women, n = 711 | ||||
| No. with outcome/No. total | 34/274 | 26/233 | 18/204 | |
| Model 1† | 1 | 0.55 (0.31–0.99) | 0.43 (0.23–0.81) | 0.03 |
| Weighted‡ | 1 | 0.65 (0.33–1.30) | 0.60 (0.28–1.29) | 0.18 |
| P for interaction§ = 0.14 (weighted‡ = 0.82) | ||||
| Race | ||||
| Caucasians, n = 1029 | ||||
| No. outcome/total No. | 42/272 | 47/366 | 44/391 | |
| Model 1† | 1 | 0.71 (0.45–1.13) | 0.57 (0.36–0.92) | 0.11 |
| Weighted ‡ | 1 | 0.90 (0.53–1.53) | 0.66 (0.37–1.19) | 0.21 |
| African Americans, n = 196‖ | ||||
| Combining tertiles 2 and 3 | Tertile 1 | Tertile 2/3 | ||
| No. outcome/total No. | 5/136 | 1/60 | – | |
| Model 1† | 1 | 0.45 (0.05–3.95) | – | 0.93 |
| Weighted‡ | 1 | 0.53 (0.05–5.31) | – | 0.02 |
| P for interaction§ = 0.99 (weighted‡ = 0.006) | ||||
| CFH genotype, rs1061170 | ||||
| TT, no high-risk alleles, n = 348 | ||||
| No. with outcome/No. total | 12/81 | 10/129 | 10/138 | |
| Model 1† | 1 | 0.45 (0.18–1.12) | 0.39 (0.16–0.98) | 0.32 |
| Weighted‡ | 1 | 0.53 (0.18–1.54) | 0.44 (0.15–1.29) | 0.41 |
| CT, 1 high-risk allele, n = 402 | ||||
| No. with outcome/No. total | 15/111 | 19/129 | 20/162 | |
| Model 1† | 1 | 0.93 (0.43–1.99) | 0.71 (0.33–1.52) | 0.31 |
| Weighted‡ | 1 | 0.95 (0.40–2.30) | 0.83 (0.32–2.17) | 0.67 |
| CC, 2 high-risk alleles, n = 127 | ||||
| No. with outcome/No. total | 9/34 | 12/51 | 11/42 | |
| Model 1† | 1 | 0.94 (0.33–2.72) | 0.91 (0.31–2.71) | 0.70 |
| Weighted‡ | 1 | 1.46 (0.45–4.66) | 0.96 (0.25–3.74) | 0.72 |
| CC/CT, 1–2 high-risk alleles, n = 529 | ||||
| No. with outcome/No. total | 24/145 | 31/180 | 31/204 | |
| Model 1† | 1 | 0.94 (0.51–1.73) | 0.74 (0.40–1.36) | 0.24 |
| Weighted‡ | 1 | 1.12 (0.55–2.26) | 0.82 (0.38–1.78) | 0.50 |
| P for interaction§ = 0.61 (weighted‡ = 0.58) | ||||
| ARMS2 genotype, rs10490924 | ||||
| GG, no high-risk alleles, n = 539 | ||||
| No. with outcome/No. total | 17/137 | 20/197 | 21/205 | |
| Model 1† | 1 | 0.71 (0.35–1.46) | 0.65 (0.32–1.33) | 0.66 |
| Weighted‡ | 1 | 0.86 (0.39–1.91) | 0.74 (0.33–1.68) | 0.92 |
| TG, 1 high-risk allele, n = 292 | ||||
| No. with outcome/No. total | 13/70 | 18/102 | 19/120 | |
| Model 1† | 1 | 0.84 (0.37–1.88) | 0.69 (0.31–1.53) | 0.36 |
| Weighted‡ | 1 | 1.05 (0.41–2.73) | 0.85 (0.31–2.33) | 0.51 |
| TT, 2 high-risk alleles, n = 46 | ||||
| No. with outcome/No. total | 6/19 | 3/10 | 1/17 | |
| Model 1† | 1 | 0.45 (0.04–4.58) | 0.07 (0.004–1.21) | 0.045 |
| Weighted‡ | 1 | 0.46 (0.05–4.39) | 0.03 (0.001–0.92) | 0.03 |
| TT/TG, 1–2 high-risk allele, n = 338 | ||||
| No. with outcome/No. total | 19/89 | 21/112 | 20/137 | |
| Model 1† | 1 | 0.75 (0.36–1.53) | 0.52 (0.25–1.07) | 0.06 |
| Weighted‡ | 1 | 0.86 (0.37–1.99) | 0.60 (0.24–01.50) | 0.15 |
| P for interaction§ = 0.14 (weighted‡ = 0.14) | ||||
Bolded values represent statistically significant results at a P value of < 0.05 or smaller.
P for trend calculated by using season-adjusted serum 25(OH)D as a continuous variable.
Model 1: adjusted for age, race, and smoking status.
Inverse probability weights applied to adjust for bias due to loss to follow-up from visit 3 to 5; n = 2 participants missing weights.
Multiplicative interactions were tested by using continuous measures of 25(OH)D, age, and ordinal measures of genotype variables.
Results for African Americans are unstable owing to low sample size. Not including one individual with incident AMD and the highest sample weight among incident cases removes the statistically significant P trend in the weighted analysis.
There was a statistically significant decreased odds of incident early AMD and large, soft drusen among incident cases of early AMD in tertile 3 compared to 1, but the significance of these findings was removed after weights were applied (Table 4). No statistically significant associations were observed with pigmentary abnormalities and incident late AMD.
Table 4.
ORs and 95% CIs for Incident AMD (Early, Soft Drusen, Pigmentary Abnormalities) and Incident Late AMD by Tertiles of 25(OH)D (n = 1225): the ARIC Study
| Model |
Tertiles of 25(OH)D, nM, Range |
P
Trend* |
||
|
Tertile 1 (10.8–53.6) |
Tertile 2 (53.7–69.3) |
Tertile 3 (69.4–173.0) |
||
| Incident, early AMD, n = 1185 | ||||
| No. outcome/total No. | 39/397 | 37/392 | 37/396 | |
| Model 1† | 1 | 0.66 (0.40–1.09) | 0.58 (0.35–0.96) | 0.22 |
| Weighted‡ | 1 | 0.84 (0.47–1.48) | 0.62 (0.34–1.14) | 0.36 |
| Large, soft drusen§, n = 1165 | ||||
| No. outcome/total No. | 28/392 | 28/387 | 24/386 | |
| Model 1 | 1 | 0.69 (0.39–1.20) | 0.52 (0.28–0.93) | 0.18 |
| Weighted | 1 | 0.79 (0.41–1.51) | 0.54 (0.27–1.09) | 0.39 |
| Large, soft drusen§, n = 1139 – further excluding those with both large, soft drusen and pigmentary abnormalities | ||||
| No. outcome/total No. | 19/383 | 18/377 | 17/379 | |
| Model 1 | 1 | 0.68 (0.35–1.35) | 0.58 (0.29–1.17) | 0.48 |
| Weighted | 1 | 0.79 (0.36–1.73) | 0.58 (0.25–1.32) | 0.63 |
| Pigmentary abnormalities§, n = 1131 | ||||
| No. outcome/total No. | 14/378 | 15/374 | 17/379 | |
| Model 1 | 1 | 0.73 (0.34–1.56) | 0.68 (0.32–1.45) | 0.49 |
| Weighted | 1 | 1.29 (0.56–2.99) | 0.99 (0.40–2.44) | 0.72 |
| Incident late AMD, n = 1225 | ||||
| No. outcome/total No. | 8/408 | 11/409 | 7/408 | |
| Model 1‖ | 1 | 1.21 (0.47–3.07) | 0.72 (0.25–2.05) | 0.54 |
| Weighted | 1 | 1.62 (0.57–4.59) | 1.27 (0.34–4.84) | 0.96 |
Bolded values represent statistically significant results at a P value of < 0.05 or smaller.
P for trend calculated by using season-adjusted serum 25(OH)D as a continuous variable.
Model 1: adjusted for age, race, and smoking status.
Inverse probability weights applied; n = 2 participants missing weights.
Large, soft drusen and pigmentary abnormalities were identified among cases of early incident AMD only. In analyses with large, soft drusen, cases of pigmentary abnormalities and no large, soft drusen were excluded. In analyses with pigmentary abnormalities, cases with large, soft drusen and no pigmentary abnormalities were excluded.
‖ Model 1 for incident late AMD adjusted only for age and smoking status. The model stability did not hold when adjustment for race was applied.
Analyses restricted to those with dementia or cognitive impairment showed similar results to those with normal cognitive functioning. The OR (95% CI), adjusted for age, race, and smoking status, for tertile 3 versus 1 was 0.55 (0.30–1.01), P trend = 0.23 and 0.59 (0.28–1.24), P trend = 0.31, respectively. Our bias analysis showed similar results to our primary findings. When participants' case status was recategorized on the basis of data from both eye photos at visit 5, the OR (95% CI) for any incident AMD in tertile 3 compared to 1 was 0.67 (0.45–1.06) and 0.79 (0.47–1.33) in unweighted and weighted analyses, respectively, with P for trends of 0.31 and 0.32.
Discussion
In this longitudinal study, we observed a statistically significant decreased odds of incident AMD among participants with high compared to low 25(OH)D concentrations during 18 years of follow-up; however, the P for trend for these analyses was not statistically significant. Inverse associations were also observed for incident early AMD and large, soft drusen among early incident cases, but not for pigmentary abnormalities or late AMD. Analyses showed no protective association between 25(OH)D and late AMD or pigmentary abnormalities; however, there were few events for either outcome and thus the risk estimates had poorer precision than examining any early AMD as a whole. This article contributes to the literature by providing a longitudinal analysis with assessment of vitamin D status before assessment of AMD incidence. Unlike the previous longitudinal studies15,16 published, our study had a biomarker for vitamin D status, 25(OH)D, that reflects vitamin D exposure from all sources (diet, supplements, and sunlight) and uses graded retinal photographs to determine disease incidence and progression.
Only two other prospective studies15,16 have examined the association between vitamin D status and incident AMD. Day et al.15 have observed no association between vitamin D status, assessed from medical chart claims, and incident AMD determined from medical record codes. These results are consistent by stage of AMD (nonneovascular and neovascular); however, unlike our study, subtype of AMD (soft drusen or pigmentary abnormalities) was not investigated. Potential exposure and outcome misclassification may explain, in part, these null findings. The second study focuses on the association between vitamin D intake from foods and supplements with the 9.4-year development of advanced AMD among participants with early or intermediate AMD.16 Although, Merle et al.16 have found a protective association between dietary vitamin D intake and incident advanced AMD, these findings are not evident with intake from dietary plus supplemental sources of vitamin D, and no data are available on 25(OH)D in blood, allowing for the contribution of vitamin D from sunlight to be considered in the analysis.
Only a few studies1,2,4,33 have specifically examined associations between vitamin D and measures of soft drusen. We previously have observed no evidence of a cross-sectional association between 25(OH)D and prevalent early AMD or prevalent soft drusen at ARIC visit 3.33 The definition for soft drusen used in our previous analysis of visit 3 ARIC data differs from the definition used to define large, soft drusen in our current study (at least one soft drusen with a diameter ≥125 μm and a grid area >500 μm). The previous grading of visit-3 retinal photographs only identifies drusen larger than ≥63 μm and does not differentiate size beyond this threshold. Differently, Parekh et al.1 have found a statistically significant lower risk of soft drusen with low 25(OH)D in a nationally representative sample, using a similar grading definition for soft drusen (at least one or more drusen >63 μm). Seddon et al.4 also have found a statistically significant inverse association between dietary intake of vitamin D and drusen size in a study of twins with discordant AMD, and Millen et al.2 have found an inverse but not statistically significant association with 25(OH)D and soft drusen in a cohort of postmenopausal women. It is possible that vitamin D mitigates development of drusen through its anti-inflammatory properties.
It is also possible that 25(OH)D concentrations are a marker for a healthy lifestyle and that residual confounding exits from such covariates as physical activity. We observed that the protective association of vitamin D on incident AMD remained in unweighted analyses adjusted for physical activity, but was removed after weights were applied. It is also possible that physical activity is a proxy measure for sunlight exposure and its inclusion in the model resulted in overadjustment.39 We were limited by not having a physical activity measurement at the same point in time as when 25(OH)D was measured. Further, the extent to which physical activity is a known risk factor for AMD is still debated.40
Our results were consistent by race when comparing tertiles 3 and 1 for Caucasians and tertiles 3 and 2 combined to tertile 1 for African Americans. The availability of only six cases of AMD among African Americans limited our ability to estimate stable ORs for this subgroup and thus the results should be interpreted with caution.
In exploratory analyses, we did not observe effect modification by either high-risk AMD SNP. Notably, we did not see evidence of a stronger protective association in those with the CC genotype, like previous findings,41 and the sample size of this study was too small to draw finite conclusions with respect to environment gene interactions. Interactions by CFH genotype do vary by single epidemiologic study (e.g., Assel et al.,42 Ho et al.43), and this likely reflects the instability of such interactions in single samples. Larger, pooled studies will be needed to answer questions regarding effect modification by race and genotype adequately.
Limitations of our data included the availability of retinal photographs in only one eye at visit 3, resulting in the potential for misclassified incident disease (false negatives). Our bias analysis suggests that inclusion of possible misclassified cases would have led to slightly attenuated risk estimates. It is possible our findings were also biased by loss to follow-up or death between visit 3 and visit 5, or selection to have retinal photographs taken at visit 5. Even though application of sampling weights attenuated the point estimates in most instances, the attenuation was not extreme. The tradeoff for correcting for bias with sampling weights was the widening of our CIs. At this time, we cannot determine if a larger sample size with greater case number would lead to significant point estimates by increasing our precision and thus tightening our CIs.
Besides sun exposure, supplemental sources of vitamin D are one of the strongest determinants of circulating 25(OH)D concentrations44; however, the Age-Related Eye Disease Study (AREDS) supplement formulation does not contain vitamin D.45 To help fill this current gap in knowledge, the ongoing Vitamin D and Omega-3 Trial (VITAL)46 has an ancillary study to examine the influence of vitamin D and omega-3 fatty acid supplementation on AMD incidence and progression, which will provide valuable information to this line of inquiry. Supportive findings of a protective effect of vitamin D supplementation on AMD should provide evidence of a causal role of vitamin D deficiency in AMD risk, but null findings could be explained by the duration of the trial (on average 5 years of follow-up), dose of the vitamin D supplement used, or the starting nutrient status of study participants.47 Blood measures for post hoc stratification analyses are only available in ∼65% of the trial participants. Further, VITAL was not originally designed to study AMD and is relying on medical record–confirmed AMD, and also subject to possible false negatives in AMD outcome misclassification.
Additional analyses in other, larger, prospective studies are needed to better understand whether having adequate vitamin D status could be yet another lifestyle factor associated with reduced risk of AMD. Of course, randomized clinical trials will be needed to determine causality. In conclusion, we observed a protective association between 25(OH)D and risk for incident AMD, primarily early AMD. These findings suggest that one's previous 25(OH)D concentrations may influence the early pathology of AMD development but need confirmation in order to better understand the implication of a person's vitamin D status to clinical ophthalmology and public health.
Supplementary Material
Acknowledgments
This work was previously presented as a poster at the Experimental Biology Annual Meeting, San Diego, California, United States, April 2–6, 2016.
The authors thank the staff and participants of the ARIC Study for their important contributions. Infrastructure was partly supported by Grant No. UL1RR025005, a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research (Bethesda, MD, USA).
Supported by the NIH National Institute on Aging Grant No. R01 AG041776, NIH National Heart, Lung, and Blood Institute (NHLBI) Grant No. R01 HL103706, and the NIH Office of Dietary Supplements Grant No. R01 HL103706-S1 and an unrestricted grant from Research to Prevent Blindness. The Atherosclerosis Risk in Communities Study has been funded in whole or in part with federal funds from the NHLBI contracts (contract Nos. HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700004I, and HHSN268201700005I), R01HL087641, R01HL086694; National Human Genome Research Institute contract U01HG004402; and NIH contract HHSN268200625226C. Infrastructure was partly supported by Grant Number UL1RR025005, a component of the National Institutes of Health and NIH Roadmap for Medical Research. Neurocognitive data are collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917 from the NIH (NHLBI, National Institute of Neurological Disorders and Stroke [NINDS], National Institute on Aging [NIA], National Institute on Deafness and Other Communication Disorders [NIDCD]), and with previous brain MRI examinations funded by R01-HL70825 from the NHLBI.
Disclosure: A.E. Millen, None; J. Nie, None; J.A. Mares, None; P.L. Lutsey, None; M.J. LaMonte, None; S.M. Meuer, None; M.W. Sahli, None; C.A. Andrews, None; B.E.K. Klein, None; R. Klein, None
References
- 1.Parekh N, Chappell RJ, Millen AE, Albert DM, Mares JA. Association between vitamin D and age-related macular degeneration in the Third National Health and Nutrition Examination Survey, 1988 through 1994. Arch Ophthalmol. 2007;125:661–669. doi: 10.1001/archopht.125.5.661. [DOI] [PubMed] [Google Scholar]
- 2.Millen AE, Voland R, Sondel SA, et al. Vitamin D status and early age-related macular degeneration in postmenopausal women. Arch Ophthalmol. 2011;129:481–489. doi: 10.1001/archophthalmol.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Golan S, Shalev V, Treister G, Chodick G, Loewenstein A. Reconsidering the connection between vitamin D levels and age-related macular degeneration. Eye (Lond) 2011;25:1122–1129. doi: 10.1038/eye.2011.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Seddon JM, Reynolds R, Shah HR, Rosner B. Smoking, dietary betaine, methionine, and vitamin D in monozygotic twins with discordant macular degeneration: epigenetic implications. Ophthalmology. 2011;118:1386–1394. doi: 10.1016/j.ophtha.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrison MA, Silveira AC, Huynh N, et al. Systems biology-based analysis implicates a novel role for vitamin D metabolism in the pathogenesis of age-related macular degeneration. Hum Genomics. 2011;5:538–568. doi: 10.1186/1479-7364-5-6-538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Graffe A, Milea D, Annweiler C, et al. Association between hypovitaminosis D and late stages of age-related macular degeneration: a case-control study. J Am Geriatr Soc. 2012;60:1367–1369. doi: 10.1111/j.1532-5415.2012.04015.x. [DOI] [PubMed] [Google Scholar]
- 7.Singh A, Falk MK, Subhi Y, Sorensen TL. The association between plasma 25-hydroxyvitamin D and subgroups in age-related macular degeneration: a cross-sectional study. PLoS One. 2013;8:e70948. doi: 10.1371/journal.pone.0070948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Itty S, Day S, Lyles KW, Stinnett SS, Vajzovic LM, Mruthyunjaya P. Vitamin D deficiency in neovascular versus nonneovascular age-related macular degeneration. Retina. 2014;34:1779–1786. doi: 10.1097/IAE.0000000000000178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim EC, Han K, Jee D. Inverse relationship between high blood 25-hydroxyvitamin D and late stage of age-related macular degeneration in a representative Korean population. Invest Ophthalmol Vis Sci. 2014;55:4823–4831. doi: 10.1167/iovs.14-14763. [DOI] [PubMed] [Google Scholar]
- 10.Cougnard-Gregoire A, Merle BM, Korobelnik JF, et al. Vitamin D deficiency in community-dwelling elderly is not associated with age-related macular degeneration. J Nutr. 2015;145:1865–1872. doi: 10.3945/jn.115.214387. [DOI] [PubMed] [Google Scholar]
- 11.Aoki A, Inoue M, Nguyen E, et al. Dietary n-3 fatty acid, alpha-tocopherol, zinc, vitamin D, vitamin C, and beta-carotene are associated with age-related macular degeneration in Japan. Sci Rep. 2016;6:20723. doi: 10.1038/srep20723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McKay GJ, Young IS, McGinty A, et al. Associations between serum vitamin D and genetic variants in vitamin D pathways and age-related macular degeneration in the European Eye Study. Ophthalmology. 2016;124:90–96. doi: 10.1016/j.ophtha.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Reed-Jones RJ, Solis GR, Lawson KA, Loya AM, Cude-Islas D, Berger CS. Vision and falls: a multidisciplinary review of the contributions of visual impairment to falls among older adults. Maturitas. 2013;75:22–28. doi: 10.1016/j.maturitas.2013.01.019. [DOI] [PubMed] [Google Scholar]
- 14.West CG, Gildengorin G, Haegerstrom-Portnoy G, Schneck ME, Lott L, Brabyn JA. Is vision function related to physical functional ability in older adults? J Am Geriatr Soc. 2002;50:136–145. doi: 10.1046/j.1532-5415.2002.50019.x. [DOI] [PubMed] [Google Scholar]
- 15.Day S, Acquah K, Platt A, Lee PP, Mruthyunjaya P, Sloan FA. Association of vitamin D deficiency and age-related macular degeneration in medicare beneficiaries. Arch Ophthalmol. 2012;130:1070–1071. doi: 10.1001/archophthalmol.2012.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Merle BMJ, Silver RE, Rosner B, Seddon JM. Associations between vitamin D intake and progression to incident advanced age-related macular degeneration. Invest Ophthalmol Vis Sci. 2017;58:4569–4578. doi: 10.1167/iovs.17-21673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ganji V, Zhang X, Tangpricha V. Serum 25-hydroxyvitamin D concentrations and prevalence estimates of hypovitaminosis D in the U.S. population based on assay-adjusted data. J Nutr. 2012;142:498–507. doi: 10.3945/jn.111.151977. [DOI] [PubMed] [Google Scholar]
- 18.The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives—the ARIC investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 19.Description of ARIC Visit 5 NCS Stage 1. The Atherosclerosis Risk in Communities Study. 2017 Available at: https://www2.cscc.unc.edu/aric/system/files/Description%20of%20V5%20stage%201%20140304.pdf Accessed June 1.
- 20.Prepared by the ARIC Carotid MRI Study Investigators; 2005. 2017. Atherosclerosis Risk in Communities (ARIC) Carotid MRI Study. Manual 1: Description and Study Management. Version 1.1. Available at: http://www.cscc.unc.edu/aric/carotid/manuals/Description_and_Study_Management.1_1.pdf Accessed March 20. [Google Scholar]
- 21.Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS) Alzheimers Dement (Amst) 2016;2:1–11. doi: 10.1016/j.dadm.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atherosclerosis Risk in Communities (ARIC) Study Research Group. Manual 7: Blood Collection. 10-15. Chapel Hill, NC: Atherosclerosis Risk in Communities (ARIC) Study Research Group; 1988. pp. 16–24. [Google Scholar]
- 23.Pols MA, Peeters PH, Bueno-De-Mesquita HB, et al. Validity and repeatability of a modified Baecke questionnaire on physical activity. Int J Epidemiol. 1995;24:381–388. doi: 10.1093/ije/24.2.381. [DOI] [PubMed] [Google Scholar]
- 24.Richardson MT, Ainsworth BE, Wu HC, Jacobs DR, Jr, Leon AS. Ability of the Atherosclerosis Risk in Communities (ARIC)/Baecke Questionnaire to assess leisure-time physical activity. Int J Epidemiol. 1995;24:685–693. doi: 10.1093/ije/24.4.685. [DOI] [PubMed] [Google Scholar]
- 25.Baecke JA, Burema J, Frijters JE. A short questionnaire for the measurement of habitual physical activity in epidemiological studies. Am J Clin Nutr. 1982;36:936–942. doi: 10.1093/ajcn/36.5.936. [DOI] [PubMed] [Google Scholar]
- 26.Holliday EG, Smith AV, Cornes BK, et al. Insights into the genetic architecture of early stage age-related macular degeneration: a genome-wide association study meta-analysis. PLoS One. 2013;8:e53830. doi: 10.1371/journal.pone.0053830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Psaty BM, O'Donnell CJ, Gudnason V, et al. Cohorts for Heart and Aging Research in Genomic Epidemiology (CHARGE) Consortium: design of prospective meta-analyses of genome-wide association studies from 5 cohorts. Circ Cardiovasc Genet. 2009;2:73–80. doi: 10.1161/CIRCGENETICS.108.829747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified Airlie House classification. ETDRS report number 10: Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1991;98(5 suppl):786–806. [PubMed] [Google Scholar]
- 29.University of Wisconsin-Madison, Ocular Epidemiology Reading Center; 2018. ARIC Neurocognitive Study. Retinal Imaging Protocol. February 11, 2011. Available at: https://www2.cscc.unc.edu/aric/sites/default/files/public/manuals/3A%20Imaging.pdf Accessed September 10. [Google Scholar]
- 30.Klein R, Meuer SM, Moss SE, Klein BE, Neider MW, Reinke J. Detection of age-related macular degeneration using a nonmydriatic digital camera and a standard film fundus camera. Arch Ophthalmol. 2004;122:1642–1646. doi: 10.1001/archopht.122.11.1642. [DOI] [PubMed] [Google Scholar]
- 31.Klein R, Davis MD, Magli YL, Segal P, Klein BE, Hubbard L. The Wisconsin age-related maculopathy grading system. Ophthalmology. 1991;98:1128–1134. doi: 10.1016/s0161-6420(91)32186-9. [DOI] [PubMed] [Google Scholar]
- 32.Lutsey PL, Eckfeldt JH, Ogagarue ER, Folsom AR, Michos ED, Gross M. The 25-hydroxyvitamin D3 C-3 epimer: distribution, correlates, and reclassification of 25-hydroxyvitamin D status in the population-based Atherosclerosis Risk in Communities Study (ARIC) Clin Chim Acta. 2015;442:75–81. doi: 10.1016/j.cca.2014.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Millen AE, Nie J, Sahli MW, et al. Vitamin D status and prevalent early age-related macular degeneration in African Americans and Caucasians: the Atherosclerosis Risk in Communities Study. J Nutr Health Aging. 2017;21:772–780. doi: 10.1007/s12603-016-0827-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.IOM (Institute of Medicine) Dietary Reference Intakes for Calcium and Vitamin D. Washington, DC: The National Academy Press;; 2011. Summary; pp. 1–14. [Google Scholar]
- 35.Cosman F, de Beur SJ, LeBoff MS, et al. Clinician's Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int. 2014;25:2359–2381. doi: 10.1007/s00198-014-2794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gottesman RF, Rawlings AM, Sharrett AR, et al. Impact of differential attrition on the association of education with cognitive change over 20 years of follow-up: the ARIC neurocognitive study. Am J Epidemiol. 2014;179:956–966. doi: 10.1093/aje/kwu020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ARIC Visit 5/NCS Analysis Manual. September 2015. 2019 Available at: https://www2.cscc.unc.edu/aric/sites/default/files/public/listings/V5%20NCS%20Analysis%20Manual_150901%20v1.pdf Accessed March 18.
- 38.SAS/STATT® 9.2 User's Guide. The SURVEYLOGISTIC Procedure (book excerpt) 2018 Available at: http://support.sas.com/documentation/cdl/en/statugsurveylogistic/61836/PDF/default/statugsurveylogistic.pdf Accessed September 14.
- 39.Kluczynski MA, Lamonte MJ, Mares JA, et al. Duration of physical activity and serum 25-hydroxyvitamin D status of postmenopausal women. Ann Epidemiol. 2011;21:440–449. doi: 10.1016/j.annepidem.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McGuinness MB, Le J, Mitchell P, et al. Physical activity and age-related macular degeneration: a systematic literature review and meta-analysis. Am J Ophthalmol. 2017;180:29–38. doi: 10.1016/j.ajo.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 41.Millen AE, Meyers KJ, Liu Z, et al. Association between vitamin D status and age-related macular degeneration by genetic risk. JAMA Ophthalmol. 2015;133:1171–1179. doi: 10.1001/jamaophthalmol.2015.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Assel MJ, Li F, Wang Y, Allen AS, Baggerly KA, Vickers AJ. Genetic polymorphisms of CFH and ARMS2 do not predict response to antioxidants and zinc in patients with age-related macular degeneration: independent statistical evaluations of data from the Age-Related Eye Disease Study. Ophthalmology. 2018;125:391–397. doi: 10.1016/j.ophtha.2017.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho L, van Leeuwen R, Witteman JC, et al. Reducing the genetic risk of age-related macular degeneration with dietary antioxidants, zinc, and omega-3 fatty acids: the Rotterdam study. Arch Ophthalmol. 2011;129:758–766. doi: 10.1001/archophthalmol.2011.141. [DOI] [PubMed] [Google Scholar]
- 44.Millen AE, Wactawski-Wende J, Pettinger M, et al. Predictors of serum 25-hydroxyvitamin D concentrations among postmenopausal women: the Women's Health Initiative Calcium plus Vitamin D clinical trial. Am J Clin Nutr. 2010;91:1324–1335. doi: 10.3945/ajcn.2009.28908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Age-Related Eye Disease Study 2 Research Group. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309:2005–2015. doi: 10.1001/jama.2013.4997. [DOI] [PubMed] [Google Scholar]
- 46.Manson JE, Bassuk SS, Lee IM, et al. The VITamin D and OmegA-3 TriaL (VITAL): rationale and design of a large randomized controlled trial of vitamin D and marine omega-3 fatty acid supplements for the primary prevention of cancer and cardiovascular disease. Contemp Clin Trials. 2012;33:159–171. doi: 10.1016/j.cct.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morris MC, Tangney CC. A potential design flaw of randomized trials of vitamin supplements. JAMA. 2011;305:1348–1349. doi: 10.1001/jama.2011.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.