Abstract
Purpose
We address the hypothesis that uncoupling protein 2 (UCP2), a cellular glucose regulator, delays physiologic retinal vascular development (PRVD) by interfering with glucose uptake through glucose transporter 1 (Glut1).
Methods
In the rat 50/10 oxygen-induced retinopathy (OIR) model, retinal Glut1 and UCP2 were measured and compared to room air (RA)-raised pups at postnatal day 14 (p14). Pups in OIR and RA received intraperitoneal genipin, an UCP2 inhibitor, or control every other day from p3 until p13. Analyses at p14 included avascular/total retinal area (AVA), Western blots of retinal UCP2 and Glut1, and immunostaining of Glut1 in retinal cryosections. Intravitreal neovascular/total retinal area (IVNV) was analyzed at p18, and electroretinograms were performed at p26. Glut1 and phosphorylated VEGFR2 (p-VEGFR2), glucose uptake, adenosine triphosphate (ATP) production, and cell proliferation were measured in human retinal microvascular endothelial cells (hRMVECs) pretreated with genipin or transfected with UCP2siRNA, Glut1siRNA, or control siRNA when incubated with VEGF or PBS.
Results
At p14, OIR pups had increased AVA with decreased Glut1 and increased UCP2 in the retina compared to RA retinas. Intraperitoneal genipin increased retinal Glut1 and reduced AVA. Compared to control, treatment with genipin or knockdown of UCP2 significantly increased Glut1, glucose uptake, ATP production, VEGF-induced p-VEGFR2 and cell proliferation in hRMVECs. Knockdown of Glut1 inhibited VEGF-induced p-VEGFR2. Genipin-treated OIR pups with decreased AVA at p14 had reduced IVNV at p18 and increased amplitudes in a- and b-waves at p26.
Conclusions
Extending PRVD by increasing retinal endothelial glucose uptake may represent a strategy to prevent severe retinopathy of prematurity and vision loss.
Keywords: angiogenesis, glucose transporter 1, oxygen induced retinopathy, VEGFR2, retinopathy of prematurity
Retinopathy of prematurity (ROP) is a leading cause of childhood blindness.1,2 Initially, preterm birth interrupts physiologic retinal vascular development (PRVD), and with additional stresses, compromises newly developed physiologic vascularity. These events lead to avascular retina, which becomes hypoxic when the infant is removed from high supplemental oxygen and stimulates overproduction of growth factors, including VEGF. Overactivated VEGF signaling through VEGF receptor 2 (VEGFR2) leads to disordered angiogenesis growing into the vitreous as intravitreal neovascularization. Intravitreal neovascularization can cause retinal detachment by fibrovascular contraction of the underlying retina. Although much research in ROP has focused on molecular mechanisms involved in regulating uncontrolled endothelial cell migration and proliferation,3–7 extending PRVD would not only reduce intravitreal neovascularization, but also enhance vision by expanding the visual field.
PRVD is regulated by retinal angiogenesis.7,8 As an active cellular event, angiogenesis requires energy support, such as adenosine triphosphate (ATP). Endothelial cells produce ATP mainly through glycolysis even in high oxygen.9,10 Sufficient ATP production is a key factor to promote tip cell formation, which occurs when PRVD is extended.11,12 Any condition that affects endothelial glycolysis, including insufficient glucose supply and uptake, might reduce endothelial cell sprouting and tip cell formation and interfere with normal angiogenesis.
We focused on glucose metabolism in retinal endothelial cells, and addressed the postulate that insufficient blood glucose uptake by retinal endothelial cells interferes with PRVD and leads to intravitreal neovascularization in ROP. Cumulative evidence indicates that inhibition of mitochondrial uncoupling protein 2 (UCP2) increases the cellular sensitivity to glucose and promotes cellular glucose uptake particularly in conditions with insufficient glucose supply.13–15 We inhibited UCP2 with its inhibitor, genipin,16,17 to test the hypothesis that inhibition of UCP2 would promote glucose uptake by retinal endothelial cells, increase cell migration and proliferation, and extend PRVD, which would then reduce subsequent intravitreal neovascularization in ROP.
Materials and Methods
Animals and Ethical Statement
Timed-pregnant Sprague-Dawley rats were purchased from Charles River (Wilmington, MA, USA). Animals had free access to food and water ad libitum and were kept on a 12-hour/12-hour light-dark cycle. All animal experimental protocols were approved by the Institutional Animal Care and Use Committee of University of Utah. All experiments were carried out in accordance with the Guidelines and Regulations for the Care and Use of Laboratory Animals by the University of Utah (Salt Lake City, UT, USA) and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Rat Oxygen-induced Retinopathy (OIR) Model and Intraperitoneal Injections
The rat OIR model used in this study has been described previously.18,19 Within 6 hours of birth, newborn Sprague-Dawley pups (male and female) and dam were placed into an oxygen chamber (Oxycycler, Biospherix, NY, USA) with controlled oxygen concentrations that cycled between 50% and 10% every 24 hours. After 14 days, pups were moved into room air (RA). Each litter had 12 to 14 pups to maintain consistency in measured outcomes in the model. Experiments were repeated in four individual litters.
In a litter, pups were randomly assigned to two groups and treated with intraperitoneal injections of genipin (10 mg/kg, Sigma-Aldrich Corp., St. Louis, MO, USA) dissolved in dimethyl sulfoxide (DMSO) and PBS (1:500) or an equal volume of vehicle (DMSO+PBS) as control beginning at postnatal day 3 (p3) and repeated every other day until p13. For pups in the OIR model, injections were performed at the end of 50% oxygen cycles and before 10% oxygen cycles to avoid interventions during the 10% oxygen cycle.
Ganzfeld Electroretinogram (ERG)
Retinal function was assessed by Ganzfeld ERG (LKC Technologies, Inc., Gaithersburg, MD, USA). Pups at p26 were fully dark-adapted overnight and subsequently handled under dim red light illumination. After anesthesia and pupil dilation with tropicamide (1%) eye drops (Bausch and Lomb, Tampa, FL, USA), pups were kept on a heating pad throughout the procedure to maintain body temperature. Subdermal needle electrodes were inserted underneath the skin at the base of the tail (ground electrode) and between the eyes on the forehead (reference electrode). Corneas were lubricated with coupling gel (GenTeal, hypromellose 0.3%; Alcon, Fort Worth, TX, USA); the objective electrode was advanced near the corneal surface, and deep red illumination was used to focus on the retina. Responses to light stimuli were elicited at luminances ranging from −4.5 to 2.2 cd s m−2 with 30 sweeps at 2-second intervals. Data were displayed as the mean amplitude (in μV) of the a-wave (as a measure of photoreceptor function) and b-wave (as a measure of bipolar cell function).
Retinal Flat Mount Preparation, Imaging, and Analysis of Peripheral Avascular Retinal Area/Total Retinal Area (AVA) and Intravitreal Neovascular/Total Retinal Area (IVNV)
After enucleation, eyes were fixed in 4% paraformaldehyde (PFA; EMS, Hatfield, PA, USA) for 2 hours and placed into PBS (containing in mM NaCl 137, KCl 2.7, Na2HPO4 8, KH2PO4, pH 7.4). To dissect the retina in each eye, the cornea, lens, and vitreous were removed and the RPE, choroid, and sclera were removed from the eyecup. Retinas were flattened onto slides. The retinal flat mounts were stained with Alex-Fluor 568-labeled isolectin GS-IB4 from Griffonia simplicifolia (2.5 μg/mL; Invitrogen, Grand Island, NY, USA) to stain the retinal vasculature. Flat mounts were imaged at ×4 magnification using an inverted fluorescence microscope (Olympus IX81; Oympus Corp. Tokyo, Japan), and individual images were joined using Metamorph 7.0 software (Molecular Devices, Inc., Sunnyvale, CA, USA). The percent of AVA and of IVNV was determined by two masked reviewers using Image J software (National Institutes of Health [NIH], Bethesda, MD, USA).
Retinal Cryosection Preparation and Staining
Anterior segments were removed from eyes after 30 minutes of fixation in 4% PFA. Eye cups were replaced into 4% PFA for an additional 1 hour and incubated overnight in 30% sucrose. After immersion in optimal cutting temperature compound (OCT; Tissue Tek; EMS, Hatfield, PA, USA), retinas were cut into 12 μm cryosections. After rinsing and blocking in blocking buffer with 5% goat serum for 1 hour at room temperature, sections were incubated with mouse anti-Glut1 (1:200; Abcam, Cambridge, MA, USA) overnight at 4°C, followed by incubation for 1 hour with FITC-conjugated goat anti-mouse (1:200; Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA), isolectin B4 (lectin, 1:500; Invitrogen) and TO-PRO-3 Iodide (1:500; Invitrogen) to stain nuclei. Sections stained without primary antibodies were used as controls. Images were captured using confocal microscopy (Olympus IX81, Tokyo, Japan) at ×20 magnification.
Cell Culture and Transfection
Human retinal microvascular endothelial cells (hRMVECs; Cell Systems, Kirkland, WA, USA) were maintained in endothelial growth medium (EGM-MV; Lonza, Walkersville, MD, USA) supplemented with 5% FBS. Cells of passages 3 to 5 were used for experiments. To knock down glucose transporter 1 (Glut1) or UCP2, hRMVECs at 70% confluence were transfected with siRNAs targeting human Glut1 gene (solute carrier family 2 member 1, SLC2A1) or human UCP2 (Applied Biosystems, Foster City, CA, USA) using lipofectamine 2000 or 3000 (Invitrogen). A silencer selective negative control siRNA (Applied Biosystems) was used as control for Glut1 or UCP2 knockdown. At 24 to 36 hours after transfection, cells were treated and used for different assays.
Glucose Uptake Assay
Human RMVECs were plated into 96-well plates at 1 × 104 cells/well and grown for 24 hours. Cells then were grown in serum-free endothelial basal medium (EBM) overnight. After three washes in warm PBS, cells were incubated in Krebs-Ringer-Phosphate-HEPES (KRPH) buffer (20 mM HEPES, 5 mM KH2PO4, 1 mM MgSO4,1 mM CaCl2, 136 mM NaCl, and 4.7 mM KCl, pH 7.4) with 2% BSA for 40 minutes. Cells then were treated with genipin (10 μM) for 2 hours before incubation with VEGF (20 ng/mL). Glucose uptake was measured after incubating with 2-NBDG (2-[N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl)Amino]-2-Deoxyglucose, 2-NBDG; 10 μM; Invitrogen) for an additional 30 minutes. DMSO and PBS were used as treatment controls for genipin and VEGF, respectively. After treatment, cells were washed in PBS three times to remove 2-NBDG that was not taken up, and then cells were measured for fluorescence at excitation/emission maxima of approximately 465/540 nm using a fluorescence microplate reader (BioTek, Winooski, VT, USA).
ATP Production Assay
Human RMVECs were plated into 6-well plates at 0.5 × 106 cells/well and grown for 48 hours until confluence. Cells then were starved in EBM medium without serum and growth factor for 3 hours and then treated with genipin (10 μM) for 2 hours. ATP concentration was measured by fluorometric ATP assay kit (Abcam) in 96-well plates.
Cell Proliferation Assay
Human RMVECs were plated into 96-well plates at a density of 5000 cells/well. At 24 hours after plating, cells were starved in serum-free media overnight, and then pretreated with genipin (10 μM) for 2 hours before incubation with VEGFA (20 ng/mL) or control PBS for another 24 hours. Cell number was measured with the Vybrant MTT cell proliferation assay kit (Invitrogen).
Western Blots
Cells or retinas were lysed in RIPA buffer with protease inhibitor cocktail (Sigma Aldrich Corp.) and orthovanadate (Thermo Fisher Scientific, Grand Island, NY, USA). Lysates were clarified by centrifugation at 16.1 × 1000g for 5 minutes at 4°C. Protein concentration in the supernatant was quantified by the bicinchoninic acid assay (BCA). A total of 20 μg of protein from each treatment was loaded into NuPAGE 4% to 12% Bis-Tris Gels (Invitrogen), transferred to a polyvinylidine fluoride (PVDF) membrane, and incubated with antibodies to phosphorylated VEGFR2 (p-VEGFR2, Y951; 1:500; Santa Cruz Biotechnology; 1:1000; Cell Signaling Technology) or Glut1 (1:1000, Abcam) at 4°C overnight. After incubating with primary antibodies, membranes then were probed with horseradish peroxidase conjugated goat anti-rabbit secondary antibody or goat anti-mouse secondary antibody (1:3000–5000, ThermoFisher Scientific) at room temperature for 1 hour. All membranes were reprobed with horseradish peroxidase conjugated β-actin (1:3000, Santa Cruz Biotechnology) as loading controls. Quantification of densitometry was performed using the licensed software UN-SCAN-IT version 7 (Silk Scientific, Inc., Orem, UT, USA).
Statistical Analysis
A mixed effects linear regression model was used to statistically analyze data collected from OIR rats for AVA and IVNV using STATA-14 software (StataCorp LLC, College Station, TX, USA). That model included fixed and random effects to account for biologic variation between litters by comparing experimental to control groups inside the same litter. The intraperitoneal injections used in this study did not contribute much variation between eyes. Only one eye from each animal was used for AVA and IVNV analysis and the fellow eye was used for protein and RNA analysis. A 2-tailed t-test was used for protein analysis and in vitro studies. Results were displayed as mean ± SEM. A minimum value of P < 0.05 was considered statistically significant. For animal studies, 16 individual pups from at least four litters were analyzed for AVA or IVNV. For ERG analyses, four to six pups were used. For immunolabeling in retinal cryosections, three to four retinal sections at 60-μm intervals were taken from one eye of three pups in each group. For protein analyses, each treatment included at least five different animals from three litters. For in vitro studies, each experimental condition included an n = 4–9 from at least two independent experiments.
Results
Delayed PRVD Associated With Decreased Retinal Glut1 in OIR
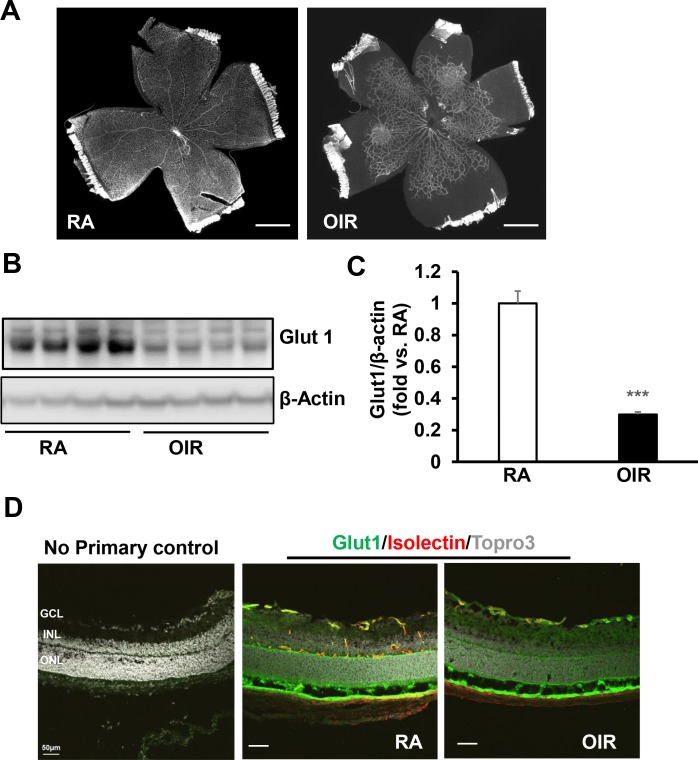
As a model of human ROP, pups in the rat OIR model have incomplete PRVD at p1419 in contrast to RA raised pups that have fully vascularized retinas (Fig. 1A). Endothelial cells use and produce energy locally through glycolysis to regulate endothelial cell proliferation, migration, and tip cell formation, which are cellular events involved in angiogenesis. The retina takes up glucose mainly through Glut1.20 Studies have shown that Glut1-mediated glucose transport is essential for postnatal development of the murine brain vasculature.12 Therefore, we postulated that delayed PRVD in OIR pups was associated with insufficient glucose uptake. To study this, Glut1 was first measured in retinal lysates from RA and OIR pups at p14. As shown in Figures 1B and 1C, compared to RA, Glut1 protein was significantly decreased in OIR. To determine if Glut1 is localized to retinal vascular EC, immunohistochemistry (IHC) for Glut1 was performed in retinas of p14 RA or OIR pups. IHC of Glut1 in retinal cryosections was mainly located in the retinal ganglion cell layer, inner plexiform layer, photoreceptor outer segments, and RPE. Glut1 also strongly colocalized with isolectin-stained retinal vascular ECs (Fig. 1D). Taken together, these data supported the notion that reduced Glut1 in retinal vascular ECs was associated with delayed PRVD in p14 OIR pups.
Figure 1.
Rat pups exposed to OIR model have delayed PRVD and decreased Glut1. (A) Representative retinal flat mounts. Scale bar: 990 μm. (B, C) Western blots of Glut1 in retinas. (B) Representative gel images and (C) quantification of densitometry (***P < 0.001 versus RA; n = 5–6), and (D) Immunohistochemistry of Glut1 in retinal cryosections (Green, Glut1; Red, isolectin, and Gray, Topro 3; n = 3) of RA and OIR pups at p14.
Decreased Retinal Glut1 is Associated With Increased UCP2 in OIR Pups
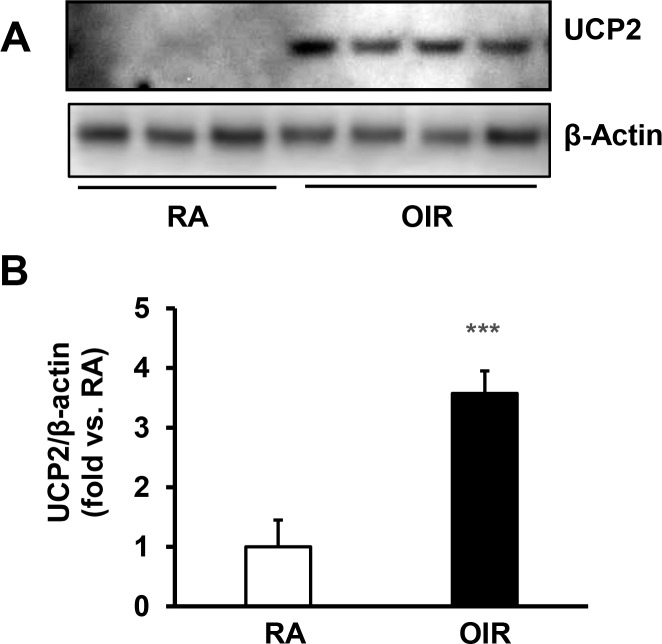
Mitochondrial UCP2 has been implicated in cellular glucose metabolism related to pathologic conditions, such as diabetic retinopathy.16 Knockdown of UCP2 significantly increases ATP production from glucose metabolism in human hepatocarcinoma cells.21 To determine if decreased retinal Glut1 is related to UCP2 expression, UCP2 protein was measured in the retinas by Western blots. Compared to RA, UCP2 was significantly increased in retinal lysates of OIR pups (Fig. 2) that had decreased Glut1 (Fig. 1B), supporting the thinking that lower Glut1 in OIR pups could be explained by increased UCP2.
Figure 2.
Pups exposed to OIR model have increased UCP2 in the retina. Western blots of UCP2 in the retina of RA and OIR pups at p14. (A) Representative gel images and (B) quantification of densitometry; ***P < 0.001 versus RA; n = 5–6.
Inhibition of UCP2 Extends PRVD by Upregulating Retinal Glut1
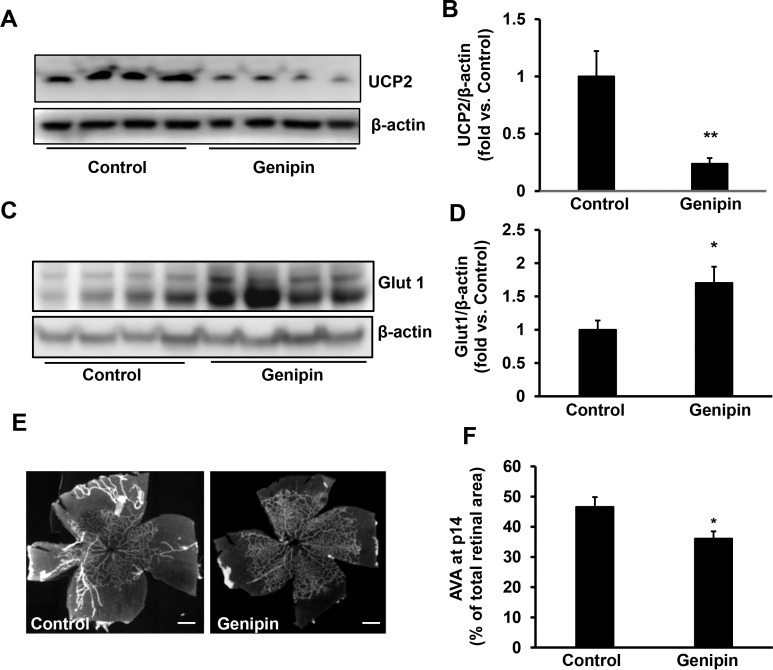
Since reduced Glut1 was associated with delayed PRVD and increased UCP2, we predicted that OIR increased retinal UCP2, which then decreased Glut1 and delayed PRVD. To test this, OIR pups were given intraperitoneal injections of genipin, an UCP2 inhibitor, or solvent control every other day from p3 to p13 during the 50% oxygen cycles. As shown in Figure 3, compared to control, inhibition of UCP2 by genipin (Figs. 3A, 3B) significantly increased Glut1 protein in the retinas of OIR pups (Figs. 3C, 3D). We then studied if this increased Glut1 had a role in extending PRVD. We predicted that genipin treatment would extend PRVD, reflected by lower AVA. Compared to control, genipin-treated pups had significantly decreased AVA measured in isolectin-stained flat mounts at p14 (Figs. 3E, 3F), supporting the prediction.
Figure 3.
Intraperitoneal genipin inhibits retinal UCP2, increases retinal Glut1, and reduces AVA of OIR pups. Western blots of (A, B) UCP2 and (C, D) Glut1 in retinas, and (E, F) AVA of p14 OIR pups received intraperitoneal injections of genipin or control from p3 to p14. (A, C) Representative gel images. (B, D) Quantification of densitometry. (E) Representative flat mount images. (F) quantification of AVA; *P < 0.05, **P < 0.01 versus. Control; n = 5–6 for Western blots and n = 14–16 for AVA.
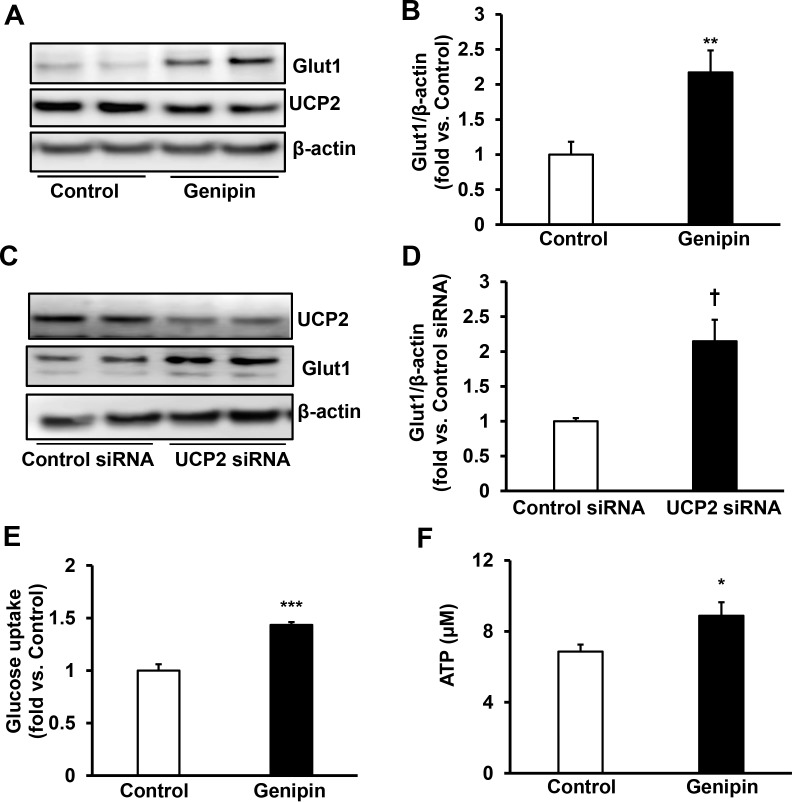
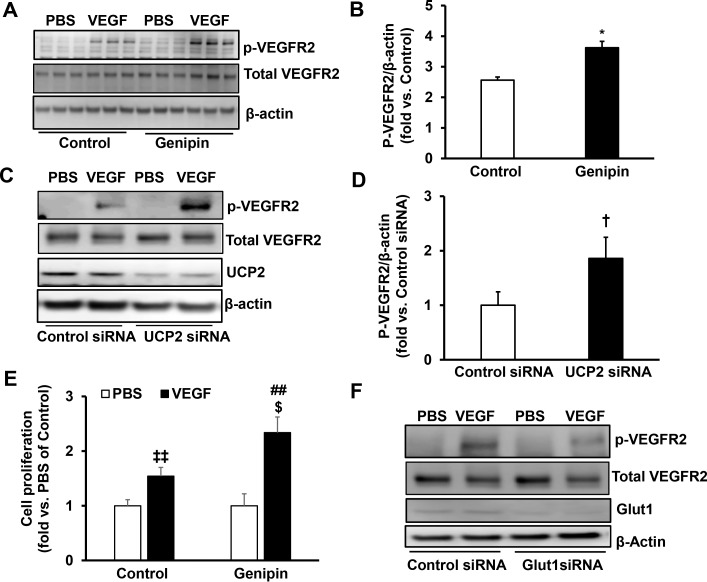
To further test the effects of inhibition of UCP2 in extending PRVD by upregulating Glut1, a series of experiments were performed in hRMVECs. We first asked if genipin treatment would increase Glut1 and, therefore, promote glucose uptake to increase ATP production. To test this hypothesis, we measured Glut1 protein by Western blot, glucose uptake by (N-[7-Nitrobenz-2-oxa-1,3-diazol-4-yl] Amino)-2-Deoxyglucose and ATP production by the fluorometric assay. Treatment with the UCP2 inhibitor, genipin, for 2 hours significantly increased Glut1 protein (Figs. 4A, 4B). To eliminate potential off-target effects of genipin treatment, Glut1 protein was measured in hRMVECs transfected with UCP2siRNA. As shown in Figures 4C and 4D, knockdown of UCP2 by siRNA significantly increased Glut1 protein, similar to genipin treatment. Genipin treatment for 30 minutes significantly increased glucose uptake (Fig. 4E) and ATP production (Fig. 4F), supporting the thinking that inhibition of UCP2 increases Glut1 protein and its mediated glucose uptake.
Figure 4.
Inhibition of UCP2 increases Glut1 and VEGF-induced glucose uptake in hRMVECs. Western blot of Glut1 and UCP2 (A, B) in hRMVECs treated with control or genipin (10 μM) for 2 hours and (C, D) in hRMVECs transfected with UCP2 siRNA or control siRNA. (A, C) Representative gel images. (B, D) Quantification of densitometry of Glut1). (E) Glucose uptake measured by (N-[7-Nitrobenz-2-oxa-1,3-diazol-4-yl]Amino)-2-Deoxyglucose and (F) ATP production in hRMVECs treated with genipin (10 μM) or control for 30 minutes (*P < 0.05, **P < 0.01, ***P < 0.001 versus. Control; †P < 0.05 versus Control siRNA; n = 4 for Western blots and n = 9 for glucose uptake and ATP production).
Variable oxygen reduced retinal VEGFR2 in rats in the OIR model compared to those in RA.22 Activation of extracellular signal-responsive kinase-1/2 (ERK1/2), a downstream signaling effector of VEGF,23 was significantly reduced in the retina of rat pups in OIR compared to those raised in RA (Supplemental Figure), supporting the thinking that reduced activation of VEGF signaling contributes to decreased angiogenesis. We then determined if increased Glut1-mediated glucose uptake from genipin treatment would increase the angiogenic activity of hRMVECs. Compared to respective controls, VEGF-induced phosphorylation of VEGFR2 (p-VEGFR2) was significantly increased in genipin-treated hRMVECs (Figs. 5A, 5B) and in hRMVECs knocked down for UCP2 by siRNA transfection compared to control (Figs. 5C, 5D). Genipin treatment increased p-VEGFR2 and hRMVEC proliferation (Fig. 5E). To determine if increased p-VEGFR2 from genipin treatment was dependent on Glut1, p-VEGFR2 was measured in hRMVECs transfected with Glut1siRNA or control siRNA. Compared to control siRNA-transfected hRMVECs, knockdown of Glut1 by Glut1 siRNA reduced VEGF-induced p-VEGFR2 in hRMVECs (Fig. 5F). Taken together, the data demonstrated that inhibition of UCP2 by genipin increases Glut1 and its mediated glucose uptake and, therefore, promotes angiogenic activity of hRMVECs through activation of VEGFR2.
Figure 5.
Inhibition of UCP2 promotes VEGFR2 activation and cell proliferation in hRMVECs via upregulation of Glut1. Western blot of p-VEGFR2 (A, B) in hRMVECs pretreated with genipin or control for 2 hours and (C, D) in hRMVECs transfected with UCP2 siRNA or control siRNA before incubation with VEGF for 15 minutes. (A, C) Representative gel images. (B, D) Quantification of densitometry). (E) cell proliferation assay in hRMVECs pretreated with genipin or control for 2 hours before incubation with VEGF for 24 hours. (F) Western blots of p-VEGFR2 in hRMVECs transfected with Glut1 siRNA or Control siRNA and treated with VEGF or PBS for 15 minutes (*P < 0.05 versus. Control; †P < 0.05 versus Control siRNA; ‡‡P < 0.01 versus Control/PBS; ##P < 0.05 versus Genipin/PBS; $P < 0.05 versus Control/VEGF; n = 3–6 for Western blots and n = 9 for cell proliferation).
Genipin-Induced PRVD Reduces IVNV and Improves ERG
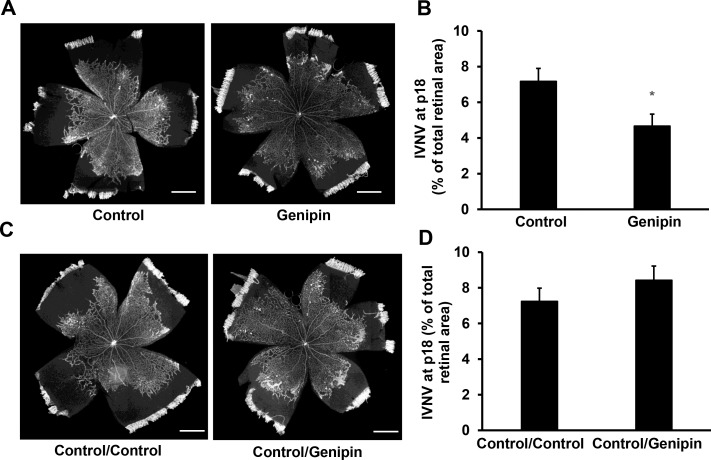
Extending PRVD reduces hypoxic retina available to stimulate pathologic angiogenesis. We predicted that genipin-treated OIR pups with less AVA, an indicator of extended PRVD, would have less IVNV. Compared to control-treated OIR pups, genipin treatment from p3 to p13 significantly reduced IVNV (Figs. 6A, 6B). To address the question if the reduced IVNV at p18 was related to reduced AVA at p14 rather than genipin treatment, IVNV was measured in OIR pups treated with genipin or control every other day from p14 to p18, instead of from p3 to p13. As shown in Figures 6C and 6D, compared to control/control treatment, late genipin treatment did not reduce IVNV in the OIR pups, supporting the thinking that increased PRVD at p14 from early genipin treatment accounted for the subsequent reduced IVNV.
Figure 6.
Intraperitoneal genipin to reduce AVA reduces intravitreal neovascularization. (A) and (B) IVNV at p18 of OIR pups received intraperitoneal injections of genipin or control from p3 to p14. (A) Representative flat mount images and (B) quantification of IVNV; *P < 0.05 versus Control. Scale bar: 990 μm. (C, D) IVNV at p18 of OIR pups treated with control or genipin from p14 to p18. (C) Representative flat mount images and (D) quantification of IVNV. Scale bar: 990 μm, n = 3–16.
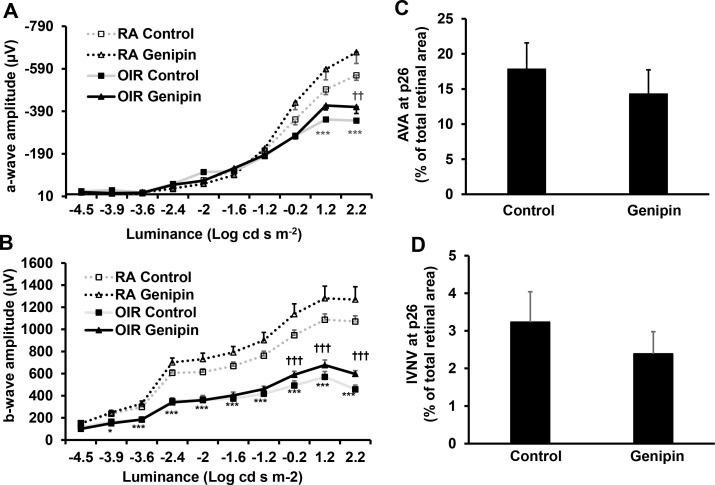
Since genipin treatment extended PRVD, we predicted that it also would improve retinal function measured by ERG. At p26, OIR pups have lower a- (Fig. 7A) and b-wave (Fig. 7B) amplitudes compared to RA, consistent with other studies.24,25 Compared to respective controls, genipin treatment significantly increased a- (Fig. 7A) and b-wave (Fig. 7B) amplitudes in OIR but not in RA pups. The OIR pups treated with genipin still maintained less AVA (Fig. 7C) and IVNV (Fig. 7D) compared to controls; however, the difference was not significant probably because the rat OIR model undergoes natural regression of IVNV and extension of PRVD.
Figure 7.
Intraperitoneal genipin increases retinal function. Ganzfeld ERG of (A) a-wave amplitude and (B) b-wave amplitudes in p26 RA and OIR pups after overnight dark adaptation and treatment with control or genipin from p3 to p13 (*P < 0.05, ***P < 0.001 versus RA/Control; ††P < 0.01, †††P < 0.001 versus OIR/Control; n = 4–6 for ERG and n = 11 for AVA and IVNV); and quantification of (C) AVA and (D) IVNV of OIR pups at p26 treated with control or genipin from p3 to p13.
Taken together, the data provided evidence that pups treated with genipin experience extended PRVD, reduced IVNV, and improved neural retinal function.
Discussion
The focus of treatment in ROP has been to inhibit pathologic intravitreal neovascularization. However, recurrent intravitreal neovascularization after anti-VEGF treatment occurs in clinic and animal studies.26,27 Therefore, only inhibiting intravitreal neovascularization without extending PRVD may not be an optimal treatment for ROP, because persistent avascular retina stimulates hypoxia-induced recurrent intravitreal neovascularization28 and impairs vision function due to loss of retinal vasculature. We studied the role of glucose uptake and energy metabolism in retinal vascular endothelial cells needed to extend PRVD and addressed the hypothesis that UCP2, a cellular glucose regulator, extended PRVD by interfering with glucose uptake through Glut1.
The retina is a metabolically active organ with high-energy demands. Glucose is the primary energy supply for the retina, although lipids also can be metabolized by retinal photoreceptors.29 The retina takes up glucose through Glut1 and generates ATP by aerobic glycolysis.20,30 Several studies have established the importance of glucose metabolism in retinal neuronal survival31 and neurovascular interactions.29,32 Recently, there is interest in the role of glycolysis in angiogenesis particularly in diseases involving aberrant vessel growth.10 Pharmacologic blockade of the glycolytic activator, phosphofructokinase-2/fructose-2,6-bisphosphatase 3 (PFKFB3) with a small molecule, 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO) reduced retinal vascular branching and radial extension and choroidal neovascularization in a mouse laser-induced model of choroidal neovascularization,33 providing evidence that glycolysis-mediated energy metabolism regulates angiogenesis.
We found that delayed PRVD in OIR pups correlated with reduced retinal Glut1 due to insufficient glucose uptake to support retinal angiogenic growth. In support of this conjecture, we also observed an association between reduced Glut1 and increased retinal UCP2. UCP2 has been involved in cellular lipid and glucose metabolism.14 Knockout of UCP2 promotes cell proliferation by stimulating cellular use of glycolysis-derived pyruvate in mouse embryonic fibroblasts.34 To test the hypothesis that insufficient glucose uptake reduced glycolytic metabolism and delayed PRVD, we treated OIR pups with the UCP2 inhibitor, genipin,16,17,35 and found inhibition of UCP2 increased retinal Glut1 protein in association with reduced AVA at p14. We also observed that in OIR, VEGF was increased but activation of the downstream signaling effector of VEGFR2, p-ERK, was decreased at p14. Maintaining activation of the VEGF signaling pathway requires VEGFR2 internalization by endocytosis and recycling,36–40 which are ATP-demanding cellular events. We further provided evidence that insufficient ATP production from reduced Glut1 reduced VEGFR2 signaling. In hRMVECs, inhibition of UCP2 by genipin increased VEGF-induced glucose uptake, ATP production, and VEGF-mediated activation of VEGFR2, whereas knockdown of Glut1 inhibited VEGFR2 activation. Our results supported the thinking that UCP2-mediated reduction in Glut1 interferes with VEGF-induced signaling and, therefore, reduces intraretinal vascularization necessary for PRVD. The importance of Glut1 expression in retinal endothelial cells also is highlighted in a study of diabetic retinopathy,41 in which hyperglycemia-induced oxidative stress disrupted glucose homeostasis by reducing Glut1 plasma membrane expression. Future studies are needed to determine if oxidative stress is involved in reduced retinal Glut1 and if UCP2 quenches reactive oxygen in OIR.
We then addressed the question whether extending PRVD by genipin treatment would reduce IVNV and improve retinal function. We observed that genipin-treated OIR pups that had less AVA at p14 had decreased IVNV at p18 and increased a- and b-wave amplitudes at p26 following overnight dark adaptation. These improved ERG amplitudes were observed in OIR pups, but not in RA pups, suggesting that UCP2 activity does not regulate retinal function in normal retinal development. However, others reported that 10- to 15-week-old UCP2 knockout mice had reduced a-waves, but normal b-waves after 1 hour of dark adaptation.42 The differences in the studies may relate to oxygen stresses in our pups and differences in animal age or possible dark adaptation time, since overnight dark adaptation is needed to measure ganglion cell function in the ERG.43 Future studies are warranted to understand the potential role of UCP2 and glucose metabolism in promoting photoreceptor maturation, neurovascular interactions, and in extending PRVD. Interestingly, room air raised pups treated with genipin every other day from p3 to p13 showed fully vascularized retinas at p14, suggesting that UCP2 is not involved in retinal vascular development. Therefore, inhibition of UCP2 may be a potentially safe target to treat ROP.
The importance of glucose homeostasis in preterm infants is recognized in neonatal care. Some studies proposed hyperglycemia as a potential risk factor for ROP.44,45 Hyperglycemia occurs in the setting of stress induced insufficient insulin secretion or relative insulin resistance.46 However, the interpretation of hyperglycemia is confounded, because it occurs in very stressed premature infants who have other conditions of prematurity that may influence the risk of ROP, and other clinical studies have not identified sufficient evidence to support a direct causal effect between high glucose and ROP.47,48 Preterm neonates, particularly those of young gestational ages or with intrauterine growth restriction, are prone to suffer hypoglycemia because of limited storage of glycogen and fat, inability to generate new glucose through gluconeogenesis by underdeveloped organs including the liver, and high metabolic demands from a relatively high metabolism rate.49–51 Preterm infants with hypoglycemia have abnormal neurodevelopment due to insufficient energy supply from glucose oxidation.50 These clinical observations emphasize the importance of glucose metabolism in preterm infants and relate to our experimental study on physiologic retinal neural and vascular development and pathologic neovascularization.
In conclusion, our study supported the line of thinking that regulating uptake of cellular glucose and glycolytic metabolism of the retinal vascular endothelial cells is important to extend PRVD and reduce retinal pathology in ROP and prematurity. Furthermore, improved glucose uptake and glycolysis may improve neural function either from improved vascularity or through other mechanisms.
Supplementary Material
Acknowledgments
Supported by the NIH Grant EY014800 and an unrestricted grant from Research to Prevent Blindness, Inc., New York, NY to the Department of Ophthalmology & Visual Sciences, University of Utah; and Knights Templar Eye Foundation Grant (HW) and NIH Grants R01EY015130 and R01EY017011 (MEH).
Disclosure: X. Han, None; J. Kong, None; M.E. Hartnett, None; H. Wang, None
References
- 1.Zin A, Gole GA. Retinopathy of prematurity-incidence today. Clin Perinatol. 2013;40:185–200. doi: 10.1016/j.clp.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Sira BI, Nissenkorn I, Kremer I. Retinopathy of prematurity; a major review. Surv Ophthalmol. 1988;33:1–16. doi: 10.1016/0039-6257(88)90068-9. [DOI] [PubMed] [Google Scholar]
- 3.Aiello LP. Clinical implications of vascular growth factors in proliferative retinopathies. Curr Opin Ophthalmol. 1997;8:19–31. doi: 10.1097/00055735-199706000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Wang H, Smith GW, Yang Z, et al. Short hairpin RNA-mediated knockdown of VEGFA in Muller cells reduces intravitreal neovascularization in a rat model of retinopathy of prematurity. Am J Pathol. 2013;183:964–974. doi: 10.1016/j.ajpath.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Yang Z, Jiang Y, et al. Quantitative analyses of retinal vascular area and density after different methods to reduce VEGF in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2014;55:737–744. doi: 10.1167/iovs.13-13429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hartnett ME. Vascular endothelial growth factor antagonist therapy for retinopathy of prematurity. Clin Perinatol. 2014;41:925–943. doi: 10.1016/j.clp.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hartnett ME. Advances in understanding and management of retinopathy of prematurity. Surv Ophthalmol. 2017;62:257–276. doi: 10.1016/j.survophthal.2016.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavallaro G, Fillipi L, Bagnoli B, et al. The pathophysiology of retinopathy of prematurity: an update of previous and recent knowledge. Acta Ophthalmol. 2014;92:2–20. doi: 10.1111/aos.12049. [DOI] [PubMed] [Google Scholar]
- 9.De Bock K, Georgiadou M, Schoors S, et al. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013. pp. 154651–663. [DOI] [PubMed]
- 10.Potente M, Carmeliet P. The link between angiogenesis and endothelial metabolism. Annu Rev Physiol. 2017;79:43–66. doi: 10.1146/annurev-physiol-021115-105134. [DOI] [PubMed] [Google Scholar]
- 11.De Bock K, Georgiadou M, Carmeliet P. Role of endothelial cell metabolism in vessel sprouting. Cell Metab. 2013;18:634–647. doi: 10.1016/j.cmet.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Teuwen LA, Geldhof V, Carmeliet P. How glucose, glutamine and fatty acid metabolism shape blood and lymph vessel development. Dev Biol. 2019;447:90–102. doi: 10.1016/j.ydbio.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 13.Parton LE, Ye CP, Coppari R, et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- 14.Diano S, Horvath TL. Mitochondrial uncoupling protein 2 (UCP2) in glucose and lipid metabolism. Trends Mol Med. 2012;18:52–58. doi: 10.1016/j.molmed.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Kong D, Vong L, Parton LE, et al. Glucose stimulation of hypothalamic MCH neurons involves K(ATP) channels, is modulated by UCP2, and regulates peripheral glucose homeostasis. Cell Metab. 2010;12:545–552. doi: 10.1016/j.cmet.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiu W, Zhou Y, Jiang L, et al. Genipin inhibits mitochondrial uncoupling protein 2 expression and ameliorates podocyte injury in diabetic mice. PLoS One. 2012;7:e41391. doi: 10.1371/journal.pone.0041391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Parton LE, Ye CP, et al. Genipin inhibits UCP2-mediated proton leak and acutely reverses obesity- and high glucose-induced beta cell dysfunction in isolated pancreatic islets. Cell Metab. 2006;3:417–427. doi: 10.1016/j.cmet.2006.04.010. [DOI] [PubMed] [Google Scholar]
- 18.Penn JS, Henry MM, Tolman BL. Exposure to alternating hypoxia and hyperoxia causes severe proliferative retinopathy in the newborn rat. Pediatr Res. 1994;36:724–731. doi: 10.1203/00006450-199412000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Penn JS, Tolman BL, Lowery LA. Variable oxygen exposure causes preretinal neovascularisation in the newborn rat. Invest Ophthalmol Vis Sci. 1993;34:576–585. [PubMed] [Google Scholar]
- 20.Busik JV, Olson LK, Grant MB, Henry DN. Glucose-induced activation of glucose uptake in cells from the inner and outer blood-retinal barrier. Invest Ophthalmol Vis Sci. 2002;43:2356–2363. [PubMed] [Google Scholar]
- 21.Vozza A, Parisi G, De Leonardis F, et al. UCP2 transports C4 metabolites out of mitochondria, regulating glucose and glutamine oxidation. Proc Natl Acad Sci U S A. 2014;111:960–965. doi: 10.1073/pnas.1317400111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Werdich XQ, McCollum GW, Rajaratnam VS, Penn JS. Variable oxygen and retinal VEGF levels: correlation with incidence and severity of pathology in a rat model of oxygen-induced retinopathy. Exp Eye Res. 2004;79:623–630. doi: 10.1016/j.exer.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 23.Bullard LE, Qi X, Penn JS. Role for extracellular signal-responsive kinase-1 and -2 in retinal angiogenesis. Invest Ophthalmol Vis Sci. 2003;44:1722–1731. doi: 10.1167/iovs.01-1193. [DOI] [PubMed] [Google Scholar]
- 24.Fulton AB, Raynaud X, Hansen RM, et al. Rod photoreceptors in infant rats with a history of oxygen exposure. Invest Ophthalmol Vis Sci. 1999;40:168–174. [PubMed] [Google Scholar]
- 25.Akula JD, Hansen RM, Martinez-Perez ME, Fulton AB. Rod Photoreceptor function predicts blood vessel abnormality in retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2007;48:4351–4359. doi: 10.1167/iovs.07-0204. [DOI] [PubMed] [Google Scholar]
- 26.Wang H. Anti-VEGF therapy in the management of retinopathy of prematurity: what we learn from representative animal models of oxygen-induced retinopathy. Eye Brain. 2016;8:81–90. doi: 10.2147/EB.S94449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hu J. Reactivation of retinopathy of prematurity after bevacizumab injection. Arch Ophthalmol. 2012;130:1000–1006. doi: 10.1001/archophthalmol.2012.592. [DOI] [PubMed] [Google Scholar]
- 28.McCloskey M, Wang H, Jiang Y, Smith GW, Strange G, Hartnett ME. Anti-VEGF antibody leads to later atypical intravitreous neovascularization and activation of angiogenic pathways in a rat model of retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2013;54:2020–2026. doi: 10.1167/iovs.13-11625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joyal JS, Sun Y, Gantner ML, et al. Retinal lipid and glucose metabolism dictates angiogenesis through the lipid sensor Ffar1. Nat Med. 2016;22:439–445. doi: 10.1038/nm.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sone H, Deo BK, Kumagai AK. Enhancement of glucose transport by vascular endothelial growth factor in retinal endothelial cells. Invest Ophthalmol Vis Sci. 2000;41:1876–1884. [PubMed] [Google Scholar]
- 31.Aït-Ali N, Fridlich R, Millet-Peul G, et al. Rod-derived cone viability factor promotes cone survival by stimulating aerobic glycolysis. Cell. 2015;161:817–832. doi: 10.1016/j.cell.2015.03.023. [DOI] [PubMed] [Google Scholar]
- 32.Joyal JS, Gantner ML, Smith LEH. Retinal energy demands control vascular supply of the retina in development and disease: the role of neuronal lipid and glucose metabolism. Prog Retin Eye Res. 2018;64:131–156. doi: 10.1016/j.preteyeres.2017.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schoors S, De Bock K, Cantelmo AR, et al. Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metab. 2014;19:37–48. doi: 10.1016/j.cmet.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 34.Pecqueur C, Alves-Guerra C, Ricquier D, Bouillaud F. UCP2, a metabolic sensor coupling glucose oxidation to mitochondrial metabolism? IUBMB Life. 2009;61:762–767. doi: 10.1002/iub.188. [DOI] [PubMed] [Google Scholar]
- 35.Ayyasamy V, Owens KM, Desouki MM, et al. Cellular model of Warburg effect identifies tumor promoting function of UCP2 in breast cancer and its suppression by genipin. PLoS One. 2011;6:e24792. doi: 10.1371/journal.pone.0024792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basagiannis D, Christoforidis S. Constitutive Endocytosis of VEGFR2 protects the receptor against shedding. J Biol Chem. 2016;291;:16892–16903. doi: 10.1074/jbc.M116.730309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gourlaouen M, Welti JC, Vasudev NS, Reynolds AR. Essential role for endocytosis in the growth factor-stimulated activation of ERK1/2 in endothelial cells. J Biol Chem. 2013;288:7467–7480. doi: 10.1074/jbc.M112.446401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu W, Shi DS, Winter JM, et al. Small GTPase ARF6 controls VEGFR2 trafficking and signaling in diabetic retinopathy. J Clin Invest. 2017;127:4569–4582. doi: 10.1172/JCI91770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trowbridge IS. Endocytosis and signals for internalization. Curr Opin Cell Biol. 1991;3:634–641. doi: 10.1016/0955-0674(91)90034-v. [DOI] [PubMed] [Google Scholar]
- 40.Wesen E, Jeffries GDM, Matson Dzebo M, Esbjorner EK. Endocytic uptake of monomeric amyloid-beta peptides is clathrin- and dynamin-independent and results in selective accumulation of Abeta(1-42) compared to Abeta(1-40) Sci Rep. 2017;7:2021. doi: 10.1038/s41598-017-02227-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fernandes R, Hosoya K, Pereira P. Reactive oxygen species downregulate glucose transport system in retinal endothelial cells. Am J Physiol Cell Physiol. 2011;300:C927–C936. doi: 10.1152/ajpcell.00140.2010. [DOI] [PubMed] [Google Scholar]
- 42.Barnstable CJ, Reddy R, Li H, Horvath TL. Mitochondrial uncoupling protein 2 (UCP2) regulates retinal ganglion cell number and survival. J Mol Neurosci. 2016;58:461–469. doi: 10.1007/s12031-016-0728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saszik SM, Robson JG, Frishman LJ. The scotopic threshold response of the dark-adapted electroretinogram of the mouse. J Physiol. 2002;543:899–916. doi: 10.1113/jphysiol.2002.019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaempf JW, Kaempf AJ, Wu Y, et al. Hyperglycemia, insulin and slower growth velocity may increase the risk of retinopathy of prematurity. J Perinatol. 2011;31:251–257. doi: 10.1038/jp.2010.152. [DOI] [PubMed] [Google Scholar]
- 45.Mohamed S, Murray JC, Dagle JM, Colaizy T. Hyperglycemia as a risk factor for the development of retinopathy of prematurity. BMC Pediatr. 2013;13:78. doi: 10.1186/1471-2431-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lemelman MB, Letourneau L, Greeley SAW. Neonatal diabetes mellitus: an update on diagnosis and management. Clin Perinatol. 2018;45:41–59. doi: 10.1016/j.clp.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Au SC, Tang SM, Rong SS, Chen LJ, Yam JC. Association between hyperglycemia and retinopathy of prematurity: a systemic review and meta-analysis. Sci Rep. 2015;5:9091. doi: 10.1038/srep09091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mohsen L, Abou-Alam M, El-Dib M, et al. A prospective study on hyperglycemia and retinopathy of prematurity. J Perinatol. 2014;34:453–457. doi: 10.1038/jp.2014.49. [DOI] [PubMed] [Google Scholar]
- 49.Singer D, Muhlfeld C. Perinatal adaptation in mammals: the impact of metabolic rate. Comp Biochem Physiol A Mol Integr Physiol. 2007;148:780–784. doi: 10.1016/j.cbpa.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 50.Sharma A, Davis A, Shekhawat PS. Hypoglycemia in the preterm neonate: etiopathogenesis, diagnosis, management and long-term outcomes. Transl Pediatr. 2017;6:335–348. doi: 10.21037/tp.2017.10.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bauer J, Werner C, Gerss J. Metabolic rate analysis of healthy preterm and full-term infants during the first weeks of life. Am J Clin Nutr. 2009;90:1517–1524. doi: 10.3945/ajcn.2009.28304. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.